Comorbidities are frequent and increasing in patients with cancer, emphasizing the importance of exploring optimal ways for uniform comorbidity registration and incorporating comorbidity management into cancer care.
Abstract
Comorbidities can have major implications for cancer care, as they might impact the timing of cancer diagnosis, compromise optimal care, affect treatment outcomes, and increase healthcare costs. Thus, it is important to comprehensively evaluate cancer comorbidities and examine trends over time. Here, we performed a systematic literature review on the prevalence and types of comorbidities for the five most common forms of cancer. Observational studies from Organisation for Economic Co-operation and Development countries published between 1990 and 2020 in English or Dutch that used routinely collected data from a representative population were included. The search yielded 3,070 articles, of which, 161 were eligible for data analyses. Multilevel analyses were performed to evaluate determinants of variation in comorbidity prevalence and trends over time. The weighted average comorbidity prevalence was 33.4%, and comorbidities were the most common in lung cancer (46.7%) and colorectal cancer (40.0%), followed by prostate cancer (28.5%), melanoma cancer (28.3%), and breast cancer (22.4%). The most common types of comorbidities were hypertension (29.7%), pulmonary diseases (15.9%), and diabetes (13.5%). After adjusting for gender, type of comorbidity index, age, data source (patient records vs. claims), and country, a significant increase in comorbidities of 0.54% per year was observed. Overall, a large and increasing proportion of the oncologic population is dealing with comorbidities, which could be used to inform and adapt treatment options to improve health outcomes and reduce healthcare costs.
Significance:
Comorbidities are frequent and increasing in patients with cancer, emphasizing the importance of exploring optimal ways for uniform comorbidity registration and incorporating comorbidity management into cancer care.
Introduction
Worldwide, 10 million patients died of cancer in 2020, whereas another 19 million patients were newly diagnosed with cancer and prevalence is expected to increase (1, 2). In addition, the number of comorbidities increases over time and doctors are more and more faced with patients with cancer managing comorbidities (3, 4). This has major implications for treatment and organization of cancer care and calls for information on prevalence and trends in cancer comorbidities. This information could inform and adapt disease management and care coordination programs to improve health outcomes and manage healthcare costs.
Comorbidity is defined as the coexistence of a disorder in addition to a primary disease of interest. Comorbidities may be a contributing factor in cancer development. For example, chronic hepatitis B increases the chance of development of liver cancer (5). In addition, comorbidities may be causally unrelated to cancer but co-occur, for example due to shared risk factors. Risk factors of cancer such as older age, smoking, and lack of physical activity are shared with other common chronic conditions (e.g., obesity, diabetes, or chronic obstructive pulmonary disease; refs. 6, 7).
There is an increased recognition of the importance of comorbidities, although major challenges remain. First, comorbidities impact cancer diagnosis. Some studies suggest that comorbidities are associated with a delay in cancer diagnosis (8, 9). Contrary, comorbidities that require regular medical visits may increase the chance of identifying cancer in an early stage (9, 10). Second, comorbidities may affect curative treatments, which compromises optimal care (11). Patients with comorbidities are less likely to receive standard cancer treatments such as surgery, chemotherapy, and radiotherapy and their chance of completing a course of cancer treatment is lower (9, 11). Third, comorbidities affect treatment outcomes. Postoperative complications, morbidity, and mortality are higher in patients with comorbidities, whereas quality of life is lower (3, 9, 11, 12). Furthermore, with the increasing subspecialization of care and surgery, providers often struggle with managing the wide spectrum of comorbidities, potentially negatively impacting outcomes (13). Fourth, comorbidities increase healthcare utilization and costs for individuals diagnosed with cancer (11, 12).
In light of these challenges, it is critical to evaluate the prevalence of different comorbidities in oncologic care to inform and adapt disease management and care coordination programs to improve health outcomes and manage healthcare costs. However, information on prevalence of cancer comorbidities is limited and fragmented, for example, aimed at specific cancer types (14–16). No large systematic review has been performed to this date. The aim of this systematic review is to infer the evidence about the prevalence of comorbidities among five common types of cancer: breast, colorectal, lung, skin, and prostate cancer. We aim to explore determinants of variation between studies and examine trends in comorbidities prevalence over time.
Materials and Methods
Following a previously written study protocol (17) based on the Centre for Reviews and Dissemination's guidance for under taking reviews in health care (18) and the Cochrane collaboration protocol template (19), an electronic search was carried out in PubMed, EMBASE, Cochrane Library, CINAHL, and Web of Science. The search strategy was tailored to each database (see Supplementary Materials and Methods) and included Medical Subject Headings (MeSH) and text word or text phrase for (i) “neoplasm,” (ii) “comorbidities,” (iii) “prevalence, index, score, measure, level, number, or scale,” and (4) “administrative claim-based or registry data.” The search was performed on the June 25, 2020.
Inclusion and exclusion criteria are presented in Table 1. We limited our scope to the five most prevalent types of cancer: breast, colorectal, lung, melanoma, and prostate cancer (20).
Table 1.
Inclusion and exclusion criteria used in the selection process.
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Studies providing data about the prevalence of comorbidities in patients diagnosed with breast, colorectal, lung, melanoma, or prostate cancer, including previously diagnosed chronic conditions | Studies not providing data about the prevalence of comorbidities in patients diagnosed with breast, colorectal, lung, melanoma, or prostate cancer |
| 2. Routinely collected prevalence data, derived from registries or health insurance claims databases | Incidental data collection or not routinely collected data (e.g., chart- or patient-based prevalence data measured for the purpose of one study) |
| 3. Population studies are representative for a broad oncologic population. Selection based on age or insurance type was permitted. | Studies restricted by type of treatment, race, presence of a certain disease or complication, survival or response to a questionnaire |
| 4. Observational studies | Case reports, randomized controlled trials, systematic reviews and meta-analyses |
| 5. Publication between January 1, 1990 and June 25, 2020 | Published before 1990 |
| 6. Published in English or Dutch | Published in other languages than English or Dutch |
| 7. Originating from an OECD-country | Published outside of an OECD-country |
Note: The 38 member countries are: Australia, Austria, Belgium, Canada, Chile, Colombia, Costa Rica, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Japan, Korea, Latvia, Lithuania, Luxembourg, Mexico, Netherlands, New Zealand, Norway, Poland, Portugal, Slovak Republic, Slovenia, Spain, Sweden, Switzerland, Turkey, United Kingdom, United States.
