ABSTRACT
The rapid increase in antibiotic resistance presents a dire situation necessitating the need for alternative therapeutic agents. Among the current alternative therapies, phage therapy (PT) is promising. This review extensively summarizes preclinical PT approaches in various in-vivo models. PT has been evaluated in several recent clinical trials. However, there are still several unanswered concerns due to a lack of appropriate regulation and pharmacokinetic data regarding the application of phages in human therapeutic procedures. In this review, we also presented the current state of PT and considered how animal models can be used to adapt these therapies for humans. The development of realistic solutions to circumvent these constraints is critical for advancing this technology.
KEYWORDS: Phage therapy, animal infection model, antimicrobial resistance, ESKAPE Pathogens, Endolysin
Introduction
Bacteriophages (phages) are natural predators of bacteria that can recognize, attack, and kill bacterial hosts without harming other bacteria or human cells. Since their independent discovery by Frederick W. Twort (1915) and Félix d’Hérelle (1917), bacteriophages have been essential in various microbiological discoveries, especially in microbial genetics. Bacteriophages, the natural predators of bacteria, have recently been discovered to be effective in modern biotechnology. They’ve been recommended as antibiotic options for numerous antibiotic-resistant bacterial strains. Phages can potentially be exploited as biocontrol agents in agriculture and the petroleum sector.
Furthermore, phages are employed as vehicles for DNA and protein vaccines, detecting pathogenic bacterial strains, and as a display system for numerous proteins and antibodies.1 Phages have played an important role in understanding several essential principles in molecular biology since their discovery in the early twentieth century. They were crucial model organisms in searching for the physical nature and function of gene, beginning with Max Delbrück’s establishment of the American Phage Working Group and extending to the explication of Francis Crick’s central dogma of molecular biology through studies of RNA transcription and protein expression in phage. Phages have been widely used in biotechnology and illuminating fundamental molecular biology concepts. Phage biology provides a wealth of recombinant DNA technologies, clinical diagnostics, and synthetic biology techniques.2
Antimicrobial resistance and phage therapy
In 2017, the World Health Organization (WHO) issued a list of 12 antibiotic-resistant priority pathogenic bacteria that threaten human health and need immediate attention. These bacteria, including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species (collectively termed ESKAPE), can cause life-threatening diseases. The absence of effective antibiotics makes public health threat causing exacerbating healthcare problems. Alternative strategies to conventional treatment are needed to curb the global threat of antibiotic resistance.3–6 One potential alternative strategy is using bacteriophages to prevent and treat antibiotic-resistant pathogens, owing to their high specificity and killing ability.7 Bacteriophages are the natural enemies of bacteria that can specifically infect and kill the host bacteria. To date, no adverse effects of bacteriophages have been reported, making them a safer therapeutic option for clinical use. The safety of phage therapy justifies its use in treating several bacterial infections.8,9
The increasing multidrug-resistant bacterial infections have spurred interest in phage therapy among physicians and scientists, and some pharma companies are also involved in phage therapy research. Phage therapy centers and recent clinical trials have produced positive results in treating patients, such as the Eliava institute in the Republic of Georgia, Military Hospitals in Belgium, and Hospitals in Wroclaw, Russia, Novosibirsk, and Poland.
Bacteriophage treatment may influence the immune-inflammatory response to bacterial infection, reduction in C-reactive protein level, and leukocyte counts, with a similar propensity of the erythrocyte-sedimentation rate that can be one of the most promising aspects of phage therapy. Unlike antibiotics, in phage therapy, repeated doses of bacteriophages are not required because bacteriophages remain in the body for longer, and their multiplication depends on the host bacterium. Notably, fewer doses of bacteriophages are needed because the number of bacteriophages increases at the infection site due to their proliferation in the bacterial host. From an immunological point of view, phage therapy is safer because bacteriophages surround our ecosystem; therefore, humans have routine exposure to bacteriophages in day-to-day life. Though bacteriophages are used as therapeutic agents in different parts of the world without any reported adverse effects, the safety of phage therapy applications needs to be evaluated.10–12 In numerous animal studies, phage therapy is secure.13–15 Uchiyama et al. found that administering mice with repeated intraperitoneal phage injections seven times per day every four days for two months had no noticeable clinical effects.13 Mice were intraperitoneally injected with phages, and neither the mice’s health nor the main organs of the mice displayed any abnormal histological changes.16 These results confirm the safety of phages.17–20
Another main concern about the safety and efficacy of phage therapy is the innate and adaptive immune response against bacteriophages. Although there are no reports of life-threatening anaphylaxis (immune) reactions during phage therapy treatment, it has been reported that immune responses could neutralize bacteriophages by producing antibodies against them. However, bacteriophage neutralization does not mean there will be a complete treatment failure. With the available sequencing technologies, bacteriophage selection and genetically-modified bacteriophages can tackle these problems. Moreover, tailored delivery systems such as lipid vesicles and nanoparticles can help bacteriophages to be sustainably released in the in-vivo environment without eliciting any immune response and neutralizing antibodies.21–27
For therapeutic applications, tailed bacteriophages (dsDNA viruses) are preferred and confined to a single order of Caudovirales. These tailed bacteriophages are diverse and are the most abundant viruses in nature. As per the recent International Committee on Taxonomy of Viruses (ICTV) classification, the order Caudovirales are further divided into 14 families, 73 subfamilies, 927 genera, and 2,814 species (https://talk.ictvonline.org/taxonomy/). All these tailed phages have a head or capsid with a dsDNA molecule; a tail with or without tail fibers. During bacterial contact, the phage makes specific contacts with surface receptors using the tail or tail fibers or both.
Bacteriophage interventions in treating Gram-positive infections
Gram-positive bacteria such as staphylococci (MRSA; Methicillin-Resistant Staphylococcus aureus), streptococci (DRSP; Drug-Resistant Streptococcus pneumoniae), and enterococci (VRE; Vancomycin-Resistant Enterococci) are prevalent and cause life-threatening infections in humans. The diseases caused by these bacteria range from mild to severe such as food poisoning to skin and soft tissue infections, endocarditis, pneumonia, osteomyelitis, sepsis, and septic shock. Some other diseases include hospital-acquired infections caused by S. aureus, ventilator-associated pneumonia caused by S. aureus, community-acquired pneumonia caused by S. pneumoniae, catheter-related infections, and urinary tract infections caused by enterococci. Given their clinical importance, antibiotic resistance among Gram-positive bacteria is worrisome. Thus, using phages to treat infections of these bacteria is a welcome intervention. Interestingly, phages targeting many Gram-positive bacteria have been isolated. Some of these are discussed below.
Staphylococcus bacteriophages
Studies on Staphylococcus-infecting bacteriophages started in the 1970s, but the in-depth molecular characterization began in the early 21st century. The increasing Staphylococcus phage studies can be attributed to the emerging staphylococcal infections that are antibiotic-resistant, in which case phage therapy is an immediate alternative. Most of the characterized Staphylococcus phages belong to the Siphoviridae family, and most of the characterized lytic phages have mutations in their lysogenic gene. As staphylococcal strains or variants are varied, a bacteriophage cocktail with broad infectivity is favorable for applications. Bacteriophage cocktails have no reported adverse effects when administered orally, topically, intranasally, intravenously, or subcutaneously. Interestingly, it has been found that many Staphylococcus phages can exhibit broad-host-range activity, infecting at least 20 strains.28–30 A study by Peng et al. 2019 showed that phages ϕMR001 and ϕSA012 could infect 101/104 and 76/104 healthcare- and community-associated MRSA strains, respectively. A study by Kishor et al.31 showed that a phage cocktail containing seven bacteriophages at 1012 PFU/ml could cure osteomyelitis in rabbits. They further reported that in the chronic group periosteal reactions, arthritis persisted, but the wound was healed, and the infection site was sterile, as shown by radiological features. A study by Leiman et al.32 showed that AB-SA01, a Staphylococcus phage cocktail containing three Myoviridae phages, could kill 94.5% of 401 clinical S. aureus in vitro. In vivo studies in mice showed that AB-SA01 had efficacy in curing acute lung infections, and no phage-resistant mutants were observed. The same AB-SA01 bacteriophage cocktail was used in human trials (first-in-humans, phase 1) by Ooi et al.,19 in which the safety and tolerability were tested in 9 patients by administering intranasally. The nine patients with recalcitrant chronic rhino sinusitis responded well to the treatment, and AB-SA01 was found to be safe up to doses of 3 × 109 PFU for 14 days, at which time 2/9 patients had eradicated S. aureus infection. In another study, Staphylococcus phages vB_Sau_Clo6, vB_Sau_CG, and K were found to infect 88%, 96%, and 86% of MRSA (n = 47) strains tested, respectively.33 Staphylococcus bacteriophage preparations are commercially available for treatment, including (I) AB-SA01 by AmpliPhi Biosciences Corp. The US, (II) Staphylococcal bacteriophage by Eliava Institute, Georgia, (III) PhagoBurn and Phosa by Pherecydes pharma, France.19–36
Streptococcus bacteriophages
Streptococcus phages are one of the least studied bacteriophages, only representing about 5% of phage genomes in the NCBI nucleotide database. Though the number of studies performed using animal models is few, we will review some of the characterized Streptococcus bacteriophages. The two pneumophages Dp-1 and Cp-1 are one of the first lytic phages isolated against S. pneumoniae that belongs to Siphoviridae and Podoviridae, respectively. The pneumoniae phage MS1 was isolated from the upper respiratory tract of the infected patient, and it was found to be closely related to Dp-1. The lytic bacteriophage A25 is a well-studied bacteriophage isolated against S. pyogenes. The novel bacteriophage, J × 01, was found to infect S. agalactiae and belongs to the family Siphoviridae. The phage M102AD was found to infect S mutants which belong to Siphoviridae and are closely related M102 phage. All the known Streptococcus thermophilus bacteriophages belong to the Siphoviridae family, but their applications are still under exploration. There is a void in Streptococcus phage research, and the number of animal studies is also scarce. Considering the infections caused by Streptococcal species in humans, there is a scope for future bacteriophage therapy research.37–42
Enterococcus bacteriophages
The enterococcal phages are mostly bound within the two species, Enterococcus faecalis and E. faecium. The characterized lytic bacteriophages infecting E. faecalis, such as ϕEF24C (Myoviridae), EFRM31 (Siphoviridae), and EFAP1 (Siphoviridae), are known to have a short lifecycle and in-vitro efficacy. The siphovirus vB_EfaS_AL3 infects E. faecalis with a genome of about 40kb. The Enterococcus bacteriophage, vB_EfaS_HEf13 infecting E. faecalis, was found to reduce the bacterial load in the human dentin ex vivo infection model. The efficacy of two Enterococci bacteriophages, vB_EfaS-Zip infecting E. faecium and vB_EfaP-Max infecting E. faecalis, was tested in in vitro collagen wound model (CWM), and it was found that the phage cocktail was effective in removing multi-species biofilms. In another study, a bacteriophage cocktail (EFDG1 AND EFDG1r) produced an additive effect against VRE E. faecalis strain. As the root canal treatment is getting tedious due to VRE E. faecalis infections, these enterococcal phages such as EFDG1, phiEF24C, IME-EF1, and EFLK1 can be used alone or as cocktails to prevent recurrent E. faecalis infections.43–47
Bacillus and Listeria bacteriophages
Other bacteriophages infecting Gram-positive bacteria include Bacillus bacteriophages and Listeria bacteriophages. The characterized Bacillus bacteriophage phi29 is one of the smallest known dsDNA podovirus. The three bacteriophages, Negev_SA, Carmel_SA, and Tavor_SA, have been shown to be highly effective against B. anthracis. A Myoviridae phage vB_BceM-HSE3 was found to reduce B. cereus infections in in-vitro models. A Listeria phage P100 was found to control Listeria monocytogenes contamination in food products. There is excellent potential for bacteriophage therapy as a therapeutic candidate against Gram-positive bacterial infections. The growing antibiotic crisis has caused the opening of phage therapy, as the drug-resistant infections caused by MRSA, DRSP, and VRE are life-threatening.48,49 Table 1 describes the recent bacteriophage-based preclinical studies performed to treat Gram-positive bacterial infections.
Table 1.
Animal studies to evaluate bacteriophages’ efficacy in treating Gram-positive bacterial infections.
| Target bacteria | Bacteriophage preparation | Model organism | Outcome | References |
|---|---|---|---|---|
| S. aureus | Five Myoviridae bacteriophages at 109 PFU/ml | Peri-prosthetic joint infections in rats |
|
50 |
| S. aureus | Two Myoviridae bacteriophages and encapsulated |
Soft-tissue infections in rats |
|
51 |
| S. aureus | One Myoviridae and one Podoviridae bacteriophage at 109 PFU/ml | Mastitis in mice |
|
52 |
| S. aureus | Two bacteriophages at 109 PFU/ml | Abscesses in mice |
|
53 |
| S. aureus | Seven bacteriophages at 1012 PFU/ml | Acute and chronic osteomyelitis in rabbits |
|
31 |
| S. aureus | Three Myoviridae phages (AB-SA01) at 108 PFU/ml | Mice |
|
35 |
| S. aureus | One Siphoviridae bacteriophage at MOI of 0.1 | Bacteremia in mice |
|
54 |
| S. aureus | One Myoviridae bacteriophage at 1010 PFU/ml | Mice |
|
55 |
| S. aureus | One phage at 1010 PFU/ml | Septicemia in mice |
|
56 |
| S. pneumoniae | Phage SP-SQ1 at 109 PFU/ml | Pneumococcal infections in mice |
|
57 |
| E. faecalis | Two bacteriophages (poloxamer) at 109 PFU/ml | Root canal infections in rat |
|
58 |
| E. faecalis | One bacteriophage at 1010 PFU/ml | Human dentin ex vivo model |
|
45 |
| E. faecalis | Two bacteriophages at 108 PFU/ml | Peritonitis mice model |
|
59 |
Bacteriophage interventions in treating Gram-negative infections
Most common pathogenic Gram-negative bacteria include E. coli, K. pneumoniae, E. cloacae, other Enterobacterials, P. aeruginosa and A. baumannii, which can cause deadly infections and mortality in humans. These bacteria cause severe local and systemic infections such as urinary tract infections, bacteremia, sepsis, bloodstream infections, respiratory tract infections, and other life-threatening nosocomial infections. The use of antibiotics in the treatment of Gram-negative infections is failing because of the intrinsic and acquired resistance toward commonly used antibiotics. The common antibiotic-resistance bacteria are carbapenem-resistant Klebsiella, carbapenem-resistant Acinetobacter, colistin-resistant E. coli and multidrug-resistant Pseudomonas.60 To overcome the bacterial resistance problem, alternative therapies are being prescribed, mainly phage therapy and a combination of bacteriophages and antibiotics. Several animal studies have been performed to establish the potential of bacteriophages in treating infections caused by Gram-negative pathogens.
