Abstract
Introduction
Twin pregnancies have significantly higher rates of perinatal morbidity and mortality compared to singleton pregnancies; current attempts to reduce perinatal mortality have been less successful in twin pregnancies. The paucity of information about modifiable risk factors for adverse neonatal outcomes in twin pregnancies, as well as independent effects of chorionicity may have contributed to this outcome. This study aimed to explore the feasibility of an observational study to identify modifiable factors associated with adverse neonatal outcomes in twin pregnancies.
Material and methods
Patients pregnant with twins at six UK hospitals between December 2019–March 2021 completed researcher‐administered questionnaires at approximately 20‐, 28‐ and 36‐weeks' gestation, recording a wide range of self‐reported social, lifestyle and demographic factors, alongside prospectively recorded clinical data from maternity records. Descriptive statistics were used to describe frequencies of exposures; logistic regression was used to determine whether factors were associated with a composite measure of adverse neonatal outcome.
Results
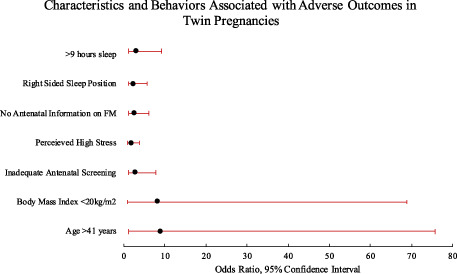
Data were collected from 65% (181/277) of eligible participants. A total of 98% (175) of participants had positive views about their participation. Some exposures, including cigarette smoking, supine sleep position and reduced fetal movements were less frequent in twin pregnancies compared to singletons, whereas fertility treatment was more common. Furthermore, different patterns of exposure were seen between monochorionic and dichorionic twins. This pilot study found some associations with adverse neonatal outcomes including: low BMI (OR 8.36, 95% CI: 1.02–68.87), maternal age ≥41 years (OR 9.0 95% CI: 1.07–75.84), maternally perceived high‐stress levels (OR 1.96, 95% CI: 1.03–3.75) and inadequate antenatal screening (OR 1.44, 95% CI: 1.01–2.06). Sleep duration ≥9 h and right‐sided going to sleep position were more frequent among pregnancies with adverse outcomes. Participants who reported receiving no information on fetal movement and reduced maternal perception of movements were more likely to have an adverse outcome, but sample size prohibited analysis based upon chorionicity.
Conclusions
An observational study of modifiable factors in twin pregnancy is feasible. Differences in the frequencies of exposures between twin and singleton pregnancies highlight the need for twin‐specific studies to identify modifiable factors and develop preventative strategies for morbidity and mortality in twin pregnancies.
Keywords: feasibility study, modifiable antenatal risk factors, neonatal morbidity and mortality, twin pregnancy
There is value in further interrogation of modifiable risk factors for neonatal adverse outcomes in twin pregnancies, developing health promotional messages, national guidance and information available for patients pregnant with twins, to mitigate the increased risk of adverse outcomes.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- GDM
gestational diabetes mellitus
- OR
odds ratio
Key message.
These data suggest value in further interrogation of modifiable risk factors for neonatal adverse outcomes in twin pregnancies, developing health promotional messages, national guidance and information available for patients pregnant with twins, to mitigate the increased risk of adverse outcome.
1. INTRODUCTION
In the UK, perinatal mortality is higher in twin pregnancies compared to singletons; there is a 3‐fold increase in stillbirth, an 8‐fold increase in neonatal death and 40% increase in preterm birth. 1 , 2 , 3 , 4 While perinatal deaths have decreased in singleton pregnancies there has been a 19% increase in stillbirth and 16% increase in neonatal death in twin pregnancies between 2016 and 2020. 5 It has been suggested that the absence of twin‐specific preventative measures in national guidance, service reviews and commissioning documents may act as a barrier to reducing perinatal mortality in twin pregnancies. 2 , 6 In addition, twins are more complex than singleton pregnancies; specifically, placental chorionicity increases perinatal mortality and morbidity in monochorionic twins, secondary to conjoining of the fetal circulations within the placenta by vascular anastomoses. 1 , 5 , 7 The recently published Confidential Enquiry into Perinatal Mortality in twin pregnancies highlighted “inadequate” care as a contributory factor in 54% of cases. 1 , 8 In addition, the evidence‐base underpinning clinical recommendations for care and/or advice in twin pregnancies may be extrapolated from research in singleton pregnancies; the validity of this generalization has been criticized. 2 , 6
Individual studies, and subsequent meta‐analysis in singletons have identified modifiable maternal characteristics, behaviors and exposures linked to late stillbirth (eg supine going‐to‐sleep position, reduced sleep duration, cigarette smoking, maternal perception of reduced fetal movements increased risk and appropriate antenatal care was protective). 9 , 10 , 11 , 12 , 13 It is unclear to what extent these findings can be extrapolated to patients with twin pregnancies. It could be that for twin pregnancies important associations may be overlooked or that associations in singletons are not transferable, ultimately meaning patients are given misleading or potentially harmful advice. The current paucity of a twin‐specific evidence‐base for modifiable risk factors for adverse neonatal outcomes, stratified by chorionicity, may reduce the impact of current attempts to prevent neonatal morbidity and mortality in twin pregnancies, and the quality of information available for patients pregnant with twins.
A reduction in the rate of neonatal morbidity and mortality in twin pregnancies is desirable due to the significant associated adverse medical, psychological and social‐economic impacts of perinatal death and related adverse neonatal outcomes. 14 , 15 , 16 The investigation of modifiable factors for stillbirth is considered a top priority for stillbirth research. 17 This supports the need for an exploratory investigation of the modifiable risk factors for perinatal morbidity and mortality in twin pregnancies. This study aimed to determine the acceptability of questionnaire study completed at several points during pregnancy to identify risk factors for adverse fetal outcomes in twin pregnancies and to identify factors associated with adverse pregnancy outcome in women with twin pregnancies which can be explored in a hypothesis‐testing study.
2. MATERIAL AND METHODS
A hypothesis‐generating study, evaluating and exploring antenatal risk factors for adverse neonatal outcomes in twin pregnancies was performed. The study aimed to explore the acceptability and feasibility of an observational study recording demographic, environmental and lifestyle factors at several time points in pregnancy. The secondary outcome was to generate hypotheses regarding potentially modifiable risk factors for neonatal adverse outcomes in twin pregnancies to be tested in a definitive study by providing information about frequency of exposures and identifying potential associations (within both monochorionic and dichorionic twin samples). The results of a definitive study could be used to develop and test clinical preventative measures for adverse neonatal outcome in twin pregnancies.
This study was conducted in six UK maternity units. Participants were recruited between December 2019 and March 2021; recruitment was temporarily paused for three months due to the COVID pandemic. Patients with singleton pregnancies, fetal congenital anomalies, maternal age less than 16 years, or who were unable to give consent were excluded from the study. Causes for stillbirth or neonatal mortality were classified using the Cause of Intrauterine and Neonatal Death in Twin Pregnancies (CoDiT) classification system; 18 any babies found to have lethal congenital abnormalities on post‐mortem examination were excluded from the final analysis. Eligible participants were given a description of the study following confirmation of twin pregnancy.
Data were collected by a researcher‐administered questionnaire, based on that used in the Midland and North of England Stillbirth Study (MiNESS) 9 (Table S1), with specific adaptations for twin pregnancies following consultation with the patient and public involvement group at the Maternal and Fetal Health Research Centre, University of Manchester. Participants completed questionnaires on paper; responses were transcribed onto an electronic database; no transcription errors were found in the 10% of records checked for accuracy. The questionnaire focused on a wide range of self‐reported social and demographic characteristics and behaviors, comprising 82 questions. Research midwives gained consent to access information from participants' maternity records; for example, body mass index (BMI), blood pressure, the number of antenatal appointments conducted, ultrasound information and pregnancy outcome data. This study adopted a cohort design. Participants were followed prospectively. Questionnaires were completed at approximately 20‐, 28‐ and 36‐weeks' gestation.
