Key Points
Question
Among patients with large-vessel occlusion acute ischemic stroke treated with mechanical thrombectomy for anterior circulation, is there a difference in periprocedural complications and 3-month functional outcome between those treated with general anesthesia vs procedural sedation?
Findings
In this multicenter randomized clinical trial including 273 patients, the proportion achieving absence of major periprocedural complications at 7 days and functional independence at 90 days was 28.2% with general anesthesia and 36.2% with procedural sedation, a difference that was not statistically significant.
Meaning
In patients with large-vessel occlusion acute ischemic stroke treated with anterior circulation thrombectomy, general anesthesia or procedural sedation did not affect periprocedural complications and functional outcomes.
This randomized clinical trial evaluates whether general anesthesia or procedural sedation for anterior circulation large-vessel occlusion acute ischemic stroke thrombectomy is associated with a difference in periprocedural complications and 3-month functional outcome.
Abstract
Importance
General anesthesia and procedural sedation are common practice for mechanical thrombectomy in acute ischemic stroke. However, risks and benefits of each strategy are unclear.
Objective
To determine whether general anesthesia or procedural sedation for anterior circulation large-vessel occlusion acute ischemic stroke thrombectomy are associated with a difference in periprocedural complications and 3-month functional outcome.
Design, Setting, and Participants
This open-label, blinded end point randomized clinical trial was conducted between August 2017 and February 2020, with final follow-up in May 2020, at 10 centers in France.
Adults with occlusion of the intracranial internal carotid artery and/or the proximal middle cerebral artery treated with thrombectomy were enrolled.
Interventions
Patients were assigned to receive general anesthesia with tracheal intubation (n = 135) or procedural sedation (n = 138).
Main Outcomes and Measures
The prespecified primary composite outcome was functional independence (a score of 0 to 2 on the modified Rankin Scale, which ranges from 0 [no neurologic disability] to 6 [death]) at 90 days and absence of major periprocedural complications (procedure-related serious adverse events, pneumonia, myocardial infarction, cardiogenic acute pulmonary edema, or malignant stroke) at 7 days.
Results
Among 273 patients evaluable for the primary outcome in the modified intention-to-treat population, 142 (52.0%) were women, and the mean (SD) age was 71.6 (13.8) years. The primary outcome occurred in 38 of 135 patients (28.2%) assigned to general anesthesia and in 50 of 138 patients (36.2%) assigned to procedural sedation (absolute difference, 8.1 percentage points; 95% CI, −2.3 to 19.1; P = .15). At 90 days, the rate of patients achieving functional independence was 33.3% (45 of 135) with general anesthesia and 39.1% (54 of 138) with procedural sedation (relative risk, 1.18; 95% CI, 0.86-1.61; P = .32). The rate of patients without major periprocedural complications at 7 days was 65.9% (89 of 135) with general anesthesia and 67.4% (93 of 138) with procedural sedation (relative risk, 1.02; 95% CI, 0.86-1.21; P = .80).
Conclusions and Relevance
In patients treated with mechanical thrombectomy for anterior circulation acute ischemic stroke, general anesthesia and procedural sedation were associated with similar rates of functional independence and major periprocedural complications.
Trial Registration
ClinicalTrials.gov Identifier: NCT03229148
Introduction
Acute ischemic stroke is a leading cause of mortality and long-term disability.1 Endovascular thrombectomy is a mainstay of treatment for eligible patients with anterior circulation large-vessel occlusion acute ischemic stroke and is included in international guidelines.2,3 There is uncertainty regarding the role of general anesthesia and procedural sedation on functional outcomes, and guidelines give no formal recommendation owing to sparse evidence.4 Previous studies have suggested that general anesthesia produces worsening of functional outcomes due to arterial hypotension; thus, preference is often given to using procedural sedation.5,6 Although procedural sedation may allow faster procedure initiation, there is uncertainty regarding possible risks, including aspiration pneumonia in patients with unprotected airways and procedural complications due to patient movement and agitation, which may alter reperfusion and affect outcomes, thus counterbalancing potential benefits.4,7 To our knowledge, 3 single-center randomized clinical trials and 1 multicenter randomized clinical trial have compared general anesthesia and procedural sedation, but all failed to show superiority for the primary end point.8,9,10,11 However, none of these trials specifically addressed the issue of periprocedural complications. We therefore sought to compare general anesthesia with procedural sedation for thrombectomy in patients with anterior cerebral circulation large-vessel occlusion stroke in a pragmatic multicenter randomized trial.
Methods
Trial Design and Oversight
We conducted the Anesthesia Management in Endovascular Therapy for Ischemic Stroke (AMETIS) trial, an investigator-initiated, multicenter, parallel-group, open-label randomized clinical trial with blinded outcome assessment that compared general anesthesia with procedural sedation in patients with large-vessel occlusion acute ischemic stroke at 10 university medical centers in France. Details of the rationale, study protocol, and statistical analysis plan have previously been published12 and are available in Supplement 1. Trial collaborators can be found in eAppendix 1 in Supplement 2. Centers were eligible to participate in the trial if they had experience in endovascular therapy (stent retriever and thrombus aspiration) and if the center had regular practice of general anesthesia and procedural sedation in routine clinical care to ensure timely application of the assigned procedure. Before the trial period, each center received on-site instruction to ensure compliance with the protocol, assessments, and standard of care. Enrolling centers can be found in eAppendix 2 in Supplement 2. The South-East I Committee for the Protection of Research Subjects approved the trial protocol and accepted a waiver of consent before randomization because eligible patients typically were not able to give informed consent and treatment was time critical. Patients or their next of kin were later required to give written informed consent to remain in the trial. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients
Patients were eligible to participate if they were 18 years or older, had an occlusion of the intracranial internal carotid artery and/or the proximal middle cerebral artery, and decision for thrombectomy according to international guidelines.2,3 Patients were excluded if they had coma or altered vigilance (defined as a score of 2 or more on the level of consciousness 1A subscale on the National Institutes of Health Stroke Scale [NIHSS]; scores range from 0 to 42, with higher score indicating greater neurological deficit) at presentation, or premorbid disability before enrollment (defined by a score of 2 or higher on the modified Rankin Scale [mRS]; scores range from 0 [no neurologic disability] to 6 [death]) assessed by the neurologist. All additional exclusion criteria are provided in eMethods 1 in Supplement 2.