Titles and abstracts were screened on eligibility by two reviewers (LD and CV) individually. Next, eligibility was assessed on the basis of full texts by the two reviewers individually. Discrepancies were resolved by discussion between the reviewers or, if no consensus was reached, a third reviewer (NS). Article screening was performed in Rayyan (21). All citations were imported into EndNote X8.2 and duplicates were discarded. Studies that used the exact same dataset were labeled as duplicate and discarded from analysis.
Methodologic quality
The quality of the studies were assessed using Hoy's risk of bias tool for prevalence studies (22). For some questions, modifications were made based on O'Sullivan (23) adjusted prevalence tool, because some questions of Hoy's tool were not relevant to our study or were not applicable to routinely collected prevalence data. The final quality assessment tool and the deviations from Hoy's or O'Sullivan's are presented in the Supplementary Materials and Methods. Three domains assessed external validity and four domains internal validity. For each domain, 1 point could be scored, with the total score ranging from 0 (lowest) to 7 (highest).
Data extraction and synthesis
A standardized extraction form was developed to systematically collect and summarize key data elements from each article and perform quality assessment. This was done individually by two reviewers (LD and CV) using Limesurvey. Answers from both reviewers were compared and differences reported.
The following data were extracted:
Prevalence of comorbidities: This was expressed as percentage of the study population having one or more comorbidities as measured by comorbidities indices [e.g., Charlson comorbidities index (CCI), Elixhauser comorbidity index (ECI)] or count/percentage of cooccurring diseases. The prevalence was extracted directly from the studies or calculated from the available information. Prevalence percentages per type of comorbidities were extracted if reported by the included studies. When the outcomes were presented for different subgroups (tumor types, ages, etc.) multiple observations were entered per study.
Type of comorbidities index: This was categorized as CCI; ECI; Cancer, Care, and Comorbidity (C3) index; and other.
Cancer characteristics: Type of cancer, metastases, and cancer subtype. A distinction was made between studies including metastases only, exclude metastasis, or no distinction. If studies only included a cancer subtype (e.g., rectal cancer as a subtype of colorectal cancer), the subtype was registered.
Study population characteristics: Age, proportion males, ethnicity, socioeconomic characteristics, and country.
Study start and duration characteristics: The start year of the study was defined as the first year of data collection in the included studies. The duration of study inclusion was calculated by the difference between start and end year.
Data source characteristics: Data sources were categorized into claims data, hospital-based routinely collected data, and other or unknown. Hospital-based routinely collected data included data from cancer registries and hospital databases.
Study quality: The sum of quality assessment items where both reviewers scored positive, ranging between 0 and 7, was used as quality indicator. In addition, reporting quality was assessed by checking if percentages of comorbidities add up to 100%.
Data analysis
To evaluate the prevalence of comorbidities in oncologic patients, uncorrected sample means and weighted averages on the percentage of patients having one or more comorbidities were calculated. Averages were weighted by study sample size using a logarithmic transformation. Mean weights were given to studies with missing sample sizes. Specific incidence of common (>10 occurrences) comorbidities were reported.
To test whether a trend in comorbidities over time was present, different multilevel linear regressions were performed: (i) unadjusted model, (ii) model adjusted for tumor type, and (iii) model adjusted for all determinants. Determinants include tumor type, type of comorbidity index, population characteristics, methodologic quality, and data source characteristics. Analyses was performed on individual observations, using multilevel regressions to correct for clustered observations belonging to the same study (24). The study identification number was added as a random intercept. To check the validity of defining study type as a data level, pooled linear regression with clustered SEs was performed.
Additional sensitivity analyses were performed to evaluate the influence of each individual determinant on the prevalence of comorbidity and the trend over time. Collinearity between start year and study duration with the different determinants was checked and defined as a Pearson coefficient above 0.7. Residual errors were plotted to check the normality assumption. IBM SPSS Statistics 25 was used for data cleaning and descriptive statistics; STATA 16 was used for the data analysis.
Data availability
The data generated in this study are publicly available in the Data Archiving and Networked Services (DANS) EASY archive at https://doi.org/10.17026/dans-zfp-ybfq and are available upon request from the corresponding author.
Results
A total of 3,070 articles remained after deduplication. Title and abstract scrutiny and full-text evaluation led to 163 eligible studies, of which, 2 articles were excluded due to identical data. Details on the selection process are displayed in Fig. 1.
Figure 1.
PRISMA-diagram displaying the study selection process.
The final set of articles included 161 studies: 47 on breast cancer, 37 on prostate cancer, 30 on colorectal cancer, 37 on lung cancer, 7 on melanoma, and 3 on multiple cancers. Table 2 presents descriptive statistical analysis; Supplementary Table S1 presents more details on the included studies. The determinant socioeconomic characteristics is not reported and used in the analyses as a result of heterogeneity of measuring and reporting this determinant in the included studies (Supplementary Table S2).
Table 2.
Descriptive statistics of 243 observations of comorbidity prevalence derived from 161 studies.
