Abstract
Genetic characterisation of circulating influenza viruses directs annual vaccine strain selection and mitigation of infection spread. We used next-generation sequencing to locally generate whole genomes from 116 A(H1N1)pdm09 and 118 A(H3N2) positive patient swabs collected across Uganda between 2010 and 2018. We recovered sequences from 92% (215/234) of the swabs, 90% (193/215) of which were whole genomes. The newly-generated sequences were genetically and phylogenetically compared to the WHO-recommended vaccines and other Africa strains sampled since 1994. Uganda strain hemagglutinin (n = 206), neuraminidase (n = 207), and matrix protein (MP, n = 213) sequences had 95.23–99.65%, 95.31–99.79%, and 95.46–100% amino acid similarity to the 2010–2020 season vaccines, respectively, with several mutated hemagglutinin antigenic, receptor binding, and N-linked glycosylation sites. Uganda influenza type-A virus strains sequenced before 2016 clustered uniquely while later strains mixed with other Africa and global strains. We are the first to report novel A(H1N1)pdm09 subclades 6B.1A.3, 6B.1A.5(a,b), and 6B.1A.6 (± T120A) that circulated in Eastern, Western, and Southern Africa in 2017–2019. Africa forms part of the global influenza ecology with high viral genetic diversity, progressive antigenic drift, and local transmissions. For a continent with inadequate health resources and where social distancing is unsustainable, vaccination is the best option. Hence, African stakeholders should prioritise routine genome sequencing and analysis to direct vaccine selection and virus control.
Subject terms: Evolution, Molecular evolution, Phylogenetics
Introduction
Novel influenza type-A viruses (IAVs) cause human respiratory infections that lead to social lockdowns, economic losses, and millions of deaths1. Genomic sequencing and characterisation of circulating IAVs are important to differentiate them from other viruses causing similar clinical symptoms for effective viral control and prevention. Seasonal influenza-related illnesses kill 290,000–650,000 people globally per year, mostly in sub-Saharan Africa2. Influenza accounts for 21.7% and 10.1% of the influenza-like illnesses (ILI) and severe acute respiratory illnesses (SARI) in Africa, respectively, and circulates all-year-round with discernible influenza peaks in North and South Africa3. Uganda's annual epidemics have two major peaks between May and November and usually constitute multiple IAV types and subtypes responsible for 13% and 6% of the ILI and SARI cases, respectively4.
Vaccination and antiviral treatment are the best ways to prevent and control viral transmission5. However, the multi-segmented IAVs continuously mutate, especially in the antigenic surface genes, hemagglutinin (HA) and neuraminidase (NA), giving rise to vaccine-escape and drug-resistant viruses6. Vaccine formulations for the Northern (NH) and Southern hemispheres (SH) are updated annually to match circulating viruses. Countries select appropriately licensed vaccines based on the genetic relatedness of their circulating viruses to the vaccines5.
Well-sampled and resource-rich countries have deep sequenced circulating IAVs using next-generation sequencing (NGS) and utilised advanced bioinformatics and phylogenetic analysis to collect sufficient data on IAV evolution patterns, drug sensitivity6, and emerging and circulating viral genetic clades7 for virus control and vaccine selection5. Influenza surveillance in Africa has improved substantially since the mid-2000s3,8,9. However, the limitation in financial resources and advanced phylogenomic analysis capacity restricts the genomic characterisation of IAVs in Africa. We systematically searched 7 databases: African Journals Online, Embase, Global Health, Google Scholar, PubMed, Scopus, and Web of Science for studies on genomic analysis of Africa IAVs. As of 30th July 2021, we found 16% (11/71) of the eligible studies sequenced and/or analyzed whole genomes (WGs) of Africa IAVs, and only 8% (5/62) of the studies that generated new viral sequences used NGS10. Only 3 Ugandan studies have sequenced WGs from 59 A(H3N2)11,12, and 19 A(H1N1)pdm0913 IAV strains sampled in 2008–2009 and 2009–2011, respectively, with sequencing conducted in the United States.
We aimed to explore the feasibility of using next-generation sequencing (NGS) in a resource-limited setting to generate WGs of Uganda IAVs sampled in 2010–2018, and compare the newly-generated sequences with vaccine strains and public Africa IAV sequences sampled in 1994–2019. We analysed the HA carrying antigenic sites which trigger host immune responses and the antiviral targets NA and matrix protein (MP). Further analysis of our newly-generated viruses for which we successfully assembled whole genomes showed evidence of intra-subtype reassortment events and reassortant A(H1N1)pmd09 and A(H3N2) strains circulating in Uganda14.
This work birthed an East African network of influenza molecular epidemiologists, which we hope to expand across Africa.
Results
Demographic characteristics of sampled patients
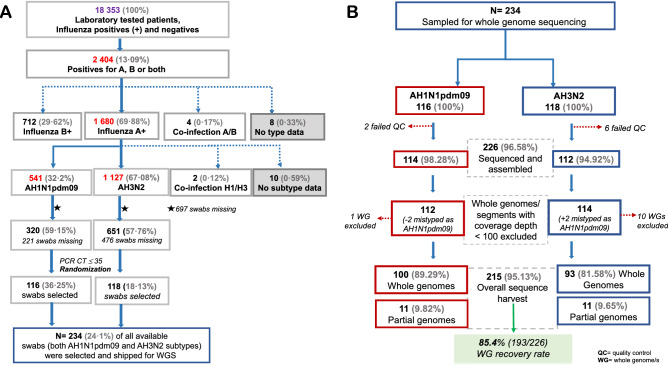
The Uganda Virus Research Institute National Influenza Centre (UVRI-NIC) laboratory tested 18,353 patients between 22nd October 2010 and 9th May 2018. Thirteen-percent (2404/18,353) were positive for influenza, 69.88% (1680/2404), 29.62% (712/2404), and 0.17% (4/2404) had influenza A, B, and A/B co-infection, respectively (Fig. 1A). IAV positives included 67.08% (1127/1680) A(H3N2), 32.2% (541/1680) A(H1N1)pdm09, and 0.12% (2/1680) AH1/H3 co-infections.
Figure 1.
Workflow of swab selection and whole genome recovery. (A) Shows how swabs were selected for influenza whole-genome sequencing (WGS). Patients diagnosed with either influenza subtypes A(H1N1)pdm09 or A(H3N2) and whose swab had a PCR CT ≤ 35 had their laboratory codes randomised based on the subtype and year of collection using the R software v3.6.3 (https://www.r-project.org). All available swabs were retrieved for years with less than fifteen swabs. The 697 swabs missing include some shipped to the Centers for Disease Control and Prevention (CDC) for routine surveillance and some lost due to an accidental failure of a freezer. The numbers are based on the UVRI-NIC laboratory dataset only, as of 9th May 2018. (B) Shows how viral samples were excluded before and after sequencing and the rate of whole genome recovery. Eight viruses [2 A(H1N1)pdm09 and 6 A(H3N2)] failed quality control (QC) before sequencing.
