Abstract
Gastric cancer, the fifth leading cause of cancer worldwide, is estimated to be responsible for approximately 1.4% of all new cancers and 1.8% of all cancer-related deaths in the United States. Despite declining incidence rates and improved survival rates, however, gastric cancer continues to disproportionately affect racial and ethnic minorities and individuals of lower socioeconomic status at higher rates than the general population. To improve outcomes globally and address disparities within the United States, continued improvements are needed in risk factor modification and biomarker development and to improve access to existing preventative measures such as genetic testing and H. pylori eradication testing, in addition to expanding upon current clinical guidelines for premalignant disease to address gaps in endoscopic surveillance and early detection.
Keywords: gastric cancer, epidemiology, risk factors, genetic syndromes, prevention
Introduction
Gastric cancer is the fifth leading cause of cancer worldwide with 1,089,103 new cases diagnosed in 2020, and with marked geographic variability in incidence (Figure 1).1 In 2022, gastric cancer is estimated to be responsible for approximately 1.4 percent of all new cancers in the United States (U.S.) and 1.8 percent of all cancer-related deaths.2 Despite declining incidence rates and improved survival rates, gastric cancer continues to disproportionately affect racial and ethnic minorities and individuals of lower socioeconomic status at higher rates than the general population.3,4 In order to improve outcomes globally and address disparities within the U.S., continued improvements are needed in risk factor modification, biomarker development, and early detection of gastric cancer lesions. This review will focus on recent advances in these areas over the past few years.
Figure 1:
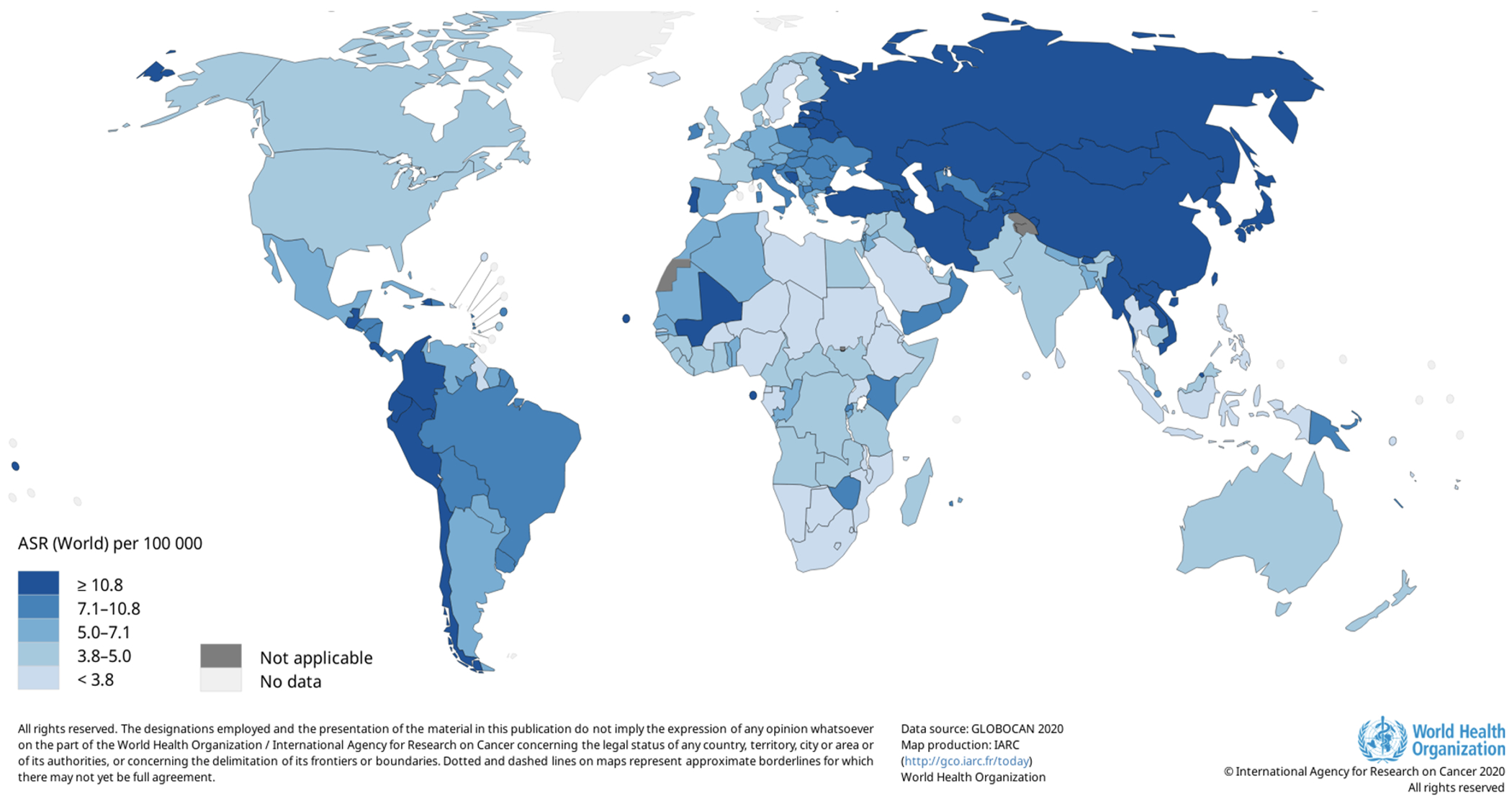
Estimated age-standardized incidence rates (World) in 2020, stomach, both sexes, all ages.
Background/Descriptive Epidemiology
In this review, we will focus on risk factors for gastric adenocarcinoma, the most common type of gastric cancer. Most gastric adenocarcinomas are thought to develop through a gradual progression from Helicobacter pylori (H. pylori)-induced chronic gastritis to atrophic gastritis, intestinal metaplasia, dysplasia, and ultimately adenocarcinoma.5 Although over half of the world’s population is infected with H. pylori, only a small subset of infected individuals progress to gastric cancer, and this process occurs over the course of around 15-20 years.6
In 2014, The Cancer Genome Atlas (TCGA) identified four molecular subtypes of gastric cancer: Epstein-Barr virus (EBV) subtype, microsatellite instability (MSI) subtype, chromosomal instability (CIN) subtype, and genomically stable subtype.7 Gastric cancer is also classified into cardia and non-cardia subtypes depending on its location (proximal or distal, respectively) in the stomach. In 2018, gastric non-cardia cancer accounted for approximately 82 percent of gastric cancer cases worldwide compared to 18 percent for cardia cases.8 Gastric cardia cancers are more likely to be associated with Epstein-Barr virus and their association with H. pylori is debated.7,9,10 Within the U.S., gastric cardia cancer tends to affect predominantly non-Hispanic White individuals, similar to esophageal adenocarcinoma, while non-cardia cancer has a greater incidence among individuals from underrepresented racial and ethnic groups and residents of lower socioeconomic status.11 Gastric cardia cancer is also more likely to be associated with diet and higher BMI.12 Both cardia and non-cardia gastric cancers have been significantly associated with male sex and tobacco smoking.13
Risk Factors
H. pylori infection
Infection with H. pylori is the most important risk factor for gastric cancer, responsible for an estimated 89% of non-cardia gastric cancers.5 H. pylori is a gram-negative bacterium that infects approximately 4.4 billion individuals worldwide, over half of the world’s population.6 In 1994, H. pylori was classified as a World Health Organization Class I carcinogen, the first bacteria to be directly associated with the development of cancer.6 H. pylori-induced gastric cancer is thought to develop through chronic inflammation leading to atrophic gastritis, gastric intestinal metaplasia, dysplasia, and ultimately gastric adenocarcinoma.14
When H. pylori infects the gastric mucosa, it induces the production of several key players in the innate and adaptive immune response, including interferon gamma (IFN-y), tumor necrosis factor-alpha (TNF-a), and several cytokines, especially interleukin 8 (IL-8) and 10 (IL-10).15 In recent years, differences in H. pylori strains have gained attention, as certain virulence factors are more closely associated with gastric cancer. For example, cytotoxin associated gene A (cagA) and more virulent forms of vacuolating cytotoxin A (vacA), are most closely associated increased gastric inflammation, persistent infection, and gastric cancer risk.16 cagA has been shown to upregulate proliferative and inflammatory pathways, interfere with tumor suppressors, and enable cells to evade the immune response, thereby promoting tumor development and growth.17 The presence of serum antibodies to H. pylori antigens and virulence factors such as cagA may contribute to gastric cancer risk. For example, a case-control study of non-cardia gastric cancer cases in China, Japan, and Korea found that seropositivity to proteins cagA, vacA, OMP, HcpC, HP0305, GroEL, NapA, HyuA, and Cad was associated with a significantly increased odds of gastric cancer (OR range, 1.29-3.26).18
Although H. pylori infection has most consistently been associated with non-cardia gastric cancer rather than cardia gastric cancer, a recent study in China also identified a significant association of H. pylori infection with cardia cancer risk. A cohort study of gastric cancer cases in China found that 62.1% of cardia gastric cancer cases and 78.5% of non-cardia cases could be attributed to H. pylori infection, with a hazard ratio of 3.06 (95% CI, 1.54-6.10) for cardia cancer cases and 5.94 (95% CI, 3.25-10.86) for non-cardia cases.9 vacA and cagA seropositivity were also associated with an increased risk of gastric cardia cancer.
Nonmodifiable Risk Factors
Race, ethnicity, age, and sex are all nonmodifiable risk factors that are associated with gastric cancer risk. Here, we consider their individual associations on gastric cancer risk in addition to their intersections.
Race and Ethnicity
Within the U.S., gastric cancer continues to disproportionately affect racial and ethnic minorities, and self-identified and electronic health record (EHR)-identified race and ethnicity have been recognized as independent risk factors associated with gastric cancer.19 From 2015 to 2019, the age-adjusted rates of new gastric cancer cases among non-Hispanic White men and women were 7.6 per 100,000 and 3.7 per 100,000 respectively, compared with 13.7 and 7.6 among American Indian men and women, 12.3 and 8.4 among Hispanic men and women, 12.8 and 7.3 among Asian and Pacific Islander men and women, and 13.4 and 7.8 among non-Hispanic Black men and women (see trends by age and race/ethnicity: Figure 2, panels A and B, respectively).2 When separated by subsite, non-Hispanic White individuals had the highest rates of cardia cancers (IR, 2.3; 95% CI, compared to Black, Hispanic, Asian and Pacific Islander, and American Indian and Alaska Native individuals (IR range 1.3-1.6) and the lowest rates of non-cardia gastric cancer (IR, 1.9; 95% CI, 1.8-1.9) compared to individuals from other racial groups (IR range 3.5-7.4).4
Figure 2:
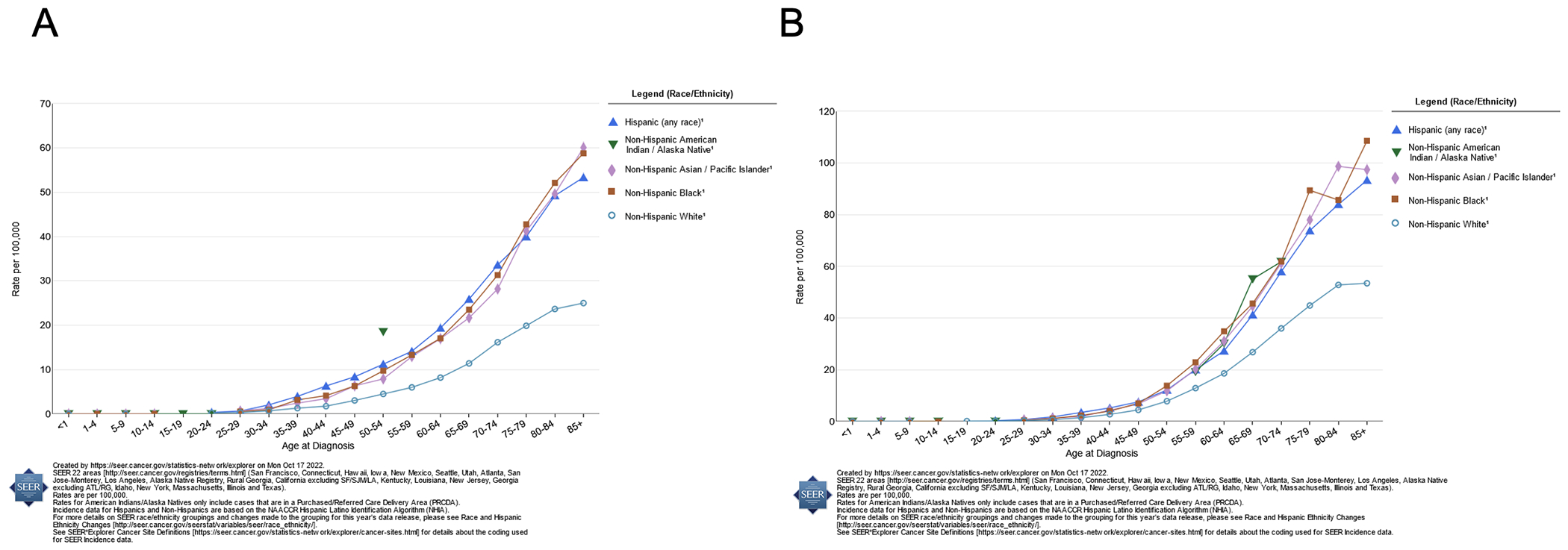
Delay-adjusted SEER stomach cancer incidence rates by age at diagnosis, 2015-2019, by race/ethnicity; panel A displays rates among women; panel B displays rates among men.
