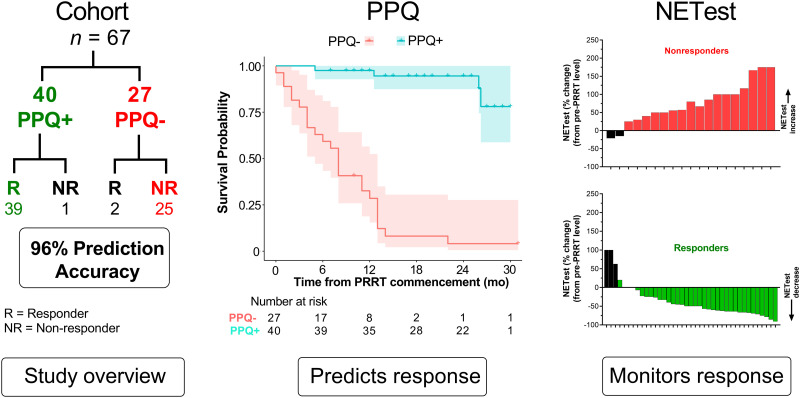
Visual Abstract
Keywords: NETest, PPQ, PRRT, NET
Abstract
Reliable biomarkers for neuroendocrine tumor (NET) management during peptide receptor radionuclide therapy (PRRT) are lacking. We validated the role of 2 circulating biomarkers: the PRRT prediction quotient (PPQ) as a predictive marker for response and the NETest as a monitoring biomarker. Furthermore, we evaluated whether tissue-based genetic alterations are effective in predicting progression-free survival (PFS). Methods: Data were prospectively collected on patients at the Memorial Sloan Kettering Cancer Center with 177Lu-DOTATATE–treated somatostatin receptor (SSTR)–positive gastroenteropancreatic and lung NETs (n = 67; median age, 66 y; 52% female; 42% pancreatic, 39% small-bowel; 78% grade 1 or 2). All cases were metastatic (89% liver) and had received 1–8 prior treatments (median, 3), including somatostatin analogs (91%), surgery (55%), or chemotherapy (49%). Treatment response included PFS. According to RECIST, version 1.1, responders had stable disease or a partial response (disease-control rate) and nonresponders had progression. Blood was collected before each cycle and at follow-up. Samples were deidentified and assayed and underwent masked analyses. The gene expression assays included RNA isolation, real-time quantitative polymerase chain reaction, and multialgorithm analyses. The PPQ (positive predicts a responder; negative predicts a nonresponder) at baseline was determined. The NETest (0–100 score) was performed. Statistics were analyzed using Mann–Whitney U testing (2-tailed) or Kaplan–Meier survival testing (PFS). In patients with archival tumor tissue, next-generation sequencing was performed through an institutional platform (Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets). Results: Forty-one patients (61%) were responders. PPQ accurately predicted 96% (64/67). The hazard ratio for prediction was 24.4 (95% CI, 8.2–72.5). Twelve-month disease control was 97% for PPQ-positive patients versus 26% for PPQ-negative patients (P < 0.0001). Median progression-free survival was not reached in those predicted to respond (PPQ-positive, n = 40) but was 8 mo in those predicted not to respond (PPQ-negative, n = 27). The NETest result in responders was 67 ± 25 at baseline and significantly (P < 0.05) decreased (−37 ± 44%) at follow-up. The NETest result in nonresponders was 44 ± 23 at baseline and significantly (P < 0.05) increased (+76% ± 56%) at progression. Overall, the NETest changes (increases or decreases) were 90% accurate. Thirty patients underwent next-generation sequencing. Tumors were microsatellite-stable, and the median mutational burden was 1.8. Alterations involved mainly the mTOR/PTEN/TSC pathway (30%). No relationship was associated with PRRT response. Conclusion: Our interim analysis confirmed that PPQ is an accurate predictor of 177Lu-DOTATATE responsiveness (radiosensitivity) and that NETest changes accurately correlated with treatment response. Tissue-based molecular genetic information had little value in PRRT prediction. Blood-based gene signatures may improve the management of patients undergoing 177Lu-DOTATATE by providing information on tumor radiosensitivity and disease course, thus allowing individualized strategies.
Peptide receptor radionuclide therapy (PRRT) is an established treatment for metastatic or nonresectable neuroendocrine tumors (NETs) that involves systemic administration of radiolabeled octreotide derivatives targeting overexpressed somatostatin receptors (SSTRs) on NETs. There are no objective means to predict therapeutic efficacy (1). Effective treatment is defined as disease stabilization and partial or complete response on structural imaging (CT or MRI). 68Ga-labeled somatostatin analog PET/CT (68Ga-SSA PET) is used to amplify diagnostic information, although response criteria remain to be defined (2–4). Treatment is effective in about 60% of cases (5); about 15%–30% of patients will exhibit disease progression during therapy, and 10%–15% will progress within 6–12 mo after treatment (2,3,5,6).
