Abstract
Rationale:
Discrimination is a risk factor and potential pathway through which social determinants such as race and sex contribute to chronic inflammation in Black Americans in middle and later adulthood. Questions remain regarding which forms of discrimination are most salient for inflammatory dysregulation, and whether there are sex-based differences in these pathways.
Objective:
This exploratory study investigates sex differences in the relationships between four forms of discrimination and inflammatory dysregulation among middle aged and older Black Americans.
Methods:
Using cross-sectionally linked data from participants in the Midlife in the United States (MIDUS II) Survey (2004–2006) and Biomarker Project (2004–2009) (N =225, ages 37–84, 67% female), this study conducted a series of multivariable regression analyses. Inflammatory burden was measured using a composite indicator comprised of five biomarkers: C-reactive protein (CRP), interleukin-6 (IL-6), fibrinogen, E-selectin, and intercellular adhesion molecule (ICAM). Discrimination measures were lifetime, daily, and chronic job discrimination and perceived inequality at work.
Results:
Black men generally reported higher levels of discrimination than Black women (3 out of 4 forms), though only sex differences in job discrimination achieved statistical significance (p <.001). In contrast, Black women exhibited more overall inflammatory burden than Black men (2.09 vs. 1.66, p =.024), particularly elevated levels of fibrinogen (p =.003). Lifetime discrimination and inequality at work were associated with higher levels of inflammatory burden, after adjusting for demographic and health factors (p =.057 and p =.029, respectively). The discrimination-inflammation relationships further varied by sex, such that more lifetime and job discrimination predicted greater inflammatory burden in Black women, but not in Black men.
Conclusion:
These findings highlight the potentially detrimental impact of discrimination and emphasize the importance of sex-specific research on biological mechanisms of health and health disparities in Black Americans.
Keywords: Discrimination, inflammation, Black Americans, sex
Introduction
Discrimination has been identified as a potential risk factor and pathway through which social determinants (e.g., race, sex, etc.) contribute to chronic inflammation and associated health outcomes. Research has outlined the detrimental impact discrimination can have on multiple physiological pathways (Cuevas et al., 2020). In particular, studies have found unfair treatment and discrimination towards Black Americans to be associated with individual measures of physiological risk (e.g., inflammation biomarkers such as C-reactive protein) (Doyle & Molix, 2014; Lewis et al., 2010) and composite measures of physiological dysregulation such as allostatic load (Brody et al., 2014; Ong et al., 2017; Upchurch et al., 2015; Van Dyke et al., 2020). These measures are posited to capture the cumulative “wear and tear” of chronic stress on the body (McEwen, 1998). Thus, relationships between discrimination and physiological dysregulation represent an important topic of investigation for researchers seeking to understand (and potentially interrupt) pathways through which social and structural inequities “get under the skin” to generate racial health disparities (Das, 2013). Questions remain regarding which forms of discrimination may be most salient for inflammatory dysregulation, and whether there are sex-based differences in these pathways.
While some studies demonstrate a link between different forms of discrimination and dysregulation across both individual (e.g., inflammation) (Doyle & Molix, 2014; Lewis et al., 2010) and multiple physiological systems (e.g., allostatic load) (Brody et al., 2014; Ong et al., 2017; Upchurch et al., 2015) among Black adults, others do not (Stepanikova et al., 2017). For example, Doyle & Molix (2014) found everyday discrimination was predictive of increased inflammation across several proinflammatory markers, including interleukin-6 (IL-6), E-selectin, and C-reactive protein (CRP) in a sample of 592 Black adults (ages 34–85 years) (Doyle & Molix, 2014). Similarly, Lewis et at al. (2010) found everyday discrimination to be associated with elevated CRP levels in a sample of 296 older Black adults (ages 65 and older; 70% female) (Lewis et al., 2010). Stepanikova et al. (2017), however, found no statistically significant association between everyday and lifetime discrimination and increased inflammation (i.e., fibrinogen, E-selectin, CRP, and IL-6) in 170 Black adults (ages 35–82 years) (Stepanikova et al., 2017). Although the findings are mixed for associations between everyday and lifetime discrimination and individual indicators of physiological dysregulation, they are more consistent in studies using composite measures of dysregulation.
Current work has implicated discrimination as a contributing factor to multisystem physiological dysregulation. Multisystem physiological dysregulation is most frequently measured as allostatic load (AL), which is a composite indicator capturing dysregulation across multiple physiological systems (e.g., sympathetic nervous system (SNS), hypothalamic-pituitary-adrenal (HPA) axis, immune system, and cardiovascular and metabolic processes) due to chronic stress (McEwen 1998). For instance, a longitudinal study of 331 rural Black adolescents by Brody and colleagues (2014), reported a proposed effect of discriminatory treatment—assessed by a revised version of the Schedule of Racist Events (SRE; Landrine & Klonoff, 1996) for use with adolescents—on higher AL levels. The 9 items in the revised SRE assessed the frequency during the previous year with which the respondent perceived specific discriminatory events such as racially based slurs and insults, disrespectful treatment from community members, physical threats, and false accusations from business employees or law enforcement officials (Brody et al., 2014). Ong et al. (2017), using a sample of 233 Black adults (ages 37–85 years), found that everyday discrimination was associated with higher allostatic load scores (Ong et al., 2017). Likewise, Upchurch et al. (2015) found that chronic exposure to everyday discrimination was predictive of higher AL levels in a community-based sample of middle-aged Black women (Upchurch et al., 2015). In a sample of 226 Black and 978 White middle-aged adults, Van Dyke and colleagues (2020) found pervasive discrimination (score of 2 vs. 0) was positively associated with greater allostatic load. Although the authors did not observe a significant race-by-discrimination interaction, the magnitude of the discrimination-AL association in their exploratory race-stratified analyses appeared to be larger for Black adults than Whites (Van Dyke et al., 2020). Collectively, these previous studies suggest that inflammation and greater overall physiological dysregulation may be a consequence of experiencing various forms of discrimination (e.g., racist events in the past year, every day) throughout the lifespan (e.g., adolescence, midlife, and old age). These effects appear particularly pronounced at midlife and older ages.
More work needs to be done on potential modifiers (such as sex) of the discrimination-inflammation relationship, as research has shown conflicting or unclear evidence (Cunningham et al., 2012; Friedman et al., 2009; Kershaw et al., 2016; Ong et al., 2017). For instance, Kershaw and colleagues (2016) found everyday discrimination, lifetime discrimination due to any attribution, and lifetime discrimination due to race/ethnicity were all significantly associated with higher IL-6 in women (multi-ethnic sample of 3099 men and 3468 women, aged 45–84 years), adjusting for sociodemographic characteristics, recent infection, anti-inflammatory medication use, and hormone replacement therapy use; however, these associations were attenuated after adjustment for BMI. In men, everyday discrimination was inversely associated with IL-6 in all adjusted models, and lifetime discrimination was unrelated to IL-6. All three discrimination measures were not associated with CRP for both women and men (Kershaw et al., 2016). In another study using a multi-racial cohort of young adults (901 Black women, 614 Black men, 958 White women, and 863 White men, aged 18–30 years), the relationship between experiences of discrimination due to race/ethnicity and CRP was positively related in both Black (1–2 discrimination experiences) and White women (3 or more discrimination experiences), adjusting for demographics, health-related and psychosocial factors. In Black men there was no association observed for 1–2 discrimination experiences, while an inverse association was observed for 3 or more discrimination experiences, adjusting for demographics and health-related factors. In White men experiences of discrimination were unrelated to inflammation (Cunningham et al., 2012). In contrast, a study of everyday discrimination, lifetime discrimination due to any attribution, and their associations with E-selectin in a sample of 804 White adults found higher levels of both forms of discrimination were associated with higher E-selectin in men but not women (Friedman et al., 2009). In contrast, Ong et al. (2017) found that the association between everyday discrimination and AL did not vary by sex in a sample of 233 Black adults, controlling for demographics, medication use, smoking, alcohol consumption, depressive symptoms, lifetime discrimination, and global perceived stress (Ong et al., 2017). A key distinction between Ong’s 2017 study and the present analysis is that Ong and colleagues focused on only one form of discrimination: everyday discrimination. Although they controlled for a second form of discrimination (lifetime), they did not probe this form of discrimination in their analyses nor discuss it at length in the findings.
