Abstract
Background and Aims
Effects of elevated CO2 (E) within a generation on photosynthesis and stomatal features have been well documented in crops; however, long-term responses to gradually elevated CO2 (Eg) and abruptly elevated CO2 (Ea) over multiple generations remain scarce.
Methods
Japonica rice plants grown in open-top chambers were tested in the first generation (F1) under Ea and in the fifth generation (F5) under Eg and Ea, as follows: Ea in F1: ambient CO2 (A) + 200 μmol mol−1; Eg in F5: an increase of A + 40 μmol mol−1 year−1 until A + 200 μmol mol−1 from 2016 to 2020; Ea in F5: A + 200 μmol mol−1 from 2016 to 2020. For multigenerational tests, the harvested seeds were grown continuously in the following year in the respective CO2 environments.
Key Results
The responses to Ea in F1 were consistent with the previous consensus, such as the occurrence of photosynthetic acclimation, stimulation of photosynthesis, and downregulation of photosynthetic physiological parameters and stomatal area. In contrast, multigenerational exposure to both Eg and Ea did not induce photosynthetic acclimation, but stimulated greater photosynthesis and had little effect on the photosynthetic physiology and stomatal traits. This suggests that E retained intergenerational effects on photosynthesis and stomatal features and that there were no multigenerational differences in the effects of Eg and Ea.
Conclusions
The present study demonstrated that projecting future changes induced by E based on the physiological responses of contemporary plants could be misleading. Thus, responses of plants to large and rapid environmental changes within a generation cannot predict the long-term response of plants to natural environmental changes over multiple generations, especially in annual herbs with short life cycles.
Keywords: Abruptly elevated CO2, gradually elevated CO2, generation, photosynthesis, japonica rice, stomatal features
INTRODUCTION
Anthropogenic pressures are rapidly changing the global environment, including the atmospheric carbon dioxide (CO2) concentration, which is now >400 μmol mol−1 (IPCC, 2022). Crops are sensitive to elevated CO2 (E), and relevant studies have become a hot topic over the last three decades because of its potentially profound impact on crop ecosystems and a strong concern for future food security (Ziska and Bunce, 2007; Lobell et al., 2011; Adachi et al., 2014; Cai et al., 2020; Ainsworth and Long, 2021).
Given the large number of E experiments implemented, changes in photosynthetic characteristics, such as stimulated photosynthesis and photosynthetic resource use efficiency, downregulated biochemical capacity, stomatal conductance and dark-adapted chlorophyll fluorescence parameters, have been investigated well and reviewed (Huxman et al., 1999; Ainsworth and Long, 2005; Leakey et al., 2009; Shanmugam et al., 2013; Zong et al., 2014; Yang et al., 2023a). Nevertheless, the response of stomatal features (stomatal density and area) to E varies among species (Bettarini et al., 1998; Ainsworth and Rogers, 2007; Haworth et al., 2015). Acclimation to E is defined as follows: the values (net photosynthetic rate and stomatal conductance) are significantly lower in plants grown under E than under ambient CO2 when both are measured at the same CO2 concentration (Drake et al., 1997; Lodge et al., 2001), and both photosynthetic acclimation and stomatal acclimation are still under discussion because of the importance of accurate prediction of future carbon uptake and storage (Smith and Dukes, 2013; Li et al., 2015; Cai et al., 2018).
Notably, almost all the above studies on crops are based on an abruptly elevated CO2 (Ea) within a generation, such as ambient CO2 + 200 μmol mol−1 in one growing season. However, CO2 levels are rising gradually; the average rate of CO2 increase was 2.4 μmol mol−1 year−1 during the last decade (https://www.esrl.noaa.gov/). Thus, small and slow environmental changes across generations might be more relevant in predicting the response to future climate change (Donelson et al., 2018). However, long-term responses to gradually elevated CO2 (Eg) and Ea over multiple generations have received little attention owing to the time, energy and funds required for initiating such projects.
Although a few studies have reported differences between Ea and Eg, they did not involve an intergenerational effect, because these studies were conducted in one growing season or on trees (Luo and Reynolds, 1999; Hui et al., 2002; Drake et al., 2016). Previous reports found that compared with exposure to Ea for a single generation, multigenerational exposure to Ea could enhance biomass further (Frenck et al., 2013; Li et al., 2019). A limited number of studies have shown that Ea decreases the response of the structural and functional community of mycorrhizal fungi; however, exposure to Eg over 21 generations, with an increase of 10 μmol mol−1 during each generation, does not change the fungal diversity and functioning (Klironomos et al., 2005). Therefore, it is reasonable to believe that the responses of plants to long-term exposure to Ea and Eg over multiple generations might differ from those to short-term exposure to Ea within a generation.
Facing human-mediated climate change, plants might adapt over generations, and plants of the next generation are likely to be different from contemporary plants, leading to long-term trends in plant fitness (Collins and Bell, 2004; Burgess and Marshall, 2014; Yin et al., 2022). Maternal environment can have substantial effects on offspring (transgenerational effects). Transgenerational adaptation is a flexible evolutionary mechanism that can result from seed modification or epigenetic variation (Lau et al., 2008; Donohue, 2009; Vráblová et al., 2018). Additionally, the adaptation of physiological traits is generally more rapid than that of morphology and growth because of the relatively few genes (Herman and Sultan, 2011; Leakey and Lau, 2012; Yin et al., 2019).
The objective of this study was to investigate photosynthesis and stomatal features in two aspects: (1) whether multigenerational exposure to E, including Ea and Eg, altered these traits compared with Ea within a generation; and (2) whether there were multigenerational differences in the effects of Ea and Eg. Thus, in this study, photosynthesis and stomatal features of japonica rice were measured under three patterns of E in single and multiple generations from 2016 to 2020: Ea within a generation, Eg over multiple generations and Ea over multiple generations.
MATERIALS AND METHODS
Site description
The experiment was conducted at the Agricultural Meteorology and Ecology Experiment Station of the Nanjing University of Information Science and Technology (32.16°N, 118.86°E) during the rice-growing seasons from 2016 to 2020. The region has a subtropical humid climate, with a mean annual temperature of 15.6 °C, mean annual precipitation of ~1100 mm, and mean annual sunshine of >1900 h. The soil texture was a silty loam with clay, silt and sand contents of 9.5, 85.2 and 5.3 %, respectively. The tilled soil had a pH of 6.1, and soil organic carbon, total N, total P and total K contents of 9.52, 1.18, 0.85 and 18.17 g kg–1, respectively, at a depth of 0–20 cm.
Experimental design
Twelve open-top chambers (OTCs) were established to simulate CO2 enrichment, with four OTCs designated as replicates for each treatment. There were three treatments of CO2 concentration, including one ambient atmospheric CO2 concentration (A) and two elevated CO2 concentrations (E): (1) maintaining A from 2016 to 2020, at ~400 μmol mol−1; (2) a ‘ramp’ of A + 40 μmol mol−1 steps, with each step lasting for one growing season from 2016 to 2020; that is, A + 40 μmol mol−1 in 2016, A + 80 μmol mol−1 in 2017, A + 120 μmol mol−1 in 2018, A + 160 μmol mol−1 in 2019 and A + 200 μmol mol−1 in 2020, which could be considered as Eg; and (3) maintaining A + 200 μmol mol−1 from 2016 to 2020, which could be considered as Ea.