Adverse neonatal outcome was defined a priori to be present when one or both twins experienced any of the following: stillbirth, neonatal death, birth before recommended by UK national guidelines (monochorionic monoamniotic twins <32 weeks' gestation, monochorionic diamniotic twins <36 weeks' gestation and dichorionic diamniotic twins <37 weeks' gestation 8 ), Apgar scores <7 at 5 min (associated with perinatal morbidity 19 , 20 ), admission to the neonatal unit after 37 weeks' gestation 8 or hypoxic ischemic encephalopathy.
2.1. Statistical analyses
Descriptive statistics were used to present the frequency of exposures, as per the Statistical Analysis Plan. 21 Univariable analyses was carried out using logistic regression and Chi‐squared test, to estimate the relation of each variable to adverse neonatal outcomes (as described above). BMI and age were analyzed as both continuous (linear) and categorical variables. In addition to odds ratios and 95% (CIs), we reported a pseudo‐R‐squared value (McFadden's 22 ) for each variable, to explore the potential value of each for inclusion in a (future) prediction model for adverse outcomes in twin pregnancies. A p‐value of <0.05 was considered statistically significant. Analyses were performed using STATA (version 14, STATACORP). Two independent assessors (IG/AH) reviewed participants' feedback about their involvement with the study. Agreement between the two investigators was determined by the Kappa test using Microsoft Excel.
2.1.1. Ethics statement
Ethical approval (19/NW/0522) and research approval (IRAS 264625) were gained on October 15, 2019. Informed consent was obtained from all research participants and all participants were given information on their right to withdrawal from participation at any time.
3. RESULTS
3.1. Feasibility outcomes
During the recruitment period, 277 eligible participants were identified. Recruitment was adversely affected by national recruitment restrictions imposed at the beginning of the Covid‐19 pandemic; otherwise between eight and 22 participants were recruited per month (Figure 1). A total of 181 participants (65%) of eligible participants were recruited: 54 monochorionic pregnancies (29.8%) and 127 (70.2%) dichorionic pregnancies, with at least one antenatal questionnaire and outcome data completed. Seventy‐eight eligible participants (28%) declined involvement and 10 (4%) withdrew consent, primarily citing time constraints; none of the participants whose babies died withdrew consent for existing data to be used. Eight (4%) participants were excluded as outcome data was not recorded: considered “lost to follow‐up” (Figure 2). No cases of neonatal mortality were due to undiagnosed congenital abnormalities. All participants completed the first questionnaire, 160 (87.9%) completed the second and 79 (43.4%) the third questionnaires. The median gestational age in the respective questionnaires was 20+3, 28+6, and 36+0 weeks' gestation. The demographic characteristics of participants are presented in Table 1.
FIGURE 1.
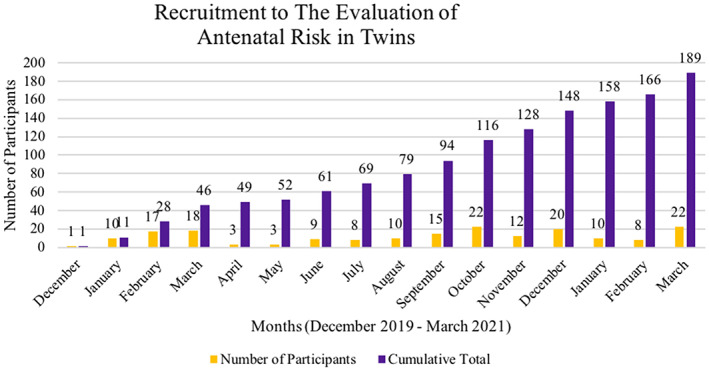
Recruitment to the evaluation of antenatal risk in twins. The recruitment levels fluctuated throughout the recruitment period. The total cumulative number of participants is show in green, and the monthly recruitment figure in blue. The red box indicates the first Covid‐19 lock down in March 2020, followed by the subsequent restrictions influencing recruitment, associated with a drop in recruitment numbers/rate over this time period.
FIGURE 2.
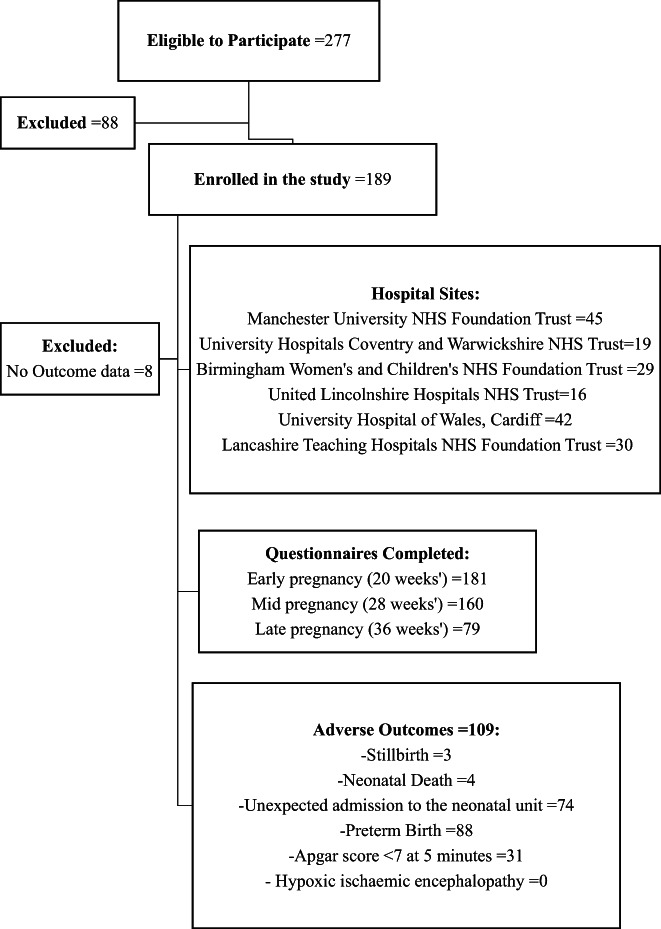
Participant flow diagram to illustrate the breakdown of recruitment, attrition and sample demographics within the study sample population. Adverse outcomes displayed are not mutually exclusive, see Table 2 for overlap. An admission to the neonatal unit after 37 weeks' gestation was considered “unexpected”. Also, preterm birth was defined as birth before recommended by NICE* guidelines (eg monochorionic monoamniotic 32 weeks' gestation, monochorionic diamniotic 36 weeks' gestation and dichorionic diamniotic 37 weeks' gestation) (NICE: National Institute for Healthcare excellence).
TABLE 1.
Demographic characteristics, social deprivation and smoking status in 181 participants with twin pregnancies.
