Abstract
Objective:
Ketamine’s effects on different dimensions of depressive symptomatology, including typical/melancholic and atypical depression, remain largely unknown. This study examined the effects of a single intravenous dose of ketamine on general depressive symptoms (measured using the Montgomery–Asberg Depression Rating Scale (MADRS), typical/melancholic symptoms (measured using the MADRS5), and atypical symptoms (measured using the Scale for Atypical Symptoms (SAS)).
Methods:
Data from 68 participants with treatment-resistant major depressive disorder (MDD) or bipolar depression were pooled from three separate, double-blind, placebo-controlled, crossover studies investigating ketamine’s efficacy in depression. MDD participants were unmedicated; bipolar participants received therapeutic-dose lithium or valproate. Clinical symptoms were collected preinfusion and up to 14 days postinfusion. Effect sizes were calculated for days 1 and 3 postinfusion. The primary measures of interest for this exploratory analysis were total MADRS, MADRS5, and SAS scores. Individual symptoms were also analyzed in an exploratory manner.
Results:
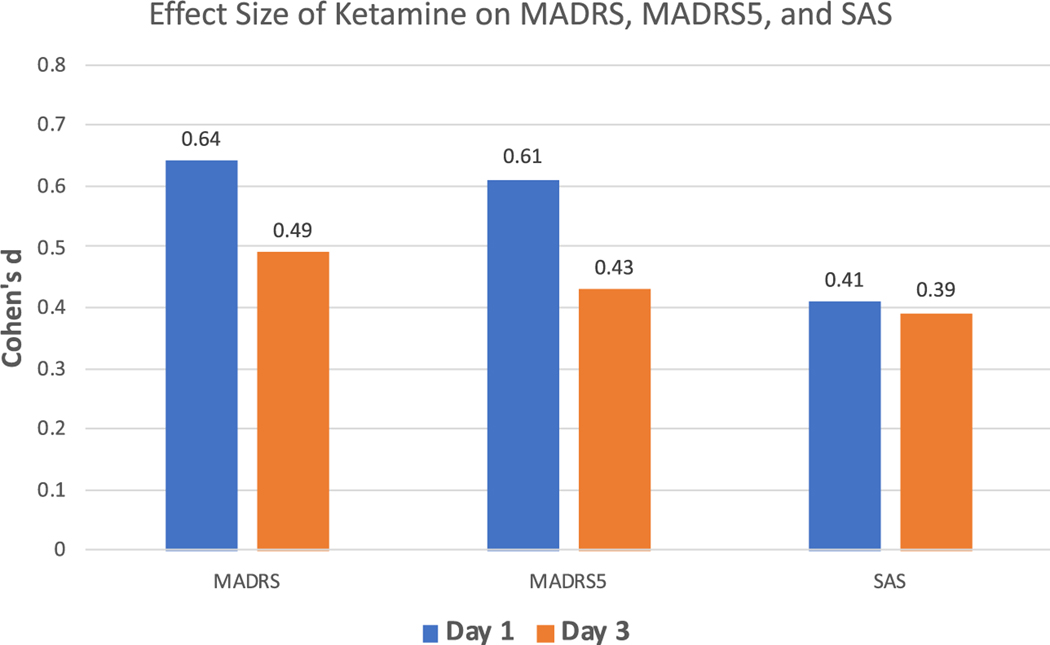
Scores improved significantly at Day 1 postinfusion (MADRS: Cohen’s d = 0.64; MADRS5: Cohen’s d = 0.61; SAS: Cohen’s d = 0.41) and continued to be significantly improved over placebo at Day 3 (MADRS: Cohen’s d = 0.49; MADRS5: Cohen’s d = 0.43; SAS: Cohen’s d = 0.39). Effect sizes were greater for typical/melancholic than atypical symptoms at Day 1 postinfusion.
Conclusion:
Ketamine appears to effectively treat both the typical/melancholic and atypical symptoms of depression, but may have early preferential effects for the former.
