Abstract
Cytokine storm syndromes (CSS) are potentially fatal hyperinflammatory states that share the underpinnings of persistent immune cell activation and uninhibited cytokine production. CSS can be genetically determined by inborn errors of immunity (i.e. familial hemophagocytic lymphohistiocytosis) or develop as a complication of infections, chronic inflammatory diseases (e.g. Still disease), or malignancies (e.g. T-cell lymphoma). Therapeutic interventions that activate the immune system such as chimeric antigen receptor T cell therapy and immune checkpoint inhibition can also trigger CSS in the setting of cancer treatment. In this review, the biology of different types of CSS are explored, and the current knowledge on the involvement of immune pathways and the contribution of host genetics are discussed. The use of animal models to study CSS is reviewed, and their relevance for human diseases is discussed. Lastly, treatment approaches for CSS are discussed with a focus on interventions that target immune cells and cytokines.
Introduction
There is nothing quite as powerful as a worldwide fatal pandemic to bring increased attention to cytokine storm syndromes (CSS) (1). From a lumper’s perspective, CSS have garnered attention from a vast variety of biomedical disciplines. CSS, including hemophagocytic lymophohistiocytosis (HLH), macrophage activation syndrome (MAS), and cytokine release syndrome (CRS), are frequently fatal end common pathways of an overly activated immune response to variety of triggers, from intracellular pathogens to hematologic malignancies to autoimmune and autoinflammatory diseases, and beyond (2). Over the last 2.5 decades, the immunology (3) and genetics (4) of CSS have been better defined using both murine models and human studies. While genetic defects in perforin-mediated cytolysis is perhaps the best studied and most common pathway resulting in CSS, defects in the inflammasome, and other pathways are being explored and better defined (5). Because of its broad clinical implications and highly translational immunology, CSS should be considered an integral aspect of even undergraduate immunology education curricula (6). Herein, the current state of the art understanding of CSS immunopathogenesis (studies of mice and humans), and the practical implications for effective therapeutics, will be presented (7). The heroic investigations of basic science immunologists, clinicians, and clinician-scientists over the last half century have championed the appropriate attention and scientific exploration to bare on this fascinating but deadly hyperinflammatory host immune response (8).
Clinical features of CSS
A hyperinflammatory immune response is at the core of CSS. No matter what the trigger or underlying condition, there are shared features among those suffering CSS. Broadly speaking, CSS is an overly exuberant immune response to a trigger frequently in the setting of a prior state of chronic inflammation and/or with an underlying genetic predisposition. Shared among CSS of multiple etiologies are clinical features secondary to the excess pro-inflammatory insults. Patients with CSS typically exhibit prolonged high fevers, cytopenias, coagulopathy, liver dysfunction, and central nervous system derangement (9). Associated laboratory and pathologic findings include hyper-ferritinemia, elevated soluble IL-2 receptor alpha (sCD25), elevated soluble haptoglobin receptor (sCD163), increased markers of inflammation (e.g. C-reactive protein), hyper-cytokinemia (e.g. IFN-γ, IL-1β, IL-6, IL-18), liver inflammation, and hemophagocytosis (5). Many of the clinical and pathologic features are utilized in various CSS diagnostic and classification criteria (10). There is no perfect set of diagnostic criteria for the various CSS of different etiologies, including HLH (11), MAS (12, 13), and even the CSS associated with severe coronavirus disease 2019 (COVID-19) (14). Broader, more disease inclusive, criteria have been proposed, but are dependent on data (e.g. hemophagocytosis) that are not always available (15). In addition, because of the severity and rapid disease progression, simplified criteria based on serum ferritin and erythrocyte sedimentation rate can be employed as timely simple screens for CSS (16).
For the purpose of this review, familial or primary HLH is defined by biallelic pathogenic variants in genes involved in the cytolytic function of T lymphocytes and natural killer cells (see section on Host genetics). MAS, also called secondary HLH by some subspecialities, is broadly defined as CSS associated with external triggers including infections, malignancies, and inflammatory diseases.
Broad array of CSS associated conditions
The importance of CSS cannot be understated, and it is likely under-recognized in children and adults across the globe (17). Whereas familial HLH is rare (1 in 50,000 live births), secondary or acquired forms of CSS may affect up to 1 in 3,000, or more, children and adults (18). Infectious agents, largely intracellular pathogens, contribute to a large percentage of CSS (Table I). The herpes virus family member is most notorious, particularly Epstein-Barr virus (EBV) and cytomegalovirus (CMV), but over 100 organisms have been reported to trigger CSS (7). Other CSS triggering pathogens include pandemic strains of influenza (19) and hemorrhagic fever viruses like dengue (20). Lassa fever, a hemorrhagic fever syndrome, is caused by an arenavirus, as is lymphocytic choriomeningitis virus (LCMV), which triggers HLH in susceptible mouse strains (21). Infections often trigger CSS in patients with underlying inflammation (e.g. rheumatic or oncologic illness), immune suppression (e.g. HIV-1/AIDS), or various genetic predispositions (e.g. familial HLH).
Table 1.
Diseases under the cytokine storm syndrome umbrella
| Cause of CSS | Example | Notable features |
|---|---|---|
| Genetic | ||
| Familial HLH | Homozygous PRF1 deficiency | Approximately half of familial HLH in North America |
| Secondary HLH | Dominant-negative STXBP2 mutation | Early-onset; often triggered by viral infection |
| Autoinflammatory | NLRC4 activating mutation | Associated with colitis |
| Primary immunodeficiency | X-linked lymphoproliferative disease (XLP1/2) | EBV-induced |
| Metabolic | Lysinic protein intolerance (SLC7A7 mutation) | Splenomegaly |
| Systemic illness | ||
| Chronic inflammation | MAS in systemic juvenile idiopathic arthritis | Rash, arthritis |
| Hematologic malignancy | T cell leukemia | Poor outcome |
| Lymphoproliferative disorder | Multicentric Castleman disease | HHV8 association |
| Infectious disease | ||
| Sepsis and septic shock | bacterial, viral and fungal pathogens | Poor NK cell function |
| Herpes virus family | Epstein-Barr virus | High mortality |
| Influenza | H1N1 | HLH gene mutations |
| Hemorrhagic fever virus | Dengue | Extreme hyperferritinemia |
| SARS-CoV-2 | COVID-19-associated ARDS and MIS-C | Severe pneumonia in ARDS; myocarditis in MIS-C |
| Other associations | ||
| CAR-T cell therapy | Cytokine release syndrome | Frequent central nervous system involvement |
| Immune dysregulation | Pregnancy | Infectious triggers common |
| Medications | Anti-CD28 monoclonal antibody | Multiple cytokine release |
Abbreviations: ARDS, acute respiratory distress syndrome; CAR-T; chimeric antigen receptor T-cell; COVID-19, coronavirus disease of 2019; EBV, Epstein-Barr virus; HHV8, human herpesvirus-8; H1N1, hemagglutinin-1 neuraminidase-1; HLH, hemophagocytic lymphohistiocytosis; MAS, macrophage activation syndrome; MIS-C, multisystem inflammatory syndrome in children; NK, natural killer; NLRC4, NOD-like receptor family CARD domain containing 4; PRF1, perforin-1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SLC7A7, solute carrier family 7 member 7 ; STXBP2, syntaxin binding protein-2.
Although a large number of autoimmune conditions have been associated with CSS/MAS, systemic lupus erythematosus (SLE) and Still disease (juvenile and adult) are most notorious (Table I). The pathogenesis of CSS in SLE is likely multi-factorial, potentially involving underlying cytopenias, increased type I IFN, and TLR triggering by self-nucleic acids, but it remains largely unknown (22). However, CSS etiology in Still disease is better understood with massive IL-18 (an IL-1 family member activated by caspase following inflammasome activation) levels integral to disease pathophysiology (23). In addition, defects in NK cell cytolysis (24) with contributing HLH associated gene defects (25) reveals some shared pathology with familial HLH. Indeed, a recent murine model combining excess IL-18 production with perforin deficiency were synergistic in causing spontaneous CSS with CD8+ T cell expansion (26). Since IL-18 in combination with IL-12 triggers IFN-γ production, this hyper-inflammatory state was responsive to IFN-γ blockade (26).
IFN-γ excess production is also associated with CSS in the setting of hematologic malignancies (Table I) (27), as is IL-6 over-production. Not only can the underlying malignancy contribute to CSS by excess IL-6 production, iatrogenic cytokine release syndrome (CRS) can result from novel therapeutics (e.g. CAR-T cell therapy and immune checkpoint inhibitors) used to treat refractory leukemias and lymphomas (Table I). CRS is also characterized by excess IL-6 and IL-1 expression, and targeted cytokine approaches in humans and murine models have proven effective in treating CRS (28-30). Solid organ malignancies can also trigger a CSS but much less frequently than malignancies of blood forming tissues (31-33).
Other less common, or perhaps less well documented, triggers of CSS are diverse in origin. Conditions/triggers associated with CSS range from cardiac bypass circuits (34, 35), to pregnancy (36), to drug-induced (37), to graft versus host disease and post-transplantation (35, 38, 39), to Castleman disease (40), to metabolic disorders (41) (Table I). However, the most well studied conditions associated with CSS/HLH are primary or familial forms of HLH (42). Familial HLH is a term often restricted to genetic defects in the perforin pathway of lymphocyte mediated cytolysis (Table I). This includes autosomal recessive mutations in PRF1, STX11, STXBP2, and UNC13D (4, 5, 42). However, homozygous defects in genes (RAB27A, LYST, AP3B1) responsible for syndromes characterized by albinism and neutrophil dysfunction (Griscelli type 2, Chediak-Higashi, Hermansky-Pudlak type 2) that are important for intracellular granule (containing melanin as well as those with perforin) function are also linked to primary HLH (4, 5). Other primary genetic contributions to CSS/HLH are defects in X-linked lymphoproliferative (XLP1 and XLP2) genes, which are associated with EBV triggering (5). Additional primary immunodeficiencies associated with CSS are individually rare, but the list of genes with defects associated with CSS is rapidly evolving (43). This includes genes important for viral control (e.g. ITK), lymphocyte activation (e.g. PIK3CD), and inflammasome activity (e.g. NLRC4, CDC42) (4, 5). Finally, gene defects resulting in inborn errors of metabolism (e.g. SLC7A7) have been linked to CSS (44). Thus, ever-expanding genetic contributions to CSS are increasingly being recognized (4, 5).
