Abstract
Background:
This study sought to determine the relative importance of a range of Bup/Nx doses compared to Bup alone in producing subjective and reinforcing effects.
Methods:
Heroin-using volunteers (n = 13) were transitioned onto daily oral hydromorphone (40 mg). Laboratory sessions assessed the reinforcing and subjective effects of intravenous (IV) doses of Bup (1.51, 2.16, 6.15, and 8.64 mg) and Bup/Nx (1.51/0.44, 2.16/0.61, 6.15/1.71, and 8.64/2.44 mg). Placebo (Pbo), heroin (25 mg) and Nx (0.3 mg) were tested as neutral, positive, and negative controls, respectively.
Results:
IV Bup alone was self-administered substantially less than IV heroin, though the two largest doses of Bup produced positive subjective effects, drug “Liking” (0–100 mm), which were comparable to heroin (mean difference: Heroin vs Bup 6.15 mg: −3.4 mm, Heroin vs Bup 8.64 mg: −11.3 mm). All indicators of abuse potential seen with IV Bup alone were substantially decreased with the addition of Nx. All Bup/Nx combinations produced ratings of aversive effects, “Bad”, which were comparable to, or greater than IV, Nx. On three of the four measures of aversive effects, the largest difference is seen with the 8.64 vs 8.64/2.44 condition.
Conclusions:
This study further demonstrates the ability of the Bup/Nx combination to deter IV use. Although none of the Bup/Nx combinations showed indications of abuse potential, formulations with larger absolute Nx, may be less abusable as they precipitate a greater degree of withdrawal.
Keywords: Intravenous, Self-administration, Buprenorphine, Opioid, Abuse liability
1. Introduction
Over the past 15 years, sublingual buprenorphine (Bup) maintenance has become one of the most commonly utilized treatments for opioid use disorder (Carrieri et al., 2006; Maxwell and McCance-Katz, 2010). Research has repeatedly shown that Bup maintenance significantly reduces the morbidity and mortality associated with opioid abuse and dependence (Mattick et al., 2008; Stancliff et al., 2013). Although Bup has reduced abuse potential in comparison to opioids that are full μ receptor agonists (Comer et al., 2008; Jasinski et al., 1978; Walsh et al., 1995), administration through rapid routes of administration [intravenous (IV), intramuscular (IM), intranasal (IN)] produces μ agonist-like effects comparable to heroin and oxycodone (Bedi et al., 1998; Comer and Collins, 2002; Comer et al., 2005, 2010; Middleton et al., 2011; Strain et al., 1997; Zacny et al., 1997).
To address concerns of Bup diversion, the opioid antagonist naloxone (Nx) was combined with Bup at a ≈4/1 ratio (Bup/Nx). The addition of Nx, which has very low sublingual/oral bioavailability, is intended to discourage misuse of Bup by parenteral routes by either precipitating withdrawal in dependent individuals or by directly antagonizing the μ agonist effects of Bup (Preston et al., 1990). Several clinical studies have demonstrated that the combined formulation (Bup/Nx) has significantly reduced abuse liability in comparison to Bup alone (Comer et al., 2010; Fudala et al., 1998; Jones et al., 2015; Mendelson et al., 1996, 1999; Stoller et al., 2001).
During early investigations of the effectiveness of this formulation, several studies focused on how varying the relative ratio of Bup to Nx affected its abuse liability (Jones et al., 2015; Mendelson et al., 1999; Preston et al., 1988). Typically, among opioid-dependent volunteers, Bup + Nx combinations produced effects that were qualitatively similar to the effects of Nx alone. Additionally, Nx dose-dependently reduced positive subjective effects, increased aversive effects, and blocked drug self-administration. Combined, this literature indicates that lower Bup/Nx ratios (i.e., larger Nx doses relative to Bup doses) are associated with less abuse potential.
Though the importance of the ratio of Bup/Nx has been demonstrated, research has yet to determine how the absolute amounts of Bup and Nx affect their reduced potential for abuse. Post-marketing surveillance has indicated that the introduction of Bup/Nx helped to reduce, but did not eliminate diversion to nonmedical routes of administration (Bruce et al., 2009; Larance et al., 2011, 2014; Lee, 2006; Vicknasingam et al., 2010). Data from these studies indicate that participants may be able to minimize the aversive consequences of IV use through repeated sequential administration of smaller doses (see Yokell et al., 2011 for a review). These data suggest that Bup/Nx formulations with lower absolute Nx content may better lend themselves to this type of diversion. Thus, the purpose of the present study was to assess the reinforcing and subjective effects of various doses of Bup and corresponding doses of Bup/Nx, maintaining the 4/1 ratio.
2. Methods
2.1. Participant selection
Participants were recruited from the New York City metropolitan area through print media advertisements. Screening consisted of: assessments of drug use, general health, and medical history, and laboratory tests (hematology, blood chemistry, and urinalysis). Participants were required to be physically and mentally healthy heroin users between the ages of 21 and 55 years, with previous IV opioid use. All participants were required to meet DSM-5 criteria for opioid use disorder and be physiologically dependent upon opioids. Potential participants were excluded from the study if they were seeking treatment for their drug use, physiologically dependent on alcohol or illicit drugs (other than opioids), or had a severe Axis I psychiatric diagnosis (other than opioid, nicotine or caffeine use disorder).
2.2. Design
Participants resided on a locked inpatient unit during the study. During the first 5–7 days after admission, participants were stabilized on oral hydromorphone (HYD) 40 mg/day (10 mg, QID). HYD maintenance was chosen in an effort to model the parameters of a previous investigation demonstrating the utility of the combined Bup/Nx formulations (Stoller et al., 2001). These parameters are also an attempt to model how Bup and Bup/Nx diversion may occur among heroin users. HYD dosing occurred at: 0700 h, 1100 h, 1700 h, and 2100 h on days during which laboratory test sessions did not occur and at: 0700 h, 1315 h, 1700h, and 2100 h on test days.
The IV challenge opioids for this study included doses of Bup alone (1.51, 2.16, 6.15 and 8.64 mg), corresponding doses of Bup/Nx in an ≈4/1 ratio (1.51/0.44, 2.16/0.61, 6.15/1.71, 8.64/2.44 mg), and neutral, positive, and negative controls [placebo (2 ml saline), heroin (25 mg), Nx (0.3 mg), respectively]. This study attempted to model the dosages of two commercially available sublingual buprenorphine products, Suboxone and Zubsolv. The labeled dosage strength of the two products is based on the weight of Bup and Nx free base. In order to avoid potential adverse effects associated with IV administration of the excipients in the commercial products, the active pharmaceutical ingredients (API) of buprenorphine and naloxone were used in the present study. Therefore, in order to calculate the amounts of the API for IV dosing of buprenorphine and naloxone in the current study, we used the following base-to-salt conversion: buprenorphine = 504.1 [hydrochloride (HCL) molecular (Mol) weight (Wt)]/467.6 (Base Mol Wt), Nx = 399.9 (HCl dihydrate Mol Wt)/327.4 (Base Mol Wt). This conversion resulted in the following dose transformations: Zubsolv: 1.4/0.36, 5.7/1.4, (API: 1.51/0.44, 6.15/1.71) and Suboxone: 2/0.5, 8/2 mg (API: 2.16/0.61, 8.64/2.44 mg). This procedure allowed us to make an accurate comparison between the two products via the intravenous route (Fischer et al., 2013). The order of dosing of all IV challenge drugs was randomized, and doses were administered under double-blind conditions.