Pseudomonas bacteriophages
Controlled phage therapy can cure drug-resistant infections in the post-antibiotic era. A study performed by Shiley et al. using an in vitro human lung model (Pseudomonas lung infection) showed antimicrobial activity. It increased interleukin 6 (IL-6) and tumor necrosis factor (TNF) production, demonstrating the antimicrobial efficacy as well as the immunological response of the phage therapy.61 P. aeruginosa is known to cause infections by forming biofilms. It has been proven that some phages can penetrate pseudomonal biofilms, which is one of the advantages of conventional therapy.62–64 A single dose of a virulent bacteriophage vB PaeP-SaPL rescues bacteremic mice infected with multidrug-resistant P. aeruginosa.65 In mice, the lung infection model of P. aeruginosa study performed by Pabary et al. showed that phages were effective in controlling the spreading of infection and can also decrease the bacterial load post-treatment.66 The combination of antibiotic-phage therapy was used to cure the relapsing periprosthetic joint infection of the knee and chronic osteomyelitis caused by MDR P. aeruginosa. The eradication of Pseudomonas infection was observed with no side effects.67 When phage OMKO1 was used with ceftazidime to treat a chronic P. aeruginosa infection of an aortic Dacron graft, a single application appeared to resolve the infection with no signs of recurrence. Other recent studies also demonstrate that bacteriophages are excellent candidates for treating P. aeruginosa infections.67–69
Acinetobacter bacteriophages
As an ESKAPE pathogen, nosocomial infections caused by MDR Acinetobacter are tedious to cure in healthcare settings. Animal studies in wound infection models of A. baumannii showed that phages, either alone or in cocktails, could reduce the bacterial load and recovery of mice/rats even with a single dose.70,71 The mice lung infection model of carbapenem-resistant A. baumannii found that after intranasal injection of bacteriophages, the mouse recovered from lethal A. baumannii infections.72 A study by Zhou et al. showed that two Myoviridae phages could recover the larvae (G. mellonella) from infections caused by carbapenem-resistant A. baumannii.73 Acinetobacter phage Abp1 was found to recover infected mice from pan-drug resistant AB, and the cytotoxicity studies showed no detectable toxicity in HeLa or THP-1 cells.74 Human phage therapy trials successfully treated A. baumannii pancreatic pseudocyst infection, indicating that the intravenous administration of phages could recover patients from A. baumannii infections.75
Other Enterobacteriaceae phages
Enterobacteriaceae includes the deadliest pathogens, and the WHO priority list contains both E. coli and K. pneumoniae at the highest rank among antibiotic-resistant bacteria. Studies showed that a single dose of bacteriophage could cure K. pneumoniae in a murine burn wound infection model.76 The phage cocktail prepared using three phages infecting E. coli, K. pneumoniae, and Enterobacter was found to cure multiple bacterial infections in a Galleria larvae model.77 In the mice lung infection model of K. pneumoniae, Cao et al. demonstrated that the administration of bacteriophages intranasally could decrease the bacterial load in the lungs and increase the survival of mice in a dose-dependent manner.78 Dufour et al. conducted a study in a mouse infection model of E. coli and administered ceftriaxone and phages separately after a two-hour post-bacterial injection. It was found that the phage-treated mice showed a 100% survival rate and demonstrated that bacteriophages have better efficacy than antibiotics in reducing the bacterial load and increasing the survival rate.79 Bacteriophage cocktails have been gaining more attention recently because of their ability to target multiple strains, species, or genera. A recent study using bacteriophages infecting E. coli, K. pneumoniae, Haemophilus influenzae, P. aeruginosa, Citrobacter freundii, and Moraxella catarrhalis showed that the prepared phage cocktails (polyvalent) have the potential to cure bacteremia in mice models. Treating mice with bacteremia after 45 min to 24 h post-infection could recover the mice from infection.80 Some other bacteriophages infecting Gram-negative bacteria including Salmonella phages, Enterobacter phages, Shigella phages, Proteus phages, Serratia phages, etc.77–84 Though phage therapy against Gram-negative bacteria shows promising results, more studies on these bacteriophages would give insights into their therapeutic effects. Table 2 describes the recent phage-based preclinical studies performed for the treatment of Gram-negative bacterial infections.
Table 2.
Animal studies to evaluate bacteriophages’ efficacy in treating Gram-negative bacterial infections.
| Target Bacteria | Bacteriophage preparation | Model Organism | Outcome | References |
|---|---|---|---|---|
| P. aeruginosa | Bacteriophage PEV20, inhalable powder at 2×107 PFU/mg | Mouse lung infection model |
|
85 |
| P. aeruginosa | Six bacteriophages at MOI of 0.05, 0.1, 1.0 | Respiratory lung infection in mice |
|
86 |
| Six bacteriophages at MOI of 25, 8 | G. mellonella |
|
86 | |
| P. aeruginosa | A bacteriophage KPP12 at 5×108 PFU | Murine infection model of keratitis |
|
87 |
| P. aeruginosa | Two bacteriophages at 1.2×109 PFU | Lung infection in Murine model |
|
66 |
| A. baumannii | One phage at 1.2×1010 PFU/ml | Rat wound infection model |
|
70 |
| A. baumannii | Four bacteriophages at 5×109 PFU | Mouse wound infection models |
|
71 |
| A. baumannii | One bacteriophage at 107, 108, 109 PFU/mouse | Immunocompromised mouse model |
|
72 |
| A. baumannii | One bacteriophage at 5×108 PFU | Mouse local and systemic infection model |
|
74 |
| K. pneumoniae | One bacteriophage at 1010 PFU/ml | Murine burn wound infection model |
|
76 |
| K. pneumoniae | One bacteriophage at 104 PFU/ml | G. mellonella infection model |
|
77 |
| K. pneumoniae | One phage at 2×109 PFU/mouse | Mice infection model |
|
78 |
| E. coli | One phage at 1×108 PFU/mouse | Intravenous mouse model |
|
88 |
| E. coli | Two phages, 536_P1 at 108 PFU/mouse |
Mice infected with a bioluminescent strain |
|
79 |
| 536_P7 108 PFU/mouse | Mice infected with a ventilator-associated strain |
|
79 | |
| E. coli | One bacteriophage at 104 PFU/ml | G. mellonella infection model |
|
77 |
In vivo animal trials of phage therapy
Nematode (Caenorhabditis elegans), common fruit fly (Drosophila melanogaster), wax moth (Galleria mellonella) and zebrafish (Danio rerio) are among the most commonly used invertebrate or lower vertebrate models for phage therapy, while chicken (Gallus gallus), rabbit (Oryctolagus cuniculus), hamster (Mesocricetus auratus) and mouse are for higher vertebrates.89
Infection in nematodes is simple because their nutritional source is bacteria; thus, pathogens primarily colonize the intestine, and phages can be delivered through the same route. Caenorhabditis elegans models have been used to evaluate the efficacy of phage therapy for Salmonella enteritidis and S. aureus infections. In both cases, bacteriophage administration increased the survival of infected larvae significantly. The health of recovered nematodes was confirmed by their ability to produce healthy progeny 100 hours after phage treatment. These results show that C. elegans can be a useful animal model for assessing the efficacy of phage therapy.90,91
Insects have a high potential among non-vertebrate infection models due to their complex innate immune system, which is very similar to mammals. D. melanogaster was used in two studies to assess the therapeutic effect of phages against P. aeruginosa infections.92 Lindberg et al.93 conducted the first study in which they investigated the pharmacokinetics and potential toxicity of phages on their own. Healthy flies were given phage solutions mixed with corn meal-dextrose medium. The presence of live bacteriophages in the lysates of the flies at various time points after treatment demonstrated that the phages survived and were not degraded in the gastrointestinal system. This suggests that oral administration can be effective in animal models, highlighting the intriguing possibility of testing phage oral administration in D. melanogaster. Furthermore, the lack of lethality after phage administration suggests that phage treatments are safe and nontoxic. Heo et al.,94 compared the effects of phage administration on P. aeruginosa infection in mice and D. melanogaster. They thought to use two infection models in order to confirm phage antibacterial activity against P. aeruginosa, which activates different virulence factors depending on the host. Given the promising potential of D. melanogaster as a simple, rapid and inexpensive animal model for studying bacterial infection and phage therapy, a guided protocol has recently been established to evaluate the antibacterial efficacy of new bacteriophages against P. aeruginosa infection in this model.95
G.mellonella is another invertebrate used for microbial infection and phage therapy. Different bacteriophages were efficiently administered in G. mellonella larvae to treat Burkholderia cepacia infection in a study by Seed et al.96 The authors also investigated whether the protective effect observed in the treated larvae was due to bacteriophage action or the host’s immune system reaction triggered by the phage injection. Heat-inactivated phages activated the immune system but did not improve larval survival, indicating that antibacterial action depended on active phage multiplication.
Interestingly, two separate studies in wax moth larvae reported the prophylactic efficacy of phage cocktails when injected or orally administered.96 In the first study, Nale et al.97 found that adding a four-phage cocktail capable of disrupting C. difficile biofilm to larvae food increased survival while preventing bacterial colonization. Forti et al.86 found that the six-phage cocktail originally used to prevent P. aeruginosa infections in G. mellonella effectively counteracted lung infections in mice. This finding demonstrated that the same bacteriophages could function in both invertebrates and vertebrates.
Zebrafish is gaining popularity as a model for studying host-bacterial interactions, particularly in its larval stage.98 The embryos’ developed innate immune system, genetic tractability, and optical transparency make them useful for studying aspects of infectious diseases unavailable in traditional animal models. Recently, some zebrafish models were created for research purposes – bacterial infections such as E. faecalis and P. aeruginosa for phage therapy.99,100
In a study using zebrafish embryos, systemic infection was accomplished by injecting bacteria into the embryos. Following circulation, phage was administered via the same route. The success of treatment was demonstrated by the increased survival of infected zebrafish embryos, their recovery from bacterial infection-induced morphology changes, and a reduction in the bacterial burden on homogenized embryos after plating. This vertebrate model validates phage therapy’s efficacy in a short (five days) and low-cost manner, demonstrating the survival and effectiveness of phages in an aquatic model delivered in the blood.89
The use of invertebrates and smaller vertebrates, like zebrafish, has many benefits for research, including decreased expense and experiment time. However, the use of higher vertebrate models to apply phage therapy to humans cannot be discarded. For instance, oral phage administration was used in birds as prophylaxis or post-infection treatment to combat infections such as salmonellosis, colibacillosis and campylobacteriosis, which are significant economic health issues for poultry worldwide.101,102 The use of encapsulated phages of specific virion regions, such as the tail spike domain, to enhance phage therapy in chickens was also investigated in some studies.103–105 Given the significance of applying phage therapy using birds as animal models, a procedure to assess phage effectiveness using a chicken embryo infected with colibacillosis was recently developed.106
Rabbits have also been used to model S. aureus wound infection and phage delivery.107 Rabbits naturally have S. aureus infections, as humans do, but unlike mice. This makes rabbits a good animal model to research the spread of these bacteria. Subcutaneous injections provided the bacterial infection, which led to abscesses. Phage delivery to bacteria was done simultaneously or right after but at the same site. Animals were slaughtered four or six days after infection to assess the bacterial load in the abscess area and the efficiency of phage therapy.89
Phage treatment was tested in a rabbit model of S. aureus infection in another study published by Kishor et al.31 and discussed by Abedon.108 Although the authors established the feasibility of phage therapy to cure bacterial illness, the rabbit model differed from the patient’s circumstance, in which bacterial infection was chronic, and phage therapy was used after traditional techniques failed. Yen et al.109 established a prophylactic impact of phages to prevent or minimize bacterial infection in newborn mouse and rabbit models infected with Vibrio cholerae.109
Nale et al.110 reported that hamsters infected with Clostridium difficile and orally treated with a phage cocktail had a higher survival rate. Temperate phages were used in this investigation due to a shortage of virulent lytic phages infecting C. difficile. As a result, it was unsuitable for therapeutic use. However, the authors demonstrated how combining multiple phage types could lessen their negative influence.110
Murine models are the most commonly employed animals for phage therapy research. Because they resemble humans, they have been utilized to demonstrate the efficacy of traditional phage therapy and to examine the interactions between phages and the host immune system.65,66,86,111,112
Overall, animal models have helped to learn more about bacteriophages’ efficacy and mechanisms of action in vivo. Invertebrates and vertebrates demonstrate the effectiveness of such medicines in less expensive, faster and more ethical ways than human clinical trials. The development of vertebrate animal models to test phages may provide a more comprehensive understanding of the mechanisms prompting host immunological and inflammatory responses to phages, which is one of the most pressing concerns about the applicability of phage therapy to people. Several ways have been attempted to increase and intensify phage activity, highlighting the promise potency of bacteriophages or their enzymes in human therapeutics. Among these are the potential to improve antibacterial action by boosting phage transport, the use of phages in cocktails, the mixing of phages with antibiotics, and the use of phages for prophylactic therapies. These strategies can be (and inexpensively) carried out in animal models before being translated into humans.89
These are all (or mostly) uncontrolled clinical trials, which limits the ability to draw clear conclusions about safety and efficacy compared to placebo-controlled blinded trials.
Furthermore, because one of the most important goals of current phage therapy is to rapidly find phages capable of counteracting bacterial infection in compassionate research, animals can gesture the safety of specific phages before patient treatment. Because the custom-made usage of phages cannot currently be regulated, phage pre-screening in animals for tailored therapeutics should at least mitigate some of the issues.113 Figure 1 shows the bacteriophage therapy in the animal infection models, and Figure 2 shows various routes of phage administration in the mice infection model.
Figure 1.
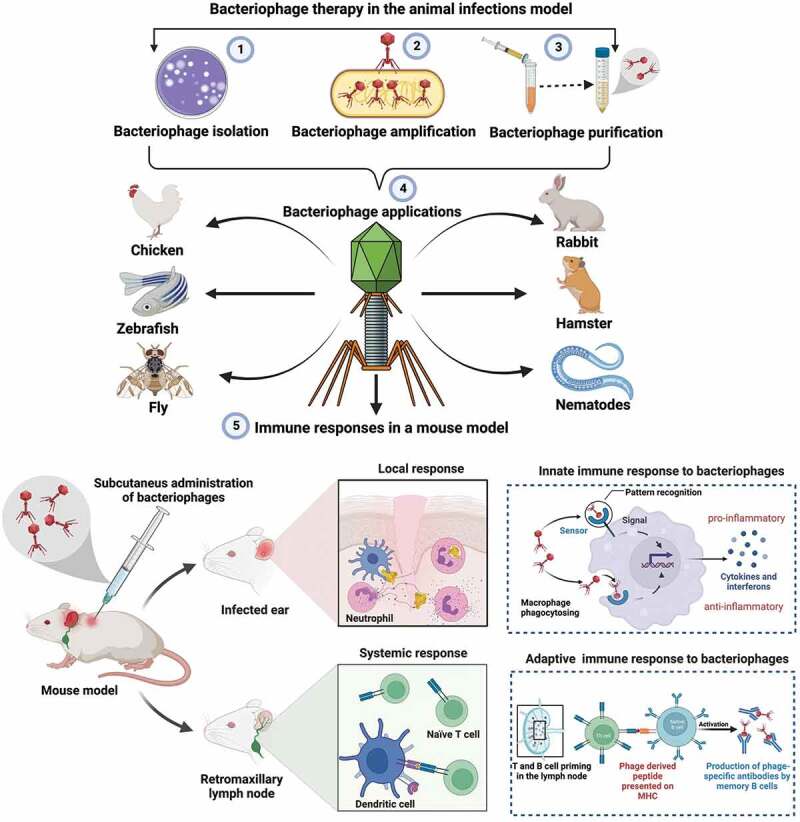
Figure shows the bacteriophage isolation process, animal infection models, and the immune responses to phages in a mouse model. The figure was created with Biorender.com.
Figure 2.
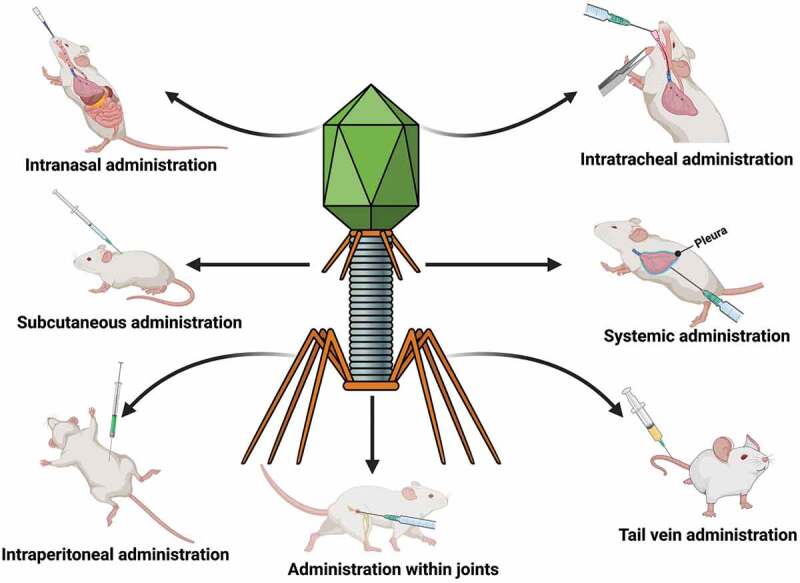
Representation of various routes of phage administration in the mice infection model. The figure was created with Biorender.com.
