Abstract
Obesity is a disease with a major negative impact on human health. However, people with obesity may not perceive their weight to be a significant problem and less than half of patients with obesity are advised by their physicians to lose weight. The purpose of this review is to highlight the importance of managing overweight and obesity by discussing the adverse consequences and impact of obesity. In summary, obesity is strongly related to >50 medical conditions, with many of them having evidence from Mendelian randomisation studies to support causality. The clinical, social and economic burdens of obesity are considerable, with these burdens potentially impacting future generations as well. This review highlights the adverse health and economic consequences of obesity and the importance of an urgent and concerted effort towards the prevention and management of obesity to reduce the burden of obesity.
Keywords: Clinical impact, economic burden, obesity
INTRODUCTION
The prevalence of obesity has increased significantly over the last two decades worldwide, including in Singapore, a multiethnic Southeast Asian country.[1,2] Recent national health surveys of adult Singaporeans suggest a continuation of this rising trend after a brief period of stabilisation.[3] Obesity, which is characterised by excessive adiposity, is not benign.
Obesity predisposes affected individuals to a large array of diseases that are often interconnected, leading to an increased risk of simple (two comorbid diseases) and complex (four or more comorbid diseases) multimorbidity in these individuals, when compared to people with healthy weight.[4] For example, in a large Finnish cohort of 114,657 people aged 16–78 years, with a mean follow-up of 12.1 years, people with obesity were five times more likely to develop simple multimorbidity and 12 times more likely to develop complex multimorbidity, with stronger associations found in people with more severe obesity.[4] This dose–response relationship between obesity and multimorbidity is also observed in other populations, including Asian populations.[5,6] In Singapore, the proportion of disability-adjusted life years, a composite measure of all health loss within a population, contributed by overweight and obesity, increased from 3.9% in 1990 to 6.4% in 2017, making it the fifth leading risk factor affecting health in Singapore.[7] Hence, obesity is a disease with a major negative impact on human health and has become a major global and regional health problem.
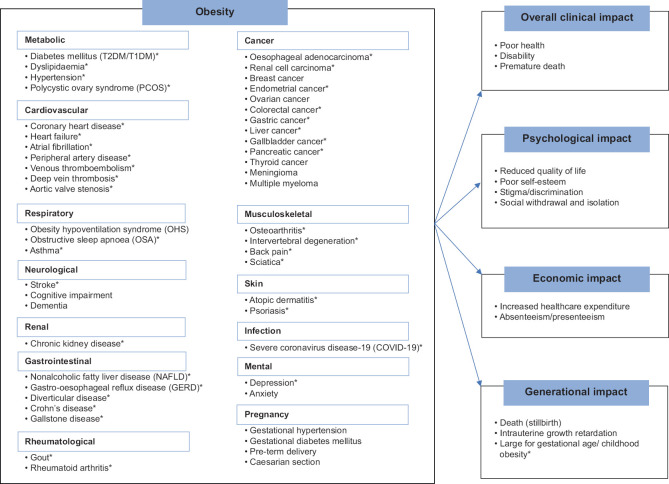
However, according to international surveys and interviews, people with obesity may not perceive their weight to be a significant problem,[8] with evidence also suggesting that less than half of patients with obesity are advised by their physicians to lose weight.[9,10] Hence, the purpose of this narrative review, as part of a series on obesity, is to highlight the importance of managing overweight and obesity by presenting and summarising the latest evidence on the adverse consequences and impact of obesity [Figure 1]. The causal role of excess adiposity on obesity-related conditions (as established by the many epidemiological evidence already described in literature) will be explored. In addition, data from Singapore, if any, will be included in the review for each section, with the review on the economic burden of overweight and obesity focusing on Singapore.
Figure 1.
Diagram shows a summary of the diseases and conditions associated with obesity and the potential impacts. *Supported by mendelian randomisation studies. T1DM: type 1 diabetes mellitus, T2DM: type 2 diabetes mellitus
EVIDENCE FOR CAUSAL ROLE OF EXCESS ADIPOSITY ON OBESITY-RELATED DISEASES
The hypertrophy of adipose tissue is associated with proinflammatory adipokine production and macrophage infiltration. In addition, the failure of adipose tissue to continually expand leads to lipotoxicity and ectopic fat deposition in lean tissues such as the heart, liver, pancreas and kidneys.[11,12] These phenomena contribute to a proinflammatory and insulin-resistant milieu and, together with increased mechanical stress due to increased adipose tissue mass,[11,13] are the main pathophysiological mechanisms responsible for the development of multiple medical conditions. Hence, there are reasonable pathways to link outcome to exposure (i.e. plausibility), one of the principles useful for establishing a causal relationship.[14]
This causal role of obesity is further supported by evidence from Mendelian randomisation (MR) studies. MR is an analytical technique involving genetic variants that are associated with exposures (risk factors such as obesity) as instrumental variables to investigate the effects of these exposures on an outcome of interest (e.g. a disease).[15] Since these genetic variants are fixed, randomly allocated at conception and temporally precede the outcome, MR is less likely to be subject to bias, confounding and reverse causation, which are frequent in conventional observational studies.[15] ’BMI’, ’obesity’, ’Mendelian randomisation’, ’Mendelian randomization’ and the outcome of interest (e.g. ’diabetes’) were used as search terms, and all studies relevant to this review were considered. These obesity-related diseases, with evidence for the causal role of obesity (plausible biological mechanisms and MR studies), and the various impacts of obesity will be discussed next.