Keywords: ketamine, atypical depression, melancholic depression, sleep, appetite
Introduction
One of the most common patterns of depressive presentation is that of atypical depression. Despite its nomenclature, atypical depression may in fact be one of the most common patterns of depression; one study identified definite atypical subtype in 30% and probable atypical subtype in 20% of both the MDD and bipolar depression populations (1). Another study of depressed out-patients presenting to a research program found that a majority (56%) demonstrated some atypical symptoms, while fewer (26%) had typical or melancholic symptoms; 14% of this sample (included in the percentages above) demonstrated symptoms of both, and 32% of the overall sample met criteria for neither (2).
The atypical concept originated in 1959 when West and Dally initially described an atypical depressive state characterized by comorbid anxiety and mood reactivity distinct from endogenous depression (3). They noted that this form of depression demonstrated greater resistance to standard antidepressant treatments, though it responded to the anti-tuberculosis drug iproniazid (a monoamine oxidase inhibitor (MAOI)) (3). Sargant similarly advocated for identifying clinical subtypes based on response to certain treatments. He identified an ‘atypical’ depression, characterized by ‘hysterical’ features, increased emotionality and reactivity, and anxious symptoms that responded to MAOIs when standard treatments, including electroconvulsive therapy (ECT), were ineffective (4).
Currently, the DSM 5 classifies atypical symptoms as a specifier, denoting that this symptom constellation is not mutually exclusive to, and may coexist with, other depressive symptom patterns (5). The atypical specifier is applied when mood reactivity (experiencing lift in mood in response to positive events) is present as well as at least two of the following: weight gain or increased appetite, hypersomnia, leaden paralysis, or extreme sensitivity to perceived interpersonal rejection resulting in social or occupational impairment (2). However, significant debate exists regarding what to consider the core constitutive symptoms of atypical depression. Two major symptomatic areas – reverse neurovegetative symptoms (i.e., hypersomnia and hyperphagia or weight gain) and mood reactivity – have typically been associated with most definitions of atypical depression (6). Nevertheless, some authors have questioned the inclusion of mood reactivity as an accurate or useful criterion for diagnosing atypical depression (7), and others have further argued against the concept’s ‘hysterical’ roots, suggesting that atypical depression may represent a personality subtype of ‘interpersonal rejection sensitivity’ (8). Other investigators have postulated that atypical depression may be a variant of bipolar II disorder, as it co-occurs more frequently with this diagnosis than with other forms of depression (9).
Results from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study – the largest real-world examination of major depressive disorder (MDD) treatment –found that atypical depression was more likely to be associated with female gender, earlier age of symptom onset, greater anxiety comorbidity, and greater symptom severity (10). More recent studies suggest that the atypical pattern is particularly robust and can primarily be distinguished from typical/ melancholic depression by sleep and appetite symptoms (11). A latent class analysis of depressive subtypes found that atypical symptoms were more strongly associated with women, higher body mass index, greater inflammation, and greater incidence of metabolic syndrome (12); in contrast, typical/melancholic symptoms were associated with lower blood pressure and hypothalamic–pituitary–adrenal (HPA) axis hyperactivity. Compared to melancholic depression, atypical symptoms have also been associated with decreased corticotropin-releasing hormone function (13, 14), lower plasma cortisol levels (11), increases in C-reactive protein and the adipokine leptin (15), and a distinct polygenic signature (16). These findings suggest that atypical depression may represent a distinct process of inflammatory and immunometabolic dysregulation (16).
While typical and atypical subtypes are often thought of as distinct presentations, a Swiss study found the co-occurrence of melancholic and atypical symptoms in almost half of a community sample of individuals experiencing a major depressive episode (17). These findings underscore the fact that different patterns of depressive symptoms (i.e., typical/melancholic versus atypical, among others), as well as their underlying biological substrates, are just as likely to coexist than present as pure form subtypes. This coexistence of different types of depressive symptoms argues for a dimensional approach to depression that can be conceptualized as a collection of related overlapping pathophysiologies and presentations (18).
