Abstract
STUDY QUESTION:
Is the use of donor oocytes in women <35 years of age associated with an increased risk of adverse perinatal outcomes compared to use of autologous oocytes?
SUMMARY ANSWER:
Among fresh assisted reproductive technology (ART) cycles performed in women under age 35, donor oocyte use is associated with a higher risk of preterm birth, low birth weight and stillbirth (when zero embryos were cryopreserved) as compared to autologous oocytes.
WHAT IS KNOWN ALREADY:
Previous studies demonstrated elevated risk of poor perinatal outcomes with donor versus autologous oocytes during ART, primarily among older women.
STUDY DESIGN, SIZE, DURATION:
Retrospective cohort study using data reported to Centers for Disease Control and Prevention’s National ART Surveillance System (NASS) during the period from 2010 to 2015 in order to best reflect advances in clinical practice. Approximately 98% of all US ART cycles are reported to NASS, and discrepancy rates were <6% for all fields evaluated in 2015.
PARTICIPANTS/MATERIALS, SETTING, METHODS:
We included all non-banking fresh and frozen ART cycles performed between 2010 and 2015 in women under age 35 using autologous or donor eggs. Cycles using cryopreserved eggs, donated embryos or a gestational carrier were excluded. Among fresh embryo transfer cycles, we calculated predicted marginal proportions to estimate the unadjusted and adjusted risk ratios (aRRs) and 95% confidence intervals (CIs) for the association between donor versus autologous oocyte use and stillbirth, spontaneous abortion, preterm delivery and low birth weight among singleton pregnancies or births. Stillbirth models were stratified by number of embryos cryopreserved. All models were adjusted for patient and treatment characteristics.
MAIN RESULTS AND THE ROLE OF CHANCE:
Among the 71 720 singleton pregnancies occurring during 2010–2015, singletons resulting from donor oocytes were more likely to be preterm (15.6% versus 11.0%; aRRs 1.39: CI 1.20–1.61) and have low birth weight(11.8% versus 8.8%; aRRs 1.34; CI 1.16–1.55) than those resulting from autologous oocytes. With zero embryos cryopreserved, donor versus autologous oocyte use was associated with increased risk for stillbirth (2.1% versus 0.6%; aRRs 3.73; CI 1.96–7.11); no association with stillbirth was found when ≥1 embryo was cryopreserved (0.54% versus 0.56%; aRR 1.15; CI 0.59–2.25).
LIMITATIONS, REASONS FOR CAUTION:
The data come from a national surveillance system and is thus limited by the accuracy of the data entered by individual providers and clinics. There may be unmeasured differences between women using donor eggs versus their own eggs that could be contributing to the reported associations. Given the large sample size, statistically significant findings may not reflect clinically important variations.
WIDER IMPLICATIONS OF THE FINDINGS:
Risks of preterm birth, low birth weight and stillbirth among singleton pregnancies using donor oocytes were increased compared to those using autologous oocytes. Further study regarding the pathophysiology of the potentially increased risks among donor oocyte recipient pregnancy is warranted.
STUDY FUNDING/COMPETING INTEREST(S):
None.
TRIAL REGISTRATION NUMBER:
N/A
Keywords: donor oocyte, preterm, low birth weight, stillbirth, young recipient, perinatal outcomes
Introduction
In the USA, donor oocyte use has been steadily increasing over the past two decades (Kawwass et al. 2013). In 2015, over 21 000 in vitro fertilization (IVF) cycles were performed using donor oocytes, and that number continues to rise (Kawwass et al. 2013; Centers for Disease Control and Prevention, 2017). Previous studies have demonstrated elevated risk of adverse pregnancy outcomes in pregnancies resulting from donor oocytes compared with autologous oocyte IVF cycles and natural conception (Masoudian et al. 2016; Kamath et al. 2017). A recent systematic review found the odds of preeclampsia and gestational hypertension to be significantly higher (2.5 and 3 times higher, respectively) in donor oocyte pregnancies compared to their autologous egg and natural conception counterparts (Masoudian et al. 2016). One possible explanation for this association is the ‘immunologic’ theory of preeclampsia that postulates foreign antigens spur an immunologic response in the recipient, creating an inflammatory milieu that can impair proper placental implantation leading to preeclampsia (Smith et al. 1997).