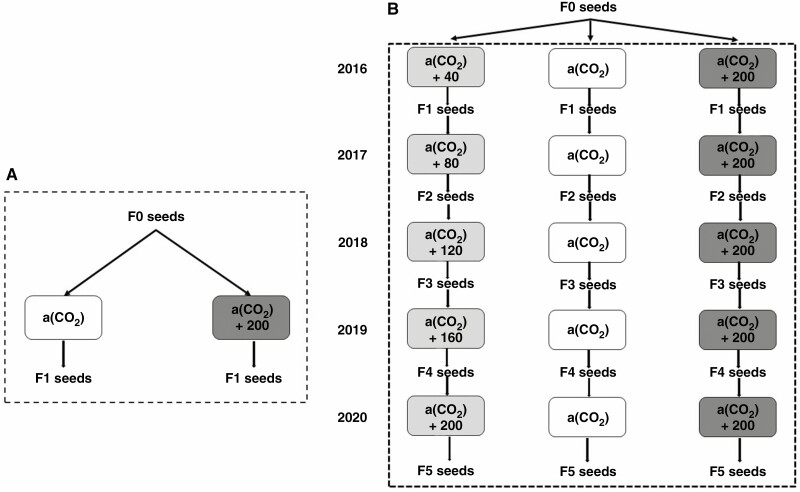
This study included the following design: (1) Ea within a generation (Ea in F1), that is, seeds grown at A and Ea in one growing season in 2019 (Fig. 1A); (2) Eg over five generations (Eg in F5), that is, seeds continuously grown at A and Eg over five generations from 2016 to 2020 (Fig. 1B); and (3) Ea over five generations (Ea in F5), that is, seeds continuously grown at A and Ea over five generations from 2016 to 2020 (Fig. 1B).
Fig. 1.
Schematic representation of the experimental design and treatments. (A) abruptly elevated CO2 within a generation (Ea in F1). (B) Gradually elevated CO2 over five generations (Eg in F5) and abruptly elevated CO2 over five generations (Ea in F5).
For the multigenerational tests, the harvested seeds were grown in the following year continuously in the respective CO2 environments (Fig. 1B). Seeds were harvested around 20 October every year and collected from maternal plants of each of the four A, Eg and Ea plots. Harvested seeds were air-dried to a constant weight (14 % moisture) and stored in a refrigerator at 4 °C before being transferred to their respective OTCs for germination and experimental trait analyses.
The OTCs were regular octagonal prism-shaped structures constructed using an aluminum alloy frame (12 m2 bottom area and 3 m height) with a highly transparent glass covering (3 mm thickness and 80 % light transmittance), and the light transmittance did not differ among the OTCs (data not shown). CO2 (GMM222; Vaisala, Helsinki, Finland) and temperature sensors were suspended in the middle of the chamber, 1.5 m above the ground, to record the CO2 concentration and temperature in the OTCs. The photosynthetic photon flux density was monitored using a micro-meteorological station (AWS800; Campbell Scientific, USA) installed in the experimental field. The details of the control system for CO2 enrichment have been described elsewhere (Yang et al., 2021). A summary of the CO2 concentrations, temperatures and photosynthetic photon flux density from 2016 to 2020 is presented in Supplementary data Table S1.
Rice cultivation
The japonica rice (Oryza sativa L.) cultivar ‘Nanjing 9108’ was grown in OTC field plots. In 2019 and 2020, pre-germinated seeds were sown on 20 May, and the rice seedlings were transplanted on 20 June. Nitrogen fertilizer was applied at a rate of 25 g m−2 using compound chemical fertilizer (N:P:K = 15 %: 15 %: 15 %) for supplying basal N and urea (N, 46 %) for top-dressing. The basal N and two top-dressings were applied at a ratio of 6:2:2 pre-transplantation (20 June 2019 and19 June 2020), early tillering (7 July 2019 and 7 July 2020) and panicle initiation (26 July 2019 and 26 July 2020). Field management, such as water supply and disease control, was performed following local practices. The rice cultivation from 2016 to 2018 was almost consistent with that during 2019 and 2020.
Gas exchange measurements
Before gas exchange measurements, a chlorophyll meter (SPAD-502; Konica Minolta Optics, Japan) was used to measure the chlorophyll content (SPAD) of eight to ten leaves per OTC in the fully expanded youngest leaves (flag leaves at the filling stage). Three leaves (in 2019) or two leaves (in 2020) with representative SPAD (values near the mean) were selected for the subsequent gas exchange measurements with an LI-Cor 6400XT Portable Photosynthesis System (Li-Cor, Lincoln, NE, USA) from 09.00 to 14.00 h on sunny days in each OTC. The measurements were conducted at the jointing stage (1–3 August 2019 and 9–13 August 2020) and the filling stage (22–25 September 2019 and 26–30 September 2020). Basal CO2 leakage in LI-Cor 6400XT usually occurs at the interface between the two sealing gaskets of the clamping chamber (Flexas et al., 2007). Therefore, we used the latex gaskets of LI-Cor 6800 (spare part number 6568-566), which has better elasticity and plasticity, to replace the foamed gaskets of LI-Cor 6400 (white, spare part number 6400-30; and black, spare part number 6400-33).
During the CO2 response curve (A–Ci, Ci: intercellular CO2 concentration) measurements in 2019 and 2020, the photosynthetic photon flux density was maintained at 1500 μmol m−2 s−1 and the leaf temperature was set to 33 °C at the jointing stage and 28 °C at the filling stage. The vapour pressure deficit was 1.77 ± 0.19 kPa in 2019 and 1.78 ± 0.25 kPa in 2020, and the flow rate for all measurements was 500 μmol s−1 in both years. For the A–Ci curve measurement, the CO2 concentration (Ca) steps were 400, 300, 200, 100, 50, 400, 500, 600, 800, 1000 and 1200 μmol mol−1 under A and 600, 500, 400, 300, 200, 100, 50, 600, 800, 1000 and 1200 μmol mol−1 under E.
The maximum rates of carboxylation (Vcmax) and electron transport (Jmax) were determined using the Farquhar, von Caemmerer and Berry models (Farquhar et al., 1980) and were rescaled to 25 °C (Vcmax25 and Jmax25) using the temperature response functions given by Bernacchi et al. (2001) and Bernacchi et al. (2003), respectively.
Leaf dark-adapted chlorophyll fluorescence
After gas exchange, leaf dark-adapted chlorophyll fluorescence parameters were estimated using a plant efficiency analyzer (Pocket PEA; Hansatech Instruments, King’s Lynn, UK) at a saturating light pulse of 3500 µmol m−2 s−1. The dark-adapted leaves were measured at predawn (~05.00 h) at the same leaf locations where the gas exchange was determined. The maximum quantum yield of photosystem II (PSII) (Fv/Fm) was calculated as (Fm − Fo)/Fm, where Fo and Fm are the minimum and maximum fluorescence intensity, respectively (Schreiber and Armond, 1978). The quantum yield for the energy dissipation (φDo) was calculated as Fo/Fm, and the quantum yield for electron transport (φEo) was calculated as (1 − Fo/Fm) × (1 − VJ), where VJ represents the relative variable fluorescence in phase J of the fluorescence induction curve (Strasser and Strasser, 1995; Tsimilli-Michael and Strasser, 2008). The performance index (PIABS) is the index that integrates different phenomena related to PSII activity (Wang et al., 2016).
