Abstract
Glutamine addiction is an important phenotype displayed in some types of cancer. In these cells, glutamine depletion results in a marked reduction in the aggressive cancer phenotype. Mesothelioma is an extremely aggressive disease that lacks effective therapy. In the present study we show that mesothelioma tumors are glutamine addicted suggesting that glutamine depletion may be a potential therapeutic strategy. We show that glutamine restriction, by removing glutamine from the medium or treatment with inhibitors that attenuate glutamine uptake (V-9302) or conversion to glutamate (CB-839), markedly reduces mesothelioma cell proliferation, spheroid formation, invasion and migration. Inhibition of the SLC1A5 glutamine importer, by knockout or treatment with V-9302, an SLC1A5 inhibitor, also markedly reduces mesothelioma cell tumor growth. A relationship between glutamine utilization and YAP1/TEAD signaling has been demonstrated in other tumor types, and the YAP1/TEAD signaling cascade is active in mesothelioma cells and drives cell survival and proliferation. We therefore assessed the impact of glutamine depletion on YAP1/TEAD signaling. We show that glutamine restriction, SLC1A5 knockdown/knockout, or treatment with V-9302 or CB-839, reduces YAP1 level, YAP1/TEAD-dependent transcription, and YAP1/TEAD target protein (e.g., CTGF, cyclin D1, COL1A2, COL3A1, etc.) levels. These changes are observed in both cells and tumors. These findings indicate that mesothelioma is a glutamine addicted cancer, show that glutamine depletion attenuates YAP1/TEAD signaling and tumor growth, and suggests that glutamine restriction may be useful as a mesothelioma treatment strategy.
Keywords: Glutamine depletion therapy, mesothelioma, SLC1A5, GLS, glutamine addiction
Introduction
The high level of glutamine in blood provides a ready source of carbon and nitrogen that can be used by cancer cells to support rapid cell prolifeation and drive tumour growth 1. Glutamine is transported into cells via the SLC1A5 transporter (ASCT2) 1. After uptake by the cell, glutamine is converted by mitochondrial glutaminases to glutamate and ammonium ion. Glutamate can then be converted to α-ketoglutarate, which enters the tricarboxylic acid cycle to generate adenosine triphoasphate through production of NADH and FADH2 2,3. There are two glutaminase enzymes, kidney-type glutaminase (GLS) and liver-type glutaminase (GLS2) 4. GLS is broadly expressed in tissues and plays an important role in cancer.
The importance of glutamine/glutamate as cancer cell survival substrates has driven studies designed to limit their availability to cancer cells 5-8 and the clinical importance of glutamine depletion therapy has been demonstrated by studies showing that inhibiting glutamine utilization attenuates patient cancer 9,10. Glutamine depletion strategies include reducing blood glutamine level, inhibiting cancer cell glutamine uptake and conversion to glutamate 9,10, and increasing cancer cell glutamate export and conversion to glutathione (GSH) 11,12. Agents that have been used to modulate intracellular glutamine/glutamate level include asparaginase (Asp) treatment which depletes blood glutamine level by deaminating glutamine 10,13, treatment with V-9302 which inhibits SLC1A5-dependent glutamine uptake, and treatment with CB-839 which inhibits GLS catalyzed conversion of glutamine to glutamate 3. Treating with one or more of these agents can markedly reduce intracellular glutamate levels 3,14,15.
YAP1/TEAD signaling is an important pro-cancer signaling pathway 16-19. YAP1 and TAZ factors interact with nuclear TEAD transcription factors to drive cancer cell survival, gene expression and tumor growth 20. YAP1/TAZ and TEAD have a role in mesothelioma where they are overexpressed and drive the malignant phenotype 21-23 and our previous study shows that YAP1/TEAD signaling is required for optimal mesothelioma cell proliferation and tumor formation 24. YAP1/TEAD signaling modulates nutrient utilization including glutamine metabolism. For example, YAP1/TEAD signaling upregulates GLS activity to facilitate glutamine conversion to glutamate 25,26 and stimulates transcription activity of genes encoding glutamine-metabolizing enzymes 26-29. In addition, silencing YAP1/TEAD signaling reduces SLC1A5 levels 27.
Mesothelioma is an aggressive/fatal cancer 30,31 that is treated by tumor-reduction surgery coupled with cisplatin/pemetrexed chemotherapy 31. This protocol, which has been in place for several decades, is only marginally successful leaving a poor clinical outcome, prolonged patient suffering and reduced life expectancy. These observations point to a pressing need for new treatment strategies. Our novel preliminary findings provide evidence that mesothelioma is a glutamine addicted cancer and that glutamine depletion reduces YAP1/TEAD pro-cancer signaling. These findings suggest that glutamine/glutamate restriction may be a viable and potent mesothelioma treatment option.
Materials and Methods
Antibodies and Reagents
RPMI1640 with L-glutamine (10-040-CV) and RPMI1640 without L-glutamine (15-040-CV) were purchased from Corning (Glendale, AZ). L-glutamine (25030-081) and 0.25% trypsin-EDTA (25200-056) were obtained from Gibco (Gaithersburg, MD). Fetal calf serum (FCS, F4135), anti-β-actin (A5441) and 4’,6-diamidino-2-phenylindole (DAPI, D9542) were purchased from Sigma (St. Louis, MO). Matrigel (354234) and BioCoat Millicell inserts (359097) were obtained from BD Bioscience (Franklin Lake, NJ). Peroxide conjugated anti-rabbit IgG (NA934V) and peroxide-conjugated anti-mouse IgG (NA931V) were purchased from GE Healthcare (Piscataway, NJ) and used at 1:5000 dilution. V-9302 (S8818) and CB-839 (S7655) were obtained from Selleck Chemicals LLC (Houston, TX). Control-siRNA (D-001206-13-05) and SLC1A5-siRNA (M-007429-01-0005) were purchased from Dharmacon (Lafayette, CO). Electroporation of siRNA was done using Amaxa Solution (VPD-1002) from Lonza (Williamsport, PA). The 8xGTIIC-Luciferase plasmid (8xGTIIC-Luc) was obtained from Addgene (Watertown, MA) and Empty vector-luciferase (EV-Luc) control plasmid, which lacks enhancer elements, was purchased from Promega (Madison, WI). Anti-SLC1A5 (8057S), anti-YAP1 (4912S), anti-YAP1-P (13008S), anti-TAZ (4883), anti-pan-TEAD (13295S), anti-CTGF (10095S), anti-CCND1 (cyclin D1, 2922S) and anti-Snail (3895S) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-Twist (ab49254), anti-Slug (ab27568) and anti-COL1A2 (ab96723) antibodies were obtained from Abcam (Waltham, MA). Anti-COL3A1 was purchased from GeneTex (Irvine, CA). The Student‘s t-test was used to assess significance. All values are presented as mean ± SEM. Asterisks indicate a significant reduction.