In addition, findings from multiple studies suggest increased risks for poor neonatal outcomes such as preterm birth and low birth weight in pregnancies resulting from donor oocytes versus pregnancies conceived with autologous eggs (Kamath et al. 2017; Boulet et al. 2018). Although biologic mechanisms for the possible association between use of donor oocytes and adverse pregnancy outcomes remains unknown, older maternal age among donor oocyte recipients may contribute to the increased risks for complications. In the general population, maternal age >35 years is associated with maternal and neonatal complications such as preeclampsia, gestational diabetes, preterm birth and cesarean delivery (Jacobsson et al. 2004; Kenny et al. 2013; Laopaiboon et al. 2014). Therefore, it is difficult to determine the degree to which adverse outcomes in donor oocyte pregnancies are due to use of donor oocytes or the advanced maternal age of the majority of recipients. Findings from one study suggest similar rates of preterm birth and low birth weight among donor oocyte pregnancies and autologous oocyte pregnancies in recipients age 35 or older (Krieg et al. 2008). Among the few studies looking at outcomes of donor oocyte pregnancies in women age <35 years, the current literature suggests inconsistent associations with low birth weight and preterm birth outcomes, with most of the studies examining lacking statistical power (Beckett and Serhal 1994; Stoop et al. 2012; Jeve et al. 2016).
We used US national assisted reproductive technology (ART) surveillance data to compare donor oocyte versus autologous oocyte cycles among women of the same age characterize pregnancy outcomes of these cycles, namely preterm birth, low birth weight, stillbirth and spontaneous abortion. Our secondary aim was to assess trends of donor oocyte use among women younger than 35 years.
Materials and Methods
Study population
The data used in this study were obtained from the Centers for Disease Control and Prevention’s National ART Surveillance System (NASS). As mandated by the Fertility Clinic Success Rates and Certification Act of 1992 (Public Law 102–493), all ART cycles performed in the USA should be reported to NASS, which includes in practice approximately 98% of all ART cycles performed in the USA. Since 1995, NASS has been collecting information on patient demographics, obstetrical and medical history, ART procedures and resulting pregnancies and births. In order to validate the data reported to NASS, a random sample of reporting clinics is visited each year by trained abstractors who compare NASS data to the medical records from the clinic. Discrepancy rates were <6% for all fields evaluated for 2015 reporting year (Centers for Disease Control and Prevention, 2017).
For the trends analysis, we included all non-banking fresh and frozen IVF cycles performed between 2000 and 2015 in women under age 35 using autologous or donor eggs (n = 903 043) and excluded cycles using cryopreserved eggs (n = 1408—only collected from 2013 onward), donated embryos (n = 3367) or a gestational carrier (n = 12 087). For the primary analysis, to account for advances in IVF procedures over time, we subsequently restricted the study population to fresh cycles initiated during 2010–2015 and resulting in a singleton pregnancy.
Study design
This is a retrospective, population-based observational cohort study that assesses the risk of poor perinatal outcomes among singleton pregnancies occurring in women under 35 who are using donor oocytes versus autologous oocytes for fresh ART cycles performed between 2010 and 2015 in order to best reflect advances in clinical practice.
Assessment of exposure and outcomes
Information about exposure (donor oocyte use) and outcomes was reported to NASS by fertility clinics. Outcomes of interest included preterm birth (birth occurring before 37 completed weeks of gestation), low birth weight (birth weight of 2499 g or less), spontaneous abortion (non-induced embryonic or fetal death or passage of products of conception before the 20th week of gestation) and stillbirth (fetal death occurring during pregnancy at 20 weeks of gestation or later) (Martin et al. 2015).
Statistical analysis
We assessed trends in the proportion of donor cycles between 2000 and 2015 using the Cochran–Armitage test. For the primary analysis, we used chi-square tests to compare the distributions of patient and treatment characteristics for donor versus autologous cycles among all cycles in women <35 and restricted to fresh cycles resulting in a singleton gestation (one fetal heart observed on ultrasound prior to 7 weeks’ gestation). We calculated predicted marginal proportions from logistic regression models to estimate the unadjusted and adjusted risk ratios (aRR) and 95% confidence intervals (CIs) for the association between use of donor oocytes and preterm birth and low birth weight (among live births) and SAB (spontaneous abortion) and stillbirth (among pregnancies). We used conditional marginals from linear regression models to calculate adjusted estimates of mean birth weight and gestational age for singleton donor versus autologous oocyte pregnancies. All models were adjusted for race, infertility diagnosis, patient age, body mass index (BMI), parity, prior spontaneous abortions, number of prior ART cycles, number of supernumerary embryos cryopreserved, number of embryos transferred and embryo stage at transfer. Models accounted for clustering by clinic. For the association between donor oocyte use and stillbirth, we found a statistically significant interaction between use of donor oocytes and number of embryos cryopreserved. An interaction term was therefore included in the model. Due to high frequencies of missing data for race/ethnicity (34%) and body mass index (18%) among all cycles, we used multiple imputation to estimate missing values for inclusion in the regression models. We used SUDAAN’s HOTDECK procedure for imputation of clustered data (Zhang et al. 2017), under the assumption of missing at random (Pedersen et al. 2017). The imputation models included state of residence, oocyte/embryo state (fresh versus frozen), infertility diagnosis, gestation weeks, parity, patient age, number of prior preterm births, number of prior spontaneous abortions and number of embryos transferred. To further account for potential differences in the underlying characteristics of women using donor versus autologous oocytes, we restricted the study population to women with no prior ART and examined associations between perinatal outcomes and use of donor oocytes. SAS version9.4 and SUDAAN 11 were used for analysis. P values <.05 were considered statistically significant.
