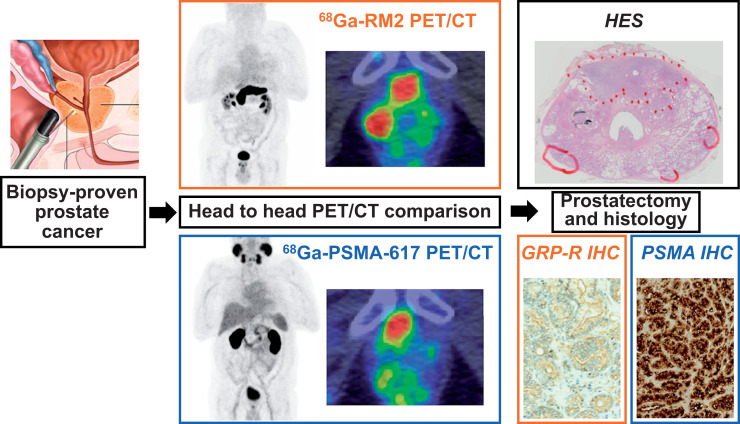
Visual Abstract
Keywords: GRP-R, PSMA, PET, prostate cancer, imaging
Abstract
Considering the wide range of therapeutic options for localized prostate cancer (e.g., active surveillance, radiation-beam therapy, focal therapy, and radical prostatectomy), accurate assessment of the aggressiveness and localization of primary prostate cancer lesions is essential for treatment decision making. National Comprehensive Cancer Network guidelines recognize prostate-specific membrane antigen (PSMA) PET/CT for use in initial staging of high-risk primary prostate cancer. The gastrin-releasing peptide receptor (GRP-R) is a neuropeptide receptor overexpressed by low-risk prostate cancer cells. We aimed to perform the first (to our knowledge) prospective head-to-head comparison of PSMA- and GRP-R–targeted imaging at initial staging to understand how PSMA PET and GRP-R PET can be used or combined in clinical practice. Methods: This was a prospective, single-center, diagnostic cross-sectional imaging study using anonymized, masked, and independent interpretations of paired PET/CT studies in 22 patients with 68Ga-PSMA-617 (a radiolabeled PSMA inhibitor) and 68Ga-RM2 (68Ga-DOTA-4-amino-1-carboxymethylpiperidine-d-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2, a radiolabeled GRP-R antagonist). We enrolled patients with newly diagnosed, biopsy-proven prostate cancer. None had received neoadjuvant hormone therapy or chemotherapy, and all underwent extended pelvic lymph node dissection. Histologic findings served as a reference. Results: On a lesion-based analysis (including lesions < 0.1 cm3), 68Ga-PSMA-617 PET/CT detected 74.3% (26/35) of all tumor lesions and 68Ga-RM2 PET/CT detected 78.1% (25/32; 1 patient could not be offered 68Ga-RM2 PET/CT). Paired examinations showed positive uptake of the 2 tracers in 21 of 32 lesions (65.6%), negative uptake in 5 of 32 lesions (15.6%), and discordant uptake in 6 of 32 lesions (18.8%). Uptake of 68Ga-PSMA-617 was higher when the International Society of Urological Pathology (ISUP) score was at least 4 versus at least 1 (P < 0.0001) or 2 (P = 0.0002). There were no significant differences in uptake between ISUP scores for 68Ga-RM2. Median 68Ga-RM2 SUVmax was significantly higher than median 68Ga-PSMA-617 SUVmax in the ISUP-2 subgroup (P = 0.01). Conclusion: 68Ga-PSMA-617 PET/CT is useful to depict higher, more clinically significant ISUP score lesions, and 68Ga-RM2 PET/CT has a higher detection rate for low-ISUP tumors. Combining PSMA PET and GRP-R PET allows for better classification of intraprostatic lesions.
Prostate cancer is the most common cancer in men and the third cause of cancer-related deaths (1). The range of therapeutic options for localized prostate cancer varies from active surveillance or focal therapy to radiation-beam therapy or radical prostatectomy, depending on the local extension and risk classification of tumor progression. Therefore, the initial assessment of primary-tumor aggressiveness is critical to treatment decision making. In combination with clinical examination, PSA level, and prostatic MRI, the risk classification of the primary tumor depends mainly on appropriate sampling by prostatic biopsies and on precise evaluation of the International Society of Urological Pathology (ISUP) score.
Prostate-specific membrane antigen (PSMA) is a type 2 glycoprotein expressed in secretory cells of prostatic epithelium. Several radiolabeled PSMA inhibitors have been developed (68Ga-PSMA-11, 68Ga-PSMA-617, 68Ga-PSMAI&T, and 18F-PSMA1007 (2)). Uptake of radiolabeled PSMA inhibitors correlates well with ISUP score and PSA level (3). Recently, National Comprehensive Cancer Network guidelines considered the use of PSMA PET/CT for the initial staging of high-risk primary prostate cancer (4). However, the ability of PSMA PET/CT to also identify lower-grade lesions is unclear.
The gastrin-releasing peptide receptor (GRP-R) is a G-protein–coupled receptor of the bombesin receptor family (5) that can be targeted with radiolabeled antagonists such as 68Ga-RM2 (68Ga-DOTA-4-amino-1-carboxymethylpiperidine-d-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2) (6), 68Ga-NeoBOMB1 (7), or 68Ga-RM26 (8) for PET imaging. Unlike PSMA, GRP-R is overexpressed in low-risk prostate cancers (low Gleason score, low prostate-specific antigen [PSA] value, and small tumor) (9–11). A study of the diagnostic performance of 68Ga-RM2 PET/CT for initial staging of prostate cancer in 41 patients reported a detection rate of 93%, a sensitivity of 98%, and a specificity of 65% (6).
