Visual Abstract
Keywords: targeted α-therapy, 223Ra, 177Lu-PSMA, metastatic castration-resistant prostate cancer, real-world practice
Abstract
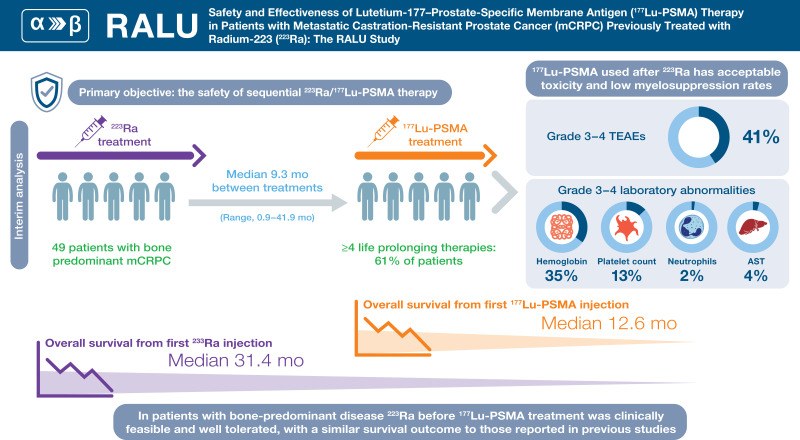
The radium lutetium (RALU) study evaluated the feasibility of sequential α- and β-emitter use in patients with bone-predominant metastatic castration-resistant prostate cancer. Methods: This preplanned interim retrospective analysis investigated safety and survival outcomes with 177Lu-PSMA in patients treated with prior 223Ra. Results: Forty-nine patients were evaluated. Patients received a median of 6 223Ra injections; 59% of patients received at least 4 177Lu-PSMA cycles. Most (69%) patients received at least 4 life-prolonging therapies before 177Lu-PSMA. Common Terminology Criteria for Adverse Events grade 3–4 treatment-emergent adverse events during 177Lu-PSMA therapy and a 30-d follow-up period included anemia (18%) and thrombocytopenia (2%). Median overall survival was 12.6 mo (95% CI, 8.8–16.1 mo) and 31.4 mo (95% CI, 25.7–37.6 mo) from starting 177Lu-PSMA or 223Ra, respectively. Conclusion: 177Lu-PSMA treatment was well tolerated in patients who had received prior 223Ra. 223Ra use before 177Lu-PSMA is feasible and can be considered for future assessment of the optimal treatment sequence.
Overall survival and quality of life in patients with bone-predominant metastatic castration-resistant prostate cancer (mCRPC) was improved by 223Ra-dichloride, a targeted α-therapy with a good safety profile (1). 223Ra therapy results in low myelosuppression rates, and recent preclinical data demonstrated its transient effect on the bone marrow without long-term effects (1,2). Therefore, earlier incorporation of 223Ra in the treatment sequence may facilitate optimal build-in of life-prolonging therapies to improve survival outcomes.
The VISION study investigated a β-emitter, 177Lu-PSMA-617, targeting PSMA-expressing cells and found prolonged overall survival and acceptable safety in heavily pretreated patients with mCRPC (3). Another 177Lu-PSMA radioligand (177Lu-PSMA-I&T) was also well tolerated, with few hematologic adverse events (AEs) of grade 3 or higher (4).
223Ra and 177Lu-PSMA regulatory approval (in some countries) for patients with mCRPC, albeit in different patient populations, prompted us to investigate the safety and survival outcomes of sequential 223Ra and 177Lu-PSMA. In VISION, 17.4% of patients received 223Ra therapy before 177Lu-PSMA without adversely affecting efficacy, but safety has not been reported for this subgroup (5). However, retrospective studies have shown that using 223Ra before 177Lu-PSMA is feasible, with acceptable safety (6,7). Moreover, 177Lu-PSMA-617 initiation at no more than 8 wk after 223Ra in patients with progressive bone-metastatic disease was effective, with acceptable safety (8). We analyzed interim data from the observational radium lutetium (RALU) study to further evaluate safety and survival for sequential 223Ra and 177Lu-PSMA therapy in patients with mCRPC.