Cell proliferation, spheroid formation, invasion and migration assays
Meso-1 and NCI-Meso-17 are malignant cell lines derived, respectively, from peritoneal and pleural mesothelioma 32,33. Routine growth was in RPMI1640 supplemented with 2 mM L-glutamine and 5% FCS. For cell growth assays, cells were plated at low density on standard plastic dishes and growth was monitored by cell counting on the days indicated in indvidual figures. Spheroid assays were used to derive highly enriched mesotheliomal cancer stem cell-like cells (MCS cells) and to measure the impact of glutamine restriction on cell function 34. In this assay, cells were plated into ultra-low attachment dishes and spheriod growth was monitored 34. Spheroids, defined as a collection of cells with diameter ≥ 25 μM, were counted at 0 - 6 d. For invasion assay, BioCoat Millicell inserts (1 cm diameter, 8 μm pores), coated with 120 μl of 250 μg/ml Matrigel (BD Biolabs) diluted in 0.01 M Tris-HCl/0.7% NaCl, were placed individually into wells of a flat-bottom 24-well plate. Cells (20,000) were seed atop the Matrigel in growth medium containing 1% FCS, while the bottom chamber contained growth medium containing 10% FCS. Cell invasion was measured from 0 - 20 h and invaded cells were detected by staining with DAPI. For migration, confluent monolayer cultures were wounded using a 10 μl pipette tip, washed to remove excess cells, and wound closure was monitored from 0 - 24 h. Cell line authenticity was confirmed by short tandem repeat analysis in the Translational Genomics Shared Service of the Greenebaum Comprehensive Cancer Center at the University of Maryland School of Medicine.
Gene knockdown
Near-confluent cells were harvested with trypsin and replated the night before electroporation. For electroporation, 1.2 million cells/group were resuspended in 100 μl of Lonza VPD-1002 nucleofection reagent (Wakersville, MD) containing 3 μg of siRNA and electroporated using an AMAXA electroporator on the T-018 setting 35,36. At 48 h, this process was repeated and the cells were permitted to recover before use in biological assays.
Luciferase assay
To measure the impact of SLC1A5 knockdown on YAP1/TEAD transcription, Meso-1 or NCI-Meso-17 cells were double electroporated with control- or SLC1A5-siRNA 37,38. At 48 h after the second electroporation 40,000 cells were seeded in 12 well plates and transfected with 1 μg of EV-Luc or YAP1/TEAD response element containing vector (8XGTIIC-Luc) using Fugene 6. DNA and transfection reagent were prepared as per the manufacturer’s protocol. After 24 h, extracts were prepared for luciferase activity assay. For inhibitor treatment 40,000 cells were plated in 12 well plates and transfected with 1 μg of EV or 8XGTIIC-Luc vector using Fugene 6 and then treated with 15 μM V-9302 or 1 μM CB-839. At 24 h extracts were prepared for luciferase activity. To characterize YAP1/TEAD transcription activity in SLC1A5 knockout cells, 40,000 Meso-1 or Meso1-SLC1A5 knockout cells were seeded in 12 well plates and transfected with 1 μg of EV or 8XGTIIC-Luc and after 24 h extracts were prepared for luciferase activity assay.
Creating SLC1A5 knockout cells
SLC1A5-specific CRISPR guide RNA, forward (5’-cacc GAG CTG TGC AAT GAA CAC TG) and reverse (5’-aaac CAG TGT TCA TTG CAC AGC TC), were identified at http://crispr.technology and cloned in the U6-driven pSpCas9(BB)-2A-Puro (PX459) V2.0 vector from Addgene (#2429). Cells were electroporated with 3 μg of plasmid using the AMAXA electroporator. At 48 h post-electroporation cells were treated with 2 μg/ml puromycin for 24 h and single cell-derived SLC1A5 knockout clones were selected by dilution cloning.
Immunoblot
Lysates were prepared in Laemmli buffer (0.063 M Tris-HCl, pH 7.5, 10% glycerol, 5% sodium dodecyl sulfate, 5% β-mercaptoethanol) and equal amounts of protein were electrophoresed on denaturing and reducing polyacrylamide gels prior for transfer to nitrocellulose. The membranes were blocked in 5% non-fat dry milk for 1 h and then incubated with primary antibodies (1:1000) followed by washing and incubation with secondary antibody (1:5000) for 2 h. Protein blots were performed a minimum of two times and a representative findings are presented.
Tumor xenografts
MCS cells, grown as spheroids on ultralow attachment plates, were prepared as a single cell suspension and 3 million cells were resuspended in 100 μl phosphate buffered saline containing 30% Matrigel and injected subcutaneously into each front flank of five eight-week-old female NOD/scid/IL-2 receptor gamma chain (NSG) knockout mice. At 7 weeks after tumor cell injection, when the tumors became palpable, treatment was initiated with 0 or 15 mg/kg V-9302, dissolved in 2% dimethyl sulfoxide in phosphate buffered saline, and delivered by intraperitoneal injection three times per week. Wild-type and SLC1A5 knockout Meso-1 tumor growth was also compared. Meso1-SLC1A5-KOc1-1-1 SLC1A5 knockout cells were injected at 3 million cells/each front flank and tumor growth was monitored in comparison to wild-type Meso-1 cells 34. Tumors were harvested and stored frozen. To prepare tumor lysates, frozen tumors were puliverized and then the powder was dissolved in Laemmli sample buffer and boiled before immunoblot. Tumor growth was monitored over ten weeks and tumor volume was measured using the formula 4/3π x (diameter/2)3. The animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Maryland Baltimore.
