Abstract
Background:
Rodent reach-to-grasp function assessment is a translationally powerful model for evaluating neurological function impairments and recovery responses. Existing assessment platforms are experimenter-dependent, costly, or low-throughput with limited output measures. Further, a direct histologic comparison of neural activation has never been conducted between any novel, automated platform and the well-established single pellet skilled reach task (SRT).
New method:
To address these technological and knowledge gaps, we designed an open-source, low-cost Automatized Reach-to-Grasp (AutoRG) pull platform that reduces experimenter interventions and variability. We assessed reach-to-grasp function in rats across seven progressively difficult stages using AutoRG. We mapped AutoRG and SRT-activated motor circuitries in the rat brain using volumetric imaging of the immediate early gene-encoded Arc (activity-regulated cytoskeleton-associated) protein.
Results:
Rats demonstrated robust forelimb reaching and pulling behavior after training in AutoRG. Reliable force versus time responses were recorded for individual reach events in real time, which were used to derive several secondary functional measures of performance. Moreover, we provide the first demonstration that for a training period of 30 min, AutoRG and SRT both engage similar neural responses in the caudal forelimb area (CFA), rostral forelimb area (RFA), and sensorimotor area (S1).
Conclusion:
AutoRG is the first low-cost, open-source pull system designed for the scale-up of volitional forelimb motor function testing and characterization of rodent reaching behavior. The similarities in neuronal activation patterns observed in the rat motor cortex after SRT and AutoRG assessments validate the AutoRG as a rigorously characterized, scalable alternative to the conventional SRT and expensive commercial systems.
Keywords: Immediate early gene, Motor circuitry, Open-source, Reach-to-grasp, Rodents, Volumetric brain imaging
1. Introduction
The kinematics of the forelimb reach is evolutionarily conserved between rats and humans (Sacrey et al., 2009; Whishaw et al., 1992). Prior work has shown higher engagement of brain motor circuits over those in the corticospinal tract in both rodents and other mammals (Alstermark et al., 2004; Yang and Lemon, 2003; Alstermark and Pettersson, 2014; Sindhurakar, 2017). Rat forelimb function tests have been used as a model for decades by neuroscientists to investigate reciprocal neuromotor and sensorimotor control pathways (Withers and Greenough, 1989; Whishaw and Tomie, 1989; Šaling, 1992; Jarratt and Hyland, 1999; Bosch-Bouju, 2014) and motor function effects of neurological pathologies such as epilepsy, neurodegenerative disorders, traumatic brain injury, ischemic stroke, and spinal cord injury (Bosch-Bouju, 2014; Whishaw and Kolb, 1988; Whishaw, 1991; McKenna and Whishaw, 1999; Arnold, 2021; Moon, 2009; Nikkhah, 1993). Several studies have focused on characterizing the biomechanics of reaching behavior, with emphasis on three distinct phases: orienting, bringing the food to the mouth, and readjusting after completion of task (Whishaw and Pellis, 1990). The single pellet reaching task (SRT) is currently the most widely used paradigm as it provides experimenters with a quantitative, well-validated, and translationally relevant assay to probe rodent forelimb skilled reaching function. In this assay, the animal must reach through an aperture in the cage wall to retrieve a food pellet. Other versions of the single-pellet task have been developed as well, including platform forelimb reaching (Miklyaeva and Whishaw, 1996), staircase forelimb reaching (Montoya, 1991), and conveyor belt reaching tests (Evenden and Robbins, 1984), among others.
Despite the pervasiveness of the single-pellet skilled reach task assay, it has several limitations. Execution of the task requires a skilled experimenter to manually place each pellet in front of the aperture, record each trial as a success or a fail, and replace the pellet. Likewise, given the importance of olfaction for this task, different experimenters may expose rats to varied olfactory cues causing unnecessary variation in assay outcomes (Whishaw and Tomie, 1989). Each rat requires the experimenter to oversee the entire reaching session, reducing throughput. Data obtained within a session is limited to binary success/fail determinations. Although more complex scoring systems have been developed, they often rely on offline video analysis and scoring, further increasing experiment time and introducing another source of subjectivity (Whishaw and Pellis, 1990; Whishaw and Coles, 1996; Irvine, 2014). Lastly, commercially available platforms are expensive, making it cost prohibitive for most research laboratories to avail multiple SRT arenas required to conduct simultaneous reach and grasp assessments in treatment animal cohorts.
With the advancement of automation and robotics in many biological fields of research, the transition to automated assays of fine motor function in rodents is an inevitable and necessary step to minimize variability and maximize the quality and fidelity of data obtained from behavioral assays (for review of automation in neuroscience, see reference (Kodandaramaiah et al., 2014)). Several researchers have sought to replace the manual work of pellet replacement and trial evaluation with automated systems, while maintaining the fundamental endpoint parameters of the SRT (Wong, 2015; Bova, 2019; Ellens, 2016; Fenrich, 2015; Fenrich, 2016; Bowles, 2021; Salameh, 2020). Automated, online scoring allows coupling of coded events with kinematic analysis using computer vision software (Bova, 2019; Ellens, 2016). The goals of these systems are often to reduce the amount of experimenter-on-rodent time needed for SRT assessments (Fenrich, 2015; Sloan, 2015). One proposal of an automated single-pellet reaching system reported plateauing performance after just 3–4 days of training using a training paradigm of 2–3 sessions per day at 100 trials per session, an intensive protocol that is made viable by automation of task execution and evaluation (Wong, 2015). While each automated version of the single-pellet SRT reduces experimenter involvement and standardizes task execution, there remains an overdependence on dichotomous metrics (i.e., success and fail-based measures) as the primary outcome of motor function assessment. The use of computer vision and machine learning for kinematic assessment expands the breadth of information gained from these assays, but the resources required for high-speed video capture and processing is costly, computing-intensive, and unable to provide direct information about force or forelimb strength, limiting accessibility to these methods (Bova, 2019; Ellens, 2016).
Due to the inherent limitations presented by the single-pellet SRT, novel automated forelimb motor tasks have been developed that provide greater insight into neuromuscular control of forelimb motor function, such as the isometric pull task (Hays, 2013). This assay utilizes a manipulandum with which the rat must interact in order to receive a reward, typically food (Wagner, 2020; Vigaru, 2013). Alterations to this basic principle include a lever-pressing manipulandum to measure forelimb speed (Hays, 2013; Swan, 2019) and a knob supination task (Meyers, 2016).
Despite the relative similarity of an isometric pull force task to the single pellet SRT, the isometric pull force task provides a plethora of secondary measures to further characterize forelimb function, of which maximal force and latency to maximal force have been previously illustrated (Sloan, 2015; Hays, 2013). Accordingly, the pull task discerns not only fine motor function (i.e., reach and grasp capability), but also muscular strength differences that can result from neurological impairment (Khodaparast, 2013). These additional measures require no post-assay video analysis and instead are embedded within the data output files of the automated system. Reaching profiles may be useful for both within-group and between-group behavioral comparisons following induction of neurological injury and throughout the recovery process (Hays, 2013). Moreover, while rodents assessed with the single-pellet SRT often display no functional impairment by week 6 post-injury, there is evidence that the pull task is more sensitive to long-term impairment as task ability persists at a reduced capacity in this same time frame (Sloan, 2015). Though these automated systems are able to reduce the amount of experimenter intervention and the time necessary to train the rats to consistent task performance, the hardware required to execute these manipulandum assays like the knob supination task and the isometric pull task are expensive and cost around $3500 per device commercially (Sindhurakar et al., 2019), which significantly limits simultaneous functional assessment of animal cohorts. Further, the cortical motor circuitry evoked by this emerging isometric pull task has not yet been histologically evaluated in comparison to the conventional single pellet SRT.
In order to increase the accessibility of a robust automated forelimb motor task and provide a simple platform for customization, we developed the first open-source, low-cost, modular version of the isometric pull task that we have termed the Automatized Reach-to-Grasp (AutoRG) task. AutoRG is a reliable yet more cost-effective forelimb motor function task compared to commercial alternatives, generates more comprehensive data than conventional single pellet skilled reach tasks, and establishes a user-friendly platform with both hardware and software modularity for lab-specific customization. We describe the fabrication, assembly, and function of our open-source AutoRG system. We provide the 3D files, a materials list, and detailed assembly instructions to guide in-house construction, as well as open-source software with standard operating protocols. To validate the performance of the open-source AutoRG system, we demonstrate the basic operation and functionality of our device by training male rats using a stage-based training protocol. Furthermore, using AutoRG, we extend the utility of the isometric pull task beyond previously described metrics to more meticulously examine novel pull task metrics that characterize rodent forelimb reach-to-grasp mechanics and reward-driven responses. Finally, we produce the first histological comparison of cortical motor circuity engaged by the isometric pull task versus the single pellet SRT using immediate early gene-encoding protein biomarkers and volumetric imaging methods.
