ABSTRACT
Purpose:
This study was designed to test the hypothesis that exposure to ivermectin in early disease prevents mortality due to COVID-19. A secondary objective was to see if the drug has any impact on the length of hospital stay among the survivors.
Methods:
It was a hospital-based retrospective case-control study conducted at a tertiary teaching hospital in India. All patients with a diagnosis of COVID-19 who were admitted between 1st April and 15th May 2021 and received inpatient care were included. Important variables like demographic details, dates of admission and discharge or death, symptoms at the time of admission, comorbidities, severity of illness at the time of admission, whether ivermectin was administered or not during the course of the illness and other treatments received as part of the standard of care were retrieved from the medical records.
Results:
Of the 965 patients who received inpatient care, 307 died during their hospital stay while 658 were successfully discharged. The proportion of cases treated with ivermectin was 17.26% among the non-survivors (53/307) and 17.93% among the survivors (118/658). The effect was statistically insignificant (crude OR = 0.954; 95% CI: 0.668–1.364, P = 0.80). Among the survivors, the median length of stay was 11 days for patients who received ivermectin (IQR: 7–15) as well as for those who did not (IQR: 7–16).
Conclusion:
This study did not show any effect of ivermectin on in-patient mortality in patients with COVID-19 and there was no effect of the drug on the length of hospital stay among the survivors.
Keywords: Case-control study, COVID-19, ivermectin, mortality
Introduction
There has been a growing interest in the potential role of the antiparasitic drug, ivermectin in the management of COVID-19, ever since it was reported to have an in-vitro activity against SARS-CoV-2.[1] Some observational studies,[2,3] a nonrandomized interventional study,[4] and some RCTs[5,6,7] have reported quicker viral clearance with the use of ivermectin. However, other studies have not found any significant benefit.[8,9,10,11] Clinical recovery and mortality benefit are clinically more relevant outcomes compared to viral clearance. An RCT of 400 patients with mild COVID-19 did not find any improvement in the time to resolution of symptoms with a five-day course of ivermectin (300 μg/kg) compared to placebo.[12]
Some observational studies[3,13,14] as well as some RCTs[6,11,15,16] have reported improved survival with ivermectin. However, another RCT reported no survival benefit.[17] A few meta-analyses of the available evidence have also noted possible reduction in mortality with this drug.[18,19,20] However, a recent review concluded that there was no survival benefit after excluding studies that were thought to have a high risk of bias.[21] The WHO has advised against the use of this drug for COVID-19 outside clinical trials.[22]
As the pandemic continues to spread and take lives worldwide and considering that ivermectin is a relatively safe and cheap drug, it is important to further investigate any possible mortality benefit of the drug in COVID-19. Given the low-case fatality rate in mild disease, very large RCTs are needed to detect with certainty any reduction in mortality. Hence, a case-control study is a pragmatic alternative. It provides a statistically efficient and logistically and economically feasible alternative for evaluating any survival benefit with the drug. This study was designed with the primary objective of estimating the association of ivermectin treatment with mortality in patients admitted with COVID-19. A secondary objective was the estimation of the extent of reduction, if any, in duration of hospital stay in survivors who received ivermectin.
Methods and Materials
Study setting
The study was conducted at a tertiary teaching hospital in eastern India.
Study design
It was a hospital-based retrospective case-control study.
Study population
All patients with a diagnosis of COVID-19 who were admitted to the hospital between 1st April and 15th May 2021 and received inpatient care were included. Patients who were admitted nominally but allowed to go on home isolation were excluded. Those patients who died during their hospital stay were selected as cases while those who were discharged alive were selected as controls. Ivermectin, taken by any patients, case or control, was the exposure of interest.
Data collection
A semi-structured proforma was used to collect data of all the patients included in the study. Important variables such as demographic data, dates of admission and discharge or death, clinical signs and symptoms at the time of admission, past medical history of comorbidities like hypertension, diabetes mellitus, cardiovascular disease, respiratory illness, chronic kidney disease and so on, severity of illness at the time of admission (mild, moderate or severe) as defined in the guidelines issued by the Ministry of Health and Family Welfare, Government of India,[23] whether ivermectin was administered or not during the course of the illness (either before or after the admission) and other treatments received as part of the standard of care were retrieved from the case notes obtained from the medical records department of the institute.
