OBJECTIVES:
To investigate neurocognitive, psychosocial, and quality of life (QoL) outcomes in children with Multisystem Inflammatory Syndrome in Children (MIS-C) seen 3–6 months after PICU admission.
DESIGN:
National prospective cohort study March 2020 to November 2021.
SETTING:
Seven PICUs in the Netherlands.
PATIENTS:
Children with MIS-C (0–17 yr) admitted to a PICU.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Children and/or parents were seen median (interquartile range [IQR] 4 mo [3–5 mo]) after PICU admission. Testing included assessment of neurocognitive, psychosocial, and QoL outcomes with reference to Dutch pre–COVID-19 general population norms. Effect sizes (Hedges’ g) were used to indicate the strengths and clinical relevance of differences: 0.2 small, 0.5 medium, and 0.8 and above large. Of 69 children with MIS-C, 49 (median age 11.6 yr [IQR 9.3–15.6 yr]) attended follow-up. General intelligence and verbal memory scores were normal compared with population norms. Twenty-nine of the 49 followed-up (59%) underwent extensive testing with worse function in domains such as visual memory, g = 1.0 (95% CI, 0.6–1.4), sustained attention, g = 2.0 (95% CI 1.4–2.4), and planning, g = 0.5 (95% CI, 0.1–0.9). The children also had more emotional and behavioral problems, g = 0.4 (95% CI 0.1–0.7), and had lower QoL scores in domains such as physical functioning g = 1.3 (95% CI 0.9–1.6), school functioning g = 1.1 (95% CI 0.7–1.4), and increased fatigue g = 0.5 (95% CI 0.1–0.9) compared with population norms. Elevated risk for posttraumatic stress disorder (PTSD) was seen in 10 of 30 children (33%) with MIS-C. Last, in the 32 parents, no elevated risk for PTSD was found.
CONCLUSIONS:
Children with MIS-C requiring PICU admission had normal overall intelligence 4 months after PICU discharge. Nevertheless, these children reported more emotional and behavioral problems, more PTSD, and worse QoL compared with general population norms. In a subset undergoing more extensive testing, we also identified irregularities in neurocognitive functions. Whether these impairments are caused by the viral or inflammatory response, the PICU admission, or COVID-19 restrictions remains to be investigated.
Keywords: child, follow-up, multisystem inflammatory syndrome in children, pediatric intensive care unit, psychology
RESEARCH IN CONTEXT.
Eighty-five percent of hospitalized children with Multisystem Inflammatory Syndrome in Children (MIS-C) after COVID-19 require PICU admission and neurological involvement is frequently observed.
In the general PICU population, neurocognitive, psychosocial, and quality of life (QoL) impairments after PICU discharge make up the Postintensive Care Syndrome in Pediatrics (PICS-P).
We sought to examine the prevalence of PICS-P in children with MIS-C 4 months after PICU admission.
WHAT THIS STUDY MEANS.
Clinicians should be aware of the PICS-P following PICU discharge.
Even though severity of illness was relatively mild with short PICU stay, children with MIS-C experience impairments in neurocognitive, psychosocial, and QoL domains 4 months after PICU discharge.
Further investigation is required for determining whether these impairments in our MIS-C population are caused by factors such as the COVID-19 infection, inflammatory response, general consequence of PICU admission and treatment, or restrictions/isolation imposed during the COVID-19 pandemic.
COVID-19 resulted in a worldwide and worrying syndrome called “Multisystem Inflammatory Syndrome in Children (MIS-C),” which is characterized by severe inflammation in multiple organs, predominantly heart, blood vessels, and brain, with risk of multiple organ failure (1). Based upon this pathogenesis, there are major concerns about possible short- and long-term sequelae of MIS-C (2). In the Netherlands, during the pandemic, 58% of hospitalized children with MIS-C were admitted to the PICU (3).
So far, the few studies of outcomes of children with MIS-C have focused mainly on inflammation and the heart (4–7). However, since there is a high frequency of neurologic involvement at the time of presentation with MIS-C (4), the question of short- and long-term sequelae arises (8–10). In particular, we wondered whether those children admitted to PICU with MIS-C were at risk of subsequent neurocognitive, psychosocial, and quality of life (QoL) impairments. Therefore, the primary aim of the Dutch, national, prospective follow-up study was to undertake neurocognitive assessment 3–6 months after PICU admission for MIS-C. The secondary aims were to assess QoL outcomes in the children with MIS-C and psychosocial outcomes in both children and their parents.
MATERIAL AND METHODS
Study Design
This study is part of a larger prospective, observational cohort study in children with COVID-19, age 0–17 years old, who presented at the emergency or outpatient department, and/or were hospitalized in the Netherlands: the clinical features of COVID-19 in Pediatric Patients study (3). The institutional review board of Leiden-Den Haag-Delft (Medical Ethics Assessment Committee Leiden-Den Haag-Delft) reviewed and approved this follow-up study (N20.043). All parents, caregivers, and/or children greater than or equal to 12 years old provided written informed consent to participate in this study.
Study Participants
All children with MIS-C (0–17 yr) admitted to one of the seven Dutch PICUs between March 2020 and June 2021 were eligible. MIS-C was defined according to the World Health Organization definition (11). Exclusion criteria included the following: child’s age at follow-up greater than or equal to 18 years (post-PICU follow-up care does not extend past the age of 17 yr), residing abroad, or being unavailable for psychological follow-up. All eligible children were invited 3–6 months after PICU admission to the multidisciplinary follow-up program at the outpatient clinic in one of the seven university hospitals in the Netherlands (12) (see also additional information in the Supplemental File, http://links.lww.com/PCC/C309).
