Abstract
Urinary arsenic concentration is often used as a biomarker of arsenic exposure. First morning void (FMV) and spot urine samples from 131 participants in southeastern Michigan were analyzed using an HPLC-IC-PMS system for six different arsenic species: arsenobetaine (AsB), arsenite (As[III]), arsenate (As[V]), methylarsonous acid MMA[III], methylarsenic acid MMA[V], and dimethylarsenic DMA[V]. Bland–Altman plots, intraclass correlation coefficients (ICCs), and Pearson correlation procedures were used to evaluate the relationship between the arsenic species in FMV and spot urine collections after normalizing the samples by specific gravity. DMA[III] and MMA[III] were not detected in any of the samples. The sum of As[III], As[V], MMA[V], and DMA[V] was designated SumAs. The ICC between SumAs in FMV and SumAs in spot samples was 0.90. The ICC showed that 90% of variation comes from between individuals and not within individuals. A significant correlation (r = 0.80 p < 0.001) was observed between FMV and spot samples for SumAs. The spot sample were a good predictor of the MMA[V] (r = 0.83 p < 0.0001), and DMA[V] (r = 0.77 p < 0.0001) in the FMV sample. These associations suggest that either FMV or spot samples can be used as an adequate bioindicator of arsenic metabolites in human urine. The benefit of using spot urine samples, instead of 24-h or FMV urine samples, is the potential reduction in budgetary and logistic requirements in epidemiological studies.
Keywords: Arsenic, Urine, First morning void urine sample, Spot urine sample, Biomarker
Introduction
The concentrations and forms of arsenic in urine are often used as biomarkers of arsenic exposure (ATSDR, 2005). Differences in urinary arsenic metabolites may stem from sampling and storage procedures (Michalke, 2003). Consequently, the study of common and consistent methodologies which can lead to reduced variance in measured levels of urinary arsenic metabolites among different individuals is critical to improve the validation process. Sample collection strategies vary depending on the purpose of the study, the logistics, and budget constraints. Three urine collection methods are common: a 24-h sample, a first morning void (FMV) sample, and a spot urine sample (Calderon et al., 1999; Smith and Steinmaus, 2000). The 24-h urine is often considered to be the most reliable sample type (Cornelius et al., 1996). However, it is infrequently used in epidemiological studies involving a large number of participants due to complex logistics. Participants have to commit to participate in the project to a greater extend to provide a 24-h sample. A FMV sample is more frequently used in epidemiological studies to evaluate arsenic exposure (Caceres et al., 2005; Karagas et al., 2001; Meza et al., 2004), because it is more feasible to collect than 24-h samples.
First morning voids are attractive because they are more concentrated, and the metabolites obtained from the analysis are known to be in contact with the bladder for a longer period. Calderon et al. (1999) showed that one FMV sample is a reasonably good estimate of exposure but Smith and Steinmaus (2000) reported that a group average of many individual spot urine samples also provides a good estimate of exposure. Researchers have also used 72-, 96- or 120-h samples (Calderon et al., 1999). Samples collected during a period of 24–120h show increased reliability when assessing within-day and between-day variation and intra-individual differences. These multi-day samples are more expensive and difficult to collect, however. This research examines the differences in arsenic metabolites in FMV and spot urine samples from an adult population in southeastern Michigan. The urine samples were analyzed for six different arsenic species: arsenobetaine (AsB), arsenite (As[III]), arsenate (As[V]), methylarsonous acid (MMA[III]), methylarsenic acid (MMA[V]), and dimethylarsenic (DMA[V]). Arsenic speciation is useful to distinguish exposure to organic and inorganic arsenic and to assess arsenic metabolism in the human body (ATSDR, 2005). The goal of the study was to evaluate arsenic species in FMV and spot urine samples as adequate biomarkers using reliability and agreement methods to assess variability. In addition, total arsenic concentration in toenails was compared to urinary arsenic to evaluate the correlation between the two biomarkers.
Methodology
Study population and sample collection
First morning void and spot urine samples were collected from a sub-sample of participants enrolled in a large case–control study of arsenic exposure and bladder cancer in 11 counties of southeastern Michigan. Project details including recruitment strategies have been published elsewhere (Meliker et al., 2010). Briefly, cases were obtained from the Michigan State Cancer Registry and were frequency-matched by age, race and gender with controls selected through a random-digit dialing procedure. The sub-sample used in this study included those individuals from the main study that had both type of samples, FMV and spot. Participants were recruited from June 2005 to May 2007. The total number of individuals was 131 of which 50 were cases and 81 were controls. Initially, individuals completed a phone interview to obtain information on medical history, life-styles habits, and water consumption patterns. Subsequently, water, toenails and urine samples were collected during visits to participants’ homes.
The research team provided two different ‘urine collection kits’ to participants along with materials and instructions for urine collection. Kit A (the FMV sample) included a plastic bag packed with an acid-washed polyethylene urine cup, antibacterial wipes, gloves, and urine collection instructions. Kit A also included a small foam cooler with dry ice into which the urine sample would be placed. Kit B (the spot sample) included only the plastic bag with collection materials with no dry ice and foam cooler. Each participant was asked to use Kit A to collect FMV sample first thing in the morning and immediately fast-freeze it by immersing the container in dry ice. FMV samples were picked up (frozen) the morning they were collected and transported to the laboratory. Participants were asked to collect spot urine samples using Kit B during field visits. Spot samples were immediately frozen in dry ice brought along by the research team and then transported to the laboratory. The FMV and spot samples were stored in the laboratory at −20°C until analysis. Each of the participants provided both spot and FMV urine samples.