Of all cancers, gastric cancer is responsible for the greatest difference in rates of death from cancer between Black and White Americans, with Black men and women dying from gastric cancer at over twice the rate of non-Hispanic White men and women.3 Notably, this disparity is restricted to non-cardia gastric tumors, which affect Black patients at higher rates (55%) compared to White patients (33%), with both groups having comparable survival rates for cardia tumors.3,4
Disparities in non-cardia gastric cancer incidence and survival by Hispanic ethnicity have also been identified. The incidence of non-cardia gastric cancer among the Hispanic White population is more than two times that among the non-Hispanic White population (5.9 per 100,000 compared to 2.1 per 100,000).20 In contrast, the incidence of cardia gastric cancer cases in the Hispanic White population (1.5 per 100,000) is lower than that in the non-Hispanic White population (2.3 per 100,000).20 Although gastric cancer incidence has been declining in almost all ethnic and racial groups, incidence rates, particularly for stage IV gastric cancer, have been increasing in Hispanic men aged 20-49 years (APC, 1.55%; 95% CI, 0.26-2.86%).21 A study of cancer-related deaths in Florida from 2008-2012 examined gastric cancer mortality rates according to major Hispanic groups (Cuban, Puerto Rican, Mexican, Central American, and South American).22 Compared to non-Hispanic White individuals, Central American men and women (male RR, 3.22; 95% CI, 2.35-4.4; female RR, 2.52; 95% CI, 1.72-3.68) and South American men and women (male RR, 3.18; 95% CI, 2.59-3.9; female RR, 3.19; 95% CI, 2.53-4.03) had the highest stomach cancer mortality rates, while Cuban men and women had the lowest mortality rates of all Hispanic groups (male RR, 1.42; 95% CI, 1.21-1.68; female RR, 1.36; 95% CI,1.1-1.66).22 These differences may reflect variation in the prevalence of H. pylori infection, socioeconomic status across different countries, and tumor classification.
More recent studies of gastric cancer incidence have also examined heterogeneity within growing Asian American subpopulations. This is particularly relevant given the rapid growth of these populations and that many of these individuals immigrate from countries with higher rates of gastric cancer than those found in the U.S. A recent study in California of adults aged 50 years and older from 2011 to 2015 found that all non-White groups were at anywhere from a 1.8 to 14.5-fold increased risk of developing non-cardia gastric cancer (IR, 3.70; 95% CI, 3.49-3.92), with Korean Americans being at greatest risk (IR, 49.0; 95% CI, 43.9-54.6).23,24 Among Asian American subgroups, incidence rates of non-cardia gastric cancer were also high among Vietnamese (23.9), Southeast Asian (21.1), Japanese (19.2), and Chinese Americans (17.6), with Filipino and South Asian Americans having comparatively lower incidence rates (6.69 and 7.75, respectively), although still almost twice the incidence rate of gastric cancer in non-Hispanic Whites.23 In contrast, the non-Hispanic White population had higher rates of cardia gastric cancer compared to non-cardia gastric cancer,23 and the non-Hispanic White incidence rate of cardia gastric cancer was surpassed only by those of Korean Americans and Japanese Americans. Despite these observed disparities in incidence rates, Asian Americans had higher rates of surgical tumor resection and survival compared to non-Hispanic whites.24
Alaska Native and American Indian populations have also been shown to have higher rates of gastric cancer, especially non-cardia cancer, compared to the U.S. White population (adjusted-IRR, 1.72; 95% CI, 1.5-1.97).4 Gastric cancer incidence rates, specifically for non-Hispanic American Indian and Alaska Native females living in urban areas, have increased by 76% from 1999-2017, compared to a decline in incidence by 22% among non-Hispanic White females.25 A study of gastric cancer incidence among American Indian and Alaska Native groups from 2005-2016 by region (Northern Plains, Alaska, Southern Plains, Pacific Coast, Eastern U.S., and Southwest U.S.) found significantly increased gastric cancer risk for all groups compared to White individuals, with populations in Alaska having the great risk (RR, 4.23; 95% CI, 3.47-5.17).26 Specifically, the prevalence of non-cardia tumors and signet ring cell carcinomas was around threefold greater among the Alaska Native population than the non-Hispanic White population from 2006-2014.27 Alaska Native individuals were also more likely to have a poorer prognosis with significantly more diagnoses of Stage IV gastric cancer (50% vs 37.6%)27 and significantly more likely to be diagnosed with gastric cancer at a younger age (<40 years) compared to White individuals (11% vs. 3%).28
Differences in gastric cancer incidence and outcomes by race and ethnicity highlight underlying disparities in exposures to risk factors including H. pylori infection, as well as structural racism and inequitable access to resources – factors that are just now starting to be explored in relation to gastric cancer. Given that H. pylori infection is the greatest risk factor for non-cardia gastric cancer, trends in gastric cancer cases by race should be contextualized within trends in H. pylori infection by race. A study of gastric cancer incidence in Tennessee found that Black patients diagnosed with gastric cancer had a greater than three times higher rate of concurrent H. pylori infection than White patients at the time of diagnosis.29 An analysis of participants in five large cohort studies in the U.S. found that Black individuals had an over twofold odds of having sero-prevalent H. pylori, and an over threefold higher odds for seropositivity to a virulent cagA-positive strain of H. pylori, as compared to White individuals, and moreover that while cagA and H. pylori seroprevalence is decreasing over time among White individuals, it is not decreasing among Black individuals .30 Immigration from countries with a higher incidence of both H. pylori infection and non-cardia gastric cancer may also contribute to observed differences in individual of different racial and ethnic backgrounds. Self-identified race and ethnicity within the U.S. do not provide a translatable basis for identifying differences in genetic predisposition to gastric cancer between groups. Disparities in exposures to other risk factors by race, such as diet, smoking, and alcohol use may also contribute to observed differences in gastric cancer risk between racial and ethnic groups. While socioeconomic status can also vary with race and ethnicity, lower socioeconomic status is associated with an increased risk of gastric cancer even within racial groups and will be discussed further in a separate section.4 Further research is needed to examine the specific interplay of these exposures with racial and ethnic identities directly in relation to gastric cancer risk.
Age
Gastric cancer risk increases with age, with most diagnoses taking place among individuals aged 65-74 (median age of diagnosis = 68) in the U.S.2 However, in certain groups, gastric cancer is being diagnosed in an increasing number of younger adults. In a study of gastric cancer patients in the southeastern U.S., Black patients presented with gastric cancer at a significantly earlier age (median age of diagnosis = 64 years) compared to white patients (72.5 years).29 Asian American patients and Alaska Native patients are also more likely to present with gastric cancer at an earlier age compared to White cancer patients.24,28
Sex
Over the past century, gastric cancer has tended to affect men at higher rates than women, with men also being more likely to die from gastric cancer than women. From 2010 to 2014, incidence rates of both cardia and non-cardia gastric cancer among men were higher than those of women across racial and ethnic groups.31 This may be explained by differential exposure to other risk factors for gastric cancer, such as alcohol and smoking. However, some recent studies demonstrate changing trends in gastric cancer incidence, particularly among young women. For example, in a large cohort study of gastric cancer incidence in the U.S. from 1995 to 2013, non-Hispanic White women under age 50 saw an increase in the number of gastric adenocarcinoma cases by 2.6% each year (95% CI, 1.7%-3.4%).32 This increased incidence rate among White women, specifically aged 25-39, was also observed from 1977 to 2006.33 Another study similarly demonstrated that rates of non-cardia gastric cancer were increasing in women under 40 years by about 2% per year between 1997 and 2014.31 These observed changes in trends have been suggested to be attributed to increasing rates of autoimmune causes of atrophic gastritis as opposed to H. pylori-induced gastritis. However, a recent study of young White women in Finland found that the majority of women with young-onset gastric cancer did have serologic evidence of H. pylori infection (68% of cases compared to 23% of controls), and that while serologic evidence of autoimmune gastritis was also associated with early-onset gastric cancer risk, it was much less common than H. pylori (8% of cases compared to 3% of controls).34 Moreover, there is now increasing evidence that autoimmune gastritis without H. pylori infection does not significantly increase risk for gastric adenocarcinoma.35 Despite possible increases the incidence of gastric cancer among younger White women, these incidence rates continue to remain lower than those for all other major racial and ethnic groups (Figure 2a).
Socioeconomic Status
Lower socioeconomic status has been associated with both increased prevalence of H. pylori seropositivity, particularly the virulent cagA+ strains, and increased risk of developing gastric cancer, especially non-cardia gastric cancer.36,37 A study of gastric cancer cases from 2000-2014 found a 1.3 times greater adjusted incidence rate ratio of gastric cancer among individuals from the lowest neighborhood socioeconomic quintile compared to those from the highest, although this variation was limited to non-cardia gastric cancer cases only.4 These disparities in cancer incidence and outcomes may be accounted for by factors more directly linked to socioeconomic status such as H. pylori infection, comorbid conditions, and long-term access to health care. Another study found reduced rates of gastric cancer among both Hispanic individuals (OR, 0.79) and non-Hispanic White individuals (OR, 0.76) in zip codes with higher neighborhood incomes (≥$45,000) compared to neighborhoods with lower median household incomes (<$45,000).19 Notably, higher (>50%) high school education rates were identified as protective among Hispanic populations (OR, 0.67; 95% CI, 0.48-0.93).19 Education and literacy are closely linked to socioeconomic status and may also be independently associated with gastric cancer incidence. A meta-analysis of participant data within the Stomach Cancer Pooling (StoP) Project, which includes case-control studies across Europe, North America, and Asia, examined gastric cancer risk and education level grouped according to the 2011 UNESCO international standard classification of education (ISCED).38,39 After adjusting for age, sex, race/ethnicity, and factors including fruit/vegetable consumption, smoking, and alcohol use, they found that intermediate and high levels of education were associated with a significantly reduced risk of gastric cancer compared to low education levels (OR, 0.68; 95% CI, 0.55-0.84 and OR, 0.60; 95% CI, 0.44-0.84, respectively), and these associations were similarly observed for cardia and non-cardia cancers.38
Diet, Obesity, Smoking, and Alcohol
Diet, nutritional deficiencies and supplementation, smoking, and alcohol use, also contribute to gastric cancer risk.