Follow-up consisted of extensive neurocognitive tests, use of validated psychosocial and QoL questionnaires in both children and their parents, and an interview by the pediatric intensivist who assessed the patient’s physical health status.
Demographics, Admission, and Follow-Up Variables
The following demographic and admission variables were prospectively collected: 1) baseline patient characteristics (i.e., sex, age at PICU admission, and body mass index); 2) comorbidities (i.e., predefined somatic or psychiatric disease; and 3) PICU admission variables (i.e., use of inotropes during admission, immunomodulation medication, respiratory support, extracorporeal membrane oxygenation, and length of stay on the PICU).
At follow-up, we collected the following data: 1) post-PICU use of medical or psychosocial care; 2) school days missed, attention problems, and subjectively experienced changes in exercise intolerance compared with pre-MIS-C; 3) eating and sleeping behavior; and 4) summary Pediatric Overall Performance Category Score and Pediatric Cerebral Performance Category Score (13). This information was collected during the pediatric intensivist interview in the follow-up clinic with both children and at least one of their parents (for interview questions, see eTable 1, http://links.lww.com/PCC/C309).
In addition, the primary outcome, neurocognitive outcome, and the secondary outcomes, psychosocial and QoL outcomes, were determined using validated, age-appropriate neurocognitive tests assessed by a psychologist and/or parent-reported and self-reported questionnaires with Dutch general population normative data (assessed before the COVID pandemic). For an extended description of the neuropsychological test battery, the questionnaires, and the informants (eTables 1 and 2 http://links.lww.com/PCC/C309).
Neurocognitive Outcomes
Neurocognitive outcomes in children with MIS-C included general intelligence (age-appropriate Wechsler scales), verbal memory (Rey auditory verbal learning test), and parent-reported executive functions (Behavior Rating Inventory of Executive Function [BRIEF]) for all participants (eTable 2, http://links.lww.com/PCC/C309). The following additional neurocognitive domains were tested in three of the seven centers: visuo-motor integration (Beery Developmental Test of Visual Motor Integration), visual memory (Rey-Osterrieth Complex Figure test), selective attention (Stroop Color Word Test), sustained attention (Bourdon Vos cancellation test), cognitive flexibility (Trail Making Test), strategy formation, and planning (both Behavioral Assessment of the Dysexecutive Syndrome in Children). Additionally, parent- and self-reported cognitive functions (Patient Reported Outcomes Measurement Information System [PROMIS]—Short Form V1.0—Cognitive Function) were assessed in this subsample.
Psychosocial and QoL Outcomes
The psychosocial outcomes assessed in children included posttraumatic stress (The Children’s Revised Impact of Event Scale) and emotional and behavioral problems (The Strengths and Difficulties Questionnaire) (eTable 1, http://links.lww.com/PCC/C309). The QoL outcomes evaluated in children included physical, emotional, social, and school functioning (Pediatric QoL Inventory), sleep (PROMIS Pediatric Short Form v1.0—Sleep-Related Impairment 8a), and fatigue (PROMIS Pediatric Short Form v2.0—Fatigue 10a).
The psychosocial outcomes assessed in parents of children with MIS-C were posttraumatic stress (posttraumatic stress disorder checklist for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), anxiety (PROMIS SF v1.0 – Anxiety 8a), depression (PROMIS SF v1.0—Depression 8b), and parental distress (Distress Thermometer for Parents).
Statistical Analyses
Analyses were performed with SPSS 28.0 for Windows (SPSS, Chicago, IL). Continuous variables are presented as mean (sd) or median (interquartile range, IQR), and categorical variables as number, proportions, and percentages. Baseline characteristics of the total MIS-C PICU population and children with MIS-C with follow-up data were compared using Mann-Whitney tests (continuous variables) and chi-square tests or Fisher’s exact tests (categorical variables). The p value for significance was taken as less than 0.05 (two-sided test), with no corrections for multiple comparisons since the work is exploratory and aimed at generating hypotheses for further, long-term studies.
Differences between neurocognitive test scores and questionnaire scores of children with MIS-C and general population norm data scores were tested with nonparametric or parametric one-sample t tests. Effect sizes (Hedges’ g) were reported to indicate the strengths and clinical relevance of the differences. Effect sizes were calculated by dividing the difference in mean scores between the groups by the pooled sd of both groups. Effect sizes were presented with 95% CIs. An effect size of 0.2 is considered small, an effect size of 0.5 is considered medium, and an effect size of 0.8 is considered large. The percentage of children with MIS-C with deviant scores is reported. In eTable 1 (http://links.lww.com/PCC/C309), all deviant scores per outcome are presented and explained.
