Abstract
For the Schistosoma mansoni flatworm pathogen, we report a structure–activity relationship of 25 derivatives of the N-phenylbenzamide compound, 1 (MMV687807), a Medicines for Malaria Venture compound for which bioactivity was originally identified in 2018. Synthesized compounds were cross-screened against the HEK 293 mammalian cells. Compounds 9 and 11 were identified as fast-acting schistosomicidal compounds whereby adult worm integrity was severely compromised within 1 h. Against HEK 293 mammalian cells, both compounds exhibited high CC50 values (9.8 ± 1.6 and 11.1 ± 0.2 μM respectively) which could translate to comfortable selectivity. When evaluated in a concentration-response format, compound 9 was active in the nanomolar range (EC50 = 80 nM), translating to a selectivity index of 123 over HEK 293 cells. The data encourage the further investigation of N-phenylbenzamides as antischistosomals.
Keywords: Antischistosomal agents, N-phenylbenzamide analogs, Schistosoma mansoni
Introduction
Schistosomiasis or bilharzia is a neglected tropical disease caused by blood-dwelling flukes of the genus Schistosoma.1 It is chronic and debilitating, and infects over 240 million people in 78 developing countries. Also, over 700 million people are at risk of acquiring the disease.2,3 In 2019, it was estimated that over 236.6 million people required preventive treatment for schistosomiasis.3 Three species are the most medically important, Schistosoma mansoni, Schistosoma haematobium and Schistosoma japonicum.1
In the absence of a vaccine, schistosomiasis control and treatment relies on a single drug, praziquantel (PZQ), which has been used in mass drug administration programs for over 40 years. PZQ is safe and active against all relevant schistosome species, and is distributed free of charge. However, the drug is not effective against juvenile worms, and does not prevent reinfection.4 The recent identification of field isolates with low sensitivity or tolerance to praziquantel, coupled with a small drug development pipeline5 highlights the urgent need for new antischistosomal drugs.
In this context of initiating drug development, the N-phenylbenzamide, compound 1 (Fig. 1), which is one of 400 compounds assembled into the Medicines for Malaria Venture’s (MMV) Pathogen box, was reported to kill S. mansoni adults6 and schistosomula (post-infective larvae)7 in vitro. Accordingly, we selected compound 1 as a starting point to derive analogs and develop a structure–activity relationship (SAR) with a view to optimizing potential antischistosomal agents.
Fig. 1.
Structure of compound 1.
To evaluate the SAR of N-phenylbenzamide derivatives against adult S. mansoni, the different substituents on the N-phenylbenzamide core scaffold were selected from a Craig plot (Fig. 2) covering all four quadrants.8 The Craig plot is a powerful tool used to predict the correlation between the physicochemical properties and the biological activity of molecules by plotting the hydrophobic character (π) of a substituent versus its electronic effect (Hammett substitution constant, σ).8 In total, 25N-phenylbenzamide derivatives were designed and synthesized.
Fig. 2.