| Characteristic | Total n = 181 (%) | Normal outcome n = 72 (%) | Adverse outcome n = 109 (%) | Odds ratio (95% confidence interval) | Monochorionic n = 54 (%) | Dichorionic n = 127 (%) |
|---|---|---|---|---|---|---|
| Maternal age | Pseudo R 2 0.0008 | p = 0.08 | ||||
| Median (range) | 32 (27–36) | 27 (23.5–32.3) | 32 (27–36) | 0.99 (0.94–1.04) | 31 (18–43) | 27 (18–50) |
| <20 years | 4 (2.2) | 0 (0) | 4 (3.7) | Incalculable | 2 (3.7) | 2 (1.6) |
| 21–25 years | 25 (13.8) | 9 (12.5) | 16 (14.7) | 1.78 (0.67–4.69) | 12 (22.2) | 13 (10.2) |
| 26–30 years | 47 (26.0) | 16 (22.2) | 31 (28.4) | 1.94 (0.87–4.31) | 9 (16.7) | 38 (29.9) |
| 31–35 years | 56 (30.9) | 28 (38.9) | 28 (25.7) | Reference | 19 (35.2) | 37 (29.1) |
| 36–40 years | 37 (20.4) | 17 (23.6) | 20 (18.4) | 1.18 (0.51–2.70) | 8 (14.8) | 29 (22.8) |
| >41 years | 10 (5.5) | 1 (1.4) | 9 (8.3) | 9 (1.07–75.84) | 2 (3.7) | 8 (6.3) |
| Missing data | 2 (1.1) | 2 (3.7) | 0 (0) | |||
| Maternal body mass index | Pseudo R 2 0.002 | p = 0.04 | ||||
| BMI Mean ± standard deviation | 27.9 ± 6.4 | 31.9 ± 5.0 | 27.7 ± 6.8 | 0.98 ± 0.94–1.03 | 26.7 ± 6.3 | 28.5 ± 6.4 |
| <20 kg/m2 | 13 (7.2) | 1 (1.4) | 12 (11.0) | 8.36 (1.02–68.87) | 7 (13.0) | 6 (4.7) |
| 20.1–25 kg/m2 | 56 (30.9) | 23 (31.9) | 33 (30.3) | Reference | 19 (35.2) | 37 (29.1) |
| 25.1–30 kg/m2 | 55 (30.4) | 22 (30.6) | 33 (30.3) | 1.05 (0.49–2.23) | 14 (25.9) | 41 (32.3) |
| 30.1–35 kg/m2 | 29 (16.0) | 16 (22.2) | 13 (11.9) | 0.57 (0.23–1.40) | 8 (14.8) | 21 (16.5) |
| >35.1 kg/m2 | 26 (14.4) | 9 (12.5) | 17 (15.6) | 1.32 (0.50–3.46) | 6 (11.1) | 20 (15.7) |
| Missing data | 2 (1.1) | 0 (0) | 2 (1.6) | |||
| Maternal ethnicity | Pseudo R 2 0.02 | p = 0.19 | ||||
| White | 136 (75.1) | 56 (77.8) | 80 (73.4) | Reference | 44 (81.5) | 92 (72.4) |
| Black/Black British | 22 (12.2) | 10 (13.9) | 12 (11.0) | 0.84 (0.34–2.08) | 3 (5.6) | 19 (15.0) |
| Asian/Asian British | 11 (6.1) | 3 (4.2) | 8 (7.3) | 1.87 (0.47–7.35) | 4 (7.4) | 7 (5.5) |
| Any other Multiple ethnic background | 9 (5.0) | 1 (1.4) | 8 (7.3) | 5.6 (0.68–46.04) | 3 (5.6) | 6 (4.7) |
| Missing data | 3 (1.7) | 0 (0) | 3 (2.4) | |||
| Parity | Pseudo R 2 0.03 | p = 0.17 | ||||
| 0 | 85 (47.0) | 47 (65.3) | 38 (34.9) | Reference | 25 (46.3) | 60 (47.2) |
| 1 | 56 (30.9) | 22 (30.6) | 34 (31.2) | 0.80 (0.40–1.61) | 17 (31.5) | 39 (30.7) |
| 2 | 24 (13.3) | 15 (20.8) | 9 (8.3) | 0.31 (0.12–0.80) | 8 (14.8) | 16 (12.6) |
| 3 | 9 (5.0) | 3 (4.2) | 6 (5.5) | 1.04 (0.24–4.44) | 2 (3.7) | 7 (5.5) |
| >4 | 7 (3.9) | 3 (4.2) | 4 (3.7) | 0.69 (0.14–3.29) | 2 (3.7) | 5 (3.9) |
| Parous | 96 (53.0) | 43 (59.7) | 53 (48.6) | 0.64 (0.35–1.17) | 29 (53.7) | 67 (52.8) |
| Marital status | Pseudo R 2 0.001 | p = 0.89 | ||||
| Single | 30 (16.6) | 13 (18.1) | 17 (15.6) | 0.81 (0.35–1.89) | 10 (18.5) | 20 (15.7) |
| Married | 86 (47.5) | 33 (45.8) | 53 (48.6) | Reference | 22 (40.7) | 64 (50.4) |
| Cohabiting | 64 (35.4) | 26 (36.1) | 38 (34.86) | 0.91 (0.47–1.76) | 21 (38.9) | 43 (33.9) |
| Missing data | 1 (0.6) | 1 (1.9) | 0 (0) | |||
| Quintiles of Deprivation Index | Pseudo R 2 0.03 | p = 0.15 | ||||
| 1 (most deprived) | 42 (23.2) | 12 (16.7) | 30 (27.5) | 2.24 (0.88–5.70) | 14 (25.9) | 28 (22.0) |
| 2 | 31 (17.1) | 9 (12.5) | 22 (20.2) | 2.19 (0.79–6.03) | 7 (13.0) | 24 (18.9) |
| 3 | 20 (11.0) | 8 (11.1) | 12 (11.0) | 2.34 (0.44–4.07) | 6 (11.1) | 14 (11.0) |
| 4 | 37 (20.4) | 19 (26.4) | 18 (16.5) | 0.85 (0.34–2.12) | 9 (16.7) | 28 (22.0) |
| 5 (least deprived) | 36 (19.9) | 17 (23.6) | 19 (7.4) | Reference | 14 (25.9) | 22 (17.3) |
| Missing data | 15 (8.3) | 4 (7.4) | 11 (8.7) | |||
| Smoking status (early pregnancy) | Pseudo R 2 0.02 | p = 0.23 | ||||
| Current smoker | 11 (6.1) | 4 (5.6) | 7 (6.4) | 1.28 (0.35–4.61) | 4 (7.4) | 7 (5.5) |
| Stopped in pregnancy | 17 (9.4) | 3 (4.2) | 14 (12.8) | 3.41 (0.93–12.53) | 6 (11.1) | 11 (8.7) |
| Stopped prior to pregnancy | 36 (19.9) | 15 (20.8) | 21 (19.3) | 1.02 (0.48–2.19) | 11 (20.4) | 25 (19.7) |
| Never smoked | 116 (64.1) | 49 (68.1) | 67 (61.5) | Reference | 33 (61.1) | 83 (65.4) |
| Missing data | 1 (0.6) | 0 (0) | 1 (0.8) | |||
| Have you changed your smoking habits in pregnancy? (early pregnancy) | Pseudo R 2 0.02 | p = 0.27 | ||||
| Yes | 23 (12.7) | 6 (8.3) | 17 (15.6) | 1.89 (0.60–5.97) | 6 (11.1) | 17 (13.4) |
| No | 35 (19.3) | 14 (19.4) | 21 (19.3) | Reference | 12 (22.2) | 23 (18.1) |
| Missing data | 7 (3.9) | 36 (66.7) | 87 (68.5) | |||
| Was this pregnancy achieved by the use of assisted reproductive technology? | Pseudo R 2 0.0067 | p = 0.20 | ||||
| Yes | 36 (19.9) | 11 (15.3) | 25 (22.9) | 1.65 (0.76–3.61) | 6 (11.1) | 30 (23.6) |
| No | 145 (80.1) | 61 (84.7) | 84 (77.1) | Reference | 48 (88.9) | 97 (76.4) |
Note: Data are presented as total values and percentages, also presented according to chorionicity. Proportions are expressed for those with complete data and known outcomes. All data presented is taken from the first questionnaire in early pregnancy (20 weeks) (n = 181).
Bold text indicates statistically significant results.
No participants expressed negative opinions or feedback about participation in the study, and 97.2% (176/178) of received comments were positive (Figure 3): comprising mostly generic positive remarks (72/178, 40%), followed by a desire to help others (61/178, 34%) and specific‐twin pregnancy comments (25/178, 14%). Less frequently, research motives (9/178, 5%), supporting future research or pregnancies (7/178, 4%), emotional reasons for participation (5/178, 3%) and indifferent (4/178, 2%) (Table S2). The Kappa value was 0.78, which suggests substantial agreement. 23 , 24
FIGURE 3.