Of the few studies that have measured multiple (two vs. three or more) forms of discrimination, most have used multi-ethnic samples. For example, studies using both the National Survey of Midlife in the United States (MIDUS) (Ong & Williams, 2019; Van Dyke et al., 2020) and the Multi-Ethnic Study of Atherosclerosis (MESA) (Whitaker et al., 2017) have examined more than one form of discrimination and their associations with health among multi-ethnic samples. While the health disparities field has been bolstered by multi-racial and comparative studies, single-race studies complement these, as they allow for a more nuanced investigation of within-group heterogeneity. Further, when factors that are unequally distributed across races are considered (e.g., socioeconomic status), a within-race approach avoids issues of residual confounding (Kaufman et al., 1997). In summary, studies examining the links between multiple forms of discrimination and more comprehensive, composite indicators of inflammatory dysregulation in Black adults, and specifically probing sex-specific differences, are critically lacking.
The current study adds to our understanding of health disparities that adversely affect Black Americans in three ways. First, it builds on prior work (Doyle & Molix, 2014; Ong et al., 2017; Stepanikova et al., 2017) by examining the relationships between multiple (four) forms of discrimination and a composite measure of inflammatory dysregulation in a sample of middle-aged and older Blacks. This approach allows for a deeper examination of the potential impact of lifetime, everyday, and workplace discrimination on inflammation in both midlife and old age. Midlife is an important phase in the life span for examining biological mechanisms of health, given it is a period of markedly rising risk for acute and chronic diseases (House et al., 2005), especially among Black Americans (Geronimus et al., 2006). Hence, there is a critical need to investigate the physiological stress pathways that lead to dysregulation of the immune system and resulting chronic inflammation in middle-aged and older Blacks. Second, this study considers whether the discrimination-inflammation relationship varies by sex. It has the potential to shed light on sex-specific biological pathways of health, given the mixed and unclear evidence in this area. Third, this study emphasizes the importance of examining intersectionality in the study of discrimination and physical health in Black Americans (Kwate & Goodman, 2015; Lewis & Van Dyke, 2018; Purdie-Vaughns & Eibach, 2008). The tendency to focus on Black Americans from a monolithic perspective is a limitation of prior research in this area. This approach does not tease apart the heterogeneity within the Black population (e.g., age, gender, socioeconomic status, sexual orientation differences) nor does it consider how within-group heterogeneity may impact discrimination exposure and associations between discrimination and physical health (Lewis & Van Dyke, 2018). For example, seminal work by Kimberlé Crenshaw and others (Crenshaw, 1989; Purdie-Vaughns & Eibach, 2008) argue that “Black women sometimes experience discrimination in ways similar to White women’s experiences; sometimes they share very similar experiences with Black men. Yet often they experience double-discrimination—the combined effects of practices [that] discriminate on the basis of race, and on the basis of sex. And sometimes, they experience discrimination as Black women—not the sum of race and sex discrimination, but as Black women” (Crenshaw, 1989, p. 149). Researchers have also noted an intersectional approach has advantages for understanding the experiences of African American men (both gay and straight) and other people who have an intersection of two or more identities (Bowleg et al., 2017).
Overall, this study had two aims: examine associations between four forms of discrimination and inflammatory dysregulation in Black Americans (Aim 1) and assess whether sex differences exist (Aim 2). We hypothesized that all four forms of discrimination would be associated with inflammatory dysregulation (Hypothesis 1). We further hypothesized that the strength of these associations would depend on sex (Hypothesis 2); however, because of the limited and contradicting findings in the literature, no specific hypotheses were made about the direction of the moderation effect of sex.
Methods
This exploratory study used cross-sectional linked data from a sample of Black Americans who participated in the second wave of the National Survey of Midlife in the United States (MIDUS II) Study (2004–2006) and Biomarker Project (2004–2009). Details of these studies are described elsewhere (Love et al., 2010; Radler, 2014; Radler & Ryff, 2010).
In brief, the MIDUS II Survey Study collected interview and self-administered questionnaire data on a variety of sociodemographic, psychosocial, and behavioral factors related to the health and well-being of midlife and older U.S. adults. The Biomarker Project recruited a subsample of 1,255 MIDUS II Survey Study participants (39.3% response rate) for an in-depth investigation of the interrelationships between biological, behavioral, and psychosocial pathways of aging-related morbidity and mortality. It included participants from both the original MIDUS sample (1995–1996) – developed using a stratified probability sampling design of English-speaking, community-residing adults from the contiguous U.S. – and members of an oversampling of Black Americans from Milwaukee, Wisconsin as part of MIDUS II (2004–2006) – identified using a stratified sampling frame based on U.S. census tracts in which at least 40% of residents were African American. The Biomarker Project subsample was comparable to the overall MIDUS II sample on most demographic and health characteristics.
The current study focused on Black Biomarker Project participants. The analytic sample included 151 Black women and 74 Black men who had complete data on all 5 inflammatory biomarkers used in our outcome variable and had completed the MIDUS II interview and self-administered questionnaire. Eleven Black adults were excluded from the analytic sample because they had insufficient inflammatory biomarker data due to partial (n=6) or missing (n=3) blood samples or were the randomly selected member of a sibling pair dropped from the analysis (n=2).The demographic and health characteristics of excluded individuals were comparable to the analytic sample according to t- and chi-squared tests.
Data Collection
Discrimination measures used in the current study were taken from the MIDUS II Survey Study self-administered questionnaire. Data for the inflammatory burden outcome measure were collected as part of the Biomarker Project, approximately 25 months after participants completed the MIDUS II Survey Study. Participants attended 2-day clinic visits where they provided biological specimens (blood, urine, saliva) for assessing multiple indicators of major biological systems. During the visit, they also completed clinical assessments (cardiovascular and heart rate variability measurements), a full medical history, detailed medication charting, and a physical exam (Ryff et al., 2019). All travel expenses were covered. All five relevant inflammatory markers were processed from fasting blood samples collected during the second day of the clinic visit according to a standardized protocol (Love et al., 2010). Health covariates were also collected in the Biomarker Project. IRB review and participant informed consent were obtained for all study components.
Measures
Inflammatory Burden
A composite score of chronic inflammatory burden was used to capture the extent of dysregulation across multiple inflammatory indicators, consistent with the measure used by Ong & Williams (2019). Scores were created by summing the number of inflammatory biomarkers, out of five, in which participants’ values fell within the highest risk quartile (Glei et al., 2013; Kang & Marks, 2014; Ong & Williams, 2019). Highest risk quartiles were used since neither overall nor sex-specific thresholds indicating clinical risk have been established for most of these inflammatory biomarkers (Kang & Marks, 2014). Quartiles for each inflammatory biomarker were established based on biomarker distributions within the full Biomarker Project sample, consistent with the approach used in previous research (Kang & Marks, 2014). The inflammatory biomarkers included in this measure were C-reactive protein (CRP; an acute inflammatory protein that increases up to 1,000-fold at sites of infection or inflammation) (Sproston & Ashworth, 2018), interleukin-6 (IL-6; a proinflammatory cytokine secreted by leukocytes in order to stimulate the immune response and synthesis of CRP) (Sproston & Ashworth, 2018), fibrinogen (an acute phase protein produced in the liver that increases during injury, infection, and inflammation), E-selectin (a cell adhesion molecule expressed as part of the inflammatory response to endothelial damage), and intercellular adhesion molecule (ICAM; is an Ig-like cell adhesion molecule expressed by several types of cell, including leukocytes and endothelial cells. ICAMs play a role in inflammatory processes, immune responses, e.g., the T-cell mediated host defense system, and are important in intracellular signaling events) (van de Stolpe & van der Saag, 1996). Potential scores ranged from 0 (no inflammatory markers in the top quartile) to 5 (all inflammatory markers in the top quartile).