The way in which different symptom constellations respond to various treatments has been an issue of long-standing interest in the study of depression. The initial identification of an atypical subtype was originally based on differential response to an MAOI. A number of studies have found that ketamine has rapid and robust – though transient – antidepressant effects in individuals with MDD and bipolar depression (19); in this context, a single intravenous infusion of subanesthetic-dose ketamine has been found to alleviate depressive symptoms within minutes, with effects lasting as long as one week. Given the differences in biological substrates elaborated above, it is therefore possible that individuals with atypical depression might also exhibit a differential response to ketamine. In a recent analysis, Wang and colleagues (20) – while not specifically examining atypical depression – demonstrated relatively greater (or more rapid) response to intravenous ketamine in individuals with non-melancholic or anxious depression.
Aims of the study
This post hoc exploratory investigation examined the effects of a single intravenous infusion of sub-anesthetic-dose (0.5 mg/kg) ketamine on atypical and typical/melancholic symptoms across a sample of individuals with treatment-resistant depression (TRD). Specifically, the study examined: (i) whether atypical depressive symptoms improved in response to ketamine; (ii) whether atypical depressive symptoms and typical/melancholic symptoms both responded to ketamine; and (iii) how specific individual symptoms – both typical/melancholic and atypical – responded to ketamine.
Material and methods
Participant selection
Data were collected from 68 in-patients who participated in one of the three independent ketamine studies conducted at the National Institute of Mental Health (NIMH) that sought to determine the safety and efficacy of ketamine versus placebo in treatment-resistant MDD or bipolar I/II depression without psychotic features; primary outcome results for the three studies have previously been reported (21–23) (Clinical Trials Identifier: NCT0088699). Participants between the ages of 18 and 65 years were admitted to the NIMH Mood and Anxiety Disorders research unit in Bethesda, Maryland, USA. All participants provided written informed consent as approved by the National Institutes of Health combined Central Nervous System Institutional Review Board. All neuropsychiatric diagnoses were based on DSM-IV-TR criteria as confirmed by both clinical interviews performed by a licensed independent psychiatric practitioner and the Structured Clinical Interview for Axis I DSM-IV Disorders, Patient Version (SCID-P) (24). All participants met criteria for a major depressive episode of at least moderate severity, defined as a Montgomery–Asberg Depres- sion Rating Scale (MADRS) total score ≥20 or ≥22 (depending on the inclusion criteria of each study) at screening and prior to each infusion. All participants were treatment-resistant, as defined by having failed to respond to at least one previous antidepressant trial. All participants were in good physical health as determined by medical examination, medical history, chest X-ray, blood laboratory tests, and urinary analysis. Exclusion criteria included pregnancy, nursing, serious suicidal ideation, and previous use of or treatment with ketamine.
Study design
All three studies were single-center, double-blind, placebo-controlled, crossover trials with order of intervention randomized; all were conducted between October 2006 and June 2015. After signing protocol-specific consent forms, all MDD participants were tapered off their current psychotropic medication regimen and remained medication-free for at least two weeks (five weeks for fluoxetine) prior to infusion. Participants with bipolar I/II depression were tapered off all psychotropic medications except lithium or valproate and maintained on therapeutic levels for at least four weeks as a witnessed prospective trial for improvement in depression. Participants in all three studies received both a single subanesthetic (0.5 mg/kg) infusion of ketamine hydrochloride over 40 min and an infusion of normal saline placebo over 40 min, two weeks apart. Independent pharmacists randomized each participant and dispensed either active or placebo solution in identical packaging. Active or placebo treatment status was not divulged to investigators, nursing staff, or clinical raters. Rating scales (see below) were administered at multiple time points, including 60 min preinfusion (baseline), at 40, 80, 120, and 230 min, and at days 1 and 3 postinfusion.