A precise, objective methodology for predicting therapeutic efficacy remains elusive (7). Primary tumor site, histopathologic grading, and SSTR imaging (particularly octreotide scanning) have limited accuracy in the prediction of responsiveness, at 60% for tumors with the highest uptake (3). In the NETTER-1 study, no difference in response was seen between patients with grade 4 uptake and those with lower uptake, such as grade 2 or 3 (5). Tumor burden, histologic grading, and the presence or intensity of 18F-FDG uptake provide prognostic information but cannot specifically predict response to PRRT (3,5). It is probable that the determinants of therapeutic efficacy are intrinsic and reflect the molecular biologic and genomic characteristics of a specific tumor. The efficacy of PRRT therefore depends on biologic parameters that determine tumor sensitivity to radionuclide therapy.
Currently, there are no specific molecular features (e.g., proliferation, mutation status, or chromosomal abnormalities) that can predict radiosensitivity (1). In other settings (e.g., breast or head-and-neck cancer), tissue transcriptomic–based or gene expression assays predicted response to external-beam radiotherapy (8–10). Such approaches require tumor tissue for evaluation, which can be obtained only by surgery or biopsy (1). Similar information from a blood-based assay would be desirable.
In 2018, we reported a circulating transcript assay (the PRRT prediction quotient [PPQ]) predicting PRRT response in gastroenteropancreatic and bronchopulmonary NETs as about 95% accurate (11,12). This study included 2 comparator cohorts: an SSA cohort and a wait-and-watch cohort. The PPQ could not predict outcome in either the SSA cohort or the wait-and-watch cohort, consistent with the proposal that the PPQ is a predictive marker specifically for PRRT. This blood-based assay comprises expression of 8 genes and captures both growth factor and metabolomic expression specifically related to oxidative stress, metabolism, and hypoxic signaling (12).
A different blood-based transcriptomic assay, the NETest, evaluates 51 NET-specific genes (13–15). This test functions as a surrogate biomarker, and changes in score, compared with before treatment (e.g., SSAs or surgery), strongly correlated with tumor progression measured with CT or MRI (16,17). Quarterly blood sampling provides a real-time evaluation of tumor status (18). A recent report on 3 independent, prospective European cohorts demonstrated the NETest to be effective (98%) in monitoring response to PRRT. NETest levels decreased in RECIST responders to treatment and remained elevated in those who progressed despite therapy (19). At the conclusion of therapy, NETest levels significantly correlated with progression-free survival (PFS).
Currently, PRRT response is evaluated with morphologic and, when possible, molecular imaging. This has well-recognized limitations, including difficulties in assessing small-volume or coalescent disease when differentiating disease stabilization from pseudoprogression (4,20–22). There is thus a need to introduce and validate alternative companion biomarkers of treatment response to define therapeutic efficacy.
In this larger prospective study, we validated the role of the PPQ as a predictive marker for PRRT efficacy in gastroenteropancreatic and bronchopulmonary NETs. The PPQ output is binary (PPQ-positive [PPQ+] and PPQ-negative [PPQ−]) and identifies those who are predicted to respond (PPQ+) versus those predicted not to respond (PPQ−) (12). We evaluated the accuracy of this output in our patient cohort to assess its utility. Thereafter, we examined the value of the NETest as a clinical monitoring biomarker for PRRT efficacy. We compared pre-PRRT levels with follow-up levels. In addition, we evaluated whether changes in NETest levels were concordant with RECIST 1.1–based status. Lastly, we evaluated whether tissue-based molecular genetic alterations (Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets [MSK-IMPACT]) (23) were effective in PFS prediction and compared this result with the blood-based PPQ in a subset for which tissue was available. Herein we report our interim analysis.
MATERIALS AND METHODS
Ethics Approval
The study was approved by the Institutional Ethics Committee at Memorial Sloan Kettering Cancer Center (approval 19-022; January 18, 2019; NCT01775072). Informed written consent was obtained from participants. All data were collected prospectively (February 2019 to May 2021).
Therapeutic Response Assessment
Response was assessed by an independent radiologist per RECIST, version 1.1. CT (or MRI) was performed at baseline (≤3 mo before PRRT) and about 2–12 mo after PRRT per the protocol (11). Forty-one of 67 (61%) patients were considered responders. Response was defined as disease control (partial or complete response to therapy or stable disease). Progression (treatment failure) was confirmation of radiologic progression, at the first scan after PRRT or earlier, if symptomatic. The latter included all who completed at least 1 PRRT cycle. Overall survival (OS) and PFS were defined as the time from PRRT commencement to death or progression, respectively. Patients alive, or alive without progression, were censored at their date of last follow-up.
Blood Sampling
Samples of blood were collected before PRRT and thereafter before each PRRT cycle (administered at intervals of ∼2 mo [2–4 cycles]) and then at follow-up (first time point, 2–3 mo after PRRT completion; second time point, from 6–9 mo to 31 mo after the last PRRT cycle). At baseline, whole blood (5 mL) was collected in EDTA-K2 tubes that included RNA-stabilization buffer and were snap-frozen. The tubes were anonymously coded and stored at −80°C within 2 h of collection (24). Randomly selected, coded blood samples were sent deidentified to Wren Laboratories (CAP8640840, CL-0704, CLIA 07D2081388) for blinded measurement.
PPQ
PPQ analysis was performed on baseline blood. Details of the PPQ, a blood-based predictive classifier, have been described (24). In brief, circulating messenger RNA involved in growth factor biology and metabolism are amplified by PCR, and expression levels are integrated with tumor grade to generate a prediction classifier summated using a logistic regression model. Samples are scored as either biomarker “positive” or “negative.” PPQ+ identifies those predicted to respond (disease stabilization or partial/complete response). PPQ− are predicted not to respond.