2.3. Sample and choice self-administration procedure
Testing consisted of two types of laboratory sessions: sample and choice. Sample and choice sessions for each IV challenge dose were completed on sequential days, with at least 24 h between different challenge doses. At approximately 0900 h, participants were brought to the laboratory to complete a sample session. Forty minutes (min) prior to drug administration, physiological monitoring began. At approximately 1000 h, participants received full doses of the IV test drug and money (U.S. $20). Over the course of the next 180 min, participants completed physiological, subjective and performance measures outlined in Table 1.
Table 1.
Sample Session Events.
| −40 | Physiological monitoring (oxygen saturation, blood pressure), Pupil Diameter, Cognitive Effects, Subjective Effects, Withdrawal |
| 0 | Sample IV drug and $20 |
| 5 | Pupil Diameter, Subjective Effects, Withdrawal |
| 15 | Pupil Diameter, Subjective Effects, Withdrawal |
| 30 | Pupil Diameter, Subjective Effects, Withdrawal |
| 45 | Pupil Diameter, Subjective Effects, Withdrawal |
| 60 | Pupil Diameter, Subjective Effects, Withdrawal |
| 90 | Pupil Diameter, Subjective Effects, Withdrawal |
| 105 | Pupil Diameter, Subjective Effects, Withdrawal |
| 120 | Pupil Diameter, Cognitive Effect, Subjective Effects, Withdrawal |
| 150 | Pupil Diameter, Subjective Effects, Withdrawal |
| 180 | Pupil Diameter, Subjective Effects, Withdrawal |
During the choice session participants completed a self-administration task to receive portions of the dose of drug or money they had sampled the previous day (0–100%, in increments of 10%). Participants could work for all or part of the sampled IV dose or money by choosing the drug or money option that were concurrently available at each trial. Thus, if the dose for that day was 20 mg, at each opportunity participants could respond for 2 mg (10% of 20 mg) or $2 (10% of $20). After a choice was made for one option, participants completed the operant task (finger presses on a computer mouse), which increased independently for each option on the following scale: 50, 100, 200, 400, 800, 1200, 1600, 2000, 2400, and 2800. The primary dependent variable in this choice procedure is the ‘break point (BP),’ at which responding for the reinforcer stops. At the end of the self-administration task (approximately 1600 h), the participant received whatever (s)he had chosen. Money was added to their study payment, and the IV drug was administered by a study physician.
2.4. Tasks and measures
Subjective Effects: Three questionnaires were used to assess subjective drug effects and opioid withdrawal symptoms. A visual analog scale (VAS) was used to assess subjective and physiological drug effects such as I feel a “Good Effect” and “High”. Participants rated each item on the scale from ‘Not at all’ (0 mm) to ‘Extremely’ (100 mm). In addition, a 5-item drug effects questionnaire (DEQ) was used to measure drug effects (strength of drug effects, good effects, bad effects, willingness to take the drug again, and drug liking) on a scale of 0 (‘No Effect’) to 4 (‘Very Strong Effect’), or −4 (‘Dislike Very Much’) to 4 (‘Like Very Much’). The Subjective Opioid Withdrawal Scale (SOWS) was used to identify the severity of opioid withdrawal symptoms (Handelsman et al., 1987).
Physiological Measures: Miosis was assessed as a physiological indicator of μ agonist effects using a NeurOptics™ Pupillometer under ambient lighting conditions. For safety, a pulse oximeter continuously monitored oxygen saturation (%SpO2) during sessions, while respiration (breaths per minute), heart rate, and blood pressure (systolic and diastolic) were measured every 5 min. For the sake of brevity, safety measures were omitted from the manuscript.
2.5. Drugs
Buprenorphine HCl powder and Nx HCl powder for IV administration were provided by INDIVIOR PLC (Richmond, VA), and heroin HCl powder was obtained from Macfarlan Smith Limited (Edinburgh, Scotland, UK). Hydromorphone HCl tablets, manufactured by Mallinckrodt Inc., (Hazelwood, MO), were purchased. A constant volume of 1 ml solution was administered at each IV dosing in order to maintain the blind. All drugs were prepared by the New York State Psychiatric Institute Pharmacy.
2.6. Statistical analyses
In response to growing concerns about the flaws of null-hypothesis significance testing, the investigators chose to utilize an estimation technique based upon the interpretation of effect size and confidence intervals (CI) rather than p values (Cumming, 2014; Ioannidis, 2005). These outcomes are thought to be more informative because they indicate the extent of uncertainty, in addition to providing the best point estimate of the dependent variable under investigation (Cumming, 2012). This proposed statistical approach focuses on identification of relatively few dependent variables and comparisons that will answer a predetermined research question. With respect to interpreting results, this approach encourages effect size estimates as the main research outcome, and interpretation of CI lengths and degree of overlap to indicate precision and degree of difference (i.e., threshold of clinical importance) between conditions (respectively). In this approach, invoking null-hypothesis significance testing is avoided, along with reporting of p values.
Because the primary objective of this study is to assess abuse potential, we have selected drug self-administration, along with subjective measures of positive and negative subjective effects, as our primary dependent measures. The reinforcing effects of the IV challenge drugs were assessed using direct mean comparisons, while subjective drug effects were analyzed as a function of mean peak/trough (maximal/minimal drug effects throughout the session). Confidence intervals were calculated at 95% of the mean, mean peak, or mean difference. The placebo (Pbo) control was set as the threshold of clinical importance for measures of drug self-administration, as is common among clinical self-administration studies (Jones and Comer, 2013). The clinical importance of positive subjective effects was assessed in comparison to the participants’ drug of choice, heroin. Measures of aversive effects were compared to the negative control, Nx, which primarily mediates the abuse deterrence of the combined Bup + Nx formulation (Preston et al., 1990). Data analyses were performed using SPSS version 21 (SPSS, 2009) and SuperANOVA (Gagnon et al., 1990).
An a priori power analysis was conducted using G*Power 3.1.7 to ensure that effects on the primary outcome measures of interest could be suitably detected. Calculations were based on our study comparing the abuse potential of Bup versus Bup + Nx among Bup-maintained heroin users (Jones et al., 2015). Using these data, we calculated a Cohen’s d effect size of 0.86 and a mean standard deviation of 500 points in progressive ratio breakpoint value. Therefore, we calculated that a total sample size of 12 completers, in this within-subjects design, would provide 87% power to detect a 433-point difference in progressive ratio breakpoint.
3. Results
3.1. Participants
Twenty-one participants were enrolled into the study between December, 2013 and October, 2015. Five participants voluntarily withdrew from the study due to: disgruntlement with the inpatient unit (n = 3), personal issues (n = 1), or inability to tolerate the IV doses (n = 1). Three participants were removed by the investigators due to: elevated liver function tests (n = 1), or behavioral issues (n = 2). Complete data sets were obtained from 13 participants for inclusion in this analysis [(Sex: 12 M, 1F; Race: 4 Caucasian, 4 African-American, 4 Multiracial, 1 Unknown/Unreported; Ethnicity: 4 Hispanic/Latino)]. The mean age of this sample was 46.9 (±6.1) years.
