Abstract
Background and Aims
Desiccation-tolerant vascular plants (DT plants) are able to tolerate the desiccation of their vegetative tissues; as a result, two untested paradigms can be found in the literature, despite contradictions to theoretical premises and empirical findings. First, it is widely accepted that DT plants form a convergent group of specialist plants to water deficit conditions. A derived paradigm is that DT plants are placed at the extreme end of stress tolerance. Here, we tested the hypotheses that DT plants (1) are in fact convergent specialists for water deficit conditions and (2) exhibit ecological strategies related to stress tolerance, conservative resource-use and survival.
Methods
We used biogeographical and functional-traits approaches to address the mentioned paradigms and assess the species’ ecological strategies. For this, 27 DT plants were used and compared to 27 phylogenetically related desiccation-sensitive vascular plants (DS plants).
Key Results
We could not confirm either of the two hypotheses. We found that despite converging in desiccation tolerance, DT plants differ in relation to the conditions in which they occur and the ecological strategies they use to deal with water deficit. We found that some DT plants exhibit advantageous responses for higher growth and resource acquisition, which are suitable responses to cope with more productive conditions or with higher disturbance. We discuss that the ability to tolerate desiccation could compensate for a drought vulnerability promoted by higher investment in growth and bring advantages to deal with quick and pronounced variation of water, rather than to drought solely.
Conclusions
DT plants are not only selected by drought as an environmental constraint. The alternative functional designs could promote the diversity of ecological strategies, which preclude their convergence to the same resources and conditions. Thus, DT plants are a heterogeneous group of plants in how they deal with drought, despite their desiccation tolerance ability.
Keywords: Biogeography, desiccation tolerance, desiccation-tolerant vascular plants, ecological strategies, drought, functional traits, resource-use, resurrection plants, species–environment relationship, stress tolerance, water deficit
INTRODUCTION
The differential performance of organisms across resources and conditions promotes patterns correlating species and the environment (Chase and Leibold, 2003). Based on this idea, biologists have historically attempted to understand ecological processes by approaching the species’ responses to the environment (Lavorel and Garnier, 2002; Funk et al., 2017). However, oversimplifying our understanding of species–environment relationships based on single responses can entail misleading assumptions. For instance, species classifications based on one trait hinders the importance of multiple trade-offs that arise from an array of traits that influence the performance of organisms (Dias et al., 2020; Shipley et al., 2016; Funk et al., 2017). This is because traits can interact and produce alternative functional designs in which species exhibit equivalent fitness for similar resources and conditions, loosening an individualized trait selection (Marks and Lechowicz, 2006; Pistón et al., 2019; Dias et al., 2020). In other words, a trait alone may not necessarily indicate the action of a selective force for species occurrence in a given habitat. Thus, inferences must be carefully drawn when correlating the species’ response to the ecological processes shaping the distribution of species across environments.
Desiccation-tolerant vascular plants (DT plants) comprise a polyphyletic group of plants able to tolerate desiccation (i.e. <13–20 % of protoplasmic water). Because of that, they are reported to be convergent specialists for water deficit conditions (e.g. Gaff, 1977; Alpert, 2000, 2005; Porembski and Barthlott, 2000; Marks et al., 2021). This paradigm is reinforced by the notion that most DT plants, from different phylogenetic lineages (i.e. convergence when less related entities seem more related than they phylogenetically are; Doolittle, 1994), have their occurrence strongly linked to ecosystems distinguished by periods of water deficit (i.e. many of those species are considered rock outcrop specialists; Porembski and Barthlott, 2000; Marks et al., 2021). Because desiccation tolerance is understood as such (Volaire, 2018), a second and derived paradigm suggests that all DT plants are placed at the extreme end of stress tolerance, with the most conservative resource-use to guarantee survival at the cost of faster growth (e.g. Alpert and Oliver, 2002; Bartels, 2005; Teodoro et al., 2021). This implies that DT plants lack advantageous traits for rapid growth in productive conditions or compensate for biomass loss. That is, DT plants would fail to compete for resources or fail to occur when drought exceeds leaves’ capacity to tolerate desiccation. These paradigms have never been tested and lead to the assumption that all DT plants are exclusively selected by water deficit playing a selective role as a stressful factor (sensuGrime, 1977). Consequently, the impact of competition and disturbance on DT plants might be overestimated (e.g. Gaff and Bole, 1986; Porembski, 2000; Alpert and Oliver, 2002).
However, specialist plants are expected to exhibit responses in which costs and benefits are supposedly more advantageous in a narrower range of resources and conditions. In other words, specialists have traits only suitable to specific ecological conditions. This ecological specialization can reflect limited plasticity or low intraspecific variability (Levins, 1962; MacArthur and Levins, 1964; Devictor et al., 2010). However, DT plants respond to the water variability with morpho-anatomical and physiological plasticity (e.g. leaf folding and accumulation of sugars during drying; Oliver et al., 2000; Porembski and Barthlott, 2000; do Nascimento et al., 2020; Marks et al., 2021; Porembski et al., 2021). They are also likely to exhibit high genetic diversity due to their occurrence in terrestrial island ecosystems (de Paula et al., 2017; Rexroth et al., 2019; Porembski et al., 2021).
Furthermore, it has been widely reported that DT plants differ in their traits, how they cope with desiccation, and the drought conditions in which they occur (e.g. Gaff and Latz, 1978; Gaff, 1986; Gaff and Bole, 1986; Meirelles et al., 1997; Marks et al., 2021). This could mean DT plants do not necessarily overlap in their ecological niche when water deficit is considered, despite their convergent desiccation tolerance response. De Paula et al. (2015) reported that some DT plants show competitive capacities in productive conditions, similar to coexisting species that cannot tolerate desiccation (i.e. desiccation-sensitive vascular plants; DS plants). Similarly, Alcantara et al. (2015) showed that DT plants are as productive as phylogenetically related DS plants when moisture conditions are favourable for their growth. These two findings agree with theoretical expectations that DT plants grow and reproduce before coexisting species do so when water is available (Scott, 2000; Bartels, 2005). Still, this might not necessarily be true for all DT plants. For example, Teodoro et al. (2021) found that a DT plant exhibited lower growth when compared to a phylogenetically related DS plant, even under high moisture conditions.
The contradictions and lack of agreement across theoretical and empirical studies highlight the need for a deeper understanding of the ecological aspects that shape the diversity and distribution of DT plants. Here, erroneous assumptions could negatively affect efforts for their conservation and potential use for biotechnological purposes. In this study, we aimed to evaluate paradigms of DT plants’ convergent ecological specialization for water deficit conditions and their placement at the extreme end of stress tolerance, attempting to test the hypotheses that (1) DT plants are convergent regarding their ecological specialization to water deficit conditions when compared to DS plants, and that (2) DT plants exhibit ecological strategies more related to stress tolerance, conservative resource-use and survival when compared to DS plants. We combined biogeographical and functional approaches to address the questions raised.