For these reasons, animal models are essential in researching possible phage therapeutics and bringing them to human medicine.89
Recent phage therapy clinical trials and status
Currently, phage therapy is only being tested in a few clinical trials, with most of them in phase I and some preparations undergoing phase II trials.114 In a recent clinical trial, bacteriophages were used for treating urinary tract infections in 113 patients undergoing transurethral resection of the prostate. The study showed the safety of bacteriophages, but bacteriophage treatment was not superior to standard-of-care antibiotic treatment, suggesting the need for extensive sample size studies with robust protocol design.115 The main reasons for the limited use of bacteriophages in therapy include the lack of proper regulatory guidelines and little public awareness.
Bacteriophage cocktails are gaining therapeutic interest because of their efficacy in killing many bacteria. Recently, a cGMP-prepared bacteriophage cocktail saved a patient’s life from an otherwise deadly A. baumannii infection.116 In another study, an 88-year-old patient suffered hospital-acquired pneumonia spurred on by carbapenem-resistant A. baumannii. A single phage in combination with tigecycline and polymyxin E was consistently administered to the patient for 16 days. The therapy cleared the pathogen, and the patient’s lung function improved clinically.117
Several recently completed clinical trials have provided important phage therapeutics lessons. One of the most illuminating clinical trials of the last decade focused on P. aeruginosa infection of burn wounds.114 The topical treatment with a fixed cocktail of 12 antipseudomonal phages was compared to 1% sulfadiazine silver emulsion cream in the open-label, controlled trial. Despite being interpreted as a negative study because it was terminated for futility before reaching full enrollment, this study provided several key lessons for future studies. The first is that the phages in the treatment regimen must be active against the treated organisms. All patients with topical isolation of P. aeruginosa from a burn were eligible to participate in the trial, regardless of whether their specific P. aeruginosa strain was susceptible to the phages in the cocktail used or not. A post-hoc analysis revealed that a subset of patients with organisms sensitive to phages in the treatment regimen benefited clinically. Second, the study demonstrated the importance of assessing phage-phage interactions before combining them in a phage cocktail and critically assessing phage stability between the production line and the bedside. To the investigators’ credit, these issues were detailed in the clinical trial report and now serve as guidance for all subsequent studies.118
Another recent clinical trial using the T4 phage to treat acute bacterial diarrhea orally in Bangladesh showed how crucial it is to comprehend pharmacokinetic and pharmacodynamic issues before beginning large-scale clinical trials.17 The goal of this study was to use T4 coliphages to treat infantile diarrhea that was thought to be brought on by enteropathogenic Escherichia coli. After an interim analysis showed no clinical benefit, the study was stopped. In this interim analysis, the researchers found that the phage regimen’s multiplicity of infections failed to consistently cause a self-sustaining replicative cycle in the gut lumen of people treated.118 Another investigation of using a fixed phage cocktail against Staphylococcus aureus bacteremia demonstrated the difficulties in studying serious infections with binary outcomes. Thirteen patients were diagnosed with Staphylococcus aureus and administered a three-phage cocktail intravenously twice daily for 14 days.119 The cocktail was safe, and all patients in the study found it safe and well-tolerated. More than half (62%) of the patients showed clinical improvement following phage therapy, while the other half had various issues unrelated to the phage. Although the study added evidence that phages could be administered parenterally to critically ill patients, the study also highlighted the difficulties. They were demonstrating advantages over current standard-of-care therapeutics. Regardless of these “failures,” the stage is now set for a new era of clinical trials to determine antimicrobial and clinical properties of phages within a framework that builds on these early findings, experiences and principles of antibiotic development that have contributed to the body of knowledge evidence supporting current antimicrobial therapy. After all is said and done, phages are just “living antibiotics.”120
A leading scientist of clinical phage therapy, professor Jean-Paul Pirnay, published the successful clinical results of the patient treated with phages. They present the case of a toddler who developed drug-resistant Pseudomonas aeruginosa sepsis following liver transplantation. For 86 days, he received intravenous bacteriophage-antibiotic combination therapy. Without antibody-mediated phage neutralization, this salvage therapy was well tolerated. It was linked to objective clinical and microbiological improvement, allowing for liver retransplantation and the complete resolution of all infections. In vitro, phage-antibiotic synergies were observed. Bacterial phage resistance did not result in therapeutic failure, which could be attributed to phage-induced virulence tradeoffs, which were investigated in various experimental models.121 Their successful clinical results show new hope and confidence in clinical phage therapy.
Another scientist who works on synthetic phages, Professor Yingfei Ma of the Shenzhen Institute of Advance Technology, Chinese Academy of Sciences research group, treated an 88-year-old Chinese man with natural phages who developed carbapenem-resistant A. baumannii pneumonia in the hospital. A personalized lytic-specific single-phage preparation, in combination with tigecycline and polymyxin E, was continuously nebulized in the patient for 16 days. The treatment was well tolerated, resulted in pathogen clearance, and improved the patient’s lung function.
This case shows phage therapy’s clinical therapeutic efficacy and safety.122
Professor Graham F. Hatfullf, a phage scientist at the University of Pittsburgh, USA, treated a 15-year-old girl with phages. Following bilateral lung transplantation, the patient with cystic fibrosis and a disseminated Mycobacterium abscessus infection was treated with a three-phage cocktail. Genome engineering and forward genetics were used to create effective lytic phage derivatives that kill the infectious M. abscessus strain. Intravenous phage treatment was well tolerated and linked to objective clinical improvements such as sternal wound closure, improved liver function, and effective improvement of infected skin nodules. In the clinical treatment, phage administration had no adverse effects.123
Professor Graham F. Hatfullf published another clinical study of phage therapy. The study presented a case of refractory cutaneous disseminated Mycobacterium chelonae infection in a patient with seronegative arthritis. The patient was successfully treated with antimicrobial, surgical, and single bacteriophage therapy. The patient developed neutralizing antibodies against the bacteriophage but has improved with negative biopsies and no evidence of bacterial resistance to the phage.124
A clinical study of phage therapy was recently reported. A male with treatment-refractory Mycobacterium abscessus pulmonary infection and severe cystic fibrosis lung disease received two mycobacteriophages intravenously. The phages were engineered to be more effective against M. abscessus and were chosen as the most effective against the subject’s bacterial isolate. Using molecular and metabolic assays in conjunction with clinical assessments, evidence of phage-induced lysis was discovered in the context of compassionate use. M. abscessus isolates showed genetic stability before and after phage treatment, with a general decline in diversity and no increased resistance to phage or antibiotics. Anti-phage neutralizing antibody titers to one phage increased over time, but this did not prevent clinical improvement during treatment. On day 379, the subject received a lung transplant, and systematic culturing of the explanted lung revealed no M. abscessus. The study described the successful results of clinical phage therapy of M. abscessus.125
Kutter et al. detailed previous clinical trials involving phage therapy, which included those conducted in Georgia and Poland.126 Two phage therapy clinical trials used as examples throughout the literature are worth mentioning: the safety of phages for treating venous leg ulcers127 and the safety and efficacy in chronic otitis.128 Rhoads and colleagues reported no adverse effects with the administration of phages in a small phase I trial in patients with venous leg ulcers.129 Wright et al. demonstrated the efficacy and safety of anti-Pseudomonal phages against MDR-P. aeruginosa-dominated late-stage recurrent otitis. These are among the first human-controlled clinical trials in the Western world.128 Several clinical trials have recently been registered (https://clinicaltrials.gov/and https://globalclinicaltrialdata.com/).
Helen et al. recently published a comprehensive review article.130 They found 13 modern clinical or safety trials published between 2005 and 2021. The trials took place in Bangladesh and/or Switzerland (n = 6), the US (n = 2), France and/or Belgium (n = 2), Australia (n = 1), Georgia (n = 1), and the United Kingdom (n = 1). Four of the 13 trials were identified as ‘phase I,’ two as ‘phase I/II,’ and seven had no formal identification. Clinical and safety studies have consistently shown that using naturally occurring phage for therapy via various delivery methods is safe. Clinical investigations also reveal that phage is effective when the appropriate amount of the proper phages is supplied to the right location to treat infections containing enough susceptible bacterial cells. However, earlier clinical trials found it difficult to match this constellation of elements. It is hoped that future trials will yield the convincing results that the field has anticipated. Meanwhile, the data on the safety and efficacy of phage therapy is deemed sufficient for continued compassionate use when antibiotics cannot meet clinical needs.131
Several uncontrolled case studies show positive clinical outcomes. On the other hand, clinical failures are likely underreported, and the few randomized controlled trials conducted have failed to demonstrate benefit. Thus, under any circumstances, no recommendation can be made to support the routine clinical use of phage therapy; much is unknown about the efficacy of phage therapy and potential reasons for failure, such as dosing, frequency of dosing, duration of treatment, routes of administration, interactions with antibiotics, interactions with other phages, the emergence of phage resistance, inadequate phage delivery, and superhost immune response. Even though more clinical research studies are required, there is a lack of (and need for) standardized assays for phage therapy and phage quantification methods, as well as a wide range of potential clinical indications, the safety and tolerability of phages, the prerequisites for safe administration of phage therapy, current regulatory pathways for expanded access, and the requirements for safe administration of phage therapy.132
The conclusion of phage therapy clinical trials is that phage treatment is generally safe, with a low incidence of adverse effects via various routes of administration. Although phage therapy appears to be a promising strategy in the fight against difficult-to-treat infections and antimicrobial resistance, high-quality trials are urgently needed to improve our understanding of the treatment’s long-term outcome.133 Significant effort is required to identify success predictors and design phage clinical trials leading to more widespread use.134
The current phage therapy status, hurdles, and limitations
Although phage therapy has shown promising efficiency in addressing infections of bacterial pathogens, it still has several limitations, including a narrow host range, a lack of relevant regulations, and the lack of pharmacokinetic data. Lin et al.135 recently provided a comprehensive review of phage therapy’s current status and limitations and summarized existing solutions for these limitations. Among others, they suggested establishing a national standard scheme for personalized phage therapy and clarifying the standard operating procedure of the clinical application of phage therapy. If these solutions are considered, there would be an improvement in the outcomes of phage therapy and clinical trials.
Bacteriophages have a limited targeting range
The bacteriophage cleavage spectrum is too narrow due to high specificity. Bacteriophages typically act on a limited number of bacteria genera and species and thus cannot target all pathogenic strains of a single bacterial species.136 Bacteriophages help treat diseases caused by a single bacterium, but clinical cases are frequent infections caused by various pathogenic bacteria. As a result, it is frequently difficult for specific bacteriophages to exert the desired therapeutic effect.136
The lysogenic phenomenon occurs when some lysogenic phages cannot lyse the host bacteria and inhibit the lytic effect of other phages on their host bacteria after integration. The viral genome replicates with the host DNA in lysogenicity, either as a free plasmid-like state or after integration into the bacterial chromosome.137 A more serious issue is that bacteriophages in the lysogenic state can transmit toxins and antibiotic resistance genes to bacteria. Unlike protein drugs, whose activity and purity can be determined using specific antibody titers, the composition of phage therapy preparations are more complex, containing both proteins and nucleic acids. As a result, assessing its quality and curative effects is difficult.138
The absence of necessary phage therapy policies
There aren’t any policies or rules regarding the use of PT in clinical settings.139 Appropriate regulatory guidelines can open doors for promoting this promising treatment. The opinions of European stakeholders were thoroughly analyzed by Verbeken et al., who also discussed the necessity of changing the regulatory framework to account for PT.140 Whether PT development takes place on an industrial or patient-specific scale, a hospital-based scale is a crucial factor to consider. They promoted the creation of a new, exclusive European regulatory framework for PT. Additionally, the potency of isolated phage preparations varies because there is no established standard for phage isolation and purification. Using bacteriophages in clinical settings is not a standard practice.135
Bacterial resistance to phages
Several studies on the emergence of bacteriophage-resistant strains suggested that if a single bacteriophage is used repetitively for a long time, bacteria evolve phage-resistant strains through natural selection.141 This is part of a long-term evolution in bacteria of anti-bacteriophage strategies such as adsorption inhibition, restriction-modification systems, injection blocking, abortion infection, superinfection immunity, and the Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR associated (CRISPR-Cas) system.142 Bacteriophage-bacteriophage interactions are reduced as a result of adsorption resistance. Bacteriophages and bacteria both die during abortion infection. CRISPR-Cas is a component of the adaptive immune system that provides bacteria and archaea adaptive immunity against foreign invaders such as plasmids and bacteriophages.143
CRISPR and Cas proteins work together to form an overall system that interferes with foreign nucleic acids in bacteria and archaea. The CRISPR-Cas system has at least two stages: adaptation, in which cells acquire new spacer sequences from exogenous DNA, and interference, in which newly acquired spacers are used to target and cleave invasive nucleic acids. By adding or deleting gaps in host cells and mutations or deletions in phage genomes, the CRISPR-Cas system contributes to the continuous evolution of bacteriophages and bacteria.137,144
Limited data on the pharmacokinetics of phages
Standardizing PT preparations is difficult because the dosage description is still unknown. Furthermore, the administration and dosage of PT directly impact its effects, making the clinical application of PT difficult. Bacteriophages are almost entirely composed of proteins and DNA or RNA, so they are easily degraded when interacting with human metabolisms, such as in the liver or stomach, when confronted by the mammals’ immune system.145 According to related pharmacokinetic studies, a quarter of bacteriophage infusions lasted 36 hours after treatment, but their effective concentration was diluted by body fluids.146
Oral administration has been the best option for both humans and animals. Furthermore, compared to other drug administration methods, it is relatively simple and comfortable, with low immunogenicity.147 Bacteriophage particles enter the systemic circulation after passing through the stomach, intestine, and intestinal mucosa during oral administration. As a result, the gastrointestinal system is regarded as the primary barrier in preventing bacteriophage infiltration of tissue.148 Furthermore, the mammalian circulatory system effectively removes bacteriophages from the blood, making maintaining sufficient bacteriophage concentrations to destroy the target bacteria difficult.149
During phage therapy, phage resistance can emerge
The potential quick growth of phage-resistant bacterial variations, which could obstruct successful treatment outcomes, is one of the critical concerns with phage therapy. According to experimental data, up to 80% of studies that focused on the intestinal milieu and 50% of studies that used sepsis models found phage-resistant variations. Three of the four clinical trials that documented the emergence of phage resistance described the observation of phage-resistant variants in human investigations. Bacteria can resist phage infection through diverse strategies, including phage receptor blockade, extracellular matrix production, competitive inhibitor production, phage DNA entry prevention, restriction-modification systems and infection prevention systems.150 Interestingly, studies have shown that bacterial mutations confer phage resistance and may also result in fitness costs in the resistant bacterium, which may be advantageous for the host. Therefore, efforts should be made to create approaches for monitoring and avoiding phage resistance.141
The immune system responses to phage therapy
Bacteriophages and their products are non-self-antigens. Thus, it is unsurprising that the immune system can recognize and launch reactions that could conceivably lessen the benefits of administering phages. Experimental studies in both animals and humans have shown immune response to phages, although there are variances depending on the phage strain, the delivery method and the amount of prior exposure. In a study, when T7 phage survival in the blood of healthy and immunocompromised mice was compared, it was discovered that phage titers remained constant for a long time in animals with severe combined immunodeficiency. 99% of phages were removed in healthy mice within 60 min of the injection. As phage titers were steady in the B-cell-deficient mice, the development of particular antibodies appeared to be the primary cause of phage clearance from blood.116