2. Materials and methods
2.1. Animals
7–10-week-old male Sprague-Dawley rats were used in this experiment. Animals were individually housed in a reversed 12 h:12 h light: dark cycle with ad libitum access to food and water. A total of 21 rats were used (n = 6 control, n = 6 single pellet skilled reaching task, n = 9 AutoRG). All experiments were approved by the University of Georgia Institutional Animal Care and Use Committee and were conducted in accordance with the NIH’s Guide for the Care and Use of Laboratory Animals.
2.2. Habituation and handling
Animals were habituated to human contact through progressive exposure to experimenters over at least 5 days prior to skilled motor training. During these habituation sessions, the rats were also familiarized with the dry pellets that were used as the reward for reaching training (20 mg chocolate flavored dustless pellets, BioServ). Prior to training, the rats were habituated to the experimental cage for 5 min per day for 2 days in the presence of pellets on the floor of the cage and in the pellet dispensing area. All habituation and experimental training sessions were run with a background white noise generator that was programmed to deliver white noise at a volume of 65 dB.
2.3. AutoRG construction and technical features
The AutoRG set-up is displayed in Fig. 1 and assembly instructions are available on GitHub (https://github.com/Braxton-goodnight/AutoRG). All electronic components were commercially sourced and are listed in Supplementary Table S-I. The larger non-electronic hardware components were 3D-printed by filament extrusion with a Dremel DigiLab 3D45 printer using polylactic acid (PLA) filament material (0.2 mm quality, 20 % infill unless otherwise stated). The smaller non-electronic hardware components that required greater resolution were 3D-printed by stereolithography (SLA) with a Formlabs Form 2 printer using a clear resin cartridge at 50 μm resolution. All 3D-printed hardware components are listed in Supplementary Table S-II.
Fig. 1.
Modular assembly of 3D-printed parts and electronic components that make up the AutoRG setup. (a) Frontal schematic of the AutoRG setup. Inset AA depicts an overview of all major 3D-printed parts, including stage quadrants, handle base, pellet dispense tower, pellet dispenser, CPX frame, pellet receptacle, positional servo casing, load cell carriage, and load cell handle adapter. (b) Picture of assembled AutoRG apparatus. Lowercase letters label individual components listed in Supplementary Tables S-I and S-II. Inset BB depicts aerial schematic and dimensions of stage and acrylic cage. (c) Wiring diagram for the electronic control system. (d) Screenshot of the AutoRG graphic user interface (GUI) showing a single pull trial.
Forelimb reach-to-grasp function and isometric pull strength was measured with a straight bar mini load cell inserted between an SLA resin handle adapter, which connected to the 1.5 mm (AF) hex key grasp handle, and an SLA resin load cell carriage, which allowed translation of the handle position along a 100 mm linear motion rail. The position of the handle/load cell apparatus was set by a positional micro servo through a series of 3D-printed linkages that connect to the load cell carriage. The load cell was wired to an analog-to-digital converter. A Circuit Playground Express microcontroller (CPX) and a CRICKIT for CPX used for central processing, were powered by a 5 V power supply and interfaced with the computer using a micro-USB to USB data/sync cable.
The soldering of a STEMMA QT/Qwiic Adapter to the CRICKIT/CPX unit is the only location where soldering is required to assemble the AutoRG and is at an unstressed junction of the system. Therefore, failure of any major electrical component (load cell, Qwiic scale ADC, servos) can be rectified by simply unplugging the dysfunctional part and plugging in a fresh, inexpensive replacement part, exemplifying the benefit of a modular hardware design.
The pellet dispenser was controlled using a continuous rotation servo plugged into the CRICKIT and positioned in an adapted version of an already available, open-source pellet dispenser (https://open-ephys.org/fed3/fed3). The pellet dispenser sat at the top of the 3D-printed pellet dispense tower. The pellet dispense tower contains an exit hole to which a rubber tube (inner diameter: 9.5 mm, outer diameter: 12.5 mm, length: 94 mm) was inserted. The other side of this tube was inserted into the PLA pellet receptacle (infill 100 %), which sat in the reaching slit of the acrylic cage on the testing side. This pellet receptacle functioned to receive the reward pellet from the tower and redirect the pellet to its small receiving bin just inside the cage, ensuring that the pellet was placed at a fixed point in front of the reaching slit with each dispense.
For experimentation, the rat was placed in a 260 mm × 131 mm plexiglass cage that is 300 mm tall and contains two 8 mm-wide, 150 mm-tall slits with an outer edge that is 20.3 mm from the edge of the cage. An 8.5 mm thick, 90 mm × 150 mm PLA block was adhered to the wall of the right slit to ensure isolated reaching of the left forelimb. The acrylic cage is open on both the top and bottom faces, and was elevated on a 52 mm-high, extrusion 3D-printed PLA stage (dimensions 170 mm × 285 mm). The experimental stage was printed as 4 smaller quadrants that snap together so that they could be printed on smaller extrusion-based 3D printers with a confined print stage.
Fig. 1a-b shows photos and schematics of the AutoRG. A diagram of the wiring is provided in Fig. 1c. Detailed instructions for complete construction, mounting, and operation of the system is also openly accessible through the Github link provided above.
2.4. AutoRG - controlling software
Two custom MATLAB (Mathworks) graphic user interfaces (GUI) were developed using the built-in MATLAB Graphic User Interface Development Environment. This software is released under the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
2.4.1. Calibration GUI
The calibration GUI was designed to calibrate the load cell for accurate load measurements and to calibrate the positional servo motor to its corresponding handle distance position for each stage. The calibration process is based on 2 wt values, typically i) 0 g, where the handle is in its native, horizontal position and ii) one value greater than 200 g, where a pre-weighed load is suspended from the handle when the apparatus is vertically positioned. The software accounts for the weight of the handle and casing itself (excluding the weight of the pre-weighed load) for the latter calibration value using an average weight across 3 independent systems to maintain the integrity of the calibration. This calibration process allows for linear interpolation of load readings within the two calibration values, which encompasses all loadings for the pull task described in the rat assays below. Further, the angle of the positional servo motor (from 0 to 180 degrees) can be inputted for each of the required handle positions, which includes − 0.30 in, 0.00 in, + 0.25 in, and + 0.50 in for our training paradigm. These calibration data are saved on file (Calibration.mat) and can be used for recalibration or in the main assay GUI.
2.4.2. Assay GUI
The primary assay GUI was designed to run the session, measure the load cell output, and deliver pellet rewards. The stage-dependent or interactive adjustment of the handle position and the trigger threshold are controlled in real time by a separate function, the update function block. The user has the option to choose the calibration.mat file manually to use pre-calibrated or newly calibrated values of the grasp handle. The update function block is a discrete MATLAB function that establishes the training parameters, namely the handle position with respect to the inner wall of the cage and the trigger threshold that must be exceeded for a successful trial. Prior to the start of a session, the user identifies the proper COM port to connect the CPX, selects the desired assay parameters (with our discrete training built in as a default protocol), and inputs other session-specific parameters, including session duration, maximum number of successes, etc. The default session parameters are 20-minute sessions and a maximum of 100 successes. For an interaction to be considered a trial, a minimum force (default: 10 g) on the load cell must be exceeded, which filters out loading noise being read by the program. All sessions begin with the handle at − 0.30 in and a trigger threshold of 10 g to re-expose the rat to the handle-reward association at the beginning of each session. Once the handle is triggered one time in this configuration, the handle and trigger threshold revert to normal stage values; this “warm-up” trial is removed from final data output matrices.
2.4.3. Customizability and modularity
The software was developed as a modular framework; the system is compatible with both discrete-stage training procedures (one of which is programmed by default) and adaptive training procedures that may be customized to fit the user’s needs. In this study, we employed a discrete, seven stage training program, adapted from a previously described training protocol (Hays, 2013), each with a predefined combination of handle position and trigger threshold. The training can also be designed using custom training protocols, including, but not limited to, alternate stages with differing parameters, adaptive stages that advance automatically based on session-read or loaded data, or machine learning systems that identify training patterns and dynamically adapt the training parameters in a continuous format. The goal of the modular framework is to create a convenient environment for developers to explore and implement training formats that improve the robustness of rat forelimb motor training while minimizing training time to stimulate the rigor at which fine motor function may be probed in preclinical studies of neurological injury. Our software is provided open source to encourage the development and subsequent sharing of innovative algorithms based on our core framework.
