Abstract
About 5–10% of all ovarian cancer cases show familial clustering, and some 15–25% of familial ovarian cancer cases are mediated by high-penetrance mutations in the BRCA1 and BRCA2 genes. Only few other genes have been identified for familial ovarian cancer.
We conducted targeted next-generation sequencing of the protein coding region of 21 candidate genes, including UTR regions, in genomic DNA samples of 48 patients with familial ovarian cancer from the Republic of Bashkortostan. We identified deleterious variants in BRCA1, BRCA2, CHEK2, MSH6 and NBN in a total of 16 patients (33%). The NBN truncating variant, p.W143X, had not previously been reported. Seven patients (15%) were carriers of the c.5266dupC variant in BRCA1, supporting a Russian origin of this founder allele. An additional 15 variants of uncertain clinical significance were observed. We conclude that our gene panel explains about one-third of familial ovarian cancer risk in the Republic of Bashkortostan.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-023-01119-z.
Keywords: Hereditary ovarian cancer, Target sequencing, Germline mutations, Pathogenic variants, Likely pathogenic variants
Introduction
Ovarian cancer (OC) is the third most common gynecological malignancy following endometrial and cervix cancers. Annually more than 295,000 new cases of the disease and 184,000 associated deaths are registered worldwide [1]. In Russia were registered 14,318 new cases of ovarian cancer and 7,616 deaths in 2018 [2]. The high mortality in ovarian cancer rate can be attributed to the asymptomatic nature of the disease in earlier stages and lack of effective screening methods [3].
Ovarian cancer is polygenic in nature. Genetic factors have an important impact on OC etiology. About 5–10% of all ovarian cancer cases are familial, and about 15–25% of hereditary ovarian cancer (HOC) cases are mediated by high-penetrance mutations in the BRCA1 and BRCA2 genes [4, 5]. According to the ClinVar database, about 3,000 and 3,400 pathogenic sequence variants (PVs) and likely pathogenic variants (LPVs) are known in BRCA1 and BRCA2 (https://www.ncbi.nlm.nih.gov/clinvar). However, additional risk genes for ovarian cancer have been identified encoding proteins involved in homology-directed repair proteins such as PALB2 [6], BRIP1 [7], RAD51C [8], RAD51D [8], or in mismatch repair such as MSH2 or MSH6 [9]. Furthermore, a major fraction of the remaining OC risk is due to sequence changes at other genomic loci with susceptibility variants of moderate to low penetrance [10]. High rates of morbidity and mortality from this cancer type indicate the need for a deeper understanding of the disease molecular genetic basis, which in turn will contribute to the development of new approaches to the diagnosis and treatment of OC.
Attractive methods for searching gene variants involved in the cancer pathogenesis are next generation sequencing (NGS) technologies, which allow the simultaneous analysis of millions of DNA samples. One of the widely used NGS technologies is targeted sequencing. This approach allows the simultaneous analysis of several genes. Using targeted sequencing, some researchers have identified the mutational spectra of genes associated with breast and/or ovarian cancer and reported pathogenic abnormalities in genes (CHEK2, ATM, NBN, RAD50, RAD51C, RAD51D, BRIP, etc.) involved in cell response to DNA damage, homologous recombination repair, cell cycle checkpoint, or apoptosis with hereditary ovarian cancer [11–13]. Pathogenic variants in genes whose protein products are involved in Fanconi Anemia (FA) signaling pathway and the mismatch repair pathway (MMR) were also identified in patients with breast cancer and ovarian cancer. In recent years, several studies have been published in which patients with hereditary breast and ovarian cancer (HBOC) were investigated using targeted sequencing not only in the BRCA1 and BRCA2 genes, but also in other candidate genes. For instance, the Ovarian Cancer Association Consortium has sequenced several dozens of candidate genes in more than 3,000 unselected ovarian cancer cases and 3,000 healthy controls [6, 7, 14]. However, there are noticeable differences in the distribution of the spectrum and frequencies of genetic variants between different regions and populations in patients with ovarian cancer, which can be associated with the accumulation of genetic disorders in the population. In this research project, we included women with a diagnosis of hereditary ovarian cancer from the Republic of Bashkortostan to determine the mutational contribution of 21 candidate genes involved in carcinogenesis to the development of ovarian cancer in our population.