Dietary Factors
Consumption of salt-preserved, smoked, and highly salted foods is associated with an increased risk of gastric cancer, likely through the induction of atrophic gastritis.14,40 Some of the decline in gastric cancer incidence over the past century may be explained by the decline in reliance on salt-preserved foods.41 A meta-analysis of 76 cohort studies found that gastric cancer risk increased by 55% with consumption of highly salted food, 15% with processed meat, and 25% with salted fish.42 An updated meta-analysis published in 2019 found that high intakes of both red and processed meats, analyzed separately, were associated with an increased risk of gastric cancer (41% and 57%, respectively), while white meat consumption was associated with a decreased risk (20%).43 Processed foods have also been recently linked to gastric cancer.44 A case-control study of patients in Brazil from 2004 to 2015 found that, even after adjusting for smoking and alcohol use, consumption of processed and ultra-processed foods was associated with an increased risk of developing gastric cancer, specifically salted bread (OR, 2.03; 95% CI, 1.3-3.18), processed meats (OR, 2.96; 95% CI, 1.82-4.81), and fried meats and fish (OR, 2.21; 95% CI, 1.13-4.3).44 There were no significant differences between associated risk ratios for cardia and non-cardia cancers.
Several different studies suggest that adherence to a diet rich in certain types of fresh fruits and vegetables is correlated with a decreased risk of gastric cancer. A pooled analysis of 15 case-controlled studies in Europe, Asia, and the Americas found that the highest and middle tertiles of citrus fruit intake, compared to the lowest, were associated with a 20% decrease in gastric cancer risk, and the protective effect of citrus fruit increased for up to three servings per week.45 After stratifying by geographical location, there was no association in studies found in North and South America, where more citrus intake originated from consumption of fruit juices.45 A prospective analysis of individuals in East Asia found that high fruit intake was associated with decreased non-cardia gastric cancer risk after adjusting for H. pylori seroprevalence, although this was more beneficial for individuals not infected with virulent, cagA-positive strains of H. pylori.46
Consumption of vegetables, especially green vegetables, has been shown to reduce the risk of developing gastric cancer by 62%.47 Interestingly, the previously discussed meta-analysis of diet and gastric cancer risk also found that consumption of white vegetables and citrus fruits significantly decreased gastric cancer risk by 33% and 10%, respectively, while consumption of tomatoes and pickled vegetables significantly increased risk by 11% and 18%.42 Pickling vegetables can introduce toxins and carcinogenic molecules such as N-nitroso compounds (also present in salt-preserved foods), and their consumption has been even more strongly associated with gastric cancer risk in other studies. An analysis of studies in both the U.S. and East Asia found that consumption of pickled vegetables increased gastric cancer risk by 52% (RR, 1.52; 95% CI, 1.37-1.68).48
An analysis of studies in the StoP Project also identified an association between consumption of allium vegetables, such as onions, garlic, and leeks, and reduced risk of gastric cancer, specifically in Asian populations (OR, 0.71; 95% CI, 0.56-0.90).49
A prospective analysis of nut products and gastric cancer in individuals from the NIH AARP Diet and Health Study identified an inverse association between the highest nut consumption (including peanuts, walnuts, and seeds) and non-cardia gastric cancer (HR, 0.73; 95% CI, 0.57-0.94).50 A similar inverse association was found for the highest peanut butter consumption (HR, 0.75; 95% CI, 0.60-0.94) with an additional decreased risk of non-cardia gastric cancer with every tablespoon.50 Calcium intake among participants within this cohort was also examined with regard to gastric cancer risk. Median total calcium intake (>881.3 mg), including both diet and supplement intake, was associated with a 23% decreased risk for non-cardia adenocarcinoma, but there was no association for cardia adenocarcinoma.51
Given its abundance in fresh citrus fruits and vegetables and potential role in inhibiting H. pylori growth, vitamin C has been studied as a possible protective factor against gastric cancer.52 Vitamin C intake was also shown to have an inverse relationship with H. pylori infection prevalence.53
A recent cross-sectional analysis of vitamin D serum levels and gastric cancer in a South Korean population found an inverse association between sufficient vitamin D concentrations (≥20 ng/mL) and gastric cancer compared to deficient vitamin D concentrations (<12 ng/mL) (OR, 0.57; 95% CI, 0.32-1.00), and a continued inverse association with a 5 ng/mL increment increase of vitamin D concentration (OR, 0.84; 95% CI, 0.72-0.98).54
Body Mass Index
Obesity, defined as body mass index (BMI) greater than 30, which is influenced by diet, lifestyle, environmental factors, and genetics, has been found to have a U-shaped association with risk of non-cardia gastric cancer. An analysis of BMI in patients with gastric cancer in the Asia Cohort Consortium found a significantly increased risk of gastric cancer, especially non-cardia, intestinal-type gastric cancer, in individuals with low BMI (<18.5 kg/m2; HR,1.15; 95% CI, 1.05-1.25) and high BMI (>27.5 kg/m2; HR, 1.12; 95% CI, 1.03-1.22).55 A similar, smaller study of gastric cancer patients in Korea found similar, nonsignificant associations between gastric cancer and lower BMI (<23 kg/m2; HR, 10.82) or higher BMI (>25 kg/m2; HR, 11.33) but only in patients without serologic evidence of H. pylori infection.56 Most recently, an analysis of over 500,000 East and Southeast Asian cohort participants replicated the U-shape finding for non-cardia gastric cancer, intestinal-type gastric cancer, and late-onset gastric cancer.55
For cardia gastric cancer, there have been more consistent findings of increasing risk with increasing BMI, particularly in American and European studies. 57 In fact, a recent analysis of the National Institutes of Health-the American Association of Retired Persons Diet and Health cohort estimated that obesity was associated with 19% of the cardia gastric cancer burden.58 Interestingly, in the large East and Southeast Asian cohort analyses described above, no associations of obesity were found with cardia gastric cancer, 55 although it is not clear why there are geographical differences in this association.
Smoking
Tobacco use has been significantly associated with both gastric cardia and non-cardia cancers.13 Across 23 studies within the StoP Project, gastric cancer was more likely to arise in smokers than nonsmokers with an odds ratio of 1.2 (95% CI, 1.09-1.32).59 Risk of gastric cancer significantly increased with number of cigarettes smoked (especially greater than 20 cigarettes per day: OR, 1.32) and duration of smoking (OR, 1.33 for 40 or more years), suggesting a dose-response relationship between the number of cigarettes smoked and extent of gastric cancer risk.59,60 Similarly, in the previously discussed follow-up study of participants in the Shandong Intervention Trial in China, smoking was associated with both an increased incidence of gastric cancer (OR, 1.72; 95% CI, 1.003-2.93) and gastric cancer-related mortality (HR, 2.01; 95% CI, 1.01-3.98).61
The interactions of smoking and H. pylori infection in increasing gastric cancer risk have also been studied in different contexts. A case-control study examining cagA status and smoking found that smokers who were seropositive for cagA had an odds ratio of 8.7 for non-cardia gastric cancer compared to an odds ratio of 3.5 for smokers who were seronegative.62 A more recent prospective cohort study determined that patients with active, but not former tobacco use, had a 33% increased risk of gastric cancer, but only when they had serological evidence of H. pylori infection.63 This suggests that smoking in combination with the presence other major risk factors such as cagA-positive H. pylori infection may synergistically increase one’s risk of developing gastric cancer.
Alcohol Use
The role of alcohol in stomach carcinogenesis has been inconsistent across studies. Several earlier case-control studies and a few cohort studies have not found a strong association between alcohol use and gastric cancer risk.60,64,65 However, in a meta-analysis of 24 studies of alcohol consumption and gastric cancer, alcohol consumption increased gastric cancer risk by 15%, with both beer and liquor consumption having significant associations with increased gastric cancer risk, but not wine, and no significant differences by subsite (cardia vs. non-cardia).42 Recently, a study of adults living in the U.S. from 1999 to 2010 demonstrated that heavy alcohol use (five or more drinks per day for a prolonged period) was associated with gastric cancer development.66 After adjusting for demographics and smoking history, individuals with heavy alcohol use were found to have an odds ratio of 3.13 (95% CI 1.15-8.64) for gastric cancer incidence (although the authors were not able to stratify by subsite).66 Taken together, these findings suggest that high levels of alcohol consumption, as opposed to any lifetime alcohol use, are associated with increased gastric cancer risk, and continued intake of certain types of alcohol (beer, liquor) may pose a greater risk than others.
Genetic Syndromes and Family History of Gastric Cancer:
Approximately 10% of gastric cancers demonstrate familial clustering, with about 1-3% of all cases arising from hereditary syndromes associated with gastric cancer.67,68 Hereditary syndromes include hereditary diffuse gastric cancer (HDGC), gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS), familial adenomatous polyposis (FAP), Lynch syndrome, Peutz-Jeughers syndrome (PJS), juvenile polyposis syndrome (JPS), and Li-Fraumeni syndrome (Table 1).
Table 1:
Genetic syndromes associated with gastric cancer
| Syndrome | Clinical features | Associated gene alterations | Lifetime risk of gastric cancer | Gastric cancer screening and prevention |
|---|---|---|---|---|
| Hereditary diffuse gastric cancer (HDGC) | Increased risk of early-onset diffuse subtype gastric cancer and lobular breast cancer | CDH1, CTNNA1, RAD51C, BRCA1, PALB2 | 33-70%69,73 | CDH1 and CTTNA genetic testing76 Prophylactic gastrectomy recommended between 18 and 40 years, endoscopic surveillance every 6-12 months if declined154 |
| Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS) | >100 polyps in the body and fundus of the stomach without duodenal and colorectal polyposis | APC promoter B1 | 13%79,160 | No current guidelines, but consider upper endoscopic surveillance at symptom onset or 15 years.161 Gastrectomy if dysplasia, or at 30-35 years or 5 years prior to youngest family member diagnosis of gastric cancer160,161 |
| Familial adenomatous polyposis (FAP) | Adenomatous and/or fundic gland polyps in stomach in addition to colon and rectal polyps | APC | 0.6-2%154 | Upper endoscopic surveillance at symptom onset or 25-30 years with specialized surveillance if dysplasia154,161 |
| Lynch Syndrome | Increased risk of intestinal subtype gastric cancer, other gastrointestinal cancers, endometrial and ovarian cancers | MLH1, MSH2, MSH6, EPCAM | 5-9%90 | Test for H. pylori and treat if present. Upper endoscopic surveillance at 30-35 years every 2-4 years, or earlier and more frequently based on family history or presence of high-risk endoscopic findings.82,161 |
| Peutz-Jeughers Syndrome (PJS) | Gastrointestinal hamartomatous polyps and mucocutaneous pigmentation | LKB1(STK11) | 29%94 | Upper endoscopic surveillance beginning at age 8-10 years, repeated every 2-3 years if polyps or resuming at 18 years if no polyps161 |
| Juvenile Polyposis Syndrome (JPS) | Gastrointestinal hamartomatous polyps | BMPR1A SMAD4 |
11-21%82,95 | Upper endoscopic surveillance beginning at age 15 every 1-3 years154 Gastrectomy in severe cases82 |
| Li-Fraumeni Syndrome | Increased risk of multiple cancer types | TP53 | 1.3-22.6%81,100,101 | No current centralized guidelines |
| Familial intestinal gastric cancer (FIGC) | Increased risk of intestinal subtype gastric cancer | Currently unknown | Currently unknown | No current centralized guidelines |
HDGC presents with early onset diffuse gastric cancer with high penetrance (33-70% by age 80) and an increased risk of lobular breast cancer.69 In about 25-40% of cases, HDGC is caused by germline mutations in CDH1, the gene encoding E-cadherin.70,71 E-cadherin is a cell adhesion molecule that plays an important role in maintaining tissue structure, and reduced expression of E-cadherin is associated with poor prognosis in multiple cancer types.72 While historically CDH1 variants have been estimated to be associated with a 40-83% lifetime risk, a recent study found that cumulative incidence of gastric cancer in CDH1 carriers is likely closer to 42% in men and 33% in women.73 Additional mutations causing variable penetrance in CDH1-negative HDGC have been identified in CTNNA1, which codes for alpha-E-catenin, as well as PALB2, RAD51C, and BRCA1, all of which are involved with recombination repair.74,75 Prophylactic gastrectomy is typically recommended for patients found to have a CDH1 mutation.76 In recent years, newer guidelines recommend CDH1 variant genetic testing for selected patients to improve gastric cancer risk stratification and determine the need for prophylactic gastrectomy versus annual endoscopic gastric surveillance.76,77 These criteria are outlined in further detail in our discussion on prevention.
