Abstract
Background
In high-income countries, standard care for primary stroke prevention in children with sickle cell anaemia and abnormal transcranial Doppler velocities results in a 92% relative risk reduction of strokes but mandates initial monthly blood transfusion. In Africa, where regular blood transfusion is not feasible for most children, we tested the hypothesis that initial moderate-dose compared to low-dose hydroxyurea decreases the incidence of strokes for children with abnormal transcranial Doppler velocities.
Methods
A double-blind, parallel-group randomised controlled trial was conducted in Nigerians 5–12 years of age with sickle cell anaemia at 3 teaching hospitals NCT02560935ClinicalTrials.gov. Randomisation utilized a permuted block allocation scheme with block sizes of 4, stratified by sex and site. Allocation was concealed from all but the pharmacists and statisticians. Participants were assigned in a 1:1 ratio to low-dose (10 mg/kg/day) or moderate-dose (20 mg/kg/day) once daily oral hydroxyurea with monthly clinical evaluation and laboratory monitoring. The primary outcome was initial stroke or transient ischaemic attack, centrally adjudicated. Secondary outcomes included all-cause hospitalisation. Analyses were done using the intention-to-treat principle. The trial was stopped early for futility after a planned minimum follow-up of 3.0 years to an actual median of 2·4 years of follow-up (IQR 2·0–2·8) for participants.
Findings
Between August 2, 2016, and June 14, 2018, 220 participants (median age 7·2 years (IQR 5·5–8·9; 114, 51·8% female;) were randomly allocated and followed for a median of 2·4 years (IQR 2·0–2·8). All participants were Nigerian. Ethnic groups included Hausa 81% (n=179), Fulani 11% (n=25), and other 7% (n=16). In the low- (n=109) and moderate-dose (n=111) hydroxyurea groups, 2·8% (3/109) and 4·5% (5/111) had strokes, with incidence rates 1·19 and 1·92 per 100 person-years respectively, incidence rate ratio 0·62 (95% confidence interval: 0·10–3·20, p=0·77). The incidence rate ratio of hospitalisation for any reason was 1·71 (95% CI, 1·15–2·57; p=0·0071), with higher incidence rates in the low-dose group. No participant had hydroxyurea stopped for myelosuppression.
Interpretation
Compared to low-dose hydroxyurea therapy, moderate-dose hydroxyurea had no difference in the stroke incidence rate. However, the moderate-dose group had lower incidence rates for all-cause hospitalisations. These findings provide an evidence-based guideline for the use of lower-dose hydroxyurea therapy for children with sickle cell anaemia at risk of stroke. Shared decision-making between the parents and the health care provider may lead to an increase in hydroxyurea dose to 20mg/kg/day if prior or subsequent increased hospitalisation rate.
Funding
National Institute of Neurological Disorders and Stroke, ClinicalTrials.gov NCT02560935, recruitment closed.
Keywords: hydroxyurea, primary stroke prevention, sickle cell anaemia, low-income setting
Introduction
The absence of stroke prevention programs in children with sickle cell anaemia living in both low-and high-resource countries results in high incidence rates of initial ischaemic strokes, recurrent strokes, and stroke-related mortality.1–3 After the completion of the Stroke Prevention Trial in Sickle Cell Anemia Trial (STOP),4 standard care in high-income settings that includes annual transcranial Doppler ultrasonography screening to identify children with abnormal velocities (≥200 cm/sec non-imaging and ≥185 cm/sec imaging methods), followed by initial monthly blood transfusion to lower maximum hemoglobin S levels from 90% to less than 30%, results in at least 92% relative risk reduction in the incidence of stroke.4 However, in Nigeria and other countries in Africa, initial monthly blood transfusion therapy for primary stroke prevention is not possible for most children.5
Approximately 50% of all infants with sickle cell anaemia in the world are born in Nigeria, 150,000 per year.6 In Nigeria, the incidence of strokes in an unscreened and untreated pediatric sickle cell anaemia population with median age 6·0 years (range 1·1 to 14·2 years) is similar to the prior incidence of strokes in the United States in children with sickle cell anaemia between 6 and 9 years of age before implementing primary stroke prevention programs, 0·88 and 0·79 strokes per 100 person-years, respectively.1,3
To address the gap for primary stroke prevention in a low-resource setting, we successfully completed a single-arm feasibility trial, Stroke Prevention in Nigeria (SPIN) (clinical trials.gov: NCT01801423), assessing the incidence of stroke in children with abnormal transcranial Doppler velocities treated with fixed moderate-dose hydroxyurea (20 mg/kg/day).7 The SPIN trial demonstrated the acceptability and safety of hydroxyurea for children with abnormal transcranial Doppler measurements. In the SPIN trial, one child had a stroke (0·76 per 100 patient-years of observation)7 compared with 14 expected strokes based on the incidence of strokes (10·7 per 100 patient-years) in the observation arm of the STOP Trial.4 Prior studies in low-income settings have shown the benefit of low-dose hydroxyurea (10 to 15 mg/kg/day) in ameliorating sickle cell disease-related complications.8–10 Given the strong preliminary data in the SPIN Trial demonstrating the benefit of moderate-dose hydroxyurea for preventing strokes in children with abnormal transcranial Doppler measurements, we tested the primary hypothesis that moderate-dose hydroxyurea (20 mg/kg/day) therapy for primary stroke prevention results in a 66% relative risk reduction (from 9 to 3 events per 100 person-years) when compared to low-dose hydroxyurea (10 mg/kg/day) therapy.
Methods
Study design and participants
The SPRING trial was a multicenter, double-blind, randomised controlled trial in children with sickle cell anaemia and abnormal transcranial Doppler velocities. Participants received either fixed low- or moderate-dose hydroxyurea.11 The protocol is available in the appendix (Supplementary Appendix, p. 1–82).
We conducted the clinical trial at three sites in northern Nigeria with a catchment area of at least 40,000 children with sickle cell anaemia.12 Ethical approval for the study was obtained from the Institutional Review Boards of Aminu Kano Teaching Hospital, Kano, Nigeria, Ministry of Health, Kano, Murtala Mohammed Specialist Hospital, Kano, Nigeria, Barau Dikko Teaching Hospital, Kaduna, Nigeria, and Vanderbilt University Medical Center, Nashville, United States. The National Agency for Food and Drug Administration and Control in Nigeria approved the trial. A Data and Safety Monitoring Board (DSMB) appointed by the National Institute of Neurological Disorders and Stroke (NINDS) reviewed serious adverse events and study progress. The DSMB included one member from Nigeria. The neurology committee had no members from Nigeria (all were from the United States or the United Kingdom).
Inclusion criteria were 5 to 12 years of age, confirmed haemoglobin SS or haemoglobin Sβ0 thalassemia (referred to as sickle cell anaemia), and abnormal transcranial Doppler defined as time-averaged mean of the maximum velocity ≥200 cm/sec, measured by two separate transcranial Doppler certified ultra-sonographers or ≥220 cm/sec measured once. We repeated the TCD velocity typically on the day of the abnormal result because we wanted to shorten the time for treatment for children at risk for strokes, improve the convenience of enrollment for the family, and decrease the likelihood of a false-positive result due to the high coefficient of variation associated with TCD velocities in children with sickle cell anemia.13 All transcranial doppler measurements were completed in asymptomatic participants on the day of their baseline clinical visit.