Chronic Discrimination
We examined four forms of chronic discrimination. Lifetime discrimination (Williams et al., 1997) was assessed with 11-items asking participants whether they had experienced several examples of major discrimination in their lifetimes because of their race, ethnicity, gender, age, religion, physical appearance, sexual orientation, or other characteristics. Examples included being discouraged from or denied opportunities, being denied or provided with inferior services, and being the target of social or police hostility. This was a general measure of discrimination in that participants did not identify which identities they attributed reported discrimination to. We created an index score indicating the number of different types of lifetime discrimination participants reported. Daily discrimination was measured with a 9-item variation of the Everyday Discrimination Scale (Kessler et al., 1999). Items assessed how frequently participants experienced various forms of routine discrimination in their daily lives. Response options ranged from 1 (often) to 4 (never). Responses were reverse coded and averaged for participants responding to at least 4 items, such that higher scores indicated more daily discrimination. Cronbach’s α =.904. Chronic job discrimination (Sternthal et al., 2011) data were collected from the portion of the sample that was currently employed or had recently worked for pay in the past ten years. Using 6-items, participants reported how frequently they were discriminated against at work, such as being watched more closely or witnessing racial slurs or jokes. Response options were coded: 4 (once a week or more), 3 (a few times a month), 2 (a few times a year or less), and 1 (never). Items were averaged for participants responding to at least half the items, such that higher scores reflected more discrimination. Cronbach’s α =.815. Perceived inequality at work was also only assessed for those employed currently or in the past ten years. Participants were asked 6-items on how much they perceived themselves to receive less work-related respect, opportunities, and satisfaction than others. Responses options ranged from 1 (a lot) to 4 (not at all). Negative items were reverse coded, and all items were averaged for participants responding to at least half the items. Higher scores reflected more perceived inequality. Cronbach’s α =.716.
Sex
Sex was self-reported as either male or female (reference group).
Demographic and Health Covariates
Demographic covariates included age and education. Age (years) was measured at the time of the Biomarker Project clinic visit. Education was categorized as no high school degree and no Graduate Equivalency Degree (GED; reference group), high school diploma or GED, some college, or bachelor’s degree. Health covariates were collected during the Biomarker Project and included tobacco use, body mass index (BMI), and five indicators (yes/no) of current physical and mental health conditions with potential to confound analyses (i.e., common, affecting >5% of the sample, and associated with inflammatory burden or individual inflammation biomarkers in preliminary analyses, p<.10). Tobacco use was self-reported regular use of cigarettes, pipes, cigars, chewing tobacco, or snuff (yes/no). BMI (kg/m2) was calculated from objective measurement of weight and height taken during the clinic visit and modeled continuously. High blood pressure (high BP) was taken from three seated readings at the clinic visit and defined as an average systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg or use of antihypertensive medications. Diabetes indicated clinic visit measurement of hemoglobin A1C ≥6.5% or fasting glucose ≥126 mg/dL or reported use of oral medications or insulin for diabetes control. Cardiovascular condition was determined by the use of prescription medications to treat medical conditions associated with the heart and circulatory system. Central nervous system medication indicated use of prescription medications affecting the central nervous system, with analgesics to relieve and control pain and sedatives most commonly reported. Depression represented a score ≥16 on the 20-item Center for Epidemiologic Studies Depression scale (CES-D) (Radloff, 1977), indicating at risk for clinical depression, or current use of prescription antidepressants (Cronbach’s α =.785).
Data Analysis
Statistical analyses were conducted in SPSS 28 (IBM Corp., Armonk, NY). Given the modest sample size and associated statistical power in this exploratory study, we investigated relationships identified as significant (p<.05) or marginally significant (p<.10) in two-tailed tests. Multiple imputation was not necessary due to the small portion of missing data (<1.2% overall; Cheema, 2014). We ran Pearson’s bivariate correlations for all chronic discrimination measures (see Supplemental Table 1). The characteristics of Black women and men were compared in descriptive analyses using two-tailed t and χ2 tests. We also compared the characteristics of working individuals (n=150) to the full sample (n=225) using one-sample t- and proportion tests.
We used a twofold approach entailing multivariable ordinary least squares regression to identify associations between multiple measures of discrimination and inflammatory burden for Black Americans, including testing for potential sex differences. First, we regressed inflammatory burden on each individual discrimination measure, modeled separately. Model sample sizes varied based on the number of participants who provided data on each chronic discrimination measure; samples sizes for the job discrimination and perceived inequality at work models were smaller (n=149 and n=150, respectively) than those for lifetime and everyday discrimination (n=224 and n=225, respectively) since only participants who had worked in the past ten years completed the relevant employment discrimination survey items. Models were calculated unadjusted for the full sample (Model 1), after accounting for a sex indicator variable (male, with female as the reference group; Model 2), and in combination with key demographic and health covariates (Model 3). Moderation of chronic discrimination-inflammatory burden relationships by sex was assessed with interaction terms (product of the sex indicator variable and each discrimination measure; Model 4). We probed the nature of these sex interaction terms, specifically identifying which groups – Black women, Black men, or both – showed evidence of significant chronic discrimination-inflammatory burden relationships, with subsequent simple slopes tests (t-tests of the slopes divided by their standard errors; Aiken et al., 1991).
Second, we regressed inflammatory burden on all four chronic discrimination measures, modeled simultaneously in supplementary analyses. This approach allowed us to examine the joint effects of all four forms of discrimination as well as the unique amount of variation attributed to each individual form of discrimination above and beyond that which is shared with other forms of discrimination. This twofold approach further distinguishes the contribution of this research from prior work.
Results
Descriptive statistics
The full sample included 225 Black participants, most of whom (86.2%) were enrolled in MIDUS as part of the oversampling of African Americans in Milwaukee. Descriptive data are presented in Table 1. The overall sample was 67.1% female (n=151) and 32.9% male (n=74), with a mean age of 53.7 years old (range = 37–85 years). Black women and men were comparable in age and education. Black women were less likely than Black men to be currently employed and use tobacco. Black women had a significantly higher average BMI and were more likely to be obese (BMI ≥ 30) than Black men. Black women reported higher rates of all five health conditions, though differences did not achieve statistical significance. Black men reported higher levels of discrimination than women; however, only sex differences in chronic job discrimination were significant (p <.001) and sex differences in daily discrimination were marginally significant (p =.061). In contrast, Black women had greater inflammatory burden than Black men overall (2.09 vs. 1.66, p =.024) and for 4 of the 5 inflammation biomarkers (i.e., CRP, IL-6, fibrinogen, and ICAM); however, only sex differences in fibrinogen were statistically significant (p =.003) and in IL-6 were marginally significant (p =.085). When comparing the full sample to the subsample who completed the work-related discrimination items (see Table 2), the full sample was slightly older (53.70 vs. 51.97 years) but comparable to the working subsample on other characteristics.
Table 1.
Participant Characteristics, By Sex. National Survey of Midlife in the United States (MIDUS) Wave II 2004–2006 and Biomarker Project 2004–2009.
| Black Women | Black Men | Sex Differences | |
|---|---|---|---|
| % or M (SD) | % or M (SD) | p * | |
| Demographics | |||
| Age (years) | 54.43 (10.79) | 52.22 (9.27) | .113 |
| Education | |||
| No HS degree & no GED | 17.2 | 16.2 | 1.000 |
| HS degree/GED | 28.5 | 31.1 | . 756 |
| Some college | 33.8 | 35.1 | .882 |
| Bachelor’s degree | 20.5 | 17.6 | .721 |
| Currently working | 57.0 | 71.6 | .041 |
| Health | |||
| Tobacco use | 24.5 | 45.9 | .002 |
| Body mass index (BMI) (kg/m2) | 33.92 (9.01) | 30.38 (6.77) | .001 |
| Normal (BMI < 25) | 11.9 | 20.3 | .110 |
| Overweight (BMI 25–29.9) | 26.5 | 32.4 | .351 |
| Obese (BMI ≥ 30) | 61.6 | 47.3 | .046 |
| Health conditions | |||
| High blood pressure | 50.3 | 39.2 | .121 |
| Diabetes | 37.7 | 33.8 | .659 |
| Cardiovascular condition | 51.0 | 40.5 | .157 |
| Central nervous system medication | 53.0 | 48.6 | .572 |
| Depression | 37.7 | 28.4 | .182 |
| Chronic discrimination measures | |||
| Lifetime discrimination | 2.87 (2.79) | 3.31 (2.93) | .272 |
| Daily discrimination | 1.58 (.66) | 1.78 (.78) | .061 |
| Chronic job discrimination | 1.56 (.57) | 1.92 (.71) | <.001 |
| Perceived inequality at work | 1.92 (.69) | 1.94 (.57) | .846 |
| Inflammatory biomarkers | |||
| Total inflammatory burden | 2.09 (1.33) | 1.66 (1.33) | .024 |
| C-reactive protein (CRP), top quartile | 43.0 | 31.1 | .109 |
| interleukin-6 (IL-6), top quartile | 46.4 | 33.8 | .085 |
| fibrinogen, top quartile | 51.0 | 29.7 | .003 |
| E-selectin, top quartile | 34.4 | 44.6 | .146 |
| Intercellular adhesion molecule (ICAM), top quartile | 34.4 | 27.0 | .290 |
| Total N | 151 (67.1%) | 74 (32.9%) |
Note. HS = high school; GED = Graduate Equivalency Degree.