Materials and methods
Patient samples
All OC patients (n = 48) originated from the Volga-Ural region but belonged to different ethnic groups from Bashkortostan, including Russians, Tatars, Bashkirs, Ukrainians, and patients of other or mixed ancestry. The average age of disease manifestation was 44 years (19–74 years). The selection criteria of patients were the characteristic generally recognized signs of likely hereditary OC: burdened family history—cases of ovarian, breast, prostate and pancreas cancers in relatives of the first and second degree of kinship; primary multiple meta—or synchronous malignant neoplasms (polyneoplasia) in the patient herself; platinum-sensitive recurrence, young age of the patient—up to 45 years in conjunction with at least one of the above diagnostic criteria, platinum-sensitive relapse. Peripheral venous blood was taken by employees of the State Autonomous Institution of Health Republican Clinical Oncology Center of the Health Ministry of the Bashkortostan Republic (Ufa). All participants of this research signed voluntary informed consent for molecular genetic studies. This work was approved by the bioethical committee of the Institute of Biochemistry and Genetics, Ufa Federal Research Center of the Russian Academy of Sciences.
Patients had different histology type of tumors but 46 were epithelial ovarian carcinomas. Of these, 30 (65%) were serous tumors; 8 (17%) were mucinous tumors; 2 (4%) were mixed epithelial tumors; 2 (4%) were undifferentiated carcinoma; 1 (2%) was a clear cell tumor; 1 (2%) was an endometrioid tumor; 1 (2%) was a squamous tumor; 1 (2%) was a Brenner’s tumor. Stromal tumors were found in 2 (4%) cases: one granulosa cell tumor (2%) and one tumor from Sertoli-Leydig cells (2%). Tumors were predominantly of a high grade (G3-G4) – 36%. A low-grade tumor (G2) was detected in 32% cases, and grading of cancer cells was not histologically determined in 32% patients. Bilateral ovarian cancer was present in 2 (4%) women with OC. Stage I of disease was established in 16% of patients; II – in 11%; III – in 70% and stage IV – in 3% of cases. Seven patients (15%) also had a personal history of breast cancer, cervical cancer or colon cancer. Metastases were detected in 43% of the patients.
Methods
Genomic DNA was isolated from peripheral white blood cells by routine phenol–chloroform extraction. To screen germline variants of the nucleotide sequence, the method of targeted next-generation sequencing was applied on the Illumina MiSeq platform using the AmpliSeq protocol with a custom panel containing primers for the synthesis of 661 amplicons covering the protein coding region of 21 candidate genes, including their UTR regions: BRCA1, BRCA2, BARD1, BRIP1, CDH1, CHEK2, EPCAM, MLH1, MRE11A, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, PTEN, RAD50, RAD51C, STK11, TP53. An additional file with used primers shows this in more detail (see Additional file 1). Then, an assessment of the reading quality and secondary processing of data was carried out in the multifunctional online service Base Space Illumina (https://basespace.illumina.com). An individual summary file was obtained for each sample under study, containing information on the number of reads, the percentage of Q30 bases, the percentage of gene coverage, the percentage of aligned reads, the level of autosomal variants colling, the number of identified SNVs, deletions, insertions relative to the reference genome, the gene regions are indicated in which changes were detected, etc. Also, for each sample,.vcf and.bam files were obtained containing complete information about all detected changes in the studied genes. For bioinformatics analysis of the nucleotide sequence variants, Illumina Variant Interpreter, ANNOVAR, SNPeff, ClinVar, gnomAD, ExAC, 1000 Genomes and ALFA services were used. Changes, detected by Targeted Next-Generation Sequencing of 21 candidate genes in hereditary ovarian cancer patients, were annotated in ANNOVAR program, using the summarize_annovar.pl script. It makes possible to compare single nucleotide substitutions with a number of specialized databases and predict the functional significance of the detected changes using in silico tools (SIFT, PolyPhen-2, LRT, Mutation Taster, Mutation Assessor, ClinVar, phyloP, GERP ++ and others) from dbNSFP v.1.3. In addition, the CADD (Combined Annotation Dependent Depletion) program was used. To estimate the population frequencies of the identified variants, we used data from the 1000 Genomes project, the Exome Aggregation Consortium and Allele Frequency Aggregator.