The majority of completers (n = 11) reported intravenous heroin use as their current route of choice (2 indicated IN). The mean duration of heroin use was 23.1 years (range: 11.5–35 yrs), and participants used an average of 6.3 bags per day (range: 1–17). Using data from the U.S. Drug Enforcement Administration (DEA, 2013) concerning the cost of heroin in NYC ($0.99 per mg pure) we estimate that a bag consists of ≈10 mg of pure heroin. In addition to daily heroin use, 13 completers were daily cigarette smokers, 8 were regular cocaine/crack users (“a few times a week”), 7 were occasional drinkers (“a few times a month” or more without meeting criteria for dependence). Five were occasional users of benzodiazepines and 4 were occasional marijuana users (“a few times a month”).
3.2. Reinforcing effects
In comparison to Pbo, only heroin produced robust self-administration behavior (Fig. 1). All doses of Bup alone elicited greater self-administration than Pbo, but there was significant overlap among the CIs of each dose with that of Pbo suggesting that the degree of difference was negligible. The greatest percentage of drug choices was elicited by the 6.15 mg dose, followed by the 8.64, 1.51 and 2.16 mg doses. However, the 8.64 mg dose produced the largest drug breakpoint value, followed by the 6.15, 1.51, and 2.16 mg doses. Table 2 displays the mean difference for each of the categories of outcome variables compared to their respective controls.
Fig. 1.
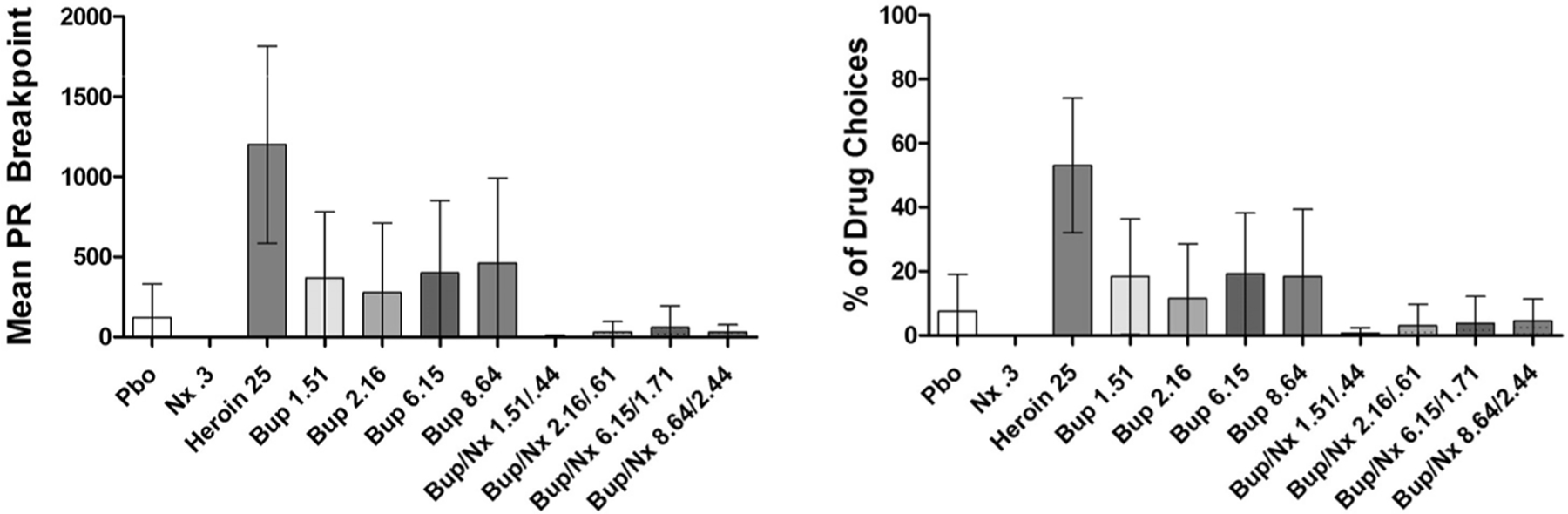
Mean (±95% CI) progressive ratio drug breakpoint and% of drug (vs money) choices.
Table 2.
Mean Rating of Drug Effects.
| IV Challenge Dose | Bup 1.51 | Bup 2.16 | Bup 6.15 | Bup 8.64 | Bup/Nx 1.51/0.44 | Bup/Nx 2.16/0.61 | Bup/Nx 6.15/1.71 | Bup/Nx 8.64/2.44 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug Self-Administration: Mean Difference vs Placebo (95% Confidence Interval) | |||||||||||||||
| - % Choice | 10.8 (−11.2 to 32.8) | 3.8 (−18.7 to 26.4) | 11.5 (−11.3 to 34.4) | 10.8 (−15.7 to 37.3) | −6.9 (−17.8 to 3.9) | −4.6 (−14.7 to 5.4) | −3.8 (−14.2 to 6.5) | −3.1 (−14.7 to 8.6) | |||||||
| - Drug Breakpoint | 246 (−243 to 735) | 153 (−355 to 662) | 277 (−249 to 803) | 338 (−276 to 953) | −119 (−324 to 86) | −92 (−293 to 109) | −62 (−279 to 156) | −92 (−299 to 115) | |||||||
| Pupil Diameter: Mean Difference vs Placebo (95% Confidence Interval) | |||||||||||||||
| - Trough (mm) | −0.3 (−0.5 to −0.1) | −1.5 (0.6 to 0.29) | −0.2 (−0.6 to 0.1) | −0.3 (−0.8 to 0.1) | −0.1 (−0.5 to 0.3) | −0.07 (−0.4 to 0.3) | −0.07 (−0.5 to 0.4) | −0.5 (−0.5 to −0.2) | |||||||
| Positive Subjective Effects: | |||||||||||||||
| Drug Effects Questionnaire (0–4): Mean Difference vs Heroin (95% Confidence Interval) | |||||||||||||||
| - Good | −1.5 (−2.5 to −0.4) | −1.4 (−2.5 to −0.2) | −0.6 (−1.8 to −0.6) | −0.7 (−1.9 to 0.5) | −1.8 (−2.8 to −0.7) | −1.6 (−2.6 to −0.6) | −1.7 (−2.6 to −0.8) | −1.7 (−2.9 to −0.5) | |||||||
| - Like | −1.2 (−2.6 to 0.3) | −1.5 (−2.9 to −0.05) | −0.5 (−1.6 to 0.7) | −0.8 (−2.0 to 0.3) | −2.2 (−3.2 to −1.1) | −1.8 (−2.8 to −0.8) | −1.9 (−2.9 to −0.9) | −2.1 (−3.3 to −0.9) | |||||||
| - Take Again | −1.0 (−2.3 to 0.3) | −1.2 (−2.3 to 0.0) | −0.3 (−1.4 to 0.8) | −0.8 (−1.8 to 0.1) | −2.1 (−3.1 to −1.1) | −1.7 (−2.8 to −0.6) | −1.8 (−2.9 to −0.8) | −1.7 (−2.9 to −0.5) | |||||||
| Visual Analog Scale (0–100): Mean Difference vs Heroin (95% Confidence Interval) | |||||||||||||||