2.5. AutoRG- animal training procedure
Animals were deprived of food 3 h prior to experiments and six rats successfully completed training. Nine rats were used for the training with the AutoRG task. After the two days of habituation, each rat was trained for two 20-minute sessions per day, for 5 days per week (interval between the sessions: approximately 1 h). The rats advanced through a series of 7 stages that were based on previous isometric pull protocols (Hays, 2013), whereby each stage progresses in difficulty by increasing either the trigger threshold or the handle position relative to the inner wall of the cage. Each stage has its own criterion to advance; once the criterion was met in a given session, the rat began the next stage in the following session. For each training session, the rats were placed in a mild food deprivation condition (no access to food for 3–5 h) and their weights were monitored to not drop over 10 % of body weight.
Stage 1. : All rats began training with the handle position set located 0.30 in. inside the acrylic cage wall (−0.30 in). The trigger threshold was set at 10 g of force. With a minimum trial threshold of 10 g, virtually any substantial interaction with the handle resulted in pellet dispensation, with the objective of priming the rat to associate the handle with the chocolate-flavored food pellet. Once the rat completed 2 consecutive sessions with a total of 60 successful trials each session, the rat advanced to Stage 2. In some early sessions, the experimenter intermittently intervened by holding a food pellet with tweezers outside the slit to draw the rat towards the handle to initiate incidental handle interaction, which facilitated the association priming process for the more inactive rats that were not engaging with the handle spontaneously. Experimenter involvement was limited to these early-stage handle-food priming sessions; late-stage training sessions and evaluation sessions were conducted with no intervention. Placement of food pellet powder on the grasp handle (as previously done in original isometric pull task training descriptions (Hays, 2013)) was also tested as a priming strategy, with less success. Both strategies relied on the rats’ use of olfaction, a well-researched strategy for rodent forelimb tasks (Whishaw and Tomie, 1989).
Stage 2. : The triggering threshold was set to 35 g, and the handle was maintained at the same position as in Stage 1 (−0.30in). The goal of Stage 2 was to shape the rat to begin pulling the handle with a force, whether that be through biting or grasping with the forelimb. Advancement to Stage 3 required the completion of a total of 40 successful trials in a single session.
Stage 3. : In this stage, the handle was retracted to 0.00in to be in line with the inner cage wall, and the trigger threshold was maintained at 35 g. Biting of the handle became more difficult in this stage since the handle resided in the narrow cage slit. By making biting more difficult, the goal of this stage was to encourage forelimb reaching of the handle. For Stages 3–7, the criterion for advancement to the next stage was 30 successful trials in a single session.
Stage 4. : The handle was retracted to 0.25 in. outside the inner wall (+0.25in) as the trigger threshold remained 35 g. Reaching was therefore required for a successful trial. Stage 4 is considered the beginning of the forelimb phase of training.
Stage 5. : The trigger threshold was increased to 65 g, which required the rat to begin applying a larger axial force to successfully complete a trial and acquire a pellet reward. The handle remained at a location of + 0.25in such that Stage 5 emphasized the strengthening of the pull.
Stage 6. : The handle was retracted to 0.50 in. outside the inner cage wall (+0.50in), which required the rat to extend its forelimb farther to produce a more isolated forelimb movement. The trigger threshold remained 65 g such that Stage 6 emphasized the extension of the reach and the isolation of the forelimb movement.
Stage 7. : The trigger threshold was increased to 120 g, making the final assay parameters 120 g for the trigger threshold and + 0.50in for the handle position. These assessment parameters are similar to those used in other stage-based pull task studies, all of which demonstrated the ability to probe forelimb motor function for neurological injury (Hays, 2013; Khodaparast, 2013; Hays, 2014; Hays, 2016).
The above-described training scheme is summarized in Supplementary Table S-III. Once Stage 7 was completed (30 successful trials in a single session), the rats were considered to have fully acquired the task. Rats were evaluated in the four sessions following the completion of Stage 7 to characterize reach-to-grasp performance.
2.6. Single Pellet Skilled Reach Task (SRT)
In order to provide a control for the upper limb circuit activation in rats (i.e. volumetric quantification of immediate early gene expression, Arc), we trained 6 rats to the single pellet reaching task (Whishaw and Pellis, 1990). Briefly, the rats were trained to grasp a single 20 mg pellet placed at 5–10 mm from a slit opening. The rats were trained in a central opening (undifferentiated paw grasping) for 5 days to successfully reach 20 pellets in less than 20 min. Once the rats were able to reach for more than 80 % of the pellets for at least 2 consecutive days, they moved on to the forced side training. For the forced side training, the rats were trained to reach from an open slit on their left, which required left paw grasping. Similar to the undifferentiated training, rats were considered trained when they were able to reach for more than 80 % of the pellets (16 pellets in less than 20 min) for at least 2 consecutive days. The food pellets were placed manually by the experimenter for each trial (fail or success).
2.7. Brain collection post-motor circuit activation
Rats that were trained to criterion outlined above on either the single pellet reach task or AutoRG were food deprived for three hours, placed in the experimental box, and allowed to reach 80–100 times. Both successful and failed reaches counted towards the target goal for this terminal motor assay. Control rats were placed in the experimental box for 30 min, approximately the time spent in the box by AutoRG- and SRT-trained rats. Animals were promptly anesthetized with 5 % isoflurane following completion of the forelimb task, and animals were maintained with intraperitoneal injections of 85 mg/kg Ketamine and 15 mg/kg Xylazine. Once an adequate plane of anesthesia was confirmed by loss of reflexes, the rats were transcardially perfused with 100 mL of cold PBS followed by 150 mL of 4 % ice-cold paraformaldehyde (PFA). The brain was then immediately extracted and immersed in 4 % PFA for 24 h. The interval between the end of the behavioral assay and the onset of perfusion was minimized to the extent possible and kept under 60 min for all animals, as this timeframe best captures the expression of the immediate early gene activity-regulated cytoskeleton-associated protein (Arc) (Renier, 2016).
2.8. Immunohistochemical staining and imaging
PFA fixed brain tissue was rinsed in PBS and stored in PBS overnight. We performed brain tissue clearing and staining using the iDISCO+ method as previously described (Renier, 2014). Briefly, brains were serially dehydrated in increasing methanol concentrations, and then washed overnight in a 66 % dichloromethane/33 % methanol solution. Brains were washed in methanol, bleached in fresh, chilled 5 % hydrogen peroxide, and rehydrated with decreasing methanol solutions. Brains were incubated in a detergent-based permeabilization solution for four days, blocked in a buffer containing 6 % goat serum for two days, and incubated with buffer containing primary antibodies for seven days (Arc, 1:1000, Synaptic Systems, Goettingen, Germany). Brains were washed and incubated with buffer containing secondary antibodies for seven days (Alexa Fluor Plus 555 IgG (H+L) Goat anti-rabbit, 1:250, Invitrogen, Waltham, Massachusetts) before again washing and serial methanol dehydration. A 3 h incubation in 66 % dichloromethane and two washes in 100 % dichloromethane completed tissue clearing and staining. Samples were stored in dibenzyl ether until imaging.
Brains were mounted ventral side down and submerged in a dibenzyl ether containing cuvette for imaging on the LaVision Biotec Ultramicroscope II light sheet. Images were acquired at 0.63x magnification at 3 micrometer thickness slices from the superior dorsal pole of the cortex to the level of the ventricles. Three-dimensional image reconstruction and cell-counting was performed in Imaris version 9.8 (Oxford Instruments, Abingdon, United Kingdom). Select slice-based analysis was performed in ImageJ (version 1.53, NIH, Bethesda, Maryland).
2.9. Statistical analysis
All statistical analyses were performed using MATLAB® statistical toolbox (Mathworks, Inc, Natick, Massachusetts.) or R version 4.1.0 (R Core Team, Vienna, Austria). The Shapiro-Wilk test was used to assess normality. Normally distributed datasets were further analyzed with a student’s t-test or analysis of variance, and Tukey’s HSD for post-hoc comparisons as appropriate. When normality was unmet, non-parametric comparisons were made using the Mann-Whitney U test or Kruskal-Wallis test, with a pairwise Wilcoxon Rank Sum test where appropriate for post-hoc comparisons. An alpha of 0.05 was used as the threshold for significance. All values reported are mean ± standard deviation, unless stated otherwise.