MATERIALS AND METHODS
Study species
DT plants can be found in all continents, except Antarctica, although the phylogenetic lineages are unevenly distributed across the globe (Marks et al., 2021). This is because desiccation tolerance of vegetative tissues re-evolved multiple times within tracheophytes due to independent evolutionary events. Desiccation tolerance is found in pteridophytes (e.g. Pteridaceae), monocots (e.g. Velloziaceae) and eudicots (e.g. Gesneriaceae; Oliver et al., 2000; Marks et al., 2021). Plants can overcome desiccation by keeping chlorophyll when desiccating (i.e. homoiochlorophyllous plants) or dismantling the photosynthetic apparatus (i.e. poikilochlorophyllous plants; Marks et al., 2021; Porembski et al., 2021). Losing chlorophyll would allow poikilochlorophyllous plants to cope with desiccation under drought conditions that homoiochlorophyllous plants are not expected to tolerate (Tuba, 2008; Oliver et al., 2020; Marks et al., 2021). Such mechanisms are considered to promote differences in the conditions and resources under which they grow (Gaff, 1977; Gaff and Latz, 1978; Meirelles et al., 1997; Oliver et al., 2020; Marks et al., 2021). Still, exceptions are found. For instance, Selaginellaceae species are homoiochlorophyllous and inhabit exposed locations where poikilochlorophyllous plants are expected to be recorded. Overall, all DT plants occur in habitats with a marked lack of soil, that are prone to quick water depletion and have high solar radiation (e.g. rock outcrops and the canopy; Marks et al., 2021).
Species selection
Assuming a higher variability between species than within them, we selected DT and DS plants based on information on functional traits available for the analyses. First, we selected 27 DT plants whose desiccation tolerance was identified by previous studies (Supplementary Data Table S1). For the DT plants, we used individuals cultivated in the glasshouses from the Botanical Garden of the University of Rostock (Germany) and the Plant Ecology Lab of the University of the State of Rio de Janeiro (Brazil). Complementarily, we included DT plants whose leaf trait information was available in de Paula et al. (2015) or in the TRY database (Kattge et al., 2020).
To promote a balanced contrast, we selected 27 DS plants considering their maximum phylogenetic relatedness with the selected DT plants, whenever possible. We also took into account the availability of leaf trait information in the above-mentioned sources (i.e. de Paula et al., 2015 and TRY database) for selection of DS plants. We selected species with the highest geographical and ecological variability possible to bring together a very heterogeneous group of DS plants with regard to water deficit conditions (i.e. generalist and specialist plants in relation to this constraint). For example, species with different distribution ranges (e.g. the widespread Juncus inflexus and the Southeastern Brazil endemic Pitcairnia azouryi; POWO, 2022), divergent habitat distributions (e.g. Pseudolaelia vellozicola, an epiphyte on Velloziaceae, and Echinochloa crus-galli, occurrence of which is extended from forests to wetlands; Porembski, 2003; POWO, 2022), and displaying different strategies to cope with drought (e.g. the succulent Prescottia montana and the annual Melinis repens; POWO, 2022).
The paradigm of DT plants’ convergent ecological specialization for water deficit
For the biogeographical approach, we performed Outlying Mean Index analysis (Dolédec et al., 2000) to identify the habitat affinities of DT and DS plants across drought-related climatic variables. This method uses the climatic information from where species occur to construct an ordination which describes species’ climatic niches. This analysis gives a marginality index (MI) and niche breadth value (NB) as descriptors of species ecological specialization. Additionally, we obtained the species’ mean niche position along the environmental gradients (NP), which describes the species’ affinity for water deficit.
We obtained species occurrence records from the databases ‘Tropicos’ (http://tropicos.org), Global Biodiversity Information Facility (GBIF; https://www.gbif.org/) and ‘Species Link’ (http://splink.cria.org.br/). We excluded duplicated, erroneous and uncertain data according to the database Plants of the World Online (POWO, 2022). Then, a presence–absence matrix for all used species was generated, identifying geographical locations, referred to as sampling units, which could contain one or more species. For every sampling unit, we assessed the environmental information regarding the following five climatic variables related to water deficit: (1) Thornthwaite’s aridity index, (2) climatic water deficit, (3) drought intensity, (4) drought frequency and (5) drought length. Higher values of Thornthwaite’s aridity index and climatic water deficit describe higher water deficit for a given location. Higher values of drought intensity, frequency and length describe more intense, frequent and extensive drought events, respectively.
To obtain Thornthwaite’s aridity index, we divided the cumulative monthly difference between precipitation and Thornthwaite’s potential evapotranspiration during the year by the modulus of the cumulative potential evapotranspiration over the same period (Thornthwaite, 1948). We calculated the climatic water deficit using absolute values of the cumulative monthly difference between precipitation and Thornthwaite’s potential evapotranspiration throughout the year (Esquivel-Muelbert et al., 2017). For drought intensity, frequency and length, a drought event was defined by a given set of consecutive dry months according to the Standardized Precipitation Evapotranspiration Index on a time scale of 1 month and within the period from January 1901 to December 2018. We estimated drought intensity as the whole-period average of cumulative Standardized Precipitation Evapotranspiration Index scores within the drought events. We assessed drought frequency by counting drought events within the period. Finally, we calculated drought length as the whole-period average of the number of months within a drought event. We derived all the climatic variables from climatic datasets obtained from the Worldclim (https://worldclim.org/) and Standardized Precipitation Evapotranspiration Index (https://spei.csic.es) databases.
For the functional approach, we applied the method proposed by Shipley et al. (2017) to predict species Ellenberg Indicator values for moisture (EIVM) throughout functional traits. We assessed the species’ EIVM by calculating their habitat affinities for the nine first ordinal Ellenberg classes for soil moisture (aquatic habitats were excluded from moisture-level gradient). We described habitat affinities based on the probability of a species being classified in a given EIVM, in which higher probabilities would describe higher affinities. Then, we generated EIVM habitat affinity curves for each species. To calculate species’ EIVM, specific leaf area (SLA), leaf dry matter content (LDMC), leaf area (LA) and seed mass (SM) were either (1) measured for DT and DS plants following the methods proposed by Pérez-Harguindeguy et al. (2013), (2) obtained from the TRY database (i.e. trait numbers 3109, 3115, 47 and 26, respectively) or (3) collected from de Paula et al. (2015). We used at least two leaf replicates from five different individuals for the trait measurements whenever possible. We assessed the functional traits using an oven (T12, Heraeus Instruments, Hanau, Germany), analytical scale (SBC33, Scaltec, Heilingenstadt, Germany), and image scanner (CanoScan LiDE 220, Canon, Amstelveen, The Netherlands). Then, we processed leaf areas in ImageJ software (Schneider et al., 2012). We considered species with trait values from at least three individuals for the species from the TRY database. Pteridophytes were not used in this analysis because they do not produce seed for SM assessment.
Ecological convergence
We used the overlapping index (OV) as a proxy for species convergence to similar ecological conditions in relation to the Outlying Mean Index analysis ordination axes and EIVM. A higher ecological overlap (i.e. higher OV values) indicates higher convergence between species. We performed multiple pairwise comparisons to estimate the OV, as described by Pastore and Calcagnì (2019). For the Outlying Mean Index analysis ordination axes, we performed kernel density estimations using species’ individuals, while for EIVM, we used the habitat affinity curves. Considering that the desiccation tolerance response can describe ecological convergence for species, DT plants were expected to exhibit higher ecological overlap with each other than when compared to DS plants.