Study measures
Overall depressive symptomatology was assessed via the MADRS, a 10-item clinician-administered scale. Symptoms associated with typical/melancholic depression were assessed via the MADRS5. The MADRS5 includes items 1 (apparent sadness), 3 (inner tension), 7 (lassitude), 8 (inability to feel), and 9 (pessimistic thoughts) from the general MADRS scale, and has been presented as a more precise measure of typical depressive symptoms (25). The Structured Interview Guide for the clinician-administered Hamilton Depression Rating Scale–Seasonal Affective Disorder Version (SIGH-SAD) was used to assess symptoms associated with atypical depression. In particular, eight of the items of the SIGH-SAD – referred to as the ‘Scale for Atypical Symptoms’ (SAS) – have been found to have an 82% sensitivity and 80% specificity for diagnosing atypical depression when using a cutoff score of 9, even in individuals without seasonal depression (26). The eight symptoms included in the SAS are social withdrawal, weight gain, increased appetite, increased eating, carbohydrate craving, hypersomnia, fatiguability (or heavy feeling), and diurnal variation (afternoon or evening slump). It should be noted that the SAS does not include items for mood reactivity or rejection sensitivity.
Statistical analysis
Total MADRS, total MADRS5, and total SAS scores were the primary measures of interest. Individual typical and atypical symptoms (assessed via the MADRS and SAS) were also examined. Specifically, additional exploratory analyses were conducted on effect sizes for all MADRS and SAS symptoms on an individual basis. Linear mixed models with restricted maximum likelihood estimation and compound symmetry covariance structure were used to examine change in individual symptoms in response to ketamine compared to placebo. Phase-specific baselines were used as a time-dependent covariate. Effects for time, drug, and their interaction were included in the model. Bonferroni-corrected simple-effects tests were used to examine omnibus effects. Cohen’s d was calculated to reflect the magnitude of change in symptoms in response to ketamine relative to placebo at days 1 and 3 postinfusion, and means and standard errors are reported. A non-zero baseline score was required for individual symptoms to be included in the analysis. Positive values in effect size indicated improvement over placebo. IBM SPSS 23.0 was used for all analyses.
Results