MATERIALS AND METHODS
The RALU study was a retrospective, multicenter medical chart review investigating the safety of 177Lu-PSMA in patients with mCRPC previously treated with 223Ra. This analysis includes patients treated in Germany. Patients were at least 18 y old with mCRPC and received at least 1 223Ra injection and subsequently at least 1 177Lu-PSMA cycle.
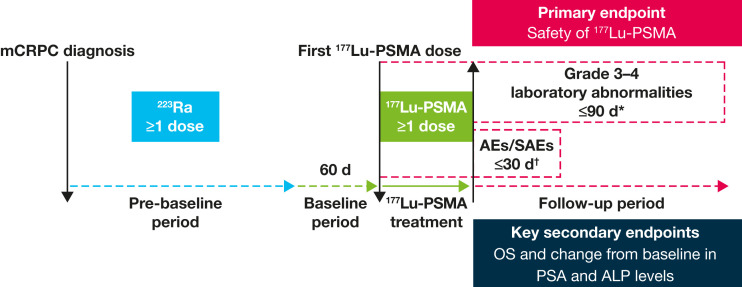
The retrospective observation period started at mCRPC diagnosis and ended either at the last available visit or death, whichever occurred first. Prebaseline, baseline, and follow-up period definitions are shown in Figure 1.
FIGURE 1.
RALU study design. *From 177Lu-PSMA start to 90 d after last dose. †From 177Lu-PSMA start to 30 d after last dose. ALP = alkaline phosphatase; OS = overall survival; PSA = prostate-specific antigen; SAEs = serious AEs.
The primary endpoint was the safety of 177Lu-PSMA after 223Ra therapy. AEs used Common Terminology Criteria for Adverse Events grading. Secondary endpoints included OS, time to next treatment, and change from baseline in serum prostate-specific antigen and alkaline phosphatase levels. AEs and grade 3–4 laboratory abnormalities were recorded as per Figure 1.
The study was conducted in accordance with relevant guidelines and regulations (supplemental methods).
RESULTS
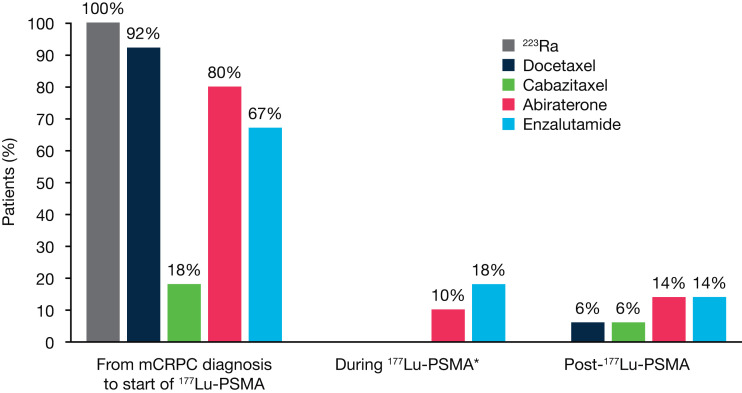
This preplanned interim analysis included medical records from 49 patients (data cutoff, January 31, 2022) (Table 1). Before 177Lu-PSMA initiation, 31% (15/49) of patients had visceral metastases, and median prostate-specific antigen and alkaline phosphatase values were 287.0 ng/mL and 142.5 U/L, respectively (Table 1). At least 1 line of taxane-based chemotherapy was received by 92% (45/49) of patients before 177Lu-PSMA initiation, with 51% (25/49) receiving taxane-based chemotherapy between 223Ra and 177Lu-PSMA (Table 1). Before starting 177Lu-PSMA, 63% (30/49) of patients received at least 4 life-prolonging therapies (docetaxel, cabazitaxel, abiraterone, enzalutamide, and 223Ra; Table 1; Fig. 2). Most patients received chemotherapy before 177Lu-PSMA; 92% received docetaxel, and 18% received cabazitaxel (Fig. 2).