The mean number of swabs sampled per subtype per year was 13 (1–18), excluding 2012, 2016, and 2018 with 2, 1 A(H1N1)pdm09, and no A(H3N2) swab available, respectively (Supplementary Table 1). Three A(H1N1)pdm09 and one A(H3N2)] sampled patients’ swabs lacked demographic data. Of the 230 swabs with data, 65.22% (150/230) and 34.78% (80/230) were from ILI and SARI cases, respectively. The number of sequenced and un-sequenced swabs were not significantly different per case, gender, age group, and geographical region per year, except Central had more swabs sequenced than other regions in 2014 and 2016 (Table 1). The mean age was not significantly different between our study and the UVRI-NIC patients (Supplementary Fig. 1).
Table 1.
Comparison of demographic characteristics of influenza A positive patients sampled by the general UVRI-NIC surveillance programme whose viral swabs were successfully or not sequenced.
| Variable | Subgroup | Total [N] | Successfully Sequenced [n%] | Un-sequenced [n%] | P-value |
|---|---|---|---|---|---|
| Subtype | H1N1pdm09 | 588 | 106 [18.03] | 482 [81.97] | < 0.0001* |
| H3N2 | 1190 | 105 [8.82] | 1085 [91.18] | ||
| H1N1 | 29 | 0 [0] | 29 [100] | ||
| Case | ILI | 1196 | 139 [11.62] | 1057 [88.38] | 0.9809 |
| SARI | 611 | 72 [11.78] | 539 [88.22] | ||
| Gender | Male | 951 | 120 [12.62] | 831 [87.38] | 0.2149 |
| Female | 856 | 91 [10.63] | 765 [89.37] | ||
| Age [years] | 1 month to < 2 | 589 | 66 [11.21] | 523 [88.79] | 0.5851 |
| 2 to < 5 | 635 | 84 [13.23] | 551 [86.77] | ||
| 5 to < 15 | 338 | 39 [11.54] | 299 [88.46] | ||
| 15 to < 50 | 222 | 20 [9.01] | 202 [90.99] | ||
| 50 to < 65 | 15 | 2 [13.33] | 13 [86.67] | ||
| ≥ 65 | 7 | 0 [0] | 7 [100] | ||
| No age data | 1 | ||||
| Sample origin, by year | |||||
| 2010 | Central | 81 | 20 [24.69] | 61 [75.31] | 0.5986 |
| Northwest | 5 | 2 [40] | 3 [60] | ||
| 2011 | Central | 106 | 29 [27.36] | 77 [72.64] | 0.5451 |
| Eastern | 1 | 0 [0] | 1 [100] | ||
| Northwest | 9 | 4 [44.44] | 5 [55.56] | ||
| Western | 8 | 1 [12.5] | 7 [87.5] | ||
| 2012 | Central | 193 | 14 [7.25] | 179 [92.75] | 0.5272 |
| Eastern | 18 | 0 [0] | 18 [100] | ||
| Northwest | 21 | 2 [9.52] | 19 [90.48] | ||
| Western | 10 | 1 [10] | 9 [90] | ||
| 2013 | Central | 67 | 14 [20.9] | 53 [79.1] | 0.9346 |
| Eastern | 3 | 1 [33.33] | 2 [66.67] | ||
| Northwest | 10 | 2 [20] | 8 [80] | ||
| Western | 4 | 1 [25] | 3 [75] | ||
| 2014 | Central | 218 | 18 [8.26] | 200 [91.74] | 0.004* |
| Eastern | 46 | 0 [0] | 46 [100] | ||
| Northwest | 35 | 1 [2.86] | 34 [97.14] | ||
| Western | 61 | 11 [18.03] | 50 [81.97] | ||
| 2015 | Central | 192 | 18 [9.38] | 174 [90.62] | 0.6795 |
| Eastern | 42 | 6 [14.29] | 36 [85.71] | ||
| Northwest | 19 | 2 [10.53] | 17 [89.47] | ||
| Western | 39 | 5 [12.82] | 34 [87.18] | ||
| 2016 | Central | 139 | 13 [9.25] | 126 [90.65] | 0.0085* |
| Eastern | 6 | 3 [50] | 3 [50] | ||
| Northwest | 15 | 0 [0] | 15 [100] | ||
| Western | 21 | 0 [0] | 21 [100] | ||
| 2017 | Central | 271 | 28 [10.33] | 243 [89.67] | 0.3299 |
| Eastern | 22 | 0 [0] | 22 [100] | ||
| Northwest | 11 | 0 [0] | 11 [100] | ||
| Western | 35 | 4 [11.43] | 31 [88.57] | ||
| 2018 | Central | 33 | 11 [33.33] | 22 [99.67] | 0.0555 |
Initially, there were 1800 positives for a single influenza A subtype (H1, H1N1pdm09 and H3N2) in the UVRI-NIC dataset. After excluding one duplicated H1N1pdm09 viral sample (ARU0583), there remained 1799 positive A samples. We included 8 successfully sequenced samples that tested and were reported as positive in the laboratory dataset (used for sample randomization) but were reported as negative in the UVRI-NIC dataset. Therefore, the demographic characteristics presented in this table were for 1807 patients recorded in the UVRI-NIC dataset. 234 of the 1807 patient’s viral swabs were sampled for whole genome sequencing, and 215 of the 234 were successfully sequenced. However, four sequenced viruses (ARU0587, EBB2521, KIB0497, and KSW0631) lacked complete demographic data so we present data for only the 211 viruses that were successfully sequenced with complete data.
Percentage number of sequenced or un-sequenced swabs are in [italics]. P-values showing significant differences between compared groups (< 0.05) are indicated in bold with a star (*).
Sequencing efficiency
All 234 sampled swabs were analysed, and their mean read counts (ranges) are reported below.
The MiSeq generated 569,435 (806–1,644,430) paired reads per sample (data not shown). Following quality control (QC), 266,020 (70,229–909,340) clean reads per sample were processed using the Iterative Refinement Meta-Assembler (IRMA), 265,868 (70,150–908,946) passed IRMA’s QC, 213,809 (1381–908,234) matched flu references, and 113,164 (777–461,172) paired reads were assembled (Supplementary Fig. 2A).
The number of assembled reads decreased with an increase in gene size. The shortest, MP and non-structural protein (NS), had 25,101 (28–91,585) and 19,341 (41–79,167) assembled reads, respectively. The NA, HA, and nucleoprotein (NP) had 18,653 (74–71,377), 14,466 (35–74,550), and 14,151 (69–74,415) reads assembled, respectively. The polymerase subunits: PA, PB2, and PB1 had 10,465 (31–56,243), 7885 (15–47,444), and 4497 (8–31,354) reads assembled, respectively (Supplementary Fig. 2B).