Dabrowska et al.,130 who evaluated the antigenicity of the proteins constituting the E. coli T4 phage head surface in humans, found that particular antibodies could be discovered in more than 80% of enrolled persons, even though none had received phage therapy. Although not fully shown, it is likely that the immune response elicited by phages has a minor or no effect on the potential bacterial killing of phage administration. Bacterial lysis happens before the induction of a particular antibody. Furthermore, with a few exceptions, phage delivery is not related to tissue damage, an increase in pro-inflammatory cytokines, or an increase in reactive oxygen species (ROS).130 Another study found that giving mice phage T4 and its head proteins intraperitoneally did not affect the production of cytokines, interferon, tumor necrosis factor, monocyte chemoattractant protein, gamma-induced monokine, granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor.151,152 Hwang et al. discovered similar results when they evaluated the safety of an E. coli phage cocktail administered orally to rats for four weeks.153 Carmody et al. investigated the efficacy of intranasal phage therapy in a mouse model of Burkholderia cenocepacia lung infection and found that bacterial density, macrophage inflammatory protein-2, and tumor necrosis factor were not increased but were considerably reduced in treated mice lungs compared to untreated controls. On the other hand, data collected in humans appear to support the notion that, even if present, the immunological response to phages is not clinically significant.154
Kaźmierczak et al. conducted a comparative immunogenicity study of two therapeutic bacteriophages, A3R and 676Z, active against Staphylococcus aureus and routinely used in patients at the Phage Therapy Unit in Poland. In a murine model, a comparison of the overall ability of whole phages to induce specific antibodies revealed typical kinetics of IgM and IgG induction by these two phages. Further research revealed that antibodies specific to ORF096 neutralize the phages’ antibacterial activity in studies of protein-specific sera. None of the studied proteins plays a particular role in the induction of specific antibodies in humans; thus, none potentially affects the effectiveness of A3R and 676Z. No evidence was found of increased specific immune responses to the investigated proteins in patients who received phage therapy.155 Phages can cause the production of neutralizing antibodies as well as phage-specific ones. However, their influence on therapeutic efficacy seemed minimal and did not prevent phage therapy from having a positive outcome. There is a “therapeutic window” because there is a difference between the specific immune response induced by high doses of parenterally administered phages (as in an animal model) and the response seen in patients treated with bacteriophages.155
Before, phages were considered spectators who only indirectly affected immunity through their effects on the mammalian microbiome. It is now known, however, that phages directly affect immunity in ways that are typically anti-inflammatory. Through phagocytosis and cytokine production, phages can influence innate immunity, but they can also affect adaptive immunity through effects on antibody production and effector polarization. Phages may thus significantly impact the outcome of bacterial infections by modulating the immune response.156
Approaches to overcome the limitations of phage therapy
Host range of bacteriophages
The problem of a limited host range can be addressed in a variety of ways, including the use of phage mixtures,157 the establishment of a phage library,158 and extensive screenings.142 A phage mixture is analogous to several drug therapies. Different bacteriophages in a mixture can infect various bacterial strains that may be present following a specific diagnosis.149 McVay treated burned mice with a bacteriophage mixture, significantly reducing their mortality.159 A phage library is an isolated phage collection. These bacteriophages have specific properties and can be used as phage preparations or as unexpanded phage reserves to match newly isolated specific target bacteria.157 The extensive screening of bacteriophages involves using a variety of hosts to identify bacteriophages that use common surface receptors to cleave a variety of pathogens, such as bacteriophages that target multiple isolates of two different pathogenic bacteria.160 It can help expand the host range of a single bacteriophage and solve the host spectrum problem by utilizing many bacteriophages.149 Expanding the host range of a single phage can also be accomplished by using genetic engineering techniques to modify a portion of the phage responsible for host binding or by cloning a second alternative or different version of these proteins involved in host binding into a single phage.161 The T7 phage, as a genetically modifiable biological nanoparticle, holds promise for biomedical imaging probes, therapeutics, drug and gene carriers, and detection tools.162 In general, a phage mixture with more than one phage type to attack bacteria may be preferable in terms of efficacy. However, a phage library may be preferable for phage-host specificity based on direct matching with a specific target pathogen.163
The exclusion of temperate bacteriophages is one of the principles for preventing lysogenicity. To eliminate the immune effects of infected lysogenic bacteria on similar bacteriophages, PT must be achieved by lytic phages that have been highly purified. Bacteriophages can encode enzymes that hydrolyze peptidoglycans, causing cell walls to degrade and infect or release offspring viruses in host cells. Endolysin is the enzyme that dissolves peptidoglycan from within. Compared to direct bacteriophage therapy, endolysin’s therapeutic effect in treating bacterial diseases is easy to evaluate. This reduces the difficulty of assessing quality. Research has recently focused on synthesizing and transforming lysin-coding genes into antimicrobial peptides, improving the original bacteriophage’s antibacterial activity.164
Implementation of relevant policies, regulations and guidelines
Several meetings on PT supervision and regulation have been held to promote the development of PT.140,165 Since their discovery, bacteriophages have been widely used in Eastern Europe and the former Soviet Union; thus, therapeutic bacteriophages have been integrated into healthcare systems.166 PT’s open policy promotes its rapid development. As a result, relevant regulatory standards must be issued on time. Furthermore, bacteriophages are isolated and purified in various ways, but all methods involve the same steps: environmental samples are collected and tested for the presence of bacteriophages. Standardized bacteriophage purification has been considered several times.167 As a result, a national standard scheme for personalized PT should be established. Furthermore, the standard operating procedure for the clinical application of PT should be clarified, including the recruitment of PT patients, the establishment of phage libraries, the isolation and identification of pathogens, the screening of effective phages for pathogens, the preparation of phage formulations, management strategies, approaches to bacteriophage preparations, the monitoring of PT efficacy, and the detection of the emergence of pathogens.168
Combination of dosage guidelines to tackle phage resistance in bacteria
Bacteriophages can be used with other antimicrobials, such as antibiotics, due to the introduction of anti-bacteriophage strains. The greatest approach to combating phage resistance is utilizing bacteriophages with antibiotics. This is also a step toward switching from antibiotic therapy to PT, hastening the growth of the PT research sector. The effectiveness of combining bacteriophages and antibiotics has been demonstrated in numerous investigations.169
Similarly, Oechslin et al. found that ciprofloxacin and a bacteriophage worked in synergy to effectively treat experimental endocarditis in rats caused by Pseudomonas aeruginosa to prevent the formation of anti-phage mutants.170 Similar to this, clinical examples showed that multidrug-resistant Staphylococcus aureus infections in radiation-exposed patients might be successfully treated with a wound healing solution including ciprofloxacin and bacteriophage polymers.117 Additionally, we know biofilm pairings can increase bacterial resistance to antibiotics, and numerous studies have demonstrated that bacteriophages and antibiotics together can lower the bacterial density in biofilms.171 Undoubtedly, several bacteriophages express anti-CRISPR proteins to circumvent the CRISPR-Cas immunity and avoid bacterial resistance. These proteins block the resistance mechanism. Prioritizing amongst bacteriophages can be employed to increase the antibacterial activity of bacteriophage combinations to limit phage resistance emergence.172 Antimicrobial peptides are innate immune components with broad-spectrum antibacterial action found in practically all organisms. Some studies had demonstrated a potent synergistic effect when bacteriolysin LysH5 and nisin were used to treat Staphylococcus aureus.173,174
The success of phage therapy, which uses bacteriophages to treat multiple drug-resistant bacterial infections, depends on the carefully chosen antibiotics used in combination therapy. Vashisth, Medhavi, et al. reported and tested the combination of different antibiotics with the bacteriophages. Using time-kill curve assays and counting the number of viable bacterial cells left after the experiment, the synergy assessment of these phages with gentamicin and tetracycline was carried out to validate this antagonistic effect. When phage-antibiotic combination groups were compared to phage-only treatment groups, a rise in bacterial turbidity was seen. This study concludes that bacteriophages’ therapeutic potential may be decreased by antibiotics targeting the bacterial protein biosynthetic machinery. Therefore, the such combination must undergo careful screening before being used in combination treatment regimens.175
Evaluation and optimization of phage delivery channels
Despite numerous advances in phage preparation for clinical applications, each route of administration presents challenges. These include phage preparation stability, target-site specific delivery, and antibody-mediated phage inactivation and clearance by the recipient’s reticuloendothelial system. Particularly, not only are phages unable to penetrate tissues, but the immune system can clear phage particles, and phage proteins are rapidly degraded by enzymes or inactivated by the stomach’s low pH. Loh et al. have briefly explained and addressed the issue of Phage delivery and encapsulation strategies before phage therapy can be considered reliable standard therapy. They further described efficient and targeted phage delivery methods, including phage encapsulation.6
Optimizing medication delivery channels must consider whether the bacteriophage can survive in the body and go to all sections of the body. If it is local, infection occurs via systemic circulation, and the bacteriophage persists in a cycle long enough to get to the afflicted location. If the phage gets delivered to the intestine, it must be consumed to survive until it enters the circulatory system.104,113,115,118,119,176,177 The encapsulation of phages in a natural biopolymer matrix, for example, is one method utilized as a barrier against the stomach environment to prevent the inactivation of bacteriophages after intake; this ensures bacteriophage effectiveness.178 Related research revealed that liposome-encapsulated phages could be efficiently maintained in the body and stay safe until they are released. Upon arrival in the stomach, the gut wall temporarily protects phages from bile salts and excretion clearance.179 Furthermore, the dose can be increased or decreased over a brief period to prevent bacteriophage inactivation or loss before antibodies reach the target microorganisms.10,180
A broader view of phage-related research
With the advent of antibiotic resistance, the number of untreatable bacterial infections has increased rapidly. The unavoidable adaptation of microbes to antibiotics, regardless of their origin (natural, semi-synthetic, or nature-inspired synthetic origin), has necessitated the need for alternative antibacterial agents, such as therapeutic phages or antimicrobial peptides.181 In-vivo efficacy of bacteriophages to cure bacterial infections has been established using animal infection models. Many preclinical studies included mice as model animals with different infection models, and the efficacy of different routes of phage administration has also been evaluated. However, most animal studies have focused on the antibacterial efficacy of bacteriophages and lack substantial studies on the immunological response of the bacteriophage. Immunological modulation of the host animals via bacteriophages may provide conclusive evidence about the therapeutic outcomes and aid the design of future treatment strategies.
Phage delivery strategies have also been employed in recent years to treat gastrointestinal tract, skin, lung, and urinary tract infections and showed better antibacterial response than bacteriophage preparations alone. However, the pharmacokinetics, immunomodulatory properties, large-scale production and sterilization are critical knowledge gaps in the use of these delivery systems in clinical applications. Apart from technological advancements, a global legal and regulatory framework is yet to be established for bacteriophages and their delivery systems.182
While studies on preparations containing one or more phage types have received much attention, the interest in phage-derived endolysins is also increasing.183 The prominent advantage of endolysin therapy is the lack of bacterial resistance. Interestingly, many studies have demonstrated the potential of endolysins as alternative therapeutic options for treating multidrug-resistant bacterial infections.184–187 Endolysins are bacteriolytic enzymes that can kill bacteria with an activity broader than the bacteriophage. However, the specificity of endolysins is still under investigation.188 Several strategies, such as molecular engineering and encapsulation of endolysins, are under exploration to improve the activity, biodistribution and half-life of endolysins.182,189 Many bacteriophage researchers believe phage therapy or phage-encoded proteins can completely replace chemical antibiotics. However, the future will likely see a co-existence of both strategies, with phage therapy as an additional weapon against the bacteria, possibly used as a combination with antibiotics.190,191 So far, such a combination therapy has tremendously succeeded in treating bacterial pathogens because the bacteria cannot simultaneously develop resistance against antibiotics and phages.
In Table 3, we provided the outcomes of endolysins administration against bacterial pathogens in some animal models. Although bacteriophage-related research has seen a renaissance over the past years, some aspects still require more attention, such as (i) the development of phage-resistant bacteria, (ii) the interaction of phages with the human immune system (during long-term treatments of infections, for example), (iii) the development of genetically modified phages for therapy, and (iv) a global regulatory framework for the production and clinical application of bacteriophages. In addition, there are limited animal studies to prove the efficacy of phage therapy in some bacteria, such as Streptococcus, Enterococcus and Mycobacterium. To establish a preclinical therapeutic efficacy, a simple animal model such as G. mellonella and C. elegans can be used for these pathogens.77,219 In Table 4, advantages and limitations of phage therapy are presented.
Table 3.
Outcomes of endolysins administration in animal models.
| Endolysin | Animal model | Target Bacteria | Outcome | References |
|---|---|---|---|---|
| LysP53 | Mice | A. baumannii |
|
185 |
| PlyC | Tested in human and mouse models | Streptococcus spp. |
|
192 |
| LysSS | Mice | A. baumannii |
|
193 |
| ClyC | Mice | S. aureus |
|
194 |
| ClyH | Mice | S. aureus |
|
195 |
| ClyF | Mice | Methicillin-resistant S. aureus |
|
196 |
| ClyJ-3 | Mice | S. pneumoniae |
|
197 |
| ClyJ | Mice | S. pneumoniae |
|
198 |
| ClyR | Mice | S. agalactiae |
|
199 |
| ClyR | Rat |
S.Sobrinus and S. mutants |
|
200 |
| ClyV | Mice | S. agalactiae |
|
201 |
| SAL200 | Rodent and Dogs | S. aureus |
|
202 |
| Cpl-1and Pal | Mice | S. pneumoniae |
|
203 |
| CPl-711 | Mice | S. pneumoniae |
|
204 |
| Ply5218 | Mice | S. suis |
|
205 |
| Ply5218 | Piglet | S. suis |
|
205 |
| PL3 and Cpl-711 | Zebrafish | S. pneumoniae |
|
206 |
| Cpl-711 | Mice | S. pneumoniae |
|
207 |
| CF-301 | Rabbit | S. aureus |
|
208 |
| CF-301 | Rat | S. aureus |
|
209 |
| S25-3LYS-his | Mice | S. aureus |
|
210 |
| ElyA1 | Mice | A. baumannii |
|
211 |
| Ply6A3 | Mice | A. baumannii |
|
212 |
| LysGH15 | Mice | S. aureus |
|
213 |
| PlyPa91 | Mice | P. aeruginosa |
|
214 |
| Plya03 and PlyPa91 | Mice | P. aeruginosa |
|
214 |
| LysRODI | Mice |
S. aureus and S. epidermis |
|
215 |
| TSPphg | Mice | S. aureus |
|
216 |
| PyS2-GN4 | Mice | P. aeruginosa |
|
217 |
| LysB | Mice | M. ulcerans |
|
218 |
Table 4.
Advantages and limitations of phage therapy.
| Advantages of phage therapy | Limitations of phage therapy |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Future perspective
Phage therapy has remarkable potential as an option for controlling antibiotic-resistant infections. However, there are many obstacles to establishing phage therapy as mainstream medicine. Here we discuss five essential strategies or challenges to be addressed to establish phage therapy in western medicine. [A] Phage socialization: Education and awareness play an important role in accepting and distributing any new therapeutic model within a population. Poor medicine literacy can lead to the misuse of medicine, mainly due to the spread of nonfactual information (as seen in antibiotic therapy). The rebirth of phage therapy is seeing increased therapeutic applications and patient and physician awareness. A strong and direct outreach is essential to capitalize on this situation in which stakeholders – researchers, physicians, policymakers, regulatory authorities, government officials, and the public should be included. Public awareness can be improved through social media platforms and regular campaigns. [B] Accessibility and availability: A successful medicine or therapy should always reach the suffering population and be available when in need. Currently, the accessibility of phage therapy is extremely low, and limited therapy centers are located in Georgia, Poland, Russia and USA. Therefore, the health care cost is high and is affordable only for the high-class population. Though compassionate-use programs are underway in many countries, including Australia, Canada, China, Japan, the UK and the USA, they are limited to critically-ill cases. Easy accessibility is also related to patient education and treatment success. [C] Regulatory framework: The establishment of legal frameworks on the production, preliminary testing, clinical trials, use, and surveillance of phage therapy will be strongly supported by public awareness, accessibility, and education. Country-wise regulatory frameworks can be developed based on medical, industrial and constitutional systems. [D] Manufacturing pipeline: The production of therapeutic phages is complicated and expensive, hindering availability and applicability. Complete phage manufacturing can be divided into small assignments such as isolation, purification, screening, testing, production, storage, and transport, which create an economic activity for the new start-ups and job opportunities for the experts. [E] Clinical trials: The lack of regulated preclinical and clinical trials is a major obstacle. Phage therapy finds more success in clinics than in corresponding clinical trials. Recently, phage therapy has been approved as an investigational new drug (IND) for life-threatening cases. Still, the modern medical research model needs approval for a successful regulatory path (in vitro discovery, animal testing, safety trials, and efficacy trials). The need for controlled clinical trials controlled by placebo or compared to standard-of-care treatment.