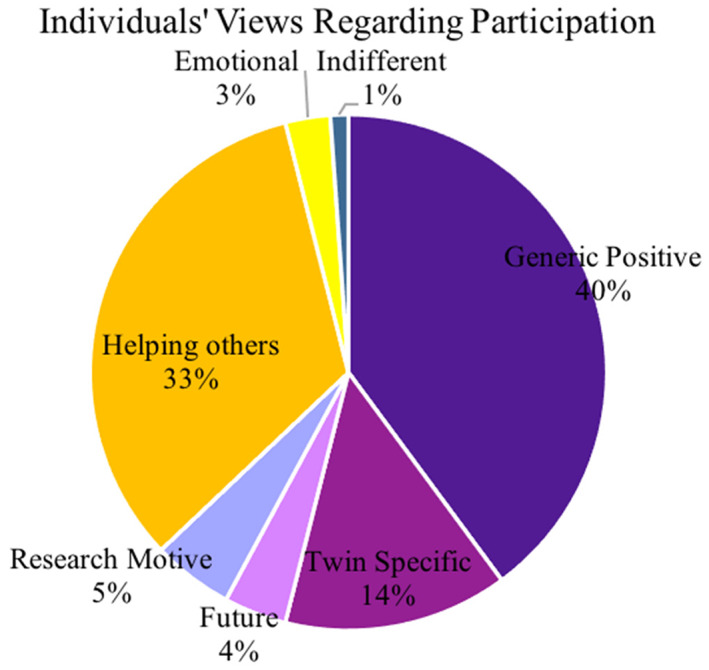
Individuals' views regarding participation. A pie chart to illustrate the frequency of different types of comments made by participants recruited into the study to explain their motivation for participating. Using directed content analysis, main groups were “Positive” “Negative” and “Indifferent”. A further analysis described maternal motives for research participation into subthemes: “Generic positive” “Twin specific” (wanting to improve resources for twin pregnancies), “Future research/pregnancies” (wanting to support research and improve future evidence‐base for pregnancy), “Research” (previous involvement or connection to research), “Help others” (desire to improve resources for other people), “Emotional” (previous loss motivation) and “Indifferent”.
3.2. Adverse outcomes
The frequency of adverse outcomes in this study was 60.2% (109/181 pregnancies) (Table 2). The most common adverse outcome was preterm birth (48.6% 88/181), followed by unexpected admission to the neonatal unit (40.9%, 74/181), and Apgar score <7 at 5 min (17.1%, 31/181). Stillbirth and neonatal death made up the two smallest groups of adverse outcomes (1.7% 3/181 and 2.2% 4/181, respectively). No pregnancies resulted in hypoxic ischemic encephalopathy. In this sample, dichorionic twins, compared to monochorionic twins had a higher frequency of preterm birth (53.5% 68/127 vs 44.4% 24/54) and a lower frequency of neonatal unit admission (36.2% 46/127 vs 50.0% 27/54). No monochorionic pregnancies resulted in stillbirth (of either/both twins), whereas 2.4% (3/127) of dichorionic pregnancies did.
TABLE 2.
Adverse outcomes experienced by participants and their frequency.
| Characteristic | Total n = 181 (%) | Monochorionic n = 54 (%) | Dichorionic n = 127 (%) |
|---|---|---|---|
| Total pregnancies | 109 (60.2) | 33 (30.3) | 75 (59.1) |
| Preterm birth | 88 (48.6) | 24 (44.4) | 68 (53.5) |
| Unexpected admission to the neonatal unit | 74 (40.9) | 27 (50.0) | 46 (36.2) |
| Apgar <7 at 5 min | 31 (17.1) | 6 (11.1) | 25 (19.7) |
| Neonatal death | 4 (2.2) | 1 (2.0) | 2 (1.6) |
| Stillbirth | 3 (1.7) | 0 (0) | 3 (2.4) |
| Hypoxic ischemic encephalopathy | 0 (0) | 0 (0) | 0 (0) |
Note: Data are presented as total values and percentages, also presented according to chorionicity. Adverse outcomes displayed are not mutually exclusive. An admission to the neonatal unit after 37 weeks' gestation was considered “unexpected”. Also, preterm birth was defined as birth before recommended by NICE guidelines (eg monochorionic monoamniotic 32 weeks' gestation, monochorionic diamniotic 36 weeks' gestation and dichorionic diamniotic 37 weeks' gestation).
In one monochorionic pregnancy, two neonatal deaths occurred due to prematurity (25+3 weeks' gestation). In four dichorionic pregnancies, three stillbirths and two neonatal deaths occurred: in one pregnancy mortality was classified as extreme prematurity which accounted for one stillbirth and one neonatal death (21+2 weeks' gestation), in another pregnancy, fetal death in utero of one twin occurred due to unknown causes at 24 weeks' gestation (the pregnancy continued to term with a live second twin). Lastly, in two separate pregnancies, a stillbirth and neonatal death occurred due to infection.
3.3. Antenatal care
The majority of participants received fewer than the recommended number of antenatal appointments for their stage of pregnancy (51.9%, 94/181), compared to those with exactly the recommended number (8.3%, 15/181) and more than recommended (26.5%, 48/181) (Table 3). Participants who had an adverse outcome were less likely to have received the recommended number of appointments (4.6% 5/109 vs 13.9% 10/72). Participants who had more than the recommended number of antenatal appointments also had a trend towards increased risk of adverse outcomes, compared to normal (odds ratio [OR] 3.33; 95% confidence interval [CI]: 0.98–11.31). A lower proportion of monochorionic pregnancies received the recommended number of antenatal appointments (3.7%, 2/54) compared to dichorionic pregnancies (10.2%, 13/127).
TABLE 3.
The total number of antenatal appointments participants received.
| Total n = 181 (%) | Normal outcome n = 72 (%) | Adverse outcome n = 109 (%) | Odds ratio (95% confidence interval) | Monochorionic n = 54 (%) | Dichorionic n = 127 (%) | |
|---|---|---|---|---|---|---|
| Antenatal appointments | Pseudo R 2 0.02 | p = 0.14 | ||||
| Less than recommended | 94 (51.9) | 42 (58.3) | 52 (47.7) | 2.48 (0.79–7.80) | 36 (66.7) | 58 (45.7) |
| Exactly recommended | 15 (8.3) | 10 (13.9) | 5 (4.6) | Reference | 2 (3.7) | 13 (10.2) |
| More than recommended | 48 (26.5) | 18 (25) | 30 (27.5) | 3.33 (0.98–11.31) | 8 (14.8) | 40 (31.5) |
| Missing data | 24 (13.3) | 8 (14.8) | 16 (12.6) | |||
Note: Data are presented as total values and percentages, also presented according to chorionicity. Antenatal appointment number was categorized into those participants who received less than the recommended number, exactly the recommended number and more than the recommended number of appointments. This information was collected by local data collectors at each questionnaire point, this information was then adjusted for by the gestation at which participants delivered and chorionicity.
Only one participant was diagnosed with gestational diabetes mellitus (GDM). Participants at risk of GDM appeared no more likely to experience an adverse outcome. However, those with risk factors who did not undergo GDM screening were more likely to have an adverse outcome (OR 12.5, 95% CI: 3.99–39.20) (Table 4).
TABLE 4.
