Abstract
Purpose
This Phase 3, multicenter study (NCT03434041) was conducted in primarily Chinese patients with treatment-resistant depression (TRD) to support the registration of esketamine nasal spray in China.
Patients and Methods
This randomized, double-blind, active-controlled study was conducted in China and the United States (US) in patients with TRD (single or recurrent episode). Eligible patients were randomized 1:1 to receive intranasal esketamine or matching placebo, each in conjunction with a newly initiated oral antidepressant (AD; duloxetine, escitalopram, sertraline, and venlafaxine extended release) (ie, esketamine plus AD or AD plus placebo). The primary endpoint, change from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) total score at Day 28, was analyzed using a mixed-effects model for repeated measures. Secondary endpoints including safety were also evaluated.
Results
Of 252 randomized patients (China, 224; US, 28), 214 completed the double-blind treatment phase. The difference between treatment groups at Day 28 was not statistically significant (difference in least-square means [95% CI]: −2.0 [−4.64, 0.55]; 2-sided p = 0.123). However, esketamine plus AD demonstrated a clinically meaningful treatment difference compared with AD plus placebo in MADRS total score at 24 hours after first dose for the study overall population and China sub-population (difference in least-square mean [95% CI]: −3.3 [−5.33, −1.33] and −2.6 [−4.64, −0.60], respectively). No new safety signals were observed.
Conclusion
Esketamine plus AD was not statistically superior to AD plus placebo in improving depressive symptoms in TRD patients at Day 28. Rapid reduction in depressive symptoms within 24 hours was observed for TRD patients treated with esketamine plus AD in the overall population and China sub-population. Safety was consistent with the established safety profile of esketamine.
Keywords: intranasal, major depressive disorder, MDD, TRD
Introduction
Major depressive disorder (MDD) is a common psychiatric disorder worldwide and a key contributor to the global burden of disease.1,2 In China, the 12-month MDD prevalence is 2.1% (approximately 30 million persons), with an estimated lifetime MDD prevalence of 3.4% (approximately 50 million persons).3 A subset of MDD patients experience treatment-resistant depression (TRD), defined as lack of clinically meaningful improvement after treatment with at least 2 different antidepressants (ADs) at adequate dosage and duration in the current depressive episode.4–7 Approximately 30% of MDD patients have TRD, and there are approximately 14.5 million TRD patients in China where there is no approved therapy for TRD.6,8–10 In patients who respond to ADs, the time to reach full effectiveness is typically 4–7 weeks. During this period, patients continue to experience symptoms and to be at risk of self-harm.7,11 Therefore, there is an unmet need for TRD intervention and novel treatments for the rapid relief of depressive symptoms.12,13
Esketamine nasal spray (SPRAVATO), the rapidly acting S-enantiomer of ketamine, is approved in conjunction with an oral AD for the treatment of adults with TRD in the United States (US) and the European Union.14,15 Marketing authorizations have also been granted in many other regions and countries. Esketamine nasal spray has also been approved for the treatment of depressive symptoms in adults with MDD with acute suicidal ideation or behavior, or in a psychiatric emergency.14,15
In a pivotal, phase 3, global, multicenter study (TRANSFORM-2, NCT02418585), esketamine nasal spray (either 56 or 84 mg) plus a newly initiated oral AD showed a statistically significant and clinically relevant improvement in depressive symptoms based on change in Montgomery-Åsberg Depression Rating Scale (MADRS)16 total score after 28 days in adult patients with TRD versus oral AD plus placebo, and clinically meaningful benefit 24 hours after the first dose.17 This phase 3, multicenter study (NCT03434041), with almost identical design features as TRANSFORM-2,17 evaluated the efficacy and safety of flexibly dosed intranasal esketamine (56 or 84 mg) plus a newly initiated oral AD for a 4-week treatment period in mainly Chinese adult patients with TRD to support regulatory requirements for registration of intranasal esketamine in China.
Materials and Methods
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. The study protocol and amendments were reviewed by ethics committees at sites in China and institutional review boards in the US (Table S1). All patients provided written informed consent before enrolling in the study.
Study Design
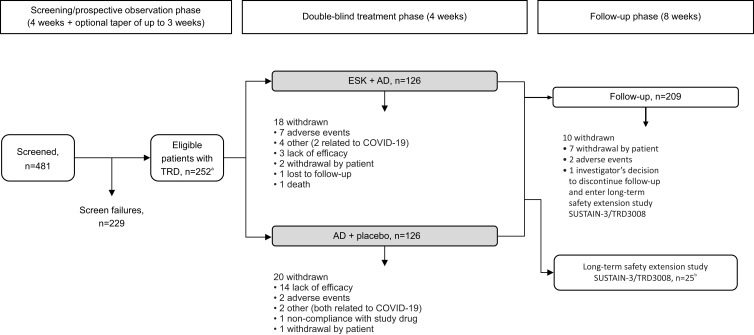
This was a phase 3, randomized, double-blind, active-controlled study conducted between June 2018 and April 2021 at 22 sites in China and 5 sites in the US to fulfil the China Health Authority regulatory requirement of an international multi-regional clinical trial. The study consisted of 3 phases: (1) a 4-week screening/prospective observational phase with an optional up to 3-week period to taper/discontinue the current AD medication; (2) a 4-week double-blind treatment phase during which study medication (esketamine or placebo) was administered at 2 treatment sessions per week on Days 1, 4, 8, 11, 15, 18, 22, and 25, plus a newly initiated oral AD administered daily; and (3) an 8-week posttreatment follow-up phase (Figure 1). After completing the double-blind treatment phase, patients enrolled in the US were eligible to enter the open-label long-term safety extension study of esketamine (SUSTAIN-3/TRD3008; NCT02782104).
Figure 1.
Patient flow diagram.
Notes: aOnly patients with non-response to at least 2 AD treatments prior to randomization were eligible to participate in the study. bTwenty-four patients entered SUSTAIN-3/TRD3008 from the double-blind treatment phase of TRD3006; 1 patient entered SUSTAIN-3/TRD3008 from the follow-up phase of TRD3006.
Abbreviations: AD, antidepressant; ESK, esketamine; TRD, treatment-resistant depression.