Data analysis
All data were entered into MS Excel using proper code for each variable. Statistical analysis was performed using Stata, version 10 (Stata Corp, Texas, USA). Continuous variables were tested for normality using Shapiro–Wilks test and expressed as mean with 95% confidence interval in the case of normal data and as median with interquartile range in the case of non-normal data. Categorical variables were expressed as percentages. T-test for difference of mean for two groups was applied after checking the equality of variance using F-test for normally distributed continuous variables. Mann–Whitney Wilcoxon U-test was applied for testing the significance of distribution of variables in the two groups. Chi-square test was applied for testing the association between two categorical variables. Further, age, which was entered as a continuous variable, was categorized into three dummy variables, namely, <25 years, 25–44 years and >45. Similarly, disease severity was categorized into three dummy variables, namely, mild, moderate and severe. Crude odds ratio with 95% confidence interval was estimated for ivermectin usage in relation to the survival and non-survival groups.
Ethical issues
As this study was based on retrospective analysis of the case notes, there was no risk of harm to the patients. To maintain anonymity, no personal identifiers were used during data collection. Ethical clearance was obtained from the Institutional Ethics Committee dated on 29/06/2021.
Results
Altogether, 1175 patients with COVID-19 were admitted between 1st April and 15th May 2021. However, 210 of these patients were excluded from the study as they were allowed to go on home leave and hence did not have adequate inpatient documentation. Of the remaining 965 patients, 307 died during their hospital stay while 658 were successfully discharged [Figure 1]. Table 1 presents the comparison of demographic and clinical factors such as signs and symptoms at the time of admission, the presence of comorbidities like hypertension, diabetes, cardiovascular diseases, asthma, chronic kidney disease and so on and disease severity on admission between those who received ivermectin and those who did not. Except cough and weakness, all factors were similarly distributed between the two groups (Chi-square test; P value > 0.05).
Figure 1.
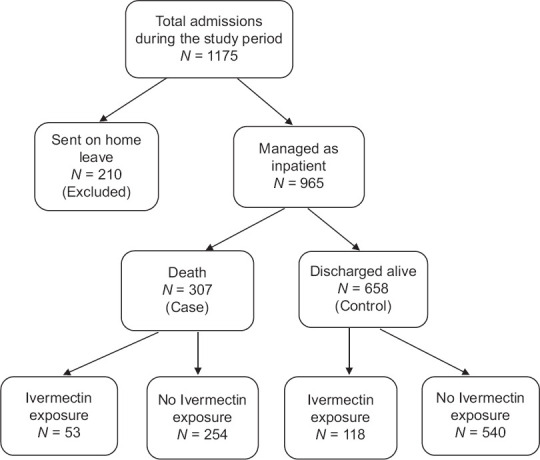
Flow chart of the study
Table 1.
Comparison of demographic and clinical factors between patients receiving and not receiving ivermectin
| Variables | Ivermectin (n=171) | No ivermectin (n=794) | Total (n=965) | Chi-square value | P |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 128 (75%) | 549 (69%) | 677 | 2.19 | 0.139 |
| Female | 43 (25%) | 245 (31%) | 288 | ||
| Age group | |||||
| <25 years | 8 (4.7%) | 18 (2.3%) | 26 | 3.17 | 0.204 |
| 25-44 | 42 (24.6%) | 207 (26.1%) | 249 | ||
| 45 above | 121 (70.7%) | 569 (71.6%) | 690 | ||
| Fever | |||||
| Yes | 115 (67.3%) | 475 (59.8%) | 590 | 3.267 | 0.071 |
| No | 56 (32.7%) | 319 (40.2%) | 375 | ||
| Shortness of breath | |||||
| Yes | 98 (57.3%) | 460 (57.9%) | 558 | 0.0255 | 0.881 |
| No | 73 (42.7%) | 334 (42.1%) | 407 | ||
| Cough | |||||
| Yes | 92 (53.8%) | 357 (45%) | 449 | 4.418 | 0.036 |
| No | 79 (48.2%) | 437 (55%) | 516 | ||
| Weakness | |||||
| Yes | 8 (4.7%) | 50 (6.3%) | 58 | 0.6527 | 0.0419 |
| No | 163 (95.3%) | 744 (93.7%) | 907 | ||
| Headache | |||||
| Yes | 11 (6.4%) | 35 (4.4%) | 46 | 1.27 | 0.26 |
| No | 160 (93.6%) | 759 (95.6%) | 919 | ||
| Hypertension | |||||
| Yes | 70 (40.9%) | 334 (42.1%) | 404 | 0.0738 | 0.786 |
| No | 101 (59.1%) | 460 (57.9%) | 561 | ||
| Diabetes | |||||
| Yes | 63 (36.8%) | 291 (36.6%) | 354 | 0.0022 | 0.960 |