Diabetes mellitus, dyslipidaemia and hypertension
As highlighted, obesity leads to insulin resistance. Additionally, elevated free fatty acid from the adipose tissues and ectopic fat deposition cause pancreatic β-cell dysfunction.[16,17] Hence, the various pathological mechanisms synergistically exacerbate the onset of type 2 diabetes mellitus (T2DM). In particular, visceral fat deposition plays an important role in the development of T2DM.[18] Hepatic fat worsens hepatic insulin resistance, while pancreatic fat affects insulin secretion and glucose tolerance.[18] In a meta-analysis of MR studies, genetically predicted higher body mass index (BMI) was consistently associated with T2DM, with a combined odds ratio (OR) of 2.03 (95% confidence interval [CI] 1.88–2.19) per 1 standard deviation increase in BMI.[19] In Singapore, multiple observational studies confirm the increased risk of T2DM with increasing BMI in all major ethnic groups,[20,21,22] although there might be ethnic-specific sensitivity to the effects of increasing adiposity.[23]
There is also increasing evidence for the role of obesity in the increased incidence of type 1 diabetes mellitus (T1DM), with studies from North America and the UK suggesting an increasing prevalence of overweight and obesity in people with T1DM.[24] It has been hypothesised that obesity-induced insulin resistance may be responsible for the accelerated loss of pancreatic β cells through excessive stimulation[25] and a chronic proinflammatory state.[26] This causal role is supported by MR studies, although the evidence is not as consistent (compared to obesity and T2DM), with high heterogeneity between the studies.[19]
Obesity is associated with dyslipidaemia, which is characterised by increased triglycerides (TGs) and free fatty acids, decreased high-density lipoprotein-cholesterol (HDL-C) with HDL dysfunction and increased low-density lipoprotein cholesterol (LDL-C), in particular, small dense LDL-C, which is particularly atherogenic.[27] The underlying mechanisms involve hepatic fat accumulation, insulin resistance and chronic inflammation.[27,28] In a recent MR study based on participants from the UK Biobank, genetically predicted higher BMI was significantly associated with dyslipidaemia (low HDL-C levels).[29] In Singapore, in a multiethnic sample of 4,723 adult participants, elevated TG was more common in people with obesity, with the prevalence of elevated TG increasing with higher BMI and waist-to-hip ratio (WHR),[20] consistent with the evidence.
Hypertension is more than twice as prevalent in people with obesity compared to people with normal weight.[30] The mechanisms for obesity-induced hypertension are varied (involving adipokines, cytokines, free fatty acids, insulin, the rennin–angiotensin–aldosterone system) and interconnected, with the final common pathways being endothelial dysfunction, extracellular fluid overload and sympathetic nervous system activation.[30,31] Hence, obesity is an established risk factor for hypertension, and its causal role is supported by MR studies,[19,32] particularly that of higher adiposity with a more unfavourable metabolic profile (higher visceral and ectopic fat).[32] In Singapore, the increased prevalence of hypertension with higher BMI and WHR is consistent, especially in males.[20]
Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is a condition in which fat accumulates in the liver in the absence of excessive alcohol consumption. Genetic variants that increase hepatic fat content have been shown to be associated with increased liver enzymes, hepatocellular damage and fibrosis, suggesting that hepatic fat accumulation mediates the development of liver fibrosis, independent of inflammation.[33] Hence, NAFLD may lead to nonalcoholic steatohepatitis (NASH), and ultimately, cirrhosis or hepatocellular carcinoma.
The prevalence of NAFLD has risen in tandem with the global epidemic of obesity, with NAFLD now being the most common cause of chronic liver disease worldwide.[34] A meta-analysis of MR studies confirms the causal effect of obesity on NAFLD,[19] with central adiposity (waist circumference) having the strongest relationship (OR 2.93, 95% CI 1.85–4.63) among the various obesity measures.[35] In Singapore, studies consistently show that participants with evidence of NAFLD have significantly higher BMI and waist circumference.[36,37]
Cardiovascular diseases
The association between obesity and increased incidence of cardiovascular diseases such as heart failure, coronary heart disease and stroke has long been established.[38] For example, based on pooled data from 97 prospective cohort studies involving 1.8 million participants, the hazard ratio (HR) for each 5 kg/m2 higher BMI was 1.27 (95% CI 1.23–1.31) for coronary heart disease and 1.18 (95% CI 1.14–1.22) for stroke after adjustment for potential confounders.[39] Additional adjustment for diabetes mellitus, hypertension and dyslipidaemia reduced the HRs to 1.15 (95% CI 1.12–1.18) and 1.04 (95% CI 1.01–1.08) for coronary heart disease and stroke, respectively, suggesting that 46% (95% CI 42%–50%) of the excess risk of BMI for coronary heart disease and 76% (95% CI 65%–91%) for stroke were mediated by these conditions,[39] which are common in people with obesity.[4,30]
Obesity itself leads to an increased risk of these cardiovascular events, likely via mechanisms such as the secretion of adipokines, proinflammatory cytokines and hypofibrinolytic factors, that together could lead to increased oxidative stress and endothelial dysfunction resulting in atherosclerosis.[40] Additionally, excessive adiposity results in haemodynamic alterations via various neurohormonal and metabolic abnormalities, causing left ventricular (LV) hypertrophy and subsequent dysfunction, leading to LV failure. LV failure, facilitated by pulmonary arterial hypertension from hypoxia due to obstructive sleep apnoea (OSA) and/or obesity hypoventilation syndrome (OHS), may subsequently lead to right ventricular failure.[41] This causal role of obesity is supported by multiple MR studies,[19,29,32] with the strongest association between BMI and heart failure, followed by BMI and coronary artery disease, then BMI and stroke.[19,32]
Another obesity-related cardiovascular disease is atrial fibrillation (AF), with evidence suggesting that obesity is an independent risk factor for AF, even after accounting for OSA.[41] Also, studies have demonstrated a strong graded association between higher BMI and the risk of persistent AF and higher BMI, with increased risk of postablation AF.[41] The mechanisms linking obesity and AF are complex and incompletely understood, with increased left atrial and ventricular abnormalities, altered haemodynamics, increased epicardial and pericardial fat, inflammation, and metabolic and neurohormonal abnormalities being the potential causal mechanisms.[41] This causal relationship is similarly supported by MR studies which consistently show that genetically predicted BMI is associated with AF.[19,32,42]
In Singapore, a longitudinal study involving 2,605 Chinese participants found that the adjusted HR for cardiovascular and stroke mortality was highest in the group with obesity (BMI ≥30 kg/m2) among those aged ≥65 years,[43] which is consistent with the association between obesity and increased incidence of cardiovascular diseases.
Obstructive sleep apnoea and hypoventilation syndrome
The increased intra-abdominal and intrathoracic pressure as a result of excessive adiposity impedes inflation of the lung, which can significantly affect the lung function, thereby leading to hypoventilation and ventilation–perfusion imbalance.[44] A constellation of obesity, daytime hypoventilation characterised by hypercapnia and hypoxaemia, and sleep-disordered breathing, without an alternative cause for hypoventilation, is known as OHS, with an estimated prevalence of 8%–20% in patients with obesity who were referred to sleep centres for evaluation of sleep-disordered breathing.[45]
The most common sleep-disordered breathing in such patients and people with obesity is OSA, as fat accumulation around the upper airways predisposes to the collapse of these airways.[44] About 50% of people with OSA have obesity, and approximately 40%–90% of people who are overweight suffer from OSA.[44,45] Consistent with epidemiological observations and genetic correlation (between OSA and BMI), an MR study shows that genetically predicted BMI is strongly associated with OSA, supporting the causal effect of BMI on OSA.[46] In Singapore, a study based on 587 Chinese participants reported that people with OSA had significantly higher BMI, and also BMI remained an important predictor of OSA after adjusting for hypertension and smoking,[47] in line with the overall evidence.