TABLE 1.
Baseline Characteristics Before Starting 177Lu-PSMA
| Characteristic | Data |
|---|---|
| Total patients | 49 (100) |
| Age (y) | 72 (57–83) |
| Eastern Cooperative Oncology Group performance status (baseline) | |
| 0 | 0 (0) |
| 1 | 36 (73) |
| 2 | 13 (27) |
| 3–4 | 0 (0) |
| Prostate-specific antigen (ng/mL) | 287.0 (20–12,229) |
| Alkaline phosphatase (U/L) | 142.5 (48–730) |
| Visceral metastatic disease | 15 (31) |
| ≥4 life-prolonging therapies* | 30 (61) |
| Novel antiandrogen therapies | |
| Abiraterone | 39 (80) |
| Enzalutamide | 33 (67) |
| Abiraterone and enzalutamide | 33 (67) |
| Number of any taxane-based chemotherapy lines† | |
| 0 | 4 (8) |
| 1 | 35 (71) |
| ≥2 | 10 (20) |
| Docetaxel | 45 (92) |
| Number of docetaxel cycles‡ | |
| 1–4 | 10 (20) |
| ≥5 | 26 (53) |
| Cabazitaxel | 9 (18) |
| Number of cabazitaxel cycles‡ | |
| 1–4 cycles | 0 (0) |
| ≥5 cycles | 5 (10) |
| Taxane-based chemotherapy between 223Ra and 177Lu-PSMA§ | 25 (51) |
Docetaxel, cabazitaxel, abiraterone, enzalutamide, and 223Ra.
Chemotherapies with same start date ± 15 d are counted as 1 line.
Not available for some patients.
After last 223Ra dose and 60 d before 177Lu-PSMA.
Qualitative data are number and percentage; continuous data are median and range.
FIGURE 2.
Use of life-prolonging therapies. *Chemotherapy was not used concomitantly with 177Lu-PSMA.
The median time from the first 223Ra and 177Lu-PSMA dose to the end of observation was 28.0 mo (range, 11.0–68.2 mo) and 8.9 mo (range, 1.4–63.9 mo), respectively. A median of 6 223Ra injections was administered, and 71% (35/49) of patients received 5–6 223Ra injections. The median 223Ra therapy duration was 4.7 mo (range, 1.0–7.0 mo). All patients received 177Lu-PSMA (177Lu-PSMA-617 [67%] or 177Lu-PSMA-I&T [33%]), and 59% (29/49) received at least 4 177Lu-PSMA cycles. The median 177Lu-PSMA therapy duration was 4.9 mo (range, 0–57.1 mo). The median time from the last 223Ra injection to the first 177Lu-PSMA dose was 9.3 mo (range, 0.9–41.9 mo). Overall, 51% (25/59) of patients received taxane-based chemotherapy during or after 223Ra and until 60 d before 177Lu-PSMA.
During 177Lu-PSMA, 92% (45/49) of patients experienced any grade of treatment-emergent AEs and 41% (20/49) experienced grade 3–4 (Supplemental Table 1). Grade 3–4 anemia and thrombocytopenia occurred in 18% (9/49) and 2% (1/49) of patients, respectively. Grade 1–2 dry mouth occurred in 27% (13/49) of patients; none had grade 3–4 dry mouth. One patient (2%) had grade 1–2 dry eye. The incidence of grade 3–4 laboratory abnormalities was highest for anemia (35% [17/49]) and thrombocytopenia (13% [6/49]) (Table 2). No grade 5 toxicities occurred.
TABLE 2.