Ecological specialization
For the Outlying Mean Index analysis results, we used MI, NB and Pearson’s measure of kurtosis (PK) over kernel density estimations for species individuals’ distribution along the two first axes of the ordination. Complementarily, we calculated PK for the species’ EIVM curves (Supplementary Data Table S2). Species ecologically more distant from the average conditions (i.e. higher MI) or with narrower niche breadth along the environmental gradients (i.e. lower NB) are expected to display higher ecological specialization. PK describes the sharpness of the peak for species’ optimal conditions. Thus, species whose optimal conditions are more restricted to certain scores of the ordination axes or EIVM (i.e. higher PK values) were regarded as more specialist in relation to moisture conditions. In this framework, it was expected that DT plants exhibit a higher MI, lower NB and higher PK when compared to DS plants.
Affinity for water deficit conditions
We used NP along the two first axes of the Outlying Mean Index analysis ordination and from the EIVM in which species exhibited the highest score (HSEIVM) to describe species’ affinity for water deficit conditions. The positive correlation between NP and the environmental variables related to water deficit indicates a higher affinity of species for such conditions, as the HSEIVM for lower EIVM reflects a higher affinity for lower soil moisture levels. Therefore, DT plants were expected to differ from DS plants by exhibiting a stronger positive correlation with the water deficit variables and higher scores for lower EIVM.
The paradigm of DT plants in the extreme end of stress tolerance
First, we conducted the globally calibrated method presented by Pierce et al. (2017) to estimate species ecological strategies according to Grime’s CSR scheme (C-selection – competitiveness, R-selection – ruderalism, S-selection – stress tolerance). In this scheme, C-selection refers to the ability of plants to compete for resources in productive habitats, R-selection denotes plants’ capacity to cope with external constraints that lead individuals to a biomass loss, and S-selection describes plants’ ability to deal with external constraints that restrict individuals’ growth. For this, we assessed the functional traits LA, SLA and LDMC as described above and used the stratefy tool to calculate species affinities for C-selection, S-selection and R-selection of the CSR scheme (Supplementary Data Table S3).
Then, we applied the procedure proposed by Westoby (1998) for estimation of the species ecological strategies within the LHS scheme (L – leaf; H – height; S – seed). SLA, plant height at maturity and SM describe the L, H and S components, respectively. We measured both SLA and SM as discussed previously. In addition, we estimated the canopy height at maturity (1) by measuring the plant height according to the procedure proposed by Pérez-Harguindeguy et al. (2013), (2) obtained from the TRY database, (3) collected from de Paula et al. (2015) or (4) using species’ voucher information. We log-transformed all the three functional traits obtained for the LHS scheme estimation. We did not use pteridophytes in the LHS estimations due to their lack of seeds for SM assessments.
Trade-offs between resource acquisition enhancing growth and resource conservation ensuring survival reflect the species’ ability to cope with environmental factors and their position within the CSR and LHS schemes (Westoby, 1998; Reich, 2014; Pierce et al., 2017). A higher score for S-selection strategy corresponds to stress tolerance, conservative resource-use and survival. For the LHS scheme, plans with low SLA, low H and higher SM can be related to stress tolerance, conservative resource-use and survival strategies (Westoby, 1998; Niinemets, 2001; Lavergne et al., 2003; Koch et al., 2004; Moles et al., 2005; Poorter and Rozendaal, 2008; Bolmgren and Eriksson, 2010). While the CSR scheme assumes that the trade-offs at the leaf level scale to whole-plant and reproductive trade-offs (Pierce et al., 2017), the LHS scheme includes direct measurement of plant reproductive strategies (Westoby, 1998). All functional traits were measured as described above.
Data analyses
First, we split the DT and DS plants into three main phylogenetic groups: pteridophytes, monocots and eudicots (Marks et al., 2021). Then, we performed analyses of covariance (ANCOVAs) to evaluate the differences between DT and DS plants. For the ANOVA’s first covariate regarding species’ ecological convergence (i.e. OV), we grouped pairwise comparisons between two DT plants as ‘desiccation-tolerance × desiccation-tolerance’ and comparisons between a DT and a DS plant as ‘desiccation-tolerance × desiccation-sensitive’. For the second covariate, we grouped pairwise comparisons within one of the three main phylogenetic groups as ‘same phylogenetic groups’ (e.g. pteridophytes × pteridophytes) and across phylogenetic groups as ‘different phylogenetic groups’ (e.g. pteridophytes × monocots). To find significant differences in PK, MI, NB, NP, C-selection, S-selection, R-selection, L, H and S between DT and DS plants, we used the species’ response to desiccation as a first covariate (i.e. desiccation-tolerant and desiccation-sensitive), and the phylogenetic groups as the second covariate (i.e. pteridophytes, monocots and eudicots). We chose this analysis to evaluate the differences between DT and DS plants while controlling for the effect of phylogenetic inertia on species scores (Blomberg and Garland, 2002). Whenever the assumptions for parametric analysis were not fulfilled, we performed Box–Cox transformations (Box and Cox, 1964) before conducting the ANCOVAs. To minimize the chance of inflating the type I error rate (Jafari and Ansari-Pour, 2019), the Bonferroni correction method was applied to the ANCOVA P-values. The Bonferroni correction was applied always comparisons were performed using different response variable as alternative proxies to investigate the same ecological question.
To analyse HSEIVM for DT and DS plants, we conducted a χ2 test to verify if the species’ highest probability of being classified to a given EIVM is independent of its response to desiccation. We chose the χ² test because it indicates if the distribution of species along EIVM is statistically related to desiccation tolerance or does not differ from what is expected by chance.
Assessment of species’ geographical distribution and to obtaing climate data, besides calculations of indices, probabilities and analyses, were performed with R software (R Core Team, 2021).
RESULTS
The paradigm of DT plants’ convergent ecological specialization for water deficit
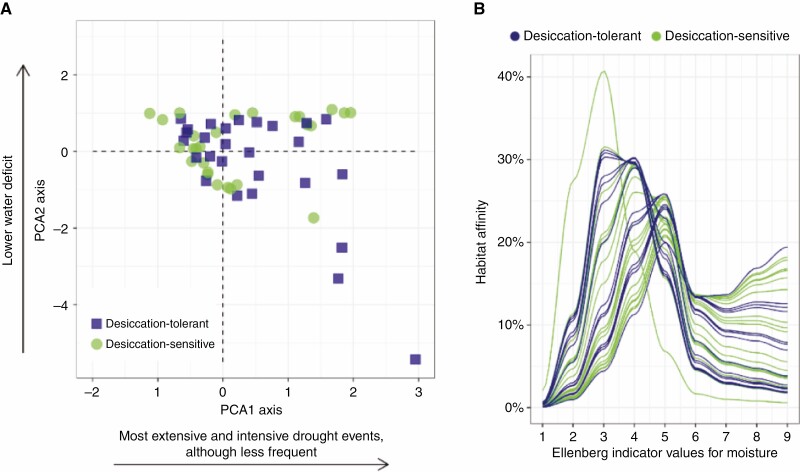
In general, we did not observe differences between DT and DS plants in the conducted analyses. The Outlying Mean Index analysis PCA1 axis was positively correlated with more extensive and intensive, although less frequent, drought events while the Outlying Mean Index analysis PCA2 axis was negatively correlated with higher water deficit (Fig. 1). Regarding species EIVM, it was not possible to delimit between DT and DS plants. Species from both functional groups were more closely related to soil moisture between the Ellenberg ordinal classes 3 and 5.