Bold test indicates p <.10.
Table 2.
Comparison of Participant Characteristics, Full Sample vs. Working Subsample.
| Full Sample | Working Subsample | Differences | |
|---|---|---|---|
| % or M (SD) | % or M (SD) | p * | |
| Demographics | |||
| Sex | |||
| Female | 67.1 | 64.0 | .471 |
| Male | 32.9 | 36.0 | .471 |
| Age (years) | 53.70 (10.35) | 51.97 (9.16) | .022 |
| Education | |||
| No HS degree & no GED | 16.9 | 16.0 | .853 |
| HS degree/GED | 29.3 | 28.7 | .936 |
| Some college | 34.2 | 33.3 | .890 |
| Bachelor’s degree | 19.6 | 22.0 | .524 |
| Health | |||
| Tobacco use | 31.6 | 32.7 | .847 |
| Body mass index (BMI) (kg/m2) | 32. 75 (8.49) | 32.64 (8.22) | .875 |
| Normal (BMI < 25) | 14.7 | 14.0 | .899 |
| Overweight (BMI 25–29.9) | 28.4 | 28.7 | 1.000 |
| Obese (BMI ≥ 30) | 56.9 | 57.3 | .980 |
| Health conditions | |||
| High blood pressure | 46.7 | 43.3 | .456 |
| Diabetes | 36.4 | 36.7 | 1.000 |
| Cardiovascular condition | 47.6 | 45.3 | .635 |
| Central nervous system medication | 51.6 | 47.3 | .335 |
| Depression | 34.7 | 30.7 | .341 |
| Chronic discrimination measures | |||
| Lifetime discrimination | 3.01 (2.84) | 2.93 (2.74) | .710 |
| Daily discrimination | 1.65 (.71) | 1.66 (.72) | .814 |
| Chronic job discrimination | 1.69 (.65) | 1.69 (.65) | .983 |
| Perceived inequality at work | 1.93 (.65) | 1.93 (.65) | 1.000 |
| Inflammatory biomarkers | |||
| Total inflammatory burden | 1.94 (1.35) | 1.84 (1.28) | .292 |
| C-reactive protein (CRP), top quartile | 39.1 | 40.7 | .757 |
| interleukin-6 (IL-6), top quartile | 42.2 | 38.7 | .427 |
| fibrinogen, top quartile | 44.0 | 40.0 | .366 |
| E-selectin, top quartile | 37.8 | 34.7 | .479 |
| Intercellular adhesion molecule (ICAM), top quartile | 32.0 | 30.0 | .662 |
| Total N | 225 (100.0%) | 150 (66.7%) |
Note. HS = high school; GED = Graduate Equivalency Degree.
Bold test indicates p <.10.
Discrimination and inflammatory burden among Black Americans
Relationships between each individual chronic discrimination scale and inflammatory burden are reported in Tables 3–6. In unadjusted Model 1, higher levels of reported lifetime discrimination (b =.079, SE =.032, p =.013) and inequality at work (b =.453, SE =.157, p =.004) were associated with more inflammatory burden, while daily and job discrimination were not.
Table 3.
Relationship Between Lifetime Discrimination and Inflammatory Burden among Black Americans, n=224.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | p * | b | SE | p * | b | SE | p * | b | SE | p * | |
| Lifetime discrimination | .079 | .032 | .013 | .084 | .031 | .007 | .058 | .030 | .057 | .121 | .037 | .001 |
| Sex (male) | ‑.475 | .188 | .012 | ‑.324 | .180 | .073 | .220 | .258 | .394 | |||
| Age | ‑.014 | .010 | .157 | ‑.016 | .010 | .092 | ||||||
| Education | ‑.041 | .090 | .646 | ‑.066 | .088 | .455 | ||||||
| Tobacco use | .482 | .201 | .018 | .438 | .197 | .028 | ||||||
| BMI | .050 | .011 | <.001 | .049 | .010 | <.001 | ||||||
| High BP | .284 | .177 | .111 | .292 | .174 | .096 | ||||||
| Diabetes | .057 | .184 | .757 | .091 | .181 | .617 | ||||||
| Cardiovascular | .218 | .196 | .267 | .235 | .193 | .223 | ||||||
| CNS medication | .309 | .184 | .094 | .322 | .181 | .076 | ||||||
| Depression | .295 | .180 | .103 | .240 | .178 | .178 | ||||||
| Interaction: Lifetime discrimination * Sex | ‑.173 | .060 | .004 | |||||||||
| Intercept | 1.719 | .130 | <.001 | 1.858 | .140 | <.001 | .380 | .683 | .579 | .403 | .672 | .550 |
|
| ||||||||||||
| R 2 | .027 | .055 | .249 | .278 | ||||||||
| F∆ R2(p) | 6.201 (.013) | 6.386 (.012) | 6.110 (<.001) | 8.401 (.004) | ||||||||
Note. BMI = body mass index (kg/m2); high BP = high blood pressure; CNS = central nervous system medication use.
Bold test indicates p <.10.
Table 6.
Relationship Between Inequality at Work and Inflammatory Burden among Black Americans, n=149.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | p * | b | SE | p * | b | SE | p * | b | SE | p * | |||
| Inequality at work | .453 | .157 | .004 | .456 | .156 | .004 | .332 | .151 | .029 | .400 | .176 | .025 | ||
| Sex (male) | ‑.306 | .212 | .152 | ‑.122 | .199 | .540 | .345 | .654 | .598 | |||||
| Age | ‑.007 | .013 | .580 | ‑.007 | .013 | .599 | ||||||||
| Education | .014 | .099 | .888 | .013 | .099 | .894 | ||||||||
| Tobacco use | .579 | .225 | .011 | .556 | .227 | .016 | ||||||||
| BMI | .044 | .012 | <.001 | .044 | .012 | <.001 | ||||||||
| High BP | .508 | .211 | .017 | .484 | .213 | .025 | ||||||||
| Diabetes | .341 | .220 | .124 | .332 | .221 | .135 | ||||||||
| Cardiovascular | .237 | .219 | .281 | .254 | .220 | .252 | ||||||||
| CNS medication | .314 | .214 | .145 | .314 | .214 | .145 | ||||||||
| Depression | .141 | .221 | .525 | .157 | .222 | .482 | ||||||||
| Interaction: Inequality at work * Sex | ‑.241 | .321 | .454 | |||||||||||
| Intercept | .959 | .319 | .003 | 1.060 | .326 | .001 | ‑.676 | .893 | .450 | ‑.813 | .913 | .375 | ||
|
| ||||||||||||||
| R 2 | .054 | .067 | .309 | .312 | ||||||||||
| F∆ R2(p) | 8.327 (.004) | 2.076 (.152) | 5.342 (<.001) | .564 (.454) | ||||||||||
Note. BMI = body mass index (kg/m2); high BP = high blood pressure; CNS = central nervous system medication use.
Bold test indicates p <.10.
Associations between lifetime discrimination (b =.084, SE =.031, p =.007) and inequality at work (b =.456, SE =.156, p =.004) and inflammatory burden remained robust after accounting for sex in Model 2. Further adjusting for demographic and health covariates in Model 3 attenuated, but did not eliminate, associations between lifetime discrimination and inequality at work and inflammatory burden (b =.058, SE =.030, p =.057 and b =.332, SE =.151, p =.029, respectively); in large part because several of the health covariates were associated with inflammatory burden. Across all models, tobacco use, and BMI were consistently associated with increased inflammatory burden, whereas relationships between other covariates and inflammatory burden were less reliably detected.
Sex differences among Black Americans
Interaction terms added in Model 4 to identify sex differences in relationships between chronic discrimination and inflammatory burden were significant in the lifetime (b =−.173, SE =.060, p =.004) and job discrimination (b =−.662, SE =.299, p =.029) models; interactions were not significant in daily discrimination and inequality at work models. Simple slopes test (see Figures 1 and 2) indicated that, when demographics and health covariates were held constant at sex-specific mean values, more lifetime and job discrimination were significantly associated or marginally associated with greater inflammatory burden for Black women (b =.121, t =3.283, p =.001 and b =.386, t =1.908, p =.058, respectively); lifetime and job discrimination were unrelated to inflammatory burden in Black men (b = −.052, t = −1.083, p =.280 and b = −.276, t = −1.248, p =.214, respectively).