Randomization and Masking
Eligible patients were randomly assigned in a 1:1 ratio to receive either general anesthesia with tracheal intubation or procedural sedation using a password-protected web-based randomization system and minimization algorithm stratified according to trial center, NIHSS score at presentation (15 or less vs more than 15), and intravenous thrombolysis. Because of the nature of the trial, local anesthesiologists and neuroradiologists who were caring for patients during thrombectomy had knowledge of the group assignments. All research staff members who were responsible for outcome assessments were blinded to allocation.
Interventions
In both groups, the choice of anesthetic agents and the thrombectomy technique (stent retriever and/or thrombus aspiration) followed local protocols and established guidelines.2,3 Systolic blood pressure had to be maintained between 140 and 180 mm Hg using fluids, vasopressor or antihypertensive therapy as necessary, and pulse oximetry higher than 94%.4 Patients assigned to general anesthesia were mechanically ventilated with the aim to maintain end tidal carbon dioxide between 30 and 35 mm Hg. For patients assigned to procedural sedation, the sedation goal was a Richmond Agitation-Sedation Scale (RASS; scores range from −5 [unresponsive] to 4 [combative]) of 0 to −3, which is a minimal to moderate sedation level.13,14 Crossover to general anesthesia with intubation was recommended if they had severe agitation, coma (RASS score of −4 to −5 despite stopping sedation), respiratory failure, or vomiting. In both groups, after completion of the procedure, anesthetic agents were immediately stopped. After general anesthesia, extubation was performed as early as possible. All other aspects of patient care, including admission to stroke unit or neurointensive care unit, followed local protocols.
Outcomes
The primary outcome was a composite of functional independence at 90 days (defined by a score of 0, 1, or 2 on the mRS) and absence of major periprocedural complications at 7 days. Data on the mRS were obtained through structured telephone interviews performed by a single certified trial investigator who was unaware of the group assignments (M.B.); data collection from caregivers or other proxies were permitted when patients were unable to complete the interview. Major complications were procedure-related serious adverse events (vessel perforation or dissection), pneumonia, myocardial infarction, cardiogenic acute pulmonary edema, and progression to malignant stroke.
Prespecified secondary outcomes included each component of the primary outcome; clinically important events during the procedure (defined as arterial hypotension, hypoxemia, aspiration, and conversion to general anesthesia); thrombectomy time metrics, including the time from angiosuite admission to successful revascularization (defined as a grade of 2b or 3, indicating reperfusion of more than 50% on the final intracranial angiogram,15 on the modified Thrombolysis in Cerebral Infarction scale; range of 0 to 3); stroke unit and hospital lengths of stay; unplanned intensive care unit admission by day 7; and the rate of death at 7 and 90 days. Additional details regarding secondary end points and definitions are provided in eMethods 2 in Supplement 2.
Research assistants who were independent of local medical teams obtained other data from medical records. The principal investigator, study site coinvestigators, and the statistician remained unaware of the group assignments until the database was locked for analysis.
Sample Size Calculation
We based the expected incidence of components of the primary composite outcome on results in previous trials (32.6% to 71% of patients had an mRS score of 0 to 2, and 85.4% to 99.6% were reported as not having major periprocedural complication; thus, approximately an incidence of 50% for the primary outcome).16,17,18,19,20 We then estimated that 248 patients would provide 90% power to detect an absolute between-group difference of 20 percentage points or greater in the primary outcome, with a 5% 2-sided type 1 error. The choice of 20% as expected difference in the primary end point was extrapolated from that reported in an earlier randomized clinical trial in patients undergoing endovascular thrombectomy.8 Assuming 10% of patients to be lost to follow-up, we aimed to enroll 270 patients with available data for assessment of the primary outcome.
Statistical Analysis
We analyzed data in the modified intention-to-treat population, which was prespecified as all patients who underwent randomization and had thrombectomy, except those for whom consent was withdrawn or unobtainable. We also analyzed a per-protocol population, which included patients from the modified intention-to-treat population except those with 1 or more protocol deviations.