Host genetics
While clinical descriptions of CSS/HLH date back almost over a century (42), and therapeutic protocols are in place (11), the immunology and genetic contributions were really only best understood over the last 2-and-a-half decades. This was sparked by the identification of homozygous defects in PRF1 as the first gene contributing to a subset of infants with primary or familial HLH (45, 46). This was later modeled in mice in Pippa Marrack’s lab (47). This landmark report demonstrated that PRF1 deficient mice uniformly succumbed to LCMV infection within 2 weeks of exposure, but it was the host immune response that yielded fatal outcomes (47). Specifically, removal of cytolytic CD8+ T lymphocytes (CTL) or blockade of interferon-gamma (IFN-γ) largely reversed mortality (47). It was later shown in both murine and human lytic lymphocytes (CTL or natural killer (NK) cells) that perforin or granzyme deficiency not only disrupted cytolysis of the target antigen presenting cell (APC), but this resulted in prolonged (5-times longer) engagement between the lytic lymphocyte and its associated APC (48, 49). It was elegantly shown that the disrupted interaction is dependent on caspase activity within the target cell (49). When this was disturbed, the prolonged interaction led to increased pro-inflammatory cytokines such as IFN-γ and tumor necrosis factor (TNF) believed to be responsible for the hyperinflammatory state (48, 49). Thus, lack of killing via the perforin pathway was directly responsible for excess immune cell cross talk resulting in inappropriately elevated pro-inflammatory cytokines believed to be responsible for the clinical features of CSS/HLH.
In addition to defects in PRF1, other homozygous mutations in genes required for perforin-mediated cytolysis were identified among infants with familial HLH (Fig. 1A). In order for preformed perforin and granzyme containing cytolytic granules to traffic along the actin cytoskeleton to the immunological synapse, dock and fuse with the cell membrane, and release perforin to form a channel into the target cell, a number of intact non-redundant gene products are necessary. Soon after the identification of homozygous PRF1 defects were identified as responsible for subsets of infants with primary HLH (46), homozygous mutations in genes required for perforin delivery to the target cell, STX11, STXBP2, UNC13D, RAB27A, LYST, and AP3B1, were linked to familial cases of HLH (4, 5, 42). Clinically, these different genetic etiologies do not all present identically in terms of the CSS/HLH severity. Graded defects in cytotoxicity determines the severity of disease observed in humans and corresponding murine models of CSS/HLH (50, 51). Moreover, CSS can develop with polygenic combinations of heterozygous defects in different genes shared in the perforin cytolytic pathway (52-54). Thus, there are multiple potential genetic contributions to primary HLH via disruption of perforin-mediated cytolysis by CTL and NK cells.
FIGURE 1.
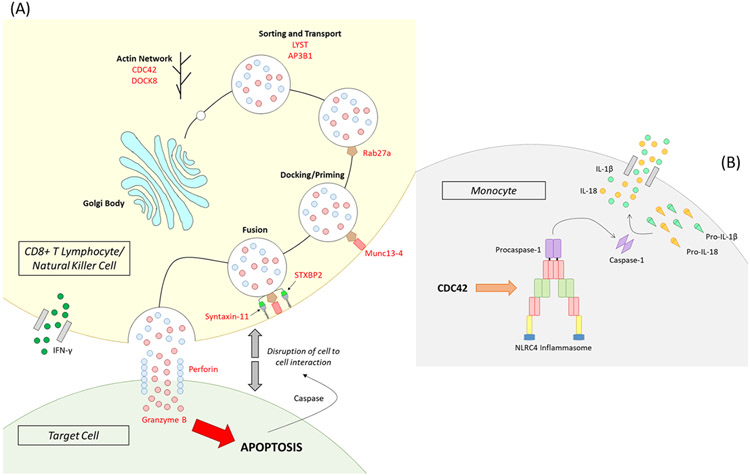
Cytolytic and autoinflammatory genetic pathways to cytokine storm syndrome. A) A cytolytic lymphocyte (top) lyses an antigen presenting cell (target cell below) via the perforin pathway by delivery of granzyme B to the target cell. Intact proteins of perforin, syntaxin 11, STXB2, Munc13-4, Rab27a, LYST, and AP3B1 are required for cytolytic granule sorting, transport, docking, priming, and fusion to the cell membrane so perforin can be released into the immunologic synapse to form a pore to deliver granzyme B resulting in caspase dependent apoptosis of the target cell. CDC42 and DOCK8 are important for trafficking of cytolytic granules along the actin cytoskeleton. B) Monocyte derived cell populations can also produce high levels of IL-1β and IL-18 when inflammasome gene products like NLRC4 are mutated or stimulated (e.g. CDC42) to activate caspase-1 from procaspase-1 resulting in conversion of pro-IL-1β and pro-IL-18 into active IL-1β and IL-18, respectively. This figure was generated by Dr. Daniel D. Reiff (University of Alabama at Birmingham).
The distinction between primary and secondary HLH, however, is becoming blurred, as heterozygous mutations in familial HLH genes have been shown to contribute to CSS beyond infancy via hyopomorphic or dominant-negative effects on perforin-mediated cytolysis (4, 25, 55). This has been most clearly demonstrated by CSS patient derived complete dominant-negative mutations in STXBP2 inhibiting fusion of perforin containing cytolytic granules to the cell membrane at the immunologic synapse (56). However, even partial dominant-negative mutations in STXBP2 can contribute to CSS in a threshold model of disease where infectious triggers (57) and/or an underlying hyper-inflammatory disease state (55) may push the pro-inflammatory environment to a point that immune regulatory mechanisms can no longer maintain a state of inflammatory homeostasis (58-60). Similarly, common heterozygous missense mutations of PRF1 that act as partial-dominant-negatives diminishing NK cell lysis (19, 61, 62) have been associated with late-onset CSS/HLH/MAS triggered by infection (e.g. H1N1 influenza) (19) or hyper-inflammatory states (e.g. Still disease) (55, 63, 64). Heterozygous missense mutations in UNC13D have also been associated with CSS in children and adults (55, 64). Interestingly, non-exonic mutations in UNC13D have also been reported in individuals with CSS (65-67), such that whole exome sequencing may overlook potential genetic defects contributing to decreased NK cell and CTL perforin mediated cytolysis (4). Intriguingly, a missense mutation in RAB27A identified in 2 unrelated individuals with CSS was shown to disrupt the interaction with MUNC13-4 (UNC13D protein), thus diminishing NK cell lytic function by delaying cytolytic granule polarization to the immunologic synapse (68). Similar to homozygous defects in perforin pathway genes (48, 49), this heterozygous HLH gene defect resulted in increased IFN-γ production likely contributing to CSS disease manifestations (68).
Notably, heterozygous defects in several different perforin pathway HLH genes have been shown to contribute to CSS pathophysiology, likely via a threshold model of disease potentially explaining why some individuals fare worse and develop a CSS when infected with the same pathogen as others (19). Therefore, heterozygous gene mutations that partially disrupt NK cell or CTL lytic function can contribute to CSS in select hyper-inflammatory states. Along these lines, mutations in familial HLH genes have been noted in those with severe COVID-19 (69) and in children with the SARS-CoV-2 post-infectious CSS, multisystem inflammatory syndrome in children (MIS-C) (70).
In addition, genes indirectly involved in trafficking cytolytic granules via their regulation of movement along the actin cytoskeleton have recently been linked to CSS, namely CDC42 (71, 72) and DOCK8 (70, 73) (Fig. 1A). Besides a putative role in perforin-mediated cytolysis, dominant mutations in CDC42 may contribute to CSS via activation of the inflammasome resulting in increased production of IL-1 and IL-18 (71, 72, 74). Dominant activating mutations in the inflammasome component gene NLRC4 have also been newly associated with CSS (75, 76) and excessive levels of IL-1 and IL-18 (77) (Fig. 1B). Hence, two established and unique immunologic pathways, perforin mediated cytolysis and inflammasome activation, contribute to the multifaceted immunology of CSS (Fig. 1) with multiple immune cell types involved in CSS development. The roles of the multiple immune cell types involved in various CSS have been illuminated using murine models of disease.
Murine models of cytokine storm syndrome
The biology of CSS is complex and the pathogenic cytokine(s) may vary depending on the underlying cause of inflammation. While profiling of human samples at various stages of CSS provides valuable insight, these studies are often limited by the availability of samples and the significant clinical heterogeneity among patients. Moreover, the results are often descriptive in nature, and a causal role of specific proinflammatory mediators remains difficult to establish. The mechanistic understanding of CSS is aided by the availability of mouse models, which also serve as important tools for preclinical evaluation of therapeutic agents. In this section, we review the use of murine models to study HLH and MAS (Fig. 2).
FIGURE 2.
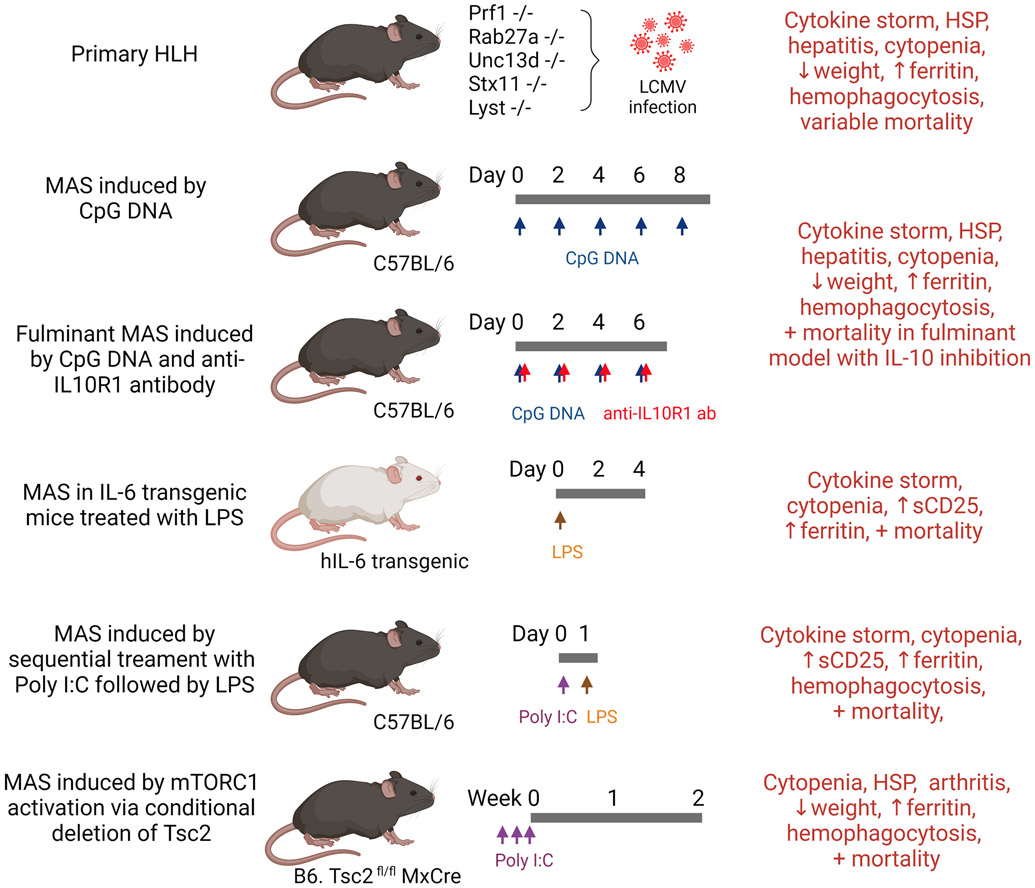
Murine models of cytokine storm syndrome. Primary HLH can be modeled by infecting mice with deficiency of PRF1, RAB27A, UNC13D, and STX11 with LCMV. Manifestations of MAS can be induced in WT mice or IL-6 transgenic mice using TLR ligands. Constitutive activation of the metabolic regulator mTORC1 by conditional deletion of Tsc2 is also shown to trigger an MAS-like disease. The typical timeline of disease development and key manifestations of MAS are displayed for each model. Abbreviations: CpG, 5'—Cytosine—phosphate—Guanine—3'; HLH, hemophagocytic lymphohistiocytosis; HSP, hepatosplenomegaly; LPS, lipopolysaccharide; MAS, macrophage activation syndrome; poly I:C, polyinosinic-polycytidylic acid; sCD25, soluble IL-2 receptor-α.