Polycystic ovary syndrome
Obesity is strongly associated with polycystic ovary syndrome (PCOS),[48] which is characterised by reproductive dysfunction (oligo-amenorrhoea, infertility), hyperandrogenism (hirsutism, acne, androgenic alopecia and biochemical hyperandrogenism) and a polycystic ovarian morphology (high antral follicle counts or increased ovarian volume).[49] Up to 88% of women with PCOS are overweight or obese,[48] with a meta-analysis showing that women with obesity had a twofold to threefold higher risk for PCOS when compared to women without obesity.[50] The pathogenesis of PCOS involves primarily insulin resistance, with the ensuing secondary hyperinsulinaemia resulting in enhanced steroidogenesis in the ovaries, particularly androgen production.[48] Hence, the insulin-resistant milieu associated with obesity can lead to the development of PCOS. This causal effect of obesity on PCOS is supported by MR studies,[32,51] with one MR study suggesting that this effect is predominantly metabolic in nature.[32] In Singapore, a study based on a multiethnic population of 389 participants reported that women with PCOS had significantly higher BMI compared to women without PCOS,[52] consistent with the evidence.
Cognitive impact and dementia
Experimental studies have shown that cellular mechanisms such as oxidative stress and inflammation can affect the brain structure and function.[53] Obesity is an established risk factor for dementia,[54] and has been associated with cognitive impairment[55,56] and decreased grey matter volume linked with executive functioning.[53,57] However, while MR studies have found causal relationships between BMI and grey matter volumes,[58] evidence for BMI and dementia has so far not been significant.[32,58,59] In Singapore, a longitudinal analysis of 1,519 cognitively normal older persons (>55 years) of Chinese ethnicity showed that central obesity was associated with a higher risk of developing mild cognitive impairment,[60] consistent with the overall evidence that higher adiposity has a negative impact on the brain.
Chronic kidney disease
Excess adiposity results in pathological processes such as lipotoxicity, inflammation, oxidative stress and activation of the renin–angiotensin–aldosterone system, leading to glomerular and tubular injuries (obesity-induced nephropathy).[61] Multiple MR studies have confirmed this causal relationship between obesity and kidney disease,[32,62,63] including one study conducted in an East Asian population using BMI-associated variants validated in East Asia.[63] In Singapore, longitudinal gain in adiposity was associated with progressive renal decline in a prospective multiethnic cohort with T2DM, suggesting that increasing adiposity would lead to adverse renal outcomes over time.[64]
Cancers
Obesity is known to be associated with 13 types of cancers: oesophageal adenocarcinoma, renal cell carcinoma, postmenopausal breast cancer, endometrial cancer, colorectal cancer, meningioma, multiple myeloma, and cancer of the gastric cardia, liver, gallbladder, pancreas, ovary and thyroid.[65] In Singapore, these obesity-associated cancers make up four out of the top five cancers affecting women (breast, colorectal, endometrial and ovarian) and two of the top five cancers affecting men (colorectal and liver).[66] Potential mechanisms of increased cancer risk in obesity include hyperinsulinaemia, chronic inflammation and oestrogen excess.[67] This causal role of obesity in cancer is supported by MR studies,[19,32] particularly for cancers of the digestive system, with all of them (oesophageal, colorectal, gastric, liver, gallbladder, pancreas) positively associated with genetically predicted BMI based on a meta-analysis of MR studies.[19]
Depression and anxiety
The prevalence of depression is much higher among people with obesity than that in the general population.[68,69] Similarly, anxiety occurs more frequently in people who are overweight or obese compared to people with normal weight, and the relationship is stronger among those who are more severely obese.[70,71,72] Research findings consistently show that people with obesity frequently suffer from psychological issues ranging from stress associated with weight-related issues, perceived weight discrimination and stigmatisation to body image dissatisfaction.[68,69] Additionally, there is evidence that the dysfunctional adipose tissues present in obesity result in metabolic abnormalities, such as altered glucocorticoid, adipokine, insulin, leptin and inflammatory signalling, which either directly or indirectly impact the control of emotions and mood.[73,74] Hence, the causal relationship between obesity and depression/anxiety is likely to have both psychological and biological components. This is supported by MR studies which demonstrate the relationship between genetically predicted BMI (and fat mass) and depression,[75,76,77] even when using a genetic instrument that omits the metabolic consequences of higher BMI.[75]
In Singapore, a study based on 83 patients with obesity at a weight management clinic reported that the prevalence of anxiety symptoms and depressive symptoms was 28% and 11%, respectively,[78] suggesting that symptoms of depression and anxiety are highly prevalent in people with obesity in Singapore and are higher than the national prevalence of depression and anxiety,[79] consistent with international data.
Severe coronavirus disease-19
The dysfunctional physiological milieu of obesity has been associated with altered lymphoid tissue integrity, shifts in leukocyte populations and proinflammatory profiles, such that immune responses and pathogen defence are impaired.[80] This is demonstrated in previous influenza outbreaks[81] and the current coronavirus disease 2019 (COVID-19) pandemic, where obesity (high adiposity) is a major risk factor for severe COVID-19 (death and hospitalisation), as supported by multiple epidemiological studies worldwide[82] and MR studies[83,84,85,86] using data from the COVID-19 Host Genetics Initiative (an international collaboration that aims to uncover the genetic determinants of outcomes related to COVID-19 susceptibility and severity). In Singapore, where the COVID-19 mortality rate is low (<0.001),[87] a subgroup analysis of younger (<60 years) COVID-19 patients found that a BMI ≥25 kg/m2 was significantly associated with the need for low-flow supplemental oxygen and mechanical ventilation,[88] consistent with observations internationally.