| No | 108 (63.2%) | 503 (63.4%) | 611 | ||
| Cardiovascular disease | |||||
| Yes | 51 (71.8%) | 254 (32%) | 305 | 0.305 | 0.581 |
| No | 120 (28.2) | 540 (68%) | 660 | ||
| Asthma | |||||
| Yes | 6 (3.5%) | 25 (3.1%) | 31 | 0.0587 | 0.809 |
| No | 165 (96.5%) | 769 (96.9%) | 934 | ||
| CKD | |||||
| Yes | 2 (1.2%) | 23 (2.9%) | 25 | 1.66 | 0.197 |
| No | 169 (98.8%) | 771 (97.1%) | 940 | ||
| Disease severity | |||||
| Mild | 48 (28.1%) | 239 (30.1%) | 287 | 0.74 | 0.689 |
| Moderate | 49 (28.6%) | 203 (25.6%) | 252 | ||
| Severe | 74 (43.3%) | 352 (44.3%) | 426 | ||
| ICU admission | |||||
| Yes | 46 (26.9%) | 221 (27.8%) | 267 | 0.0612 | 0.0643 |
| No | 125 (73.1%) | 573 (72.2%) | 698 |
Table 2 presents the comparison of various drugs administered between patients who were treated with ivermectin and those who were not. Altogether, 171 (17.7%) received ivermectin in addition to other medicines such as antibiotics (168), steroids (161), remdesvir (83), toclizumab (4) and itolizumab (1). The remaining 794 who did not receive ivermectin were also treated with the above medicines as per the institutional protocol and the clinical judgement of the treating team. Usage of all drugs between the two groups was comparable (Chi-square test; P value > 0.05).
Table 2.
Comparison of usage of various drugs between patients receiving and not receiving ivermectin
| Variables | Ivermectin (n=171) | No ivermectin (n=794) | Total (n=965) | Chi-square value | P |
|---|---|---|---|---|---|
| Antibiotic | |||||
| Yes | 168 (98.2) | 778 (98%) | 946 | 0.0946 | 0.824 |
| No | 3 (1.8) | 16 (2%) | 19 | ||
| Steroid | |||||
| Yes | 161 (97.7%) | 731 (97.1) | 892 | 0.876 | 0.349 |
| No | 10 (2.3%) | 63 (2.9%) | 73 | ||
| Remdesvir | |||||
| Yes | 83 (48.5) | 352 (44.3%) | 435 | 1.0051 | 0.316 |
| No | 88 (51.5) | 442 (55. 7%) | 530 | ||
| Toclizumab | |||||
| Yes | 4 (2.3%) | 38 (4.8%) | 42 | 2.02 | 0.155 |
| No | 167 (97.7%) | 756 (95.2%) | 923 | ||
| Itlizumab | |||||
| Yes | 1 (0.6%) | 11 (1.4%) | 12 | 0.7343 | 0.391 |
| No | 170 (99.4%) | 783 (98.6%) | 953 |
Table 3 presents the effect of ivermectin on survival. The proportion of cases treated with ivermectin was 17.26% among the non-survivors and 17.93% among the survivors. The effect was statistically insignificant (crude OR = 0.954; 95% CI: 0.668–1.364, P = 0.80).
Table 3.
Association of ivermectin with survival
| Ivermectin | Case (non-survivors) (n=307) | Control (survivors) (n=658) | Total (n=965) | Odds ratio (95% CI) | Chi-square value | P |
|---|---|---|---|---|---|---|
| Yes | 53 (17.26%) | 118 (17.93%) | 171 | 0.95 (0.67-1.36)* | 0.0643 | 0.800 |
| No | 254 | 540 | 794 | 0.96 (0.66-1.39)** | 0.0529 | 0.819 |
*Crude OR; **Adjusted OR
A logistic regression analysis was performed with bootstrapping after 50 replications of the random data set to estimate the effect of ivermectin on the outcome, that is survival status after controlling for each of the confounders. Even after adjusting for the confounders, the effect was statistically not significant (adjusted OR = 0.96; 95% CI: 0.66–1.39, P = 0.819). The logistic regression model was found to fit well (Wald Chi-square with 5 d.f. = 41.52, P = 0.00001). The AIC was 1155.44 and BIC was −29.33.
Effect of ivermectin on duration of hospital stay among the survivors
Table 4 presents the duration of hospital stay in days. It was tested for normality using Shapiro–Wilk W test. It was found to be non-normally distributed (z-statistic = 10.261, P value = 0.0001). Hence, Mann–Whitney Wilcoxon U-test was applied to compare the distribution of hospital stay between the survivors who received ivermectin and those who did not. There was no significant difference between the two groups (P = 0.8759).
