Abstract
Introduction:
The diagnosis of Wilson disease (WD) is plagued by biochemical and clinical uncertainties. Thus, calculated parameters have been proposed. This study aimed to: (a) compare the diagnostic values of non-caeruloplasmin copper (NCC), NCC percentage (NCC%), copper-caeruloplasmin ratio (CCR) and adjusted copper in WD; and (b) derive and evaluate a discriminant function in diagnosing WD.
Methods:
A total of 213 subjects across all ages who were investigated for WD were recruited. WD was confirmed in 55 patients, and the rest were WD free. Based on serum copper and caeruloplasmin values, NCC, NCC%, CCR and adjusted copper were calculated for each subject. A function was derived using discriminant analysis, and the cut-off value was determined through receiver operating characteristic analysis. Classification accuracy was found by cross-tabulation.
Results:
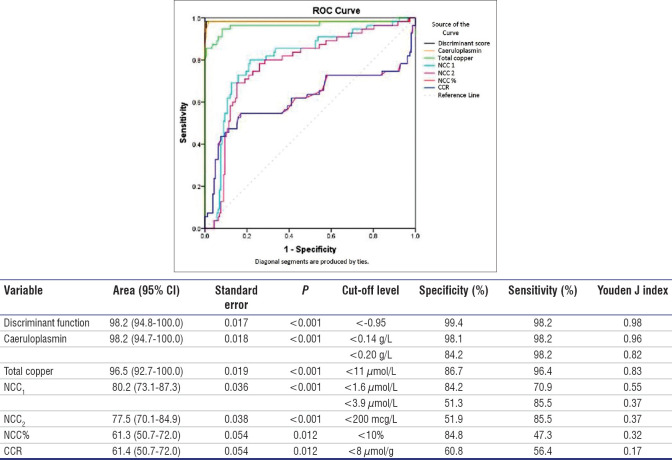
Caeruloplasmin, total copper, NCC, NCC%, CCR, adjusted copper and discriminant function were significantly lower in WD compared to non-WD. Discriminant function showed the best diagnostic specificity (99.4%), sensitivity (98.2%) and classification accuracy (99.1%). Caeruloplasmin levels <0.14 g/L showed higher accuracy than the recommended 0.20 g/L cut-off value (97.7% vs. 87.8%). Similarly, molar NCC below the European cut-off of 1.6 umol/L showed higher accuracy than the American cut-off of 3.9 umol/L (80.3% vs. 59.6%) (P < 0.001). NCC%, mass NCC, CCR and adjusted copper showed poorer performances.
Conclusion:
Discriminant function differentiates WD from non-WD with excellent specificity, sensitivity and accuracy. Performance of serum caeruloplasmin <0.14 g/L was better than that of <0.20 g/L. NCC, NCC%, CCR and adjusted copper are not helpful in diagnosing WD.
Keywords: Caeruloplasmin, discriminant function, free copper, non-caeruloplasmin copper, Wilson disease
INTRODUCTION
Copper is an important metalloenzyme cofactor involved in cellular respiration, iron oxidation, dopamine metabolism, antioxidation and connective-tissue formation.[1,2] Up to 90% of copper in the blood is bound to caeruloplasmin, with the rest distributed among albumin, transcuprein and histidine, leaving 2%–5% in its free biologically active form.[1,3,4] An average diet sufficiently meets daily copper requirements. Once absorbed from the gut, copper is transported by albumin and histidine to its main regulating organ, the liver.[3] There, it is incorporated by a P-type ATP7B metal transporter into apocaeruloplasmin to form holocaeruloplasmin. The latter then carries copper back into circulation[4] or gets excreted through bile when hepatic copper content exceeds 1 μmol/L.[5] It is through the balance between gut absorption and biliary excretion that copper is tightly regulated to ensure no free copper exists in cytosols.[6]
When in excess, the biologically active free copper results in cellular injury owing to its pro-oxidative and radical-producing properties.[1,2,4,7] Free copper crosses the blood-brain barrier 1,000 times higher than caeruloplasmin-bound copper,[1] although the exact mechanism of its transport remains elusive because the amount of ATP7B transporters are much less than that of ATP7A transporters in the brain.[8]
Caeruloplasmin is a blue α-2 glycoprotein of 132,000 Da molecular weight[9,10] coded by genes on chromosome 3.[11] It is largely involved in copper transportation but also plays a part in haematopoiesis where it oxidises ferrous to its ferric form, allowing iron to be transported by transferrin.[1,6] It is understood that one molecule of caeruloplasmin binds 6-8 atoms of copper of which six are unexchangeable,[12,13] and the rest are loosely bound or readily adsorbed.[12] The number of copper atoms bound to caeruloplasmin is, however, heterogeneous.[14,15] Based on this six copper-atoms-per-caeruloplasmin assumption, it is deduced that one caeruloplasmin molecule comprises 0.3% copper.[2,13] In clinically relevant terms, it is divided into apocaeruloplasmin and holocaeruloplasmin. Apocaeruloplasmin does not carry any copper and is the inactive form of caeruloplasmin, possessing <1% of holocaeruloplasmin's oxidase activity.[11,16]