| - Good | −24.5 (−46.1 to −2.8) | −23.2 (−52.5 to 6.0) | −7.9 (−39.7 to 23.9) | −2.2 (−37.3 to 32.9) | −34.3 (−58.2 to −10.4) | −25.2 (−53.5 to 3.1) | −28.5 (−47.8 to −9.2) | −20.4 (−47.8 to −9.2) | |||||||
| - High | −22.7 (−39.2 to −6.2) | −20.9 (−44.7 to 2.8) | −3.4 (−33.3 to 26.6) | −11.3 (−43.1 to 20.5) | −21.5 (−45.9 to 3.0) | −25.8 (−48.7 to −2.8) | −22.1 (−44.4 to 0.2) | −19.4 (−48.6 to 9.8) | |||||||
| - Liked the Choice | −13.8 (−41.1 to 13.6) | −21.3 (−51.6 to 8.96) | −5.39 (−40.1 to 29.3) | −14.8 (−41.8 to 12.1) | −35.8 (−60.5 to −11.1) | −32.9 (−59.7 to −6.09) | −26.5 (−48.8 to −4.26) | −22.5 (−56.4 to 11.5) | |||||||
| - Would Pay | −6.3 (−10.2 to −2.4) | −5.7 (−11.5 to 0.1) | −2.9 (−8.1 to 2.2) | −4.5 (−10.0 to 1.1) | −8.5 (−12.9 to −4.0) | −8.3 (−13.0 to −3.6) | −8.0 (−12.3 to −3.6) | −7.4 (−12.1 to −2.6) | |||||||
| Aversive Subjective Effects | |||||||||||||||
| Drug Effects Questionnaire (0–4): Mean Difference vs Naloxone (95% Confidence Interval) | |||||||||||||||
| - Bad | −2.1 (−3.2 to 1.0) | −1.8 (−2.9 to −0.7) | −1.8 (−3.0 to −0.5) | −1.3 (−2.6 to −0.1) | 0.3 (−1.0 to 1.6) | 0 (−1.5 to 1.5) | 0.5 (−0.5 to 1.4) | 0.8 (−0.3 to 1.8) | |||||||
| Subjective Opioid Withdrawal Scale (0–64): Mean Difference (95% Confidence Interval) | |||||||||||||||
| - Sum Score | −18.4 (−32.0 to −4.8) | −20.9 (−34.0 to −7.8) | −19.9 (−32.9 to −6.9) | −19.7 (−33.8 to −5.6) | −7.9 (−18.3 to 2.4) | −4.4 (−18.4 to 9.6) | −4.7 (−15.3 to 5.9) | 4.6 (−6.8 to 15.9) | |||||||
| Visual Analog Scale (0–100): Mean Difference vs Naloxone (95% Confidence Interval) | |||||||||||||||
| - Anxious | −3.8 (−28.0 to 20.3) | −5.1 (−26.9 to 16.7) | −16.3 (−37.3 to 4.7) | −5.5 (−34.8 to 23.8) | −10.5 (−31.9 to 10.8) | −6.6 (−25.7 to 12.4) | 17.7 (−2.0 to 37.3) | 2.6 (−12.2 to 17.4) | |||||||
| - Bad | −38.2 (−62.6 to −13.7) | −40.9 (−63.9 to −18.0) | −42.6 (−67.1 to −18.1) | −40.3 (−68.3 to −12.3) | −7.3 (−41.0 to 26.4) | 5.8 (−25.7 to 37.4) | 5.6 (−19.0 to 30.2) | 20.5 (−7.1 to 48.1) | |||||||
| - Nauseated | −28.5 (−48.7 to −8.3) | −28.8 (−48.7 to −8.9) | −30.6 (−49.2 to −12.0) | −22.2 (−39.1 to −5.3) | −10.2 (−34.8 to 14.5) | −5.2 (−32.5 to 22.2) | −11.5 (−30.8 to 7.8) | 3.5 (−12.7 to 19.7) | |||||||
The addition of Nx reduced Bup self-administration to below Pbo levels (Fig. 1). Comparisons of mean difference between each Bup and its respective Bup + Nx dose are shown in Table 3. The degree of difference was similar across each of the 4 Bup vs Bup/Nx comparisons. However, the greatest decrease in drug breakpoint following the addition of Nx was observed with the 8.64 mg dose (vs 8.64/2.44) followed by the 1.51, 6.15 and 2.16 mg doses. When examined as a function of percentage of drug choices, the greatest decrease was seen with the 1.51 mg dose (vs 1.51/.44) followed by the 6.15, 8.64, and 2.16 mg doses.
Table 3.
Buprenorphine vs Buprenorphine + Naloxone Comparisons.
| IV Challenge Dose | Bup 1.51 Vs Bup/Nx 1.51/0.44 | Bup 2.16 Vs Bup/Nx 2.16/0.61 | Bup 6.15 Vs Bup/Nx 6.15/1.71 | Bup 8.64 Vs Bup/Nx 8.64/2.44 | ||
|---|---|---|---|---|---|---|
| Drug Self-Administration: Mean Difference (95% Confidence Interval) | ||||||
| - % Drug Choices | 17.7 (−0.29 to 35.7) | 8.46 (−10.9 to 27.9) | 15.4 (−3.9 to 34.7) | 13.8 (−10.0 to 37.7) | ||
| - Drug Breakpoint | 365.4 (−46.9 to 777.7) | 246.2 (−201.1 to 693.4) | 338.5 (−133.2 to 810.1) | 430.8 (−120.5 to 982.1) | ||
|
Positive Subjective Effects
| ||||||
| Drug Effects Questionnaire (0–4): Mean Difference (95% Confidence Interval) | ||||||