Fig. 1.
Species’ correlation to water deficit, by their response to desiccation. (A) Species distribution in relation to the two first axes of the outlying mean index analysis. (B) Species’ habitat affinities in relation to Ellenberg indicator values for moisture.
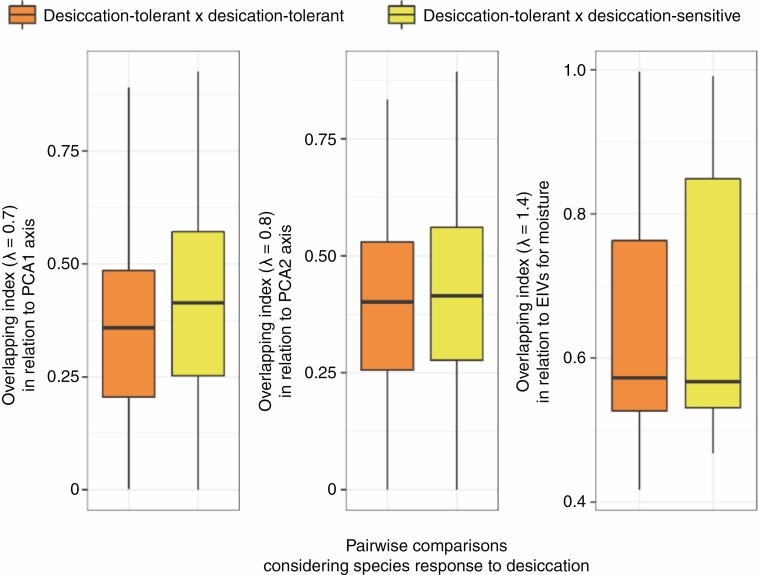
In relation to the occurrence of species’ individuals along the first axis of the Outlying Mean Index analysis ordination, we found a significantly higher ecological overlapping (OVPCA1: F = 14.9631, adjusted P = 0.0003; Fig. 2; Supplementary Data Table S4) in desiccation-tolerance × desiccation-sensitive comparisons (OVPCA1 μ = 0.4124) than in desiccation-tolerance × desiccation-tolerance comparisons (OVPCA1 μ = 0.3616). However, no significant difference was found when the axis PCA2 was considered (OVPCA2: F = 5.5153, adjusted P = 0.057). Similarly, the ecological overlap between DT plants was not significantly different from the ecological overlap between DT and DS plants regarding their EIVM (OVEIVM: F = 0.5014, adjusted P = 1).
Fig. 2.
Species’ convergence in relation to water deficit, according to the outlying mean index analysis and Ellenberg indicator values for moisture (EIVM), in which the pairwise overlapping index between desiccation-tolerant and desiccation-sensitive vascular plants is compared to the pairwise overlapping index between desiccation-tolerant species.
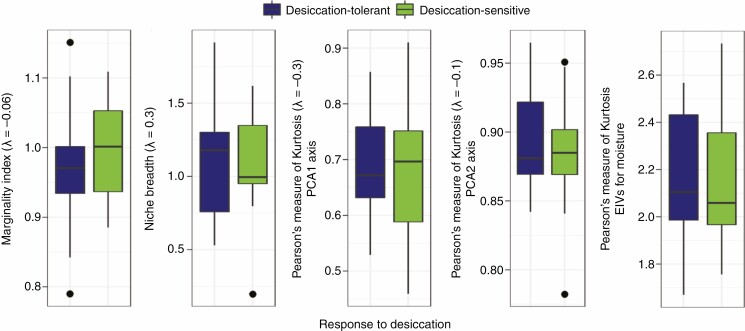
We did not find a significant effect of species response to desiccation on the overall variability in species distribution shape along the two first Outlying Mean Index analysis axes (PKPCA1: F = 0.1516, adjusted P = 1; PKPCA2: F = 0.9352, adjusted P = 1), species ecological distance from the average conditions (MI: F = 1.4029, adjusted P = 1) and species niche breadth along the environmental gradients (NB: F = 0.0093, adjusted P = 1; Fig. 3; Supplementary Data Table S5). In addition, we did not find a significant difference in the habitat affinities between DT and DS plants along a soil moisture gradient (PKEIVM: F = 0.0513, d.f. = 31, adjusted P = 1).
Fig. 3.
Comparisons of species ecological specialization along water deficit gradients according to outlying mean index analysis and Ellenberg indicator values for moisture (EIVM), in relation to their response to desiccation.
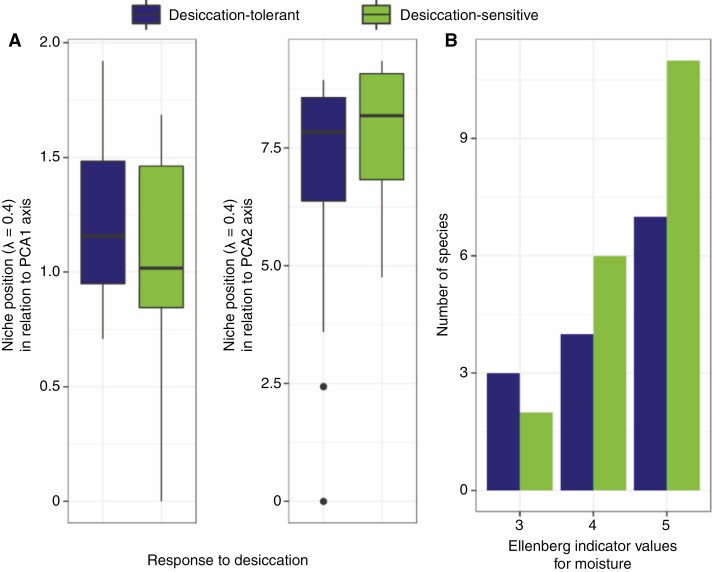
The niche position along the two first Outlying Mean Index analysis axes between DT and DS plants was not significantly different (NPPCA1: F = 1.4899, adjusted P-value = 0.4560; NPPCA2: F = 2.7050, adjusted P = 0.2126; Fig. 4). Besides, the species’ HSEIVM was independent of the species strategies to cope with desiccation (HSEIVM: χ2 = 0.7485, P = 0.7866).
Fig. 4.
Comparisons of species affinity for water deficit according to outlying mean index analysis and Ellenberg indicator values for moisture, in relation to their response to desiccation. (A) Species’ niche position in relation to the outlying mean index analysis two first axes. (B) Number of species by the Ellenberg indicator value in which their higher habitat affinity was registered.