Wilson disease (WD) is a rare autosomal recessive disorder most commonly caused by a missense mutation of the ATP7B gene that encodes the ATPase responsible for copper-caeruloplasmin incorporation.[8,13] It affects about 30 per million population with higher rates in liver/neurology clinics, and in Asian population where consanguinity is apparent.[3,8,13,17] Prevalence of heterozygous carriers is estimated to be at one out of 7,000 individuals.[8] The main pathology lies in the failure to incorporate copper into apocaeruloplasmin, resulting in reduced holocaeruloplasmin and increased free copper in the liver. As free copper saturates hepatocytes, it spills into the circulation and deposits in other organs, such as the iris, brain, kidneys, joints and erythrocytes.[3,4,13] Being a pro-oxidant, deposition of free copper in these organs result in hepatic injury, Kayser-Fleischer (KF) rings, neuro-psychiatric disorders, renal failure and haemolytic anaemia as seen in WD. KF rings are almost always found in patients with WD presenting with neurological symptoms, whereas only half of patients presenting with hepatic disease elicit this ophthalmic finding.[13] Paediatric patients tend to present with fulminant hepatic failure, whereas adolescents and adults are more likely to present with neurologic features, such as tremors, dysarthria and psychiatric conditions.[8] Chelating agents such as penicillamine and trientine are the mainstay of treatment although ultimately liver transplant is curative.[3,18]
Diagnosing WD requires both clinical and biochemical parameters. In patients with suspected liver disease, the presence of KF rings plus a biochemical triad of low caeruloplasmin, low total copper and increased urinary copper are sufficient to diagnose WD.[3,8] A diagnostic scoring system incorporating clinical features, biochemical findings and mutation analysis has also been developed by the Working Party at the 8th International Meeting on Wilson Disease, Leipzig 2001.[18,19] However, diagnosis is not straightforward because not all patients present with typical features and biochemical markers are often within normal limits. Apart from this, analytical issues of the biochemical assays also contribute.[15] Direct free copper measurement serves to be a valuable diagnostic tool but is unfortunately cumbersome and not widely available.
To overcome the diagnostic difficulties of WD, multiple derived parameters to calculate the free copper fraction have been suggested. One such calculation that is widely used is the non-caeruloplasmin copper (NCC). This is obtained by subtracting caeruloplasmin-bound copper from total copper concentration. NCC is assumed to represent free copper. However, because of physiologically impossible negative values, alternative parameters have been proposed. These include NCC percentage (NCC%), copper-caeruloplasmin ratio (CCR) and adjusted copper. NCC% is also affected by negative values and the latter two have the advantage of overcoming negative values and negating the need for age or gender-specific values. However, their clinical utility in WD are unascertained.
Therefore, this study was undertaken to compare the diagnostic values of NCC, NCC%, CCR and adjusted copper in WD. The clinical utility of a multivariate discriminant analysis in differentiating WD was also evaluated.
METHODS
A total of 213 patients across all ages who presented to University of Malaya Medical Centre (UMMC) between 1 January 2015 and 31 December 2017 with unexplained hepatic, neurological or neuropsychiatric presentations and/or who were worked up for WD were recruited as the primary cohort [Figure 1]. Serum caeruloplasmin and total copper results were retrieved from the laboratory information system. Each patient's electronic medical record was accessed from the hospital information system and reviewed for clinical history and final diagnosis. Diagnosis of WD was made on the basis of phenotypic classification, clinical features and laboratory results [Table 1].[19] Patients who were finally diagnosed with WD were included in the WD group, whereas patients who were not given a final diagnosis of WD were grouped as non-WD. Test results of WD patients at presentation and during follow-up, including those on chelation therapy, were included in the data collection process. To ensure representativeness of serum copper in follow-up patients with WD, results exceeding the reference change value (RCV) of either caeruloplasmin (RCV >18%) or total copper (RCV >25%) were excluded from the analysis.[20]
Figure 1.
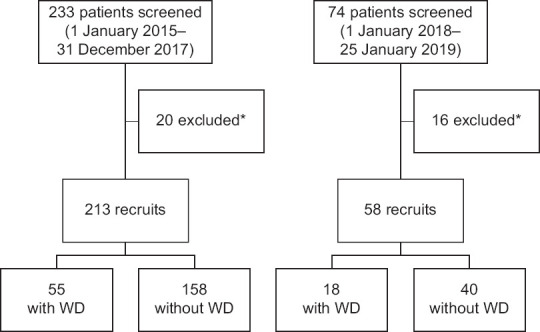
Flowchart shows the patient selection process in (a) the primary cohort and (b) the validation cohort. *Follow-up patients with serial serum copper and/or serum caeruloplasmin values exceeding reference change value (RCV). WD: Wilson disease
Table 1.