NETest
Details of the methodology, mathematic analysis, and validation of the NETest have been published (18,24). Briefly, it comprises a 2-step protocol (RNA isolation/cDNA production and quantitative polymerase chain reaction) from whole blood. Samples were assayed at a clinically certified laboratory (Wren Laboratories). The results are expressed as an activity index (NETest score) from 0 to 100 (11,24). The upper limit of normality is 20 (16).
Statistical Analysis
Prism (version 9.0; GraphPad Software) for Microsoft Windows and MedCalc Statistical Software (version 20.009; MedCalc Software) were used (11,24). The efficacy of PRRT was defined per the RECIST 1.1 evaluation of best response as either disease control rate (partial response + complete response + stable disease) or progression, as previously described (11,24). The accuracy of PPQ was assessed by evaluating the concordance between PPQ prediction and outcomes, including response and OS. The accuracy of NETest changes (increase or decrease) was evaluated comparing baseline with follow-up levels. Intergroup analyses were undertaken using 2-tailed nonparametric tests (Mann–Whitney U test or Wilcoxon signed-rank test) as applicable. The Fisher exact test was used to compare proportions, such as response rates and pretreatment groups. Survival rates (PFS and OS) were estimated using a Kaplan–Meier estimator. Log-rank tests were used to compare survival curves, whereas hazard ratios (HRs) were calculated in a Cox model to assess the impact of candidate factors on survival. The association between PFS and OS was measured using the Pearson r. Statistical significance was defined as a P value of less than 0.05 (2-sided). Data are presented as mean ± SD. Ninety-five percent CIs are included when appropriate.
RESULTS
Patients
The cohort (including gastroenteropancreatic and bronchopulmonary NETs) included 100 patients recruited to date, 67 of whom had completed the PRRT treatment evaluations and are reported here. Clinical characteristics are in Table 1. Median age was 66 y (range, 26–88 y), 52% were female, 42% had pancreatic and 39% small-bowel NETs, and 78% had grade 1 or 2 disease. All had metastatic disease (100%) and had received 1–8 prior treatments (median, 3), including somatostatin analogs (SSAs, 91%), surgery (55%), or chemotherapy (49%, capecitabine and temozolomide or platinum-based therapy). Twenty-six were categorized as heavily pretreated. Forty-one were categorized as having received standard pretreatments. Heavy pretreatments (median, 4; range, 2–8) included chemotherapy, 177Lu-DOTATATE (n = 2), and a somatostatin antagonist (177Lu-satoreotide, n = 6) (25)). Standard pretreatments (median, 2; range, 1–4) typically comprised surgery and SSAs, or SSAs and liver-directed therapy. Table 1 includes a breakdown of the PPQ+ and PPQ− cohorts. No significant differences were identified except that the PPQ− cohort was significantly older (median age, 72 y [range, 30–88] vs. 62 y [range, 26–86 y]; P = 0.01) and had more bone involvement (70% vs. 45%, P = 0.048).
TABLE 1.
Demographics
| Evaluable patients | Total cohort | PPQ+ | PPQ− |
|---|---|---|---|
| Total patients (n) | 67 | 40 | 27 |
| Age (y) | 66 (26–88) | 62 (26–86) | 72 (30–88)* |
| Sex | |||
| Male | 32 | 19 | 13 |
| Female | 35 | 21 | 14 |
| Time since diagnosis (mo) | 65 (5–213) (mean ± SD, 68 ± 50) | 74 (5–213) (mean ± SD, 75 ± 48) | 39 (7–185) (mean ± SD, 56 ± 53) |
| Time from start of PRRT to final assessment (mo) | 14 (1–31) | 24 (5–30) | 8 (0–31)* |
| NET origin | |||
| Bronchopulmonary | 3 (5%) | 1 | 2 |
| Typical carcinoids | 2 | 1 | 1 |
| Atypical carcinoids | 0 | ||
| Carcinoids not otherwise specified | 0 | ||
| High-grade (mixed adenocarcinoma and small cell lung carcinoma) | 1 | 1 | |
| Gastroenteropancreatic | 61 (91%) | 38 (95%) | 23 (85%) |
| Pancreas | 28 | 19 | 9 |
| Small intestine | 26 | 17 | 9 |
| Appendix | 1 | 0 | 1 |
| Rectum | 6 | 2 | 4 |
| Cancer of unknown primary | 1 | 0 | 1 |
| Renal | 2 | 1 | 1 |
| Gastroenteropancreatic NETs, tumor grade | |||
| 1 (Ki-67, 0%–2%) | 17 (28%) | 11 | 6 |
| 2 (Ki-67, 3%–20%) | 35 (57%) | 22 | 13 |
| 3 (Ki-67, >20%) | 8 (13%) | 4 | 4 |
| Nonspecified (well-differentiated) | 1 (2%) | 1 | 0 |
| Clinical stage IV at enrollment | 67(100%) | 40 (100%) | 27 (100%) |
| Liver | 58 | 32 | 26 |
| Lymph nodes | 51 | 27 | 24 |
| Bone | 37 | 18 | 19† |
| Peritoneum | 21 | 10 | 11 |
| Lung | 11 | 5 | 6 |
| Other sites (e.g., adrenal, pleura, pericardium) | 23 | 12 | 11 |
| Previous therapy | |||
| Surgery | 37 (55%) | 26 (65%) | 11 (41%) |
| Somatostatin analogs | 61 (91%) | 37 (93%) | 24 (89%) |
| Pharmacotherapy | 57 (85%) | 31 (77%) | 26 (96%) |
| Capecitabine and temozolomide | 21 | 12 | 9 |
| Chemotherapy (platinum/dacarbazine) | 12 | 8 | 4 |
| Everolimus | 15 | 7 | 8 |
| Sunitinib | 3 | 1 | 2 |
| Others (axitinib, cabozatinib, denosumab, zoledronic acid, and levatinib) | 6 | 3 | 3 |
| Other therapies | 38 (57%) | 21 (53%) | 17 (63%) |
| PRRT | 8‡ | 4 (3)§ | 4 (3)§ |
| Radiotherapy | 6 | 1 | 5 |
| Liver-directed therapies∥ | 24 | 16 | 8 |
P < 0.05 vs. PPQ+ (Mann–Whitney U test).