In addition to urine samples, toenail samples were also collected by researchers during field visits. Participants were asked to clip toenails with materials provided by the research team using the methodology described by Slotnick et al. (2007).
Sample preparation and analysis
Urine samples were quickly thawed using a water bath. After they achieved room temperature, samples were filtered using a 0.22 μm syringe filter and no preservatives were added. Specific gravity measures were taken immediately after samples were thawed and reached room temperature; freezing urine samples apparently does not change the specific gravity (Nermell et al., 2008). Specific gravity measures provide a measure of dissolved material and particles concentration in urine (Flasar, 2008). Specific gravity measurements were conducted with a pycnometer (Fisher Scientific) using the density of water as a reference. Three weight measurements were taken using a pycnometer: the empty flask, flask with water, and flask with urine sample. The weight of the urine was obtained by subtracting the weight of the empty flask and its specific gravity was calculated by dividing the urine by the weight of the water previously obtained. Samples with specific gravity above 1.03 or below 1.01 were considered too concentrated or too diluted and were excluded from the analysis (n = 2) (Teass et al., 2003). Concentrations were adjusted to the mean specific gravity of the samples (1.018 g/ml).
Immediately after filtration, samples were analyzed using a High Performance Liquid Chromatography-Inductively Coupled Plasma-Mass Spectrometry (HPLC-ICP-MS) system following an adaptation protocol from Le et al. (2000). Urine samples were filtered through a 5μm 250mm × 4.6 mm column (Phenomenex, Torrance, CA, USA). The mobile phase contained 4% (v/v) methanol, 5 mM tetrabutylammonium hydroxide (TBAH), 10 mM ammonium phosphate at pH 9.5. The HPLC system (Alltech) was coupled to an ICP-MS (Agilent Technologies Model 7500c) unit. Detection limits following the method were: AsB, 0.06μg/L; As[III], 0.112μg/L; As[V], 0.147 μg/L; MMA[III], 0.138 μg/L; MMA[V], 0.117 μg/L; and DMA[V], 0.076 μg/L, with recovery rates ranging between 96% and 105%, respectively. Urine certified reference material (National Institute for Environmental Studies, NIES NO. 18, Tsukuba, Ibaraki, Japan) was prepared by dissolving the appropriate amount of urine powder in water to obtain 69 ± 12 μg/L AsB, 36 ± 9 μg/L DMA and 137 ± 11 μg/L total As. A 2mgL−1 germanium internal standard solution was prepared from the stock Ge solution (Inorganic Ventures, 1000mgL−1 Ge) by dilution with the same mobile phase being used in the analytical column.
Toenail samples, after being collected, were washed and dried overnight in a 60°C oven. Following the drying procedure, toenails were digested using Optima HNO3 (Fisher Chemical) and Suprapur H2O2 (Merk). Toenail samples were analyzed for total arsenic using ICP-MS and calibration standards were prepared prior to analysis. A complete description of toenail sample preparation and analysis has been described in Slotnick et al. (2007).
Statistical analysis
Descriptive statistics were calculated for urinary arsenic metabolites as well as for the participants’ attribute data. Histograms and normal probability plots revealed deviations from normal distribution for all urinary arsenic metabolites and toenail arsenic concentrations. Log10-transformations were therefore applied to the data before performing statistical analysis. No difference was found between spot samples collected in the morning and those collected in the afternoon. For this paper, the sum of As[III], As[V], MMA[V] and DMA[V] was designated SumAs. The sum of As[III] and As[V] was used as the total of inorganic arsenic (InAs) because it provides a more stable measure of inorganic arsenic in urine, since these two species may interconvert while in urine. MMA[III] was not detected in any of the samples. MMA and DMA will refer to the pentavalent species of MMA and DMA, unless otherwise stated. Concentrations below the detection limit were set to the limit of detection divided by the square root of two. Values below the detection limit were labeled “BDL” in tables and figures. The relative proportion of arsenic in each species (%InAs, %MMA, and %DMA) was calculated by dividing the concentration of arsenic in each species by the concentration of InAs, MMA, and DMA combined.
The association of each arsenic species with variables such as disease status, gender, age, and smoking was assessed using univariate analyses. In order to evaluate reliability for each species in FMV samples and spot samples, the intraclass correlation coefficient (ICC) was calculated. The ICC was used to estimate the correlation of each species among individuals within the same group of samples (FMV or spot) and it is different from the Pearson correlation coefficient where the variables of interest are modeled as two distinct traits. The ICC shows how much of the variance comes from between subjects and not within subjects. To evaluate the degree of agreement, a Bland–Altman plot of the difference between the urinary arsenic concentration of both sample types against the mean of both sample types (FMV-spot ±2SD) was constructed (Bland and Altman, 1986). Pearson correlation coefficients (r) were generated to compare arsenic concentration and species in each sampling method. Pearson correlation coefficients were also generated to compare arsenic in toenails with total urinary arsenic metabolites for each sample type. The use of non-parametric analyses did not change or improve the correlation coefficients. Pearson correlation coefficients and ICC were estimated on log-transformed levels that accounted for urine dilution using specific gravity, and on the samples not adjusted for specific gravity.
The ICC and Pearson correlation coefficient were performed for all individual species and SumAs. The Bland–Altman plot was performed for SumAs. Since no significant differences among disease status, gender, age, and smoking status were seen these groups were combined for the correlation coefficients presented here. All statistical analyses were run using SAS statistical software, version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Demographic information and arsenic average concentrations for participants who provided urine samples are shown in Table 1. A total of 131 FMV and corresponding spot samples were used in this study. The majority of participants included in this research were men (86%) and the group’s average age was 65.7; the age distribution reflects the fact that bladder cancer is predominantly a disease of elderly Caucasian men. Table 1 also shows SumAs for all groups. Cases and ever smokers have a slightly higher arsenic concentration in both samples, FMV and spot, than controls and never smokers.