Figure 1. Lifetime Discrimination-Inflammatory Burden for Black Women and Men.
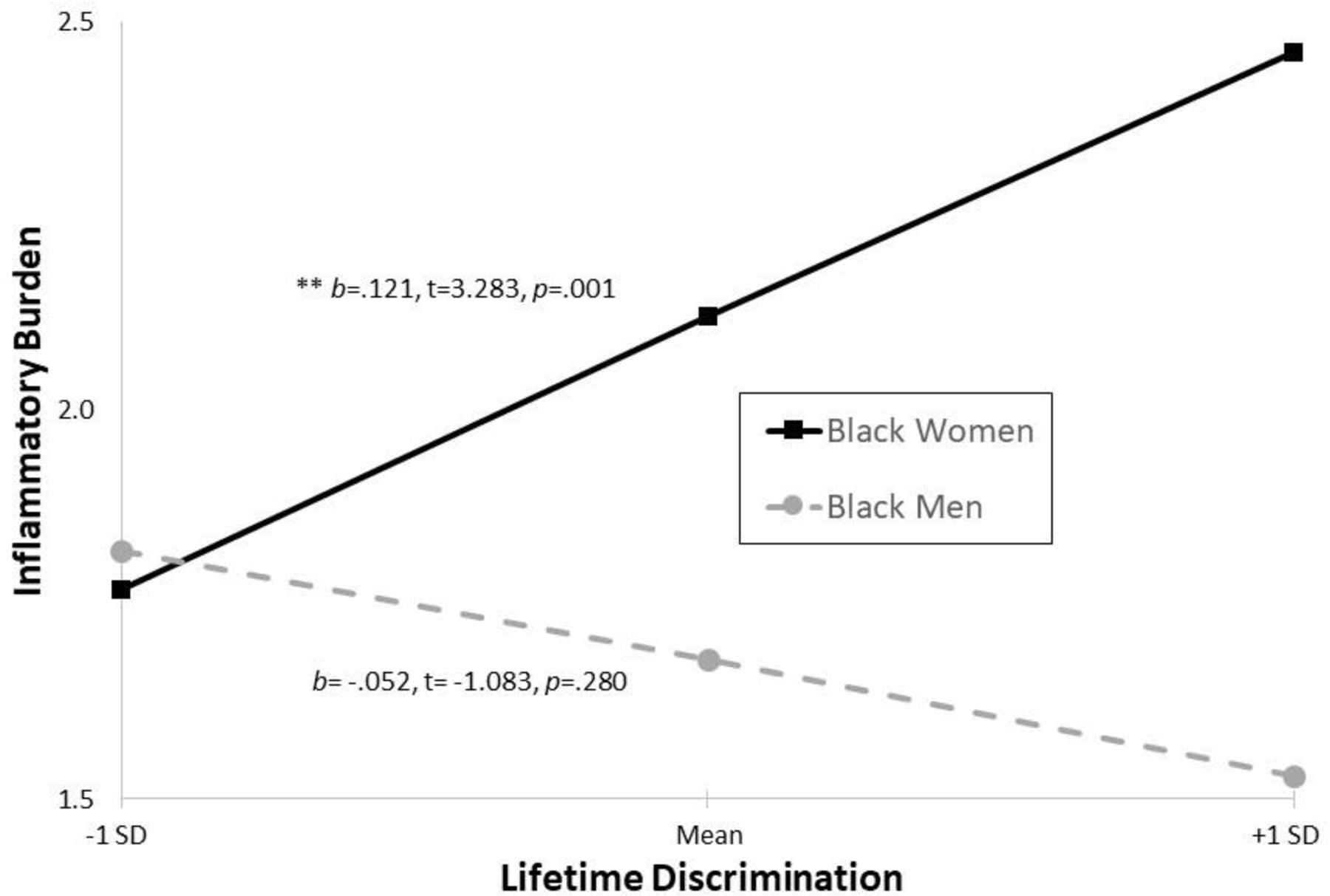
Note. Covariates held constant at sex-specific mean values.
Figure 2. Job Discrimination-Inflammatory Burden for Black Women and Men.
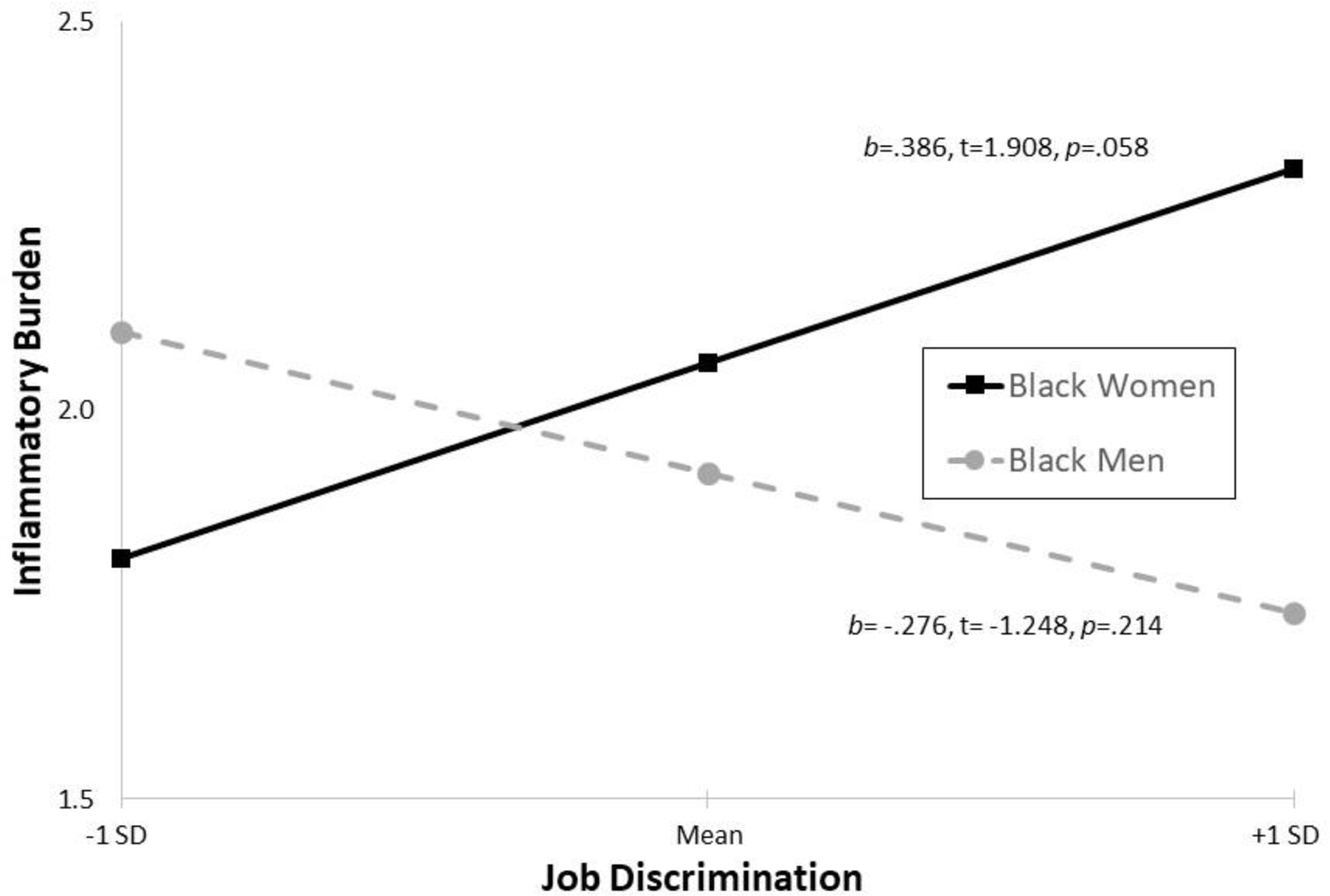
Note. Covariates held constant at sex-specific mean values.