3. Results
3.1. AutoRG characterization
An overview of the AutoRG apparatus and wiring schematic is provided in Fig. 1 and a brief video of the apparatus in use in Video 1. Assembly of the AutoRG apparatus requires printing of the custom parts, ordering of the commercial parts, and following the provided, detailed assembly instructions. The cost of the commercial components to produce one unit is less than $150; additional costs include the price of the 3D printing material used for the custom parts, which includes resin cartridges for the SLA-printed parts and the PLA spools for the extrusion-printed parts. With openly available STL files that encode the designs for the 3D-printed parts, components can be scaled, adjusted, or mirrored as needed. The customizability and low cost of the open-source system affords greater flexibility in study design and more cost-effective scalability compared to established commercial systems.
The primary GUI, displayed in Fig. 1d, allows the user to connect the CPX to the computer, input session information (e.g., rat and experimenter identification), change the session parameters from default (session duration, maximum successes, initial trigger threshold, and initial handle position), and start/stop the session. Session data is automatically saved to the computer. A continuous force trace of the load cell’s reading is provided, as well as an indicator for the trial initiation threshold and the success threshold. An example trial is displayed in the plot of Fig. 1d. After the software is downloaded from Github and initialized for the user’s computer (for which a detailed protocol is provided), the user can interact solely with the GUI to execute the assay and calibrate the system. Therefore, no MATLAB or other software experience is required to use the AutoRG system. Blank and active GUIs for both assay execution (GUI1) and system calibration (GUI_scale_calibration) are displayed in Supplementary Fig. S1.
The force readings captured by the AutoRG system are not affected by changes in handle position, whether fully extended into the cage or fully retracted (Fig. 2a). A sub-one percent change in standard deviation across all tested handle positions demonstrates the AutoRG’s precision, and a negligibly variant mean force across handle positions facilitates cross-stage comparisons of the rat’s reaching behavior. The AutoRG is also linear throughout its relevant working range (0–300 g) and accurate (Fig. 2b). The weight reported by the system correlated well with the actual force applied. The AutoRG also demonstrated stability following extensive usage (Fig. 2c). Despite 1500 pulls with 225 g of force, the system’s force readouts demonstrated insignificant variance from those recorded immediately following a calibration. The AutoRG system appropriately dispenses pellets in 94.9 % of activations (Fig. 2d). The majority of activations resulted in one pellet being dispensed, and in an overwhelming majority of cases (>90 %), either one or two pellets were dispensed.
Fig. 2.
AutoRG accurately reads the applied force and reliably reinforces successful trials. (a) Experiments with an independently verified 227 g force demonstrated the accuracy of the AutoRG force measurement system across various handle positions. Readouts deviated minimally from 227 g, and accuracy was not affected by the position of the handle. (b) Independently verified forces ranging from 0 to 300 g, the relevant range of pull forces for rats in an isometric pull task, were accurately measured by AutoRG. (c) AutoRG calibration is consistent and stable after extensive use. (d) AutoRG dispenses 1–3 pellets approximately 95 % of the time, allowing for reliable, automated conditioning of rodent reaching behavior.
Six rats (out of nine) successfully progressed through seven distinct stages, each increasing in difficulty (Fig. 3a). The seven-stage training protocol was sufficient to achieve consistent forelimb reaching behavior; the mean maximum force achieved was significantly higher at Stage 7 than at Stage 4 (Fig. 3b). In Stage 1, rats took an average of 10.2 sessions (± 4.2 sessions, Fig. 3c). Once the association between the handle and the chocolate pellet was established in Stage 1, rats passed Stages 2 and 3 quickly, spending an average of 1.5 and 2.3 sessions in each stage, respectively. Stage 4 marks the first stage where the handle is retracted outside of the cage wall and is, consequently, the first stage where rats cannot bite—and must reach for—the handle. Most rats took less than four sessions to pass Stage 4, while a single rat took nine sessions (3.8 ± 3.2 sessions). Rats passed Stage 5 quickly (1.2 ± 0.4 sessions). At Stages 6 and 7, there was significant variability in the number of sessions required to pass. Some rats completed the stage in a single session, while others took up to 12. Overall, rats completed training in 20–40 sessions, with a mean of 30.2 sessions over 15.1 days.
Fig. 3.
AutoRG facilitated a 7-stage automated training of rats over 4 weeks, on average. (a) Schematics showing the handle position and force threshold for the critical Stages 1, 4, 6, and 7. Plots on the left show individual rat progression (gray) and average rat progression (black) as the percentage of the stage’s passing criterion, defined as (number of successes achieved in session) / (minimum number of successes required to pass stage). 100 % is defined as ≥ 60 successes for Stage 1 and ≥ 30 successes for Stages 4, 6, and 7. Colored dots indicate one or more rats finishing the stage. Graphs indicate mean and SEM. (b) Distribution of pull forces at stage 4 (mid-training, first stage of forced reaching - blue) and stage 7 (trained rats - green). The median force is indicated with a dashed line. (c) Cumulative bar graph showing the time spent at each stage for each rat. The number of sessions until completion of training varied from 19 to 40 sessions, with a mean of 30.2 sessions.
Regardless of the number of sessions needed for each rat to acquire this reaching skill, rats that successfully passed Stage 7 continued to reliably reach in subsequent sessions, allowing for a granular, quantitative analysis of specific reaching behaviors. In Fig. 4, selected variables from the force vs. time graphs of successful trials after passing Stage 7 demonstrated the ability of AutoRG to capture reaching styles and mechanics without expensive, high-speed video equipment/programs. Qualitative analysis of five randomly selected trials depicts heterogeneity in the path to surpassing the 120-gram success threshold (Fig. 4a). Several measures can be defined and calculated from the force vs. time reaching profile; a representative trace annotated with key characteristics is displayed in Fig. 4b. Peaks, defined mathematically as a local maximum, indicate distinct pulling motions. The maximum force is the single highest force recorded over the two second trial window and is the amplitude of the maximum force peak. We define exertion graphically as the area under the curve and mathematically as the integral of the force vs. time graph. The latency to first success is the duration of time, in seconds, between trial initiation and a recorded force greater than 120 g.
Fig. 4.
AutoRG quantified the reaching style of trained rats during successful trials. (a) Five randomly chosen force vs. time graphs of successful trials demonstrate different pulling behaviors. (b) Annotated representative graph highlights defined variables. Peaks are annotated in blue, the maximum force in orange, the area under the curve in gray, and the latency to first success in green. (c-f) Histograms of the number of peaks, maximum force (in grams), area under the curve (in grams*second), and latency to first success (in seconds), n = 796 successful trials after passing stage 7. Black curves represent probability functions.
In successful trials, rats often made multiple, distinct reaching motions (Fig. 4c). In a plurality of trials, rats pulled four times. Rats pulled once in 6 % of all successes and pulled eight times in 1 % of these trials. Similar variation is seen in the maximum force, area under the curve, and the latency to first success (Fig. 4d-f). The maximum force was in the peri-threshold range of 120–150 g in 37.3 % of successful trials. The maximum force exceeded 200 g in 16.1 % of trials. The area under the curve is slightly right skewed, with a median value of 80.5 g*second. A majority of the exertion in successful trials occurred in the first second after contacting the handle (47.3 g*s in time window 0–1 s, 32.5 g*s in time window 1–2 s). In one-third of successes, rats were engaged with the handle at the end of the trial window and in 5.7 % of cases recorded a force greater than 120 g. The mean full width at half max across all peaks was 0.167 s, with the broadest peak of each trial spanning a mean of 0.287 s. Peaks were closely grouped; the mean interval between peaks was 0.269 s and only 4.4 % of trials had an average time between peaks greater than 0.5 s.
We questioned whether the wide range of variables that characterized the successful rat’s reach in Fig. 4 had patterns of latent, phenotypically similar behavior. Unsupervised clustering algorithms (including k-means, Gaussian mixture models, and hierarchical clustering schemes) and dimensionality reduction approaches (including principal component analysis and t-distributed stochastic neighbor embedding) were apparently uninformative. Additional exploratory analyses of successful trials are shown in Supplementary Fig. S2.