We successfully sequenced and assembled viral genes from 96.58% (226/234) of the swabs (Fig. 1B). Eleven viral WGs with a depth of coverage < 100 were excluded, leaving 215 viruses. 89.77% (193/215) of these were WGs, spanning 100% and > 96.7% nucleotides in the coding sequences (CDS) and complete genome of A/California/7/2009(H1N1) and A/Perth/16/2009(H3N2) vaccine viruses. Our overall WG recovery rate was 85.4% (193/226). The remaining 10.23% (22/215) viruses had complete CDS for 2–7 genes. Two viruses sampled as A(H1N1)pdm09 matched IRMA’s A(H3N2) references and were included in the A(H3N2) analysis. All newly-generated 215 virus sequences were submitted in a publicly accessible database, GISAID EpiFlu™ (https://www.gisaid.org/), under accessions EPIISL498819–EPIISL498931 [A(H1N1pdm09)], and EPIISL498934–EPIISL499037 [A(H3N2)].
Antigenic drift among Uganda IAVs
Uganda IAV HA1 proteins continuously drifted away from the 2010–2020 vaccines (Supplementary Table 2). For seasons when formulations differed, Uganda A(H3N2) strains had 1–2 extra unique amino acid (aa) substitutions when compared to the Southern (SH) than the Northern hemisphere (NH) vaccine strains. Since Uganda’s largest part lies north of the equator, the substitutions described below are relative to NH and SNH vaccines (shared by NH and SH) for the sampled 2010–2018 [A(H1N1)pdm09] and 2010–2017 [A(H3N2)] seasons.
We observed 18 unique aa substitutions across the five antigenic sites15,16 amongst the 107 A(H1N1)pdm09 strains (Supplementary Table 2A). Ranking from the most variable, the main antigenic sites Ca2, Sa, Sb, Ca1, and Cb had 6, 5, 4, 2, and 1 unique aa substitutions, respectively. Substitution S164T, S185T, S203T, and H138R and S74R were the most frequent at site Sa, Sb, Ca1, and Ca2, respectively. All 2010–2016 viruses had S203T, and 90% (27/30) of the 2017–2018 viruses had S164T and S74R.
There were 92 unique aa substitutions across the five antigenic sites16,17 amongst the 99 A(H3N2) strains (Supplementary Table 2B). The antigenic sites B, A, D, C, and E had 24, 22, 17, 16, and 13 unique aa substitutions, respectively. Substitution K144N, P194L, H311Q, S96N, and K62E was the most frequent at site A, B, C, D, and E, respectively. Forty-seven percent (47/99) and 41.41% (41/99) of the 2010–2017 strains had V186G and N145S, respectively.
Uganda A(H1N1)pdm09 strains had mutated receptor binding sites (RBS, H138Q/R, S190V, and D222E) and S164T that alter the glycosylation motif at sites 162–1647. Uganda A(H3N2) strains had more aa substitutions affecting the RBS [130-loop (T135K, A/S138S/A, I/R140K, R140I), 150-loop (Q/H156H/Q), 190-helix (I192V, P194L, A196T, Q197H/R, A/S/A/P/S198S/A/P/S/P), 220-loop (N/D225D/N, F/Y219S)], and those creating (S45N, A128T, K160T) and removing [N45S, N122S/D, N144K/S, T/N128A, T135K] potential N-linked glycosylation sites (Supplementary Table 2B).
Subgroup analysis showed differences of 1–4 and 1–32 unique aa substitutions at antigenic sites of A(H1N1)pdm09 and A(H3N2) strains, respectively, sampled from different cases, gender, age groups, and geographical regions relative to each subtype vaccines (Supplementary Tables 3, 4).
Amino acid similarity of complete HA, NA, and MP protein sequences of Uganda IAVs to vaccine strains
The complete HA (H1), NA (N1), and MP protein sequences of Uganda A(H1N1)pdm09 strains had 94, 81, and 21 unique aa substitutions and a mean amino acid similarity of 98.09 (96.99–99.65%), 98.2 (96.8–99.79%), and 99.17 (97.73–100%), respectively, compared to A/California/7/2009(H1N1), A/Michigan/45/2015(H1N1), and A/Brisbane/02/2018(H1N1) vaccines (Supplementary Table 5). All N1 proteins lacked the neuraminidase inhibitors (NAIs) resistance substitution H275Y. However, 7.55% (8/106) had T362I (n = 1), I117M (n = 2), Y155H (n = 2), and V234I (n = 3) associated with reduced susceptibility to NAIs in vitro18.
The complete HA (H3), NA (N2), and MP protein sequences of Uganda A(H3N2) strains had 160, 118, and 31 unique aa substitutions, and mean amino acid similarity of 97.47 (95.23–99.29%), 97.64 (95.31–99.79%), and 99 (95.46–100%), respectively, compared to A/Perth/16/2009(H3N2), A/Victoria/361/2011(H3N2), A/Texas/50/2012(H3N2), A/Switzerland/9715293/2013(H3N2), A/Hong Kong/4801/2014(H3N2), A/Singapore/INFIMH-16-0019/2016(H3N2), A/Switzerland/8060/2017(H3N2), A/South Australia/34/2019(H3N2), and A/Kansas/14/2017(H3N2) vaccines (Supplementary Table 6). N2 proteins lacked the NAI-resistance H274Y (N2 numbering), but 18.8% (19/101) carried Y155F (n = 3), and E/D221D/K/E (n = 16) that reduce susceptibility to NAIs18.
All A(H1N1)pdm09 and A(H3N2) M2 proteins had the primary adamantine-resistance marker (S31N), and 7.7% (8/104) of A(H3N2) had secondary V27A relative to adamantine-susceptible A/New York/392/2004(H3N2) strain (Supplementary Fig. 3). All Uganda IAVs M2 proteins had aa substitutions (L3I, L4F, T5F, E6K, V7I, E8C, and T9R) fixed in their extracellular N-terminal, a region that supports M2-antibody interactions19.
The influenza surveillance webtool (FluSurver) identified aa substitutions in complete HA and NA proteins reported to alter host specificity and cause mild/strong drug resistance, respectively, and aa substitutions in both proteins that could potentially alter viral virulence, antigenic drift, glycosylation, and sites of interactions (Supplementary Tables 5, 6).
