Abstract
Little is known about a regulatory role of CaMKK2 for hematopoietic stem (HSC) and progenitor (HPC) cell function. To assess this, we used Camkk2−/− and wild type (WT) control mouse bone marrow (BM) cells. BM cells were collected/processed and compared under hypoxia (3% oxygen; physioxia) vs. ambient air (~21% oxygen). Subjecting cells collected to ambient air, even for a few minutes, causes a stress that we termed Extra Physiological Shock/Stress (EPHOSS) that causes differentiation of HSCs and HPCs. We consider physioxia collection/processing a more relevant way to assess HSC/HPC numbers and function, as the cells remain in an oxygen tension closer physiologic conditions. Camkk2−/− cells collected/processed at 3% oxygen had positive and negative effects respectively on HSCs (by engraftment using competitive transplantation with congenic donor and competitor cells and lethally irradiated congenic recipient mice), and HPCs (by colony forming assays of CFU-GM, BFU-E, and CFU-GEMM) compared to WT cells processed in ambient air. Thus, with cells collected/processed under physioxia, and therefore never exposed and naïve to ambient air conditions, CaMKK2 not only appears to act as an HSC to HPC differentiation fate determinant, but as we found for other intracellular mediators, the Camkk−/− mouse BM cells were relatively resistant to effects of EPHOSS. This information is of potential use for modulation of WT BM HSCs and HPCs for future clinical advantage.
Keywords: CaMKK2, Hypoxia cell collection/processing, CaMKK2 knockout mice, Hematopoietic stem (HSCs) and progenitor (HPCs) cells, Phenotypic and functional assessment of HSCs and HPCs
Introduction
Regulation of hematopoiesis and hematopoietic stem (HSCs) and progenitor (HPCs) cells is mediated in large part by cytokines, chemokines, and additional growth factors (GFs) [1, 2]. This regulation is influenced by receptors on the cell surface of the HSCs, HPCs, and other mature cells through intracellular activation of effector molecules in a positive or negative fashion [1–3]. While much is known about intracellular mediators, there is still much more to be uncovered, for optimal undertaking of HSC and HPC regulation especially under their collection and processing under physioxia conditions of low oxygen (5%) tension, for potential future clinical use.
Calcium (Ca2+) binds its primary intracellular receptor, calmodulin, to trigger downstream pathways involving Ca2+/calmodulin-dependent kinase kinase 2 (CaMKK2) in certain cell types [4–13]. However, very little is known of a role for CaMKK2 in the regulation of HSCs and HPCs [14, 15], nothing was known when cells were collected/processed under low (3%; physioxia) oxygen tensions, which we believe is a more relevant physiological means to assess a role for CaMKK2 in regulation of hematopoiesis [16]. CaMKK2 is involved in energy balance, adiposity, and glucose homeostasis in non-hematopoietic cells [5]. In hematopoietic cells collected and studied in non-physiological oxygen conditions of ambient air (~21% oxygen tension) Camkk2 was found to regulate myeloid cells functions relevant for the inflammatory response and tumor-induced immunosuppression [17–19]. While functions of Camkk2 have been suggested in terms of its potential for therapeutic intervention [5], for this to be involved in hematopoiesis and HSCs and HPCs, it is necessary to better understand how Camkk2 influences growth of HSCs and HPCs in physioxia. To this end we used Camkk2 knockout (Camkk2−/−) mouse bone marrow (BM) cells [13] collected/processed in hypoxia (3% oxygen) vs. ambient air, as previously published by us to assess relative resistance to the effects of Extra Physiological Shock/Stress (EPHOSS), and effects on HSC to HPC differentiation fate decisions [16, 20–23] for wild-type (WT) control mice [16] and previously for certain mouse knock-out BM cells, such as HIF-1α−/−, cyclophilin D−/−, p53−/− and miR21−/−, where some were found to be relatively resistant to EPHOSS [16]. This comparison with hypoxia collected/processed cells has not previously been reported for Camkk2−/− mice.
Collection/processing of mouse bone marrow (BM) cells at physioxia (3% oxygen) offers a more physiologic atmosphere similar to that present in BM in vivo [16], and thus a likely more relevant view of how HSCs and HPCs would behave in their normal in vivo BM microenvironment.
Methods
Mice
Animal studies were approved by Indiana University School of Medicine (IUSM) Institutional Animal Care and Use Committee (IACUC) and all experiments were performed in compliance with NIH guidelines on the use and care of laboratory and experimental animals. Camkk2−/− mice were previously generated through the targeted deletion of exons 2-4 of the mouse Camkk2 gene [24]. Wild-type (WT) and CaMkk2−/− mice (both on a C57BL/6 strain background; WT also on a BoyJ or F1 background) were housed in the IUSM Laboratory Animal Resource Center (Indianapolis, IN) under 12 h light/dark cycle. Food and water were provided ad libitum.