Incidence of Grade 3–4 Laboratory Abnormalities Measured from 177Lu-PSMA Start to 90 Days After Last Dose
| Abnormality | Patients (n) | Incidence (n) |
|---|---|---|
| Hemoglobin | 49 | 17 (35%) |
| Platelet count | 47 | 6 (13%)* |
| Neutrophils | 49 | 1 (2%) |
| Aspartate aminotransferase | 49 | 2 (4%) |
Four of 6 had low platelets at baseline, with further reductions at follow-up (1 had chemotherapy before 177Lu-PSMA); 1 of 6 had normal platelets at baseline, with reductions seen after chemotherapy and at follow-up; 1 of 6 had normal platelets at baseline, with reduction at follow-up.
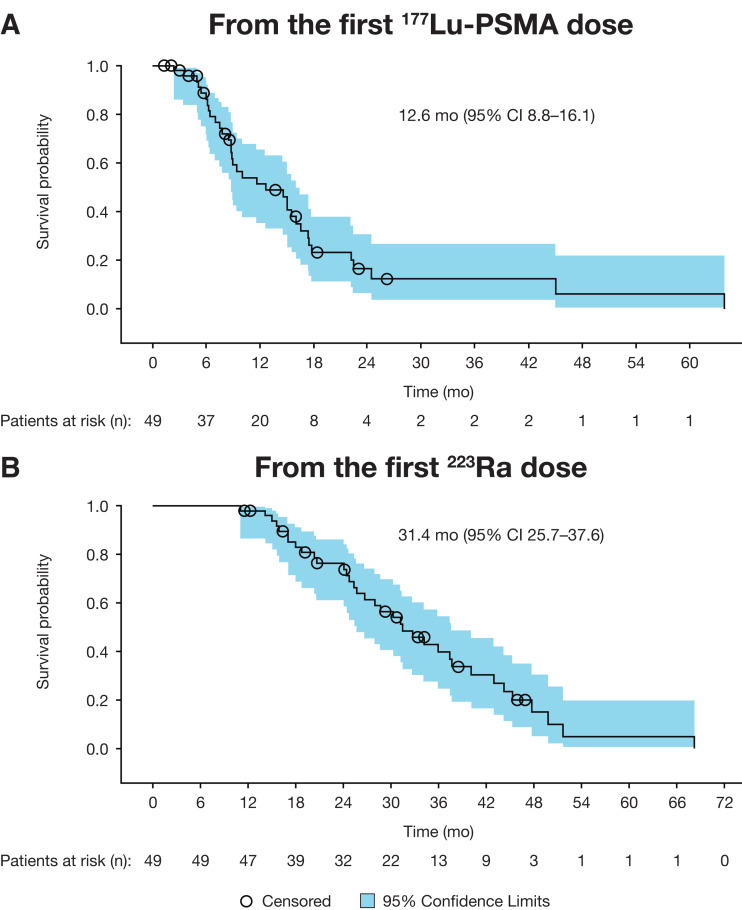
Median overall survival was 12.6 mo (95% CI, 8.8–16.1 mo) and 31.4 mo (95% CI, 25.7–37.6 mo) from the first dose of 177Lu-PSMA and 223Ra, respectively (Fig. 3). During 177Lu-PSMA, 39% and 29% of patients had at least a 30% or 50% decline in prostate-specific antigen (best response), respectively; corresponding alkaline phosphatase declines were 6% and 4%.
FIGURE 3.
Kaplan–Meier plots for overall survival calculated from first 177Lu-PSMA (A) and 223Ra (B) dose.
DISCUSSION
Randomized trials have demonstrated low myelosuppression rates in patients with mCRPC receiving 223Ra or 177Lu-PSMA (1,3,9). However, chemotherapy and advanced disease affecting bone marrow function can increase myelosuppression rates in this setting (10–12). Therefore, in real-world practice, radiopharmaceutical therapy after prior chemotherapy may result in more serious hematologic AEs.
177Lu-PSMA after 223Ra treatment had an acceptable safety profile. Notably, this was despite the heavy pretreatment of the patient population, with more than 90% of patients having received chemotherapy in addition to 223Ra and 177Lu-PSMA. Grade 3–4 anemia and thrombocytopenia incidences were 18% and 2%, respectively, consistent with the retrospective analysis of patients receiving the 223Ra and 177Lu-PSMA sequence in the real-world REASSURE study (15% and 4%, respectively) (6). When 177Lu-PSMA was given within 8 wk of 223Ra, the incidence of anemia of at least grade 3 was similar (18%), but the rates of leukopenia and thrombocytopenia of at least grade 3 were higher than reported here (14% vs. 0 and 21% vs. 2%, respectively) (8).