In preclinical work, we compared in vitro GRP-R and PSMA expression in primary prostate cancer samples by means of 111In-RM2 and 111In-PSMA-617. Our results suggested that PSMA- and GRP-R–based imaging may have a complementary role in fully characterizing the local extent and aggressiveness of prostate cancer (with GRP-R being a valuable target in patients with a low metastatic risk and PSMA being a valuable target in patients with a higher risk (12)).
Additionally, a pilot clinical study using 68Ga-PSMA-11 PET/CT and 68Ga-RM2 PET/CT on 8 patients also suggested a complementary role for these imaging modalities in the initial staging of prostate cancer (13).
Here, we present a prospective head-to-head comparison between 68Ga-PSMA-617 PET/CT and 68Ga-RM2 PET/CT for the initial assessment of localized primary prostate cancer tumors. Our primary objective was to assess uptake intensity (SUVmax) with 68Ga-PSMA-617 and 68Ga-RM2 PET/CT at the level of prostatic lesions and to compare SUVmax between ISUP score categories. Secondary objectives were to compare 68Ga-PSMA-617 and 68Ga-RM2 uptake stratified by ISUP score, to compare SUVmax at 2 acquisition times (60 and 120 min after injection), and to evaluate for an association between the immunohistochemistry scores of the targets (PSMA and GRP-R) and 68Ga-PSMA-617 and 68Ga-RM2 uptake.
MATERIALS AND METHODS
Study Design and Participants
This was a prospective, single-center, diagnostic cross-sectional imaging study using anonymized, masked, and independent interpretations of paired 68Ga-PSMA-617 PET/CT and 68Ga-RM2 PET/CT scans (EudraCT 2017-000490-36, NCT03604757). 68Ga-PSMA-617 PET/CT and 68Ga-RM2 PET/CT were performed before prostatectomy in no specific order and with no consideration of patient characteristics. We prospectively enrolled 22 patients with newly diagnosed, biopsy-proven prostate cancer. The French ethical committee approved this study (approval 2017/62), and all subjects gave written informed consent.
The inclusion criteria were an age greater than 18 y, a diagnosis of prostate cancer confirmed by biopsy, and an indication for prostatectomy. No patient had received neoadjuvant hormone therapy or chemotherapy. All patients underwent extended pelvic lymph node dissection.
Radiopharmaceuticals and PET/CT Protocol
68Ga-PSMA-617 and 68Ga-RM2 were produced according to our previous description (12), with minor modifications (68Ga was used as the radionuclide, and 10 μg of PSMA-617 were used). The Discovery RX PET/CT device (GE Healthcare) at the University Hospital of Bordeaux was used. Whole-body PET/CT images were acquired from vertex to mid thighs, with 2.5-min emission scans per bed position, at 60 and 120 min after intravenous administration of 2 MBq/kg (range, 80–200 MBq) of 68Ga-PSMA-617 or 68Ga-RM2. Images were reconstructed using an ordered-subset expectation maximization algorithm with 2 iterations and 21 subsets (matrix size, 256 × 256; 47 slices corresponding to a 15.6-cm transaxial field of view; voxel size, 2.376 × 2.376 × 3.27 mm). The CT acquisition was performed for attenuation correction, in helical mode, using 120 kV, mAs modulation to optimally reduce the dose, and a 512 × 512 matrix (voxel size, 0.9766 × 0.9766 × 2 mm).
PET/CT Image Analysis
PET/CT and multiparametric MR (when available) images were analyzed using Pmod software (version 3.5; PMOD Technologies LLC). A manual registration was performed between each modality, using a linear transformation, to aid visual analysis and accurate positioning of the tumor lesion. Then, manual segmentation was performed by 2 experienced nuclear physicians masked to the histologic findings, radiopharmaceutical, and patient characteristics. Supravesical sections were removed because of physiologic renal uptake of 68Ga-PSMA-617 and physiologic pancreatic uptake of 68Ga-RM2. A consensus was reached in cases of discrepancy between the 2 interpretations. Uptake of 68Ga-PSMA-617 and 68Ga-RM2 was quantified according to SUVmax and described for each lesion.
Histology
Prostatectomy samples were fixed and embedded in paraffin blocks. Tissue slices 5 μm thick were stained with hematoxylin, eosin, and saffron, and an experienced pathologist manually surrounded tumor lesions under microscopic examination and reported the ISUP score and size of each lesion. Lesions smaller than 0.1 cm3 were included in the analysis. Histologic samples were then digitized using a slide scanner (NDP.scan; Hamamatsu). The obtained images were arranged and reoriented to facilitate comparison between histology and PET imaging.
Immunohistochemistry
The immunohistochemical study was performed as previously described for GRP-R (14) and PSMA (15). Immunohistochemistry results were expressed as an immunoreactive score (IRS) that considered staining intensity and the percentage of stained tumor cells, as previously described (14). The final IRS score (product of staining intensity score and percentage-of-positive-cells score) thus ranged from 0 to 12. No PSMA or GRPR expression was categorized as IRS 0–1, weak PSMA or GRP-R expression was categorized as IRS 2–3, moderate PSMA or GRP-R expression was categorized as IRS 4–8, and strong PSMA or GRP-R expression was categorized as IRS 9–12. Immunohistochemistry results were dichotomized into 2 groups: low PSMA or GRP-R expression (absent/weak expression) and high PSMA or GRP-R expression (moderate/strong expression).