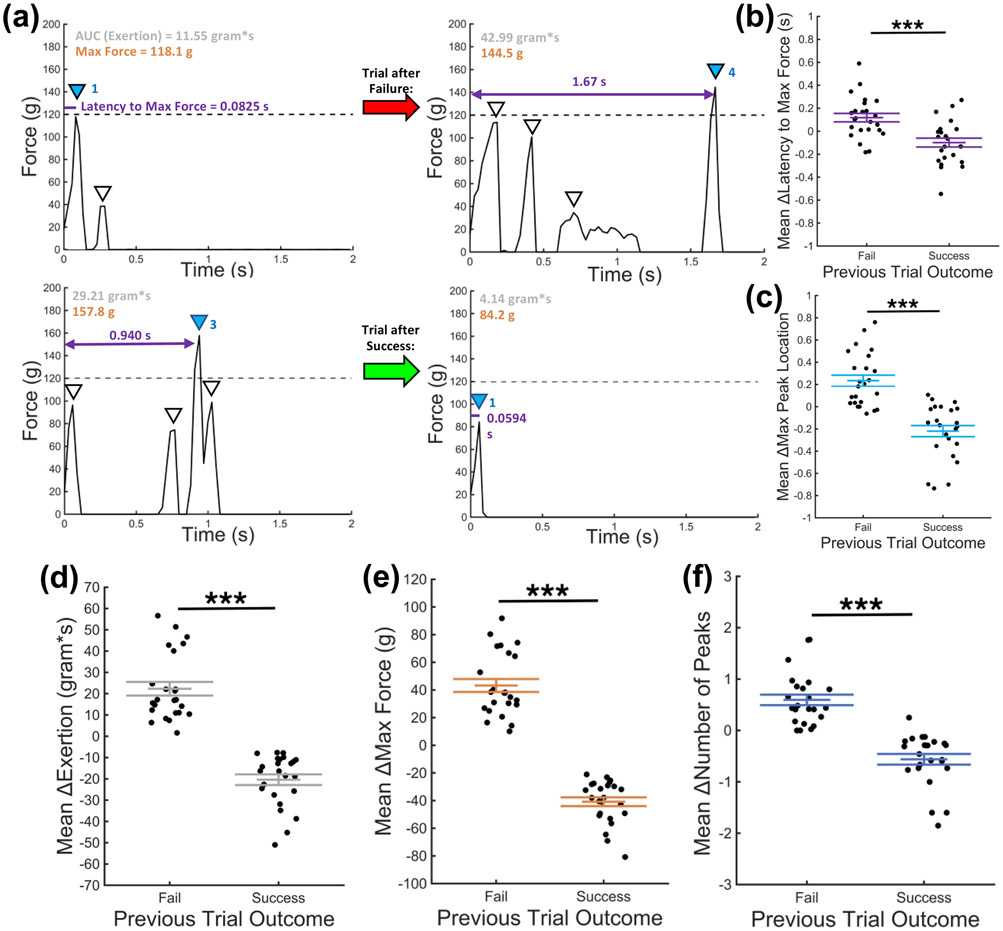
Characteristics extracted from the individual trace of the force vs. graph trial can be analyzed across many interactions with the handle, providing insight into the reward-dependent behavior of rodents to inform the development of custom training protocols. Fig. 5a depicts representative changes in critical trace characteristics following a failure (top) or success (bottom). In absolute terms, rats exerted less effort following a failure than following a success (31.7 ± 11.8 g*s vs. 77.7 ± 24.2 g*s, respectively). Notably, rats altered their effort based on the previous trial’s effort and the outcome. If the (n) trial was a failure, rats exerted a mean of 22.3 g*s more effort in the (n + 1) trial (p < 0.001, Fig. 5d). Conversely, if the (n) trial was a success, rats exerted a mean of 20.4 g*s less force in the (n + 1 trial). This trend was consistent across the maximum force achieved and the number of peaks (Fig. 5e-f). The change in maximum force and the change in number of peaks was positive following a failure and negative following a success (Maximum Force: +43.2 ± 22.9 g post-failure, −40.8 ± 15.6 g post-success; Number of peaks: +0.60 ± 0.50 post-failure, −0.56 ± 0.51 post-success; all p < 0.001). Rat- and session-specific data is presented in Supplementary Fig. S3, where changes in force in response to previous trial outcome can be tracked.
Fig. 5.
Trained rats exhibited outcome-induced adaptation of force, pulling frequency, and latency to success. (a) Representative trials demonstrate changes in behavior trends following a failed trial (top) or a successful trial (bottom). (b-f). Changes in pulling behavior, relative to the previous trial, when the previous trial resulted in either a failure or a success. (b) Change in latency to the trial maximum force, where positive values indicate a longer latency from trial onset. (c) The maximum force peak occurs later in trials following a failure than trials following success. (d-f) The area under the curve, maximum force, and number of peaks increases in the (n + 1) trial when the (n) trial was a failure and decreases when (n) was a success. N = 24 sessions for all panels, significance levels: *** p < 0.001.
The modulation of intertrial characteristics, apparently in response to previous trial outcome, persisted across other characteristics that were extracted from the force vs. time reaching profile. Rats took longer to attain the maximum force in trials following a failure, relative to the previous (fail) trial (Fig. 5b). This contrasted with behavior after a previous successful trial, in which the time from the first contact with the handle to the maximum force reached was essentially unchanged (p < 0.001). In the reaching profile, multiple peaks were seen (Fig. 4c). The peaks were numbered in chronological order, starting from the earliest and counting upwards. Each peak within a trial were described by characteristics unique to that peak (i.e.: amplitude, width, position on the x-axis, etc.), and therefore, the AutoRG can easily identify the location of the maximum force peak. Interestingly, the position of the max force peak comes later in the trial following a failure and earlier following a previous success (p < 0.001, Fig. 5c).
3.2. Visualization of reach and grasp activated brain motor circuitry
While the forelimb activity can be mechanically measured and represented, a visualization of the corresponding neuronal activity patterns that translate into the observed motion of the forelimb has so far not been characterized (Fig. 6). In order to achieve this, we labeled Arc activity in the brain. Arc is a cytoskeletal protein that is upregulated in neurons immediately following neuronal firing. Intensity-based heatmaps of Arc expression in the cerebrum of rats thirty- to sixty-minutes following no behavioral assay (control), single-pellet SRT, or AutoRG are displayed in Fig. 6b. Qualitatively, both SRT and AutoRG animals demonstrated greatly increased Arc expression in the regions responsible for left forelimb motor function: the right caudal forelimb area (CFA), rostral forelimb area (RFA), and the somatosensory cortex (S). Activation through the cortical layers approached a Gaussian distribution in all three groups, with peaks centered around Layer IV (Fig. 6e). Following smoothing and binning, Arc fluorescence intensity between SRT-control and AutoRG-control was compared pixel-by-pixel (Fig. 6f). SRT and AutoRG both displayed increased Arc expression across the imaged brains compared to control. Between SRT and AutoRG animals, Arc expression was found to be higher in regions corresponding to the primary and secondary motor cortices in AutoRG rats, whereas a relative increase in Arc expression was seen in the region of the somatosensory cortex in SRT animals (Fig. 6d).
Fig. 6.
AutoRG training elicits the activation of neuronal circuitry in the motor cortex. (a) The experimental timeline of the terminal motor assay which elicited Arc expression. (b) Heatmaps of Arc expression in the dorsal-ventral (top) and anterior-posterior (bottom) planes. Warmer colors indicate higher levels of Arc expression. (c) Representative image denoting defined regions of interest: blue (rostral forelimb area, RFA), green (caudal forelimb area, CFA), yellow (somatosensory cortex, S), white line (bregma). (d) Heatmap demonstrating differences in Arc intensity between AutoRG and SRT. Purple indicates greater Arc intensity in the AutoRG animals in that area, red indicates greater Arc intensity in SRT animals. (e) Arc expression as a function of cortical depth in control (blue), SRT (yellow), and AutoRG (orange). (f) Change in Arc expression, relative to control, across the imaged brain. Pixels were binned and compared to their spatial counterpart. Dashed black lines indicate differences in Arc expression, + /− 1 % change omitted from the graph. (g) The number of Arc+ cells in the caudal forelimb area, rostral forelimb area, and somatosensory cortex in control, SRT, and AutoRG animals. (h) The ratio of Arc+ expression in the right:left hemispheres in the CFA, RFA, and S of control, SRT, and AutoRG animals. Significance levels: * p < 0.05, ** p < 0.01.