| All | Lung | Breast | Prostate | Colorectal | Melanoma | Multiplea | |
|---|---|---|---|---|---|---|---|
| N | N | N | N | N | N | N | |
| N studies | 161 | 37 | 47 | 37 | 30 | 7 | 3 |
| N observationsb | 243 | 53 | 67 | 56 | 54 | 10 | 3 |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
|---|---|---|---|---|---|---|---|
| Age groupsc | |||||||
| - Age below 45 | 146 (60.1) | 33 (62.3) | 35 (52.2) | 32 (57.1) | 37 (68.5) | 8 (80) | 1 (33.3) |
| - Age 45–59 | 151 (62.1) | 34 (64.2) | 36 (53.7) | 33 (58.9) | 39 (72.2) | 8 (80) | 1 (33.3) |
| - Age 60–69 | 225 (92.6) | 53 (100) | 58 (86.6) | 50 (89.3) | 51 (94.4) | 10 (100) | 3 (100) |
| - Age 70–79 | 220 (90.5) | 53 (100) | 58 (86.6) | 49 (87.5) | 51 (94.4) | 7 (70) | 2 (66.7) |
| - Age 80 or above | 216 (88.9) | 53 (100) | 59 (88.1) | 44 (78.6) | 51 (94.4) | 7 (70) | 2 (66.7) |
| Presence of subtype (yes) | 74 (30.5) | 27 (50.9) | 19 (28.4) | 2 (3.6) | 21 (38.9) | 5 (50) | — |
| Reporting quality check (valid) | 187 (77) | 45 (84.9) | 51 (76.1) | 44 (78.6) | 36 (66.7) | 8 (80) | 3 (100) |
| Country | |||||||
| Australia | 9 (3.7) | 3 (5.7) | — | — | 6 (11.1) | — | — |
| Canada | 11 (4.5) | 2 (3.8) | 4 (6) | 2 (3.6) | 3 (5.6) | — | — |
| Denmark | 16 (6.6) | 4 (7.5) | 2 (3) | 6 (10.7) | 2 (3.7) | 2 (20) | — |
| Finland | 1 (0.4) | — | 1 (1.5) | — | — | — | — |
| France | 3 (1.2) | — | 1 (1.5) | — | 2 (3.7) | — | — |
| Germany | 1 (0.4) | 1 (1.9) | — | — | — | — | — |
| Italy | 2 (0.8) | 1 (1.9) | 1 (1.5) | — | — | — | — |
| Japan | 2 (0.8) | 1 (1.9) | — | — | 1 (1.9) | — | — |
| The Netherlands | 16 (6.6) | 2 (3.8) | — | 4 (7.1) | 10 (18.5) | — | — |
| New Zealand | 3 (1.2) | — | 3 (4.5) | — | — | — | — |
| Norway | 6 (2.5) | 3 (5.7) | 1 (1.5) | 1 (1.8) | 1 (1.9) | — | — |
| Spain | 3 (1.2) | — | 3 (4.5) | — | — | — | — |
| Sweden | 17 (7) | 2 (3.8) | 1 (1.5) | 14 (25) | — | — | — |
| UK | 27 (11.1) | 6 (11.3) | 13 (19.4) | — | 8 (14.8) | — | — |
| USA | 126 (51.9) | 28 (52.8) | 37 (55.2) | 29 (51.8) | 21 (38.9) | 8 (80) | 3 (100) |
| Type of data | |||||||
| Hospital-based routinely collected datad | 147 (60.5) | 32 (60.4) | 38 (56.7) | 36 (64.3) | 36 (66.7) | 5 (50) | — |
| Claims data | 91 (37.4) | 21 (39.6) | 26 (38.8) | 20 (35.7) | 16 (29.6) | 5 (50) | 3 (100) |
| Other/unknowne | 5 (2.1) | — | 3 (4.5) | — | 2 (3.7) | — | — |
| Metastases | |||||||
| No distinction | 175 (72) | 43 (81.1) | 51 (76.1) | 31 (55.4) | 40 (74.1) | 7 (70) | 3 (100) |
| Metastases excluded | 53 (21.8) | 7 (13.2) | 12 (17.9) | 20 (35.7) | 12 (22.2) | 2 (20) | — |
| Only metastases | 15 (6.2) | 3 (5.7) | 4 (6) | 5 (8.9) | 2 (3.7) | 1 (10) | — |
| Type of comorbidities index | |||||||
| Charlson comorbidity index | 198 (81.5) | 42 (79.2) | 55 (82.1) | 48 (85.7) | 42 (77.8) | 8 (80) | 3 (100) |
| Elixhauser comorbidity index | 15 (6.2) | 7 (13.2) | 3 (4.5) | 2 (3.6) | 3 (5.6) | — | — |
| C3 index | 3 (1.2) | — | 2 (3) | — | 1 (1.9) | — | — |
| Other | 27 (11.1) | 4 (7.5) | 7 (10.4) | 6 (10.7) | 8 (14.8) | 2 (20) | — |
| Missing comorbidities percentage | 34 (14) | 7 (13.2) | 8 (11.9) | 7 (12.5) | 10 (18.5) | 2 (20) | 0 (0) |
| Missing start year | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0 (0) |
| Missing study duration | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0 (0) |
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean(95% CI) | |
|---|---|---|---|---|---|---|---|
| Mean % comorbidities | 33.6 (31.1–36.0) | 46.9 (41.9–51.9) | 22.3 (18.6–26.1) | 29.1 (25.4–32.9) | 39.7 (35.0–44.3) | 31.3 (10.2–52.4) | 38.5 (24.2–52.7) |
| Weighted mean % comorbiditiesf | 33.4 (31.0–35.8) | 46.7 (41.6–51.7) | 22.4 (18.8–26.0) | 28.5 (24.9–32.2) | 40.0 (35.4–44.6) | 28.3 (8.5–48.1) | 39.4 (26.3–52.4) |
| Start year | 2002.0 (2001.1–2002.9) | 2003.1 (2001.2–2004.9) | 2002.3 (2000.4–2004.1) | 1999.7 (1998.0–2001.4) | 2002.7 (2000.9–2004.5) | 2004.9 (1999.5–2010.3) | 1998.7 (1993.5–2003.8) |
| Study duration (years) | 6.03 (5.59–6.57) | 5.38 (4.35–6.40) | 5.60 (4.45–6.75) | 7.52 (6.31–8.73) | 5.50 (4.47–6.53) | 7.00 (4.39–9.61) | 6.00 (-0.57 -12.56) |
| Validity score (0–7) | 5.81 (5.68–5.94) | 6.09 (5.86–6.33) | 5.58 (5.38–5.78) | 6.02 (5.79–6.24) | 5.63 (5.24–6.02) | 5.50 (4.73–6.27) | 6.00 (6.00–6.00) |
| Proportion male | 0.51 (0.46–0.56) | 0.58 (0.54–0.61) | 0.02 (−0.01–0.06) | 1.00 (1.00–1.00) | 0.52 (0.50–0.53) | 0.56 (0.51–0.62) | 0.52 (0.51–0.54) |
| Proportion Caucasian | 0.82 (0.80–0.84) | 0.85 (0.83–0.88) | 0.80 (0.76–0.85) | 0.76 (0.73–0.82) | 0.81 (0.75–0.88) | 0.96 (0.91–1.00) | 0.80 (0.70–0.90) |
| Study sample size | 44569.5 (30287.7–58851.2) | 62152.2 (14272.7–110031.7) | 48291.8 (11224.0–85359.6) | 52359.2 (28136.6–76581.7) | 21790.4 (11958.4–31622.3) | 22791.6 (−13305.9 – 58889.2) | 117142.3 (-147648.0– 381932.6) |
aThe category multiple includes observations that make no distinction between the tumor types and can therefore not be presented within the categories of the individual tumor types.
bA study can report comorbidities for different subgroups (tumor types, ages, etc.), which we considered as separate observations. Analyses were performed with the individual observations of subgroup comorbidity prevalence.
cObservations can be classified into multiple age groups (e.g., studies that include ages 60–80 are included in age groups 60–69 and 70–79). The observations by age are thus not mutually exclusive.
dThis data includes data from cancer registries and hospital databases.
eThe data source is either hospital-based routinely collected data or claims data, however, it is unknown which of the two.
fAverages were weighted by subgroup sample size using a logarithmic transformation. Mean weights were given to studies with missing sample sizes.