RESULTS
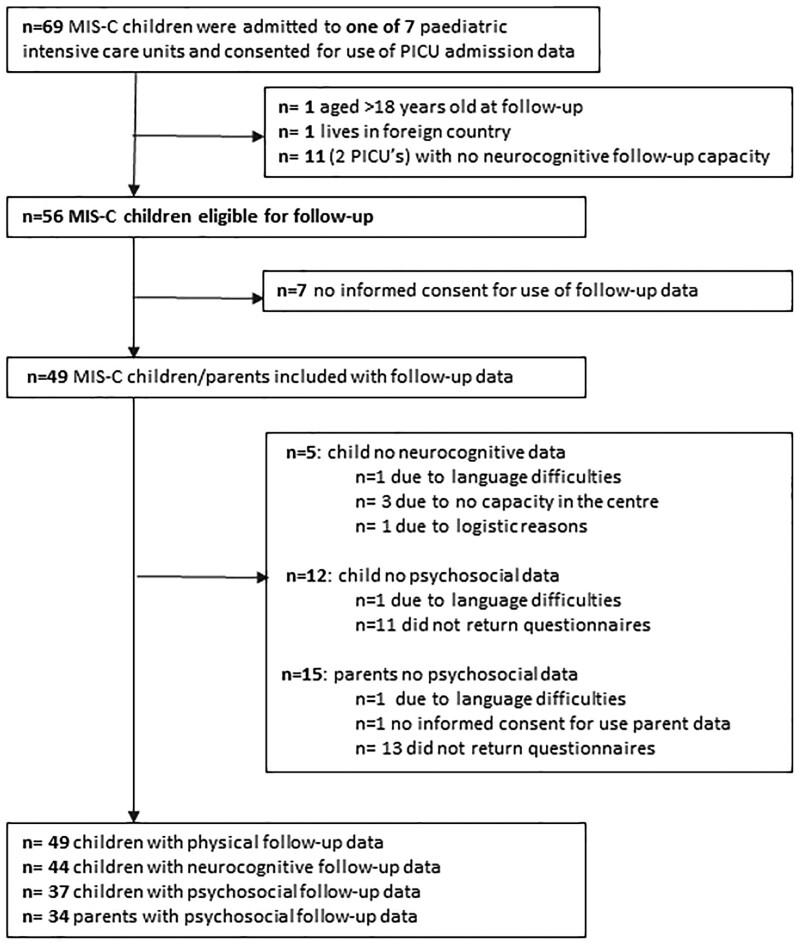
Between March 2020 and June 2021, 69 children with MIS-C were admitted to the seven participating Dutch PICUs (Table 1). Forty-nine of the children (82%) received inotropic support, and five (7%) required invasive ventilation. The median length of stay was 4 days (IQR 3–6 d). There were no deaths. Overall, of the 69 children with MIS-C, 49 were available, and the families agreed to the study (Fig. 1). Forty-four of these 49 children underwent neurocognitive testing and psychosocial assessment, with median age 11.6 years (IQR 9.3–14.4 yr) (full age range 3.7–17.2 yr). A subset of 29 children with MIS-C received an extensive neurocognitive test battery. Their age at follow-up and length of PICU stay were comparable with that of the total sample of 44.
TABLE 1.
Demographical and PICU Characteristics of Children With Multisystem Inflammatory Syndrome in Children
| Demographical and PICU Variables | All PICU MIS-C, N = 69 | Included PICU MIS-C, N = 49 |
|---|---|---|
| Age at admission, yr, median (IQR) | 11.2 (8.9–14.3) | 11.2 (9.0–13.8) |
| Sex male, n (%) | 41/67 (59) | 30/49 (61) |
| Body mass index, kg/m2, median (IQR) | n = 55; 17.8 (16.0–22.4) | n = 35; 17.7 (15.7–22.4) |
| Number of patients with comorbidities, n (%) | 22/57 (39) | 15/39 (38) |
| Asthma | 7 (12) | 5 (13) |
| Diabetes | 1 (2) | 1 (3) |
| Immunodeficiency | 2 (4) | 1 (3) |
| Cardiac disease | 0 (0) | 0 (0) |
| Other comorbidity | 12 (21) | 8 (21) |
| Inotropes during admission, n (%) | 49/60 (82) | 39/46 (85) |
| Immunomodulation medications, n (%) | ||
| Immunoglobines | 65 (94) | 47 (96) |
| Steroids | 46 (67) | 35 (71) |
| Aspirin/nonsteroidal anti-inflammatory drugs | 51 (74) | 35 (71) |
| Invasive respiratory support | 5 (7) | 2 (4) |
| Noninvasive respiratory supporta, n (%) | ||
| Low flow nasal cannulab | 28 (41) | 20 (41) |
| Non-rebreathing mask | 8 (12) | 7 (14) |
| High flow nasal cannula | 11 (16) | 8 (16) |
| Continuous positive airway pressure/bilevel positive airway pressure | 2 (3) | 0 (0) |
| No respiratory support, n (%) | 28 (41) | 21 (43) |
| Extracorporeal membrane oxygenation, n (%) | 1 (1) | 0 (0) |
| Length of PICU stay, median days (IQR) | 4.0 (3.0–5.5) | 4.0 (3.0–6.0) |
| Mortality, n (%) | 0 (0) | 0 (0) |
IQR = interquartile range; MIS-C = Multisystem Inflammatory Syndrome in Children.
Multiple options possible per patient.
Until maximum of 2 L/min.
Figure 1.
Patient flow. MIS-C = Multisystem Inflammatory Syndrome in Children.
Regarding PICU follow-up, all 49 children with MIS-C reported either none or mild overall disability at follow-up. Four children (8%) had neurological symptoms, including one child with small fiber polyneuropathy and the others with severe headaches. Compared with the pre-MIS-C state, 21 children (43%) reported subjective experience of impaired exercise tolerance, four children (8%) reported worse eating behavior, and 10 children (20%) had worse sleep behavior (eTable 3, http://links.lww.com/PCC/C309). Children with MIS-C missed a median 11 days (IQR 5–21.5 d) at school after PICU discharge.