Diagnostic criteria of Wilson disease in recruits.
| Diagnostic criteria | Wilson disease | Non-Wilson disease |
|---|---|---|
| Phenotype (≥1) | ||
| Hepatic | + | + |
|
| ||
| Neurologic | + | + |
|
| ||
| Other | + | + |
|
| ||
| Clinical feature | ||
|
| ||
| KF rings (slit lamp examination) | ± | − |
|
| ||
| Laboratory values | ||
|
| ||
| Serum caeruloplasmin | <0.2 g/L | ≥0.2 g/L |
|
| ||
| Urinary copper | >2×ULN | ≤2×ULN (normal) |
|
| ||
| Urinary copper, post penicillamine (if done) | >5×ULN | ≤5×ULN (normal) |
|
| ||
| Liver biopsy (if done) | + | − |
KF: Kayser-Fleischer, ULN: upper limit of normal
To validate the equation derived by discriminant analysis, a second set of recruits (n = 58) was extracted between 1 January 2018 and 25 January 2019 using the processes described before.
Serum caeruloplasmin was measured by an immunoturbidometric assay using goat polyclonal antibodies specific for caeruloplasmin on Abbot ARCHITECT ci4100 (Abbott Diagnostics, Lake Forest, IL, USA). Sample for total plasma copper was collected in a royal blue-top K2 EDTA BD Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and analysed using inductively coupled plasma mass spectrometry (ICP-MS) on NexION (Perkin Elmer Inc, Shelton, USA).
Plasma free copper values were calculated using the following formulae:
-
(a)
NCC in molar units (NCC1) (μmol/L)[5,21]
= total copper (μmol/L) – [47 × caeruloplasmin (g/L)]
-
(b)
NCC in mass units (NCC2) (mcg/L)[18] = total copper (mcg/L) – caeruloplasmin-bound copper (mcg/L)
-
(c)
NCC% (%)[4] = [NCC (μmol/L)/total copper (μmol/L)] × 100
-
(d)
CCR (μmol/g)[14] = [total copper (μmol/L) × 0.132]/caeruloplasmin (g/L)
The formula for adjusted copper was derived using total copper and caeruloplasmin values of patients without WD (n = 158).[15] Using the same concept by Barth et al. in deriving adjusted calcium formula and taking the mean of copper's reference range value as 17.5 μmol/L, the adjusted copper formula below was derived[22]:
Adjusted copper (μmol/L) = total copper (μmol/L) – [63.5 × caeruloplasmin (g/L)] + 17.5
The discriminant function was constructed on the basis of the canonical discriminant function coefficient values obtained from discriminant analysis. After the receiver operating characteristic (ROC) curve analysis, the value giving the best specificity and sensitivity was taken as the cut-off value to discriminate WD from non-WD. A cut-off value lying between the WD and non-WD groups’ centroids is considered valid. The discriminant function is an absolute value without a particular reportable unit.
Discriminant function = −3 + [14 × caeruloplasmin (g/L)] + [0.017 × copper (μmol/L)]
The study was carried out in accordance with the principles of the Declaration of Helsinki and received approval from UMMC's ethics board.
All data were analysed on IBM SPSS Statistics version 23.0 for Windows (IBM Corp, Armonk, NY, USA). Data were tested for normality using the Shapiro-Wilk test. Normally distributed data were described as mean ± standard deviation values and nonparametric data as median ± interquartile range values. To assess the diagnostic utility of the calculated parameters in WD, ROC curve analysis and cross-tabulations were conducted. A P value of <0.05 was considered significant for all analysed data.
RESULTS
A total of 213 patients with a median age of 35 years (range 20–48 years) with male majority (60.6%) were included in the study. Of the 213 recruits, 55 (25.8%) were diagnosed with WD and the remaining 158 (74.2%) were free from the disease. The two major clinical features prompting investigation for WD were hepatic (e.g. fulminant hepatic failure, chronic active hepatitis, cirrhosis) and neurologic (e.g. Parkinsonism, movement disorders). Of the 55 patients with WD, 17 (30.9%) had KF rings, 36 (65.5%) did not have KF rings and 2 (3.6%) were not assessed for KF rings because of early demise. Caeruloplasmin, total copper, NCC1, NCC2, NCC%, CCR and discriminant function medians were significantly lower in the WD group than in the non-WD group. Age and adjusted copper medians were not significantly different between the groups. Descriptive summary of WD and non-WD groups are shown in Table 2.
Table 2.