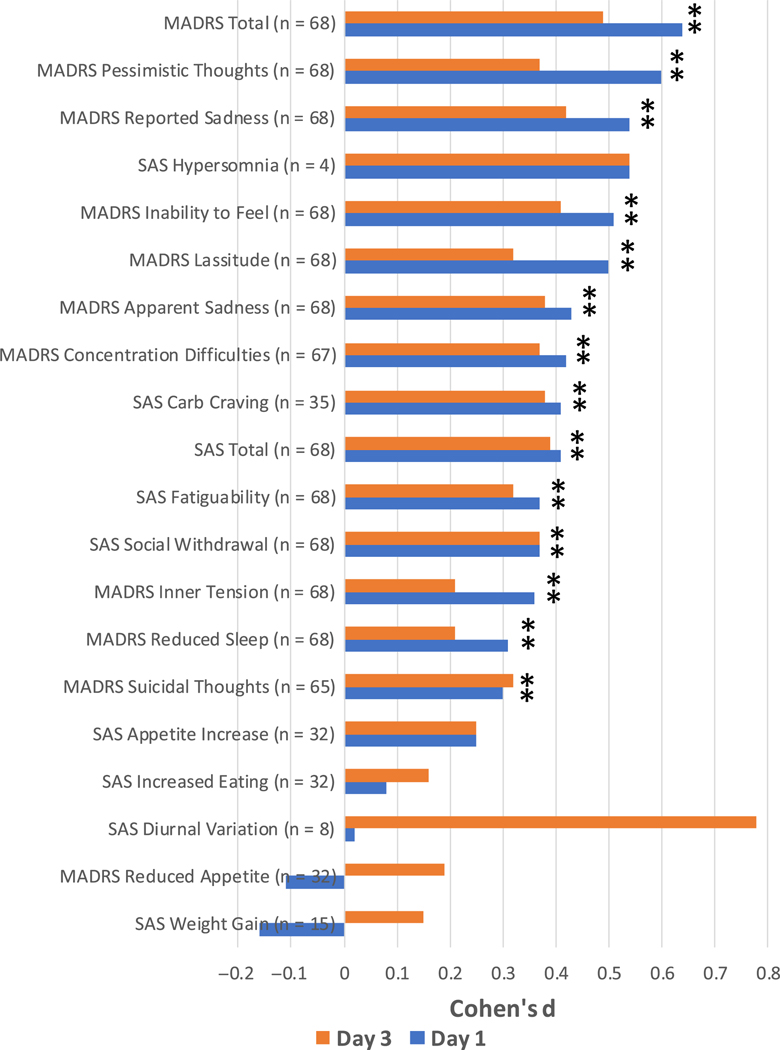
Clinical characteristics for the 68 participants are summarized in Table 1. Baseline SAS scores indicated that 26 of the 68 participants (38.2%) met criteria for atypical depression (specifically, SAS score ≥ 9). Statistically significant improvement in MADRS (placebo: 30.9 (1.1), ketamine: 21.7 (1.1); Cohen’s d = 0.64; n = 68), MADRS5 (placebo: 17.2 (0.7), ketamine: 11.6 (0.7); Cohen’s d = 0.61; n = 68), and SAS (placebo: 6.7 (0.3), ketamine: 5.0 (0.3); Cohen’s d = 0.41; n = 68) total scores was observed at Day 1 postketamine administration; the effect size was greatest for the MADRS and MADRS5 compared to the SAS (see Fig. 1). Compared to placebo, the most robust, statistically significant (P < 0.05, two-tailed, uncorrected for multiplicity) improvements were seen in the following individual MADRS items: pessimistic thoughts (placebo: 3.6 (0.2), ketamine: 2.2 (0.2); Cohen’s d = 0.60, n = 68), reported sadness (placebo: 4.0 (0.2), ketamine: 2.7 (0.2); Cohen’s d = 0.54, n = 68), and inability to feel (placebo: 3.7 (0.2), ketamine: 2.5 (0.2); Cohen’s d = 0.51, n = 68) (Fig. 2a) as well as the following individual SAS items: carbohydrate craving (placebo: 0.9 (0.1), ketamine: 0.3 (0.2); Cohen’s d = 0.41, n = 35), social withdrawal (placebo: 2.4 (0.1), ketamine: 1.7 (0.1); Cohen’s d = 0.37, n = 68), and fatiguability (placebo: 3.2 (0.2), ketamine: 2.4 (0.1); Cohen’s d = 0.37, n = 68) (Fig. 2a).
Table 1.
Demographic and clinical characteristics of participants receiving ketamine
| Characteristics | Participants (n = 68) mean (SD) |
|---|---|
| Age | 42.0 (11.8) |
| Body mass index | 29.1 (6.5) |
| Age of onset, years | 17.0 (7.0) |
| Length of iIlness, years | 25.1 (12.0) |
| Length of current depressive episode, months | 22.8 (35.4) |
| Baseline | |
| MADRS | 33.5 (4.8) |
| MADRS5 | 18.8 (2.6) |
| SIGH-SAD, SAS | 8.4 (3.1) |
| N (%) | |
| Gender, female | 41 (60.3) |
| Race, white | 61 (89.7) |
| College education | 31 (45.6) |
| Diagnosis (bipolar depression) | 39 (57.4) |
| SAS atypical depression (≥9), baseline | 26 (38.2) |
MADRS: Montgomery–Asberg Depression Rating Scale; SIGH-SAD: The Structured Interview Guide for the Hamilton Depression Rating Scale–Seasonal Affective Disorder Version; SAS: Scale for Atypical Symptoms.
Fig. 1.
Effect size of ketamine on MADRS, MADRS5, and SAS total scores. MADRS, Montgomery–Asberg Depression Rating Scale; SAS, Scale for Atypical Symptoms.