| - Good | 0.31 (−0.32 to 0.93) | 0.23 (−0.21 to 0.67) | 1.08 (0.40 to 1.75) | 1.00 (0.51 to 1.49) | ||
| - Like | 1.00 (0.26 to 1.74) | 0.31 (−0.69 to 1.31) | 1.46 (0.78 to 2.14) | 1.62 (0.85 to 2.38) | ||
| - Take Again | 1.08 (0.28 to 1.88) | 0.54 (−0.34 to 1.42) | 1.54 (0.63 to 2.45) | 0.85 (0.07 to 1.620) | ||
| Visual Analog Scale (0–100): Mean Difference (95% Confidence Interval) | ||||||
| - Good | 9.85 (−0.74 to 20.44) | 2.00 (−6.79 to 10.79) | 20.62 (−1.73 to 42.69) | 18.23 (−1.42 to 37.88) | ||
| - High | −1.23 (−20.93 to 18.47) | 4.85 (−17.85 to 27.55) | 18.69 (2.85 to 34.54) | 8.08 (−7.48 to 23.63) | ||
| - Liked the Choice | 22.1 (1.84 to 42.3) | 11.6 (−4.66 to 27.9) | 21.1 (-0.15 to 42.5) | 7.62 (−14.0 to 29.2) | ||
| - Would Pay | 2.15 (−0.37 to 4.344) | 2.62 (−0.99 to 6.22) | 5.08 (1.07 to 9.09) | 2.92 (−1.56 to 7.41) | ||
| Aversive Subjective Effects | ||||||
| Drug Effects Questionnaire (0–4): Mean Difference (95% Confidence Interval) | ||||||
| - Bad | −2.39 (−3.45 to −1.32) | −1.85 (−2.83 to −0.86) | −2.23 (−3.49 to −0.97) | −2.08 (−3.19 to −0.96) | ||
| Subjective Opioid Withdrawal Scale (0–64): Mean Difference(95% Confidence Interval) | ||||||
| - Sum Score | −10.5 (−20.0 to −0.89) | −16.5 (−27.3 to −5.76) | −15.2 (−21.1 to −9.36) | −24.3 (−34.7 to −13.9) | ||
| Visual Analog Scale (0–100): Mean Difference(95% Confidence Interval) | ||||||
| - Bad | −30.8 (−56.5 to −5.17) | −46.8 (−68.1 to −25.5) | −48.2 (−75.1 to −21.3) | −60.8 (−85.1 to −36.6) | ||
| - Nauseated | −18.4 (−39.2 to 2.39) | −23.7 (−42.5 to −4.87) | −19.1 (−40.9 to 2.81) | −25.8 (−44.2 to −7.31) | ||
3.3. Positive subjective effects
Mean peak VAS assessments of “Good,” “Liked the Choice,” “I Would Pay,” and “High” greatly increased following IV heroin administration (vs Pbo). Similar results were found on DEQ measures of “Would Take Again,” “Good,” and “Like.” The Bup 6.15 mg and 8.64 mg doses also elicited robust ratings on these measures, greater than Pbo and often equivalent to that of heroin (Fig. 2). The two smaller Bup doses shared more CI overlap with Pbo. Across these measures, the smallest differences from heroin were consistently observed with the 6.15 mg dose, followed by the 8.64, 2.16 and 1.51 mg doses (Table 2). The addition of Nx to these doses reduced ratings on these measures to Pbo levels. Comparisons of mean differences between each Bup dose and its respective Bup + Nx dose revealed the greatest degree of differences with the two larger doses. Across the seven measures assessed, the greatest decrease seen with the addition of naloxone was observed with the 6.15 mg dose (vs 6.15/1.71 mg).
Fig. 2.
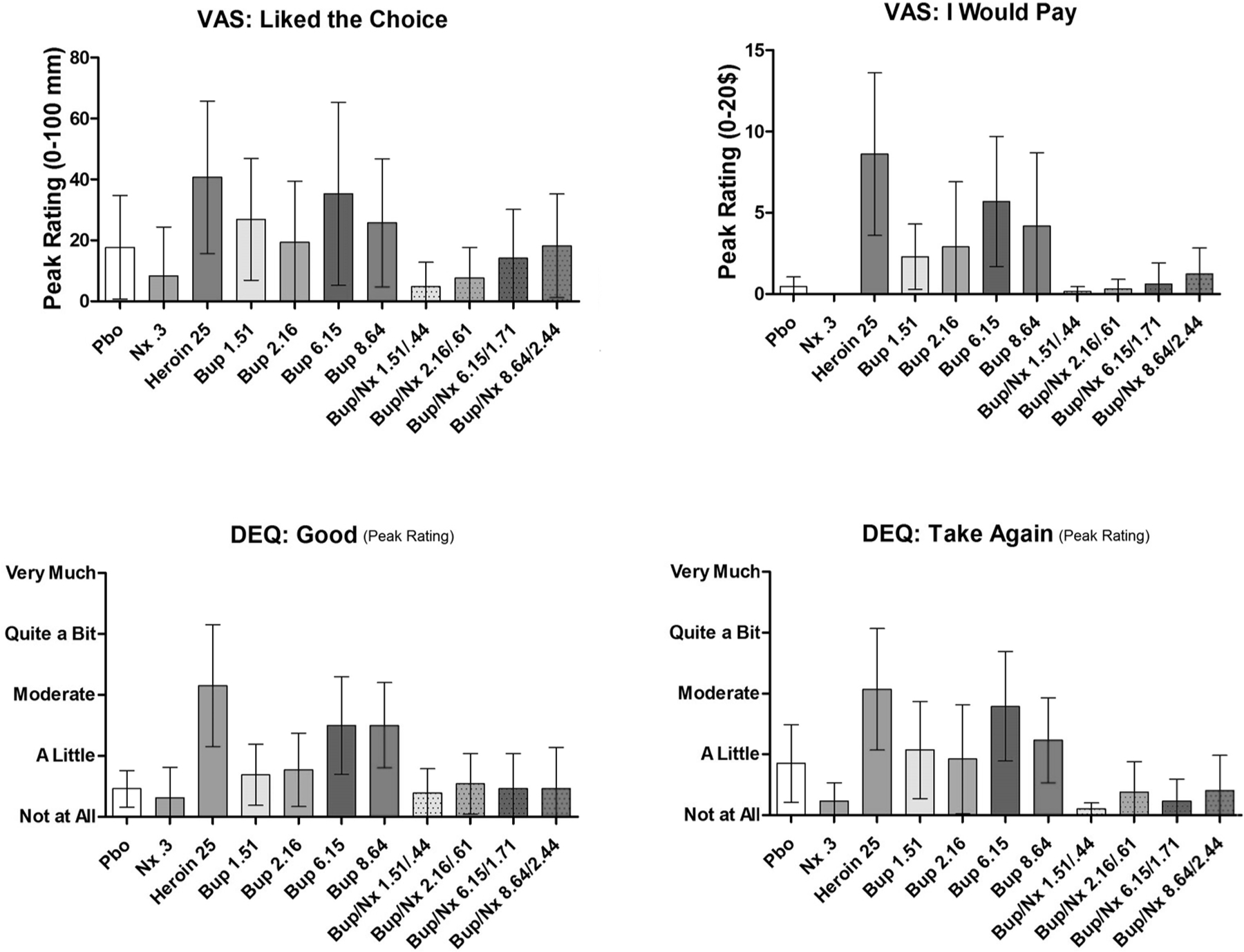
Mean peak (±95% CI) visual analog scale (VAS) and drug effects questionnaire (DEQ) ratings of: “Liking,” “Good,” “Would Take Again,” and “Would Pay.”.
3.4. Aversive subjective effects
As expected, IV Nx produced robust subjective reports of: “Bad” (VAS and DEQ), “Nauseated,” and opioid withdrawal (SOWS score). All doses of IV Bup alone produced minimal aversive effects that were significantly less than those produced by Nx (Fig. 3). Meanwhile, all Bup/Nx doses produced ratings on these measures that were similar to Nx alone, all showing a similar degree of CI overlap in mean ratings. On three of the four measures of aversive subjective effects, the largest differences were observed with the 8.64 vs 8.64/2.44 condition. Comparisons of mean differences between each Bup and its respective Bup/Nx dose revealed minimal differences among the doses.
Fig. 3.