To better quantify the region-specific changes in Arc activity, Arc+ cells were counted in the RFA (blue), CFA (green), and somatosensory cortex (yellow, Fig. 6c). The number of Arc positive cells in the CFA (p = 0.0065) and the RFA (p = 0.026) was significantly higher in AutoRG rats compared to control (Fig. 6g). AutoRG animals had a greater number of Arc+ cells in the CFA compared to SRT animals (1367 ± 984 vs. 384 ± 107, respectively), but this finding was not statistically significant (p = 0.0762). No statistically significant difference was seen in the number of Arc+ cells in the somatosensory cortex (p = 0.1425). The ratio of Arc+ cells in the right compared to the left hemispheres of the same animals was calculated in Fig. 6h. No statistically significant differences were seen in the CFA or the RFA, but there was preferential activation of the right compared to the left hemisphere in the somatosensory cortex of AutoRG rats compared to control (p = 0.045).
We questioned whether the significant increases in Arc+ cells seen in the CFA and RFA of AutoRG rats could have resulted from an accumulation of lifetime reaching behavior, as opposed to task- and session-specific effects. There were no significant correlations observed between the total number of left forelimb reaches over an animal’s lifespan and the number of Arc+ cells seen following terminal motor assay in the CFA, RFA, or somatosensory cortex (Supplementary Fig. S4).
4. Discussion
We have developed the AutoRG as an automated, open-source, user-friendly rodent forelimb motor assay. The provided software code, 3D-printer files, and assembly protocols allow labs without technical or engineering experience to implement a reliable forelimb behavior task that improves throughput, reduces exogenous variability, and increases the granularity of data obtained about forelimb function. The AutoRG is comparable to traditional single pellet skilled reaching assays behaviorally and cellularly with the added benefit of real-time performance analysis throughout the time course of the session and more comprehensive data output at the session endpoint. Further, we characterized reaching behavior that may inform future training and assessment paradigms.
The AutoRG system displays reliability in pellet reward delivery and pull force measurement across an envelope of device settings, applied forces, and wear conditions (Fig. 2). 3D printing of structural components of the AutoRG and plug-and-play cables allow customizability, upgrades, or replacements to system components without the need for tools or significant downtime. Future iterations of the AutoRG can include a rationally designed olfactory cue near the handle or the addition of flashes or auditory tones to capitalize on the rodent’s high olfactory drive or to more quickly establish operant conditioning type associations (Whishaw and Tomie, 1989; Hays, 2013).
Ideally, only a single pellet should be dispensed per successful reach. We show in Fig. 2d that one pellet is dispensed in a majority of the activations (53.5 %) and pellet(s) are dispensed 94.9 % of the time, but we also note that a pellet fails to be dispensed in approximately 5.1 % of activations. This result is in-line with other lab-designed, automated forelimb tasks, where a pellet fails to be dispensed between 2.5 % (Wong, 2015) and 12.8 % (Ellens, 2016) of the time. Although commercial pellet dispensers have reported failure rates below 1 % (Hasz and Redish, 2018; Mei, 2020), we sought to balance the simplicity and material cost of the pellet dispenser with precision in the number of pellets dispensed. We ultimately opted to implement a simple, rotating disk design where pellets would only be dispensed when the holes of the upper and lower disks were aligned, which can result in the release of 2–3 pellets and rarely can result in the release of no pellets. The dispensation of multiple pellets would be concerning for automated SRT designs in which the pellet is the reaching object itself, as this can alter forelimb biomechanics during reaching. However, it is of less concern in the AutoRG where the pellet is simply a reward for reaching a separate pull bar and where beyond Stage 1 in the early shaping of the animal’s association with the pull bar, the infallible delivery of the food pellet is less important. The modular and open-source nature of the AutoRG allow end-users to implement a feedback loop with an IR-based beam break sensor or weight sensor if individual experimental demands justify the added cost and complexity of such systems.
This study used a discrete, seven-stage training protocol to validate the capabilities of the AutoRG. The AutoRG successfully trained six out of nine rats. This passing rate is comparable to single-pellet skilled reaching (4 out of 6 in this study), to the 76–100 % reported in prior studies of isometric pull force tasks (Fenrich, 2015; Hays, 2013; Becker, 2016), and to the 65–75 % reported in other assays of automated forelimb motor testing (Ellens, 2016; Sloan, 2015; Butensky, 2017). Two rats that failed AutoRG training passed stage 6 (>65 g of force at furthest handle position from cage) but were unable to complete training due to difficulties on Stage 7 (>120 g of force at furthest handle position), leading us to speculate whether individualized success thresholds are appropriate. While an average of 30 sessions of twice-daily training led to a supra-threshold pull in approximately half of trials, we do not contend that this training scheme is optimal or that the rats in this study are maximally trained.
The training scheme described herein was adapted from the previously described discrete-stage protocol (Hays, 2013) such that each progressive stage increments toward the final assay parameters (+0.50 in outside inner wall of cage, 120 g trigger threshold) by increasing either the trigger threshold burden or the distance from the inner wall of the cage, but not both in a single stage progression, unlike the origin discrete-stage protocol. Through this methodology, we were able to achieve multiple goals simultaneously. First, we were able to identify the stage progressions (i.e., the parameter changes) that most challenged the rat cohort (e.g., the transitions to Stage 4, Stage 6, and Stage 7, which required a mean of 3.8, 5.2, and 5.2 sessions to pass, respectively) and which least challenged the cohort (e.g., the transitions to Stage 2 and 5, which required a mean of 1.5 and 1.2 sessions to pass, respectively) (Supplementary Table S-III). This information is essential to inform the development of a more simplified, more streamlined discrete-stage training scheme, and the data may also be used for future training schemes that employ real-time modulation of handle position and trigger threshold driven by pre-programmed pattern recognition and/or machine learning processes.
The seven-stage protocol served as a basic protocol that allowed us to demonstrate the functional capability of the AutoRG prototype across a range of assay parameters and elicit consistent, high-volume forelimb reaching and grasping in rodents. Indeed, optimized protocols have been proposed (Becker, 2016) and may be implemented into the modular “update function” block of the software. Additional training sessions have also been reported to increase the success rate, and real-time modulation of the success threshold can accelerate training as well (Hays, 2013; Meyers, 2019). The software was designed with modularity in mind to explicitly facilitate implementation of alternative training protocols. Likewise, as rats display strong spontaneous forelimb laterality preference, the freely available 3D component designs make mirroring the system for right-handed testing trivial and can further improve training yield (Whishaw, 1992). Despite a non-optimized training scheme, the shift in force distributions from stage 4 to stage 7 is compelling evidence that this design of the AutoRG works to interrogate forelimb motor function as intended (Fig. 3b).
In the context of replacing traditional forelimb motor assays, the AutoRG provides distinct behavioral advantages over conventional assessments, while targeting similar areas of neural circuitry. Histological data obtained after optical clearing, staining for the immediate early gene-encoded Arc protein, and volumetric whole-brain imaging demonstrates that training with the AutoRG causes increased Arc expression in the primary and secondary motor cortices. Prior work has validated Arc expression as a region- and task-specific marker of motor learning, where acutely increased Arc expression is directly associated with enrichment of persistent firing patterns in neuronal subpopulations in the task activated motor circuit (Hosp, 2013; Cao, 2015; Ren, 2014). Heatmaps of Arc expression in Fig. 6b are consistent with the brain regions known to control forelimb motor function (Hall and Lindholm, 1974; Neafsey and Sievert, 1982). Increases in Arc-expressing neurons in the caudal and rostral forelimb areas following training on the AutoRG reflects consolidation and strengthening of forelimb-specific motor circuits, compared to untrained animals.
Although no statistically significant differences were observed in the extent of neural circuit activation between AutoRG and skilled reach task animals (p = 0.0762), there was a trend towards increased Arc expression in AutoRG animals. The data in Fig. 6 leads to three hypothesis-generating interpretations: the SRT and AutoRG equally activate the cortical forelimb motor areas, AutoRG animals display increased neuronal activity by accumulating changes of long-term potentiation over a greater quantity of lifetime forelimb reaches, or a fundamental difference between the SRT and AutoRG tasks causes increased neuronal activation in AutoRG-trained animals. AutoRG animals did reach more than SRT animals over their lifespan (1750.7 ± 379.1 vs 459 ± 59.6 reaches); selective strengthening of the forelimb motor circuit might, therefore, represent volume-dependency as opposed to task-specificity. Besides the logistical challenges that would result from training rats four-fold more on the SRT, prior work has shown that most new postsynaptic dendritic spines, indicative of plasticity, form one hour after exposure to a novel motor task and plateau after four days of training, calling into question the plastic benefits one could expect by extending the duration of non-novel training (Xu, 2009). Although limited by the small sample size (n = 6), there is no clear trend in Supplementary Fig. S4 suggestive of a positive correlation between number of lifetime reaches and degree of motor circuit activation. While this study was neither designed nor powered to detect superiority, the histological data validates, at minimum, that the AutoRG spatially activates the same cortical areas as the SRT and with similar intensity.