Ethical approval
This project was approved by the Institutional Review Board at CDC.
Results
Trends in donor oocyte cycles
Among all ART cycles performed in the USA in women under age 35 between 2000 and 2015 (n = 886 181), the percentage that used donor oocytes has remained nearly constant for the past 15 years, ranging between 2.5 and 3.0% (Fig. 1). The absolute number of donor cycles performed in women under the age of 35 in the USA increased from 1132 in 2000 to 2050 in 2015.
Figure 1.
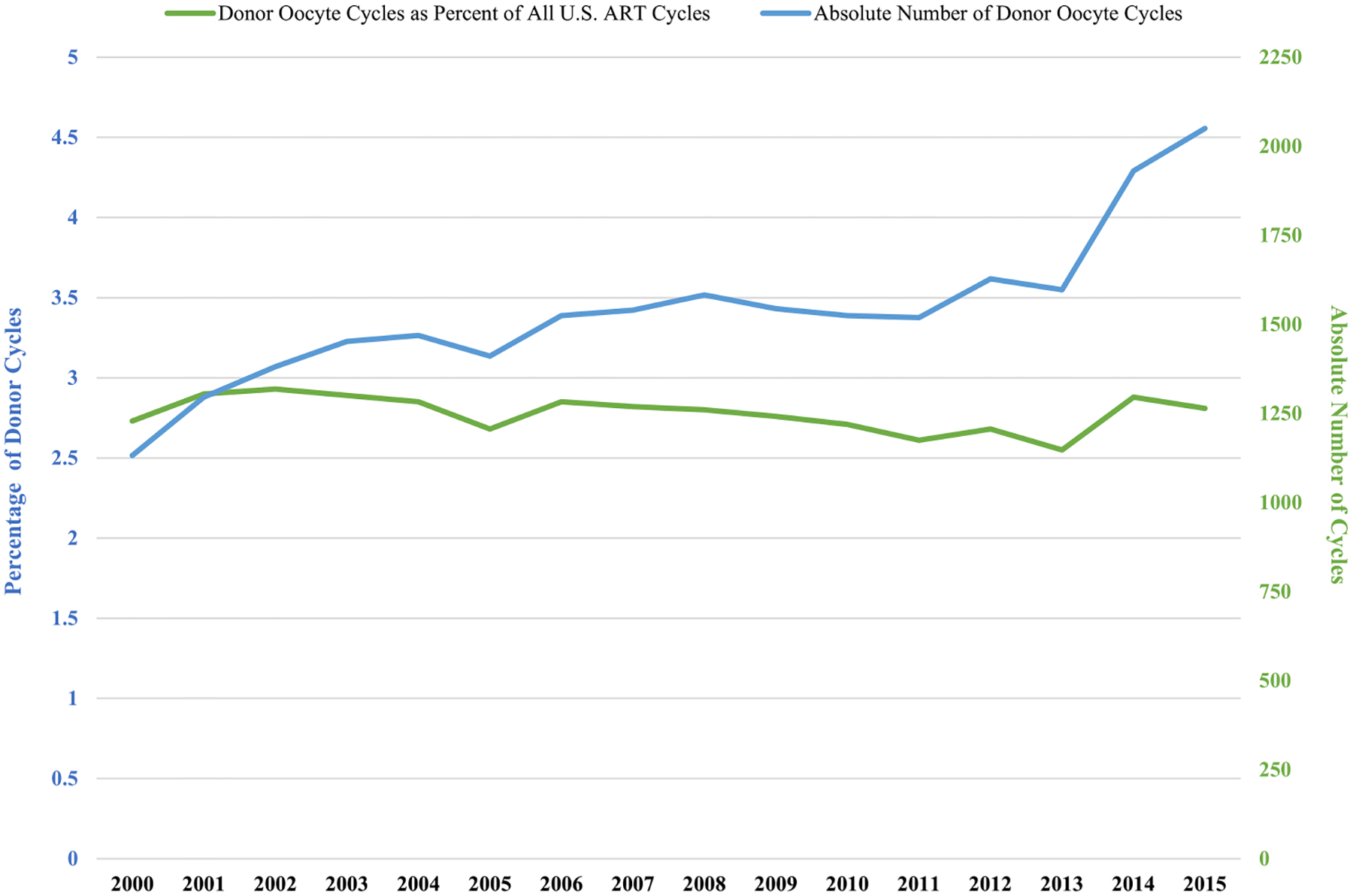
The absolute number of donor oocyte cycles and donor oocyte cycles as percent of all US ART cycles among women <35 years old.