Temporal and spatial divergence of Uganda IAVs
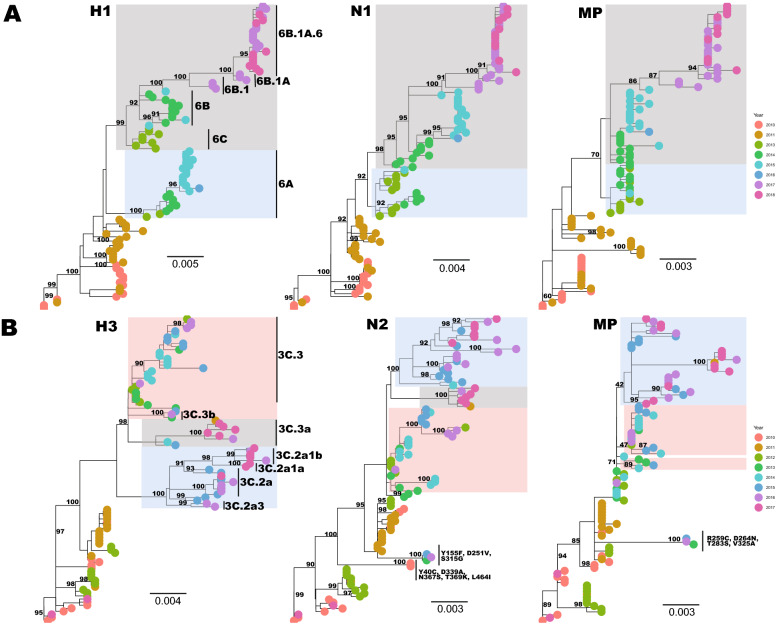
Uganda IAV strains phylogenetically clustered according to their year of sampling, with multiple lineages circulating annually (Fig. 2). Two major H1 lineages co-circulated; lineage 1 (shaded blue) with strains belonging to clade 6A and lineage 2 (shaded grey) with clade 6C, 6B, 6B.1, 6B.1A, 6B.1A.6 strains circulated in 2013–2016 and 2013–2018, respectively (Fig. 2A). The N1 and MP phylogenies showed similar lineages 1 and 2 emerged in 2013 and 2014, respectively, and lineage 2 dominated in 2016–2018.
Figure 2.
Phylogenies showing the temporal divergence of the HA, NA and MP genes of Uganda A(H1N1)pdm09 (A) and A(H3N2) (B) influenza viruses sampled from 2010 to 2018. Trees were rooted using the oldest sequence in the dataset. Shaded clusters are the two and three major co-circulating lineages observed since 2013 and 2012 for A(H1N1)pdm09 and A(H3N2) viruses, respectively. The third A(H3N2) lineage (with one 2011 and 2016–2017 viruses) disappeared in the MP phylogeny.
The dominant H3 lineage 1 (pink) contained clade 3C.3b and 3C.3 strains that circulated from 2013 to 2016. Lineages 2 (grey) had clade 3C.3a and lineage 3 (blue) had clade 3C.2a, 3C.2a3, and 3C.2a1(a, b) strains that emerged in 2014 and 2015, respectively, and co-circulated through 2017. We observed two long-branched clusters (bootstrap = 100%); cluster 1 (KSW0659 and KSW0643, sampled in 2010) and cluster 2 (TOR0492, TOR1664, and NSY0304, sampled in 2013–2016) in the N2 and MP phylogeny, with 3–5 unique amino acid (9–13 nucleotides) substitutions absent in other Uganda strains (Fig. 2B).
Virus strains sampled from different geographical sites mixed in all phylogenies (Supplementary Fig. 4).
Viral clades circulating in Uganda
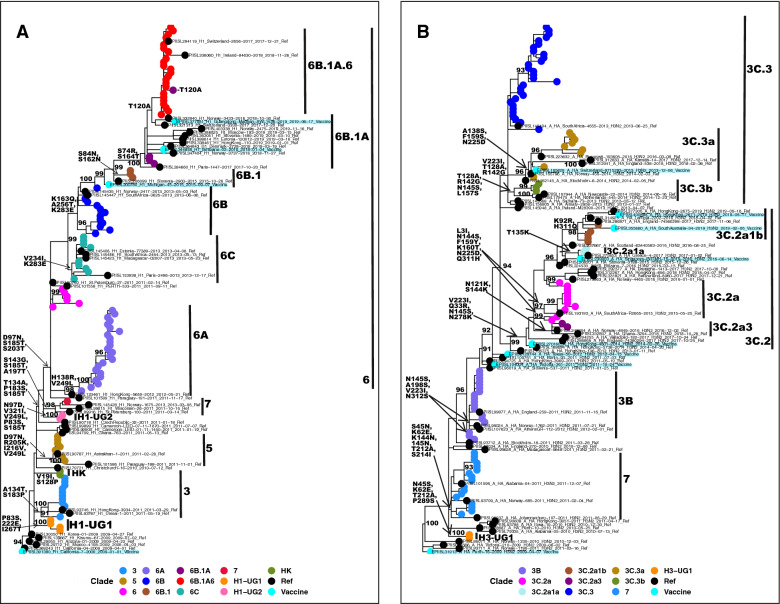
Uganda A(H1N1)pdm09 strains belonged to five global clades (A/Hong Kong/2212/2010(H1N1)-HK, 3, 5, 6, and 7) (Fig. 3A and Supplementary Fig. 5). The HK clade circulated in 2010 and had aa substitutions V19I, N97D, and S128P. Two novel clades H1-UG1 with P83S, D222E, and I267T, and H1-UG2 with T134A, P183S, and S185T, circulated in 2010 and 2011, respectively. Clade 3 had 2010–2011 strains with A134T and S183P. Clade 5 had 2011 strains with D97N, R205K, I216V, and V249L. One 2011 strain from Kisenyi clustered with A/St.Petersburgh/100/2011(H1N1) in clade 7 with A197T, S143G, and K163I. Clade 6 viruses with D97N, S185T, and S203T dominated since 2012 and diverged into 6A, 6B, and 6C. A novel subclade 6B.1A.6 with T120A emerged in November 2017 and dominated through 2018.
Figure 3.
Genetic clades of influenza A viruses that previously circulated in Uganda during the 2010–2018 seasons. All labelled clades (indicated by black bars) were inferred based on the signature amino acid substitutions in the HA1 protein indicated on the tree trunk in bold. (A) Shows clades for 2010–2018 A(H1N1)pdm09 viruses. Novel clades H1-UG1 and H1-UG2 are indicated. Genetic clade 6 diverged into 6A, 6B, and 6C. All clade 3, 5, and 7 viruses were collected from Entebbe and Kampala (Central Uganda) and circulated in 2010–2011. (B) Shows clades for 2010–2017 A(H3N2) viruses. A novel clade H3-UG1 is indicated. Clade 3 persisted in all 9 years. A similar figure with the full sequence names is provided in Supplementary Fig. 5.