After ANNOVAR annotation, a search for pathogenic variants was conducted that may represent driver mutations in the development of ovarian cancer. This further analysis included the use of custom filters, based on the following criteria:
The selection of variants located in exons and splicing sites,
Selection of potentially functionally significant genetic variants: truncating variants (frameshift, stop gained and splice variants) and nonsynonymous single nucleotide substitutions,
Selection of variants with frequency no more than 1%, according to 1000 Genomes, the Exome Aggregation Consortium and Allele Frequency Aggregator. Previously undescribed variants with unknown frequency were not rejected if they had potential functional significance. Verification of all selected nucleotide sequence changes was carried out using Sanger sequencing. The frequencies of identified variants were calculated as the ratio of the samples number with the variant to the total number of samples.
Results
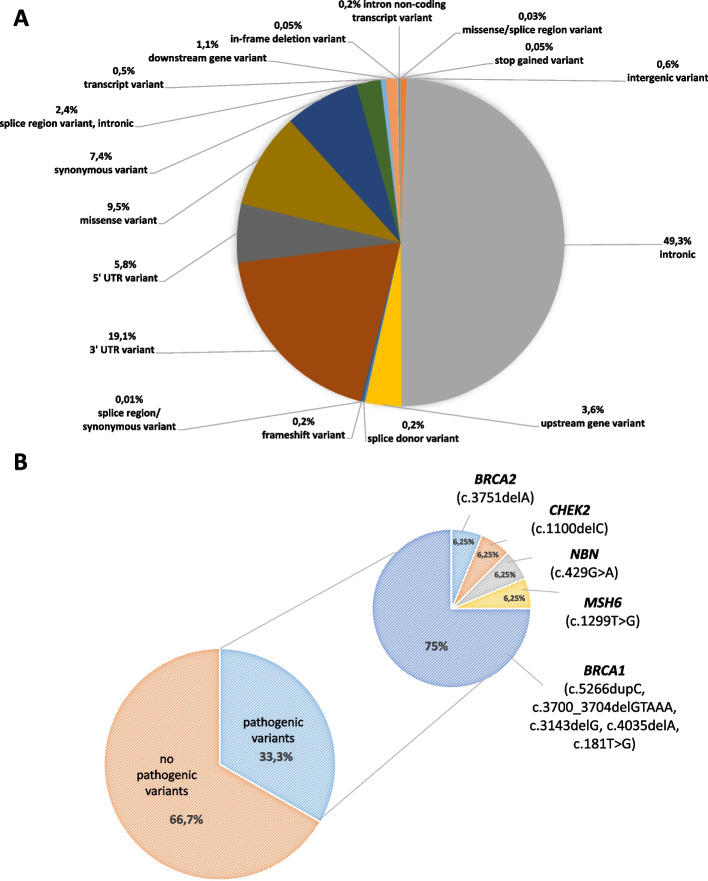
By sequencing 21 candidate genes in 48 ovarian cancer patient samples, an average of 181 variants (range: 122–226) were detected per patient. Most of the variants (on average 88 variants per patient) were identified in the intronic region of the genes; variants of the 3’-UTR (on average 33 variants in patient) were also often found. Any variants of the 5’-UTR; missense; synonymous; upstream gene; splice region/intron variants also were detected in all patients. The distribution of identified variants among different portions of the respective genes is illustrated in Fig. 1A.
Fig. 1.
A Spectrum of variants detected by targeted next-generation sequencing in hereditary ovarian cancer cases. B Distribution of patients with and without pathogenic or likely pathogenic variants
Pathogenic (PVs) and likely pathogenic variants (LPVs) of BRCA1, BRCA2, CHEK2, MSH6 and NBN genes were detected in 16/48 patients (33%). The vast majority of PVs/LPVs were found in BRCA1, in 25% of the patients (12/48). In one patient we observed PVs/LPVs in CHEK2, in one case in BRCA2, in one patient in NBN and in one case in MSH6. No pathogenic or likely pathogenic variants were found in BARD1, BRIP1, CDH1, EPCAM, MLH1, MRE11A, MSH2, NBN, PALB2, PMS2, PTEN, RAD50, RAD51C, STK11, or TP53. The distribution of pathogenic or likely pathogenic variants in candidate genes is illustrated in Fig. 1B.
Loss-of-function variants
Functional disease-causing variants (frameshift, stop gain and one deleterious missense variants) were found in 33% of patients. In total, 9 different loss-of-function variants were detected in 16 DNA samples (Table 1). By far the most frequently mutated gene was BRCA1 with the founder mutation c.5266dupC in seven cases, the variant c.3143delG in two patients, two further PVs (c.4035delA, c.3700_3704delGTAAA) in one patient each, and the variant c.181T >G (encoding the RING finger substitution p.Cys61Gly) in one case. In the BRCA2 gene we found one pathogenic variant (c.3751dupA). In the CHEK2 gene we detected one patient with the founder mutation c.1100delC. In the NBN gene we detected a novel truncating variant (c.429G>A, p.W143X). One further truncating variant was identified in MSH6 (c.1299T>G, p.Y433X) (Table 1).