Table 1.
Demographic characteristics and average total arsenic concentrations (SumAs) of FMV, spot urine samples, and toenails.
| Variable | n | % | SumAsa | FMV urine (μg/L) | SumAsa | Spot urine (μg/L) | [As] | Toenails (μg/g) |
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Ab mean | Gc mean | Ab mean | Gc mean | Ab mean | Gc mean | |||
|
| ||||||||
| All individuals | 131 | 100 | 6.9 | 4.7 | 8.0 | 5.7 | 0.20 | 0.13 |
| Disease status | ||||||||
| Case | 50 | 38.2 | 7.6 | 5.1 | 8.3 | 6.3 | 0.25 | 0.16 |
| Control | 81 | 61.8 | 6.4 | 4.5 | 7.8 | 5.4 | 0.16 | 0.12 |
| Gender | ||||||||
| Female | 18 | 13.7 | 6.9 | 3.8 | 8.0 | 5.0 | 0.26 | 0.14 |
| Male | 113 | 86.3 | 6.7 | 4.9 | 8.2 | 5.8 | 0.19 | 0.13 |
| Age | ||||||||
| <65 | 50 | 38.2 | 7.6 | 5.1 | 9.0 | 6.3 | 0.24 | 0.17 |
| 65–75 | 55 | 42.0 | 6.5 | 4.4 | 7.3 | 5.4 | 0.19 | 0.12 |
| >75 | 26 | 19.8 | 6.1 | 4.8 | 7.2 | 5.4 | 0.13 | 0.10 |
| Smoking | ||||||||
| Never | 43 | 32.8 | 6.3 | 4.7 | 7.8 | 5.6 | 0.18 | 0.13 |
| Ever | 87 | 66.4 | 7.2 | 4.8 | 8.1 | 6.0 | 0.20 | 0.13 |
| Race | ||||||||
| White | 123 | 93.9 | 6.8 | 4.7 | 7.8 | 5.6 | 0.20 | 0.13 |
| Other | 8 | 6.1 | 8.4 | 5.5 | 11.3 | 7.0 | 0.18 | 0.12 |
SumAs = As[III] + As[V] +MMA[V]+DMA[V].
Arithmetic mean adjusted by specific gravity.
Geometric mean adjusted by specific gravity.
Table 2 shows the distribution of concentrations for each species in FMV and spot samples before and after specific gravity adjustment. The average storage time for samples was 41 days (0–189 days). MMA[III] was not detected in any of the samples. Overall, spot urine samples had more detectable arsenic concentrations of all species than FMV samples. After specific gravity adjustment, median values for first morning voids of As[III], As[V], MMA, DMA and AsB were BDL, BDL, 0.7μg/L, 3.5μg/L, and 2.4μg/L, respectively. After specific gravity adjustment, median values for spot samples of As[III], As[V], MMA, DMA and AsB were 0.26μg/L, BDL, 0.9μg/L, 3.9μg/L, and 2.6μg/L, respectively.
Table 2.
Statistical distributions of concentrations of each arsenic species for FMV and spot urine samples.
| Arsenic | FMV unadjusted (|xg/L)a | FMV adjusted (μg/L)a,d | FMV %BDLb | Spot unadjusted (μg/L)a | Spot adjusted (μg/L)a,d | Spot %BDLb |
|---|---|---|---|---|---|---|
|
| ||||||
| InAsc | BDL(BDL-0.5) | 0.1 (BDL-0.6) | 57 | 0.3 (0.1–0.7) | 0.5 (0.1–0.9) | 34 |
| MMA | 0.5 (0.3–2.1) | 0.7 (0.4–2.8) | 6 | 0.8 (0.4–1.6) | 0.9 (0.6–1.5) | 4 |
| DMA | 3.2 (1.9–5.7) | 3.5 (2.1–5.8) | 1 | 3.3 (2.1–6.4) | 3.9 (2.2–6.4) | 1 |
| AsB | 2.3 (1.2–8.3) | 2.4 (1.0–8.7) | 0 | 2.6 (0.8–8.7) | 2.6 (0.9–8.1) | 1 |
[Median(25th, 75th percentiles)].
BDL.
InAs = As[III] + As[V].
Adjusted to the mean specific gravity (1.018 g/ml).
To evaluate the degree of agreement, a Bland–Altman plot was constructed (Fig. 1). The mean difference in SumAs between samples was −1.11 μg/L (95% CI −1.89, −0.33). The Bland–Altman plot showed a high degree of agreement with 92% of samples within the limits of agreements determined by two standard deviations. This indicates that there is little difference between FMV and spot urine levels for 92% of the samples. Table 3 shows the Pearson correlation coefficients and ICC for associations of each arsenic species between FMV and spot samples. Arsenic concentrations (SumAs) between spot and FMV samples were strongly correlated (r = 0.80) (Fig. 2). Individual arsenic species were also correlated. Methylated metabolites, MMA and DMA showed a higher correlation between FMV and spot samples than inorganic arsenic (As[III] and As[V]). Spot and FMV samples were the most correlated for MMA (r = 0.83), and DMA was also strongly correlated (r = 0.77). The ICC between SumAs in FMV and SumAs in spot samples was 0.90. The ICC shows that 90% of variation comes from between individuals and not within individuals. ICCs were 0.88, 0.91, 0.91 for InAs, MMA and DMA, respectively.
Fig. 1.