Study Population
The study population included adults aged 18–64 years, who met the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for single-episode MDD or recurrent MDD without psychotic features, based on clinical assessment and confirmed by the Mini International Neuropsychiatric Interview (MINI). TRD was defined as lack of clinically meaningful improvement after treatment with at least 2 different ADs at adequate dose and duration (≥6 weeks) in the current depressive episode. At the start of the 4-week screening/prospective observational phase, patients were required to have had documented nonresponse (≤25% improvement) to treatment with 1–5 oral ADs at adequate dose and duration based on the Massachusetts General Hospital – Antidepressant Treatment Response Questionnaire (MGH-ATRQ) for the current depressive episode and confirmed by documented records. In addition, patients were currently receiving a different oral AD (on the MGH-ATRQ), to which they had been adherent for at least the previous 2 weeks at or above the minimum therapeutic dose. Patients who were non-responders to their current oral AD(s) were eligible for randomization if all other entry criteria were met. Nonresponse at the end of the screening/prospective observational phase was defined as ≤25% improvement in the MADRS total score from Week 1 to Week 4 and a MADRS total score of ≥28 on Week 2 and Week 4 during the screening/prospective observational phase.
Patients with previous nonresponse to esketamine or ketamine, nonresponse to all oral ADs for this study (described below), or nonresponse to electroconvulsive therapy were excluded. Patients who had received vagal nerve stimulation or deep brain stimulation in the current depressive episode were also excluded. Other key exclusion criteria were current or prior diagnosis of a psychotic disorder or MDD with psychotic features, bipolar or related disorders, current obsessive compulsive disorder, intellectual disability, autism spectrum disorder, borderline, antisocial, histrionic, or narcissistic personality disorder; current or recent (past 6 months) homicidal ideation/intent or suicidal ideation with intent to act or history of suicidal behavior within the past year; and a recent history (past 6 months) of moderate or severe substance or alcohol use disorder, including a lifetime history of ketamine, phencyclidine, lysergic acid diethylamide, or 3,4-methylenedioxy-methamphetamine hallucinogen-related use disorder.
Randomization and Blinding
Central randomization was implemented in this study. A computer-generated randomization schedule was used to randomly assign patients in a 1:1 ratio to receive double-blind treatment with either intranasal esketamine or matching placebo, each in combination with a newly initiated open-label oral AD. Randomization was balanced by using randomly permuted blocks and was stratified by country (China or US), class of oral AD (serotonin and norepinephrine reuptake inhibitor [SNRI] or selective serotonin reuptake inhibitor [SSRI]), and consent to biomarker evaluation (Yes or No). Investigators, patients, site personnel, clinical team, and those assessing outcomes or analyzing the data were blind to the treatment assignment.
Intranasal Study Drugs and Newly Initiated Oral ADs
Each device delivered 2 sprays of either esketamine (a total of 28 mg of esketamine base) or placebo. Intranasal esketamine or placebo devices had identical appearance and packaging. All patients received training and practiced spraying (into the air, not intranasally) using a demonstration intranasal device that was filled with placebo solution before the first administration. Patients self-administered intranasal esketamine or placebo at the clinical site under the direct supervision of the investigator/designee. On Day 1, esketamine was started at 56 mg. On Days 4, 8, 11, 15, 18, 22, and 25, the investigator judged, based on efficacy and tolerability, whether to increase the dose of esketamine to 84 mg or to maintain the dose at 56 mg.
Open-label oral AD medication (duloxetine, escitalopram, sertraline, and venlafaxine extended release as active comparator) was assigned by the investigator based on review of MGH-ATRQ and relevant prior AD medication information and was one to which the patient had not previously been nonresponsive, in the current depressive episode, and to which the patient had not been previously intolerant (lifetime). Dosing of the oral AD began on Day 1 and followed a protocol-specific titration schedule.
Efficacy Assessments
Efficacy assessments included (1) MADRS, a clinician-rated measure of depression severity,16 and scored by independent, remote (by phone), blinded MADRS raters to ensure an unbiased efficacy evaluation; (2) Sheehan Disability Scale (SDS), a patient-reported measure of functional impairment and associated disability;18,19 (3) Clinical Global Impression of Severity (CGI-S), a clinician-rated measure of illness severity;20 (4) 7-Item Generalized Anxiety Disorder Scale (GAD-7), a patient-reported measure of anxiety symptoms;21 and (5) 5-level EQ-5D (EQ-5D-5L), a patient-reported measure of health outcome.22
The primary endpoint was change in MADRS total score (7-day recall) from baseline to Day 28 (end of double-blind treatment phase). Two key secondary endpoints were (1) change from baseline in MADRS total score (24-hour recall) at 24 hours post first dose (Day 2)23 and (2) change from baseline in SDS total score at Day 28. Other secondary endpoints were change from baseline to Day 28 in CGI-S, GAD-7, and EQ-5D-5L, respectively.
Pharmacokinetic (PK) Assessments
PK was evaluated in the China population only. Venous blood samples (approximately 4 mL each) were collected for PK analysis at predose, 40 minutes, 2 hours, and 6 hours postdose on Day 25. Plasma esketamine concentrations were measured in each sample.
Safety Assessments
Safety evaluation was based on reported adverse events (AEs), clinical laboratory tests, vital sign measurements, physical examinations, body weight, height, electrocardiograms (ECGs), pulse oximetry, and nasal examinations. Other safety evaluations included the Columbia-Suicide Severity Rating Scale (C-SSRS) to assess potential suicidal ideation and behavior,24 the Clinician-Administered Dissociative States Scale (CADSS) to assess dissociative symptoms,25 and the 20-Item Physician Withdrawal Checklist (PWC-20) to assess potential withdrawal symptoms following cessation of intranasal study medication.26
Statistical Analysis
Sample Size Determination
Planned sample size was calculated assuming a treatment difference for the double-blind treatment phase of 6.5 points in MADRS total score between esketamine and the active comparator, a standard deviation (SD) of 12, a 1-sided significance level of 0.025, and a dropout rate of 25%. The treatment difference and SD used in this calculation were based on results from a Phase 2 study evaluating intranasal esketamine for TRD27 and on clinical judgement. Approximately 117 patients were to be randomized to each treatment group to achieve greater than 90% power, with a sample size of 105 Chinese patients per treatment arm (n = 210 total) plus 12 non-Chinese patients per treatment arm (n = 24 total).
Analysis Sets
Patients were classified into the following analysis sets: all randomized, efficacy, safety, follow-up, and pharmacokinetic. The all randomized analysis set included all randomized patients regardless of whether treatment was received. The efficacy analysis set (ie, full analysis set), included all randomized patients who received at least 1 dose of intranasal study medication and 1 dose of oral AD medication during the double-blind treatment phase. The safety analysis set included all randomized patients who received at least 1 dose of intranasal study medication or 1 dose of oral AD medication during the double-blind treatment phase. The follow-up analysis set included all patients who entered the follow-up phase. The pharmacokinetic analysis set included all patients who received at least 1 dose of intranasal study medication and had at least 1 post-treatment sample collected during treatment.