Fig. 2.
Effect size of ketamine on MADRS and SAS individual items at days 1 and 3. MADRS, Montgomery–Asberg Depression Rating Scale; SAS, Scale for Atypical Symptoms. *Indicates P ≤ 0.05, not Bonferroni corrected.
At Day 3 postketamine administration, significant improvements continued to be seen in MADRS (placebo: 30.5 (1.1), ketamine: 23.3 (1.1); Cohen’s d = 0.49; n = 68), MADRS5 (placebo: 16.8 (0.7), ketamine: 12.8 (0.7); Cohen’s d = 0.043; n = 68), and SAS (placebo: 6.7 (0.3), ketamine: 5.0 (0.3); Cohen’s d = 0.39; n = 68) total scores (see Fig. 1). The most robust, statistically significant (P < 0.05) improvements in individual symptoms were observed for four MADRS items: reported sadness (placebo: 4.0 (0.2), ketamine: 3.0 (0.2); Cohen’s d = 0.42, n = 68), inability to feel (placebo: 3.7 (0.2), ketamine: 2.8 (0.2); Cohen’s d = 0.41, n = 68), concentration difficulties (placebo: 3.6 (0.1), ketamine: 2.7 (0.1); Cohen’s d = 0.37, n = 67), and apparent sadness (placebo: 3.4 (0.2), ketamine: 2.4 (0.2); Cohen’s d = 0.38, n = 68) (Fig. 2b). The statistically significant (P < 0.05) SAS items that remained most improved were social withdrawal (placebo: 2.4 (0.1), ketamine: 1.7 (0.1); Cohen’s d = 0.37, n = 68), carbohydrate craving (placebo: 0.8 (0.1), ketamine: 0.3 (0.2); Cohen’s d = 0.38, n = 35), and fatiguability (placebo: 3.2 (0.2), ketamine: 2.5 (0.1); Cohen’s d = 0.32, n = 68). SAS scores from Day 1 to Day 3 were stable, with consistent improvements in the three top individual symptoms (carbohydrate craving, social withdrawal, and fatiguability). It should be noted that the SAS items hypersomnia and diurnal variation showed a large effect at both time points, but because few participants reported these symptoms at baseline, these effects were not statistically significant.
When analyzed by diagnosis, ketamine had no significantly different effects in individuals with MDD versus bipolar depression on any of the study measures.
Discussion
Statistically significant rapid improvements in both typical/melancholic and atypical depressive symptoms were seen in response to a single administration of subanesthetic-dose intravenous ketamine versus placebo in this group of treatment-resistant participants with either MDD or bipolar depression. Although ketamine appeared to yield greater effect sizes for typical/melancholic symptoms at the earlier time point (Day 1), effect sizes for both typical/melancholic and atypical symptoms were nearly equivalent at Day 3. Furthermore, the effect size for atypical symptoms appeared stable at both Day 1 and Day 3. In this way, ketamine appears to be more similar to MAOIs than to other standard antidepressants, demonstrating effectiveness in treating both typical/melancholic and atypical symptoms. The results also suggest that typical/ melancholic symptoms may be more readily responsive to ketamine at the earlier time point (Day 1), with gradual tempering of that response over time (by Day 3). These differences in response trajectory may indicate a divergent mechanism underlying response for each symptom dimension. In this context, the inflammatory and immunometabolic processes that hypothetically underlie the atypical symptom dimension may be more resistant to ketamine’s rapid effects at Day 1.
In terms of individual symptoms, the typical symptoms on which ketamine had the greatest effect were pessimistic thoughts, reported sadness, inability to feel, and lassitude. The three atypical symptoms on which ketamine had the greatest effect were carbohydrate craving, fatiguability, and social withdrawal. Interestingly, ketamine demonstrated relatively smaller effects on sleep and appetite symptoms, though it could be argued that changes in sleep and appetite may not have manifested over the short time frame of the analysis (three days).