Measurement of stomatal parameters
A section of the abaxial epidermis measuring ~1 cm2 was imprinted from the middle of the leaf using clear nail varnish. Stomatal density (the number of stomata per unit leaf area) was observed using a microscope (XD-MDI; Xindi, Shanghai, China), and the area of the stomatal apparatus (the size of the guard cells and the pore) was calculated using the image of stomata with ImageJ, a publicly available Java-based image processing program developed at the US National Institutes of Health (http://rsb.info.nih.gov/ij/). The stomatal density of each segment was determined for the five fields. The areas of five stomatal apparatuses were observed in each field. The stomatal apparatus area index (the ratio of stomatal area to unit leaf area) in each field was calculated as the stomatal density multiplied by the stomatal apparatus area. The measurement of stomatal parameters within generation mentioned above refers to our recent study (Yang et al., 2021).
Leaf mass and nitrogen content per unit area
Leaf area measurements were performed using an area meter (Li-3100; Li-Cor, Lincoln, NE, USA). Subsequently, the leaves were oven-dried at 105 °C for 30 min, then at 80 °C to a constant weight, and weighed. The leaf nitrogen concentration was measured using a CHNOS Elemental Analyzer (MOD Vario El III, Germany). Finally, leaf mass per unit leaf area (LMA; in grams per square metre) and nitrogen content per unit leaf area (Na; in grams per square metre) were calculated using the above data.
Photosynthetic resource use efficiency
Water use efficiency (WUE) was defined as the net photosynthetic rate at growth CO2 concentration (Agrowth) at saturating light intensity (1500 μmol m−2 s−1) divided by the transpiration rate (Tr). Likewise, photosynthetic nitrogen use efficiency (PNUE) was defined as Agrowth divided by Na.
Statistical analysis
An ANOVA with Tukey’s HSD post-hoc test was applied to analyse the difference in a given item between the treatments. The A–Ci curves were fitted using the ‘plantecophys’ package of R software. Linear regression was used to quantify the relationships between variables. Statistical analyses were performed using IBM SPSS 20.0 (SPSS, Chicago, IL, USA) and Microsoft Excel 2016. Statistical significance was set at P < 0.05.
RESULTS
Gas exchange parameters
In all treatments (Ea in F1 and Eg and Ea in F5), E dramatically stimulated the net photosynthetic rate at growth CO2 concentrations (Agrowth) at the jointing and filling stages (P < 0.05; Table 1). The stimulation of the average Agrowth was 21, 32 and 33 % at Ea in F1, Eg in F5 and Ea in F5, respectively, across the two stages.
Table 1.
Gas exchange parameters measured at growth CO2 concentration under Ea in F1 and Eg and Ea in F5
| Generation | Stage | CO2 |
A
growth
(μmol m−2 s−1) |
g
s
(mol m−2 s−1) |
T
r
(mmol m−2 s−1) |
V
cmax25
(μmol m−2 s−1) |
J
max25
(μmol m−2 s−1) |
|---|---|---|---|---|---|---|---|
| F1 | Jointing | A | 29.6 (0.5) b | 0.338 (0.014) a | 5.52 (0.21) a | 115.7 (2.3) a | 139.6 (3.5) a |
| E a | 33.1 (0.8) a | 0.254 (0.020) b | 4.69 (0.32) b | 103.2 (2.3) b | 131.0 (2.4) b | ||
| Filling | A | 17.3 (0.4) b | 0.148 (0.007) a | 2.60 (0.09) a | 82.0 (1.8) a | 145.3 (3.4) a | |
| E a | 22.3 (0.4) a | 0.133 (0.007) a | 2.31 (0.08) b | 75.2 (1.0) b | 134.0 (3.6) b | ||
| F5 | Jointing | A | 25.3 (0.4) b | 0.211 (0.012) a | 4.57 (0.27) a | 102.9 (2.1) a | 129.8 (2.9) a |
| E g | 32.0 (0.6) a | 0.203 (0.014) a | 3.84 (0.17) a | 100.5 (1.3) a | 128.0 (3.0) a | ||
| E a | 31.9 (0.5) a | 0.207 (0.019) a | 3.97 (0.22) a | 96.1 (2.4) a | 127.0 (2.9) a | ||
| Filling | A | 15.9 (0.5) b | 0.140 (0.005) a | 2.22 (0.09) a | 69.4 (2.0) a | 123.7 (3.2) a | |
| E g | 21.9 (0.9) a | 0.121 (0.010) a | 2.04 (0.15) a | 69.3 (2.3) a | 129.8 (3.8) a | ||
| E a | 22.1 (0.4) a | 0.116 (0.007) a | 1.95 (0.09) a | 69.3 (1.7) a | 133.6 (2.9) a |
Abbreviations: Agrowth, net photosynthetic rate at growth CO2 concentration; gs, stomatal conductance to CO2; Jmax25, the maximum electron transport rate at 25 °C; Tr, transpiration rate; Vcmax25, the maximum rate of Rubisco carboxylation at 25 °C. Values are the mean (s.e.m.), n = 4. Different lowercase letters indicate significant differences among the CO2 treatments (P < 0.05).
However, the remaining gas exchange parameters exhibited distinct CO2 responses in F1 and F5. In F1, Ea significantly decreased gs at the jointing stage and Tr, Vcmax25 and Jmax25 at two stages (P < 0.05), with only a non-significant reduction in gs at the filling stage (P > 0.05). In contrast, Eg and Ea in F5 did not affect these traits at the two stages (P > 0.05), despite a tendency for a reduction in gs and Tr (Table 1). Furthermore, all gas exchange parameters in F5 did not differ between Eg and Ea (Table 1).
Photosynthetic and stomatal acclimation
In F1, the net photosynthetic rate (An) was significantly lower under Ea than under A at different Ci conditions (P < 0.05), with the exception of the lowest Ci concentration at the jointing and filling stages (Fig. 2A, B). However, in F5, An did not differ at all Ci concentrations under Eg and Ea at two stages (P > 0.05; Fig. 2C, D). The results clearly showed the occurrence of photosynthetic acclimation under Ea in F1, but not under Eg and Ea in F5.
Fig. 2.
The A–Ci curves of Ea in F1 (A, B), Eg and Ea in F5 (C, D) at the jointing (A, C) and filling stages (B, D). Each point represents the mean of four replicates, and bars represent the s.e.m. *P < 0.05 and **P < 0.01; ns, no significant difference. Panels A and B were taken from our previous paper (Yang et al., 2021).
Compared with A in F1, gs was lower under Ea only when Ci was <400 μmol mol−1 at the jointing stage (P < 0.05; Supplementary data Fig. S1A), whereas gs did not differ at high Ci at the jointing stage and at all Ci levels at the filling stage between the CO2 treatments (P > 0.05; Supplementary data Fig. S1A, B). Likewise, Eg and Ea in F5 did not alter gs in all Ci conditions at two stages (P > 0.05), except for two Ci concentrations at the filling stage (Supplementary data Fig. S1C, D). This suggests that stomatal acclimation rarely occurs under E, regardless of the E pattern (Egvs Ea) and generation (F1 vs F5).
Leaf traits
The LMA was not significantly affected by Ea in F1 at the jointing and filling stages, and a slightly downward trend was observed, whereas Eg and Ea in F5 significantly or slightly increased LMA at the two stages (Table 2). Compared with A in F1, Na was lower under Ea, with significant or non-significant differences; however, Na was slightly higher but non-significant under Eg and Ea than under A in F5 at the two stages (Table 2). Additionally, LMA and Na in F5 did not show significant difference between Eg and Ea.
Table 2.
