Abstract
The term “nanotechnology” was coined by Norio Taniguchi in the 1970s to describe the manipulation of materials at the nano (10−9) scale, and the term “nanomedicine” was put forward by Eric Drexler and Robert Freitas Jr. in the 1990s to signify the application of nanotechnology in medicine. Nanomedicine encompasses a variety of systems including nanoparticles, nanofibers, surface nano-patterning, nanoporous matrices, and nanoscale coatings. Of these, nanoparticle-based applications in drug formulations and delivery have emerged as the most utilized nanomedicine system. This review aims to present a comprehensive assessment of nanomedicine approaches in vascular diseases, emphasizing particle designs, therapeutic effects, and current state-of-the-art. The expected advantages of utilizing nanoparticles for drug delivery stem from the particle’s ability to (1) protect the drug from plasma-induced deactivation; (2) optimize drug pharmacokinetics and biodistribution; (3) enhance drug delivery to the disease site via passive and active mechanisms; (4) modulate drug release mechanisms via diffusion, degradation, and other unique stimuli-triggered processes; and (5) biodegrade or get eliminated safely from the body. Several nanoparticle systems encapsulating a variety of payloads have shown these advantages in vascular drug delivery applications in preclinical evaluation. At the same time, new challenges have emerged regarding discrepancy between expected and actual fate of nanoparticles in vivo, manufacturing barriers of complex nanoparticle designs, and issues of toxicity and immune response, which have limited successful clinical translation of vascular nanomedicine systems. In this context, this review will discuss challenges and opportunities to advance the field of vascular nanomedicine.
Keywords: nanomedicine, vascular, targeting, drug delivery, animal models
Vascular pathologies, such as atherosclerosis, myocardial infarction, stroke, deep vein thrombosis (DVT), and pulmonary embolism (PE), are leading causes of morbidities and mortalities in the world.1–3 Therefore, significant preclinical and clinical research efforts are focused toward these diseases as well as their diagnosis, prevention, and treatment. Disease pathogenesis for these involves a variety of cellular and biomolecular entities within the vascular compartment, which often present reciprocal mechanisms of inflammation and thrombosis, precipitating in vessel occlusion and tissue injury. Therefore, current therapeutic approaches are directed toward one or more cellular or molecular component of these mechanisms. Most clinical approaches utilize systemic oral or intravenous (bolus or transcatheter infusion) delivery of therapeutics (e.g., anticoagulants, antiplatelet agents, fibrinolytics, and anti-inflammatory molecules) in a highly regulated regimen. Such direct systemic drug administration can lead to several unwanted effects4–8:
Suboptimal circulation time and bioavailability of drugs due to plasma-induced deactivation by circulating inhibitor molecules.
Drug clearance into nonspecific tissues leading to suboptimal concentration at target disease site in the vasculature.
Systemic and off-target drug actions that cause harmful side effects including coagulopathy, neurotoxicity, nephrotoxicity, and hemorrhage.
Such problems can be possibly resolved by spatiotemporally restricting drug delivery and action at therapeutically effective doses at the target vascular pathology site. An excellent example for such “targeted” drug action is represented by the design and applications of drug eluting stents (DESs), where polymer coatings applied on the stent metal frame act as a matrix for locally sustained release of anti-inflammatory drugs such as paclitaxel and sirolimus to prevent restenosis of stented blood vessel.9–11 However, interventional procedures like angioplasty and stenting are expensive, necessitate specialized expertise in terms of personnel and facilities, and may not be applicable or accessible to many patients in various vascular disease scenarios or within required treatment windows.12–15 Therefore, pharmacotherapeutic approaches that can be administered relatively easily, and can localize and enhance drug availability and activity at the vascular disease site, can provide significant advantages in treating many disease scenarios. This is where “nanomedicine”-based approaches present significant promise.16 Although the concept of manipulating matter at very small scale was put forward by Richard Feynman in the 1950s,17 the term “nanotechnology” was actually coined by NorioTaniguchi in the 1970s18 to describe the manipulation of materialsat the nano(10−9) scale, and buildingonthat theterm “nanomedicine” was put forward by Eric Drexler and Robert Freitas Jr. in the 1990s to signify the application of nanotechnology in medicine.19,20 Within this field, the technologies and applications in treating vascular pathologies predominantly involve either direct bioconjugation or chemical modification of drug molecules to enhance their disease site-specific availability and activity,21–25 or the utilization of particulate delivery platforms (i.e., nanoparticles and microparticles) to encapsulate the drugs for disease site-directed delivery via passive and active mechanisms.26 To elucidate and review these approaches, it is first necessary to provide a summary description of the cellular and molecular components of vascular disease pathology. To this end, the next section will describe the major cellular and molecular players in the thromboinflammatory niche of major vascular diseases. Building on that, subsequent sections will describe various designs and experimental findings regarding nanomedicine systems directed at treating these pathological conditions, together with a discussion of opportunities and challenges. For convenience of the readers, Table 1 provides acronyms, abbreviations, and corresponding full names of molecules, motifs, cells, materials, and terminologies used throughout the descriptions.
Table 1.
Alphabetical list of acronyms/abbreviations and corresponding full name of molecules, entities, and terminologies used throughout the article
| Abbreviation/Acronym | Corresponding full name |
|---|---|
| AGD | Alanine-glycine-aspartic acid |
| ALI | Acute lung injury |
| CAM | Cell adhesion molecule |
| CCR | CC-chemokine receptor |
| CGNKRTRGC | Cysteine-cysteine-asparagine-lysine-arginine-threonine-arginine-glycine-cysteine (Lyp-1) |
| CLIO | Cross-linked iron oxide |
| CQQHHLGGAKQAGDV | Cysteine-glutamine-glutamine histidi ne-histidine-leucine-glycine-glycine-alanine-lysine-glutamine-alanine-glycine-aspartic acid-valine |
| CREKA | Cysteine-arginine-glutamic acid-lysine-alanine |
| DES | Drug-eluting stent |
| DNA | Deoxyribonucleic acid |
| DVT | Deep vein thrombosis |
| EC | Endothelial cell |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| FeCl3 | Ferric chloride |
| Fg | Fibrinogen |
| Gd | Gadolinium |
| GP | Glycoprotein |
| GFPRGFPAGGC | Glycine-phenylalanine-proline-argi-nine-glycine-phenylalanine-proline-alanine-glycine-glycine-cysteine |
| HDL | High-density lipoprotein |
| HIS | Poly L-histidine |
| ICAM | Intracellular cell adhesion molecule |
| IFN | Interferon |
| IL | Interleukin |
| ITDGEATDSG | Isoleucine-threonine-aspartic acid-glycine-glutamic acid-alanine-threonine-aspartic acid-serine-glycine |
| Kd | Dissociation constant |
| KZWXLPX | Lysine-hydrophobic amino-acid-tryptophan-any amino acid-leucine-proline-any amino acid |
| LDL | Low-density lipoprotein |
| LMWH | Low-molecular-weight heparin |
| MCP | Monocyte chemoattractant protein |
| MION | Monocrystalline iron oxide nanoparticles |
| MMP | Matrix metalloproteinase |
| MRI | Magnetic resonance imaging |
| MSR | Macrophage scavenger receptor |
| NET | Neutrophil extracellular traps |
| NIR | Near infrared |
| NK | Nattokinase |
| NNQKIVNLKEKVAQLEA | Asparagine-asparagine-glutamine-lysine-isoleucine-valine-asparagine-leucine-lysine-glutamic acid-lysine-valine-alanine-glutamine-leucine-glutamic acid-alanine |
| NNSKSHT | Arginine-arginine-serine-lysine-serine-histidine-threonine |
| NO | Nitric oxide |
| NP | Nanoparticle |
| PAD | Peptidyl arginine deaminase |
| PDGF | Platelet-derived growth factor |
| PDPA | Poly(2-diisopropylaminoethyl methacrylate |
| PE | Pulmonary embolism |
| PECAM | Platelet-endothelial cell adhesion molecule |
| PEG | Polyethylene glycol |
| PET | Positron emission tomography |
| PFC | Perfluorocarbon |
| PGA | Polyglutamic acid |
| PLGA | Poly-lactic-co-glycolic acid |
| PLGD | Polyglycerol dendrimer |
| PLL | Poly(L-lysine) |
| PLL-PLA | Poly-L-lysine-co-poly-lactic acid |
| PS | Phosphatidyl serine |
| PSAPEG | Poly(sebacic acid)-co-PEG |
| PSGL | P-selectin glycoprotein ligand |
| PVA | Polyvinyl alcohol |
| QD | Quantum dot |
| RANTES | Regulated upon activation normal T cell expressed and secreted |
| RBC | Red blood cell |
| RGD | Arginine-glycine-aspartic acid |
| RNA | Ribonucleic acid |
| SAK | Staphylokinase |
| scFv | Single-chain variable fragment |
| scuPA | Single-chain urokinase-type plasminogen activator |
| SK | Streptokinase |
| SMC | Smooth muscle cells |
| SPIO | Superparamagnetic iron oxide |
| TGF | Transforming growth factor |
| TLTYTWS | Threonine-leucine-threonine-tyrosine-threonine-tryptophan-serine |
| TM | Thrombomodulin |
| tPA | Tissue plasminogen activator |
| TFPI | Tissue factor pathway inhibitor |
| UK | Urokinase |
| uPA | Urokinase-type plasminogen activator |
| VCAM | Vascular cell adhesion molecule |
| VHPKQHR | Valine-histidine-proline-lysine-glutamine-histidine-arginine |
| VHSPNKK | Valine-histidine-serine-proline-arginine-arginine-lysine-lysine |
| vWF | von Willebrand factor |
| WBC | White blood cell |
Major Cellular and Molecular Components in Vascular Disease
Blood is a fluid connective tissue composed of cellular components, namely, red blood cells (RBCs), white blood cells (WBCs), and platelets suspended in an aqueous plasma phase rich in various proteins (predominantly albumin, immunoglobulins, and fibrinogen), coagulation factors, salts, and ions. In the circulatory system, blood flows through blood vessels, namely, arteries, veins, and capillaries, whose luminal wall is lined by endothelial cells (ECs) sitting on a subendothelial matrix composed predominantly of collagen (types I, III, and IV).27,28 Healthy ECs present a dense covering of carbohydrate-rich brush-like polymers on their luminal (i.e., blood exposed) surface, termed the “glycocalyx,” which renders important thromboresistant properties by virtue of sterically hindering protein adsorption and platelet adhesion.29,30 Furthermore, the glycocalyx allows binding of several anticoagulant molecules such as antithrombin, heparin cofactor II, thrombomodulin (TM), and tissue factor pathway inhibitor (TFPI), all of which play unique roles in downregulating various outputs of the coagulation process. These important protective functions of the endothelial lining (with its glycocalyx), cumulatively, prevent clotting of blood at the vessel wall (i.e., thrombus formation) and thus prevent vessel occlusion. However, various vascular disease scenarios can injure and denude this endothelial lining, and this can lead to prothrombotic mechanisms.
One prime example is atherosclerosis, which is an inflammatory vascular disease of arteries where increased concentration of low-density lipoprotein (LDL) cholesterol in plasma and dysregulated cholesterol metabolism can injure and inflame the luminal endothelium and induces expression of several leukocyte adhesion molecules like P-selectin, E-selectin, intercellular cell adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and integrin αLβ2.31–33 Leukocytes, predominantly monocytes and neutrophils, do not bind to healthy endothelium but can bind to diseased ECs via these adhesion proteins, which then primes ECs for further active inflammatory mechanisms like transcytosis into subendothelial intima, transformation of monocytes to macrophages, macrophage, neutrophil, and endothelial secretion of various inflammatory chemokines and cytokines. Leukocyte adhesion, rolling, and transmigration into intima are also mediated by mechanisms involving CC-chemokine receptor 1 (CCR1), CCR2, CCR5, and CXC-chemokine receptor 2 (CXCR2). Transcytosed lesional macrophages can ingest modified LDLs via scavenger receptors and transform over time into lipid-rich foam cells. Platelets, which are traditionally considered to be significant players in the advanced thrombotic events associated with later stages of atherosclerosis (e.g., hemorrhagic, eroded, or ruptured plaques), are now also implicated in several biomolecular events during the initial stages of atherosclerotic lesion development and progression.34–38 For example, diseased and injured endothelium can secrete von Willebrand factor (VWF) from their Weibel-Palade bodies and platelets can bind to this locally secreted and deposited VWF via the glycoprotein Ibα (GPIbα) component of the platelet surface receptor complex GPIb–IX–V.37,38 Thus, endothelial VWF as well as P-selectin can promote platelet tethering and rolling via interaction with platelet GPIbα and P-selectin glycoprotein ligand-1 (PSGL-1).36–40 Platelets can also mediate the binding of leukocytes to diseased endothelium and thus the activated platelet–endothelium–leukocyte triad forms the major inflammatory phenotype in this disease. Activated integrin αIIbβ3, present on the platelet surface at high density (50,000–80,000 per cell), assumes the ligand-binding form upon platelet activation and binds its natural ligand fibrinogen (Fg), as mediated by molecular interactions with arginine–glycine–aspartic acid (RGD) and AGD-based peptide sequences.41–45 Integrin αVβ3 is principally a fibronectin/vitronectin receptor that helps extracellular matrix (ECM) anchorage and focal adhesion of ECs. However, in activated proinflammatory environment, αVβ3 and αIIbβ3 can interact with each other via RGD-mediated bridging by Fg to secure the attachment-activated platelets on inflamed endothelium at the atherosclerotic lesion site.46–48 These platelet–endothelium–leukocyte interactions as well as macrophagic ingestion of lipids result in a cascade of chronic inflammatory events. These involve recruitment, activation, and infiltration of more inflammatory cells, secretion of matrix degrading enzymes (e.g., matrix metalloproteinases or MMPs, and neutrophil elastase), degradation of collagenous matrix, and migration of smooth muscle cells (SMCs) and fibroblasts. Proinflammatory biomolecules like interleukin-1β (IL-1β), regulated upon activation normal T cell expressed and secreted (RANTES), monocyte chemoattractant protein-1 (MCP-1) and macrophage colony-stimulating factor (M-CSF) secreted by activated platelets, endothelium, and leukocytes facilitate inflammatory cell recruitment, lesion-associated cellular transformations, and plaque progression. Activated platelets also secrete PDGF (platelet-derived growth factor) and TGF-β (transforming growth factor-β) that stimulate endothelial and SMC migration, proliferation, new blood vessel formation, and matrix synthesis within the atherosclerotic lesion site. Matrix protein (e.g., collagen) secretion by SMCs and fibroblasts counterbalance matrix degradation, and subsequent development of a lipidic necrotic core in the subendothelial region covered by a fibrous collagenous cap. In vulnerable atherosclerotic lesions, matrix degradation is upregulated due to high MMP and elastase activity, while matrix production is downregulated (e.g., by signaling mechanisms of interferon gamma [IFN-γ] produced by T-cells). In the presence of stress and hemodynamic alterations, ruptures and fissures develop in such “high-risk” plaques causing intraplaque hemorrhage or exposure of the subendothelial collagen and necrotic lipidic core to flowing blood. This results in more platelet adhesion, activation, and aggregation via Fg-to-αIIbβ3 and P-selectin-to-PSGL-1 interactions, and the status transforms into a complex thrombotic (i.e., atherothrombosis) phase. The phosphatidyl serine (PS)-rich activated platelet surface allows colocalization and activation of coagulation factors (e.g., FIXa + FVIIIa in tenase complex, and FXa + FVa in prothrombinase complex) and cofactors (e.g., Ca2+ ion) from blood, promoting coagulation cascade stimulation and amplification, thereby leading to high levels of thrombin generation. The thrombin converts local Fg into fibrin which assembles and cross-links in presence of activated FXIIIa to form an occlusive clot that restricts blood flow.37,38,49,50 Such thrombo-occlusive events occurring in the coronary artery leads to myocardial infarction (i.e., heart attack) and in the carotid artery leads to stroke, which remain the two highest mortality factors in the global population.
While atherosclerosis and atherothrombosis occur in the relatively high shear regions of the arteries, another distinct mechanism with some overlapping cellular and molecular components occurs in the low shear regions of the veins. Especially near the valves in the vein, the low flow and eddies in circulation can lead to endothelial dysfunction, activation, and subsequent adhesion of platelets and leukocytes (predominantly neutrophils and monocytes). Here, the intercellular interactions can prime neutrophils to become activated and these active neutrophils (and also reportedly some macrophages) can decondense and secrete their DNA triggered by unique histone citrullination mechanisms involving intracellular myeloperoxidase, peptidyl arginine deaminase 4 (PAD-4), and elastase activities.51–55 These extracellularly secreted decondensed DNA (e.g., neutrophil extracellular traps or NETs) can arrest and activate more platelets, and trigger coagulation mechanisms to enhance thrombin generation and fibrin deposition.56–58 These thromboinflammatory mechanisms are characteristic in DVT and have also been recently implicated in the microvascular thrombosis in acute lung injury (ALI) as well as cancer-associated thrombosis. Venous thrombi can also dislodge, travel via circulation, and occlude distal vascular beds, for example, PE that occurs post-DVT or trauma and in cancer patients. Fig. 1 shows schematics of characteristic components of thrombotic and thromboinflammatory mechanisms typically seen in various vascular disease pathologies.
Fig. 1.