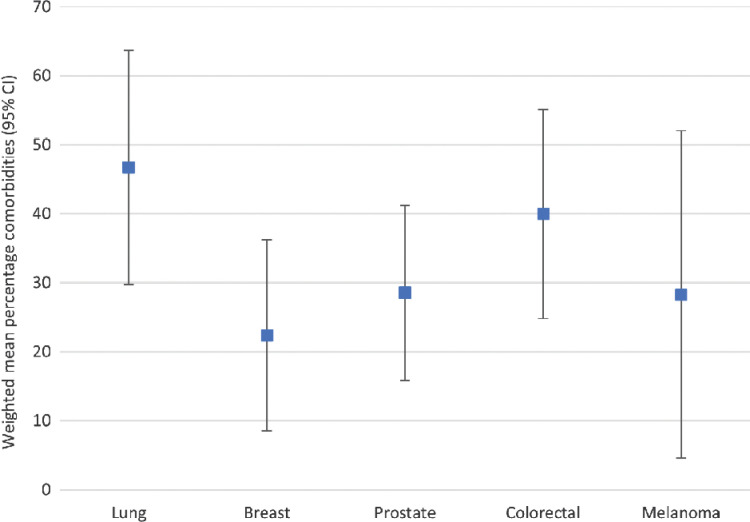
Twenty-six studies did not report the percentage of one or more comorbidities, but reported either percentage of two or more comorbidities or only reported data on types of comorbidities. The 161 studies rendered 243 observations, as some studies reported comorbidity prevalence data for, among others, multiple age groups or tumor types. Figure 2 presents the mean percentage of comorbidities per tumor type. The overall weighted average percentage of patients with one or more comorbidities is 33.4% [95% confidence interval (CI), 31.0–35.8], which is 46.7% (95% CI, 41.6–51.7) for lung cancer, 40.0% (95% CI, 35.4–44.6) for colorectal cancer, 28.5% (95% CI, 24.9–32.2) for prostate cancer, 28.3% (95% CI, 8.5–48.1) for melanoma, and 22.4% (85% CI, 18.8–26.0) for breast cancer.
Figure 2.
Error plot of the weighted mean percentage of comorbidities for the different tumor types. Averages were weighted by study sample size using a logarithmic transformation. Mean weights were given to studies with missing sample sizes.
Thirty-two studies reported individual types of comorbidities (Table 3). The most common comorbidity was hypertension (29.7%) followed by pulmonary diseases (15.9%) and diabetes (13.5%). For lung, breast, and prostate cancer, these comorbidities were also the most common. For colorectal cancer, the most common comorbidity was hypertension followed by renal diseases and diabetes. For melanoma, only one study presented comorbidities. The most common comorbidity was diabetes with followed by other malignancies and pulmonary disease. Table 3 present percentages and confidence intervals of the most common types of comorbidities.
Table 3.
Prevalence of types of comorbidities in the included studies.
| All | Lung | Breast | Prostate | Colorectal | Melanoma | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N a | Mean % (95% CI) | N a | Mean % (95% CI) | N a | Mean % (95% CI) | N a | Mean % (95% CI) | N a | Mean % (95% CI) | N a | Mean % (95% CI) | |
| N studies | 32 | 8 | 8 | 6 | 9 | 1 | ||||||
| N observationsb | 65 | 15 | 11 | 12 | 25 | 2 | ||||||
| Hypertension | 26 | 29.68 (23.02–36.35) | 4 | 37.01 (−8.92 – 82.94) | 3 | 31.57 (-25.64 – 88.78) | 4 | 41.77 (28.80–54.74) | 15 | 24.13 (17.91–30.35) | — | — |
| Pulmonary disease (including COPD) | 63 | 15.85 (12.18–19.53) | 14 | 35.64 (26.38–44.90) | 11 | 8.12 (6.06–10.17) | 12 | 7.37 (3.67–11.07) | 24 | 12.10 (8.09–16.10) | 2 | 15.95 (14.04–17.86) |
| Diabetes | 65 | 13.47 (11.55–15.39) | 15 | 17.55 (13.15–21.95) | 11 | 11.52 (6.71–16.33) | 12 | 8.90 (4.08–13.71) | 25 | 13.39 (10.56–16.21) | 2 | 21.95 (11.15–32.75) |
| Other malignancies | 32 | 11.89 (9.47–14.31) | 8 | 16.08 (9.91–22.24) | 3 | 4.95 (1.84–8.06) | 6 | 4.60 (−0.28 – 9.47) | 13 | 13.3 (10.91–15.70) | 2 | 18.25 (-3.99 – 40.49) |
| Heart failure | 47 | 8.60 (6.92–10.89) | 14 | 12.17 (9.03–15.32) | 10 | 5.08 (2.46–7.70) | 8 | 6.23 (1.14–11.32) | 13 | 8.57 (5.14–11.99) | 2 | 11.00 (-0.04 - 0.26) |
| Renal disease | 41 | 6.55 (1.68–11.41) | 13 | 5.40 (2.87–7.93) | 10 | 2.20 (1.01–3.39) | 7 | 3.95 (0.01–7.89) | 9 | 13.98 (-10.86 – 38.82) | 2 | 11.35 (5.63–17.07) |
| Cerebrovascular diseases | 36 | 6.24 (4.90–7.58) | 11 | 7.57 (5.16–9.99) | 8 | 3.95 (1.82–6.08) | 3 | 4.43 (−2.37 – 11.24) | 12 | 5.77 (3.23–8.32) | 2 | 13.60 (-4.19 – 31.39) |
| Myocardial infarction | 31 | 2.69 (1.95–3.43) | 9 | 4.48 (2.58–6.392.48) | 8 | 1.37 (0.44–2.30) | 4 | 1.55 (0.68–2.42) | 8 | 2.33 (1.24–3.43) | 2 | 3.60 (-9.11 – 16.31) |
| Rheuma | 31 | 1.92 (1.47–2.38) | 6 | 2.93 (1.04–4.82) | 7 | 1.80 (0.88–2.73) | 7 | 1.03 (0.34–1.72) | 9 | 1.75 (1.12–2.37) | 2 | 3.20 (1.92–4.47) |
| Peptic ulcer | 36 | 1.59 (0.91–2.27) | 9 | 1.81 (0.76–2.86) | 9 | 0.75 (0.26–1.23) | 7 | 1.09 (−0.41 – 2.59) | 9 | 2.62 (0.04–5.19) | 2 | 1.50 (-3.58 – 6.58) |
| Liver disease | 34 | 1.52 (0.64–2.40) | 11 | 2.66 (−0.01 – 5.31) | 6 | 0.33 (0.15–0.50) | 6 | 0.51 (0.20–0.83) | 9 | 1.22 (0.09–2.34) | 2 | 3.25 (2.61–3.89) |
| Dementia | 34 | 1.17 (0.79–1.55) | 11 | 1.03 (0.68–1.38) | 7 | 1.21 (0.36–2.06) | 8 | 0.98 (−0.56 – 2.51) | 8 | 1.52 (0.77–2.27) | — | — |
Note: Bold percentages present the top 3 comorbidities within the tumor type categories.