Descriptive data between Wilson disease (WD) and non-WD groups.
| Parameter | WD (n=55) | Non-WD (n=158) | P † | ||
|---|---|---|---|---|---|
|
|
|
||||
| Median (IQR) | n (%)* | Median (IQR) | n (%)* | ||
| Demographics | |||||
|
| |||||
| Age (yr) | 34 (20 to 43) | - | 36 (20 to 51) | - | 0.160 |
|
| |||||
| Male gender | - | 33 (60.0) | - | 96 (60.8) | 0.921 |
|
| |||||
| Clinical presentation | |||||
|
| |||||
| Hepatic | - | 22 (40.0) | - | 65 (41.1) | <0.001 |
|
| |||||
| Neurologic | - | 17 (30.9) | - | 68 (43.0) | |
|
| |||||
| Neuropsychiatric | - | 7 (12.7) | - | 20 (12.7) | |
|
| |||||
| Combination | - | 9 (16.4) | - | 0 (0.0) | |
|
| |||||
| None, e.g., donors, family screening | - | 0 (0.0) | - | 5 (3.2) | |
|
| |||||
| Biochemical feature | |||||
|
| |||||
| Caeruloplasmin (g/L) | 0.04 (0.03 to 0.07) | - | 0.25 (0.22 to 0.29) | - | <0.001 |
|
| |||||
| Total copper (µmol/L) | 2.70 (1.26 to 4.85) | - | 15.91 (12.80 to 18.60) | - | <0.001 |
|
| |||||
| NCC1 (µmol/L) | 0.19 (−0.34 to 2.09) | - | 3.94 (2.51 to 6.15) | - | <0.001 |
|
| |||||
| NCC2 (mcg/L) | 7.10 (−26.75 to 117.62) | - | 204.17 (119.80 to 337.45) | - | <0.001 |
|
| |||||
| NCC% (%) | 12 (−20 to 39) | - | 26 (19 to 33) | - | 0.012 |
|
| |||||
| CCR (µmol/g) | 7.04 (5.19 to 10.19) | - | 8.39 (7.62 to 9.24) | - | 0.012 |
|
| |||||
| Adjusted copper (µmol/L) | 17.12 (16.54 to 17.83) | - | 17.51 (15.97 to 18.96) | - | 0.697 |
|
| |||||
| Discriminant function | −2.35 (−2.55 to −1.94) | - | 0.79 (0.37 to 1.44) | - | <0.001 |
*Percentage calculated based on total within group. †Medians based on Mann-Whitney U test and frequencies based on Pearson’s chi-square test. Value is significant at P<0.05. CCR: copper-caeruloplasmin ratio, IQR: interquartile range, NCC%: non-caeruloplasmin copper percentage, NCC1: non-caeruloplasmin copper in molar unit, NCC2: non-caeruloplasmin copper in mass unit
Figure 2 shows a strong direct relationship between total copper and caeruloplasmin (R2 = 0.763) with WD subjects largely concentrated at the lower end of the regression. Spearman bivariate analysis showed significantly strong negative correlation between WD with discriminant function (−0.731, P < 0.001), caeruloplasmin (−0.731, P < 0.001) and total copper (−0.705, P < 0.001). All other variables showed weak correlations with WD [Table 3]. In view of poor correlation and similar distribution between groups, adjusted copper was omitted from subsequent analyses.
Figure 2.
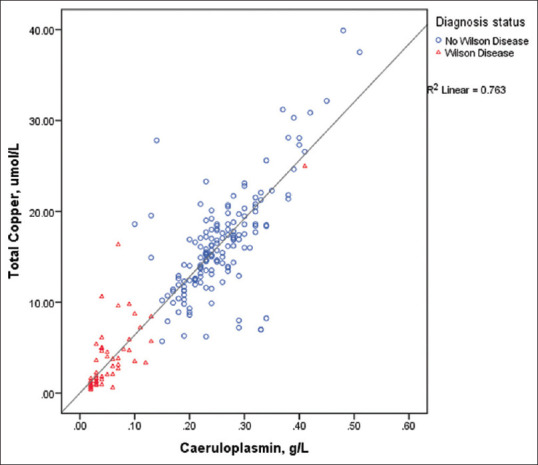
Scatter plot shows serum total copper against serum caeruloplasmin with samples labelled according to diagnosis.
Table 3.
Spearman correlation study between Wilson disease and eight laboratory parameters.