The paradigm of DT plants in the extreme end of stress tolerance
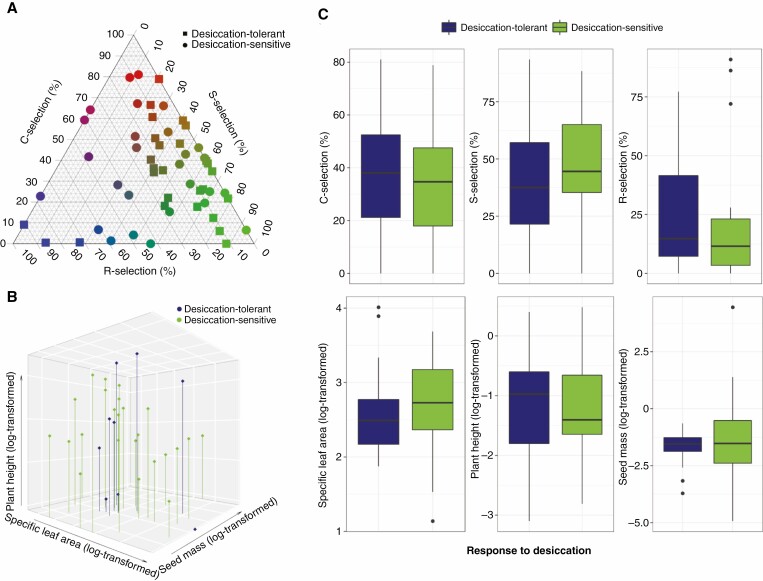
It was not possible to observe a distinction between DT and DS plants either in the CSR scheme or in the LHS scheme (Fig. 5; Supplementary Data Table S6). No significant effect of species response to desiccation was found on the variability of their relative proportion of C-selection (F = 0.3771, adjusted P = 1), S-selection (F = 2.31, adjusted P = 0.4044) and R-selection (F = 1.3553, adjusted P = 0.7497). The species’ SLA did not differ between DT and DS plants (L: F = 0.9837, adjusted P = 0.9783), as well as plants’ H (H: F = 0.018, adjusted P = 1) and SM (S: F = 0.156, adjusted P = 1).
Fig. 5.
Ecological strategies of desiccation-tolerant and desiccation-sensitive vascular plants according to Grime’s CSR and Westoby’s LHS schemes. (A) Species mean position in the CSR scheme. (B) Species mean position in the LHS scheme. (C) Comparisons of the species’ relative proportion of C-selection, S-selection, R-selection, specific leaf area, plant height and seed mass in relation to species response to desiccation.
DISCUSSION
We did not find a convergent ecological specialization to water deficit conditions for the DT plants. They also did not exhibit ecological strategies more related to stress tolerance, conservative resource-use and survival than DS plants. We suggest that species are far more complex than having their ecological niches defined only by one response to the environment, as ecosystems do not have just one prominent process exclusively explaining species occupancy.
DT plants were not a homogeneous group in any of the aspects covered by this study. The DT plants’ convergence in their response to desiccation should be carefully used to underpin assumptions of species convergence in other regards (Fig. 6). Convergence depends on the specific response or aspect of the environment in question (Winemiller et al., 2015; Funk et al., 2017; Pistón et al., 2019). For example, because of their ability to tolerate desiccation, all desiccation-tolerant plants can cope with drought (i.e. water deficit restricting growth of DP plants). However, it does not mean that all DT plants have the same fitness when facing drought (i.e. water deficit promoting biomass loss, such as when leaves cannot rehydrate or completely re-green).
Fig. 6.
The diversity of desiccation-tolerant vascular plants, in relation to their phylogenetic and geographical distribution, morphology and ecological strategies.
We should not neglect the statistical improbability of different evolutionary events generating the same complex genetic outcome (Gould, 1970). Some DT plants construct small, expensive and long-lived leaves with slow returns on biomass investment (e.g. the higher S-selection in most monocots). Others invest in long-lived leaves with a higher light interception, which is advantageous in productive conditions (e.g. higher C-selection in many pteridophytes and eudicots). Adding more heterogeneity, it is also possible to find DT plants with cheap photosynthetic tissues in which the carbon returns on biomass investment are higher, favouring short productive opportunities (e.g. the higher R-selection in some Poaceae lineages).
The different ways that DT plant use to perceive and cope with drought can be reflected in the environmental conditions in which they occur. For instance, the poikilochlorophyllous Afrotrilepis pilosa and Vellozia plicata grow in fully exposed habitats on granite outcrops (Porembski et al., 2021), while the homoiochlorophyllous Doryopteris collina and Ramonda myconi inhabit more shaded and sheltered habitats on the same type of outcrops (Meirelles et al., 1997; Fernández-Marín et al., 2020). Our results showed that A. pilosa and V. plicata have a higher relative proportion of stress tolerance (66 and 65 %, respectively) than D. collina and R. myconi (24 and 21 %, respectively). Thus, despite converging in their desiccation tolerance, these species do not converge in water deficit conditions in which they are found and in how they deal with water availability. Such differences can also be observed within the same phylogenetic lineages in which species share the same mechanism to tolerate desiccation. For example, in contrast to V. plicata, Barbacenia gounelleana exhibited an acquisitive resource-use, a CS/CSR strategy (C : S : R = 34 : 41 : 24 %), and had its occurrence related to the most humid sites among all DT plants. Similarly, five DT pteridophytes registered a higher relative proportion for C-selection (Adiantum latifolium, D. collina, D. varians, Polypodium interjectum, Po. vulgare), while three showed higher scores for S-selection (Anemia ferruginea, Asplenium ceterach, As. trichomanes).
We observed that the moisture conditions in which the DT plants had their HSEIVM varied from fresh soils of average dampness (e.g. Microchloa kunthii and Trilepis lhotzkiana) to dry rather than moist ground (e.g. Oropetium aristatum and V. plicata). These findings can be linked to the fact that some DT plants exhibit advantageous responses for higher growth and resource acquisition (e.g. higher LA and SLA; Reich et al., 1998; Niinemets, 2001; Poorter and Rozendaal, 2008). Such responses would reflect suitable ecological strategies to also cope with more productive conditions or with higher disturbance (e.g. higher relative percentage of C and R strategies, higher H, and smaller SM; Westoby, 1998; Pierce et al., 2017). Therefore, our results displace DT plants from the extreme end of stress tolerance and reveals the relevance of other ecological processes shaping the distribution of DT plants.
The higher capacity to deal with productive and disturbance conditions can increase DT plants’ vulnerability to the negative effects of drought (Reich, 2014). However, their ability to tolerate desiccation could compensate for the drought vulnerability promoted by higher investment in growth. The idea of a trait interaction to mitigate the negative effects of drought can be found in previous studies. For instance, DT plants display folded leaves while drying, reducing photooxidative damage promoted by light incidence over desiccated tissues (Porembski and Barthlott, 2000; Porembski et al., 2021). This means that DT plants exhibit a greater leaf area for light capture when water is available, giving them higher competitive abilities for resources. However, they decrease their exposed surface area when water is unavailable, increasing stress tolerance capacity. Other traits support this response. For example, leaf folding correlates with a venation structure that avoids irreversible damage in their hydraulic system during leaf folding (i.e. parallel nervature in monocots or net-like venation pattern in Gesneriaceae; Kampowski et al., 2018; Porembski et al., 2021). Besides leaf folding during desiccation and its appropriate venation structure, many DT monocot species also develop a velamen radicum (e.g. in Velloziaceae species). The velamen radicum increases water capture and storage (Porembski and Barthlott, 2000; Oliveira et al., 2005; Zotz et al., 2017; Porembski et al., 2021), reducing DT plants’ exposure to water deficit conditions and improving their competitive abilities (Oliveira et al., 2005).