Craig plot of Hammett substitution constants (σ) and hydrophobicity (π).
Scheme 1 shows the synthetic route for N-phenylbenzamide derivatives using modified procedures reported by Tegeli and Larsen, et al.9,10.
Scheme 1.
Reagents and conditions: (a) 3,4-dimethoxyaniline, EDCI, DMAP, DMF, 50 °C, 72 h for compound 4; (b) appropriate aromatic amine, EDCI, DMAP, DCM, 0 °C – 30 °C, 19–24 h for compounds 6 – 18 and 21 – 30.
| 4 R1 = R2 = OMe, R3 = OH, R4 = H, R5 = Cl | 15 R1 = R3 = R4 = R5 = H, R2 = CF3 |
| 6 R1 = R2 = R3 = R5 = H, R4 = CF3 | 16 R1 = R3 = R5 = H, R2 = CF3, R4 = F |
| 7 R1 = R2 = Me, R3 = R5 = H, R4 = CF3 | 17 R1 = R2 = OMe, R3 = R5 = H, R4 = F |
| 8 R1 = R2 = OMe, R3 = R5 = H, R4 = CF3 | 18 R1 = R3 = R5 = H, R2 = CF3, R4 = CN |
| 9 R1 = R2 = Cl, R3 = R5 = H, R4 = CF3 | 21 R1 = R2 = Me, R3 = R5 = H, R4 = F |
| 10 R1 = R4 = CF3, R2 = R3 = R5 = H | 22 R1 = R2 = Cl, R3 = R5 = H, R4 = F |
| 11 R1 = R3 = R5 = H, R2 = R4 = CF3 | 23 R1 = R2 = R3 = R5 = H, R4 = F |
| 12 R1 = R2 = F, R3 = R5 = H, R4 = CF3 | 24 R1 = R2 = F, R3 = R4 = R5 = H |
| 13 R1 = R2 = R4 = F, R3 = R5 = H | 25 R1 = R2 = Cl, R3 = R4 = R5 = H |
| 14 R1 = R2 = Me, R3 = R4 = R5 = H | 26 R1 = R2 = OMe, R3 = R4 = R5 = H |
| 27 R1 = R2 = Me, R4 = CN, R3 = R5 = H | 29 R1 = R2 = H, R4 = CN, R3 = R5 = H |
| 28 R1 = R2 = OMe, R4 = CN, R3 = R5 = H | 30 R1 = R2 = F, R4 = CN, R3 = R5 = H |
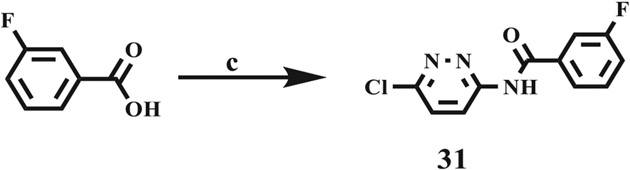
Scheme 2 shows the synthetic route for the N-(6-chloropyridazin-3-yl)-3-fluorobenzamide derivative using a modified procedure reported by Savjani, et al.11
Scheme 2.
Reagents and conditions: (c) 6-chloro-3-aminopyridazine (dissolved in methanol), DCC, DMAP, DCM, 0° C – rt, 24 h.
Coupling of 3,4-dimethoxyaniline with 5-chlorosalisylic acid gave compound 4. Acid-amine coupling of 4-(trifluoromethyl)benzoic acid with different substituted anilines gave compounds 6–12. The compounds 13, 16, 17, and 21 – 23 were obtained via coupling of 4-fluorobenzoic acid with different substituted anilines. Compound 18 was prepared from the reaction of 4-cyanobenzoic acid with 3-(tri-fluoromethyl)aniline. Compounds 14, 15 and 24 – 26 were realized by the reaction of benzoic acid with different substituted anilines. Compounds 27–30 were prepared from 4-cyanobenzoic acid and substituted anilines. Compound 31 was synthesized by the reaction of 3-Fluorobenzoic acid with 6-chloro-3-aminopyridazine.
The target compounds were evaluated for antischistosomal activity at 5 μM against adult S. mansoni. The antischistosomal activity was recorded visually as a function of time (1, 5, 24 and 48 h) using a constrained nomenclature of descriptors that convey the many responses to chemical insult that the schistosome flatworm is capable of. Each descriptor is awarded a value and these are added up to provide a ‘severity score’ that ranges from zero (no activity) to four (maximum activity).12,13 The severity score data are presented in Table 1, whereas the underlying descriptor data are found in the Supporting Information. The most potent compounds in the severity score assay were also evaluated at 24 h over five concentrations (5–0.02 μM) in a worm motility assay (WormAssay) whereby motility was considered an indicator of parasite viability. Motility was plotted as a function of compound concentration to generate EC50 data (Table 1).
Table 1.
In vitro activity of N-phenylbenzamide analogs against adult S. mansoni and HEK 293 cells.
| Code | Chemical structure | Adult S. mansoni Severity scores (5 μM)a |
Adult S. mansoni EC50 (μM)b |
HEK 293 CC50 (μM)c |
SId | |||
|---|---|---|---|---|---|---|---|---|
| 1 h | 5 h | 24 h | 48 h | |||||
| 4 |

|
0 | 1 | 1 | 1 | - | > 20 | - |
| 6 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 7 |

|
2 | 2 | 3 | 3 | 3.7 | > 20 | > 5.4 |
| 8 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 9 |

|
3 | 3 | 4 | 4 | 0.08 | 9.8 ± 1.6 | 123 |
| 10 |

|
1 | 1 | 2 | 3 | 1.25 | > 20 | > 16 |
| 11 |

|
3 | 3 | 3 | 4 | 1.10 | 11.1 ± 0.2 | 10.1 |
| 12 |

|
2 | 2 | 2 | 3 | 3.80 | 14.6 ± 2.0 | 3.84 |
| 13 |

|
1 | 1 | 0 | 0 | - | > 20 | - |
| 14 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 15 |

|
1 | 1 | 0 | 0 | - | > 20 | - |
| 16 |

|
1 | 0 | 0 | 0 | - | > 20 | - |
| 17 |

|
1 | 0 | 0 | 0 | - | > 20 | - |
| 18 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 21 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 22 |

|
2 | 2 | 2 | 2 | - | > 20 | - |
| 23 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 24 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 25 |

|
2 | 2 | 2 | 2 | - | > 20 | - |
| 26 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 27 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 28 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 29 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 30 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
| 31 |