Other diseases
Other diseases with established epidemiological and strong MR evidence include asthma,[32] gastro-oesophageal reflux disease,[19,32] diverticular disease,[19,32] gallstone disease,[19,32] Crohn's disease,[19,89] osteoarthritis,[32,90] intervertebral degeneration (including back pain and sciatica),[91,92] peripheral arterial disease,[19,32] venous thromboembolism,[19,32] deep vein thrombosis,[19,32] aortic valve stenosis,[19] atopic dermatitis,[93] psoriasis,[32] gout[32] and rheumatoid arthritis.[32]
IMPACT ON MOTHER AND CHILD
Maternal obesity has been associated with adverse outcomes, including increased mortality, for both mother and child.[94] Mothers with obesity are more likely to develop pregnancy complications such as gestational hypertension, preeclampsia, gestational diabetes mellitus and thromboembolic disease, with a higher risk of preterm delivery, caesarian section, stillbirth, intrauterine growth retardation and foetus that is large for gestational age.[94,95,96] In an MR study, genetically elevated maternal BMI was associated with higher offspring birthweight, supporting a causal relationship.[97] These adverse outcomes can lead to complications and disability, with increased birth weight being associated with childhood adiposity and metabolic disorders during life.[94] Hence, the impact of obesity may extend beyond the current generation (mother) to the next generation (child). In Singapore, findings from the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) study, a prospective mother–offspring birth cohort, showed that pre-pregnancy BMI and maternal obesity were associated with child size and adiposity[98] and childhood obesity,[99] respectively, confirming the generational impact of obesity.
PSYCHOSOCIAL IMPACT
Obesity negatively impacts health-related quality of life, with greater degrees of obesity associated with greater impairments.[100,101,102] While most individuals often report significant difficulties with physical and occupational functioning, many also experience problems with social functioning, such as social withdrawal[103] and social isolation.[104] This could be due to perceived negative attitudes and discrimination towards people with obesity, increased self-consciousness and self-blame for being overweight.[69,104] People with obesity, especially females, are often dissatisfied with their body image, which is exacerbated by society's expectation of thinness, with the degree of dissatisfaction positively correlated with the amount of excess weight.[102,105,106] Taken together, obesity can negatively impact an individual's self-esteem,[69] thereby affecting self-efficacy,[107] possibly resulting in a vicious circle and downward spiral.
ECONOMIC IMPACT
There are substantially higher healthcare utilisation and medical costs among people who are overweight or obese due to treatment of medical conditions caused by excess adiposity and for direct obesity treatments, which may include weight loss surgeries and medications.[108] In addition to direct medical costs, there is also an increase in indirect costs as a result of increased absenteeism (workdays missed due to illness or injury) and presenteeism (reduced productivity while working).[109]
The economic burden of overweight and obesity has been well described and quantified in North America,[108,110] Europe,[111,112,113,114] Brazil,[115] Australia,[116,117] China[118] and Saudi Arabia,[119] with an estimated cost of 0.8%–2.4% of gross domestic product (GDP) in 2019 based on eight countries.[120] As for Singapore, a recent study has attempted to quantify the economic burden of overweight and obesity.[121] Using econometric methods and cross-sectional data from the Singapore Epidemiology of Eye Diseases (SEED) cohort, which includes measured height and weight, self-reported healthcare utilisation and absenteeism/presenteeism (based on a modified version of the Work Productivity Activity Impairment questionnaire), the incremental per capita and aggregate direct and indirect costs of excess weight among a multiethnic population of older adults (aged 40–80) were estimated.
Among Chinese, individuals who were overweight missed one additional workday per year compared to those who were of normal weight. Individuals in the obese category had SGD720 per year greater medical expenditures, but missed workdays were not statistically different from those in the normal weight category. Among Indians, differences were not significant between normal and overweight categories, but Indians in the obese category incurred an additional SGD310 per year in absenteeism costs than those of normal weight. For Malays, no significant differences by BMI category were identified.[121]
In aggregate, the predicted total medical expenditures attributable to overweight and obesity in Singapore were estimated to be SGD178 million, representing 1.6% of Singapore's total healthcare expenditures (SGD11,300 million in 2019). This figure is on the low end of published estimates, which range between 2.9% and 9.7% of the total healthcare spending.[121] Including absenteeism increases this estimate to SGD261 million, although this estimate is likely to be conservative because it does not include costs for presenteeism (reduced productivity while working), retraining, injuries or other costs resulting from excess weight in the workplace.[121] By ethnicity, Malays are responsible for 19% of the total costs of excess weight, even though they make up only 12% of the Singapore population.[121] This disproportionate burden is consistent with the disproportionately higher rates of overweight and obesity among Malays.[3]
LIMITATIONS OF REVIEW
First, this review discussed mainly the impact of adult obesity, with a focus on diseases that have shown a strong relationship with obesity, especially those supported by MR studies. Hence, the impact of paediatric obesity is not discussed and not all diseases have been covered. Second, not all the diseases supported by evidence from MR studies were discussed in detail. Nonetheless, they are listed in the section ’Other diseases’ and included in Figure 1. Third, interventional studies which show significant weight loss-improving health outcomes were not discussed. These studies, by showing the positive impact of weight loss, would have further supported the negative impact of obesity. Lastly, while there are some MR studies based on Asian cohorts, the majority of MR studies were based on genetic data derived from individuals of European ancestry or large cohorts that were predominantly European. Hence, the inference of causality may be limited in non-European populations.
CONCLUSION
As discussed, obesity is strongly related to more than 50 medical conditions [summarised in Figure 1], with evidence from MR studies to support causality for many of these conditions. Based on the 2017 Global Burden of Diseases study, the top four causes (cardiovascular diseases, cancers, musculoskeletal disorders and mental disorders) of disability-adjusted life years in Singapore account for more than 50% of the total burden,[7] with many of the common conditions in these four causes related to obesity. Hence, the clinical, social and economic burdens of obesity are considerable, potentially impacting future generations as well. This review, therefore, highlights the importance of an urgent and concerted effort towards the prevention and management of obesity to reduce the burden of obesity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–96. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Epidemiology & Disease Control Division and Policy, Research & Surveillance Group, Ministry of Health and Health Promotion Board, Singapore. National Population Health Survey 2020 (Household Interview and Health Examination) [Last accessed on 15 Feb 2023]. Available from: https://www.moh.gov.sg/docs/librariesprovider5/default-document-library/nphs-2020-survey-report.pdf .