GAPPS is a recently characterized autosomal dominant syndrome associated with gastric adenocarcinoma. First described in 2012, GAPPS is now understood to be caused by a mutation in the APC promoter gene with a penetrance of about 13% in carriers.69,78,79 Diagnosed patients typically have greater than 100 polyps limited to the body and fundus of the stomach in the absence of duodenal and colorectal polyposis.80 Endoscopic screening may begin at 15 years or earlier if symptomatic, with continued surveillance anywhere between six months and three years, although screening guidelines are not currently well established.81 Notably, both GAPPS and CDH1 mutations are not associated with H. pylori infection, potentially representing an alternate pathway of gastric carcinogenesis.
FAP is an autosomal dominant syndrome caused by a germline mutation in the APC gene, which presents with numerous colonic and rectal polyps beginning in childhood or adolescence. FAP has classically been associated with a 100% risk of colorectal cancer and minimal risk of gastric cancer in the U.S. (~0.6%), similar to that of the general population.82,83 However, recent studies suggest that this risk may be greater. For example, a study of 767 patients with FAP found that 1.3% of patients developed gastric cancer between 2012 and 2016, despite annual or more frequent esophagogastroduodenoscopy (EGD) surveillance.84 These tumors may develop from gastric adenomatous or fundic gland polyps, which are commonly found in patients with FAP, but the exact mechanism by which this occurs is poorly understood.85 Recent studies have identified an increased incidence of high grade dysplasia in both fundic gland polyps and gastric adenomas, especially polyps that are large in size.84,86 Upper gastrointestinal tumor screening typically begins at 20-25 years or at the onset of findings of colonic polyps. Repeat endoscopic surveillance ranges from every three to five years and frequency depends on the number, size, and histological findings of duodenal polyps.87
Of all the hereditary syndromes associated with gastric cancer, Lynch syndrome is the most common, affecting approximately 1 in 279 individuals in the general population.88 Lynch syndrome is caused by a number of mutations in genes responsible for DNA mismatch repair including MLH1, MSH2, MSH6, and EPCAM.81 Resultant mutations and/or hypermethylation of these genes generate microsatellite instability, which increases the risk of developing colorectal and intestinal-type gastric cancers in addition to extraintestinal cancers such as ovarian and endometrial cancers.89 Affected individuals have a 5-9% lifetime risk of gastric cancer.90 One study found that in individuals with Lynch syndrome, gastric cancer incidence was independently associated with male sex, older age, MLH1 or MSH2 mutations, and the number of first-degree relatives with gastric cancer.91 Patients with several of these risk factors may benefit from more frequent endoscopic gastric cancer surveillance.
PJS, which presents with gastrointestinal hamartomatous polyps and mucocutaneous pigmented lesions, is caused by a mutation in the tumor suppressor gene LKB1 (STK11).92 Patients have a significantly increased risk of breast, uterine, lung, and gastrointestinal cancers, especially gastric cancer.93 PJS confers a 29% lifetime risk of gastric cancer (median age of diagnosis = 30) and routine EGD screening begins at age 8 and is repeated every 2-3 years.94
JPS is an autosomal dominant syndrome that presents in childhood with multiple hamartomatous gastrointestinal polyps and a 38% - 68% risk of colorectal cancer and 11% - 21% lifetime risk of upper gastrointestinal (duodenal and diffuse or intestinal gastric) cancers (median age of diagnosis, 58).82,95 Mutations in BMPR1A and SMAD4 (MADH4, DPC4), both members of the TGF-beta signaling pathway, have been shown to be associated with JPS.96 Specifically, carriers of SMAD4 mutations are more likely to develop severe gastric polyposis and progress to gastric cancer.97
Li-Fraumeni Syndrome is a rare condition caused by mutations in the TP53 tumor suppressor gene. Mutations may be inherited in an autosomal dominant fashion; however, between 7 and 20% of these germline mutations may be de novo.98 It is associated with gastric cancer in addition to breast cancer, sarcomas, brain masses, skin cancer, lung cancer, and adrenal tumors.99 Risk of gastric cancer in family members with inherited Li-Fraumeni syndrome has been variable across different studies, ranging from 1.3% to 22.6%.81,100,101
Familial intestinal gastric cancer (FIGC) is another type of autosomal dominant inherited gastric cancer risk syndrome. Currently, the genetic basis for this syndrome is unknown. Candidate causative genes include those involved in DNA repair, including BRCA1 and BRCA2.
Mutations in BRCA1 and BRCA2, two genes encoding DNA repair enzymes in homologous recombination, demonstrate an autosomal dominant pattern of inheritance in familial hereditary breast and ovarian cancer (HBOC). Germline mutations in BRCA1 and BRCA2 have also been associated with gastric cancer, although the increased risk associated with these mutations has not yet been well-delineated.102 In a study of families with inheritance patterns similar to FIGC and HDGC, in addition to BRCA1 and BRCA2, PALB2, a gene also involved in homologous recombination, and ATM, a gene involved in DNA repair, were also associated with these patterns.69
Although different modifiable and nonmodifiable risk factors are likely to increase gastric cancer risk in genetically predisposed individuals, further investigation is necessary to understand their contributions to this risk. Individuals with known mutations associated with gastric cancer and additional independent risk factors for gastric cancer will likely benefit from earlier and more frequent interventions such as endoscopic surveillance or surgical prophylaxis. More specific screening guidelines are needed to address these nuances.
In the absence of a defined genetic syndrome or known mutation, a family history of gastric cancer also confers an increased risk of an individual developing gastric cancer. An analysis of pathogenic variants in gastric cancer found that 19.8% of individuals with gastric cancer had a relevant germline mutation.103 In a clinical trial in Korea of H. pylori eradication among individuals with a first-degree family history of gastric cancer, H. pylori treatment was associated with a 55% reduced risk of developing gastric cancer compared to placebo and 73% reduced risk of gastric cancer compared to those with persistent H. pylori.104 This suggests that H. pylori eradication is even more critical to preventing gastric cancer in patients with a family history of gastric cancer.
Prevention
A study of healthcare cost in the U.S. found that total annual healthcare cost of gastric cancer per patient was greater than the cost of colorectal, liver, or lung cancer, per patient, at over 45,000 dollars.105 Prevention of gastric cancer has the potential to be highly cost-effective and mitigate financial burdens to both patients and healthcare systems. Given the heterogeneity found in gastric cancer, prevention necessitates a multifaceted approach, including adherence to H. pylori treatment and eradication guidelines, improved understanding of the drivers of eradication failure and relevant next steps, and more specific guidelines to identify and effectively screen individuals at higher risk of progression across the spectrum of H. pylori-associated disease.
H. pylori Testing, Treatment, and Eradication
H. pylori infection is the greatest risk factor for developing gastric cancer; as a result, testing for and treating H. pylori are essential components of gastric cancer prevention. Common testing approaches include histopathological staining of endoscopic biopsies, as well as non-invasive methods such as stool antigen tests, urea breath tests, and antibody testing. A Cochrane review of non-invasive H. pylori testing identified that urea breath tests had the greatest diagnostic accuracy of all of the non-invasive options, although false negatives can occur with all tests for active infection when patients are on antibiotics or taking proton pump inhibitors.106
Trials of H. pylori eradication therapy have found a significant reduction in gastric cancer incidence (RR, 0.54; 95% CI, 0.40-0.72) and gastric cancer-related deaths (RR, 0.61, 95% CI, 0.40-0.92).107,108 Following diagnosis, H. pylori treatment reduces risk of gastric cancer but does not bring this risk to zero.109 A retrospective cohort study of patients within the Veterans Affairs hospital system found that patients who were treated for H. pylori still had a non-significantly increased risk of gastric cancer (SHR, 1.16; 95% CI, 0.74-1.83), but those who additionally had eradication testing demonstrating successful treatment of their H. pylori infection had a significantly reduced risk of gastric cancer, compared to individuals who did not have successful eradication of their bacteria (SHR, 0.24; 95% CI, 0.15-0.41).109 In a randomized controlled clinical trial of H. pylori eradication in first-degree relatives of patients with gastric cancer, 1.2% of participants in the H. pylori treatment group developed gastric cancer compared to 2.7% of the placebo group over the follow-up period of about 9 years.110 Moreover, of the participants who developed gastric cancer in the treatment group, 50% had persistent H. pylori infection.110 Taken together, these findings demonstrate that individuals with persistent H. pylori infection are at a particularly increased risk of developing gastric cancer.
The 2017 ACG guidelines recommend that all patients infected with H. pylori be treated with appropriate combination therapy and undergo eradication testing following treatment completion, reflecting the importance of reducing persistent H. pylori infection.111 However, eradication testing and follow-up treatment in the event of repeat infection positivity do not always take place. In a retrospective cohort study of 152 patients with H. pylori-associated peptic ulcer disease, only 44% of patients received eradication testing.112 In that cohort, predictors of eradication testing included outpatient status at the time of H. pylori diagnosis as well as follow-up care with a gastroenterologist.112 These findings highlight inconsistent eradication testing practices between specialties and the potential for gaps in care after hospital discharge. These findings also illustrate the need for more consistent eradication testing and system-based approaches to ensure that H. pylori eradication testing is completed after treatment, such as implementing automated testing reminders in the electronic health record.
H. pylori antibiotic resistance also contributes to eradication failure and persistence of infection. A meta-analysis of 178 studies of the geographic distribution of H. pylori antibiotic resistance identified at least 10% overall prevalence of H. pylori resistance to clarithromycin, metronidazole, and levofloxacin across all World Health Organization regions.113 Local antibiotic resistance rates should be closely monitored given these regional differences, especially when planning and implementing therapies for large-scale eradication programs in areas where populations are at increased risk of gastric cancer.114
Host factors may also contribute to treatment failures. For example, a meta-analysis of 57 studies examining H. pylori eradication found that individuals with CYP2C19 polymorphism enhanced metabolizer phenotypes were 82% more likely to experience H. pylori eradication failure than those with wild-type phenotypes (OR, 1.82; 95% CI, 1.52-2.19). CYP2C19 polymorphisms that enhance drug metabolism are prevalent among individuals across racial and ethnic groups within the U.S., and can be clinically tested.115 In addition to genetic host factors, which can be costly to test for, incomplete understanding and education regarding treatment regimens, inconsistent healthcare follow-up, and treatment-related adverse effects may also contribute to antibiotic resistance. Active awareness and consideration of these contributors to antibiotic resistance and continued communication and follow-up between patients and healthcare professionals may improve H. pylori treatment-related preventative efforts. Taken together, ensuring completion of eradication testing in combination with improved assessments of antibiotic resistance on both individual and regional levels are essential to preventing future cases of gastric cancer.