Sample characteristics
Overall, there were 71 720 singleton pregnancies resulting from fresh ART cycles during 2010–2015. Of those, 2105 resulted from donor oocytes and 69 615 resulted from autologous oocytes (Table I). Compared with women using autologous oocytes, a higher proportion of women using donor oocytes were 30–34 years of age (75.6% versus71.6%, P < 0.0001). The donor oocyte group had a higher proportion of diminished ovarian reserve diagnoses as compared to the autologous group (56.7% versus 8.5%, P < 0.0001). Among pregnancies following donor oocyte cycles, 17.5% had one prior ART cycle and 33.9% had two or more prior ART cycles (P < 0.0001). The autologous group had a lower rate of prior ART cycles: 15.3% had one prior cycle and 13.5% had two or more prior ART cycles (P < 0.0001). There was also a higher percentage of embryos transferred on Day 5/6 in the donor oocyte versus the autologous group (81.4% versus 67.2%, P < 0.0001). Approximately 42.2% of oocyte donors were between the ages of 25 and 29, 28.8% of the donors were under age 25 and 17.3% were between 30 and 34 years of age (data not shown). Only3.1% of the donor oocytes came from women age 35 or older. The donor age was unknown in 8.7% of the pregnancies. Otherwise, the donor and autologous characteristics were generally similar, although many comparisons were statistically different due to large sample size.
Table I.
Patient and clinical characteristics of singleton pregnancies resulting from fresh donor oocyte versus autologous oocyte cycles, in women <35 years of age, USA, 2010–2015.
| Donor oocyte recipients | Autologous oocyte patients | P value | |||
|---|---|---|---|---|---|
| Characteristics N = 71 720 | N | Percent (%) | N | Percent (%) | |
| Age | |||||
| <25 | 42 | 2.0 | 1497 | 2.2 | 0.0003 |
| 25–29 | 471 | 22.4 | 18271 | 26.3 | |
| 30–34 | 1592 | 75.6 | 49 847 | 71.6 | |
| Race/ethnicity | |||||
| Non-Hispanic White | 1059 | 50.3 | 34547 | 49.6 | 0.0884 |
| Non-Hispanic Black | 50 | 2.4 | 2385 | 3.4 | |
| Asian/Pacific Islander | 159 | 7.6 | 4995 | 7.2 | |
| Hispanic | 125 | 5.9 | 3685 | 5.3 | |
| Other | * | <1 | 110 | 0.2 | |
| Missing | * | −34.0 | 23 893 | 34.3 | |
| Infertility diagnosis | |||||
| Tubal factor | 107 | 5.1 | 9687 | 13.9 | <.0001 |
| Endometriosis | 187 | 8.9 | 7990 | 11.5 | 0.0002 |
| Uterine factor | 59 | 2.8 | 2294 | 3.3 | 0.2115 |
| disorder | 153 | 7.3 | 15 084 | 21.7 | <.0001 |
| Diminished ovarian reserve | 1194 | 56.7 | 5947 | 8.5 | <.0001 |
| Male factor | 350 | 16.6 | 30 020 | 43.1 | <.0001 |
| Unexplained | 97 | 4.6 | 11 005 | 15.8 | <.0001 |
| BMI (kg/m 2 ) | |||||
| <18.5 | 59 | 2.8 | 1846 | 2.7 | <.0001 |
| 18.5–24.9 | 953 | 45.3 | 33 374 | 47.9 | |
| 25.0–29.9 | 364 | 17.3 | 13 166 | 18.9 | |
| ≥30 | 254 | 12.1 | 9641 | 13.9 | |
| Missing | 475 | 22.6 | 11 588 | 16.7 | |
| Number of prior pregnancies | |||||
| 0 | 1188 | 57.8 | 39 331 | 56.7 | <.0001 |
| 1 | 412 | 20.0 | 17288 | 24.9 | |
| ≥2 | 456 | 22.2 | 12 803 | 18.4 | |
| Number of prior spontaneous abortions | |||||
| 0 | 1597 | 78.0 | 54 356 | 78.5 | <.0001 |
| 1 | 268 | 13.1 | 10591 | 15.3 | |
| ≥2 | 183 | 8.9 | 4342 | 6.3 | |
| Number of prior preterm births | |||||