Murine models of primary HLH
Primary or familial HLH is caused by genetic defects that impair the exocytosis of cytotoxic granules, which result in defective CD8+ T cell- and NK cell-directed cytotoxicity (78, 79). Perforin (encoded by PRF1) is a glycoprotein that forms channels on the target cell membrane to allow entry of granzyme and other cytotoxic proteins. Biallelic mutations in PRF1 represent the most common cause of familial HLH in humans, and, fittingly, perforin deficiency in mice is the first described model of HLH (47).
Perforin-deficient mice develop normally and features of HLH occur only after infection with lymphocytic choriomeningitic virus (LCMV) (47). This is congruent with the observations in patients with HLH as disease onset is often precipitated by infections. Following LCMV infection, perforin-deficient mice develop the hallmarks of HLH including splenomegaly, pancytopenia, hypertriglyceridemia, hypofibrinogenemia, and cytokine storm. Fulminant hemophagocytosis is observed in tissues including the liver, spleen, and bone marrow (47). In the absence of perforin, activated CD8+ T cells fail to eliminate antigen-presenting cells (APC) and thereby creates a vicious cycle of reciprocal immune activation (80). The pathologic features of this model are largely prevented by depletion of CD8+ T lymphocytes or neutralization of IFN-γ (47). These findings strongly support the rationale of targeting T lymphocytes and IFN-γ in the treatment of HLH.
Murine models of UNC13D deficiency (Jinx mice), RAB27A deficiency (ashen mice), LYST deficiency (souris mice) and STX11 deficiency are also used to study the biology of familial HLH (51, 81-83) (Fig. 2). Similar to perforin-deficient mice, these strains show normal growth and development at baseline. In the setting of LCMV infection, however, they exhibit impaired viral clearance and develop features of HLH including hypothermia, splenomegaly, pancytopenia, hypertriglyceridemia, and cytokine storm. The severity of disease in RAB27A-deficient mice and STX11-deficient mice is milder compared to PRF1-deficient mice, which is in line with the later disease onset in patients with the corresponding mutations (51).
The aforementioned mouse strains are typically healthy until immune cell activation is triggered by LCMV. Spontaneous disease onset is noted in perforin-deficient mice with dendritic cell-specific deficiency of Fas (84). The interaction between Fas and Fas-ligand induces apoptotic cell death of activated immune cells. These data further show that timely elimination of APC by CTL is essential to prevent the development of hyperinflammation and cytokine storm.
CpG-DNA induced model of MAS
MAS is a CSS induced by intrinsic factors (i.e. Still disease and malignancy) or extrinsic factors (i.e. infections and drugs). To model MAS in mice, researchers often use Toll-like receptor (TLR) ligands to elicit systemic inflammation and cytokine storm. Behrens et al. described a model of MAS using repeated injection of the TLR9 ligand CpG DNA (85). Recurrent activation of TLR9 in this model results in cytopenia, hepatosplenomegaly, hepatitis, hyperferritinemia, and hemophagocytosis (85). The production of IFN-γ is required for the development of anemia, thrombocytopenia and hepatitis in this model. Interestingly, neither the absence of B and T lymphocytes (Rag2−/− mice) nor depletion of NK cells affects disease development, although some clinical features are attenuated in Rag2−/− Il2rg−/− mice with complete absence of B lymphocytes, T lymphocytes, and NK cells.
Instead, CpG DNA-induced MAS appears to be more dependent on myeloid cells. The excess production of IFN-γ is dependent on IL-12 primarily produced by inflammatory monocytes (86, 87). Prominent extramedullary myelopoiesis and accumulation of inflammatory monocytes are evident in CpG-treated mice and features of MAS are reversed with monocyte/macrophage depletion using clodronate-containing liposomes or antibodies to macrophage colony-stimulating factor (87, 88). CpG-induced MAS is also associated with increased production of IL-10, which is observed in patients with MAS (89). This cytokine plays a protective role in the hyperinflammatory response, as IL-10 blockade leads to fulminant MAS and lethal disease in CpG-treated mice (85). Curiously, host microbiota also contribute to the development of cytokine storm in this model, as the manifestations of MAS are significantly attenuated in germ-free mice and in wild-type (WT) mice treated with broad spectrum antibiotics (90).
LPS-induced MAS in IL-6 transgenic mice
IL-6 possesses multiple proinflammatory roles, and elevated IL-6 levels in the peripheral blood is associated with different types of CSS. While transgenic mice engineered to overproduce human IL-6 do not show evidence of MAS at baseline, Strippoli et al. demonstrated that a single dose of the TLR4 ligand lipopolysaccharide (LPS) or TLR3 ligand Poly I:C is sufficient to induce hyperinflammation and early mortality in these mice (91). IL-6 transgenic mice treated with LPS exhibit anemia, neutropenia, hyperferritinemia, and a cytokine storm that includes high levels of TNF, IL-1β, and IL-18. Hemophagocytosis has not been described in this model. Development of the MAS-like syndrome in IL-6 transgenic mice also requires IFN-γ; neutralizing antibodies to IFN-γ reduced ferritin levels and improved survival and body weight recovery (92).
Other models of MAS induced by TLR agonists
Another TLR-driven model of MAS was established by Wang et al. to mimic infection-associated MAS. Sequential treatment with a viral TLR agonist (Poly I:C or R837, a TLR7 agonist) followed by a non-lethal dose of LPS, but not in the reversed order, elicits a lethal MAS-like disease characterized by pancytopenia, hyperferritinemia, and hemophagocytosis (93). Curiously, IFN-γ is dispensable in this model (93).
Chronic activation of Tlr7 alone is also sufficient to drive the development of anemia, thrombocytopenia, and hemophagocytosis in mice (94). Studies using a transgenic strain that overexpresses Tlr7 identified a population of monocyte-derived inflammatory macrophages that are responsible for the manifestations of cytopenia and hemophagocytosis. Signaling through interferon regulatory factor-5 (Irf5) downstream of Tlr7 is essential for the development of these macrophages. Interestingly, polymorphisms in IRF5 are associated with MAS susceptibility in patients with pediatric Still disease (95).
The connection between TLR signaling, LCMV infection, and the development of MAS is reinforced by a study by Ohyagi and colleagues (96). However, they found that the uptake of erythrocytes by monocyte-derived dendritic cells is essential for the production of IL-10, which inhibits the hyperinflammatory response. This notion supports the finding that human hemophagocytes exhibit a transcriptomic signature of alternative activation (97). Further studies are needed to determine the role of hemophagocytes in CSS (98).
MAS driven by hyperactive metabolic pathways
In the model of MAS driven by sequential treatment Poly I:C followed by LPS, Wang et al. demonstrated that the inflammatory response was dependent on cellular immunometabolism. Inhibition of glycolysis by 2-deoxyglucose, but not inhibition of individual cytokines, is sufficient to block the development of the lethal MAS-like disease (93). Supporting this view, constitutive activation of the metabolic regulator mechanist target of rapamycin complex 1 (mTORC1) via conditional deletion of Tsc2 can drive the development of an MAS-like disease with cytopenia, hepatosplenomegaly, increase ferritin levels and fulminant hemophagocytosis (99). Because mTORC1 signals downstream of multiple cytokines, this pathway may serve as a nexus for input from multiple proinflammatory mediators. Interestingly, MAS is also associated with inborn errors of cellular metabolism in humans (41, 100).
Heterogeneity in the murine models of CSS and relevance to human disease
The murine models of HLH and MAS share the pathognomonic features of cytokine storm, hepatosplenomegaly, cytopenia and hemophagocytosis. There are also clear differences among these models, which is, perhaps, expected given the heterogenous causes of CSS in humans, despite the overlapping clinical manifestations. Therefore, lessons from these models are helpful in determining the mechanism and appropriate intervention for the different types of CSS.
A key discrepancy between the mouse models is the role of IFN-γ. Excess production of IFN-γ is a hallmark of familial HLH and MAS in patients, and blockade of IFN-γ is effective for the treatment of familial HLH refractory to other interventions (101, 102). IFN-γ is critically important for disease development in the perforin-deficiency model of HLH, CpG-induced MAS, and LPS-induced MAS in IL-6 transgenic mice (47, 85, 92). Overproduction of IFN-γ alone is sufficient to drive hemophagocytosis and anemia (103). However, repeated TLR9 activation in combination with IL-10 receptor blockade can trigger fulminant MAS in the absence of IFN-γ signaling (86). MAS secondary to sequential administration of poly I:C and LPS is also unresponsive to IFN-γ blockade (93). The development of CSS in the absence of IFN-γ is particularly relevant in rare cases of HLH that develop in patients lacking the IFN-γ receptor (104).
Another difference among the murine models is the role of activated T lymphocytes. Ineffective killing of activated lymphocytes by CD8+ T cells and NK cells is central to the biology of HLH as illustrated by the perforin-deficiency model (47, 80). In contrast, neither T lymphocytes nor NK cells are required in the development of MAS triggered by CpG or Poly I:C / LPS (85, 93). Mononuclear phagocytes including monocytes, macrophages, and dendritic cells appear to play a more prominent in the models of CSS induced by TLR ligands. These observations further suggest the existence of multiple pathways of hyperinflammation that can lead to similar clinical manifestations.
Given the involvement of multiple cytokines in CSS, therapeutic approaches that modulate common mediators of these cytokines may be more effective than focusing on a single target with variable involvement. Indeed, inhibitors of Janus kinases (JAK) are increasing used in the treatment of HLH as they can inhibit the signaling pathways of IFN-γ among many other cytokines and growth factors (105). In the perforin-deficiency model of HLH and fulminant MAS induced by CpG and IL-10 blockade, treatment with ruxolitinib showed additional protective effects beyond IFN-γ inhibition, including reductions in splenomegaly, CD8+ T cell activation, and neutrophil activation (106). A recent study further illustrate that combined treatment with ruxolitinib and anti-IFN-γ antibodies may be even more effective for the treatment of familial HLH in mice (107).