The identification of gestational diabetes mellitus (GDM), screening and past medical history of participants.
| Characteristic | Total n = 181 (%) | Normal outcome n = 72 (%) | Adverse outcome n = 109 (%) | Odds ratio (95% confidence interval) | Monochorionic n = 54 (%) | Dichorionic n = 127 (%) |
|---|---|---|---|---|---|---|
| GDM | Pseudo R 2 0.00 | p = Incalculable | ||||
| GDM diagnosed | 1 (0.6) | 0 (0) | 1 (0.9) | Incalculable | 0 (0) | 1 (0.8) |
| GDM not diagnosed | 180 (99.4) | 72 (100.0) | 108 (99.1) | Reference | 54 (100.0) | 126 (99.2) |
| Participants with GDM risk factors | Pseudo R 2 0.003 | p = 0.39 | ||||
| Risk factors | 71 (39.2) | 31 (43.1) | 40 (36.7) | 0.77 (0.42–1.41) | 21 (38.9) | 50 (39.4) |
| No risk factors | 110 (60.8) | 41 (56.9) | 69 (63.3) | Reference | 33 (61.1) | 77 (60.6) |
| Participants with GDM risk factors, who had screening | Pseudo R 2 0.10 | p < 0.001 | ||||
| Screened | 35 (19.3) | 25 (34.7) | 10 (9.2) | 0.24 (0.10–0.54) | 7 (13.0) | 28 (22.0) |
| Unscreened | 36 (19.9) | 6 (8.3) | 30 (27.5) | 3.0 (1.14–7.74) | 14 (25.9) | 22 (17.3) |
| No risk factors (does not need screening) | 110 (60.8) | 41 (56.9) | 69 (63.3) | Reference | 33 (61.1) | 77 (60.6) |
| Past medical history | Pseudo R 2 0.005 | p = 0.55 | ||||
| No previous health issues | 112 (61.9) | 46 (63.9) | 66 (60.6) | Reference | 33 (61.1) | 79 (62.2) |
| Physical health history | 45 (24.9) | 18 (25.0) | 27 (24.8) | 1.05 (0.52–2.12) | 15 (27.8) | 30 (23.6) |
| Mental health history | 18 (9.9) | 5 (6.9) | 13 (11.9) | 1.81 (0.60–5.43) | 5 (9.3) | 13 (10.2) |
| Missing data | 6 (3.3) | 1 (1.9) | 5 (3.9) | |||
Note: Data are presented as total values and percentages, also presented according to chorionicity. Risk factors for GDM were assessed using NICE guidance (ethnicity with a prevalence of diabetes, BMI ≥30 kg/m2, proteinuria, previous GDM or macrosomic baby ≥4.5 kg). Three categories of past medical history were created from the first questionnaire, by participants who answered yes to previous physical health or mental health issues, participants with no previous health issues were considered as the “no previous health issues” group. Participants were asked about their physical health history “anemia, asthma, cervical or uterine surgery, diabetes, epilepsy, congenital heart condition, rheumatic heart condition, hyper‐/hypotension, hyper‐/hypothyroid, inflammatory bowel syndrome, polycystic ovarian syndrome, renal disease, sickle cell anemia, systemic lupus, thalassemia, thrombophilia, renal infections, uterine abnormality, thrombosis, other medical issue” and mental health history “depression, psychiatric, other mental health issue”.
Bold text indicates statistically significant results.
3.4. Perceived social stress
Participants who reported higher levels of perceived social stress throughout pregnancy had an increased risk of adverse outcomes (OR 1.96, 95% CI: 1.03–3.75) (Table 5). Those with dichorionic pregnancies more frequently had high perceived stress scores, compared to monochorionic pregnancies (59.8% 76/127 vs 24.1% 13/54). Perceived stress scores reduced as pregnancy progressed; median 14 (early pregnancy) to 11 (late pregnancy).
TABLE 5.
Perceived social stress as an antenatal risk factor for adverse outcomes in participants.
| Characteristic | Total n = 181 (%) | Normal outcome n = 72 (%) | Adverse outcome n = 109 (%) | Odds ratio (95% confidence interval) | Monochorionic n = 54 (%) | Dichorionic n = 127 (%) |
|---|---|---|---|---|---|---|
| Early pregnancy | Median (IQR) 14 (8) | Pseudo R 2 0.006 | p = 0.24 | |||
| Low stress score | 101 (55.8) | 44 (61.1) | 57 (52.3) | Reference | 38 (72.2) | 63 (49.6) |
| High stress score | 80 (44.2) | 28 (38.9) | 52 (47.7) | 1.43 (0.78–2.62) | 16 (29.6) | 64 (50.4) |
| Mid‐pregnancy | Median (IQR) 14 (7) | Pseudo R 2 0.01 | p = 0.13 | |||
| Low stress score | 69 (38.1) | 42 (59.7) | 51 (46.8) | Reference | 33 (61.1) | 60 (47.2) |
| High stress score | 84 (46.4) | 30 (41.7) | 58 (53.2) | 1.59 (0.87–2.90) | 21 (38.9) | 67 (52.8) |
| Missing data | 28 (15.5) | |||||
| Late pregnancy | Median (IQR) 11 (7) | Pseudo R 2 0.18 | p = 0.82 | |||
| Low stress score | 58 (32.0) | 38 (52.8) | 20 (18.3) | Reference | 9 (16.7) | 49 (38.6) |
| High stress score | 16 (8.8) | 10 (13.9) | 6 (5.5) | 1.14 (0.36–3.59) | 3 (5.6) | 13 (10.2) |
| Missing data | 107 (59.1) | 42 (77.8) | 65 (51.2) | |||
| Overall social stress | Median 15 (IQR) (6) | Pseudo R 2 0.02 | p = 0.04 | |||
| Low stress score | 117 (64.6) | 53 (73.6) | 64 (58.7) | Reference | 41 (75.9) | 51 (40.2) |
| High stress score | 64 (35.4) | 19 (26.4) | 45 (41.3) | 1.96 (1.03–3.75) | 13 (24.1) | 76 (59.8) |
Note: Data are presented as total values and percentages, also presented according to chorionicity; the median is expressed with the interquartile range (IQR). Early pregnancy (20 weeks) (n = 181), mid‐pregnancy (28 weeks) (n = 160), late pregnancy (36 weeks) (n = 79) approximately. The perceived stress scale (a 10‐item questionnaire, a tool used to measure individual stress levels, questions have a score 0–4, values are added) was used to define perceived social stress, participants with values ≥15 were considered to have a high stress score at all gestations. The overall social stress score was assessed by adding available scores from each questionnaire and dividing by the number of questionnaires available (accounting for some participants not completing the last questionnaire).
3.5. Sleep position
There was a change in frequencies of reported sleep position as the pregnancy progressed (Figure 4); fewer participants fell asleep on their front (32.0% 58/181 before pregnancy vs 0% from mid‐pregnancy), and supine (10.6% 19/181 vs 0.6% at the same time points). Throughout all gestations, falling to sleep on the left side was the most commonly reported sleep position (51.0% n = 80 mid‐pregnancy); in late pregnancy, there was a trend of normal outcomes, compared to adverse outcomes with left‐sided going to sleep position (45.8% 33/72 vs 14.7% 16/109). The most commonly reported waking up position at the beginning of pregnancy was the right side (35.4%) which was associated with an increased risk of adverse outcomes (OR 2.50, 95% CI: 1.11–5.64). Waking up in variable sleep positions was associated with a lower risk of adverse outcome (OR 0.11, 95% CI: 0.01–0.92). Most participants reported changing position “lots of times” during the night (54.7% 99/181); in late pregnancy, participants who reported changing position “lots of times” had a higher frequency of normal outcomes, compared to adverse outcomes (47.2% 34/72 vs 11.9% 13/109). More participants reported receiving their advice on sleep habits from a midwife (12.2%) or the internet (9.4%), than from a friend or relative (3.9%) and a hospital doctor (3.3%), 117 (64.6%) did not answer.
FIGURE 4.
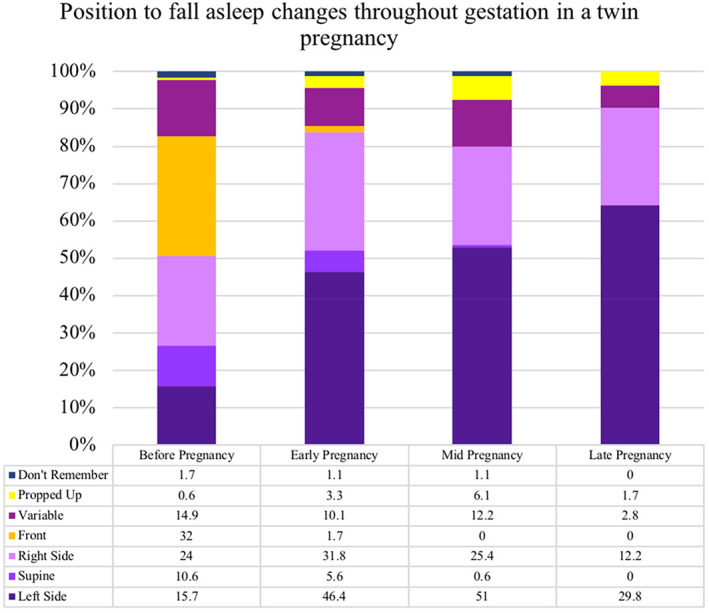
Position to fall asleep changes throughout gestation in a twin pregnancy. A bar chart to illustrate the respondents falling to sleep position at progressive gestational ages in twin pregnancies. Early pregnancy (20 weeks) (n = 181), mid‐pregnancy (28 weeks) (n = 160), late pregnancy (36 weeks) (n = 79) approximately. The frequency of some sleep positions (back, front) decreases at increasing gestation, whereas some positions (left, right) increase as pregnancy progresses.