Alternatively, some DT plants might not have their occurrence restricted to the existence of drought as an external constraint that limits productivity. For those species, drought leads to a disturbance, where individuals lose biomass caused by irreversible damage, or being perceived by species as a secondary selective agent (Grime, 1977; Wilson and Lee, 2000; Pierce et al., 2017). This may be the case for the two annual plants O. aristatum and O. thomaeum (Porembski et al., 2021), which had high relative proportions of ruderalism (72 and 86.2 %, respectively). Drought leading to a disturbance for these species is corroborated by their high SLA, besides low H and SM (O. aristatum: SLA = 49, H = 83 and SM = 0.042; O. thomaeum: SLA = 55.2, H = 45 and SM = 0.225). Desiccation tolerance would allow their leaves to survive quick water shortages, when drought acts as a stressful factor. However, their leaf traits favour quicker returns on biomass investment and shorter life cycles, reaching reproduction before long-term droughts lead to disturbance. This is compatible with the fact that these species occur in geographical regions with more extensive and intensive drought events in the dry season and short dry periods in the wet season. Therefore, the alternative functional designs within DT plants could promote the diversity of ecological strategies found among them and suggest that their occurrence is not driven by a common selective force, such as water deficit as a stressful constraint.
Rather than solely water deficit, we suggest that desiccation tolerance could bring advantages to dealing with the quick and pronounced variation in water availability. This is supported by the almost absence of DT plants in deserts (Fahmy et al., 2006; Porembski et al., 2021) and their strong correlation with habitats characterized by marked moisture fluctuations (i.e. rock outcrops; Porembski and Barthlott, 2000; Gaff and Oliver, 2013). Our results are in agreement with these expectations. Although we did not assess moisture fluctuations, we found a low average probability of occurrence of DT plants under extreme dryness (0.32 % according to species EIVM).
The higher temporal variation in the availability of a given resource is expected to benefit species with a more generalist response to different levels of this resource (Lynch and Gabriel, 1987; Sexton et al., 2017). This is because species from highly variable environments are expected to mitigate the selective pressures promoted by the circumstances to which they are subjected (Wilson and Yoshimura, 1994; Callaway et al., 2003). For example, a habitat in which organisms experience enough water to grow, but cannot avoid the negative effects of drought periods, is considered to favour species that can cope with water abundance and deficiency. In this case, DT plants exhibit traits that allow compatible growth and reproduction when water is available, plus the survival of photosynthetic tissues when water is unavailable (Oliver and Bewley, 1996; Zhang et al., 2018; do Nascimento et al., 2020). This is consistent with the plastic responses of DT plants to water availability (do Nascimento et al., 2020) and their need to hold a positive carbon balance under repeated desiccation–rehydration cycles (Alpert, 2005). Therefore, the occurrence of DT plants in such habitats could imply ecological strategies that fit both contrasting environmental situations they experience instead of an exclusive adaptation for only one facet of the environment.
Our results showed that the view of DT plants as a homogeneous group of plants in how they deal with drought and the simple correlation of desiccation tolerance with water deficit might be a mistaken generalization. Considerable knowledge about DT plants has been gathered, and paradigms have been consolidated. However, we need new studies that challenge the accepted paradigms that might be constraining scientific progress regarding the ecological aspects of DT plants. We argue that alternative functional designs should be considered when the responses of DT plants to water deficit conditions are investigated. Also, we encourage new studies that seek for a better understanding of how our results might vary within the most diverse phylogenetic lineages. Besides drought promoting environmental stress, we suggest that the low water availability intensifying competition between plants or long droughts leading to biomass loss might have also played an important evolutionary role for some DT species. Among vascular plants, desiccation tolerance has independently re-evolved multiple times, and it is therefore plausible that the importance of those evolutionary processes changes according to the distinct phylogenetic lineages in which DT plants are found. A better understanding of the responses of DT plants to the ecological processes that shape their occurrences across environments would substantially aid the development of more robust ecological assumptions for these species.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following.
Table S1: list of desiccation-tolerant vascular plants and phylogenetically related desiccation-sensitive plants used as model species in this study.
Table S2: ecological specialization and water deficit affinity scores for species.
Table S3: ecological specialization and water deficit affinity scores for species.
Table S4: ANCOVA summary table for species’ ecological convergence.
Table S5: ANCOVA summary table for species’ ecological specialization and water deficit affinity scores.
Table S6. ANCOVA summary table for determining significant differences between desiccation-tolerant and desiccation-sensitive plants in relation to their ecological strategies, when controlling for their phylogenetic groups.
ACKNOWLEDGEMENTS
We thank Andressa Fraga, Beatriz Prado, Gisela Cheoo, Julius Köhler, Raissa Freitas, Yan César and Yan Nunes for their intellectual and fieldwork contributions to our study, as to the anonymous peer-reviewers for their valuable comments on the manuscript. We also thank the staff members of SMAC-RJ, INEA-RJ, and Bondinho do Pão de Açúcar for their logistic help.
Contributor Information
Luiz Bondi, Department of Botany, University of Rostock, Rostock, Germany; Department of Ecology, State University of Rio de Janeiro (UERJ), Rio de Janeiro, Brazil.
Luiza F A de Paula, Department of Genetics, Ecology and Evolution, Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil.
Bruno H P Rosado, Department of Ecology, State University of Rio de Janeiro (UERJ), Rio de Janeiro, Brazil.
Stefan Porembski, Department of Botany, University of Rostock, Rostock, Germany.
FUNDING
This research was supported by the Deutscher Akademischer Austauschdienst (DAAD) [Grant number Fund. prog. no. 57440921 to L.B.]; and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [Grant number 88887.569558/2020-00 to L.F.A.P.].