| 0 | 2001 | 97.9 | 67 305 | 97.3 | 0.105 |
| ≥1 | 43 | 2.1 | 1861 | 2.7 | |
| Number of prior term births | |||||
| 0 | 1668 | 81.3 | 55 601 | 80.2 | <.0001 |
| 1 | 250 | 12.2 | 11 033 | 15.9 | |
| ≥2 | 134 | 6.5 | 2682 | 3.9 | |
| Number of prior ART cycles | |||||
| 0 | 1023 | 48.6 | 49 503 | 71.2 | <.0001 |
| 1 | 368 | 17.5 | 10 638 | 15.3 | |
| ≥2 | 712 | 33.9 | 9418 | 13.5 | |
| Use of ICSI | |||||
| Yes | 1653 | 78.5 | 53 466 | 76.9 | 0.0848 |
| No | 452 | 21.5 | 16041 | 23.1 | |
| Use of assisted hatching | |||||
| Yes | 390 | 18.5 | 16981 | 24.4 | <.0001 |
| No | 1715 | 81.5 | 52 634 | 75.6 | |
| Embryo stage at transfer | |||||
| Day 2/3 | 357 | 17.0 | 21 695 | 31.2 | <.0001 |
| Day 5/6 | 1714 | 81.4 | 46 747 | 67.2 | |
| Other | 34 | 1.6 | 1173 | 1.7 | |
| Number of embryos transferred | |||||
| 1 | 763 | 36.3 | 24229 | 34.8 | <.0001 |
| 2 | 1269 | 60.3 | 41 028 | 58.9 | |
| ≥3 | 73 | 3.5 | 4358 | 6.3 | |
| Number of supernumerary embryos cryopreserved | |||||
| 0 | 431 | 20.6 | 25 381 | 36.6 | <.0001 |
| 1 to 2 | 435 | 20.7 | 16 192 | 23.3 | |
| 3 to 4 | 431 | 20.6 | 12 385 | 17.9 | |
| ≥5 | 800 | 38.2 | 15 427 | 22.2 | |
| Elective single embryo transfer | |||||
| Yes | 699 | 34.3 | 19 198 | 29.7 | <.0001 |
| No | 1342 | 65.8 | 45 386 | 70.3 | |
| Cycle resulted in live birth? | |||||
| Yes | 1883 | 89.5 | 62 071 | 89.2 | 0.6727 |
| No | 222 | 10.6 | 7544 | 10.8 | |
Data suppressed due to cell count <5
Perinatal outcomes
Among singleton live births from fresh cycles in women <35 years old, the proportion of preterm birth was 15.7% for donor oocytes versus 11.2% for autologous oocytes (P < 0.0001) (Table II). Similarly, the proportion of low birth weight among donor oocyte live births was11.8%; in the autologous oocyte group, it was 8.8% (P < 0.0001). After adjustment, use of donor oocytes was associated with an increased risk of preterm birth (aRR 1.39, 95% CI 1.20–1.61) and low birth weight (aRR 1.34, 95% CI 1.16–1.55) compared to autologous cycles in women under 35 years of age. The adjusted mean gestational age for the donor oocyte group was 38.6 weeks (standard deviation (SD) ± .07 weeks), and the adjusted mean gestational age for the autologous oocyte group was 38.8 weeks (SD ± 01 weeks, P = 0.001). The adjusted mean birth weight for the donor oocyte group (3231 g (SD ± 18 g) was not significantly different than the adjusted mean birth weight for the autologous oocyte group (3235 g (SD ± 4 g), P = 0.835). The number of supernumerary embryos cryopreserved modified the association between donor oocytes and stillbirth. When no supernumerary embryos were available for cryopreservation, use of donor oocytes was associated with an increased risk for stillbirth (aRR 3.73, 95% CI 1.96–7.11). When at least one supernumerary embryo was cryopreserved, the association was no longer significant (aRR 1.15, 95% CI 0.59–2.25). We found no association between the use of donor oocytes and spontaneous abortion in women under age 35 (aRR 0.97, 95% CI 0.83–1.13). When restricted to singleton pregnancies among women with no prior IVF cycles, effect estimates were similar to those observed for the full study population (Supplementary Table I).