- 4.Kivimäki M, Strandberg T, Pentti J, Nyberg ST, Frank P, Jokela M, et al. Body-mass index and risk of obesity-related complex multimorbidity: An observational multicohort study. Lancet Diabetes Endocrinol. 2022;10:253–63. doi: 10.1016/S2213-8587(22)00033-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Tan NC, Jafar TH. Ethnic variation, socioeconomic status, and factors associated with cardio-metabolic multi-morbidity among uncontrolled hypertension in multiethnic Singapore. J Hum Hypertens. 2022;36:218–27. doi: 10.1038/s41371-020-00457-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee HA, Park H. Comorbidity network analysis related to obesity in middle-aged and older adults: Findings from Korean population-based survey data. Epidemiol Health. 2021;43:e2021018. doi: 10.4178/epih.e2021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epidemiology and Disease Control Division, Ministry of Health, Singapore; Institute for Health Metrics and Evaluation. The Burden of Disease in Singapore, 1990-2017: An Overview of the Global Burden of Disease Study 2017 Results. Seattle, WA: IHME; 2019. [Google Scholar]
- 8.McHale CT, Laidlaw AH, Cecil JE. Primary care patient and practitioner views of weight and weight-related discussion: A mixed-methods study. BMJ Open. 2020;10:e034023. doi: 10.1136/bmjopen-2019-034023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caterson ID, Alfadda AA, Auerbach P, Coutinho W, Cuevas A, Dicker D, et al. Gaps to bridge: Misalignment between perception, reality and actions in obesity. Diabetes Obes Metab. 2019;21:1914–24. doi: 10.1111/dom.13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blackburn M, Stathi A, Keogh E, Eccleston C. Raising the topic of weight in general practice: Perspectives of GPs and primary care nurses. BMJ Open. 2015;5:e008546. doi: 10.1136/bmjopen-2015-008546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, et al. Obesity. Nat Rev Dis Primers. 2017;3:17034. doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 12.Guerreiro VA, Carvalho D, Freitas P. Obesity, adipose tissue, and inflammation answered in questions. J Obes. 2022;2022:2252516. doi: 10.1155/2022/2252516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upadhyay J, Farr O, Perakakis N, Ghaly W, Mantzoros C. Obesity as a disease. Med Clin North Am. 2018;102:13–33. doi: 10.1016/j.mcna.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Howick J, Glasziou P, Aronson JK. The evolution of evidence hierarchies: What can Bradford Hill's ’guidelines for causation’ contribute? J R Soc Med. 2009;102:186–94. doi: 10.1258/jrsm.2009.090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith GD, Ebrahim S. ’Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 16.Capurso C, Capurso A. From excess adiposity to insulin resistance: The role of free fatty acids. Vascul Pharmacol. 2012;57:91–7. doi: 10.1016/j.vph.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–33. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Taylor R. Pathogenesis of type 2 diabetes: Tracing the reverse route from cure to cause. Diabetologia. 2008;51:1781–9. doi: 10.1007/s00125-008-1116-7. [DOI] [PubMed] [Google Scholar]
- 19.Larsson SC, Burgess S. Causal role of high body mass index in multiple chronic diseases: A systematic review and meta-analysis of Mendelian randomization studies. BMC Med. 2021;19:320. doi: 10.1186/s12916-021-02188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deurenberg-Yap M, Chew SK, Lin VF, Tan BY, van Staveren WA, Deurenberg P. Relationships between indices of obesity and its co-morbidities in multi-ethnic Singapore. Int J Obes Relat Metab Disord. 2001;25:1554–62. doi: 10.1038/sj.ijo.0801739. [DOI] [PubMed] [Google Scholar]
- 21.Odegaard AO, Koh WP, Vazquez G, Arakawa K, Lee HP, Yu MC, et al. BMI and diabetes risk in Singaporean Chinese. Diabetes Care. 2009;32:1104–6. doi: 10.2337/dc08-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan JCY, Chee ML, Tan NYQ, Cheng CY, Wong TY, Sabanayagam C. Differential effect of body mass index on the incidence of diabetes and diabetic retinopathy in two Asian populations. Nutr Diabetes. 2018;8:16. doi: 10.1038/s41387-018-0018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoo CM, Sairazi S, Taslim S, Gardner D, Wu Y, Lee J, et al. Ethnicity modifies the relationships of insulin resistance, inflammation, and adiponectin with obesity in a multiethnic Asian population. Diabetes Care. 2011;34:1120–6. doi: 10.2337/dc10-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantley NW, Lonnen K, Kyrou I, Tahrani AA, Kahal H. The association between overweight/obesity and double diabetes in adults with type 1 diabetes; a cross-sectional study. BMC Endocr Disord. 2021;21:187. doi: 10.1186/s12902-021-00851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkin TJ. The convergence of type 1 and type 2 diabetes in childhood: The accelerator hypothesis. Pediatr Diabetes. 2012;13:334–9. doi: 10.1111/j.1399-5448.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- 26.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun Rev. 2014;13:981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients. 2013;5:1218–40. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bays HE, Toth PP, Kris-Etherton PM, Abate N, Aronne LJ, Brown WV, et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:304–83. doi: 10.1016/j.jacl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 29.He C, Zhang M, Li J, Wang Y, Chen L, Qi B, et al. Novel insights into the consequences of obesity: A phenotype-wide Mendelian randomization study. Eur J Hum Genet. 2022;30:540–6. doi: 10.1038/s41431-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotsis V, Jordan J, Micic D, Finer N, Leitner DR, Toplak H, et al. Obesity and cardiovascular risk: A call for action from the European Society of Hypertension Working Group of Obesity, Diabetes and the High-risk Patient and European Association for the Study of Obesity: Part A: Mechanisms of obesity induced hypertension, diabetes and dyslipidemia and practice guidelines for treatment. J Hypertens. 2018;36:1427–40. doi: 10.1097/HJH.0000000000001730. [DOI] [PubMed] [Google Scholar]
- 31.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, et al. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment: A position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich) 2013;15:14–33. doi: 10.1111/jch.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin S, Tyrrell J, Thomas EL, Bown MJ, Wood AR, Beaumont RN, et al. Disease consequences of higher adiposity uncoupled from its adverse metabolic effects using Mendelian randomisation. Elife. 2022;11:e72452. doi: 10.7554/eLife.72452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dongiovanni P, Stender S, Pietrelli A, Mancina RM, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283:356–70. doi: 10.1111/joim.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 35.Xie J, Huang H, Liu Z, Li Y, Yu C, Xu L, et al. The associations between modifiable risk factors and nonalcoholic fatty liver disease: A comprehensive Mendelian randomization study. Hepatology. 2022 doi: 10.1002/hep.32728. [DOI] [PubMed] [Google Scholar]