The median overall survival from the start of 177Lu-PSMA or 223Ra therapy (12.6 and 31.4 mo, respectively) corresponded to that reported in REASSURE (13.2 and 28.0 mo, respectively) (6). In patients with mCRPC who underwent 177Lu-PSMA therapy in the WARMTH study, overall survival was longer in patients with bone metastases receiving prior 223Ra than in those who did not (16 vs. 12 mo in patients with 6–20 bone lesions, P = 0.038, and 11 vs. 7 mo in patients with diffuse involvement, P = 0.034) (12).
This study’s strength is underlined by broad inclusion criteria and high-quality data with few missing datapoints. Accordingly, we could effectively evaluate 177Lu-PSMA safety in patients with a history of 223Ra therapy who received chemotherapy, before or after 223Ra treatment. Nevertheless, a retrospective study design may have contributed to a patient selection bias due to the preset outcomes of interest. Other limitations include retrospective AE grading, lack of ascertainment of 177Lu-PSMA doses and schedules, and a lack of comparison to patients who were not pretreated with 223Ra. Despite small patient numbers, patients were managed and treated in high-volume German nuclear medicine centers with extensive 223Ra and 177Lu-PSMA experience.
CONCLUSION
This retrospective cohort study demonstrated that, for patients with bone-predominant mCRPC who were receiving 223Ra in routine care, subsequent 177Lu-PSMA treatment was clinically feasible and well tolerated, with limited myelosuppression. Survival outcomes reflected those of previous reports. Therefore, in patients with bone-predominant mCRPC, 223Ra use before 177Lu-PSMA can be considered in future assessments of the optimal sequence for life-prolonging therapies.
DISCLOSURE
Cancer Communications and Consultancy Ltd., Cheshire, U.K., provided medical writing assistance (funded by Bayer). Dr. Lila Adnane (Bayer) provided editorial assistance. Kambiz Rahbar receives honoraria from Advanced Accelerator Applications (AAA) and Bayer and has a consultancy/advisory role with ABX GmbH, ABX-CRO, Bayer, and AAA. Markus Essler has a consultancy/advisory role with Bayer, AAA, and Ipsen and receives travel funds from Ipsen. Matthias Eiber owns stocks or has other ownership interests in Novartis and Telix Pharmaceuticals; has a consultancy/advisory role with Blue Earth Diagnostics, ABX Advanced Biochemical Compounds, Janssen Oncology, Telix Pharmaceuticals, and Novartis; receives research funding from Siemens, ABX Advanced Biochemical Compounds, Blue Earth Diagnostics, and Bayer; has a patent application for rhPSMA; and receives travel funds from Bayer Schering Pharma. Christian la Fougère has a consultancy/advisory role with Novartis, EUSA-Pharma, Ipsen, Oncodesign, and Sirtex Medical and receives research funding from Oncovision. Vikas Prasad receives honoraria from AAA; has a consultancy/advisory role with Bayer; and receives research funding from Ipsen. Wolfgang Fendler receives honoraria from Parexel and AAA; has a consultancy/advisory role with Janssen, Calyx, and Bayer; and receives research funding from SOFIE. Philipp Rassek is an employee of Porterhouse Group AG Paracelsus Kliniken. Helmut Dittmann has a consultancy/advisory role with Bayer, Ipsen, and Eisai AG. Ralph Bundschuh receives honoraria from Eisai AG and has a consultancy/advisory role with Bayer. Kim Pabst receives a Junior Clinician Scientist Stipend from the University Medicine Essen Clinician Scientist Academy (sponsor: Faculty of Medicine and Deutsche Forschungsgemeinschaft) and research funding from Bayer. Milena Kurtinecz, and Frank Verholen are employees of Bayer. Oliver Sartor has a consultancy/advisory