Taken together, murine models of HLH and MAS have significantly improved our mechanistic understanding of CSS. They help to demonstrate the multifaceted immunology of CSS, ranging from defective perforin mediated cytolysis to dominantly active inflammasomopathies to non-genetic infection triggers. These models not only provide a platform to study the different pathways that contribute to hyperinflammation, but they allow for evaluation of novel therapeutics. However, researchers should always be mindful of the potential translational gap between animal models and human disease. The phase 1 clinical trial on the CD28 superantagonist TGN1412, which appeared safe in murine and non-human primate models but resulted in rapid development of a severe CSS in healthy volunteers, is an important reminder that differences in host biology can be associated with very different outcomes (108).
Treatment of CSS
Treatment of CSS is focused on removing the inciting trigger(s) and calming the hyperinflammatory response. With the many different paths that lead to CSS, the choice treatment should be based on available evidence and tailored to each patient. Evaluation of novel therapeutic options should consider the lessons learned from decades of failure in the search of more effective treatment for sepsis (109). The cause of CSS, contribution of host genetics, severity and heterogeneity of clinical manifestations as well as utility of animal models for the particular form of CSS should be taken into consideration for treatment selection and clinical trial design.
Immune cells as therapeutic targets
For familial HLH, cytolytic lymphocytes (CTL and NK cells) are primary drivers of disease secondary to their defective lysis of APC/target cells resulting in prolonged engagement and excess proinflammatory cytokine production (e.g. IFN-γ). Recently, increased percentages of activated CD8+ T cells (CD4dim, CD38high, HLA-DR+) have been noted in both HLH (110) and MAS (111) patient populations. This provides additional rationale for the use of etoposide, an inhibitor of topoisomerase II, to target proliferating lymphocytes in HLH (Table II) (11). The glucocorticoid, dexamethasone (good central nervous system penetration), is given with etoposide as a broadly immunosuppressant targeting most all immune cell types (11). For refractory HLH requiring salvage therapy, almetuzumab (anti-CD52; targets lymphocytes and myeloid cells for depletion) (112) and anti-thymocyte globlulin (ATG; targets T lymphocytes for depletion) (113) have been employed to control familial HLH prior to stem cell transplantation (Table II).
Table 2.
Immunologic therapeutics used in treatment of cytokine storm syndrome
| Therapeutic agent | Target | Mechanism of action | Application |
|---|---|---|---|
| Etoposide (topoisomerase II inhibitor) | T lymphocytes | Inhibits cell proliferation | HLH |
| Almetuzumab (anti-CD52) | Lymphocytes, monocytes | Depletes leukocytes | HLH |
| Rituximab (anti-CD20 mAb) | B lymphocytes | Depletes B lymphocytes | EBV-MAS |
| Anti-thymocyte globulin (ATG) | T lymphocytes | Depletes T lymphocytes | HLH |
| Cyclosporin A (calcineurin inhibitor) | IL-2, IFN-γ, others | Inhibits cell proliferation and effector functions | HLH, MAS |
| Anakinra (rhIL-1Ra) | IL-1 | Blocks IL-1 from receptor binding | MAS, CRS |
| Tocilizumab (anti-IL-6R mAb) | IL-6 | Blocks IL-6 from receptor binding | MAS, CRS |
| Emapalumab (anti-IFN-γ mAb) | IFN-γ | Neutralizes IFN-γ | HLH, MAS |
| Tadekinig alfa (rhIL-18BP) | IL-18 | Blocks IL-18 from receptor binding | NLRC4-MAS, XIAP |
| Ruxolitinib (JAK1/2 inhibitor) | IFN-γ, IL-6, IL-12, others | Inhibits cytokine signaling | HLH, MAS, severe COVID-19 |
| Plasmapheresis | Multiple cytokines | Remove proinflammatory mediators | severe COVID-19 |
| Dexamethasone (glucocorticoids) | Multiple cytokines | Broad immunosuppression | Most CSS |
| Intravenous immunoglobulin (IVIG) | Multiple cytokines | unknown | MAS, MIS-C |
Abbreviations: BP, binding protein; COVID-19, coronavirus disease of 2019; CRS, cytokine release syndrome; EBV, Epstein-Barr virus; HLH, hemophagocytic lymphohistiocytosis; IFN, interferon; JAK, Janus kinase; mAb, monoclonal antibody; MAS, macrophage activation syndrome; MIS-C, multisystem inflammatory syndrome in children; NLRC4, NOD-like receptor family CARD domain containing 4; Ra, receptor antagonist; rh, recombinant human; XIAP, X-linked inhibitor of apoptosis protein.
Another cellular target for treating HLH includes rituximab (anti-CD20; targets B lymphocytes for depletion) specifically for HLH in the setting of EBV infection (Table II). The rationale derives in part from the fact that EBV generally targets B cells (T cells can also be infected), but most of the benefit of rituximab in treating EBV triggered HLH has been noted in the primary immunodeficiency syndromes, such as XLP (114). Rituximab has the most survival benefit in treating EBV associated HLH when the serum ferritin is ≤1,000 ng/mL and the EBV viral load is ≤1,500 copies/mL (114). Nevertheless, survival in the setting of EBV HLH is not ideal. Rituximab therapy has also been used to treat thrombotic microangiopathy (TMA), a potential coincident condition associated with MAS/HLH with poor survival (115). In addition, eculizumab (anti-C5, complement) may improve TMA survival by preventing terminal complement activation on endothelial cells (116). The use of biologics targeting various cell types is often carried out in conjunction with etoposide-based protocols, which in the best of centers still yields mortality rates near 40% (117). Targeting cytokines broadly via the nuclear factor of activated T cells (NFAT) inhibitor, cyclosporin A has been dropped from the etoposide-based protocols for increased side effects without additional benefit (117). However, glucocorticoids remain a frequent mainstay of CSS therapy and work in part by inhibiting cytokine production (Table II).
Cytokines as therapeutic targets
Another approach to treating HLH/CSS, which may in part target cytokines via anti-cytokine antibodies, is intravenous immunoglobulin (IVIg) (Table II). This has primarily found success in non-EBV infection triggered CSS (118). Targeting cytokines broadly with plasmapheresis in the setting of CSS has also been reported as beneficial (119). More recently, the targeting of individual pro-inflammatory cytokines has gained acceptance in treating CSS. Initially, targeting tumor necrosis factor (TNF) was anecdotally reported to sometimes help or to hinder treatment of CSS in various case reports/series (17). While targeting TNF has not gained traction, targeting IL-1 in the setting of CSS has gained wider acceptance (Table II).
The first reported beneficial use of IL-1 blockade with the recombinant human IL-1 receptor antagonist (rhIL-1Ra) anakinra for severe CSS was in a child with a histiocytic disorder (120). Anakinra was seen as an attractive therapeutic in the setting of CSS because of its recombinant nature, short half-life (4-6 hours), quick action, large therapeutic window, and prior established safety profile (121, 122). Anakinra has been particularly useful in treating rheumatic triggers of MAS/CSS, particularly in those with Still disease (123, 124). Anakinra may also play a role in CSS associated with sepsis (122). And while malignancy associated HLH/CSS is associated with high mortality rates (125), anakinra has anecdotally been reported to benefit some in this setting (126). Most recently, anakinra has also notably improved survival in the setting of severe COVID-19 CSS (127).
While early during the pandemic blockade of IL-6 appeared to improve survival of severe COVID-19 CSS, meta-analyses of multiple clinical trials have been less enthusiastic about the benefit of anti-IL-6 or anti-IL-6R mAb approaches for hospitalized COVID-19 patients (128). However, IL-6 blockade with tocilizumab (anti-IL-6R mAb) has received United States Food and Drug Administration (FDA) approval in treating iatrogenic CRS associated with chimeric T cell treatment for refractory hematopoietic malignancies (129) (Table II). The FDA has also recently approved of emapalumab (anti-IFN-γ mAb) for primary/familial HLH (102) (Table II), bringing the murine model full circle (47). IFN-γ over-expression occurs in the setting of familial HLH as the result of decreased perforin-mediated cytolysis, but IFN-γ is not the only increased cytokine noted (49). This perhaps explains the benefit of adding JAK inhibition (Table II) to anti-IFN-γ mAb therapy in treating CSS/HLH (107). JAK inhibition with baricitinib has also been touted to treat severe COVID-19 CSS (130). While the coronavirus is the trigger for the deadly COVID-19 CSS (131), the appropriate cytokine(s) to target for this pandemic remains unclear (132).
Conclusions
CSS are gaining wider recognition as deadly complications from a wide variety of genetic, infectious, rheumatic, oncologic, and other conditions, ranging from familial HLH to EBV to Still disease to T cell lymphoma to Castleman disease (7, 8, 17). While there are multiple potential CSS triggers with genetic contributions, including worldwide pandemics (19, 69), the defective host immune response is largely responsible for the inflammatory mortality, best characterized in the setting of familial HLH (47). Following identification of the genes responsible for familial HLH in humans, genetic murine models have been established to explore the pathophysiology of CSS/HLH (21). Defects in perforin-mediated cytolysis, though, are not just restricted to familial HLH, but also contribute to the much more common secondary forms of HLH/MAS/CSS (19, 55, 64, 70). Ineffective NK cell and CTL lysis of targets cell via perforin result in prolonged engagement of the lytic lymphocyte with its target cell producing excess pro-inflammatory cytokines believed responsible for the multi-organ dysfunction of CSS (48, 49). Other immunologic pathways can also be disturbed in CSS (5).
More recently, gene defects resulting in constitutive inflammasome activation have been identified (4, 5). Mutations in CDC42 (75, 76) and NLRC4 are associated with CSS characterized by high levels of the inflammasome product, IL-18 (133). IL-18 as a target to treat autoinflammatory CSS is currently being explored (134). Another, inflammasome product, IL-1, is already a well-established target in treating various forms of CSS, including Still disease (2). Targeting of other individual cytokines have received FDA approval for various CSS (IL-6 blockade for CRS, IFN-γ blockade for familial HLH) (102, 129). Broader cytokine inhibition with JAK inhibitors is also being explored for treatment of CSS/HLH (135). Again, murine models have proven valuable to study this therapeutic approach (136), as well as murine models exploring non-hereditary forms of CSS (85). Beyond broad immunosuppression with glucocorticoids and chemotherapy (etoposide), targeted cytokine approaches are proving efficacious and less toxic in saving lives of those afflicted with CSS (7, 8). As we continue to expand our knowledge of the multifaceted immunology of various CSS scenarios via murine and human studies (3), we will be able to provide a precision medicine approach to treating the frequently fatal entity of CSS (137).