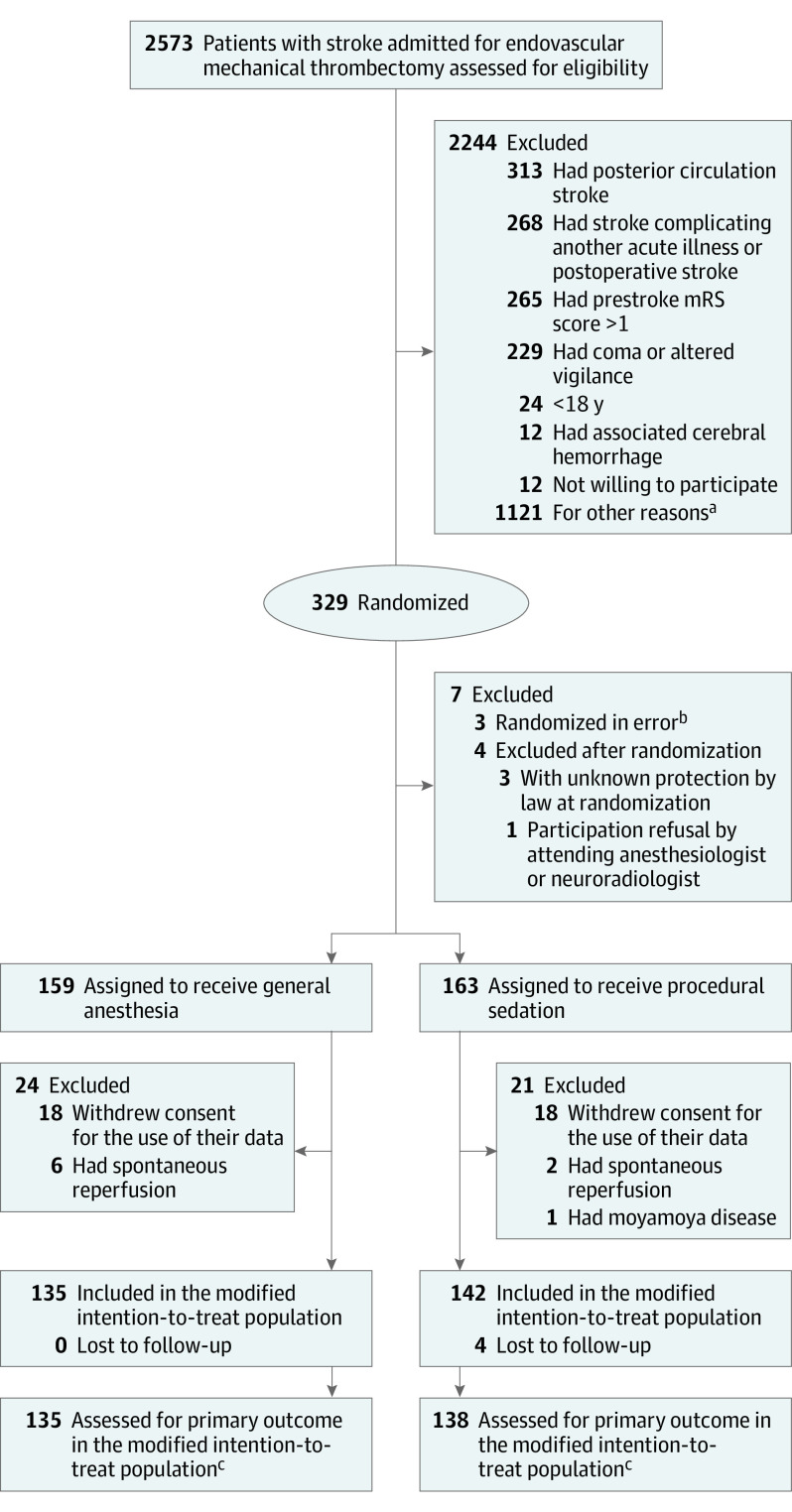
On March 7, 2019, after 270 patients had been enrolled, 44 patients were excluded (22 in each group) because they withdrew consent, had spontaneous revascularization, or were found to be ineligible. The study protocol was amended accordingly in July 2019, and 59 additional patients were randomly assigned to a study group to obtain the full sample, of which 47 (24 in the general anesthesia group and 23 in the procedural sedation group) were evaluable for assessment of the primary outcome (Figure 1).
Figure 1. Randomization and Treatment of the Patients.
mRS indicates modified Rankin Scale.
aOther reasons include anterior cerebral artery or nonaccessible distal middle cerebral artery occlusion, investigation team not available (night shifts or weekends), inclusion in other trial, or protected by law.
bThree randomization errors occurred without patient assignment due to informatic issues.
cOn March 7, 2019, after 270 patients had been enrolled, 44 patients were excluded (22 in the general anesthesia group and 22 in the procedural sedation group) because patients withdrew consent, had spontaneous revascularization, or were found to be ineligible. The study protocol was then amended accordingly in July 2019, and 59 additional patients were randomly assigned to a study group to obtain the full sample, including 47 patients (24 in the general anesthesia group and 23 in the procedural sedation group) who were evaluable for assessment of the primary outcome.
The primary outcome was compared between the 2 groups using an unadjusted χ2 test. Other binary outcomes were tested using an unadjusted χ2 or Fisher exact tests. Results are presented as absolute differences and relative risks with 95% CIs. Multiple logistic mixed regression was used to identify prespecified covariates with a known relationship to the primary outcome in addition to the stratification variables. Multicollinearity between variables was assessed by computing the variance inflation factor and using the Farrar-Glauber test. Adjusted analyses were performed with robust random-effect Poisson generalized linear mixed model regression with robust variance for binary outcomes, ordinal logistic mixed regression for mRS,21 and linear mixed regression for continuous outcomes, with center as a random effect. Time to death was compared between the 2 groups using the Kaplan-Meier method. The marginal Cox proportional hazards model was used to estimate hazard ratios and 95% CIs.
Prespecified subgroup analyses were used to evaluate the difference between randomization groups on the primary outcome. Interaction terms in the random-effects regression model were used to test for heterogeneity of effect between subgroups.