a N are number of observations.
bA study can report comorbidities for different subgroups (tumor types, ages, etc.), which we considered as separate observations. Analyses were performed with the individual observations of subgroup comorbidity prevalence.
Table 4 present the multilevel models. In the unadjusted model (model 1), no significant trend in comorbidities was found. This result is unaffected after adjusting for different tumor types (model 2). When adjusting for all determinants (model 3), a significant positive trend is found over time, predicting a yearly increase in comorbidities of 0.54%. These indicate that, ceteris paribus, comorbidity incidence increases by 5.4% per decade. Proportion Caucasians was removed from the model because of the low number of included observations (n = 85). The model is presented in Supplementary Table S3. The robustness check of using a multilevel model is presented in Supplementary Table S4, revealing a comparable yearly increase in comorbidity prevalence of 0.56% and an R2 of 0.68 when adjusting for all determinants.
Table 4.
Multilevel regression models for percentage of comorbidities over time.
| Coefficient (SE) | P value | |
|---|---|---|
| Model 1: Unadjusted multilevel model for percentage of comorbidities over timea | ||
| Start year | −0.19 (0.24) | 0.439 |
| Study duration | −0.28 (0.39) | 0.466 |
| Constant | 38.74*** (5.92) | 0.000 |
| Model 2: Multilevel model for percentage of comorbidities over time adjusted for tumor typesb | ||
| Tumor type | ||
| Multipled | (baseline) | |
| Melanoma | −6.50 (9.70) | 0.502 |
| Prostate | −8.73 (7.16) | 0.223 |
| Lung | 13.21 (7.39) | 0.074 |
| Breast | −12.65 (7.34) | 0.085 |
| Colorectal | 1.28 (7.39) | 0.863 |
| Start year | −0.21 (0.20) | 0.304 |
| Study duration | −0.21 (0.33) | 0.519 |
| Constant | 41.99*** (8.37) | 0.000 |
| Model 3: Multilevel model for percentage of comorbidities adjusted for all determinantsc | ||
| Country | ||
| Australia | (baseline) | |
| Canada | −3.25 (4.29) | 0.448 |
| Denmark | 18.30*** (5.17) | 0.000 |
| France | 0.64 (12.12) | 0.958 |
| Italy | 4.60 (11.78) | 0.696 |
| Japan | −18.98 (11.33) | 0.094 |
| The Netherlands | 31.40*** (5.84) | 0.000 |
| New Zealand | 5.61 (8.55) | 0.512 |
| Norway | −1.88 (4.45) | 0.673 |
| Spain | 12.94 (10.98) | 0.238 |
| Sweden | 16.05** (5.42) | 0.003 |
| UK | 5.81 (3.88) | 0.134 |
| USA | 11.13** (4.28) | 0.009 |
| Data type | ||
| Hospital-based routinely collected data | (baseline) | |
| Claims data | 11.62*** (2.58) | 0.000 |
| Other/unknown | 9.16 (9.22) | 0.320 |
| Tumor type | ||
| Multipled | (baseline) | |
| Breast | 3.93 (6.5) | 0.545 |
| Lung | 12.96* (5.47) | 0.018 |
| Prostate | −19.64** (6.24) | 0.002 |
| Colorectal | 3.87 (5.49) | 0.481 |
| Melanoma | −4.24 (7.03) | 0.547 |
| Metastatic | ||
| No distinction | (baseline) | |
| Metastasis only | 0.9 (2.02) | 0.657 |
| Metastasis excluded | 1.57 (2.64) | 0.553 |
| Index category | ||
| Charlson comorbidity index | (baseline) | |
| Elixhauser comorbidity index | 14.95*** (2.68) | 0.000 |
| C3 index | 18.27*** (5.54) | 0.001 |
| Other | −5.19 (5.85) | 0.374 |
| Age groupe | ||
| Age below 45 | −5.77* (2.41) | 0.017 |
| Age 45–59 | −1.83 (2.52) | 0.467 |
| Age 60–69 | 0.32 (2.54) | 0.899 |
| Age 70–79 | 5.26 (2.79) | 0.059 |
| Age 80 or above | 8.94*** (2.78) | 0.001 |
| Presence of subtype | ||
| No | (baseline) | |
| Yes | 1.56 (1.98) | 0.429 |
| Proportion male | 29.43*** (7.37) | 0.000 |
| Reporting quality | ||
| Valid | (baseline) | |
| Not valid | 4.76 (4.11) | 0.247 |
| Validity score | 1.75 (1.23) | 0.154 |
| Start year | 0.54** (0.18) | 0.004 |
| Study duration | 0.10 (0.31) | 0.740 |
| Constant | −26.11* (12.07) | 0.030 |
Note: Significant: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
aObservations = 208, number of groups = 140, log likelihood = −869.06.
bObservations = 208, number of groups = 140, log likelihood = −822.30.
cObservations = 199, number of groups = 137, log likelihood = −721.43.
dThe category multiple includes observations that make no distinction between the tumor types and can therefore not be presented within the categories of the individual tumor types.
eObservations can be classified into multiple age groups (e.g., studies that include ages 60–80 are included in age groups 60–69 and 70–79). The observations by age are thus not mutually exclusive. Therefore, dummy variables were entered in the model per category.