Flow Cytometry
For analyzing HSCs and HPCs phenotype in mouse BM, cells were aliquoted at a concentration of ~2-3 × 106 cells per tube, washed in PBS, incubated in fluorescently-conjugated anti-mouse antibody cocktail for 20 min at room temperature, washed in PBS, and then fixed in 1.5% formaldehyde. Samples were analyzed on an LSRII flow cytometer (BD Biosciences). Single color compensation and isotype controls were included for each experiment. Data analysis was performed using FlowJo 7.6.3 software (TreeStar, WA, USA). Percent of each population was used to calculate absolute numbers of each population per femur. Phenotyping markers used were FITC-mouse lineage cocktail (CD3, Gr-1, CD11b, CD45R, Ter119; BioLegend; cat. # 133302), PE-CF594-anti-Ly6A/E (a.k.a. Sca1; clone D7; BD Biosciences), APC-H7-anti-CD117 (a.k.a. cKit; clone 2B8; BD Biosciences), APC-anti-CD135 (a.k.a. Flt3; clone A2F10.1; BD Biosciences), PE-anti-CD34 (clone RAM34; BD Biosciences), and PerCP-Cy™5.5-anti-CD16/CD32 (a.k.a. FcγII/IIIR; clone 2.4G2; BD Biosciences). HSC and HPC populations [16, 22, 23, 25] were defined as follows: LSK cells Lin− Sca1+ cKit+, long-term (LT)-HSC: LSK Flt3− CD34−, short-term (ST)-HSC: LSK Flt3− CD34+, multipotent progenitor (MPP): LSK Flt3+ CD34+, common myeloid progenitor (CMP): Lin− Sca1− cKit+ (LK) FcγII/IIIRlo CD34+, granulocyte-macrophage progenitor (GMP): LK FcγII/nIRhi CD34+, and megakaryocyte-erythrocyte progenitor (MEP): LK FcγII/IIIR− CD34−/lo. For all antibodies used in these studies, the validation for the relevant species and applications can be found on the indicated manufacturer’s website.
Engraftment Studies
Camkk2−/− and WT mice (n = 4 per group) on a C57BL/6 background (CD45.2 + CD45.1−) were sacrificed and BM was harvested by flushing with DPBS in a 3% O2, 5% CO2 hypoxia chamber [16]. Half of the harvested BM cells from each mouse were split in the hypoxia chamber and moved to ambient 21% O2 and allowed to equilibrate in ambient air for 2 h [16]. Cells from 4 donor mice per group were combined to make donor pools. Cells were transplanted by injecting 200,000 BM donor cells via tail vein to lethally-irradiated recipient F1 mice (dual CD45.2 + CD45.1+). Recipient mice of hypoxia isolated cells were injected within the 3% O2, 5% CO2 hypoxia chamber using a special device so that the cells are not exposed to ambient air during the injection procedure [16], while recipients of ambient air exposed cells were injected in ambient air [16]. For all mice, a second tail vein injection was performed within 2 h of initial injection to transplant 200,000 competitor cells (competitive transplant setting), which were BM cells from BoyJ background mice (CD45.2-CD45.1+). At months 1, 2, 4, and 6 post-transplant, peripheral blood (PB) was taken from all recipient mice via submandibular bleeding. At month 6, mice were euthanized post PB collection, and BM from one femur per recipient was harvested by flushing. For PB samples, red blood cells were lysed with 1X RBC Lysis Buffer (Biolegend) and cells were washed twice with PBS. For all samples, cells were stained with anti-CD45.1 (clone A20, BD Biosciences) and anti-CD45.2 (clone 104, BD Biosciences). BM cells were also stained with anti-B220 (clone RA3-6B2, BioLegend), anti-CD3e (clone 145-2C11, BD Biosciences), and anti-CD11b (clone M1/70, BD Biosciences). Cells were incubated for 20 min at room temperature with antibody cocktail, washed in PBS, and then fixed in 1.5% formaldehyde. Samples were analyzed on an LSRII flow cytometer (BD Biosciences). Chimerism was reported as % CD45.2 + CD45.1− relative to all cells expressing donor mouse CD45 for PB cells and as % CD45.2 + CD45.1− for BM cells. Myeloid/Lymphoid ratios were reported as the number of CD11b + cells divided by the sum of B220+ cells plus CD3e + cells.
Colony Formation in Vitro to Assess Numbers of Functional HPCs in Camkk2−/− Mice and Wildtype (WT) Control Mice
BM cells were harvested from femur(s) by flushing with DPBS (BioWhittaker). Whole bone marrow cells were plated at 5 × 104 cells/ml in 1% methylcellulose/Iscove’s Modified Dulbecco’s Medium (IMDM) with 30% FBS (Corning), 1u/ml erythropoietin (Amgen), 5%(v/v) poke-weed mitogen spleen cell conditioned medium (PWMSCM), 50 ng rmSCF (R&D Systems), and 0.1 mM Hemin (Sigma). Cultures were incubated for 6-7 days at 37 °C in a 5% CO2/5% lowered oxygen tension humidified environment while cells were collected at 3% or ambient air (~21%) oxygen. The plates for colonies from both 3% and ~ 21% oxygen were incubated at 5% oxygen as we have previously used this oxygen tension for plating to maximize detection of colony numbers. Cultures were scored for colony forming unit (CFU) – granulocyte (G), macrophage (M), burst forming unit (BFU)-erythroid (E)/colony forming unit (CFU) - granulocyte, erythrocyte, macrophage, megakaryocyte (GEMM) [16, 21–23, 25].