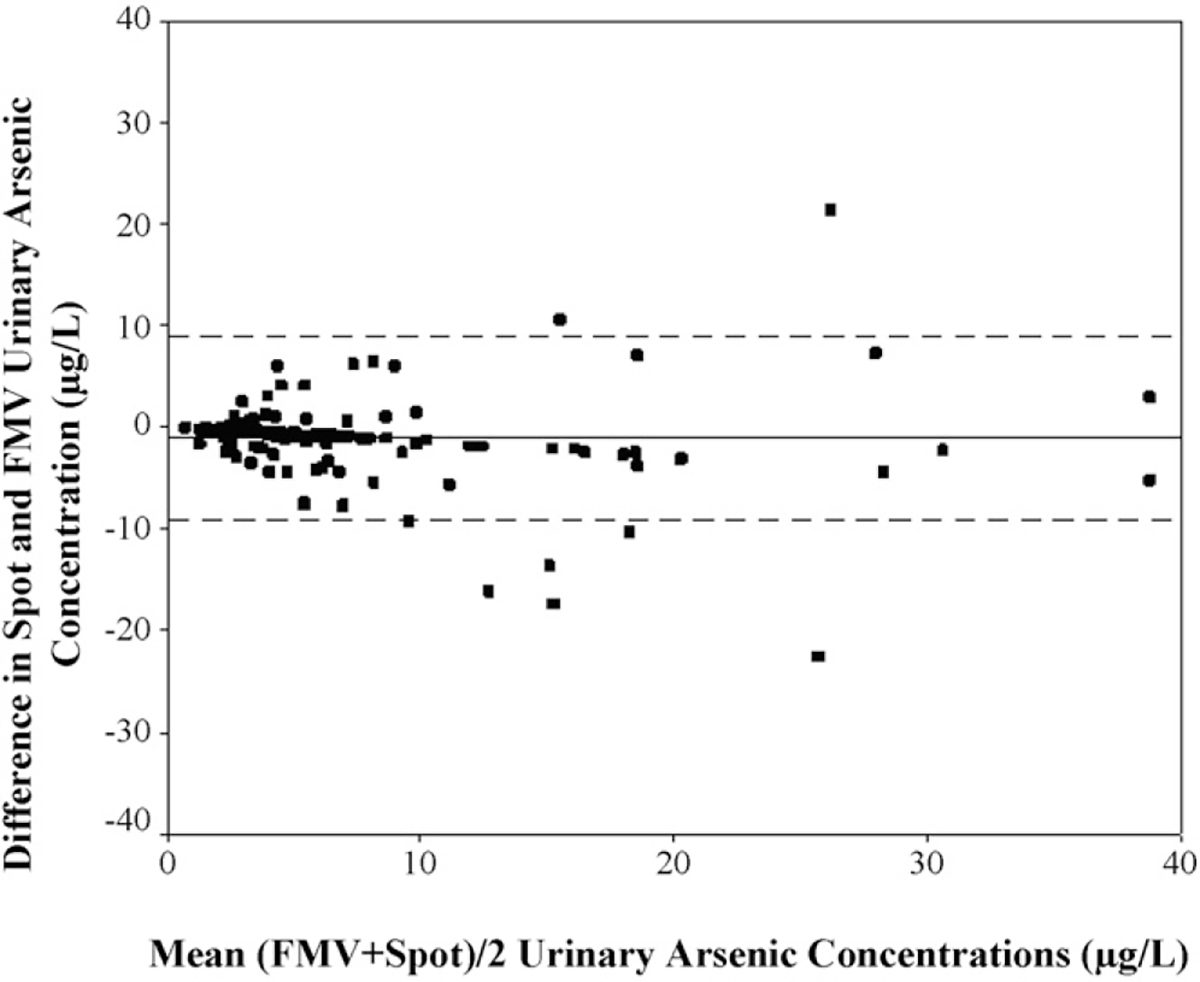
Bland–Altman plot of difference between FMV and spot SumAs (μg/L) urine samples (n = 131).
Table 3.
Pearson correlation coefficients and ICC between FMV and spot samples for each arsenic species.
| FMV correlated with spot sample | Pearson’s correlation coefficient before SGa adjustment | Pearson’s correlation coefficient after SGa adjustment | ICC | p-valued |
|---|---|---|---|---|
|
| ||||
| SumAsb | 0.76 | 0.82 | 0.90 | <0.0001 |
| InAsc | 0.63 | 0.72 | 0.88 | <0.0001 |
| DMA | 0.76 | 0.78 | 0.91 | <0.0001 |
| MMA | 0.78 | 0.83 | 0.89 | <0.0001 |
| AsB | 0.81 | 0.84 | 0.93 | <0.0001 |
Specific gravity.
SumAs = As[III] + As[V] +MMA[V]+DMA[V].
InAs = As[III] + As[V].
p-values for correlations and ICCs.
Fig. 2.