Results
Glutamine depletion reduces the mesotheliom cancer phenotype
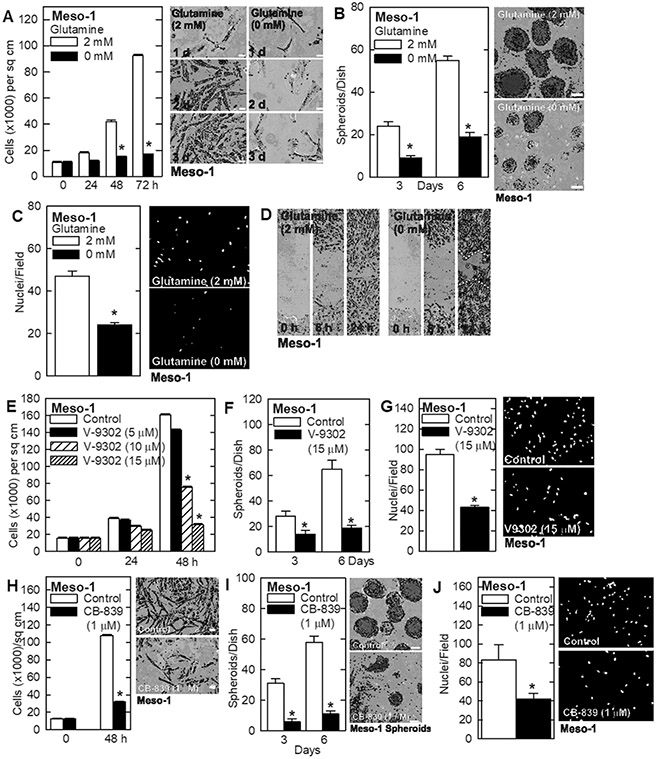
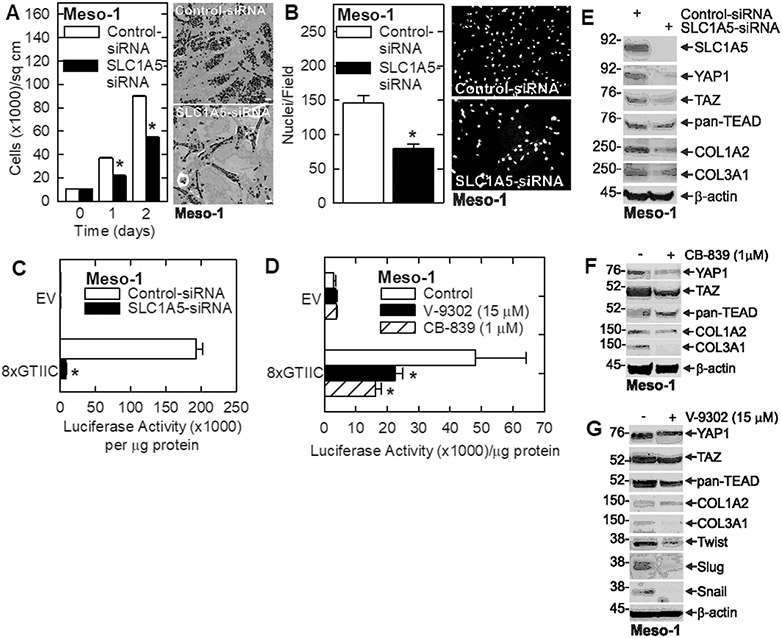
Glutamine addiction is an important property of various cancer types that can be exploited therapeutically 2,3. A major goal of this study is to assess the response of mesothelioma cancer cells to glutamine restriction. To assess the role of glutamine in maintaining the mesothelioma cancer cell phenotype, we grew Meso-1 cells in medium containing 0 or 2 mM glutamine and monitored the impact on cancer endpoints. We observed a marked reduction in cell proliferation (Fig. 1A), spheroid formation (Fig. 1B), invasion through matrigel (Fig. 1C) and migration (Fig. 1D) in glutamine-free conditions. To confirm that the cells require glutamine to maintain the aggressive cancer phenotype, we treated with V-9302, which inhibits SLC1A5, a plasma membrane transporter that imports glutamine 39, and CB-839 which inhibits GLS, the enzyme that converts glutamine to glutamate 11. Treatment with V-9302 produces a dose-dependent decrease in cell proliferation (Fig. 1E), inhibits spheroid formation (Fig. 1F) and reduces invasion through matrigel (Fig. 1G). Treatment with CB-839 also reduces these cancer cell characteristics (Fig. 1H/I/J). We also measured the impact of SLC1A5 knockdown on the cancer phenotype. Treatment of Meso-1 cells with SLC1A5-siRNA reduced cell proliferation (Fig 2A) and invasion (Fig. 2B). These findings strongly suggest that mesothelioma cancer cells require glutamine to maintain the aggressive cancer phenotype and are glutamine addicted.
Fig. 1.
Glutamine is essential to maintain an aggressive mesothelioma cell phenotype. A/B Meso-1 cells were grown as monolayer cultures in regular cell culture dishes or as cancer stem-like cell-enriched spheroids in ultra-low attachment dishes. The absence of glutamine reduces proliferation and spheroid growth. C/D Absence of glutamine reduces Meso-1 cell invasion through matrigel and wound closure. E/F/G Treatment with the glutamine transporter inhibitor, V-9302, reduces Meso- 1 cell growth, spheroid formation and invasion. H/I/J Treatment with the glutaminase (GLS) inhibitor, CB-839, reduces Meso-1 cell growth, spheroid formation and cell invasion. Cell growth, spheroid formation and invasion were monitored as outlined in Materials and Methods. The values are mean ± SEM an asterisk indicates a significant reduction in response compares to control, n = 3, p < 0.05. Bars = 50 microns in all panels.
Fig. 2.
Knockdown of the SLC1A5 glutamine transporter reduces the Meso-1 cell cancer phenotype. A/B Meso-1 cells treated with 3 μg of control- or SLC1A5-siRNA and cell growth and matrigel invasion were monitored. C Meso-1 cells were electroporated with 3 μg of control- or SLC1A5-siRNA and then transfected with 1 μg of EV-Luc or 8xGTIIC-Luc (encodes TEAD response elements) plasmids and luciferase activity was monitored at 24 h. D Meso-1 cells were transfected with 1 μg of EV-Luc or 8xGTIIC luciferase plasmids and then treated with the indicated level of V-9302 or CB839 and at 24 h luciferase activity was measured. E/F/G Effect of SLC1A5 knockdown and CB-839 or V-9302 treatment on SLC1A5 and YAP1/TEAD signaling proteins in Meso-1 cells. The values are mean ± SEM and the asterisks indicate a significant reduction compared to control, n = 3, p < 0.05. Bars = 50 microns in all panels. Cell growth and invasion were monitored as outlined in Materials and Methods.
Role of YAP1/TEAD signaling
YAP1/TEAD signaling has been reported to be impacted by alterations in glutamine metabolism 25,40 and so we monitored the impact of SLC1A5 knockdown, and treatment wtih V-9302 and CB-839, on YAP1/TEAD dependent transcription. Meso-1 cells were transfected with EV-Luc or a YAP1/TEAD transcription reporter plamid (8xGTIIC-Luc) encoding the TEAD response element linked to the luciferase gene 41,42. Fig. 2C shows that SLC1A5 knockdown reduces YAP1/TEAD dependent transcription, as does treatment with V-9302 or CB-839 (Fig. 2D). To better understand this regulation, we examined the effect of SLC1A5 knockdown on expression of Hippo signaling related proteins 24,43. Fig. 2E shows that YAP1, TAZ and pan-TEAD protein levels are reduced in response to SLC1A5 knockdown and that this is associated with a reduction in YAP1/TEAD regulated collagen gene (COL1A2 and COL3A1) expression. Consistent with these findings, treatment with CB-839 reduces YAP1, TAZ, COL1A2 and COL3A1 levels (Fig. 2F), while V-9302 treatment reduces YAP1, TAZ, pan-TEAD, and COL3A1, but not COL1A2 (Fig. 2G). We also show that V-9302 treatment reduces the levels of the Twist, Slug and Snail sugggesting that EMT (epithelial mesenchymal transition) is attenuated (Fig. 2G).