Collection/Processing of Mouse BM Cells in Hypoxia/Physioxia (5% Oxygen)
We have previously shown in detail that collection/processing of mouse BM cells in hypoxia (3% oxygen) vs. in ambient air (~21% O2) allows for increased numbers of WT HSCs by phenotypic analysis and engrafting capability in a competitive transplant assay of lethally-irradiated recipients (dual F1, CD45.2+/CD45.1+ mice) with donor and competitor respectively CD45.2+ and CD45.1 (Boy J) mice [16]. The engrafting assay to detect functional HSCs through chimerism analysis of donor cells, used a 1:1 ratio of donor and competitor cells. Both Camkk2−/− and WT mice were on a CD45.2+ mouse strain background. Mouse BM cells were first collected in 3% oxygen in a hypoxic chamber [16] then split into two parts, with half remaining in hypoxia for processing and the other half being moved to normoxia (~21%; oxygen) for 2 h before further processing. After the 2 h acclimation, both hypoxia and normoxia cells were cultured for CFU HPC assays at lowered (5% oxygen) [16, 23]. All reagents and supplied used in hypoxia were first equilibrated in the 3% hypoxia chamber for at least 16 h prior to their use. For the transplant experiments, hypoxia collected BM cells were injected i.v. to recipient mice in the hypoxia chamber with a special restraint device so that the donor hypoxia collected BM are not subjected to ambient air while allowing survival of recipient [16, 23].
Results
As mentioned in the Methods Section, collection/processing of mouse BM cells in hypoxia/physioxia (3% oxygen) picks up differences in HSCs and HPCs compared to BM cells exposed to normoxia (~2%1 oxygen), which may be more relevant in vivo effects than that seen by other publications [16, 20–23]. Hence, WT and Camkk2−/− BM cells were comparatively analyzed at 3% vs. ambient air (~21%) oxygen levels to pick up potential differences in phenotypic and functional HSCs and HPCs.
Phenotypic Analysis of WT and Camkk2−/− Mouse BM Cells
We analyzed numbers of rigorously defined phenotyped long-term (LT)-HSCs, short-term (ST)-HSCs, multipotential progenitor (MPP) common myeloid progenitor (CMP), granulocyte macrophage progenitor (GMP) and myeloid erythroid progenitor (MEP) cells (Fig. 1). As reported in previous papers [16, 21–23], there are significantly greater numbers of WT LT-HSCs when BM cells are collected/processed in low (3%) oxygen (Fig. 1A). Of interest, in air processing, there was no significant differences in Camkk2−/− vs. WT LT-HSCs, and no significant differences in Camkk2−/− cells between those collected/processed in hypoxia than in air, and the Camkk2−/− cells were significantly decreased in hypoxia collection/processing than that of the WT LT-HSCs and similar to air and hypoxia collected Camkk2−/− cells. This data suggests that CaMKK2 might serve as a positive regulator of phenotyped LT-HSCs under ambient air collection and processing, and under these conditions the genetic deletion of Camkk2 may negatively impact the reservoir of quiescent LT-HSC. Further, the reduced sensitivity of Camkk2−/− cells to the air exposure also pinpoints CaMKK2 as a relevant component of the mechanism responsible for the loss of LT-HSC associated with the collection of BM cells in the presence of air. Except for numbers of Camkk2−/− CMP cells where there were slightly greater numbers than those of WT cells than in hypoxia (Fig. 1D) none of the other progenitors (Fig. 1B–F) showed significant differences. In in vivo relevant oxygen collection conditions (3% oxygen), however, phenotypical analysis of HSCs/HPCs while of some use may not necessarily recapitulate the functional activities of these cells [26]. Hence, it is extremely important to assess competitive engrafting capacity of these cells to detect functional activity of engrafting HSCs, in the context of physioxia.
Fig. 1.