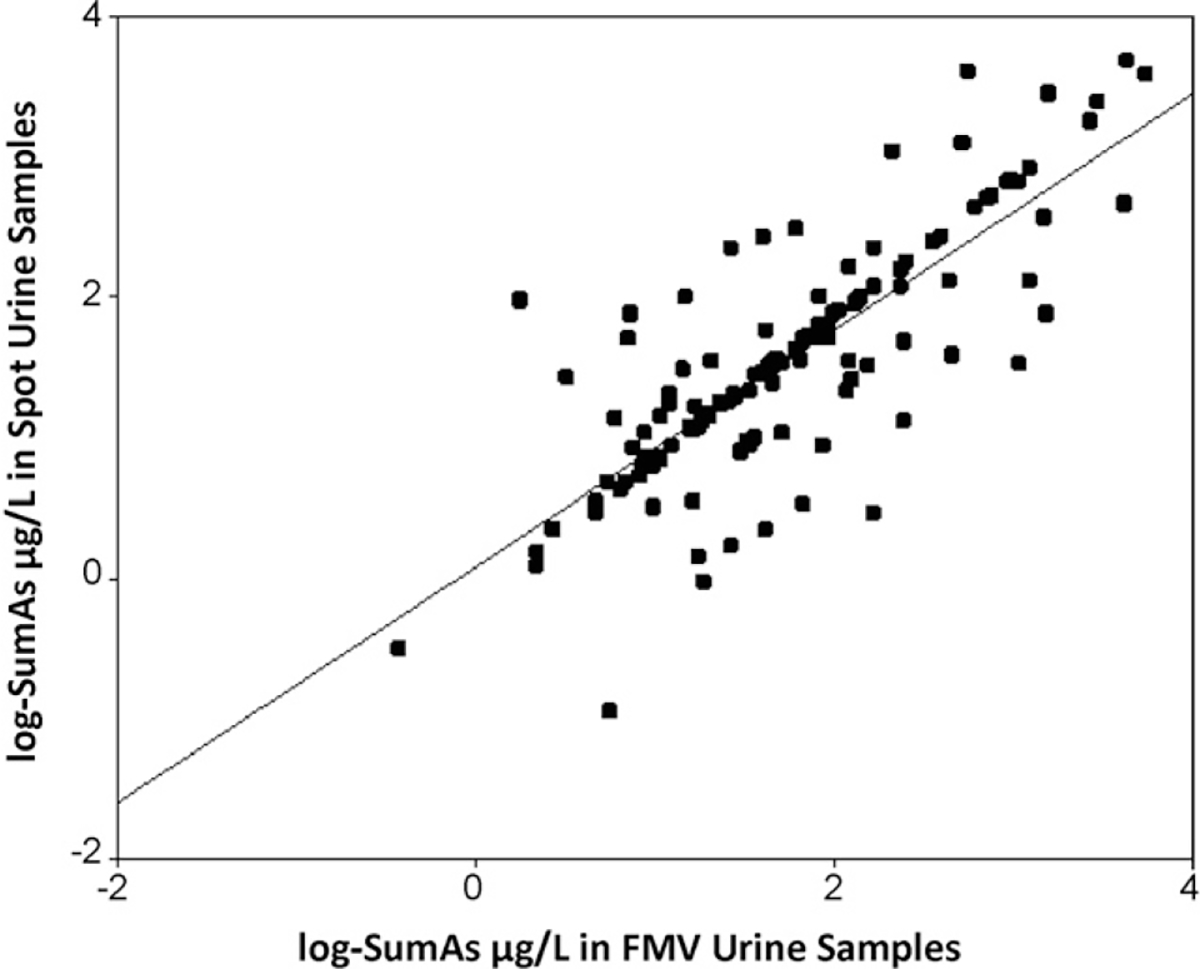
Correlation between FMV and spot samples for log10-SumAs.
Given the interest in arsenic methylation, the urinary arsenic profile for the 131 individuals was determined using FMV and spot samples separately and is presented in Table 4. The relative proportion of FMV samples for %InAs, %MMA, and %DMA were 7.9%, 16.0%, and 76.0%, respectively. The relative proportion of spot samples for %InAs, %MMA, and %DMA were 9.6%, 18.1%, and 72.3%, respectively. Pearson correlation coefficients between FMV and spot samples for %InAs, %MMA, and %DMA were significant (∞ = 0.05) (results not shown).
Table 4.
Proportion of arsenic species in FMV and spot urine samples.
| Variable | N | % | %InAs FMV | %InAs SPOT | %DMA FMV | %DMA SPOT | %MMA FMV | %MMA SPOT |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| All | 131 | 100 | 7.9 | 9.6 | 76.0 | 72.3 | 16.0 | 18.1 |
| Bladder cancer | ||||||||
| Cases | 50 | 38.2 | 7.0 | 9.4 | 77.7 | 73.0 | 15.2 | 17.5 |
| Controls | 81 | 61.8 | 8.6 | 9.9 | 74.9 | 71.7 | 16.6 | 18.4 |
| Gender | ||||||||
| Women | 18 | 13.7 | 9.7 | 10.2 | 76.0 | 72.9 | 14.3 | 16.9 |
| Men | 113 | 86.3 | 7.7 | 9.6 | 75.9 | 72.1 | 16.3 | 18.3 |
| Age | ||||||||
| <65 | 50 | 38.2 | 8.2 | 9.8 | 75.9 | 71.8 | 16.0 | 18.4 |
| 65–75 | 56 | 42.0 | 8.7 | 10.1 | 74.7 | 70.9 | 16.2 | 19.0 |
| >75 | 26 | 19.8 | 6.0 | 8.5 | 79.0 | 76.1 | 15.0 | 15.4 |
| Smoking | ||||||||
| Never | 43 | 32.8 | 8.2 | 8.9 | 75.5 | 72.2 | 16.2 | 18.8 |
| Ever | 88 | 66.4 | 7.9 | 9.9 | 76.2 | 72.5 | 15.9 | 17.6 |
| Race | ||||||||
| White | 123 | 93.9 | 7.9 | 9.6 | 76.2 | 72.3 | 16.1 | 18.0 |
| Other | 8 | 6.1 | 8.5 | 9.6 | 75.7 | 71.6 | 15.6 | 18.3 |
In order to compare biomarkers of arsenic exposure, toenail arsenic levels were compared with spot and FMV urine samples (Table 5). Spot samples and FMV samples were significantly correlated to arsenic in toenails. However, the correlation for FMV samples was higher than for spot samples. In addition, arsenic concentrations in toenails were compared with arsenic concentrations in FMV samples and spot samples. All correlations were positively significant and similar between toenail total arsenic concentrations and both FMV and spot urine arsenic species, with the exception of AsB which showed a small negative association.