Supplementary analyses
Supplementary analyses were also conducted examining relationships between all four chronic discrimination measures modeled simultaneously and inflammatory burden (Supplemental Table 2). In unadjusted Model 1, higher levels of reported lifetime discrimination (b =.084, SE =.047, p =.087) and inequality at work (b =.424, SE =.171, p =.014) were associated with more inflammatory burden, while daily and job discrimination were not, consistent with our main analyses described above. Associations between lifetime discrimination and inequality at work and inflammatory burden remained robust after accounting for sex in Model 2, but both were attenuated after adjusting for demographic and health covariates in Model 3 such that only the association between inequality at work and inflammatory burden remained marginally significant (b =.295, SE =.163, p =.072). Multicollinearity prevented examination of moderation by sex in this analysis.
Summary
When examined individually, lifetime discrimination and inequality at work predicted higher levels of inflammatory burden, adjusting for sex, other demographics, and health covariates. Analyses testing whether relationships between discrimination and inflammatory burden were moderated by sex (i.e., interactions) revealed significant sex differences, such that more lifetime and job discrimination were associated with greater inflammatory burden in Black women, but not in Black men. When modeled simultaneously in supplementary analyses, only inequality at work was marginally associated with inflammatory burden after accounting for the variance shared by the other chronic discrimination measures, demographics, and health covariates.
Discussion
The goals of this study were two-fold: (1) to examine the relationships between four forms of discrimination (lifetime, daily, and job discrimination, and inequality at work) and a composite measure of inflammatory dysregulation among middle age and older Black adults; and (2) to investigate whether sex differences may exist. Three key findings are important. First, Black men generally reported higher levels of discrimination than women (3 out of 4 forms), though only differences in reported job discrimination achieved statistical significance. In contrast, Black women had greater overall inflammatory burden than men, particularly elevated levels of fibrinogen. Second, higher levels of reported lifetime discrimination and inequality at work were associated with more inflammatory burden in both unadjusted and adjusted models. Third, sex-modified associations between lifetime and job discrimination and inflammatory burden, such that more lifetime and job discrimination predicted greater inflammatory burden in Black women but not in Black men. Collectively, these findings suggest Black adults who experience specific forms of discrimination may be at great risk for physiological dysregulation. Further, the findings demonstrate that the discrimination-inflammation link varies by sex, such that discrimination may be a more salient risk factor for chronic inflammation in Black women than in Black men. This appears to be the case even though Black men report higher levels of discrimination. These findings highlight the potential consequences of discrimination in the lives of Black Americans and further illustrate that middle age and older Black women may be at an increased risk for discrimination-related inflammation and associated adverse health outcomes.
These findings are consistent with studies that have shown discrimination is linked with greater chronic inflammation among women, particularly Black women. For example, using a community sample of Black women ages 30–50, Nuru-Jeter et al. (2013) found a positive association between racial/ethnic discrimination and elevated levels of pro-inflammatory biomarkers (IL-6, TNFα, hsCRP) among women reporting higher vs. lower levels of Anticipatory Racism Threat (p<.05) (Nuru-Jeter et al., 2013). In the Coronary Artery Risk Development in Young Adults (CARDIA) study, Black women who experienced one or two episodes of racial/ethnic discrimination in six specified domains (compared to those reporting no experiences of discrimination) also had higher levels of inflammation (as measured by CRP), adjusting for blood pressure, plasma total cholesterol, triglycerides, homeostatic model assessment for insulin resistance (HOMA-IR), age, education, community, social desirability, and personal control. However, this association was not observed in men or White women (Cunningham et al., 2012). In another longitudinal study involving 2,490 women from racially diverse backgrounds (Black, White, Japanese and Hispanic), Beatty-Moody (2014) found that in non-obese women (BMI less than 30), higher perceived everyday discrimination was associated with higher CRP levels over a 7-year period (Beatty-Moody et al., 2014). Ratner and colleagues (2013) have further shown that perceived stigmatization of one’s racial group as being more devalued by society was predictive of increased IL-6 in Black and Latina women, ages 18–44 years. This same study also found no association between everyday discrimination and increased IL-6 (Ratner et al., 2013).
The inconsistencies in findings across studies may be partly explained by differences in the discrimination measures used. Specifically, the approach to measuring discrimination in the Nuru-Jeter et al. (2013) study was to explicitly ask about experiences of racial and ethnic discrimination (Nuru-Jeter et al., 2013), and in the Cunningham (2012) study participants were asked had they ever experienced racial/ethnic discrimination in six different domains: “at school, getting a job, at work, getting housing, getting medical care, on the street or in a public setting, and at home.” In comparison, the lifetime discrimination measure used in this study (Kessler et al., 1999; Williams et al., 1997) inquiries about discriminatory experiences due to any attribution (e.g., race, ethnicity, gender, age, or religion). Thus, a respondent’s response to a question about general unfair treatment must first be endorsed before asking follow-up questions about attribution. Scholars have noted these two approaches make different assumptions about how best to query respondents and, thus, have unique strengths and limitations (see Lewis et al., 2015 for a review). One strength of the Lifetime and Everyday Discrimination scales (Williams et al., 1997), the Perceived Inequality at Work scale, and, to a lesser degree, the Chronic Job Discrimination scale used in this study is that respondents were asked to recall and report experiences of discrimination but were not simultaneously required to identify the cause. Accordingly, these measures entailed a lower level of cognitive challenge. However, a key limitation of these measures is that the number of respondents reporting mistreatment due exclusively to race or ethnicity cannot be determined (Williams, 1999). Thus future research should contrast the two approaches – those that ask about global experiences of discrimination versus those that ask about racial and ethnic discrimination (Lewis et al., 2015) – and assess their associations with key indicators of physiological dysregulation among Black adults.
The inconsistencies when comparing the current findings with those previously reported may further be due to the characteristics of the samples and the age cohorts included. A key limitation of the current literature is the use of sex-specific subpopulations, including women anticipating a racial threat (Nuru-Jeter et al., 2013) and non-obese women (Beatty-Moody et al., 2014). The present study used a community-based sample of Black adults and thus may be more representative of Black women nationwide. Moreover, our sample focused on midlife and older ages, with a range of 37 to 85 years. In contrast, Ratner and colleagues (2013) used a sample of Black and Latina women ages 18–44 years. Hence, they looked at early adulthood and midlife (Ratner et al., 2013), which may differ in the cumulative effects of discrimination on physiologic systems. More research on this topic is needed with large, nationally representative samples of Black Americans and longitudinal data that facilitate investigation of how relationships between discrimination and inflammation may change over the life course.
In support of this point, evidence shows that experiences of discrimination vary based on the time period in the life course that they are experienced (Gee et al., 2012). For example, Gee and colleagues (2007) report that age discrimination in the workplace varies as women move from being young job seekers to mid-career employees to retirees (Gee et al., 2007). The authors argue that a key implication of these age-patterned exposures is that the frequency and forms of discrimination are likely to change over the life course. Our findings lend support to and extend this idea through the lens of intersectionality. The middle-aged and older Black women in our sample who reported higher lifetime and job discrimination had consistently greater inflammatory burden. Thus, the effects of discrimination related to race, age, and sex, may reverberate across the life course and reinforce one another (Gee et al., 2012) such that Black women are put at a greater disadvantage.
Exposure to discrimination, whether racial or not, is linked to worse health outcomes in Black women. For instance, “high vs. low” perceived racial discrimination has been associated with lower kidney function (eGFR) over time among Black women, ages 30–64 years (Beydoun et al., 2017). This same study also found “medium vs. low” perceived racial and gender discrimination were both significantly related to worse kidney function at follow-up (see supplemental analyses Table S2); these associations remained significant after adjustment for lifestyle (smoking and drug use), health-related (e.g., self-rated health, BMI, hypertension, and diabetes), and psychosocial (depressive symptoms) factors, although the effect sizes were attenuated (Beydoun et al., 2017). Black women (ages 25–50 years) reporting frequent nonracial discrimination (e.g., due to gender, age, etc.) versus those reporting no exposure to discrimination (as measured by the 6-item Everyday Discrimination Scale) had higher odds of hypertension, adjusting for age, education, BMI, and instrumental and emotional support) (Roberts et al., 2007).