Uganda A(H3N2) strains belonged to two global clades 3 and 7 (Fig. 3B) and co-circulated in 2010–2012. A novel clade H3-UG1 with L183H, T212A, S214I, and P289S circulated in 2010. The major clade 3 had strains with N145S and V223I and diverged into 3B, 3C.2, and 3C.3. New subclades 3C.2a1a with T135K and 3C.2a1b with K92R and H311Q dominated in May–June and May–November 2017, respectively.
Based on our dataset, similar clades circulated in Uganda and other African countries, except for A(H1N1)pdm09 clade 7, HK, and H1-UG2 (Supplementary Table 7). However, our recent systematic review showed that clade 7-like strains also circulated in Kenya, Tanzania, and South Africa between 2010 and 201210.
Phylogenetic relatedness of Africa IAVs
We define a group as a highly-supported phylogenetic cluster (bootstrap ≥ 90%) with at least three Uganda IAV nucleotide sequences.
Uganda A(H1N1)pdm09 strains collected before 2016 clustered uniquely towards the root, while the 2017–2018 strains mixed with Eastern, Central, Western, and Southern Africa strains (Supplementary Figs. 6–8). The H1, N1, and MP phylogeny contained 6, 5, and 1 group, with 50% (3/6), 60% (3/5), and 100% of the groups unique to Uganda, respectively (Supplementary Table 8A).
Uganda A(H3N2) strains collected in 2008–2016 and some before April 2017 clustered uniquely closest to the root (Supplementary Figs. 9–11). Notably, the 2008–2009 Makerere Walter Reed project (MWRP)11 and our newly-generated strain sequences clustered separately. The H3, N2, and MP phylogeny had 8, 10, and 2 groups, respectively, and 50% (4/8) H3 (circulated in different years) and 40% (4/10) N2 groups had only Uganda strains (Supplementary Table 8B).
If not clustered alone, Uganda strains grouped with strains from neighboring Kenya, Tanzania, Madagascar, and Congo. Interestingly, four unique H3 lineages (bootstrap ≥ 90%); Kenya (n = 1), Congo (n = 1), and West Africa (n = 2) co-circulated in 2019 (not shown). Virus group details are provided in Supplementary Table 8.
Discussion
Our study demonstrated the feasibility of NGS whole-genome sequencing of IAVs in a resource-limited setting. Analysis of the newly-generated HA, NA, and MP sequences highlighted a continuous antigenic drift, multiple introductions, and local transmission of A(H1N1)pdm09 and A(H3N2) viruses in Uganda. All Uganda strains lacked the neuraminidase inhibitors resistance marker (H275Y) but had the adamantine-resistance marker (S31N) in their MP proteins. Although we analyzed only the HA, NA, and MP, additional amino acid substitutions such as I38T/F/M in the endonuclease domain of the Polymerase PA protein have also been shown to give rise to antiviral resistance20–22. Africa IAVs were genetically similar, but unique viral lineages (bootstrap ≥ 90%) circulated and persisted in Uganda and other countries for 1–3 years.
We successfully recovered 96.58% (226/234) genomes and 85.4% (193/226) WGs directly from frozen human swabs. Our WG recovery rate is comparable to the 82–88% reported in developed countries23,24 and better than in Scotland (47.3%)25 and Mexico (66%)26. 10–20% of frozen swabs fail NGS due to RNA degradation during storage and pre-sequencing analysis. The shortest genes, MP and NS, were sequenced at a greater depth and the least for the polymerase segment 2 (PB1) as previously reported in Mexico and France23,26.
Sequence analysis showed most amino acid substitutions affected the antigenic sites Sa and Ca2 of A(H1N1)pdm09 and sites A and B of A(H3N2) viruses. Substitutions S164T (Sa) and S74R (Ca2) observed in 90% of Uganda A(H1N1)pdm09 strains also dominated in Kenya in 2017–201827. The globally frequent S203T at site Ca1 has no known function28. Five-percent (4/77) of the 2010–2016 Uganda A(H1N1)pdm09 strains had the RBS D222E, speculated to increase infection severity28. Amino acid substitutions in the H1 (P100S, S220T, I338V) and N1 (V106I, N248D) detected earlier in 2009–2011 among Uganda A(H1N1)pdm09 strains relative to A/California/7/2009(H1N1)13 persisted through 2017. All 2010–2013 Uganda A(H3N2) strains had mutated antigenic sites also observed in Kenya29. It would be interesting to do antigenic and phenotypic analysis to assess the effect of the observed aa substitutions on vaccine responses and viral characteristics (virulence, pathogenicity, and transmissibility), respectively.
Uganda IAV strains lacked the NA H274Y or H275Y substitutions confirming previous reports of 98% of global IAVs being sensitive to NAIs6. However, some N1 proteins carried permissive aa substitutions V234I, N369K, and V241I known to counteract the effect of H275Y. These aa substitutions enhance the NA surface expression and enzymatic activity, hence increasing viral fitness. Uganda IAVs NA active and catalytic sites30 were highly conserved indicating a lack of pharmacological selection pressure, which confirms the low NAIs intake in Uganda due to their privatization.
Uganda A(H1N1)pdm09 strains formed steeper ladder-like phylogenies than A(H3N2), which could result from sampling effects and/or directional selection due to immune escape31. Reassortment (gene exchange) within influenza viruses of the same subtype results in genetically unique strains which also affect the history and structure of the phylogenies32–34. Our recent whole-genome analysis using a Bayesian coalescent reassortant constant population model35 confirmed reassortment among Uganda A(H1N1)pmd09 (0.1237–0.4255) and A(H3N2) strains (0.00912–0.0355 events/lineage/year)14. Further whole-genome analysis to identify additional evolutionary processes driving the observed genetic variations is ongoing.
Phylogenetic analysis revealed the circulation of multiple viral lineages and clades in Uganda as observed worldwide7,11,36. New viral lineages and clades were observed in-between seasons indicating multiple viral introductions into Uganda per year. Novel subclades 6B.1A.6 [A(H1N1)pdm09] and 3C.2a1a and 3C. 2a1b [A(H3N2)] emerged in Uganda in 2017. Our detailed review of previous studies showed that clade 3C.3a emerged outside Africa in 2013, and later circulated in Burkina Faso, Ghana, Senegal, South Africa, Ethiopia, Tanzania, Madagascar, Cameroon, and Nigeria in 201410. Here, we show evidence that a clade 3C.3a-like strain (KSY0906_A_HA-H3_Uganda_UVRI_Kisenyi_003_2011-10-19) was first sampled in 2011 and similar strains circulated in 2014 through 2017 in Uganda. While its subclade 3C.3a1 continued to circulate outside Africa through 2021, making it the longest circulating H3 subclade10,37. Strikingly, we observed A(H1N1)pdm09 strains belonging to novel subclades 6B.1A.3, 6B.1A.5a, 6B.1A.5b, and 6B.1A.6 (± T120A) sampled during 2018, 2019, 2017–2018, and 2017–2018 seasons, respectively, in Eastern, Western, and Southern Africa (Supplementary Table 7), but were never reported before10. Uganda IAV strains from all geographical regions mixed, showing widespread local viral transmissions.