Complete case analysis was performed for all outcomes. We did not compensate for dropouts. A 2-sided P value less than .05 was considered for statistical significance. No correction for multiple testing was applied in the analysis of secondary outcomes, except for the components of the composite primary end point. All analyses were generated with Stata version 15.0 (StataCorp).
Results
Patients
From August 31, 2017, through February 8, 2020, a total of 2573 patients were assessed for eligibility; a total of 329 patients were enrolled. Of these, 273 patients met the criteria for inclusion in the modified intention-to-treat population (135 assigned to general anesthesia and 138 assigned to procedural sedation) (Figure 1).
The demographic and baseline characteristics of the patients were well balanced in the treatment groups (Table 1; eTable 1 in Supplement 2). Among 273 patients evaluable for the primary outcome in the modified intention-to-treat population, 142 (52.0%) were women, and the mean (SD) age was 71.6 (13.8) years. The median (IQR) NIHSS score of the population was 16 (11-20). The percentage of patients who received intravenous thrombolysis and the time from stroke onset to angiosuite admission were similar in both groups.
Table 1. Baseline Patient and Procedure Characteristics in the Modified Intention-to-Treat Population.
| Characteristic | No. (%) | |
|---|---|---|
| General anesthesia (n = 135) | Procedural sedation (n = 138) | |
| Age, mean (SD), y | 72.0 (13.2) | 71.3 (14.4) |
| Sex | ||
| Female | 70 (51.9) | 72 (52.2) |
| Male | 65 (48.1) | 66 (47.8) |
| NIHSS score | ||
| Median (IQR)a | 16 (11-20) | 15 (11-20) |
| >15b | 70 (51.9) | 68 (49.3) |
| Intravenous thrombolysisb | 62 (45.9) | 70 (50.7) |
| Transfer from another hospitalc | 69 (51.1) | 63 (45.7) |
| Wake-up or unwitnessed stroked | 46 (34.0) | 40 (29.0) |
| Left hemisphere stroke | 66 (48.9) | 74 (53.6) |
| Prestroke disabilitye | 23 (17.6) | 14 (10.5) |
| ASPECTS score, median (IQR)f | 8 (7-9) | 8 (7-9) |
| Location of artery occlusion | ||
| Intracranial internal carotid artery | 18 (13.3) | 28 (20.3) |
| Middle cerebral artery M1 segment | 86 (63.7) | 84 (60.9) |
| Middle cerebral artery M2 segment | 31 (23.0) | 26 (18.8) |
| Tandem lesiong | 23 (18.0) | 23 (17.2) |
| Duration, median (IQR), min | ||
| From angiosuite to groin punctureh | 11 (8-18) | 9 (4-15) |
| From groin puncture to reperfusioni | 35 (25-58) | 41 (24-62) |
| From angiosuite to reperfusionj | 50 (35-72) | 48 (32-72) |
| From angiosuite to end of procedurek | 61 (41-90) | 60 (38-87) |
| Successful reperfusionl | 115 (85.2) | 107 (77.6) |
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomography Score; mTICI, modified Thrombolysis in Cerebral Infarction; NIHSS, National Institutes of Health Stroke Scale.
Scores on the NIHSS range from 0 to 42, with higher scores indicating a more severe deficit.
Stratification variable.
Transfer from another hospital to stroke centers for thrombectomy.
Wake-up or unwitnessed strokes are strokes of unknown time onset.
Prestroke disability was defined as a prestroke score on the modified Rankin Scale of 2 or higher. Scores on the modified Rankin Scale range from 0 (no neurologic disability) to 6 (death), with higher scores indicating more severe functional disability. Data were obtained during follow-up from the patient or next-of-kin interview. Data were available for 131 patients in the general anesthesia group and 134 patients in the procedural sedation group.
The ASPECTS is an imaging measure of the extent of ischemic stroke. Scores range from 0 to 10, with higher scores indicating a smaller infarct core. Magnetic resonance imaging ASPECTS were calculated if this was the only qualifying modality. Baseline scores were available for 127 patients in the general anesthesia group and 126 patients in the procedural sedation group.
Tandem lesion means association of an extracranial internal carotid artery pathology (ipsilateral significant stenosis or occlusion) and intracerebral anterior circulation large-vessel occlusion.
Data were missing for 3 patients in the general anesthesia group and 5 patients in the procedural sedation group.
Data on the duration from groin puncture to reperfusion (if any) were available for 120 of 123 patients with any reperfusion (mTICI of 1 to 3) in the general anesthesia group and 121 of 126 patients with any reperfusion (mTICI of 1 to 3) in the procedural sedation group.
Reperfusion was assessed using the mTICI reperfusion score ranging from 0 (no perfusion) to 3 (full perfusion with filling of all distal branches). Data were missing for 1 patient in the procedural sedation group.
Data were missing for 3 patients in the procedural sedation group.
Successful reperfusion was defined as an mTICI score of 2b or 3, indicating reperfusion of more than 50% of the affected territory.
The median (IQR) time from angiosuite admission to groin puncture was 11 (8-18) minutes in the general anesthesia group and 9 (4-15) minutes in the procedural sedation group; the median (IQR) time from angiosuite admission to reperfusion was 50 (35-72) minutes and 48 (32-72) minutes, respectively. Successful reperfusion was achieved in 115 patients (85.2%) assigned to general anesthesia and 107 patients (77.6%) assigned to procedural sedation (relative risk, 0.91; 95% CI, 0.81-1.02; P = .11). Radiological intervention characteristics are described in eTable 2 in Supplement 2. General anesthesia and procedural sedation characteristics are described in eTable 3 in Supplement 2.