Table 1.
Loss-of-function variants in familial ovarian cancer patients from Bashkortostan
| Gene | Variant | Exon | Protein change | Zygo-sity | Type of variant | ClinVar |
|---|---|---|---|---|---|---|
| BRCA1 | c.181T>G | 4/23 | p.Cys61Gly | Het | missense | pathogenic |
| BRCA1 | c.3143delG | 10/23 | p.Gly1048ValfsTer14 | Het | frameshift | pathogenic |
| BRCA1 | c.3143delG | 10/23 | p.Gly1048ValfsTer14 | Het | frameshift | pathogenic |
| BRCA1 |
c.3700_3704 delGTAAA |
10/23 | p.Val1234GlnfsTer8 | Het | frameshift | pathogenic |
| BRCA1 | c.4035delA | 10/23 | p.Glu1346LysfsTer20 | Het | frameshift | pathogenic |
| BRCA1 | c.5266dupC | 19/23 | p.Gln1756ProfsTer74 | Het | frameshift | pathogenic |
| BRCA1 | c.5266dupC | 19/23 | p.Gln1756ProfsTer74 | Het | frameshift | pathogenic |
| BRCA1 | c.5266dupC | 19/23 | p.Gln1756ProfsTer74 | Het | frameshift | pathogenic |
| BRCA1 | c.5266dupC | 19/23 | p.Gln1756ProfsTer74 | Het | frameshift | pathogenic |
| BRCA1 | c.5266dupC | 19/23 | p.Gln1756ProfsTer74 | Het | frameshift | pathogenic |
| BRCA1 | c.5266dupC | 19/23 | p.Gln1756ProfsTer74 | Het | frameshift | pathogenic |
| BRCA1 | c.5266dupC | 19/23 | p.Gln1756ProfsTer74 | Het | frameshift | pathogenic |
| BRCA2 | c.3751dupA | 11/27 | p.Thr1251AsnfsTer14 | Het | frameshift | pathogenic |
| CHEK2 | c.1100delC | 11/15 | p.Thr367MetfsTer15 | Het | frameshift | pathogenic |
| MSH6 | c.1299T >G | 4/10 | p.Tyr433Ter | Het | stop gained | pathogenic |
| NBN | c.429G >A | 4/16 | p.Trp143Ter | Het | stop gained | pathogenic |
The seven patients heterozygous for the c.5266dupC mutation were diagnosed with serous (5/7), clear cell (1/7) and squamous cell (1/7) carcinomas. Two patients with c.5266dupC additionally had cervical cancer and/or breast cancer, as well as vaginal, omental and liver metastases. Five c.5266dupC carriers were of Russian origin and two carriers of Tatar origin. One of the two patients with BRCA1*c.3143delG also had breast cancer. The patient with the MSH6*p.Y433X truncation also had endometrial cancer, this patient also harbored unclassified variants in MUTYH and BRCA2. The patient with CHEK2*c.1100delC was identified with serous carcinoma. The clinical data of these and the remaining PV carriers are summarized in Table 2.
Table 2.