Exclusion criteria were a prior overt stroke (a focal neurological deficit of acute onset) based on history, focal neurological deficit on standardised neurological examination, or concern for moderate or severe neurological deficit based on a positive “10 questions” screening,14 and haemoglobin less than 6 g/dL. No participants were prescribed hydroxyurea prior to the trial. All participant’s guardians provided written informed consent prior to screening and enrollment, and children age 7 and above provided their assent. The clinical features of the population screened for eligibility are described elsewhere.11,15
Randomisation and masking:
Eligible children with sickle cell anaemia and abnormal transcranial Doppler velocities were randomly assigned to receive either low- or moderate-dose once daily oral hydroxyurea. Randomisation allocation tables were constructed by study statisticians using a permuted block allocation scheme, based on block sizes of four, stratified by sex, within site. The tables were loaded into REDCap and used to randomize participants (1:1) by the unblinded study pharmacist after determining eligibility criteria. The participants went to the trial pharmacists who distributed the trial medication to the guardian. Allocation was concealed from all other study personnel except the statisticians because of the requirement to prepare interim reports for the DSMB. REDCap allows access to certain fields to be restricted.
Procedures
For participants, research visits with a history, physical examination, and laboratory values were obtained monthly. Transcranial doppler ultrasound was completed at trial entry, 3 months, 12 months, and upon exit from the trial. Transcranial Doppler measurement in the trial served as a biomarker for hydroxyurea therapy response. The dosing scheme was weight-dependent on 100 mg, 250 mg, and 500 mg hydroxyurea capsules. Hydroxyurea was supplied by Bond Chemical, Ibadan, Nigeria.
The planned doses of treatment were approximately 10 mg/kg/day or 20 mg/kg/day of oral hydroxyurea daily for a minimum of 3 years. Participants were to be withdrawn if unable to tolerate hydroxyurea therapy or myelosuppression was suspected based on the defined parameters and was unresponsive to maneuvers described below.
The site investigators, the principal investigators, research staff at each site, and trial pharmacists met weekly to discuss any laboratory or clinical assessment of hydroxyurea-related toxicity or clinical trial defined adverse events and serious adverse events. The two study monitors assessed the completed blood cell counts weekly and discussed the results on the weekly conference calls with the study site team investigators from all three sites and the leadership of the data coordinating center. If myelosuppression secondary to hydroxyurea had occurred, the medical monitors would have been unmasked and a lower dose of hydroxyurea provided. An additional second weekly meeting occurred between the study coordinators and the leadership of the data coordinating center to ensure appropriate follow-up for any laboratory concerns, serious adverse event monitoring that required hospitalisation, or serious adverse events including death, stroke, or transient ischemic attacks. Hemoglobin F level was done at baseline, then annually, and upon exit. Follow-up visits were requested to repeat any laboratory measurements outside of the expected range, which was based on prior laboratory results performed in the cohort; the expected ranges were updated twice in the trial to include the upper and lower expected limit. (Supplementary Appendix, Table S1 pages 83–87).
Outcomes:
The primary endpoint was the occurrence of an initial clinical stroke or transient ischaemic attack, based on the World Health Organisation definition.16 Pediatric National Institutes of Health Stroke Scale evaluations after a possible stroke were recorded with a video; the Neurology Committee reviewed the neurological examination video and case report forms of all suspected strokes and were blinded to group assignment.
The prespecified secondary outcome measure was hospitalisations for any cause. Events were centrally adjudicated without knowledge of treatment assignment. Each hospitalisation and unscheduled outpatient visit were adjudicated for the primary reason for admission in the following hierarchal and mutually exclusive categories: stroke, acute chest syndrome, pain, or fever (temperature ≥ 38°C), with each event assigned only one primary reason. The acute vaso-occlusive event was either pain or acute chest syndrome. Malaria was diagnosed as per the World Health Organisation and local study site clinical practice. Participants could have malaria with any of the primary reasons for admission.
The adjudication process for the primary cause of hospitalization includes the following algorithm:
If the patient presents with neurological deficits confirmed to be a stroke or transient ischemic attack and acute chest syndrome, pain, fever, or other symptoms, then the primary cause of the hospitalization is stroke/transient ischemic attack.
If the patient presents with acute chest syndrome, pain, fever, other symptoms, and no indication of neurological deficits for stroke or transient ischemic attack, then the primary cause of the hospitalization is acute chest syndrome.
If the patient presents with pain, fever, other symptoms, and no acute chest syndrome or indication of neurological deficits for stroke or transient ischemic attack, then the primary cause of the hospitalization is pain.
If the patient presents with fever and other symptoms, and no pain, acute chest syndrome, or indication of neurological deficits for stroke or transient ischemic attack, then the primary cause of the hospitalization is fever.
If the patient presents with other symptoms and no fever, pain, acute chest syndrome, or indication of neurological deficits for stroke or transient ischemic attack, then the primary cause of the hospitalization is other. The primary reason should be provided after adjudication, along with all applicable secondary reasons for hospitalization.
The adjudication process to determine the primary reason for admission was: 1) Local site investigator, pediatrician, hematologist, and study coordinator decided the primary and secondary reason; 2) Study contact principal investigator independently reviewed the reason for admission; 3) If the primary reason for admission was concordant between the study site team and contact principal investigator no further discussion occurred; 3) If discordant, the research teams from all three sites, plus the contact principal investigator determined the primary cause of hospitalization based on consensus (approximately 10 team members reviewed every discordant primary reason for admission).
For the duration of the study, participant hospitalizations were documented. When available, a de-identified source document was uploaded for review. The adjudication of all parties was documented via REDCap.
Definitions:
Pain requiring hospitalisation: a SCA-associated pain episode requiring admission to the hospital and treatment with opioids. Emergency department visits were not included in this definition due to the variation in outpatient pain management practices and to capture only acute severe pain events. Headaches treated with opioids were also not included in this definition due to the difference in the proposed pathophysiology of headaches as opposed to acute vaso-occlusive pain.
Fever: Temperature greater than or equal to 110.4°F or 38°C.
Acute chest syndrome: presence of at least 2 of the following criteria,17 including positive chest signs: temperature greater than 38°C, increased respiratory rate of greater than the 90th percentile for age,18 positive chest pain or pulmonary auscultatory findings, increased oxygen requirement (SpO2 drop ≥3% from a documented steady-state value on room air) 19, and a new radio-density on chest roentgenogram. A chest X-ray was done as available. A diagnosis of pneumonia will be considered an ACS episode. All cases of acute vaso-occlusive pain and ACS episodes were adjudicated with the site pediatrician, hematologists, and principal investigator on the research team to ensure a uniform definition.
Malaria: Based on World Health Organization and local study site guidelines, a fever (greater than or equal to 110.4°F or 38°C) and positive light microscopy, a rapid diagnostic test that can detect P. falciparum completed (depending on test availability).
A post-hoc secondary outcome of acute pain managed only at home was assessed without knowledge of the treatment assignment or clinical outcome. The diagnoses of acute pain managed only at home was based on whether the guardians provided a history during the monthly research visit of administering a non-steroidal anti-inflammatory drug (ibuprofen, diclofenac) alone or with both paracetamol and codeine for SCA-related pain excluding headache. Neither the guardian providing the history, nor the research coordinators, were aware that the interim medical history of pain medication administered in the prior 30 days would be used to determine an acute pain event at home.
Participants received monthly laboratory monitoring to assess for myelosuppression throughout the trial. Myelosuppression possibly related to hydroxyurea was defined as an absolute neutrophil count <1000 × 109/L or a platelet count <80 × 109/L. All interim laboratory values were reviewed weekly via teleconference conference with the trial leadership, medical monitors, and lead research coordinators from each site. Reticulocyte assessment was not done because of inadequate quality control measures. Adherence was measured with mean cell volume, hydroxyurea pill counts returned to the pharmacists monthly, and haemoglobin F levels assessed annually.
The research team and the institutional Nigerian Ethics Committee did not believe an untreated (placebo) group of children with abnormal TCD velocities was ethical because of this group’s established high risk of strokes. Upon initiation of the trial, limited data from controlled clinical trials were available to assess the safety of hydroxyurea, particularly regarding potentially increasing the incidence of severe adverse events in children living in Africa. The Data Safety Monitoring Board recommended a comparison group of children with sickle cell anaemia that completed study screening procedures, had normal or conditional transcranial Doppler velocities as per STOP criteria,4 were not treated with hydroxyurea, and agreed to be followed prospectively for at least 36 months (n=220). The vital status of the participants in the comparison group was assessed monthly via phone calls and at least one study visit per year. Participants in the comparison group could crossover to the therapy group if, during routine care, annual transcranial Doppler measurements were ≥200 cm/sec, and all other inclusion criteria were met.