Table II.
Perinatal outcomes for singleton pregnancies resulting from fresh donor and autologous oocytes in women <35 years of age, USA, 2010–2015.
| # of total Donor oocyte Cycles = 2 105 | # of total Autologous oocyte Cycles = 69 615 | |||||||
|---|---|---|---|---|---|---|---|---|
| Donor oocyte recipient | Autologous oocyte patient | |||||||
| N = 71 720 | % | N | % | N | Risk ratio (unadjusted) | 95% confidence interval | Adjusted risk ratio* | 95% confidence interval |
| Preterm birth (<37 completed weeks) a | 15.64 | 293 | 11.03 | 6827 | 1.42 | 1.24–1.62 | 1.39 | 1.20–1.61 |
| Low birth weight < 2500g a | 11.76 | 216 | 8.77 | 5360 | 1.34 | 1.18–1.53 | 1.34 | 1.16–1.55 |
| Stillbirth b | 0.86 | 18 | 0.58 | 403 | ||||
| 0 embryos cryopreserved | 2.09 | 9 | 0.62 | 157 | 3.38 | 1.80–6.32 | 3.73 | 1.96–7.11 |
| ≥1 embryos cryopreserved | 0.54 | 9 | 0.56 | 245 | 0.97 | 0.51–1.86 | 1.15 | 0.59–2.25 |
| Spontaneous abortion | 8.79 | 185 | 9.03 | 6288 | 0.97 | 0.84–1.12 | 0.97 | 0.83–1.13 |
Variables adjusted include race, infertility diagnosis, patient age, BMI, parity, prior spontaneous abortion, prior ART use, number of supernumerary cryopreserved embryos, embryo stage at transfer and number of embryos transferred.
Denominator for preterm birth and low birth weight calculations is live births among women <35 years of age using ART, 2010–2015
Denominator for stillbirth calculations is pregnancies in women <35 years of age using ART, 2010–2015
BMI: body mass index
Discussion
Using data from a national surveillance system, we found that the percentage of donor oocyte cycles among US women <35 years of age was nearly constant between 2000 and 2015 ranging from 2.5 to3.0%. However, since the overall number of ART cycles performed in women under age 35 has increased, the absolute number of donor oocyte cycles performed in women under age 35 also increased over the study period. Donor oocyte use among women of all ages has also increased (Kawwass et al. 2013). Nevertheless, we can conclude that the proportions of women using autologous or donor oocytes in the under 35 age category between 2000 and 2015 have remained similar.
Among singleton live births resulting from fresh cycles in women under 35 years of age, donor oocyte use was associated with a higher risk of preterm birth and low birth weight delivery as compared to autologous oocyte use. However, only adjusted mean birth weights were significantly different between the two study groups. While on average both donor and autologous oocyte neonates born to women under 35 were term and of normal birthweight, our results suggest an increased likelihood of preterm or low birthweight delivery among donor oocyte recipients.
Donor oocytes have been identified as an independent risk factor for preeclampsia and pregnancy-induced hypertension (Odegard et al. 2000; Flenady et al. 2011; Morgan 2016; Kenny and Kell 2017). The increased risk of preeclampsia and pregnancy-induced hypertension in donor oocyte cycles may explain our findings of higher rates of preterm birth and low birth weight among donor oocyte cycles; however, we were unable to assess these factors as data on preeclampsia and hypertension are not available in NASS. There is also evidence that underlying infertility is an independent risk factor for preterm birth and low birth weight (Basso and Baird 2003). This may account for some of the baseline elevated risks of adverse perinatal outcomes among IVF pregnancies when compared with spontaneously conceived infants (Jacobsson et al. 2004). However, when comparing donor oocyte cycles to autologous oocyte cycles, donor oocyte neonates are still at a higher risk for preterm birth and low birth weight (Adams et al. 2015) which may be explained by variations in implantation or placentation associated with subfertility or other factors.