Neurocognitive Outcomes
In the 44 children with neurocognitive testing, general intelligence scores, verbal memory scores, and parent-reported executive functions (BRIEF questionnaire) were comparable with the general population norms (Table 2).
TABLE 2.
Neurocognitive Outcomes in Children With Multisystem Inflammatory Syndrome in Children
| Outcome Variable | n | MIS-C, Mean (sd) | Test mean (sd) | p vs Norm | Effect Size (95% CI) | % MIS-C With Deviant Scorec |
|---|---|---|---|---|---|---|
| General intelligence index scoresa | ||||||
| Total intelligence quotientd | 44 | 99.0 (16.1) | 100 (15) | 0.70 | 0.1 (–0.2 to 0.4) | 18 |
| Verbal comprehension indexd | 43 | 99.8 (16.6) | 100 (15) | 0.93 | 0.0 (–03 to 0.3) | 28 |
| Visual spatial index | 26 | 94.5 (10.3) | 100 (15) | 0.02 | 0.4 (0.0–0.8) | 8 |
| Fluid reasoning index | 26 | 100.4 (13.9) | 100 (15) | 0.83 | 0.0 (–0.4 to 0.4) | 4 |
| Working memory index | 26 | 99.3 (12.2) | 100 (15) | 0.44 | 0.1 (–0.3 to 0.4) | 8 |
| Processing speed index | 26 | 99.0 (15.0) | 100 (15) | 0.54 | 0.1 (–0.3 to 0.5) | 15 |
| Cognitive competence index | 23 | 97.9 (13.3) | 100 (15) | 0.51 | 0.2 (–0.3 to 0.6) | 9 |
| Visuo-motor integrationa | 26 | 94.0 (13.7) | 100 (15) | 0.14 | 0.4 (0.0–0.8) | 19 |
| Memorya | ||||||
| Verbal memory, immediate | 38 | –0.05 (1.2) | 0 (1) | 0.81 | 0.1 (–0.4 to 0.3) | 26 |
| Verbal memory, delayed | 38 | –0.15 (1.4) | 0 (1) | 0.53 | 0.2 (–0.2 to 0.5) | 21 |
| Visual memory, immediate | 24 | –0.99 (1.3) | 0 (1) | < 0.001 | 1.0 (0.6–1.4) | 50 |
| Visual memory, delayed | 24 | –1.02 (1.3) | 0 (1) | < 0.001 | 1.0 (0.6–1.4) | 42 |
| Executive functionsa | ||||||
| Selective attention | 23 | 0.05 (1.3) | 0 (1) | 0.43 | 0.1 (–0.4 to 0.5) | 30 |
| Sustained attention, response time | 19 | –2.00 (2.0) | 0 (1) | < 0.001 | 2.0 (1.4–2.4) | 68 |
| Sustained attention, tempo fluctuations | 19 | –5.03 (5.1) | 0 (1) | < 0.001 | 3.7 (3.2–4.2) | 84 |
| Cognitive flexibility | 23 | –0.17 (0.7) | 0 (1) | 0.13 | 0.2 (–0.2 to 0.6) | 13 |
| Strategy formation | 26 | –0.14 (1.3) | 0 (1) | 0.29 | 0.1 (–0.3 to 0.5) | 19 |
| Planning deficits | 18 | –0.51 (0.9) | 0 (1) | 0.03 | 0.5 (–0.1 to 0.9) | 33 |
| Parent-reported cognitive and executive functionsc | ||||||
| Behavior regulation indexb | 31 | 46.5 (13.4) | 50 (10) | 0.15 | 0.4 (0.0–0.7) | 10 |
| Metacognition indexb | 29 | 45.1 (13.2) | 50 (10) | 0.06 | 0.5 (0.1–0.9) | 10 |
| Total Executive Function scoreb | 31 | 45.7 (14.0) | 50 (10) | 0.10 | 0.5 (0.1–0.8) | 6 |
| Patient Reported Outcomes Measurement Information System Cognitive Functionsa | 17 | 46.8 (5.8) | 50 (10) | 0.04 | 0.2 (–0.2 to 0.8) | 35 |
MIS-C = Multisystem Inflammatory Syndrome in Children.
Higher score = better functioning.
Lower score = better functioning.
See eTable 2 (http://links.lww.com/PCC/C309) for cut-off scores indicating deviant scores.
Mean total intelligence quotient of subset of n = 29 extensively tested children = 98.7 (sd 13.8); mean verbal comprehension index = 98.1 (sd 15.0).
In the subset of 29 more extensively tested children, we observed worse scores compared with norms for the following: visual spatial index with a medium effect size g = 0.4 (95% CI 0.0–0.8), immediate and delayed visual memory both with large effect sizes g = 1.0 (95% CI 0.6–1.4), sustained attention response time and tempo fluctuations both with large effect sizes g greater than 2.0, planning deficits with a medium effect size g = 0.5 (95% CI 0.6–1.4), and parent-reported cognitive function scores with a small effect size g = 0.2 (95% CI –0.2 to 0.8).