Finally, H. pylori reinfection should also be considered when confirming eradication, especially in individuals more likely to be exposed to H. pylori. A meta-analysis of 132 studies of H. pylori reinfection found that the global annual rate of recurrence for H. pylori infection after confirmed eradication was 4.3% (95% CI, 4-5%) and that this rate was inversely related to the human development index (HDI), a statistical index combining life expectancy, education, and per capita income.116
Chemoprevention
As previously discussed, H. pylori eradication therapy can reduce the risk of developing gastric cancer. Aspirin use has also been associated with a reduction in risk of multiple gastrointestinal cancers, including gastric cancer, especially intestinal-type non-cardia gastric cancer.117,118 A recent study of 316 individuals with gastric cancer within the Nurses’ Health Study and Health Professionals Follow-up Study in the U.S. found that aspirin use at least twice per week was associated with a reduction in gastric cancer risk among women (HR, 0.52; 95% CI, 0.37-0.73), but not men.119
Some studies have also investigated the interaction of aspirin with H. pylori, finding that aspirin use is significantly associated with a reduction in gastric cancer risk in H. pylori-positive patients, but not among H. pylori-negative patients.120,121 Additionally, a study of a cohort of over 63,000 patients in Hong Kong who had undergone successful eradication of H. pylori between 2003 and 2012 found that aspirin use following eradication was associated with a decreased risk of developing gastric cancer (HR, 0.3; 95% CI, 0.15-0.61).122 A significantly greater reduction in risk of gastric cancer was also observed with increasing aspirin dosage, frequency, and regimen duration. However, this reduction in gastric cancer risk was not observed in a similar study of this cohort in association with non-aspirin nonsteroidal anti-inflammatory drugs.123
Vitamin and garlic supplementation have also been explored as potential chemoprevention in a large randomized controlled trial in a high-incidence area of China. During 22.3 years of follow-up, the Shandong Intervention Trial found that 7.3 years of vitamin (C, E, and selenium) supplementation was associated with a significant reduction in both gastric cancer incidence and mortality (OR, 0.64; 95% CI, 0.46-0.91; and HR, 0.48; 95% CI, 0.31-0.75).124 In this same trial, 7.3 years of oral garlic extract and oil supplementation was not significantly associated with a reduction in gastric cancer incidence (HR, 0.81; 95% CI, 0.57-1.13), but was associated with a significant reduction in gastric cancer mortality (HR, 0.66; 95% CI, 0.43-1.00), although these trends only became noticeable after 12 and 14.7 years of follow-up, respectively.124 A further analysis of this trial exploring effect modification by lifestyle factors found that garlic supplementation was only associated with a reduction in gastric cancer mortality in participants with H. pylori infection who did not consume alcohol (P for interaction = 0.04).61
Endoscopic Surveillance
Endoscopic screening and surveillance in individuals at increased risk of developing gastric cancer is the primary screening method for gastric cancer prevention. In areas of the world such as East Asia, recent studies have shown mass gastric cancer endoscopic surveillance programs to be effective in reducing both gastric cancer incidence and mortality.125,126 Currently, no endoscopic screening for individuals at average risk of gastric cancer is standard in the U.S. A modeling study found that upper EGD at the time of colorectal cancer screening colonoscopy in the U.S., with follow-up EGD every three years only in the presence of intestinal metaplasia or higher-grade pathologic lesions, was cost-effective for Asian American, Hispanic White, and non-Hispanic Blacks patients, but not for non-Hispanic White patients.127
Gastric intestinal metaplasia (GIM), characterized by the growth of intestinal-like epithelium in the gastric mucosa, is thought to be a precursor lesion to gastric dysplasia and cancer, with prevalence ranging from around five percent to greater than 20 percent in the U.S. and greater than 20 percent in regions with a greater incidence of H. pylori infection, such as China.126,128,129 Since GIM is a histopathologic diagnosis requiring endoscopic biopsy for diagnosis, the prevalence of GIM may be difficult to estimate given the lack of universal endoscopic screening. Within the U.S., the prevalence of GIM varies among racial and ethnic groups, with Asian American, Black, and Hispanic White individuals having a twofold or greater prevalence compared to non-Hispanic White individuals.128,130,131
An analysis of over 25,000 patients with GIM across 10 studies found an incidence rate of approximately 12.4 cases of gastric cancer per 10,000 patient-years (95% CI, 10.7-14.3).132,133 In a cohort of patients in California, the presence of extensive GIM on endoscopy was associated with a five-to-sevenfold greater risk of cancer progression compared to findings of focal GIM.134 Furthermore, a recent study of GIM in a western European population with a generally low incidence of gastric cancer found that patients with GIM identified in both the antrum and body of stomach were more likely to exhibit disease progression (3.1% of cases) compared to patients with GIM found in one location of the stomach (0.4% of cases).135 Additionally, incomplete GIM is associated with a higher risk of progression to gastric cancer compared to complete GIM.136 A 10-year study of 81 patients with complete GIM and 11 patients with incomplete GIM found that while no patients with complete GIM experienced disease progression, 50% of patients with incomplete GIM progressed to dysplasia or cancer.137 The Operative Link on Gastric Intestinal Metaplasia (OLGIM) assessment is a tool used to stratify patients at risk of progression to gastric cancer by staging the severity of histopathologic GIM findings from endoscopic biopsies.138 A prospective study utilizing OLGIM for endoscopic surveillance of patients with GIM in Singapore found that both patients with intermediate (OLGIM II) and high-risk GIM (OLGIM III-IV) were at an increased risk of developing high grade dysplasia and Stage I gastric adenocarcinoma (HR, 7.34; 95% CI, 1.60-33.7 and HR, 20.77; 95% CI, 5.04-85.6, respectively).139 Furthermore, patients with both intermediate/high-risk GIM who had a smoking history of 20 or more pack years were at an even greater risk of developing gastric cancer (HR, 3.69; 95% CI, 1.03-13.2).139 These findings support the utility of multifaceted risk stratification systems that incorporate multiple modifiable and nonmodifiable risk factors.
For patients found to have GIM on endoscopy, the most recent AGA clinical screening guidelines recommend H. pylori testing and eradication, but do not recommend routine endoscopic surveillance.140 However, they do acknowledge that certain patient populations (racial and ethnic minorities, immigrants, patients with a family history of gastric cancer) may be at increased risk of developing gastric cancer and could elect for endoscopic surveillance, potentially after 3-5 years.140 One study found that among patients with GIM in the U.S., Hispanic patients had the greatest risk of progressing to gastric adenocarcinoma compared to other racial and ethnic groups.134 Although only an estimated 0.5% -2.7% of patients with GIM develop gastric adenocarcinoma annually, this is comparable to that of patients diagnosed with Barrett’s esophagus who develop esophageal adenocarcinoma, estimated around 0.12-0.5% annually.132,133,141–143 In contrast, the screening guidelines for Barrett’s esophagus have been well-defined and recommend continued surveillance every 3-5 years, even in the absence of dysplasia.144,145
Improving gastric cancer surveillance guidelines for individuals with known hereditary cancer syndromes who are at increased risk of developing gastric cancer is another actionable component of gastric cancer prevention. For example, a retrospective analysis of 217 patients with Lynch syndrome found that five patients developed upper gastrointestinal (duodenal or gastric) cancers that were detected by surveillance, and four of these cancers were Stage 1 and occurred within two years of previous endoscopy.146 Given these findings, for patients with Lynch syndrome, these researchers recommend H. pylori testing and treatment if needed at the time of Lynch syndrome diagnosis, in the form of non-invasive testing for individuals under 30 and endoscopic testing for those over 30.147 They also suggest performing upper gastrointestinal endoscopic surveillance concurrently with colonoscopy beginning at age 30, or at least 2-5 years prior to the diagnosis of an upper gastrointestinal cancer in a family member if earlier.147
Circulating Biomarkers
Circulating biomarkers for gastric cancer prevention offer the potential of a noninvasive approach to identifying individuals at greatest risk of progressing to gastric cancer. Some of these biomarkers have already been employed in the clinical setting in areas with a higher regional incidence of gastric cancer.
Numerous studies have explored the association of serum antibodies to H. pylori proteins and risk of gastric cancer, and a recent meta-analysis of nine studies found that five H. pylori virulence factors were significantly associated with non-cardia gastric cancer risk: cagA (OR, 3.22; 95% CI, 2.10-4.94); HP 0305 (OR, 1.72; 95% CI, 1.32-2.25); HyuA (OR, 1.42; 95% CI 1.13-1.79); Omp (OR, 1.83, 1.30-2.58); and vacA (OR, 2.05; 95% CI, 1.67-2.52).148 Notably, the meta-analysis found no significant associations between any of the antigens and risk of cardia gastric cancer.
The ABC method, which combines serum H. pylori antibodies with pepsinogen-defined chronic atrophic gastritis, was first developed by Miki et al. in Japan and has been used to risk stratify patients for further gastric cancer screening in East Asia. 149,150 A recent study sought to utilize antibody responses to specific H. pylori antibodies to create a new predictive model in three East Asian cohorts, and found that in addition to pepsinogen-defined chronic atrophic gastritis, adding antibody responses to the H. pylori proteins HP 0305, HP 1564, and UreA, along with gender and age, resulted in a model with a greater area under the curve (AUC) than that of the ABC method alone (AUC, 73.79%; 95% CI, 75.37%-82.35%; vs. AUC, 68.75%; 95% CI, 65.91-71.58; p for difference <0.001).151
Using an untargeted global profiling platform to assess 1,000 metabolites in pre-diagnostic plasma, a recent case-control study in Shanghai of 250 incident gastric cancer cases and one-to-one matched controls identified eighteen metabolites that were significantly associated with gastric cancer.152 One of these metabolites, methylmalonic acid, is elevated in B12 deficiency. Vitamin B12 deficiency is linked with both gastric cancer and atrophic gastritis, a potential precursor lesion to gastric cancer.153
Personalized Medicine
Employing a more personalized approach to assessing risk that includes tailored genetic testing poses another opportunity for improved risk stratification of patients more likely to develop gastric cancer. Genetic testing for gastric cancer has focused on the diffuse histopathologic type. As discussed earlier, CDH1 may be used as a marker for HDGC. The Blair criteria for HDGC recommend CDH1 testing, followed by CTNNA1 testing if CDH1 testing is negative, for patients who meet any of the following family (first or second-degree blood relatives) criteria: ≥2 cases of gastric cancer in the family regardless of age, with at least one diffuse gastric cancer (DGC), ≥1 case of DGC at any age, and ≥1 case of lobular breast cancer at age <70 years, in different family members, ≥2 cases of lobular breast cancer in family members <50 years of age.76 Individual criteria include a diagnosis of DGC at age <50 years, at any age in individuals of Māori ethnicity or personal or family history of cleft lip or palate, or history of DGC and lobular breast cancer or bilateral lobular breast cancer diagnosed at age <70 years.76 For patients who carry a germline CDH1 mutation, the National Comprehensive Cancer Network (NCCN) guidelines recommend prophylactic total gastrectomy between 18 and 40 years of age, with opportunities for endoscopic surveillance every 6-12 months for patients who do not wish to undergo surgery.154 However, endoscopic surveillance may not always identify precursor gastric lesions.155 In the future, we anticipate that genetic testing and related preventive strategies will be broadened to include other known genetic contributors to gastric cancer risk for both diffuse and intestinal types. Mixed models of susceptibility incorporating multiple hereditary and non-hereditary risk factors, such as those that have been developed in breast cancer, will further advance risk stratification in gastric cancer.156
While genetic testing is a promising clinical tool for cancer prevention, we must also acknowledge the disparities that exist both in the accessibility of this testing to all patients and in the clinical decision making of who gets tested. Significant racial and ethnic disparities have been identified regarding referrals made for breast cancer-related genetic counseling for non-Hispanic Black patients compared to non-Hispanic White patients.157 Barriers to genetic counseling and hereditary cancer testing include patient and provider awareness of testing options, stress surrounding testing, disparate insurance coverage of testing, reduced availability of family medical history, and lower levels of trust in the health system among racial and ethnic minorities.158 Increased awareness of these disparities in addition to interventions can improve access to these screening tools for individuals at increased risk of developing gastric cancer, which can in turn reduce risk of disease progression.