- 36.Goh GB, Kwan C, Lim SY, Venkatanarasimha NK, Abu-Bakar R, Krishnamoorthy TL, et al. Perceptions of non-alcoholic fatty liver disease – An Asian community-based study. Gastroenterol Rep (Oxf) 2016;4:131–5. doi: 10.1093/gastro/gov047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen K, Sng WK, Quah JH, Liu J, Chong BY, Lee HK, et al. Clinical spectrum of non-alcoholic fatty liver disease in patients with diabetes mellitus. PLoS One. 2020;15:e0236977. doi: 10.1371/journal.pone.0236977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 39.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, et al. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects) Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383:970–83. doi: 10.1016/S0140-6736(13)61836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 41.Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: Effects of weight loss and exercise. J Am Coll Cardiol. 2017;70:2022–35. doi: 10.1016/j.jacc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Ardissino M, Reddy RK, Slob EAW, Patel KHK, Ryan DK, Gill D, et al. Sleep disordered breathing, obesity and atrial fibrillation: A Mendelian randomisation study. Genes (Basel) 2022;13:104. doi: 10.3390/genes13010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng TP, Jin A, Chow KY, Feng L, Nyunt MSZ, Yap KB. Age-dependent relationships between body mass index and mortality: Singapore longitudinal ageing study. PLoS One. 2017;12:e0180818. doi: 10.1371/journal.pone.0180818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brock JM, Billeter A, Muller-Stich BP, Herth F. Obesity and the lung: What we know today. Respiration. 2020;99:856–66. doi: 10.1159/000509735. [DOI] [PubMed] [Google Scholar]
- 45.Masa JF, Pépin JL, Borel JC, Mokhlesi B, Murphy PB, Sánchez-Quiroga Maria Á. Obesity hypoventilation syndrome. Eur Respir Rev. 2019;28:180097. doi: 10.1183/16000617.0097-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strausz S, Ruotsalainen S, Ollila HM, Karjalainen J, Kiiskinen T, Reeve M, et al. Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Eur Respir J. 2021;57:2003091. doi: 10.1183/13993003.03091-2020. [DOI] [PubMed] [Google Scholar]
- 47.Chan PF, Tai BC, Loo G, Koo CY, Ong TH, Yeo TC, et al. Optimal body mass index cut-offs for identification of patients with coronary artery disease at high risk of obstructive sleep apnoea. Heart Lung Circ. 2016;25:847–54. doi: 10.1016/j.hlc.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Barber TM, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 2021;95:531–41. doi: 10.1111/cen.14421. [DOI] [PubMed] [Google Scholar]
- 49.The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 50.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2012;18:618–37. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 51.Brower MA, Hai Y, Jones MR, Guo X, Chen YI, Rotter JI, et al. Bidirectional Mendelian randomization to explore the causal relationships between body mass index and polycystic ovary syndrome. Hum Reprod. 2019;34:127–36. doi: 10.1093/humrep/dey343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neubronner SA, Indran IR, Chan YH, Thu AWP, Yong EL. Effect of body mass index (BMI) on phenotypic features of polycystic ovary syndrome (PCOS) in Singapore women: A prospective cross-sectional study. BMC Womens Health. 2021;21:135. doi: 10.1186/s12905-021-01277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Dalaeen A, Al-Domi H. Does obesity put your brain at risk? Diabetes Metab Syndr. 2022;16:102444. doi: 10.1016/j.dsx.2022.102444. [DOI] [PubMed] [Google Scholar]
- 54.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obes Rev. 2011;12:e426–37. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 55.Talaei M, Feng L, Barrenetxea J, Yuan JM, Pan A, Koh WP. Adiposity, weight change, and risk of cognitive impairment: The Singapore Chinese health study. J Alzheimers Dis. 2020;74:319–29. doi: 10.3233/JAD-191052. [DOI] [PubMed] [Google Scholar]
- 56.Moh MC, Low S, Ng TP, Wang J, Ang SF, Tan C, et al. Association of traditional and novel measures of central obesity with cognitive performance in older multi-ethnic Asians with type 2 diabetes. Clin Obes. 2020;10:e12352. doi: 10.1111/cob.12352. [DOI] [PubMed] [Google Scholar]
- 57.García-García I, Michaud A, Dadar M, Zeighami Y, Neseliler S, Collins DL, et al. Neuroanatomical differences in obesity: Meta-analytic findings and their validation in an independent dataset. Int J Obes (Lond) 2019;43:943–51. doi: 10.1038/s41366-018-0164-4. [DOI] [PubMed] [Google Scholar]
- 58.Mulugeta A, Lumsden A, Hypponen E. Unlocking the causal link of metabolically different adiposity subtypes with brain volumes and the risks of dementia and stroke: A Mendelian randomization study. Neurobiol Aging. 2021;102:161–9. doi: 10.1016/j.neurobiolaging.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee S, Walter S, Kauwe JSK, Saykin AJ, Bennett DA, Larson EB, et al. Genetically predicted body mass index and Alzheimer's disease-related phenotypes in three large samples: Mendelian randomization analyses. Alzheimers Dement. 2015;11:1439–51. doi: 10.1016/j.jalz.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ng TP, Feng L, Nyunt MS, Feng L, Gao Q, Lim ML, et al. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: Follow-up of the Singapore longitudinal ageing study cohort. JAMA Neurol. 2016;73:456–63. doi: 10.1001/jamaneurol.2015.4899. [DOI] [PubMed] [Google Scholar]
- 61.Wang M, Wang Z, Chen Y, Dong Y. Kidney damage caused by obesity and its feasible treatment drugs. Int J Mol Sci. 2022;23:747. doi: 10.3390/ijms23020747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu X, Eales JM, Jiang X, Sanderson E, Drzal M, Saluja S, et al. Contributions of obesity to kidney health and disease-Insights from mendelian randomisation and the human kidney transcriptomics. Cardiovasc Res. 2022;118:3151–61. doi: 10.1093/cvr/cvab357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye C, Kong L, Zhao Z, Li M, Wang S, Lin H, et al. Causal associations of obesity with chronic kidney disease and arterial stiffness: A Mendelian randomization study. J Clin Endocrinol Metab. 2022;107:e825–35. doi: 10.1210/clinem/dgab633. [DOI] [PubMed] [Google Scholar]
- 64.Moh MC, Sum CF, Tavintharan S, Ang K, Kwan PY, Lee SBM, et al. Gain in adiposity over 3 years is associated with progressive renal decline in multi-ethnic South-east Asians with type 2 diabetes. J Diabetes. 2019;11:316–25. doi: 10.1111/1753-0407.12848. [DOI] [PubMed] [Google Scholar]
- 65.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer — Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singapore Ministry of Health. Top 5 leading cancers among Singapore residents. [Last accessed on 31 Jan 2023]. Available from: https://data.gov.sg/dataset/to p-5-leading-cancers .