Intra-trial analysis of the trained rat’s successful reaches displayed in Fig. 4 and Supplementary Fig. S2 define a range of expected reaching behaviors with AutoRG use. There are many phenotypically different paths to a supra-threshold force that cannot be explained by differences in individual or session. Qualitative and quantitative analysis of the intra-trial characteristics (e.g. individual representative traces (Fig. 4a), max force histograms (Fig. 4d), average force vs. time curve) resemble findings that have previously been reported (Hays, 2013), reinforcing that the behavior described here is universal to the task and not an artifact of this AutoRG design. This finding may reflect an inherent degree of biological variability, where rats can actively regulate the magnitude of motor variability in response to reward rates (Dhawale, 2019). Prior studies of forelimb reach behavior have found that variability in the forelimb and digit trajectories decreases with task learning, albeit incompletely (Bova et al., 2021; Nica, 2018), and rats display increased behavioral variability in the context of environmental uncertainty (Tervo, 2014). Rats may yet be undertrained or underexposed to the apparatus at stage 7 settings, and additional sessions on the AutoRG could further refine reaching behavior. Intra-trial characteristics shown in Fig. 4 and Supplementary Fig. S2 represent the capability of the AutoRG platform to provide granular data for analysis; the precise behavioral or biological implications of changes in these variables is beyond the scope of this paper and represents an avenue for future study.
Analysis of inter-trial reaching behavior after completion of training displays striking trends that are easily quantified and stereotyped. Following a failed trial, rats increase their number of pulls, maximum force, and vigor of interaction with the handle (area under the curve) relative to the previous trial. This trend is statistically significant, comparable in magnitude bidirectionally (i.e.: rats increase their effort similarly following a failure as they decrease following a success), and robust across all rats and sessions. Analyses of forelimb reaching tasks typically depend on summary statistics pooled from individual trials. However, the notion that each trial can be considered equal to and independent from any other within a session is an oversimplification of cumulative reach and grasp activity. Any evaluation of rat behavior on the isometric pull force task must be informed by long-range analysis across trials. The AutoRG system innately tracks chronological trial-by-trial data, facilitating analysis informed by the previous outcome and potentially unlocking a rich, novel avenue for interpreting rat forelimb motor mechanics.
Traditionally, forelimb reaching tasks, and isometric pull tasks in particular, have been largely used to study motor impairment in neurological injury models. However, though traditional reach tasks such as the SRT have been translated to motor learning and cognition studies, the emerging, high-throughput automated systems such as the isometric pull task have yet to be fully realized as tools that can be widely employed in other areas of neuroscience. The increasing prevalence of automated behavioral assays makes AutoRG ideally suited for use in combination with either head-fixed or freely moving rodents undergoing electrophysiology recordings, closed-loop stimulation, in vivo microscopy, or chemo- and optogenetic manipulation. While detailed scoring systems have been developed for the SRT, they require significant offline, experimenter-intensive video scoring (Whishaw et al., 2008). In comparison, the AutoRG system seamlessly records and outputs all data, which can be mined to detect subtle changes in motor behavior that might arise in early phases of neurodegenerative diseases like Parkinsonism or Alzheimer’s (Vecchia, 2018). The use of AutoRG can also be extended to study addiction, the neuroeconomics of volitional behavior, and the encoding of motor learning (Glimcher, 2003; Steiner and Redish, 2014).
The AutoRG presents an attractive, scalable, and affordable solution to the problem of reproducibility in science (Collins and Tabak, 2014; Goodman et al., 2016;). Our platform is designed to be affordable to spec, easy to assemble, and requires little specialized knowledge to use. The combination of these traits, coupled with complete automation once the rat is placed onto the platform, allows a single experimenter to manage multiple AutoRG units simultaneously. While the enhanced throughput due to parallel evaluation is indeed a feature of all automated systems, the use of expensive commercial systems presents a financial limitation to the number of units that can be purchased for simultaneous use. Increased throughput of behavioral assessment allows cohorts of animals to be tested simultaneously, thus maintaining identical conditions for all animals within a cohort. The decrease in experimenter time commitment ameliorates the rate-limiting step of behavioral testing and presents an opportunity to increase the number of animals per study, enhancing statistical power and better accounting for inherent biological variability.
5. Conclusion
AutoRG has been demonstrated as a reliable, open-source platform that can facilitate the scale-up of rodent forelimb motor function studies at a lower cost than existing commercial isometric pull systems, with numerous customizable features. The similar activation of the motor cortex by AutoRG in comparison to the gold standard SRT verifies the feasibility of using AutoRG as an alternative motor function task. With newly identified secondary metrics derived from the force versus time trace, analysis of such metrics affords the opportunity to better characterize rodent reaching behavior and reward-driven behavior on a more individualized basis.
Supplementary Material
Acknowledgments
This work is supported by funding from the National Institutes of Health, Bethesda, MD, USA; and National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA.
Footnotes
CRediT authorship contribution statement
Rameen Forghani: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Braxton Goodnight: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Charles-Francois Vincent Latchoumane: Conceptualization, Methodology, Software, Validation, Formal analysis, Writing- original draft, Writing – review & editing, Visualization, Supervision, Project administration. Lohitash Karumbaiah: Conceptualization, Methodology, Formal analysis, Resources, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
None.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jneumeth.2023.109798.
Data Availability
Data will be made available on request.
References
- Alstermark B, Ogawa J, Isa T, 2004. Lack of monosynaptic corticomotoneuronal EPSPs in Rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J. Neurophysiol 91 (4), 1832–1839. [DOI] [PubMed] [Google Scholar]
- Alstermark B,B, Pettersson LG, 2014. Skilled reaching and grasping in the rat: lacking effect of corticospinal lesion. Front. Neurol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold BM, et al. , 2021. Prolonged acute intermittent hypoxia improves forelimb reach-to-grasp function in a rat model of chronic cervical spinal cord injury. Exp. Neurol 340. [DOI] [PubMed] [Google Scholar]
- Becker AM, et al. , 2016. An automated task for the training and assessment of distal forelimb function in a mouse model of ischemic stroke. J. Neurosci. Methods 258, 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Bouju C, et al. , 2014. Reduced reach-related modulation of motor thalamus neural activity in a rat model of parkinson’s disease. J. Neurosci 34 (48). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova A, et al. , 2019. Automated rat single-pellet reaching with 3-dimensional reconstruction of paw and digit trajectories. J. Vis. Exp. JoVE 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova A, Ferris K, Leventhal DK, 2021. Evolution of gross forelimb and fine digit kinematics during skilled reaching acquisition in rats. eNeuro 8 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles S, et al. , 2021. Closed-loop automated reaching apparatus (CLARA) for interrogating complex motor behaviors. J. Neural Eng 18 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butensky SD, et al. , 2017. The knob supination task: a semi-automated method for assessing forelimb function in rats. JoVE 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao VY, et al. , 2015. Motor learning consolidates arc-expressing neuronal ensembles in secondary motor cortex. Neuron 86 (6), 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, Tabak LA, 2014. Policy: NIH plans to enhance reproducibility. Nature 505, 612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawale AK, et al. , 2019. Adaptive regulation of motor variability. Curr. Biol 29 (21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellens DJ, et al. , 2016. An automated rat single pellet reaching system with high-speed video capture. J. Neurosci. Methods 271, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, Robbins TW, 1984. Effects of unilateral 6-Hydroxydopamine lesions of the caudate-putamen on skilled forepaw use in the rat. Behav. Brain Res 14 (1), 61–68. [DOI] [PubMed] [Google Scholar]
- Fenrich KK, et al. , 2015. Improved single pellet grasping using automated ad libitum full-time training robot. Behav. Brain Res 281, 137–148. [DOI] [PubMed] [Google Scholar]
- Fenrich KK, et al. , 2016. Single pellet grasping following cervical spinal cord injury in adult rat using an automated full-time training robot. Behav. Brain Res 299, 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW, 2003. The neurobiology of visual-saccadic decision making. Annu. Rev. Neurosci 26, 133–179. [DOI] [PubMed] [Google Scholar]