The model shows that comorbidities are more prevalent in lung cancer and less prevalent in prostate cancer. Denmark, the Netherlands, Sweden, United Kingdom, and the United States report significantly higher comorbidity incidence. Furthermore, the age group 80 or above, and proportion males display higher comorbidity rates. Finally, the use of claims data and the use of ECI or C3 index is associated with higher comorbidity rates. Cancer characteristics (e.g., time of measurement, metastasis, specific tumors) do not significantly affect comorbidity incidence, nor do we find an effect of study quality. The level of comorbidities is significantly lower for age below 45. Residuals of the models were normally distributed and no collinearity was found between the determinants, the start year of the study and the study duration.
Additional sensitivity analysis reveal difference in trends of comorbidity over time for differ tumor types (Supplementary Table S5; Supplementary Fig. S1), therefore tumor type is added to every sensitivity analyses. The individual determinants of country, data type, comorbidity index, age, and proportion male affect comorbidity prevalence (Supplementary Table S6). The sensitivity analyses reveal that the switch between a nonsignificant negative time trend in the unadjusted regression to a significant positive trend in the full model is predominantly mediated by type of country, type of data source, and age (Supplementary Table S7).
Discussion
This review sought to infer the evidence on the prevalence of comorbidities among oncologic patients and distinguish differences between the five most common types of cancer: breast, colorectal, lung, skin, and prostate cancer. In addition, we explored determinants of variation between studies and examined trends in prevalence of comorbidities.
We found that the weighted average prevalence of comorbidities in all five cancer types together is 33.4%. Comorbidities seem most common in patients with lung and colorectal cancer, with 46.7% and 40.0%, respectively. This is followed by prostate cancer with 28.5%, melanoma with 28.3%, and breast cancer with 22.4%. However, large variation existed between the data from the different studies. This variation can partly be explained by characteristics of the patient population. However, it can also partly be explained by study characteristics as country, kind of measurement tools, and type of data. After adjusting for all determinants, a significant increase in comorbidities of 0.54% per year was found. The most common type of comorbidity was hypertension, followed by pulmonary diseases and diabetes.
Previous literature
Previous studies have reported variance in the prevalence of comorbidities for different tumor types. Lee and colleagues performed a systematic review on articles between 1990 and 2009 about the impact of comorbidity on chemotherapy use and outcomes in patients with cancer. They reported a range of 0.4% to 90% of patients with cancer with comorbidities, the highest frequency among patients with lung (35%), breast (20%), or colorectal cancers (20%; ref. 25). A review article by Sarfati and colleagues on the impact of comorbidity on cancer and its treatment stated that some cancers, such as lung, are strongly associated with risk factors (e.g., age and lifestyle) related to other chronic conditions (chronic obstructive pulmonary disease and congestive heart failure; refs. 11, 26). For other cancers, for example breast and prostate cancer this association is less strong (11). For instance, obesity in premenopausal women may reduce the risk of breast cancer whereas the reverse is true for postmenopausal women (27). For prostate cancer, obesity is associated with reduced risk of nonaggressive prostate cancer but increased risk of aggressive prostate cancer (28). A report on the status of cancer in 1975 to 2010 in the United States by Edwards and colleagues is consistent with our findings (26). They reported a comparable prevalence of comorbidities in patients with breast and prostate cancers, higher frequencies in patients with lung cancer and intermediate frequencies for patients with colorectal cancer. Edwards and colleagues additionally reported diabetes, chronic obstructive pulmonary disease, and congestive heart failure as the most common comorbidities for breast, colorectum, lung, and prostate cancer (26). The prevalence is higher in comparison to a population without cancer. Fowler and colleagues found hypertension, COPD, and diabetes as the most common comorbidities for colon and lung cancer (29). Edwards and colleagues used categories from the CCI, which does not include hypertension, whereas Fowler and colleagues added additional comorbidities to the CCI.
Previous studies also supported the increase of comorbidities for specific tumor types over time. Leersum and colleagues found an increase in comorbidities from 47% to 62% over a time period from 1995 to 2010 in patients with colorectal cancer (30). Aarts and colleagues found an increase from 55% to 76% over a time period from 1995 to 2012 for patients with small cell lung cancer (31). Both studies are performed in the Netherlands and the same comorbidity index and hospital-based routinely collected data were used during the entire periods. These indicate an increase in comorbidity that is not influenced by the type of index, country, or type of data. A different contributing factor of increase in comorbidities is the aging population, as the prevalence of multimorbidity in the general population increases with age (32, 33).
We found substantial levels of variation between the included studies. Another review from Sarfati found that no gold standard existed for measuring comorbidities in oncologic patients (34). Approaches of measuring comorbidities varied based on the study questions, patient population, and available data. Our study revealed that different study characteristics impacted the prevalence of comorbidities in oncologic patients: type of data, country, and type of comorbidity index did matter.
The finding in our study that the use of claims data results in higher levels of comorbidities is in line with literature. Claims data are constructed for administrative and reimbursement purposes, lack detail on the comorbidities, and are at risk for upcoding and misclassification (35, 36). In addition, the assignment of codes is open to differences in interpretation, which might result in variability in coding practices (36, 37). Full medical records or claims data correcting for upcoding (e.g., by ruling out codes if they appear only ones or multiple times but only within a 30-day window) might provide the best insight in the prevalence and burden of comorbidities (35, 38, 39).
Little is known about differences in comorbidities in oncologic patients between countries. Potential differences in prevalence of comorbidities between countries could (partly) be explained by international inconsistencies in the coding and registration of comorbidities (40). Previous studies did suggest that the United States has higher rates of multimorbidity and higher healthcare spending in comparison to other countries (41, 42). However, our study showed the highest percentages of comorbidities in the Netherlands, Sweden, and Denmark. One factor that may explain high intercountry variability is differences in registration and claims systems (43). For example, the number of diagnostic-related groups (DRG) differs from over 4,000 in the Netherlands to about a 1,000 in Germany, Sweden, and Austria (44). This may result in critical differences in how comorbidities are measured between countries, reducing intercountry comparability. However, this may only explain intercountry differences in claim-based comorbidity assessment.
Strength and limitations
The main strengths of this study are that we systematically gathered and summarized the literature on the prevalence of comorbidities. Our review is in line with previous studies, however, adds knowledge on comorbidity trends for a broad oncologic population, heterogeneity between studies, and determinants that impact comorbidity prevalence. To our knowledge, this is the first study to explore variation between studies regarding the prevalence of comorbidities. Another strength is that our literature search was limited to the five most common types of cancers whereas our search string in the study protocol was broader. This probably ensures that no studies are missed.