3.6. Fetal movements
Participants who reported not receiving any information about fetal movements in early pregnancy (23.8%, 43/181) had an increased risk of adverse outcomes, compared to those who received verbal advice (OR 2.69, 95% CI: 1.18–6.10) (Table 6). Participants who reported reduced fetal movements at any point were not more likely to experience an adverse outcome. Participants who reported an increase in strength, frequency or vigorousness of fetal movements in mid‐pregnancy had a higher frequency of normal outcomes, compared to adverse outcomes (increased strength 73.6% 53/72 vs 60.6% 66/109; increased frequency 58.3% 42/72 vs 51.4% 56/109; vigorous movements 76.4% 55/72 vs 61.5% 67/109). There was no association between maternal feeling of fetal hiccups or feeling contractions and adverse perinatal outcome.
TABLE 6.
Maternal perception of fetal movements in twin pregnancies in mid‐pregnancy.
| Characteristic | Total n = 181 (%) | Normal outcome n = 72 (%) | Adverse outcome n = 109 (%) | Odds ratio (95% confidence interval) | Monochorionic n = 54 (%) | Dichorionic n = 127 (%) |
|---|---|---|---|---|---|---|
| Did you receive any information about fetal movements in early pregnancy? | Pseudo R 2 0.03 | p = 0.07 | ||||
| No information was given | 43 (23.8) | 11 (15.3) | 32 (29.4) | 2.69 (1.18–6.10) | 14 (25.9) | 29 (22.8) |
| Verbal information | 77 (42.5) | 37 (51.4) | 40 (36.7) | Reference | 28 (51.9) | 49 (38.6) |
| Written information | 39 (21.5) | 14 (19.4) | 25 (22.9) | 1.65 (0.75–3.65) | 7 (13.0) | 32 (25.2) |
| Other | 9 (5.0) | 5 (6.9) | 4 (3.7) | 0.74 (0.18–2.97) | 2 (3.7) | 7 (5.5) |
| Missing data | 13 (7.2) | 3 (5.6) | 10 (7.9) | |||
| Experienced fetal hiccups | Pseudo R 2 0.04 | p = 0.25 | ||||
| Once | 7 (3.9) | 4 (5.6) | 3 (2.8) | 1.74 (0.29–10.52) | 4 (7.4) | 3 (2.4) |
| Daily | 6 (3.3) | 2 (2.8) | 4 (3.7) | 0.81 (0.32–2.08) | 2 (3.7) | 4 (3.1) |
| Occasionally | 29 (16.0) | 15 (20.8) | 14 (12.8) | 0.65 (0.13–3.27) | 5 (9.3) | 24 (18.9) |
| Unsure | 24 (13.3) | 5 (6.9) | 19 (17.4) | 3.30 (1.04–10.47) | 10 (18.5) | 14 (11.0) |
| No | 46 (25.4) | 20 (27.8) | 26 (21.1) | Reference | 14 (25.9) | 29 (22.8) |
| Missing data | 69 (38.1) | 19 (35.2) | 53 (41.7) | |||
| Perception of strength of fetal movements | Pseudo R 2 0.02 | p = 0.02 | ||||
| Increase | 119 (65.8) | 53 (73.6) | 66 (60.6) | 0.66 (0.28–1.53) | 37 (68.5) | 82 (64.6) |
| Decrease | 5 (2.8) | 4 (5.6) | 1 (0.9) | 0.13 (0.01–1.34) | 2 (3.7) | 3 (2.4) |
| Stay the same | 29 (16.0) | 10 (13.9) | 19 (17.4) | Reference | 9 (16.7) | 20 (15.7) |
| Unsure | 7 (3.9) | 3 (4.2) | 4 (3.7) | 0.70 (0.13–3.77) | 0 (0) | 7 (5.5) |
| Missing data | 21 (11.6) | 6 (11.1) | 15 (11.8) | |||
| Perception of frequency of fetal movements | Pseudo R 2 0.001 | p = 0.97 | ||||
| Increase | 98 (54.1) | 42 (58.3) | 56 (51.4) | 1.08 (0.53–2.17) | 31 (57.4) | 67 (52.8) |
| Decrease | 8 (4.4) | 3 (4.2) | 5 (4.6) | 1.35 (0.29–6.30) | 3 (5.6) | 5 (3.9) |
| Stay the same | 47 (26.0) | 21 (29.2) | 26 (23.9) | Reference | 13 (24.1) | 34 (26.8) |
| Unsure | 6 (3.3) | 3 (4.2) | 3 (2.8) | 0.81 (0.15–4.42) | 1 (1.9) | 5 (3.9) |
| Missing data | 22 (12.2) | 6 (11.1) | 16 (12.6) | |||
| Experienced periods of vigorous fetal movement | Pseudo R 2 0.02 | p = 0.54 | ||||
| Yes | 122 (67.4) | 55 (76.4) | 67 (61.5) | 0.79 (0.38–1.67) | 37 (68.5) | 85 (66.9) |
| No | 38 (21.0) | 15 (20.8) | 23 (21.1) | Reference | 11 (20.4) | 27 (21.3) |
| Missing data | 21 (11.6) | 6 (11.1) | 15 (11.8) | |||
| Experienced at least one episode of reduced fetal movements | Pseudo R 2 0.004 | p = 0.34 | ||||
| Yes | 48 (26.5) | 24 (33.3) | 24 (22.0) | 0.72 (0.36–1.42) | 11 (20.4) | 37 (29.1) |
| No | 110 (60.8) | 46 (63.9) | 64 (58.7) | Reference | 37 (68.5) | 73 (57.5) |
| Missing data | 23 (12.7) | 6 (11.1) | 17 (13.4) | |||
Note: Data are presented as total values and percentages, also presented according to chorionicity. Early pregnancy (20 weeks) (n = 181), mid‐pregnancy (28 weeks) (n = 160) approximately. Participants were asked “In the last two weeks did the strength/frequency of your baby's movements alter?” questions were repeated in all three questionnaires.
Bold text indicates statistically significant results.
4. DISCUSSION
This pilot study demonstrates the feasibility of the study design for evaluating associations of potentially modifiable factors and risk of adverse neonatal outcome in twin pregnancies. It identifies areas of potential interest that should be investigated in a larger definitive study. Importantly, this study suggests that there may be differences between risk factors associated with adverse neonatal outcomes in patients pregnant with singletons and those pregnant with twins and between dichorionic and monochorionic twins. However, this study contains fewer monochorionic twin pregnancies compared to dichorionic counterparts; this makes the independent effects of chorionicity difficult to assess objectively here. It is important that any prospective study includes a large enough sample of monochorionic twins so that the adverse perinatal/neonatal events may be suitably investigated.
The participation rate (68.2%) was similar to the Auckland Stillbirth study (72%), and the Sydney stillbirth study (67%), and greater than MiNESS (45%); 9 , 10 , 13 implying patients pregnant with twins are willing to participate in research relating to pregnancy outcome. The proportion of dichorionic vs monochorionic twins was consistent with that reported nationally 25 , 26 suggesting that the study design and recruitment are likely to have produced generalizable results.