Table 4.
Comparison of duration of hospital stay (in days) among survived COVID-19 patients
| Ivermectin | n | Hospital stay (in days) Median (IQR) | MW U-test statistics | P |
|---|---|---|---|---|
| Yes | 118 | 11 (7-15) | 0.152 | 0.8759 |
| No | 540 | 11 (7-16) |
Discussion
Putting the results in context
The study did not find any association between ivermectin use and survival in patients with COVID-19. This is in agreement with the existing WHO guidelines that do not advocate the use of this drug outside of clinical trials.[22]
An RCT of 400 patients with mild COVID-19 did not find any improvement in the time to resolution of symptoms with a 5-day course of ivermectin (300 μg/kg).[12] More recently, an RCT of 490 patients with mild-to-moderate COVID-19 with comorbidities did not find any reduction in progression to severe disease with a 5-day course of ivermectin 0.4 mg/kg body weight.[24] RCTs evaluating the effect of ivermectin on mortality have given conflicting results. Some of the earlier meta-analyses had concluded a survival benefit with the drug.[18,19,20] However, a recent review that excluded trials thought to have a high risk of bias found no survival benefit.[22] It could be argued that with the very low-case fatality rates reported with mild and moderate COVID-19, the RCTs were insufficiently powered to detect any potential survival benefit with the drug. Therefore, a case-control study is a pragmatic design to study any possible association between survival and the usage of ivermectin in early COVID-19. The authors feel that the findings of this study will be a useful contribution to the current body of evidence on the effect of ivermectin on mortality in COVID-19. A secondary objective of this study was to see if there was any reduction in the length of hospital stay among the survivors who had received ivermectin. No difference was observed in the duration of hospital stay between patients who received ivermectin and those who did not. Thus, no recommendation can be made on the basis of this study for the use of ivermectin in COVID-19.
Possible explanation for the lack of demonstrable efficacy
Despite the demonstration of in-vitro antiviral activity of ivermectin against SARS-COV-II, a compelling evidence of its clinical utility in COVID-19 remains elusive. This is probably because the concentration of the drug at which its in-vitro antiviral activity was noted[1] is difficult to achieve with pharmacological doses.[25] Doses as high as 90–120 mg/dose have been demonstrated to be safe in human beings and remain potential subjects of future research for their role in the treatment of COVID-19.[26]
Limitations
The study has some obvious limitations. As it was a case-control study, its results cannot substitute the findings of high-quality RCTs but only complement them. As it was based on retrospective case note analysis, the accuracy of the data was dependent upon the documentations done by the treating teams. As many of the patients had already received the drug prior to their admission, the possibility of a recall bias cannot be ruled out. Moreover, the regime used by the patients was not standardized even though 12 mg tablets given once daily for 3–5 days was the most commonly used regimen in adults and even found a place in the national guidelines in India for a brief period.[27]
Conclusion
This study did not show any effect of ivermectin on in-patient mortality in patients with COVID-19. Similarly, there was no effect of the drug on the length of hospital stay among the survivors. Thus, on the basis of this study, no recommendation can be made for its use in COVID-19. Large multicentric RCTs using larger doses of ivermectin should be conducted to further evaluate its potential role in the treatment of the disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:1–4. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam MT, Murshed R, Bhiuyan E, Saber S, Alam RF, Robin RC. A case series of 100 COVID-19 positive patients treated with combination of ivermectin and doxycycline. J Bangladesh Coll Phys Surg. 2020;38:10–5. [Google Scholar]
- 3.Khan MSI, Khan MSI, Debnath CR, Nath PN, Mahtab MA, Nabeka H, et al. Ivermectin treatment may improve the prognosis of patients with COVID-19. Arch Bronconeumol. 2020;56:828–30. doi: 10.1016/j.arbr.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espitia-Hernandez G, Munguia L, Diaz-Chiguer D, Lopez-Elizalde R, Jimenez-Ponce F. Effects of ivermectin-azithromycin-cholecalciferol combined therapy on COVID-19 infected patients:A proof of concept study. Biomed Res. 2020;31:129–33. [Google Scholar]