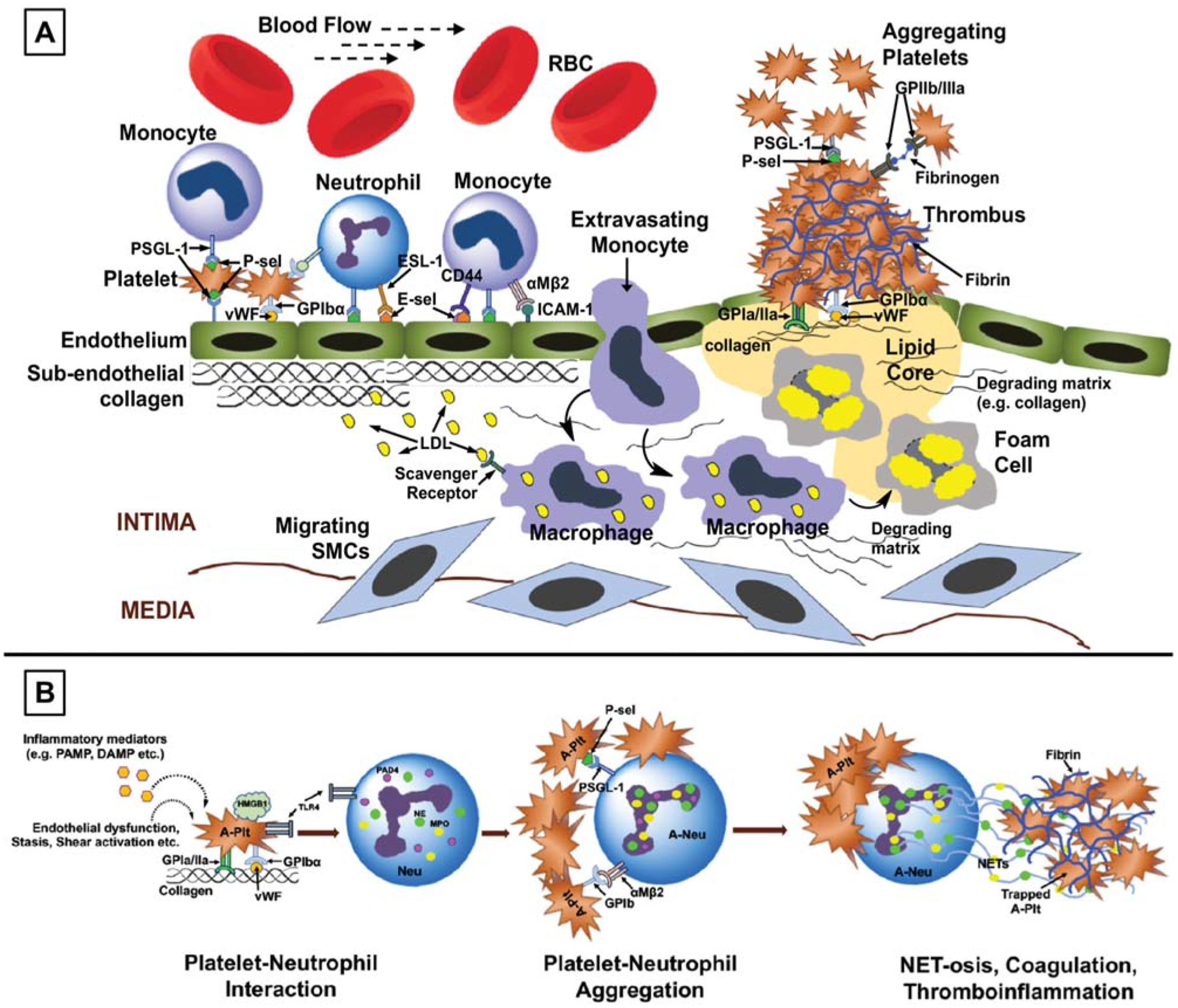
Schematic diagrams for cellular and molecular components in (A) atherosclerosis and atherothrombosis and (B) thromboinflammation (e.g., deep vein thrombosis, sepsis, and lung injury) pathologies in vascular diseases. RBC, red blood cell; P-sel, P-selectin; PSGL-1, P-selectin glycoprotein ligand 1; E-sel, E-selectin; vWF, von Willebrand factor; ICAM, intercellular adhesion molecule; LDL, low-density lipoprotein; SMC, smooth muscle cell; PAMP, pathogen-associated molecular patterns; DAMP, damage-associated molecular patterns; HMGB-1, high mobility group box 1 protein; A-plt, activated platelet; TLR 4, toll-like receptor 4; PAD 4, peptidylarginine deiminase 4; NE, neutrophil elastase; MPO, myeloperoxidase; Neu, neutrophil; NET, neutrophil extracellular trap.
Based on the earlier-described cellular and molecular components of vascular diseases, it becomes intuitively evident that therapeutic approaches that can downregulate or inhibit the proinflammatory and prothrombotic molecules on ECs, leukocytes, and platelets disrupt the various intercellular interactions, mitigate inflammatory signals, inhibit coagula-tory pathways, and rapidly degrade occlusive blood clots, and can have significant benefit in treating the various conditions. Thus, the drug molecules for such treatment span the categories of receptor inhibiting molecules and antibodies, gene therapy for receptor downregulation and signaling pathway inhibition, anticoagulant molecules, and fibrinolytic agents. In addition, with the purpose of detection and diagnosis of the pathologic conditions, different contrast agents and imaging probes can be directed toward the cellular and molecular entities. Therefore, nanomedicine strategies for these disease scenarios have focused on direct chemical modification as well as particle-based formulation of these drugs and imaging agents in various ways to enhance pharmacokinetic profile, favorable biodistribution, disease site-selective delivery, and site-localized function. The following sections will provide salient examples of such approaches and technologies.
Direct Modification of Therapeutic Agents
A potential issue for many drug molecules directly administered in the vascular compartment (e.g., via intravenous administration) is their nonspecific interaction with plasma proteins as well as specific interactions with inhibitors in circulating plasma, and rapid clearance through liver and kidney, all of which reduce drug circulation lifetime and bioavailability. One of the earliest strategies to reduce these effects was put forward by Helmut Ringsdorf during the 1970s, which involved conjugation of the drug molecule to high-molecular-weight water-soluble macromolecules that may form a protective shield around the drug molecule while keeping it in circulation.59–61 Furthermore, drug conjugation to the polymer can be mediated by chemical bonds (e.g., amide, orthoester, ester, anhydride, carbonate, and urethane) that can be cleaved by mechanisms such as enzymatic and/or pH-sensitive reactions to release active drugs for subsequent action. The “Ringsdorf model” for polymer-conjugated prodrug system has been attempted on several drug molecules in treatment of vascular diseases, especially fibrinolytic drugs and anticoagulant molecules. For example, PEG (polyethylene glycol)-based modification (PEGylation) of fibrinolytic agents such as tissue plasminogen activator (tPA), streptokinase (SK), urokinase (UK), and staphylokinase (SAK) has been reported.62–66 In some studies, it was found that the “protective” property of PEG-SK conjugates could be improved by increasing the molecular weight of PEG.63 PEGylated systems of truncated SK variants with promising in vitro fibrinolytic activity and enhanced in vivo circulation residence time have also been reported recently, although the in vivo fibrinolytic capacity of these systems were not reported.67 In preclinical ex vivo studies using beagles, PEG-conjugated urokinase was able to maintain fibrinolytic capacity for long periods of time (i.e., prolonged bioactivity in plasma), while free urokinase rapidly lost its activity in plasma due to inhibitor effects.64 Recombinant tPA has also been conjugated to PEG and the PEG-tPA product was reported to show partial enhancement of circulation lifetime and bioactivity compared with free tPA in canine models.62 The PEGylation strategy has also been reported for anticoagulant molecules. For example, PEG has been conjugated to the thrombin inhibitor hirudin and these PEG-hirudin systems have been evaluated in vitro, in vivo, and even in clinical studies in human patients. The studies showed that PEG-hirudin maintains its anticoagulant activity while providing a substantially longer circulation lifetime compared to unconjugated hirudin.68,69 In another interesting design, the anticoagulant anionic macromolecule heparin was conjugated to a polyarginine-modified cationic form of the fibrinolytic tPA, such that the tPA can be released in the presence of the cationic heparin antagonist protamine.70 The purpose of this design was to impart charge-induced drug release and the system was tested in vitro with promising result, but has not been reported in any in vivo evaluation. The antiproliferative drug paclitaxel (clinically used in DESs for mitigating restenosis and intimal hyperplasia) has been conjugated also to polymers such as polyglutamic acid (PGA) to result in products like Xyotax that have been studied for cancer treatment, but such designs also may find use in vascular antiproliferative therapies. Other direct chemical or structural modification of therapeutic molecules involve strategies to enhance targeting and selectivity of drug delivery (and action) at the disease site by recombinant modifications or conjugation of specific antibodies, antibody fragments, and peptide ligands. For example, the direct modification of tPA with anti-ICAM antibody to enable specific delivery of the drug to inflamed endothelium has been reported and this system demonstrated targeted fibrinolytic efficacy in vivo in lung embolism in mouse models.71 In a parallel design, tPA has been conjugated to RBCs to render long circulation time as well as incorporation into nascent clots for clot-specific fibrinolytic activity.72–74 Compared with free tPA, the RBC-tPA system has shown the ability to allow “prophylactic fibrinolysis” in vivo in mouse models of pulmonary thrombosis when administered before vascular injury, although it did not show efficient lytic ability when administered 10 minutes after vascular injury. In an approach to direct fibrinolytic drugs to platelet-rich thrombi, urokinase was modified with a monoclonal antibody 7E3 (abciximab) that binds to integrin αIIbβ3 on the surface of platelets.75 The urokinase-7E3 system showed significantly enhanced binding to αIIbβ3-coated substrates in vitro and enhanced plasmin generation on such substrates, and in ex vivo studies this system showed enhanced targeted fibrinolytic and antiplatelet ability at lower concentrations compared with free urokinase. A similar design rationale and experimental findings were reported where a SAK mutant was engineered to bear the tripeptide sequence of RGD that is known to have binding capability to many cell surface integrins, including platelet αIIbβ3.76 The SK-RGD system showed enhanced platelet targeting and fibrinolytic ability in vitro and also demonstrated the ability to render efficient clot lysis as well as reduced reocclusion incidents in vivo in porcine coronary artery balloon injury model.
In another approach, a recombinant system was constructed by fusing urokinase-type plasminogen activator (uPA) with single-chain variable fragment (scFv), an antibody to platelet–endothelial cell adhesion molecule (PECAM).77 Compared with unconjugated uPA, the anti-PECAM scFv-uPA showed enhanced ability to target cerebral arterial vasculature and lyse clots effectively in vivo in a mouse model, demonstrating its potential in treating cerebrovascular occlusions (e.g., in ischemic stroke). In a related study, the anti-PECAM scFv-uPA construct was further modified via recombinant techniques to include a thrombin-activatable domain, such that the anti-PECAM scFv could render targeted delivery to clot site endothelium while clot-localized high thrombin levels could cleave the uPA for clot site-specific action.78 This modified system demonstrated minimal effect on circulating fibrinogen and an enhanced targeted fibrinolytic affect in pulmonary reperfusion injury in mice. A similar recombinant fusion construct was recently developed with an scFv directed at platelet αIIb instead of PECAM, so as to target clot-associated platelets instead of endothelium.79 This novel construct, termed “PLT/uPA-T,” showed binding ability to both quiescent and active platelets as well as fibrinolytic ability in microfluidic assays in vitro. The PLT/uPA-T construct also showed the ability to lyse nascent clots in a thrombin-responsive manner but not preexisting clots in transgenic (for human GP αIIb) mouse models of ferric chloride (FeCl3)-induced carotid artery thrombosis in vivo, thus showing promise for thromboprophylaxis. In another scFv-based approach, a direct FXa inhibitor namely tick anticoagulant peptide (TAP) was conjugated to an scFv directed at fibrin (scFv59D8), and this construct showed targeted anticoagulant activity in human blood-based assays in vitro.80 In yet another approach, single-chain urokinase plasminogen activator (scuPA) was conjugated to an scFv directed toward platelet glycoprotein αIIbβ3, such that the scFv-scuPA system was able to prophylactically prevent carotid thrombosis in vivo in mice, in a targeted fashion without affecting the systemic hemostasis status (measured by tail-bleeding assays).81 In further refinement, platelet αIIbβ3-directed scFv was conjugated to a recombinant microplasminogen engineered to be activated by thrombin, such that thrombin-triggered release of platelet-targeted microplasminogen could enable local plasmin generation and clot lysis in mouse models of mesenteric thrombosis and PE.82
An alternate “clot-targeted triggerable release” design for fibrinolytic agent tPA was also recently reported, where tPA was camouflaged by albumin by two different strategies, one by conjugating anionically modified tPA (modified by low-molecular-weight heparin [LMWH]) with cationically modified albumin (modified by protamine) and the other by conjugating albumin to tPA via a thrombin-cleavable peptide linker sequence GFPRGFPAGGC.83 An additional component of this design was potential decoration of the albumin-based camouflage shell with clot-homing ligands (e.g., the platelet αIIbβ3 targeting peptide CQQHHLGGAKQAGDV derived from fibrinogen) such that upon homing to the clot, the shedding of the albumin camouflage shell could be rendered by heparin dosing (to reverse heparin–protamine association) or thrombin-triggered cleavage of the linker, leading to clot-localized release of tPA. In vitro, these systems have shown ability to bind to platelets and in vivo these systems have shown the ability to render fibrinolytic activity at levels equivalent to free tPA but with reduced systemic side-effects. In another recent approach, a prodrug nanoparticle comprising the antiplatelet and antithrombotic drug diosgenin conjugated directly to PEG was reported, where the prodrug nanoparticle was shown to be safe toward cell viability in vitro and to be capable of reducing thrombus burden in vivo in a cerebral artery thrombosis model in mice.84 Fig. 2 shows schematic representations of the various design approaches involving direct modification of vascular therapeutic molecules, discussed earlier. While these systems allow for longer circulation lifetimes and potential targeting of clot-relevant cellular and molecular components, such direct modification can also potentially pose issues of affecting the bioactivity and stability of the actual drug molecules. Also, transforming drug molecules into larger high-molecular-weight systems by modification with other proteins and polymers may sterically limit their diffusion and permeation capabilities in larger compact clots. An alternative strategy to protect the drugs in circulation, increase residence time and potentially achieve targeted delivery (and release) at the clot site, is to package these in micro- and nanoparticulate vehicles, as reviewed in the subsequent sections.
Fig. 2.
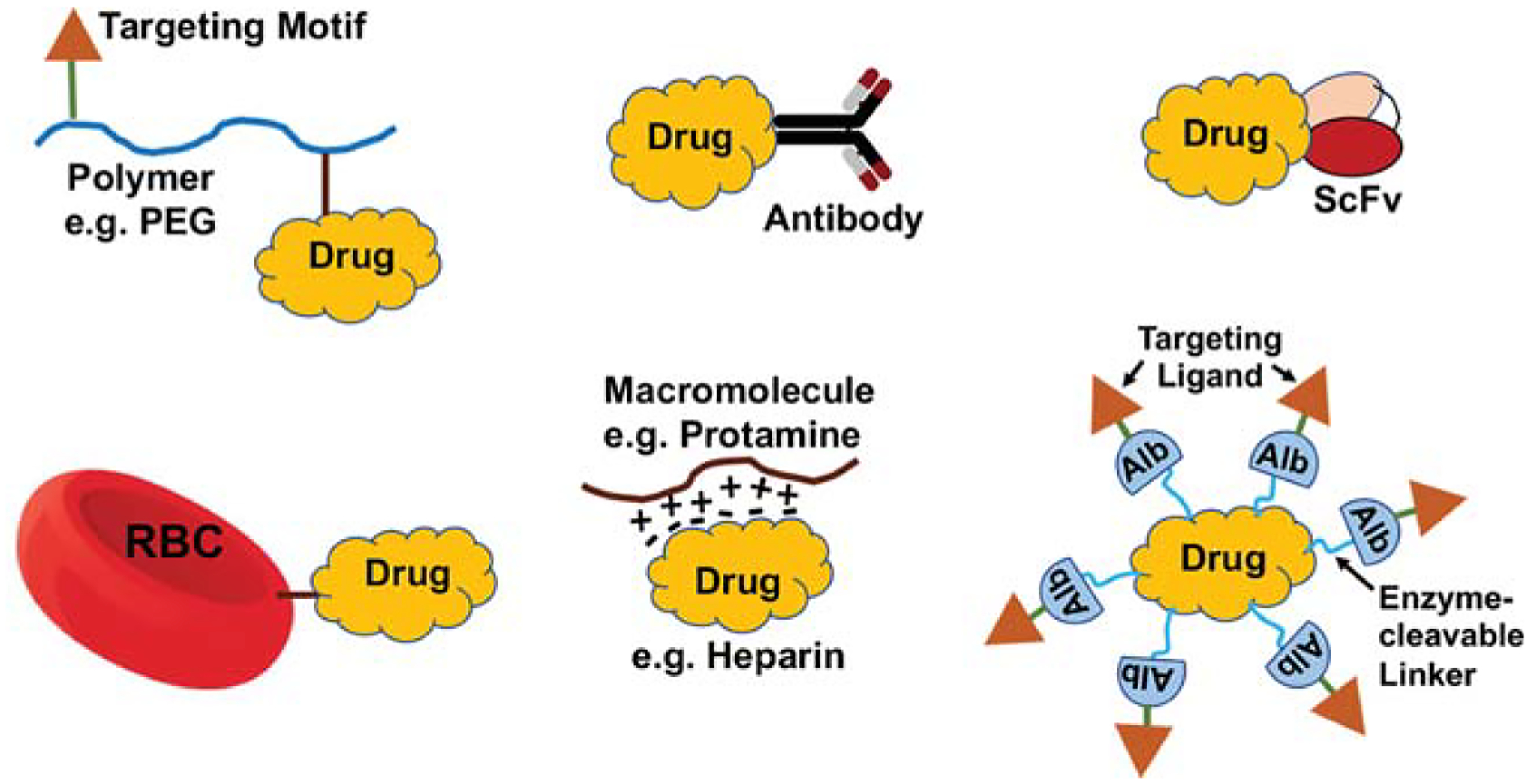
Schematic representations of various design approaches involving direct modification of therapeutic molecules in the development of vascular nanomedicine. PEG, polyethylene glycol; Alb, albumin.