Cross-Sectional Analysis of PET Signal and Histology
For each tumor lesion, SUVmax for 68Ga-PSMA-617 and 68Ga-RM2 was compared with histology (cancer or noncancer area), allowing determination of concordance and discordance of findings.
Cross-Sectional Analysis of PET Signal and Immunohistochemistry Staining
For each tumor lesion, SUVmax for 668Ga-PSMA-617 and 68Ga-RM2 SUVmax was compared with the immunohistochemistry score for the whole tumor compartment.
Statistical Analysis
The sample size was fixed at 6 patients for each metastatic risk group defined at enrollment, before surgery (ISUP-1 and cT1-T2a and PSA < 10 ng/mL, Briganti < 5%; ISUP-2 or cT2b or PSA = 10–20 ng/mL; ISUP-3 or cT2b or PSA = 10–20 ng/mL; ISUP4–5 or cT2c or PSA > 20 ng/mL).
Quantitative variables are described as mean and SD, median and first to third quartiles, or minimum to maximum. Qualitative variables are described as frequency and percentage. SUVmax was compared at the patient level in normal tissues and at the lesion level in pathologic tissues. All comparisons of SUVmax (between ISUP scores, radiopharmaceuticals, and acquisition times) used univariable mixed linear regression models, including a random intercept to consider the intrapatient correlation (with a variance component structure). The models’ hypotheses (normality and heteroscedasticity of residuals) were systematically checked, leading us to transform SUVmax in pathologic tissues by the natural logarithm. Exponential of parameters estimated with these last models can be expressed as a multiplicative factor: less than 1 means a decreased value compared with the other group, and more than 1 means an increased value compared with the other group.
Comparisons of SUVmax in normal and pathologic tissues between 60 and 120 min were performed first to select the adequate acquisition time for other analyses. Comparison of SUVmax between negative and positive immunochemistry scores used nonparametric Wilcoxon tests. For the 2 primary outcomes only, if the global statistical test was significant at 2.5%, 2-by-2 comparison tests between ISUP scores were interpreted using a 0.4% significance level (Bonferroni method). Statistical analyses used SAS software (version 9.4; SAS Institute).
RESULTS
Radiopharmaceuticals, Patient Characteristics, and Lesion Characteristics
Twenty-two men with newly diagnosed prostate cancer were enrolled in the study between April 25, 2018, and November 19, 2019. The demographic and clinicopathologic characteristics of the population are presented in Table 1. The median interval between the 2 PET/CT examinations was 6 d (Q1–Q3, 3–8 d). The median interval between the last PET/CT examination and surgery was 6 d (Q1–Q3, 1–15 d). Nine (41%) patients underwent 68Ga-PSMA-617 PET/CT first, and 13 (59%) patients underwent 68Ga-RM2 PET/CT first. One patient could not undergo 68Ga-RM2 PET/CT. The median injected activity was 167.2 MBq (range, 118.7–210.2 MBq) for 68Ga-PSMA-617 and 149.5 MBq (range, 84.5–198.5 MBq) for 68Ga-RM2. All images were acquired at 1 and 2 h after injection, except in 1 patient, who underwent 68Ga-RM2 imaging at 1 h only.
TABLE 1.
Patient Characteristics (n = 22)
| Variable | At diagnosis | Histopathology |
|---|---|---|
| ISUP score (n) | ||
| 1 (Gleason 6) | 5 (22.7%) | 1 (4.5%) |
| 2 (Gleason 7 [3 + 4]) | 6 (27.3%) | 9 (40.9%) |
| 3 (Gleason 7 [4 + 3]) | 4 (18.2%) | 2 (9.1%) |
| 4 (Gleason 8) | 2 (9.1%) | 3 (13.6%) |
| 5 (Gleason > 8) | 5 (22.7%) | 7 (31.8%) |
| TNM stage (n) | ||
| T2a | 19 (86.4%) | 0 (0.0%) |
| T2c | 3 (13.6%) | 6 (27.3%) |
| T3a | 0 (0.0%) | 11 (50.0%) |
| T3b | 0 (0.0%) | 5 (22.7%) |
| N0 | 0 (0.0%) | 20 (90.9%) |
| N1 | 0 (0.0%) | 1 (4.5%) |
| Nx | 22 (100.0%) | 1 (4.5%) |
| Age (y) | ||
| Mean | 64.0 (SD, 5.9) | |
| Median | 65 (Q1–Q3, 59–68) | |
| Minimum–maximum | 52–75 | |
| PSA (ng/mL) | ||
| Mean | 8.3 (SD, 4.0) | |
| Median | 7 (Q1–Q3, 6–9) | |
| Minimum–maximum | 3–21 |
Thirty-five lesions (including lesions < 0.1 cm3) were identified by histology on prostatectomy samples: 9 ISUP-1 (25.7%), 13 ISUP-2 (37.1%), 3 ISUP-3 (8.6%), 3 ISUP-4 (8.6%), and 7 ISUP-5 (20.0%).