| Parameter | Spearman correlation with WD | P* |
|---|---|---|
| Discriminant function | −0.731 | <0.001 |
|
| ||
| Caeruloplasmin | −0.731 | <0.001 |
|
| ||
| Total copper | −0.705 | <0.001 |
|
| ||
| NCC1 | −0.458 | <0.001 |
|
| ||
| NCC2 | −0.417 | <0.001 |
|
| ||
| NCC% | −0.172 | 0.012 |
|
| ||
| CCR | −0.172 | 0.012 |
|
| ||
| Adjusted copper | −0.027 | 0.699 |
*Two-tailed significance. CCR: copper-caeruloplasmin ratio, NCC%: non-caeruloplasmin copper percentage, NCC1: non-caeruloplasmin copper in molar unit, NCC2: non-caeruloplasmin copper in mass unit, WD: Wilson disease
From ROC curve analysis, discriminant function and caeruloplasmin shared the highest area under the curve (AUC) of 0.982 followed closely by total copper (0.965). All other parameters had lower AUCs ranging between 0.613 and 0.802 with high standard errors [Figure 3]. Caeruloplasmin <0.14 g/L gave a better specificity of 98.1% than the 84.2% specificity given by the 0.20 g/L cut-off value with similar sensitivity. As for total copper, the cut-off level that gave the best specificity and sensitivity coincided with its lower reference limit of 11 μmol/L; At this cut-off value, the specificity and sensitivity for WD were 86.7% and 96.4% respectively. Taking advantage of low caeruloplasmin and low total copper in predicting WD, a discriminant analysis combining the two parameters into a single function was undertaken. A cut-off value of less than −0.95 yielded the best specificity of 99.4% and sensitivity of 98.2% in predicting WD. This cut-off value lay between the two groups’ centroids of −2.29 and 0.80.
Figure 3.
Receiver operating characteristic (ROC) curve shows the analysis of discriminant function, caeruloplasmin, total copper, NCC1, NCC2, NCC% and CCR. Specificity and sensitivity are reported based on recommended cut-offs or reference limits. CCR: copper-caeruloplasmin ratio, NCC%: non-caeruloplasmin copper percentage, NCC1: non-caeruloplasmin copper in molar unit, NCC2: non-caeruloplasmin copper in mass unit
The European recommended cut-off for NCC1 of 1.6 μmol/L produced a specificity of 84.2% and sensitivity of 70.9% in diagnosing WD. The American recommended cut-off of 3.9 μmol/L improved the sensitivity to 85.5% whereas reducing the specificity to 51.3%. As for NCC2, the recommended cut-off value of <200 mcg/L gave a poor specificity of 51.9% and sensitivity of 85.5%. NCC% and CCR showed poorer specificities and sensitivities at their recommended cut-off values [Figure 3].
Using the specific cut-off values for each parameter, patients were grouped into WD and non-WD and cross-tabulated against actual WD statuses [Table 4]. Discriminant function showed the best accuracy of 99.1% where 98.2% of patients with WD and 99.4% of patients without WD were correctly classified. Caeruloplasmin at a cut-off limit of 0.14 g/L fared better than the 0.20 g/L cut-off limit with an overall accuracy of 97.7% and 87.8%, respectively. Total copper at levels <11 μmol/L correctly classified 96.4% of patients with WD and 86.7% of patients with non-WD, giving an overall accuracy of 89.2%.
Table 4.
Accuracy of each parameter in correctly classifying patients into WD and non-WD.
| Parameter | No. of patients |
n (%) Accuracy (TP + TN) |
P* | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Correct classification | Wrong classification | |||||
|
|
|
|||||
| WD (TP) | Non-WD (TN) | WD (FP) | Non-WD (FN) | |||
| Discriminant function (<−0.95) | 54 | 157 | 1 | 1 | 99.1 | <0.001 |
|
| ||||||
| Caeruloplasmin (<0.14 g/L) | 54 | 154 | 4 | 1 | 97.7 | <0.001 |
|
| ||||||
| Caeruloplasmin (<0.20 g/L) | 54 | 133 | 25 | 1 | 87.8 | <0.001 |
|
| ||||||
| Total copper (<11 µmol/L) | 53 | 137 | 21 | 2 | 89.2 | <0.001 |
|
| ||||||
| NCC1 (<1.6 µmol/L) | 38 | 133 | 25 | 17 | 80.3 | <0.001 |
|
| ||||||
| NCC1 (<3.9 µmol/L) | 47 | 80 | 78 | 8 | 59.6 | <0.001 |
|
| ||||||
| NCC% (<10%) | 26 | 134 | 24 | 29 | 75.1 | <0.001 |
|
| ||||||
| NCC2 (<200 mcg/L) | 47 | 83 | 75 | 8 | 61.0 | <0.001 |
|
| ||||||
| CCR (<8 µmol/g) | 31 | 96 | 62 | 24 | 59.6 | 0.027 |
*Based on Pearson’s chi-square test. Value is significant at 0.05. CCR: copper-caeruloplasmin ratio, FN: false negative, FP: false positive, NCC%: non-caeruloplasmin copper percentage, NCC1: non-caeruloplasmin copper in molar unit, NCC2: non-caeruloplasmin copper in mass unit, TN: true negative, TP: true positive, WD: Wilson disease
Between the European and American recommended cut-off values for NCC1, the former had a better accuracy of 80.3% than the higher American cut-off value of 3.9 μmol/L. All other parameters such as NCC%, NCC2 and CCR had low accuracies and varying performances in classifying patients according to their WD states.