Effects of elevated CO2 on leaf traits in F1 and F5
| Generation | Stage | CO2 | LMA (g m−2) |
N
a
(g m−2) |
|---|---|---|---|---|
| F1 | Jointing | A | 56.0 ± 0.9 a | 2.11 ± 0.04 a |
| E a | 55.8 ± 0.7 a | 2.06 ± 0.02 a | ||
| Filling | A | 66.2 ± 1.1 a | 1.68 ± 0.04 a | |
| E a | 65.4 ± 1.2 a | 1.58 ± 0.04 b | ||
| F5 | Jointing | A | 49.3 ± 0.7 a | 1.63 ± 0.04 a |
| E g | 50.7 ± 1.0 a | 1.67 ± 0.03 a | ||
| E a | 51.8 ± 0.8 a | 1.67 ± 0.03 a | ||
| Filling | A | 61.0 ± 2.0 b | 1.37 ± 0.05 a | |
| E g | 67.7 ± 1.4 a | 1.47 ± 0.03 a | ||
| E a | 66.9 ± 1.0 a | 1.42 ± 0.03 a |
Abbreviations: LMA, leaf mass per unit leaf area; Na, leaf nitrogen content per unit leaf area. Values are the mean ± s.e.m. (n = 4). Different lowercase letters indicate significant differences among CO2 treatments (P < 0.05). The values of F1 were taken from our previous paper (Yang et al., 2021).
Photosynthetic nitrogen and water use efficiency
Independent of single and multiple generations, E significantly promoted PNUE (Fig. 3A, B) and WUE (Fig. 3C, D). The average stimulation in PNUE was 26, 26 and 29 % under Ea in F1, Eg in F5 and Ea in F5, respectively, across the two stages. Accordingly, the average stimulation in WUE was 41, 51 and 52 % under Ea in F1, Eg in F5 and Ea in F5, respectively.
Fig. 3.
Photosynthetic nitrogen use efficiency (PNUE) and water use efficiency (WUE) of Ea in F1 (A, C) and Eg and Ea in F5 (B, D) at the jointing and filling stages. Different lowercase letters indicate significant differences among the bars (values are shown as the mean + s.e.m., P < 0.05, n = 4).
Stomatal features
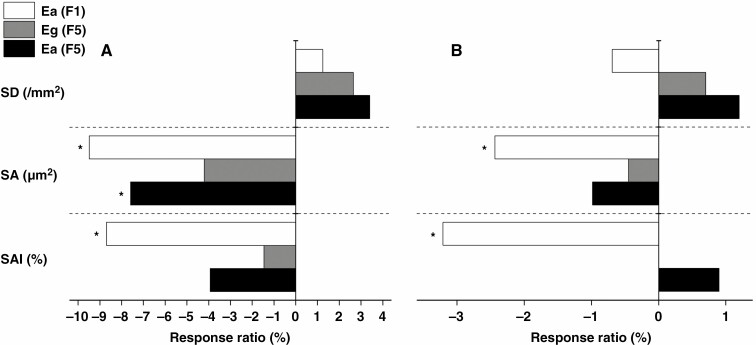
In F1, stomatal apparatus area (SA) and stomatal apparatus area index (SAI) were significantly lower under Ea than under A at the two stages, whereas stomatal density (SD) did not change between CO2 treatments (Table 3). In contrast to Ea in F1, Eg and Ea in F5 had no effects on SD or on SA and SAI at the two stages, with the exception of decreased SA under Ea at the jointing stage (Table 3; Fig. 4). Notably, all stomatal parameters in F5 were similar between Eg and Ea, and the magnitude of change was always larger under Ea than under Eg (Table 3; Fig. 4).
Table 3.
Effects of elevated CO2 on stomatal parameters in F1 and F5
| Generation | Stage | CO2 | SD (mm−2) | SA (μm2) | SAI (%) |
|---|---|---|---|---|---|
| F1 | Jointing | A | 532 ± 5 a | 302.5 ± 1.2 a | 16.1 ± 0.1 a |
| E a | 538 ± 3 a | 273.8 ± 0.7 b | 14.7 ± 0.1 b | ||
| Heading | A | 724 ± 6 a | 258.5 ± 0.8 a | 18.7 ± 0.2 a | |
| E a | 719 ± 4 a | 252.2 ± 0.9 b | 18.1 ± 0.1 b | ||
| F5 | Jointing | A | 514 ± 9 a | 133.1 ± 2.1 a | 6.9 ± 0.2 a |
| E g | 528 ± 10 a | 127.5 ± 1.7 ab | 6.8 ± 0.2 a | ||
| E a | 532 ± 10 a | 123.0 ± 1.8 b | 6.6 ± 0.1 a | ||
| Filling | A | 702 ± 12 a | 111.8 ± 1.7 a | 7.8 ± 0.2 a | |
| E g | 706 ± 12 a | 111.3 ± 1.6 a | 7.8 ± 0.2 a | ||
| E a | 710 ± 11 a | 111.0 ± 1.4 a | 7.9 ± 0.2 a |
Abbreviations: SA, stomatal apparatus area; SAI, stomatal apparatus area index; SD, stomatal density. Values are the mean ± s.e.m. (n = 4). Different lowercase letters indicate significant differences among CO2 treatments (P < 0.05).
Fig. 4.
The response ratio of stomatal density (SD), stomatal apparatus area (SA) and stomatal apparatus area index (SAI) under Ea in F1 and under Eg and Ea in F5 at the jointing (A) and filling (B) stages. *P < 0.05 (n = 4). SAI = SD × SA ×10−4 ×100 %. Response ratio = (E − A)/A, where A (ambient CO2) and E (elevated CO2) are from the same generation (F1 or F5).
Chlorophyll fluorescence of dark-adapted leaves
Although the magnitude of the chlorophyll fluorescence parameters did not show significant differences among the CO2 treatments in F1 and F5, the directions of these parameters differed between F1 and F5 (Supplementary data Table S2). For example, the maximum quantum yield of PSII (Fv/Fm), quantum yield for electron transport (φEo) and performance index (PIABS) had negative effects, whereas the quantum yield for energy dissipation (φDo) had a positive effect under Ea in F1 at the two stages. However, these traits did not exhibit consistent directions under Eg and Ea in F5.
DISCUSSION
Almost all growth chamber, greenhouse and field experiments have investigated the response of crops to Ea only within a generation; for example, ambient CO2 + 200 μmol mol−1 in one growing season (Wand et al., 1999; Ainsworth and Long, 2005; Leuzinger and Hättenschwiler, 2013; Ainsworth and Long, 2021), as shown in Fig. 1A in the present study. However, multigenerational exposure to E might alter plant physiology and growth in ways that cannot be predicted from single-generation studies, especially for C3 species that are most sensitive to changes in the atmospheric CO2 concentration (Ward and Kelly, 2004). Hence, our study provides new insights into the effects of E over multiple generations on photosynthesis and stomatal features in japonica rice. Two patterns of E were used in this study: Eg and Ea.