LITERATURE CITED
- Alcantara S, de Mello-Silva R, Teodoro GS, Drequeceler K, Ackerly DD, Oliveira RS.. 2015. Carbon assimilation and habitat segregation in resurrection plants: a comparison between desiccation- and non-desiccation-tolerant species of Neotropical Velloziaceae (Pandanales). Functional Ecology 29: 1499–1512. [Google Scholar]
- Alpert P. 2000. The discovery, scope, and puzzle of desiccation tolerance in plants. Plant Ecology 151: 5–17. [Google Scholar]
- Alpert P. 2005. The limits and frontiers of desiccation-tolerant life. Integrative and Comparative Biology 45: 685–695. doi: 10.1093/icb/45.5.685. [DOI] [PubMed] [Google Scholar]
- Alpert P, Oliver MJ.. 2002. Drying without dying. In: Black M, Pritchard HW, eds. Desiccation and survival in plants: drying without dying. Wallingford: CABI Publishing, 3–43. [Google Scholar]
- Bartels D. 2005. Desiccation tolerance studied in the resurrection plant Craterostigma plantagineum. Integrative and Comparative Biology 45: 696–701. doi: 10.1093/icb/45.5.696. [DOI] [PubMed] [Google Scholar]
- Blomberg SP, Garland T.. 2002. Tempo and mode in evolution: Phylogenetic inertia, adaptation and comparative methods. Journal of Evolutionary Biology 15: 899–910. doi: 10.1046/j.1420-9101.2002.00472.x. [DOI] [Google Scholar]
- Box GE, Cox DR.. 1964. An analysis of transformations. Journal of the Royal Statistical Society: Series B (Methodological) 26: 211–243. [Google Scholar]
- Bolmgren K, Eriksson O.. 2010. Seed mass and the evolution of fleshy fruits in angiosperms. Oikos 119: 707–718. doi: 10.1111/j.1600-0706.2009.17944.x [DOI] [Google Scholar]
- Callaway RM, Pennings SC, Richards CL.. 2003. Phenotypic plasticity and interactions among plants. Ecology 84: 1115–1128. doi: 10.1890/0012-9658(2003)084[1115:ppaiap]2.0.co;2. [DOI] [Google Scholar]
- Chase JM, Leibold MA.. 2003. Ecological Niches linking classical and contemporary approaches. Chicago: The University of Chicago Press. [Google Scholar]
- Devictor V, Clavel J, Julliard R, et al. 2010. Defining and measuring ecological specialization. Journal of Applied Ecology 47: 15–25. doi: 10.1111/j.1365-2664.2009.01744.x. [DOI] [Google Scholar]
- Dias ATC, Rosado BHP, De Bello F, Pistón N, De Mattos EA.. 2020. Alternative plant designs: consequences for community assembly and ecosystem functioning. Annals of Botany 125: 391–398. doi: 10.1093/aob/mcz180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle RF. 1994. Convergent evolution: the need to be explicit. Trends in Biochemical Sciences 19: 15–18. doi: 10.1016/0968-0004(94)90167-8 [DOI] [PubMed] [Google Scholar]
- Dolédec S, Chessel D, Gimaret-Carpentier C.. 2000. Niche separation in community analysis: a new method. Ecology 81: 2914–2927. doi: 10.1890/0012-9658(2000)081[2914:nsicaa]2.0.co;2. [DOI] [Google Scholar]
- Esquivel-Muelbert A, Galbraith D, Dexter KG, et al. 2017. Biogeographic distributions of neotropical trees reflect their directly measured drought tolerances. Scientific Reports 7: 20170821. doi: 10.1038/s41598-017-08105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy GM, Gaff DF, El-Ghani MMA.. 2006. Does Egypt represent an ecological limit to desiccation tolerant plants? Qatar University Science Journal 26: 91–100. [Google Scholar]
- Fernández-Marín B, Nadal M, Gago J, et al. 2020. Born to revive: molecular and physiological mechanisms of double tolerance in a paleotropical and resurrection plant. New Phytologist 226: 741–759. doi: 10.1111/nph.16464. [DOI] [PubMed] [Google Scholar]
- Funk JL, Larson JE, Ames GM, et al. 2017. Revisiting the Holy Grail: using plant functional traits to understand ecological processes. Biological Reviews 92: 1156–1173. doi: 10.1111/brv.12275. [DOI] [PubMed] [Google Scholar]
- Gaff DF. 1977. Desiccation tolerant vascular plants of Southern Africa. Oecologia 31: 95–109. doi: 10.1007/bf00348713. [DOI] [PubMed] [Google Scholar]
- Gaff DF. 1986. Desiccation tolerant ‘resurrection’ grasses from Kenya and West Africa. Oecologia 70: 118–120. doi: 10.1007/bf00377119. [DOI] [PubMed] [Google Scholar]
- Gaff DF, Bole PV.. 1986. Resurrection grasses in India. Oecologia 71: 159–160. doi: 10.1007/bf00377337. [DOI] [PubMed] [Google Scholar]
- Gaff DF, Latz PK.. 1978. The occurrence of resurrection plants in the Australian flora. Australian Journal of Botany 26: 485–492. doi: 10.1071/bt9780485. [DOI] [Google Scholar]
- Gaff DF, Oliver M.. 2013. The evolution of desiccation tolerance in angiosperm plants: a rare yet common phenomenon. Functional Plant Biology 40: 315–328. doi: 10.1071/fp12321. [DOI] [PubMed] [Google Scholar]
- Gould SJ. 1970. Dollo on Dollo’s law: irreversibility and the status of evolutionary laws. Journal of the History of Biology 3: 189–212. doi: 10.1007/bf00137351. [DOI] [PubMed] [Google Scholar]
- Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. The American Naturalist 111: 1169–1194. doi: 10.1086/283244. [DOI] [Google Scholar]
- Jafari M, Ansari-Pour N.. 2019. Why, when and how to adjust your P values? Cell Journal 20: 604–607. doi: 10.22074/cellj.2019.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampowski T, Demandt S, Poppinga S, Speck T.. 2018. Kinematical, structural and mechanical adaptations to desiccation in poikilohydric Ramonda myconi (Gesneriaceae). Frontiers in Plant Science 871: 1–17. doi: 10.3389/fpls.2018.01701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattge J, Bönisch G, Díaz S, et al. 2020. TRY plant trait database – Enhanced coverage and open access. Global Change Biology 26: 119–188. doi: 10.1111/gcb.14904. [DOI] [PubMed] [Google Scholar]
- Koch GW, Sillett SC, Jennings GM, Davis SD.. 2004. The limits of tree height. Nature 428: 851–854. doi: 10.1038/nature02417. [DOI] [PubMed] [Google Scholar]
- Lavergne S, Garnier E, Debussche M.. 2003. Do rock endemic and widespread plant species differ under the Leaf-Height-Seed plant ecology strategy scheme? Ecology Letters 6: 398–404. doi: 10.1046/j.1461-0248.2003.00456.x. [DOI] [Google Scholar]
- Levins R. 1962. Theory of fitness in a heterogeneous environment. I. The fitness set and adaptive function. The American Naturalist 96: 361–373. doi: 10.1086/282245. [DOI] [Google Scholar]
- Lavorel S, Garnier E.. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology 16: 545–556. doi: 10.1046/j.1365-2435.2002.00664.x. [DOI] [Google Scholar]
- Lynch M, Gabriel W.. 1987. Environmental tolerance. The American Naturalist 129: 2831172–2831303. doi: 10.1086/284635. [DOI] [Google Scholar]
- MacArthur R, Levins R.. 1964. Competition, habitat selection, and character displacement in a patchy environment. Proceedings of the National Academy of Sciences of the United States of America 51: 1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks RA, Farrant JM, McLetchie DN, VanBuren R.. 2021. Unexplored dimensions of variability in vegetative desiccation tolerance. American Journal of Botany 108: 1–13. doi: 10.1002/ajb2.1588. [DOI] [PubMed] [Google Scholar]
- Marks CO, Lechowicz MJ.. 2006. Alternative designs and the evolution of functional diversity. American Naturalist 167: 55–66. doi: 10.1086/498276. [DOI] [PubMed] [Google Scholar]
- Meirelles ST, De Mattos EA, Da Silva AC.. 1997. Potential desiccation tolerant vascular plants from southeastern Brazil. Polish Journal of Environmental Studies 4: 17–21. [Google Scholar]