Statistical analysis:
To test our primary hypothesis, our sample size was based in part on the results of our SPIN feasibility trial demonstrating a drop to less than abnormal velocities (<200 cm/sec) in approximately 66% (15 of 23) of participants after three months on moderate-dose hydroxyurea therapy.7 In the STOP trial, the incidence rate of stroke in the untreated and the monthly blood transfusion group of children with abnormal transcranial Doppler measurements was 10·7 and 0·97 events per 100 person-years, respectively.4 Two additional conservative assumptions were made. First, low-dose hydroxyurea would have some benefit, reducing the stroke incidence to below 10·7 events per 100 person-years in the untreated group in the STOP trial. Second, moderate-dose hydroxyurea would be beneficial, but would not be as effective as regular blood transfusion therapy arm in the STOP trial.11
We anticipated a recruitment window of 2 years, a minimum follow-up time of 3 years, and a 9% loss to follow-up in each group per year. Using Fisher’s exact test, a sample size of 220 (110 patients per arm) provided at least 90% power to detect a 66% relative risk reduction of the incidence rate of stroke for the moderate-dose arm, assuming 9 events per 100 patient-years for the low-dose arm versus 3 events per 100 patient-years for the moderate-dose arm, with two-sided type I error, and an alpha level of 0·05.
For the primary hypothesis and all outcomes, the intention-to-treat principle was used to compare incidence rates between the two treatment groups. We summarised descriptive statistics using median and interquartile range (IQR) for continuous data and percentages for categorical data. The comparison of baseline and exit characteristics between the low- and moderate-dose groups was performed using the Mann-Whitney test for continuous variables and Pearson’s chi-square test for categorical variables. Paired Wilcoxon rank-sum tests were performed to examine the change in laboratory values from baseline to 3 months and from baseline to 12 and 24 months. All tests used a 2-tailed probability, with P-values <0·05 considered statistically significant. Seven postulated stroke risk factors (age, sex, baseline haemoglobin,3 baseline haemoglobin F level, transcranial Doppler velocities ≥ 200 cm/sec at baseline and 3 months,20 and random assignment to fixed low- and moderate-dose hydroxyurea) were evaluated with Cox regression, with each model including group assignment and another risk factor. The small number of strokes precluded constructing a model with all risk factors. A recently introduced, but not validated, surrogate marker of hydroxyurea efficacy in Africa was introduced and assessed after the completion of the trial, defined as haemoglobin level ≥9·0 g/dl or a haemoglobin F level ≥20%.21 All analyses were conducted in SPSS version 26. The SPRING trial is registered on ClinicalTrials.gov number, NCT02560935.
Role of the funding source:
The sponsors did not have any role in the design and conduct of the study: collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Results
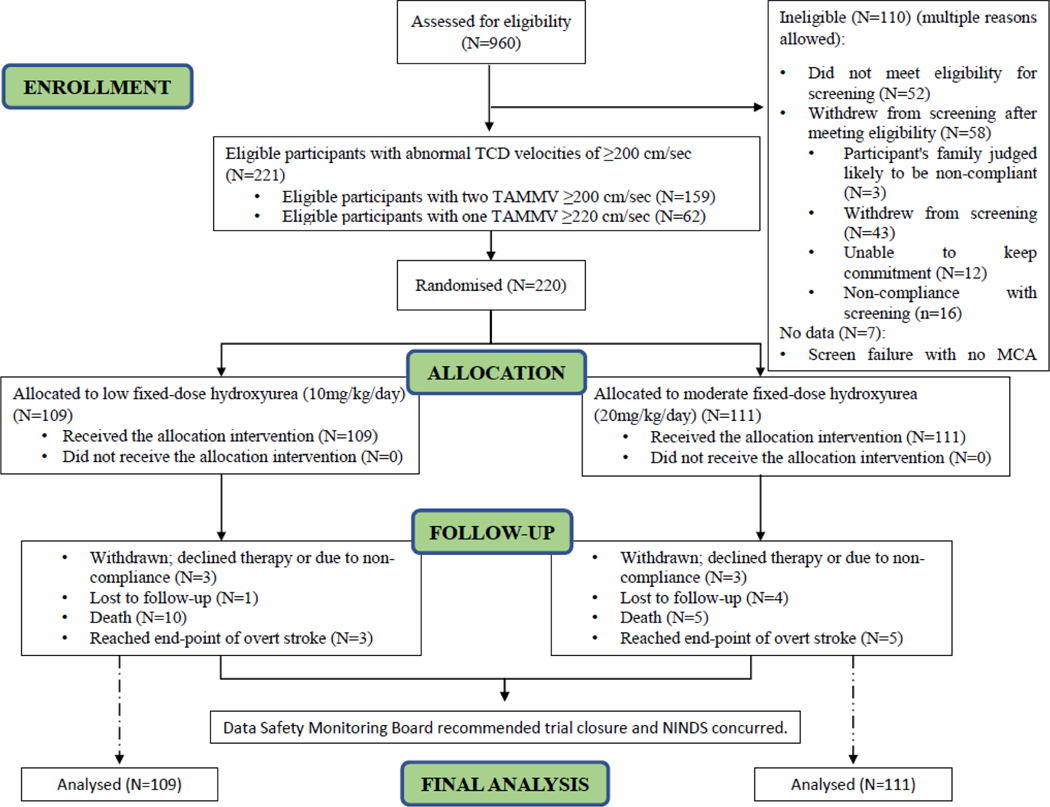
The first and last participants were enrolled on August 2, 2016, and June 14, 2018, respectively (for specific site enrollment, see Table S2, Supplementary Appendix, p. 88). Among 960 participants who were screened for eligibility in the trial, a total of 220 children were randomly assigned to low- (n=109) or moderate-dose (n=111) hydroxyurea, Table 1 (median age: 7·2 years, IQR 5·5–8·9), 114 (51·8%) females). Among the group of children with abnormal velocities, two ultrasonographers independently measured abnormal velocity measurements ≥200 cm/sec and < 200 cm/sec in 159 participants, and one ultrasonographer measured one abnormal velocity ≥220 cm/sec in 61 participants. The mean monthly visits in the two therapy groups were 27·1and 27·6 in the low- and moderate-dose groups, respectively. The median number of monthly visits was 29·0 in both groups (IQR 23·0–34·0). The median follow-up of the treatment group participants was 2·4 years (IQR: 2·0–2·8) because the trial was stopped early by the NINDS. During the trial, 2·7% (6/220) participants withdrew, 3 participants from each group, Figure 1.
Table 1.