Generally, there is a paucity of research exploring views and experiences of participating in research during the perinatal period. 17 It is possible that the sensitive subject matter may have reduced participation rates, but maternal opinion of participation was overwhelmingly positive, similar to other studies of this nature. 9 , 13 , 27 , 28 Those with negative opinions of research may have declined participation, thus their views are not represented here. Previously, non‐participation in research has not reflected an objection to participation per se, but rather barriers to, or misunderstanding of, the nature or process of the project itself. 29 Nevertheless, this study has highlighted important themes from patients pregnant with twins participating in research, including specific motives relating to helping other patients with twins and improving the information available to support future research; it may be possible to devise recruitment strategies aligned with these themes. It is also important to consider the views and experiences of specific groups of patients who may have lower rates of participation (such as those with socioeconomic deprivation, domestic abuse and minority ethnic groups 29 , 30 ); this is particularly important in certain ethnic minority groups with higher rates of twin pregnancy (West‐African populations 1/50 births 31 ).
Some observed associations here were similar to those in singleton pregnancies, for example, patients with risk factors for GDM but who were not screened or who had high perceived social stress had increased adverse outcomes. Although singletons have an increased risk of stillbirth and low birthweight with a supine going‐to‐sleep position, this exposure was less frequent amoung women who had a twin pregnancy. After 28‐weeks' gestation women with a twin pregnancy had a reduced frequency of supine going‐to‐sleep position compared to singletons (0.6% vs 6.5%), front (0% vs 1%), and an increased number of participants who reported sleeping propped‐up (6.1% vs 3.1%). 22 Therefore, associations between adverse pregnancy outcomes and supine going‐to‐sleep position are less likely to be seen. Compared to historic singletons (studied as “control” participants in the original MiNESS) there were similar rates of increased strength of fetal movements (65.8% vs 62.8%), but increased fetal movement frequency (54.1% vs 34.8%) and reduced rates of perceived fetal hiccups (22.7% vs 62.9%) suggesting that maternal perception of fetal movements may differ in twin, compared with singleton pregnancies. 10 , 29 , 32
This study was the largest prospective study that has attempted to collect detailed information about modifiable antenatal risk factors for adverse neonatal outcomes in twin pregnancies. Within the timeframe and adjusting for the effects of a recent global pandemic, the study recruited at a reasonable rate. Overall, eight participants (4%) were “lost to follow‐up’” which may have been an effect of the pandemic increasing difficulties in contacting individuals. Participants included in the study demonstrated profiles of background, marital status, parity, BMI and sociodemographic characteristics broadly consistent with that published locally and nationally, suggests that the sample is representative of a wider population of patients pregnant with twins. 32
To investigate modifiable risk factors for perinatal morbidity and mortality in twin pregnancies, a cohort design is the most appropriate study methodology and encourages exploration at different gestational ages. However, fewer than 50% of the participants completed the third questionnaire (late‐pregnancy). The attrition observed could be attributed to birth before 36 weeks: the mean gestational age at birth was 35+3 weeks (SD 2.8 days). This reduces the reliability of associations explored in late pregnancy; the sample size for a large‐scale study would need to be inflated accordingly. In total, 30 (16.5%) participants gave birth between 34 and 36 weeks, 17 of which were monochorionic twins. One means to increase the response rate would be to complete the final questionnaire at 34‐weeks' gestation. Additionally, by focusing on women who attend twin clinics, the continuity of care observed may increase the response rate at later gestations.
This study uses a similar questionnaire to other studies of this phenomenon in singleton pregnancies, which facilitates the comparison of results. 9 , 10 , 13 It is important to recognize this pilot study was not designed to determine independent associations; the statistical power of this study intended only to indicate feasibility and to confirm areas of interest for a future study. Therefore, assessing the impact of exposures on monochorionic and dichorionic twin pregnancy outcomes separately was not possible. However, this study has shown that the frequencies of exposures (most notably including fertility treatment) are not the same between these groups, suggesting the benefit of breaking groups down by chorionicity in a future study. A limitation of the study design is the potential existence of any unidentified confounding factors, such as the potential for recall bias, for example, sleep position. This study attempted to minimize recall bias in several ways: (i) the potential for bias towards specific questions was reduced by using a structured questionnaire, (ii) participants in the study were pregnant at the time they completed the questionnaires and therefore, their experiences and answers would not have been biased by knowledge of the outcome of their pregnancy and (iii) participants were asked the same series of questions, reducing the potential for variation between participants.
The findings of this hypothesis‐generating study suggest that twin pregnancies may have different modifiable antenatal risk factors for adverse neonatal outcome, compared to singletons. With the exception of patients who had risk factors for GDM but were not screened the individual variables displayed limited predictive value for adverse outcome. A future study should involve a greater number of participants to support the exploration of associations between maternal factors and adverse neonatal outcomes in twin pregnancy and according to chorionicty. A multivariable analysis of factors is needed to attempt to gain understanding of the relation between variables and adverse neonatal outcomes. Where interest is in identifying factors, which affect the chance of having an adverse outcome, this will involve identifying an appropriate adjustment set to control for confounding between each factor and the outcome. To identify factors with an OR of 2 (which would be considered relevant for practical implications) the sample size needed in a future study to detect such a relation between adverse neonatal outcomes and (i) perceived social stress would require 358 participants, (ii) BMI ≤20 kg/m2 would require 1022 participants and (iii) ART for conception would need 554 participants. Future studies should also include inflation in recruitment, to account for the loss to follow‐up and preterm births. In addition, future research is required to investigate potential causality and any additional underlying mechanisms that contribute to adverse neonatal outcomes in twin pregnancies.
5. CONCLUSION
This study has added to the current existing body of knowledge regarding potential relation between modifiable risk factors for adverse outcomes in twin pregnancies. These preliminary data suggest that current health promotional messages, national guidance and information available for patients pregnant with twins through extrapolation from singleton pregnancy research, may not accurately mitigate risk. This study demonstrates the feasibility of an observational study in this population, and the value of further interrogation of relation between modifiable factors and twin perinatal morbidity and mortality. The information generated from a larger study should be used to support the introduction of twin‐specific preventative measures for morbidity and mortality, aiming to reduce the occurrence of neonatal morbidity and mortality at a similar rate to that observed in singleton pregnancies. 5 , 7
AUTHOR CONTRIBUTIONS
AEPH and MDK conceived the study, the study protocol and statistical analysis plan was drafted by AEPH, MDK, LEH and JW. ET and AEPH conducted the study and coordinated recruitment. Data analysis was conducted by IG, JW, AEPH, LE H and MDK. All authors participated in the interpretation of results and drafting of the manuscript.