The dynamics of 68Ga-PSMA-617 and 68Ga-RM2 uptake were then analyzed in normal and pathologic prostatic tissues. In the normal prostate, the median SUVmax with 68Ga-RM2 was 3.20 (Q1–Q3, 2.40–3.80) at 1 h and 2.40 (range, 1.85–3.85) at 2 h. 68Ga-RM2 uptake was significantly lower at 2 h (β = −0.59; 95% CI, −0.95 to −0.24; P = 0.003). For 68Ga-PSMA-617 in the normal prostate, the median SUVmax was 2.55 (range, 2.20–3.40) at 1 h and 2.50 (range, 2.00–3.10) at 2 h, with no differences between the 2 acquisition times (β = −0.10; 95% CI, −0.31–0.10; P = 0.31).
In tumor areas, the median SUVmax with 68Ga-RM2 was 5.20 (range, 3.30–8.30) at 1 h and 5.40 (Q1–Q3, 3.75–7.90) at 2 h (exp(b) = 0.99; 95% CI, 0.81–1.23; P = 0.96). For 68Ga-PSMA-617 uptake in tumor lesions, the median SUVmax was 4.20 (range, 3.00–6.10) at 1 h and 4.10 (range, 2.90–7.30) at 2 h, with no significant differences between the 2 acquisition times (eβ = 1.00; 95% CI, 0.78–1.30; P = 0.98).
Therefore, given the lower uptake of 68Ga-RM2 in normal prostate tissue at 2 h and the equivalent uptake in tumor lesions at 1 and 2 h, an analysis was conducted using PET/CT data obtained 2 h after injection. For 68Ga-PSMA-617, as no differences in uptake were seen either in normal prostate or in tumor area, the 1-h uptake time recommended by the joint guidelines of the European Association of Nuclear Medicine and the Society of Nuclear Medicine and Molecular Imaging for 68Ga-PSMA PET/CT was applied (16).
Lesion-Based PET/CT Imaging
Of the 35 prostatic lesions evaluated with 68Ga-PSMA-617 PET/CT, 26 (74.3%) were detected. Undetected lesions had an ISUP score of no more than 2 (6 ISUP-1 and 3 ISUP-2).
Of the 32 prostatic lesions evaluated with 68Ga-RM2 PET/CT, 25 (78.1%) were detected by 68Ga-RM2 PET/CT. The undetected lesions included 4 that were ISUP-1, 2 that were ISUP-2, and 1 that was ISUP-4 (Table 2).
TABLE 2.
ISUP-Based Stratification of Lesions Detected by 68Ga-PSMA-617 PET/CT or 68Ga-RM2 PET/CT
| Lesion on imaging | Total | ISUP-1 | ISUP-2 | ISUP-3 | ≥ISUP-4 |
|---|---|---|---|---|---|
| 68Ga-PSMA-617 PET/CT* | |||||
| n | 35 | 9 | 13 | 3 | 10 |
| No | 9 (25.7%) | 6 (66.7%) | 3 (23.1%) | ||
| Yes | 26 (74.3%) | 3 (33.3%) | 10 (76.9%) | 3 (100%) | 10 (100%) |
| 68Ga-RM2 PET/CT† | |||||
| n‡ | 32 (3) | 8 (1) | 13 | 3 | 8 (2) |
| No | 7 (21.9%) | 4 (50.0%) | 2 (15.4%) | 1 (12.5%) | |
| Yes | 25 (78.1%) | 4 (50.0%) | 11 (84.6%) | 3 (100%) | 7 (87.5%) |
60 min after intravenous administration.
120 min after intravenous administration.
One lesion is missing for patient who did not benefit from 68Ga-RM2 PET/CT, and 2 other missing lesions correspond to failure of PET/CT device at 2 h after injection for another patient.
Concordance and Discordance in PET/CT Imaging
Twenty-one (65.6%) of 32 histology-proven lesions (whatever their volume) showed uptake of both 68Ga-PSMA-617 and 68Ga-RM2, 4 (12.5%) were seen only on 68Ga-RM2, 2 (6.3%) were seen only on 68Ga-PSMA-617, and 5 (15.6%) were negative on both 68Ga-PSMA-617 and 68Ga-RM2.
Association with Pathologic Parameters
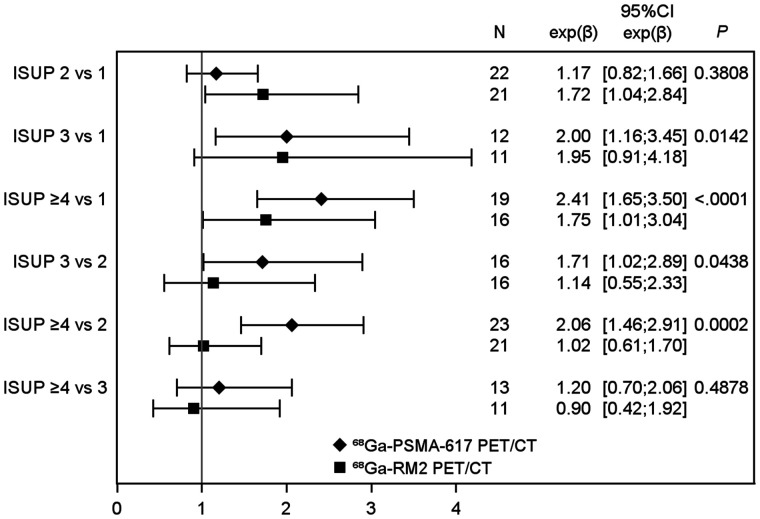
Regarding uptake of the radiopharmaceuticals according to histology parameters, 68Ga-PSMA-617 SUVmax differed according to ISUP score (P = 0.003), with a higher SUVmax for increasing ISUP scores (Tables 2 and 3). Especially, uptake of 68Ga-PSMA-617 was higher for ISUP scores of at least 4 than for an ISUP score of 1 (eβ = 2.41; 95% CI, 1.65–3.50; P < 0.0001) or 2 (eβ = 2.06; 95% CI, 1.46–2.91; P = 0.002).