P < 0.05 (χ2).
Six previously treated with JR-11.
Treated with JR-11.
Including chemoembolization, radioembolization, and selective internal radiation therapy.
Qualitative data are number and percentage; continuous data are median and range.
Thirty (16 heavily pretreated, 14 with standard pretreatments) had next-generation tumor sequencing per MSK-IMPACT. This tool identifies actionable alterations in 341 key cancer genes and separately detects chromosomal abnormalities, such as 18q loss (23,26).
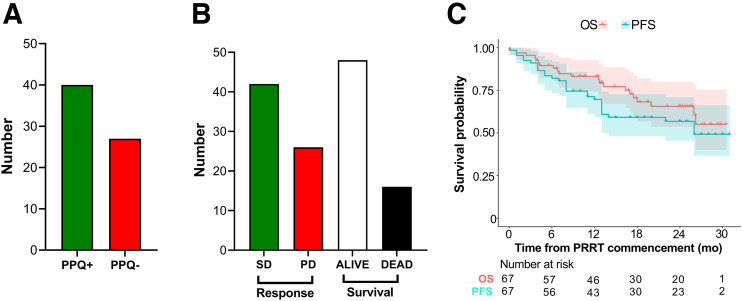
Forty (60%) were PPQ+ (predicted to respond to PRRT) (Fig. 1A). Treatment response (disease control rate) occurred in 41 (61%) of the 67 individuals, and 46 were alive at the time of evaluation (Fig. 1B). The median PFS (mPFS) was 26 mo and median OS was not reached (Fig. 1C). The 12-mo survival rates were 70% (PFS) and 83% (OS), respectively. The median pre-PRRT NETest score was 53 (range, 20–100).
FIGURE 1.
Response and baseline characteristics. (A) PPQ status before treatment. (B) Absolute response and survival in cohort. (C) Data showing that mPFS and mOS were not reached. Shading represents 95% CI for each survival curve. mOS = median overall survival; PD = progressive disease; SD = stable disease.
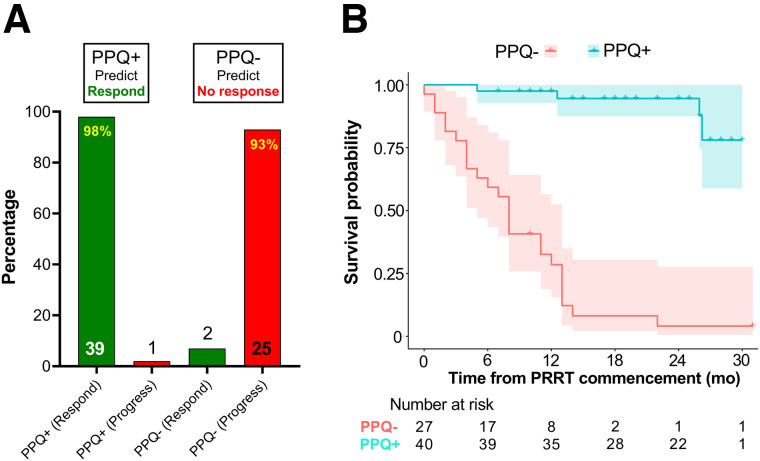
PPQ
Forty were PPQ+. Thirty-nine (98%) responded to PRRT (Fig. 2A). Twenty-seven were PPQ−. Twenty-five (93%) progressed despite PRRT. The overall predictive accuracy was 96% (64/67). Patients who were PPQ− had a risk of progression or death 24 times higher than patients who were PPQ+ (HR, 24.4; 95% CI, 8.2–72.5).
FIGURE 2.
Relationship between PPQ and response. (A) Thirty-nine PPQ+ patients responded, whereas 1 patient progressed. Twenty-five PPQ− patients developed disease progression despite PPRT; 2 PPQ− patients responded to therapy. (B) mPFS was not reached for PPQ+ patients but was 8 mo for PPQ− patients. Difference was statistically significant (log-rank test, P < 0.0001). HR for the biomarker was 24. Shading represents 95% CI for each survival curve.