Primary Outcome
The primary composite outcome of functional independence at 90 days and absence of any major periprocedural complication occurring by day 7 was attained by 38 patients (28.2%) assigned to general anesthesia and by 50 (36.2%) assigned to procedural sedation (absolute difference, 8.1 percentage points; 95% CI, −2.3 to 19.1; relative risk, 1.29; 95% CI, 0.91-1.82; P = .15) (Table 2).
Table 2. Efficacy and Safety Outcomes in the Modified Intention-to-Treat Population.
| Outcome | No. (%) | Standardized difference (95% CI) | P value | ||
|---|---|---|---|---|---|
| General anesthesia (n = 135) | Procedural sedation (n = 138) | Absolute difference | Relative risk | ||
| Primary outcome | |||||
| Primary composite outcome | 38 (28.2) | 50 (36.2) | 8.1 (−2.3 to 19.1) | 1.29 (0.91 to 1.82) | .15 |
| Components of primary outcomea | |||||
| Functional independence at 90 db | 45 (33.3) | 54 (39.1) | 6 (−6 to 17) | 1.18 (0.86 to 1.61) | .32 |
| Absence of any major periprocedural complications at 7 d | 89 (65.9) | 93 (67.4) | 1 (−10 to 13) | 1.02 (0.86 to 1.21) | .80 |
| Secondary outcomes | |||||
| Procedure-related serious adverse eventsc | 9 (6.7) | 5 (3.6) | −3 (−8.2 to 2.1) | 0.54 (0.19 to 1.58) | .26 |
| Pneumonia | 26 (19.3) | 28 (20.4) | 1 (−8 to 11) | 1.06 (0.66 to 1.71) | .81 |
| Myocardial infarction | 0 | 1 (0.7) | 0 (−1 to 2) | NA | .32 |
| Cardiogenic pulmonary edema | 11 (8.2) | 18 (13.0) | 5 (−2 to 12) | 1.60 (0.79 to 3.26) | .19 |
| Progression to malignant stroke | 11 (8.2) | 6 (4.4) | −4 (−10 to 2) | 0.53 (0.20 to 1.40) | .19 |
| Ordinal score on the mRS at 90 d, median (IQR) | 3 (2-5) | 3 (2-4) | 0 (−1 to 1) | 0.80 (0.53 to 1.22) | .34 |
| Recovery at 90 d | |||||
| Excellentd | 20 (14.8) | 26 (18.8) | 4 (−5 to 13) | 1.27 (0.75 to 2.17) | .37 |
| Goode | 31 (23.0) | 36 (26.1) | 3 (−7 to 13) | 1.14 (0.75 to 1.72) | .55 |
| Moderatef | 79 (58.5) | 88 (63.8) | 5 (−6 to 17) | 1.09 (0.90 to 1.32) | .37 |
| Score on utility-weighted modified Rankin scale, mean (SD)g | 4.9 (3.5) | 5.4 (3.4) | 0.5 (−0.3 to 1.3) | 0.5 (−0.3 to 1.3) | .24 |
| Symptomatic intracranial hemorrhage | 21 (15.6) | 20 (14.5) | −1 (−10 to 7) | 0.93 (0.53 to 1.64) | .81 |
| Death | |||||
| At 7 d | 12 (8.9) | 12 (8.7) | 0 (−7 to 7) | 0.99 (0.46 to 2.10) | .96 |
| At 90 d | 25 (18.5) | 23 (16.7) | −2 (−11 to 7) | 0.90 (0.54 to 1.51) | .69 |
| NIHSS score, median (IQR) | |||||
| At day 1h | 9 (3-19) | 8 (3-17) | −1 (−4 to 2) | −1 (−4 to 2) | .47 |
| At day 7i | 3 (0-10) | 3 (1-10) | 0 (−2 to 2) | 0 (−2 to 2) | .85 |
| Duration of stay, median (IQR), d | |||||
| In stroke unitj | 4 (2-7) | 4 (2-6) | 0 (−1 to 1) | 0 (−1 to 1) | .44 |
| In hospitalk | 13 (8-21) | 11 (7-18) | −2 (−5 to 1) | −2 (−5 to 1) | .09 |
| Clinically important events | |||||
| Hypotensionl | 118 (87.4) | 62 (44.9) | −42 (−52 to −32) | 0.51 (0.42 to 0.63) | <.001 |
| Hypoxemiam | 7 (5.2) | 13 (9.4) | 4 (−2 to 10) | 1.82 (0.75 to 4.41) | .19 |
| Severe hypoxemian | 3 (2.2) | 4 (2.9) | 0 (−3 to 4) | 1.30 (0.30 to 5.72) | .72 |
| Aspiration | 1 (0.7) | 3 (2.2) | 1 (−1 to 4) | 2.93 (0.31 to 27.86) | .32 |
| Conversion to general anesthesia | NA | 15 (10.9) | NA | NA | NA |
Abbreviations: mRS, modified Rankin Scale; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale.
P values and 95% CIs were corrected with Hochberg type I error inflation.
Functional independence at 90 days was defined as an mRS score of 0 to 2.
Procedure-related serious adverse events were arterial dissection or perforation.
Excellent recovery at 90 days defined as an mRS score of 0 to 1.
Good recovery defined with sliding dichotomy responder analysis relating day 90 mRS with baseline NIHSS score: an mRS score of 0 for NIHSS scores of 7 or less, mRS scores of 0 to 1 for NIHSS scores of 8 to 14, and mRS scores of 0 to 2 for NIHSS scores greater than 14.