Efficacy Analysis
Statistical analysis tests were conducted at a 2-sided 0.05 level of significance unless otherwise noted. A serial gatekeeping (fixed sequence) approach was applied to adjust for multiplicity and to strongly control type I error across the primary and the 2 key secondary efficacy endpoints. The 2 key secondary endpoints were analyzed sequentially and considered statistically significant at the 2-sided 0.05 level only if the endpoint individually and previous endpoints in the hierarchy, including the primary endpoint, were significant at the 2-sided 0.05 level.
For the primary efficacy analysis, change from baseline in MADRS total score at Day 28 in the double-blind phase was analyzed using a mixed-effects model for repeated measures (MMRM) based on observed case data. The model included the baseline MADRS total score as a covariate and treatment, country, class of AD (SNRI or SSRI), day, and day-by-treatment interaction as fixed effects. The key secondary endpoints and other secondary endpoint (ie, CGI-S) were analyzed using a model similar to the one described for the primary efficacy analysis. The change from baseline in GAD-7 total score at Day 28 was analyzed using an analysis of covariance (ANCOVA) model with treatment, country, and class of AD (SNRI or SSRI) as factors, and the baseline score as the covariate. Descriptive statistics of actual values and changes from baseline by treatment group were provided for EQ-5D-5L.
PK Analyses
Plasma esketamine concentrations were listed for all Chinese patients by esketamine dose and study day. Descriptive statistics were used to summarize esketamine concentrations at each sampling timepoint.
Safety Analysis
The number of patients with treatment-emergent AEs (TEAEs), serious AEs (SAEs), and TEAEs that led to discontinuation of study medication was summarized by system organ class and preferred term. Descriptive statistics were provided for clinical laboratory tests, vital signs, ECG values, and CADSS. For PWC-20, the proportion of patients with withdrawal symptoms at the end of the double-blind treatment phase or during follow-up was presented by treatment group, and symptoms at follow-up were compared to the last intranasal dosing visit and summarized. C-SSRS was used to assess the occurrence, severity, and frequency of suicide-related ideation and behaviors during the double-blind period and follow-up phase. Changes in nasal examination findings from baseline for each examination were listed for the double-blind phase by treatment group.
Results
Study Population
Of 481 patients screened, 252 (n = 224, China; n = 28, US) were randomized 1:1 to receive esketamine plus AD (n = 126) or AD plus placebo (n = 126); of these, 214 (84.9%) completed the double-blind treatment phase (Figure 1). All patients were treated with either 56 mg of esketamine nasal spray or matching placebo nasal spray on Day 1. On Day 4, 40.2% of patients in the esketamine group had their dose increased to 84 mg. On Day 25, the last dosing day, 85.2% of patients in the esketamine group were on 84 mg. In the esketamine plus AD group, the most common reason for withdrawal was AEs (n = 7, 5.6%), followed by “other” reason for withdrawal (n = 4, 3.2%). In the AD plus placebo group, the most common reason for withdrawal was lack of efficacy (n = 14, 11.1%), followed by AEs and “other” reason for withdrawal, respectively, each with 2 (1.6%) patients. A total of 209 patients entered the follow-up phase, and 199 (95.2%) completed the entire 8-week follow-up phase. A total of 25 patients from the US continued into the long-term safety extension study of esketamine (SUSTAIN-3/TRD3008, NCT02782104).
Esketamine plus AD and AD plus placebo groups were comparable based on demographic and baseline clinical characteristics (Table 1, Table S2), with similar mean age (36.9 vs 37.8 years), gender mix (male: 53.2% vs 56.3%), disease severity (mean MADRS total scores: 36.5 vs 35.9), and mean duration of the current depressive episode (225.3 vs 218.6 weeks) (Table 1). Most patients (≥81%) in each treatment group received intranasal study medication on all 8 dosing days during the double-blind phase. Most patients (≥85%) in each group were exposed to intranasal study medication for at least 22 days.
Table 1.
Demographic and Baseline Clinical Characteristicsa
| Characteristic | Esketamine Plus AD (n=124) | AD Plus Placebo (n=126) | Total (n=250) |
|---|---|---|---|
| Age, years – mean (SD) | 36.9 (12.04) | 37.8 (12.36) | 37.3 (12.19) |
| Sex – n (%) | |||
| Male | 66 (53.2) | 71 (56.3) | 137 (54.8) |
| Female | 58 (46.8) | 55 (43.7) | 113 (45.2) |
| Race – n (%) | |||
| Asian | 110 (88.7) | 112 (88.9) | 222 (88.8) |
| White | 12 (9.7) | 9 (7.1) | 21 (8.4) |
| Black or African American | 1 (0.8) | 4 (3.2) | 5 (2.0) |
| Multiple | 1 (0.8) | 0 (0) | 1 (0.4) |
| Not reported | 0 (0) | 1 (0.8) | 1 (0.4) |
| Country – n (%) | |||
| China | 110 (88.7) | 112 (88.9) | 222 (88.8) |
| United States | 14 (11.3) | 14 (11.1) | 28 (11.2) |
| BMI, calculated as kg/m2– mean (SD) | 24.8 (4.98) | 24.2 (4.32) | 24.5 (4.66) |
| Employment status – n (%)b | |||
| Any type of employment | 86 (69.4) | 79 (62.7) | 165 (66.0) |
| Any type of unemployment | 27 (21.8) | 38 (30.2) | 65 (26.0) |
| Other | 11 (8.9) | 9 (7.1) | 20 (8.0) |
| Age when diagnosed with MDD, years – mean (SD) | 27.2 (11.53) | 28.2 (11.87) | 27.7 (11.69) |
| Duration of current episode, weeks – mean (SD) | 225.3 (317.75) | 218.6 (274.20) | 221.9 (296.02) |
| MADRS total score – mean (SD) | 36.5 (5.21) | 35.9 (4.50) | 36.2 (4.87) |
| CGI-S – mean (SD) | 5.1 (0.61) | 5.2 (0.68) | 5.1 (0.65) |
| CGI-S category – n (%) | |||
| Mildly ill | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Moderately ill | 15 (12.1) | 16 (12.7) | 31 (12.4) |
| Markedly ill | 81 (65.3) | 74 (58.7) | 155 (62.0) |
| Severely ill | 27 (21.8) | 33 (26.2) | 60 (24.0) |
| Most extremely ill | 0 (0) | 2 (1.6) | 2 (0.8) |
| Class of oral AD – n (%) | |||
| SNRI | 68 (54.8) | 69 (54.8) | 137 (54.8) |
| SSRI | 56 (45.2) | 57 (45.2) | 113 (45.2) |
| Oral AD – n (%) | |||
| Duloxetine | 36 (29.0) | 40 (31.7) | 76 (30.4) |
| Escitalopram | 30 (24.2) | 34 (27.0) | 64 (25.6) |
| Venlafaxine extended release | 31 (25.0) | 29 (23.0) | 60 (24.0) |
| Sertraline | 27 (21.8) | 23 (18.3) | 50 (20.0) |
| Number of previous treatment failures in current episode – n (%)c | |||
| 1 | 38 (30.6) | 38 (30.2) | 76 (30.4) |
| 2 | 46 (37.1) | 47 (37.3) | 93 (37.2) |
| 3 | 29 (23.4) | 31 (24.6) | 60 (24.0) |
| 4 | 7 (5.6) | 8 (6.3) | 15 (6.0) |
| 5 | 4 (3.2) | 2 (1.6) | 6 (2.4) |
Notes: aData generated from the efficacy analysis set. bAny type of employment includes any category containing “employed”, sheltered work, housewife or dependent husband, and student; any type of unemployment includes any category containing “unemployed”; “other” includes retired or no information available. cNumber of AD medications with nonresponse (defined as ≤25% improvement) taken for at least 6 weeks during the current episode as obtained from MGH-ATRQ at screening.