Baseline characteristics of the SPRING participants (N=220) randomly allocated to fixed low and moderate hydroxyurea groups
| Characteristics* | Low-dose group (n = 109) | Moderate-dose group (n = 111) |
|---|---|---|
| Age at start of hydroxyurea, years | 7·4 (5·7 – 9·6) | 7·0 (5·5 – 8·4) |
| Female sex | 56/109 (51·4) | 58/111 (52·.3) |
| Ethnicity | ||
| Hausa | 89 (81.7) | 90 (81.1) |
| Fulani | 14 (12.8) | 11 (9.9) |
| Other | 6 (5.5) | 10 (9.0) |
| Height, cm | 115·3 (108·4 – 125·0) | 114·0 (106·0 – 121·8) |
| Weight, kg | 18·0 (15·5 – 20·7) | 17·0 (15·0 – 20·0) |
| BMI, kg/m2 | 13·5 (12·7 – 14·4) | 13·3 (12·5 – 14·3) |
| Transcranial Doppler velocity at screening, cm/sec | 207·0 (203·0 – 222·5) | 206·0 (203·0 – 221·0) |
| Total haemoglobin, g/dl | 7·0 (6·4 – 7·6) | 7·1 (6·6 – 7·7) |
| White blood cell count, 103/mm3 | 14·2 (11·4 – 18·8) | 14·3 (11·8 – 17·5) |
| Platelet count, 103/mm3 | 447·0 (345·5 – 522·0) | 414·0 (285·0 – 496·0) |
| Absolute neutrophil count, mm3 (n=219) | 6969·2 (5195·2 – 9076·6) | 6628·5 (5310·8 – 8382·0) |
| Systolic blood pressure, mm Hg | 100·0 (90·0 – 100·0) | 100·0 (90·0 – 100·0) |
| Mean cell volume, fl | 86·0 (81·8 – 90·0) | 86·2 (79·9 – 90·6) |
| Percent haemoglobin F, (n=219) | 8·5 (5·2 – 12·1) | 8·2 (6·1 – 12·3) |
| Percent haemoglobin S, (n=217) | 81·3 (76·7 – 84·8) | 82·6 (78·1 – 86·0) |
Median (inter-quartile range) for continuous variables and counts (percentages) for categorical variables
Figure 1.
Trial profile
The mean prescribed doses for the low- and moderate-dose groups were 10·8 mg/kg/day and 20·6 mg/kg/day, respectively. The prescribed minimum and maximum doses were 9·5 and 15·4 mg/kg/day in the low-dose hydroxyurea group. In the moderate-dose hydroxyurea group, the prescribed minimum and maximum doses were 19·3 and 23·9 mg/kg/day. A maximum of three capsules was provided for treatment.
The trial was one of the first hydroxyurea randomized controlled trials initiated in Africa. A comparison group was created to determine whether hydroxyurea therapy was associated with serious adverse events. A total of 220 children with sickle cell anaemia and transcranial Doppler measurements <200 cm/sec were enrolled in the comparison group. Nine crossed over because of elevated transcranial Doppler measurement ≥200 cm/sec to the therapy groups and were randomly assigned. The median follow-up of the participants in the comparison group was 2·8 years (IQR 1·5–3·1). In the comparison group, two participants developed strokes within 2 months after their last transcranial Doppler of 134 cm/sec and 157 cm/sec, respectively.
At a planned interim analysis in August 2019, no difference was found in stroke incidence rates between the two groups. Subsequently, a futility analysis was conducted using conditional power (the chance of finding a difference if the study was completed). Conditional power was 6·0%, under the pre-specified decision boundary of 30%. Based on these results, the NINDS Clinical Trials leadership stopped the trial because of futility on November 6, 2019. The last contact for all participants was March 12, 2020.
Eight children had strokes, three in the low-dose and five in the moderate-dose group. One child with an abnormal velocity ≥220 cm/sec had a stroke within two weeks after starting hydroxyurea therapy. Two children with a stroke presented with seizures and unilateral or bilateral weakness, Table 2. Six presented with hemiparesis, three of whom also had facial weakness; one also had a visual field defect, Table 2. The expected stroke event rate for the low- and moderate-dose group was 9 and 3 per 100 person-years; however, the actual stroke rates in the low- and moderate-dose hydroxyurea groups were 1·19 and 1·92 per 100 person-years, respectively, an incidence rate ratio of 0·62 (95% CI, 0·10–3·20; p=0·77). Strokes occurred at the following time points: 13% (1 of 8) at 6 months, 63% (5 of 8) at 12 months, and 88% (7 of 8) at 24 months after starting hydroxyurea therapy.
Table 2.
Characteristics of therapy group participants who experienced strokes during the trial.
| Patient No. | Time from Beginning Hydroxyurea to Endpoint (mo.) | Endpoint Classification and Description† | Age (yrs.) | Mean Dose Hydroxyurea (mg/kg/day) | Steady-State Hb* (g/dl) | Hb at Endpoint (g/dl) | MCV* at Baseline (fl) | MCV at Endpoint (fl) | TCD* at baseline (cm/sec) | TCD at 3-Months (cm/sec) |
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed Low-dose Group | ||||||||||
| 1 | 8.2 | Stroke: Slurred speech, right hemiparesis, face, and arm more than leg. PedNIHSS=5 | 9.1 | 11.2 | 6.7 | 6.7 | 76.7 | 90.1 | 309.0 | 291.0 |
| 2 | 12.2 | Stroke: Left hemiparesis, leg more than arm. PedNIHSS=3 | 10.0 | 9.8 | 7.1 | 7.0 | 91.7 | 92.8 | 293.0 | 232.0 |
| 3 | 10.0 | Stroke: Right face, arm, and leg weakness. PedNIHSS=4 | 7.4 | 9.8 | 8.3 | 7.5 | 90.0 | 96.0 | 231.0 | 218.0 |
| Fixed Moderate-dose Group | ||||||||||
| 4 | 0.3 | Stroke: Right hemiparesis, arm more than leg. PedNIHSS=3 | 6.2 | 22.6 | 7.1 | N/A | 96.2 | N/A | 204.0 | N/A |
| 5 | 10.4 | Stroke: Left hemiparesis, face and arm more than leg. Left visual field cut. PedNIHSS=5 | 8.3 | 20.4 | 6.7 | 8.6 | 85.1 | 108.6 | 243.0 | 247.0 |
| 6 | 8.0 | Stroke: Left hemiparesis, arm more than leg. PedNIHSS=2 | 12.4 | 19.9 | 6.0 | 7.8 | 83.4 | 118.4 | 202.0 | 143.0 |
| 7 | 14.8 | Stroke: Left-sided seizure, with persistent hemiparesis, face, and arm more than leg. PedNIHSS=7 | 5.1 | 23.5 | 8.1 | 8.4 | 88.8 | 119.7 | 232.0 | 254.0 |
| 8 | 30.7 | Stroke: Seizure, global aphasia, bilateral weakness, right more than left. Bilateral strokes in setting of e. coli sepsis. PedNIHSS=26 | 6.5 | 20.0 | 7.4 | 10.9 | 85.7 | 88.2 | 204.0 | 187.0 |
All endpoint evaluations with the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) occurred at 24 hours or more after symptom onset.
Risk factors associated with a stroke included transcranial Doppler velocities at baseline (hazard ratio=1·04; 95% CI, 1·02–1·06; p=0·00013), and at three months (hazard ratio=1·05; 95% CI, 1·03 – 1·08; p<0·0001), controlling for the treatment group (Table S3, Supplementary Appendix, p. 88). A higher proportion of strokes occurred when baseline transcranial velocities were ≥220 cm/sec, 8·5% of participants (5/59), compared with < 220 cm/sec,1·9% (3/161), p=0·034. After excluding the participant with a stroke before 3 months, similarly, a higher proportion of strokes occurred when the transcranial velocities were ≥ 220 cm/sec compared with < 220 cm/sec at 3 months, 50% (4/8) and 1·5% (3/205), respectively, p<0·0001 (Table S4, Supplementary Appendix, p. 89). The Pearson correlation between the baseline and threemonth velocities was 0·29 (p<0·0001).