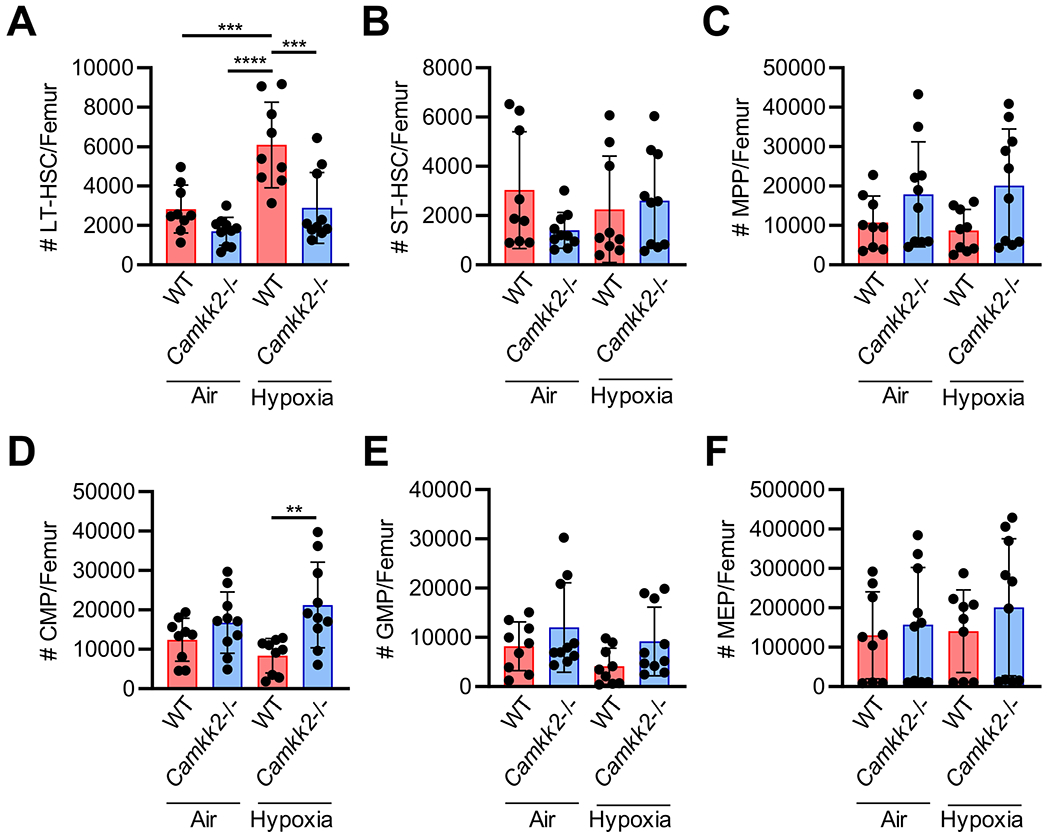
Phenotypic analysis of Camkk2−/− bone marrow hematopoietic stem (HSC) and progenitor (HPC) cells isolated under hypoxia then processed under ambient air (21% O2) versus hypoxia (3% O2). In a hypoxic glove box (acclimated to 3% O2 for 18 hours) BM from femurs of 8 week-old wildtype (WT) or Camkk2−/− mice was flushed in sterile PBS, counted, and split in half so that one half remained under hypoxia and the other half was removed from hypoxia and acclimated to ambient air for 2 h. Nucleated cells were then analyzed by flow cytometry to determine the number of HSCs and HPCs. (A) Long-term (LT)-HSC were defined as Lin− Sca1+ cKit+ (LSK) Flt3− CD34−. (B) Short-term (ST)-HSC were defined as LSK Flt3− CD34+. (C) Multipotent progenitors (MPP) were defined as LSK Flt+ CD34+. (D) Common myeloid progenitors (CMP) were defined as Lin− Sca1− cKit+ (LK) FcγII/IIIRlo CD34+. (E) Granulocyte-macrophage progenitors (GMP) were defined as LK FcγII/IIIRhi CD34+. (F) Megakaryocyte-erythrocyte progenitors (MEP) were defined as LK FcγII/IIIR− CD34−/lo. (A-F) Data are pooled from three independent experiments, 3-4 mice each (n = 9-10 total per group). Stats: two-way ANOVA and post-hoc Tukey’s multiple comparisons. **p < 0.01; ***p < 0.001;****p < 0.0001
Functional Analysis of WT and Camkk2−/− HSC BM as Assessed by Competitive Transplant Studies
As shown in Fig. 2, BM chimerism of WT cells collected/processed in air were compared to that of Camkk2−/− cells in hypoxia (3% oxygen; more physioxic conditions). Not shown in Fig. 2 is the WT hypoxia information, as in all previous studies [16, 20–23], the WT hypoxia engrafting HSCs were always about 2-fold increased for WT hypoxia vs. WT air collected/processed BM cells, and we were limited by recipient mouse numbers. PB cells were collected from these engrafted mice at months 1 (Fig. 2A), 2 (Fig. 2B), 4 (Fig. 2C), and 6 (Fig. 2D), and also from BM at month 6 (Fig. 1E). Months 1-6 PB showed enhanced engraftment of Camkk2−/− cells at hypoxia vs. ambient air compared to that of WT cells collected/processed at ambient air, with similar results for month 6 BM cells (Fig. 2E). Moreover, the Camkk2−/− BM was resistant to effects of ambient air EPHOSS. Thus, with differences between phenotypic LT-HSCs (Fig. 1A) where CaMKK2 showed a positive regulatory effect on LT-HSCs, analysis of the functional engraftment of short (months 1 and 2) and longer term engraftment (4-6 months; Fig. 2A–E), the functionally engrafting HSCs collected/processed in hypoxia demonstrate that CaMKK2 has a negative regulatory effect on HSC function. Further, this data suggests that CaMKK2 has a role as a fate determinant in the context of extra physiologic stress, where it is apparently required for the EPHOSS induced rapid differentiation of HSCs. Thus, phenotypic and engrafting HSCs show divergent effects and the functional activity of HSCs collected in ambient air are not nearly fully functional for Camkk2−/− engrafting capability. Also, BM from month 6 Camkk2−/− engrafting cells (Fig. 2F) show a significant decrease in ambient air and a trend to decrease of Camkk2−/− in hypoxia compared to WT cells in ambient air in the myeloid/lymphoid ratio, demonstrating a myeloid skew associated with CaMKK2. The selective expression of CaMKK2 in the myeloid lineage further supports this hypothesis, suggesting that CaMKK2 may have a key role in the development and differentiation programs of myeloid cells [15, 17]. Since function outweighs phenotype when cells are collected/processed in hypoxia (3% oxygen; physioxia), we suggest that CaMKK2 has a negative regulatory role on functional engrafting HSCs [16, 20–23, 25]. Accordingly, genetic deletion of Camkk2 or its functional blocking by small molecules inhibitors may be leveraged to stimulate HSC engraftment and blood cell regeneration [15].