Abbreviations: AD, antidepressant; BMI, body mass index; CGI-S, Clinical Global Impression of Severity; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder; MGH-ATRQ, Massachusetts General Hospital – Antidepressant Treatment Response Questionnaire; SD, standard deviation; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Efficacy
MADRS Primary Efficacy Analysis and Follow-Up Results
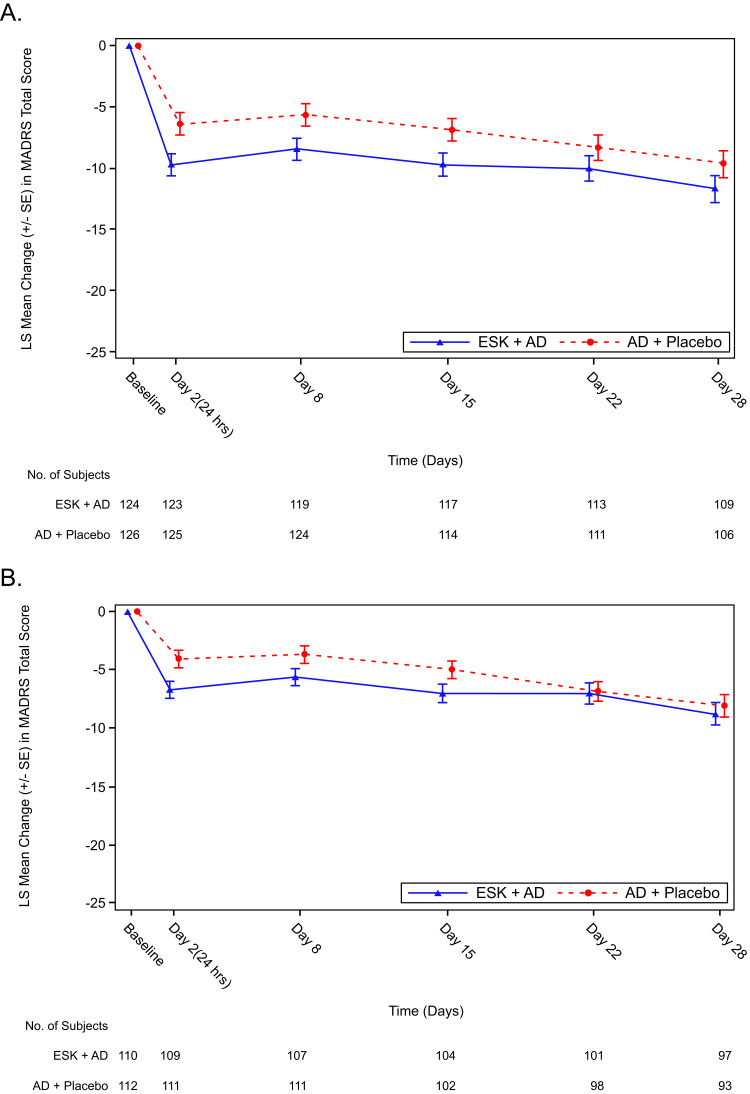
The mean (SD) MADRS total score at baseline was 36.5 (5.21) for the esketamine plus AD group and 35.9 (4.50) for the AD plus placebo group, and at Day 28 was 26.5 (10.33) and 27.9 (10.04), respectively (Table 2). The mean change (SD) in MADRS total score from baseline at Day 28 was -10.1 (10.80) for the esketamine plus AD group and −8.1 (10.26) for the AD plus placebo group. The least squares (LS) mean difference (95% confidence interval [CI]) at Day 28 between the two treatment groups analyzed by MMRM was −2.0 (−4.64, 0.55); this difference was not statistically significant (2-sided p = 0.123). In the China population (n = 222), the LS mean difference (95% CI) was -0.7 (−3.35, 1.94). The MADRS change over time in the overall population and China population during the double-blind treatment phase is shown in Figure 2. China patients continued to the 8-week follow-up phase, during which the mean MADRS total score continuously improved. Patients who were previously on esketamine showed a larger reduction in MADRS total score compared with those who were previously on placebo, with a mean score at week 8 of 20.5 versus 24.6, respectively (Figure S1).
Table 2.
Change in MADRS Total Scorea from Baseline to Day 28 in the Double-Blind Treatment Phase
| Esketamine Plus AD | AD Plus Placebo | |
|---|---|---|
| Baseline | ||
| N | 124 | 126 |
| Mean (SD) | 36.5 (5.21) | 35.9 (4.50) |
| Median (range) | 36.0 (25, 50) | 36.0 (27, 48) |
| Day 28 | ||
| N | 109 | 106 |
| Mean (SD) | 26.5 (10.33) | 27.9 (10.04) |
| Median (range) | 28.0 (0, 43) | 30.0 (0, 44) |
| Change from baseline to Day 28 | ||
| N | 109 | 106 |
| Mean (SD) | −10.1 (10.80) | −8.1 (10.26) |
| Median (range) | −7.0 (−42, 10) | −6.0 (−38, 8) |
| MMRM analysisb | ||
| Difference of LS means (SE) | −2.0 (1.32) | |
| 95% CI on difference | −4.64, 0.55 | |
| 2-sided p-value | 0.123 | |
Notes: aMADRS total score ranges from 0 to 60; a higher score indicates a more severe condition. Negative change in score indicates improvement. bTest for treatment effect is based on MMRM analysis with change from baseline as the response variable and the fixed effect model terms for treatment (esketamine plus AD, AD plus placebo), day, country, class of oral AD (SNRI or SSRI), and treatment-by-day, and baseline value as a covariate. A negative difference favors esketamine.