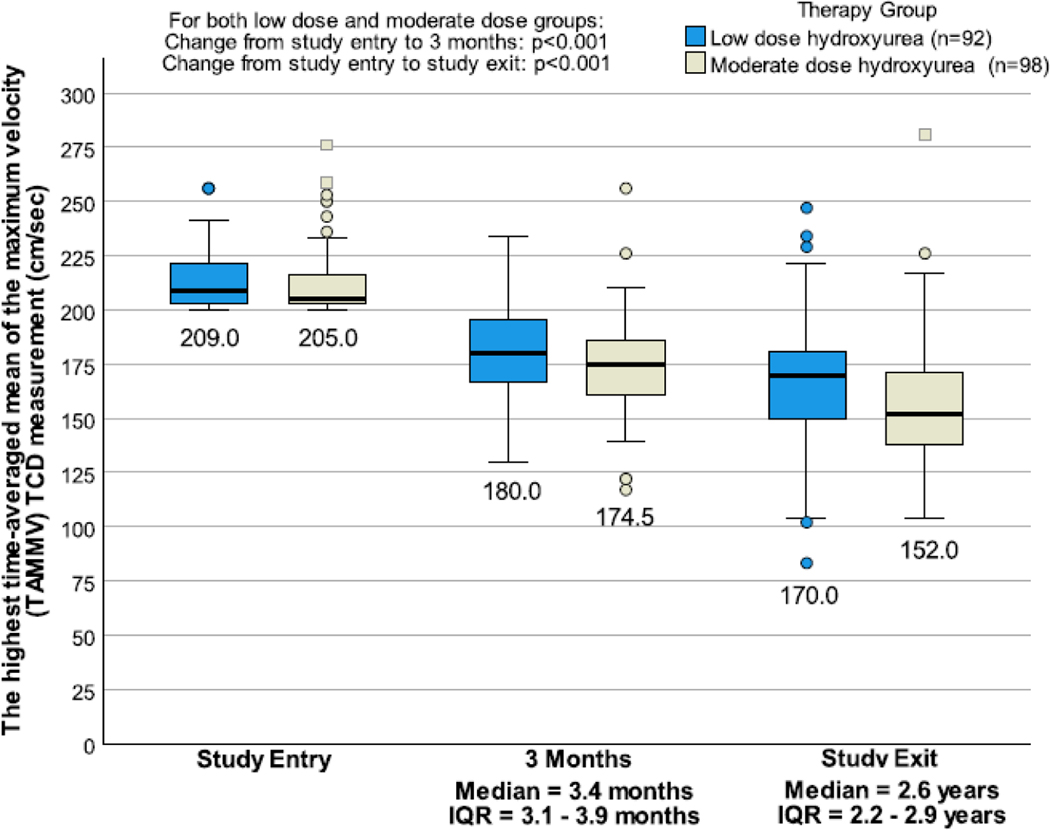
Transcranial Doppler velocities dropped to normal levels (<170 cm/sec) at three months in the low- and moderate-dose groups in 29·3% (27/92) and 39·8% (39/98) (p=0·13) and at exit in 48·9% (45/92) and 71·4% (70/98), respectively (p=0·0015), Figure 2. Upon trial exit, 29·4% (32/109) and 66·7% (74/111) of the low- and moderate-dose groups had either a haemoglobin level ≥9·0 g/dl or a haemoglobin F level ≥20%, respectively, which is a surrogate endpoint of hydroxyurea efficacy, with no difference in the incidence rate of strokes (p=0·26). Additional laboratory values demonstrating the anticipated hydroxyurea response from baseline to exit are shown in Figure S1, Supplementary Appendix, p. 90. As a measure of adherence, returned pills during the trial were 5·4% and 4·8% in the low- and moderate-dose groups, respectively, p=0·14. Mean increase in mean cell volume from baseline to exit increased by 1·5 fl and 7·2 fl in the low- and moderate-dose groups, respectively, p<0·0001. The median hemoglobin F level increased by 1·9% (IQR 0·0–5·2%) and 10·0% (IQR 3·4–13·8) from baseline to exit in the low-and moderate-dose groups, respectively.
Figure 2.
Boxplots of transcranial Doppler measurements at baseline, 3 months, and at exit, by therapy group
Death occurred in both the low-dose and moderate-dose groups, but not with a greater incidence rate than in the comparison group. No deaths were treatment-related. A total of 10 deaths (3·98 per 100 person-years), 5 deaths (1·92 per 100 person-years), and 9 deaths (1·81 per 100 person-years) occurred in the low-dose, moderate-dose, and the comparison group, respectively. One child in the moderate-dose group died within a week of a stroke. The mortality incidence rate ratio when comparing the children treated with low- and moderate-dose hydroxyurea to the non-abnormal transcranial Doppler group (no hydroxyurea therapy, n= 211) was 2·20 (95% CI, 0·80–6·10) and 1·06 (95% CI, 0·28 – 3·51); p=0·14 and 0·998, respectively (Table S5, Supplementary Appendix, p. 89).
The incidence rates of all-cause hospitalisation and hospitalisation for an acute vaso-occlusive event (acute pain or acute chest syndrome) were higher in the low-dose than in the moderate-dose group, incidence rate ratios 1·71 (95% CI, 1·15 – 2·57; p=0·0071) and 1·88 (95% CI, 1·14– 3·16; p=0·012) respectively, Table 3.
Table 3.
Primary and secondary outcomes for SPRING Trial participants randomly allocated to fixed low- (N=109) and fixed moderate-dose (N=111) hydroxyurea groups.
| Outcome | Number of events | Incidence rate§ | Incidence rate ratio# (95% CI) | P value* |
|---|---|---|---|---|
|
| ||||
| Primary outcome | ||||
| Ischaemic strokes | 0·62 (0·10 – 3·20) | 0·768 | ||
| Low-dose group | 3 | 1·19 | ||
| Moderate-dose group | 5 | 1·92 | ||
| Death outcome | ||||
| Death during trial | 2·08 (0·65 – 7·74) | 0·185 | ||
| Low-dose group | 10 | 3·98 | ||
| Moderate-dose group | 5 | 1·92 | ||
| Secondary outcome - hospitalisation | ||||
| Hospitalisation for any reason | 1·71 (1·15 – 2·57) | 0·0071 | ||
| Low-dose group | 69 | 27·43 | ||
| Moderate-dose group | 42 | 16·08 | ||
| Vaso-occlusive event † | 1·88 (1·14 – 3·16) | 0·012 | ||
| Low-dose group | 47 | 18·69 | ||
| Moderate-dose group | 26 | 9·96 | ||
| Acute chest syndrome | 4·15 (0·83 – 40·14) | 0·097 | ||
| Low-dose group | 8 | 3·18 | ||
| Moderate-dose group | 2 | 0·77 | ||
| Acute vaso-occlusive pain | 1·69 (0·99 – 2·93) | 0·055 | ||
| Low-dose group | 39 | 15·51 | ||
| Moderate-dose group | 24 | 9·19 | ||
| Malaria | 1·62 (0·97 – 2·72) | 0·065 | ||
| Low-dose group | 42 | 19·89 | ||
| Moderate-dose group | 27 | 16·08 | ||
| Osteomyelitis | 4·15 (0·41 – 204·50) | 0·352 | ||
| Low-dose group | 4 | 1·59 | ||
| Moderate-dose group | 1 | 0·38 | ||
| Secondary outcome - hospitalisation or outpatient visit | ||||
| Vaso-occlusive event | 1·98 (1·35 – 2·93) | <0·001 | ||
| Low-dose group | 82 | 32·60 | ||
| Moderate-dose group | 43 | 16·47 | ||
| Acute chest syndrome | 5·19 (1·11 – 48·73) | 0·033 | ||
| Low-dose group | 10 | 3·98 | ||
| Moderate-dose group | 2 | 0·77 | ||
| Vaso-occlusive pain | 1·82 (1·23 – 2·74) | 0·0022 | ||
| Low-dose group | 72 | 28·63 | ||
| Moderate-dose group | 41 | 15·70 | ||
| Malaria | 1·24 (0·80 – 1·93) | 0·360 | ||
| Low-dose group | 49 | 19·48 | ||
| Moderate-dose group | 41 | 15·70 | ||
| Transfusion | 1·52 (0·71 – 3·34) | 0·322 | ||
| Low-dose group | 19 | 7·55 | ||
| Moderate-dose group | 13 | 4·98 | ||
| Secondary outcome - home medication administered at least once in 30 days | ||||
| Vaso-occlusive pain – medication at home | 1·33 (1·11 – 1·59) | 0·0023 | ||
| Low-dose group | 289 | 114·90 | ||
| Moderate-dose group | 226 | 86·54 | ||
| Malaria – medication at home | 1·24 (1·00 – 1·55) | 0·049 | ||
| Low-dose group | 188 | 74·78 | ||
| Moderate-dose group | 157 | 60·12 | ||
Incidence rate ratio reference group is moderate hydroxyurea, the denominator of the incidence rate ratio.