Three influential meta-analyses have reported that E stimulates photosynthesis by 13–35 % in crops (Ainsworth and Long, 2005; Ainsworth and Rogers, 2007; Leakey et al., 2009). Our results agree with the conclusion, as observed under Ea in F1 with a 21 % stimulation in Agrowth (Table 1). Likewise, Eg and Ea in F5 markedly increased Agrowth by 32 and 33 %, respectively (Table 1). This suggests that E-induced stimulation of photosynthesis is a certain fact, regardless of the pattern of E (Egvs Ea) and the generation (F1 vs F5) (Saban et al., 2019). Interestingly, in contrast to F1, in which the remaining gas exchange parameters decreased under Ea, which was consistent with the previous consensus (Ainsworth and Long, 2005; Wang et al., 2013; Hu et al., 2022), Eg and Ea in F5 did not affect these traits (Table 1). This indicates that transgenerational effects can attenuate the negative impact of E on photosynthetic physiological traits. Additionally, the larger stimulation in Agrowth in F5 than in F1 was attributed to the lower reduction in gs, owing to less CO2 limitation at constant external CO2 concentrations (Supplementary data Fig. S2). The reduction in leaf nitrogen can be explained by the decreased transpiration-driven mass flow under E (McDonald et al., 2002; McGrath and Lobell, 2013), which matched the relationships between Tr and Na under Ea in F1. Likewise, the absence of reduction in Tr and Na also occurred simultaneously under Eg and Ea in F5 (Tables 1 and 2).
Stomatal structure is an embodiment of the ability of plants to adapt to the environment (Kondamudi et al., 2016). An early study using fossil leaves showed that the evolution of leaf-form with the development of stomatal characteristics is causally associated with the change in atmospheric CO2 over time (Beerling et al., 2001). In the present study, stomatal features exhibited different responses not only between F1 and F5 but also between Ea and Eg. To be specific, Ea in F1 decreased SA and SAI at the two stages, Ea in F5 merely decreased SA at the jointing stage, and Eg in F5 did not affect stomatal traits at two stages (Fig. 4). This suggests that compared with a single generation, multigenerational exposure to E had little effect on stomatal features, especially under the Eg pattern. Thus, generations regulate the adaptation of stomata to a CO2-enriched environment. Furthermore, SA determines the response of gs to E (Ainsworth and Rogers, 2007; Yang et al., 2021), and this relationship was also observed under Eg and Ea over multiple generations (Supplementary data Fig. S3).
Photosynthetic and stomatal acclimation in CO2-enriched environments is a major problem that increases the complexity of models for predicting future carbon uptake and storage (Martinez-Carrasco et al., 2005; Li et al., 2012; Cai et al., 2018; Smith and Keenan, 2020). The vast majority of experiments have proved the existence of photosynthetic acclimation under E (Sage, 1994; Leakey et al., 2009; Hu et al., 2022), as shown under Ea in F1. However, photosynthetic acclimation was not detected under Eg and Ea in F5 (Fig. 2C, D). Previous studies have demonstrated that photosynthetic acclimation is accompanied by a reduction in Vcmax, Jmax, Na, Fv/Fm and φEo (Hymus et al., 2001; Ainsworth and Long, 2005; Chen et al., 2005; Lawlor and Tezara, 2009; Wilhelm and Selmar, 2011). This was consistent with the present findings for Ea in F1 but not for Vcmax and Jmax under Eg and Ea in F5 (Tables 1 and 2; Fig. 2; Supplementary data Table S2). Furthermore, stomatal acclimation rarely occurred in all treatments (Supplementary data Fig. S1). These results suggest that compared with a single generation, multigenerational exposure to E, including Eg and Ea, altered the ability for acclimation of photosynthesis, but not for acclimation of stomata.
PNUE and WUE were promoted by E (Fig. 3); however, the mechanism differed between generations. In F1, the stimulated PNUE (or WUE) was attributed to increased Agrowth and decreased Na (or Tr) (Tables 1 and 2). In contrast, in F5, only the increased Agrowth, not the decreased Na (or Tr), accounted for the stimulated PNUE (or WUE). A limited study found that multigenerational exposure to Ea could modulate gs, thereby attenuating the negative impact of drought stress on WUE in wheat (Li et al., 2017). In the present study, the larger WUE stimulation over multiple generations than for a single generation supported and broadened this finding in both Ea and Eg conditions.
To our knowledge, the present study is the first to compare and illustrate the extent and direction of the changes in photosynthesis and stomatal features under Ea and Eg over multiple generations relative to those under Ea within a generation. As discussed above, the effects of Ea on plants within a generation have been well documented and are consistent with our results. To date, only a single study has reported that fungal species richness and mycorrhizal fungal taxa decreased under Ea, but not under Eg over 21 generations (Klironomos et al., 2005). This observation seemed to support our finding that Eg in F5 had little effect on plant physiological traits. Several studies supported the results of Ea in F5, which showed that there was a lack of photosynthetic acclimation under Ea over multiple generations (Bezemer and Jones, 2012; Haworth et al., 2015). Furthermore, some studies of natural CO2 springs (surrounding volcanic degassing vents) might correspond to the treatment of Ea in F5 (Miglietta et al., 1993). For instance, no difference in leaf nitrogen for monocotyledonous species and a trend of reduced photosynthetic acclimation was found in natural CO2 springs (long-term exposure, >10 years; Onoda et al., 2007, 2009; Watson-Lazowski et al., 2016), possibly because multigenerational plants increased nitrogen uptake from inorganic nitrogen pools in the soil (Ueda et al., 2017).
A meta-analysis of responses to E in natural CO2 springs showed that photosynthetic physiological parameters decreased in CO2-enriched environments (Saban et al., 2019); however, this result was not observed in the present study. The discrepancy between natural CO2 springs and the present study might be based on the fact that sufficient water and nitrogen are available in agricultural ecosystems. Thus, transgenerational adaptation might differ between natural and human-managed agricultural ecosystems. Here, we considered the changes in gs as an example of a plant adaptation response. Owing to sufficient water availability in the paddy field in the present study, the reduction in gs induced by E was alleviated over generations, primarily as an adaptive response to maintain photosynthesis at relatively high levels. In another study, gs was decreased further by maternal E relative to maternal A over 7 years in a desert, primarily as an adaptive response to conserve water (Grossman and Rice, 2014).
Unexpectedly, multigenerational exposure to Eg and Ea resulted in similar responses in almost all tested traits. The maternal environment is generally assumed to change seed quality (Potvin and Tousignant, 1996; Huxman et al., 1998; Lau et al., 2008). Such differentiation between Eg and Ea across generations did occur, such as seed nitrogen content (Yang et al., 2023b); however, the difference in seed quality did not explain the similar responsiveness. Nevertheless, we speculate that the mechanisms of Eg and Ea over multiple generations might be different. Under Eg in F5, plants were likely to adapt gradually to stepwise changes in CO2 levels from one generation to the next; that is, owing to the smaller increase in CO2 level, an increase of 40 μmol mol−1 based on A could not result in a significant effect in the first generation (Liu et al., 2020). Likewise, an increase of 40 μmol mol−1 based on A + 40 μmol mol−1 could not result in a significant effect in the second generation either, and the adaption continued to the fifth generation, wherein the CO2 level reached A + 200 μmol mol−1. But under Ea in F5, plants could ‘remember’ the maternal environment and use environmental ‘memory’ to adapt when experiencing the recurrent environments (Bruce et al., 2007; Avramova, 2015; Liu et al., 2019); that is, when experiencing Ea in F1, such a large and rapid change in the CO2 level altered photosynthetic and stomatal traits; however, when several subsequent generations were continuously grown in the same CO2 environment, plants could ‘remember’ the maternal CO2 environment and adjust to adapt. Such transgenerational ‘memory’ of the ancestral environment might result from epigenetic variation, such as DNA methylation, contributing to phenotype expression in offspring (Heard and Martienssen, 2014), as shown in a recent study suggesting that DNA methylation might contribute to plant adaptation to multigenerational exposure to future atmospheric CO2 (Saban et al., 2020). Thus, DNA methylation for plants across generations requires further investigation in CO2-enriched environments.