- 67.Mili N, Paschou SA, Goulis DG, Dimopoulos MA, Lambrinoudaki I, Psaltopoulou T. Obesity, metabolic syndrome, and cancer: Pathophysiological and therapeutic associations. Endocrine. 2021;74:478–97. doi: 10.1007/s12020-021-02884-x. [DOI] [PubMed] [Google Scholar]
- 68.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–30. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarwer DB, Polonsky HM. The psychosocial burden of obesity. Endocrinol Metab Clin North Am. 2016;45:677–88. doi: 10.1016/j.ecl.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amiri S, Behnezhad S. Obesity and anxiety symptoms: A systematic review and meta-analysis. Neuropsychiatr. 2019;33:72–89. doi: 10.1007/s40211-019-0302-9. [DOI] [PubMed] [Google Scholar]
- 71.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–9. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 72.Gariepy G, Nitka D, Schmitz N. The association between obesity and anxiety disorders in the population: A systematic review and meta-analysis. Int J Obes (Lond) 2010;34:407–19. doi: 10.1038/ijo.2009.252. [DOI] [PubMed] [Google Scholar]
- 73.Hryhorczuk C, Sharma S, Fulton SE. Metabolic disturbances connecting obesity and depression. Front Neurosci. 2013;7:177. doi: 10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fulton S, Decarie-Spain L, Fioramonti X, Guiard B, Nakajima S. The menace of obesity to depression and anxiety prevalence. Trends Endocrinol Metab. 2022;33:18–35. doi: 10.1016/j.tem.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Tyrrell J, Mulugeta A, Wood AR, Zhou A, Beaumont RN, Tuke MA, et al. Using genetics to understand the causal influence of higher BMI on depression. Int J Epidemiol. 2018;48:834–48. doi: 10.1093/ije/dyy223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartwig FP, Bowden J, Loret de Mola C, Tovo-Rodrigues L, Davey Smith G, Horta BL. Body mass index and psychiatric disorders: A Mendelian randomization study. Sci Rep. 2016;6:32730. doi: 10.1038/srep32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Speed MS, Jefsen OH, Børglum AD, Speed D, Østergaard SD. Investigating the association between body fat and depression via Mendelian randomization. Transl Psychiatry. 2019;9:184. doi: 10.1038/s41398-019-0516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ho CS, Lu Y, Ndukwe N, Chew MW, Shabbir A, So JB, et al. Symptoms of anxiety and depression in obese Singaporeans: A preliminary study. East Asian Arch Psychiatry. 2018;28:3–8. [PubMed] [Google Scholar]
- 79.Subramaniam M, Abdin E, Vaingankar JA, Shafie S, Chua BY, Sambasivam R, et al. Tracking the mental health of a nation: Prevalence and correlates of mental disorders in the second Singapore mental health study. Epidemiol Psychiatr Sci. 2019;29:e29. doi: 10.1017/S2045796019000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7:66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: Obesity and 2009 pandemic influenza A (H1N1) Clin Infect Dis. 2011;52:301–12. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- 82.Ho JSY, Fernando DI, Chan MY, Sia CH. Obesity in COVID-19: A systematic review and meta-analysis. Ann Acad Med Singap. 2020;49:996–1008. [PubMed] [Google Scholar]
- 83.Qu HQ, Qu J, Glessner J, Hakonarson H. Mendelian randomization study of obesity and type 2 diabetes in hospitalized COVID-19 patients. Metabolism. 2022;129:155156. doi: 10.1016/j.metabol.2022.155156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freuer D, Linseisen J, Meisinger C. Impact of body composition on COVID-19 susceptibility and severity: A two-sample multivariable Mendelian randomization study. Metabolism. 2021;118:154732. doi: 10.1016/j.metabol.2021.154732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao M, Wang Q, Piernas C, Astbury NM, Jebb SA, Holmes MV, et al. Associations between body composition, fat distribution and metabolic consequences of excess adiposity with severe COVID-19 outcomes: Observational study and Mendelian randomisation analysis. Int J Obes (Lond) 2022;46:943–50. doi: 10.1038/s41366-021-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li S, Hua X. Modifiable lifestyle factors and severe COVID-19 risk: A Mendelian randomisation study. BMC Med Genomics. 2021;14:38. doi: 10.1186/s12920-021-00887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.World Health Organisation. WHO (COVID-19) [Last accessed on 19 Nov 2022]. Available from: https://covid19.who.int/region/wpro/country/sg .
- 88.Ong SWX, Young BE, Leo YS, Lye DC. Association of higher body mass index with severe coronavirus disease 2019 (COVID-19) in younger patients. Clin Infect Dis. 2020;71:2300–2. doi: 10.1093/cid/ciaa548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carreras-Torres R, Ibanez-Sanz G, Obon-Santacana M, Duell EJ, Moreno V. Identifying environmental risk factors for inflammatory bowel diseases: A Mendelian randomization study. Sci Rep. 2020;10:19273. doi: 10.1038/s41598-020-76361-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gill D, Karhunen V, Malik R, Dichgans M, Sofat N. Cardiometabolic traits mediating the effect of education on osteoarthritis risk: A Mendelian randomization study. Osteoarthritis Cartilage. 2021;29:365–71. doi: 10.1016/j.joca.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou J, Mi J, Peng Y, Han H, Liu Z. Causal associations of obesity with the intervertebral degeneration, low back pain, and sciatica: A two-sample Mendelian randomization study. Front Endocrinol (Lausanne) 2021;12:740200. doi: 10.3389/fendo.2021.740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elgaeva EE, Tsepilov Y, Freidin MB, Williams FMK, Aulchenko Y, Suri P. ISSLS Prize in Clinical Science 2020. Examining causal effects of body mass index on back pain: A Mendelian randomization study. Eur Spine J. 2020;29:686–91. doi: 10.1007/s00586-019-06224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yew YW, Loh M, Thng STG, Chambers JC. Investigating causal relationships between Body Mass Index and risk of atopic dermatitis: A Mendelian randomization analysis. Sci Rep. 2020;10:15279. doi: 10.1038/s41598-020-72301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tersigni C, Neri C, D’Ippolito S, Garofalo S, Martino C, Lanzone A, et al. Impact of maternal obesity on the risk of preterm delivery: Insights into pathogenic mechanisms. J Matern Fetal Neonatal Med. 2022;35:3216–21. doi: 10.1080/14767058.2020.1817370. [DOI] [PubMed] [Google Scholar]