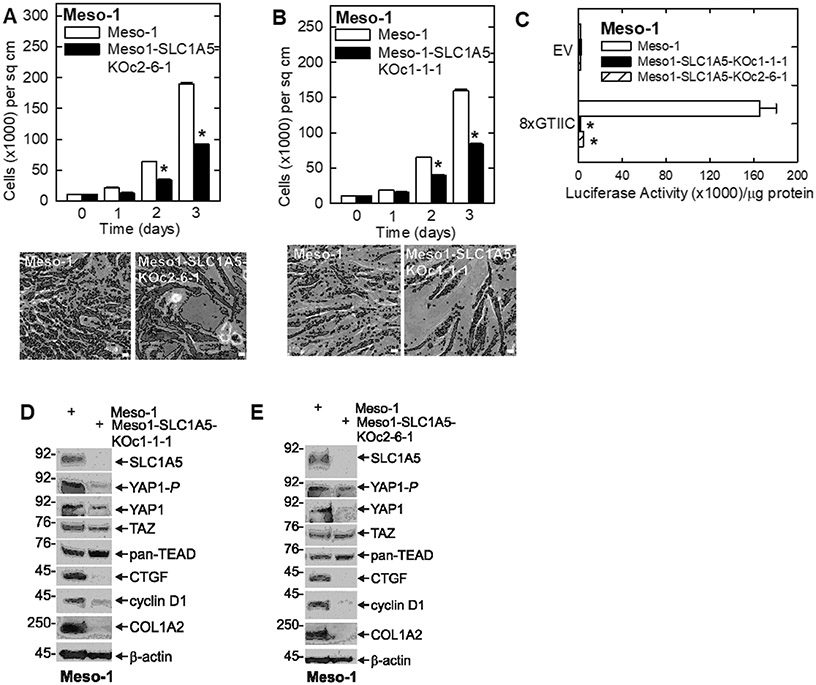
We next examined the impact of SLC1A5 knockout on the cancer cell phenotype and expression of YAP1/TEAD signaling proteins. We constructed SLC1A5 knockout clones using CRISPR/Cas9. Fig. 3A/B shows that proliferation of the SLC1A5 knockout cell lines (Meso1-SLC1A5-KOc2-6-1 and Meso1-SLC1A5-KOc1-1-1) is markedly reduced compared to wild-type Meso-1 cells. Moreover, as shown in Fig. 3C, SLC1A5 knockout results in a marked reduction in YAP1/TEAD transcription as measured by the reduction in 8xGTIIC-Luc activity. We also compared the impact of SLC1A5 knockout on expression of YAP1/TAZ/TEAD and selected downstream targets. Fig. 3D/E show a reduction in YAP1 and YAP1-P in both SLC1A5 knockout cell clones. TAZ levels are also slighty decreased, but pan-TEAD levels are increased. It is possible that the increase in pan-TEAD level is an effort by the cells to compensate for the loss of YAP1 signaling. Among the downstream target genes, CTGF, cyclin D1 and COL1A2 are reduced. These findings indicate that SLC1A5 knockout reduces YAP1/TEAD level and YAP1/TEAD dependent transcription and that this is associated with a reduction in some YAP1/TEAD target proteins.
Fig. 3.
SLC1A5 knockout reduces Meso-1 cell growth and YAP1/TEAD signaling. A/B Cell growth assays show that two independent Meso-1 SLC1A5 knockout cell lines display reduced cell proliferation. C Wild-type Meso-1 cells and two SLC1A5 knockout Meso-1 cell lines were transferted with 1 μg of EV-Luc or 8xGTIIC luciferase reporter vector and after 24 h extracts were prepared to monitor luciferase activity. D/E Extracts of wild-type and SLC1A5 knockout Meso-1 cells were prepared and assayed for the level of YAP1/TEAD and related proteins. The values are mean ± SEM and asterisks indicate a significant reduction in response compares to control, n = 3, p < 0.05. Bars = 50 microns in all panels. Cell growth was monitored a described in Materials and Methods.
Glutamine depletion suppresses the cancer phenotype in NCI-Meso-17 cells
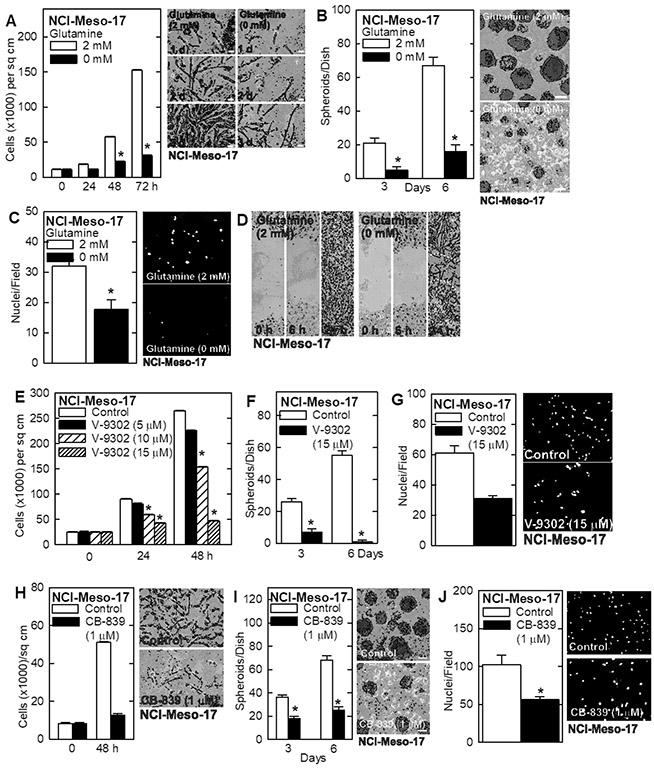
It is important to confirm these findings in a second mesothelioma cell line. We selected NCI-Meso-17 cells which is an aggressive tumor-forming cell line derived from pleural mesothelioma 33. Glutamine restriction reduces NCI-Meso-17 cell proliferation (Fig. 4A) and ability to form cancer stem-like cell enriched spheroids (Fig. 4B). Glutamine restriction also reduces the ability of these cells to invade matrigel (Fig. 4C) and migrate in monolayer culture (Fig. 4D). In addition, V-9302 treatment produces a dose-dependent reduction in cell proliferation (Fig. 4E), spheroid formation (Fig. 4F) and matrigel invasion (Fig. 4G). We repeated these experiments with the GLS inhibitor, CB-839, and found a similar reduction in proliferation, spheroid formation and matrigel invasion (Fig. 4H/I/J).