- Moles AT, Ackerly DD, Webb CO, et al. 2005. Factors that shape seed mass evolution. Proceedings of the National Academy of Sciences of the United States of America 102: 10540–10544. doi: 10.1073/pnas.0501473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Nascimento A, Suguiyama VF, Sanches RFE, et al. 2020. Barbacenia graminifolia, a resurrection plant with high capacity of water retention. Flora 267: 151604. doi: 10.1016/j.flora.2020.151604. [DOI] [Google Scholar]
- Niinemets U. 2001. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82: 453–469. doi: 10.1890/0012-9658(2001)082[0453:gsccol]2.0.co;2. [DOI] [Google Scholar]
- Oliveira RS, Dawson TE, Burgess SSO.. 2005. Evidence for direct water absorption by the shoot of the desiccation-tolerant plant Vellozia flavicans in the savannas of central Brazil. Journal of Tropical Ecology 21: 585–588. doi: 10.1017/s0266467405002658. [DOI] [Google Scholar]
- Oliver MJ, Bewley JD.. 1996. Desiccation-tolerance of plant tissues: a mechanistic overview. Horticultural Reviews 18: 171–213. [Google Scholar]
- Oliver MJ, Farrant JM, Hilhorst HWM, Mundree S, Williams B, Bewley JD.. 2020. Desiccation tolerance: avoiding cellular damage during drying and rehydration. Annual Review of Plant Biology 71: 435–460. doi: 10.1146/annurev-arplant-071219-105542. [DOI] [PubMed] [Google Scholar]
- Oliver MJ, Tuba Z, Mishler BD.. 2000. The evolution of vegetative desiccation tolerance in land plants. Plant Ecology 151: 85–100. [Google Scholar]
- Pastore M, Calcagnì A.. 2019. Measuring distribution similarities between samples: a distribution-free overlapping index. Frontiers in Psychology 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula LFA, Leal BSS, Rexroth J, Porembski S, Palma-Silva C.. 2017. Transferability of microsatellite loci to Vellozia plicata (Velloziaceae), a widespread species on Brazilian inselbergs. Revista Brasileira de Botanica 40: 1071–1075. doi: 10.1007/s40415-017-0396-x. [DOI] [Google Scholar]
- de Paula LFA, Negreiros D, Azevedo LO, Fernandes RL, Stehmann JR, Silveira FAO.. 2015. Functional ecology as a missing link for conservation of a resource-limited flora in the Atlantic forest. Biodiversity and Conservation 24: 2239–2253. doi: 10.1007/s10531-015-0904-x. [DOI] [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. 2013. New handbook for standardized measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. doi: 10.1071/BT12225. [DOI] [Google Scholar]
- Pierce S, Negreiros D, Cerabolini BEL, et al. 2017. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Functional Ecology 31: 444–457. [Google Scholar]
- Pistón N, de Bello F, Dias ATC, et al. 2019. Multidimensional ecological analyses demonstrate how interactions between functional traits shape fitness and life history strategies. Journal of Ecology 107: 2317. doi: 10.1111/1365-2745.13190. [DOI] [Google Scholar]
- Poorter LD, Rozendaal MA.. 2008. Leaf size and leaf display of thirty-eight tropical tree species. Oecologia 158: 35–46. doi: 10.1007/s00442-008-1131-x. [DOI] [PubMed] [Google Scholar]
- Porembski S, Barthlott W.. 2000. Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecology 151: 19–28. [Google Scholar]
- Porembski S. 2000. The invasibility of tropical granite outcrops (‘inselbergs’) by exotic weeds. Journal of the Royal Society of Western Australia 83: 131–137. [Google Scholar]
- Porembski S. 2003. Epiphytic orchids on arborescent Velloziaceae and Cyperaceae: Extremes of phorophyte specialisation. Nordic Journal of Botany 23: 505–512. doi: 10.1111/j.1756-1051.2003.tb00424.x. [DOI] [Google Scholar]
- Porembski S, Rexroth J, Weising K, et al. 2021. An overview on desiccation-tolerant mat-forming monocotyledons on tropical inselbergs. Flora 285: 151953. doi: 10.1016/j.flora.2021.151953 [DOI] [Google Scholar]
- POWO. 2022. Plants of the world online. Kew: Royal Botanic Gardens. http://www.plantsoftheworldonline.org/. Accessed 2 February 2022. [Google Scholar]
- R Core Team. 2021. R: a language and environment for statistical computing, R software. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. doi: 10.1111/1365-2745.12211. [DOI] [Google Scholar]
- Reich PB, Tjoelker MG, Walters MB, Vanderklein DW, Buschena C.. 1998. Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Functional Ecology 12: 327–338. doi: 10.1046/j.1365-2435.1998.00208.x. [DOI] [Google Scholar]
- Rexroth J, Krebes L, Wöhrmann T, et al. 2019. New microsatellite markers for Xerophyta dasylirioides (Velloziaceae), an endemic species on Malagasy inselbergs. Applications in Plant Sciences 7: 1–5. doi: 10.1002/aps3.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. 2000. Resurrection plants and the secrets of eternal leaf. Annals of Botany 85: 159–166. doi: 10.1006/anbo.1999.1006 [DOI] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW.. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton JP, Montiel J, Shay JE, Stephens MR, Slatyer RA.. 2017. Evolution of ecological niche breadth. Annual Review of Ecology, Evolution, and Systematics 48: 183–206. doi: 10.1146/annurev-ecolsys-110316-023003. [DOI] [Google Scholar]
- Shipley B, Belluau M, Kühn I, et al. 2017. Predicting habitat affinities of plant species using commonly measured functional traits. Journal of Vegetation Science 28: 1082–1095. doi: 10.1111/jvs.12554. [DOI] [Google Scholar]
- Shipley B, De Bello F, Cornelissen JHC, Laliberté E, Laughlin DC, Reich PB.. 2016. Reinforcing loose foundation stones in trait-based plant ecology. Oecologia 180: 923–931. doi: 10.1007/s00442-016-3549-x. [DOI] [PubMed] [Google Scholar]
- Teodoro GS, Costa PB, Brum M, et al. 2021. Desiccation tolerance implies costs to productivity but allows survival under extreme drought conditions in Velloziaceae species in campos rupestres. Environmental and Experimental Botany 189: 104556. doi: 10.1016/j.envexpbot.2021.104556. [DOI] [Google Scholar]
- Thornthwaite CW. 1948. An approach toward a rational classification of climate. Geographical Review 38: 55–94. doi: 10.2307/210739. [DOI] [Google Scholar]
- Tuba Z. 2008. Notes on the poikilochlorophyllous desiccation-tolerant plants. Acta Biologica Szegediensis 52: 111–113. [Google Scholar]
- Volaire F. 2018. A unified framework of plant adaptive strategies to drought: Crossing scales and disciplines. Global Change Biology 24: 2929–2938. doi: 10.1111/gcb.14062. [DOI] [PubMed] [Google Scholar]
- Westoby M. 1998. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant and Soil 199: 213–227. doi: 10.1023/A:1004327224729. [DOI] [Google Scholar]
- Wilson BJ, Lee WG.. 2000. C - S - R triangle theory: community-level predictions, tests, evaluation of criticisms and relation to other theories. Oikos 91: 77–96. doi: 10.1034/j.1600-0706.2000.910107.x. [DOI] [Google Scholar]
- Wilson DS, Yoshimura J.. 1994. On the coexistence of specialists and generalists. The American Naturalist 144: 692–707. [Google Scholar]
- Winemiller KO, Fitzgerald DB, Bower LM, Pianka ER.. 2015. Functional traits, convergent evolution, and periodic tables of niches. Ecology Letters 18: 737–751. doi: 10.1111/ele.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Shan L, Li Y.. 2018. Prolonged dry periods between rainfall events shorten the growth period of the resurrection plant Reaumuria soongorica. Ecology and Evolution 8: 920–927. doi: 10.1002/ece3.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotz G, Schickenberg N, Albach D.. 2017. The velamen radicum is common among terrestrial monocotyledons. Annals of Botany 120: 625–632. doi: 10.1093/aob/mcx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.