Fig. 2.

Engraftment advantage for Camkk2−/− HSC/HPC isolated in hypoxia or exposed to ambient air. A-G) WT and/or CaMkk2−/− mice (n = 4 per group; 8 week-old) on a C57BL/6 (CD45.2 + CD45.1−) background were sacrificed and BM was harvested in a 3% O2, 5% CO2 chamber (CaMkk2−/− Hypoxia). Half of BM cells from each mouse were split to ambient air 21% O2 and allowed to equilibrate for 2 h. BM cells from respective donor genotypes were combined to make donor pools. Cells were transplanted by injecting 200,000 whole BM donor cells via tail vein to recipient F1 mice (CD45.2 + CD45.1+). “CaMkk2−/− Hypoxia” cells were injected into recipient mice within the 3% O2, 5% CO2 chamber [16], while cells from the “WT Air” and “CaMkk2−/− Air” condition were injected in ambient air. For all mice, a second tail vein injection was then done in ambient air to transplant 200,000 competitor cells, which were BM cells from BoyJ background mice (CD45.2−CD45.1+). A-D) At months 1, 2, 4, and 6 post injection, blood was collected from recipient mice. Red blood cells were lysed, and cells were stained for CD45.2 and CD45.1 cell surface expression and analyzed by flow cytometry. Donor chimerism is shown as a percentage of cells that are CD45.2 + CD45.1− relative to all CD45 expressing cells in the peripheral blood. E-F) At month 6 post-injection, BM cells were harvested from one femur per recipient mouse and cells were stained for donor chimerism (CD45.2 + CD45.1− relative to total BM cells) and myeloid/lymphoid ratio (CD11b+/ sum (B220+ and CD3e+). Stats: 1-way ANOVA and post-hoc Tukey’s multiple comparisons. *p < 0.05; **p < 0.01; ***p < 0.001
Functional Analysis of HPCs from Camkk2−/− and WT BM in Hypoxia Vs. Ambient Air as Assessed by HPC CFU Analysis
While there were no significant differences in total nucleated BM cells between the WT control and Camkk2−/− cells (Fig. 3A), CFU-GM, BFU-E, and CFU-GEMM, as noted before [16] show decreased numbers of WT HPCs when collected/processed in air (with CFU-GM in this instance demonstrating a strong trend to decrease in hypoxia (Fig. 3B–D). Camkk2−/− CFU-GM (Fig. 3B) in air showed increased numbers compared to WT cells in air, suggesting CaMKK2 is a negative regulator of CFU-GM in extra physiologic conditions, with no significant change for BFU-E and CFU-GEMM. However, CFU-GM, BFU-E and CFU-GEMM of Camkk2−/− HPCs collected/processed in hypoxia vs. ambient air show significant decreases in CFU-GM, BFU-E, and CFU-GEMM compared to those exposed to ambient air (Fig. 3B–D). The CFU-GM under ambient air conditions is similar to reports of others [14]. Importantly, and in contrast to ambient air isolated cells, functional HPC CFU numbers were modestly but insignificantly decreased in Camkk2−/− compared to WT when isolated under physioxia. This suggests that CaMKK2 acts as a positive regulator of HPCs or has little regulatory effect on HPCs in physioxia, and it also demonstrates that CaMKK2 plays a role in HPC fate determination specifically in the presence of extra physiologic oxygen stress. Phenotypic HPCs (Fig. 1) showed contrasting changes compared to functional HPCs. This may in part be due to those HPCs detected by phenotype not being exactly equivalent to those HPCs defined by colony assay, and it does demonstrate that phenotype identification of HPCs does not in this case equate with HPCs detected by function. It has recently been reported [19] that CaMKK2 during ambient air conditions regulates the response to reactive oxygen species (ROS). ROS has been implicated in ambient air vs. hypoxic collection/processing of mouse BM HSCs/HPCs [16, 23].
Fig. 3.