Conclusion
Antibiotic resistance is one of the major global healthcare threats endangering the efficacy of available antibiotics. Exploring bacteriophage therapy as a standard clinical strategy to treat infections could be a way out of this crisis. The contribution of animal studies in the approval of medicinal drugs or therapeutics is inevitable. This review sheds light on using animal models to evaluate the efficacy of phages as therapeutics and the necessary regulations in transforming phage research from in vitro to preclinical-clinical trials and medical products. Considering the importance of bacteriophages in molecular biology, diagnostics and drug delivery, this review summarizes the applications of phages in therapy and vaccinology. Though our understanding of phage genomes has been improvised in the technological era, a lot needs to be studied about the immunological and pharmacological aspects for which animal models will be inevitable. We also observed some obstacles in establishing phage therapy in the 21st century and addressed them with possible solutions to improve the future of phage therapy research.
Acknowledgments
The authors (FMK, VSG, GKO, and NO) sincerely thank Prof. Hongping Wei and Prof. Hang Yang (Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan, China) for their guidance. The figures were created with Biorender.com.
Funding Statement
This work is supported by a Research Fund for International Young Scientists from the National Natural Science Foundation of China (NSFC) to Dr. Fazal Mehmood Khan of Grant number: 32250410292.This work is financially supported by the National Natural Science Foundation of China [81960353 and 82172238], the Science and Technology Support Project of Guizhou Province [2020-1Y332], Sanming Project of Medicine in Shenzhen[SZSM202111020], Science and Technology Cooperation Project of Zunyi Science and Technology Bureau [HZ-2019-50] and Health Commission Science and Technology Fund Project of Guizhou Province [gzwjkj2019-1-151].
Authors’ contributions
FMK, PM, and VSG conceptualized the idea and data collection and wrote the original draft. NM, GKO, and NO reviewed and edited the manuscript. TA and GH conceptualized the idea and reviewed and edited the final draft. FMK and GH also contributed funds for this work. All the authors read and approved the final version for publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Haq IU, Chaudhry WN, Akhtar MN, Andleeb S, Qadri I.. Bacteriophages and their implications on future biotechnology: a review. Virol J. 2012;9(1):1–23. doi: 10.1186/1743-422X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown N, Cox C.. Bacteriophage use in molecular biology and biotechnology. Bacterioph Therapy Biol Technol. 2021; 465–506. [Google Scholar]
- 3.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N. WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018. Mar;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 4.Srinivasiah S, Bhavsar J, Thapar K, Liles M, Schoenfeld T, Wommack KE. Phages across the biosphere: contrasts of viruses in soil and aquatic environments. Res Microbiol. 2008;159(5):349–57. doi: 10.1016/j.resmic.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Oyejobi GK, Sule WF, Akinde SB, Khan FM, Ogolla F. Multidrug-resistant enteric bacteria in Nigeria and potential use of bacteriophages as biocontrol. Sci Total Environ. 2022;824:153842. doi: 10.1016/j.scitotenv.2022.153842. [DOI] [PubMed] [Google Scholar]
- 6.Loh B, Gondil VS, Manohar P, Khan FM, Yang H, Leptihn S. Encapsulation and delivery of therapeutic phages. Appl Environ Microbiol. 2021;87(5):e01979-20. doi: 10.1128/AEM.01979-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.São-José C, Costa AR, Melo LD. Bacteriophages and their lytic enzymes as alternative antibacterial therapies in the age of antibiotic resistance. Front Microbiol. 2022;13:978. doi: 10.3389/fmicb.2022.884176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin DM, Koskella B, Lin HC. Phage therapy: an alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. 2017;8(3):162. doi: 10.4292/wjgpt.v8.i3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirnay J, Verbeken G, Ceyssens P-J, Huys I, De Vos D, Ameloot C, Fauconnier A. The magistral phage. Viruses. 2018;10(2):64. doi: 10.3390/v10020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Górski A, Międzybrodzki R, Weber-Dąbrowska B, Fortuna W, Letkiewicz S, Rogóż P, Jończyk-Matysiak E, Dąbrowska K, Majewska J, Borysowski J. Phage therapy: combating infections with potential for evolving from merely a treatment for complications to targeting diseases. Front Microbiol. 2016;7:1515. doi: 10.3389/fmicb.2016.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henein A. What are the limitations on the wider therapeutic use of phage? Bacteriophage. 2013;3(2):e24872. doi: 10.4161/bact.24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pouillot F, Chomton M, Blois H, Courroux C, Noelig J, Bidet P, Bingen E, Bonacorsi S. Efficacy of bacteriophage therapy in experimental sepsis and meningitis caused by a clone O25b: h4-ST131 Escherichia coli strain producing CTX-M-15. Antimicrob Agents Chemother. 2012;56(7):3568–75. doi: 10.1128/AAC.06330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchiyama J, Rashel M, Takemura I, Wakiguchi H, Matsuzaki S. In silico and in vivo evaluation of bacteriophage φEF24C, a candidate for treatment of Enterococcus faecalis infections. Appl Environ Microbiol. 2008;74(13):4149–63. doi: 10.1128/AEM.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shivshetty N, Hosamani R, Ahmed L, Oli AK, Sannauallah S, Sharanbassappa S, Patil SA, Kelmani CR.. Experimental protection of diabetic mice against Lethal P. aeruginosa infection by bacteriophage. Biomed Res Int. 2014;2014:793242. doi: 10.1155/2014/793242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochieng’oduor JM, Onkoba N, Maloba F, Arodi WO, Nyachieo A. Efficacy of lytic Staphylococcus aureus bacteriophage against multidrug-resistant Staphylococcus aureus in mice. J Infect Dev Ctries. 2016;10(11):1208–13. doi: 10.3855/jidc.7931. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Guo G, Sun E, Song J, Yang L, Zhu L, Liang W, Hua L, Peng Z, Tang X, et al. Isolation of a T7-like lytic Pasteurella bacteriophage vB_pmup_phb01 and its potential use in therapy against Pasteurella multocida infections. Viruses. 2019;11(1):86. 10.3390/v11010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarker SA, Sultana S, Reuteler G, Moine D, Descombes P, Charton F, Bourdin G, McCallin S, Ngom-Bru C, Neville T, Akter M, Huq S, Qadri F, Talukdar K, Kassam M, Delley M, Loiseau C, Deng Y, El Aidy S, Berger B, Brüssow H.. Oral phage therapy of acute bacterial diarrhea tith cwo poliphage Preparations: arandomized trial in children from Bangladesh. EBioMedicine. 2016. Jan 5;4:124–37. doi: 10.1016/j.ebiom.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gindin M, Febvre HP, Rao S, Wallace TC, Weir TL. Bacteriophage for gastrointestinal health (PHAGE) study: evaluating the safety and tolerability of supplemental bacteriophage consumption. J Am Coll Nutr. 2019;38(1):68–75. doi: 10.1080/07315724.2018.1483783. [DOI] [PubMed] [Google Scholar]
- 19.Ooi ML, Drilling AJ, Morales S, Fong S, Moraitis S, Macias-Valle L, Vreugde S, Psaltis AJ, Wormald P-J. Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to Staphylococcus aureus. JAMA Otolaryngol–Head Neck Surg. 2019;145(8):723–29. doi: 10.1001/jamaoto.2019.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovic Fabijan A, Lin RCY, Ho J, Maddocks S, Ben Zakour NL, Iredell JR, Khalid A, Venturini C, Chard R, Morales S, et al. Westmead Bacteriophage Therapy Team. 2020. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol. 2020;5(3):465–72. doi: 10.1038/s41564-019-0634-z. [DOI] [PubMed] [Google Scholar]
- 21.Westwater C, Kasman LM, Schofield DA, Werner PA, Dolan JW, Schmidt MG, Norris JS. Use of genetically engineered phage to deliver antimicrobial agents to bacteria: an alternative therapy for treatment of bacterial infections. Antimicrob Agents Chemother. 2003;47(4):1301–07. doi: 10.1128/AAC.47.4.1301-1307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Łusiak-Szelachowska M, Żaczek M, Weber-Dabrowska B, Międzybrodzki R, Kłak M, Fortuna W, Letkiewicz S, Rogóż P, Szufnarowski K, Jończyk-Matysiak E, et al. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol. 2014;27(6):295–304. doi: 10.1089/vim.2013.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speck P, Smithyman A, Millard A. Safety and efficacy of phage therapy via the intravenous route. FEMS Microbiol Lett. 2016;363(3):fnv242. doi: 10.1093/femsle/fnv242. [DOI] [PubMed] [Google Scholar]
- 24.Żaczek M, Łusiak-Szelachowska M, Jończyk-Matysiak E, Weber-Dąbrowska B, Międzybrodzki R, Owczarek B, Kopciuch A, Fortuna W, Rogóż P, Górski A. Antibody production in response to staphylococcal MS-1 phage cocktail in patients undergoing phage therapy. Front Microbiol. 2016;7:1681. doi: 10.3389/fmicb.2016.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roach DR, Debarbieux L. Phage therapy: awakening a sleeping giant. Emerg Top Life Sci. 2017;1(1):93–103. doi: 10.1042/ETLS20170002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abedon ST. Bacteriophage clinical use as antibacterial “drugs”: utility and precedent. Microbiol Spectr. 2017 Aug;5(4). doi: 10.1128/microbiolspec.BAD-0003-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gondil VS, Chhibber S. Evading antibody mediated inactivation of bacteriophages using delivery systems. J Virol Curr Res. 2017;1:555–74. doi: 10.19080/JOJIV.2017.01.555574. [DOI] [Google Scholar]
- 28.Lu TK, Koeris MS. The next generation of bacteriophage therapy. Current opinion in microbiology. 2011;14(5):524–31. doi: 10.1016/j.mib.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 29.Azam AH, Tanji Y. Peculiarities of Staphylococcus aureus phages and their possible application in phage therapy. Appl Microbiol Biotechnol. 2019;103(11):4279–89. doi: 10.1007/s00253-019-09810-2. [DOI] [PubMed] [Google Scholar]
- 30.Moller AG, Lindsay JA, Read TD. Determinants of phage host range in Staphylococcus species. Appl Environ Microbiol. 2019;85(11):e00209–19. doi: 10.1128/AEM.00209-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kishor C, Mishra R, Saraf S, Kumar M, Srivastav A, Nath G. Phage therapy of staphylococcal chronic osteomyelitis in experimental animal model. Indian J Med Res. 2016;143(1):87. doi: 10.4103/0971-5916.178615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leiman PG, Kanamaru S, Mesyanzhinov VV, Arisaka F, Rossmann MG. Structure and morphogenesis of bacteriophage T4. Cell Mol Life Sci. 2003;60(11):2356–70. doi: 10.1007/s00018-003-3072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abatángelo V, Peressutti Bacci N, Boncompain CA, Amadio AA, Carrasco S, Suárez CA, Morbidoni HR. Broad-range lytic bacteriophages that kill Staphylococcus aureus local field strains. PloS One. 2017;12(7):e0181671. doi: 10.1371/journal.pone.0181671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper CJ, Koonjan S, Nilsson AS. Enhancing whole phage therapy and their derived antimicrobial enzymes through complex formulation. Pharmaceuticals. 2018;11(2):34. doi: 10.3390/ph11020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehman SM, Mearns G, Rankin D, Cole R, Smrekar F, Branston S, Morales S. Design and preclinical development of a phage product for the treatment of antibiotic-resistant Staphylococcus aureus infections. Viruses. 2019;11(1):88. doi: 10.3390/v11010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng C, Hanawa T, Azam AH, LeBlanc C, Ung P, Matsuda T, Onishi H, Miyanaga K, Tanji Y. Silviavirus phage ɸMR003 displays a broad host range against methicillin-resistant Staphylococcus aureus of human origin. Appl Microbiol Biotechnol. 2019;103(18):7751–65. doi: 10.1007/s00253-019-10039-2. [DOI] [PubMed] [Google Scholar]
- 37.Delisle AL, Guo M, Chalmers NI, Barcak GJ, Rousseau GM, Moineau S. Biology and genome sequence of Streptococcus mutans phage M102AD. Appl Environ Microbiol. 2012;78(7):2264–71. doi: 10.1128/AEM.07726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai Q, Zhang W, Yang Y, Tang F, Nguyen X, Liu G, Lu C. Characterization and genome sequencing of a novel bacteriophage infecting Streptococcus agalactiae with high similarity to a phage from Streptococcus pyogenes. Arch Virol. 2013;158(8):1733–41. doi: 10.1007/s00705-013-1667-x. [DOI] [PubMed] [Google Scholar]
- 39.Leprohon P, Gingras H, Ouennane S, Moineau S, Ouellette M. A genomic approach to understand interactions between Streptococcus pneumoniae and its bacteriophages. BMC Genomics. 2015;16(1):1–13. doi: 10.1186/s12864-015-2134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witold K, Sabri M, Gingras H, Ouellette M, Tremblay DM, Moineau S. Complete Genome Sequence of Streptococcus pneumoniae Virulent Phage MS1. Microbiol Resour Announc. 2017;5(28). doi: 10.1128/genomeA.00333-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonnell B, Mahony J, Hanemaaijer L, Neve H, Noben J-P, Lugli GA, Ventura M, Kouwen TR, van Sinderen D. Global survey and genome exploration of bacteriophages infecting the lactic acid bacterium Streptococcus thermophilus. Front Microbiol. 2017;8:1754. doi: 10.3389/fmicb.2017.01754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McShan WM, McCullor KA, Nguyen SV, Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Braunstein M, Rood JI. The bacteriophages of Streptococcus pyogenes. Microbiol Spectrum. 2019;7(3):7.3. 8. doi: 10.1128/microbiolspec.GPP3-0059-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duerkop BA, Palmer KL, Horsburgh MJ.. Enterococcal bacteriophages and genome defense. In: Gilmore MS, Clewell DB, Ike Y, Shankar N, editors. Enterococci: From commensals to leading causes of drug resistant infection [Internet]. Boston: Massachusetts Eye and Ear Infirmary; 2014. Feb 11. [PubMed] [Google Scholar]
- 44.Khalifa L, Gelman D, Shlezinger M, Dessal AL, Coppenhagen-Glazer S, Beyth N, Hazan R. Defeating antibiotic-and phage-resistant Enterococcus faecalis using a phage cocktail in vitro and in a clot model. Front Microbiol. 2018;9:326. doi: 10.3389/fmicb.2018.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee D, Im J, Na H, Ryu S, Yun C-H, Han SH. The novel Enterococcus phage vB_EfaS_HEf13 has broad lytic activity against clinical isolates of Enterococcus faecalis. Front Microbiol. 2019;10:2877. doi: 10.3389/fmicb.2019.02877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melo LD, Ferreira R, Costa AR, Oliveira H, Azeredo J. Efficacy and safety assessment of two enterococci phages in an in vitro biofilm wound model. Sci Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-43115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan Y, Zhao F, Wang L, Tan D, Cong C, Li X, Xu Y. Complete genome analysis of the novel Enterococcus faecalis phage vB_efas_al3. Arch Virol. 2019;164(10):2599–603. doi: 10.1007/s00705-019-04341-7 [DOI] [PubMed] [Google Scholar]
- 48.Alkalay S, Sternberg S, Coppenhagen-Glazer S, Hazan R.. Complete genome sequences of three bacillus anthracis bacteriophages. Genome Announc. 2018. Jan 4;6(1):e01164–17. doi: 10.1128/genomeA.01164-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng Q, Yuan Y. Characterization of a novel phage infecting the pathogenic multidrug-resistant Bacillus cereus and functional analysis of its endolysin. Appl Microbiol Biotechnol. 2018;102(18):7901–12. doi: 10.1007/s00253-018-9219-7. [DOI] [PubMed] [Google Scholar]