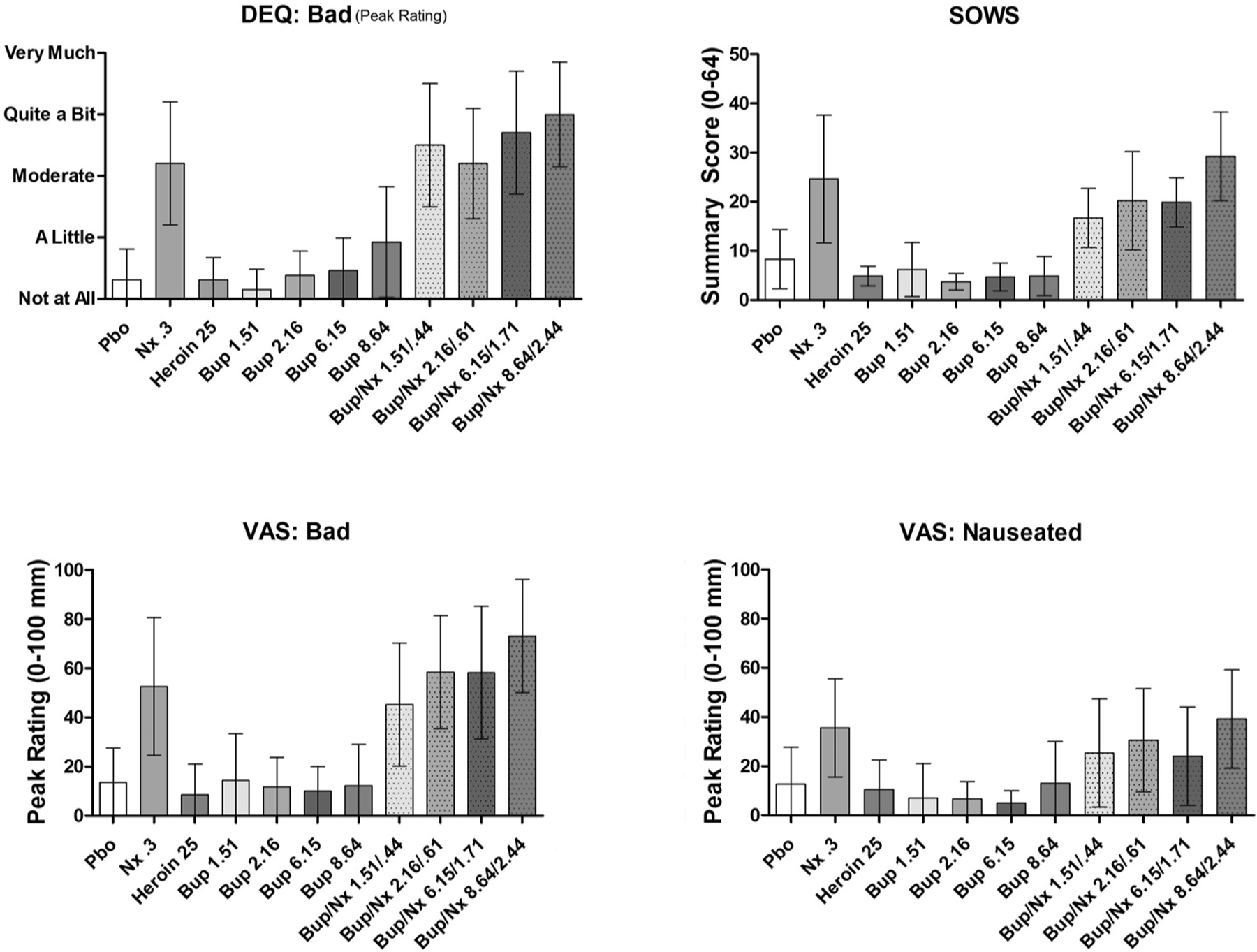
Mean peak (±95% CI) VAS and DEQ ratings of: “Bad,” “Nauseated,” and subjective opioid withdrawal.
3.5. Time course of subjective effects
Shown in Fig. 4 are the time to onset and dissipation of “Good” and “Bad” effects for the highest dose of IV Bup and Bup/Nx throughout the 3-h session (control drugs and lower doses of Bup and Bup/Nx were not included to simplify the figures). Typically, the positive subjective effects of IV Bup alone reached peak levels within 15 min following administration. Ratings remained elevated (vs baseline), for approximately one hour, with dose-dependent dissipation of drug effects. The two lower Bup/Nx doses typically showed minimal increases in positive subjective effects (typically < 10 mm). The two larger Bup/Nx doses only began to increase positive ratings 45 min post administration. For Bup alone, ratings of “Bad” effects were typically minimal throughout the session. For Bup/Nx combinations, aversive effects peaked within 5 min post administration and began to decrease within 10 min, but remained elevated for 45–60 min after administration.
Fig. 4.
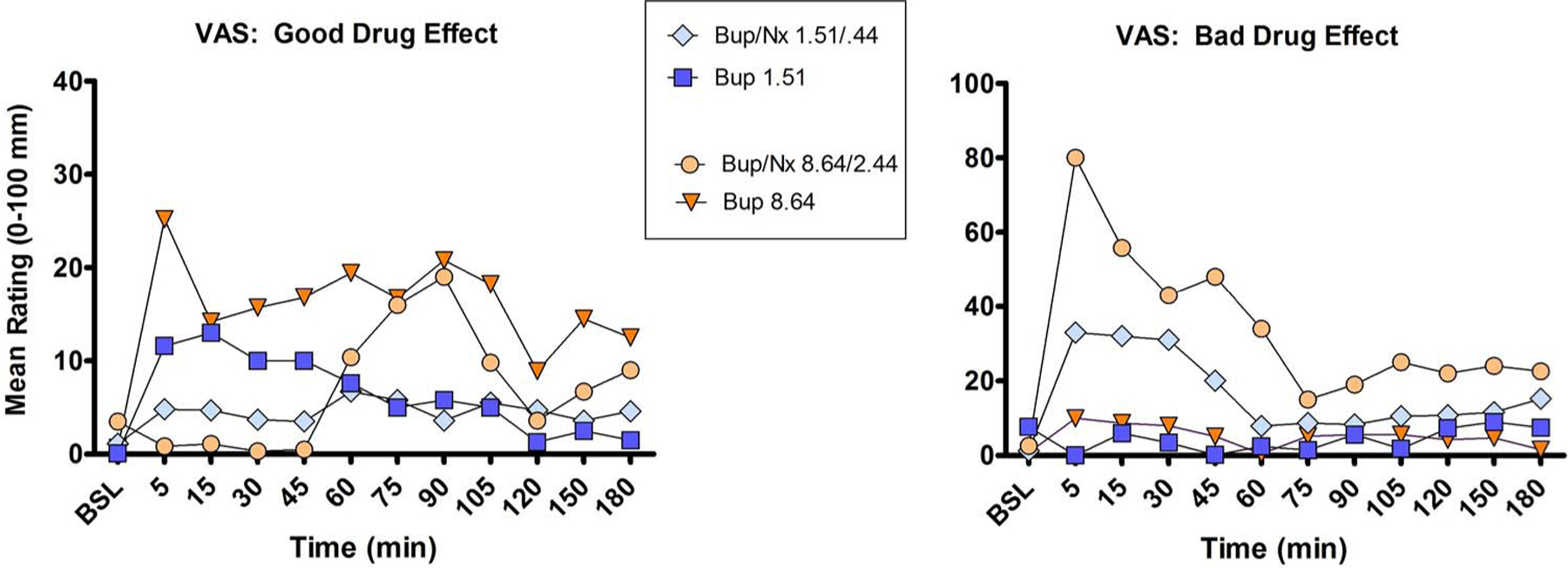
Mean VAS ratings of “Good” and “Bad” drug effects at each time point throughout the sample session.