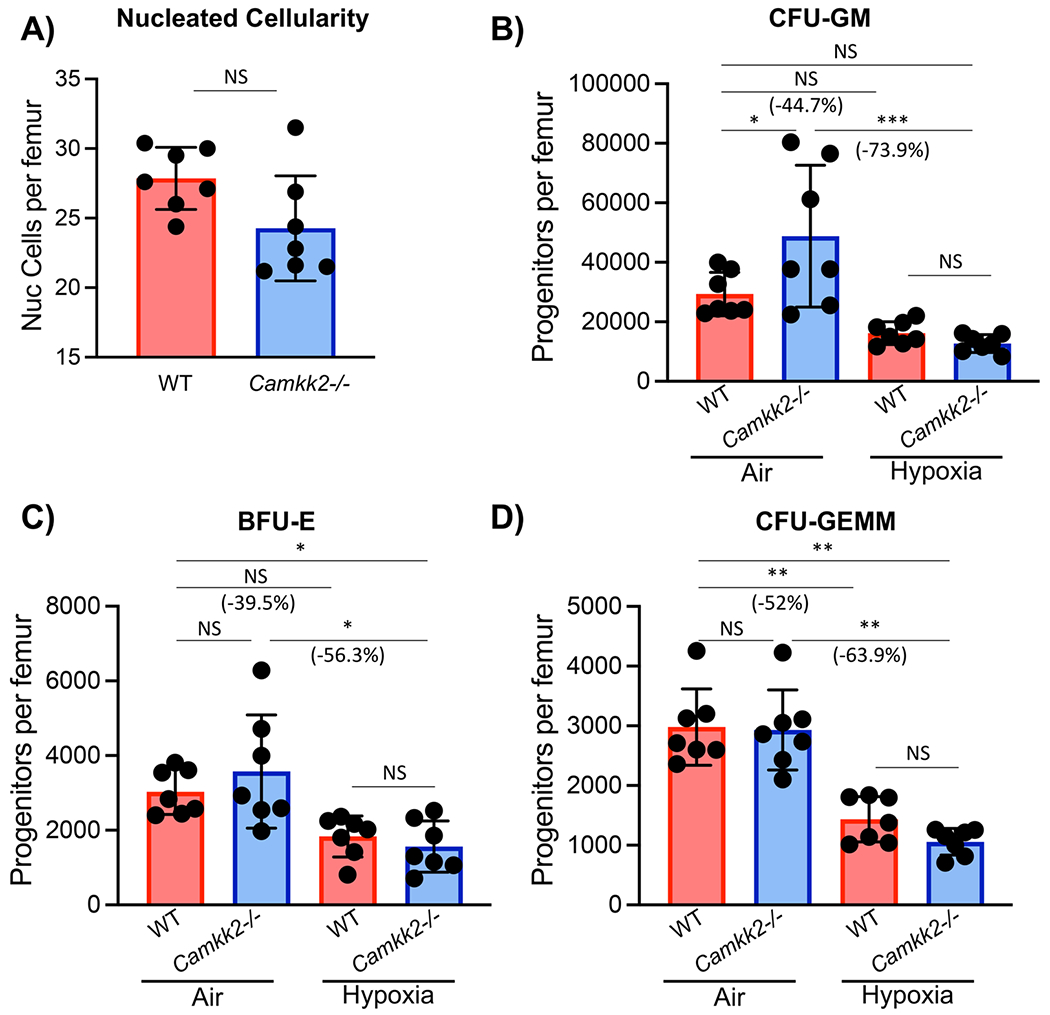
Nucleated cellularity and in vitro colony formation by wildtype (WT) and CaMkk2−/− bone marrow hematopoietic progenitor cells (HPC) isolated under hypoxia (3% O2) and then plated in ambient air (21% O2) versus hypoxia (3% O2). In a hypoxic glove box bone marrow from the femur of WT or KO mice was harvested in sterile PBS, counted, and split in half so that one half remained under hypoxia and the other half was acclimated to ambient air for 2 hours. Nucleated cells were counted and methylcellulose cultures established to enumerate CFU-GM, BFU-E, and CFU-GEMM. Cells were plated at 5 × 104 nucleated cells per ml containing fetal bovine serum (30% v/v), PWMSCM (5% vol/vol), mSCF (50 ng/ml), erythropoietin (1 U/ml), and Hemin (0.1 mM). Cultures were incubated for 6 days at 5%CO2/5% oxygen in a humidified environment. All culture reagents used to plate cells remaining in hypoxia were acclimated in the hypoxia chamber for 16-18 h. Results are shown for 2 separate experiments with a total n = 7. (A) Nucleated cellularity, (B) CFU-GM, (C), BFU-E, and (D) CFU-GEMM are shown. Statistical analysis was performed using t-tests (A) or two-way ANOVA with Tukey post hoc tests (B-D). *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
Very little is known about a role for CaMKK2 as a signal transduction factor in regulation of HSCs and HPCs, and the available data indicate a role for this kinase in mechanisms regulating quiescence and differentiation of HSC [15] under ambient air collection/processing of cells. Further, nothing is known regarding this in context of collection/processing these cells in hypoxia/physioxia assessed during in vivo oxygen conditions for normal WT BM [16, 19, 22, 23] and mobilized PB [21], vs. ambient air conditions. Collection/processing in low oxygen was also used to evaluate P53−/−, Hif1a−/−, Cyclophilin D−/−, the hypoximer, Mir210−/− [16] and fanconia anemia (Fanca−/− and Fancc−/− mice [22]. Herein, we suggest that CaMKK2 controls responsiveness of LT-HSC to supraphysiological levels of oxygen, and deletion of this kinase prevents loss of LT-HSC during the collection step (Fig. 1). Moreover, our data indicate that under physioxia, CaMKK2 acts as a negative regulator for functionally engrafting and regeneration of HSCs (Fig. 2). Under physiologically relevant physioxia, CaMKK2 acts as a positive regulator of functional CFU-GM, BFU-E, and CFU-GEMM. This negative regulation of CaMKK2 for functional engrafting HSCs assessed by engraftment in a competitive setting (Fig. 2A–E) and positive regulation of CaMKK2 for functional HPCs (CFU-GM, BFU-E and CFU-GEMM) (Fig. 3A–D) suggest that CaMKK2 under physioxia can serve as an HSC to HPC differentiation fate determinant, having negative and positive effects respectively on HSCs and HPCs. Moreover, similar to some intracellular signaling knockout mice, CaMKK2 deficient HSCs appear resistant to effects of EPHOSS. This work demonstrates the need to compare WT and gene −/− mouse studies by collection/processing cells in low (3%) oxygen/physioxia vs. ambient air to get an accurate read-out of how these cells likely behave in an in vivo relevant condition of low oxygen that mimics the lowered oxygen tension in BM [16].