Fisher’s exact test
Vaso-occlusive events include both acute chest syndrome and severe acute pain events that required hospitalisation. §Cumulative person-years in the low-dose and moderate dose groups were 251.52 and 261.14, respectively. Incidence rate per 100 person-years.
Most acute pain episodes occurred at home and not in a hospital or an outpatient visit with a health care provider. An acute pain episode at home occurred in 66·9% (73 of 109) and 72.9% (81 of 111) of the low- and moderate-dose groups, respectively. The incidence of acute vaso-occlusive pain at home for those in the low- and moderate-dose hydroxyurea group was 114·9 and 86·5 person-years, with an incidence rate ratio of 1·33 (95% CI, 1·11–1·59; p=0·0023), Table 3. Health care provider treatment of acute vaso-occlusive pain (inpatient or outpatient setting) was higher in the low-dose when compared to the moderate-dose group, the incidence rate ratio of 1·82 (95% CI, 1·23–2·74; p=0·0022), Table 3. No participant in either group had hydroxyurea stopped for myelosuppression or drug-related toxicity, and no participant required dose reductions. The baseline and exit hematology laboratory values per treatment group are displayed Table S6, Supplementary Appendix, p. 89.
Discussion
The SPRING trial is the first randomised controlled trial demonstrating the benefits of initial hydroxyurea therapy for primary stroke prevention with no associated increase in hydroxyurea-related toxicities or death. Compared to low-dose hydroxyurea therapy, moderate-dose had no difference in the stroke incidence rate. In both hydroxyurea treatment groups, the stroke incidence rate was comparable to the STOP trial’s initial monthly transfusion arm of approximately 1 stroke per 100 person-years.4 However, moderate-dose hydroxyurea was associated with lower incidence rates of all-cause hospitalizations, vaso-occlusive events requiring hospitalizations, and home management of acute vaso-occlusive pain events.
A significant strength of the SPRING Trial was the initial focus on the sustainability of the stroke prevention teams after completion of the trial. The main obstacles to sustainability were the household poverty level of most children attending the clinic coupled with a predominantly self-pay health system. In Nigeria, approximately 53% of families, 82.5 million live below the international poverty line of approximately US $1.90 per day.22 Thus, many families cannot afford the cost of a complete blood cell count for hydroxyurea myeloid suppression surveillance with a cost of ~US $3.00 in Kano, Nigeria,
To address the sustainability of the trial results after completion, we identified a Nigerian pharmaceutical company that produces hydroxyurea at a subsidized cost (Bond Chemical, Ibadan, Nigeria) of $0.16 per 500 mg capsule. Additionally, we 1) implemented task-shifting from physicians to nurses for continued free of charge TCD screening; 2) provided free of charge hydroxyurea for children with abnormal TCD velocities or pre-existing strokes; and 3) created state-supported stroke prevention teams at the teaching hospitals.23 Together, these strategies ensured a seamless transition between completing the trial and implementing state-government-supported primary and secondary stroke prevention programs in the three Nigerian states.12
The precise biological mechanism of hydroxyurea’s beneficial effects for preventing ischaemic strokes in children with abnormal transcranial Doppler velocities remains unclear. Plausible factors contributing to hydroxyurea’s effect in primary stroke prevention are multifactorial. They include increasing haemoglobin and haemoglobin F levels,24 haemoglobin oxygen saturation percentage,25 and nitric oxide via cGMP and cAMP levels,26,27 while simultaneously decreasing white blood cell count, red blood cell count, and reticulocyte counts, and reducing red blood cell adhesion to the endothelium.24
In a systematic review of the literature, before 28 and after cessation of the SPRING Trial,29 there has been no randomised controlled trial demonstrating the benefit or harm of initial hydroxyurea treatment for abnormal transcranial Doppler measurements in either a low-middle income or high-income setting. However, the team had significant evidence that a moderate-dose of hydroxyurea was effective in reducing the transcranial Doppler, based on the single-arm SPIN Trial using moderate-dose hydroxyurea and pooled analysis of 7 studies before the trial indicating hydroxyurea therapy decreases transcranial Doppler velocities, with a mean decrease in velocities of 25 cm/second.28 Together, these data were sufficient to initiate a randomised control trial for primary stroke prevention with initial low- and moderate-dose hydroxyurea.
Before the trial, the Nigerian trial pediatricians were at equipoise as to whether low- or moderate-dose hydroxyurea should be used for primary stroke prevention. Collectively the pediatricians were unwilling to include a maximum tolerated dose of hydroxyurea arm in the trial. The basis for their strong opinion was preliminary data from the SPIN trial demonstrating the benefit of moderate-dose hydroxyurea for primary stroke prevention,7 and their concern about the complex medical care and relatively expensive laboratory monitoring required to titrate children to the maximum tolerated dose in a setting where nurses were providing the majority of clinical care without a pediatrician. After the SPRING Trial was initiated, the results of the SCD hydroxyurea trial conducted in Angola, Uganda, and the Democratic Republic of Congo in children with sickle cell anaemia were published.30 In this trial, the mean maximum tolerated hydroxyurea dose was 22·5 mg/kg/day, which was not clinically different from the mean moderate hydroxyurea dose in the SPRING Trial, 20·6 mg/kg/day, and the fixed-dose of 20 mg/kg/day in the SPIN Trial. In the completed hydroxyurea trial of 600 children with sickle anaemia, 5·1% of the participants had hematology laboratory dose-limiting toxic effects30 compared to no participants in the SPRING Trial or SPIN Trial. Also, after the SPRING trial was completed, Lagunju et al. described the results of the largest and most extensive experience with initial hydroxyurea therapy for primary stroke prevention in children with abnormal transcranial Doppler velocities.31 In this observational study conducted in Ibadan, Nigeria, 396 children with HbSS and abnormal transcranial velocities were treated with a maximum tolerated dose of hydroxyurea. Two strokes occurred in this cohort with a stroke incidence of 0·08 per 100 patient-years. Equally important, in this observational study, the mean hydroxyurea dose was 23·7 mg/kg/day; however, no myelosuppression occurred resulting in the cessation of hydroxyurea therapy.
Three key trial outcomes will change clinical practice and decrease the gap in primary stroke prevention in Nigeria and elsewhere in Africa. First, the absence of myelosuppression in either treatment group is important. At the SPRING trial sites, complete blood cell counts for children on hydroxyurea have been reduced from monthly to biannually, per the new local standard. This change in monitoring dramatically reduces costs and burdens for families.
The second change in regional practice is the initial and 3-month transcranial Doppler velocities as biomarkers demonstrating hydroxyurea response. Both Doppler velocities were independently associated with future strokes. In the extended STOP trial, a threshold of ≥220 cm/sec conferred high-risk for subsequent strokes.20 All strokes (6 of 6) that occurred in an extended evaluation of STOP participants were in participants with initial velocities ≥220 cm/sec.20 Thus, if the initial transcranial Doppler velocity is ≥220 cm/sec, stroke risk is high, and at least moderate rather than low-dose hydroxyurea should be considered. At a minimum, the transcranial Doppler study should be repeated in 3 months. If the 3-month velocity remains ≥220 cm/sec, an evaluation should be considered for adherence, increasing the hydroxyurea dose from low to moderate or moderate to the maximum tolerated dose, or both. Furthermore, the statistical relationship between strokes and the magnitude of the TCD velocity confirms the importance of the TCD velocity as a risk factor for overt stroke in the Nigerian population, as in the STOP Trial.