Incorporation of Therapeutic Agents in Nanoparticles without Clot-Targeting Mechanisms
Due to the issues associated with direct systemic delivery of vascular therapeutic agents as well as potential challenges associated with direct drug modifications, significant research has been directed in the past two decades toward incorporation of the agents within microscale and nanoscale drug carrier systems. To this end, studies have extensively focused on using particulate drug carriers such as liposomes, polymeric particles, lipoprotein particles, micelles, quantum dots (QDs), gold particles, dendrimers, ultrasound-sensitive bubbles iron oxide particles, and nanogels (Fig. 3).
Fig. 3.
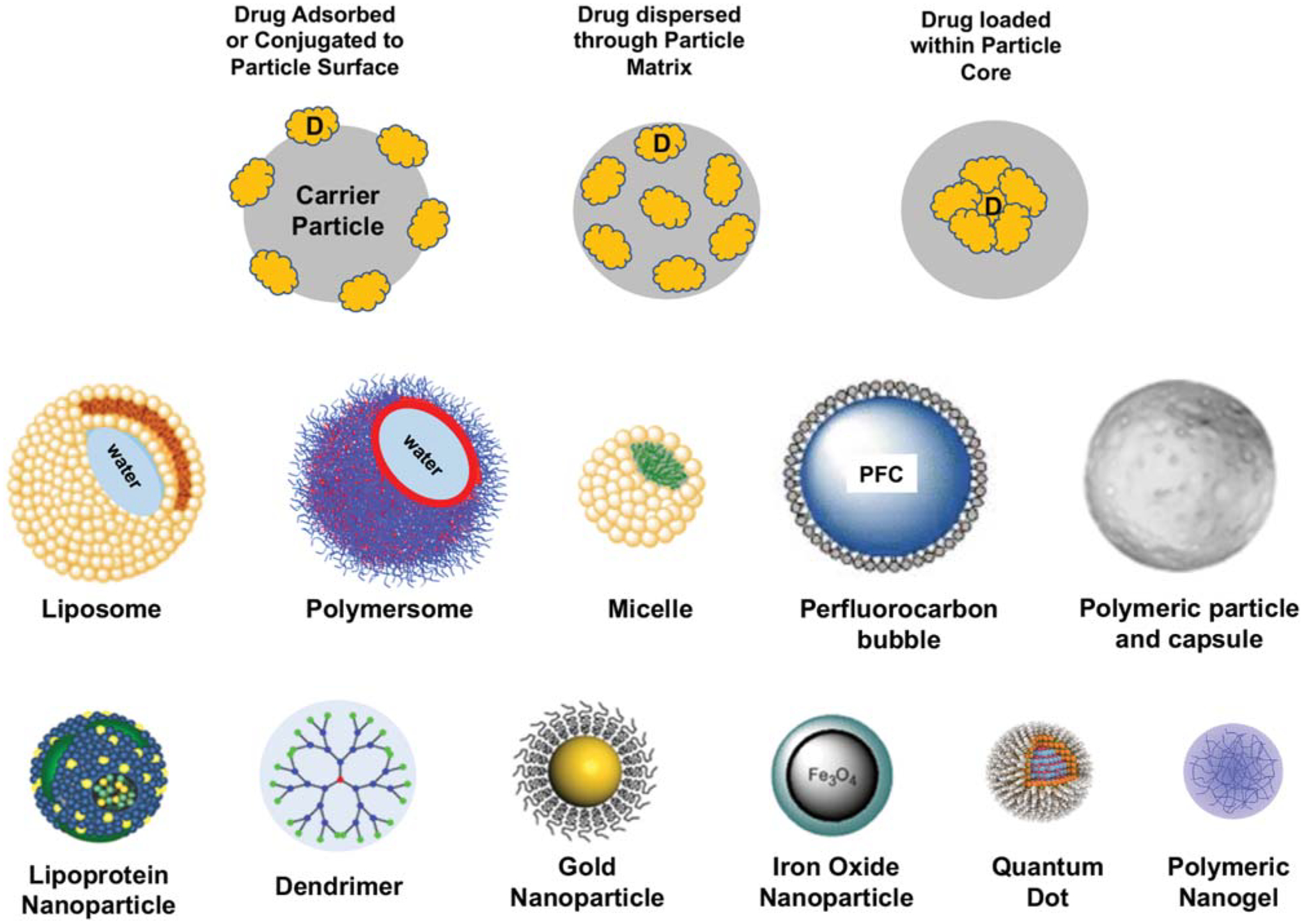
Schematic representations of various design approaches involving particle-based loading of drug (or imaging probes) in the development of vascular nanomedicine along with representative designs of characteristic nanoparticle systems used as particulate vehicles.
Liposomes are amphiphilic phospholipid-based vesicular structures originally reported by Sir Alec Bangham, where the phospholipids self-assemble to form particles with a lipidic (hydrophobic) shell and an aqueous core.85,86 These systems can technically encapsulate both lipophilic and hydrophilic drugs in their lipid membrane and aqueous core volume fractions, respectively. For amphiphilic phospholipid molecules, liposome formation is thermodynamically for a molecular packing fraction (v/al) of approximately 1, where “v” is the hydrophobic volume, “a” is the hydrophilic surface area, and “l” is the hydrophobic length. In an aqueous environment, the amphiphilic molecules tend to form planar lamellar bilayer assemblies that ultimately fold into spherical vesicles containing a lipidic shell and aqueous core.87 Such vesicle morphologies can have single lamellar shell (unilamellar) or multiple concentric shells (multilamellar). Liposome size ranges may vary from approximately 50 nm to a few microns in diameter. Large microscale multilamellar vesicles may be morphologically transformed into nanoscale (diameter of 50–200 nm) unilamellar vesicles by nano-extrusion or sonication. Moreover, modifications to the liposome outer surface using hydrophilic polymers such as PEG can impart steric hindrance to coronal adsorption of blood proteins (i.e., opsonization) and macrophagic uptake, thus rendering “stealth” properties that avoid rapid macrophagic clearance from circulation.88 This results in enhancing the circulatory lifetime of liposomes and their payload.89 This liposome approach has been clinically utilized in the formulation of cancer drugs such as Doxil, Daunosome, and Myocet, thereby making liposomes popular choices in examining drug delivery to other diseases including vascular.90 Accordingly, various vascular drugs, especially fibrinolytic agents, have been encapsulated in liposomes for in vivo delivery. The earliest studies with such formulations demonstrated enhanced circulation residence time and in vivo efficacy of the drugs, for example, of the fibrinolytic drugs SK and tPA, when encapsulated within liposomes compared with direct administration.91–93 Subsequent studies showed the ability of liposome-encapsulated SK formulations to improve therapeutic efficacy in vivo in rabbit and canine models of vascular occlusion.94–96 In related studies, Erdoğan et al demonstrated using radiolabeled SK in a rabbit jugular vein thrombosis model that lipid-based vesicular systems (liposomes, niosomes, and sphingosomes) are capable of encapsulating SK and making it bioavailable in thrombus at levels similar to free SK administration intravenously.97 During the late 1980s and 1990s, recombinant tPA was approved as the fibrinolytic drug of choice replacing SK in the United States and United Kingdom, due to relatively higher affinity of tPA for “fibrin” (i.e., clot) compared with “fibrinogen.” However, a significant challenge with tPA is its rapid inhibition by plasminogen activator inhibitor type 1 (PAI-1) present in plasma, leading to its circulation half-life being approximately 5 minutes.98 Consequently, liposomal encapsulation of tPA has been studied to enhance his circulation residence time.99 Studies with liposome-encapsulated tPA has shown that the drug can be encapsulated in PEGylated liposomes at an entrapment efficiency of approximately 20 to 40%, its circulation lifetime can be increased by four- to fivefold compared with free tPA (tested in vivo), and tPA released from such liposomes can render effective fibrinolysis in vitro and in vivo. Liposomes have also been assessed for encapsulation and delivery for other vascular therapeutics (e.g., anticoagulants), DNA/RNA, and molecular imaging agents. For example, LMWH has been incorporated in cationically charged liposomes for delivery via oral, subcutaneous, and inhalation route to explore enhancement of bioavailability of the drug.100 In another interesting approach, LMWH-loaded liposomes were immobilized on stents to reduce the risk of in-stent thrombosis.101 Liposomes have also been used to deliver DNA and siRNA in preclinical treatment evaluation in vascular diseases,102–107 although most of these approaches use liposome surface decorated with targeting fusigenic or cell penetrating peptides, as described in the next section. Besides fibrinolytic drugs, anticoagulants and gene therapy agents, liposomes have also utilized for imaging of vascular diseases by encapsulating imaging contrast agents such as the MRI probe gadolinium (Gd), by either direct loading of Gd salts or by lipid conjugation of Gd chelates.108–110 Analogous to amphiphilic lipid-based liposomal systems, amphiphilic block copolymer-based vesicular systems (polymersomes)111–113 can also be used to encapsulate and deliver vascular therapeutics, although the limited investigations in this field have utilized “active targeting” of polymersomes, as described later in this article.
While amphiphilic lipids and block copolymer molecules with packing parameter closer to 1 (i.e., cylindrical shape) form vesicles (i.e., liposomes and polymersomes), those with packing parameter less than 0.5 tend to form compact micelles with a hydrophobic core and a hydrophilic shell. Micellar structures have been researched for encapsulation and delivery of hydrophobic cancer drugs, but their study in vascular drug delivery has been limited. For example, PEG-polycation micelles have been studied for gene delivery applications to atherosclerotic lesions using rabbit models.114 Strategies based on micelles, directed at diseased or dysregulated endothelial components of sites of atherosclerosis and thrombosis, have recently been described.115,116 Majority of such strategies require micelle surface modification involving ligand-based active targeting, which is discussed in the subsequent section. Among other encapsulating particle systems, polymer-based solid micro- and nanoparticles have remained important components in vascular drug delivery applications.12,13,117,118 Using various phase segregation techniques, polymer-based solid nanoparticle carriers have been manufactured from biocompatible and biodegradable polymers such as poly-lactic-co-glycolic acid (PLGA), PEG, and polyvinyl alcohol (PVA) and these particle systems have been studied for encapsulation and delivery of fibrinolytic agents like SK and tPA, anticoagulant molecules like heparin, and antiproliferative agents like probucol, rapamycin, and paclitaxel that areknown to reduce restenosis, with promising outcomes in preclinical models and limited clinical studies.94,95,119–129 Ultrasound-sensitive bubbles (e.g., those made of perfluorocarbon encapsulated within a lipidic or polymeric shell) are approved in the clinic for cardiac imaging.130,131 Similar microbubbles have been studied for combined vascular therapeutic and diagnostic (i.e., theranostic) applications, where the drug-loaded bubble can be tracked by ultrasound image guidance and then destabilized by focused ultrasound for site-selective drug release. For example, PVA-based bubbles have been loaded with the gas nitric oxide (NO) that has vasodilatory and thrombo-resistant functions, for image-guided NO delivery to diseased vasculature.131 Similar bubbles have also been described for delivery of DNA, double-stranded RNA and oligonucleotides, recombinant proteins, growth factors, and fibrinolytic agents.132–136 Specifically, for fibrinolytic strategies, ultrasound-induced mechanical disruption of clots combined with ultrasound-triggered fibrinolytic delivery has led to strategies of “sonothrombolysis.” Another important class of nanostructures that have been extensively studied for drug delivery, including delivery of vascular therapeutics, is “dendrimers” which are highly branched polymeric nanostructures that may be synthesized by “convergent” or “divergent” chemical techniques.137,138 A payload of genes, drugs, and imaging agents can be loaded in the dendrimer core, the branching zone as well as in the branch extremities.139 In an early study, the fibrinolytic SK was bioconjugated to polyglycerol dendrimer (PGLD), and these PGLD-SK systems could be immobilized in the wells of ELISA plates via amide conjugation.140 These immobilized PGLD-SK assays showed fibrinolytic activity in vitro with blood samples but were not reported in any in vivo evaluation. Recently, a poly(L-lysine)-based dendrimer system has been reported that can be loaded with the fibrinolytic drug nattokinase (NK) and these NK/PLL nanocomposite particles were shown to have safety toward hemolysis and cell viability, with conserved enzymatic activity in vitro.141 In related studies, the same researchers have also reported on incorporating NK in dendrimers made from PEGylated polyglutamic acid, and this dendrimer system has shown low toxicity and excellent thrombolytic ability in vitro and in vivo.142 Besides these, dendrimers also have been used for ligand-mediated active targeting in vascular drug delivery, as examined in the subsequent section.
Besides lipid- and polymer-based nanoparticle systems, inorganic nanoparticles, especially gold (Au) and iron oxide systems, have been studied in drug delivery and imaging in vascular diseases extensively.143–148 Colloidal solid gold nanoparticles can be synthesized by reduction of chloroauric acid in the presence of a stabilizing agent, but also hollow gold nanostructures fabricated by galvanic replacement techniques have been extensively studied as carrier vehicles for drug delivery and imaging, and for near infrared (NIR) wavelength-triggered plasmonic properties that allow photothermally induced drug delivery.149–153 Gold nanoparticles have been reported for cell-specific imaging, and for image-guided targeted drug delivery in cardiovascular diseases.154,155 For example, particles having Au core coated with Apolipoprotein A-1 and phospholipids were shown to be internalized by atherosclerosis-relevant macrophages in ApoE −/− mice, thereby enabling enhanced molecular imaging of lesion-associated macrophage burden, extent of calcification, and stenosis.156 Au nanoparticles were surface-modified by a peptide sequence that can be specifically degraded by MMPs and with an NIR fluorescence dye Cy5.5 in another study, to be used as a molecular imaging probe at sites of high MMP activity in atherosclerotic lesions.157 Au nanoparticles can also enable photothermal ablation properties, and this has been utilized in recanalization of atherosclerotic plaques in coronary arteries in human postmortem ex vivo specimens.158 Superparamagnetic iron oxide (SPIO) nanoparticles are categorized based on their hydrodynamic diameter, for example, oral-SPIO (300 nm–3.5 μm), standard-SPIO (SSPIO, 60–150 nm), ultrasmall-SPIO (USPIO, 5–40 nm), and monocrystalline iron oxide NPs (MION).159 Moreover, MIONs containing a chemically cross-linked polysaccharide (e.g., dextran) shell are designated cross-linked iron oxide (CLIO) particles.160,161 Various SPIO systems have been researched as contrast agents in MRI-based cellular and molecular imaging of vascular diseases.162–171 Drug loading of SPIO systems can lead to efficient theranostic constructs, as demonstrated for the delivery of various antithrombotic and anticoagulant agents.172,173 Iron oxide nanoparticles have also been incorporated along with paclitaxel within PLGA particles to form drug-loaded magnetic constructs, which could be guided by an induced magnetic field for carotid artery site-directed triggered release of the antiproliferative drug.174 Other molecular imaging probes have also been combined with iron oxide nanoparticles to enable multimodal vascular imaging with enhanced resolution and sensitivity.175–178 QDs are another class of inorganic nanoparticles that have undergone substantial research in vascular imaging. QDs are semiconductor nanocrystals (e.g., particles with a cadmium selenide core with a zinc sulfide shell) whose fluorescent properties vary with size and composition, and they are sufficiently electron dense to also allow electron microscopy.179 The in vivo pharmacokinetics, biodistribution, and safety of QDs continueto be debated180–183; however, they have been studied in vascular drug delivery and imaging applications. As an example, QDs have been incorporated in high-density lipoprotein (HDL)-based plaque-targeting nanoparticles for imaging of atherosclerotic plaques.184 Similar HDL particles have also incorporated MRI contrast agents, and these were assessed for targeted MR imaging of atherosclerotic plaques.185–187 Ligand modification of QDs has also been assessed for active targeting to vascular disease sites, as discussed in the next section.