- 95.Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity—A determinant of perinatal and offspring outcomes? Nat Rev Endocrinol. 2012;8:679–88. doi: 10.1038/nrendo.2012.176. [DOI] [PubMed] [Google Scholar]
- 96.Shin D, Song WO. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J Matern Fetal Neonatal Med. 2015;28:1679–86. doi: 10.3109/14767058.2014.964675. [DOI] [PubMed] [Google Scholar]
- 97.Tyrrell J, Richmond RC, Palmer TM, Feenstra B, Rangarajan J, Metrustry S, et al. Genetic evidence for causal relationships between maternal obesity-related traits and birth weight. JAMA. 2016;315:1129–40. doi: 10.1001/jama.2016.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin X, Aris IM, Tint MT, Soh SE, Godfrey KM, Yeo GS, et al. Ethnic differences in effects of maternal pre-pregnancy and pregnancy adiposity on offspring size and adiposity. J Clin Endocrinol Metab. 2015;100:3641–50. doi: 10.1210/jc.2015-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aris IM, Bernard JY, Chen LW, Tint MT, Pang WW, Soh SE, et al. Modifiable risk factors in the first 1000 days for subsequent risk of childhood overweight in an Asian cohort: Significance of parental overweight status. Int J Obes (Lond) 2018;42:44–51. doi: 10.1038/ijo.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sarwer DB, Lavery M, Spitzer JC. A review of the relationships between extreme obesity, quality of life, and sexual function. Obes Surg. 2012;22:668–76. doi: 10.1007/s11695-012-0588-1. [DOI] [PubMed] [Google Scholar]
- 101.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev. 2001;2:173–82. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 102.Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev. 2001;2:219–29. doi: 10.1046/j.1467-789x.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 103.Rotenberg KJ, Bharathi C, Davies H, Finch T. Obesity and the social withdrawal syndrome. Eat Behav. 2017;26:167–70. doi: 10.1016/j.eatbeh.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 104.Jung FU, Luck-Sikorski C. Overweight and lonely. A representative study on loneliness in obese people and its determinants? Obes Facts. 2019;12:440–7. doi: 10.1159/000500095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarwer DB, Wadden TA, Foster GD. Assessment of body image dissatisfaction in obese women: Specificity, severity, and clinical significance. J Consult Clin Psychol. 1998;66:651–4. doi: 10.1037//0022-006x.66.4.651. [DOI] [PubMed] [Google Scholar]
- 106.Weinberger NA, Kersting A, Riedel-Heller SG, Luck-Sikorski C. Body dissatisfaction in individuals with obesity compared to normal-weight individuals: A systematic review and meta-analysis. Obes Facts. 2016;9:424–41. doi: 10.1159/000454837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Valentin Ayala R, Bernstein J. Changing weight management self-efficacy among obese Puerto Rican adults: A quantitative study using a health coaching intervention. Intrnet j. allied health sci. pract. 2020;18:Article 9. doi: 10.46743/1540-580X/2020.1867. [Google Scholar]
- 108.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: Payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 109.Finkelstein EA, DiBonaventura M, Burgess SM, Hale BC. The costs of obesity in the workplace. J Occup Environ Med. 2010;52:971–6. doi: 10.1097/JOM.0b013e3181f274d2. [DOI] [PubMed] [Google Scholar]
- 110.Anis AH, Zhang W, Bansback N, Guh DP, Amarsi Z, Birmingham CL. Obesity and overweight in Canada: An updated cost-of-illness study. Obes Rev. 2010;11:31–40. doi: 10.1111/j.1467-789X.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 111.Lette M, Bemelmans WJ, Breda J, Slobbe LC, Dias J, Boshuizen HC. Health care costs attributable to overweight calculated in a standardized way for three European countries. Eur J Health Econ. 2016;17:61–9. doi: 10.1007/s10198-014-0655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lehnert T, Streltchenia P, Konnopka A, Riedel-Heller SG, König HH. Health burden and costs of obesity and overweight in Germany: An update. Eur J Health Econ. 2015;16:957–67. doi: 10.1007/s10198-014-0645-x. [DOI] [PubMed] [Google Scholar]
- 113.Odegaard K, Borg S, Persson U, Svensson M. The Swedish cost burden of overweight and obesity – evaluated with the PAR approach and a statistical modelling approach. Int J Pediatr Obes. 2008;3(Suppl 1):51–7. doi: 10.1080/17477160801897067. [DOI] [PubMed] [Google Scholar]
- 114.Effertz T, Engel S, Verheyen F, Linder R. The costs and consequences of obesity in Germany: A new approach from a prevalence and life-cycle perspective. Eur J Health Econ. 2016;17:1141–58. doi: 10.1007/s10198-015-0751-4. [DOI] [PubMed] [Google Scholar]
- 115.de Oliveira ML, Santos LM, da Silva EN. Direct healthcare cost of obesity in brazil: An application of the cost-of-illness method from the perspective of the public health system in 2011. PLoS One. 2015;10:e0121160. doi: 10.1371/journal.pone.0121160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Colagiuri S, Lee CM, Colagiuri R, Magliano D, Shaw JE, Zimmet PZ, et al. The cost of overweight and obesity in Australia. Med J Aust. 2010;192:260–4. doi: 10.5694/j.1326-5377.2010.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 117.Lee CMY, Goode B, Nørtoft E, Shaw JE, Magliano DJ, Colagiuri S. The cost of diabetes and obesity in Australia. J Med Econ. 2018;21:1001–5. doi: 10.1080/13696998.2018.1497641. [DOI] [PubMed] [Google Scholar]
- 118.Qin X, Pan J. The medical cost attributable to obesity and overweight in China: Estimation based on longitudinal surveys. Health Econ. 2016;25:1291–311. doi: 10.1002/hec.3217. [DOI] [PubMed] [Google Scholar]
- 119.Malkin JD, Baid D, Alsukait RF, Alghaith T, Alluhidan M, Alabdulkarim H, et al. The economic burden of overweight and obesity in Saudi Arabia. PLoS One. 2022;17:e0264993. doi: 10.1371/journal.pone.0264993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Okunogbe A, Nugent R, Spencer G, Ralston J, Wilding J. Economic impacts of overweight and obesity: Current and future estimates for eight countries. BMJ Glob Health. 2021;6:e006351. doi: 10.1136/bmjgh-2021-006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Junxing C, Huynh VA, Lamoureux E, Tham KW, Finkelstein EA. Economic burden of excess weight among older adults in Singapore: A cross-sectional study. BMJ Open. 2022;12:e064357. doi: 10.1136/bmjopen-2022-064357. [DOI] [PMC free article] [PubMed] [Google Scholar]