Acknowledgments
P.Y.L. is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) K08-AR074562, the Rheumatology Research Foundation Investigator Award and K Supplement Award, Charles H. Hood Foundation Child Health Research Award, and Arthritis National Research Foundation All Arthritis Grant. R.Q.C. is supported by the Arthritis Foundation, Alabama Chapter Endowed Chair in Pediatric Rheumatology at the University of Alabama (UAB) Heersink School of Medicine (SOM). His work was funded by grants for the Kaul Pediatric Research Institute, the Histiocyte Association, Sobi, and the UAB Heersink SOM and Precision Medicine Institute. The funders had no role in study design, data collection and interpretation, or decision to submit the work for publication.
Abbreviations used in this article:
- APC
antigen presenting cell
- CAR-T
chimeric antigen receptor T cell
- CMV
cytomegalovirus
- CRS
cytokine release syndrome
- COVID-19
coronavirus disease 2019
- CSS
cytokine storm syndrome
- CTL
cytolytic T lymphocyte
- EBV
Epstein-Barr virus
- FDA
Food and Drug Administration
- HLH
hemophagocytic lymphohistiocytosis
- IFN-γ
interferon-gamma
- IVIg,
intravenous immunoglobulin
- JAK
Janus kinase
- LCMV
lymphocytic choriomeningitis virus
- mAb
monoclonal antibody
- MAS
macrophage activation syndrome
- MIS-C
multisystem inflammatory syndrome in children
- NFAT
nuclear factor of activated T cells
- NK
natural killer
- Ra
receptor antagonist
- rh
recombinant human
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- TLR
toll-like receptor
- TMA
thrombotic microangiopathy
- TNF
tumor necrosis factor
- XLP
X-linked lymphoproliferative
- WT
wild-type
Footnotes
Disclosures
P.Y.L. has consulted for Exo Therapeutics and Brickell Bio, and he receives royalties from Up-to-Date. R.Q.C. has received grant support from Sobi and served as a consultant to Novartis, Pfizer, Sironax, and Sobi.
References
- 1.Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF, Caricchio R, Mahmud S, Hazen MM, Halyabar O, Hoyt KJ, Han J, Grom AA, Gattorno M, Ravelli A, De Benedetti F, Behrens EM, Cron RQ, and Nigrovic PA. 2020. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol 72: 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson LA, and Cron RQ. 2020. Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis in Childhood Inflammatory Disorders: Diagnosis and Management. Paediatr Drugs 22: 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crayne CB, Albeituni S, Nichols KE, and Cron RQ. 2019. The Immunology of Macrophage Activation Syndrome. Front Immunol 10: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulert GS, and Cron RQ. 2020. The genetics of macrophage activation syndrome. Genes Immun 21: 169–181. [DOI] [PubMed] [Google Scholar]
- 5.Canna SW, and Cron RQ. 2020. Highways to hell: mechanism based management of Cytokine Storm Syndromes. J Allergy Clin Immunol 146: 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter E, Amiel E, Bose N, Bottaro A, Carr WH, Swanson-Mungerson M, Varga SM, and Jameson JM. 2021. American Association of Immunologists Recommendations for an Undergraduate Course in Immunology. Immunohorizons 5: 448–465. [DOI] [PubMed] [Google Scholar]
- 7.Cron RQ, Goyal G, and Chatham WW. 2022. Cytokine Storm Syndrome. Annu Rev Med, in press. [DOI] [PubMed] [Google Scholar]
- 8.Fajgenbaum DC, and June CH. 2020. Cytokine Storm. N Engl J Med 383: 2255–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cron RQ, Davi S, Minoia F, and Ravelli A. 2015. Clinical features and correct diagnosis of macrophage activation syndrome. Expert Rev Clin Immunol 11: 1043–1053. [DOI] [PubMed] [Google Scholar]
- 10.Ravelli A, Davi S, Minoia F, Martini A, and Cron RQ. 2015. Macrophage Activation Syndrome. Hematol Oncol Clin North Am 29: 927–941. [DOI] [PubMed] [Google Scholar]
- 11.Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, and Janka G. 2007. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 48: 124–131. [DOI] [PubMed] [Google Scholar]
- 12.Minoia F, Bovis F, Davi S, Horne A, Fischbach M, Frosch M, Huber A, Jelusic M, Sawhney S, McCurdy DK, Silva CA, Rigante D, Unsal E, Ruperto N, Martini A, Cron RQ, Ravelli A, t. C. A. Pediatric Rheumatology International Trials Organization, t. P. R. C. S. G. Rheumatology Research Alliance, and S. the Histiocyte. 2019. Development and initial validation of the MS score for diagnosis of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Ann Rheum Dis 78: 1357–1362. [DOI] [PubMed] [Google Scholar]
- 13.Ravelli A, Minoia F, Davi S, Horne A, Bovis F, Pistorio A, Arico M, Avcin T, Behrens EM, De Benedetti F, Filipovic L, Grom AA, Henter JI, Ilowite NT, Jordan MB, Khubchandani R, Kitoh T, Lehmberg K, Lovell DJ, Miettunen P, Nichols KE, Ozen S, Pachlopnik Schmid J, Ramanan AV, Russo R, Schneider R, Sterba G, Uziel Y, Wallace C, Wouters C, Wulffraat N, Demirkaya E, Brunner HI, Martini A, Ruperto N, and Cron RQ. 2016. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis 75: 481–489. [DOI] [PubMed] [Google Scholar]
- 14.Cron RQ, Schulert GS, and Tattersall RS. 2020. Defining the scourge of COVID-19 hyperinflammatory syndrome. Lancet Rheumatol 2: e727–e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, Coppo P, and Hejblum G. 2014. Development and validation of a score for the diagnosis of reactive hemophagocytic syndrome (HScore). Arthritis Rheumatol 66: 2613–2620. [DOI] [PubMed] [Google Scholar]
- 16.Eloseily EMA, Minoia F, Crayne CB, Beukelman T, Ravelli A, and Cron RQ. 2019. Ferritin to Erythrocyte Sedimentation Rate Ratio: Simple Measure to Identify Macrophage Activation Syndrome in Systemic Juvenile Idiopathic Arthritis. ACR Open Rheumatol 1: 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crayne C, and Cron RQ. 2019. Pediatric macrophage activation syndrome, recognizing the tip of the Iceberg. Eur J Rheumatol 7: S13–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan MB, Allen CE, Weitzman S, Filipovich AH, and McClain KL. 2011. How I treat hemophagocytic lymphohistiocytosis. Blood 118: 4041–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulert GS, Zhang M, Fall N, Husami A, Kissell D, Hanosh A, Zhang K, Davis K, Jentzen JM, Napolitano L, Siddiqui J, Smith LB, Harms PW, Grom AA, and Cron RQ. 2016. Whole-Exome Sequencing Reveals Mutations in Genes Linked to Hemophagocytic Lymphohistiocytosis and Macrophage Activation Syndrome in Fatal Cases of H1N1 Influenza. J Infect Dis 213: 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cron RQ, Behrens EM, Shakoory B, Ramanan AV, and Chatham WW. 2015. Does Viral Hemorrhagic Fever Represent Reactive Hemophagocytic Syndrome? J Rheumatol 42: 1078–1080. [DOI] [PubMed] [Google Scholar]
- 21.Brisse E, Wouters CH, and Matthys P. 2015. Hemophagocytic lymphohistiocytosis (HLH): A heterogeneous spectrum of cytokine-driven immune disorders. Cytokine Growth Factor Rev 26: 263–280. [DOI] [PubMed] [Google Scholar]
- 22.Schulert GS 2022. The Big Bad Wolf: Macrophage Activation Syndrome in Childhood-Onset Systemic Lupus Erythematosus. J Rheumatol 49: 1082–1084. [DOI] [PubMed] [Google Scholar]
- 23.Schulert GS, and Canna SW. 2018. Convergent pathways of the hyperferritinemic syndromes. Int Immunol 30: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grom AA 2004. Natural killer cell dysfunction: A common pathway in systemic-onset juvenile rheumatoid arthritis, macrophage activation syndrome, and hemophagocytic lymphohistiocytosis? Arthritis Rheum 50: 689–698. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman KM, Linghu B, Szustakowski JD, Husami A, Yang F, Zhang K, Filipovich AH, Fall N, Harley JB, Nirmala NR, and Grom AA. 2014. Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheumatol 66: 3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsoukas P, Rapp E, Van Der Kraak L, Weiss ES, Dang V, Schneider C, Klein E, Picarsic J, Salcedo R, Stewart CA, and Canna SW. 2020. Interleukin-18 and cytotoxic impairment are independent and synergistic causes of murine virus-induced hyperinflammation. Blood 136: 2162–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siebert S, Amos N, Williams BD, and Lawson TM. 2007. Cytokine production by hepatic anaplastic large-cell lymphoma presenting as a rheumatic syndrome. Semin Arthritis Rheum 37: 63–67. [DOI] [PubMed] [Google Scholar]
- 28.Diorio C, Vatsayan A, Talleur AC, Annesley C, Jaroscak JJ, Shalabi H, Ombrello AK, Hudspeth M, Maude SL, Gardner RA, and Shah NN. 2022. Anakinra utilization in refractory pediatric CAR T-cell associated toxicities. Blood Adv 6: 3398–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, and Sadelain M. 2018. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 24: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, and Grupp SA. 2014. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li XY, Zhu SM, Li XY, Dong RS, Zhang AA, Li SJ, and Geng YL. 2022. Reactive Hemophagocytic Lymphohistiocytosis Secondary to Ovarian Adenocarcinoma: A Rare Case Report. J Inflamm Res 15: 5121–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qureshi M, Alabd A, Behling E, Schwarting R, and Haroldson K. 2022. Acute Liver Failure in Hemophagocytic Lymphohistiocytosis Secondary to Metastatic Renal Cell Carcinoma: A Diagnostic Dilemma. Cureus 14: e23455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou YS, Cui YC, Yin MJ, Xie QW, Shen ZL, Shi HX, Ye YJ, and Liang B. 2021. Gastric cancer complicated with hemophagocytic lymphohistiocytosis: case report and a brief review. J Gastrointest Oncol 12: 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takei K, Motoyoshi N, Sakamoto K, and Kitamoto T. 2019. Marchiafava-Bignami disease with haemophagocytic lymphohistiocytosis as a postoperative complication of cardiac surgery. Case Rep 12: e230368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson CP, Jagdale A, Walcott G, Iwase H, Foote JB, Cron RQ, Hara H, Cleveland DC, and Cooper DKC. 2021. A perspective on the potential detrimental role of inflammation in pig orthotopic heart xenotransplantation. Xenotransplantation 28: e12687. [DOI] [PubMed] [Google Scholar]