Fig. 4.
Glutamine is essential to maintain the cancer phenotype of NCI-Meso-17 cells. A/B NCI-Meso-17 cells were grown as monolayer or spheroid (MCS cell enriched) cultures. Treatment with glutamine-free medium reduces proliferation and spheriod growth. C/D NCI-Meso-17 cells were grown in glutamine-free or 2 mM glutamine containing medium. Treatment with glutamine-free medium reduces invasion and migration. E/F/G Treatment of NCI-Meso-17 cells with V-9302 reduces cell growth, spheroid formation and cell invasion. H/I/J Treatment of NCI-Meso-17 cells with CB-839 (GLS inhibitor) reduces cell growth, spheroid formation and cell invasion. The values are mean ± SEM and asterisks indicate a significant change reduction as compared to control, n = 3, p < 0.05. Bars = 50 microns in all panels. Cell growth, spheroid formation, invasion and migratoin were monitored as outlined in Materials and Methods.
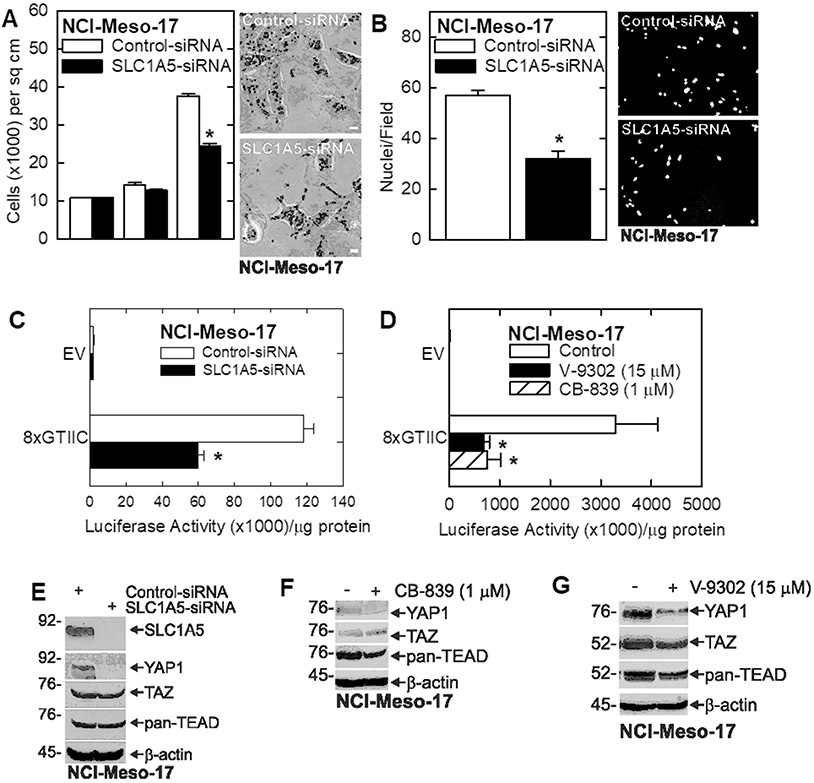
To assess the impact of SLC1A5 knockdown on the cancer phenotype, we treated NCI-Meso-17 cells with SCL1A5-siRNA. SLC1A5 knockdown causes NCI-Meso-17 cells to grow more slowly (Fig. 5A) and invade matrigel less efficiently (Fig. 5B). In addition, SLC1A5 knockdown (Fig. 5C), or treatment with V-9302 or CB-839 (Fig. 5D), reduces YAP1/TEAD-dependent transcription and this is associated with reduced YAP1 levels with variable change in TAZ and pan-TEAD levels (Fig. 5E/F/G). The reduction in YAP1 level in these cells most likely accounts for the reduction in YAP1/TEAD-dependent transcription.
Fig. 5.
Knockdown of the SLC1A5 glutamine transporter reduces the NCI-Meso-17 cell cancer phenotype. A/B NCI-Meso-17 cells were treated 3 μg of control- or SLC1A5-siRNA and after 48 h cell growth and matrigel invasive were monitored. C NCI-Meso-17 cells were electroporated with 3 μg of control- or SLC1A5-siRNA and then transfected with 1 μg of EV-Luc or 8xGTIIC-Luc. At 24 h extracts were prepared to monitor luciferase activity. D NCI-Mesoo-17 cells were transfected with 1 μg of EV-Luc or 8xGTIIC-Luc and then treated with or V-9302 or CB-839. At 24 h extracts were prepared to monitor luciferase activity. E/F/G NCI-Meso-17 cells were treated with control- or SLC1A5-siRNA, or with V-9302 (SLC1A5 inhibitor) or CB-839 (GLS inhibitor), and the impact on YAP1/TEAD signaling proteins was monitored by immunoblot. The values are mean ± SEM and asterisks indicate a significant reduction compared to control, n = 3, p < 0.05. Bars = 50 microns in all panels.
Impact of glutamine depletion on tumor formation
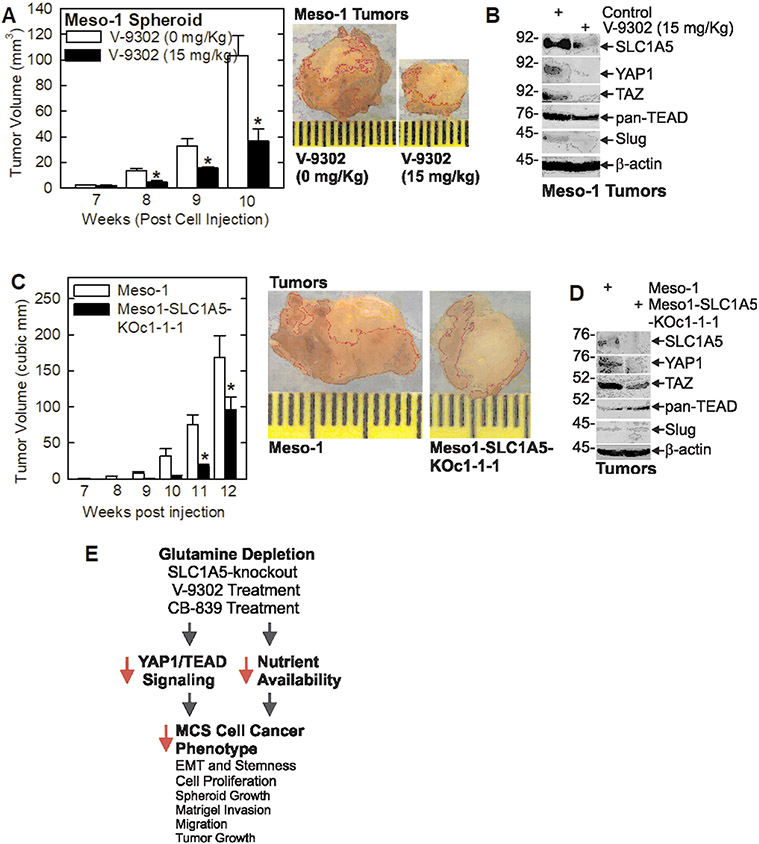
We next monitored the impact of V-9302 treatment and SLC1A5 knockout on tumor growth. Spheroid-derived Meso-1 cells were injected into each front flank in NSG mice and when tumors became palpable treatment was initiated with V-9302. Fig. 6A/B show that V-9302 treatment causes a marked reduction in tumor formation. Moreover, this is associated with reduced levels of SLC1A5, YAP1, TAZ, pan-TEAD and the Slug EMT marker. Characteriztaion of SLC1A5 knockout cells (Meso1-SLC1A5-KOc1-1-1) (Fig. 6C/D) revealed a marked reduction in tumor growth which was associated with reduced SLC1A5, YAP1 and TAZ, and reduced Slug levels. Pan-TEAD levels are increased in this case, perhaps to compensate for the loss of YAP1/TAZ stimulation.