Clinical data of patients with identified loss-of-function variants
| TruncatingVariant | Other variants identified | Histology | Subtype | Grade | Other cancers in the patient | Metastasis |
|---|---|---|---|---|---|---|
|
BRCA1 c.5266dupC |
– | epithelial | serous | Gx | – | – |
| – | epithelial | clear cell | G2 | – | – | |
| – | epithelial | serous | G2 | – | – | |
| – | epithelial | serous | G2 | – | omental metastases | |
|
BRCA2 c.4043G>C |
epithelial | squamous cell | G3 | cervical cancer; breast cancer | liver metastases | |
| – | epithelial | serous | G4 | breast cancer | – | |
| – | epithelial | serous | Gx | – | vaginal metastases | |
|
BRCA1 c.3700_3704delGTAAA |
ATM c.2149C>T | epithelial | serous | G4 | – | omental and mesenteric metastases |
|
BRCA1 c.3143delG |
PALB2 c.1486G>C |
epithelial | serous | G2 | – | – |
| – | epithelial | serous | G2 | breast cancer | lymph node metastases | |
|
BRCA1 c.4035delA |
– | epithelial | serous | G3 | – | omental metastases |
|
BRCA1 c.181 T > G |
– | epithelial | mucinous | G2/G3 | – | omental metastases |
|
BRCA2 c.3751dupA |
– | epithelial | serous | G2 | – | – |
|
CHEK2 c.1100delC |
ATM c.6028A>G |
epithelial | serous | Gx | – | – |
|
NBN c.429G > A |
– | epithelial | serous | Gx | – | – |
|
MSH6 c.1299 T > G |
BRCA2 c.5624A>C; MUTYH c.985G>A |
epithelial | mixed | G2/G3 | endometrial cancer | – |
Variants of unknown clinical significance
We additionally identified 15 rare variants of uncertain significance, including six novel missense variants, that were located in BRCA2 (3), PALB2 (2), ATM (3), NBN (2), MRE11 (2), MSH6 (1), and MUTYH (2) genes (Table 3). Six of these variants (BRCA2*p.Cys1348Ser, BRCA2*p.Lys1875Thr, PALB2*p.Asp496His, ATM*p.Arg717Trp, ATM*p.Arg2010Gly, MUTYH*c.985G>A) were found together with truncating variants in BRCA1, CHEK2 or MSH6, respectively, making them less likely to constitute the driver of carcinogenesis. The MUTYH*c.1187G>A variant, encoding p.Gly396Asp (rs36053993), was considered potentially pathogenic in the biallelic state [15] but the patient here was heterozygous only. One patient had three variants of uncertain significance in BRCA2, MRE11 and NBN, illustrating the challenge to identify a causal variant among rare missense substitutions of different DNA repair genes found in the same patient.
Table 3.
Rare missense substitutions of different DNA repair genes
| Gene | Variant | Location | Type of variant | Protein change | Pathogenic variants in other genes | Databases | ||
|---|---|---|---|---|---|---|---|---|
| dbSNP | ClinVar | gnomAD MAF | ||||||
| ATM | c.2149C > T | ex.14 | Missense | p.(Arg717Trp) |
BRCA1 c.3700_3704delGTAAA |
rs147515380 | Uncertain significance | 0.00003 |
| ATM | c.6022A > G | ex.41 | Missense | p.(Ile2008Val) | – | rs2084586855 | Uncertain significance | – |
| ATM | c.6028A > G | ex.41 | Missense | p.(Arg2010Gly) | CHEK2c.1100delC | – | Uncertain significance | – |
| BRCA2 | c.3968A > G | ex.11 | Missense | p.(Lys1323Arg) | – | – | – | – |
| BRCA2 | c.4043G > C | ex.11 | Missense | p.(Cys1348Ser) |
BRCA1 c.5266dupC |
– | – | – |
| BRCA2 | c.5624A > C | ex.11 | Missense | p.(Lys1875Thr) |
MSH6 c.1299 T > G |
rs587782583 | Uncertain significance | 0.00001 |
| MRE11 | c.1480G > A | ex.13 | Missense | p.(Glu494Lys) | – | rs104895016 | Conflicting interpretations of pathogenicity | 0.002 |
| MRE11 | c.1492G > A | ex. 13 | Missense | p.(Asp498Asn) | – | rs564511708 | Uncertain significance | 0.002 |
| MSH6 | c.926C > G | ex.4 | Missense | p.(Ser309Cys) | – | rs544222338 | Conflicting interpretations of pathogenicity | 0.002 |
| MUTYH | c.985G > A | ex.11 | Missense | p.(Val329Met) |
MSH6 c.1299 T > G |
rs147718169 | Conflicting interpretations of pathogenicity | 0.00008 |
| MUTYH | c.1187G > A | ex.13 | Missense, Splice region | p.(Gly396Asp) | – | rs36053993 | Likely pathogenic | 0.003 |
| NBN | c.515T > C | ex.5 | Missense | p.(Val172Ala) | – | – | – | – |
| NBN | c.1912T > C | ex. 12 | Missense, Splice region | p.(Ser638Pro) | – | rs199657566 | Uncertain significance | 0.00003 |
| PALB2 | c.315G > C | ex. 4 | Missense | p.(Glu105Asp) | – | rs515726108 | Uncertain significance | 0.00003 |
| PALB2 | c.1486G > C | ex.4 | Missense | p.(Asp496His) |
BRCA1 c.3143delG |
– | – | – |
Discussion