- 50.Morris JL, Letson HL, Elliott L, Grant AL, Wilkinson M, Hazratwala K, McEwen P. Evaluation of bacteriophage as an adjunct therapy for treatment of peri-prosthetic joint infection caused by Staphylococcus aureus. PloS One. 2019;14(12):e0226574. doi: 10.1371/journal.pone.0226574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chhibber S, Shukla A, Kaur S. Transfersomal phage cocktail is an effective treatment against methicillin-resistant Staphylococcus aureus-mediated skin and soft tissue infections. Antimicrob Agents Chemother. 2017;61(10):e02146-16. doi: 10.1128/AAC.02146-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geng H, Zou W, Zhang M, Xu L, Liu F, Li X, Wang L, Xu Y. Evaluation of phage therapy in the treatment of Staphylococcus aureus-induced mastitis in mice. Folia Microbiol (Praha). 2020;65(2):339–51. doi: 10.1007/s12223-019-00729-9. [DOI] [PubMed] [Google Scholar]
- 53.Capparelli R, Parlato M, Borriello G, Salvatore P, Iannelli D. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob Agents Chemother. 2007;51(8):2765–73. doi: 10.1128/AAC.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsuzaki S, Yasuda M, Nishikawa H, Kuroda M, Ujihara T, Shuin T, Shen Y, Jin Z, Fujimoto S, Nasimuzzaman M, et al. Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage φMR11. J Infect Dis. 2003;187(4):613–24. doi: 10.1086/374001. [DOI] [PubMed] [Google Scholar]
- 55.Hsieh SE, Lo HH, Chen ST, Lee MC, Tseng YH.. Wide host range and strong lytic activity of Staphylococcus aureus lytic phage Stau2. Appl Environ Microbiol. 2011. Feb;77(3):756–61. doi: 10.1128/AEM.01848-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takemura-Uchiyama I, Uchiyama J, Osanai M, Morimoto N, Asagiri T, Ujihara T, Daibata M, Sugiura T, Matsuzaki S. Experimental phage therapy against lethal lung-derived septicemia caused by Staphylococcus aureus in mice. Microb Infect. 2014;16(6):512–17. doi: 10.1016/j.micinf.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Almaghrabi MK. Isolation and characterisation of bacteriophages that infect capsulated Streptococcus pneumonia [Thesis]. University of Leicester; 2013. https://hdl.handle.net/2381/28085 [Google Scholar]
- 58.Shlezinger M, Friedman M, Houri-Haddad Y, Hazan R, Beyth N. Phages in a thermoreversible sustained-release formulation targeting E. faecalis in vitro and in vivo. PloS One. 2019;14(7):e0219599. doi: 10.1371/journal.pone.0219599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gelman D, Beyth S, Lerer V, Adler K, Poradosu-Cohen R, Coppenhagen-Glazer S, Hazan R. Combined bacteriophages and antibiotics as an efficient therapy against VRE Enterococcus faecalis in a mouse model. Res Microbiol. 2018;169(9):531–39. doi: 10.1016/j.resmic.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Manohar P, Shanthini T, Ayyanar R, Bozdogan B, Wilson A, Tamhankar AJ, Nachimuthu R, Lopes BS. The distribution of carbapenem-and colistin-resistance in Gram-negative bacteria from the Tamil Nadu region in India. J Med Microbiol. 2017;66(7):874–83. doi: 10.1099/jmm.0.000508. [DOI] [PubMed] [Google Scholar]
- 61.Shiley JR, Comfort KK, Robinson JB. Immunogenicity and antimicrobial effectiveness of Pseudomonas aeruginosa specific bacteriophage in a human lung in vitro model. Appl Microbiol Biotechnol. 2017;101(21):7977–85. doi: 10.1007/s00253-017-8504-1. [DOI] [PubMed] [Google Scholar]
- 62.Fong SA, Drilling A, Morales S, Cornet ME, Woodworth BA, Fokkens WJ, Psaltis AJ, Vreugde S, Wormald P-J. Activity of bacteriophages in removing biofilms of Pseudomonas aeruginosa isolates from chronic rhinosinusitis patients. Front Cell Infect Microbiol. 2017;7:418. doi: 10.3389/fcimb.2017.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waters EM, Neill DR, Kaman B, Sahota JS, Clokie MRJ, Winstanley C, Kadioglu A. Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa. Thorax. 2017;72(7):666–67. doi: 10.1136/thoraxjnl-2016-209265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shafique M, Alvi IA, Abbas Z, Ur Rehman S. Assessment of biofilm removal capacity of a broad host range bacteriophage JHP against Pseudomonas aeruginosa. Apmis. 2017;125(6):579–84. doi: 10.1111/apm.12691. [DOI] [PubMed] [Google Scholar]
- 65.Alvi IA, Asif M, Tabassum R, Aslam R, Abbas Z, Rehman SU. RLP, a bacteriophage of the family Podoviridae, rescues mice from bacteremia caused by multi-drug-resistant Pseudomonas aeruginosa. Arch Virol. 2020;165(6):1289–97. doi: 10.1007/s00705-020-04601-x [DOI] [PubMed] [Google Scholar]
- 66.Pabary R, Singh C, Morales S, Bush A, Alshafi K, Bilton D, Alton EWFW, Smithyman A, Davies JC. Antipseudomonal bacteriophage reduces infective burden and inflammatory response in murine lung. Antimicrob Agents Chemother. 2016;60(2):744–51. doi: 10.1128/AAC.01426-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tkhilaishvili T, Winkler T, Müller M, Perka C, Trampuz A. Bacteriophages as adjuvant to antibiotics for the treatment of periprosthetic joint infection caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019;64(1):e00924-19. doi: 10.1128/AAC.00924-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA, Narayan D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health. 2018;2018(1):60–66. doi: 10.1093/emph/eoy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ong SP, Azam AH, Sasahara T, Miyanaga K, Tanji Y. Characterization of Pseudomonas lytic phages and their application as a cocktail with antibiotics in controlling Pseudomonas aeruginosa. J Biosci Bioeng. 2020;129(6):693–99. doi: 10.1016/j.jbiosc.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Kusradze I, Karumidze N, Rigvava S, Dvalidze T, Katsitadze M, Amiranashvili I, Goderdzishvili M. Characterization and testing the efficiency of Acinetobacter baumannii phage vB-GEC_Ab-M-G7 as an antibacterial agent. Front Microbiol. 2016;7:1590. doi: 10.3389/fmicb.2016.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Regeimbal JM, Jacobs AC, Corey BW, Henry MS, Thompson MG, Pavlicek RL, Quinones J, Hannah RM, Ghebremedhin M, Crane NJ, et al. Personalized therapeutic cocktail of wild environmental phages rescues mice from Acinetobacter baumannii wound infections. Antimicrob Agents Chemother. 2016;60(10):5806–16. doi: 10.1128/AAC.02877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hua Y, Luo T, Yang Y, Dong D, Wang R, Wang Y, Xu M, Guo X, Hu F, He P. Phage therapy as a promising new treatment for lung infection caused by carbapenem-resistant Acinetobacter baumannii in mice. Front Microbiol. 2018;8:2659. doi: 10.3389/fmicb.2017.02659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou W, Feng Y, Zong Z. Two new lytic bacteriophages of the Myoviridae family against carbapenem-resistant Acinetobacter baumannii. Front Microbiol. 2018;9:850. doi: 10.3389/fmicb.2018.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin S, Huang G, Zhang Y, Jiang B, Yang Z, Dong Z, You B, Yuan Z, Hu F, Zhao Y, et al. Phage Abp1 rescues human cells and mice from infection by pan-drug resistant Acinetobacter baumannii. Cell Physiol Biochem. 2017;44(6):2337–45. doi: 10.1159/000486117. [DOI] [PubMed] [Google Scholar]
- 75.Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017;61(10):e00954-17. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumari S, Harjai K, Chhibber S. Bacteriophage versus antimicrobial agents for the treatment of murine burn wound infection caused by Klebsiella pneumoniae B5055. J Med Microbiol. 2011;60(2):205–10. doi: 10.1099/jmm.0.018580-0. [DOI] [PubMed] [Google Scholar]
- 77.Manohar P, Nachimuthu R, Lopes BS. The therapeutic potential of bacteriophages targeting gram-negative bacteria using Galleria mellonella infection model. BMC Microbiol. 2018;18(1):1–11. doi: 10.1186/s12866-018-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hsieh SE, Lo HH, Chen ST, Lee MC, Tseng YH.. Wide host range and strong lytic activity of Staphylococcus aureus lytic phage Stau2. Appl Environ Microbiol. 2011. Feb;77(3):756–61. doi: 10.1128/AEM.01848-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dufour N, Debarbieux L, Fromentin M, Ricard J-D. Treatment of highly virulent extraintestinal pathogenic Escherichia coli pneumonia with bacteriophages. Crit Care Med. 2015;43(6):e190–98. doi: 10.1097/CCM.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 80.Kaabi SAG, Musafer HK. An experimental mouse model for phage therapy of bacterial pathogens causing bacteremia. Microb Pathog. 2019;137:103770. doi: 10.1016/j.micpath.2019.103770. [DOI] [PubMed] [Google Scholar]
- 81.Matsushita K, Uchiyama J, Kato S-I, Ujihara T, Hoshiba H, Sugihara S, Muraoka A, Wakiguchi H, Matsuzaki S. Morphological and genetic analysis of three bacteriophages of Serratia marcescens isolated from environmental water. FEMS Microbiol Lett. 2009;291(2):201–08. doi: 10.1111/j.1574-6968.2008.01455.x. [DOI] [PubMed] [Google Scholar]
- 82.Mai V, Ukhanova M, Reinhard MK, Li M, Sulakvelidze A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage. 2015;5(4):e1088124. doi: 10.1080/21597081.2015.1088124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alves DR, Nzakizwanayo J, Dedi C, Olympiou C, Hanin A, Kot W, Hansen L, Lametsch R, Gahan CGM, Schellenberger P, et al. Genomic and ecogenomic characterization of Proteus mirabilis bacteriophages. Front Microbiol. 2019;10:1783. doi: 10.3389/fmicb.2019.01783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dallal MMS, Nikkhahi F, Alimohammadi M, Douraghi M, Rajabi Z, Foroushani AR, Azimi A, Fardsanei F. Phage therapy as an approach to control Salmonella enterica serotype enteritidis infection in mice. Rev Soc Bras Med Trop. 2019;52:e20190290. doi: 10.1590/0037-8682-0290-2019. [DOI] [PubMed] [Google Scholar]
- 85.Chang RYK, Chen K, Wang J, Wallin M, Britton W, Morales S, Kutter E, Li J, Chan H-K. Proof-of-principle study in a murine lung infection model of antipseudomonal activity of phage PEV20 in a dry-powder formulation. Antimicrob Agents Chemother. 2018;62(2):e01714–17. doi: 10.1128/AAC.01714-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Forti F, Roach DR, Cafora M, Pasini ME, Horner DS, Fiscarelli EV, Rossitto M, Cariani L, Briani F, Debarbieux L, et al. Design of a broad-range bacteriophage cocktail that reduces Pseudomonas aeruginosa biofilms and treats acute infections in two animal models. Antimicrob Agents Chemother. 2018;62(6):e02573–17 doi: 10.1128/AAC.02573-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fukuda K, Ishida W, Uchiyama J, Rashel M, Kato S-I, Morita T, Muraoka A, Sumi T, Matsuzaki S, Daibata M, et al. Pseudomonas aeruginosa keratitis in mice: effects of topical bacteriophage KPP12 administration. PloS One. 2012;7(10):e47742. doi: 10.1371/journal.pone.0047742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schneider G, Szentes N, Horváth M, Dorn Á, Cox A, Nagy G, Doffkay Z, Maróti G, Rákhely G, Kovács T. Kinetics of targeted phage rescue in a mouse model of systemic Escherichia coli K1. Biomed Res Int. 2018;2018:2018. doi: 10.1155/2018/7569645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brix A, Cafora M, Aureli M, Pistocchi A. Animal models to translate phage therapy to human medicine. Int J Mol Sci. 2020;21(10):3715. doi: 10.3390/ijms21103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Augustine J, Gopalakrishnan MV, Bhat SG. Application of ΦSP-1 and ΦSP-3 as a therapeutic strategy against Salmonella Enteritidis infection using Caenorhabditis elegans as model organism. FEMS Microbiol Lett. 2014;356(1):113–17. doi: 10.1111/1574-6968.12493. [DOI] [PubMed] [Google Scholar]
- 91.Głowacka-Rutkowska A, Gozdek A, Empel J, Gawor J, Żuchniewicz K, Kozińska A, Dębski J, Gromadka R, Łobocka M. The ability of lytic staphylococcal podovirus vB_saup_phiago1. 3 to coexist in equilibrium with its host facilitates the selection of host mutants of attenuated virulence but does not preclude the phage antistaphylococcal activity in a nematode infection model. Front Microbiol. 2018;9:3227. doi: 10.3389/fmicb.2018.03227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster—from microbial recognition to whole-organism physiology. Nat Rev Immunol. 2014;14(12):796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lindberg HM, McKean KA, Wang I-N. Phage fitness may help predict phage therapy efficacy. Bacteriophage. 2014;4(4):e964081. 10.4161/21597073.2014.964081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao F, Wang X, Wang L, Li Z, Che J, Wang L, Li X, Cao Z, Zhang J, Jin L, Xu Y.. Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. Biomed Res Int. 2015;2015:752930. doi: 10.1155/2015/752930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heo YJ, Lee YR, Jung HH, Lee J, Ko G, Cho YH.. Antibacterial efficacy of phages against Pseudomonas aeruginosa infections in mice and Drosophila melanogaster. Antimicrob Agents Chemother. 2009. Jun;53(6):2469–74. doi: 10.1128/AAC.01646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seed KD, Dennis JJ. Experimental bacteriophage therapy increases survival of Galleria mellonella larvae infected with clinically relevant strains of the Burkholderia cepacia complex. Antimicrob Agents Chemother. 2009;53(5):2205–08. doi: 10.1128/AAC.01166-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nale JY, Chutia M, Carr P, Hickenbotham PT, Clokie MRJ. ‘Get in early’; biofilm and wax moth (Galleria mellonella) models reveal new insights into the therapeutic potential of Clostridium difficile bacteriophages. Front Microbiol. 2016;7:1383. doi: 10.3389/fmicb.2016.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Neely MN, Pfeifer JD, Caparon M. Streptococcus-zebrafish model of bacterial pathogenesis. Infect Immun. 2002;70(7):3904–14. doi: 10.1128/IAI.70.7.3904-3914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Al-Zubidi M, Widziolek M, Court EK, Gains AF, Smith RE, Ansbro K, Alrafaie A, Evans C, Murdoch C, Mesnage S, et al. Identification of novel bacteriophages with therapeutic potential that target Enterococcus faecalis. Infect Immun. 2019;87(11):512–19. doi: 10.1128/IAI.00512-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cafora M, Deflorian G, Forti F, Ferrari L, Binelli G, Briani F, Ghisotti D, Pistocchi A. Phage therapy against Pseudomonas aeruginosa infections in a cystic fibrosis zebrafish model. Sci Rep. 2019;9(1):1527. doi: 10.1038/s41598-018-37636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahmadi M, Karimi Torshizi MA, Rahimi S, Dennehy JJ. Prophylactic bacteriophage administration more effective than post-infection administration in reducing Salmonella enterica serovar Enteritidis shedding in quail. Front Microbiol. 2016;7:1253. doi: 10.3389/fmicb.2016.01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wernicki A, Nowaczek A, Urban-Chmiel R. Bacteriophage therapy to combat bacterial infections in poultry. Virol J. 2017;14(1):1–13. doi: 10.1186/s12985-017-0849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Colom J, Cano-Sarabia M, Otero J, Aríñez-Soriano J, Cortés P, Maspoch D, Llagostera M. Microencapsulation with alginate/CaCO3: a strategy for improved phage therapy. Sci Rep. 2017;7(1):1–10. doi: 10.1038/srep41441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Colom J, Cano-Sarabia M, Otero J, Cortés P, Maspoch D, Llagostera M. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl Environ Microbiol. 2015;81(14):4841–49. doi: 10.1128/AEM.00812-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Waseh S, Hanifi-Moghaddam P, Coleman R, Masotti M, Ryan S, Foss M, MacKenzie R, Henry M, Szymanski CM, Tanha J, et al. Orally administered P22 phage tailspike protein reduces Salmonella colonization in chickens: prospects of a novel therapy against bacterial infections. PloS One. 2010;5(11):e13904. doi: 10.1371/journal.pone.0013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jang HJ, Bae HW, Cho YH.. Exploitation of Drosophila infection models to evaluate antibacterial efficacy of phages. Methods Mol Biol. 2019;1898:183–190. doi: 10.1007/978-1-4939-8940-9_15. [DOI] [PubMed] [Google Scholar]