Incorporation of Therapeutic Agents in Nanoparticles with Active Clot-Targeting Mechanisms
Several nanoparticle systems described previously in the context of packaging of vascular therapeutics have also been studied to engineer “actively targeted” drug delivery systems where the nanoparticles can utilize specific molecular interactions to anchor at vascular disease sites. The molecular interactions can be mediated by antibodies and their fragments, proteins, peptides, etc., such that these can act as ligands to specific receptors that are exposed or expressed or upregulated at the site of vascular pathologies. Decoration of the nanoparticle surface with such ligands can be achieved by noncovalent or covalent bioconjugation techniques. The motivation for such molecular targeting is to enhance the specificity and selectivity of payload (drug) localization, and also in some cases utilize receptor-mediated internalization of drug-loaded nanoparticles within disease-specific cells.188,189 Regarding noncovalent strategies to modify nanoparticle surface, ligands are adsorbed mostly via physical interactions (e.g., hydrophobic, affinity-based, and charge-based) with the particle surface. For example, in one study, polystyrene particles were coated with antibodies directed at P-selectin and E-selectin using adsorbed bacterial protein A molecules as spacers.190 P- and E-selectins are upregulated on stimulated platelets, ECs, and monocytes in vascular inflammation and thrombosis, and therefore surface decoration of nanoparticles with antibodies directed at them can enable active targeting to such vascular disease sites. In another study, chitosan particles were coated with antiamyloid monoclonal antibodies to target amyloid β-protein deposits in cerebral vasculature of mice.191 Other nanoparticles, such as liposomes, latex beads, and albumin particles, have been surface-decorated noncovalently with recombinant GPIbα (rGPIbα) and rGPα2β1 to bind VWF and collagen, respectively, to allow potential targeting to vascular injury sites.192–194 Affinity-based interaction between avidin and biotin is another strategy that has been extensively studied for decorating nanoparticle surface in vascular targeting. Avidin (and Streptavidin), a glycosylated positively charged protein with high affinity toward biotin (dissociation constant Kd of 10−15 M),195,196 has been utilized to engineer ligand-decorated nanoparticles for drug delivery by incubating avidin-modified particles with biotinylated ligands, or vice versa. Using this approach, RBCs have been decorated with fibrinolytic molecules (e.g., tPA), and various nanoparticle systems have been decorated with antibodies targeted to various cell adhesion molecules (CAMs) in vascular disease.197 Contrary to such noncovalent techniques, covalent techniques for bioconjugation involve specific chemical reactions between ligands to appropriate reactive groups on the nanoparticles. Common bioconjugation reactions in this aspect are amide linkages (reaction between amine and carboxyl motifs), hydrazine linkages (reaction between hydrazide and aldehyde motifs), sulfhydryl-mediated linkages (reaction between sulfhydryl group and maleimide, sulfone, acetamide, or pyridyl groups), and alkyne-azide–based cycloaddition (“click”) reactions.198 Other unique bioconjugation techniques involve enzyme-catalyzed reactions, for example, Sortase A-catalyzed reaction with motifs bearing the peptide sequence LPETG199 and transglutaminase-catalyzed cross-linking of peptide motifs bearing lysine and glutamine residues.200 The noncovalent or covalent strategies can be used to conjugate ligands on preformed particles, or by pre-reacting to constituent macromolecules first followed by assembly of such molecules into nano- and microparticles.
Utilizing the various surface-modification techniques described earlier, several nanoparticle constructs have been described for vascular disease-specific delivery of therapeutics and imaging agents. For example, in one approach echogenic liposomes were surface-decorated with antibodies specific to fibrinogen, fibrin, and intercellular adhesion molecule-1 (ICAM-1), to permit ultrasound-induced cavitation for thrombolytic agent delivery (sonothrombolysis).201–203 In a similar approach, lipid-shell based gas-filled microbubbles were surface-decorated with scFv directed at platelet αIIbβ3 to enable clot-specific targeting and ultrasound-based imaging, and image-guided assessment of thrombolysis after urokinase administration.204 Liposomes have also been surface-modified with antibodies directed to LDL receptors LOX-1 for atherosclerotic lesion-targeted delivery of contrast agents in molecular imaging.205 In another liposomal technology, named LipoCardium, targeted delivery of anti-inflammatory prostaglandins to atherosclerotic sites was achieved using liposomes surface decorated with antibodies specific for VCAM-1.206 Besides decoration with antibodies, scFv fragments have also been utilized for active targeting of other nanoparticles to vascular disease sites. For example, elastin-like protein (ELP)-based micelles were surface decorated with platelet αIIbβ3-specific scFv and anticoagulant effector molecule TM using Sortase A-catalyzed bioconjugation, and the resultant scFv/TM-ELP micelles were capable of targeting platelet-rich thrombi in vivo to reduce thrombus growth via TM-mediated activation of protein C pathway of anticoagulation (FVa and FVIIIa inhibition).207 The Sortase A-catalyzed conjugation of platelet αIIbβ3-specific scFv was also reported for surface-decoration of ultra-small iron oxide nanoparticles for contrast-enhanced MRI of carotid artery thrombosis in mice.208 Similar scFv was also used to conjugate copper (64Cu) chelating cage amine systems for effective PET imaging of platelet-rich thrombi in vivo.209
Besides antibodies and antibody fragments, peptide ligands have also been used for surface-modification of drug delivery particles. For example, small peptides that can bind to VWF, collagen, integrin αIIbβ3, and P-selectin on stimulated platelets have been used to surface-modify liposomes, to allow targeting to sites of endothelial injury, endothelial denudation, platelet activation, and thrombosis in vascular pathologies.210–220 Therefore, these liposomal systems can be potentially utilized for spatio-temporally targeted drug delivery to sites of vascular disease and dysregulation. Besides liposomes, other lipidic and polymeric micelles have also been ligand-modified for vascularly targeted drug delivery. For example, micelles were surface-decorated with antibodies toward macrophage scavenger receptors (MSR) and loaded with Gd chelates or fluorescent probes to selectively target atherosclerotic lesions in ApoE −/− mice, for molecular imaging of the disease.221,222 In another approach, Gd-loaded PEG-lipid micelles were surface-decorated with antibodies directed at oxidized LDL lipoproteins in atherosclerotic plaques, for targeted molecular imaging of the plaque.223 Similar Gd-loaded micelles surface-decorated with anti-CD36 antibodies were able to target lesion-associated macrophages in plaques.224 In another report, lipid-polymer hybrid particles were decorated with a peptide sequence KZWXLPX (Z: hydrophobic amino acid, X: any amino acid) and these constructs termed “nanoburrs” were able to actively bind exposed collagen IV at arterial injury sites for delivery of antiproliferative drugs to modulate SMC activity.225,226 In yet another approach, a 9-amino acid sequence CGNKRTRGC (Lyp-1) was used to modify the surface of micelles to bind p32 receptors in atherosclerotic plaques, and CREKA peptide was similarly used to bind fibrin–fibronectin clots for enhanced targeting of atherosclerotic plaques in vivo.227,228
Antibody and peptide ligand decorations on solid polymeric particles as well on polymer capsules have also been studied for targeted therapeutic delivery to vascular pathologies. For example, anti-ICAM-1 antibodies were conjugated to PLGA nanoparticles for specific targeting of inflamed ECs in vascular disease.229,230 Anti-VCAM-1 antibodies were similarly used to modify the surface of microparticles and nanoparticles made of poly(sebacic acid)-co-PEG (PSAPEG) to enable atherosclerotic lesion-targeted delivery in ApoE −/− mice.231 Instead of antibodies, peptide ligands have also used for vascular targeting of nanoparticles. For example, surface decoration with a fibrinogen-derived peptide sequence NNQKIVNLKEKVAQLEA was utilized to target polystyrene particles to ICAM-1 in mouse models.232 Another ICAM-1-specific peptide sequence ITDGEATDSG (also known as LABL) has been reported for surface decoration of DNA complex nanoparticles and PLGA nanoparticles to demonstrate in vitro gene delivery to ICAM-1 expressing cells.233,234 MRI contrast-enhancing nanoparticles, such as iron oxide particles, monocrystalline magnetic particles, and Gd-containing systems, have been described that are surface-decorated with VCAM-binding peptide sequences like cyclic VHSPNKK, VHPKQHR, and cyclic NNSKSHT for molecular imaging of atherosclerotic lesions.235–237 In another approach, poly-L-lysine-co-poly-lactic acid copolymer (PLL-PLA) nanoparticles were surface-modified with RGD peptides to achieve co-localization with active platelets (via binding of platelet αIIbβ3) at the site of traumatic vascular injury and deliver anti-inflammatory drugs.238,239 Surface decoration with RGD or AGD peptides for vascular targeting has been reported also using RBCs, latex beads, or albumin particles as the drug carrier vehicle.240–245 HDL nanoparticles that are already amenable to natural uptake into atherosclerotic lesions due to lipoprotein transports have been further decorated with RGD ligands for active targeting to inflamed vascular sites.246 In the area of polymer capsules, recent work has described calcium carbonate (CaCO3)-templated submicron diameter polymer capsule structures made of poly(2-diisopropylaminoethyl methacrylate) (PDPA) and PEG, and stabilized with poly L-histidine (HIS), and these PDPA-HIS capsules were further surface-decorated with collagen IV targeting peptide TLTYTWS using “click” chemistry to enable targeting to exposed atherosclerotic plaque.247 Vascular targeting ligands have also been used for decorating ultrasound-sensitive micro- and nanobubbles to direct them toward cell-surface receptor moieties like CAMs (e.g., ICAM and VCAM) and integrins (e.g., αVβ3), to achieve targeted therapy and molecular imaging of vascular inflammation and atherosclerosis.248–250 In related work, similar bubbles with shells bearing maleimido-4(p-phenylbutyrate)-phospholipid were surface-conjugated using therapeutic antibody Abciximab (ReoPro by Eli Lilly) to target platelet integrin αIIbβ3 for enhanced molecular imaging of platelet-rich thrombi.251 Other nanoparticles like dendrimers have also been investigated for active targeting to vascular disease, for example, by decorating them with cyclic RGD peptides for targeting endothelial integrin αVβ3 and also loading with radioactive Bromine (76Br) for positron emission tomography (PET)-based targeted molecular imaging of ischemic tissue in mice.252 Several other ligands relevant to disease-specific biomarkers and cellular phenotypes have been utilized for active targeting of dendrimers to vascular pathologies.253–255
Among inorganic nanostructures for vascularly targeted delivery, cross-linked dextran-coated iron oxide (CLIO) nanoparticles have been surface-modified with peptides and ligands that can target CAMs and clot-associated fibrin to enable targeting to inflammatory, angiogenic, and thrombotic regions in atherosclerosis for contrast-enhanced MR imaging.161 Such particles have also been surface-decorated using ligands specific for VCAM-1, P-selectin, and platelet integrin αIIbβ3, for contrast-enhanced targeted MRI of atherosclerosis and thrombosis.256 Using another approach, SPIOs were surface-modified by Annexin V to enable specific interaction with anionic lipids (e.g., phosphatidylserine) on outer membrane leaflets of apoptotic cells and thus achieve selective targeting of such particles to “foam cells” in atheromatous plaque in rabbits for T2-weighted MRI.257 QDs have also been decorated with anti-CAM antibodies directed to VCAM, ICAM, PECAM, etc. for in vivo optical imaging of atherosclerotic lesions.258,259 Additional approaches for surface engineering of QDs for targeted optical imaging of vascular disease-specific markers comprise targeting to oxidized LDL receptor CD36, phosphatidylserine-exposing cells, and plaque-relevant MMPs.260,261 As an alternative to direct targeting of QDs to the vascular disease site markers, they have also been used as “payloads” within other nanoparticle systems to achieve multimodal imaging of vascular disease sites. For example, QDs were loaded within paramagnetic micelles targeted to macrophagic scavenger receptors and also within HDL nanoparticles, for combined optical and MR imaging of atherosclerosis.184,262 In another approach, gold nanoparticles were conjugated to QDs via a proteolytically degradable peptide sequence so that in the “conjugated” state the QD luminescence was quenched, whereas the luminescence was significantly enhanced once the conjugate was enzymatically cleaved.263 Such unique strategies can potentially allow probing of proteolytic enzyme activities that are typical of atherosclerotic lesions.
Current State-of-the-Art in Vascular Nanomedicine Systems and Future Opportunities
As evident from the technologies and applications described in the previous sections, the field of “nanotechnology and nanomedicine” has opened up unique ways to achieve disease site-directed delivery of various therapeutic and imaging agents as singular or combination payloads in vascular pathologies (Fig. 4). Such localized delivery may possibly overcome issues of bioavailability and narrow therapeutic window for many therapeutic molecules by enhancing disease-localized concentrations with lower dose overall, thereby maximizing treatment effects in target tissue while avoiding systemic off-target side-effects. Concepts around local delivery in the cardiovascular domain arose decades ago through successful utilization of perivascular delivery systems in animal models.264,265 For such applications, polymeric matrix systems loaded with heparin were placed around rat carotid arteries during balloon angioplasty, achieving sustained local release of drug for specific time periods and thereby reduce postprocedural arterial restenosis and occlusion. Similar polymeric matrices that incorporated ECs to provide a source of endogenous vasoregulatory molecules were reportedly capable of reducing neointimal hyperplasia in rat and pig models of vascular injury.266,267 During the last three decades, other “site-localized delivery” systems have been engineered for vascular therapies, including intraluminal, intramural, and stent-based devices, that have demonstrated high efficiency in rendering target site-localized therapeutic effect while reducing nonspecific distribution and off-target side-effects associated with systemic delivery.268 Nanotechnology has enabled further refinement of such devices. As an example, silver nanoparticles have been incorporated in implantable and intravascular devices to prevent bacterial adhesion, growth, and biofilm development.269 Catheters as well as stents have been modified with carbon nanotubes for mechanical enhancement and drug delivery functions.270,271 Furthermore, stent designs have been refined with nanoscale fabrication and texturing techniques to allow enhanced drug loading, favorable biointeractions with tissues, and programmed drug release. Stents have also been modified with coatings containing drug-loaded nanoparticles for sustained drug delivery following endovascular procedures.272–274 Nanomaterials are also being explored in fabrication of artificial vascular grafts and conduits to impart thromboresistant and infection-resistant properties.275,276
Fig. 4.
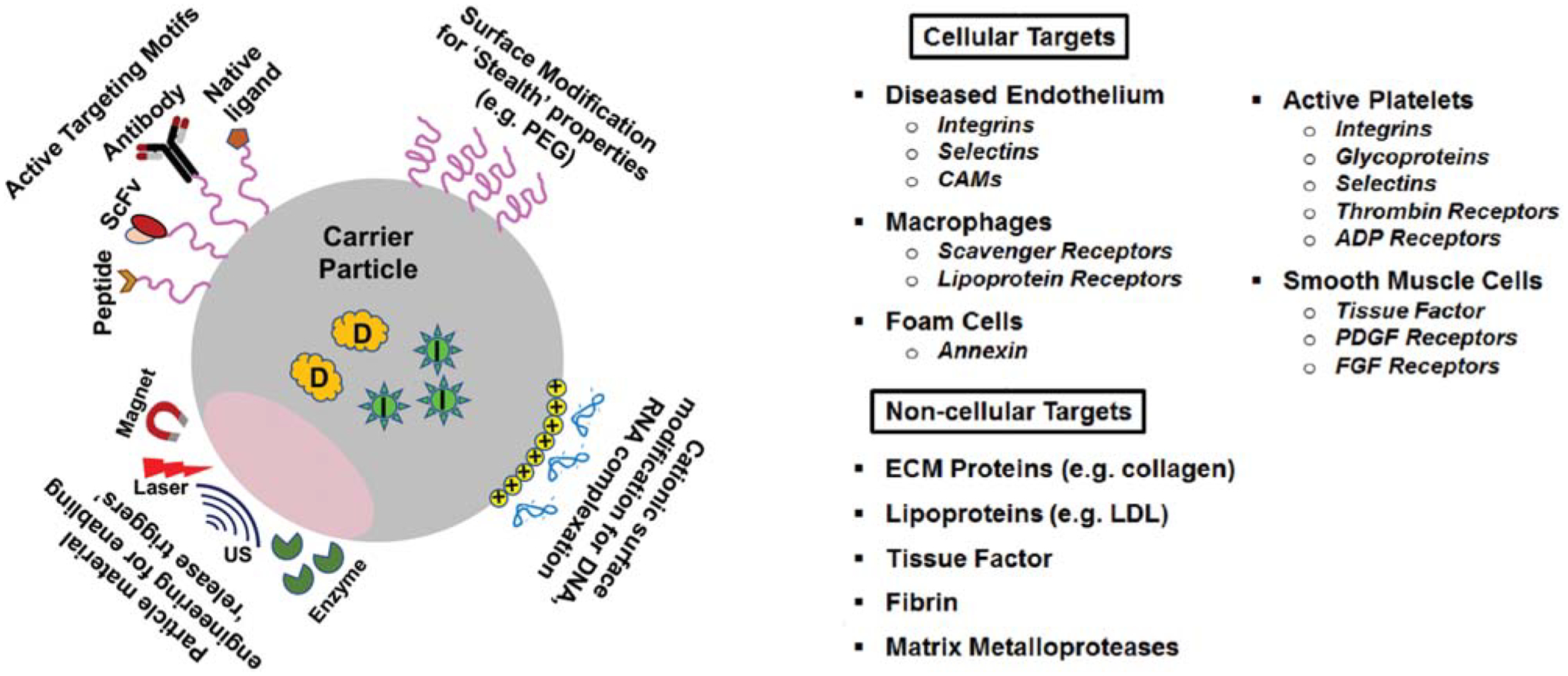
Schematic representations of various design approaches in engineering of nanoparticles for enabling surface “stealth” properties, active targeting to vascular disease-relevant proteins and cells, loading of charged payload (e.g., DNA, RNA) and stimuli-triggered release mechanisms, along with list of cellular and noncellular targets that such engineered nanoparticles have been directed to. US, ultrasound; ADP, adenosine diphosphate; CAM, cell adhesion molecule; PDGF, platelet-derived growth factor; FGF, fibroblast growth factor.