To overcome data overfitting, the discriminant function was evaluated on the validation cohort of 58 subjects. Serum caeruloplasmin was also evaluated in view of its close performance to the discriminant function. Both discriminant function and caeruloplasmin at the 0.14 g/L cut-off shared the same classification accuracy of 100% in which all 18 patients with WD and 40 patients without WD were correctly classified [Table 5].
Table 5.
Accuracy of the discriminant function in correctly classifying patients into Wilson disease (WD) and non-WD in the validation cohort.
| Variable (cut-off level) | Predicted diagnosis | Actual diagnosis | Accuracy (%) | |
|---|---|---|---|---|
|
| ||||
| WD (n=18) | Non-WD (n=40) | |||
| Discriminant function (<−0.95) | WD | 18 | 0 | 100 |
|
| ||||
| Non-WD | 0 | 40 | ||
|
| ||||
| Caeruloplasmin (<0.14 g/L) | WD | 18 | 0 | 100 |
|
| ||||
| Non-WD | 0 | 40 | ||
|
| ||||
| Caeruloplasmin (<0.20 g/L) | WD | 18 | 5 | 91.4 |
|
| ||||
| Non-WD | 0 | 35 | ||
DISCUSSION
Diagnosing WD is a challenge to both clinical and laboratory physicians. Although believed to be pathognomonic of WD, KF rings are only present in 44%–62% of patients with WD at the point of diagnosis and can also be found in chronic and neonatal cholestasis.[3] Furthermore, hepatic presentations of WD are often clinically and biochemically indistinguishable from viral hepatitis, fatty liver disease and autoimmune hepatitis.[3] Neurological manifestations of Menkes disease may also mimic the extrapyramidal signs of WD.[15]
Multivariate discriminant analysis has been applied to discriminate the differences among groups of diseases and to allocate new observations into the established groups.[23,24] In this study, a linear discriminant function has been constructed using serum caeruloplasmin and total copper data from patients with and without WD. When applied case-wise, the formula effectively differentiated WD from non-WD with a sensitivity of 98.2%. Unlike previous mathematical formulae, this formula seeks to differentiate between the two conditions. When the first two canonical functions (representing almost 99% of the total dispersion) were plotted [Figure 2], it is evident that the two groups can be clearly differentiated. Once calculated, the formula can be conveniently incorporated into a programmable calculator or computer spreadsheet, allowing the user to insert the necessary parameters and obtain a provisional diagnosis.
In this validation cohort of the study, both the discriminant function and caeruloplasmin below 0.14 g/L showed 100% classification accuracy [Table 5]. However, the small sample size of 58 recruits may not reflect the true effect magnitude of the function. Therefore, in view of its higher specificity (99.4% vs. 98.1%) [Figure 3], the authors propose using the discriminant function as an adjunct diagnostic tool, especially in patients with indeterminate/borderline features where a low serum caeruloplasmin level alone is insufficient to diagnose WD.
Biochemically, low serum caeruloplasmin, low serum total copper and elevated urine copper are diagnostic of WD, but often these levels are within normal limits in patients with WD.[15] Low caeruloplasmin is not exclusive to WD because caeruloplasmin levels are decreased also in neonates due to reduced apocaeruloplasmin synthesis,[16,25] protein-losing enteropathy, nephrotic syndrome, malnutrition, severe liver disease and copper-deficient parenteral nutrition.[3,13] Heterozygotes for the ATP7B mutation may also have normal levels of caeruloplasmin.[4,13,15] Elevated serum caeruloplasmin levels are found in inflammation, infection, malignancy and oestrogenic states thus reducing the sensitivity of low caeruloplasmin in identifying WD.[3,6,26] The reference limit of caeruloplasmin also varies between laboratories. In the American Association for the Study of Liver Diseases guidelines, a serum caeruloplasmin concentration below 0.20 g/L is considered one of the diagnostic criteria for WD.[3] But in European guidelines, a lower cut-off of <0.10 g/L has been proposed in the WD scoring system.[18] Mak et al. reported that a serum caeruloplasmin concentration of 0.14 g/L performed better than a medical decision threshold of 0.20 g/L.[17] Similarly, in our study, the lower caeruloplasmin cut-off of 0.14 g/L showed better sensitivity and specificity of 98.2% and 98.1%, respectively.