Fig. 6.
Inhibition of SLC1A5 activity suppresses mesothelioma tumor growth. A/B Meso-1 cells, grown as spheroids, were injected (3 million cells/site) into each front flank in NSG mice (5 mice/group). When tumors were first detected, at 7 weeks, treatment was initiated three times/week with 0 or 15 mg/Kg V-9302 (SLC1A5 inhibitor). Tumor size was monitored weekly and at 10 weeks the tumors were harvested and imaged, and total tumor lysate was prepared to detect expression of the indicated markers. C/D Wild-type and SLC1A5 knockout (Meso1-SLC1A5-KOc1-1-1) Meso-1 cells, grown as spheroids, were injected into each front flank (3 million cells/site) in NSG mice (5 mice/group). Tumor size was measured weekly and at 12 weeks the tumors were harvested and imaged, and total cell lysate was prepared to detect expression of the indicated markers. Values are mean ± SEM and asterisks indicate a significant reduction as compared to control, n = 10 tumors, p < 0.05. E Schematic describing the impact of glutamine restriction on human mesothelioma cells and tumors. The model is described in the Discussion.
Discussion
Glutamine metabolism in cancer
Glutamine is the most abundant amino acid in blood and as such serves as an important metabolic substrate to maintain tissues. Moreover, glutamine is essential for cancer cell survival and proliferation in a range of cancer types. This is because glutamine can serve as a substrate to fuel multiple metabolic pathways including the Krebs cycle, redox homeostasis, and synthesis of cellular building blocks including nucleic acids, fatty acids, GSH and amino acids 44. Moreover, enhanced glutaminolysis has been linked to the accumulation of oncogenic metabolites including 2-Hydroxyglutarate, succinate and fumarate 44. The importance of glutamine has led to consideration of glutamine depletion as a strategy to treat a range of cancers including melanoma 45, lung 46, pancreatic 47, breast 48, prostate 49 and acute myeloid leukemia 9,10,50-52.
Mesothelioma is a glutamine addicted cancer
Mesothelioma is a highly aggressive and fatal cancer 30,31. Treatment involves tumor-reduction surgery, cisplatin/pemetrexed chemotherapy 31 and/or immune therapy 53-55. However, these strategies are only marginally successful leaving an extremely poor clinical outcome and limited life expectancy. These observations point to a significant need for new treatment strategies. Although, as noted above, the use of glutamine depletion strategies has been reported for a range of leukemias and solid tumor types, to our knowledge no manuscripts have reported glutamine depletion studies using mesothelioma cancer models.
Our present studies suggest that mesothelioma is a glutamine addicted cancer. This is based on glutamine depletion and pharmacologic inhibitor studies showing that removing glutamine from the cell culture medium, or treating with glutamine uptake inhibitor or glutaminase inhibitor, suppress mesothelioma cell proliferation, spheroid formation, invasion and migration. Moreover, treatment with V-9302, a SLC1A5 inhibitor, which inhibits cancer cell uptake of glutamine, markedly reduce tumor xenograft growth. SLC1A5 knockout also produced a marked reduction in tumor growth. These findings suggest that glutamine depletion therapy may benefit mesothelioma patients.
YAP1/TEAD signaling changes in glutamine depleted cells
YAP1/TEAD signaling is a centrally important pro-cancer signaling pathway 16 and is often highly activated cancer cells 17-19. YAP1 and TAZ factors interact with nuclear TEAD transcription factors to drive cancer cell survival-related gene expression and tumor growth 20 and are considered important cancer therapy targets 20. YAP1/TAZ and TEAD have a role in mesothelioma where they are overexpressed and drive the malignant phenotype 21-23. Our previous study shows that YAP1/TEAD signaling is required for optimal mesothelioma cell proliferation and tumor formation 24. We now show that glutamine depletion reduces mesothelioma cell YAP1/TEAD signaling. YAP1 level is reduced in SLC1A5 knockdown cells and in cells treated with agents that inhibit glutamine uptake (V-9302) and conversion to glutamate (CB-839). Using a TEAD transcription promoter linked to luciferase, we showed that these treatments reduce YAP1/TEAD dependent transcription and that this is associated with reduced levels of selected downstream YAP1/TEAD target proteins (CTGF, COL1A2, COL3A1). Similar changes were observed in SLC1A5 knockout cells. Although we do not yet know the detailed mechanism, these findings suggest that glutamine starvation communicates to the cell that proliferation is not permitted thereby reducing YAP1/TEAD signaling. We note that that these responses are observed in both Meso-1 and NCI-Meso-17 cells, suggesting that these responses represent a common mode of regulation in multiple mesothelioma tumor cell lines. An interesting feature is that manipulations that target SLC1A5 (SLC1A5 knockdown and V-9302 treatment) are more effective than treatment with CB-839 which targets GLS activity.
These findings are consistent with reports in other cancers that glutamine restriction modifies signaling cascades related to nutrient deprivation and cell proliferation. Prominent among these responses are changes in YAP1/TEAD signaling 25,40. Moreover, YAP1/TEAD signaling modulates nutrient utilization including glutamine metabolism. For example, YAP1/TEAD signaling upregulates GLS activity to facilitate glutamine conversion to glutamate 25,26, stimulates transcription activity of genes encoding glutamine-metabolizing enzymes 26-29 and increases transcription of SLC1A5 to enhance cellular glutamine uptake 27. In addition, silencing YAP1/TEAD signaling reduces SLC1A5 levels 27. Our previous studies show that YAP1/TEAD signaling is an important survival pathway in mesothelioma 24 and the present studies show that YAP1/TEAD signaling is reduced by inhibiting glutamine utilization.