In the context of nanoparticle and microparticle systems for vascular drug delivery, recent studies have emphasized the emerging role of “biophysical” parameters such as shape, size, and elasticity in refining the design and enhancing the intravascular performance of the particles. For example, several recent computational and experimental studies have established that particle geometry plays a significant role in their distribution in the vascular compartment under the hemodynamic flow environment. These evaluations have identified that size of particles play important roles in their margination capabilities through the RBC volume as well as in their interaction with the target surface at the vascular wall. Computational and experimental studies have indicated that micro-scale particles have a higher margination probability through the flowing RBC volume compared with nanoscale particles.277–281 Related studies have also indicated that particles of nonspherical geometry (e.g., ellipsoids, rods, and discs) have a higher probability of margination from flowing blood RBC volume toward the vascular wall and higher surface area of interaction at the wall that can benefit stable binding and retention for achieving enhanced wall-localized drug delivery under hemodynamic flow environment.282–291 Additionally, particle stiffness (i.e., elasticity) has been shown to affect their margination and adhesion behavior in flow.281,292–294 In fact, an optimal example of this is seen in naturewherebi-concavelargesize (~8 μm diameter) highly flexible RBCs tend to congregate toward the center of the flow volume while biconvex smaller (~2 μm diameter) stiffer platelets tend to marginate more toward the blood vessel wall.295–299 Building on these findings, in recent years, unique designs and manufacturing approaches have focused on development of nano- and microparticles with tailored geometries and stiffness to customize their transport, interaction, circulation residence time, and targeting properties within the vascular compartment.300–310 Such biophysical parameters can potentially be combined with ligand-based active targeting strategies on particle platforms to construct drug delivery systems possessing enhanced capabilities of targeting, anchorage, and site-specific delivery. Furthermore, in the context of active targeting of drug delivery particles to vascular disease sites, several studies have demonstrated the benefits of “heteromultivalent ligand modification” of the particles where ligand motifs directed at multiple types of targets are codecorated on the particle surface to enhance selectivity and anchorage strength under hemodynamic flow environment.213,219,306,311 Such findings continue to present exciting opportunities to further explore the interplay between particle biophysical and biochemical factors, blood flow environment, and vascular topology to fine tune drug delivery particle design toward specific vascular nanomedicine applications.312,313
While the majority of particulate delivery systems achieve release of encapsulated payload via diffusion and degradation/dissolution-mediated mechanisms,314,315 robust research has also been focused on incorporating unique “triggered release” mechanisms in various drug delivery systems. In such designs, drug release is achieved by chemical and/or physical triggers of particle destabilization in the disease-specific environment. Such triggering can be from internal stimuli such as pH, enzyme action, temperature, and shear forces or external stimuli such as focused ultrasound, NIR irradiation, and magnetic impulses.135,136,201,202,219,316–328 These unique “triggered release” approaches continue to add exciting refinements and attributes in the area of vascular nanomedicine, which can be leveraged to enhance delivery efficiency and release kinetics and therapeutic effects site selectively in various vascular pathologies.
Conclusion
Nanotechnology provides an efficient way to achieve improved delivery of drug molecules and imaging agents at sites of vascular disease, via packaging within micro- and nanoparticle vehicles that can be administered in circulation and can traverse through various transport and interaction barriers in blood flow to passively accumulate or actively anchor at target vascular sites. The success of these approaches depends on optimization of a variety of factors, including effective encapsulation of the payload within the particles, minimization of pre-target release of the payload, maintenance of sufficient circulation time periods to allow interaction with target cells and tissues, efficient margination (if needed) through flowing blood volume toward vascular wall, passive accumulation or active binding at the target site under hemodynamic conditions, efficient payload release via diffusion or site-specific triggers, and safe elimination of the vehicle (or its components) via metabolism, biodegradation, and excretion. As evident from the comprehensive descriptions in previous sections, meeting all these criteria optimally is quite challenging. At the same time, the ability to refine biophysical and biochemical design parameters as well as appropriate animal models and new and improved analysis techniques have allowed significant advancement toward optimization of vascular nanomedicine systems. Such advancements may allow design of vascular nanomedicine to customize toward specific disease treatment requirements, instead of using universal design criteria. For efficient clinical translation, vascular nanomedicine research should also focus on robust analysis of cost–benefit ratios, quality control, and regulatory hurdles for these technologies.
Footnotes
Conflicts of interest
Dr. Sen Gupta has a patent US 9107845 licensed to Haima Therapeutics, and a patent US 9107963 issued. Dr. Sen Gupta has no other conflicts of interest, relevant financial activities, and relationships.
References
- 1.Heart Disease Facts. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/heartdisease/facts.htm. Accessed November 2, 2018 [Google Scholar]
- 2.Cardiovascular Disease. World Health Organization. Available at: http://www.who.int/cardiovascular_diseases/en/. Accessed November 2, 2018 [Google Scholar]
- 3.Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation 2018;137(12):e67–e492 [DOI] [PubMed] [Google Scholar]
- 4.Phillips DR, Conley PB, Sinha U, Andre P. Therapeutic approaches in arterial thrombosis. J Thromb Haemost 2005;3(08):1577–1589 [DOI] [PubMed] [Google Scholar]
- 5.Gurbel PA, Serebruany VL. Oral platelet IIb/IIIa inhibitors: from attractive theory to clinical failures. J Thromb Thrombolysis 2000;10(03):217–220 [DOI] [PubMed] [Google Scholar]
- 6.Marzilli M. From the experimental myocardial infarction to the clinical acute myocardial infarction: limitations of thrombolytic therapy. Int J Cardiol 1995;49(Suppl):S71–S75 [DOI] [PubMed] [Google Scholar]
- 7.Greineder CF, Howard MD, Carnemolla R, Cines DB, Muzykantov VR. Advanced drug delivery systems for antithrombotic agents. Blood 2013;122(09):1565–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebeiz AG, Granger CB, Simoons ML. Incidence and management of complications of fibrinolytic, antiplatelet, and anticoagulant therapy. Fundamental and Clinical Cardiology 2005;52:375–395 [Google Scholar]
- 9.Wessely R. New drug-eluting stent concepts. Nat Rev Cardiol 2010;7(04):194–203 [DOI] [PubMed] [Google Scholar]
- 10.Puranik AS, Dawson ER, Peppas NA. Recent advances in drug eluting stents. Int J Pharm 2013;441(1–2):665–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefanini GG, Holmes DR Jr. Drug-eluting coronary-artery stents. N Engl J Med 2013;368(03):254–265 [DOI] [PubMed] [Google Scholar]
- 12.Brieger D, Topol E. Local drug delivery systems and prevention of restenosis. Cardiovasc Res 1997;35(03):405–413 [DOI] [PubMed] [Google Scholar]
- 13.Eccleston DS, Lincoff AM. Catheter-based drug delivery for rest-enosis. Adv Drug Deliv Rev 1997;24:31–43 [Google Scholar]
- 14.Fattori R, Piva T. Drug-eluting stents in vascular intervention. Lancet 2003;361(9353):247–249 [DOI] [PubMed] [Google Scholar]
- 15.Torchilin VP. Targeting of drugs and drug carriers within the cardiovascular system. Adv Drug Deliv Rev 1995;17:75–101 [Google Scholar]
- 16.Lanza GM, Marsh JN, Hu G, et al. Rationale for a nanomedicine approach to thrombolytic therapy. Stroke 2010;41(10, Suppl): S42–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feynman RP. There’s plenty of room at the bottom. Transcript of talk given on Dec 29, 1959 to the American Physical Society. Available at: http://calteches.library.caltech.edu/1976/1/1960Bottom.pdf. Accessed April 2, 2019
- 18.Taniguchi N. On the basic concept of ‘nano-technology’. Proceedings of the International Conference on Production Engineering Tokyo, Part II, Japan Society of Precision Engineering; 1974:5–10 [Google Scholar]
- 19.Freitas RA Jr. Nanomedicine, Volume I: Basic Capabilities. Austin, TX: Landes Biosciences; 1999 [Google Scholar]
- 20.Drexler KE. Nanosystems: Molecular Machinery, Manufacturing, and Computation. New York, NY: John Wiley & Sons, Inc; 1992 [Google Scholar]
- 21.Zolot RS, Basu S, Million RP. Antibody-drug conjugates. Nat Rev Drug Discov 2013;12(04):259–260 [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Palasubramaniam J, Gkanatsas Y, et al. Towards effective and safe thrombolysis and thromboprophylaxis: preclinical testing of a novel antibody-targeted recombinant plasminogen activator directed against activated platelets. Circ Res 2014;114(07):1083–1093 [DOI] [PubMed] [Google Scholar]
- 23.Dong N, Da Cunha V, Citkowicz A, et al. P-selectin-targeting of the fibrin selective thrombolytic Desmodus rotundus salivary plasminogen activator alpha1. Thromb Haemost 2004;92(05):956–965 [DOI] [PubMed] [Google Scholar]
- 24.Diamond SL. Engineering design of optimal strategies for blood clot dissolution. Annu Rev Biomed Eng 1999;1:427–462 [DOI] [PubMed] [Google Scholar]
- 25.Thomas AC, Campbell JH. Targeted delivery of heparin and LMWH using a fibrin antibody prevents restenosis. Atherosclerosis 2004;176(01):73–81 [DOI] [PubMed] [Google Scholar]
- 26.Gupta AS. Nanomedicine approaches in vascular disease: a review. Nanomedicine (Lond) 2011;7(06):763–779 [DOI] [PubMed] [Google Scholar]
- 27.Watson SP. Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Curr Pharm Des 2009;15(12):1358–1372 [DOI] [PubMed] [Google Scholar]
- 28.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol 2014;15(12):771–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007;454(03):345–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 2014;69(07):777–784 [DOI] [PubMed] [Google Scholar]
- 31.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340(02):115–126 [DOI] [PubMed] [Google Scholar]
- 32.Lusis AJ. Atherosclerosis. Nature 2000;407(6801):233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Libby P. Inflammation in atherosclerosis. Nature 2002;420(6917):868–874 [DOI] [PubMed] [Google Scholar]
- 34.Massberg S, Brand K, Grüner S, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med 2002;196(07):887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost 2003;1(09):1897–1905 [DOI] [PubMed] [Google Scholar]
- 36.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol 2003;23(12):2131–2137 [DOI] [PubMed] [Google Scholar]
- 37.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest 2005;115(12):3378–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002;8(11):1227–1234 [DOI] [PubMed] [Google Scholar]
- 39.Frenette PS, Denis CV, Weiss L, et al. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J Exp Med 2000;191(08):1413–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.André P. P-selectin in haemostasis. Br J Haematol 2004;126(03):298–306 [DOI] [PubMed] [Google Scholar]
- 41.Byzova TV, Plow EF. Networking in the hemostatic system. Integrin alphaIIbbeta3 binds prothrombin and influences its activation. J Biol Chem 1997;272(43):27183–27188 [DOI] [PubMed] [Google Scholar]
- 42.Cierniewski CS, Byzova T, Papierak M, et al. Peptide ligands can bind to distinct sites in integrin alphaIIbbeta3 and elicit different functional responses. J Biol Chem 1999;274(24):16923–16932 [DOI] [PubMed] [Google Scholar]
- 43.Deckmyn H, Ulrichts H, Van de Walle G, et al. Platelet antigens and their functions. Vox Sang 2002;87:S105–S111 [DOI] [PubMed] [Google Scholar]
- 44.Pytela R, Pierschbacher MD, Ginsberg MH, Plow EF, Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp–specific adhesion receptors. Science 1986;231(4745):1559–1562 [DOI] [PubMed] [Google Scholar]
- 45.D’Souza SE, Ginsberg MH, Matsueda GR, Plow EF. A discrete sequence in a platelet integrin is involved in ligand recognition. Nature 1991;350(6313):66–68 [DOI] [PubMed] [Google Scholar]
- 46.Verheul HM, Jorna AS, Hoekman K, Broxterman HJ, Gebbink MF, Pinedo HM. Vascular endothelial growth factor-stimulated endothelial cells promote adhesion and activation of platelets. Blood 2000;96(13):4216–4221 [PubMed] [Google Scholar]
- 47.Stouffer GA, Pathak A, Zhao R, Huang J. Down but not out: new insights into the role of alphaVbeta3 integrins in vascular healing. Arterioscler Thromb Vasc Biol 2005;25(07):1309–1310 [DOI] [PubMed] [Google Scholar]
- 48.Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPI-balpha. J Exp Med 1998;187(03):329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005;46(06):937–954 [DOI] [PubMed] [Google Scholar]
- 50.Mastenbroek TG, van Geffen JP, Heemskerk JW, Cosemans JM. Acute and persistent platelet and coagulant activities in atherothrombosis. J Thromb Haemost 2015;13(Suppl 1):S272–S280 [DOI] [PubMed] [Google Scholar]
- 51.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev 2007;21(02):99–111 [DOI] [PubMed] [Google Scholar]
- 52.Totani L, Evangelista V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arterioscler Thromb Vasc Biol 2010;30(12):2357–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Brühl M-L, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012;209(04):819–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J, Kim K, Barazia A, Tseng A, Cho J. Platelet-neutrophil interactions under thromboinflammatory conditions. Cell Mol Life Sci 2015;72(14):2627–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolberg AS, Rosendaal FR, Weitz JI, et al. Venous thrombosis. Nat Rev Dis Primers 2015;1:15006. [DOI] [PubMed] [Google Scholar]
- 56.Gould TJ, Vu TT, Swystun LL, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol 2014;34(09):1977–1984 [DOI] [PubMed] [Google Scholar]
- 57.Sørensen OE, Borregaard N. Neutrophil extracellular traps - the dark side of neutrophils. J Clin Invest 2016;126(05):1612–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dyer MR, Chen Q, Haldeman S, et al. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci Rep 2018;8(01):2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ringsdorf H. Structure and properties of pharmacologically active polymers. J Poly Sci: Polymer Symposia 1975;51:135–153 [Google Scholar]
- 60.Elvira C, Gallardo A, Roman JS, Cifuentes A. Covalent polymer-drug conjugates. Molecules 2005;10(01):114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer 2006;6(09):688–701 [DOI] [PubMed] [Google Scholar]
- 62.Berger H Jr, Pizzo SV. Preparation of polyethylene glycol-tissue plasminogen activator adducts that retain functional activity: characteristics and behavior in three animal species. Blood 1988;71(06):1641–1647 [PubMed] [Google Scholar]
- 63.Rajagopalan S, Gonias SL, Pizzo SV. A nonantigenic covalent streptokinase-polyethylene glycol complex with plasminogen activator function. J Clin Invest 1985;75(02):413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakuragawa N, Shimizu K, Kondo K, Kondo S, Niwa M. Studies on the effect of PEG-modified urokinase on coagulation-fibrinolysis using beagles. Thromb Res 1986;41(05):627–635 [DOI] [PubMed] [Google Scholar]
- 65.Moreadith RW, Collen D. Clinical development of PEGylated recombinant staphylokinase (PEG-Sak) for bolus thrombolytic treatment of patients with acute myocardial infarction. Adv Drug Deliv Rev 2003;55(10):1337–1345 [DOI] [PubMed] [Google Scholar]
- 66.Collen D, Sinnaeve P, Demarsin E, et al. Polyethylene glycol-derivatized cysteine-substitution variants of recombinant staphylokinase for single-bolus treatment of acute myocardial infarction. Circulation 2000;102(15):1766–1772 [DOI] [PubMed] [Google Scholar]
- 67.Sawhney P, Katare K, Sahni G. PEGylation of truncated streptokinase leads to formulation of a useful drug with ameliorated attributes. PLoS One 2016;11(05):e0155831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pöschel KA, Bucha E, Esslinger H-U, et al. Pharmacodynamics and pharmacokinetics of polyethylene glycol-hirudin in patients with chronic renal failure. Kidney Int 2000;58(06):2478–2484 [DOI] [PubMed] [Google Scholar]