We found that serum total copper at the lower reference limit of 11 μmol/L had a sensitivity and specificity of 96.4% and 86.7%, respectively, in diagnosing WD. WD is a disease of copper overload but serum total copper is usually decreased in proportion to caeruloplasmin. However, serum total copper may be elevated in acute liver failure of WD because of the release of copper from tissue stores.[3] Normal or elevated total serum copper in the presence of low caeruloplasmin levels indicate an increase in NCC. Direct measurement of free copper is therefore superior as elevated free copper is specific to WD and levels reduce in response to chelators, making it an excellent treatment monitoring tool in WD.[3,7,13] Unfortunately, its analysis which involves ultrafiltration and ICP-MS is cumbersome and not widely available.[7]
Free copper or NCC can be calculated using a formula based on the assumption that copper constitutes 0.3% of a caeruloplasmin molecule and that the nonfree fraction of copper is bound to caeruloplasmin alone.[1] NCC is expressed in either molecular weight or mass units — the former being more widely used in SI-unit-reporting regions. There is a significant disparity between the NCC reference values quoted in European and American guidelines (<1.6 and <3.9 μmol/L, respectively). Our study found that NCC at the European cut-off value fared better in specificity than the American cut-off (84.2% vs. 51.3%). It was also not surprising that NCC2 showed similar sensitivity to NCC1 at the American cut-off level of 3.9 μmol/L. The poorer performance of NCC is probably due to the heterogeneity in copper content and the existence of multiple structural forms of caeruloplasmin.[12,25,27]
Another issue with NCC is the physiologically impossible negative results in many patients. A study by Twomey et al. reported >20% of negative NCC values across three different facilities in the United Kingdom and that a single cut-off value to diagnose WD is not transferable between laboratories.[5] Similarly, we observed negative NCC values in 17.8% of all subjects (25.5% of WD and 15.2% of non-WD subjects) (data not shown). This could be due to overestimation of caeruloplasmin by turbidoimmunometric method, where both apo- and holocaeruloplasmins are measured.[5,11] Note that NCC only assumes copper-containing holocaeruloplasmin, and not apocaeruloplasmin in its calculation.
Realising the flaws of NCC, several other derived parameters were suggested. NCC%, which considers the percentage of calculated free copper to total copper, is one such parameter.[4,21] However, because it involves calculated NCC as its numerator, physiologically impossible negative values persist to be a problem. CCR, which is the ratio of total copper to caeruloplasmin, has also been suggested.[14] Although this parameter overcomes issues of negative values and age/gender-specific cut-offs, it has not been largely studied in WD. When copper is adjusted for caeruloplasmin, a strong agreement with NCC values is found.[15] This implies that adjusted copper is a clinically equivalent parameter to NCC but its utility in WD remains to be validated. In our study, these calculated parameters showed poor sensitivity and poor specificity in diagnosing WD. Accuracy was also poor in discriminating WD from non-WD.
This study was not without limitations. Owing to disease rarity, sample sizes of both primary and validation cohorts were small, and data collection was done retrospectively in a single referral centre. As genetic studies were not available, patients who did not meet the diagnostic requirements of WD were diagnosed on the basis of the attending physician's clinical suspicion.
In conclusion, this study shows that a set of linear discriminant function based on serum caeruloplasmin and serum total copper can effectively differentiate WD from non-WD with a high degree of specificity, sensitivity and accuracy. However, this needs to be verified on a large cohort of patients. Serum caeruloplasmin at the 0.14 g/L cut-off level performs better than the 0.20 g/L cut-off in diagnosing WD. Calculated parameters including NCC (both molar and mass units), NCC%, CCR and adjusted copper are not helpful diagnostics in WD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Catalani S, Paganelli M, Gilberti ME, Rozzini L, Lanfranchi F, Padovani A, et al. Free copper in serum: An analytical challenge and its possible applications. J Trace Elem Med Biol. 2018;45:176–80. doi: 10.1016/j.jtemb.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Ferenci P. Metabolic Aspects of Chronic Liver Disease. 2008 [Google Scholar]
- 3.Roberts EA, Schilsky ML American Association for Study of Liver Diseases (AASLD) Diagnosis and treatment of Wilson disease: An update. Hepatology. 2008;47:2089–111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- 4.Twomey PJ, Wierzbicki AS, House IM, Viljoen A, Reynolds TM. Percentage non-caeruloplasmin bound copper. Clin Biochem. 2007;40:749–50. doi: 10.1016/j.clinbiochem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Twomey PJ, Viljoen A, Reynolds TM, Wierzbicki AS. Non-ceruloplasmin-bound copper in routine clinical practice in different laboratories. J Trace Elem Med Biol. 2008;22:50–3. doi: 10.1016/j.jtemb.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Arredondo M, Núñez MT. Iron and copper metabolism. Mol Aspects Med. 2005;26:313–27. doi: 10.1016/j.mam.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 7.McMillin GA, Travis JJ, Hunt JW. Direct measurement of free copper in serum or plasma ultrafiltrate. Am J Clin Pathol. 2009;131:160–5. doi: 10.1309/AJCP7Z9KBFINVGYF. [DOI] [PubMed] [Google Scholar]