Future Directions
Most people living with H. pylori infection do not develop gastric cancer. More detailed, research-driven guidelines are necessary to identify those individuals who, within this population, are at an increased risk of cancer, and develop personalized testing, treatment, and surveillance strategies while ensuring equitable resource allocation and access to these options. This includes the development of multifactorial algorithms incorporating serum biomarkers, exposures, family history, and demographic information to predict gastric cancer risk to create a more personalized approach to screening and surveillance. These algorithms could help focus resources such as endoscopy on patients most likely to benefit from invasive testing. Infection with H. pylori may also contribute to dysbiosis within the gastrointestinal tract, which can promote progression of H. pylori-associated disease (gastritis, metaplasia, dysplasia, and cancer).159
It is currently unclear exactly why H. pylori persistence occurs in some patients, although it may be attributed to infection with more virulent strains of H. pylori, such as cagA-positive strains, and/or increased antibiotic resistance. Persistent H. pylori infection can be identified and addressed through eradication testing; however, as discussed earlier, eradication testing is often not performed. This could be due to several reasons, such as a lack of reminders for healthcare practitioners to set up eradication testing, losing patients to follow-up due to logistical and/or financial reasons, and variable applications of existing guidelines. Designing systems level interventions can address this discrepancy, and potentially reduce the risk of developing gastric cancer that accompanies persistent infection.
Conclusions
While the incidence of gastric cancer has been declining within the U.S. and worldwide, the overall high mortality rates and existing disparities in both incidence and mortality suggest the need for delineating and implementing risk-stratified prevention strategies, which would be highly cost-effective. Future research should include developing multifactorial mixed models of nonmodifiable and modifiable risk factors for gastric cancer to identify patients at greatest risk of progressing to gastric cancer. Finally, future interventions should improve access to existing preventative measures such as genetic testing and H. pylori eradication testing in addition to expanding upon current clinical guidelines for premalignant disease to address gaps in endoscopic surveillance.
Acknowledgements
This work was supported in part by the National Institutes of Health (NIH) grants P20 CA251657 (to M. Epplein and K.S. Garman) and Duke Cancer Institute P30 CA014236 (to M. Epplein and K.S. Garman), as well as the Eugene A. Stead Scholarship through the Stead Fellowship Endowment Fund (to P. Alagesan).
Footnotes
The authors declare no potential conflicts of interest
References
- 1.Cancer IAfRo. Stomach Fact Sheet. The Global Cancer Observatory. [cited 2022 February 22]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf
- 2.Institute NC. Cancer Stat Facts: Stomach Cancer. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. [cited 2022 March 13]. Available from: https://seer.cancer.gov/statfacts/html/stomach.html
- 3.Giaquinto AN, Miller KD, Tossas KY, Winn RA, Jemal A, Siegel RL. Cancer statistics for African American/Black People 2022. CA Cancer J Clin 2022;72:202–229. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Tao L, Murphy JD, Camargo MC, Oren E, Valasek MA, et al. Race/Ethnicity-, Socioeconomic Status-, and Anatomic Subsite-Specific Risks for Gastric Cancer. Gastroenterology 2019;156:59–62.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis 2012;13:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420–429. [DOI] [PubMed] [Google Scholar]
- 7.Network CGAR. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 2020;69:1564–1571. [DOI] [PubMed] [Google Scholar]
- 9.Yang L, Kartsonaki C, Yao P, de Martel C, Plummer M, Chapman D, et al. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health 2021;6:e888–e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer causes & control : CCC 2011;22:375–87. [DOI] [PubMed] [Google Scholar]
- 11.Network CGAR, University AWGA, Agency BC, Hospital BaWs, Institute B, University B, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung H, Siegel RL, Torre LA, Pearson-Stuttard J, Islami F, Fedewa SA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin 2019;69:88–112. [DOI] [PubMed] [Google Scholar]
- 13.Gu J, Chen R, Wang SM, Li M, Fan Z, Li X, et al. Prediction Models for Gastric Cancer Risk in the General Population: A Systematic Review. Cancer Prev Res (Phila) 2022;15:309–318. [DOI] [PubMed] [Google Scholar]
- 14.Correa P Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735–40. [PubMed] [Google Scholar]
- 15.Pinto-Santini D, Salama NR. The biology of Helicobacter pylori infection, a major risk factor for gastric adenocarcinoma. Cancer Epidemiol Biomarkers Prev 2005;14:1853–8. [DOI] [PubMed] [Google Scholar]
- 16.Chmiela M, Kupcinskas J. Review: Pathogenesis of Helicobacter pylori infection. Helicobacter 2019;24 Suppl 1:e12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amieva M, Peek RM. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 2016;150:64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai H, Ye F, Michel A, Murphy G, Sasazuki S, Taylor PR, et al. Helicobacter pylori blood biomarker for gastric cancer risk in East Asia. International journal of epidemiology 2016;45:774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong E, Duan L, Wu BU. Racial and Ethnic Minorities at Increased Risk for Gastric Cancer in a Regional US Population Study. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2017;15:511–517. [DOI] [PubMed] [Google Scholar]
- 20.Miller KD, Ortiz AP, Pinheiro PS, Bandi P, Minihan A, Fuchs HE, et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J Clin 2021;71:466–487. [DOI] [PubMed] [Google Scholar]
- 21.Merchant SJ, Kim J, Choi AH, Sun V, Chao J, Nelson R. A rising trend in the incidence of advanced gastric cancer in young Hispanic men. Gastric Cancer 2017;20:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinheiro PS, Callahan KE, Siegel RL, Jin H, Morris CR, Trapido EJ, et al. Cancer Mortality in Hispanic Ethnic Groups. Cancer Epidemiol Biomarkers Prev 2017;26:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah SC, McKinley M, Gupta S, Peek RM, Martinez ME, Gomez SL. Population-Based Analysis of Differences in Gastric Cancer Incidence Among Races and Ethnicities in Individuals Age 50 Years and Older. Gastroenterology 2020;159:1705–1714.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang RJ, Sharp N, Talamoa RO, Ji HP, Hwang JH, Palaniappan LP. One Size Does Not Fit All: Marked Heterogeneity in Incidence of and Survival from Gastric Cancer among Asian American Subgroups. Cancer Epidemiol Biomarkers Prev 2020;29:903–909. [DOI] [PubMed] [Google Scholar]
- 25.Melkonian SC, Jim MA, Pete D, Poel A, Dominguez AE, Echo-Hawk A, et al. Cancer disparities among non-Hispanic urban American Indian and Alaska Native populations in the United States, 1999-2017. Cancer 2022;128:1626–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melkonian SC, Pete D, Jim MA, Haverkamp D, Wiggins CL, Bruce MG, et al. Gastric Cancer Among American Indian and Alaska Native Populations in the United States, 2005-2016. Am J Gastroenterol 2020;115:1989–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinson HA, Shelby NJ, Alberts SR, Olnes MJ. Gastric cancer in Alaska Native people: A cancer health disparity. World J Gastroenterol 2018;24:2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nolen LD, Bressler S, Vindigni SM, Miller K, Nash S. Gastric Cancer in Alaska Native and American Indian People Living in Alaska, 1990-2017. Clin Transl Gastroenterol 2021;12:e00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsao MW, Delozier OM, Stiles ZE, Magnotti LJ, Behrman SW, Deneve JL, et al. The impact of race and socioeconomic status on the presentation, management and outcomes for gastric cancer patients: Analysis from a metropolitan area in the southeast United States. J Surg Oncol 2020;121:494–502. [DOI] [PubMed] [Google Scholar]
- 30.Varga MG, Butt J, Blot WJ, Le Marchand L, Haiman CA, Chen Y, et al. Racial differences in Helicobacter pylori CagA sero-prevalence in a consortium of adult cohorts in the United States. Cancer Epidemiol Biomarkers Prev 2020;29:2084–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islami F, DeSantis CE, Jemal A. Incidence Trends of Esophageal and Gastric Cancer Subtypes by Race, Ethnicity, and Age in the United States, 1997-2014. Clin Gastroenterol Hepatol 2019;17:429–439. [DOI] [PubMed] [Google Scholar]
- 32.Anderson WF, Rabkin CS, Turner N, Fraumeni JF, Rosenberg PS, Camargo MC. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst 2018;110:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson WF, Camargo MC, Fraumeni JF, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA 2010;303:1723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butt J, Lehtinen M, Öhman H, Waterboer T, Epplein M. Association of Helicobacter pylori and Autoimmune Gastritis With Stomach Cancer in a Cohort of Young Finnish Women. Gastroenterology 2022;163:305–307.e4. [DOI] [PubMed] [Google Scholar]
- 35.Goldenring J No H. pylori, no adenocarcinoma for patients with autoimmune gastritis. Gut 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uthman OA, Jadidi E, Moradi T. Socioeconomic position and incidence of gastric cancer: a systematic review and meta-analysis. J Epidemiol Community Health 2013;67:854–60. [DOI] [PubMed] [Google Scholar]
- 37.Epplein M, Cohen SS, Sonderman JS, Zheng W, Williams SM, Blot WJ, et al. Neighborhood socio-economic characteristics, African ancestry, and Helicobacter pylori sero-prevalence. Cancer Causes Control 2012;23:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rota M, Alicandro G, Pelucchi C, Bonzi R, Bertuccio P, Hu J, et al. Education and gastric cancer risk-An individual participant data meta-analysis in the StoP project consortium. Int J Cancer 2020;146:671–681. [DOI] [PubMed] [Google Scholar]
- 39.Pelucchi C, Lunet N, Boccia S, Zhang ZF, Praud D, Boffetta P, et al. The stomach cancer pooling (StoP) project: study design and presentation. Eur J Cancer Prev 2015;24:16–23. [DOI] [PubMed] [Google Scholar]
- 40.Joossens JV, Hill MJ, Elliott P, Stamler R, Lesaffre E, Dyer A, et al. Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J Epidemiol 1996;25:494–504. [DOI] [PubMed] [Google Scholar]
- 41.Salt Tsugane S., salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci 2005;96:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer 2015;51:2820–32. [DOI] [PubMed] [Google Scholar]
- 43.Kim SR, Kim K, Lee SA, Kwon SO, Lee JK, Keum N, et al. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose⁻Response Meta-Analysis. Nutrients 2019;11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peres SV, Silva DRM, Coimbra FJF, Fagundes MA, Auzier JJN, Pelosof AG, et al. Consumption of processed and ultra-processed foods by patients with stomach adenocarcinoma: a multicentric case-control study in the Amazon and southeast regions of Brazil. Cancer Causes Control 2022;33:889–898. [DOI] [PubMed] [Google Scholar]
- 45.Bertuccio P, Alicandro G, Rota M, Pelucchi C, Bonzi R, Galeone C, et al. Citrus fruit intake and gastric cancer: The stomach cancer pooling (StoP) project consortium. Int J Cancer 2019;144:2936–2944. [DOI] [PubMed] [Google Scholar]