The mPFS in PPQ+ was not reached (Fig. 2B). At 12 mo, 98% of PPQ+ patients were progression-free. The mPFS in the PPQ− cohort was 8 mo. At 12 mo, 29% were progression-free. All but 1 of 7 who were stable at 12 mo eventually developed progressive disease (by 23 mo).
The OS rate at 12 mo was 83%. OS (12 mo) was higher in PPQ+ (98%) than in PPQ− individuals (60%, P < 0.0001, log-rank test). Separately, 54% of heavily pretreated patients were PPQ+, compared with 63% of those with few prior treatments. Of the 6 previously treated with PRRT, 4 exhibited stable disease. Three were PPQ+; the 2 progressors were both PPQ−.
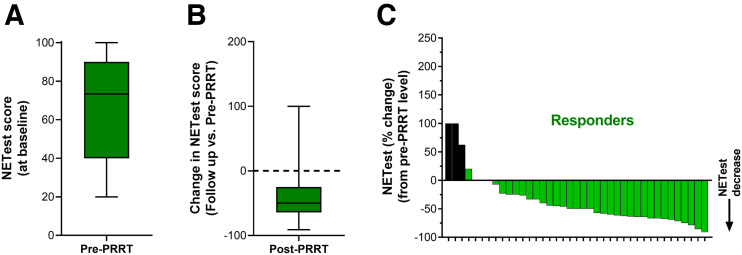
NETest and PRRT Response
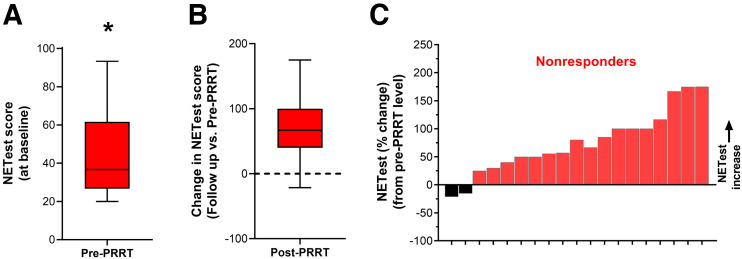
The NETest was elevated in all before therapy (58 ± 27). In responders, baseline NETest levels were 67 ± 25 (Fig. 3A). At follow-up, levels were significantly decreased by −37% ± 44% (P = 0.0002, Fig. 3B). This is consistent with the assay measuring a response to therapy (decrease in tumor activity). The waterfall plot indicates that 90% (n = 35) exhibited a stabilization or decrease in the NETest with PRRT (Fig. 3C). In nonresponders, baseline levels were 44 ± 23 (Fig. 4A). This was significantly (P = 0.0005) lower than baseline scores in responders. At follow-up, levels were significantly increased (+76% ± 56%, P = 0.0002; Fig. 4B). The waterfall plot analysis (Fig. 4C) indicated that 89% (n = 17 of 19 evaluable) exhibited an increase.
FIGURE 3.
NETest in responders. (A) Baseline (pre-PRRT) NETest levels were 67 ± 25. (B) NETest levels decreased by −37% ± 44% after PRRT (P = 0.0002). (C) Waterfall plot demonstrates decrease in NETest from baseline in individual responders. Black bars identify changes in score that were associated with posttreatment NETest levels > 40.
FIGURE 4.
NETest in nonresponders. (A) Baseline (pre-PRRT) NETest levels were 44 ± 23. (B) NETest levels increased by +76% ± 56% after PRRT (P = 0.0002). (C) Waterfall plot demonstrates increase in NETest from baseline in individual nonresponders. Black bars identify changes in score that were associated with posttreatment NETest levels > 40. *P = 0.0005 vs. responders.
A subanalysis showed similar response rates in those with fewer prior treatments (26/41, 63%) and those heavily pretreated (15/26, 58%; P = 0.80, Fisher test). Moreover, there were no differences in PFS (P = 0.66, log-rank test). The 12-mo OS was 80% in the heavily pretreated cohort and 85% in patients with fewer prior treatments (P = 0.42, log-rank test). Pretreatment NETest scores were similar in both groups (57 ± 27 in patients with fewer prior treatments vs. 60 ± 27 in the heavily pretreated patients; P = 0.77, Mann–Whitney test). In heavily pretreated patients, the decrease in NETest scores (−9% ± 71%) was similar to that in patients with fewer prior treatments (+5% ± 73%; P = 0.34, Mann–Whitney test). In patients who received prior PRRT (n = 8), responders exhibited a median NETest change of −66.5; in those with progression, the change was +56%.
Molecular Genetic Evaluation (MSK-IMPACT) and Response
Thirty patients (45%) had tumor tissue molecular genetic testing; 16 were heavily pretreated, and 14 were not. All were microsatellite-stable. Tumor mutational burden was low (median, 1.8 mutations; range, 0–29.8). Overall, 11 (37%) had 0–1 mutations whereas 19 exhibited multiple mutations (63%). The most common alterations were in the mTOR/PTEN/TSC pathway (n = 9) and MEN-1 (n = 8). Six (75%) with MEN-1 mutations also had alterations in the mTOR/PTEN/TSC pathway. Separately, DAXX was mutated in 3 different patients, whereas ATRX was mutated in 1. Chromosomal losses were noted in 7 (23%). These included 11q losses in 2 (grade 3 renal NET and mixed adenocarcinoma and small cell lung carcinoma) and 18q losses in 5 (3 small-bowel NETs, 2 rectal NETs). Thirteen (81%) of 16 with heavy pretreatment and 11 of 14 (79%) with standard pretreatment exhibited a tumor mutational burden below the detection cutoff. The average number of mutations or chromosomal abnormalities was also similar in both (3.5 alterations per patient vs. 8.4 for the heavy pretreatment vs. the standard pretreatment, respectively). No apparent relationship was identified between mutations (burden or number) and the number of prior treatments.