It makes sense that we would see an increased risk of stillbirth among pregnancies resulting from donor oocytes based on the literature. One theory suggests that donor oocytes, being foreign material, incite an immune reaction in the recipient (Levron et al. 2014). Lack of immunologic tolerance to the foreign DNA of the embryo can lead to poor placental implantation and the consequences that follow, such as gestational hypertension and preeclampsia, among other placental pathology. Hypertensive disorders of pregnancy, preeclampsia and other forms of placental pathology have all been linked to stillbirth (Hovatta et al. 1983; Korteweg et al. 2009; Levron et al. 2014; Stillbirth Collaborative Research Network Writing, 2011; van der Hoorn et al. 2010). Our results regarding the risk of stillbirth among donor oocyte pregnancies were not so clear, though. The interaction between number of supernumerary embryos cryopreserved and use of donor oocytes for the model predicting stillbirth risk was an interesting and unexpected finding. The reason for the 3-fold increase in stillbirth risk in the absence of available supernumerary embryos is not clear. It is possible that women with no embryos to freeze had low quality oocytes or embryos, which could lead to increased risk for stillbirth. However, since we did not see the same association in the autologous group, other related factors that uniquely affect donor oocyte pregnancies but could not be explored in the present study may explain this finding.
Our study investigated adverse perinatal outcomes, in a large cohort of women under age 35, thereby reducing potential confounding by factors related to older maternal age and adding to the limited literature on donor oocyte outcomes in younger women undergoing IVF. Another strength of this study is the quality of the data and the high compliance of clinics with mandatory data reporting. Annual data validation procedures help to evaluate data discrepancies and maintain the integrity of the NASS data. Discrepancy rates were <6% for all fields evaluated in 2015 reporting year (Centers for Disease Control and Prevention, 2017). Our finding that donor oocyte use is associated with elevated risks of preterm birth and low birth weight is consistent with other studies that include women of all ages (Dude et al. 2016; Savasi et al. 2016; Kamath et al. 2017).
Our data were obtained from a national surveillance system and thus are limited by the accuracy of the data inputted by individual providers and clinics. It is possible that the increased risk for poor perinatal outcomes in women under age 35 using donor eggs could be due to residual confounding. While we controlled for potential confounders that we could identify, there may be unmeasured differences between women using donor eggs versus their own eggs that are not included in NASS that might explain the reported associations, particularly those for preterm birth and low birth weight, where the adjusted risk ratios were less than 1.5. Finally, we were unable to account for correlation among multiple cycles contributed by a single patient, although we did account for clinic-level clustering.
Overall, our findings suggest that risks associated with donor oocyte use persist even in young recipients. Additional studies are needed to elucidate the pathophysiology and immunologic mechanisms that may contribute to these risks, specifically the increased risk of stillbirth among women with no embryos available for cryopreservation warrants further study. Linking NASS with the National Vital Statistics System data would allow studying the effect of donor oocyte use on gestational pathology, such as gestational hypertension, preeclampsia, eclampsia and gestational diabetes.
Supplementary Material
Funding
There was no grant funding source for this project. Funding for conference presentation was provided by the Marianne Ruby Award through the Emory Department of Gynecology and Obstetrics.
Footnotes
Supplementary data
Supplementary data are available at Human Reproduction online.
Conflict of interest
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. All authors declare no conflicts of interest.
References
- 102–493 PL. Fertility Clinic Success Rate and Certification Act of 1992. 1992. [PubMed]
- Adams DH, Clark RA, Davies MJ, de Lacey S. A meta-analysis of neonatal health outcomes from oocyte donation. J Dev Orig Health Dis 2015;1–16. [DOI] [PubMed] [Google Scholar]
- Basso O, Baird DD. Infertility and preterm delivery, birthweight, and caesarean section: a study within the Danish National Birth Cohort. Hum Reprod 2003;18:2478–2484. [DOI] [PubMed] [Google Scholar]
- Beckett V, Serhal P. The evolution and outcome of pregnancies from oocyte donation. Hum Reprod 1994;9:2444. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Kawwass JF, Crawford S, Davies MJ, Kissin DM. Preterm birth and small-for-gestational age in singleton in vitro fertilization births using donor oocytes. Am J Epidemiol 2018. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Assisted Reproductive Technology National Summary Report. In: In American Society for Reproductive Medicine SfART. US Dept of Health and Human Services, 2015, 2017 [Google Scholar]