- 46.Wang T, Cai H, Sasazuki S, Tsugane S, Zheng W, Cho ER, et al. Fruit and vegetable consumption, Helicobacter pylori antibodies, and gastric cancer risk: A pooled analysis of prospective studies in China, Japan, and Korea. International journal of cancer Journal international du cancer 2017;140:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferro A, Costa AR, Morais S, Bertuccio P, Rota M, Pelucchi C, et al. Fruits and vegetables intake and gastric cancer risk: A pooled analysis within the Stomach cancer Pooling Project. Int J Cancer 2020;147:3090–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren JS, Kamangar F, Forman D, Islami F. Pickled food and risk of gastric cancer--a systematic review and meta-analysis of English and Chinese literature. Cancer Epidemiol Biomarkers Prev 2012;21:905–15. [DOI] [PubMed] [Google Scholar]
- 49.Dalmartello M, Turati F, Zhang ZF, Lunet N, Rota M, Bonzi R, et al. Allium vegetables intake and the risk of gastric cancer in the Stomach cancer Pooling (StoP) Project. Br J Cancer 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashemian M, Murphy G, Etemadi A, Dawsey SM, Liao LM, Abnet CC. Nut and peanut butter consumption and the risk of esophageal and gastric cancer subtypes. Am J Clin Nutr 2017;106:858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah SC, Dai Q, Zhu X, Peek RM Jr., Smalley W, Roumie C, et al. Associations between calcium and magnesium intake and the risk of incident gastric cancer: A prospective cohort analysis of the National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study. Int J Cancer 2020;146:2999–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang HM, Wakisaka N, Maeda O, Yamamoto T. Vitamin C inhibits the growth of a bacterial risk factor for gastric carcinoma: Helicobacter pylori. Cancer 1997;80:1897–903. [PubMed] [Google Scholar]
- 53.Cardenas VM, Graham DY. Smoking and Helicobacter pylori infection in a sample of U.S. adults. Epidemiology 2005;16:586–90. [DOI] [PubMed] [Google Scholar]
- 54.Kwak JH, Paik JK. Vitamin D Status and Gastric Cancer: A Cross-Sectional Study in Koreans. Nutrients 2020;12: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jang J, Lee S, Ko KP, Abe SK, Rahman MS, Saito E, et al. Association between Body Mass Index and Risk of Gastric Cancer by Anatomic and Histologic Subtypes in Over 500,000 East and Southeast Asian Cohort Participants. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2022;31:1727–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jang J, Cho EJ, Hwang Y, Weiderpass E, Ahn C, Choi J, et al. Association between Body Mass Index and Gastric Cancer Risk According to Effect Modification by Helicobacter pylori Infection. Cancer Res Treat 2019;51:1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric Cancer 2015;18:23–32. [DOI] [PubMed] [Google Scholar]
- 58.Wang SM, Katki HA, Graubard BI, Kahle LL, Chaturvedi A, Matthews CE, et al. Population Attributable Risks of Subtypes of Esophageal and Gastric Cancers in the United States. The American journal of gastroenterology 2021;116:1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Praud D, Rota M, Pelucchi C, Bertuccio P, Rosso T, Galeone C, et al. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev 2018;27:124–133. [DOI] [PubMed] [Google Scholar]
- 60.Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut 2010;59:39–48. [DOI] [PubMed] [Google Scholar]
- 61.Guo Y, Li ZX, Zhang JY, Ma JL, Zhang L, Zhang Y, et al. Association Between Lifestyle Factors, Vitamin and Garlic Supplementation, and Gastric Cancer Outcomes: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open 2020;3:e206628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang XQ, Yan H, Terry PD, Wang JS, Cheng L, Wu WA, et al. Interactions between CagA and smoking in gastric cancer. World J Gastroenterol 2011;17:3330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butt J, Varga MG, Wang T, Tsugane S, Shimazu T, Zheng W, et al. Smoking, Helicobacter pylori serology, and gastric cancer risk in prospective studies from China, Japan, and Korea. Cancer Prev Res (Phila) 2019;12:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States). Cancer Causes Control 2001;12:721–32. [DOI] [PubMed] [Google Scholar]
- 65.Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollenbeck AR, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007;165:1424–33. [DOI] [PubMed] [Google Scholar]
- 66.Laszkowska M, Rodriguez S, Kim J, Hur C. Heavy Alcohol Use Is Associated With Gastric Cancer: Analysis of the National Health and Nutrition Examination Survey From 1999 to 2010. Am J Gastroenterol 2021;116:1083–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zanghieri G, Di Gregorio C, Sacchetti C, Fante R, Sassatelli R, Cannizzo G, et al. Familial occurrence of gastric cancer in the 2-year experience of a population-based registry. Cancer 1990;66:2047–51. [DOI] [PubMed] [Google Scholar]
- 68.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Pelaez J, Barbosa-Matos R, São José C, Sousa S, Gullo I, Hoogerbrugge N, et al. Gastric cancer genetic predisposition and clinical presentations: Established heritable causes and potential candidate genes. Eur J Med Genet 2022;65:104401. [DOI] [PubMed] [Google Scholar]
- 70.Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010;47:436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998;392:402–5. [DOI] [PubMed] [Google Scholar]
- 72.Schurig W [Multiple carcinomas of the gastrointestinal tract. (Large intestine polyposis and carcinogenesis)]. Zentralbl Chir 1968;93:1538–43. [PubMed] [Google Scholar]
- 73.Roberts ME, Ranola JMO, Marshall ML, Susswein LR, Graceffo S, Bohnert K, et al. Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol 2019;5:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahasrabudhe R, Lott P, Bohorquez M, Toal T, Estrada AP, Suarez JJ, et al. Germline Mutations in PALB2, BRCA1, and RAD51C, Which Regulate DNA Recombination Repair, in Patients With Gastric Cancer. Gastroenterology 2017;152:983–986.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Majewski IJ, Kluijt I, Cats A, Scerri TS, de Jong D, Kluin RJ, et al. An α-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J Pathol 2013;229:621–9. [DOI] [PubMed] [Google Scholar]
- 76.Blair VR, McLeod M, Carneiro F, Coit DG, D’Addario JL, van Dieren JM, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 2020;21:e386–e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gullo I, van der Post RS, Carneiro F. Recent advances in the pathology of heritable gastric cancer syndromes. Histopathology 2021;78:125–147. [DOI] [PubMed] [Google Scholar]
- 78.Li J, Woods SL, Healey S, Beesley J, Chen X, Lee JS, et al. Point Mutations in Exon 1B of APC Reveal Gastric Adenocarcinoma and Proximal Polyposis of the Stomach as a Familial Adenomatous Polyposis Variant. Am J Hum Genet 2016;98:830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Worthley DL, Phillips KD, Wayte N, Schrader KA, Healey S, Kaurah P, et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut 2012;61:774–9. [DOI] [PubMed] [Google Scholar]
- 80.Rudloff U Gastric adenocarcinoma and proximal polyposis of the stomach: diagnosis and clinical perspectives. Clin Exp Gastroenterol 2018;11:447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim W, Kidambi T, Lin J, Idos G. Genetic Syndromes Associated with Gastric Cancer. Gastrointest Endosc Clin N Am 2022;32:147–162. [DOI] [PubMed] [Google Scholar]
- 82.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223–62; quiz 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet 1988;1:1149–51. [DOI] [PubMed] [Google Scholar]
- 84.Mankaney G, Leone P, Cruise M, LaGuardia L, O’Malley M, Bhatt A, et al. Gastric cancer in FAP: a concerning rise in incidence. Fam Cancer 2017;16:371–376. [DOI] [PubMed] [Google Scholar]
- 85.Garrean S, Hering J, Saied A, Jani J, Espat NJ. Gastric adenocarcinoma arising from fundic gland polyps in a patient with familial adenomatous polyposis syndrome. Am Surg 2008;74:79–83. [PubMed] [Google Scholar]
- 86.Martin I, Roos VH, Anele C, Walton SJ, Cuthill V, Suzuki N, et al. Gastric adenomas and their management in familial adenomatous polyposis. Endoscopy 2021;53:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 1989;2:783–5. [DOI] [PubMed] [Google Scholar]
- 88.Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, et al. Prevalence and Penetrance of Major Genes and Polygenes for Colorectal Cancer. Cancer Epidemiol Biomarkers Prev 2017;26:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Niessen RC, Hofstra RM, Westers H, Ligtenberg MJ, Kooi K, Jager PO, et al. Germline hypermethylation of MLH1 and EPCAM deletions are a frequent cause of Lynch syndrome. Genes Chromosomes Cancer 2009;48:737–44. [DOI] [PubMed] [Google Scholar]
- 90.Capelle LG, Van Grieken NC, Lingsma HF, Steyerberg EW, Klokman WJ, Bruno MJ, et al. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology 2010;138:487–92. [DOI] [PubMed] [Google Scholar]
- 91.Kim J, Braun D, Ukaegbu C, Dhingra TG, Kastrinos F, Parmigiani G, et al. Clinical Factors Associated With Gastric Cancer in Individuals With Lynch Syndrome. Clin Gastroenterol Hepatol 2020;18:830–837.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 1998;18:38–43. [DOI] [PubMed] [Google Scholar]
- 93.Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000;119:1447–53. [DOI] [PubMed] [Google Scholar]
- 94.Giardiello FM, Trimbath JD. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol 2006;4:408–15. [DOI] [PubMed] [Google Scholar]
- 95.Brosens LA, Langeveld D, van Hattem WA, Giardiello FM, Offerhaus GJ. Juvenile polyposis syndrome. World J Gastroenterol 2011;17:4839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Howe JR, Sayed MG, Ahmed AF, Ringold J, Larsen-Haidle J, Merg A, et al. The prevalence of MADH4 and BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. J Med Genet 2004;41:484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Latchford AR, Neale K, Phillips RK, Clark SK. Juvenile polyposis syndrome: a study of genotype, phenotype, and long-term outcome. Dis Colon Rectum 2012;55:1038–43. [DOI] [PubMed] [Google Scholar]
- 98.Gonzalez KD, Buzin CH, Noltner KA, Gu D, Li W, Malkin D, et al. High frequency of de novo mutations in Li-Fraumeni syndrome. J Med Genet 2009;46:689–93. [DOI] [PubMed] [Google Scholar]
- 99.Hisada M, Garber JE, Fung CY, Fraumeni JF, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst 1998;90:606–11. [DOI] [PubMed] [Google Scholar]
- 100.Masciari S, Dewanwala A, Stoffel EM, Lauwers GY, Zheng H, Achatz MI, et al. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med 2011;13:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McBride KA, Ballinger ML, Killick E, Kirk J, Tattersall MH, Eeles RA, et al. Li-Fraumeni syndrome: cancer risk assessment and clinical management. Nat Rev Clin Oncol 2014;11:260–71. [DOI] [PubMed] [Google Scholar]
- 102.Alexandrov LB, Nik-Zainal S, Siu HC, Leung SY, Stratton MR. A mutational signature in gastric cancer suggests therapeutic strategies. Nat Commun 2015;6:8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ku GY, Kemel Y, Maron SB, Chou JF, Ravichandran V, Shameer Z, et al. Prevalence of Germline Alterations on Targeted Tumor-Normal Sequencing of Esophagogastric Cancer. JAMA Netw Open 2021;4:e2114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rustgi SD, Oh A, Hur C. Testing and Treating Helicobacter pylori Infection in Individuals With Family History of Gastric Cancer is Cost-effective. Gastroenterology 2021;161:2051–2052.e4. [DOI] [PubMed] [Google Scholar]