role with Bayer, Sanofi, AstraZeneca, Dendreon, Constellation Pharmaceuticals, AAA, Pfizer, Bristol-Myers Squibb, Bavarian Nordic, EMD Serono, Astellas Pharma, Progenics, Blue Earth Diagnostics, Myovant Sciences, Myriad Genetics, Novartis, Clarity Pharmaceuticals, Fusion Pharmaceuticals, Isotopen Technologien, Janssen, Noxopharm, Clovis Oncology, Noria Therapeutics, Point Biopharma, TeneoBio, Telix Pharmaceuticals, and Theragnostics; receives travel funds from Bayer, Johnson & Johnson, Sanofi, AstraZeneca, and Progenics; provides expert testimony for Sanofi; owns stocks or has other ownership interests in Lilly, GlaxoSmithKline, Abbvie, Cardinal Health, United Health Group, PSMA Therapeutics, Clarity Pharmaceuticals, Noria Therapeutics, and Clovis Oncology; and receives research funding from Bayer, Sanofi, Endocyte, Merck, InVitae, Constellation Pharmaceuticals, AAA, AstraZeneca, Dendreon, SOTIO, Janssen, and Progenics. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is it safe to use 177Lu-PSMA to treat patients with mCRPC if they have previously received 223Ra?
PERTINENT FINDINGS: Low rates of overall and hematologic AEs indicated an acceptable safety profile for this treatment sequence. Median OS was 12.6 and 31.4 mo from the first dose of 177Lu-PSMA and 223Ra, respectively, and 39% of patients had at least a 30% decline in prostate-specific antigen.
IMPLICATIONS FOR PATIENT CARE: Introduction of 223Ra early in the treatment sequence in patients with bone-predominant mCRPC and subsequent treatment with 177Lu-PSMA is feasible, well tolerated, and effective.
REFERENCES
- 1. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. [DOI] [PubMed] [Google Scholar]
- 2. Parker CC, Coleman RE, Sartor O, et al. Three-year safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases from phase 3 randomized alpharadin in symptomatic prostate cancer trial. Eur Urol. 2018;73:427–435. [DOI] [PubMed] [Google Scholar]
- 3. Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heck MM, Tauber R, Schwaiger S, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2019;75:920–926. [DOI] [PubMed] [Google Scholar]
- 5. Vaishampayan N, Morris MJ, Krause BJ, et al. [177Lu]Lu-PSMA-617 in PSMA-positive metastatic castration-resistant prostate cancer: prior and concomitant treatment subgroup analyses of the VISION trial. J Clin Oncol. 2022;40(16_Suppl):5001. [Google Scholar]
- 6. Sartor O, la Fougere C, Essler M, et al. Lutetium-177-prostate-specific membrane antigen ligand after radium-223 treatment in men with bone-metastatic castration-resistant prostate cancer: real-world clinical experience. J Nucl Med. 2021;63:410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmadzadehfar H, Zimbelmann S, Yordanova A, et al. Radioligand therapy of metastatic prostate cancer using 177Lu-PSMA-617 after radiation exposure to 223Ra-dichloride. Oncotarget. 2017;8:55567–55574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baumgarten J, Groener D, Nguyen Ngoc C, et al. Safety and efficacy of 177lutetium-PSMA-617 radioligand therapy shortly after failing 223radium-dichloride. Cancers (Basel). 2022;14:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. [DOI] [PubMed] [Google Scholar]
- 10. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. [DOI] [PubMed] [Google Scholar]
- 11. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. [DOI] [PubMed] [Google Scholar]
- 12. Ahmadzadehfar H, Matern R, Baum RP, et al. The impact of the extent of the bone involvement on overall survival and toxicity in mCRPC patients receiving [177Lu]Lu-PSMA-617: a WARMTH multicentre study. Eur J Nucl Med Mol Imaging. 2021;48:4067–4076. [DOI] [PubMed] [Google Scholar]