|
0 | 0 | 0 | 0 | - | > 20 | - |
Each compound was screened twice as a singleton and representative data are shown.
24 h time point assay.
Human embryonic kidney cell lines; 48 h time point assay.
SI, selectivity index, i.e., HEK 293 CC50/WormAssay EC50.
Based on the data presented in Table 1, a number of SAR attributes could be identified. Electron withdrawing groups on the meta and para positions on the left hand side phenyl (dichlorination in 9) induced strong degenerative changes, including death, by 48 h with associated severity scores of 3 and 4. This is in contrast to the electron donating methoxy groups in 8 which was inactive. Interestingly, regio-isomerism on the left hand side phenyl appears consequential to antischistosomal activity. Specifically, shifting the position of the trifluoromethyl substituent from the para position in 10 to the meta position (11) increased activity, whereby the latter compound induced higher severity scores more rapidly which manifested as obvious degenerative changes by 48 h. Disubstitution (either with 2Cl or 2F) at the meta and para positions of the left hand side phenyl (9 and 12), while maintaining a highly electron withdrawing CF3 on the right hand side phenyl, appeared to preserve activity, albeit that the 2Cl group was the more active, whereby the worms had already died by 48 h.
When the CF3 on the right hand side phenyl was replaced with a F, a substituent with a weaker electron-withdrawing capacity (see Craig plot in Fig. 2), activity was essentially compromised (12 vs 13), apart from a transient decrease in worm motility at the two early time points for 13. Even with a highly electron-withdrawing group on the right hand side phenyl, highly electron donating groups on the left hand side phenyl, e.g., 8, eliminated activity. The removal of a CF3 from the right hand side phenyl, while maintaining the dimethyl substitution on the left hand side phenyl abolished activity (7 vs 14).
Compound 7 is particularly interesting because it retains substantial potency despite possessing electron releasing groups on the left hand side phenyl (7 vs 8). More SAR investigations are required to understand whether or not disubstitution/monosubstitution with other electron donating groups on the left hand side phenyl (such as OH and NH2), while maintaining a CF3 on the right hand side phenyl, would preserve activity.
On replacement of the CF3 with a fluoro on the right hand side phenyl (11 vs 16), activity was essentially abolished. The same replacement to derive 22 from 9 led to a decrease in activity whereby the strong degenerative and eventually lethal changes (severity score of 4) noted for 9 were replaced with uncoordinated and slowed motility (score of 2). Also, the replacement of the CF3 with a cyano group on the right hand side phenyl (11 vs 18) abolished activity. This was also true for analogs possessing a cyano group on the right hand side phenyl with variations on the left hand side phenyl, i.e., 27–30.
We also introduced a pyridazine ring system in molecule 31 in an effort to introduce novelty and positively modulate physicochemical parameters such as solubility and cLogP. Unfortunately, these changes resulted in an inactive compound. Future studies could incorporate the substituents drawn from compounds 9 and 11 (most active), into the pyridazine and phenyl rings of 31 with the hope that this could preserve activity. Last, it is also important to note that simplification of the structures by removing substituents from the right hand side phenyl (e.g., 14, 15 and 24–26) abolished activity, albeit some activity was retained for 25 whereby the worms had decreased motility and were unable to adhere to the surface of the well. Likewise, the removal of substituents on the left hand side phenyl, e.g., 6 and 23, yielded inactive compounds. In summary, it seems that electron withdrawing groups on the left hand side phenyl (CF3, 2Cl or 2F) and the right hand side phenyl (as CF3) resulted in active compounds (Fig. 3). The hierarchy of potency on the left hand side phenyl was as follows: 2Cl < 2F < unsubstituted. As for the right hand side phenyl, potency progressed as CF3 < F < unsubstituted. The combined effects of the most active Craig plot substituents on the two phenyls resulted in compound 9, which was the most potent compound. This is consistent with previous investigations of this compound class.14
Fig. 3.
Summary of the antischistosomal SAR for the N-phenylbenzamide scaffold.