- 105.Casamayor M, Morlock R, Maeda H, Ajani J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience 2018;12:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Best LM, Takwoingi Y, Siddique S, Selladurai A, Gandhi A, Low B, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev 2018;3:CD012080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 2020;69:2113–2121. [DOI] [PubMed] [Google Scholar]
- 108.Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012;104:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumar S, Metz DC, Ellenberg S, Kaplan DE, Goldberg DS. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology 2020;158:527–536.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choi IJ, Kim CG, Lee JY, Kim YI, Kook MC, Park B, et al. Family History of Gastric Cancer and Helicobacter pylori Treatment. N Engl J Med 2020;382:427–436. [DOI] [PubMed] [Google Scholar]
- 111.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017;112:212–239. [DOI] [PubMed] [Google Scholar]
- 112.Feder R, Posner S, Qin Y, Zheng J, Chow SC, Garman KS. Helicobacter pylori-associated peptic ulcer disease: A retrospective analysis of post-treatment testing practices. Helicobacter 2018;23:e12540. [DOI] [PubMed] [Google Scholar]
- 113.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018;155:1372–1382.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut 2020;69:2093–2112. [DOI] [PubMed] [Google Scholar]
- 115.Shah SC, Tepler A, Chung CP, Suarez G, Peek RM, Hung A, et al. Host Genetic Determinants Associated With Helicobacter pylori Eradication Treatment Failure: A Systematic Review and Meta-analysis. Gastroenterology 2021;161:1443–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu Y, Wan JH, Li XY, Zhu Y, Graham DY, Lu NH. Systematic review with meta-analysis: the global recurrence rate of Helicobacter pylori. Aliment Pharmacol Ther 2017;46:773–779. [DOI] [PubMed] [Google Scholar]
- 117.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol 2012;23:1403–15. [DOI] [PubMed] [Google Scholar]
- 118.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol 2012;13:518–27. [DOI] [PubMed] [Google Scholar]
- 119.Kwon S, Ma W, Drew DA, Klempner SJ, Leonardo BM, Flynn JJ, et al. Association Between Aspirin Use and Gastric Adenocarcinoma: A Prospective Cohort Study. Cancer Prev Res (Phila) 2022;15:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Akre K, Ekström AM, Signorello LB, Hansson LE, Nyrén O. Aspirin and risk for gastric cancer: a population-based case-control study in Sweden. Br J Cancer 2001;84:965–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ye X, Fu J, Yang Y, Gao Y, Liu L, Chen S. Frequency-risk and duration-risk relationships between aspirin use and gastric cancer: a systematic review and meta-analysis. PLoS One 2013;8:e71522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheung KS, Chan EW, Wong AYS, Chen L, Seto WK, Wong ICK, et al. Aspirin and Risk of Gastric Cancer After Helicobacter pylori Eradication: A Territory-Wide Study. J Natl Cancer Inst 2018;110:743–749. [DOI] [PubMed] [Google Scholar]
- 123.Li B, Cheung KS, Wong IY, Leung WK, Law S. Nonaspirin nonsteroidal anti-inflammatory drugs and gastric cancer risk after Helicobacter pylori eradication: A territory-wide study. Cancer 2021;127:1805–1815. [DOI] [PubMed] [Google Scholar]
- 124.Li WQ, Zhang JY, Ma JL, Li ZX, Zhang L, Zhang Y, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ 2019;366:l5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen R, Liu Y, Song G, Li B, Zhao D, Hua Z, et al. Effectiveness of one-time endoscopic screening programme in prevention of upper gastrointestinal cancer in China: a multicentre population-based cohort study. Gut 2021;70:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li WQ, Qin XX, Li ZX, Wang LH, Liu ZC, Fan XH, et al. Beneficial effects of endoscopic screening on gastric cancer and optimal screening interval: a population-based study. Endoscopy 2022;54:848–858. [DOI] [PubMed] [Google Scholar]
- 127.Saumoy M, Schneider Y, Shen N, Kahaleh M, Sharaiha RZ, Shah SC. Cost Effectiveness of Gastric Cancer Screening According to Race and Ethnicity. Gastroenterology 2018;155:648–660. [DOI] [PubMed] [Google Scholar]
- 128.Huang RJ, Ende AR, Singla A, Higa JT, Choi AY, Lee AB, et al. Prevalence, risk factors, and surveillance patterns for gastric intestinal metaplasia among patients undergoing upper endoscopy with biopsy. Gastrointest Endosc 2020;91:70–77.e1. [DOI] [PubMed] [Google Scholar]
- 129.Altayar O, Davitkov P, Shah SC, Gawron AJ, Morgan DR, Turner K, et al. AGA Technical Review on Gastric Intestinal Metaplasia-Epidemiology and Risk Factors. Gastroenterology 2020;158:732–744.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nguyen TH, Tan MC, Liu Y, Rugge M, Thrift AP, El-Serag HB. Prevalence of Gastric Intestinal Metaplasia in a Multiethnic US Veterans Population. Clin Gastroenterol Hepatol 2021;19:269–276.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Genta RM, Turner KO, Sonnenberg A. Demographic and socioeconomic influences on Helicobacter pylori gastritis and its pre-neoplastic lesions amongst US residents. Aliment Pharmacol Ther 2017;46:322–330. [DOI] [PubMed] [Google Scholar]
- 132.Reddy KM, Chang JI, Shi JM, Wu BU. Risk of Gastric Cancer Among Patients With Intestinal Metaplasia of the Stomach in a US Integrated Health Care System. Clin Gastroenterol Hepatol 2016;14:1420–5. [DOI] [PubMed] [Google Scholar]
- 133.Gawron AJ, Shah SC, Altayar O, Davitkov P, Morgan D, Turner K, et al. AGA Technical Review on Gastric Intestinal Metaplasia-Natural History and Clinical Outcomes. Gastroenterology 2020;158:705–731.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li D, Bautista MC, Jiang SF, Daryani P, Brackett M, Armstrong MA, et al. Risks and Predictors of Gastric Adenocarcinoma in Patients with Gastric Intestinal Metaplasia and Dysplasia: A Population-Based Study. Am J Gastroenterol 2016;111:1104–13. [DOI] [PubMed] [Google Scholar]
- 135.O’Connor A, Bowden A, Farrell E, Weininger J, Crowther S, McNamara D, et al. Risk of Progression of Gastric Intestinal Metaplasia Is Significantly Greater When Detected in Both Antrum and Body. Dig Dis Sci 2021;66:3470–3475. [DOI] [PubMed] [Google Scholar]
- 136.González CA, Sanz-Anquela JM, Companioni O, Bonet C, Berdasco M, López C, et al. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: results of the Spanish follow-up multicenter study. J Gastroenterol Hepatol 2016;31:953–8. [DOI] [PubMed] [Google Scholar]
- 137.Pittayanon R, Rerknimitr R, Klaikaew N, Sanpavat A, Chaithongrat S, Mahachai V, et al. The risk of gastric cancer in patients with gastric intestinal metaplasia in 5-year follow-up. Aliment Pharmacol Ther 2017;46:40–45. [DOI] [PubMed] [Google Scholar]
- 138.Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010;71:1150–8. [DOI] [PubMed] [Google Scholar]
- 139.Lee JWJ, Zhu F, Srivastava S, Tsao SK, Khor C, Ho KY, et al. Severity of gastric intestinal metaplasia predicts the risk of gastric cancer: a prospective multicentre cohort study (GCEP). Gut 2022;71:854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gupta S, Li D, El Serag HB, Davitkov P, Altayar O, Sultan S, et al. AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology 2020;158:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Allen JE, Desai M, Roumans CAM, Vennalaganti S, Vennalaganti P, Bansal A, et al. Low Risk of Progression of Barrett’s Esophagus to Neoplasia in Women. J Clin Gastroenterol 2021;55:321–326. [DOI] [PubMed] [Google Scholar]
- 142.Chandrasekar VT, Hamade N, Desai M, Rai T, Gorrepati VS, Jegadeesan R, et al. Significantly lower annual rates of neoplastic progression in short- compared to long-segment non-dysplastic Barrett’s esophagus: a systematic review and meta-analysis. Endoscopy 2019;51:665–672. [DOI] [PubMed] [Google Scholar]
- 143.Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 2011;365:1375–83. [DOI] [PubMed] [Google Scholar]
- 144.Shaheen NJ, Falk GW, Iyer PG, Gerson LB, Gastroenterology ACo. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shaheen NJ, Falk GW, Iyer PG, Souza RF, Yadlapati RH, Sauer BG, et al. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am J Gastroenterol 2022;117:559–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kumar S, Dudzik CM, Reed M, Long JM, Wangensteen KJ, Katona BW. Upper Endoscopic Surveillance in Lynch Syndrome Detects Gastric and Duodenal Adenocarcinomas. Cancer Prev Res (Phila) 2020;13:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kumar S, Farha N, Burke CA, Katona BW. Upper Gastrointestinal Cancer Surveillance in Lynch Syndrome. Cancers (Basel) 2022;14: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.El Hafa F, Wang T, Ndifor VM, Jin G. Association between Helicobacter pylori antibodies determined by multiplex serology and gastric cancer risk: A meta-analysis. Helicobacter 2022;27:e12881. [DOI] [PubMed] [Google Scholar]
- 149.Miki K Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels - “ABC method”. Proceedings of the Japan Academy Series B, Physical and biological sciences 2011;87:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. International journal of cancer Journal international du cancer 2004;109:138–43. [DOI] [PubMed] [Google Scholar]
- 151.Murphy JD, Olshan AF, Lin FC, Troester MA, Nichols HB, Butt J, et al. A Predictive Model of Noncardia Gastric Adenocarcinoma Risk Using Antibody Response to Helicobacter pylori Proteins and Pepsinogen. Cancer Epidemiol Biomarkers Prev 2022;31:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shu X, Cai H, Lan Q, Cai Q, Ji BT, Zheng W, et al. A Prospective Investigation of Circulating Metabolome Identifies Potential Biomarkers for Gastric Cancer Risk. Cancer Epidemiol Biomarkers Prev 2021;30:1634–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miranti EH, Stolzenberg-Solomon R, Weinstein SJ, Selhub J, Männistö S, Taylor PR, et al. Low vitamin B12 increases risk of gastric cancer: A prospective study of one-carbon metabolism nutrients and risk of upper gastrointestinal tract cancer. Int J Cancer 2017;141:1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.NCCN. NCCN Clinical Practice Guidelines in Oncology. Gastric Cancer. National Comprehensive Cancer Network; 2022. January 11, 2022. Accessed October 25, 2022. [Google Scholar]
- 155.Lim YC, di Pietro M, O’Donovan M, Richardson S, Debiram I, Dwerryhouse S, et al. Prospective cohort study assessing outcomes of patients from families fulfilling criteria for hereditary diffuse gastric cancer undergoing endoscopic surveillance. Gastrointest Endosc 2014;80:78–87. [DOI] [PubMed] [Google Scholar]
- 156.Lee A, Mavaddat N, Wilcox AN, Cunningham AP, Carver T, Hartley S, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med 2019;21:1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peterson JM, Pepin A, Thomas R, Biagi T, Stark E, Sparks AD, et al. Racial disparities in breast cancer hereditary risk assessment referrals. J Genet Couns 2020;29:587–593. [DOI] [PubMed] [Google Scholar]
- 158.Williams CD, Bullard AJ, O’Leary M, Thomas R, Redding TS, Goldstein K. Racial/Ethnic Disparities in BRCA Counseling and Testing: a Narrative Review. J Racial Ethn Health Disparities 2019;6:570–583. [DOI] [PubMed] [Google Scholar]
- 159.Guo Y, Zhang Y, Gerhard M, Gao JJ, Mejias-Luque R, Zhang L, et al. Effect of. Gut 2020;69:1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tacheci I, Repak R, Podhola M, Benesova L, Cyrany J, Bures J, et al. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS) - A Helicobacter-opposite point. Best Pract Res Clin Gastroenterol 2021;50–51:101728. [DOI] [PubMed] [Google Scholar]
- 161.NCCN. NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Colorectal. National Comprehensive Cancer Network; 2022. June 8, 2022. Accessed October 26, 2022. [Google Scholar]