We acknowledge some limitations. First, we limited our inclusion criteria to the use of data from health claims and registries based on ICD codes. This results in the possibility that some diagnoses have been missed or results are overestimated due to upcoding in claims data. On the other hand, the use of administrative data has enabled us to analyze prevalence based on large populations increasing the generalizability of the results. Second, we use the occurrence of one or more comorbidities as outcome variable, which does not take into account prevalence of multiple comorbidities simultaneously and its increase over time. However, different types of comorbidity indices cannot be compared on this dimension. Third, heterogeneity in the definitions and coding of types of comorbidities in the articles included in our review might affect prevalence and relative importance of specific comorbidities. Fourth, the model adjusting for all determinants (model 3) risks overfitting the data, where too many determinants are added with respect to the number of observations. Because of this risk, caution is needed when interpreting the results, especially of individual determinants. However, a general effect on the main variables of interest can be generated and the sensitivity analyses further explores, and substantiates the main findings. It remains unknown to what extent the remaining unexplained variance relates to heterogeneity in measuring and reporting or other unobserved study characteristics. Finally, the quality assessment form was tailored to the purpose of our review. However, this specific quality assessment form has not been validated.
Implications
With increasing comorbidity prevalence, adjustment of clinical pathways may become increasingly important in the future. This study provides a starting point to benchmark and monitor comorbidity prevalence between countries and within countries, as well as to spur further research into implications of increased comorbidity burden on clinical decision making. However, we found high unexplained variation in comorbidity prevalence between studies, potentially due to definition and registration heterogeneity. This emphasizes the importance for a gold standard for definition and registration of comorbidities. Implicitly, a trade-off between accuracy and efficiency may be present: although medical records may be more comprehensive and accurate, it may require additional administrative expenditures to disclose information on comorbidities. Routinely collected data may therefore be a less costly alternative to estimate comorbidity prevalence. Two rival indices are commonly used: the ECI and CCI. Although the ECI is argued to match or outperform the CCI, most studies included in this review report the CCI only (45–47). One issue of the CCI is that weights tend to be recalibrated over time, reducing intertemporal comparability. We would argue for an international definition of the ECI as well as a tool to translate the ECI to CCI, so both measures can be reported and applied. The use of standardized registration and measurement tools for comorbidities ensures that differences among countries, trends over time or differences between tumor types can be studied more thoroughly.
Our study contributes to discussions regarding centralizing specialized cancer care, driven by evidence that high volumes improve treatment outcomes (48–50). An increasing comorbidity prevalence may be orthogonal to the trend of increased specialization, as this may require a more generalist approach requiring professional expertise from other departments and organizations. These emphasize the importance to include the high and increasing comorbidity prevalence in debates on centralization of care and the importance to stimulate and facilitate collaborations between different healthcare organizations.
Increasing comorbidity prevalence also affects treatment costs. Although some reimbursement systems adjust for comorbidities, others do not. Reimbursement systems require standardized comorbidity measurement and adequate payment adjustment to counteract perverse incentives such as cherry picking, adverse selection, and upcoding (44). This is a promising area for future research.
Conclusion
In this systematic review we have gathered and summarized the current literature on the prevalence of comorbidities. We find that as substantial proportion of patients have at least one comorbidity, which comorbidities increase over time, and that large differences in measurement methods, databases used, and reported comorbidities in studies exist.
These findings underline the importance of comorbidities management in cancer care, given that such a large proportion of the oncological population deals with more diseases at once. These high and rising numbers could be included in discussions on optimizing clinical pathways and centralizing specialized oncologic care. However, there is a great extent of variation between reported comorbidities in studies, revealing uniformity in measuring and reporting comorbidities is lacking and needs improvement.
Supplementary Material
Supplementary tables and figure
Search strings for the literature search and the quality assesment tools
Acknowledgments
No funding was received for this research.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Authors' Disclosures
No author disclosures were reported.
Authors' Contributions
C.E. Vrinzen: Conceptualization, data curation, formal analysis, validation, investigation, visualization, methodology, writing–original draft, project administration. L. Delfgou: Conceptualization, data curation, formal analysis, validation, investigation, methodology, project administration, writing–review and editing. N. Stadhouders: Conceptualization, formal analysis, supervision, validation, investigation, methodology, project administration, writing–review and editing. R.P. Hermens: Supervision, validation, writing–review and editing. M.A. Merkx: Supervision, writing–review and editing. H.J. Bloemendal: Supervision, writing–review and editing. P.P. Jeurissen: Conceptualization, supervision, validation, methodology, writing–review and editing.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO). Cancer Tomorrow. A tool that predicts the future cancer incidence and mortality burden worldwide from the current estimates in 2020 up until 2040; 2020.
- 3. Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol 2000;35:181–200. [DOI] [PubMed] [Google Scholar]
- 4. Wedding U, Roehrig B, Klippstein A, Steiner P, Schaeffer T, Pientka L, et al. Comorbidity in patients with cancer: prevalence and severity measured by cumulative illness rating scale. Crit Rev Oncol Hematol 2007;61:269–76. [DOI] [PubMed] [Google Scholar]
- 5. Raffetti E, Fattovich G, Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: a systematic review and meta-analysis. Liver Int 2016;36:1239–51. [DOI] [PubMed] [Google Scholar]
- 6. Gerteis JID, Deitz D, LeRoy L, Ricciardi R, Miller T, Basu J. Multiple chronic conditions chartbook. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [Google Scholar]
- 7. Jang JS, Choi YY, Lee WK, Choi JE, Cha SI, Kim YJ, et al. Telomere length and the risk of lung cancer. Cancer Sci 2008;99:1385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gonzalez EC, Ferrante JM, Van Durme DJ, Pal N, Roetzheim RG. Comorbid illness and the early detection of cancer. South Med J 2001;94:913–20. [PubMed] [Google Scholar]
- 9. Søgaard M, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol 2013;5(Suppl):3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaeth PA, Satariano WA, Ragland DR. Limiting comorbid conditions and breast cancer stage at diagnosis. J Gerontol A Biol Sci Med Sci 2000;55:M593–600. [DOI] [PubMed] [Google Scholar]
- 11. Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin 2016;66:337–50. [DOI] [PubMed] [Google Scholar]
- 12. Genther DJ, Gourin CG. Effect of comorbidity on short-term outcomes and cost of care after head and neck cancer surgery in the elderly. Head Neck 2015;37:685–93. [DOI] [PubMed] [Google Scholar]
- 13. Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging Population; Board on Health Care Services; Institute of Medicine; Levit L BE, Nass S, et al., editors. Delivering high-quality cancer care: charting a new course for a system in crisis. Washington (DC): National Academies Press; 2013. [PubMed] [Google Scholar]
- 14. Grose D, Morrison DS, Devereux G, Jones R, Sharma D, Selby C, et al. Comorbidities in lung cancer: prevalence, severity and links with socioeconomic status and treatment. Postgrad Med J 2014;90:305–10. [DOI] [PubMed] [Google Scholar]
- 15. Eytan DF, Blackford AL, Eisele DW, Fakhry C. Prevalence of comorbidities among older head and neck cancer survivors in the United States. Otolaryngol Head Neck Surg 2019;160:85–92. [DOI] [PubMed] [Google Scholar]
- 16. Chia VM, O'Malley CD, Danese MD, Lindquist KJ, Gleeson ML, Kelsh MA, et al. Prevalence and incidence of comorbidities in elderly women with ovarian cancer. Gynecol Oncol 2013;129:346–52. [DOI] [PubMed] [Google Scholar]
- 17. Delfgou L, Stadhouders N. The prevalence of comorbidities among patients with cancer based on administrative data: a systematic review protocol 2020 scientific center for quality in healthcare; 2020.