The relationship between PPQ, genetic abnormalities, and outcome (12-mo PFS) are summarized in Table 2. First, no relationship was identified between tumor mutational burden and response (45% response in those with 0–1 mutations vs. 58% response in those with >1 mutation; P = 0.71, Fisher test). The PPQ biomarker was similarly distributed (PPQ+ in 5/11 or 45% of patients with 0–1 mutations vs. 10–19 or 53% of those with >1 mutation; P > 0.99, Fisher test) in these groups.
TABLE 2.
MSK-IMPACT, PPQ, and Outcomes
| Category | n | Responder | Nonresponder | mPFS (mo) | 12-mo PFS | PPQ+ | PPQ− |
|---|---|---|---|---|---|---|---|
| 0–1 mutations | 11 | 5 | 6 | 11 | 46% | 5 | 6 |
| >1 mutation | 19 | 11 | 8 | 26 | 79% | 10 | 9* |
| No chromosomal losses | 23 | 15 | 8 | Not reached | 74% | 14 | 9† |
| Chromosomal losses‡ | 7 | 1 | 6 | 11 | 43% | 1 | 6 |
| mTOR/PTEN/TSC mutations | 9 | 6 | 3 | 26 | 78% | 6 | 3 |
| MEN-1 mutations | 8 | 5 | 3 | 26 | 75% | 5 | 3 |
One patient responded but was PPQ−. Patient had 6 mutations, including PSM1, PMS2, BC2L11, INPP4A, NFE2L2, and PPP2R1A.
This is same patient as above.
11q (n = 2) or 18q (n = 5) losses.
Likewise, no relationship with response was noted between those with MEN-1 mutations and those with no MEN-1 (63% vs. 50%; P = 0.69, Fisher test) or mTOR pathway mutations (67% vs. 48%; P = 0.44, Fisher test). Similar proportions of those with MEN-1 mutations or alterations in the mTOR pathway were PPQ+ (63% and 67%, respectively). All DAXX were PPQ+ and responded. The patient with an ATRX mutation was PPQ− and did not respond.
Chromosomal loss, in contrast, was associated with a poorer 12-mo PFS (43% vs. 74%; P = 0.03, log-rank test). All 5 with a Chr18q loss were PPQ−. None responded; the mPFS was 11 mo. Of note, 4 (80%) also perished, suggesting Chr18q loss to be a marker of poor prognosis.
DISCUSSION
The current study evaluated 2 blood-derived signatures as predictors and monitors of PRRT efficacy. The study, an independent, prospective U.S. study, validated the PPQ as a predictor of PRRT response and the NETest as an effective treatment monitor. Both tools were clinically valuable irrespective of previous treatments incurred, such as platinum-based chemotherapy or prior PRRT. Abnormalities, either common cancer-associated mutations or NET chromosomal abnormalities, as measured in tumor tissue (MSK-IMPACT) exhibited little value as predictors and did not consistently correlate with the PPQ-predictive output except, potentially, Chr18q loss. We are not, however, able to identify a causal relationship since none of the 8 genes that define the PPQ signature are encoded on Chr18q. At this point, we are of the opinion that any relationship between PPQ, Chr18q loss, and outcome may reflect a correlation between PFS and OS. Indeed, 18 of 26 (69%) subjects undergoing progression ultimately died within 20 mo of follow-up. Our assessment also identified no confounding variables that might be linked to PPQ, but we note that the PPQ− cohort was older than the PPQ+ cohort and had more bone involvement.
A critical unmet need in PRRT is to predict who will benefit so as to provide personalized, effective, and economically viable therapy. Prior studies have focused on clinical parameters such as staging, or biomarkers such as chromogranin A, to predict outcome. All provided limited information. Grading of SSTR expression by imaging has some value but cannot predict more than 60% of responses for lesions with a Krenning score of 4 (uptake greater than in the spleen or kidneys) by octreotide scanning. For SSTR PET, higher uptake (mean SUVmax and tumor SUVmax/liver SUVaverage) correlated with the therapy response, but not all patients with intense uptake respond to treatment (3,7,27).
Our study had several strengths. It was an independent, prospective study undertaken at a U.S. center of excellence. We report our analysis of the first 67 subjects. This cohort is large and includes heavily pretreated individuals. The study is ongoing, and our final target recruitment is 150 patients. A weakness of the study is that only 45% of patients have tissue molecular data. This reflects the difficulties engendered by the coronavirus disease 2019 pandemic, which significantly impacted how we treat and manage our cancer patients (hospital visits). Nevertheless, despite the inherent difficulties in follow-up and tissue acquisition during the pandemic, our study results are highly significant and provide a clear evaluation of how these 2 blood biomarkers might manage PRRT.