Overall, these studies show that Black women exposed to higher levels of discrimination are more likely to experience adverse health outcomes, including greater inflammation (Beatty-Moody et al., 2014; Beydoun et al., 2017; Cunningham et al., 2012; Nuru-Jeter et al., 2013; Roberts et al., 2007). These findings are independent of demographics, health-related or psychosocial factors (e.g., social desirability, mastery, receiving social support), and are robust regardless of the measure of discrimination (racial/ethnic, nonracial, gender, everyday, etc.) or study design (sample characteristics, age cohort included, or period of the life course). Our findings likewise show that Black women exposed to higher levels of discrimination (lifetime and job) have greater inflammation, independent of demographic and health-related factors. Further highlighting the importance of investigating multiple forms of discrimination on health outcomes among Black women.
Limitations
First, our discrimination measures were based on self-report and did not include assessments of institutional or structural racism (e.g., residential segregation, criminal justice system bias, racial profiling and police brutality). This limited our ability to effectively assess the health consequences of discrimination occurring at multiple levels of influence. Second, our study included a relatively modest sample of Black men (n=76) and a smaller working-only sample of men (n=56). Thus, we were unable to detect weak effect sizes or small sex-based differences in associations between discrimination and inflammatory burden. Therefore, additional research in this area using larger samples of Black men and working individuals is warranted. Third, the sample’s older age range may be an important consideration with our findings. Theoretical and empirical research suggests that chronic or daily exposure to stressors cumulatively weathers the body over the life course, particularly in Black women (Geronimus et al., 2010). Perhaps by the latter half of the lifespan, inflammatory systems are dysregulated and unresponsive to current or recent exposure to chronic discrimination. Replicating this study with a younger age cohort or with longitudinal data may clarify whether relationships between chronic discrimination and inflammatory burden are consistent or vary across different stages of the lifespan. Finally, we did not weight the results to be nationally representative because of the variable sampling strategies used in MIDUS II and the Biomarker Project.
Conclusions
This study sheds light on the biological underpinnings of exposure to multiple forms of discrimination and sex-based differences in the discrimination-inflammation relationship. To our knowledge, the present analysis is among the first to consider associations between four different forms of discrimination and a composite measure of inflammatory burden and potential sex differences within a sample of middle-aged and older U.S. Blacks. Although the mechanisms underlying the observed associations in men and women have yet to be disentangled, these findings add to the growing literature linking discrimination to inflammation in Black women and highlights the critical need for more sex-specific research in this area.
Supplementary Material
Table 4.
Relationship Between Daily Discrimination and Inflammatory Burden among Black Americans, n=225.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | p * | b | SE | p * | b | SE | p * | b | SE | p * | |
| Daily discrimination | .133 | .128 | .298 | .174 | .127 | .173 | .054 | .120 | .653 | .197 | .152 | .196 |
| Sex (male) | ‑.465 | .191 | .016 | ‑.297 | .183 | .107 | .328 | .450 | .466 | |||
| Age | ‑.012 | .010 | .213 | ‑.013 | .010 | .204 | ||||||
| Education | ‑.005 | .087 | .958 | ‑.007 | .087 | .936 | ||||||
| Tobacco use | .525 | .203 | .010 | .493 | .204 | .016 | ||||||
| BMI | .053 | .011 | <.001 | .053 | .011 | <.001 | ||||||
| High BP | .328 | .178 | .066 | .321 | .177 | .072 | ||||||
| Diabetes | .042 | .185 | .820 | .048 | .184 | .794 | ||||||
| Cardiovascular | .169 | .196 | .390 | .189 | .196 | .335 | ||||||
| CNS medication | .346 | .184 | .062 | .368 | .184 | .047 | ||||||
| Depression | .286 | .181 | .115 | .277 | .180 | .126 | ||||||
| Interaction: Daily discrimination * Sex | ‑.363 | .238 | .130 | |||||||||
| Intercept | 1.732 | .229 | <.001 | 1.817 | .229 | <.001 | .202 | .709 | .776 | ‑.015 | .721 | .984 |
|
| ||||||||||||
| R 2 | .005 | .031 | .236 | .244 | ||||||||
| F∆ R2(p) | 1.086 (.298) | 5.924 (.016) | 6.345 (<.001) | 2.312 (.130) | ||||||||
Note. BMI = body mass index (kg/m2); high BP = high blood pressure; CNS = central nervous system medication use.
Bold test indicates p <.10.
Table 5.
Relationship Between Job Discrimination and Inflammatory Burden among Black Americans, n=150.
| Model 1 | Model 2 | Model 3 | Model 4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | p * | b | SE | p * | b | SE | p * | b | SE | p * | ||
| Job discrimination | .064 | .161 | .692 | .126 | .166 | .449 | .085 | .152 | .576 | .386 | .202 | .058 | |
| Sex (male) | ‑.316 | .225 | .162 | ‑.128 | .210 | .544 | 1.029 | .563 | .069 | ||||
| Age | ‑.011 | .013 | .380 | ‑.013 | .012 | .314 | |||||||
| Education | .012 | .100 | .908 | ‑.039 | .101 | .704 | |||||||
| Tobacco use | .629 | .225 | .006 | .584 | .223 | .010 | |||||||
| BMI | .044 | .012 | <.001 | .044 | .012 | <.001 | |||||||
| High BP | .591 | .209 | .005 | .557 | .206 | .008 | |||||||
| Diabetes | .356 | .223 | .114 | .304 | .222 | .173 | |||||||
| Cardiovascular | .227 | .221 | .307 | .245 | .218 | .264 | |||||||
| CNS medication | .312 | .217 | .153 | .307 | .214 | .155 | |||||||
| Depression | .243 | .218 | .266 | .223 | .215 | .302 | |||||||
| Interaction: Job discrimination * Sex | ‑.662 | .299 | .029 | ||||||||||
| Intercept | 1.732 | .291 | <.001 | 1.740 | .290 | <.001 | ‑.047 | .888 | .957 | ‑.327 | .885 | .713 | |
|
| |||||||||||||
| R 2 | .001 | .014 | .290 | .315 | |||||||||
| F∆ R2(p) | .158 (.692) | 1.980 (.162) | 5.962 (<.001) | 4.893 (.029) | |||||||||
Note. BMI = body mass index (kg/m2); high BP = high blood pressure; CNS = central nervous system medication use.
Bold test indicates p <.10.
Highlights.
In general, Black men report higher levels of discrimination than Black women
Overall, Black women report more inflammatory burden compared to Black men
Lifetime discrimination and work inequality are associated with greater inflammation
The discrimination-inflammation relationship varies by sex
Lifetime and job discrimination are linked to greater inflammation in Black women
Acknowledgements:
Roland Thorpe for his feedback.
Funding:
This work was supported by a grant to DRB from the Alzheimer’s Association (AARFD-21-852652) and NIA (1 K01 AG068376-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest:
None.