- 18. Centre for Reviews and Dissemination. The CRD's guidance for undertaking reviews in health care. New York: University of York; 2009. [Google Scholar]
- 19. The Cochrane Collaboration. Protocol template (Protocol). 2013.
- 20. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 21. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65:934–9. [DOI] [PubMed] [Google Scholar]
- 23. O'Sullivan JW, Albasri A, Nicholson BD, Perera R, Aronson JK, Roberts N, et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open 2018;8:e018557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernández-Castilla B, Jamshidi L, Declercq L, Beretvas SN, Onghena P. Van den Noortgate W. The application of meta-analytic (multi-level) models with multiple random effects: a systematic review. Behav Res Methods 2020;52:2031–52. [DOI] [PubMed] [Google Scholar]
- 25. Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol 2011;29:106–17. [DOI] [PubMed] [Google Scholar]
- 26. Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120:1290–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast 2004;13:85–92. [DOI] [PubMed] [Google Scholar]
- 28. Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev 2007;29:88–97. [DOI] [PubMed] [Google Scholar]
- 29. Fowler H, Belot A, Ellis L, Maringe C, Luque-Fernandez MA, Njagi EN, et al. Comorbidity prevalence among cancer patients: a population-based cohort study of four cancers. BMC Cancer 2020;20:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Leersum NJ, Janssen-Heijnen ML, Wouters MW, Rutten HJ, Coebergh JW, Tollenaar RA, et al. Increasing prevalence of comorbidity in patients with colorectal cancer in the South of the Netherlands 1995–2010. Int J Cancer 2013;132:2157–63. [DOI] [PubMed] [Google Scholar]
- 31. Aarts MJ, Aerts JG, van den Borne BE, Biesma B, Lemmens VE, Kloover JS. Comorbidity in patients with small-cell lung cancer: trends and prognostic impact. Clin Lung Cancer 2015;16:282–91. [DOI] [PubMed] [Google Scholar]
- 32. Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J 2014;44:1055–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc 2015;16:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarfati D. Review of methods used to measure comorbidity in cancer populations: no gold standard exists. J Clin Epidemiol 2012;65:924–33. [DOI] [PubMed] [Google Scholar]
- 35. Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care 2006;44:921–8. [DOI] [PubMed] [Google Scholar]
- 36. Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care 2002;40(8 Suppl):Iv–26–35. [DOI] [PubMed] [Google Scholar]
- 37. Nimptsch U. Disease-specific trends of comorbidity coding and implications for risk adjustment in hospital administrative data. Health Serv Res 2016;51:981–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen G, Lix L, Tu K, Hemmelgarn BR, Campbell NR, McAlister FA, et al. Influence of using different databases and 'look back' intervals to define comorbidity profiles for patients with newly diagnosed hypertension: implications for health services researchers. PLoS One 2016;11:e0162074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–67. [DOI] [PubMed] [Google Scholar]
- 40. Lüchtenborg M, Morris EJA, Tataru D, Coupland VH, Smith A, Milne RL, et al. Investigation of the international comparability of population-based routine hospital data set derived comorbidity scores for patients with lung cancer. Thorax 2018;73:339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fisher ES, Whaley FS, Krushat WM, Malenka DJ, Fleming C, Baron JA, et al. The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. Am J Public Health 1992;82:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hernández B, Voll S, Lewis NA, McCrory C, White A, Stirland L, et al. Comparisons of disease cluster patterns, prevalence and health factors in the USA, Canada, England and Ireland. BMC Public Health 2021;21:1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mihailovic N, Kocic S, Jakovljevic M. Review of diagnosis-related group-based financing of hospital care. Health Serv Res Manag Epidemiol 2016;3:2333392816647892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Busse R, Geissler A, Aaviksoo A, Cots F, Häkkinen U, Kobel C, et al. Diagnosis related groups in Europe: moving towards transparency, efficiency, and quality in hospitals? BMJ 2013;346:f3197. [DOI] [PubMed] [Google Scholar]
- 45. Ladha KS, Zhao K, Quraishi SA, Kurth T, Eikermann M, Kaafarani HMA, et al. The Deyo-Charlson and Elixhauser-van Walraven Comorbidity Indices as predictors of mortality in critically ill patients. BMJ Open 2015;5:e008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li B, Evans D, Faris P, Dean S, Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res 2008;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Menendez ME, Neuhaus V, van Dijk CN, Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res 2014;472:2878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huo YR, Phan K, Morris DL, Liauw W. Systematic review and a meta-analysis of hospital and surgeon volume/outcome relationships in colorectal cancer surgery. J Gastrointest Oncol 2017;8:534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raphael MJ, Siemens R, Peng Y, Vera-Badillo FE, Booth CM. Volume of systemic cancer therapy delivery and outcomes of patients with solid tumors: A systematic review and methodologic evaluation of the literature. Journal of Cancer Policy 2020;23:100215. [Google Scholar]
- 50. Trinh QD, Bjartell A, Freedland SJ, Hollenbeck BK, Hu JC, Shariat SF, et al. A systematic review of the volume-outcome relationship for radical prostatectomy. Eur Urol 2013;64:786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables and figure
Search strings for the literature search and the quality assesment tools
Data Availability Statement
The data generated in this study are publicly available in the Data Archiving and Networked Services (DANS) EASY archive at https://doi.org/10.17026/dans-zfp-ybfq and are available upon request from the corresponding author.