The third change in regional practice is Nigerian pediatricians that participated in the trial now take an acute vaso-occlusive pain history specifically focused on acute pain at home and pain resulting in a subsequent health care visit before and after starting hydroxyurea for primary stroke prevention. If there is a high incidence rate of vaso-occlusive events (acute pain or acute chest syndrome), shared decision-making between the guardian and the health care provider regarding the preferred hydroxyurea dose should be initiated.
A perceived major weakness of the trial is the absence of brain MRI to confirm a stroke, as was the case in the STOP Trial. However, in the STOP Trial, the presence of a stroke was based on a clinical diagnosis of all 11 participants after stroke adjudication, which an MRI of the brain confirmed.4 Further, the World Health Organisation’s definition of a stroke does not include neuroimaging, an acceptable clinical endpoint in the American Society of Hematology Guidelines. 29 Further, a clinical diagnosis of stroke as the primary outcome measure has been applied in a NIH-sponsored sickle cell anaemia randomized controlled stroke prevention trial conducted in high-income countries.32 Lastly, every month, participants were evaluated inperson with a standardised questionnaire to detect neurological impairment in children living in Africa,14 coupled with a standardised neurological examination to detect strokes. A video of the neurological examination was centrally adjudicated for the presence of a stroke for any child with concern for stroke with a detailed recording of the neurological examination, Table 2. Together, these strategies decreased the likelihood of an undetected and untreated stroke being missed in between the 30-day schedule research visits. Another limitation was the absence of magnetic resonance angiography to detect cerebral vasculopathy, an exclusion criterion in one primary stroke prevention trial based in the United States33 that was not applied in this trial. However, only approximately 20% of children with sickle cell anemia and abnormal velocities have magnetic resonance angiography-defined severe vasculopathy.34
An inherent limitation in any clinical trial, including ours, is the inability to address the durability of the treatment effect. To address the long-term efficacy of low-dose hydroxyurea therapy for primary stroke prevention, we have initiated a stroke registry (ClinicalTrials.gov NCT04800809) to follow the neurological outcome of all the participants now that the SPRING trial has been completed.
Primary stroke prevention with initial low-dose hydroxyurea therapy and biannual complete blood cell count is a new evidence-based strategy for children with sickle cell anemia living in Africa. For those children at risk for strokes with increased rates of all-cause hospitalizations, vaso-occlusive pain events at home or requiring a physician visit, fixed moderate-dose hydroxyurea therapy and biannual complete blood counts should be considered after an informed discussion with the parents.
Supplementary Material
Research in context:
Primary stroke prevention in children with sickle cell anaemia is effective when coupled with transcranial Doppler screening and initial blood transfusion therapy for children with abnormal velocities. In Africa, where regular blood transfusion is not feasible for most children, we tested the hypothesis that moderate-dose compared to low-dose hydroxyurea decreases the incidence of strokes for children with abnormal transcranial Doppler velocities. The trial’s results have clinical implications for hundreds of thousands of children living in Africa with sickle cell anaemia.
Evidence before this study:
In a systematic review of the literature, prior to, and after cessation of the SPRING Trial, we found no randomised controlled trial demonstrating the benefit or harm of initial hydroxyurea treatment for abnormal transcranial Doppler measurements in either a low-middle income or high-income setting. Our Nigerian feasibility single-arm trial that began in 2013 (SPIN trial, clinical trials.gov:NCT01801423), coupled with pooled analysis prior to the start of the trial, demonstrated hydroxyurea lowered transcranial Doppler velocities and prevented strokes in a high-risk population. We searched PubMed for articles published before January 1, 2014, for the medical subject heading terms “sickle cell”, “stroke”, “brain infarction”, and “primary prevention”, and we searched titles and abstracts for the terms “stroke”, “prevention”, “sickle cell anemia”, “sickle cell anaemia”, “sickle cell disorders”, “sickle cell disease”, “sickle cell disorder”, and “sickle cell diseases”. We also searched ClinicalTrials.gov and reference lists in published articles. We refined references because of quality and relevance (qualifying as a scoping rather than a systematic literature review). We completed the same search strategy after completion of the trial on April 1, 2021.
Added value of this study
The trial demonstrated for the first time that in children with sickle cell anaemia with abnormal transcranial Doppler velocities, fixed-low dose (10mg/kg/day) and moderate dose hydroxyurea (20mg/kg/day) have equivalent stroke incidence rates. Further, no laboratory monitoring is required for assessment of myelosuppression. The study also demonstrated for the first time that in Africa, an increase in hydroxyurea dosage from low to moderate dose decreases the rate of in-hospital vaso-occlusive episodes and acute pain events at home.
Implications of all the available evidence
The results of this trial have changed clinical practice in three states in Nigeria, with an estimated 40,000 children with sickle cell anaemia at risk for strokes. As standard care, these children now undergo transcranial Doppler assessments, and if abnormal, the state provides hydroxyurea free-of-charge at an initial fixed low-dose of 10mg/kg/day. If prior or future acute vaso-occlusive episodes (pain or acute chest syndrome) are frequent, shared decision-making between the guardians and the health care provider may lead to an increase in hydroxyurea dose to 20mg/kg/day.
Acknowledgments:
We are posthumously thankful to our certified radiologist Shehi Ali, MBBS, who tirelessly worked to certify physicians and nurses to conduct the transcranial Doppler assessments in Nigeria. Without his effort, this trial would not have been completed. We are grateful to our research coordinators and study personnel who tirelessly coordinated the trial in Kano, Nigeria and Kaduna, Nigeria. We are grateful to our research coordinators and study personnel who tirelessly coordinated the trial in Kano, Nigeria and Kaduna, Nigeria. We appreciate Bilya Sani Musa, Mustafa Nateqi, Khadijah Bulama, Murtala Umar, Charity Dooshima Agba-Dooyum, Fahad Usman, Abdulrasheed Sani, Awwal Gambo, Jamila Ibrahim, Gloria Bahago, Jamil Galadanci, Leshana Saint Jean, and Jennifer C. Beck for facilitating administrative tasks required for the successful conduct of this study.
Funding:
Research reported in this publication was supported by the National Institutes of Health Grant #1R01NS094041, K24HL147017, NCT 03380351, NIH/NCATS UL1 TR000445, 1K43TW011583, and the generous donation of the Phillips family. The sponsors did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the National Institute of Health.
Conflict of Interests:
Dr. Kirkham received royalties from the MacKeith Press as a result of her contributions to the Cerebrovascular Disease and Stroke in Children book. Dr. Kirkham received consulting fees from Global Blood Therapeutics for TCD training for HOPE Kids and KOPE Kids 2. Dr. Kirkham received a payment from Johnson & Johnson for a presentation in October 2019, and from Bluebird Bio for a presentation in December 2019. Dr. Kirkham received payment for a speaker’s bureau in November 2019. Dr. Kirkham received payment for an institution discretionary for medicolegal. Dr. Kirkham received support for a European Paediatric Neurology meeting in Athens, in September 2019, from BIAL. Dr. Kirkham was part of a group who received a DWL transcranial Doppler from Global Blood Therapeutics for training purposes in HOPE Kids 2.
Dr. Trevathan served as a member of the NINDS/NIH advisory council.
Dr. DeBaun and his institution are the sponsor of two externally funded research investigator-initiated projects. Global Blood Therapeutics (GBT) will provide funding for the cost of the clinical studies. GBT will not be a co-sponsor of either study. Dr. DeBaun is not receiving any compensation for the conduct of these two-investigator initiated observational studies. Dr. DeBaun is a member of the Global Blood Therapeutics advisory board for a proposed randomized controlled trial for which he receives compensation. Dr. DeBaun is the steering committee for a Novartis-sponsored phase II trial to prevent priapism in men. Dr. DeBaun was a medical advisor for the development of the CTX001 Early Economic Model. Dr. DeBaun provided medical input on the economic model as part of an expert reference group for Vertex/CRISPR CTX001 Early Economic Model in 2020. Dr. DeBaun provided a one-time consultation to the Formal Pharmaceutical company about sickle cell disease in 2021.