- 5.Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, et al. A five day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–6. doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmud R, Rahman MM, Alam I, Ahmed KGU, Kabir AKMH, Sayeed SKJB, et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms:A randomized trial. J Int Med Res. 2021;49:3000605211013550. doi: 10.1177/03000605211013550. doi:10.1177/03000605211013550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, et al. Ivermectin shows clinical benefits in mild to moderate COVID19:A randomised controlled double-blind, dose-response study in Lagos. QJM-Int J Med. 2021;114:80–8. doi: 10.1093/qjmed/hcab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camprubi D, Almuedo-Riera A, Martí-Soler H, Soriano A, Hurtado JC, Subirà C, et al. Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients. PLoS One. 2020;15:e0242184. doi: 10.1371/journal.pone.0242184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury A, Shahbaz M, Karim M, Islam J, Dan G, Shuixiang H. A comparative study on ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID-19 patients. EJMO. 2021;5:63–70. [Google Scholar]
- 10.Mohan A, Tiwari P, Suri TM, Mittal S, Patel A, Jain A, et al. Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV):A single-centre randomized, placebo-controlled trial. J Infect Chemother. 2021;27:1743–9. doi: 10.1016/j.jiac.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravikirti , Roy R, Pattadar C, Raj R, Agarwal N, Biswas B, et al. Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19:A double-blind randomized placebo controlled trial in eastern India. J Pharm Pharm Sci. 2021;24:343–50. doi: 10.18433/jpps32105. [DOI] [PubMed] [Google Scholar]
- 12.López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19:A randomized clinical trial. JAMA. 2021;325:1426–35. doi: 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hector C, Roberto H. Safety and efficacy of the combined use of ivermectin, dexamethasone, enoxaparin and aspirin against COVID-19 –The I. D. E. A. Protocol. J Clin Trials. 2021;11:1–6. [Google Scholar]
- 14.Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter J-J. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019-The ICON study. CHEST. 2020. [[Last accessed on 2020 Dec 17]]. Available from: https://journal.chestnet.org/action/showPdf?pii=S0012-3692%2820%2934898-4 . [DOI] [PMC free article] [PubMed]
- 15.Hashim HA, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, Abdulamir AS. Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv. 2020.10.14. doi:10.1101/2020.10.26.20219345. [Google Scholar]
- 16.Niaee MS, Namdar P, Allami A, Zolghadr L, Javadi A, Karampour A, et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients:A randomized multi-center clinical trial. Asian Pac J Trop Med. 2021;14:266–73. [Google Scholar]
- 17.Gonzalez JLB, González Gámez M, Enciso EAM, Esparza-Maldonado RJ, Hernanez-Palacios D, Duenas-Campos S. Efficacy and safety of Ivermectin and Hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial. medRxiv. 2021 doi: 10.3390/idr14020020. doi:10.1101/2021.02.18.21252037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill A, Abdulamir A, Ahmed S, Asghar A, Babalola OE, Basri R, et al. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. Research Square. 2021 doi:10.21203/rs.3.rs-148845/v1. [Google Scholar]
- 19.Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, et al. Ivermectin for prevention and treatment of COVID-19 infection:A systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021;28:e434–60. doi: 10.1097/MJT.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karale S, Bansal V, Makadia J, Tayyeb M, Khan H, Ghanta SS, et al. A meta-analysis of mortality, need for ICU admission, use of mechanical ventilation and adverse effects with ivermectin use in COVID-19 patients. medRxiv. 2021 doi:10.1101/2021.04.30.21256415. [Google Scholar]
- 21.Hill A, Mirchandani M, Pilkington V. Ivermectin for COVID-19:Addressing potential bias and medical fraud. Open Forum Infect Dis. 2022;9:1–3. doi: 10.1093/ofid/ofab645. doi:10.1093/ofid/ofab645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamontagne F, Agoritsas T, Macdonald H, Leo Y, Diaz J, Agarwal A, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Health and Family Welfare (Government of India). Clinical Management Protocol: COVID-19 (Version 4), June 27, 2020. Available from: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19dated27062020.pdf .
- 24.Lim SCL, Hor CP, Tay KH. Effect of ivermectin on disease progression among adults with mild to moderate COVID-19 and comorbidities. JAMA Intern Med. 2022;182:426–35. doi: 10.1001/jamainternmed.2022.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peña-Silva R, Duffull SB, Steer AC, Jaramillo-Rincon SX, Gwee A, Zhu X. Pharmacokinetic considerations on the repurposing of ivermectin for treatment of COVID-19. Br J Clin Pharmacol. 2021;87:1589–90. doi: 10.1111/bcp.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42:1122–33. doi: 10.1177/009127002401382731. [DOI] [PubMed] [Google Scholar]
- 27.Ministry of Health and Family Welfare (Government of India) Clinical Management Protocol for COVID-19 (Version 6), May 24, 2021 [Google Scholar]