Table 5.
Association between urine arsenic species and total arsenic concentration in toenails.
| FMV and Spot sample species correlated with total arsenic in toenail samples | Pearson’s correlation coefficient | p-value |
|---|---|---|
|
| ||
| SumAsFMV | 0.45 | <0.0001 |
| SumAsSPOT | 0.38 | <0.0001 |
| InAsFMV | 0.40 | <0.0001 |
| InAsSPOT | 0.31 | <0.0001 |
| MMAfmv | 0.42 | <0.0001 |
| MMASPOT | 0.40 | <0.0001 |
| DMAfmv | 0.43 | <0.0001 |
| DMASPOT | 0.35 | <0.0001 |
| AsBFMV | −0.10 | <0.0001 |
| AsBSPOT | −0.15 | <0.0001 |
Discussion
Our analysis of 131 paired urine samples shows that arsenic species in spot urine samples are adequate biomarkers to measure arsenic exposure. To our knowledge, this is the first reported study evaluating FMV vs. spot urine samples with a large sample size where 99% of participants had detectable total arsenic concentrations. Furthermore, this research measured several urinary arsenic metabolites in order to better compare sampling methods. Although 24-h samples are considered the most reliable sample type (Cornelius et al., 1996), the search for less-expensive and more logistically efficient methods is always a goal. Spot sample collection, instead of FMV, will decrease budgetary and logistical issues.
Dilution variation adjustments and arsenic metabolism are often discussed as confounders in biomarker validation. Data interpretation of 24-h, FMV, and spot urine samples are influenced by factors such as urine concentration and volume. To avoid biased results due to variation in urine dilution, an adjustment by urinary creatinine concentration is usually performed (Hinwood et al., 2002). Creatinine analysis is performed on the basis that its excretion is constant from the body. However, creatinine is formed by creatine and its excretion is related to muscle mass and meat intake (Hall Moran et al., 2001; Worsfold et al., 1999). Studies have shown that urinary creatinine excretion varies by age, gender, body size, and diet (Boeniger et al., 1993; Suwazono et al., 2005). As a result, some studies have proposed the use of specific gravity instead of creatinine for normalization purposes, because of the strong correlation between specific gravity and creatinine (Moore et al., 1997; Parikh et al., 2002).
Our results show slightly more detectable arsenic concentrations in spot samples than in FMV samples before and after specific gravity adjustment. There are some studies evaluating dilution adjustments and urinary arsenic but not sample type. Nermell et al. (2008) evaluated specific gravity vs. creatinine and urinary arsenic in a Bangladeshi population (n = 1466). They concluded that specific gravity may be influenced by age, gender, and body size but the influence of these is less than for creatinine. Suwazono et al. (2005) evaluated creatinine vs. specific gravity and urinary cadmium in Swedish populations (n = 993). They also concluded that urinary cadmium adjusted for creatinine is more affected by age, gender, body size, and meat intake than is specific gravity.
The Bland–Altman plot showed a high degree of agreement between sample types indicating that spot samples are adequate to evaluate arsenic exposure. In A addition to the strong correlations presented here, the ICCs for SumAs, InAs, MMA, DMA, and AsB indicate that most of the variance between FMV and spot urine samples is due to subject-to-subject variability. The same results are true for methylation patterns where just ~10% of the variation is due to other sources. A previous study where two or three samples were collected over time from the same individuals revealed that besides within-person variation, laboratory imprecision makes a substantial contribution to the total variance not due to between-subject variability (Steinmaus et al., 2005). They estimated that ~45% of variance in their analysis was due to laboratory imprecision. Another study that evaluated intra-individual variability, Concha et al. (2002), found considerable between-person variability in the urinary excretion while little day-to-day variation was seen among arsenic metabolites. An important consideration when evaluating variability is the population level of exposure. This population is exposed to low-to-moderate levels of arsenic (≤50μg/L). Drastic changes in arsenic metabolism may not be expected and arsenic excretion may be happening at a constant rate. In populations with a constant arsenic exposure a single urine sample may provide useful information to assess arsenic exposure and individual arsenic metabolism (Navas-Acien et al., 2009).
A moderate correlation was observed between urinary arsenic (SumAs) and arsenic in toenails (Table 5). Even though urinary arsenic is a short term biomarker and arsenic in toenails is a longer term biomarker, the fact that arsenic concentrations of public and private water sources are stable over time (Steinmaus et al., 2005) may influence the correlation between the two biomarkers. Total arsenic concentrations in toenails were found significantly correlated with those of both FMV and spot samples. FMV vs. total toenail concentrations were more strongly correlated than spot vs. toenails concentrations. However, when outlying FMV samples were removed from the analysis, the correlation was lower (r = 0.39), very similar to the correlation for arsenic toenails vs. spot samples (r = 0.38). The correlations of each urinary arsenic species, including arsenobetaine, with total arsenic concentration in toenails also confirm that toenail arsenic reflects mostly inorganic arsenic. Urine reflects exposures occurring in the past days or weeks while toenails may reflect a longer period of exposure (NRC, 2001). Arsenobetaine appear to undergo little or no metabolism and therefore is excreted without any significant changes in structure (Borak and Hosgood, 2007). AsB is excreted after 2 or 3 days following ingestion, therefore it is reflected in urine but may not be reflected in the toenail biomarker since the latter reflects a longer period of more chronic exposure, presumably, mostly inorganic arsenic. Further, inorganic arsenic is thought to bind to keratin proteins in nails (Mandal et al., 2003; NRC, 1999). The correlation of arsenic in urine and toenails is notable considering the lifetimes of these biomarkers and the many variables that can moderate the arsenic burden in each biomarker (Slotnick et al., 2007). Diet, arsenobetaine excretion, and its correlation with toenails in this population will be assessed further in future research.