- 36.Wilson-Morkeh H, Frise C, and Youngstein T. 2022. Haemophagocytic lymphohistiocytosis in pregnancy. Obstet Med 15: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramanan AV, and Schneider R. 2003. Macrophage activation syndrome following initiation of etanercept in a child with systemic onset juvenile rheumatoid arthritis. J Rheumatol 30: 401–403. [PubMed] [Google Scholar]
- 38.Chesner J, Schiano TD, Fiel MI, and Crismale JF. 2021. Hemophagocytic lymphohistiocytosis occurring after liver transplantation: A case series and review of the literature. Clin Transplant 35: e14392. [DOI] [PubMed] [Google Scholar]
- 39.Xu Z, and Otrock ZK. 2022. Extracorporeal photopheresis: A case of graft-versus-host-disease and hemophagocytic lymphohistiocytosis following liver transplantation. Transfusion 62: 2409–2413. [DOI] [PubMed] [Google Scholar]
- 40.Fajgenbaum DC 2018. Novel insights and therapeutic approaches in idiopathic multicentric Castleman disease. Blood 132: 2323–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogier de Baulny H, Schiff M, and Dionisi-Vici C. 2012. Lysinuric protein intolerance (LPI): a multi organ disease by far more complex than a classic urea cycle disorder. Mol Genet Metab 106: 12–17. [DOI] [PubMed] [Google Scholar]
- 42.Janka GE 2012. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med 63: 233–246. [DOI] [PubMed] [Google Scholar]
- 43.Chinn IK, Eckstein OS, Peckham-Gregory EC, Goldberg BR, Forbes LR, Nicholas SK, Mace EM, Vogel TP, Abhyankar HA, Diaz MI, Heslop HE, Krance RA, Martinez CA, Nguyen TC, Bashir DA, Goldman JR, Stray-Pedersen A, Pedroza LA, Poli MC, Aldave-Becerra JC, McGhee SA, Al-Herz W, Chamdin A, Coban-Akdemir ZH, Jhangiani SN, Muzny DM, Cao TN, Hong DN, Gibbs RA, Lupski JR, Orange JS, McClain KL, and Allen CE. 2018. Genetic and mechanistic diversity in pediatric hemophagocytic lymphohistiocytosis. Blood 132: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gadoury-Levesque V, Dong L, Su R, Chen J, Zhang K, Risma KA, Marsh RA, and Sun M. 2020. Frequency and spectrum of disease-causing variants in 1892 patients with suspected genetic HLH disorders. Blood Adv 4: 2578–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Behrens EM, and Cron RQ. 2015. Kill or be killed. J Immunol 194: 5041–5043. [DOI] [PubMed] [Google Scholar]
- 46.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint Basile G, and Kumar V. 1999. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 286: 1957–1959. [DOI] [PubMed] [Google Scholar]
- 47.Jordan MB, Hildeman D, Kappler J, and Marrack P. 2004. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood 104: 735–743. [DOI] [PubMed] [Google Scholar]
- 48.Anft M, Netter P, Urlaub D, Prager I, Schaffner S, and Watzl C. 2020. NK cell detachment from target cells is regulated by successful cytotoxicity and influences cytokine production. Cell Mol Immunol 17: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jenkins MR, Rudd-Schmidt JA, Lopez JA, Ramsbottom KM, Mannering SI, Andrews DM, Voskoboinik I, and Trapani JA. 2015. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J Exp Med 212: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jessen B, Kogl T, Sepulveda FE, de Saint Basile G, Aichele P, and Ehl S. 2013. Graded defects in cytotoxicity determine severity of hemophagocytic lymphohistiocytosis in humans and mice. Front Immunol 4: 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sepulveda FE, Debeurme F, Menasche G, Kurowska M, Cote M, Pachlopnik Schmid J, Fischer A, and de Saint Basile G. 2013. Distinct severity of HLH in both human and murine mutants with complete loss of cytotoxic effector PRF1, RAB27A, and STX11. Blood 121: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sepulveda FE, Garrigue A, Maschalidi S, Garfa-Traore M, Menasche G, Fischer A, and de Saint Basile G. 2016. Polygenic mutations in the cytotoxicity pathway increase susceptibility to develop HLH immunopathology in mice. Blood 127: 2113–2121. [DOI] [PubMed] [Google Scholar]
- 53.Steen EA, Hermiston ML, Nichols KE, and Meyer LK. 2021. Digenic Inheritance: Evidence and Gaps in Hemophagocytic Lymphohistiocytosis. Front Immunol 12: 777851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang K, Chandrakasan S, Chapman H, Valencia CA, Husami A, Kissell D, Johnson JA, and Filipovich AH. 2014. Synergistic defects of different molecules in the cytotoxic pathway lead to clinical familial hemophagocytic lymphohistiocytosis. Blood 124: 1331–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M, Behrens EM, Atkinson TP, Shakoory B, Grom AA, and Cron RQ. 2014. Genetic defects in cytolysis in macrophage activation syndrome. Curr Rheumatol Rep 16: 439–446. [DOI] [PubMed] [Google Scholar]
- 56.Spessott WA, Sanmillan ML, McCormick ME, Patel N, Villanueva J, Zhang K, Nichols KE, and Giraudo CG. 2015. Hemophagocytic lymphohistiocytosis caused by dominant-negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion. Blood 125: 1566–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reiff DD, Zhang M, Smitherman EA, Mannion ML, Stoll ML, Weiser P, and Cron RQ. 2022. A Rare STXBP2 Mutation in Severe COVID-19 and Secondary Cytokine Storm Syndrome. Life (Basel) 12: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brisse E, Wouters CH, and Matthys P. 2016. Advances in the pathogenesis of primary and secondary haemophagocytic lymphohistiocytosis: differences and similarities. Br J Haematol 174: 203–217. [DOI] [PubMed] [Google Scholar]
- 59.de Saint Basile G, Sepulveda FE, Maschalidi S, and Fischer A. 2015. Cytotoxic granule secretion by lymphocytes and its link to immune homeostasis. F1000Res 4: 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strippoli R, Caiello I, and De Benedetti F. 2013. Reaching the threshold: a multilayer pathogenesis of macrophage activation syndrome. J Rheumatol 40: 761–767. [DOI] [PubMed] [Google Scholar]
- 61.House IG, Thia K, Brennan AJ, Tothill R, Dobrovic A, Yeh WZ, Saffery R, Chatterton Z, Trapani JA, and Voskoboinik I. 2015. Heterozygosity for the common perforin mutation, p.A91V, impairs the cytotoxicity of primary natural killer cells from healthy individuals. Immunol Cell Biol 93: 575–580. [DOI] [PubMed] [Google Scholar]
- 62.Risma KA, Frayer RW, Filipovich AH, and Sumegi J. 2006. Aberrant maturation of mutant perforin underlies the clinical diversity of hemophagocytic lymphohistiocytosis. J Clin Invest 116: 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vastert SJ, van Wijk R, D'Urbano LE, de Vooght KM, de Jager W, Ravelli A, Magni-Manzoni S, Insalaco A, Cortis E, van Solinge WW, Prakken BJ, Wulffraat NM, de Benedetti F, and Kuis W. 2010. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford) 49: 441–449. [DOI] [PubMed] [Google Scholar]
- 64.Zhang K, Jordan MB, Marsh RA, Johnson JA, Kissell D, Meller J, Villanueva J, Risma KA, Wei Q, Klein PS, and Filipovich AH. 2011. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial hemophagocytic lymphohistiocytosis. Blood 118: 5794–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cichocki F, Schlums H, Li H, Stache V, Holmes T, Lenvik TR, Chiang SC, Miller JS, Meeths M, Anderson SK, and Bryceson YT. 2014. Transcriptional regulation of Munc13-4 expression in cytotoxic lymphocytes is disrupted by an intronic mutation associated with a primary immunodeficiency. J Exp Med 211: 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meeths M, Chiang SC, Wood SM, Entesarian M, Schlums H, Bang B, Nordenskjold E, Bjorklund C, Jakovljevic G, Jazbec J, Hasle H, Holmqvist BM, Rajic L, Pfeifer S, Rosthoj S, Sabel M, Salmi TT, Stokland T, Winiarski J, Ljunggren HG, Fadeel B, Nordenskjold M, Henter JI, and Bryceson YT. 2011. Familial hemophagocytic lymphohistiocytosis type 3 (FHL3) caused by deep intronic mutation and inversion in UNC13D. Blood 118: 5783–5793. [DOI] [PubMed] [Google Scholar]
- 67.Schulert GS, Zhang M, Husami A, Fall N, Brunner H, Zhang K, Cron RQ, and Grom AA. 2018. Brief Report: Novel UNC13D Intronic Variant Disrupting an NF-kappaB Enhancer in a Patient With Recurrent Macrophage Activation Syndrome and Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol 70: 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang M, Bracaglia C, Prencipe G, Bemrich-Stolz CJ, Beukelman T, Dimmitt RA, Chatham WW, Zhang K, Li H, Walter MR, de Benedetti F, Grom AA, and Cron RQ. 2016. A heterozygous RAB27A mutation associated with delayed cytolytic granule polarization and hemophagocytic lymphohistiocytosis. J Immunol 196: 2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schulert GS, Blum SA, and Cron RQ. 2021. Host genetics of pediatric SARS-CoV-2 COVID-19 and multisystem inflammatory syndrome in children. Curr Opin Pediatr 33: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vagrecha A, Zhang M, Acharya S, Lozinsky S, Singer A, Levine C, Al-Ghafry M, Fein Levy C, and Cron RQ. 2022. Hemophagocytic Lymphohistiocytosis Gene Variants in Multisystem Inflammatory Syndrome in Children. Biology (Basel) 11: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coppola S, Insalaco A, Zara E, Di Rocco M, Marafon DP, Spadaro F, Pannone L, Farina L, Pasquini L, Martinelli S, De Benedetti F, and Tartaglia M. 2022. Mutations at the C-terminus of CDC42 cause distinct hematopoietic and autoinflammatory disorders. J Allergy Clin Immunol 150: 223–228. [DOI] [PubMed] [Google Scholar]