Our results under Ea in F1 (most experiments conducted) and Eg and Ea in F5 (actual cases in future climate) provide experimental evidence that E retains intergenerational effects on photosynthesis and stomatal features, whereas there were no multigenerational differences in the effects of Eg and Ea. Briefly, the responses to Ea in F1 were consistent with the previous consensus, such as the occurrence of photosynthetic acclimation, stimulation of photosynthesis and downregulation of photosynthetic physiological parameters and stomatal area. In contrast, Eg and Ea in F5 did not induce photosynthetic acclimation but stimulated greater photosynthesis and had little effect on photosynthetic physiology and stomatal traits. Hence, projecting future changes induced by E based on the physiological responses of contemporary plants could be misleading. Furthermore, the results from large and rapid environmental changes within a generation cannot accurately predict the effects of natural environmental changes on plants over multiple generations, especially in annual herbs with short life cycles.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: basic information on CO2 concentration, temperature and photosynthetic photon flux density from 2016 to 2020. Table S2: the responses of dark-adapted chlorophyll fluorescence parameters to Ea in F1 and to Eg and Ea in F5. Fig. S1: the responses of stomatal conductance to intercellular CO2 under Ea in F1 and Eg and Ea in F5 at the jointing and filling stages. Fig. S2: relationship of the response ratio of net photosynthetic rate against the response ratio of stomatal conductance at growth CO2 concentration. Fig. S3: correlation between stomatal conductance and stomatal apparatus area under Eg and Ea in F5.
ACKNOWLEDGEMENTS
We express thanks to Cailin Wang of the Jiangsu Academy of Agricultural Sciences for the provision with rice seeds and M.S. Chunyan You of the Bureau of Agriculture and Rural Affairs of Pukou District, Nanjing City for help with field measurements. Many thanks also go to Beijing Tian Hang Hua Chuang Technology Co., Ltd for their technical assistance with the CO2 concentration control system.
Contributor Information
Kai Yang, State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China; University of Chinese Academy of Sciences, Beijing, China.
Yao Huang, State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China; University of Chinese Academy of Sciences, Beijing, China.
Jingrui Yang, State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China; University of Chinese Academy of Sciences, Beijing, China.
Chunhua Lv, State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China; University of Chinese Academy of Sciences, Beijing, China.
Zhenghua Hu, Collaborative Innovation Center on Forecast and Evaluation of Meteorological Disasters, School of Applied Meteorology, Nanjing University of Information Science & Technology, Nanjing, China.
Lingfei Yu, State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China.
Wenjuan Sun, State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China.
FUNDING
This work was supported by the National Natural Science Foundation of China (41530533 and 42171064) and the Strategic Priority Research Program of the Chinese Academy of Sciences, China (XDA26010103).
LITERATURE CITED
- Adachi M, Hasegawa T, Fukayama H, et al. 2014. Soil and water warming accelerates phenology and down-regulation of leaf photosynthesis of rice plants grown under free-air CO2 enrichment (FACE). Plant and Cell Physiology 55: 370–380. doi: 10.1093/pcp/pcu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Long SP.. 2005. What have we learned from 15 years of free‐air CO2 enrichment (FACE)? A meta‐analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165: 351–372. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Long SPP.. 2021. 30 years of free-air carbon dioxide enrichment (FACE): what have we learned about future crop productivity and its potential for adaptation? Global Change Biology 27: 27–49. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A.. 2007. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell & Environment 30: 258–270. [DOI] [PubMed] [Google Scholar]
- Avramova Z. 2015. Transcriptional ‘memory’ of a stress: transient chromatin and memory (epigenetic) marks at stress‐response genes. The Plant Journal 83: 149–159. doi: 10.1111/tpj.12832. [DOI] [PubMed] [Google Scholar]
- Beerling DJ, Osborne CP, Chaloner WG.. 2001. Evolution of leaf-form in land plants linked to atmospheric CO2 decline in the Late Palaeozoic era. Nature 410: 352–354. doi: 10.1038/35066546. [DOI] [PubMed] [Google Scholar]
- Bernacchi C, Singsaas E, Pimentel C, Portis A Jr, Long SP.. 2001. Improved temperature response functions for models of Rubisco‐limited photosynthesis. Plant, Cell & Environment 24: 253–259. [Google Scholar]
- Bernacchi CJ, Pimentel C, Long SP.. 2003. In vivo temperature response functions of parameters required to model RuBP‐limited photosynthesis. Plant, Cell & Environment 26: 1419–1430. [Google Scholar]
- Bettarini I, Vaccari FP, Miglietta F.. 1998. Elevated CO2 concentrations and stomatal density: observations from 17 plant species growing in a CO2 spring in central Italy. Global Change Biology 4: 17–22. doi: 10.1046/j.1365-2486.1998.00098.x. [DOI] [Google Scholar]
- Bezemer TM, Jones TH.. 2012. The effects of CO2 and nutrient enrichment on photosynthesis and growth of Poa annua in two consecutive generations. Ecological Research 27: 873–882. doi: 10.1007/s11284-012-0961-5. [DOI] [Google Scholar]
- Bruce TJ, Matthes MC, Napier JA, Pickett JA.. 2007. Stressful ‘memories’ of plants: evidence and possible mechanisms. Plant Science 173: 603–608. doi: 10.1016/j.plantsci.2007.09.002. [DOI] [Google Scholar]
- Burgess SC, Marshall DJ.. 2014. Adaptive parental effects: the importance of estimating environmental predictability and offspring fitness appropriately. Oikos 123: 769–776. doi: 10.1111/oik.01235. [DOI] [Google Scholar]
- Cai C, Li G, Yang H, et al. 2018. Do all leaf photosynthesis parameters of rice acclimate to elevated CO2, elevated temperature, and their combination, in FACE environments? Global Change Biology 24: 1685–1707. [DOI] [PubMed] [Google Scholar]
- Cai C, Li G, Di L, et al. 2020. The acclimation of leaf photosynthesis of wheat and rice to seasonal temperature changes in T-FACE environments. Global Change Biology 26: 539–556. [DOI] [PubMed] [Google Scholar]
- Chen G-Y, Yong Z-H, Liao Y, et al. 2005. Photosynthetic acclimation in rice leaves to free-air CO2 enrichment related to both ribulose-1,5-bisphosphate carboxylation limitation and ribulose-1,5-bisphosphate regeneration limitation. Plant and Cell Physiology 46: 1036–1045. doi: 10.1093/pcp/pci113. [DOI] [PubMed] [Google Scholar]
- Collins S, Bell G.. 2004. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature 431: 566–569. doi: 10.1038/nature02945. [DOI] [PubMed] [Google Scholar]
- Donelson JM, Salinas S, Munday PL, Shama LN.. 2018. Transgenerational plasticity and climate change experiments: where do we go from here? Global Change Biology 24: 13–34. [DOI] [PubMed] [Google Scholar]