The present pilot study aimed to investigate the mutational spectrum of 21 candidate genes in 48 patients with likely hereditary ovarian cancer and to identify major contributing genes in the hitherto uncharacterized population of Bashkortostan. The results show that about one-third of these ovarian cancer cases from the Volga-Ural region can be explained by a truncating mutation in one of these genes, most notably BRCA1. Of note, the common truncating variant c.5266dupC accounted for about 1 in 7 ovarian cancer cases in our cohort, supporting its predominant role and proposed origin in Russia [16]. A previous breast cancer study from Bashkortostan identified c.5266dupC in some 4% of breast cancer patients [17], indicating a three– to fourfold enrichment of pathogenic BRCA1 variants in ovarian cancer compared to breast cancer from the same population. This is consistent with previous comparative studies in Slavic breast and ovarian cancer patients, e.g. in Belarus [18]. Our high frequency of c.5266dupC is also consistent with a previous study of Suspitsin et al. who found this variant in 9.7% of ovarian cancer cases from the North-West and 17.2% ovarian cancer patients from the South of Russia [19]. The latter matches our findings and is clinically important because BRCA1-deficient ovarian carcinomas are particularly vulnerable against platinum-based therapy as well as PARP1 inhibitors [20–22]. Three of our BRCA1 mutation carriers also had breast cancer (and one additional cervical cancer), and one MSH6 mutation carrier also had endometrial cancer, in line with the known role of these genes in different types of DNA repair and cancer predisposition.
Apart from BRCA1, BRCA2 and MSH6, no clearly pathogenic variant was identified in other established ovarian cancer genes tested. We identified one patient with a well-known truncating variant in CHEK2. Although CHEK2 variants have been proposed to predispose to ovarian cancer [23] and the c.1100delC variant has been previously reported in two Russian ovarian cancer patients [24], there is insufficient evidence at present to conclude that CHEK2 contributes to ovarian cancer risk as it does in breast cancer [24]. An additional missense variant of ATM in this patient was of uncertain clinical significance. We furthermore identified a novel truncating variant in NBN, another candidate gene for ovarian cancer. NBN encodes Nibrin, the Nijmegen Breakage Syndrome protein, which recognizes DNA double-strand breaks and modulates homologous recombinational repair [25, 26]. It is unclear whether NBN represents an ovarian cancer susceptibility gene [27–30], though a lack of the MRE11-RAD50-NBN complex has been reported in almost half of epithelial ovarian cancers [31]. However, such deficiency may occur by somatic inactivation and much larger case–control association studies will be needed to finally resolve the role of NBN germline variants in the etiology of this cancer.
Apart from the uncertain role of some of the candidate genes selected for panel testing, the interpretation of missense variants is another challenge that will need to be addressed in the future. Unclassified variants have been found in several patients here, including one patient with three such variants. Such rare variants could make a significant contribution to those two-thirds of hereditary ovarian cancer patients that are not explained by PVs in the currently tested genes. However, it is not possible at present to assign a risk estimate to any of these single variants nor to their potentially synergistic combination.
In summary, this is the first report of multi-gene panel testing for germline variants among cancer patients from Bashkortostan. This study has identified BRCA1 as the main contributor to the familial ovarian cancer risk in this country and has uncovered novel variants in additional genes that will deserve consideration in further studies of hereditary ovarian cancer.
Supplementary Information
Additional file 1. List of primers to cover protein-coding and UTR regions selected for targeted NGS sequencing for the AmpliSeq Illumina panel.
Acknowledgements
Targeted Next-Generation sequencing was funded by Russian Foundation for Basic Research (18-29-09129), Ministry of Science and Higher Education of Russian Federation (№075-03-2021-193/5) and Ministry of Science and Higher Education of Russian Federation (FZWU-2020-0027). The Russian-German collaboration was further supported by the German Research Foundation (Do761/15-1). We thank all patients who took part in this research work and all scientists, clinicians, oncologists and technicians who enabled this work to be carried out.