A previous European consortium that included 3 centers—Erasmus University, IEO Milan, and Bad Berka—examined the predictive utility of PPQ (11). This study included 2 comparator cohorts: an SSA cohort and a wait-and-watch cohort. The PPQ could not predict outcome in either of these cohorts. This finding is consistent with the proposal that the PPQ is a predictive marker specifically for PRRT. Although this study comprises a single treatment arm, future studies (e.g., NCT05247905) will evaluate whether the PPQ can predict outcomes after PRRT versus treatment with capecitabine and temozolomide.
The biomarker was initially developed in an Italian cohort. In 2 separate follow-up cohorts, from 2 ENETS centers of excellence, the PPQ was 95% accurate. The overall median PFS was not reached in PPQ+ versus PPQ− patients (10–14 mo; HR, 18–77; P < 0.0001). In the current study, the overall PPQ-predictive accuracy was 96% in the Memorial Sloan Kettering Cancer Center cohort. Prior treatment had no impact on the utility of PPQ as a predictive marker. The mPFS of those detected as PPQ− was 12 mo but was not reached for those who were PPQ+. The HR of 24.4 (95% CI, 8.2–72.5) confirmed the impact of this biomarker. PPQ accurately predicted response, irrespective of prior PRRT or heavy pretreatment, and provided utility as a stratification marker for PRRT.
We also evaluated the NETest to monitor treatment efficacy. We focused on pretreatment and follow-up blood results. The NETest was 90% accurate for determining PRRT response (stabilization or a decrease in PRRT responders vs. an increase in nonresponders). This confirms an earlier study showing a 90.2% accuracy in the 3 European cohorts (19). In the European studies, the NETest score had an average −29% ± 26% decrease in responders and a +73% ± 11% increase in nonresponders (pre-NETest to follow-up scores). Our results were similar: a −37% ± 44% decrease in responders and a +76% ± 56% increase in nonresponders. Our study validates the NETest as an accurate monitor of treatment response. Although a small proportion (<10%) will not exhibit clinically actionable changes, the NETest provides real-time clinical value for most. Moreover, this biomarker may add valuable information to current imaging protocols for response evaluation. Imaging accuracy is problematic because of frequent pseudoprogression or slow response. Of further interest is our observation that patients who responded to PRRT exhibited higher pretreatment NETest levels. The basis for this finding requires further investigation, but the finding indicates an intriguing potential utility for this biomarker in treatment stratification. An elevated NETest may identify the molecular hallmarks of treatment responsiveness.
To our knowledge, this is the first study to assess the relationship between genetic alterations and PRRT. A recent review identified the absence of any such peer-reviewed data and highlighted the importance of determining whether such relationships exist (1). In our study, 30 patients (45%) had tissue blocks available for MSK-IMPACT evaluation. All were microsatellite-stable and had few mutational or chromosomal abnormalities, as expected in NETs (28,29). No relationship between mutations, regardless of type (mTOR/MEN-1), and outcome (PFS/OS) was identified, suggesting that these have little predictive value in PRRT. This is supported by the absence of a relationship between mutations and PPQ; the latter is predictive in 96% of cases, compared with less than 50% for mutations. Of note, chromosomal losses (Chr 11q/18q) were associated with a poorer outcome. Individuals with such abnormalities failed to respond to PRRT, and a large proportion (70%) died during follow-up. This is consistent with a prognostic rather than predictive value; 18q loss is associated with metastatic spread and poorer outcomes (30).
In summary, this interim analysis confirmed the PPQ as an effective, accurate (96%) predictor of 177Lu-DOTATATE-PRRT, unaffected by prior PRRT or chemotherapy, and consistent with a role as a radiation-responsive multigenomic blood biomarker. This study confirmed that NETest scores decreased in 177Lu-DOTATATE responders. In contrast, in nonresponders, scores increased. Blood-based gene signatures may enhance the management of patients undergoing 177Lu-DOTATATE therapy by providing information on tumor radiosensitivity and early disease course, thus allowing individualized strategies.
CONCLUSION
We have independently validated 2 blood-based gene signatures and found them to be effective, noninvasive tools that can enhance the management of patients who undergo 177Lu-DOTATATE therapy.
DISCLOSURE
PPQ/NETest measurements were provided pro bono by Wren Laboratories. This study was supported in part by NIH/NCI Cancer Center support grant P30 CA008748. Lisa Bodei is a nonremunerated consultant for AAA, ITM, Clovis Oncology, Curium, and Iba and receives research funding from AAA. Nitya Raj is on the advisory boards for Ipsen Pharma, HRA Pharma, Progenics Pharmaceuticals, and AAA. Simone Krebs is supported in part by the NIH/NCI Paul Calabresi Career Development Award for Clinical Oncology (K12 CA184746). Diane Reidy-Lagunes receives research funding from Merck, Ipsen, and Novartis and is a consultant for Novartis, Lexicon, and AAA. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Are validated liquid biopsies that can predict or monitor PRRT a critical unmet need in NET management?
PERTINENT FINDINGS: Two blood-based biomarkers were evaluated in a prospective study (n = 67). The PPQ was an accurate (96%) predictor of 177Lu-DOTATATE response, and NETest changes correctly (90%) correlated with treatment response.