Although MMA[III] and DMA[III] have been reported in human urine (Aposhian et al., 2000; Valenzuela et al., 2005) most epidemiological studies have recorded only As[III], As[V], MMA[V], and DMA[V] (Loffredo et al., 2003; Steinmaus et al., 2006) since the detection of the trivalent species is very difficult if samples are not analyzed immediately (Gong et al., 2001; Mandal et al., 2001). Moreover, in populations exposed to low levels of arsenic, they may be undetectable since MMA[III] and DMA[III] have been observed only in areas where residents have endemically been exposed to high levels of arsenic (>100μg/L) in drinking water (e.g. Mexico, India). Recently, a model has been proposed which questions the formation of MMA[III] and DMA[III] as significant intermediates during arsenic metabolism in humans (Slejkovec et al., 2008). The levels of pentavalent methylated species were highly correlated between FMV and spot samples, especially MMA. MMA[V] excretion has been related to an increased risk of arsenic-related diseases such as bladder and skin cancer (ATSDR, 2005; Chen et al., 2003; Hopenhayn-Rich et al., 1996; Steinmaus et al., 2006).
Two different pathways have been proposed to explain arsenic metabolism. The first one, described by Cullen and Reimer (1986) explains arsenic biotransformation in two stages: oxidation/reduction followed by methylation reactions. The second pathway was described more by Hayakawa et al. (2005). The main difference between the two is that Hayakawa et al.’s model involves preferential formation of trivalent methylated species before the pentavalent methylated species. At the present time, the precise mechanisms of arsenic biotransformation and the enzymes involved are still unclear. The urinary arsenic profiles observed in this study do not provide confirmatory evidence that arsenic metabolism follows either the Cullen and Reimer (1986) or Hayakawa et al. (2005) biotransformation pathways. However, collection of urine samples using the methods described here, combined with genetic information may provide insight on the metabolic processes.
In conclusion, results presented here suggest that FMV and spot samples can be used without preference when evaluating arsenic exposure in epidemiological studies. Spot urine samples, as opposed to 24-h or FMV urine samples, are simpler to obtain and less-expensive to collect, making them a good biomarker candidate for epidemiological studies. Nonetheless, factors such as laboratory imprecision and stability of methylation patterns may result in some within-individual variability.
References
- Agency for Toxic Substances and Disease Registry A, 2005. Toxicological Profile for Arsenic. [PubMed] [Google Scholar]
- Aposhian H,Gurzau E,Le XC,Gurzau A,Healy S,Lu X,Ma M,Yip L,Zakharyan R, Maiorino R, et al. , 2000. Occurrence of monomethylarsonous acid in urine of humans exposed to inorganic arsenic. Chem. Res. Toxicol. 12, 693–697. [DOI] [PubMed] [Google Scholar]
- Bland J, Altman D, 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307–310. [PubMed] [Google Scholar]
- Boeniger M, Lowry L, Rosenberg J, 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. AIHA J. (Fairfax, VA) 54, 201–212. [DOI] [PubMed] [Google Scholar]
- Borak J, Hosgood H, 2007. Seafood arsenic: implications for human risk assessment. Regul. Toxicol. Pharmacol. 47, 204–212. [DOI] [PubMed] [Google Scholar]
- Caceres D, Pino P, Montesinos M, Atalah E, Amigo H, Loomis D, 2005. Exposure to inorganic arsenic in drinking water and total urinary arsenic concentration in a Chilean population. Environ. Res. 98, 151–159. [DOI] [PubMed] [Google Scholar]
- Calderon R, Huggens E, Le XC, Schreinemachers D, Thomas DJ, 1999. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ. Health Perspect. 107, 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Su H-J, Guo Y-L, Hsueh Y-M, Smith T, Ryan L, Lee M-S, Christiani D, 2003. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Controls 14, 303–310. [DOI] [PubMed] [Google Scholar]
- Concha G, Vogler G, Nermell B, Vahter M, 2002. Intra-individual variation in the metabolism of inorganic arsenic. Int. Arch. Occup. Environ. Health 75, 576–580. [DOI] [PubMed] [Google Scholar]
- Cornelius R, Heinzow B, Herber R, Christensen JM, Poulson O, Sabbiobi E, Templeton D, Vahter M, Vesterberg O, 1996. Sample collection guidelines for trace elements in blood and urine. J. Trace Elem. Med. Biol. 10, 103–127. [DOI] [PubMed] [Google Scholar]
- Cullen W, Reimer K, 1986. Arsenic speciation in the environment. Chem. Rev. 89, 713–764. [Google Scholar]
- Flasar C, 2008. What is specific gravity? Nursing 38, 14. [DOI] [PubMed] [Google Scholar]
- Gong Z, Lu X, Cullen W, Le XC, 2001. Unstable trivalent arsenic metabolites, monomethylarsonous acid and dimethylarsinous acid. J. Anal. Atom. Spectrom. 16, 1409–1413. [Google Scholar]
- Hall Moran V, Leathard H, Coley J, 2001. Urinary hormones levels during the natural menstrual cycle: the effect of age. J. Endocrinol. 170, 157–164. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Kobayashi Y, Cui X, Hirano S, 2005. A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase CYT19. Arch. Toxicol. 79, 183–191. [DOI] [PubMed] [Google Scholar]