The most active compounds from the severity score assay were assessed for a concentration-dependent (from 5 to 0.02 μM) reduction in worm motility at 24 h using a WormAssay. Compound 9 was the most active (EC50 = 0.08 μM) whereas 11, 10, 7 and 12 had low micromolar EC50 values of 1.10, 1.25, 3.70 and 3.80 μM, respectively (Table 1). Against HEK 293 cells after 48 h, those compounds that were the most active against the parasites also registered CC50 values of < 20 μM, i.e., 9 (CC50 = 9.8 ± 1.6 μM), 11 (CC50 = 11.1 ± 0.2 μM) and 12 (CC50 = 14.6 ± 2.0 μM) (Table 1). However, for 9, the associated selectivity index was relatively high (SI = 128) which encourages further medicinal chemistry expansion of these compounds.
In conclusion, SAR studies were conducted around compound 1 leading to the generation of several new N-phenylbenzamide derivatives. Compound 9 emerged as the most promising antischistosomal (EC50 = 0.08 μM). Further, 9 had a comfortable selectivity index value of 123. Further exploration of much stronger electron withdrawing groups (e.g., NO2, CF3SO2) around the N-phenylbenzamide scaffold to potentially enhance activity is ongoing in our laboratories.
Supplementary Material
Acknowledgements
This study was financially supported by ARES Trading S.A., an affiliate of Merck KGaA, Darmstadt, Germany. We gratefully acknowledge Prof. Kelly Chibale at the Drug Discovery and Development Centre (H3D) in the Department of Chemistry of the University of Cape Town for his assistance in conducting spectroscopic experiments. The Department of Chemistry of the University of Zambia is also gratefully acknowledged for the support (P.M.C.). Maintenance of the S. mansoni life-cycle was supported, in part, by a NIH-NIAID R21AI156554 grant to C.R.C. among other principal investigators. Also, adult worms were harvested from LVG Golden Syrian hamsters which were provided by the NIAID Schistosomiasis Resource Center of the Biomedical Research Institute (Rockville, MD) under the NIH-NIAID Contract HHSN272201700014I.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data (experimental details for synthetic protocols, chemical characterisation of compounds, and biological procedures). Supplementary data to this article can be found online at https://doi.org/10.1016/j.bmcl.2023.129164.
Data availability
Data will be made available on request.
References
- 1.Schistosomiasis, https://www.cdc.gov/dpdx/schistosomiasis/index.html (accessed 2022-11-22).
- 2.Schistosomiasis (Bilharzia). https://www.who.int/health-topics/schistosomiasis (accessed 2022-11-22).
- 3.WHO factsheet. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed 2022-11-22).
- 4.Spangenberg T. Alternatives to praziquantel for the prevention and control of schistosomiasis. ACS Infect Dis. 2020;7:1–4. 10.1021/acsinfecdis.0c00542. [DOI] [PubMed] [Google Scholar]
- 5.Cheuka PM. Drug discovery and target identification against schistosomiasis: a reality check on progress and future prospects. Curr Top in Med Chem. 2022;22:1595–1610. [DOI] [PubMed] [Google Scholar]
- 6.Pasche V, Laleu B, Keiser J. Early antischistosomal leads identified from in Vitro and in Vivo screening of the medicines for malaria venture pathogen box. ACS Infect Dis. 2018;5:1–9. 10.1021/acsinfecdis.8b00220. [DOI] [PubMed] [Google Scholar]
- 7.Maccesi M, Aguiar PHN, Pasche V, et al. Multi-center screening of the pathogen box collection for schistosomiasis drug discovery. Parasit Vect. 2019;12:1–10. 10.1186/s13071-019-3747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapoor Y, Kumar K. Quantitative structure activity relationship in drug design: an overview. SF J Pharm Anal Chem. 2019;2:1–13. [Google Scholar]
- 9.Tegeli VS. Synthesis of ester and amide derivatives of certain NSAIDS. Solapur University; 2015. [Google Scholar]
- 10.Larsen BJ, Rosano RJ, Ford-hutchinson TA, Reitz AB, Wrobel JE. A method for C2 acylation of 1,3-indandiones. Tetrahedron. 2018;74:2762–2768. 10.1016/j.tet.2018.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savjani JK, Mulamkattil S, Variya B, Patel S. Molecular docking, synthesis and biological screening of mefenamic acid derivatives as anti-inflammatory agents. Eur J Pharmacol. 2017;801:28–34. 10.1016/j.ejphar.2017.02.051. [DOI] [PubMed] [Google Scholar]
- 12.Long T, Neitz RJ, Beasley R, et al. Structure-bioactivity relationship for benzimidazole thiophene inhibitors of polo-like kinase 1 (PLK1), a potential drug target in Schistosoma mansoni. PLoS Neglect Trop Dis. 2016;10:1–21. 10.1371/journal.pntd.0004356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buskes MJ, Clements M, Bachovchin KA, et al. Structure-bioactivity relationships of lapatinib derived analogs against Schistosoma mansoni. ACS Med Chem Lett. 2020;11:258–265. 10.1021/acsmedchemlett.9b00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan N, Dätwyler P, Ernst B, et al. Activities of N, N′-Diarylurea MMV665852 analogs against Schistosoma mansoni. Antimicrob Agents Chemother. 2015;59:1935–1941. 10.1128/AAC.04463-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.