TABLE 3.
Comparison of 68Ga-PSMA-617 and 68Ga-RM2 Uptake with ISUP Score
| Median SUVmax | ||
|---|---|---|
| ISUP score | 68Ga-RM2 | 68Ga-PSMA-617 |
| 1 | 3.45 (2.50–4.70) | 3.00 (2.60–3.50) |
| 2 | 6.30 (5.30–7.50) | 3.60 (3.40–4.50) |
| 3 | 8.30 (3.80–9.80) | 6.80 (5.10–7.10) |
| ≥4 | 7.35 (3.25–9.05) | 7.45 (5.90–12.50) |
Data in parentheses are Q1–Q3.
There were no significant differences in uptake between ISUP scores for 68Ga-RM2 (P = 0.11).
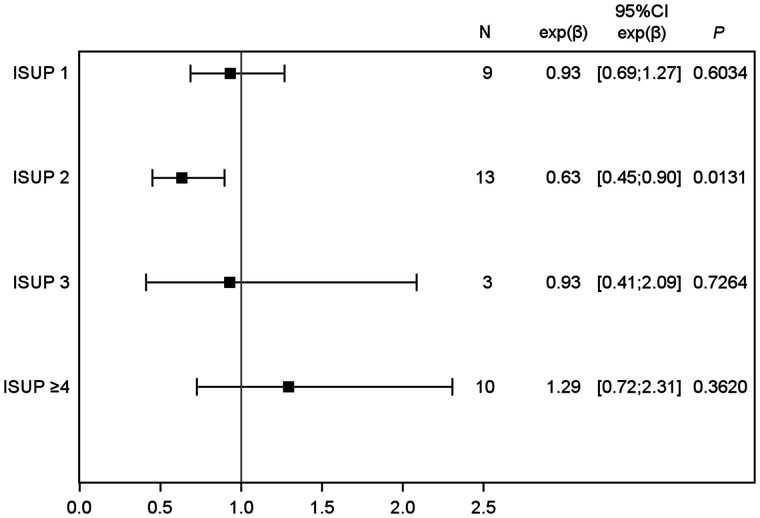
Median 68Ga-RM2 SUVmax was significantly higher than median 68Ga-PSMA-617 SUVmax in the ISUP-2 subgroup (6.30 [Q1–Q3, 5.30–7.50] vs. 3.60 [Q1–Q3, 3.40–4.50], P = 0.01). In other ISUP groups, no differences in uptake were seen between 68Ga-PSMA-617 and 68Ga-RM2 (Table 3; Figs. 1 and 2).
FIGURE 1.
Comparison of 68Ga-PSMA-617 and 68Ga-RM2 uptake with ISUP score. Estimates of >1 and <1 indicated higher and lower SUVmax in higher ISUP, respectively.
FIGURE 2.
68Ga-PSMA-617 SUVmax compared with 68Ga-RM2 SUVmax, according to ISUP groups. Estimates of >1 and <1 indicated higher and lower SUVmax, respectively, with 68Ga-PSMA-617. For ISUP ≥ 4 group, when patient who had only 68Ga-PSMA-617 was excluded, values were 1.32 (95% CI, 0.72–2.44) (P = 0.3459).
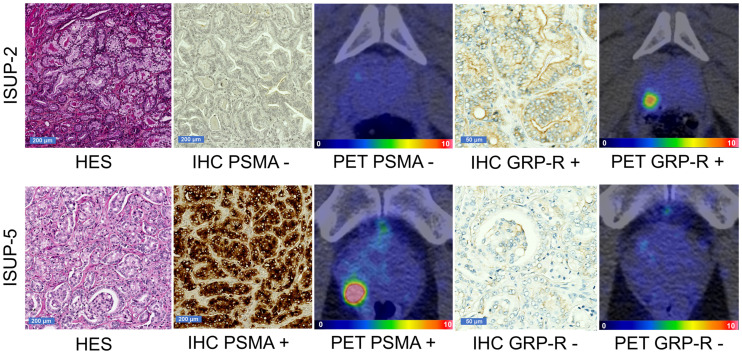
Immunochemistry was also conducted on prostatectomy samples from patients included in this study. Sixteen samples were available for GRP-R staining and 18 for PSMA staining (the remaining samples were considered noncontributive by the pathologist and were excluded from the analyses). GRP-R staining was considered positive (IRS ≥ 4) in 11 (68.8%) of 16 lesions. The median GRP-R IRS score was 4 (Q1–Q3, 3–6). The PSMA IRS was considered positive (IRS ≥ 4) in 15 (83.3%) of 18 lesions. The median PSMA IRS score was 11 (interquartile range, 6–12). The median 68Ga-RM2 SUVmax was 6.40 (interquartile range, 3.70–7.50) in samples low for GRP-R, versus 7.35 (interquartile range, 5.30–9.00) for samples positive for GRP-R (P = 0.50) (Fig. 3; Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). The median 68Ga-PSMA-617 SUVmax was 3.60 (Q1–Q3, 3.00–5.30) for PSMA-negative samples and 6.80 (Q1–Q3, 4.50–8.50) for PSMA-positive samples (P = 0.12) (Fig. 3; Supplemental Fig. 2).