While further studies are warranted, this information presents new data, not previously published and could unlock more information on a role for CaMKK2 in regulation of HSCs and HPCs, and possible uses of small CaMKK2 molecule reagents to assess their effects and inhibitors of CaMKK2, such as Profeta [27], on in vivo and ex-vivo effects of hematopoiesis by injecting them into WT mice, and for effects on ex-vivo expansion on physiologically relevant numbers of HSCs and HPCs. This may eventually lead to useful translational means to modulate mouse, and later human hematopoiesis, for normal and malignant and non-malignant hematopoiesis.
Funding
These studies were supported by NIH R35 HL139599 (Outstanding Investigator Award) and U54 DK 106846 (Cooperative Center of Excellence in Hematology (CCEH)) to H.E.B. J.R. was supported as a post-doctoral fellow on T32 DK 007519 to H.E.B. U.S. was supported by R01AR068332 (NIAMS/NIH). LR was supported by NIH 2U19AI067798-16.
Footnotes
Code Availability Not applicable.
Ethics Approval No problems with ethics approval.
Consent to Participate All authors consented to participate.
Consent for Publication All authors consented to publish this paper.
Conflicts of Interest/Competing Interests No COI from any co-authors.
Data Availability
All data is present in this paper, and material is available upon request.
References
- 1.Shaheen M & Broxmeyer HE (2013). Principles of cytokine signaling. In: Hematology: Basic principles and practice, 6th edition (Ed. Hoffman R, Benz EJ Jr., Silberstein LE, Heslop H, Weitz JI, & Anastasi J). Elsevier Saunders, Philadelphia, PA. Chapter 14. pp. 136–146. [Google Scholar]
- 2.Broxmeyer HE & Capitano ML (2022). Cytokines/Chemokines/Other Growth Regulators and Their Receptors. Hematology: Basic Principles and Practice, Eighth Edition (Ed. Hoffman R, et al. ). Accepted, and in print 2022. [Google Scholar]
- 3.Shaheen M & Broxmeyer HE (2018). Cytokine/receptor families and signal transduction. In: Hematology: Basic principles and practice. 7th edition (Ed. Hoffman R, Benz E, Silberstein L, Heslop H, Weitz JI, and Anastasi J, Salama ME, & Abutalib SA). Chapter 16. Pages 163–175. [Google Scholar]
- 4.Marcelo KL, Means AR, & York B (2016). The Ca(2+)/Calmodulin/CaMKK2 Axis: Nature’s metabolic CaMshaft. Trends in Endocrinology and Metabolism: TEM, 27(10), 706–718. 10.1016/j.tem.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Racioppi L, & Means AR (2012). Calcium/calmodulin-dependent protein kinase kinase 2: Roles in signaling and pathophysiology. The Journal of Biological Chemistry, 287(38), 31658–31665. 10.1074/jbc.R112.356485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan Y, Zhou XE, Xu HE, & Melcher K (2018). Structure and physiological regulation of AMPK. International Journal of Molecular Sciences, 19(11), 3534. 10.3390/ijms19113534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams JN, & Sankar U (2019). CaMKK2 signaling in metabolism and skeletal disease: A new Axis with therapeutic potential. Current Osteoporosis Reports, 17(4), 169–177. 10.1007/s11914-019-00518-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dengler F (2020). Activation of AMPK under hypoxia: Many roads leading to Rome. International Journal of Molecular Sciences, 21(7), 2428. 10.3390/ijms21072428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacquel A, Luciano F, Robert G, & Auberger P (2018). Implication and regulation of AMPK during physiological and pathological myeloid differentiation. International Journal of Molecular Sciences, 19(10), 2991. 10.3390/ijms19102991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JN, Kambrath AV, Patel RB, Kang KS, Mével E, Li Y, Cheng YH, Pucylowski AJ, Hassert MA, Voor MJ, Kacena MA, Thompson WR, Warden SJ, Burr DB, Allen MR, Robling AG, & Sankar U (2018). Inhibition of CaMKK2 enhances fracture healing by stimulating Indian hedgehog signaling and accelerating endochondral ossification. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research, 33(5), 930–944. 10.1002/jbmr.3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sankar U, Pritchard ZJ, & Voor MJ (2016). Micro-computed tomography assisted distal femur metaphyseal blunt punch compression for determining trabecular bone strength in mice. Journal of Biomechanics, 49(7), 1233–1237. 10.1016/j.jbiomech.2016.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pritchard ZJ, Cary RL, Yang C, Novack DV, Voor MJ, & Sankar U (2015). Inhibition of CaMKK2 reverses age-associated decline in bone mass. Bone, 75, 120–127. 10.1016/j.bone.2015.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cary RL, Waddell S, Racioppi L, Long F, Novack DV, Voor MJ, & Sankar U (2013). Inhibition of Ca2+/calmodulin-dependent protein kinase kinase 2 stimulates osteoblast formation and inhibits osteoclast differentiation. Journal of Bone and Mineral Research : the Official Journal of the American Society for Bone and Mineral Research, 28(7), 1599–1610. 