FUNDING INFORMATION
This study was funded by a bursary funded jointly by the British Maternal Fetal Medicine Society and the Twins Trust awarded to Professor Heazell and Kilby.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
REFERENCES
- 1. Draper ES, Gallimore ID, Kurinczuk JJ, Kenyon S. Maternal, Newborn and Infant Clinical Outcome Review Programme MBRRACE‐UK Perinatal Confidential Enquiry Stillbirths and Neonatal Deaths in twin pregnancies. 2021. [cited 2021 November 11]. Available from: https://www.hqip.org.uk/national‐programmes
- 2. Windmill H. Reducing Stillbirths in Multiple Pregnancies and the NHS Stillbirth “Care Bundle.” TAMBA/Twins Trust. 2018. [cited 2021 November 11]. Available from: https://twinstrust.org/asset/$EA170AD6‐F8A5‐4F67‐A85CE9A6CDE68EB7/
- 3. Chepkin S, Prince S, Johnston T, et al. Learning from standardised reviews when babies die. National Perinatal Mortality Review Tool First Annual Report. 2019. [cited 2022 May 22]. Available from: https://www.npeu.ox.ac.uk/assets/downloads/pmrt/reports/PMRT%20Report%202019%20v1.0.pdf
- 4. Kilby MD, Gibson JL, Ville Y. Falling perinatal mortality in twins in the UK: organisational success or chance? BJOG. 2019;126:341‐347. [DOI] [PubMed] [Google Scholar]
- 5. Draper E, Gallimore I, Smith L, et al. MBBRACE‐UK Perinatal Mortality Surveillance Report UK Perinatal Deaths for Births from January to December 2020. 2022. [cited 2022 October 14]. Available from: https://www.npeu.ox.ac.uk/assets/downloads/mbrrace‐uk/reports/perinatal‐surveillance‐report‐2020/MBRRACE‐UK_Perinatal_Surveillance_Report_2020.pdf
- 6. Fox R, McMullen S, Windmill H. Maternity services and multiple births. A joint report by NCT and the twins and multiple births association. Maternity services and multiple births. NCT and Tamba. 2015. [cited 2022 May 25]. Available from: https://twinstrust.org/static/30985846‐9bc2‐4660‐a641642828419b9e/Maternity‐Services‐for‐Multiples‐Tamba‐NCT‐Joint‐Report‐r2‐2.pdf
- 7. National Institute for Health and Care Excellence . Twin and triplet pregnancy. Guidance. NICE. 2019. [cited 2021 November 21]. Available from: https://www.nice.org.uk/guidance/ng137/resources/twin‐and‐triplet‐pregnancy‐pdf‐66141724389829
- 8. Draper ES, Gallimore ID, Smith LK, et al. Maternal, Newborn and Infant Clinical Outcome Review Programme MBRRACE‐UK Perinatal Mortality Surveillance Report. 2019. [cited 2022 May 21]. Available from: www.hqip.org.uk/national‐programmes
- 9. Platts J, Mitchell EA, Stacey T, et al. The Midland and north of England stillbirth study (MiNESS). BMC Pregnancy Childbirth. 2014;14:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stacey T, Thompson JMD, Mitchell EA, Ekeroma AJ, Zuccollo JM, McCowan LME. The Auckland stillbirth study, a case‐control study exploring modifiable risk factors for third trimester stillbirth: methods and rationale. ANZJOG. 2011;51:3‐8. [DOI] [PubMed] [Google Scholar]
- 11. Cronin R, Minglan L, Thompson J, et al. An individual participant data meta‐analysis of maternal going‐to‐sleep position, interactions with fetal vulnerability and the risk of late stillbirth. EClinicalMedicine. 2019;10:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson J, Wilson J, Bradford B, et al. A better understanding of the association between maternal perception of foetal movements and late stillbirth—findings from an individual participant data meta‐analysis. BMC Med. 2021;19:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordon A, Raynes‐Greenow C, Bond D, Morris J, Rawlinson W, Jeffery H. Sleep position, fetal growth restriction, and late‐pregnancy stillbirth: the Sydney stillbirth study. Obstet Gynecol. 2015;125:347‐355. [DOI] [PubMed] [Google Scholar]
- 14. Flenady V, Wojcieszek AM, Middleton P, et al. Stillbirths: recall to action in high‐income countries. Lancet. 2016;387:691‐702. [DOI] [PubMed] [Google Scholar]
- 15. Mistry H, Heazell AEP, Vincent O, Roberts T. A structured review and exploration of the healthcare costs associated with stillbirth and a subsequent pregnancy in England and Wales. BMC Pregnancy Childbirth. 2013;13:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heazell AEP, Siassakos D, Blencowe H, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387:604‐616. [DOI] [PubMed] [Google Scholar]
- 17. Heazell AEP, Whitworth MK, Whitcombe J, et al. Research priorities for stillbirth: process overview and results from UK Stillbirth Priority Setting Partnership. Ultrasound Obstet Gynecol. 2015;46:641‐647. [DOI] [PubMed] [Google Scholar]
- 18. Gulati N, Mackie FL, Cox P, et al. Cause of intrauterine and neonatal death in twin pregnancies (CoDiT): development of a novel classification system. BJOG. 2020;127:1507‐1515. [DOI] [PubMed] [Google Scholar]
- 19. Razaz N, Cnattingius S, Joseph K. Association between Apgar scores of 7 to 9 and neonatal mortality and morbidity: population‐based cohort study of term infants in Sweden. BMJ. 2019;365:1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Institute for Health and Care Excellence . Intrapartum care for healthy women and babies. Nice. 2014. [cited 2022 May 13]. Available from: www.nice.org.uk/guidance/cg190 [PubMed]
- 21. Heazell A, Kilby M. Statistical Analysis Plan Version 1.0. University of Manchester; 2021. doi: 10.48420/16912771.v2 [DOI] [Google Scholar]
- 22. Stacey T, Tennant P, McCowan L, et al. Gestational diabetes and the risk of late stillbirth: a case‐control study from England, UK. BJOG. 2019;126:973‐982. [DOI] [PubMed] [Google Scholar]
- 23. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159. [PubMed] [Google Scholar]
- 24. Rigby A. Statistical methods in epidemiology. V. Towards an understanding of the kappa coefficient. Disabil Rehabil. 2000;22:339‐344. [DOI] [PubMed] [Google Scholar]
- 25. Birth characteristics in England and Wales ‐ Office for Ntional Statistics. [cited 2022 May 19]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2020
- 26. Kilby M, Bricker L. Management of monochorionic twin pregnancy: green‐top guideline No. 51. BJOG. 2016;124:e1‐e45. doi: 10.1111/1471-0528.14188 [DOI] [PubMed] [Google Scholar]
- 27. McCowan LME, Thompson JMD, Cronin RS, et al. Going to sleep in the supine position is a modifiable risk factor for late pregnancy stillbirth; findings from the New Zealand multicentre stillbirth case‐control study. PLoS One. 2017;12:e0179396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stacey T, Thompson JMD, Mitchell EA, Ekeroma AJ, Zuccollo JM, McCowan LME. Association between maternal sleep practices and risk of late stillbirth: a case‐control study. BMJ. 2011;342:3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Budd J, Stacey T, Martin B, Roberts D, Heazell AEP. Women's experiences of being invited to participate in a case‐control study of stillbirth ‐ findings from the Midlands and North of England stillbirth study. BMC Pregnancy Childbirth. 2018;18:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Origlia Ikhilor P, Hasenberg G, Kurth E, Asefaw F, Pehlke‐Milde J, Cignacco E. Communication barriers in maternity care of allophone migrants: experiences of women, healthcare professionals, and intercultural interpreters. J Adv Nurs. 2019;75:2200‐2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santana DS, Surita FG, Cecatti JG. Multiple pregnancy: epidemiology and association with maternal and perinatal morbidity. Rev Bras Ginecol Obstet. 2018;40:554‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heazell A, Budd J, Smith LK, et al. Associations between social and behavioural factors and the risk of late stillbirth ‐ findings from the Midland and North of England stillbirth case‐control study. BJOG. 2021;128:704‐713. [DOI] [PubMed] [Google Scholar]
Supporting information
Table S1
Table S2
ACKNOWLEDGMENTS
In addition, we are grateful for the NIHR Clinical Research Network for their support for the research midwives and nurses who recruited participants. We would like to thank the following people: Chloe O'Hara, Julie Lowe (Birmingham Women's and Children's Hospital NHS Foundation Trust), Katrina Rigby, Julie Earnshaw, Krishna Panchal, Cheryl Wyatt (Lancashire Teaching Hospitals NHS Trust), Sally Hammon, Christine Hughes (Manchester University NHS Foundation Trust), Dr Habiba Kapaya, Amanda Roper (United Lincolnshire Hospitals NHS Trust), Hannah Speirs, Natalie Morris, Dr Soma Mukherjee (University Hospitals Coventry and Warwickshire NHS Trust) Maryanne Bray, Denise Goodman, Vikki Keeping, Dr Amy Robb and Kate Siddall (University Hospital of Wales – Cardiff and Vale Health Board) for their efforts in recruitment and retention of participants.
Greatholder I, Tomlinson E, Wilkinson J, Higgins LE, Kilby MD, Heazell AEP. Evaluating antenatal risk in twin pregnancies—A feasibility study to identify modifiable factors associated with adverse pregnancy outcomes. Acta Obstet Gynecol Scand. 2023;102:585‐596. doi: 10.1111/aogs.14540
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