Moderate recovery at 90 days defined as an mRS score of 0 to 3.
The utility-weighted mRS scores range from 0 (death) to 10 (no symptoms or disability). The following weights are assigned to scores 0 through 6 on the mRS: 10.0, 9.1, 7.6, 6.5, 3.3, 0, and 0, respectively.
Data were missing for 3 patients in the general anesthesia group and 8 patients in the procedural sedation group.
Data were missing for 28 patients in the general anesthesia group and 25 patients in the procedural sedation group.
Data were missing for 1 patient in the procedural sedation group.
Data were missing for 3 patients in the general anesthesia group.
Hypotension was defined as 1 episode of systolic blood pressure of 120 mm Hg or less during the prespecified time points of blood pressure measurement during mechanical thrombectomy.
Hypoxemia was defined as 1 episode of pulsatile oximetry of 92% or less during the prespecified time points of pulsatile oximetry measurement during mechanical thrombectomy.
Severe hypoxemia was defined as pulsatile oximetry of 88% or less during the prespecified time points of pulsatile oximetry measurement during mechanical thrombectomy.
Secondary and Safety Outcomes
Predefined secondary and safety outcomes are presented in Table 2. The rates of functional independence at 90 days were 45 of 135 patients (33.3%) in the general anesthesia group and 54 of 138 patients (39.1%) in the procedural sedation group (relative risk, 1.18; 95% CI, 0.86-1.61; P = .32) (Figure 2); the rates of patients without major periprocedural complications were 89 of 135 patients (65.9%) and 93 of 138 patients (67.4%), respectively (relative risk, 1.02; 95% CI, 0.86-1.21; P = .80). There was a significant relationship between major periprocedural complications and functional independence at 90 days (odds ratio, 6.81; 95% CI, 3.40-13.60; P < .001).
Figure 2. Distribution of Functional Outcomes at 90 Days in the Modified Intention-to-Treat Population.
Shown is the distribution of scores for disability on the modified Rankin Scale (mRS) among patients in the general anesthesia group and the procedural sedation group in the modified intention-to-treat population (A) and in subgroups defined according to major periprocedural complications (B). Scores for disability range from 0 to 6, with 0 indicating no neurologic deficit; 1, no clinically significant disability; 2, slight disability; 3, moderate disability requiring some help; 4, moderately severe disability; 5, severe disability; and 6, death. Functional independence was defined as an mRS score of 0, 1, or 2. Major periprocedural complications were procedure-related serious adverse events (vessel perforation or dissection), pneumonia, myocardial infarction, cardiogenic acute pulmonary edema, or progression to malignant stroke within 7 days after endovascular thrombectomy. There was significant heterogeneity according to the presence or absence of major periprocedural complications and functional independence at 90 days (P for interaction < .001).
The percentage of patients with hypotension was 87.4% (118 of 135) in the general anesthesia group and 44.9% (62 of 138) in the procedural sedation group (P < .001). Fifteen patients (10.9%) assigned to procedural sedation crossed over to general anesthesia. Death within 90 days occurred in 48 of 273 patients (17.6%) in the trial population, including 25 in the general anesthesia group and 23 in the procedural sedation group (eFigure in Supplement 2).
The primary, secondary and safety outcomes were unaffected after adjustment for stratification variables and covariates (eTables 4 to 6 in Supplement 2). Analyses of the primary and secondary outcomes in the per-protocol population showed similar results to those in the modified intention-to-treat population (eTables 7 and 8 in Supplement 2).
Subgroup Analyses
Subgroup analyses for the primary end point are presented in Figure 3. There was evidence of a differential effect among patients older than or younger than 70 years.
Figure 3. Prespecified Subgroup Analyses of the Primary Outcome in the Modified Intention-to-Treat Population.

The forest plot shows the risk ratio for the primary outcome (functional independence at 90 days and absence of major periprocedural complications at 7 days after endovascular thrombectomy) in 10 prespecified subgroups. ASPECTS indicates Alberta Stroke Program Early Computed Tomography Score; NIHSS, National Institutes of Health Stroke Scale.
Discussion
In patients with anterior circulation large-vessel occlusion acute ischemic stroke who were eligible for thrombectomy, outcomes for functional independence at 90 days and the absence of major periprocedural complications at 7 days were similar between those who received general anesthesia and procedural sedation.
The proportion of patients with functional independence at 90 days that was observed in our trial was comparable with that of previous randomized trials of thrombectomy (36.2%)16,21,22 but was lower than that reported in a pooled patient-level analysis that aggregated a total of 368 patients from 3 previous single-center randomized trials of general anesthesia vs procedural sedation for thrombectomy (42.2%).23 However, functional independence was the primary outcome in only one of these trials. Although recommended by current guidelines,2,3 it is possible that the worse outcomes in our trial compared with previous randomized trials were related to the high proportion of patients (41% in our trial) enrolled beyond the 6-hour window from stroke onset or had an unknown time of onset (wake-up or unwitnessed stroke). Moreover, despite this reflects practice under real-life conditions, 48% of the patients in our trial were transferred from another hospital, which may have contributed to a longer time from stroke onset to treatment initiation.