Footnotes
Ethics Approval and Consent to Participate: The study that generated the data was IRB approved by Vanderbilt University Medical Center and the local sites in Nigeria, and all participants provided informed consent.
Data Sharing: Fully anonymised data may be shared upon request from any qualified investigator with approved study and data transfer agreement between Vanderbilt University Medical Center and the Institution.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdullahi SU, DeBaun MR, Jordan LC, Rodeghier M, Galadanci NA. Stroke Recurrence in Nigerian Children With Sickle Cell Disease: Evidence for a Secondary Stroke Prevention Trial. Pediatr Neurol 2019; 95: 73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkaran B, Char G, Morris JS, Thomas PW, Serjeant BE, Serjeant GR. Stroke in a cohort of patients with homozygous sickle cell disease. J Pediatr 1992; 120(3): 360–6. [DOI] [PubMed] [Google Scholar]
- 3.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular Accidents in Sickle Cell Disease: Rates and Risk Factors. Blood 1998; 91(1): 288–94. [PubMed] [Google Scholar]
- 4.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 1998; 339(1): 5–11. [DOI] [PubMed] [Google Scholar]
- 5.Lagunju IA, Brown BJ, Sodeinde OO. Chronic blood transfusion for primary and secondary stroke prevention in Nigerian children with sickle cell disease: a 5-year appraisal. Pediatr Blood Cancer 2013; 60(12): 1940–5. [DOI] [PubMed] [Google Scholar]
- 6.Assembly WH. Sickle-cell anaemia: report by the Secretariat. Geneva, Switzerland: World Health Organization; 2006. www.who.int/iris/handle/10665/20890. Accessed 7 June 2021. [Google Scholar]
- 7.Galadanci NA, Abdullahi SU, Ali Abubakar S, et al. Moderate fixed-dose hydroxyurea for primary prevention of strokes in Nigerian children with sickle cell disease: Final results of the SPIN trial. Am J Hematol 2020; 95(9): E247–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel DK, Mashon RS, Patel S, Das BS, Purohit P, Bishwal SC. Low dose hydroxyurea is effective in reducing the incidence of painful crisis and frequency of blood transfusion in sickle cell anemia patients from eastern India. Hemoglobin 2012; 36(5): 409–20. [DOI] [PubMed] [Google Scholar]
- 9.Svarch E, Machín S, Nieves RM, Mancia de Reyes AG, Navarrete M, Rodríguez H. Hydroxyurea treatment in children with sickle cell anemia in Central America and the Caribbean countries. Pediatr Blood Cancer 2006; 47(1): 111–2. [DOI] [PubMed] [Google Scholar]
- 10.Jain DL, Apte M, Colah R, et al. Efficacy of fixed low dose hydroxyurea in Indian children with sickle cell anemia: a single centre experience. Indian Pediatr 2013; 50(10): 929–33. [DOI] [PubMed] [Google Scholar]
- 11.Abdullahi SU, Wudil BJ, Bello-Manga H, et al. Primary prevention of stroke in children with sickle cell anemia in sub-Saharan Africa: rationale and design of phase III randomized clinical trial. Pediatr Hematol Oncol 2021; 38(1): 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galadanci AA, Galadanci NA, Jibir BW, et al. Approximately 40 000 children with sickle cell anemia require screening with TCD and treating with hydroxyurea for stroke prevention in three states in northern Nigeria. Am J Hematol 2019; 94(11): E305–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galadanci NA, Umar Abdullahi S, Vance LD, et al. Feasibility trial for primary stroke prevention in children with sickle cell anemia in Nigeria (SPIN trial). Am J Hematol 2017; 92(8): 780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mung’ala-Odera V, Meehan R, Njuguna P, et al. Validity and reliability of the ‘Ten Questions’ questionnaire for detecting moderate to severe neurological impairment in children aged 6–9 years in rural Kenya. Neuroepidemiology 2004; 23(1–2): 67–72. [DOI] [PubMed] [Google Scholar]
- 15.Bello-Manga H, Galadanci AA, Abdullahi S, et al. Low educational level of head of household, as a proxy for poverty, is associated with severe anaemia among children with sickle cell disease living in a low-resource setting: evidence from the SPRING trial. Br J Haematol 2020; 190(6): 939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ 1980; 58(1): 113–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Oppong SA, Asare EV, Olayemi E, et al. Multidisciplinary care results in similar maternal and perinatal mortality rates for women with and without SCD in a low-resource setting. Am J Hematol 2019; 94(2): 223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 2011; 377(9770): 1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rackoff WR, Kunkel N, Silber JH, Asakura T, Ohene-Frempong K. Pulse oximetry and factors associated with hemoglobin oxygen desaturation in children with sickle cell disease. Blood 1993; 81(12): 3422–7. [PubMed] [Google Scholar]
- 20.Lee MT, Piomelli S, Granger S, et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood 2006; 108(3): 847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John CC, Opoka RO, Latham TS, et al. Hydroxyurea Dose Escalation for Sickle Cell Anemia in Sub-Saharan Africa. N Engl J Med 2020; 382(26): 2524–33. [DOI] [PubMed] [Google Scholar]
- 22.Sattar S. Poverty & Equity Brief Sub-Saharan Africa Nigeria April 2020. World Bank Group; 2020: https://databank.worldbank.org/data/download/poverty/33EF03BB-9722-4AE2-ABC7-AA2972D68AFE/Global_POVEQ_NGA.pdf. [Google Scholar]
- 23.Ghafuri DL, Abdullahi SU, Dambatta AH, et al. Establishing Sickle Cell Disease Stroke Prevention Teams in Africa is Feasible: Program Evaluation Using the RE-AIM Framework. J Pediatr Hematol Oncol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010; 115(26): 5300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pashankar FD, Manwani D, Lee MT, Green NS. Hydroxyurea Improves Oxygen Saturation in Children With Sickle Cell Disease. J Pediatr Hematol Oncol 2015; 37(3): 242–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nahavandi M, Tavakkoli F, Wyche MQ, Perlin E, Winter WP, Castro O. Nitric oxide and cyclic GMP levels in sickle cell patients receiving hydroxyurea. Br J Haematol 2002; 119(3): 855–7. [DOI] [PubMed] [Google Scholar]
- 27.Cokic VP, Beleslin-Cokic BB, Tomic M, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea induces the eNOS-cGMP pathway in endothelial cells. Blood 2006; 108(1): 184–91. [DOI] [PubMed] [Google Scholar]
- 28.DeBaun MR, Kirkham FJ. Central nervous system complications and management in sickle cell disease. Blood 2016; 127(7): 829–38. [DOI] [PubMed] [Google Scholar]
- 29.DeBaun MR, Jordan LC, King AA, et al. American Society of Hematology 2020 guidelines for sickle cell disease: prevention, diagnosis, and treatment of cerebrovascular disease in children and adults. Blood Advances 2020; 4(8): 1554–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tshilolo L, Tomlinson G, Williams TN, et al. Hydroxyurea for Children with Sickle Cell Anemia in SubSaharan Africa. N Engl J Med 2019; 380(2): 121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagunju IA, Labaeka A, Ibeh JN, Orimadegun AE, Brown BJ, Sodeinde OO. Transcranial Doppler screening in Nigerian children with sickle cell disease: A 10-year longitudinal study on the SPPIBA cohort. Pediatr Blood Cancer 2021; 68(4): e28906. [DOI] [PubMed] [Google Scholar]
- 32.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med 2014; 371(8): 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet 2016; 387(10019): 661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abboud MR, Cure J, Granger S, et al. Magnetic resonance angiography in children with sickle cell disease and abnormal transcranial Doppler ultrasonography findings enrolled in the STOP study. Blood 2004; 103(7): 2822–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.