- 69.Pöschel KA, Bucha E, Esslinger H-U, et al. Anticoagulant efficacy of PEG-Hirudin in patients on maintenance hemodialysis. Kidney Int 2004;65(02):666–674 [DOI] [PubMed] [Google Scholar]
- 70.Absar S, Choi S, Yang VC, Kwon YM. Heparin-triggered release of camouflaged tissue plasminogen activator for targeted thrombolysis. J Control Release 2012;157(01):46–54 [DOI] [PubMed] [Google Scholar]
- 71.Murciano J-C, Muro S, Koniaris L, et al. ICAM-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood 2003;101(10):3977–3984 [DOI] [PubMed] [Google Scholar]
- 72.Ganguly K, Krasik T, Medinilla S, et al. Blood clearance and activity of erythrocyte-coupled fibrinolytics. J Pharmacol Exp Ther 2005;312(03):1106–1113 [DOI] [PubMed] [Google Scholar]
- 73.Gersh KC, Zaitsev S, Cines DB, Muzykantov V, Weisel JW. Flow-dependent channel formation in clots by an erythrocyte-bound fibrinolytic agent. Blood 2011;117(18):4964–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol 2003;21(08):891–896 [DOI] [PubMed] [Google Scholar]
- 75.Bode C, Meinhardt G, Runge MS, et al. Platelet-targeted fibrinolysis enhances clot lysis and inhibits platelet aggregation. Circulation 1991;84(02):805–813 [DOI] [PubMed] [Google Scholar]
- 76.Chen H, Mo W, Zhang Y, et al. Functional properties of a novel mutant of staphylokinase with platelet-targeted fibrinolysis and antiplatelet aggregation activities. Eur J Pharmacol 2007;566(1–3):137–144 [DOI] [PubMed] [Google Scholar]
- 77.Danielyan K, Ding B-S, Gottstein C, Cines DB, Muzykantov VR. Deliveryofanti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema. J Pharmacol Exp Ther 2007;321(03):947–952 [DOI] [PubMed] [Google Scholar]
- 78.Ding B-S, Hong N, Murciano J-C, et al. Prophylactic thrombolysis by thrombin-activated latent prourokinase targeted to PECAM-1 in the pulmonary vasculature. Blood 2008;111(04):1999–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fuentes RE, Zaitsev S, Ahn HS, et al. A chimeric platelet-targeted urokinase prodrug selectively blocks new thrombus formation. J Clin Invest 2016;126(02):483–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagemeyer CE, Tomic I, Jaminet P, et al. Fibrin-targeted direct factor Xa inhibition: construction and characterization of a recombinant factor Xa inhibitor composed of an anti-fibrin single-chain antibody and tick anticoagulant peptide. Thromb Haemost 2004;92(01):47–53 [DOI] [PubMed] [Google Scholar]
- 81.Huang T, Li N, Gao J. Recent strategies on targeted delivery of thrombolytics. Asian J Pharm Sci 2019. Available at: 10.1016/j.ajps.2018.12.004. Accessed April 2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonnard T, Tennant Z, Niego B, et al. Novel thrombolytic drug based on thrombin cleavable microplasminogen coupled to a single-chain antibody specific for activated GPIIb/IIIa. J Am Heart Assoc 2017;6(02):e004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Absar S, Kwon YM, Ahsan F. Bio-responsive delivery of tissue plasminogen activator for localized thrombolysis. J Control Release 2014;177:42–50 [DOI] [PubMed] [Google Scholar]
- 84.Wei Z, Xin G, Wang H, et al. The diosgenin prodrug nanoparticles with pH-responsive as a drug delivery system uniquely prevents thrombosis without increased bleeding risk. Nanomedicine (Lond) 2018;14(03):673–684 [DOI] [PubMed] [Google Scholar]
- 85.Lasic DD, Papahadjopaulos D, eds. Medical Applications of Liposomes. Amsterdam, The Netherlands: Elsevier; 1998 [Google Scholar]
- 86.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 2005;4(02):145–160 [DOI] [PubMed] [Google Scholar]
- 87.Israelachvili J. Intermolecular and Surface Forces. 2nd ed. New York, NY: Harcourt Brace & Company; 1998 [Google Scholar]
- 88.Ceh BWinterhalter M, Frederik PM, et al. Stealth® liposomes: from theory to product. Adv Drug Deliv Rev 1997;24:165–177 [Google Scholar]
- 89.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 2006;1(03):297–315 [PMC free article] [PubMed] [Google Scholar]
- 90.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med 2012;63:185–198 [DOI] [PubMed] [Google Scholar]
- 91.Nguyen PD, O’Rear EA, Johnson AE, Patterson E, Whitsett TL, Bhakta R. Accelerated thrombolysis and reperfusion in a canine model of myocardial infarction by liposomal encapsulation of streptokinase. Circ Res 1990;66(03):875–878 [DOI] [PubMed] [Google Scholar]
- 92.Heeremans JM, Prevost R, Bekkers ME, et al. Thrombolytic treatment with tissue-type plasminogen activator (t-PA) containing liposomes in rabbits: a comparison with free t-PA. Thromb Haemost 1995;73(03):488–494 [PubMed] [Google Scholar]
- 93.Kim I-S, Choi H-G, Choi H-S, Kim BK, Kim CK. Prolonged systemic delivery of streptokinase using liposome. Arch Pharm Res 1998;21(03):248–252 [DOI] [PubMed] [Google Scholar]
- 94.Leach JK, O’Rear EA, Patterson E, Miao Y, Johnson AE. Accelerated thrombolysis in a rabbit model of carotid artery thrombosis with liposome-encapsulated and microencapsulated streptokinase. Thromb Haemost 2003;90(01):64–70 [PubMed] [Google Scholar]
- 95.Leach JK, Patterson E, O’Rear EA. Improving thrombolysis with encapsulated plasminogen activators and clinical relevance to myocardial infarction and stroke. Clin Hemorheol Microcirc 2004;30(3–4):225–228 [PubMed] [Google Scholar]
- 96.Perkins WR, Vaughan DE, Plavin SR, et al. Streptokinase entrapment in interdigitation-fusion liposomes improves thrombolysis in an experimental rabbit model. Thromb Haemost 1997;77(06):1174–1178 [PubMed] [Google Scholar]
- 97.Erdoğan S, Özer AY, Volkan B, Caner B, Bilgili H. Thrombus localization by using streptokinase containing vesicular systems. Drug Deliv 2006;13(04):303–309 [DOI] [PubMed] [Google Scholar]
- 98.El-Sherbiny IM, Elkholi IE, Yacoub MH. Tissue plasminogen activator-based clot busting: Controlled delivery approaches. Glob Cardiol Sci Pract 2014;2014(03):336–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim J-Y, Kim J-K, Park J-S, Byun Y, Kim CK. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials 2009;30(29):5751–5756 [DOI] [PubMed] [Google Scholar]
- 100.Li H, Chen Y, Deng Y, Wang Y, Ke X, Ci T. Effects of surface charge of lowmolecular weight heparin-modified cationicliposomesondrug efficacy and toxicity. Drug Dev Ind Pharm 2017;43(07):1163–1172 [DOI] [PubMed] [Google Scholar]
- 101.Koromila G, Michanetzis GPA, Missirlis YF, Antimisiaris SG. Heparin incorporating liposomes as a delivery system of heparin from PET-covered metallic stents: effect on haemocompatibility. Biomaterials 2006;27(12):2525–2533 [DOI] [PubMed] [Google Scholar]
- 102.Dzau VJ, Mann MJ, Morishita R, Kaneda Y. Fusigenic viral liposome for gene therapy in cardiovascular diseases. Proc Natl Acad Sci U S A 1996;93(21):11421–11425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nabel EG. Gene therapy for cardiovascular disease. Circulation 1995;91(02):541–548 [DOI] [PubMed] [Google Scholar]
- 104.Morishita R, Gibbons GH, Kaneda Y, Ogihara T, Dzau VJ. Novel and effective gene transfer technique for study of vascular renin angiotensin system. J Clin Invest 1993;91(06):2580–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leclerc G, Gal D, Takeshita S, Nikol S, Weir L, Isner JM. Percutaneous arterial gene transfer in a rabbit model. Efficiency in normal and balloon-dilated atherosclerotic arteries. J Clin Invest 1992;90(03):936–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim CS, Chapman GD, Gammon RS, et al. Direct in vivo gene transfer into the coronary and peripheral vasculatures of the intact dog. Circulation 1991;83(06):2007–2011 [DOI] [PubMed] [Google Scholar]
- 107.Chapman GD, Lim CS, Gammon RS, et al. Gene transfer into coronary arteries of intact animals with a percutaneous balloon catheter. Circ Res 1992;71(01):27–33 [DOI] [PubMed] [Google Scholar]
- 108.Zheng J, Liu J, Dunne M, Jaffray DA, Allen C. In vivo performance of a liposomal vascular contrast agent for CT and MR-based image guidance applications. Pharm Res 2007;24(06):1193–1201 [DOI] [PubMed] [Google Scholar]
- 109.Maiseyeu A, Mihai G, Kampfrath T, et al. Gadolinium-containing phosphatidylserine liposomes for molecular imaging of atherosclerosis. J Lipid Res 2009;50(11):2157–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mulder WJ, Douma K, Koning GA, et al. Liposome-enhanced MRI of neointimal lesions in the ApoE-KO mouse. Magn Reson Med 2006;55(05):1170–1174 [DOI] [PubMed] [Google Scholar]
- 111.Discher DE, Ahmed F. Polymersomes. Annu Rev Biomed Eng 2006;8:323–341 [DOI] [PubMed] [Google Scholar]
- 112.Lee JS, Feijen J. Polymersomes for drug delivery: design, formation and characterization. J Control Release 2012;161(02):473–483 [DOI] [PubMed] [Google Scholar]
- 113.Massignani M, Lomas H, Battaglia G. Polymersomes: a synthetic biological approach to encapsulation and delivery. Adv Polym Sci 2010;229:115–154 [Google Scholar]
- 114.Akagi D, Oba M, Koyama H, et al. Biocompatible micellar nano-vectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther 2007;14(13):1029–1038 [DOI] [PubMed] [Google Scholar]
- 115.Ding B-S, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv 2006;6(02):98–112 [DOI] [PubMed] [Google Scholar]
- 116.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res 2007;24(01):1–16 [DOI] [PubMed] [Google Scholar]
- 117.Lincoff AM, Topol EJ, Ellis SG. Local drug delivery for the prevention of restenosis. Fact, fancy, and future. Circulation 1994;90(04):2070–2084 [DOI] [PubMed] [Google Scholar]
- 118.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 2001;70(1–2):1–20 [DOI] [PubMed] [Google Scholar]
- 119.Yang Z, Birkenhauer P, Julmy F, et al. Sustained release of heparin from polymeric particles for inhibition of human vascular smooth muscle cell proliferation. J Control Release 1999;60(2–3):269–277 [DOI] [PubMed] [Google Scholar]
- 120.Chung T-W, Wang SS, Tsai W-J. Accelerating thrombolysis with chitosan-coated plasminogen activators encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles. Biomaterials 2008;29(02):228–237 [DOI] [PubMed] [Google Scholar]
- 121.Klugherz BD, Meneveau N, Chen W, et al. Sustained intramural retention and regional redistribution following local vascular delivery of Polylactic-coglycolic acid and liposomal nanoparticulate formulations containing probucol. J Cardiovasc Pharmacol Ther 1999;4(03):167–174 [DOI] [PubMed] [Google Scholar]
- 122.Kolodgie FD, John M, Khurana C, et al. Sustained reduction of instent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation 2002;106(10):1195–1198 [DOI] [PubMed] [Google Scholar]
- 123.Westedt U, Kalinowski M, Wittmar M, et al. Poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) nanoparticles for local delivery of paclitaxel for restenosis treatment. J Control Release 2007;119(01):41–51 [DOI] [PubMed] [Google Scholar]
- 124.Deshpande D, Devalapally H, Amiji M. Enhancement in antiproliferative effects of paclitaxel in aortic smooth muscle cells upon co-administration with ceramide using biodegradable polymeric nanoparticles. Pharm Res 2008;25(08):1936–1947 [DOI] [PubMed] [Google Scholar]
- 125.Zou W, Cao G, Xi Y, Zhang N. New approach for local delivery of rapamycin by bioadhesive PLGA-Carbopol nanoparticles. Drug Deliv 2009;16(01):15–23 [DOI] [PubMed] [Google Scholar]
- 126.Zweers ML, Engbers GH, Grijpma DW, Feijen J. Release of anti-restenosis drugs from poly(ethylene oxide)-poly(DL-lactic-co-glycolic acid) nanoparticles. J Control Release 2006;114(03): 317–324 [DOI] [PubMed] [Google Scholar]
- 127.Luderer F, Löbler M, Rohm HW, et al. Biodegradable sirolimus-loaded poly(lactide) nanoparticles as drug delivery system for the prevention of in-stent restenosis in coronary stent application. J Biomater Appl 2011;25(08):851–875 [DOI] [PubMed] [Google Scholar]
- 128.Nakano K, Egashira K, Masuda S, et al. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology: efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. JACC Cardiovasc Interv 2009;2(04):277–283 [DOI] [PubMed] [Google Scholar]
- 129.Margolis J, McDonald J, Heuser R, et al. Systemic nanoparticle paclitaxel (nab-paclitaxel) for in-stent restenosis I (SNAPIST-I): a first-in-human safety and dose-finding study. Clin Cardiol 2007;30(04):165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Unger E, Matsunaga TO, Schumann PA, et al. Microbubbles in molecular imaging and therapy. Medicamundi 2003;47:58–65 [Google Scholar]
- 131.Cavalieri F, Finelli I, Tortora M, et al. Polymer microbubbles as diagnostic and therapeutic gas delivery device. Chem Mater 2008;20:3254–3258 [Google Scholar]
- 132.Tsivgoulis G, Culp WC, Alexandrov AV. Ultrasound enhanced thrombolysis in acute arterial ischemia. Ultrasonics 2008;48(04):303–311 [DOI] [PubMed] [Google Scholar]
- 133.Rubiera M, Alexandrov AV. Sonothrombolysis in the management of acute ischemic stroke. Am J Cardiovasc Drugs 2010;10(01):5–10 [DOI] [PubMed] [Google Scholar]
- 134.Mayer CR, Bekeredjian R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv Drug Deliv Rev 2008;60(10):1177–1192 [DOI] [PubMed] [Google Scholar]
- 135.Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK. Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thromb Res 2009;124(03):306–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tiukinhoy-Laing SD, Buchanan K, Parikh D, et al. Fibrin targeting of tissue plasminogen activator-loaded echogenic liposomes. J Drug Target 2007;15(02):109–114 [DOI] [PubMed] [Google Scholar]
- 137.Tomalia DA. Starburst dendrimers—nanoscopic supermolecules according to dendritic rules and principles. Macromol Symp 1996;101:243–255 [Google Scholar]
- 138.Hawker CJ, Fréchet JMJ. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc 1990;112:7638–7647 [Google Scholar]
- 139.Svenson S, Tomalia DA. Dendrimers in biomedical applications–reflections on the field. Adv Drug Deliv Rev 2005;57(15):2106–2129 [DOI] [PubMed] [Google Scholar]
- 140.Fernandes EG, de Queiroz AA, Abraham GA, San Román J. Antithrombogenic properties of bioconjugate streptokinasepolyglycerol dendrimers. J Mater Sci Mater Med 2006;17(02):105–111 [DOI] [PubMed] [Google Scholar]
- 141.Wu C, Gao C, Lü S, et al. Construction of polylysine dendrimer nanocomposites carrying nattokinase and their application in thrombolysis. J Biomed Mater Res A 2018;106(02):440–449 [DOI] [PubMed] [Google Scholar]
- 142.Zhang S-F, Lü S, Gao C, et al. Multiarm-polyethylene glycol-polyglutamic acid peptide dendrimer: design, synthesis, and dissolving thrombus. J Biomed Mater Res A 2018;106(06):1687–1696 [DOI] [PubMed] [Google Scholar]
- 143.Chen PC, Mwakwari SC, Oyelere AK. Gold nanoparticles: from nanomedicine to nanosensing. Nanotechnol Sci Appl 2008;1:45–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sperling RA, Rivera Gil P, Zhang F, Zanella M, Parak WJ. Biological applications of gold nanoparticles. Chem Soc Rev 2008;37(09):1896–1908 [DOI] [PubMed] [Google Scholar]
- 145.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 2008;23(03):217–228 [DOI] [PubMed] [Google Scholar]
- 146.Rosen JE, Chan L, Shieh DB, Gu FX. Iron oxide nanoparticles for targeted cancer imaging and diagnostics. Nanomedicine (Lond) 2012;8(03):275–290 [DOI] [PubMed] [Google Scholar]
- 147.Tassa C, Shaw SY, Weissleder R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc Chem Res 2011;44(10):842–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yen SK, Padmanabhan P, Selvan ST. Multifunctional iron oxide nanoparticles for diagnostics, therapy and macromolecule delivery. Theranostics 2013;3(12):986–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brust M, Walker M, Bethell D, et al. Synthesis of thiol derivatized gold nanoparticles in a two phase liquid-liquid system. J Chem Soc Chem Commun 1994;7:801–802 [Google Scholar]
- 150.Sun Y, Xia Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002;298(5601):2176–2179 [DOI] [PubMed] [Google Scholar]
- 151.Vekilov PG. Gold nanoparticles: grown in a crystal. Nat Nanotechnol 2011;6(02):82–83 [DOI] [PubMed] [Google Scholar]
- 152.Au L, Lu X, Xia Y. A comparative study of galvanic replacement reactions involving Ag nanocubes and AuCl2− or AuCl4−. Adv Mater 2008;20(13):2517–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choi Y, Hong S, Liu L, Kim SK, Park S. Galvanically replaced hollow Au-Ag nanospheres: study of their surface plasmon resonance. Langmuir 2012;28(16):6670–6676 [DOI] [PubMed] [Google Scholar]
- 154.Spivak MY, Bubnov RV, Yemets IM, Lazarenko LM, Tymoshok NO, Ulberg ZR. Gold nanoparticles - the theranostic challenge for PPPM: nanocardiology application. EPMA J 2013;4(01):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang B, Yantsen E, Larson T, et al. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Lett 2009;9(06):2212–2217 [DOI] [PubMed] [Google Scholar]
- 156.Cormode DP, Roessl E, Thran A, et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology 2010;256(03):774–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee S, Cha EJ, Park K, et al. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew Chem Int Ed Engl 2008;47(15):2804–2807 [DOI] [PubMed] [Google Scholar]