- 72.Lam MT, Coppola S, Krumbach OHF, Prencipe G, Insalaco A, Cifaldi C, Brigida I, Zara E, Scala S, Di Cesare S, Martinelli S, Di Rocco M, Pascarella A, Niceta M, Pantaleoni F, Ciolfi A, Netter P, Carisey AF, Diehl M, Akbarzadeh M, Conti F, Merli P, Pastore A, Levi Mortera S, Camerini S, Farina L, Buchholzer M, Pannone L, Cao TN, Coban-Akdemir ZH, Jhangiani SN, Muzny DM, Gibbs RA, Basso-Ricci L, Chiriaco M, Dvorsky R, Putignani L, Carsetti R, Janning P, Stray-Pedersen A, Erichsen HC, Horne A, Bryceson YT, Torralba-Raga L, Ramme K, Rosti V, Bracaglia C, Messia V, Palma P, Finocchi A, Locatelli F, Chinn IK, Lupski JR, Mace EM, Cancrini C, Aiuti A, Ahmadian MR, Orange JS, De Benedetti F, and Tartaglia M. 2019. A novel disorder involving dyshematopoiesis, inflammation, and HLH due to aberrant CDC42 function. J Exp Med 216: 2778–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang M, Cron RR, Absher D, Atkinson P, Chatham WW, and Cron RQ. 2020. Characterization of DOCK8 as a novel gene associated with hemophagocytic lymphohistiocytosis (abstract). J Immunol 204: 145.144. [Google Scholar]
- 74.Nishitani-Isa M, Mukai K, Honda Y, Nihira H, Tanaka T, Shibata H, Kodama K, Hiejima E, Izawa K, Kawasaki Y, Osawa M, Katata Y, Onodera S, Watanabe T, Uchida T, Kure S, Takita J, Ohara O, Saito MK, Nishikomori R, Taguchi T, Sasahara Y, and Yasumi T. 2022. Trapping of CDC42 C-terminal variants in the Golgi drives pyrin inflammasome hyperactivation. J Exp Med 219: e20211889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, DiMattia MA, Zaal KJ, Sanchez GA, Kim H, Chapelle D, Plass N, Huang Y, Villarino AV, Biancotto A, Fleisher TA, Duncan JA, O'Shea JJ, Benseler S, Grom A, Deng Z, Laxer RM, and Goldbach-Mansky R. 2014. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet 46: 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romberg N, Al Moussawi K, Nelson-Williams C, Stiegler AL, Loring E, Choi M, Overton J, Meffre E, Khokha MK, Huttner AJ, West B, Podoltsev NA, Boggon TJ, Kazmierczak BI, and Lifton RP. 2014. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet 46: 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weiss ES, Girard-Guyonvarc'h C, Holzinger D, de Jesus AA, Tariq Z, Picarsic J, Schiffrin EJ, Foell D, Grom AA, Ammann S, Ehl S, Hoshino T, Goldbach-Mansky R, Gabay C, and Canna SW. 2018. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood 131: 1442–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morimoto A, Nakazawa Y, and Ishii E. 2016. Hemophagocytic lymphohistiocytosis: Pathogenesis, diagnosis, and management. Pediatr Int 58: 817–825. [DOI] [PubMed] [Google Scholar]
- 79.Canna SW, and Marsh RA. 2020. Pediatric hemophagocytic lymphohistiocytosis. Blood 135: 1332–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Terrell CE, and Jordan MB. 2013. Perforin deficiency impairs a critical immunoregulatory loop involving murine CD8(+) T cells and dendritic cells. Blood 121: 5184–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jessen B, Maul-Pavicic A, Ufheil H, Vraetz T, Enders A, Lehmberg K, Langler A, Gross-Wieltsch U, Bay A, Kaya Z, Bryceson YT, Koscielniak E, Badawy S, Davies G, Hufnagel M, Schmitt-Graeff A, Aichele P, Zur Stadt U, Schwarz K, and Ehl S. 2011. Subtle differences in CTL cytotoxicity determine susceptibility to hemophagocytic lymphohistiocytosis in mice and humans with Chediak-Higashi syndrome. Blood 118: 4620–4629. [DOI] [PubMed] [Google Scholar]
- 82.Pachlopnik Schmid J, Ho CH, Diana J, Pivert G, Lehuen A, Geissmann F, Fischer A, and de Saint Basile G. 2008. A Griscelli syndrome type 2 murine model of hemophagocytic lymphohistiocytosis (HLH). Eur J Immunol 38: 3219–3225. [DOI] [PubMed] [Google Scholar]
- 83.Crozat K, Hoebe K, Ugolini S, Hong NA, Janssen E, Rutschmann S, Mudd S, Sovath S, Vivier E, and Beutler B. 2007. Jinx, an MCMV susceptibility phenotype caused by disruption of Unc13d: a mouse model of type 3 familial hemophagocytic lymphohistiocytosis. J Exp Med 204: 853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen M, Felix K, and Wang J. 2012. Critical role for perforin and Fas-dependent killing of dendritic cells in the control of inflammation. Blood 119: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M, Kambayashi T, and Koretzky GA. 2011. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest 121: 2264–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Canna SW, Wrobel J, Chu N, Kreiger PA, Paessler M, and Behrens EM. 2013. Interferon-gamma mediates anemia but is dispensable for fulminant toll-like receptor 9-induced macrophage activation syndrome and hemophagocytosis in mice. Arthritis Rheum 65: 1764–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weaver LK, Chu N, and Behrens EM. 2016. TLR9-mediated inflammation drives a Ccr2-independent peripheral monocytosis through enhanced extramedullary monocytopoiesis. Proc Natl Acad Sci U S A 113: 10944–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahajan S, Decker CE, Yang Z, Veis D, Mellins ED, and Faccio R. 2019. Plcgamma2/Tmem178 dependent pathway in myeloid cells modulates the pathogenesis of cytokine storm syndrome. J Autoimmun 100: 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Y, Kong F, Wang S, Yu M, Xu Y, Kang J, Tu S, and Li F. 2021. Increased levels of serum interleukin-10 are associated with poor outcome in adult hemophagocytic lymphohistiocytosis patients. Orphanet J Rare Dis 16: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weaver LK, Minichino D, Biswas C, Chu N, Lee JJ, Bittinger K, Albeituni S, Nichols KE, and Behrens EM. 2019. Microbiota-dependent signals are required to sustain TLR-mediated immune responses. JCI Insight 4: e124370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Strippoli R, Carvello F, Scianaro R, De Pasquale L, Vivarelli M, Petrini S, Bracci-Laudiero L, and De Benedetti F. 2012. Amplification of the response to Toll-like receptor ligands by prolonged exposure to interleukin-6 in mice: implication for the pathogenesis of macrophage activation syndrome. Arthritis and rheumatism 64: 1680–1688. [DOI] [PubMed] [Google Scholar]
- 92.Prencipe G, Caiello I, Pascarella A, Grom AA, Bracaglia C, Chatel L, Ferlin WG, Marasco E, Strippoli R, de Min C, and De Benedetti F. 2018. Neutralization of IFN-gamma reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J Allergy Clin Immunol 141: 1439–1449. [DOI] [PubMed] [Google Scholar]
- 93.Wang A, Pope SD, Weinstein JS, Yu S, Zhang C, Booth CJ, and Medzhitov R. 2019. Specific sequences of infectious challenge lead to secondary hemophagocytic lymphohistiocytosis-like disease in mice. Proc Natl Acad Sci U S A 116: 2200–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Akilesh HM, Buechler MB, Duggan JM, Hahn WO, Matta B, Sun X, Gessay G, Whalen E, Mason M, Presnell SR, Elkon KB, Lacy-Hulbert A, Barnes BJ, Pepper M, and Hamerman JA. 2019. Chronic TLR7 and TLR9 signaling drives anemia via differentiation of specialized hemophagocytes. Science 363: eaao5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yanagimachi M, Naruto T, Miyamae T, Hara T, Kikuchi M, Hara R, Imagawa T, Mori M, Sato H, Goto H, and Yokota S. 2011. Association of IRF5 polymorphisms with susceptibility to macrophage activation syndrome in patients with juvenile idiopathic arthritis. J Rheumatol 38: 769–774. [DOI] [PubMed] [Google Scholar]
- 96.Ohyagi H, Onai N, Sato T, Yotsumoto S, Liu J, Akiba H, Yagita H, Atarashi K, Honda K, Roers A, Muller W, Kurabayashi K, Hosoi-Amaike M, Takahashi N, Hirokawa M, Matsushima K, Sawada K, and Ohteki T. 2013. Monocyte-derived dendritic cells perform hemophagocytosis to fine-tune excessive immune responses. Immunity 39: 584–598. [DOI] [PubMed] [Google Scholar]
- 97.Canna SW, Costa-Reis P, Bernal WE, Chu N, Sullivan KE, Paessler ME, and Behrens EM. 2014. Brief report: alternative activation of laser-captured murine hemophagocytes. Arthritis Rheumatol 66: 1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Behrens EM 2008. Macrophage activation syndrome in rheumatic disease: what is the role of the antigen presenting cell? Autoimmun Rev 7: 305–308. [DOI] [PubMed] [Google Scholar]
- 99.Huang Z, You X, Chen L, Du Y, Brodeur K, Jee H, Wang Q, Linder G, Darbousset R, Cunin P, Chang MH, Wactor A, Wauford BM, Todd MJC, Wei K, Li Y, Levescot A, Iwakura Y, Pascual V, Baldwin NE, Quartier P, Li T, Gianatasio MT, Hasserjian RP, Henderson LA, Sykes DB, Mellins ED, Canna SW, Charles JF, Nigrovic PA, and Lee PY. 2022. mTORC1 links pathology in experimental models of Still's disease and macrophage activation syndrome. Nat Commun 13: 6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gokce M, Unal O, Hismi B, Gumruk F, Coskun T, Balta G, Unal S, Cetin M, Kalkanoglu-Sivri HS, Dursun A, and Tokatli A. 2012. Secondary hemophagocytosis in 3 patients with organic acidemia involving propionate metabolism. Pediatr Hematol Oncol 29: 92–98. [DOI] [PubMed] [Google Scholar]
- 101.Bracaglia C, de Graaf K, Pires Marafon D, Guilhot F, Ferlin W, Prencipe G, Caiello I, Davi S, Schulert G, Ravelli A, Grom AA, de Min C, and De Benedetti F. 2017. Elevated circulating levels of interferon-gamma and interferon-gamma-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Annals of the rheumatic diseases 76: 166–172. [DOI] [PubMed] [Google Scholar]
- 102.Locatelli F, Jordan MB, Allen C, Cesaro S, Rizzari C, Rao A, Degar B, Garrington TP, Sevilla J, Putti MC, Fagioli F, Ahlmann M, Dapena Diaz JL, Henry M, De Benedetti F, Grom A, Lapeyre G, Jacqmin P, Ballabio M, and de Min C. 2020. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N Engl J Med 382: 1811–1822. [DOI] [PubMed] [Google Scholar]
- 103.Zoller EE, Lykens JE, Terrell CE, Aliberti J, Filipovich AH, Henson PM, and Jordan MB. 2011. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med 208: 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tesi B, Sieni E, Neves C, Romano F, Cetica V, Cordeiro AI, Chiang S, Schlums H, Galli L, Avenali S, Tondo A, Canessa C, Henter JI, Nordenskjold M, Hsu AP, Holland SM, Neves JF, Azzari C, and Bryceson YT. 2015. Hemophagocytic lymphohistiocytosis in 2 patients with underlying IFN-gamma receptor deficiency. J Allergy Clin Immunol 135: 1638–1641. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Q, Zhao YZ, Ma HH, Wang D, Cui L, Li WJ, Wei A, Wang CJ, Wang TY, Li ZG, and Zhang R. 2022. A study of ruxolitinib response-based stratified treatment for pediatric hemophagocytic lymphohistiocytosis. Blood 139: 3493–3504. [DOI] [PubMed] [Google Scholar]