FIGURE 3.
Representative GRP-R and PSMA immunohistochemistry with corresponding 68Ga-RM2 and 68Ga-PSMA-617 PET/CT images from 2 patients. (Top) Hematoxylin, eosin, and saffron staining of ISUP-2 sample (×5 magnification) with negative PSMA immunohistochemistry (×5 magnification), negative 68Ga-PSMA-617 PET/CT, positive GRP-R immunohistochemistry (×20 magnification), and positive 68Ga-RM2 PET/CT. (Bottom) Hematoxylin, eosin, and saffron staining of ISUP-5 sample (×5 magnification) with positive PSMA immunohistochemistry (×5 magnification), positive 68Ga-PSMA-617 PET/CT, negative GRP-R immunohistochemistry (×20 magnification), and negative 68Ga-RM2 PET/CT. HES = hematoxylin, eosin, and saffron; IHC = immunohistochemistry. Intensity-scale bars indicate SUV.
DISCUSSION
Several radiopharmaceuticals have been developed to help in the staging of prostate cancer. 11C-acetate, marking lipid metabolism, cannot reliably distinguish between benign prostatic hyperplasia and prostate tumors. Moreover, the radiolabeled amino acid 18F-fluciclovine has not shown good diagnostic performance for characterization of primary lesions (17). Finally, 11C- and 18F-choline, also marking lipid metabolism, have shown lower sensitivity than multiparametric MRI for primary detection of prostate cancer (18). Thus, improvements in current molecular imaging for prostate cancer appear necessary to initially assess the aggressiveness of the primary tumor.
PSMA and GRP-R are differently overexpressed in prostate cancer, raising hope for precise molecular imaging of tumor lesions within the prostate gland. Few studies have prospectively investigated the role of these radiopharmaceuticals at initial staging, before surgery. In a prospective study enrolling 56 patients with intermediate-grade prostate cancer before prostatectomy, PSMA PET was to be accurate in detecting intraprostatic lesions that had an ISUP score of at least 2. In contrast, the detection rate of PSMA PET was low for ISUP-1 lesions. Touijer et al. prospectively investigated 68Ga-RM2 PET/CT in 16 patients before radical prostatectomy. The performance of 68Ga-RM2 PET/CT imaging did not significantly differ from that of multiparametric MRI in terms of sensitivity, specificity, and accuracy (19). Therefore, the objective of this work was to perform a head-to-head comparison of PSMA and GRP-R targeting, covering various metastatic risks, at the initial staging of prostate cancer using 68Ga-PSMA-617 and 68Ga-RM2 radiopharmaceuticals. Our aim was to better understand how PSMA PET and GRP-R PET can map progression risk and how they might be used or combined in clinical practice. Because of the exploratory nature of this study, we were not aiming at assessing the diagnostic performance of the radiopharmaceuticals.
An interesting finding of our work was the lower uptake of 68Ga-RM2 in nonpathologic prostate tissue at 2 h after injection despite equivalent results on tumor lesion uptake at 1 and 2 h. This result can be extracted from preclinical studies (20) but has never, to our knowledge, been translated into PET/CT studies. This observation suggests that results from previous studies using a time point of 1 h after injection for 68Ga-RM2 PET/CT imaging are not optimal. Surprisingly, uptake of 68Ga-PSMA-617 was similar between 1 and 2 h—a result that contrasts with a previous publication that reported increasing uptake between 1 and 3 h, but the study populations were different (21).
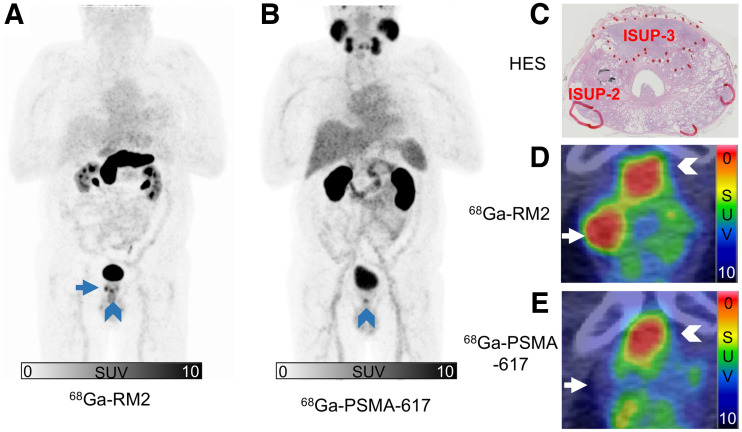
On a lesion-based analysis and using histologic results as a reference, detection of primary lesions by 68Ga-RM2 PET/CT was fairly good, compared with 68Ga-PSMA-617 PET/CT. A previous study evaluating the diagnostic potential of 68Ga-RM2 PET/CT for primary prostate cancer found a higher sensitivity of 68Ga-RM2 PET/CT than found in our work (0.98) (6). This difference can be explained by exclusion of all lesions 0.1 cm3 or smaller. When these very small lesions, which are below the spatial resolution of PET scanners, were removed for our study population, 68Ga-RM2 PET/CT detected 86% of lesions and 68Ga-PSMA-617 PET/CT detected 83% of lesions. The high uptake of 68Ga-PSMA-617 in tumor lesions of high ISUP score correlates with the known efficacy of PSMA imaging of intraprostatic tumors in patients with newly diagnosed high-risk prostate cancer (3). Additionally, 68Ga-RM2 PET/CT outperformed 68Ga-PSMA-617 PET/CT for the detection of ISUP-2 lesions (Fig. 4).