- Hinwood AL, Sim MR, de Klerk N, Drummer O, Gerostamoulos J, Bastone EE, 2002.Are 24-hour urine samples and creatinine adjustment required for analysis of inorganic arsenic in urine in population studies? Environ. Res. 88, 219–224. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs M, Smith A, Kalman D, Moore L, 1996. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ. Health Perspect. 104, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagas MR, Le XC, Morris S, Blum J, Lu X, Spate V, Carey M, Stannard V, Klaue B, Tosteston T, 2001. Markers of low level arsenic exposure for evaluating human cancer risks in a US population. Int. J. Occup. Med. Environ. Health 14, 171–175. [PubMed] [Google Scholar]
- Le XC, Lu X, Ma M, Cullen W, Aposhian HV, Zheng B, 2000. Speciation of key arsenic metabolic intermediates in human urine. Anal. Chem. 72, 5172–5177. [DOI] [PubMed] [Google Scholar]
- Loffredo C, Aposhian H, Cebrian M, Yamauchi H, Silbergeld E, 2003. Variability in human metabolism of arsenic. Environ. Res. 92, 85–91. [DOI] [PubMed] [Google Scholar]
- Mandal B, Ogra Y, Suzuki K, 2001. Identification of dimethylarsinous and monomethyarsonous acids in human urine of the arsenic-affected areas in West Bengal, India. Chem. Res. Toxicol. 14, 371–378. [DOI] [PubMed] [Google Scholar]
- Mandal B, Ogra Y, Suzuki K, 2003. Speciation of arsenic in human nail and hair from arsenic-affected area by HPLC-inductively coupled plasma mass spectrometry. Toxicol. Appl. Pharmacol. 189, 73–83. [DOI] [PubMed] [Google Scholar]
- Meliker JR, Slotnick MJ, Avrusking GA, Schottenfeld D, Jacquez GM, Wilson ML, Goovaerts P, Franzblau A, Nriagu JO, 2010. Lifetime Exposure to Arsenic in Drinking Water and Bladder Cancer: a Population-Based Case-Control Study in Michigan. Cancer Causes Control In Print, USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza M, Kopplin M, Burgess J, Gandolfi A, 2004. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ. Res. 96, 119–126. [DOI] [PubMed] [Google Scholar]
- Michalke B, 2003. Element speciation definitions, analytical methodology, and some examples. Ecotoxicol. Environ. Saf. 56, 122–139. [DOI] [PubMed] [Google Scholar]
- Moore R, Hirata-Dulas C, Kasiske B, 1997. Use of urine specific gravity to improve screening for albuminuria. Kidney Int. 52, 240–243. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Umans J, Howard BV, Goessler W, Francesconi K, Crainiceanu C, Silbergeld E, Guallar E, 2009. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Hearth Study. Environ. Health Perspect. 117, 1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 1999. In: Water SoAiD (Ed.), Arsenic in Drinking Water. National Academy Press, Washington, DC. [Google Scholar]
- National Research Council, 2001. In: Water SoAiD (Ed.), Arsenic in Drinking Water 2001 Update. National Academy Press, pp. 15–23. [Google Scholar]
- Nermell B, Lindberg AL, Rahman M, Berglund M, Persson LA, Arifeen SE, Vahter M, 2008. Urinary arsenic concentration adjustment factors and malnutrition. Environ. Res. 106, 212–218. [DOI] [PubMed] [Google Scholar]
- Parikh C, Gyamlani G, Carvounis C, 2002. Screening for microalbuminuria simplified by urine specific gravity. Am. J. Nephrol. 22, 315–319. [DOI] [PubMed] [Google Scholar]
- Slejkovec Z, Falnoga I, Goessler W, van Elteren J, Raml R, Podgornik H, Cernelc P, 2008. Analytical artifacts in the speciation of arsenic in clinical samples. Anal. Chim. Acta 607, 83–91. [DOI] [PubMed] [Google Scholar]
- Slotnick M, Meliker J, Nriagu J, 2007. Intra-individual variability in toenails arsenic concentrations in a Michigan population, USA. J. Expo. Sci. Environ. Epidemiol. 18, 1–9. [DOI] [PubMed] [Google Scholar]
- Smith AH, Steinmaus C, 2000. Arsenic in urine and drinking water. Environ. Health Perspect, A494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Kalman D, Attallah R, Smith AH, 2005. Intraindividual variability in arsenic methylation in a US population. Cancer Epidemiol. Biomarkers Prev. 14, 919–924. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Bates M, Yuan Y, Kalman D, Attallah R, Rey O, Biggs M, Hopenhayn C, Moore L, Hoang B, et al. , 2006. Arsenic methylation and bladder cancer risk in case–control studies in Argentina and the United States. J. Occup. Environ. Med. 48, 478–488. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, Akesson A, Alfven T, Jarup L, Vahter M, 2005. Creatinine concentrations versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers 10, 117–126. [DOI] [PubMed] [Google Scholar]
- Teass A, Biagini R, DeBord G, Hull R, 2003. Application of biological monitoring methods. In: US Department of Health and Human Services PS, Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health (Eds.), NIOSH Manual of Analytical Methods, fourth edition. Eller PM, Cincinnati, OH. [Google Scholar]
- Valenzuela O, Borja-Aburto H, Garcia-Vargas G, Cruz-Gonzalez M, Garcia-Montalvo E, Calderon-Aranda E, Del Razo L, 2005. Urinary trivalent methylated species in a population exposed to inorganic arsenic. Environ. Health Perspect. 113, 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsfold M, Davie MW, Haddaway MJ, 1999. Age-related changes in body composition, hydroxyproline, and creatinine excretion in normal women. Calcif. Tissue Int. 64, 40–44. [DOI] [PubMed] [Google Scholar]