The discrepancy between overall age-adequate total intelligence and parent-reported executive function scores and significant deviant scores for neurocognitive domains such as visual memory, attention, and planning is illustrated in eFigure 1 (http://links.lww.com/PCC/C309). In addition, eFigure 1 (http://links.lww.com/PCC/C309) also shows all outcomes by individual neurocognitive domain scores, as exhibiting either age-adequate or age-deviant scores.
Psychosocial and QoL Outcomes
Parents reported more emotional and behavioral problems in the children with MIS-C with a small effect size g = 0.4 (95% CI –0.1 to 0.7) compared with our general population norms (Table 3). The children reported emotional and behavioral problems that were comparable with the prevalence observed in population norms (Table 3). However, regarding prosocial behavior (i.e., a child’s ability to get along well with peers), compared with normative data, the children had significantly better scores (eTable 4, http://links.lww.com/PCC/C309). Eight of 37 parents (22%) reported an elevated risk for posttraumatic stress disorder (PTSD) in their child with MIS-C. Ten of 30 children (33%) reported an elevated risk for PTSD in themselves. One of 32 parents (3%) reported an elevated risk for PTSD in themselves and less depressive symptoms compared with general population norms were reported (Table 3).
TABLE 3.
Psychosocial Outcomes in Children With Multisystem Inflammatory Syndrome in Children and Their Parents
| Outcome Variable | n | MIS-C | n | Norm Data | p | Effect Size (95% CI) | % MIS-C With Deviant Scoreb |
|---|---|---|---|---|---|---|---|
| Psychosocial outcomes in children with MIS-C | |||||||
| Posttraumatic stressa | |||||||
| Total score (parent-reported) | 36 | 17.4 (15.7) | 37 | 20.2 (14.1)c | 0.30 | 0.2 (–0.3 to 0.6) | |
| Elevated risk for PTSD (parent-reported) | 37 | 22% | 16%c | ||||
| Total score (self-reported) | 29 | 20.4 (18.0) | 214 | 19.5 (13.1)c | 0.78 | 0.1 (–0.3 to 0.5) | |
| Elevated risk for PTSD (self-reported) | 30 | 33% | |||||
| Emotional and behavioral problemsa | |||||||
| Total difficulties (parent-reported) | 36 | 0.4 (1.0) | 1947 | 0 (1)d | 0.04 | 0.4 (–0.1 to 0.7) | 33 |
| Total difficulties (self-reported) | 20 | 10.0 (5.6) | 437 | 8.1 (4.8)e | 0.33 | 0.4 (–0.1 to 0.8) | 25 |
| Psychosocial outcomes in parents of children with MIS-C | |||||||
| Posttraumatic stressa | |||||||
| PTSD checklist for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, indication PTSD | 32 | 3% | 7%e | ||||
| Anxiety and depressiona | |||||||
| Anxiety symptoms | 34 | 49.8 (9.1) | 1002 | 49.9 (10.1)e | 0.88 | 0.0 (–0.3 to 0.4) | 29 |
| Depressive symptoms | 34 | 46.6 (9.7) | 1002 | 49.6 (10.0)e | 0.03 | 0.3 (0.0–0.6) | 24 |
| Parental distressa | |||||||
| Overall distress | 29 | 2.6 (3.2) | 1421 | 3.4 (2.7)e | 0.17 | 0.3 (–0.1 to 0.7) | 24 |
MIS-C = Multisystem Inflammatory Syndrome in Children, PTSD = posttraumatic stress disorder.
Higher score = worse (more problems or symptoms).
See eTable 1 (http://links.lww.com/PCC/C309) for norm data sample explanation and cut-off scores indicating deviant scores.
Norm population is trauma-exposed children and adolescents.
z scores based on specific sex/age groups from the Dutch general population.
Norm population is the Dutch general population.
See eTable 4 (http://links.lww.com/PCC/C309) for the posttraumatic stress and emotional and behavioral subscale scores of children with MIS-C.
Regarding QoL, children with MIS-C reported significantly worse physical functioning with a large effect size g = 1.3 (95% CI 0.9–1.6), worse school functioning with a large effect size g = 1.1 (95% CI 0.7–1.4), and increased fatigue with a medium effect size g = 0.5 (95% CI 0.1–0.9) compared with general population norms (Table 4).
TABLE 4.
Quality of Life in Children With Multisystem Inflammatory Syndrome in Children
| Outcome Variable | n | MIS-C | n | Norm Data | p | Effect Size (95% CI) | % MIS-C With Deviant Scored |
|---|---|---|---|---|---|---|---|
| Health-related quality of life | |||||||
| Physical functioning, mean z score (sd)a | 36 | –1.3 (1.8) | 966 | 0 (1)c | < 0.001 | 1.3 (0.9–1.6) | 50 |
| Emotional functioning, mean z score (sd)a | 36 | –0.4 (1.3) | 966 | 0 (1) | 0.07 | 0.4 (0.1–0.7) | 25 |
| Social functioning, mean z score (sd)a | 36 | –0.1 (1.2) | 966 | 0 (1) | 0.50 | 0.1 (–0.2 to 0.4) | 14 |
| School functioning, mean z score (sd)a | 36 | –1.1 (1.7) | 966 | 0 (1) | < 0.001 | 1.1 (0.7–1.4) | 47 |
| Patient Reported Outcomes Measurement Information System short forms | |||||||
| Fatigue, mean T score (sd)b | 26 | 45.9 (11.7) | 527 | 39.8 (12.1) | 0.01 | 0.5 (0.1–0.9) | 46 |
| Sleep-related impairment, mean T score (sd)b | 24 | 49.9 (10.1) | 527 | 47.5 (10.0) | 0.26 | 0.2 (–0.6 to 0.2) | 50 |
MIS-C = Multisystem Inflammatory Syndrome in Children.