Abbreviations: AD, antidepressant; LS, least squares; MADRS, Montgomery-Åsberg Depression Rating Scale; MMRM, mixed model for repeated measures; SD, standard deviation; SE, standard error; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Figure 2.
LS mean change in MADRS total score over timea in the double-blind treatment phase in the overall population (A) and China population (B).
Note: aLS mean and SE were based on MMRM analysis with change from baseline as the response variable and the fixed effect model terms for treatment (esketamine plus AD, AD plus placebo), day, country, class of oral AD (SNRI or SSRI), and treatment-by-day, and baseline value as a covariate. Negative change in score indicates improvement.
Abbreviations: AD, antidepressant; ESK, esketamine; LS, least squares; MADRS, Montgomery-Åsberg Depression Rating Scale; MMRM, mixed model for repeated measures; SE, standard error; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Key Secondary Efficacy Analyses
The 2 key secondary endpoints were designed to assess the effect of esketamine on the rapid reduction of depressive symptoms and on patient-reported functioning and associated disability. Based on the predefined testing sequence of the primary and key secondary endpoints, the change from baseline in MADRS total score at 24 hours (Day 2) and the change from baseline in SDS total score at Day 28 could not be formally tested. The change in MADRS total score at 24 hours numerically favored esketamine plus AD over AD plus placebo. Using an MMRM model, the LS mean difference (95% CI) between the 2 treatment groups was −3.3 (−5.33, −1.33) (Table 3). In the China population, the LS mean difference (95% CI) between esketamine plus AD and AD plus placebo was −2.6 (−4.64, −0.60). The LS mean difference (95% CI) of change in SDS total score from baseline to Day 28 between the 2 treatment groups was −1.0 (−2.96, 0.97) based on MMRM analysis (Table 3). In the China population, the LS mean difference (95% CI) between the 2 treatment groups was −0.1 (−2.09, 1.93).
Table 3.
Key Secondary Efficacy Endpoints Assessed in the Double-Blind Treatment Phase
| Esketamine Plus AD | AD Plus Placebo | |
|---|---|---|
| Change in MADRS total scorea from baseline to Day 2 (24 hours) | ||
| Baseline | ||
| N | 124 | 126 |
| Mean (SD) | 36.5 (5.21) | 35.9 (4.50) |
| Median (range) | 36.0 (25, 50) | 36.0 (27, 48) |
| Day 2 (24 hours) | ||
| N | 123 | 125 |
| Mean (SD) | 28.5 (9.39) | 31.5 (8.30) |
| Median (range) | 30.0 (0, 47) | 33.0 (1, 49) |
| Change from baseline to Day 2 (24 hours) | ||
| N | 123 | 125 |
| Mean (SD) | −8.0 (9.01) | −4.4 (7.66) |
| Median (range) | −6.0 (−38, 13) | −3.0 (−31, 12) |
| MMRM analysisb | ||
| Difference of LS means (SE) | −3.3 (1.02) | |
| 95% CI on difference | −5.33, −1.33 | |
| Change in SDS total scorec from baseline to Day 28 | ||
| Baseline | ||
| N | 119 | 122 |
| Mean (SD) | 22.9 (5.18) | 22.5 (5.20) |
| Median (range) | 24.0 (6, 30) | 23.5 (8, 30) |
| Day 28 | ||
| N | 100 | 99 |
| Mean (SD) | 16.6 (8.34) | 17.1 (8.31) |
| Median (range) | 17.0 (0, 30) | 18.0 (0, 30) |
| Change from baseline to Day 28 | ||
| N | 99 | 98 |
| Mean (SD) | −6.3 (7.54) | −5.3 (7.03) |
| Median (range) | −4.0 (−30, 6) | −4.0 (−22, 10) |
| MMRM analysisb | ||
| Difference of LS means (SE) | −1.0 (1.00) | |
| 95% CI on difference | −2.96, 0.97 | |
Notes: aMADRS total score ranges from 0 to 60; a higher score indicates a more severe condition. Negative change in score indicates improvement. bTest for treatment effect is based on MMRM analysis with change from baseline as the response variable and the fixed effect model terms for treatment (esketamine plus AD, AD plus placebo), day, country, class of oral AD (SNRI or SSRI), and treatment-by-day, and baseline value as a covariate. A negative difference favors esketamine. cSDS total score ranges from 0 to 30; a higher score indicates greater impairment. Negative change in score indicates improvement.
Abbreviations: AD, antidepressant; CI, confidence interval; LS, least squares; MADRS, Montgomery-Åsberg Depression Rating Scale; MMRM, mixed model for repeated measures; SD, standard deviation; SDS, Sheehan Disability Scale; SE, standard error; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Other Secondary Efficacy Analyses: CGI-S, GAD-7, and EQ-5D-5L
At Day 28, the LS mean difference (95% CI) of change from baseline in CGI-S between esketamine plus AD and AD plus placebo was −0.2 (−0.46, 0.10). CGI-S frequency distribution at baseline and Day 28 is shown in Figure S2. Severity of depression decreased during the treatment phase in both treatment groups with 29.2–30.9% of patients in the normal/borderline/mild category at Day 28.
Mean GAD-7 total scores improved from baseline to the endpoint of the double-blind phase in both treatment groups: mean (SD) changes from baseline were −4.3 (5.65) in the esketamine plus AD group and −2.9 (5.28) in the AD plus placebo group (Table S3). Using an ANCOVA model, the LS mean difference (95% CI) of change in GAD-7 total score from baseline to Day 28 between treatment groups was −1.6 (−2.78, −0.36), in favor of the esketamine plus AD group (Table S3).
The percentage of patients who reported problems in each of the 5 individual dimensions in the EQ-5D-5L assessment decreased from baseline to Day 28 of the double-blind phase in both treatment groups (Figure S3).