This study is associated with several strengths and limitations. Strengths include the following: (i) subject-level data were combined from three independent ketamine trials, resulting in a relatively large sample size; and (ii) all participants were well-characterized in-patients with high interrater reliability for the studied measures of interest. Potential limitations include: (i) the secondary/post hoc design; (ii) the pooling of MDD and bipolar depression participants; (iii) the pooling of unmedicated (MDD) and medicated (lithium or valproate-maintained bipolar depression) participants; (iv) the absence of patients who received repeated doses of ketamine (which may affect response differently than the single-dose administration used in the current study); (v) the fact that the SAS – the tool used here for atypical depressive symptoms – has limitations for assessing atypical symptoms; (vi) the limited number of SAS symptoms reported at baseline, which may have suppressed estimates of effect size for SAS total scores as well as individual symptoms; and (vii) the relative lack of minorities in the study sample, which limited our ability to generalize the findings to different ethnic backgrounds.
Future investigations should be conducted to replicate these results in a prospective manner. Additional studies examining the difference between MDD and bipolar depression as it relates to typical/melancholic and atypical symptoms would help improve our understanding of the atypical concept. It would be of particular interest to see whether differences in the trajectory of response of typical/melancholic versus atypical symptoms hold up in a larger prospective sample and over repeated ketamine administrations. Finally, it will be important to continue to elucidate the biological mechanisms underlying the distinction between typical/melancholic and atypical symptoms, with a particular focus on the HPA axis, inflammation, and immunometabolic processes.
Significant outcomes.
The rapid-acting glutamatergic modulator ketamine effectively relieved many symptoms of depression.
Ketamine effectively treated both typical/melancholic and atypical depressive symptoms; however, at Day 1, the effect size was larger for treating typical/melancholic symptoms.
Ketamine had the smallest effect on appetite- and sleep-related depressive symptoms.
Limitations.
This study was a post hoc secondary analysis of data drawn from previously conducted studies.
Participants were pooled across diagnoses (major depressive disorder (MDD) and bipolar depression) and medication state (MDD patients were unmedicated; bipolar patients were receiving stable doses of lithium or valproate).
The exploratory nature of the analysis requires prospective replication before firm conclusions can be drawn.
Acknowledgements
Funding for this work was supported in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002927; NCT0088699), by a NARSAD Independent Investigator grant (to CAZ), and by a Brain & Behavior Mood Disorders Research Award (to CAZ). This research was made possible through the National Institutes of Health (NIH) Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, Elsevier, and other private donors (to SJP).
Dr. Zarate is listed as a coinventor on a patent for the use of ketamine in major depression and suicidal ideation; as a coinventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a coinventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government.
Footnotes
Conflict of interest
All other authors have no conflict of interest to disclose, financial or otherwise.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/acps.13216.