Uganda A(H1N1)pdm09 and A(H3N2) strains collected before 2015 and 2016, respectively, phylogenetically clustered distinctively from most Africa strains which could be due to insufficient viral sequencing in earlier years across Africa. Strains collected later were genetically similar to those from Eastern, Central, Southern, and Western Africa. Notably, all 2010–2018 Uganda strains clustered with global strains highlighting Uganda as part of the global influenza ecology.
The coronavirus disease 2019 (COVID-19) pandemic revealed virus exchange between Uganda and other countries through air and border travel. Currently, we are doing phylogeographic analysis on our newly-generated and public global IAV genomes to identify origins of viruses migrating to Uganda and Africa, and the continent's contribution to the global influenza migration network.
This is the first and largest study to sequence Uganda A(H1N1)pdm09 and A(H3N2) virus whole genomes (WGs) locally, and extensively describe the genetic diversity and evolution patterns of Africa IAVs since 1994. Our work expands on previous influenza sequencing and characterisation efforts of African researchers10 and highlights the feasibility of next-generation whole-genome sequencing for real-time disease surveillance and detection of emerging strains in Africa. Our newly-generated sequences add significantly to public Africa IAV genomes. However, we sequenced pre-collected swabs with a biased geographical sampling. Therefore, we did not consider geographical location in the swab randomisation.
Due to financial constraints, we sampled only 24.1% (234/971) of available swabs, 8.12% (19/234) of which failed quality control and NGS. Although we generated WGs, we analysed only the HA, NA, and MP genes from a few strains sequenced per case, gender, age group, and geographical region per year. Therefore, our results do not give a comprehensive description of the genetic diversity of Uganda and Africa IAVs.
Although NGS technologies can improve influenza surveillance in Africa, their implementation presents several challenges in analysing, reporting, and management of their big data. Fortunately, African researchers can leverage freely available automated pipelines38–40 for NGS data quality control, assembly, and variant calling for both segmented and non-segmented viruses. However, these pipelines are validated using sequences sampled from outside Africa. As shown here, genomic differences among strains sampled from different geographical regions and times cannot be ignored, hence African researchers will need to strengthen their existing Bioinformatics capacity to customize and/or develop region-specific tools.
Our study provides a platform for larger studies and highlights the potential of molecular surveillance to improve viral detection and disease management. Existing African surveillance programmes should prioritize routine sequencing and genome analysis to monitor circulating IAVs. Our findings will inform Uganda’s public health, use of NAIs prophylactic treatment, and decision to design vaccination programs, especially for high-risk groups like children, pregnant mothers, and the elderly.
Methods
Influenza surveillance design and source of swabs
The Uganda Virus Research Institute National Influenza Centre (UVRI-NIC) in Entebbe implements a clinic and hospital-based surveillance at thirteen peri-urban and densely populated sites in seven districts across Northwest, Western, Central, and Eastern Uganda (Supplementary Fig. 12)4.
Nasal and oropharyngeal swabs and demographic data were collected from ILI and SARI patients in 2010–20184. The swabs were tested, typed for influenza A and B, and the IAV positives subtyped for seasonal [A(H1N1) and A(H3N2)] and pandemic A(H1N1)pdm09 influenza using the Centers for Disease Control and Prevention’s (CDC) real-time reverse-transcription polymerase chain reaction (rRT-PCR) protocols and primers (Atlanta, Georgia)41.
Swab selection for whole-genome sequencing
We aimed to sequence 100 whole genomes (WGs) per subtype [A(H1N1)pdm09 and A(H3N2)] due to financial constraints. Available swabs from 971 IAV patients with real-time PCR cycle threshold (CT ≤ 35) were stratified by subtype and year of collection, randomised, and then selected every first fifteen swabs per strata. Twenty-four percent (234/971) of the swabs [116 A(H1N1)pdm09 and 118 A(H3N2)] were sequenced at a KEMRI-Wellcome Trust Programme collaborating laboratory in Kilifi, Kenya.
Viral RNA isolation and amplification
Viral ribonucleic acid (RNA) was extracted from 140 μL swab sample using the QIAamp Viral RNA Mini extraction kit and manufacturer’s protocol (Qiagen, Hilden, Germany). The RNA was reverse transcribed and the whole genome amplified using the multi-segment real-time polymerase chain reaction (M-RTPCR)24 and universal IAV Uni/Inf primers at standardised thermocycling conditions (Supplementary methods)36.
Next-generation sequencing
The M-RTPCR amplicon libraries were prepared using the Nextera XT DNA library preparation kit and protocol (Illumina, San Diego, California, USA), cleaned using the 0.8 × AMPure XP beads, quantified on the Qubit 3.0 fluorometer using the dsDNA High sensitivity kit (Invitrogen, Carlsbad, California, USA). Library size distributions were assessed using the Agilent Technology 2100 Bioanalyzer and the High Sensitivity DNA kit (Agilent Technologies, Santa Clara, California, USA). Samples with a broad fragment size spectrum (> 250 bp) were normalized manually to 2 nM. 5 μL per sample library were pooled, denatured using Sodium Hydroxide (NaOH), and diluted to 12.5 pmol. Diluted libraries were spiked with 5% Phi-X control (Illumina, San Diego, CA, USA) and sequenced using the Illumina MiSeq (Illumina Inc., San Diego, California, USA) generating 2 × 250 bp paired reads per sample.
We assessed sequencing efficiency based on the depth of coverage and number of gene segments recovered per swab.
Sequence quality control and assembly
Raw reads were de-duplicated and decontaminated, and clean reads assembled using the reference-based FLU module of the Iterative Refinement Meta-Assembler (IRMA) v0.6.742 at default settings (Supplementary methods). The A(H1N1)pdm09 and A(H3N2) virus assemblies were compared with A/California/7/2009(H1N1) and A/Perth/16/2009(H3N2) vaccine viruses, respectively.
The newly-generated sequences were deposited in the Global Initiative on Sharing All Influenza Data database (GISAID EpiFlu™, https://www.gisaid.org/) under accessions EPIISL498819-EPIISL498931 [A(H1N1)pdm09] and EPIISL498934-EPIISL499037 [A(H3N2)].
Genetic characterisation of surface and matrix proteins
Gene sequences for the Southern and Northern Hemispheres or both (SNH) vaccine and clade reference viruses per subtype were downloaded from the GISAID EpiFlu database43 (https://www.gisaid.org/; accessed on 26th February 2020). Uganda and vaccine virus sequences per subtype were aligned using MUSCLE v3.8.155144.