- Donohue K. 2009. Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society B: Biological Sciences 364: 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake BG, GonzalezMeler MA, Long SP.. 1997. More efficient plants: a consequence of rising atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology 48: 609–639. doi: 10.1146/annurev.arplant.48.1.609. [DOI] [PubMed] [Google Scholar]
- Drake JE, Macdonald CA, Tjoelker MG, et al. 2016. Short-term carbon cycling responses of a mature eucalypt woodland to gradual stepwise enrichment of atmospheric CO2 concentration. Global Change Biology 22: 380–390. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA.. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- Flexas J, Díaz-Espejo A, Berry JA, et al. 2007. Analysis of leakage in IRGA’s leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. Journal of Experimental Botany 58: 1533–1543. [DOI] [PubMed] [Google Scholar]
- Frenck G, van der Linden L, Mikkelsen TN, Brix H, Jørgensen RB.. 2013. Response to multi-generational selection under elevated CO2 in two temperature regimes suggests enhanced carbon assimilation and increased reproductive output in Brassica napus L. Ecology and Evolution 3: 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman JD, Rice KJ.. 2014. Contemporary evolution of an invasive grass in response to elevated atmospheric CO2 at a Mojave Desert FACE site. Ecology Letters 17: 710–716. doi: 10.1111/ele.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth M, Moser G, Raschi A, Kammann C, Grünhage L, Müller C.. 2015. Carbon dioxide fertilisation and supressed respiration induce enhanced spring biomass production in a mixed species temperate meadow exposed to moderate carbon dioxide enrichment. Functional Plant Biology 43: 26–39. [DOI] [PubMed] [Google Scholar]
- Heard E, Martienssen RA.. 2014. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157: 95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JJ, Sultan SE.. 2011. Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Frontiers in Plant Science 2: 102. doi: 10.3389/fpls.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chen W, Tong K, et al. 2022. Response of rice growth and leaf physiology to elevated CO2 concentrations: a meta-analysis of 20-year FACE studies. Science of the Total Environment 807: 151017. doi: 10.1016/j.scitotenv.2021.151017. [DOI] [PubMed] [Google Scholar]
- Hui DF, Sims DA, Johnson DW, Cheng WX, Luo YQ.. 2002. Effects of gradual versus step increases in carbon dioxide on Plantago photosynthesis and growth in a microcosm study. Environmental and Experimental Botany 47: 51–66. doi: 10.1016/s0098-8472(01)00112-5. [DOI] [Google Scholar]
- Huxman TE, Hamerlynck EP, Jordan DN, Salsman KJ, Smith SD.. 1998. The effects of parental CO2 environment on seed quality and subsequent seedling performance in Bromus rubens. Oecologia 114: 202–208. doi: 10.1007/s004420050437. [DOI] [PubMed] [Google Scholar]
- Huxman TE, Hamerlynck EP, Smith SD.. 1999. Reproductive allocation and seed production in Bromus madritensis ssp. rubens at elevated atmospheric CO2. Functional Ecology 13: 769–777. [Google Scholar]
- Hymus GJ, Baker NR, Long SP.. 2001. Growth in elevated CO2 can both increase and decrease photochemistry and photoinhibition of photosynthesis in a predictable manner. Dactylis glomerata grown in two levels of nitrogen nutrition. Plant Physiology 127: 1204–1211. doi: 10.1104/pp.010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. 2022. Climate change 2022: impacts, adaptation, and vulnerability. In: Pörtner H-O, Roberts DC, Tignor M, et al., eds. Contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press,653. [Google Scholar]
- Klironomos JN, Allen MF, Rillig MC, et al. 2005. Abrupt rise in atmospheric CO2 overestimates community response in a model plant–soil system. Nature 433: 621–624. doi: 10.1038/nature03268. [DOI] [PubMed] [Google Scholar]
- Kondamudi R, Swamy KN, Rao YV, et al. 2016. Gas exchange, carbon balance and stomatal traits in wild and cultivated rice (Oryza sativa L.) genotypes. Acta Physiologiae Plantarum 38: 160. [Google Scholar]
- Lau JA, Peiffer J, Reich PB, Tiffin P.. 2008. Transgenerational effects of global environmental change: long-term CO2 and nitrogen treatments influence offspring growth response to elevated CO2. Oecologia 158: 141–150. doi: 10.1007/s00442-008-1127-6. [DOI] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W.. 2009. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Annals of Botany 103: 561–579. doi: 10.1093/aob/mcn244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey ADB, Lau JA.. 2012. Evolutionary context for understanding and manipulating plant responses to past, present and future atmospheric CO2. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 613–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR.. 2009. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. Journal of Experimental Botany 60: 2859–2876. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- Leuzinger S, Hättenschwiler S.. 2013. Beyond global change: lessons from 25 years of CO2 research. Oecologia 171: 639–651. doi: 10.1007/s00442-012-2584-5. [DOI] [PubMed] [Google Scholar]
- Li G, Lin L, Dong Y, et al. 2012. Testing two models for the estimation of leaf stomatal conductance in four greenhouse crops cucumber, chrysanthemum, tulip and lilium. Agricultural and Forest Meteorology 165: 92–103. doi: 10.1016/j.agrformet.2012.06.004. [DOI] [Google Scholar]
- Li T, Hasegawa T, Yin X, et al. 2015. Uncertainties in predicting rice yield by current crop models under a wide range of climatic conditions. Global Change Biology 21: 1328–1341. doi: 10.1111/gcb.12758. [DOI] [PubMed] [Google Scholar]
- Li X, Khan A, Lv Z, Fang L, Jiang D, Liu F.. 2019. Effect of multigenerational exposure to elevated atmospheric CO2 concentration on grain quality in wheat. Environmental and Experimental Botany 157: 310–319. [Google Scholar]
- Li Y, Li X, Yu J, Liu F.. 2017. Effect of the transgenerational exposure to elevated CO2 on the drought response of winter wheat: stomatal control and water use efficiency. Environmental and Experimental Botany 136: 78–84. doi: 10.1016/j.envexpbot.2017.01.006. [DOI] [Google Scholar]
- Liu C, Hu ZH, Yu LF, Chen ST, Liu XM.. 2020. Responses of photosynthetic characteristics and growth in rice and winter wheat to different elevated CO2 concentrations. Photosynthetica 58: 1130–1140. doi: 10.32615/ps.2020.066. [DOI] [Google Scholar]
- Liu Y, Schwalm CR, Samuels-Crow KE, Ogle K.. 2019. Ecological memory of daily carbon exchange across the globe and its importance in drylands. Ecology Letters 22: 1806–1816. doi: 10.1111/ele.13363. [DOI] [PubMed] [Google Scholar]
- Lobell DB, Schlenker W, Costa-Roberts J.. 2011. Climate trends and global crop production since 1980. Science 333: 616–620. doi: 10.1126/science.1204531. [DOI] [PubMed] [Google Scholar]
- Lodge RJ, Dijkstra P, Drake BG, Morison JIL.. 2001. Stomatal acclimation to increased CO2 concentration in a Florida scrub oak species Quercus myrtifolia Willd. Plant Cell and Environment 24: 77–88. [Google Scholar]
- Luo YQ, Reynolds JF.. 1999. Validity of extrapolating field CO2 experiments to predict carbon sequestration in natural ecosystems. Ecology 80: 1568–1583. doi: 10.1890/0012-9658(1999)080[1568:voefce]2.0.co;2. [DOI] [Google Scholar]