References
- Aiken L, West S, & Reno R (1991). Multiple regression: Testing and interpreting interactions Sage. [Google Scholar]
- Beatty-Moody DL, Matthews KA, Bromberger JT, & Brown C (2014). Everyday discrimination prospectively predicts inflammation across 7-years in racially diverse midlife women: Study of Women’s Health Across the Nation. J Soc Issues, 70(2), 298–314. 10.1111/josi.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Poggi-Burke A, Zonderman AB, Rostant OS, Evans MK, & Crews DC (2017). Perceived discrimination and longitudinal change in kidney function among urban adults. Psychosom Med, 79(7), 824–834. 10.1097/psy.0000000000000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowleg L, del Río-González AM, Holt SL, Pérez C, Massie JS, Mandell JE, & Boone A, C. (2017). Intersectional epistemologies of ignorance: How behavioral and social science research shapes what we know, think we know, and don’t know about us Black men’s sexualities. JSR, 54(4–5), 577–603. 10.1080/00224499.2017.1295300 [DOI] [PubMed] [Google Scholar]
- Brody GH, Lei MK, Chae DH, Yu T, Kogan SM, & Beach SRH (2014). Perceived discrimination among African American adolescents and allostatic load: A longitudinal analysis with buffering effects. Child Dev, 85(3), 989–1002. 10.1111/cdev.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenshaw K (1989). Demarginalizing the intersection of race and sex: A Black feminist critique of antidiscrimination doctrine, feminist theory and antiracist politics. University of Chicago Legal Forum, 8, 139–167. [Google Scholar]
- Cuevas AG, Ong AD, Carvalho K, Ho T, Chan SW, Allen JD, Chen R, Rodgers J, Biba U, & Williams DR (2020). Discrimination and systemic inflammation: A critical review and synthesis. Brain Behav Immun, 89, 465–479. 10.1016/j.bbi.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe CI, & Berkman LF (2012). Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the cardia cohort of 4 us communities. Soc Sci Med, 75(5), 922–931. 10.1016/j.socscimed.2012.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A (2013). How does race get “under the skin”?: Inflammation, weathering, and metabolic problems in late life. Soc Sci Med, 77, 75–83. 10.1016/j.socscimed.2012.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DM, & Molix L (2014). Perceived discrimination as a stressor for close relationships: Identifying psychological and physiological pathways. J Behav Med, 37(6), 1134–1144. 10.1007/s10865-014-9563-8 [DOI] [PubMed] [Google Scholar]
- Friedman EM, Williams DR, Singer BH, & Ryff CD (2009). Chronic discrimination predicts higher circulating levels of e-selectin in a national sample: The MIDUS study. Brain Behav Immun, 23(5), 684–692. 10.1016/j.bbi.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee GC, Pavalko EK, & Long JS (2007). Age, cohort and perceived age discrimination: Using the life course to assess self-reported age discrimination. Soc Forces, 86(1), 265–290. http://www.jstor.org/stable/4495036 [Google Scholar]
- Gee GC, Walsemann KM, & Brondolo E (2012). A life course perspective on how racism may be related to health inequities. Am J Public Health, 102(5), 967–974. 10.2105/AJPH.2012.300666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, & Bound J (2006). “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. Am J Public Health, 96(5), 826–833. 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, & Cruz TD (2010). Do us Black women experience stress-related accelerated biological aging?: A novel theory and first population-based test of Black-White differences in telomere length. Hum Nat, 21(1), 19–38. 10.1007/s12110-010-9078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Shkolnikov VM, Jdanov D, Shkolnikova M, Vaupel JW, & Weinstein M (2013). Perceived stress and biological risk: Is the link stronger in Russians than in Taiwanese and Americans? Stress, 16(4), 411–420. 10.3109/10253890.2013.789015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Lantz PM, & Herd P (2005). Continuity and change in the social stratification of aging and health over the life course: Evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans’ changing lives study). J Gerontol B Psychol Sci Soc Sci, 60 Spec No 2(Special_Issue_2), 15–26. 10.1093/geronb/60.special_issue_2.s15 [DOI] [PubMed] [Google Scholar]
- Kang S, & Marks NF (2014). Filial caregiving is associated with greater neuroendocrine dysfunction: Evidence from the 2005 National Survey of Midlife in the United States. SAGE Open Medicine, 2, 2050312113520152. 10.1177/2050312113520152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw KN, Lewis TT, Diez Roux AV, Jenny NS, Liu K, Penedo FJ, & Carnethon MR (2016). Self-reported experiences of discrimination and inflammation among men and women: The multi-ethnic study of atherosclerosis. Health Psychol, 35(4), 343–350. 10.1037/hea0000331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, & Williams DR (1999). The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J Health Soc Behav, 40(3), 208–230. https://www.ncbi.nlm.nih.gov/pubmed/10513145 [PubMed] [Google Scholar]
- Kwate NO, & Goodman MS (2015). Racism at the intersections: Gender and socioeconomic differences in the experience of racism among African Americans. Am J Orthopsychiatry, 85(5), 397–408. 10.1037/ort0000086 [DOI] [PubMed] [Google Scholar]
- Lewis TT, Aiello AE, Leurgans S, Kelly J, & Barnes LL (2010). Self-reported experiences of everyday discrimination are associated with elevated c-reactive protein levels in older African-American adults. Brain Behav Immun, 24(3), 438–443. 10.1016/j.bbi.2009.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Cogburn CD, & Williams DR (2015). Self-reported experiences of discrimination and health: Scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psychol, 11(1), 407–440. 10.1146/annurev-clinpsy-032814-112728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, & Van Dyke ME (2018). Discrimination and the health of African Americans: The potential importance of intersectionalities. Curr Dir Psychol Sci, 27(3), 176–182. 10.1177/0963721418770442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, & Ryff CD (2010). Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. J Aging Health, 22(8), 1059–1080. 10.1177/0898264310374355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci, 840, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- Nuru-Jeter A, Chae DH, Price M, Telesford J, Mendoza-Denton R, & Woods-Giscombe C (2013). Anticipatory racism threat and superwoman schema: Elucidating the relationship between racial discrimination and chronic inflammation. Circulation, 128(suppl_22), A9550. 10.1161/circ.128.suppl_22.A9550 [DOI] [Google Scholar]
- Ong AD, & Williams DR (2019). Lifetime discrimination, global sleep quality, and inflammation burden in a multiethnic sample of middle-aged adults. Cultur Divers Ethnic Minor Psychol, 25(1), 82–90. 10.1037/cdp0000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AD, Williams DR, Nwizu U, & Gruenewald TL (2017). Everyday unfair treatment and multisystem biological dysregulation in African American adults. Cultur Divers Ethnic Minor Psychol, 23(1), 27–35. 10.1037/cdp0000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdie-Vaughns V, & Eibach RP (2008). Intersectional invisibility: The distinctive advantages and disadvantages of multiple subordinate-group identities. Sex Roles, 59(5), 377–391. [Google Scholar]
- Radler BT (2014). The Midlife in the United States (MIDUS) series: A national longitudinal study of health and well-being. Open health data, 2(1), e3. 10.5334/ohd.ai [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radler BT, & Ryff CD (2010). Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. J Aging Health, 22(3), 307–331. 10.1177/0898264309358617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Ratner KG, Halim ML, & Amodio DM (2013). Perceived stigmatization, ingroup pride, and immune and endocrine activity:Evidence from a community sample of Black and Latina women. Soc Psychol Personal Sci, 4(1), 82–91. 10.1177/1948550612443715 [DOI] [Google Scholar]
- Roberts CB, Vines AI, Kaufman JS, & James SA (2007). Cross-sectional association between perceived discrimination and hypertension in African-American men and women: The pitt county study. American Journal of Epidemiology, 167(5), 624–632. 10.1093/aje/kwm334 [DOI] [PubMed] [Google Scholar]
- Ryff CD, Seeman T, & Weinstein M (2019). Midlife in the United States (MIDUS 2): Biomarker project, 2004–2009 Inter-university Consortium for Political and Social Research [distributor] 10.3886/ICPSR29282.v9 [DOI]
- Sproston NR, & Ashworth JJ (2018). Role of c-reactive protein at sites of inflammation and infection. Front Immunol, 9, 754–754. 10.3389/fimmu.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanikova I, Bateman LB, & Oates GR (2017). Systemic inflammation in midlife: Race, socioeconomic status, and perceived discrimination. Am J Prev Med, 52(1s1), S63–s76. 10.1016/j.amepre.2016.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternthal MJ, Slopen N, & Williams DR (2011). Racial disparities in health: How much does stress really matter? Du Bois Rev, 8(1), 95–113. 10.1017/S1742058X11000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch DM, Stein J, Greendale GA, Chyu L, Tseng CH, Huang MH, Lewis TT, Kravitz HM, & Seeman T (2015). A longitudinal investigation of race, socioeconomic status, and psychosocial mediators of allostatic load in midlife women: Findings from the Study of Women’s Health Across the Nation. Psychosom Med, 77(4), 402–412. 10.1097/psy.0000000000000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Stolpe A, & van der Saag PT (1996). Intercellular adhesion molecule-1. J Mol Med (Berl), 74(1), 13–33. 10.1007/bf00202069 [DOI] [PubMed] [Google Scholar]
- Van Dyke ME, Baumhofer NK, Slopen N, Mujahid MS, Clark CR, Williams DR, & Lewis TT (2020). Pervasive discrimination and allostatic load in African American and White adults. Psychosom Med, 82(3), 316–323. 10.1097/psy.0000000000000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker KM, Everson-Rose SA, Pankow JS, Rodriguez CJ, Lewis TT, Kershaw KN, Diez Roux AV, & Lutsey PL (2017). Experiences of discrimination and incident type 2 diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Epidemiol, 186(4), 445–455. 10.1093/aje/kwx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR (1999). Race, socioeconomic status, and health the added effects of racism and discrimination. Ann N Y Acad Sci, 896(1), 173–188. [DOI] [PubMed] [Google Scholar]
- Williams DR, Yan Y, Jackson JS, & Anderson NB (1997). Racial differences in physical and mental health: Socio-economic status, stress and discrimination. J Health Psychol, 2(3), 335–351. 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