IMPLICATIONS FOR PATIENT CARE: Blood-based gene signatures may enhance the management of patients undergoing 177Lu-DOTATATE by providing information on tumor radiosensitivity and disease course, thus allowing individualized strategies.
REFERENCES
- 1. Bodei L, Schöder H, Baum RP, et al. Molecular profiling of neuroendocrine tumours to predict response and toxicity to peptide receptor radionuclide therapy. Lancet Oncol. 2020;21:e431–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. [DOI] [PubMed] [Google Scholar]
- 3. Kwekkeboom DJ, Kam BL, van Essen M, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–R73. [DOI] [PubMed] [Google Scholar]
- 4. Liberini V, Huellner MW, Grimaldi S, et al. The challenge of evaluating response to peptide receptor radionuclide therapy in gastroenteropancreatic neuroendocrine tumors: the present and the future. Diagnostics (Basel). 2020;10:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baum RP, Kulkarni HR, Singh A, et al. Results and adverse events of personalized peptide receptor radionuclide therapy with 90yttrium and 177lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget. 2018;9:16932–16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Albertelli M, Dotto A, Di Dato C, et al. PRRT: identikit of the perfect patient. Rev Endocr Metab Disord. 2021;22:563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eschrich SA, Fulp WJ, Pawitan Y, et al. Validation of a radiosensitivity molecular signature in breast cancer. Clin Cancer Res. 2012;18:5134–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009;75:489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim HS, Kim SC, Kim SJ, et al. Identification of a radiosensitivity signature using integrative metaanalysis of published microarray data for NCI-60 cancer cells. BMC Genomics. 2012;13:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bodei L, Kidd MS, Singh A, et al. PRRT genomic signature in blood for prediction of 177Lu-octreotate efficacy. Eur J Nucl Med Mol Imaging. 2018;45:1155–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kidd M, Modlin IM. Therapy: the role of liquid biopsies to manage and predict PRRT for NETs. Nat Rev Gastroenterol Hepatol. 2017;14:331–332. [DOI] [PubMed] [Google Scholar]
- 13. Modlin I, Drozdov I, Kidd M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS One. 2013;8:e63364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen F, Zhang Y, Gibbons DL, et al. Pan-cancer molecular classes transcending tumor lineage across 32 cancer types, multiple data platforms, and over 10,000 cases. Clin Cancer Res. 2018;24:2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pacak K, Kidd M, Meuter L, Modlin IM. A novel liquid biopsy (NETest) identifies paragangliomas and pheochromocytomas with high accuracy. Endocr Relat Cancer. 2021;28:731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu E, Paulson S, Gulati A, et al. Assessment of NETest clinical utility in a US registry-based study. Oncologist. 2019;24:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Öberg K, Califano A, Strosberg JR, et al. A meta-analysis of the accuracy of a neuroendocrine tumor mRNA genomic biomarker (NETest) in blood. Ann Oncol. 2020;31:202–212. [DOI] [PubMed] [Google Scholar]
- 18. Kidd M, Drozdov I, Modlin I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr Relat Cancer. 2015;22:561–575. [DOI] [PubMed] [Google Scholar]
- 19. Bodei L, Kidd MS, Singh A, et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: the NETest. Eur J Nucl Med Mol Imaging. 2020;47:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bodei L, Sundin A, Kidd M, Prasad V, Modlin IM. The status of neuroendocrine tumor imaging: from darkness to light? Neuroendocrinology. 2015;101:1–17. [DOI] [PubMed] [Google Scholar]
- 21. Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. [DOI] [PubMed] [Google Scholar]
- 22. Brabander T, van der Zwan WA, Teunissen JJM, et al. Pitfalls in the response evaluation after peptide receptor radionuclide therapy with [177Lu-DOTA0,Tyr3]octreotate. Endocr Relat Cancer. 2017;24:243–251. [DOI] [PubMed] [Google Scholar]
- 23. Cheng DT, Prasad M, Chekaluk Y, et al. Comprehensive detection of germline variants by MSK-IMPACT, a clinical diagnostic platform for solid tumor molecular oncology and concurrent cancer predisposition testing. BMC Med Genomics. 2017;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bodei L, Kidd M, Modlin IM, et al. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2016;43:839–851. [DOI] [PubMed] [Google Scholar]
- 25. Reidy-Lagunes D, Pandit-Taskar N, O’Donoghue JA, et al. Phase I trial of well-differentiated neuroendocrine tumors (NETs) with radiolabeled somatostatin antagonist 177Lu-satoreotide tetraxetan. Clin Cancer Res. 2019;25:6939–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ortega C, Wong RKS, Schaefferkoetter J, et al. Quantitative 68Ga-DOTATATE PET/CT parameters for the prediction of therapy response in patients with progressive metastatic neuroendocrine tumors treated with 177Lu-DOTATATE. J Nucl Med. 2021;62:1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simbolo M, Vicentini C, Mafficini A, et al. Mutational and copy number asset of primary sporadic neuroendocrine tumors of the small intestine. Virchows Arch. 2018;473:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scarpa A, Chang DK, Nones K, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65–71. [DOI] [PubMed] [Google Scholar]
- 30. Hofving T, Elias E, Rehammar A, et al. SMAD4 haploinsufficiency in small intestinal neuroendocrine tumors. BMC Cancer. 2021;21:101. [DOI] [PMC free article] [PubMed] [Google Scholar]