Data availability statement
The data that support the findings of this study are available from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Robertson HA, Lam RW, Stewart JN, Yatham LN, Tam EM, Zis AP. Atypical depressive symptoms and clusters in unipolar and bipolar depression. Acta Psychiatr Scand 1996;94:421–427. [DOI] [PubMed] [Google Scholar]
- 2.Nierenberg AA, Alpert JE, Pava J. Course and treatment of atypical depression. J Clin Psychiatry 1998;59(Suppl 18):5–9. [PubMed] [Google Scholar]
- 3.West ED, Dally PJ. Effects of iproniazid in depressive syndromes. Br Med J 1959;1:1491–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sargant W. Some newer drugs in the treatment of depression and their relation to other somatic treatments. Psychosomatics 1960;1:14–17. [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edn. Arlington, VA: Author; 2013. [Google Scholar]
- 6.Angst J, Gamma A, Sellaro R, Zhang H, Merikangas KR. Toward validation of atypical depression in the community: results of the Zurich cohort study. J Affect Disord 2002;72:125–138. [DOI] [PubMed] [Google Scholar]
- 7.Thase ME. Atypical depression: useful concept, but it’s time to revise the DSM-IV criteria. Neuropsychopharmacology 2009;34:2633–2641. [DOI] [PubMed] [Google Scholar]
- 8.Parker GB, Thase ME. Atypical depression: a valid sub-type? J Clin Psychiatry 2007;68:e08. [DOI] [PubMed] [Google Scholar]
- 9.Akiskal HS, Benazzi F. Atypical depression: a variant of bipolar II or a bridge between unipolar and bipolar II? J Affect Disord 2005;84:209–217. [DOI] [PubMed] [Google Scholar]
- 10.Novick JS, Stewart JW, Wisniewski SR et al. Clinical and demographic features of atypical depression in outpatients with major depressive disorder: preliminary findings from STAR*D. J Clin Psychiatry 2005;66:1002–1011. [DOI] [PubMed] [Google Scholar]
- 11.Lamers F, Vogelzangs N, Merikangas KR, De Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 2013;18:692–699. [DOI] [PubMed] [Google Scholar]
- 12.Lamers F, De Jonge P, Nolen WA et al. Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry 2010;71:1582–1589. [DOI] [PubMed] [Google Scholar]
- 13.Gold PW, Chrousos GP. Melancholic and atypical subtypes of depression represent distinct pathophysiological entities: CRH, neural circuits, and the diathesis for anxiety and depression. Mol Psychiatry 2013;18:632–634. [DOI] [PubMed] [Google Scholar]
- 14.Joseph-Vanderpool JR, Rosenthal NE, Chrousos GP et al. Abnormal pituitary-adrenal responses to corticotropin-releasing hormone in patients with seasonal affective disorder: clinical and pathophysiological implications. J Clin Endocrinol Metab 1991;72:1382–1387. [DOI] [PubMed] [Google Scholar]
- 15.Milaneschi Y, Lamers F, Bot M, Drent ML, Penninx BW. Leptin dysregulation is specifically associated with major depression with atypical features: evidence for a mechanism connecting obesity and depression. Biol Psychiatry 2015;81:807–814. [DOI] [PubMed] [Google Scholar]
- 16.Milaneschi Y, Lamers F, Peyrot WJ et al. Genetic association of major depression with atypical features and obesity-related immunometabolic dysregulations. JAMA Psychiatry 2017;74:1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angst J, Gamma A, Benazzi F, Ajdacic V, Rossler W. Melancholia and atypical depression in the Zurich study: epidemiology clinical characteristics, course, comorbidity and personality. Acta Psychiatr Scand Suppl 2007;433:72–84. [DOI] [PubMed] [Google Scholar]
- 18.Benazzi F. Various forms of depression. Dialogues Clin Neurosci 2006;8:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishimoto T, Chawla JM, Hagi K et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 2016;46:1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Zhou Y, Zheng W et al. Association between depression subtypes and response to repeated-dose intravenous ketamine. Acta Psychiatr Scand 2019;140: 446–457. [DOI] [PubMed] [Google Scholar]
- 21.Diazgranados N, Ibrahim L, Brutsche NE et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 2010;67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarate CA Jr, Brutsche NE, Ibrahim L et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 2012;71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nugent AC, Ballard ED, Gould TD et al. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry 2019;24:1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: Author; 2000. [Google Scholar]
- 25.Bech P, Allerup P, Larsen ER, Csillag C, Licht RW. The Hamilton Depression Scale (HAM-D) and the Montgomery-Asberg Depression Scale (MADRS). A psychometric re-analysis of the European genome-based therapeutic drugs for depression study using Rasch analysis. Psychiatry Res 2014;217:226–232. [DOI] [PubMed] [Google Scholar]
- 26.Thuile J, Even C, Musa C, Friedman S, Rouillon F. Clinical correlates of atypical depression and validation of the French version of the Scale for Atypical Symptoms (SAS). J Affect Disord. 2009;118:113–117. [DOI] [PubMed] [Google Scholar]