Lower scores = worse quality of life (QoL).
Higher scores = worse QoL scores (more sleep impairments and/or fatigue).
z scores norms for parent-reported (5–7 yr) and self-reported (8–17 yr) based on Dutch general population.
See eTable 1 (http://links.lww.com/PCC/C309) for norm data sample explanation and cut-off scores indicating deviant scores.
DISCUSSION
In this Dutch, nationwide, prospective follow-up study of children with MIS-C (and their parents), we have focused on the short-term neurocognitive, psychosocial, and QoL outcomes in those previously admitted to the PICU. In comparison with general follow-up of PICU cohorts, our population of MIS-C cases were infrequently invasively ventilated, and the main reason for acute admission was use of vasopressors.
At follow-up, between 3 and 6 months later, we had three main findings in our MIS-C cohort of children and parents. First, we failed to find any evidence of affected general intelligence scores or verbal memory scores, but, instead, there was evidence of subtle irregularities in visual memory and executive functions. Second, parents also reported that their child had emotional and behavioral problems. Last, these children had worse QoL (e.g., worse school and physical functioning and increased fatigue) and an increased risk for PTSD compared with population normative data. The short-term impact of the PICU admission of the child on parents appeared to be minor.
Neurocognitive Outcomes
In this study, the objectively tested general intelligence and verbal memory outcomes in children with MIS-C were comparable with the Dutch normative data. Interestingly, these age-adequate full scale intelligence scores in children with MIS-C contrast with the lower intelligence scores reported in recent meta-analyses in the general PICU population (14–16). We also found that the parent-reported executive function scores (BRIEF questionnaire) were comparable with the general Dutch population, which, again, contrasts with the previously reported worse parent-reported executive functions in the general PICU population (17). An explanation for the current MIS-C cohort with better overall cognitive function might be the short length of stay in the PICU during acute illness. In the general PICU population, prolonged length of stay on the PICU is associated with worse cognitive functioning (14, 16).
When unraveling neurocognitive functioning in a more extensively tested sample of children with MIS-C, deviations from the normative data with medium to large effect sizes were found in specific neurocognitive domains, including visual spatial intelligence, visual memory, sustained attention, and planning skills. More in depth, general intelligence represents a reliable predictor for global intellectual functioning, and the normal verbal comprehension scores indicate a normally developed application of knowledge involving verbal concept formation, reasoning, and expression. In contrast, our children with MIS-C scored worse than the expected normative data on visual spatial index, which means that their ability to evaluate visual details and to understand visual spatial relationships seems to be affected. Moreover, their normal working memory abilities for processing visual and verbal information are in contrast with their affected delayed visual memory abilities. It is unknown whether these neurocognitive irregularities might be the result of neurological involvement as part of MIS-C or the critical illness or some general association with treatments used in the PICU. The latter two reasons have been described as factors in PICS-P (18).
In children with MIS-C, the impaired delayed visual memory might have led to the worse functional experience at school (i.e., as assessed by paying attention in class, remembering things, keeping up with schoolwork) and cognitive dysfunction. Alternatively, worse school and cognitive function could be secondary to school closures and social restrictions during (partial) lockdowns and curfews as part of the COVID-19 pandemic regulations in the Netherlands during the period of our study (19).
Psychosocial Outcomes
One in three parents reported emotional and behavioral problems in their child with MIS-C. This prevalence is similar to the reported range (22–29%) in the general PICU population (15). Also for the elevated risk for PTSD, the 33% prevalence in the children with MIS-C group was comparable with the prevalence of 10–30% PTSD reported in a meta-analysis of general PICU population studies (15).
In a review regarding worse psychologic and psychiatric outcomes after PICU admission, septic illness and longer length of PICU stay have been found to be independent predictors (20). Comparable with MIS-C, sepsis involves different inflammatory, immunologic, hormonal, and metabolic pathways, and the systemic inflammation in the brain might therefore be identical. On the other hand, our observed short length of PICU stay contradicts our findings of worse psychosocial outcomes in children with MIS-C, which are comparable with those reported in the general PICU population. One explanation might be that the continuously stressful and unpredictable nature of life during the COVID-19 pandemic combined with cerebral effects of the systemic inflammation might be responsible for these psychosocial impairments. Especially since previous stressful life events and post-PICU stress responses are predictors for emotional and behavioral problems and PTSD in children after PICU admission (21, 22).
Surprisingly, parents reported comparable risk for PTSD to our general population norms. Although MIS-C was an unknown acute syndrome requiring PICU admission of their child, only 3% of the parents exhibited an elevated risk for subclinical PTSD. This is remarkably low compared with previous studies in which 24–30% of parents of critically ill children were at risk for subclinical PTSD (21). A possible explanation is the media attention for MIS-C. Parents might have felt more supported (in and outside the hospital), which stimulated their natural recovery. On the other hand, longer term follow-up might reveal delayed reactions since an acute PICU admission of a child is a known risk factor for long-term psychosocial problems in parents (21).