- 106.Das R, Guan P, Sprague L, Verbist K, Tedrick P, An QA, Cheng C, Kurachi M, Levine R, Wherry EJ, Canna SW, Behrens EM, and Nichols KE. 2016. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood 127: 1666–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joly JA, Vallee A, Bourdin B, Bourbonnais S, Patey N, Gaboury L, Theoret Y, and Decaluwe H 2023. Combined IFN-gamma and JAK inhibition to treat hemophagocytic lymphohistiocytosis in mice. J Allergy Clin Immunol 151: 247–259. [DOI] [PubMed] [Google Scholar]
- 108.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, and Panoskaltsis N. 2006. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355: 1018–1028. [DOI] [PubMed] [Google Scholar]
- 109.Cavaillon JM, Singer M, and Skirecki T. 2020. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol Med 12: e10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chaturvedi V, Marsh RA, Zoref-Lorenz A, Owsley E, Chaturvedi V, Nguyen TC, Goldman JR, Henry MM, Greenberg JN, Ladisch S, Hermiston ML, Jeng M, Naqvi A, Allen CE, Wong HR, and Jordan MB. 2021. T-cell activation profiles distinguish hemophagocytic lymphohistiocytosis and early sepsis. Blood 137: 2337–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Matteis A, Colucci M, Rossi MN, Caiello I, Merli P, Tumino N, Bertaina V, Pardeo M, Bracaglia C, Locatelli F, De Benedetti F, and Prencipe G. 2022. Expansion of CD4dimCD8+ T cells characterizes macrophage activation syndrome and other secondary HLH. Blood 140: 262–273. [DOI] [PubMed] [Google Scholar]
- 112.Marsh RA, Allen CE, McClain KL, Weinstein JL, Kanter J, Skiles J, Lee ND, Khan SP, Lawrence J, Mo JQ, Bleesing JJ, Filipovich AH, and Jordan MB. 2013. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer 60: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mahlaoui N, Ouachee-Chardin M, de Saint Basile G, Neven B, Picard C, Blanche S, and Fischer A. 2007. Immunotherapy of familial hemophagocytic lymphohistiocytosis with antithymocyte globulins: a single-center retrospective report of 38 patients. Pediatrics 120: e622–e628. [DOI] [PubMed] [Google Scholar]
- 114.Chellapandian D, Das R, Zelley K, Wiener SJ, Zhao H, Teachey DT, Nichols KE, and E.-H. R. S. Group. 2013. Treatment of Epstein Barr virus-induced haemophagocytic lymphohistiocytosis with rituximab-containing chemo-immunotherapeutic regimens. Br J Haematol 162: 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Minoia F, Tibaldi J, Muratore V, Gallizzi R, Bracaglia C, Arduini A, Comak E, Vougiouka O, Trauzeddel R, Filocamo G, Mastrangelo A, Micalizzi C, Kasapcopur O, Unsal E, Kitoh T, Tsitsami E, Kostik M, Schmid JP, Prader S, Laube G, Maritsi D, Jelusic M, Shenoi S, Vastert S, Ardissino G, Cron RQ, Ravelli A, and M. A. s. W. G. o. t. P. R. E. Society. 2021. Thrombotic Microangiopathy Associated with Macrophage Activation Syndrome: A Multinational Study of 23 Patients. J Pediatr 235: 196–202. [DOI] [PubMed] [Google Scholar]
- 116.Zhang R, Zhou M, Qi J, Miao W, Zhang Z, Wu D, and Han Y. 2020. Efficacy and Safety of Eculizumab in the Treatment of Transplant-Associated Thrombotic Microangiopathy: A Systematic Review and Meta-Analysis. Front Immunol 11: 564647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bergsten E, Horne A, Arico M, Astigarraga I, Egeler RM, Filipovich AH, Ishii E, Janka G, Ladisch S, Lehmberg K, McClain KL, Minkov M, Montgomery S, Nanduri V, Rosso D, and Henter JI. 2017. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood 130: 2728–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Larroche C, Bruneel F, Andre MH, Bader-Meunier B, Baruchel A, Tribout B, Genereau T, Zunic P, and T. Comite d'Evaluation et de Diffusion des Innovation. 2000. [Intravenously administered gamma-globulins in reactive hemaphagocytic syndrome. Multicenter study to assess their importance, by the immunoglobulins group of experts of CEDIT of the AP-HP]. Ann Med Interne (Paris) 151: 533–539. [PubMed] [Google Scholar]
- 119.Demirkol D, Yildizdas D, Bayrakci B, Karapinar B, Kendirli T, Koroglu TF, Dursun O, Erkek N, Gedik H, Citak A, Kesici S, Karabocuoglu M, Carcillo JA, and H. L. H. M. A. S. C. C. S. G. Turkish Secondary. 2012. Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: what is the treatment? Crit Care 16: R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Behrens EM, Kreiger PA, Cherian S, and Cron RQ. 2006. Interleukin 1 receptor antagonist to treat cytophagic histiocytic panniculitis with secondary hemophagocytic lymphohistiocytosis. J Rheumatol 33: 2081–2084. [PubMed] [Google Scholar]
- 121.Mehta P, Cron RQ, Hartwell J, Manson JJ, and Tattersall RS. 2020. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol 2: e358–e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, and Opal SM. 2016. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med 44: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eloseily EM, Weiser P, Crayne CB, Haines H, Mannion ML, Stoll ML, Beukelman T, Atkinson TP, and Cron RQ. 2020. Benefit of Anakinra in Treating Pediatric Secondary Hemophagocytic Lymphohistiocytosis. Arthritis Rheumatol 72: 326–334. [DOI] [PubMed] [Google Scholar]
- 124.Miettunen PM, Narendran A, Jayanthan A, Behrens EM, and Cron RQ. 2011. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology (Oxford) 50: 417–419. [DOI] [PubMed] [Google Scholar]
- 125.Jiang JG, Liu CJ, Yeh CM, Yang CF, Liu YC, Wang HY, Ko PS, Chen PM, Yu YB, Gau JP, and Tsai CK. 2022. Prognostic factors in patients with bone marrow hemophagocytosis and its association with hematologic malignancies. Hematol Oncol, in press. [DOI] [PubMed] [Google Scholar]
- 126.Bami S, Vagrecha A, Soberman D, Badawi M, Cannone D, Lipton JM, Cron RQ, and Levy CF. 2020. The use of anakinra in the treatment of secondary hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 67: e28581. [DOI] [PubMed] [Google Scholar]
- 127.Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, Kalomenidis I, Chrysos G, Angheben A, Kainis I, Alexiou Z, Castelli F, Serino FS, Tsilika M, Bakakos P, Nicastri E, Tzavara V, Kostis E, Dagna L, Koufargyris P, Dimakou K, Savvanis S, Tzatzagou G, Chini M, Cavalli G, Bassetti M, Katrini K, Kotsis V, Tsoukalas G, Selmi C, Bliziotis I, Samarkos M, Doumas M, Ktena S, Masgala A, Papanikolaou I, Kosmidou M, Myrodia DM, Argyraki A, Cardellino CS, Koliakou K, Katsigianni EI, Rapti V, Giannitsioti E, Cingolani A, Micha S, Akinosoglou K, Liatsis-Douvitsas O, Symbardi S, Gatselis N, Mouktaroudi M, Ippolito G, Florou E, Kotsaki A, Netea MG, Eugen-Olsen J, Kyprianou M, Panagopoulos P, Dalekos GN, and Giamarellos-Bourboulis EJ. 2021. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 27: 1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Group, W. H. O. R. E. A. f. C.-T. W., Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, Savovic J, Tierney J, Baron G, Benbenishty JS, Berry LR, Broman N, Cavalcanti AB, Colman R, De Buyser SL, Derde LPG, Domingo P, Omar SF, Fernandez-Cruz A, Feuth T, Garcia F, Garcia-Vicuna R, Gonzalez-Alvaro I, Gordon AC, Haynes R, Hermine O, Horby PW, Horick NK, Kumar K, Lambrecht BN, Landray MJ, Leal L, Lederer DJ, Lorenzi E, Mariette X, Merchante N, Misnan NA, Mohan SV, Nivens MC, Oksi J, Perez-Molina JA, Pizov R, Porcher R, Postma S, Rajasuriar R, Ramanan AV, Ravaud P, Reid PD, Rutgers A, Sancho-Lopez A, Seto TB, Sivapalasingam S, Soin AS, Staplin N, Stone JH, Strohbehn GW, Sunden-Cullberg J, Torre-Cisneros J, Tsai LW, van Hoogstraten H, van Meerten T, Veiga VC, Westerweel PE, Murthy S, Diaz JV, Marshall JC, and Sterne JAC. 2021. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 326: 499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, Przepiorka D, Farrell AT, and Pazdur R. 2018. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist 23: 943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cron RQ 2022. No perfect therapy for the imperfect COVID-19 cytokine storm. Lancet Rheumatol 4: e308–e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cron RQ 2020. Coronavirus is the trigger but the immune response is deadly. Lancet Rheumatol 2: e370–e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cron RQ 2021. COVID-19 cytokine storm: targeting the appropriate cytokine. Lancet Rheumatol 3: e236–e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Canna SW, Girard C, Malle L, de Jesus A, Romberg N, Kelsen J, Surrey LF, Russo P, Sleight A, Schiffrin E, Gabay C, Goldbach-Mansky R, and Behrens EM. 2017. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol 139: 1698–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yasin S, Fall N, Brown RA, Henderlight M, Canna SW, Girard-Guyonvarc'h C, Gabay C, Grom AA, and Schulert GS. 2020. IL-18 as a biomarker linking systemic juvenile idiopathic arthritis and macrophage activation syndrome. Rheumatology (Oxford) 59: 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Keenan C, Nichols KE, and Albeituni S. 2021. Use of the JAK Inhibitor Ruxolitinib in the Treatment of Hemophagocytic Lymphohistiocytosis. Front Immunol 12: 614704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meyer LK, Verbist KC, Albeituni S, Scull BP, Bassett RC, Stroh AN, Tillman H, Allen CE, Hermiston ML, and Nichols KE. 2020. JAK/STAT pathway inhibition sensitizes CD8 T cells to dexamethasone-induced apoptosis in hyperinflammation. Blood 136: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Behrens EM, and Koretzky GA. 2017. Review: Cytokine Storm Syndrome: Looking Toward the Precision Medicine Era. Arthritis Rheumatol 69: 1135–1143. [DOI] [PubMed] [Google Scholar]