PK
Mean esketamine concentrations were highest in samples collected at 40 minutes postdose and declined over time (Table S4). Mean concentrations were slightly lower for the 56 mg (n = 13) dose group compared with the 84 mg (n = 75) dose group (101 ng/mL versus 108 ng/mL, respectively) at the 40-minute sampling timepoint, whereas dose-related differences in mean concentrations were observed at 2 hours and 6 hours postdose.
Safety
TEAEs
Overall, 120 (95.2%) esketamine-treated patients and 89 (70.6%) placebo-treated patients experienced 1 or more TEAEs in the double-blind phase (Table 4). A total of 117 (92.9%) esketamine-treated patients and 55 (43.7%) placebo-treated patients experienced 1 or more TEAEs that were considered by the investigator at least possibly related to the intranasal drug.
Table 4.
Safety Summary During the Double-Blind Treatment Phase
| Esketamine Plus AD (n=126) | AD Plus Placebo (n=126) | |
|---|---|---|
| TEAEs | 120 (95.2) | 89 (70.6) |
| Most common (≥10% of patients in either group) | ||
| Dizziness | 97 (77.0) | 25 (19.8) |
| Dissociation | 75 (59.5) | 8 (6.3) |
| Nausea | 53 (42.1) | 17 (13.5) |
| Blood pressure increased | 38 (30.2) | 13 (10.3) |
| Hypoesthesia | 25 (19.8) | 1 (0.8) |
| Vomiting | 23 (18.3) | 2 (1.6) |
| Vision blurred | 23 (18.3) | 1 (0.8) |
| Somnolence | 20 (15.9) | 11 (8.7) |
| Headache | 16 (12.7) | 14 (11.1) |
| Dysgeusia | 14 (11.1) | 6 (4.8) |
| TEAEs possibly related to intranasal druga | 117 (92.9) | 55 (43.7) |
| TEAEs leading to deathb | 1 (0.8) | 0 (0) |
| Treatment-emergent SAEs | 3 (2.4) | 3 (2.4) |
| Completed suicideb | 1 (0.8) | 0 (0) |
| Depression | 1 (0.8) | 3 (2.4) |
| Suicide attempt | 1 (0.8) | 0 (0) |
| TEAEs leading to discontinuation of intranasal study medicationc | 7 (5.6) | 2 (1.6) |
| Nausea | 2 (1.6) | 1 (0.8) |
| Vomiting | 2 (1.6) | 0 (0) |
| Dissociation | 2 (1.6) | 0 (0) |
| Dizziness | 2 (1.6) | 2 (1.6) |
| Depressional suicide | 1 (0.8) | 0 (0) |
| Headache | 1 (0.8) | 0 (0) |
| Asthenia | 1 (0.8) | 0 (0) |
| Blood pressure increased | 1 (0.8) | 0 (0) |
| Decreased appetite | 1 (0.8) | 0 (0) |
| Myalgia | 1 (0.8) | 0 (0) |
| Diarrhea | 0 (0) | 1 (0.8) |
| Parosmia | 0 (0) | 1 (0.8) |
| Insomnia | 0 (0) | 2 (1.6) |
Notes: All data are n (%). Incidence is based on the number of patients experiencing at least one AE, not the number of events. aStudy drug relationships of possible, probable, and very likely are included in this category. bSame patient. cAn AE that started in the double-blind treatment phase and resulted in discontinuation in the follow-up phase is counted as treatment-emergent in the double-blind treatment phase.
Abbreviations: AD, antidepressant; AE, adverse event; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
During the double-blind treatment phase, the most common TEAEs (reported by ≥10% of patients) in the esketamine plus AD group were dizziness (77.0%), dissociation (59.5%), nausea (42.1%), increased blood pressure (30.2%), hypoesthesia (19.8%), vomiting (18.3%), vision blurred (18.3%), somnolence (15.9%), headache (12.7%), and dysgeusia (11.1%); the most common TEAEs in the AD plus placebo group were dizziness (19.8%), nausea (13.5%), headache (11.1%), and blood pressure increased (10.3%) (Table 4). Most TEAEs were mild or moderate in severity. Overall, most TEAEs of dissociation, dizziness, vertigo, and increased blood pressure occurred on the day of dosing, and most of these events resolved on the same day (Table S5).
There was 1 death (0.8%) in the esketamine plus AD group during the double-blind phase (Table 4). The patient completed suicide on Day 4 after the first intranasal dose. The investigator considered the event possibly related to esketamine treatment. However, there were several contributing factors (eg, underlying severe depressive condition with worsening symptoms since the start of a major depressive episode despite several pharmacological treatments, prior high suicidal risk, and changes of medications in the last month), and the sponsor assessed the event as not related to esketamine treatment. One patient in the esketamine plus AD group with history of suicidal ideation and self-injury had a suicide attempt (aborted suicidal attempt) one day after Day 1 dosing, which was deemed by the investigator as doubtfully related to intranasal treatment. During the double-blind phase, 4 patients (1 in the esketamine plus AD group and 3 in the AD plus placebo group) experienced a SAE related to the underlying depression (Table 4).
Seven (5.6%) patients in the esketamine plus AD group and 2 (1.6%) in the AD plus placebo group experienced TEAEs leading to discontinuation of intranasal study medication (Table 4). The most common TEAEs leading to intranasal study medication discontinuation in esketamine-treated patients were nausea, vomiting, dissociation, and dizziness, all reported for 2 (1.6%) patients each.
Vital Signs and Physical Findings
Across all intranasal dosing days, mean systolic blood pressure and diastolic blood pressure values in the esketamine plus AD group increased at 40-minute postdose and subsequently returned close to predose values at 1.5-hour postdose (Figure S4). The mean (SD) maximum increase in systolic blood pressure from the predose timepoint was 13.2 (11.14) mmHg in the esketamine plus AD group and 5.4 (9.29) mmHg in the AD plus placebo group; the mean (SD) maximum increase in diastolic blood pressure from the predose timepoint was 10.2 (8.35) mmHg in the esketamine plus AD group and 4.7 (6.62) mmHg in the AD plus placebo group.
There were no clinically important findings in either treatment group for clinical laboratory evaluations, ECGs, nasal examinations, heart rate, pulse rate, pulse oximetry, respiratory rate, or ECG parameters from average predose over time during the double-blind phase.
C-SSRS
At baseline, 67.5% of patients in the esketamine plus AD group and 73.2% in the AD plus placebo group had no suicidal ideation or behavior based on C-SSRS. The percentage of patients reporting no suicidal ideation or behavior increased from baseline to the double-blind endpoint (Figure S5). At each post-baseline assessment, most patients in either treatment group reported a score of 0 indicating no events of suicidal ideation or behavior. During the double-blind treatment phase, 12 esketamine-treated patients and 14 placebo-treated patients had treatment-emergent post-baseline suicidal ideation versus baseline; 1 patient in the esketamine plus AD group had treatment-emergent post-baseline suicidal behavior versus baseline.