- 107.Wills QF, Kerrigan C, Soothill JS. Experimental bacteriophage protection against Staphylococcus aureus abscesses in a rabbit model. Antimicrob Agents Chemother. 2005;49(3):1220–21. doi: 10.1128/AAC.49.3.1220-1221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abedon ST. Commentary: phage therapy of staphylococcal chronic osteomyelitis in experimental animal model. Front Microbiol. 2016 Aug 10;7:1251. doi: 10.3389/fmicb.2016.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yen M, Cairns LS, Camilli A. A cocktail of three virulent bacteriophages prevents Vibrio cholerae infection in animal models. Nat Commun. 2017;8(1):1–7. doi: 10.1038/ncomms14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nale JY, Spencer J, Hargreaves KR, Buckley AM, Trzepiński P, Douce GR, Clokie MRJ. Bacteriophage combinations significantly reduce Clostridium difficile growth in vitro and proliferation in vivo. Antimicrob Agents Chemother. 2016;60(2):968–81. doi: 10.1128/AAC.01774-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, Balloy V, Touqui L. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010;201(7):1096–104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 112.Leshkasheli L, Kutateladze M, Balarjishvili N, Bolkvadze D, Save J, Oechslin F, Que Y-A, Resch G. Efficacy of newly isolated and highly potent bacteriophages in a mouse model of extensively drug-resistant Acinetobacter baumannii bacteraemia. J Global Antimicrob Resist. 2019;19:255–61. doi: 10.1016/j.jgar.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 113.Melo LD, Oliveira H, Pires DP, Dabrowska K, Azeredo J. Phage therapy efficacy: a review of the last 10 years of preclinical studies. Crit Rev Microbiol. 2020;46(1):78–99. doi: 10.1080/1040841X.2020.1729695. [DOI] [PubMed] [Google Scholar]
- 114.Jault P, Leclerc T, Jennes S, Pirnay JP, Que Y-A, Resch G, Rousseau AF, Ravat F, Carsin H, Le Floch R, et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect Dis. 2019;19(1):35–45. doi: 10.1016/S1473-3099(18)30482-1. [DOI] [PubMed] [Google Scholar]
- 115.Leitner L, Ujmajuridze A, Chanishvili N, Goderdzishvili M, Chkonia I, Rigvava S, Chkhotua A, Changashvili G, McCallin S, Schneider MP, et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial. Lancet Infect Dis. 2021;21(3):427–36. doi: 10.1016/S1473-3099(20)30330-3. [DOI] [PubMed] [Google Scholar]
- 116.Srivastava AS, Kaido T, Carrier E. Immunological factors that affect the in vivo fate of T7 phage in the mouse. J Virol Methods. 2004;115(1):99–104. doi: 10.1016/j.jviromet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 117.Jikia D, Chkhaidze N, Imedashvili E, Mgaloblishvili I, Tsitlanadze G, Katsarava R, Glenn Morris J, Sulakvelidze A. The use of a novel biodegradable preparation capable of the sustained release of bacteriophages and ciprofloxacin, in the complex treatment of multidrug-resistant Staphylococcus aureus-infected local radiation injuries caused by exposure to Sr90. Clin Exp Dermatol: Clin Dermatol. 2005;30(1):23–26. doi: 10.1111/j.1365-2230.2004.01600.x. [DOI] [PubMed] [Google Scholar]
- 118.Hatfull GF, Dedrick RM, Schooley RT. Phage therapy for antibiotic-resistant bacterial infections. Annu Rev Med. 2022;73(1):197–211. doi: 10.1146/annurev-med-080219-122208. [DOI] [PubMed] [Google Scholar]
- 119.Petrovic Fabijan A, Lin RCY, Ho J, Maddocks S, Ben Zakour NL, Iredell JR, Khalid A, Venturini C, Chard R, Morales S, et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol. 2020;5(3):465–72. doi: 10.1038/s41564-019-0634-z. [DOI] [PubMed] [Google Scholar]
- 120.Schooley RT, Strathdee S. Treat phage like living antibiotics. Nat Microbiol. 2020;5(3):391–92. doi: 10.1038/s41564-019-0666-4. [DOI] [PubMed] [Google Scholar]
- 121.Van Nieuwenhuyse B, Van der Linden D, Chatzis O, Lood C, Wagemans J, Lavigne R, Schroven K, Paeshuyse J, de Magnée C, Sokal E, et al. Bacteriophage-antibiotic combination therapy against extensively drug-resistant Pseudomonas aeruginosa infection to allow liver transplantation in a toddler. Nat Commun. 2022;13(1):5725. doi: 10.1038/s41467-022-33294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan X, Chen H, Zhang M, Zhao Y, Jiang Y, Liu X, Huang W, Ma Y. Clinical experience of personalized phage therapy against carbapenem-resistant Acinetobacter baumannii lung infection in a patient with chronic obstructive pulmonary disease. Front Cell Infect Microbiol. 2021;11:631585. doi: 10.3389/fcimb.2021.631585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dedrick RM, Guerrero-Bustamante CA, Garlena RA, Russell DA, Ford K, Harris K, Gilmour KC, Soothill J, Jacobs-Sera D, Schooley RT, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019;25(5):730–33. doi: 10.1038/s41591-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Little JS, Dedrick RM, Freeman KG, Cristinziano M, Smith BE, Benson CA, Jhaveri TA, Baden LR, Solomon DA, Hatfull GF. Bacteriophage treatment of disseminated cutaneous Mycobacterium chelonae infection. Nat Commun. 2022;13(1):1–7. doi: 10.1038/s41467-022-29689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Llamas MA, van der Sar AM. Assessing Pseudomonas virulence with nonmammalian host: zebrafish. Methods Mol Biol. 2014;1149:709–21. doi: 10.1007/978-1-4939-0473-0_55. [DOI] [PubMed] [Google Scholar]
- 126.Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon S. Phage therapy in clinical practice: treatment of human infections. curr Pharmac Biotechnol. 2010;11(1):69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 127.Roach DR, Leung CY, Henry M, Morello E, Singh D, Di Santo JP, Weitz JS, Debarbieux L. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe. 2017;22(1):38–47. e4. doi: 10.1016/j.chom.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 128.Wright A, Hawkins CH, Änggård EE, Harper DR. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa ; a preliminary report of efficacy. Clin Otolaryngol. 2009;34(4):349–57. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 129.Rhoads D, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care. 2009;18(6):237–43. doi: 10.12968/jowc.2009.18.6.42801. [DOI] [PubMed] [Google Scholar]
- 130.Dąbrowska K, Miernikiewicz P, Piotrowicz A, Hodyra K, Owczarek B, Lecion D, Kaźmierczak Z, Letarov A, Górski A. Immunogenicity studies of proteins forming the T4 phage head surface. J Virol. 2014;88(21):12551–57. doi: 10.1128/JVI.02043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stacey HJ, De Soir S, Jones JD. The safety and efficacy of phage therapy: a systematic review of clinical and safety trials. Antibiotics. 2022;11(10):1340. doi: 10.3390/antibiotics11101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suh GA, Lodise TP, Tamma PD, Knisely JM, Alexander J, Aslam S, Barton KD, Bizzell E, Totten KMC, Campbell JL, et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob Agents Chemother. 2022;66(3):e02071-21 doi: 10.1128/aac.02071-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Uyttebroek S, Chen B, Onsea J, Ruythooren F, Debaveye Y, Devolder D, Spriet I, Depypere M, Wagemans J, Lavigne R, et al. Safety and efficacy of phage therapy in difficult-to-treat infections: a systematic review. Lancet Infect Dis. 2022;22(8):e208–20. doi: 10.1016/S1473-3099(21)00612-5. [DOI] [PubMed] [Google Scholar]
- 134.Nick JA, Dedrick RM, Gray AL, Vladar EK, Smith BE, Freeman KG, Malcolm KC, Epperson LE, Hasan NA, Hendrix J, Callahan K, Walton K, Vestal B, Wheeler E, Rysavy NM, Poch K, Caceres S, Lovell VK, Hisert KB, de Moura VC, Chatterjee D, De P, Weakly N, Martiniano SL, Lynch DA, Daley CL, Strong M, Jia F, Hatfull GF, Davidson RM.. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell. 2022. May 26;185(11):1860–1874.e12. doi: 10.1016/j.cell.2022.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin J, Du F, Long M, Li P. Limitations of phage therapy and corresponding optimization strategies: a review. Molecules. 2022;27(6):1857. doi: 10.3390/molecules27061857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gill JJ, Hyman P. Phage choice, isolation, and preparation for phage therapy. Curr Pharm Biotechnol. 2010;11(1):2–14. doi: 10.2174/138920110790725311. [DOI] [PubMed] [Google Scholar]
- 137.Carascal MB, Dela Cruz-Papa DM, Remenyi R, Cruz MCB, Destura RV. Phage revolution against multidrug-resistant clinical pathogens in Southeast Asia. Front Microbiol. 2022;13:34. doi: 10.3389/fmicb.2022.820572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tsonos J, Vandenheuvel D, Briers Y, De Greve H, Hernalsteens J-P, Lavigne R. Hurdles in bacteriophage therapy: deconstructing the parameters. Vet Microbiol. 2014;171(3–4):460–69. doi: 10.1016/j.vetmic.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 139.Fauconnier A. Phage therapy regulation: from night to dawn. Viruses. 2019;11(4):352. 10.3390/v11040352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Verbeken G, Pirnay J-P, Lavigne R, Jennes S, De Vos D, Casteels M, Huys I. Call for a dedicated European legal framework for bacteriophage therapy. Arch Immunol Ther Exp (Warsz). 2014;62(2):117–29. doi: 10.1007/s00005-014-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oechslin F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses. 2018;10(7):351. doi: 10.3390/v10070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hyman P, Abedon ST. Bacteriophage host range and bacterial resistance. Adv Appl Microbiol. 2010;70:217–48. [DOI] [PubMed] [Google Scholar]
- 143.Rath D, Amlinger L, Rath A, Lundgren M. The CRISPR-Cas immune system: biology, mechanisms and applications. Biochimie. 2015;117:119–28. doi: 10.1016/j.biochi.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 144.Deveau H, Garneau JE, Moineau S. CRISPR/Cas system and its role in phage-bacteria interactions. Annu Rev Microbiol. 2010;64(1):475–93. doi: 10.1146/annurev.micro.112408.134123. [DOI] [PubMed] [Google Scholar]
- 145.Merril CR. Interaction of bacteriophages with animals. Cambridge, UK: Cambridge University Press; 2008. p. 332–52. [Google Scholar]
- 146.Gill J, Pacan JC, Carson ME, Leslie KE, Griffiths MW, Sabour PM. Efficacy and pharmacokinetics of bacteriophage therapy in treatment of subclinical Staphylococcus aureus mastitis in lactating dairy cattle. Antimicrob Agents Chemother. 2006;50(9):2912–18. doi: 10.1128/AAC.01630-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zelasko S, Gorski A, Dabrowska K. Delivering phage therapy per os: benefits and barriers. Expert Rev Anti Infect Ther. 2017;15(2):167–79. doi: 10.1080/14787210.2017.1265447. [DOI] [PubMed] [Google Scholar]
- 148.Huh H, Wong S, St. Jean J, Slavcev R. Bacteriophage interactions with mammalian tissue: therapeutic applications. Adv Drug Deliv Rev. 2019;145:4–17. doi: 10.1016/j.addr.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 149.Goodridge LD. Designing phage therapeutics. Curr Pharm Biotechnol. 2010;11(1):15–27. doi: 10.2174/138920110790725348. [DOI] [PubMed] [Google Scholar]
- 150.Labrie SJ, Samson JE, Moineau S. Nat Rev Microbiol. 2010;8(5):317–27. 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- 151.Park K, Cha K, Myung H. Observation of inflammatory responses in mice orally fed with bacteriophage T 7. J Appl Microbiol. 2014;117(3):627–33. doi: 10.1111/jam.12565. [DOI] [PubMed] [Google Scholar]
- 152.Miernikiewicz P, Dąbrowska K, Piotrowicz A, Owczarek B, Wojas-Turek J, Kicielińska J, Rossowska J, Pajtasz-Piasecka E, Hodyra K, Macegoniuk K, et al. T4 phage and its head surface proteins do not stimulate inflammatory mediator production. PloS One. 2013;8(8):e71036. doi: 10.1371/journal.pone.0071036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hwang J-Y, Kim J-E, Song Y-J, Park J-H. Safety of using Escherichia coli bacteriophages as a sanitizing agent based on inflammatory responses in rats. Food Sci Biotechnol. 2016;25(1):355–60. doi: 10.1007/s10068-016-0050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carmody LA, Gill J, Summer E, Sajjan U, Gonzalez C, Young R, LiPuma J. Efficacy of bacteriophage therapy in a model of Burkholderia cenocepacia pulmonary infection. J Infect Dis. 2010;201(2):264–71. doi: 10.1086/649227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kaźmierczak Z, Majewska J, Miernikiewicz P, Międzybrodzki R, Nowak S, Harhala M, Lecion D, Kęska W, Owczarek B, Ciekot J, et al. Immune response to therapeutic staphylococcal bacteriophages in mammals: kinetics of induction, immunogenic structural proteins, natural and induced antibodies. Front Immunol. 2021;12:2073. doi: 10.3389/fimmu.2021.639570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van Belleghem JD, Dąbrowska K, Vaneechoutte M, Barr J, Bollyky P. Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses. 2018;11(1):10. doi: 10.3390/v11010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Balogh B, Jones J, Iriarte F, Momol M. Phage therapy for plant disease control. curr Pharmac Biotechnol. 2010;11(1):48–57. doi: 10.2174/138920110790725302. [DOI] [PubMed] [Google Scholar]
- 158.Tetz GV, Ruggles KV, Zhou H, Heguy A, Tsirigos A, Tetz V. Bacteriophages as potential new mammalian pathogens. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-07278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McVay CS, Velásquez M, Fralick JA. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob Agents Chemother. 2007;51(6):1934–38. doi: 10.1128/AAC.01028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Viscardi M, Perugini AG, Auriemma C, Capuano F, Morabito S, Kim K-P, Loessner MJ, Iovane G. Isolation and characterisation of two novel coliphages with high potential to control antibiotic-resistant pathogenic Escherichia coli (EHEC and EPEC). Int J Antimicrob Agents. 2008;31(2):152–57. doi: 10.1016/j.ijantimicag.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 161.Lenneman BR, Fernbach J, Loessner MJ, Lu TK, Kilcher S. Enhancing phage therapy through synthetic biology and genome engineering. Curr Opin Biotechnol. 2021;68:151–59. doi: 10.1016/j.copbio.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yue H, Li Y, Yang M, Mao C. T7 Phage as an Emerging Nanobiomaterial with Genetically Tunable Target Specificity. Adv Sci. 2022;9(4):2103645. doi: 10.1002/advs.202103645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Omidfar K, Daneshpour M. Advances in phage display technology for drug discovery. Expert Opin Drug Discov. 2015;10(6):651–69. doi: 10.1517/17460441.2015.1037738. [DOI] [PubMed] [Google Scholar]
- 164.Peng S-Y, You R-I, Lai M-J, Lin N-T, Chen L-K, Chang K-C. Highly potent antimicrobial modified peptides derived from the Acinetobacter baumannii phage endolysin LysAB2. Sci Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-11832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pelfrene E, Willebrand E, Cavaleiro Sanches A, Sebris Z, Cavaleri M. Bacteriophage therapy: a regulatory perspective. J Antimicrob Chemother. 2016;71(8):2071–74. doi: 10.1093/jac/dkw083. [DOI] [PubMed] [Google Scholar]