Amino acid substitutions in the major antigenic sites (A, B, C, D, and E) of A(H1N1)pdm0915 and A(H3N2)16 virus HA1 proteins were identified manually, while those in the complete HA, NA, and MP proteins and their functions were identified using the influenza surveillance (FluSurver) webtool (http://flusurver.bii.a-star.edu.sg; accessed on 24th September 2021) (Supplementary methods).
Phylogenetic analysis and clade classification
Uganda HA, NA, and MP gene sequences per subtype were aligned and maximum-likelihood trees reconstructed using IQtree v1.6.1145 with a GTR + G4 model and 1000 bootstraps. Trees were rooted using the oldest sequence in the dataset and visualized in ggtree v2.4.146 and Figtree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/).
Uganda, clade references, and vaccine virus sequences per subtype were aligned and maximum-likelihood trees reconstructed as above. Viral sequences were classified into genetic clades based on signature amino acid substitutions in their HA protein HA1 subunits7.
For Africa analysis, HA, NA, and MP gene sequences were downloaded from GISAID, accessed on 27th February 2020. Sequences with > 100 bps shorter or longer than the actual gene size and ambiguous “N” bases were excluded. The remaining A(H1N1)pdm09 (496 H1, 443 N1, and 278 MP) and A(H3N2) (718 H3, 675 N2, and 439 MP) sequences were aligned using a codon-aware aligner (https://github.com/veg/hyphy-analyses/tree/master/codon-msa) and maximum-likelihood trees reconstructed as above. Accessions for sequences analysed are provided in Supplementary Table 9.
Statistical analysis
We used the Chi-Square or Fisher’s Exact test (as appropriate) and the Wilcoxon rank-sum test in R v3.6.3 (https://www.r-project.org), to compare differences in the categorical and continuous patient demographics between successfully sequenced and un-sequenced swabs, respectively.
Ethics
This study was approved by the Makerere University School of Biomedical Sciences Research and Ethics Committee (SBS-REC) (ref: SBS-577) and the Uganda National Council of Science and Technology (UNCST) (ref: HS2519).
Our study was nested in the National Influenza Surveillance Programme at the Uganda Virus Research Institute (UVRI) where patients’ consent and assent were verbally obtained before sample collection. Since our study used archived patient clinical samples collected from the surveillance programme and presented “minimal risk” of harm to patients, we obtained a waiver of consent through the Makerere University SBS-REC. Permission to use archived samples was granted by Prof. Julius Lutwama, director of the Department of Arbovirology and Emerging and Re-Emerging Viral Infections at UVRI.
Supplementary Information
Acknowledgements
We extend our gratitude to the effort and contribution of the surveillance clinical officers and nurses at the sentinel sites, and the laboratory team at the Department of Arbovirology Emerging and Re-Emerging Infectious Diseases (UVRI) in sample and data collection, processing, archiving, and permitting us to use them. We gratefully acknowledge the authors from the originating laboratories responsible for obtaining the specimens and the submitting laboratories where genetic sequence data were generated and shared via the GISAID EpiFlu Initiative, on which this research is based. All submitters of data may be contacted directly via http://www.gisaid.org.
Author contributions
G.N. proposed the idea and designed the study with supervision from S.D.W.F., J.M.K., and D.P.K., N.O., J.T.K., and J.J.L. manage the UVRI-NIC influenza surveillance and provided both the samples and patient epidemiological data. D.C.O., Z.R.D., C.N.A., and D.J.N. hosted, provided protocol, and trained G.N. on laboratory and whole-genome sequencing procedures. G.N. analysed the data with guidance from S.D.W.F., interpreted results, and wrote the manuscript. G.N. with guidance from R.G. did the statistical analysis and interpretation. S.D.W.F. and D.J. guided on result presentation. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication, and G.N. and S.D.W.F. verified the underlying data. All authors revised and edited the manuscript’s preceding drafts and approved the final manuscript.
Funding
This work was supported by the Makerere University-Uganda Virus Research Institute Centre of Excellence for Infection and Immunity Research and Training (MUII) Programme. MUII is supported through the Developing Excellence in Leadership, Training and Science (DELTAS) Africa Initiative (Grant no. 107743). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS), Alliance for Accelerating Excellence in Science in Africa (AESA), and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (Grant no. 107743) and the UK Government. The work was also funded in part by a Wellcome Trust grant (102975).
Data availability
All sequences generated in this study with their respective metadata were submitted to GISAID EpiFlu (https://www.gisaid.org/) under accessions EPIISL498819–EPIISL498931 [A(H1N1pdm09)], and EPIISL498934–EPIISL499037 [A(H3N2)].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Simon D. W. Frost and Julius J. Lutwama.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-30667-z.
References
- 1.Taubenberger JK, Morens DM. Influenza: The once and future pandemic. Public Health Rep. 2010;125(Suppl 3):16–26. [PMC free article] [PubMed] [Google Scholar]
- 2.Iuliano AD, et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radin JM, et al. Influenza surveillance in 15 countries in Africa, 2006–2010. J. Infect. Dis. 2012;206(Suppl 1):S14–S21. doi: 10.1093/infdis/jis606. [DOI] [PubMed] [Google Scholar]
- 4.Lutwama JJ, et al. Clinic- and hospital-based sentinel influenza surveillance, Uganda 2007–2010. J. Infect. Dis. 2012;206(Suppl 1):S87–S93. doi: 10.1093/infdis/jis578. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Recommendations for influenza vaccine composition. https://www.who.int/teams/control-of-neglected-tropical-diseases/yaws/diagnosis-and-treatment/global-influenza-programme (2021).
- 6.Hussain M, Galvin HD, Haw TY, Nutsford AN, Husain M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017;10:121–134. doi: 10.2147/IDR.S105473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ECDC. Influenza Virus Characterisation Reports, summary Europe. European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/seasonal-influenza/surveillance-and-disease-data/influenza-virus-characterisation (2010).
- 8.Green A. Progress in influenza surveillance in Africa. Lancet Lond. Engl. 2018;391:1345–1346. doi: 10.1016/S0140-6736(18)30713-X. [DOI] [PubMed] [Google Scholar]
- 9.Igboh LS, et al. Influenza surveillance capacity improvements in Africa during 2011–2017. Influenza Other Respir. Viruses. 2021;15:495–505. doi: 10.1111/irv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabakooza G, Galiwango R, Frost SDW, Kateete DP, Kitayimbwa JM. Molecular epidemiology and evolutionary dynamics of human influenza type-A viruses in Africa: A systematic review. Microorganisms. 2022;10:900. doi: 10.3390/microorganisms10050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byarugaba DK, et al. Molecular epidemiology of influenza A/H3N2 viruses circulating in Uganda. PLoS One. 2011;6:e27803. doi: 10.1371/journal.pone.0027803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaira, B. B. Detection and characterization of human influenza A virus isolates from patients attending Kayunga and Mulago Hospitals in Uganda. Semantic Sch. MSc Thesis (2011).