- Goodman SN, Fanelli D, Ioannidis JPA, 2016. What does research reproducibility mean? Sci. Trans. Med 8 (341). [DOI] [PubMed] [Google Scholar]
- Hall RD, Lindholm EP, 1974. Organization of motor and somatosensory neocortex in the albino rat. Brain Res. 66 (1), 23–38. [Google Scholar]
- Hasz BM, Redish AD, 2018. Deliberation and procedural automation on a two-step task for rats. Front. Integr. Neurosci 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, et al. , 2013. The isometric pull task: a novel automated method for quantifying forelimb force generation in rats. J. Neurosci. Methods 212 (2), 329–337. [DOI] [PubMed] [Google Scholar]
- Hays SA, et al. , 2013. “The bradykinesia assessment task: an automated method to measure forelimb speed in rodents. J. Neurosci. Methods 214 (1), 52–61. [DOI] [PubMed] [Google Scholar]
- Hays SA, et al. , 2014. The timing and amount of vagus nerve stimulation during rehabilitative training affect post-stroke recovery of forelimb strength. Neuroreport 25 (9), 682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, et al. , 2016. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol. Aging 43, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp JA, et al. , 2013. Region and task-specific activation of Arc in primary motor cortex of rats following motor skill learning. Neuroscience 250, 557–564. [DOI] [PubMed] [Google Scholar]
- Irvine KA, et al. , 2014. The irvine, beatties, and bresnahan (IBB) forelimb recovery scale: an assessment of reliability and validity. Front. Neurol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarratt H, Hyland B, 1999. Neuronal activity in rat red nucleus during forelimb reach-to-grasp movements. Neuroscience 88 (2), 629–642. [DOI] [PubMed] [Google Scholar]
- Khodaparast N, et al. , 2013. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol. Dis 60, 80–88. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Whishaw IQ, 1999. Complete compensation in skilled reaching success with associated impairments in limb synergies, after dorsal column lesion in the rat. J. Neurosci 19 (5), 1885–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J, et al. , 2020. Automated radial 8-arm maze: a voluntary and stress-free behavior test to assess spatial learning and memory in mice. Behav. Brain Res 381. [DOI] [PubMed] [Google Scholar]
- Meyers E, et al. , 2016. The supination assessment task: an automated method for quantifying forelimb rotational function in rats. J. Neurosci. Methods 266, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EC, et al. , 2019. Enhancing plasticity in central networks improves motor and sensory recovery after nerve damage. Nat. Commun 10 (1), 5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklyaeva EI, Whishaw IQ, 1996. HemiParkinson analogue rats display active support in good limbs versus passive support in bad limbs on a skilled reaching task of variable height. Behav. Neurosci 110 (1), 117–125. [DOI] [PubMed] [Google Scholar]
- Montoya CP, et al. , 1991. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J. Neurosci. Methods 36 (2–3), 219–228. [DOI] [PubMed] [Google Scholar]
- Moon SK, et al. , 2009. Both compensation and recovery of skilled reaching following small photothrombotic stroke to motor cortex in the rat. Exp. Neurol 218 (1), 145–153. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Sievert C, 1982. A second forelimb motor area exists in rat frontal cortex. Brain Res. 232 (1), 151–156. [DOI] [PubMed] [Google Scholar]
- Nica I, et al. , 2018. Automated assessment of endpoint and kinematic features of skilled reaching in rats. Front. Behav. Neurosci 11, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkhah G, et al. , 1993. Restoration of complex sensorimotor behavior and skilled forelimb use by a modified nigral cell suspension transplantation approach in the rat parkinson model. Neuroscience 56 (1), 33–43. [DOI] [PubMed] [Google Scholar]
- , 2015Open Science Collaboration, Estimating the reproducibility of psychological science, Science, 349, 2015. [DOI] [PubMed] [Google Scholar]
- Kodandaramaiah S, Boyden E, and Forest C, 2014. In vivo robotics: the automation of neuroscience and other intact-system biological fields, Ann. N.Y. Acad. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, et al. , 2014. Arc regulates experience-dependent persistent firing patterns in frontal cortex. J. Neurosci 34 (19), 6583–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, et al. , 2014. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159 (4), 896–910. [DOI] [PubMed] [Google Scholar]
- Renier N, et al. , 2016. Mapping of brain activity by automated volume analysis of immediate early genes. Cell 165 (7), 1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacrey LAR, Alaverdashvili M, Wishaw IQ, 2009. Similar hand shaping in reaching-for-food (skilled reaching) in rats and humans provides evidence of homology in release, collection, and manipulation movements. Behav. Brain Res 204 (1), 153–161. [DOI] [PubMed] [Google Scholar]
- Salameh G, et al. , 2020. The home-cage automated skilled reaching apparatus (HASRA): individualized training of group-housed mice in a single pellet reaching task. eNeuro 7 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šaling M, et al. , 1992. Reaching behavior in the rat: absence of forelimb peripheral input. Physiol. Behav 51 (6), 1151–1156. [DOI] [PubMed] [Google Scholar]
- Sindhurakar A, et al. , 2017. An automated test of rat forelimb supination quantifies motor function loss and recovery after corticospinal injury. Neurorehabil. Neural Repair 31 (2), 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhurakar A, Butensky SD, Carmel JB, 2019. Automated forelimb tasks for rodents: current advantages and limitations, and future promise. Neurorehabil. Neural Repair 33 (7), 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan AM, et al. , 2015. A within-animal comparison of skilled forelimb assessments in rats. PLoS One 10 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AP, Redish AD, 2014. Behavioral and neurophysiological correlates of regret in rat decision-making on a neuroeconomic task. Nat. Neurosci 17 (7), 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan CB, et al. , 2019. Beta frequency oscillations in the subthalamic nucleus are not sufficient for the development of symptoms of parkinsonian bradykinesia/akinesia in rats. eNeuro 6 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DGR, et al. , 2014. Behavioral variability through stochastic choice and its gating by anterior cingulate cortex. Cell 159 (1), 21–32. [DOI] [PubMed] [Google Scholar]
- Vecchia DD, et al. , 2018. Effects of ketamine on vocal impairment, gait changes, and anhedonia induced by bilateral 6-OHDA infusion into the substantia nigra pars compacta in rats: Therapeutic implications for Parkinson’s disease. Behav. Brain Res 342, 1–10. [DOI] [PubMed] [Google Scholar]
- Vigaru BC, et al. , 2013. A robotic platform to assess, guide and perturb rat forelimb movements. IEEE Trans. Neural Syst. Rehabil. Eng 21 (5), 796–805. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, et al. , 2020. Skilled reaching tasks for head-fixed mice using a robotic manipulandum. Nat. Protoc 15 (3), 1237–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, et al. , 1991. The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, videorecording, and movement notation analysis. Behav. Brain Res 42 (1), 77–91. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, 1992. Lateralization and reaching skill related: results and implications from a large sample of Long-Evans rats. Behav. Brain Res 52 (1), 45–48. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Coles BLK, 1996. Varieties of paw and digit movement during spontaneous food handling in rats: postures, bimanual coordination, preferences, and the effect of forelimb cortex lesions. Behav. Brain Res 77 (1–2), 135–148. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Kolb B, 1988. Sparing of skilled forelimb reaching and corticospinal projections after neonatal motor cortex removal or hemidecortication in the rat: support for the Kennard doctrine. Brain Res. 451 (1), 97–114. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, 1990. The structure of skilled forelimb reaching in the rat: A proximally driven movement with a single distal rotatory component. Behav. Brain Res 41 (1), 49–59. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA, 1989. Olfaction directs skilled forelimb reaching in the rat. Behav. Brain Res 32 (1), 11–21. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM, Gorny BP, 1992. Skilled reaching in rats and humans: evidence for parallel development or homology. Behav. Brain Res 47 (1), 59–70. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Whishaw P, Gorny B, 2008. The structure of skilled forelimb reaching in the rat: a movement rating scale. J. Vis. Exp. JoVE 18 (18), 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers GS, Greenough WT, 1989. Reach training selectively alters dendritic branching in subpopulations of layer II–III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia 27 (1), 61–69. [DOI] [PubMed] [Google Scholar]
- Wong CC, et al. , 2015. An automated behavioral box to assess forelimb function in rats. J. Neurosci. Methods 246, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, et al. , 2009. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 460, 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HW, Lemon RN, 2003. An electron microscopic examination of the corticospinal projection to the cervical spinal cord in the rat: lack of evidence for cortico-motoneuronal synapses. Exp. Brain Res 149 (4), 458–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.