Glutamine starvation and tumor growth
We also examined the impact of glutamine depletion using a xenograft tumor model. We show that both SLC1A5 knockout and treatment with V-9302 reduces tumor formation. A minimum 50% reduction in tumor size is observed in each case. Analysis of tumor extracts reveal a substantial and consistent reduction in YAP1 signaling. V-9302 treated cells display reduced levels of SLC1A5, YAP1, TAZ and pan-TEAD and reduced levels of the EMT marker, Slug. SLC1A5 knockout tumors display reduced SLC1A5, YAP1, TAZ and Slug levels. In this case pan-TEAD levels are elevated, perhaps as a compensatory mechanism. These studies confirm that mesothelioma tumor growth is highly suppressed by inhibition of SL1CA5 activity either, using an inhibitor or by SLC1A5 knockout, and that this reduction is associated with reduced YAP1/TEAD signaling.
As shown in the model in Fig. 6E, our studies suggest that that mesothelioma is a glutamine addicted cancer and that depletion of glutamine in the cell culture medium, inhibition or knockdown/knockout of the SLC1A5 glutamine importer, or inhibition of glutaminase, the enzyme that converts glutamine to glutamate, reduces nutrient availability to MCS cells and attenuates YAP1/TEAD signaling. The nutrient deprivation and the accompanying reduction in YAP1/TEAD signaling results in a reduction in cell proliferation, spheroid growth, invasion, migration and tumor growth.
Glutamine is the most abundant amino acid in blood that serves as an important substrate to cancer cells for production of cell substrates and energy. Asparaginase converts asparagine and glutamine to aspartate and glutamate, respectively, thereby decreasing plasma concentrations of asparagine and glutamine which ultimately reduces glutamine availability to the cancer cells 56. Recent studies support the use of asparaginase and asparaginase derivatives as cancer therapeutics 9,10,52,57,58. Mesothelioma is a highly aggressive and treatment-resistant cancer, and our findings suggest that asparaginase-dependent glutamine depletion may be a useful mesothelioma treatment strategy.
Acknowledgements
This work was supported by a gift from the Kazan McClain Partners’ Foundation to RLE. Warren Naselsky was supported by the Cancer Biology T32 Training Grant (T32 CA154274) an International Lung Cancer Foundation Young Investigator Award, and a 2021 iMIG Young Investigator Microgrant Award sponsored Kazan, McClain, Satterley & Greenwood PLC. John J. Newland was also supported by the Cancer Biology T32 Training Grant (T32 CA154274). This work utilized the Translational Genomics Shared Service of the Greenebaum Comprehensive Cancer Center (P30 CA134274) at the University of Maryland School of Medicine.
Support:
National Institutes of Health (R01 CA211909) to Richard L. Eckert and utilized the facilities of the Greenebaum Comprehensive Cancer Center (P30 CA134274) at the University of Maryland School of Medicine.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Data Sharing
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Literature Cited
- 1.Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreadith RW, Lehninger AL. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J Biol Chem. 1984;259(10):6215–6221. [PubMed] [Google Scholar]
- 3.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16(10):619–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–159. [DOI] [PubMed] [Google Scholar]
- 5.Yuneva MO, Fan TW, Allen TD, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15(2):157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao P, Tchernyshyov I, Chang TC, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458(7239):762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu W, Zhang C, Wu R, Sun Y, Levine A, Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A. 2010;107(16):7455–7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emadi A Exploiting AML vulnerability: glutamine dependency. Blood. 2015;126(11):1269–1270. [DOI] [PubMed] [Google Scholar]
- 10.Emadi A, Zokaee H, Sausville EA. Asparaginase in the treatment of non-ALL hematologic malignancies. Cancer Chemother Pharmacol. 2014;73(5):875–883. [DOI] [PubMed] [Google Scholar]
- 11.Gross MI, Demo SD, Dennison JB, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13(4):890–901. [DOI] [PubMed] [Google Scholar]
- 12.Boysen G, Jamshidi-Parsian A, Davis MA, et al. Glutaminase inhibitor CB-839 increases radiation sensitivity of lung tumor cells and human lung tumor xenografts in mice. Int J Radiat Biol. 2019;95(4):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rau RE, Dreyer Z, Choi MR, et al. Outcome of pediatric patients with acute lymphoblastic leukemia/lymphoblastic lymphoma with hypersensitivity to pegaspargase treated with PEGylated Erwinia asparaginase, pegcrisantaspase: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2018;65(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero R, Sayin VI, Davidson SM, et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med. 2017;23(11):1362–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayin VI, LeBoeuf SE, Singh SX, et al. Activation of the NRF2 antioxidant program generates an imbalance in central carbon metabolism in cancer. Elife. 2017;6:e28083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246–257. [DOI] [PubMed] [Google Scholar]
- 17.Fisher ML, Ciavattone N, Grun D, Adhikary G, Eckert RL. Sulforaphane reduces YAP/Np63alpha signaling to reduce cancer stem cell survival and tumor formation. Oncotarget. 2017;8(43):73407–73418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher ML, Grun D, Adhikary G, Xu W, Eckert RL. Inhibition of YAP function overcomes BRAF inhibitor resistance in melanoma cancer stem cells. Oncotarget. 2017;8(66):110257–110272. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Song S, Ajani JA, Honjo S, et al. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 2014;74(15):4170–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanconato F, Battilana G, Cordenonsi M, Piccolo S. YAP/TAZ as therapeutic targets in cancer. Curr Opin Pharmacol. 2016;29:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekido Y Molecular pathogenesis of malignant mesothelioma. Carcinogenesis. 2013;34(7):1413–1419. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita A, Sato T, Mukai S, et al. TAZ activation by Hippo pathway dysregulation induces cytokine gene expression and promotes mesothelial cell transformation. Oncogene. 2018, 38:1966–1978. [DOI] [PubMed] [Google Scholar]