4. Discussion
This study examined the abuse liability of various doses of intravenous Bup and Nx at an ≈4/1 ratio. Specifically, we examined whether reinforcing, positive and negative subjective effects would vary if the amount of Bup and Nx differed while the ratio remained unchanged. Only the positive control of IV heroin acted as a robust reinforcer. All four doses of Bup alone (1.51, 2.16, 6.15, and 8.64) were self-administered more than Pbo, but these differences were not statistically significant. The addition of Nx led to almost complete attenuation of self-administration. When the investigators examined the degree of difference between Bup and its respective Bup/Nx dose, only modest differences were found.
Although Bup on its own was self-administered only slightly more than Pbo, the two larger doses did often produce reports of positive subjective effects that were significantly greater than Pbo, comparable to those seen with IV heroin. Of all the Bup doses, the 6.15 mg Bup dose appeared to produce the most consistent positive subjective effects (across the most measures), though the degree of difference between the 6.15 and 8.64 doses was minimal. These data suggest that the positive subjective effects of Bup plateau, supporting several other clinical studies reporting a ceiling effect to Bup’s μ agonist effects (Jasinski et al., 1978; Jones et al., 2015; Walsh et al., 1994; Umbricht et al., 2004). When Bup was combined with Nx, these effects returned to Pbo levels. The degree of difference between each Bup dose and its respective Bup + Nx dose was typically much greater for the two largest Bup doses. However, with the minimal positive subjective effects evoked by the two smallest doses, we may be observing a floor effect with respect to the attenuating actions of Nx.
The aversive effects of Bup alone were minimal, while each of the Bup/Nx combinations produced aversive effects comparable to Nx. Across the various measures of aversive subjective effects, the degree of increase in ratings between Bup and Bup/Nx was similar. Increases in subjective reports of “Bad” drug effect and measures of opioid withdrawal replicate previous studies in which parenteral Bup/Nx was administered to opioid-dependent populations (Doyon, 2004; Medelson et al., 1999; Stoller et al., 2001).
Interestingly, although the aversive effects of Bup alone were minimal and peak ratings of positive subjective effects for the two larger doses were often as robust as those produced by heroin, the degree of self-administration of these doses appeared more like Pbo than heroin. Other investigations have shown that under full agonist maintenance, parenteral Bup can produce notable positive subjective effects (Mendelson et al., 1999; Stoller et al., 2001), but under these maintenance conditions it is not self-administered (Comer et al., 2008). These data demonstrate that the reinforcing effects of opioids are not necessarily causally related only to their euphoric/positive subjective effects, but reflective of the combined positive and negative subjective components of the drug experience. This highlights the importance of a comprehensive assessment of subjective effects to understanding motivations behind the abuse liability of a drug (Lamb et al., 1991).
The time course of drug effects revealed that the positive subjective effects of IV Bup alone were relatively rapid and long lasting. With the addition of Nx, these initial positive effects appeared to be blunted in a dose-dependent manner, until Nx dissipated. Similarly, the aversive effects associated with Bup/Nx administration had a rapid onset, but began to decrease after approximately 45 min in a dose-dependent manner. These data suggest that the abuse deterrence of the combined formulation is attributable to both direct antagonism of Bup’s positive subjective effects by Nx and Nx-precipitated withdrawal symptoms. The time course of drug effects also suggests that both of these effects are more robust with larger absolute amounts of Nx. Therefore, combined Bup + Nx formulations with a greater absolute amount of Nx may be better at deterring IV, IN and IM abuse. This conclusion is additionally supported by the data indicating that Bup’s positive subjective effects plateau, possibly making formulations with lower Nx amounts more appealing to those with the intention to use in a nonmedical manner (for a review see Walsh and Eissenberg, 2003). As Nx has a significantly shorter half-life than Bup, sequential parenteral administration of smaller Bup/Nx doses may allow users to somewhat avoid Nx-precipitated withdrawal while the rewarding effects of the Bup increase. Though no positive subjective effects of smaller Bup/Nx doses were observed under the current single-administration conditions, future studies should examine a cumulative effect of multiple doses.
In sum, the addition of a respective ¼ dose of Nx to each of the IV Bup challenge drugs resulted in almost complete attenuation of reinforcing and positive subjective effects. The finding of significantly reduced abuse liability of Bup in combination with Nx, affirms the effectiveness of the abuse-deterrent formulation via the IV route for all doses tested. Therefore, in any situation where it is medically suitable, prescribing should strongly favor formulations with Nx. Bup’s risk of overdose is relatively low, even when administered through nonmedical routes. Nonetheless, administration as directed remains a significant deterrent against the transmission of HIV and HCV (Alter, 2002; Sullivan and Fiellin, 2005). In the current study, the two larger IV Bup doses tested appeared to have greater potential for abuse, though previous studies suggest that effects such as this will plateau (Walsh et al., 1994; Umbricht et al., 2004). Concerning direct comparison of the relative effectiveness of Bup + Nx, larger absolute amounts of Nx may be a more effective deterrent to parenteral routes of use, as they appear to precipitate more severe withdrawal. However, this assertion is complicated by Bup’s unique receptor pharmacology and how its time course of effects interact with Nx.
Supplementary Material
Acknowledgements
The medical assistance of Janet Murray and Claudia Tindall, along with the technical assistance and Johnathan Vogelman, Rachel Luba, and Andrew Segoshi is gratefully acknowledged.
Funding sources
Over the past three years all of the authors (with the exception of Drs. Jones and Metz) have received compensation (in the form of partial salary support) from investigator-initiated studies supported by Reckitt-Benckiser Pharmaceuticals, Schering-Plough Corporation, Johnson & Johnson Pharmaceutical Research & Development, Endo Pharmaceuticals, and MediciNova. In addition, SDC has also served as a consultant to the following companies: Grunenthal USA, Guidepoint Global, Mallinckrodt, Neuromed, Orexo, Pfizer, and Salix.
Footnotes
Conflicts of interest
This study was supported by an unrestricted, unsolicited investigator-initiated grant to SDC from INDIVIOR PLC. The study sponsor had no role in study design, data collection, analysis and interpretation, the writing of the manuscript, or the decision to submit this manuscript for publication, but did review the report for scientific accuracy. Financial support for the preparation of this manuscript was provided by the National Institute on Drug Abuse grant K01DA030446 to JDJ.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2017.06.033.