CADSS
In the esketamine plus AD group, the mean total and component CADSS scores peaked at 40-minute postdose and generally returned to predose values at 1.5-hour postdose (Figure S6). The peak mean CADSS total score at the 40-minute timepoint in esketamine-treated patients decreased over time with consecutive doses. More esketamine-treated patients (n = 91, 72.8%) had an increase greater than 4 in CADSS total score from predose at any time during the double-blind phase compared with placebo-treated patients (n = 6, 4.8%).
PWC-20
The changes in withdrawal symptoms as assessed by the PWC-20 after cessation of treatment with esketamine plus AD were consistent with observed changes in symptoms of depression and anxiety. No clear evidence of withdrawal was observed 2 weeks after cessation of treatment with esketamine plus AD.
Discussion
This active-controlled study investigating the efficacy and safety of esketamine versus placebo, each in combination with a newly initiated oral AD, in predominantly Chinese patients with TRD did not achieve statistical significance for the primary endpoint, change in MADRS total score from baseline to Day 28, although a 2-point difference was observed. The average 2-point difference from placebo at endpoint in short-term studies has been used to establish the clinical relevance of an AD against placebo.28,29 Although the first key secondary endpoint could not be formally evaluated due to the prespecified testing hierarchy, esketamine-treated patients experienced clinically important, rapid reduction in depressive symptoms within 24 hours after the first intranasal dose (LS mean difference [95% CI]: −3.3 [−5.33, −1.33]). A rapid reduction in depressive symptoms was also observed in the China population (LS mean difference [95% CI]: −2.6 [−4.64, −0.60]). These data are consistent with previous findings from esketamine studies for TRD and MDD with acute suicidal ideation or behavior,30,31 and reflect the characteristics of a rapid-acting antidepressant (ie, efficacy generally demonstrated within hours to 1 week).32 Efficacy data in this study are in contrast to the effects of standard oral ADs, which typically require several weeks before conferring clinical benefit.
In contrast to a previous report in a global population,17 this study conducted in a primarily Chinese population did not demonstrate a therapeutic effect after 4-week double-blind treatment with esketamine. The observed 2-point difference in MADRS total score was driven primarily by data from US patients. A smaller magnitude of change was observed in the China subgroup compared with the US subgroup and global phase 3 TRD studies.17,33
After completion of the study, a number of factors were explored, including demographic and clinical characteristics, treatment history, cultural and ethnic differences in a Western versus Chinese population, PK, and study execution, that may have contributed to the failure of this study. There were no obvious factors identified to explain the difference in the primary efficacy outcome between China and US subgroups, and global phase 3 TRD studies.17,33 With respect to PK, esketamine concentrations exhibited the expected dose-dependent differences between the 56 mg and 84 mg doses and were similar to those observed in the global phase 3 TRD studies.17,33
The use of remote blinded independent raters to administer the MADRS assessment by telephone was hypothesized as a potential contributing factor. Since independent remote raters did not know individual subjects and their baseline characteristics well and were unable to see the participants in order to judge affect or change in affect during the rating process, the use of independent remote raters may have reduced the sensitivity of detecting change in depressive symptoms. Remote independent rating had not been used in prior MDD registrational trials in China. According to an earlier pilot study in the China population,34 remote raters tended to rate patients slightly higher at the same visit than site-based raters, especially when patients demonstrated lower overall symptom severity. However, this finding was not observed in another similar pilot study in a US population, which demonstrated that remote blinded ratings were comparable to site-based ratings in this US population with TRD at all timepoints.35 This may be partly due to cultural and ethnic differences in a Western versus Chinese population in how patients express their depressive symptoms, especially to a stranger by telephone.
In addition, the hypothesis that remote rating may be less sensitive to change in depression may be supported by the observed outcomes in some PROs included in this study. In a post-hoc analysis of the percentage of patients with clinically meaningful improvement in MADRS and patient-reported outcomes (PROs) (GAD-7, EQ-5D-5L, and EQ-VAS) in the China population at Day 28, the PROs results appeared to favor esketamine over placebo (Figure S7), whereas similar response rates were seen for the clinician-rated MADRS for both treatment groups. This suggests that patients may have experienced some improvement not captured by the remote clinical rating.
Another potential factor was the heterogeneity of site experiences. In a post-hoc analysis of MADRS change over time in the China population, higher enrolling sites (Figure S8) showed a numerically greater treatment difference (favoring esketamine) in MADRS total score compared with lower enrolling sites. Higher enrolling sites tended to have more experience with MDD clinical trials and/or the treatment and management of patients with TRD compared with lower enrolling sites.
Safety was consistent with the established safety profile of esketamine, and no new safety signal was identified. The 2 cases of serious suicide-related events (completed suicide and suicide attempt) in the esketamine plus AD group were considered unrelated to esketamine treatment due to the underlying disease and prior high suicidal risk or history of self-injury. There were no clinically important findings observed during the double-blind phase or in the follow-up phase in either treatment group for clinical laboratory evaluations, nasal examinations, ECGs, or assessment by the C-SSRS, the CADSS, or the PWC-20.
Several limitations of the study merit comment. As esketamine has known transient dissociative effects that are difficult to blind (the dissociative effects are not possible to mimic adequately using an active placebo), these specific treatment-emergent events could have biased the staff who provided and observed the dosing. Therefore, to ensure an unbiased efficacy evaluation, independent, remote (by telephone), blinded MADRS raters were used to assess the treatment response. However, the use of remote raters may have reduced the sensitivity of detecting change in depressive symptoms as discussed above. In addition, as this was a flexible-dose study, dose–response relationships could not be evaluated because direct comparisons between dose groups could not be made.
Conclusions
Results from the primary efficacy analysis showed that treatment with esketamine plus a newly initiated oral AD did not demonstrate statistical superiority for improvement in depressive symptoms compared with treatment with a newly initiated oral AD plus placebo at Day 28 in patients with TRD. Although the key secondary endpoints could not be formally evaluated due to the testing hierarchy, rapid reduction in depressive symptoms within 24 hours was observed for the patients treated with esketamine plus a newly initiated oral AD in the overall population and China population. Safety findings, including types and incidences of TEAEs, SAEs, and TEAEs leading to treatment discontinuation, as well as laboratory assessments, vital sign measurements, and scale data (including CADSS, C-SSRS, and PWC-20), were consistent with the established safety profile of esketamine assessed in the completed global phase 3 studies and no new safety signal was identified.