FIGURE 4.
68Ga-RM2 maximum-intensity projection (A); 68Ga-PSMA-617 maximum-intensity projection (B); hematoxylin, eosin, and saffron staining of histologic slice from prostatectomy of patient 7 with manual demarcation of tumor lesions (C); 68Ga-RM2 transaxial PET/CT image (D); and 68Ga-PSMA-617 transaxial PET/CT image (E). Anterior ISUP-3 lesion and right basal ISUP-2 lesion were seen on histology, with 2 small lesions < 0.1 cm3 (C). 68Ga-RM2 PET/CT and 68Ga-PSMA-617 PET/CT showed similar uptake on ISUP-3 lesion: SUVmax was 6.7 for 68Ga-RM2 and 6.8 for 68Ga-PSMA-617 (arrowheads). 68Ga-RM2 was the only radiopharmaceutical able to detect ISUP-2 lesion well (arrows): SUVmax was 7.3 for 68Ga-RM2 and 3.4 for 68Ga-PSMA-617. HES = hematoxylin, eosin, and saffron.
Therefore, to better classify intraprostatic lesions, we propose that both PSMA PET and GRP-R PET be performed, as discordant uptake occurs in 6 of 32 (18.8%) lesions. We suggest that PSMA PET be performed first for staging high-risk lesions. Next, the addition of GRP-R PET would allow a more extensive characterization of lower-risk prostate cancer lesions. Indeed, a low 68Ga-PSMA-617 uptake associated with a high 68Ga-RM2 uptake would suggest a low-grade prostatic tumor lesion. This double-PET strategy might also be used for guidance of biopsies to decrease the discordance rate between biopsy staging and final staging on prostatectomy samples (22). Finally, the possibility of precision detection and characterization of intraprostatic lesions opens new avenues for radiotherapy planning or focal treatments.
68Ga-PSMA-617 PET/CT was the only imaging modality able to detect the single metastatic lymph node confirmed by histology (ISUP-5) in our study. No significant uptake in this lymph node was seen on 68Ga-RM2 PET/CT or on previously ordered 18F-choline PET/CT (23). This result illustrates the higher sensitivity of PSMA PET for depicting metastatic disease in high-risk or recurrent prostate cancer (24).
Overall, most intraprostatic lesions were detected by PSMA or GRP-R PET. It should be stressed, however, that there still were some lesions (5/32, 15.6%) unseen by both modalities.
Results from this molecular imaging PET study were consolidated by GRP-R and PSMA immunohistochemistry conducted on surgical samples. A meaningfully higher tracer uptake was seen on immunohistochemistry-positive samples for PSMA, but this was not confirmed statistically. Other immunohistochemistry scores should also be considered (11).
A limitation of our monocentric phase II institutional study is obviously the limited number of patients enrolled. The small sample size may have led to underpowered results. Moreover, the SUV of 68Ga-PSMA-617 might not be directly transferable to the 68Ga-PSMA-11 used in clinics. Finally, visual analysis between histology and PET imaging can be suboptimal. Methods for accurate spatial registration of PET images and histopathologic images, using fiducial markers, have been developed (25) and deserve to be implemented.
CONCLUSION
This prospective head-to-head comparison showed the remarkable potential of the combination of 68Ga-RM2 PET/CT and 68Ga-PSMA-617 PET/CT to evaluate different aspects of prostate cancer biology. 68Ga-PSMA-617 PET/CT is useful to depict lesions with a higher, more clinically significant, ISUP score. 68Ga-RM2 has a higher detection rate than 68Ga-PSMA-617 in lower ISUP scores but uptake similar to that of 68Ga-PSMA-617 in higher ISUP scores. Importantly, almost 20% of lesions were seen only on GRP-R PET (∼13%) or PSMA PET (∼6%), revealing the complementary role of these imaging procedures. Combining PSMA PET and GRP-R PET allows better classification of intraprostatic lesions.
DISCLOSURE
This was an investigator-initiated trial with institutional academic funding. The study was funded and promoted by the University Hospital of Bordeaux (grant AOI 2016; recipient, Clément Morgat). Life Molecular Imaging provided the RM2 precursor and the reference compound but had no role in the study design. Clément Morgat reports consulting activities for IRE Elit and research support from IRE Elit and Life Molecular Imaging outside the submitted work. The corresponding author had full access to all data and final responsibility to submit for publication. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
We thank all the patients for their participation, and we thank the entire staff of the PET research center (Pessac, France), whose hard work made this study possible. Sandrine Fouchet is warmly thanked for all her administrative work. We also want to thank Frédérique Sgoifo and Tiziri Aoudjit for data management.
KEY POINTS
QUESTION: What is the role of GRP-R targeting in initial staging of localized prostate cancer in the context of PSMA PET/CT?
PERTINENT FINDINGS: In a prospective, head-to head comparison of 22 paired PET/CT examinations using 68Ga-RM2 (a radiolabeled GRP-R antagonist) and 68Ga-PSMA-617, the median 68Ga-RM2 SUVmax was significantly higher than the median 68Ga-PSMA-617 SUVmax in the ISUP-2 subgroup. As expected, 68Ga-PSMA-617 PET/CT was useful for initial staging of tumors with a high ISUP score.