- Centers for Disease Control and Prevention ASfRM, Society for Assisted Reproductive Technology. 2015 Assisted Reproductive Technology National Summary Report. Atlanta: GA, 2017 [Google Scholar]
- Dude AM, Yeh JS, Muasher SJ. Donor oocytes are associated with preterm birth when compared to fresh autologous in vitro fertilization cycles in singleton pregnancies. Fertil Steril 2016;106: 660–665. [DOI] [PubMed] [Google Scholar]
- Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, Coory M, Gordon A, Ellwood D, McIntyre HD et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet 2011;377:1331–1340. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Lipasti A, Rapola J, Karjalainen O. Causes of stillbirth: a clinicopathological study of 243 patients. Br J Obstet Gynaecol 1983;90:691–696. [DOI] [PubMed] [Google Scholar]
- Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol 2004;104:727–733. [DOI] [PubMed] [Google Scholar]
- Jeve YB, Potdar N, Opoku A, Khare M. Three-arm age-matched retrospective cohort study of obstetric outcomes of donor oocyte pregnancies. Int J Gynaecol Obstet 2016;133:156–158. [DOI] [PubMed] [Google Scholar]
- Kamath MS, Antonisamy B, Mascarenhas M, Sunkara SK. High-risk of preterm birth and low birth weight after oocyte donation IVF: analysis of 133,785 live births. Reprod Biomed Online 2017;35: 318–324. [DOI] [PubMed] [Google Scholar]
- Kawwass JF, Monsour M, Crawford S, Kissin DM, Session DR, Kulkarni AD, Jamieson DJ, National ARTSSG. Trends and outcomes for donor oocyte cycles in the United States, 2000–2010. JAMA 2013;310:2426–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny LC, Kell DB. Immunological tolerance, pregnancy, and preeclampsia: the roles of semen microbes and the father. Front Med (Lausanne) 2017;4:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny LC, Lavender T, McNamee R, O’Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One 2013;8: e56583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korteweg FJ, Erwich JJ, Holm JP, Ravise JM, van der Meer J, Veeger NJ, Timmer A. Diverse placental pathologies as the main causes of fetal death. Obstet Gynecol 2009;114:809–817. [DOI] [PubMed] [Google Scholar]
- Krieg SA, Henne MB, Westphal LM. Obstetric outcomes in donor oocyte pregnancies compared with advanced maternal age in in vitro fertilization pregnancies. Fertil Steril 2008;90:65–70. [DOI] [PubMed] [Google Scholar]
- Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel JP, Souza JP, Gulmezoglu AM, WHOMSoMNHR N. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG 2014;121:49–56. [DOI] [PubMed] [Google Scholar]
- Levron Y, Dviri M, Segol I, Yerushalmi GM, Hourvitz A, Orvieto R, Mazaki-Tovi S, Yinon Y. The ‘immunologic theory’ of preeclampsia revisited: a lesson from donor oocyte gestations. Am J Obstet Gynecol 2014;211:383 e381–383 e385. [DOI] [PubMed] [Google Scholar]
- Martin J, Osterman M, Kirmeyer S, Gregory E. Measuring gestational age in vital statistics data: transitioning to the obstetric estimate. Natl Vital Stat Rep 2015;64:1–10. [PubMed] [Google Scholar]
- Masoudian P, Nasr A, de Nanassy J, Fung-Kee-Fung K, Bainbridge SA, El Demellawy D. Oocyte donation pregnancies and the risk of preeclampsia or gestational hypertension: a systematic review and metaanalysis. Am J Obstet Gynecol 2016;214:328–339. [DOI] [PubMed] [Google Scholar]
- Morgan TK. Role of the placenta in preterm birth: a review. Am JPerinatol 2016;33:258–266. [DOI] [PubMed] [Google Scholar]
- Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol 2000;96:950–955. [PubMed] [Google Scholar]
- Pedersen AB, Mikkelsen EM, Cronin-Fenton D, Kristensen NR, Pham TM, Pedersen L, Petersen I. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol 2017;9:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savasi VM, Mandia L, Laoreti A, Cetin I. Maternal and fetal outcomes in oocyte donation pregnancies. Hum Reprod Update 2016;22: 620–633. [DOI] [PubMed] [Google Scholar]
- Smith GN, Walker M, Tessier JL, Millar KG. Increased incidence of preeclampsia in women conceiving by intrauterine insemination with donor versus partner sperm for treatment of primary infertility. Am J Obstet Gynecol 1997;177:455–458. [DOI] [PubMed] [Google Scholar]
- Stillbirth Collaborative Research Network Writing G. Causes of death among stillbirths. JAMA 2011;306:2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop D, Baumgarten M, Haentjens P, Polyzos NP, De Vos M, Verheyen G, Camus M, Devroey P. Obstetric outcome in donor oocyte pregnancies: a matched-pair analysis. Reprod Biol Endocrinol 2012;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn ML, Lashley EE, Bianchi DW, Claas FH, Schonkeren CM, Scherjon SA. Clinical and immunologic aspects of egg donation pregnancies: a systematic review. Hum Reprod Update 2010;16:704–712. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Crawford S, Boulet S, Monsour M, Cohen B, McKane P, Freeman K. Using multiple imputation to address missing values of hierarchical data. J Mod Appl Stat Methods 2017;16:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