- 158.Lukianova-Hleb EY, Mrochek AG, Lapotko DO. Method for disruption and re-canalization of atherosclerotic plaques in coronary vessels with photothermal bubbles generated around gold nanoparticles. Lasers Surg Med 2009;41(03):240–247 [DOI] [PubMed] [Google Scholar]
- 159.Karaagac O, Kockar H, Beyaz S, et al. A simple way to synthesize superparamagnetic iron oxide nanoparticles in air atmosphere: iron ion concentration effect. IEEE Trans Magn 2010;46:3978–3983 [Google Scholar]
- 160.Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology 1990;175(02):489–493 [DOI] [PubMed] [Google Scholar]
- 161.McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliv Rev 2008;60(11):1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sosnovik DE, Nahrendorf M, Weissleder R. Magnetic nanoparticles for MR imaging: agents, techniques and cardiovascular applications. Basic Res Cardiol 2008;103(02):122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bjørnerud A, Johansson L. The utility of superparamagnetic contrast agents in MRI: theoretical consideration and applications in the cardiovascular system. NMR Biomed 2004;17(07):465–477 [DOI] [PubMed] [Google Scholar]
- 164.Yilmaz A, Dengler MA, van der Kuip H, et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J 2013;34(06):462–475 [DOI] [PubMed] [Google Scholar]
- 165.Jaffer FA, Nahrendorf M, Sosnovik D, Kelly KA, Aikawa E, Weissleder R. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol Imaging 2006;5(02):85–92 [PubMed] [Google Scholar]
- 166.Trivedi RA, U-King-Im JM, Graves MJ, et al. In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke 2004;35(07):1631–1635 [DOI] [PubMed] [Google Scholar]
- 167.Tang TY, Muller KH, Graves MJ, et al. Iron oxide particles for atheroma imaging. Arterioscler Thromb Vasc Biol 2009;29(07): 1001–1008 [DOI] [PubMed] [Google Scholar]
- 168.Schmitz SA, Taupitz M, Wagner S, Wolf KJ, Beyersdorff D, Hamm B. Magnetic resonance imaging of atherosclerotic plaques using superparamagnetic iron oxide particles. J Magn Reson Imaging 2001;14(04):355–361 [DOI] [PubMed] [Google Scholar]
- 169.Morishige K, Kacher DF, Libby P, et al. High-resolution magnetic resonance imaging enhanced with superparamagnetic nanoparticles measures macrophage burden in atherosclerosis. Circulation 2010;122(17):1707–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sigovan M, Boussel L, Sulaiman A, et al. Rapid-clearance iron nanoparticles for inflammation imaging of atherosclerotic plaque: initial experience in animal model. Radiology 2009;252(02):401–409 [DOI] [PubMed] [Google Scholar]
- 171.Kawahara I, Nakamoto M, Kitagawa N, et al. Potential of magnetic resonance plaque imaging using superparamagnetic particles of iron oxide for the detection of carotid plaque. Neurol Med Chir (Tokyo) 2008;48(04):157–161, discussion 161–162 [DOI] [PubMed] [Google Scholar]
- 172.Erdem SS, Sazonova IY, Hara T, Jaffer FA, McCarthy JR. Detection and treatment of intravascular thrombi with magnetofluorescent nanoparticles. Methods Enzymol 2012;508:191–209 [DOI] [PubMed] [Google Scholar]
- 173.Wuang SC, Neoh KG, Kang E-T, et al. Heparinized magnetic nanoparticles: in vitro assessment for biomedical applications. Adv Funct Mater 2006;16:1723–1730 [Google Scholar]
- 174.Chorny M, Fishbein I, Yellen BB, et al. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc Natl Acad Sci U S A 2010;107(18):8346–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sharma R, Kwon S. New applications of nanoparticles in cardiovascular imaging. J Exp Nanosci 2007;2:115–126 [Google Scholar]
- 176.Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. Nanotechnology in medical imaging: probe design and applications. Arterioscler Thromb Vasc Biol 2009;29(07):992–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sinusas AJ, Bengel F, Nahrendorf M, et al. Multimodality cardiovascular molecular imaging, Part I. Circ Cardiovasc Imaging 2008;1(03):244–256 [DOI] [PubMed] [Google Scholar]
- 178.Nahrendorf M, Sosnovik DE, French BA, et al. Multimodality cardiovascular molecular imaging, Part II. Circ Cardiovasc Imaging 2009;2(01):56–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Smith AM, Gao X, Nie S. Quantum dot nanocrystals for in vivo molecular and cellular imaging. Photochem Photobiol 2004;80(03):377–385 [DOI] [PubMed] [Google Scholar]
- 180.Douma K, Prinzen L, Slaaf DW, et al. Nanoparticles for optical molecular imaging of atherosclerosis. Small 2009;5(05):544–557 [DOI] [PubMed] [Google Scholar]
- 181.Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett 2009;9(06):2354–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tang Y, Han S, Liu H, et al. The role of surface chemistry in determining in vivo biodistribution and toxicity of CdSe/ZnS core-shell quantum dots. Biomaterials 2013;34(34):8741–8755 [DOI] [PubMed] [Google Scholar]
- 183.Hauck TS, Anderson RE, Fischer HC, Newbigging S, Chan WC. In vivo quantum-dot toxicity assessment. Small 2010;6(01):138–144 [DOI] [PubMed] [Google Scholar]
- 184.Skajaa T, Cormode DP, Falk E, Mulder WJ, Fisher EA, Fayad ZA. High-density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis. Arterioscler Thromb Vasc Biol 2010;30(02):169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc 2004;126(50):16316–16317 [DOI] [PubMed] [Google Scholar]
- 186.Frias JC, Ma Y, Williams KJ, Fayad ZA, Fisher EA. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett 2006;6(10):2220–2224 [DOI] [PubMed] [Google Scholar]
- 187.Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 2010;148(02):135–146 [DOI] [PubMed] [Google Scholar]
- 188.Hirsjärvi S, Passirani C, Benoit JP. Passive and active tumour targeting with nanocarriers. Curr Drug Discov Technol 2011;8(03):188–196 [DOI] [PubMed] [Google Scholar]
- 189.Huynh NT, Roger E, Lautram N, Benoît JP, Passirani C. The rise and rise of stealth nanocarriers for cancer therapy: passive versus active targeting. Nanomedicine (Lond) 2010;5(09):1415–1433 [DOI] [PubMed] [Google Scholar]
- 190.Blackwell JE, Dagia NM, Dickerson JB, Berg EL, Goetz DJ. Ligand coated nanosphere adhesion to E- and P-selectin under static and flow conditions. Ann Biomed Eng 2001;29(06):523–533 [DOI] [PubMed] [Google Scholar]
- 191.Jancsó G, Domoki F, Sántha P, et al. Beta-amyloid (1–42) peptide impairs blood-brain barrier function after intracarotid infusion in rats. Neurosci Lett 1998;253(02):139–141 [DOI] [PubMed] [Google Scholar]
- 192.Nishiya T, Kainoh M, Murata M, Handa M, Ikeda Y. Reconstitution of adhesive properties of human platelets in liposomes carrying both recombinant glycoproteins Ia/IIa and Ib alpha under flow conditions: specific synergy of receptor-ligand interactions. Blood 2002;100(01):136–142 [DOI] [PubMed] [Google Scholar]
- 193.Wada T, Okamura Y, Takeoka S, et al. Deformability and adhesive force of artificial platelets measured by atomic force microscopy. J Biorheol 2009;23(01):35–40 [Google Scholar]
- 194.Nishiya T, Kainoh M, Murata M, Handa M, Ikeda Y. Platelet interactions with liposomes carrying recombinant platelet membrane glycoproteins or fibrinogen: approach to platelet substitutes. Artif Cells Blood Substit Immobil Biotechnol 2001;29(06):453–464 [DOI] [PubMed] [Google Scholar]
- 195.Wilchek M, Bayer EA, Livnah O. Essentials of biorecognition: the (strept)avidin-biotin system as a model for protein-protein and protein-ligand interaction. Immunol Lett 2006;103(01):27–32 [DOI] [PubMed] [Google Scholar]
- 196.Lesch HP, Kaikkonen MU, Pikkarainen JT, Ylä-Herttuala S. Avidinbiotin technology in targeted therapy. Expert Opin Drug Deliv 2010;7(05):551–564 [DOI] [PubMed] [Google Scholar]
- 197.Muzykantov V Targeted Drug Delivery to Endothelial Adhesion Molecules. ISRN Vascular Medicine; 2013; Article ID 916254. Available at: 10.1155/2013/916254. Accessed April 2, 2019 [DOI] [Google Scholar]
- 198.Sen Gupta A, von Recum HA. Bioconjugation strategies: lipids, liposomes, polymersomes, and microbubbles. In: Narain R, ed. Chemistry of Bioconjugates: Synthesis, Characterization, and Biomedical Applications. Hoboken, NJ: Wiley; 2014 [Google Scholar]
- 199.Leung MKM, Hagemeyer CE, Johnston APR, et al. Bio-click chemistry: enzymatic functionalization of PEGylated capsules for targeting applications. Angew Chem Int Ed Engl 2012;51(29):7132–7136 [DOI] [PubMed] [Google Scholar]
- 200.Walper SA, Turner KB, Medintz IL. Enzymatic bioconjugation of nanoparticles: developing specificity and control. Curr Opin Biotechnol 2015;34:232–241 [DOI] [PubMed] [Google Scholar]
- 201.Demos SM, Alkan-Onyuksel H, Kane BJ, et al. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J Am Coll Cardiol 1999;33(03):867–875 [DOI] [PubMed] [Google Scholar]
- 202.Klegerman ME, Zou Y, McPherson DD. Fibrin targeting of echogenic liposomes with inactivated tissue plasminogen activator. J Liposome Res 2008;18(02):95–112 [DOI] [PubMed] [Google Scholar]
- 203.Huang S-L. Liposomes in ultrasonic drug and gene delivery. Adv Drug Deliv Rev 2008;60(10):1167–1176 [DOI] [PubMed] [Google Scholar]
- 204.Wang X, Hagemeyer CE, Hohmann JD, et al. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation 2012;125(25):3117–3126 [DOI] [PubMed] [Google Scholar]
- 205.Li D, Patel AR, Klibanov AL, et al. Molecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonance. Circ Cardiovasc Imaging 2010;3(04):464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Homem de Bittencourt PI Jr, Lagranha DJ, Maslinkiewicz A, et al. LipoCardium: endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis 2007;193(02):245–258 [DOI] [PubMed] [Google Scholar]
- 207.Kim W, Haller C, Dai E, et al. Targeted antithrombotic protein micelles. Angew Chem Int Ed Engl 2015;54(05):1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ta HT, Li Z, Hagemeyer CE, et al. Molecular imaging of activated platelets via antibody-targeted ultra-small iron oxide nanoparticles displaying unique dual MRI contrast. Biomaterials 2017;134:31–42 [DOI] [PubMed] [Google Scholar]
- 209.Alt K, Paterson BM, Ardipradja K, et al. Single-chain antibody conjugated to a cage amine chelator and labeled with positron-emitting copper-64 for diagnostic imaging of activated platelets. Mol Pharm 2014;11(08):2855–2863 [DOI] [PubMed] [Google Scholar]
- 210.Gupta AS, Huang G, Lestini BJ, Sagnella S, Kottke-Marchant K, Marchant RE. RGD-modified liposomes targeted to activated platelets as a potential vascular drug delivery system. Thromb Haemost 2005;93(01):106–114 [DOI] [PubMed] [Google Scholar]
- 211.Huang G, Zhou Z, Srinivasan R, et al. Affinity manipulation of surface-conjugated RGD peptide to modulate binding of liposomes to activated platelets. Biomaterials 2008;29(11):1676–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Srinivasan R, Marchant RE, Gupta AS. In vitro and in vivo platelet targeting by cyclic RGD-modified liposomes. J Biomed Mater Res A 2010;93(03):1004–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Modery CL, Ravikumar M, Wong TL, Dzuricky MJ, Durongkaveroj N, Sen Gupta A. Heteromultivalent liposomal nanoconstructs for enhanced targeting and shear-stable binding to active platelets for site-selective vascular drug delivery. Biomaterials 2011;32(35):9504–9514 [DOI] [PubMed] [Google Scholar]
- 214.Ravikumar M, Modery CL, Wong TL, Dzuricky M, Sen Gupta A. Mimicking adhesive functionalities of blood platelets using ligand-decorated liposomes. Bioconjug Chem 2012;23(06):1266–1275 [DOI] [PubMed] [Google Scholar]
- 215.Ravikumar M, Modery CL, Wong TL, Gupta AS. Peptide-decorated liposomes promote arrest and aggregation of activated platelets under flow on vascular injury relevant protein surfaces in vitro. Biomacromolecules 2012;13(05):1495–1502 [DOI] [PubMed] [Google Scholar]
- 216.Modery-Pawlowski CL, Tian LL, Ravikumar M, Wong TL, Sen Gupta A. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials 2013;34(12):3031–3041 [DOI] [PubMed] [Google Scholar]
- 217.Haji-Valizadeh HN, Modery-Pawlowski CL, Sen Gupta A. A FVIII-derived peptide enables von Willebrand factor (VWF)-binding of artificial platelet nanoconstructs without interfering with VWF-adhesion of natural platelets. Nanoscale 2014;6(09):4765–4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shukla M, Sekhon UD, Betapudi V, et al. In vitro characterization of SynthoPlate™ (synthetic platelet) technology and its in vivo evaluation in severely thrombocytopenic mice. J Thromb Haemost 2017;15(02):375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pawlowski CL, Li W, Sun M, et al. Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials 2017;128:94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hickman DA, Pawlowski CL, Shevitz A, et al. Intravenous synthetic platelet (SynthoPlate) nanoconstructs reduce bleeding and improve ‘golden hour’ survival in a porcine model of traumatic arterial hemorrhage. Sci Rep 2018;8(01):3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mulder WJM, Strijkers GJ, Briley-Saboe KC, et al. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn Reson Med 2007;58(06):1164–1170 [DOI] [PubMed] [Google Scholar]
- 222.Lipinski MJ, Amirbekian V, Frias JC, et al. MRI to detect atherosclerosis with gadolinium-containing immunomicelles targeting the macrophage scavenger receptor. Magn Reson Med 2006;56(03):601–610 [DOI] [PubMed] [Google Scholar]
- 223.Briley-Saebo KC, Shaw PX, Mulder WJM, et al. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation 2008;117(25):3206–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Amirbekian V, Lipinski MJ, Frias JC, et al. MR imaging of human atherosclerosis using immunomicelles molecularly targeted to macrophages. J Cardiovasc Magn Reson 2009;11(Suppl 1):83 [Google Scholar]
- 225.Chan JM, Zhang L, Tong R, et al. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc Natl Acad Sci U S A 2010;107(05):2213–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chan JM, Rhee JW, Drum CL, et al. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid-polymeric nanoparticles. Proc Natl Acad Sci U S A 2011;108(48):19347–19352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Peters D, Kastantin M, Kotamraju VR, et al. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci U S A 2009;106(24):9815–9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hamzah J, Kotamraju VR, Seo JW, et al. Specific penetration and accumulation of a homing peptide within atherosclerotic plaques of apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A 2011;108(17):7154–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Koning GA, Schiffelers RM, Storm G. Endothelial cells at inflammatory sites as target for therapeutic intervention. Endothelium 2002;9(03):161–171 [DOI] [PubMed] [Google Scholar]
- 230.Muro S, Dziubla T, Qiu W, et al. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J Pharmacol Exp Ther 2006;317(03):1161–1169 [DOI] [PubMed] [Google Scholar]
- 231.Deosarkar SP, Malgor R, Fu J, Kohn LD, Hanes J, Goetz DJ. Polymeric particles conjugated with a ligand to VCAM-1 exhibit selective, avid, and focal adhesion to sites of atherosclerosis. Biotechnol Bioeng 2008;101(02):400–407 [DOI] [PubMed] [Google Scholar]
- 232.Garnacho C, Serrano D, Muro S. A fibrinogen-derived peptide provides intercellular adhesion molecule-1-specific targeting and intraendothelial transport of polymer nanocarriers in human cell cultures and mice. J Pharmacol Exp Ther 2012;340(03):638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Khondee S, Baoum A, Siahaan TJ, Berkland C. Calcium condensed LABL-TAT complexes effectively target gene delivery to ICAM-1 expressing cells. Mol Pharm 2011;8(03):788–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang N, Chittasupho C, Duangrat C, Siahaan TJ, Berkland C. PLGA nanoparticle–peptide conjugate effectively targets intercellular cell-adhesion molecule-1. Bioconjug Chem 2008;19(01):145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res 2005;96(03):327–336 [DOI] [PubMed] [Google Scholar]
- 236.Nahrendorf M, Jaffer FA, Kelly KA, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation 2006;114(14):1504–1511 [DOI] [PubMed] [Google Scholar]
- 237.Burtea C, Laurent S, Port M, et al. Magnetic resonance molecular imaging of vascular cell adhesion molecule-1 expression in inflammatory lesions using a peptide-vectorized paramagnetic imaging probe. J Med Chem 2009;52(15):4725–4742 [DOI] [PubMed] [Google Scholar]
- 238.Bertram JP, Williams CA, Robinson R, Segal SS, Flynn NT, Lavik EB. Intravenous hemostat: nanotechnology to halt bleeding. Sci Transl Med 2009;1(11):11ra22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hubbard WB, Lashoff-Sullivan MM, Lavik EB, et al. Steroid-loaded hemostatic nanoparticles combat lung injury after blast trauma. ACS Macro Lett 2015;4(04):387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Coller BS, Springer KT, Beer JH, et al. Thromboerythrocytes. In vitro studies of a potential autologous, semi-artificial alternative to platelet transfusions. J Clin Invest 1992;89(02):546–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Levi M, Friederich PW, Middleton S, et al. Fibrinogen-coated albumin microcapsules reduce bleeding in severely thrombocytopenic rabbits. Nat Med 1999;5(01):107–111 [DOI] [PubMed] [Google Scholar]