QoL Outcomes
In accordance with general PICU population studies, at follow-up, children with MIS-C had worse physical QoL than general population norm. In the general PICU population, lower physical QoL has been associated with older age, longer length of stay on the PICU, and increased disease severity (14, 15). Regarding children with MIS-C, the length of PICU stay was short, and as reported in other cohort studies, the cardiac function normalized in most MIS-C patients (4, 6). Nevertheless, the worse experienced physical functioning and increased fatigue reported in our study were also found in other follow-up studies of physical capacity of children with MIS-C. Penner et al (4) showed that children with MIS-C experienced severe physical functioning despite normalization of biochemical and inflammation markers at follow-up. Capone et al (6) reported persistent fatigue with regular activities in children at 8 weeks of follow-up despite normalized myocardial function. Possible causes for the experience of worse physical functioning might therefore be related to the pathogenesis of MIS-C (severe multiple organ inflammation), treatment during PICU admission, or activity restrictions after hospital discharge due to the cardiac involvement. Moreover, the social restrictions of the COVID-19 pandemic with limited opportunities for physical activity for all children might also have hampered children with MIS-C to be physically active, thereby resulting in physical sequelae (22).
Taking all the above together, the main strengths of this study are the type of follow-up carried out (i.e., national, standardized, and prospective), the low risk of selection bias, and the extensive neurocognitive, psychological, and QoL test battery. However, not all our centers could carry out detailed testing. One potential limitation is that we did not have a non-MIS-C COVID-19 pandemic control group. Therefore, our normative general population data might not reflect what was normal during the pandemic. Also, we did not use validated exercise capacity tests such as the 6-minute walk tests along with follow-up lung function testing in our assessment of physical activity and function—these should be considered in the future.
CONCLUSIONS
In this national follow-up study of children admitted to the PICU with MIS-C during the COVID-19 pandemic, we have assessed neurocognitive, psychosocial, and QoL outcomes 3–6 months after admission. Overall, general intelligence in children with MIS-C was normal. However, these children experienced worse school functioning. Some neurocognitive function irregularities were found. These children experienced more emotional and behavioral problems and an elevated risk for PTSD compared with the corresponding norm populations. It is unknown whether this is the effect of the social restrictions during the COVID pandemic or the direct result of MIS-C and/or the PICU admission as described within the PICS-P framework. This report indicates the need for longer term follow-up of these children with inclusion of a control group.
ACKNOWLEDGMENTS
We thank the children and their parents participating in this study. We acknowledge the work of the psychologists from all university centers, in particular Dr. N. Tamis who tested half of the Multisystem Inflammatory Syndrome in Children cohort. We also acknowledge the help of Dr. M. Königs, Prof. Dr. J. Oosterlaan, and J. Dunk. We also thank all COVID-19 in Pediatric Patients study researchers: Erasmus MC: Miriam Mooij, Rianne Oostenbrink, Pieter Fraaij; AUMC: Simone Hashimoto, Caroline Brackel, Mariken Gruppen, Taco Kuijpers, Merlijn vd Berg, Martijn vd Kuip, Suzanne Terheggen-Lagro; Maastricht UMC+: Manouk van der Steen, Michiel Bannier; Leids Universitair Medisch Centrum: Gertjan Lugthart, David Slotboom, Anne Verbeek, Danielle Brinkman, Petra Hissink Muller, Erik von Asmuth; Universitair Medisch Centrum Groningen: Liesbeth Scholvinck, Wineke Armbrust, Elizabeth Legger; Albert Schweitzer Ziekenhuis: Ankie Lebon, Radboud UMC: Koen van Aerde, Ronald Petru, Saskia de Wildt; Amphia Ziekenhuis: Sanne Hammer; Máxima MC: Lonneke van Onzenoort; Zuyderland Medisch Centrum: Han Hendriks; Isala: Jolita Bekhof; Spaarne Gasthuis: Marlies van Houten; Bernhoven: Jan van der Linden; Franciscus Gasthuis & Vlietland: Gerdien Tramper; Maasstad Ziekenhuis: Michael Groeneweg, Xandra van den Tweel; Hagaziekenhuis (Juliana kinderziekenhuis): Esther Peeters, Denise Rook, Mirjam van Veen; HMC: Jantien Bolt; Groene Hart Ziekenhuis: Helma van Gameren; Meander Medisch Centrum: Margot Ernst-Kruis; UMC Utrecht: Joanne Wildenbeest, Joris van Montfrans, Tom Wolfs, Bas Vastert; Alrijne Ziekenhuis: Anjali Kooter-Bechan; Martini Ziekenhuis: Arvid Kamps; Bravis ziekenhuis: Stephanie de Crom, Christiaan van Woerden; Catharina Ziekenhuis: Carien Miedema; Prinses Máxima Centrum voor Kinderoncologie: Wim Tissing; Slingeland Ziekenhuis: Monique Jacobs, Elisabeth-TweeStedenziekenhuis: Charlie Obihara; Gelre Ziekenhuizen: Annemarie Oudshoorn; St Jansdal Ziekenhuis: Annette Vernooij; Canisius-Wilhelmina Ziekenhuis: Ingeborg Barts; Dijklander Ziekenhuis: Yolande Thomasse; Ommelander Ziekenhuis Groningen: Bettina Auffarth; ZorgSaam Ziekenhuis: Joyce Goris; BovenIJ Ziekenhuis: Venje Boonstra; Curacao Medical Center: Lindy Janssen, Shirley Lo-A-Njoe; Deventer Ziekenhuis: Jenneke Homan-van der Veen; Elkerliek Ziekenhuis: Marianne Faber, Mijke Breukels; Zaans Medisch Centrum: Maarten Rijpert, Leontien van der Aa; and Tergooi ziekenhuizen: Karin Miedema.