A particular feature of this trial, although unusual in mechanical thrombectomy trials, was the use of a primary outcome that was a composite of functional independence and major periprocedural complications rather than functional outcome on the mRS. A potential risk of procedural sedation is arterial perforation or dissection due to patient movement. In the current trial, procedure-related serious adverse events occurred in a similar proportion of patients who received general anesthesia or procedural sedation and was similar to that observed in previous trials.9,11,19,24 Unprotected airways in patients with impaired consciousness may promote aspiration pneumonia,25 which is associated with worse outcomes.26 The rates of patients who had pneumonia in our trial were similar to that observed in previous trials (18.6% vs 19.5%, respectively)23 and did not differ between those who received general anesthesia and procedural sedation. However, although major procedure-related adverse events and periprocedural complications may influence successful reperfusion or contribute to the worsening of stroke, the relative importance of each component on long-term neurologic outcome may be different.
Intraprocedural arterial hypotension has been repeatedly associated with altered neurologic outcomes following acute ischemic stroke27; however, the optimal blood pressure levels during endovascular therapy remains unknown. A complicating factor in the interpretation of the association between blood pressure deficits and outcome is the heterogeneity of hypotension definitions in previous studies, making cross-study comparisons difficult. Given the risk of arterial hypotension, investigators have questioned the use of general anesthesia for thrombectomy. The definition of intraprocedural hypotension in our trial was supported by literature.8 The results of our trial that the rate of hypotension with general anesthesia was twice as high as that with procedural sedation corroborate those of the recent General Anesthesia vs Sedation for Acute Stroke Treatment (GASS) trial.11 In that trial, the authors used a standardized protocol for blood pressure management; however, the cumulative duration of hypotension and rates of functional independence were similar between general anesthesia and sedation. The current trial showed a potential disadvantage of general anesthesia in the subgroup of patients 70 years or older, a patient population with a higher risk of significant hypotension and adverse postoperative outcomes.28
An individual data analysis of 3 previous single-center randomized trials reported more functional independence at 3 months with general anesthesia than sedation (mRS score of 0 to 2 at 90 days: 49.2% vs 35.1%).23 One possible explanation for why the findings of the current trial differ may be the inclusion of patients with higher NIHSS scores in these trials, indicating greater neurological deficit or altered consciousness in some patients that can have had benefit from tracheal intubation and airway protection. Other potential explanations include the infrequent use of procedural sedation before study inception, which may have conferred potential advantage to general anesthesia, and single-center designs. Importantly, functional independence was the primary outcome in only 1 of 3 trials.
The strengths of our trial include the variety of stroke centers, reflecting different practice patterns, and the pragmatic protocol that aimed at maintaining routine practice except for the use of general anesthesia or procedural sedation. On-site or online specific training was not provided before or during the trial period. Sites embedded the trial within routine clinical care due to simple interventions consistent with local protocols.
Limitations
Limitations must also be considered. First, our trial was intended to be pragmatic, and the anesthesia protocol was based on individual clinician expertise rather than guided by a standardized protocol. Although this reflects clinical practice, it allowed for potential differences in dose adjustments and adverse events. However, this is unlikely to have affected the results, as illustrated in the GASS trial.11 Second, although study investigators were asked to maintain systolic blood pressure between 140 and 180 mm Hg, substantial variability in blood pressure control across study sites cannot be excluded. However, such variability is difficult to prevent in multicenter trials, especially in an emergency context. Third, eligibility for thrombectomy was established by the neurologists and neuroradiologists at the trial sites with possible inclusion of patients with prestroke disability or perioperative stroke that may have influenced outcomes. However, the per-protocol analysis that excluded these patients found similar results. Fourth, the relatively small sample size may have caused potential between-group differences to be missed. Fifth, the observed between-group effect was lower than the anticipated absolute risk reduction of 20 percentage points. The expected effect size used for power calculation was consistent with that reported in an earlier randomized clinical trial8 and recent findings of a pooled patient-level analysis23 but could have been overestimated. Thus, the trial may not be powered enough to detect small but clinically important treatment effects for the comparison of general anesthesia with procedural sedation. With a 28% rate in the general anesthesia group and 36% in the sedation group (ie, rate difference of 8%), a total of 1476 patients would have been needed to detect this difference between the 2 groups with a 5% 2-sided type 1 error and 90% power. Sixth, generalizability to populations not included in the trial, such as those with higher NIHSS scores or to centers without experience with general anesthesia or procedural sedation for mechanical thrombectomy, remains to be evaluated. Seventh, thrombectomy has been available for certain patients who were not included in our trial, which may have introduced a selection bias.
Conclusions
In conclusion, our trial showed that among patients with anterior circulation large-vessel occlusion acute ischemic stroke, general anesthesia and procedural sedation for mechanical thrombectomy were associated with similar rates of functional independence and major periprocedural complications.
Trial Protocol
eAppendix 1. AMETIS Trial Collaborators
eAppendix 2. Enrolling Centers and Patient Recruitment
eMethods 1. Inclusion and Exclusion Criteria
eMethods 2. Prespecified Secondary Outcome Measures
eFigure. Kaplan-Meier 90-Day Mortality Curve
eTable 1. Baseline Patient and Stroke Characteristics
eTable 2. Radiological Intervention Characteristics
eTable 3. General Anesthesia and Procedural Sedation Characteristics
eTable 4. Univariable and Multivariable Analysis of Factors Associated With the Primary Outcome
eTable 5. Unadjusted and Adjusted Efficacy Outcomes
eTable 6. unadjusted and Adjusted Safety Variables
eTable 7. Per-Protocol Baseline Patient and Stroke Characteristics
eTable 8. Per-Protocol Efficacy Analysis
eReferences.