References
- Alter MJ, 2002. Prevention of spread of hepatitis C. Hepatolology 36, S93–S98. [DOI] [PubMed] [Google Scholar]
- Bedi NS, Ray R, Jain R, Dhar NK, 1998. Abuse liability of buprenorphine-a study among experienced drug users. Indian J. Physiol. Pharmacol 42, 95–100. [PubMed] [Google Scholar]
- Bruce RD, Govindasamy S, Sylla L, Kamarulzaman A, Altice FL, 2009. Lack of reduction in buprenorphine injection after introduction of co-formulated buprenorphine/naloxone to the Malaysian market. Am. J. Drug Alcohol Abuse 35, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri MP, Amass L, Lucas GM, Vlahov D, Wodak A, Woody GE, 2006. Buprenorphine use: the international experience. Clin. Infect. Dis 43, S197–S215. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, 2002. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J. Pharmacol. Exp. Ther 303, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Walker EA, 2005. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J. Pharmacol. Exp. Ther 315, 1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ, 2008. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology 33, 1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Manubay J, Amass L, Cooper ZD, Saccone P, Kleber HD, 2010. Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers. Addiction 105, 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming G, 2012. Understanding the New Statistics: Effect Sizes, Confidence Intervals, and Meta-Analysis NY, Routledge New York. [Google Scholar]
- Cumming G, 2014. The new statistics: why and how. Psychol. Sci 25, 7–29. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration, 2013. Heroin Domestic Monitor Program (Available at: http://info.publicintelligence.net/DEA-HeroinDMP-2011.pdf. Accessed 15 November 2015).
- Doyon S, 2004. Opioids. In: Tintinalli JE, Kelen GD, Stapczynski JS, Ma OJ, Cline DM (Eds.), Emergency Medicine: A Comprehensive Study Guide, 6th ed. McGraw-Hill, New York, pp. 1071–1075. [Google Scholar]
- Fischer A, Jönsson M, Hjelmström P, 2013. Pharmaceutical and pharmacokinetic characterization of a novel sublingual buprenorphine/naloxone tablet formulation in healthy volunteers. Drug Dev. Ind. Pharm 41, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Yu E, Macfadden W, Boardman C, Chiang CN, 1998. Effects of buprenorphine and naloxone in morphine-stabilized opioid addicts. Drug Alcohol Depend 50, 1–8. [DOI] [PubMed] [Google Scholar]
- Gagnon J, Roth JM, Carroll M, Haycock KA, Plamondon J, Feldman DS, Simpson J, 1990. Superanova accessible general linear modeling. Yale J. Biol. Med 63, 191–192. [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD, 1987. Two new rating scales for opiate withdrawal. Am. J. Drug Alcohol Abuse 13, 293–308. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD, 1978. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch. Gen. Psychiatry 35, 501–516. [DOI] [PubMed] [Google Scholar]
- Jones JD, Comer SD, 2013. A review of human drug self-administration procedures. Behav. Pharmacol 24, 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Vosburg SK, Manubay JM, Mogali S, Metz V, Comer SD, 2015. Abuse potential of intranasal buprenorphine versus buprenorphine/naloxone in buprenorphine-maintained heroin users. Addict. Biol 20, 784–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, Goldberg SR, 1991. The reinforcing and subjective effects of morphine in post-addicts: a dose-response study. J. Pharmacol. Exp. Ther 259, 1165–1173. [PubMed] [Google Scholar]
- Larance B, Degenhardt L, Lintzeris N, Bell J, Winstock A, Dietze P, Mattick R, Ali R, Horyniak D, 2011. Post-marketing surveillance of buprenorphine-naloxone in Australia Diversion, injection and adherence with supervised dosing. Drug Alcohol Depend 118, 265–273. [DOI] [PubMed] [Google Scholar]
- Larance B, Lintzeris N, Ali R, Dietze P, Mattick R, Jenkinson R, White N, Degenhardt L, 2014. The diversion and injection of a buprenorphine-naloxone soluble film formulation. Drug Alcohol Depend 136, 21–27. [DOI] [PubMed] [Google Scholar]
- Lee CE, 2006. Tackling subutex abuse in Singapore. Singapore Med. J 47, 919–921. [PubMed] [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M, 2008. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev 16, CD002207. [DOI] [PubMed] [Google Scholar]
- Maxwell JC, McCance-Katz EF, 2010. Indicators of buprenorphine and methadone use and abuse: what do we know? Am. J. Addict 19, 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Fernandez I, Welm S, Melby AK, Baggott MJ, 1996. Buprenorphine and naloxone interactions in opiate-dependent volunteers. Clin. Pharmacol. Ther 60, 105–114. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Welm S, Baggott M, Fernandez I, Melby AK, Nath RP, 1999. Buprenorphine and naloxone combinations: the effects of three dose ratios in morphine-stabilized: opiate-dependent volunteers. Psychopharmacology (Berl.) 141, 37–46. [DOI] [PubMed] [Google Scholar]
- Middleton LS, Nuzzo PA, Lofwall MR, Moody DE, Walsh SL, 2011. The pharmacodynamic and pharmacokinetic profile of intranasal crushed buprenorphine and buprenorphine/naloxone tablets in opioid abusers. Addiction 106, 1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA, 1988. Buprenorphine and naloxone alone and in combination in opioid-dependent humans. Psychopharmacology (Berl.) 94 (4), 484–490. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA, 1990. Effects of sublingually given naloxone in opioid-dependent human volunteers. Drug Alcohol Depend 25, 27–34. [DOI] [PubMed] [Google Scholar]
- SPSS I, 2009. SPSS 18.0.0 for Windows Illinois, Chicago. [Google Scholar]
- Stoller KB, Bigelow GE, Walsh SL, Strain EC, 2001. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology (Berl.) 154, 230–242. [DOI] [PubMed] [Google Scholar]
- Strain EC, Walsh SL, Preston KL, Liebson IA, Bigelow GE, 1997. The effects of buprenorphine in buprenorphine-maintained volunteers. Psychopharmacology (Berl.) 129, 329–338. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Fiellin DA, 2005. Buprenorphine: its role in preventing HIV transmission and improving the care of HIV-infected patients with opioid dependence. Clin. Infect. Dis 41, 891–896. [DOI] [PubMed] [Google Scholar]
- Umbricht A, Huestis MA, Cone EJ, Preston KL, 2004. Effects of high-dose intravenous buprenorphine in experienced opioid abusers. J. Clin. Psychopharmacol 24, 479–487. [DOI] [PubMed] [Google Scholar]
- Vicknasingam B, Mazlan M, Schottenfeld RS, Chawarski MC, 2010. Injection of buprenorphine and buprenorphine/naloxone tablets in Malaysia. Drug Alcohol Depend 111, 44–49. [DOI] [PubMed] [Google Scholar]
- Walsh S, Eissenberg T, 2003. The clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinic. Drug Alcohol Depend 70, S13–S27. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE, 1994. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin. Pharmacol. Ther 55, 569–580. [DOI] [PubMed] [Google Scholar]
- Walsh S, Preston K, Bigelow G, Stitzer M, 1995. Acute administration of buprenorphine in humans: partial agonist and blockage effect. J. Pharmacol. Exp. Ther 274, 361–372. [PubMed] [Google Scholar]
- Yokell MA, Zaller ND, Green TC, Rich JD, 2011. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use: an international review. Curr. Drug Abuse Rev 4, 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Conley K, Galinkin J, 1997. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J. Pharmacol. Exp. Ther 282, 1187–1197. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