- Martinez-Carrasco R, Perez P, Morcuende R.. 2005. Interactive effects of elevated CO2, temperature and nitrogen on photosynthesis of wheat grown under temperature gradient tunnels. Environmental and Experimental Botany 54: 49–59. doi: 10.1016/j.envexpbot.2004.05.004. [DOI] [Google Scholar]
- McDonald EP, Erickson JE, Kruger EL.. 2002. Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Functional Plant Biology 29: 1115–1120. doi: 10.1071/fp02007. [DOI] [PubMed] [Google Scholar]
- McGrath JM, Lobell DB.. 2013. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant, Cell and Environment 36: 697–705. [DOI] [PubMed] [Google Scholar]
- Miglietta F, Raschi A, Bettarini I, et al. 1993. Natural CO2 springs in Italy: a resource for examining long‐term response of vegetation to rising atmospheric CO2 concentrations. Plant, Cell & Environment 16: 873–878. [Google Scholar]
- Onoda Y, Hirose T, Hikosaka K.. 2007. Effect of elevated CO2 levels on leaf starch, nitrogen and photosynthesis of plants growing at three natural CO2 springs in Japan. Ecological Research 22: 475–484. [Google Scholar]
- Onoda Y, Hirose T, Hikosaka K.. 2009. Does leaf photosynthesis adapt to CO2-enriched environments? An experiment on plants originating from three natural CO2 springs. New Phytologist 182: 698–709. doi: 10.1111/j.1469-8137.2009.02786.x. [DOI] [PubMed] [Google Scholar]
- Potvin C, Tousignant D.. 1996. Evolutionary consequences of simulated global change: genetic adaptation or adaptive phenotypic plasticity. Oecologia 108: 683–693. doi: 10.1007/bf00329043. [DOI] [PubMed] [Google Scholar]
- Saban JM, Chapman MA, Taylor G.. 2019. FACE facts hold for multiple generations; evidence from natural CO2 springs. Global Change Biology 25: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban JM, Watson-Lazowski A, Chapman MA, Taylor G.. 2020. The methylome is altered for plants in a high CO2 world: insights into the response of a wild plant population to multigenerational exposure to elevated atmospheric [CO2]. Global Change Biology 26: 6474–6492. doi: 10.1111/gcb.15249. [DOI] [PubMed] [Google Scholar]
- Sage RF. 1994. Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosynthesis Research 39: 351–368. doi: 10.1007/bf00014591. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Armond PA.. 1978. Heat-induced changes of chlorophyll fluorescence in isolated chloroplasts and related heat-damage at the pigment level. Biochimica et Biophysica Acta (BBA) - Bioenergetics 502: 138–151. [DOI] [PubMed] [Google Scholar]
- Shanmugam S, Kjaer KH, Ottosen C-O, Rosenqvist E, Sharma DK, Wollenweber B.. 2013. The alleviating effect of elevated CO2 on heat stress susceptibility of two wheat (Triticum aestivum L.) cultivars. Journal of Agronomy and Crop Science 199: 340–350. [Google Scholar]
- Smith NG, Dukes JS.. 2013. Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Global Change Biology 19: 45–63. doi: 10.1111/j.1365-2486.2012.02797.x. [DOI] [PubMed] [Google Scholar]
- Smith NG, Keenan TF.. 2020. Mechanisms underlying leaf photosynthetic acclimation to warming and elevated CO2 as inferred from least-cost optimality theory. Global Change Biology 26: 5202–5216. doi: 10.1111/gcb.15212. [DOI] [PubMed] [Google Scholar]
- Strasser BJ, Strasser RJ.. 1995. Measuring fast fluorescence transients to address environmental questions: the JIP test. In: Mathis P, ed. Photosynthesis: from light to biosphere. Dordrecht: Kluwer Academic, 997–980. [Google Scholar]
- Tsimilli-Michael M, Strasser RJ.. 2008. In vivo assessment of stress impact on plant’s vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants. In: Varma A, ed. Mycorrhiza: genetics and molecular biology, eco-function, biotechnology, ecophysiology, and structure and systematics. Berlin: Springer, 679–703. [Google Scholar]
- Ueda MU, Onoda Y, Kamiyama C, Hikosaka K.. 2017. Decades-long effects of high CO2 concentration on soil nitrogen dynamics at a natural CO2 spring. Ecological Research 32: 215–225. doi: 10.1007/s11284-016-1432-1. [DOI] [Google Scholar]
- Vráblová M, Hronková M, Vrábl D, Kubásek J, Šantrůček J.. 2018. Light intensity-regulated stomatal development in three generations of Lepidium sativum. Environmental and Experimental Botany 156: 316–324. [Google Scholar]
- Wand SJE, Midgley GF, Jones MH, Curtis PS.. 1999. Responses of wild C4 and C3 grass (Poaceae) species to elevated atmospheric CO2 concentration: a meta-analytic test of current theories and perceptions. Global Change Biology 5: 723–741. doi: 10.1046/j.1365-2486.1999.00265.x. [DOI] [Google Scholar]
- Wang L, Feng Z, Schjoerring JK.. 2013. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): a meta-analytic test of current hypotheses. Agriculture, Ecosystems & Environment 178: 57–63. [Google Scholar]
- Wang YW, Xu C, Lv CF, et al. 2016. Chlorophyll a fluorescence analysis of high-yield rice (Oryza sativa L.) LYPJ during leaf senescence. Photosynthetica 54: 422–429. doi: 10.1007/s11099-016-0185-y. [DOI] [Google Scholar]
- Ward JK, Kelly JK.. 2004. Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecology Letters 7: 427–440. doi: 10.1111/j.1461-0248.2004.00589.x. [DOI] [Google Scholar]
- Watson-Lazowski A, Lin Y, Miglietta F, Edwards RJ, Chapman MA, Taylor G.. 2016. Plant adaptation or acclimation to rising CO2? Insight from first multigenerational RNA-Seq transcriptome. Global Change Biology 22: 3760–3773. doi: 10.1111/gcb.13322. [DOI] [PubMed] [Google Scholar]
- Wilhelm C, Selmar D.. 2011. Energy dissipation is an essential mechanism to sustain the viability of plants: the physiological limits of improved photosynthesis. Journal of Plant Physiology 168: 79–87. doi: 10.1016/j.jplph.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Yang K, Yang J, Lv C, et al. 2021. Reduced mesophyll conductance induces photosynthetic acclimation of japonica rice under elevated CO2. Environmental and Experimental Botany 190: 104590. doi: 10.1016/j.envexpbot.2021.104590. [DOI] [Google Scholar]
- Yang K, Huang Y, Yang J, et al. 2023a. The determiner of photosynthetic acclimation induced by biochemical limitation under elevated CO2 in japonica rice. Journal of Plant Physiology 280: 153889. doi: 10.1016/j.jplph.2022.153889. [DOI] [PubMed] [Google Scholar]
- Yang K, Huang Y, Lv C, et al. 2023b. Does elevated CO2 cause human malnutrition? A new understanding from small and slow CO2 change across generations in rice grain quality. Environmental and Experimental Botany 2023: 105236. [Google Scholar]
- Yin J, Zhou M, Lin Z, Li QQ, Zhang Y-Y.. 2019. Transgenerational effects benefit offspring across diverse environments: a meta-analysis in plants and animals. Ecology Letters 22: 1976–1986. doi: 10.1111/ele.13373. [DOI] [PubMed] [Google Scholar]
- Yin J, Lin X, Yao J, Li QQ, Zhang Y-Y.. 2022. Genotypic variation of transgenerational plasticity can be explained by environmental predictability at origins. Oikos 2022: 09006. doi: 10.1111/oik.09006. [DOI] [Google Scholar]
- Ziska LH, Bunce JA.. 2007. Predicting the impact of changing CO2 on crop yields: some thoughts on food. New Phytologist 175: 607–618. doi: 10.1111/j.1469-8137.2007.02180.x. [DOI] [PubMed] [Google Scholar]
- Zong YZ, Wang WF, Xue QW, Shangguan ZP.. 2014. Interactive effects of elevated CO2 and drought on photosynthetic capacity and PSII performance in maize. Photosynthetica 52: 63–70. doi: 10.1007/s11099-014-0009-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.