- 13.Byarugaba DK, et al. Whole-genome analysis of influenza A(H1N1)pdm09 viruses isolated in Uganda from 2009 to 2011. Influenza Other Respir. Viruses. 2016;10:486–492. doi: 10.1111/irv.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabakooza G, et al. Whole-genome analysis to determine the rate and patterns of intra-subtype reassortment among influenza type-A viruses in Africa. Virus Evol. 2022;8:veac005. doi: 10.1093/ve/veac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. The antigenic structure of the influenza virus A/PR/8/ 34 hemagglutinin (H1 subtype) Cell. 1982;31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 16.Wiley DC, Skehel JJ. The structure and function of the haemagglutinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- 17.Lee M-S, Chen JS-E. Predicting antigenic variants of influenza A/H3N2 viruses. Emerg. Infect. Dis. 2004;10:1385–1390. doi: 10.3201/eid1008.040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir. Ther. 2012;17:159–173. doi: 10.3851/IMP2067. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Li H, Chen Y-H. N-terminus of M2 protein could induce antibodies with inhibitory activity against influenza virus replication. FEMS Immunol. Med. Microbiol. 2003;35:141–146. doi: 10.1016/S0928-8244(03)00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones JC, et al. Identification of the I38T PA substitution as a resistance marker for next-generation influenza virus endonuclease inhibitors. MBio. 2018;9:e00430-18. doi: 10.1128/mBio.00430-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden FG, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N. Engl. J. Med. 2018;379:913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- 22.Omoto S, et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci. Rep. 2018;8:9633. doi: 10.1038/s41598-018-27890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon B, et al. Whole genome sequencing of A(H3N2) influenza viruses reveals variants associated with severity during the 2016–2017 season. Viruses. 2019;11:108. doi: 10.3390/v11020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou B, et al. Multiplex reverse transcription-PCR for simultaneous surveillance of influenza A and B viruses. J. Clin. Microbiol. 2017;55:3492–3501. doi: 10.1128/JCM.00957-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein EJ, et al. Integrating patient and whole-genome sequencing data to provide insights into the epidemiology of seasonal influenza A(H3N2) viruses. Microb. Genom. 2018;4:e000137. doi: 10.1099/mgen.0.000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escalera-Zamudio M, et al. Efficient whole genome sequencing of influenza A viruses. bioRxiv. 2019 doi: 10.1101/749234. [DOI] [Google Scholar]
- 27.Opanda S, Bulimo W, Gachara G, Ekuttan C, Amukoye E. Assessing antigenic drift and phylogeny of influenza A (H1N1) pdm09 virus in Kenya using HA1 sub-unit of the hemagglutinin gene. PLoS One. 2020;15:e0228029. doi: 10.1371/journal.pone.0228029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goka EA, Vallely PJ, Mutton KJ, Klapper PE. Mutations associated with severity of the pandemic influenza A(H1N1)pdm09 in humans: A systematic review and meta-analysis of epidemiological evidence. Arch. Virol. 2014;159:3167–3183. doi: 10.1007/s00705-014-2179-z. [DOI] [PubMed] [Google Scholar]
- 29.Nyang’au EM, Bulimo WD, Mobegi V, Opanda S, Magiri E. Genetic analysis of HA1 domain of influenza A/H3N2 viruses isolated in Kenya during the 2007–2013 seasons reveal significant divergence from WHO-recommended vaccine strains. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;95:413–420. doi: 10.1016/j.ijid.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 30.McAuley JL, Gilbertson BP, Trifkovic S, Brown LE, McKimm-Breschkin JL. Influenza virus neuraminidase structure and functions. Front. Microbiol. 2019;10:39. doi: 10.3389/fmicb.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volz EM, Koelle K, Bedford T. Viral phylodynamics. PLoS Comput. Biol. 2013;9:e1002947. doi: 10.1371/journal.pcbi.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonsen L, et al. The genesis and spread of reassortment human influenza A/H3N2 viruses conferring adamantane resistance. Mol. Biol. Evol. 2007;24:1811–1820. doi: 10.1093/molbev/msm103. [DOI] [PubMed] [Google Scholar]
- 33.Nelson MI, et al. Multiple reassortment events in the evolutionary history of H1N1 influenza A virus since 1918. PLoS Pathog. 2008;4:e1000012. doi: 10.1371/journal.ppat.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rambaut A, et al. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;453:615–619. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller NF, Stolz U, Dudas G, Stadler T, Vaughan TG. Bayesian inference of reassortment networks reveals fitness benefits of reassortment in human influenza viruses. Proc. Natl. Acad. Sci. 2020;117:17104–17111. doi: 10.1073/pnas.1918304117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owuor DC, et al. Genetic characterization of influenza A(H3N2) viruses circulating in coastal Kenya, 2009–2017. Influenza Other Respir. Viruses. 2020;14:320–330. doi: 10.1111/irv.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadfield J, et al. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajami NJ, Wong MC, Ross MC, Lloyd RE, Petrosino JF. Maximal viral information recovery from sequence data using VirMAP. Nat. Commun. 2018;9:3205. doi: 10.1038/s41467-018-05658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vilsker M, et al. Genome detective: An automated system for virus identification from high-throughput sequencing data. Bioinformatics (Oxf., Engl.) 2019;35:871–873. doi: 10.1093/bioinformatics/bty695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner DD, et al. VPipe: An automated bioinformatics platform for assembly and management of viral next-generation sequencing data. Microbiol. Spectr. 2022;10:e0256421. doi: 10.1128/spectrum.02564-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.C.D.C. Protocol of real time RTPCR for influenza A(H1N1). The WHO Collaborating Centre for influenza at CDC. C.D.C.; 2009. [Google Scholar]
- 42.Shepard SS, et al. Viral deep sequencing needs an adaptive approach: IRMA, the iterative refinement meta-assembler. BMC Genom. 2016;17:708. doi: 10.1186/s12864-016-3030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shu Y, McCauley J. GISAID: Global initiative on sharing all influenza data—From vision to reality. Eurosurveillance. 2017;22:30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu G, Smith DK, Zhu H, Guan Y, Lam TT-Y. ggtree: An r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequences generated in this study with their respective metadata were submitted to GISAID EpiFlu (https://www.gisaid.org/) under accessions EPIISL498819–EPIISL498931 [A(H1N1pdm09)], and EPIISL498934–EPIISL499037 [A(H3N2)].