- 242.Okamura Y, Takeoka S, Teramura Y, et al. Hemostatic effects of fibrinogen gamma-chain dodecapeptide-conjugated polymerized albumin particles in vitro and in vivo. Transfusion 2005;45(07):1221–1228 [DOI] [PubMed] [Google Scholar]
- 243.Okamura Y, Fujie T, Nogawa M, et al. Haemostatic effects of polymerized albumin particles carrying fibrinogen gamma-chain dodecapeptide as platelet substitutes in severely thrombocytopenic rabbits. Transfus Med 2008;18(03):158–166 [DOI] [PubMed] [Google Scholar]
- 244.Takeoka S, Okamura Y, Teramura Y, et al. Function of fibrinogen gamma-chain dodecapeptide-conjugated latex beads under flow. Biochem Biophys Res Commun 2003;312(03):773–779 [DOI] [PubMed] [Google Scholar]
- 245.Okamura Y, Handa M, Suzuki H, Ikeda Y, Takeoka S. New strategy of platelet substitutes for enhancing platelet aggregation at high shear rates: cooperative effects of a mixed system of fibrinogen gamma-chain dodecapeptide- or glycoprotein Ibalpha-conjugated latex beads under flow conditions. J Artif Organs 2006;9(04):251–258 [DOI] [PubMed] [Google Scholar]
- 246.Chen W, Jarzyna PA, van Tilborg GA, et al. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. FASEB J 2010;24(06):1689–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Richardson JJ, Choy MY, Guo J, et al. Polymer capsules for plaque-targeted in vivo delivery. Adv Mater 2016;28(35):7703–7707 [DOI] [PubMed] [Google Scholar]
- 248.Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 2007;116(03):276–284 [DOI] [PubMed] [Google Scholar]
- 249.Lindner JR. Molecular imaging of cardiovascular disease with contrast-enhanced ultrasonography. Nat Rev Cardiol 2009;6(07):475–481 [DOI] [PubMed] [Google Scholar]
- 250.Villanueva FS, Wagner WR. Ultrasound molecular imaging of cardiovascular disease. Nat Clin Pract Cardiovasc Med 2008;5(Suppl 2):S26–S32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Alonso A, Della Martina A, Stroick M, et al. Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke 2007;38(05):1508–151 [DOI] [PubMed] [Google Scholar]
- 252.Hagooly A, Almutairi A, Rossin R, et al. Evaluation of a RGD-dendrimer labeled with 76Br in hindlimb ischemia mouse model. J Nucl Med 2008;49(Suppl 1):184 [Google Scholar]
- 253.Taite LJ, West JL. Poly(ethylene glycol)-lysine dendrimers for targeted delivery of nitric oxide. J Biomat Sci Polym Ed 2006;17(10):1159–1172 [Google Scholar]
- 254.Makowski M, Jansen C, Botnar R, et al. Molecular MRI of atherosclerosis: from mouse to man. Medicamundi 2010;54:14 [Google Scholar]
- 255.Thukkani AK, Glaus C, Welch MJ. Molecular imaging of vascular inflammation with nanoparticles. Curr Cardiovasc Imaging Rep 2010;3(03):151–161 [Google Scholar]
- 256.McAteer MA, Akhtar AM, von Zur Muhlen C, Choudhury RP. An approach to molecular imaging of atherosclerosis, thrombosis, and vascular inflammation using microparticles of iron oxide. Atherosclerosis 2010;209(01):18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Smith BR, Heverhagen J, Knopp M, et al. Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI). Biomed Micro-devices 2007;9(05):719–727 [DOI] [PubMed] [Google Scholar]
- 258.Ferrara DE, Glaus C, Taylor WR. Targeting vascular epitopes using quantum dots. Nanoparticles in biomedical imaging. Fundamental Biomed Tech 2008;102:443–461 [Google Scholar]
- 259.Jayagopal A, Russ PK, Haselton FR. Surface engineering of quantum dots for in vivo vascular imaging. Bioconjug Chem 2007;18(05):1424–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kahn E, Vejux A, Ménétrier F, et al. Analysis of CD36 expression on human monocytic cells and atherosclerotic tissue sections with quantum dots: investigation by flow cytometry and spectral imaging microscopy. Anal Quant Cytol Histol 2006;28(01):14–26 [PubMed] [Google Scholar]
- 261.Prinzen L, Miserus RJ, Dirksen A, et al. Optical and magnetic resonance imaging of cell death and platelet activation using annexin A5-functionalized quantum dots. Nano Lett 2007;7(01):93–100 [DOI] [PubMed] [Google Scholar]
- 262.Mulder WJ, Koole R, Brandwijk RJ, et al. Quantum dots with a paramagnetic coating as a bimodal molecular imaging probe. Nano Lett 2006;6(01):1–6 [DOI] [PubMed] [Google Scholar]
- 263.Chang E, Miller JS, Sun J, et al. Protease-activated quantum dot probes. Biochem Biophys Res Commun 2005;334(04):1317–1321 [DOI] [PubMed] [Google Scholar]
- 264.Edelman ER, Adams DH, Karnovsky MJ. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A 1990;87(10):3773–3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Villa AE, Guzman LA, Chen W, Golomb G, Levy RJ, Topol EJ. Local delivery of dexamethasone for prevention of neointimal proliferation in a rat model of balloon angioplasty. J Clin Invest 1994;93(03):1243–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nathan A, Nugent MA, Edelman ER. Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc Natl Acad Sci U S A 1995;92(18):8130–8134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nugent HM, Rogers C, Edelman ER. Endothelial implants inhibit intimal hyperplasia after porcine angioplasty. Circ Res 1999;84(04):384–391 [DOI] [PubMed] [Google Scholar]
- 268.Wilensky RL, March KL, Gradus-Pizlo I, et al. Regional and arterial localization of radioactive microparticles after local delivery by unsupported or supported porous balloon catheters. Am Heart J 1995;129(05):852–859 [DOI] [PubMed] [Google Scholar]
- 269.Zhang L, Keogh S, Rickard CM. Reducing the risk of infection associated with vascular access devices through nanotechnology: a perspective. Int J Nanomedicine 2013;8:4453–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Harris DL, Graffagnini MJ. Nanomaterials in medical devices: a snapshot of markets, technologies and companies. Nanotechnol Law Business 2007;4:415–422 [Google Scholar]
- 271.Martinez AW, Chaikof EL. Microfabrication and nanotechnology in stent design. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2011;3(03):256–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.McDowell G, Slevin M, Krupinski J. Nanotechnology for the treatment of coronary in stent restenosis: a clinical perspective. Vasc Cell 2011;3(01):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yin R-X, Yang D-Z, Wu J-Z. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics 2014;4(02):175–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Goh D, Tan A, Farhatnia Y, Rajadas J, Alavijeh MS, Seifalian AM. Nanotechnology-based gene-eluting stents. Mol Pharm 2013;10(04):1279–1298 [DOI] [PubMed] [Google Scholar]
- 275.Sarkar S, Schmitz-Rixen T, Hamilton G, Seifalian AM. Achieving the ideal properties for vascular bypass grafts using a tissue engineered approach: a review. Med Biol Eng Comput 2007;45(04):327–336 [DOI] [PubMed] [Google Scholar]
- 276.de Mel A, Naghavi N, Cousins BG, et al. Nitric oxide-eluting nanocomposite for cardiovascular implants. J Mater Sci Mater Med 2014;25(03):917–929 [DOI] [PubMed] [Google Scholar]
- 277.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials 2010;31(06):1392–1402 [DOI] [PubMed] [Google Scholar]
- 278.Toy R, Hayden E, Shoup C, Baskaran H, Karathanasis E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 2011;22(11):115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Ann Biomed Eng 2005;33(02):179–190 [DOI] [PubMed] [Google Scholar]
- 280.Lee T-R, Choi M, Kopacz AM, Yun SH, Liu WK, Decuzzi P. On the near-wall accumulation of injectable particles in the microcirculation: smaller is not better. Sci Rep 2013;3:2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Müller K, Fedosov DA, Gompper G. Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci Rep 2014;4:4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Tao L, Hu W, Liu Y, Huang G, Sumer BD, Gao J. Shape-specific polymeric nanomedicine: emerging opportunities and challenges. Exp Biol Med (Maywood) 2011;236(01):20–29 [DOI] [PubMed] [Google Scholar]
- 283.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release 2007;121(1–2):3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shah S, Liu Y, Hu W, Gao J. Modeling particle shape-dependent dynamics in nanomedicine. J Nanosci Nanotechnol 2011;11(02):919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 2015;33(09):941–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Pillai JD, Dunn SS, Napier ME, DeSimone JM. Novel platforms for vascular carriers with controlled geometry. IUBMB Life 2011;63(08):596–606 [DOI] [PubMed] [Google Scholar]
- 287.Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials 2006;27(30):5307–5314 [DOI] [PubMed] [Google Scholar]
- 288.Doshi N, Prabhakarpandian B, Rea-Ramsey A, Pant K, Sundaram S, Mitragotri S. Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. J Control Release 2010;146(02):196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Vahidkhah K, Bagchi P. Microparticle shape effects on margination, near-wall dynamics and adhesion in a three-dimensional simulation of red blood cell suspension. Soft Matter 2015;11(11):2097–2109 [DOI] [PubMed] [Google Scholar]
- 290.Fish MB, Thompson AJ, Fromen CA, Eniola-Adefeso O. Emergence and utility of non-spherical particles in biomedicine. Ind Eng Chem Res 2015;54(16):4043–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Thompson AJ, Mastria EM, Eniola-Adefeso O. The margination propensity of ellipsoidal micro/nanoparticles to the endothelium in human blood flow. Biomaterials 2013;34(23):5863–5871 [DOI] [PubMed] [Google Scholar]
- 292.Anselmo AC, Zhang M, Kumar S, et al. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 2015;9(03):3169–3177 [DOI] [PubMed] [Google Scholar]
- 293.Fish MB, Fromen CA, Lopez-Cazares G, et al. Exploring deformable particles in vascular-targeted drug delivery: softer is only sometimes better. Biomaterials 2017;124:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gutierrez M, Ojeda LS, Eniola-Adefeso O. Vascular-targeted particle binding efficacy in the presence of rigid red blood cells: Implications for performance in diseased blood. Biomicrofluidics 2018;12(04):042217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Aarts PA, van den Broek SA, Prins GW, Kuiken GD, Sixma JJ, Heethaar RM. Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arteriosclerosis 1988;8(06):819–824 [DOI] [PubMed] [Google Scholar]
- 296.Skorczewski T, Erickson LC, Fogelson AL. Platelet motion near a vessel wall or thrombus surface in two-dimensional whole blood simulations. Biophys J 2013;104(08):1764–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tokarev AA, Butylin AA, Ermakova EA, Shnol EE, Panasenko GP, Ataullakhanov FI. Finite platelet size could be responsible for platelet margination effect. Biophys J 2011;101(08):1835–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kumar A, Graham MD. Margination and segregation in confined flows of blood and other multicomponent suspensions. Soft Matter 2012;8(10):10536–10548 [DOI] [PubMed] [Google Scholar]
- 299.Vahidkhah K, Diamond SL, Bagchi P. Platelet dynamics in three-dimensional simulation of whole blood. Biophys J 2014;106(11):2529–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Perry JL, Herlihy KP, Napier ME, Desimone JM. PRINT: a novel platform toward shape and size specific nanoparticle theranostics. Acc Chem Res 2011;44(10):990–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Daum N, Tscheka C, Neumeyer A, Schneider M. Novel approaches for drug delivery systems in nanomedicine: effects of particle design and shape. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2012;4(01):52–65 [DOI] [PubMed] [Google Scholar]
- 302.Caldorera-Moore M, Guimard N, Shi L, Roy K. Designer nano-particles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin Drug Deliv 2010;7(04):479–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater 2009;8(01):15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Champion JA, Katare YK, Mitragotri S. Making polymeric micro-and nanoparticles of complex shapes. Proc Natl Acad Sci U S A 2007;104(29):11901–11904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Doshi N, Orje JN, Molins B, Smith JW, Mitragotri S, Ruggeri ZM. Platelet mimetic particles for targeting thrombi in flowing blood. Adv Mater 2012;24(28):3864–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Anselmo AC, Modery-Pawlowski CL, Menegatti S, et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 2014;8(11):11243–11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wibroe PP, Anselmo AC, Nilsson PH, et al. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat Nanotechnol 2017;12(06):589–594 [DOI] [PubMed] [Google Scholar]
- 308.Pan DC, Myerson JW, Brenner JS, et al. Nanoparticle properties modulate their attachment and effect on carrier red blood cells. Sci Rep 2018;8(01):1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Myerson JW, Braender B, Mcpherson O, et al. Flexible nanoparticles reach sterically obscure endothelial targets inaccessible to rigid nanoparticles. Adv Mater 2018;30(32):e1802373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Brenner JS, Pan DC, Myerson JW, et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat Commun 2018;9(01):2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Modery-Pawlowski CL, Gupta AS. Heteromultivalent ligand-decoration for actively targeted nanomedicine. Biomaterials 2014;35(09):2568–2579 [DOI] [PubMed] [Google Scholar]
- 312.Myerson JW, Anselmo AC, Liu Y, et al. Non-affinity factors modulating vascular targeting of nano- and microcarriers. Adv Drug Del Rev 2016;99(Pt A):97–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Parhiz H, Khoshnejad M, Myerson JW, et al. Unintended effects of drug carriers: Big issues of small particles. Adv Drug Deliv Rev 2018;130:90–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Siepmann J, Siepmann F. Modeling of diffusion controlled drug delivery. J Control Release 2012;161(02):351–362 [DOI] [PubMed] [Google Scholar]
- 315.Peppas NA, Narasimhan B. Mathematical models in drug delivery: how modeling has shaped the way we design new drug delivery systems. J Control Release 2014;190:75–81 [DOI] [PubMed] [Google Scholar]
- 316.Shum P, Kim JM, Thompson DH. Phototriggering of liposomal drug delivery systems. Adv Drug Deliv Rev 2001;53(03):273–284 [DOI] [PubMed] [Google Scholar]
- 317.Schmaljohann D Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev 2006;58(15):1655–1670 [DOI] [PubMed] [Google Scholar]
- 318.Marsh JN, Senpan A, Hu G, et al. Fibrin-targeted perfluorocarbon nanoparticles for targeted thrombolysis. Nanomedicine (Lond) 2007;2(04):533–543 [DOI] [PubMed] [Google Scholar]
- 319.Meng F, Zhong Z, Feijen J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules 2009;10(02):197–209 [DOI] [PubMed] [Google Scholar]
- 320.Ma Y-H, Wu S-Y, Wu T, Chang YJ, Hua MY, Chen JP. Magnetically targeted thrombolysis with recombinant tissue plasminogen activator bound to polyacrylic acid-coated nanoparticles. Biomaterials 2009;30(19):3343–3351 [DOI] [PubMed] [Google Scholar]
- 321.Bi F, Zhang J, Su Y, Tang YC, Liu JN. Chemical conjugation of urokinase to magnetic nanoparticles for targeted thrombolysis. Biomaterials 2009;30(28):5125–5130 [DOI] [PubMed] [Google Scholar]
- 322.Paasonen L, Sipilä T, Subrizi A, et al. Gold-embedded photosensitive liposomes for drug delivery: triggering mechanism and intracellular release. J Control Release 2010;147(01):136–143 [DOI] [PubMed] [Google Scholar]
- 323.Korin N, Kanapathipillai M, Matthews BD, et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 2012;337(6095):738–742 [DOI] [PubMed] [Google Scholar]
- 324.Holme MN, Fedotenko IA, Abegg D, et al. Shear-stress sensitive lenticular vesicles for targeted drug delivery. Nat Nanotechnol 2012;7(08):536–543 [DOI] [PubMed] [Google Scholar]
- 325.Lin KY, Kwong GA, Warren AD, Wood DK, Bhatia SN. Nanoparticles that sense thrombin activity as synthetic urinary biomarkers of thrombosis. ACS Nano 2013;7(10):9001–9009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Lin KY, Lo JH, Consul N, Kwong GA, Bhatia SN. Self-titrating anticoagulant nanocomplexes that restore homeostatic regulation of the coagulation cascade. ACS Nano 2014;8(09):8776–8785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Hansen AH, Mouritsen OG, Arouri A. Enzymatic action of phospholipase A2 on liposomal drug delivery systems. Int J Pharm 2015;491(1–2):49–57 [DOI] [PubMed] [Google Scholar]
- 328.Tasci TO, Disharoon D, Schoeman RM, et al. Enhanced fibrinolysis with magnetically powered colloidal microwheels. Small 2017;13(36). Doi: 10.1002/smll.201700954 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