IMPLICATIONS FOR PATIENT CARE: Combining PSMA PET and GRP-R PET allows better classification of intraprostatic lesions.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Schwarzenboeck SM, Rauscher I, Bluemel C, et al. PSMA ligands for PET imaging of prostate cancer. J Nucl Med. 2017;58:1545–1552. [DOI] [PubMed] [Google Scholar]
- 3. Uprimny C, Kroiss AS, Decristoforo C, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44:941–949. [DOI] [PubMed] [Google Scholar]
- 4. Acute lymphoblastic leukemia. National Comprehensive Cancer Network website. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459. Published 2022. Accessed December 5, 2022.
- 5. Mansi R, Fleischmann A, Mäcke HR, Reubi JC. Targeting GRPR in urological cancers: from basic research to clinical application. Nat Rev Urol. 2013;10:235–244. [DOI] [PubMed] [Google Scholar]
- 6. Duan H, Baratto L, Fan RE, et al. Correlation of 68Ga-RM2 PET with postsurgery histopathology findings in patients with newly diagnosed intermediate- or high-risk prostate cancer. J Nucl Med. 2022;63:1829–1835. [DOI] [PubMed] [Google Scholar]
- 7. Nock BA, Kaloudi A, Lymperis E, et al. Theranostic perspectives in prostate cancer with the gastrin-releasing peptide receptor antagonist NeoBOMB1: preclinical and first clinical results. J Nucl Med. 2017;58:75–80. [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Niu G, Fan X, et al. PET using a GRPR antagonist 68Ga-RM26 in healthy volunteers and prostate cancer patients. J Nucl Med. 2018;59:922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beer M, Montani M, Gerhardt J, et al. Profiling gastrin-releasing peptide receptor in prostate tissues: clinical implications and molecular correlates. Prostate. 2012;72:318–325. [DOI] [PubMed] [Google Scholar]
- 10. Körner M, Waser B, Rehmann R, Reubi JC. Early over-expression of GRP receptors in prostatic carcinogenesis. Prostate. 2014;74:217–224. [DOI] [PubMed] [Google Scholar]
- 11. Faviana P, Boldrini L, Erba PA, et al. Gastrin-releasing peptide receptor in low grade prostate cancer: can it be a better predictor than prostate-specific membrane antigen? Front Oncol. 2021;11:650249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schollhammer R, De Clermont Gallerande H, Yacoub M, et al. Comparison of the radiolabeled PSMA-inhibitor 111In-PSMA-617 and the radiolabeled GRP-R antagonist 111In-RM2 in primary prostate cancer samples. EJNMMI Res. 2019;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fassbender TF, Schiller F, Zamboglou C, et al. Voxel-based comparison of [68Ga]Ga-RM2-PET/CT and [68Ga]Ga-PSMA-11-PET/CT with histopathology for diagnosis of primary prostate cancer. EJNMMI Res. 2020;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morgat C, MacGrogan G, Brouste V, et al. Expression of gastrin-releasing peptide receptor in breast cancer and its association with pathologic, biologic, and clinical parameters: a study of 1,432 primary tumors. J Nucl Med. 2017;58:1401–1407. [DOI] [PubMed] [Google Scholar]
- 15. Woythal N, Arsenic R, Kempkensteffen C, et al. Immunohistochemical validation of PSMA expression measured by 68Ga-PSMA PET/CT in primary prostate cancer. J Nucl Med. 2018;59:238–243. [DOI] [PubMed] [Google Scholar]
- 16. Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging—version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 17. Parent EE, Schuster DM. Update on 18F-fluciclovine PET for prostate cancer imaging. J Nucl Med. 2018;59:733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nitsch S, Hakenberg OW, Heuschkel M, et al. Evaluation of prostate cancer with 11C- and 18F-choline PET/CT: diagnosis and initial Staging. J Nucl Med. 2016(suppl 3);57:38S–42S. [DOI] [PubMed] [Google Scholar]
- 19. Touijer KA, Michaud L, Alvarez HAV, et al. Prospective study of the radiolabeled GRPR antagonist BAY86-7548 for positron emission tomography/computed tomography imaging of newly diagnosed prostate cancer. Eur Urol Oncol. 2019;2:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mansi R, Wang X, Forrer F, et al. Development of a potent DOTA-conjugated bombesin antagonist for targeting GRPr-positive tumours. Eur J Nucl Med Mol Imaging. 2011;38:97–107. [DOI] [PubMed] [Google Scholar]
- 21. Afshar-Oromieh A, Hetzheim H, Kratochwil C, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56:1697–1705. [DOI] [PubMed] [Google Scholar]
- 22. Qiu D-X, Li J, Zhang J-W, et al. Dual-tracer PET/CT-targeted, mpMRI-targeted, systematic biopsy, and combined biopsy for the diagnosis of prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2022;49:2821–2832. [DOI] [PubMed] [Google Scholar]
- 23. Schollhammer R, de Clermont Gallerande H, Robert G, et al. 68Ga-PSMA-617 compared with 68Ga-RM2 and 18F-Fcholine PET/CT for the initial staging of high-risk prostate cancer. Clin Nucl Med. 2019;44:e535–e536. [DOI] [PubMed] [Google Scholar]
- 24. Minamimoto R, Hancock S, Schneider B, et al. Pilot comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in patients with biochemically recurrent prostate cancer. J Nucl Med. 2016;57:557–562. [DOI] [PubMed] [Google Scholar]
- 25. Puri T, Chalkidou A, Henley-Smith R, et al. A method for accurate spatial registration of PET images and histopathology slices. EJNMMI Res. 2015;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]