- 8.Poujois A, Woimant F. Wilson's disease: A 2017 update. Clin Res Hepatol Gastroenterol. 2018;42:512–20. doi: 10.1016/j.clinre.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Magdoff-Fairchild B, Lovell FM, Low BW. An x-ray crystallographic study of ceruloplasmin. Determination of molecular weight. J Biol Chem. 1969;244:3497–9. [PubMed] [Google Scholar]
- 10.Takahashi N, Ortel TL, Putnam FW. Single-chain structure of human ceruloplasmin: The complete amino acid sequence of the whole molecule. Proc Natl Acad Sci U S A. 1984;81:390–4. doi: 10.1073/pnas.81.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macintyre G, Gutfreund KS, Martin WR, Camicioli R, Cox DW. Value of an enzymatic assay for the determination of serum ceruloplasmin. J Lab Clin Med. 2004;144:294–301. doi: 10.1016/j.lab.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Natelson S, Natelson EA. Principles of Applied Clinical Chemistry. Plenum Press: New York; 1980. Ceruloplasmin: Copper metabolism; 30 pp. [Google Scholar]
- 13.Walshe JM. Clinical Investigations Standing Committee of the Association of Clinical Biochemists. Wilson’s disease: The importance of measuring serum caeruloplasmin non-immunologically. Ann Clin Biochem. 2003;40:115–21. doi: 10.1258/000456303763046021. [DOI] [PubMed] [Google Scholar]
- 14.Twomey PJ, Viljoen A, House IM, Reynolds TM, Wierzbicki AS. Copper: caeruloplasmin ratio. J Clin Pathol. 2007;60:441–2. doi: 10.1136/jcp.2006.041756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twomey PJ, Viljoen A, House IM, Reynolds TM, Wierzbicki AS. Adjusting copper concentrations for caeruloplasmin levels in routine clinical practice. J Clin Pathol. 2006;59:867–9. doi: 10.1136/jcp.2005.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda I, Pearson T, Holtzman NA. Determination of apoceruloplasmin by radioimmunoassay in nutritional copper deficiency, Menkes’ kinky hair syndrome, Wilson's disease, and umbilical cord blood. Pediatr Res. 1974;8:821–4. doi: 10.1203/00006450-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Mak CM, Lam CW, Tam S. Diagnostic accuracy of serum ceruloplasmin in Wilson disease: Determination of sensitivity and specificity by ROC curve analysis among ATP7B-genotyped subjects. Clin Chem. 2008;54:1356–62. doi: 10.1373/clinchem.2008.103432. [DOI] [PubMed] [Google Scholar]
- 18.European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J Hepatol. 2012;56:671–85. doi: 10.1016/j.jhep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Ferenci P, Caca K, Loudianos G, Mieli-Vergani G, Tanner S, Sternlieb I, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003;23:139–42. doi: 10.1034/j.1600-0676.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 20.Quality requirements. Desirable Biological Variation Database specifications. In: Westgard QC [online] [Last accessed on 02 Jan 2019]. Availablefrom: https://www.westgard.com/biodatabase1.htm .
- 21.Duncan A, Yacoubian C, Beetham R, Catchpole A, Bullock D. The role of calculated non-caeruloplasmin-bound copper in Wilson's disease. Ann Clin Biochem. 2017;54:649–54. doi: 10.1177/0004563216676843. [DOI] [PubMed] [Google Scholar]
- 22.Barth JH, Fiddy JB, Payne RB. Adjustment of serum total calcium for albumin concentration: Effects of non-linearity and of regression differences between laboratories. Ann Clin Biochem. 1996;33:55–8. doi: 10.1177/000456329603300108. [DOI] [PubMed] [Google Scholar]
- 23.Urrechaga E, Aguirre U, Izquierdo S. Multivariable discriminant analysis for the differential diagnosis of microcytic anemia. Anemia. 2013;2013:457834. doi: 10.1155/2013/457834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castaldo G, Oriani G, Lofrano MM, Cimino L, Topa M, Budillon G, et al. Differential diagnosis between hepatocellular carcinoma and cirrhosis through a discriminant function based on results for serum analytes. Clin Chem. 1996;42:1263–9. [PubMed] [Google Scholar]
- 25.Sato M, Schilsky ML, Stockert RJ, Morell AG, Sternlieb I. Detection of multiple forms of human ceruloplasmin. A novel Mr 200,000 form. J Biol Chem. 1990;265:2533–7. [PubMed] [Google Scholar]
- 26.Hirano K, Ogihara T, Ogihara H, Hiroi M, Hasegawa M, Tamai H. Identification of apo- and holo-forms of ceruloplasmin in patients with Wilson's disease using native polyacrylamide gel electrophoresis. Clin Biochem. 2005;38:9–12. doi: 10.1016/j.clinbiochem.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita K, Liang CJ, Funakoshi S, Kobata A. Structural studies of asparagine-linked sugar chains of human ceruloplasmin. Structural characteristics of the triantennary complex type sugar chains of human plasma glycoproteins. J Biol Chem. 1981;256:1283–9. [PubMed] [Google Scholar]