10.1002/jbmr.1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teng EC, Racioppi L, & Means AR (2011). A cell-intrinsic role for CaMKK2 in granulocyte lineage commitment and differentiation. Journal of Leukocyte Biology, 90(5), 897–909. 10.1189/jlb.0311152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racioppi L, Lento W, Huang W, Arvai S, Doan PL, Harris JR, Marcon F, Nakaya HI, Liu Y, & Chao N (2017). Calcium/calmodulin-dependent kinase kinase 2 regulates hematopoietic stem and progenitor cell regeneration. Cell Death & Disease, 8(10), e3076. 10.1038/cddis.2017.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantel CR, O’Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, Brustovetsky N, Srour EF, Lee MR, Messina-Graham S, Haas DM, Falah N, Kapur R, Pelus LM, Bardeesy N, Fitamant J, Ivan M, Kim KS, & Broxmeyer HE (2015). Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell, 161(7), 1553–1565. 10.1016/jxell.2015.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Racioppi L, Noeldner PK, Lin F, Arvai S, & Means AR (2012). Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. The Journal of Biological Chemistry, 287(14), 11579–11591. 10.1074/jbc.M111.336032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racioppi L, Nelson ER, Huang W, Mukherjee D, Lawrence SA, Lento W, Masci AM, Jiao Y, Park S, York B, Liu Y, Baek AE, Drewry DH, Zuercher WJ, Bertani FR, Businaro L, Geradts J, Hall A, Means AR, … McDonnell DP (2019). CaMKK2 in myeloid cells is a key regulator of the immune-suppressive microenvironment in breast cancer. Nature Communications, 10(1), 2450. 10.1038/s41467-019-10424-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Liu Y, Luz A, Berrong M, Meyer JN, Zou Y, Swann E, Sundaramoorthy P, Kang Y, Jauhari S, Lento W, Chao N, & Racioppi L (2021). Calcium/calmodulin dependent protein kinase kinase 2 regulates the expansion of tumor-induced myeloid-derived suppressor cells. Frontiers in Immunology, 12, 754083. 10.3389/fimmu.2021.754083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broxmeyer HE, O’Leary HA, Huang X, & Mantel C (2015). The importance of hypoxia and extra physiologic oxygen shock/stress for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex vivo. Current Opinion in Hematology, 22(4), 273–278. 10.1097/M0H.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aljoufi A, Cooper S, & Broxmeyer HE (2020). Collection and processing of mobilized mouse peripheral blood at lowered oxygen tension yields enhanced numbers of hematopoietic stem cells. Stem Cell Reviews and Reports, 16(5), 946–953. 10.1007/s12015-020-10021-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broxmeyer HE, Capitano ML, Cooper S, Potchanant ES, & Clapp DW (2021). Numbers of long-term hematopoietic stem cells from bone marrow of fanca and fancc knockout mice can be greatly enhanced by their collection and processing in physioxia conditions. Blood Cells, Molecules & Diseases, 86, 102492. 10.1016/j.bcmd.2020.102492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capitano ML, Mohamad SF, Cooper S, Guo B, Huang X, Gunawan AM, Sampson C, Ropa J, Srour EF, Orschell CM, & Broxmeyer HE (2021). Mitigating oxygen stress enhances aged mouse hematopoietic stem cell numbers and function. The Journal of Clinical Investigation, 131(1), e140177. 10.1172/JCI140177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, & Means AR (2008). Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metabolism, 7(5), 377–388. 10.1016/j.cmet.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 25.Broxmeyer HE, Hoggatt J, O’Leary HA, Mantel C, Chitteti BR, Cooper S, Messina-Graham S, Hangoc G, Farag S, Rohrabaugh SL, Ou X, Speth J, Pelus LM, Srour EF, & Campbell TB (2012). Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nature Medicine, 18(12), 1786–1796. 10.1038/nm.2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Yao C, Teng Y, Jiang R, Huang X, Liu S, Wan J, Broxmeyer HE, & Guo B (2019). Phorbol ester induced ex vivo expansion of rigorously-defined phenotypic but not functional human cord blood hematopoietic stem cells: A cautionary tale demonstrating that phenotype does not always recapitulate stem cell function. Leukemia, 33(12), 2962–2966. 10.1038/s41375-019-0528-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Profeta GS, Dos Reis CV, Santiago A, Godoi P, Fala AM, Wells CI, Sartori R, Salmazo A, Ramos PZ, Massirer KB, Elkins JM, Drewry DH, Gileadi O, & Couñago RM (2019). Binding and structural analyses of potent inhibitors of the human Ca2+/calmodulin dependent protein kinase kinase 2 (CAMKK2) identified from a collection of commercially-available kinase inhibitors. Scientific Reports, 9(1), 16452. 10.1038/s41598-019-52795-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data is present in this paper, and material is available upon request.