Supplementary Material
Footnotes
*See also p. 341.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Dr. Buddingh’s institution received funding from Leids Universitair Fonds/ Bontiusstichting and ZonMw. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Drs. Otten and Buysse contributed equally as cofirst authors.
REFERENCES
- 1.McArdle AJ, Vito O, Patel H, et al. ; BATS Consortium: Treatment of multisystem inflammatory syndrome in children. N Engl J Med 2021; 385:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou SHY, Beghi E, Helbok R, et al. : Global incidence of neurological manifestations among patients hospitalized with COVID-19-A report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open 2021; 4:e2112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buddingh EP, Mooij MG, von Asmuth EGJ: COPP Studie LUMC - Landelijk COVID-19 onderzoek bij kinderen, 2020. Available at: https://www.covidkids.nl/. Accessed January 9, 2023
- 4.Penner J, Abdel-Mannan O, Grant K, et al. ; GOSH PIMS-TS MDT Group: 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: A retrospective cohort study. Lancet Child Adolesc Health 2021; 5:473–482 [DOI] [PubMed] [Google Scholar]
- 5.Farooqi KM, Chan A, Weller RJ, et al. : Longitudinal outcomes for multisystem inflammatory syndrome in children. Pediatrics 2021; 148:e2021051155. [DOI] [PubMed] [Google Scholar]
- 6.Capone CA, Misra N, Ganigara M, et al. : Six month follow-up of patients with multi-system inflammatory syndrome in children. Pediatrics 2021; 148:e2021050973. [DOI] [PubMed] [Google Scholar]
- 7.Davies P, du Pre P, Lillie J, et al. : One-year outcomes of critical care patients post-COVID-19 multisystem inflammatory syndrome in children. JAMA Pediatr 2021; 175:1281–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaRovere KL, Riggs BJ, Poussaint TY, et al. ; Overcoming COVID-19 Investigators: Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol 2021; 78:536–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JE, Asfour A, Sewell TB, et al. : Neurological issues in children with COVID-19. Neurosci Lett 2021; 743:135567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schober ME, Pavia AT, Bohnsack JF: Neurologic manifestations of COVID-19 in children: Emerging pathophysiologic insights. Pediatr Crit Care Med 2021; 22:655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman S, Godfred-Cato S, Gorman R, et al. : Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19: World Health Organization, 2020. Available at: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed January 9, 2023
- 12.Buysse C, Knoester H: Richtlijn follow-up van kinderen naopname op een intensive care, Versie 1.0 2017. Available at: https://www.nvk.nl/themas/kwaliteit/richtlijnen/richtlijn?componentid=6881283&tagtitles=Intensive%2BCare. Accessed January 9, 2023
- 13.Jayaram N, McNally B, Tang F, et al. : Survival after out-of-hospital cardiac arrest in children. J Am Heart Assoc 2015; 4:e002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hordijk JA, Verbruggen SC, Buysse CM, et al. : Neurocognitive functioning and health-related quality of life of children after pediatric intensive care admission: A systematic review. Qual Life Res 2022; 31:2601–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko MSM, Poh PF, Heng KYC, et al. : Assessment of long-term psychological outcomes after pediatric intensive care unit admission: A systematic review and meta-analysis. JAMA Pediatr 2022; 176:e215767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Sonnaville ESV, Knigs M, van Leijden O, et al. : Intelligence outcome of pediatric intensive care unit survivors: A systematic meta-analysis and meta-regression. BMC Med 2022; 20:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verstraete S, Verbruggen SC, Hordijk JA, et al. : Long-term developmental effects of withholding parenteral nutrition for 1 week in the paediatric intensive care unit: A 2-year follow-up of the PEPaNIC international, randomised, controlled trial. Lancet Respir Med 2019; 7:141–153 [DOI] [PubMed] [Google Scholar]
- 18.Watson RS, Choong K, Colville G, et al. : Life after critical illness in children-toward an understanding of pediatric post-intensive care syndrome. J Pediatr 2018; 198:16–24 [DOI] [PubMed] [Google Scholar]
- 19.Panda PK, Gupta J, Chowdhury SR, et al. : Psychological and behavioral impact of lockdown and quarantine measures for COVID-19 pandemic on children, adolescents and caregivers: A systematic review and meta-analysis. J Trop Pediatr 2021; 67:fmaa122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes-Junior LC, Rosa M, Lima RAG: Psychological and psychiatric outcomes following PICU admission: A systematic review of cohort studies. Pediatr Crit Care Med 2018; 19:e58–e67 [DOI] [PubMed] [Google Scholar]
- 21.Bronner MB, Peek N, Knoester H, et al. : Course and predictors of posttraumatic stress disorder in parents after pediatric intensive care treatment of their child. J Pediatr Psychol 2010; 35:966–974 [DOI] [PubMed] [Google Scholar]
- 22.Guan H, Okely AD, Aguilar-Farias N, et al. : Promoting healthy movement behaviours among children during the COVID-19 pandemic. Lancet Child Adolesc Health 2020; 4:416–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.