- 23.Zhang WQ, Dai YY, Hsu PC, et al. Targeting YAP in malignant pleural mesothelioma. J Cell Mol Med. 2017, 21:2663–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kandasamy S, Adhikary G, Rorke EA, et al. The YAP1 Signaling Inhibitors, Verteporfin and CA3, Suppress the Mesothelioma Cancer Stem Cell Phenotype. Mol Cancer Res. 2020;18(3):343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Zhao H, Li Y, et al. The role of YAP/TAZ activity in cancer metabolic reprogramming. Mol Cancer. 2018;17(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertero T, Oldham WM, Cottrill KA, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126(9):3313–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards DN, Ngwa VM, Wang S, et al. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci Signal. 2017;10(508):eaan4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du K, Hyun J, Premont RT, et al. Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Activation of Hepatic Stellate Cells. Gastroenterology. 2018;154(5):1465–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang CS, Stampouloglou E, Kingston NM, Zhang L, Monti S, Varelas X. Glutamine-utilizing transaminases are a metabolic vulnerability of TAZ/YAP-activated cancer cells. EMBO Rep. 2018;19(6):e43577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao S, Jin S, Cao J, et al. Advances in malignant peritoneal mesothelioma. Int J Colorectal Dis. 2015;30(1):1–10. [DOI] [PubMed] [Google Scholar]
- 31.Hassan R, Alexander R, Antman K, et al. Current treatment options and biology of peritoneal mesothelioma: meeting summary of the first NIH peritoneal mesothelioma conference. Ann Oncol. 2006;17(11):1615–1619. [DOI] [PubMed] [Google Scholar]
- 32.Varghese S, Whipple R, Martin SS, Alexander HR. Multipotent cancer stem cells derived from human malignant peritoneal mesothelioma promote tumorigenesis. PLoS One. 2012;7(12):e52825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalra N, Zhang J, Thomas A, et al. Mesothelioma patient derived tumor xenografts with defined BAP1 mutations that mimic the molecular characteristics of human malignant mesothelioma. BMC Cancer. 2015;15(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adhikary G, Grun D, Alexander HR, et al. Transglutaminase is a mesothelioma cancer stem cell survival protein that is required for tumor formation. Oncotarget. 2018;9(77):34495–34505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adhikary G, Chew YC, Reece EA, Eckert RL. PKC-delta and -eta, MEKK-1, MEK-6, MEK-3, and p38-delta Are Essential Mediators of the Response of Normal Human Epidermal Keratinocytes to Differentiating Agents. J Invest Dermatol. 2010, 130:2017–2030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 36.Chew YC, Adhikary G, Wilson GM, Reece EA, Eckert RL. PKCdelta suppresses keratinocyte proliferation by increasing p21CIP1 level by a KLF4-dependent mechanism. J Biol Chem. 2011;286:28771–28782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Shrestha S, Adhikary G, Xu W, Kandasamy S, Eckert RL. ACTL6A suppresses p21(Cip1) expression to enhance the epidermal squamous cell carcinoma phenotype. Oncogene. 2020;39(36):5855–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrestha S, Adhikary G, Naselsky W, Xu W, Friedberg JS, Eckert RL. ACTL6A suppresses p21(Cip1) tumor suppressor expression to maintain an aggressive mesothelioma cancer cell phenotype. Oncogenesis. 2021;10(10):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulte ML, Fu A, Zhao P, et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med. 2018;24(2):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koo JH, Guan KL. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018;28(2):196–206. [DOI] [PubMed] [Google Scholar]
- 41.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. [DOI] [PubMed] [Google Scholar]
- 42.Kegelman CD, Mason DE, Dawahare JH, et al. Skeletal cell YAP and TAZ combinatorially promote bone development. FASEB J. 2018;32(5):2706–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mickle M, Adhikary G, Shrestha S, Xu W, Eckert RL. VGLL4 inhibits YAP1/TEAD signaling to suppress the epidermal squamous cell carcinoma cancer phenotype. Mol Carcinog. 2021;60(7):497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma S, Agnihotri N, Kumar S. Targeting fuel pocket of cancer cell metabolism: A focus on glutaminolysis. Biochem Pharmacol. 2022;198:114943. [DOI] [PubMed] [Google Scholar]
- 45.Ratnikov BI, Scott DA, Osterman AL, Smith JW, Ronai ZA. Metabolic rewiring in melanoma. Oncogene. 2017;36(2):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanhove K, Derveaux E, Graulus GJ, et al. Glutamine Addiction and Therapeutic Strategies in Lung Cancer. Int J Mol Sci. 2019;20(2):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Słotwiński R, Lech G, Słotwińska SM. Molecular aspects of pancreatic cancer: focus on reprogrammed metabolism in a nutrient-deficient environment and potential therapeutic targets. Cent Eur J Immunol. 2021;46(2):258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delgir S, Bastami M, Ilkhani K, Safi A, Seif F, Alivand MR. The pathways related to glutamine metabolism, glutamine inhibitors and their implication for improving the efficiency of chemotherapy in triple-negative breast cancer. Mutat Res Rev Mutat Res. 2021;787:108366. [DOI] [PubMed] [Google Scholar]
- 49.Fidelito G, Watt MJ, Taylor RA. Personalized Medicine for Prostate Cancer: Is Targeting Metabolism a Reality? Front Oncol. 2021;11:778761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emadi A, Jun SA, Tsukamoto T, Fathi AT, Minden MD, Dang CV. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Exp Hematol. 2014;42(4):247–251. [DOI] [PubMed] [Google Scholar]
- 51.Kapadia B, Shetty AC, Bollino D, et al. Translatome changes in acute myeloid leukemia cells post exposure to pegcrisantaspase and venetoclax. Exp Hematol. 2022;108:55–63. [DOI] [PubMed] [Google Scholar]
- 52.Emadi A, Kapadia B, Bollino D, et al. Venetoclax and pegcrisantaspase for complex karyotype acute myeloid leukemia. Leukemia. 2021;35(7):1907–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mansfield AS. Immune checkpoint inhibition in malignant mesothelioma: Does it have a future? Lung Cancer. 2017;105:49–51. [DOI] [PubMed] [Google Scholar]
- 54.Nowak AK, Stockler MR, Byrne MJ. Assessing quality of life during chemotherapy for pleural mesothelioma: feasibility, validity, and results of using the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire and Lung Cancer Module. J Clin Oncol. 2004;22(15):3172–3180. [DOI] [PubMed] [Google Scholar]
- 55.Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20(2):239–253. [DOI] [PubMed] [Google Scholar]
- 56.Beckett A, Gervais D. What makes a good new therapeutic L-asparaginase? World J Microbiol Biotechnol. 2019;35(10):152. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Zhang L, Liu X, et al. Efficacy and Safety of a Pegasparaginase-Based Chemotherapy Regimen vs an L-asparaginase-Based Chemotherapy Regimen for Newly Diagnosed Advanced Extranodal Natural Killer/T-Cell Lymphoma: A Randomized Clinical Trial. JAMA Oncol. 2022;8(7):1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao C, Ma X, Zhang Z, et al. Asparaginase Erwinia chrysanthemi for acute lymphoblastic leukemia and lymphoblastic lymphoma. Drugs Today (Barc). 2022;58(6):261–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.