Abbreviations
- CADD
Combined Annotation Dependent Depletion
- DNA
Deoxyribonucleic acid
- FA
Fanconi Anemia
- HBOC
Hereditary breast and ovarian cancer
- HOC
Hereditary ovarian cancer
- LPV
Likely pathogenic variant
- MMR
Mismatch repair
- NGS
Next-generation sequencing
- OC
Ovarian cancer
- PV
Pathogenic variant
- SNV
Single-nucleotide variant
- UTR
Untranslated region
Authors’ contributions
DP took part in the preparation, conduct and bioinformatic analysis of data of targeted sequencing of DNA samples and was a major contributor in writing the manuscript. EM isolated and prepared DNA samples for inclusion in subsequent exome sequencing. YV took part in the preparation and conduct of targeted sequencing of DNA samples. DS analyzed and interpreted patient data to include in the study. RF analyzed and interpreted patient data to include in the study. AN took part in the preparation and conduct of targeted sequencing of DNA samples. RV took part in bioinformatic analysis of targeted sequencing results. NB took part in data analysis. TD took part in data analysis and was a major contributor in writing the manuscript. EK took part in bioinformatic analysis of targeted sequencing results and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The design of the research, collection of blood samples, the Targeted Next-Generation sequencing, analysis and interpretation of data was funded by the Ministry of Science and Higher Education of Russian Federation (№075-03-2021-193/5), Ministry of Science and Higher Education of Russian Federation (075-15-2021-595), and RF grant MK-3208.2022.1.4.
Availability of data and materials
Raw data of Targeted Next-Generation Sequencing available at the link https://www.ncbi.nlm.nih.gov/sra/PRJNA906939.
Declarations
Ethics approval and consent to participate
This work was approved by the Committee on Biomedical Ethics at the Institute of Biochemistry and Genetics, Ufa Federal Research Center of the Russian Academy of Sciences.
Consent for publication
All study participants gave informed consent to participate.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
D. S. Prokofyeva, Email: dager-glaid@yandex.ru
T. Dörk, Email: doerk.thilo@mh-hannover.de
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Kaprin AD, Starinskiy VV, Petrova GV. The state of cancer care for the population of Russia in 2018. Moscow Scientific Research Institute named after P.A. Herzen: Branch of the FSBI “National Medical Research Center of Radiology” Ministry of Health of Russia; 2019. ISBN 978-5-85502-251-3. https://glavonco.ru/cancer_register/%D0%97%D0%B0%D0%B1%D0%BE%D0%BB_2018_%D0%AD%D0%BB%D0%B5%D0%BA%D1%82%D1%80.pdf.
- 3.Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS. Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol. 2015 doi: 10.1097/AOG.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramus SJ, Harrington PA, Pye C, et al. Contribution of BRCA1 and BRCA2 mutations to inherited ovarian cancer. Hum Mutat. 2007. 10.1002/humu.20599. [DOI] [PubMed]
- 5.Kast K, Rhiem K, Wappenschmidt B, Hahnen E, et al. Prevalence of BRCA1/2 germline mutations in 21 401 families with breast and ovarian cancer. J Med Genet. 2016 doi: 10.1136/jmedgenet-2015-103672. [DOI] [PubMed] [Google Scholar]
- 6.Song H, Dicks EM, Tyrer J, et al. Population-based targeted sequencing of 54 candidate genes identifies PALB2 as a susceptibility gene for high-grade serous ovarian cancer. J Med Genet. 2020 doi: 10.1136/jmedgenet-2019-106739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramus SJ, Song H, Dicks E, Tyrer JP, Rosenthal AN et al. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J Natl Cancer Inst. 2015. 10.1093/jnci/djv214. [DOI] [PMC free article] [PubMed]
- 8.Song H, Dicks E, Ramus SJ, et al. Contribution of Germline Mutations in the RAD51B, RAD51C, and RAD51D Genes to Ovarian Cancer in the Population. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.61.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song H, Cicek MS, Dicks E, et al. The contribution of deleterious germline mutations in BRCA1, BRCA2 and the mismatch repair genes to ovarian cancer in the population. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones MR, Kamara D, Karlan BY, Pharoah PDP, Gayther SA. Genetic epidemiology of ovarian cancer and prospects for polygenic risk prediction. Gynecol Oncol. 2017 doi: 10.1016/j.ygyno.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Buys SS, Sandbach JF, Gammon A, Patel G, Kidd J, et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer. 2017 doi: 10.1002/cncr.30498. [DOI] [PubMed] [Google Scholar]