Group Information. The ANARLF Network and the AMETIS Study Group
Data Sharing Statement
References
- 1.Mendelson SJ, Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review. JAMA. 2021;325(11):1088-1098. doi: 10.1001/jama.2020.26867 [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 3.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)–European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke: endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talke PO, Sharma D, Heyer EJ, Bergese SD, Blackham KA, Stevens RD. Republished: Society for Neuroscience in Anesthesiology and Critical Care expert consensus statement: anesthetic management of endovascular treatment for acute ischemic stroke. Stroke. 2014;45(8):e138-e150. doi: 10.1161/STROKEAHA.113.003412 [DOI] [PubMed] [Google Scholar]
- 5.Wijayatilake DS, Ratnayake G, Ragavan D. Anaesthesia for neuroradiology: thrombectomy: ‘one small step for man, one giant leap for anaesthesia’. Curr Opin Anaesthesiol. 2016;29(5):568-575. doi: 10.1097/ACO.0000000000000377 [DOI] [PubMed] [Google Scholar]
- 6.Campbell BCV, van Zwam WH, Goyal M, et al. ; HERMES collaborators . Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. 2018;17(1):47-53. doi: 10.1016/S1474-4422(17)30407-6 [DOI] [PubMed] [Google Scholar]
- 7.Goldhoorn RB, Bernsen MLE, Hofmeijer J, et al. ; MR CLEAN Registry Investigators . Anesthetic management during endovascular treatment of acute ischemic stroke in the MR CLEAN Registry. Neurology. 2020;94(1):e97-e106. doi: 10.1212/WNL.0000000000008674 [DOI] [PubMed] [Google Scholar]
- 8.Schönenberger S, Uhlmann L, Hacke W, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316(19):1986-1996. doi: 10.1001/jama.2016.16623 [DOI] [PubMed] [Google Scholar]
- 9.Löwhagen Hendén P, Rentzos A, Karlsson JE, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke Trial (Anesthesia During Stroke). Stroke. 2017;48(6):1601-1607. doi: 10.1161/STROKEAHA.117.016554 [DOI] [PubMed] [Google Scholar]
- 10.Simonsen CZ, Yoo AJ, Sørensen LH, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018;75(4):470-477. doi: 10.1001/jamaneurol.2017.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurice A, Eugène F, Ronzière T, et al. ; GASS (General Anesthesia versus Sedation for Acute Stroke Treatment) Study Group and the French Society of Anesthesiologists (SFAR) Research Network . General anesthesia versus sedation, both with hemodynamic control, during intraarterial treatment for stroke: the GASS randomized trial. Anesthesiology. 2022;136(4):567-576. doi: 10.1097/ALN.0000000000004142 [DOI] [PubMed] [Google Scholar]
- 12.Chabanne R, Fernandez-Canal C, Degos V, et al. ; ANARLF Network and the AMETIS study group . Sedation versus general anaesthesia in endovascular therapy for anterior circulation acute ischaemic stroke: the multicentre randomised controlled AMETIS trial study protocol. BMJ Open. 2019;9(9):e027561. doi: 10.1136/bmjopen-2018-027561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. doi: 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 14.Chanques G, Jaber S, Barbotte E, et al. Validation of the French translated Richmond Vigilance-Agitation Scale. Ann Fr Anesth Reanim. 2006;25(7):696-701. doi: 10.1016/j.annfar.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 15.Zaidat OO, Yoo AJ, Khatri P, et al. ; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization working group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650-2663. doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 17.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 18.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 19.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 20.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 21.Martins SO, Mont’Alverne F, Rebello LC, et al. ; RESILIENT Investigators . Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. 2020;382(24):2316-2326. doi: 10.1056/NEJMoa2000120 [DOI] [PubMed] [Google Scholar]
- 22.Yang P, Zhang Y, Zhang L, et al. ; DIRECT-MT Investigators . Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi: 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 23.Schönenberger S, Hendén PL, Simonsen CZ, et al. Association of general anesthesia vs procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA. 2019;322(13):1283-1293. doi: 10.1001/jama.2019.11455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 25.Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med. 2019;380(7):651-663. doi: 10.1056/NEJMra1714562 [DOI] [PubMed] [Google Scholar]
- 26.Hannawi Y, Hannawi B, Rao CPV, Suarez JI, Bershad EM. Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc Dis. 2013;35(5):430-443. doi: 10.1159/000350199 [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen M, Schönenberger S, Hendèn PL, et al. ; SAGA collaborators . Blood pressure thresholds and neurologic outcomes after endovascular therapy for acute ischemic stroke: an analysis of individual patient data from 3 randomized clinical trials. JAMA Neurol. 2020;77(5):622-631. doi: 10.1001/jamaneurol.2019.4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devereaux PJ, Yang H, Yusuf S, et al. ; POISE Study Group . Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847. doi: 10.1016/S0140-6736(08)60601-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. AMETIS Trial Collaborators
eAppendix 2. Enrolling Centers and Patient Recruitment
eMethods 1. Inclusion and Exclusion Criteria
eMethods 2. Prespecified Secondary Outcome Measures
eFigure. Kaplan-Meier 90-Day Mortality Curve
eTable 1. Baseline Patient and Stroke Characteristics
eTable 2. Radiological Intervention Characteristics
eTable 3. General Anesthesia and Procedural Sedation Characteristics
eTable 4. Univariable and Multivariable Analysis of Factors Associated With the Primary Outcome
eTable 5. Unadjusted and Adjusted Efficacy Outcomes
eTable 6. unadjusted and Adjusted Safety Variables
eTable 7. Per-Protocol Baseline Patient and Stroke Characteristics
eTable 8. Per-Protocol Efficacy Analysis
eReferences.
Group Information. The ANARLF Network and the AMETIS Study Group
Data Sharing Statement