- 166.Furfaro LL, Payne MS, Chang BJ. Bacteriophage therapy: clinical trials and regulatory hurdles. Front Cell Infect Microbiol. 2018;8:376. doi: 10.3389/fcimb.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Luong T, Salabarria A-C, Edwards RA, Roach DR. Standardized bacteriophage purification for personalized phage therapy. Nat Protoc. 2020;15(9):2867–90. doi: 10.1038/s41596-020-0346-0. [DOI] [PubMed] [Google Scholar]
- 168.Cui Z, Guo X, Feng T, Li L. Exploring the whole standard operating procedure for phage therapy in clinical practice. J Transl Med. 2019;17(1):1–7. doi: 10.1186/s12967-019-2120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Morrisette T, Kebriaei R, Lev KL, Morales S, Rybak MJ. Bacteriophage therapeutics: a primer for clinicians on phage-antibiotic combinations. Pharmacotherapy: J Hum Pharmacol Drug Therapy. 2020;40(2):153–68. doi: 10.1002/phar.2358. [DOI] [PubMed] [Google Scholar]
- 170.Oechslin F, Piccardi P, Mancini S, Gabard J, Moreillon P, Entenza JM, Resch G, Que Y-A. Synergistic interaction between phage therapy and antibiotics clears Pseudomonas aeruginosa infection in endocarditis and reduces virulence. J Infect Dis. 2017;215(5):703–12. doi: 10.1093/infdis/jiw632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Akturk E, Oliveira H, Santos SB, Costa S, Kuyumcu S, Melo LDR, Azeredo J. Synergistic action of phage and antibiotics: parameters to enhance the killing efficacy against mono and dual-species biofilms. Antibiotics. 2019;8(3):103. doi: 10.3390/antibiotics8030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gayder S, Parcey M, Nesbitt D, Castle AJ, Svircev AM. Population dynamics between Erwinia amylovora, Pantoea agglomerans and bacteriophages: exploiting synergy and competition to improve phage cocktail efficacy. Microorganisms. 2020;8(9):1449. doi: 10.3390/microorganisms8091449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.García P, Martínez B, Rodríguez L, Rodríguez A. Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. Int J Food Microbiol. 2010;141(3):151–55. doi: 10.1016/j.ijfoodmicro.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 174.Titze I, Krömker V. Antimicrobial activity of a phage mixture and a lactic acid bacterium against Staphylococcus aureus from bovine mastitis. Vet Sci. 2020;7(1):31. doi: 10.3390/vetsci7010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Aslam S, Lampley E, Wooten D, Karris M, Benson C, Strathdee S, Schooley RT.. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. Open Forum Infect Dis. 2020. Aug 27;7(9):ofaa389. doi: 10.1093/ofid/ofaa389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vashisth M, Yashveer S, Anand T, Virmani N, Bera BC, Vaid RK.. Antibiotics targeting bacterial protein synthesis reduce the lytic activity of bacteriophages. Virus Res. 2022. Nov;321:198909. doi: 10.1016/j.virusres.2022.198909. [DOI] [PubMed] [Google Scholar]
- 177.Ma Y, Pacan JC, Wang Q, Xu Y, Huang X, Korenevsky A, Sabour PM. Microencapsulation of bacteriophage Felix O1 into chitosan-alginate microspheres for oral delivery. Appl Environ Microbiol. 2008;74(15):4799–805. doi: 10.1128/AEM.00246-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dini C, Islan GA, Castro GR. Characterization and stability analysis of biopolymeric matrices designed for phage-controlled release. Appl Biochem Biotechnol. 2014;174(6):2031–47. doi: 10.1007/s12010-014-1152-3. [DOI] [PubMed] [Google Scholar]
- 179.Otero J, García-Rodríguez A, Cano-Sarabia M, Maspoch D, Marcos R, Cortés P, Llagostera M. Biodistribution of liposome-encapsulated bacteriophages and their transcytosis during oral phage therapy. Front Microbiol. 2019;10:689. doi: 10.3389/fmicb.2019.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Abedon ST, Thomas-Abedon C. Phage therapy pharmacology. Curr Pharm Biotechnol. 2010;11(1):28–47. doi: 10.2174/138920110790725410. [DOI] [PubMed] [Google Scholar]
- 181.Kaur P, Gondil VS, Chhibber S. A novel wound dressing consisting of PVA-SA hybrid hydrogel membrane for topical delivery of bacteriophages and antibiotics. Int J Pharm. 2019;572:118779. doi: 10.1016/j.ijpharm.2019.118779. [DOI] [PubMed] [Google Scholar]
- 182.Gondil VS, Chhibber S. Bacteriophage and endolysin encapsulation systems: a promising strategy to improve therapeutic outcomes. Front Pharmacol. 2021;12:675440. doi: 10.3389/fphar.2021.675440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fischetti VA. Development of phage lysins as novel therapeutics: a historical perspective. Viruses. 2018;10(6):310. doi: 10.3390/v10060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Khan FM, Gondil VS, Li C, Jiang M, Li J, Yu J, Wei H, Yang H. A novel Acinetobacter baumannii bacteriophage endolysin LysAB54 with high antibacterial activity against multiple Gram-negative microbes. Front Cell Infect Microbiol. 2021;11:637313. doi: 10.3389/fcimb.2021.637313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li C, Jiang M, Khan FM, Zhao X, Wang G, Zhou W, Li J, Yu J, Li Y, Wei H, et al. Intrinsic antimicrobial peptide facilitates a new broad-spectrum lysin LysP53 to kill Acinetobacter baumannii in vitro and in a mouse burn infection model. ACS Infect Dis. 2021;7(12):3336–44. doi: 10.1021/acsinfecdis.1c00497. [DOI] [PubMed] [Google Scholar]
- 186.Waddell TE, Franklin K, Mazzocco A, Johnson RP.. Preparation and characterization of anti-phage serum. Methods Mol Biol. 2009;501:287–92. doi: 10.1007/978-1-60327-164-6_24. [DOI] [PubMed] [Google Scholar]
- 187.Abdelrahman F, Easwaran M, Daramola OI, Ragab S, Lynch S, Oduselu TJ, Khan FM, Ayobami A, Adnan F, Torrents E, et al. Phage-encoded endolysins. Antibiotics. 2021;10(2):124. doi: 10.3390/antibiotics10020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gondil VS, Harjai K, Chhibber S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int J Antimicrob Agents. 2020;55(2):105844. doi: 10.1016/j.ijantimicag.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 189.Seijsing J, Sobieraj AM, Keller N, Shen Y, Zinkernagel AS, Loessner MJ, Schmelcher M. Improved biodistribution and extended serum half-life of a bacteriophage endolysin by albumin binding domain fusion. Front Microbiol. 2018;9:2927. doi: 10.3389/fmicb.2018.02927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tagliaferri TL, Jansen M, Horz H-P. Fighting pathogenic bacteria on two fronts: phages and antibiotics as combined strategy. Front Cell Infect Microbiol. 2019;9:22. doi: 10.3389/fcimb.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gondil VS, Khan FM, Mehra N, Kumar D, Khullar A, Sharma T, Sharma A, Mehta R, Yang H. Clinical potential of bacteriophage and endolysin based therapeutics: A futuristic approach. In: Arora PK, editor. Microbial products for health, environment and agriculture. Singapore: Springer Singapore; 2021. p. 39–58. doi: 10.1007/978-981-16-1947-2_3. [DOI] [Google Scholar]
- 192.Harhala MA, Gembara K, Nelson DC, Miernikiewicz P, Dąbrowska K. Immunogenicity of Endolysin PlyC. Antibiotics. 2022;11(7):966. doi: 10.3390/antibiotics11070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim S, Lee D-W, Jin J-S, Kim J. Antimicrobial activity of LysSS, a novel phage endolysin, against Acinetobacter baumannii and Pseudomonas aeruginosa. J Global Antimicrob Resist. 2020;22:32–39. doi: 10.1016/j.jgar.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 194.Li X, Wang S, Nyaruaba R, Liu H, Yang H, Wei H. A highly active chimeric lysin with a calcium-enhanced bactericidal activity against Staphylococcus aureus in vitro and in vivo. Antibiotics. 2021;10(4):461. doi: 10.3390/antibiotics10040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang H, Zhang Y, Yu J, Huang Y, Zhang X-E, Wei H. Novel chimeric lysin with high-level antimicrobial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo. Antimicrob Agents Chemother. 2014;58(1):536–42. doi: 10.1128/AAC.01793-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yang H, Zhang H, Wang J, Yu J, Wei H. A novel chimeric lysin with robust antibacterial activity against planktonic and biofilm methicillin-resistant Staphylococcus aureus. Sci Rep. 2017;7(1):1–13. doi: 10.1038/srep40182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang H, Luo D, Etobayeva I, Li X, Gong Y, Wang S, Li Q, Xu P, Yin W, He J, et al. Linker editing of pneumococcal lysin ClyJ conveys improved bactericidal activity. Antimicrob Agents Chemother. 2020;64(2):e01610–19. doi: 10.1128/AAC.01610-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang H, Gong Y, Zhang H, Etobayeva I, Miernikiewicz P, Luo D, Li X, Zhang X, Dąbrowska K, Nelson DC, et al. ClyJ is a novel pneumococcal chimeric lysin with a cysteine-and histidine-dependent amidohydrolase/peptidase catalytic domain. Antimicrob Agents Chemother. 2019;63(4):e02043-18 doi: 10.1128/AAC.02043-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang H, Linden SB, Wang J, Yu J, Nelson DC, Wei H. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci Rep. 2015;5(1):1–12. doi: 10.1038/srep17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xu J, Yang H, Bi Y, Li W, Wei H, Li Y. Activity of the chimeric lysin ClyR against common Gram-positive oral microbes and its anticaries efficacy in rat models. Viruses. Viruses. 2018;10(7):380. 10.3390/v10070380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang L, Luo D, Gondil VS, Gong Y, Jia M, Yan D, He J, Hu S, Yang H, Wei H. Construction and characterization of a chimeric lysin ClyV with improved bactericidal activity against Streptococcus agalactiae in vitro and in vivo. Appl Microbiol Biotechnol. 2020;104(4):1609–19. doi: 10.1007/s00253-019-10325-z. [DOI] [PubMed] [Google Scholar]
- 202.Jun SY, Jung GM, Yoon SJ, Choi Y-J, Koh WS, Moon KS, Kang SH. Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob Agents Chemother. 2014;58(4):2084–88. doi: 10.1128/AAC.02232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Harhala M, Nelson D, Miernikiewicz P, Heselpoth R, Brzezicka B, Majewska J, Linden S, Shang X, Szymczak A, Lecion D, et al. Safety studies of pneumococcal endolysins Cpl-1 and Pal. Viruses; Viruses. 2018;10(11):638. doi: 10.3390/v10110638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Corsini B, Díez-Martínez R, Aguinagalde L, González-Camacho F, García-Fernández E, Letrado P, García P, Yuste J. Chemotherapy with phage lysins reduces pneumococcal colonization of the respiratory tract. Antimicrob Agents Chemother. 2018;62(6):e02212–17. doi: 10.1128/AAC.02212-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang Z, Ma J, Wang J, Yang D, Kong L, Fu Q, Cheng Y, Wang H, Yan Y, Sun J. Application of the phage lysin Ply5218 in the treatment of Streptococcus suis infection in piglets. Viruses. Viruses. 2019;11(8):715. doi: 10.3390/v11080715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vázquez R, García P. Synergy between two chimeric lysins to kill Streptococcus pneumoniae. Front Microbiol. 2019;10:1251. doi: 10.3389/fmicb.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Letrado P, Corsini B, Díez-Martínez R, Bustamante N, Yuste JE, García P. Bactericidal synergism between antibiotics and phage endolysin Cpl-711 to kill multidrug-resistant pneumococcus. Future Microbiol. 2018;13(11):1215–23. doi: 10.2217/fmb-2018-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shah SU, Xiong YQ, Abdelhady W, Iwaz J, Pak Y, Schuch R, Cassino C, Lehoux D, Bayer AS. Effect of the lysin exebacase on cardiac vegetation progression in a rabbit model of methicillin-resistant Staphylococcus aureus endocarditis as determined by echocardiography. Antimicrob Agents Chemother. 2020;64(7):e00482-20. doi: 10.1128/AAC.00482-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Karau MJ, Schmidt-Malan SM, Yan Q, Greenwood-Quaintance KE, Mandrekar J, Lehoux D, Schuch R, Cassino C, Patel R. Exebacase in addition to daptomycin is more active than daptomycin or exebacase alone in methicillin-resistant Staphylococcus aureus osteomyelitis in rats. Antimicrob Agents Chemother. 2019;63(10):e01235-19. doi: 10.1128/AAC.01235-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Imanishi I, Uchiyama J, Tsukui T, Hisatsune J, Ide K, Matsuzaki S, Sugai M, Nishifuji K. Therapeutic potential of an endolysin derived from kayvirus S25-3 for staphylococcal impetigo. Viruses. 2019;11(9):769. doi: 10.3390/v11090769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Blasco L, Ambroa A, Trastoy R, Bleriot I, Moscoso M, Fernández-Garcia L, Perez-Nadales E, Fernández-Cuenca F, Torre-Cisneros J, Oteo-Iglesias J, et al. In vitro and in vivo efficacy of combinations of colistin and different endolysins against clinical strains of multi-drug resistant pathogens. Sci Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-64145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wu M, Hu K, Xie Y, Liu Y, Mu D, Guo H, Zhang Z, Zhang Y, Chang D, Shi Y. A novel phage PD-6A3, and its endolysin Ply6A3, with extended lytic activity against Acinetobacter baumannii. Front Microbiol. 2019;9:3302. doi: 10.3389/fmicb.2018.03302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cheng M, Zhang L, Zhang H, Li X, Wang Y, Xia F, Wang B, Cai R, Guo Z, Zhang Y, et al. An ointment consisting of the phage lysin LysGH15 and apigenin for decolonization of methicillin-resistant Staphylococcus aureus from skin wounds. Viruses. 2018;10(5):244. doi: 10.3390/v10050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Raz A, Serrano A, Hernandez A, Euler CW, Fischetti VA. Isolation of phage lysins that effectively kill Pseudomonas aeruginosa in mouse models of lung and skin infection. Antimicrob Agents Chemother. 2019;63(7):e00024-19. doi: 10.1128/AAC.00024-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gutiérrez D, Garrido V, Fernández L, Portilla S, Rodríguez A, Grilló MJ, García P. Phage lytic protein LysRODI prevents staphylococcal mastitis in mice. Front Microbiol. 2020;11:7. doi: 10.3389/fmicb.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang F, Ji X, Li Q, Zhang G, Peng J, Hai J, Zhang Y, Ci B, Li H, Xiong Y, et al. Tspphg Lysin from the extremophilic thermus bacteriophage TSP4 as a potential antimicrobial agent against both gram-negative and gram-positive pathogenic bacteria. Viruses. 2020;12(2):192. doi: 10.3390/v12020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Heselpoth RD, Euler CW, Schuch R, Fischetti VA. Lysocins: bioengineered antimicrobials that deliver lysins across the outer membrane of Gram-negative bacteria. Antimicrob Agents Chemother. 2019;63(6):e00342-19. doi: 10.1128/AAC.00342-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fraga AG, Trigo G, Murthy RK, Akhtar S, Hebbur M, Pacheco AR, Dominguez J, Silva-Gomes R, Gonçalves CM, Oliveira H, et al. Antimicrobial activity of Mycobacteriophage D29 Lysin B during Mycobacterium ulcerans infection. PLoS Negl Trop Dis. 2019;13(8):e0007113 doi: 10.1371/journal.pntd.0007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Manohar P, Loh B, Elangovan N, Loganathan A, Nachimuthu R, Leptihn S. A multiwell-plate Caenorhabditis elegans assay for assessing the therapeutic potential of Bacteriophages against Clinical Pathogens. Microbiol Spectrum. 2022;10(1):e01393-21. doi: 10.1128/spectrum.01393-21. [DOI] [PMC free article] [PubMed] [Google Scholar]