- 12.Tedaldi G, Tebaldi M, Zampiga V, Danesi R, et al. Multiple-gene panel analysis in a case series of 255 women with hereditary breast and ovarian cancer. Oncotarget. 2017 doi: 10.18632/oncotarget.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav S, Reeves A, Campian S, Paine A, Zakalik D. Outcomes of retesting BRCA negative patients using multigene panels. Fam Cancer. 2017 doi: 10.1007/s10689-016-9956-7. [DOI] [PubMed] [Google Scholar]
- 14.Dicks E, Song H, Ramus SJ, et al. Germline whole exome sequencing and large-scale replication identifies FANCM as a likely high grade serous ovarian cancer susceptibility gene. Oncotarget. 2017 doi: 10.18632/oncotarget.15871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT. Inherited variants of MYH associated with somatic G:CT: a mutations in colorectal tumors. Nat Genet. 2002. 10.1038/ng828. [DOI] [PubMed]
- 16.Hamel N, Feng BJ, Foretova L, et al. On the origin and diffusion of BRCA1 c.5266dupC (5382insC) in European populations. Eur J Hum Genet. 2011 doi: 10.1038/ejhg.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermisheva MA, Zinnamullina GF, Gantsev ShKh, Kochanova VA, Popov OS, Dörk T, Khusnutdinova EK. Frequency of 5382insC mutation of the BRCA1 gene. Vopr Onkol. 2008;54(1):31–3. [PubMed] [Google Scholar]
- 18.Bogdanova NV, Antonenkova NN, Rogov YI, Karstens JH, Hillemanns P, Dörk T. High frequency and allele-specific differences of BRCA1 founder mutations in breast cancer and ovarian cancer patients from Belarus. Clin Genet. 2010 doi: 10.1111/j.1399-0004.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 19.Suspitsin EN, Sherina NY, Ponomariova DN, et al. High frequency of BRCA1, but not CHEK2 or NBS1 (NBN), founder mutations in Russian ovarian cancer patients. Hered Cancer Clin Pract. 2009 doi: 10.1186/1897-4287-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorodnova TV, Sokolenko AP, Ivantsov AO, et al. High response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutation. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokolenko AP, Bizin IV, Preobrazhenskaya EV, et al. Molecular profiles of BRCA1-associated ovarian cancer treated by platinum-based therapy: analysis of primary, residual and relapsed tumors. Int J Cancer. 2020 doi: 10.1002/ijc.32776. [DOI] [PubMed] [Google Scholar]
- 23.Szymanska-Pasternak J, Szymanska A, Medrek K, et al. CHEK2 variants predispose to benign, borderline and low-grade invasive ovarian tumors. Gynecol Oncol. 2006 doi: 10.1016/j.ygyno.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Krylova NY, Ponomariova DN, Sherina NY, Ogorodnikova NY, et al. CHEK2 1100delC mutation in Russian ovarian cancer patients. Hereditary Cancer Clin Pract. 2007;5(3):1–4. doi: 10.1186/1897-4287-5-3-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavin MF. ATM and the Mre11 complex combine to recognize and signal DNA double-strand breaks. Oncogene. 2007 doi: 10.1038/sj.onc.1210880. [DOI] [PubMed] [Google Scholar]
- 26.van den Bosch M, Bree RT, Lowndes NF. The MRN complex: coordinating and mediating the response to broken chromosomes. EMBO Rep. 2003 doi: 10.1038/sj.embor.embor925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koczkowska M, Krawczynska N, Stukan M, et al. Spectrum and prevalence of pathogenic variants in ovarian cancer susceptibility genes in a group of 333 patients. Cancers (Basel) 2018 doi: 10.3390/cancers10110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krivokuca A, Boljevic I, Jovandic S, et al. Germline mutations in cancer susceptibility genes in high grade serous ovarian cancer in Serbia. J Hum Genet. 2019 doi: 10.1038/s10038-019-0562-z. [DOI] [PubMed] [Google Scholar]
- 29.Kurian AW, Hughes E, Handorf EA, Gutin A, Allen B, Hartman A-R, Hall MJ. Breast and ovarian cancer penetrance estimates derived from germline multiple-gene sequencing results in women. JCO Precis Oncol. 2017 doi: 10.1200/PO.16.00066. [DOI] [PubMed] [Google Scholar]
- 30.Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt S, Samartzis EP, Zimmermann AK, et al. Lack of MRE11-RAD50-NBS1 (MRN) complex detection occurs frequently in low-grade epithelial ovarian cancer. BMC Cancer. 2017 doi: 10.1186/s12885-016-3026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. List of primers to cover protein-coding and UTR regions selected for targeted NGS sequencing for the AmpliSeq Illumina panel.
Data Availability Statement
Raw data of Targeted Next-Generation Sequencing available at the link https://www.ncbi.nlm.nih.gov/sra/PRJNA906939.