Acknowledgments
The authors thank all patients for their participation in this study and acknowledge the collaboration and commitment of the investigators and their staff. Writing assistance was provided by Jennifer DiNieri, PhD, of System One. Additional editorial support was provided by Harry Ma, PhD, of Janssen Global Services, LLC.
Funding Statement
The study was funded by Janssen Research & Development, LLC.
Data Sharing Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access [YODA] Project site at http://yoda.yale.edu.
Disclosure
Xu Chen and Gang Wang declare no conflicts of interest in this work. Xuan Hou, Daisy Bai, Rosanne Lane, Chong Zhang, Carla Canuso, and Dong-Jing Fu are employees of Janssen Research & Development, LLC and may own stock or stock options in Johnson & Johnson.
References
- 1.Baldessarini RJ, Forte A, Selle V, et al. Morbidity in Depressive Disorders. Psychother Psychosom. 2017;86(2):65–72. doi: 10.1159/000448661 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Depression Fact Sheet; 2021. Available from: http://www.who.int/en/news-room/fact-sheets/detail/depression. Accessed July 11, 2022.
- 3.Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: A cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi: 10.1016/S2215-0366(18)30511-X [DOI] [PubMed] [Google Scholar]
- 4.Gaynes B, Asher G, Gartlehner, G et al. Definition of Treatment-Resistant Depression in the Medicare Population [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018. [PubMed] [Google Scholar]
- 5.Al-Harbi KS. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–388. doi: 10.2147/PPA.S29716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. doi: 10.1016/S0006-3223(03)00231-2 [DOI] [PubMed] [Google Scholar]
- 7.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- 8.Fife D, Feng Y, Wang MY, et al. Epidemiology of pharmaceutically treated depression and treatment resistant depression in Taiwan. Psychiatry Res. 2017;252:277–283. doi: 10.1016/j.psychres.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 9.Shram MJ, Sellers EM, Romach MK. Oral ketamine as a positive control in human abuse potential studies. Drug Alcohol Depend. 2011;114:185–193. doi: 10.1016/j.drugalcdep.2010.10.002 [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Wu T, Dong S, et al. One-year incidence rate of treatment resistant depression (TRD) and treatment characteristics in China. J Affect Disord. 2022;305:77–84. doi: 10.1016/j.jad.2022.02.054 [DOI] [PubMed] [Google Scholar]
- 11.Saveanu R, Etkin A, Duchemin AM, et al. The international Study to Predict Optimized Treatment in Depression (iSPOT-D): Outcomes from the acute phase of antidepressant treatment. J Psychiatr Res. 2015;61:1–12. doi: 10.1016/j.jpsychires.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 12.DeWilde KE, Levitch CF, Murrough JW, et al. The promise of ketamine for treatment-resistant depression: Current evidence and future directions. Ann N Y Acad Sci. 2015;1345:47–58. doi: 10.1111/nyas.12646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: Potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SPRAVATO [Prescribing Information]. Janssen Pharmaceuticals, Inc, Titusville, NJ; 2020. Available from: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SPRAVATO-pi.pdf. Accessed July 11, 2022. [Google Scholar]
- 15.SPRAVATO [EPAR – Product Information]. Janssen-Cilag International NV, Beerse, Belgium; 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/spravato-epar-product-information_en.pdf. Accessed July 11, 2022. [Google Scholar]
- 16.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 17.Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: A randomized double-blind active-controlled study. Am J Psychiatry. 2019;176:428–438. doi: 10.1176/appi.ajp.2019.19020172 [DOI] [PubMed] [Google Scholar]
- 18.Leon AC, Olfson M, Portera L, et al. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27:93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD [DOI] [PubMed] [Google Scholar]
- 19.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95. doi: 10.1097/00004850-199606003-00015 [DOI] [PubMed] [Google Scholar]
- 20.Guy W, Ed. Clinical Global Impressions, In: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD, U.S: Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 21.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 22.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson KM, Devine JM, Ho KF, et al. Evidence to support Montgomery-Asberg Depression Rating Scale administration every 24 hours to assess rapid onset of treatment response. J Clin Psychiatry. 2016;77:1681–1686. doi: 10.4088/JCP.15m10253 [DOI] [PubMed] [Google Scholar]
- 24.Posner K, Oquendo MA, Gould M, et al. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/ajp.2007.164.7.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902 [DOI] [PubMed] [Google Scholar]
- 26.Rickels K, Garcia-Espana F, Mandos LA, et al. Physician Withdrawal Checklist (PWC-20). J Clin Psychopharmacol. 2008;28:447–451. doi: 10.1097/JCP.0b013e31817efbac [DOI] [PubMed] [Google Scholar]
- 27.Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry. 2018;75:139–148. doi: 10.1001/jamapsychiatry.2017.3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melander H, Salmonson T, Abadie E, et al. A regulatory Apologia--A review of placebo-controlled studies in regulatory submissions of new-generation antidepressants. Eur Neuropsychopharmacol. 2008;18:623–627. doi: 10.1016/j.euroneuro.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 29.Montgomery SA, Möller HJ. Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol. 2009;24:111–118. doi: 10.1097/YIC.0b013e32832a8eb2 [DOI] [PubMed] [Google Scholar]
- 30.Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: Double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191. doi: 10.4088/JCP.19m13191 [DOI] [PubMed] [Google Scholar]
- 31.Ionescu DF, Fu DJ, Qiu X, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: Results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol. 2021;24(1):22–31. doi: 10.1093/ijnp/pyaa068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration. Draft Guidance “Major Depressive Disorder: Developing Drugs for Treatment Guidance for Industry”. 2018. Available from: https://www.fda.gov/media/113988/download. Accessed February 9, 2023.
- 33.Fedgchin M, Trivedi M, Daly EJ, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: Results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019;22:616–630. doi: 10.1093/ijnp/pyz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G, Si T, Xu X, et al. Independent telephone-based assessment of depressive symptoms in China. Presented at The International Society for CNS Clinical Trials and Methodology (ISCTM) Autumn Conference, Marina Del Ray, CA. 2018. [Google Scholar]
- 35.Targum SD, Daly E, Fedgchin M, et al. Comparability of blinded remote and site-based assessments of response to adjunctive esketamine or placebo nasal spray in patients with treatment resistant depression. J Psychiatr Res. 2019;111:68–73. doi: 10.1016/j.jpsychires.2019.01.017 [DOI] [PubMed] [Google Scholar]