Abstract
Background
There are no prediction models for bile leakage associated with subtotal cholecystectomy (STC). Therefore, this study aimed to generate a multivariable prediction model for post-STC bile leakage and evaluate its overall performance.
Methods
We analysed prospectively managed data of patients who underwent STC by a single consultant surgeon between 14 May 2013 and 21 December 2021. STC was schematised into four variants with five subvariants and classified broadly as closed-tract or open-tract STC. A contingency table was used to detect independent risk factors for bile leakage. A multiple logistic regression analysis was used to generate a model. Discrimination and calibration statistics were computed to assess the accuracy of the model.
Results
A total of 81 patients underwent the STC procedure. Twenty-eight patients (35%) developed bile leakage. Of these, 18 patients (64%) required secondary surgical intervention. Multivariable logistic regression revealed two independent predictors of post-STC bile leak: open-tract STC (odds ratio [OR], 7.07; 95% confidence interval [CI], 2.191–25.89; P = 0.0170) and acute cholecystitis (OR, 5.449; 95% CI, 1.584–23.48; P = 0.0121). The area under the receiver-operating characteristic curve was 82.11% (95% CI, 72.87–91.34; P < 0.0001). Tjur’s pseudo-R2 was 0.3189 and the Hosmer–Lemeshow goodness-of-fit statistic was 4.916 (P = 0.7665).
Conclusions
Open-tract STC and acute cholecystitis are the most reliable predictors of bile leakage associated with STC. Future prospective, multicentre studies with higher statistical power are needed to generate more specific and externally validated prediction models for post-STC bile leaks.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-023-10049-2.
Keywords: Subtotal cholecystectomy, Bile leak, Risk factors, Logistic regression, Multivariable model, Prediction
Total cholecystectomy is the definitive treatment for symptomatic and complicated cholecystolithiasis. However, recent nationwide studies have demonstrated a shift toward subtotal cholecystectomy (STC) in the United States [1] and England [2] over the past two decades despite the spectrum of adverse effects associated with it [3–5].
Postoperative bile leakage is a significant adverse outcome of STC that affects up to 20% of patients who undergo this surgery [6–8]. Although some bile leaks spontaneously resolve, this postoperative event is associated with high morbidity rates and prolonged hospitalisation [3–8]. Additional investigations and multiple procedures, including endoscopic retrograde cholangiopancreatography (ERCP), ultrasound-guided drain insertion, and reoperations, are often required to manage ongoing bile leaks and their sequelae.
Several authors have postulated that patient characteristics and surgical techniques may influence the incidence of bile leak after STC, but this has not yet been established [3, 4, 9–12]. After analysing 100 case series published between January 1985 and June 2022 (Supplementary Material, Section 1), it was apparent that identifying the predictors of bile leaks associated with STC was not a focus, as it was attempted by only one author [13]. Consequently, there is limited knowledge of the risk factors for post-STC bile leaks, and there are no available preoperative or intraoperative assessment scales to identify those who are most at risk [14].
Identifying factors associated with bile leaks would enable surgeons to prepare and optimise postoperative monitoring to ensure the early detection of leaks and mitigate the risk of post-STC bile leaks via targeted perioperative interventions. Additionally, accurate predictive models can inform the shared decision-making process with patients and ultimately improve the quality and transparency of communication and the consent process for cholecystectomy [15].
This study aimed to generate a multivariable logistic regression model to detect and quantitatively characterise the predictors of bile leak associated with STC. The objectives of this study were to characterise the cohort of patients who underwent STC, stratify patients by the occurrence of post-STC bile leakage, identify independent preoperative and intraoperative clinical factors associated with post-STC bile leakage, and model the statistical relationships of the predictors and post-STC bile leak.
Materials and methods
Study setting, design, and patient selection
The study was conducted in a university acute care hospital, which is a tertiary academic centre for hepatobiliary surgery. The emergency general surgery unit was the destination for patients with acute biliary events requiring an urgent cholecystectomy during the index admission or an elective cholecystectomy after a course of conservative treatment, ERCP or tube cholecystostomy, and temporary discharge.
We analysed a prospectively managed clinical data database of all adult patients who underwent an urgent or elective STC by a single attending surgeon at the University Hospital between 14 May 2013 and 21 December 2021. All patients treated between 2020 and 2021 were free of coronavirus disease 2019 (COVID-19) at the time of surgery. The postoperative follow-up was longitudinal, and the patients were reviewed in the wards and outpatient clinics. All perioperative and long-term clinical outcomes were regularly gathered, and the final follow-up was conducted via electronic review of the patient charts on 23 June 2022.
Ethical considerations and reporting guidance
All procedures were performed and all patients were managed according to the ethical standards of the institution’s general surgery department. This included a comprehensive preoperative discussion, a second opinion from a hepatobiliary surgery consultant before conversion to STC, and consultations with other subspecialists in the event of postoperative complications. The Institutional Clinical Audit Management Board reviewed, approved, and registered the study protocol (registration number: 10817). The study is reported according to the preferred reporting of case series in surgery (PROCESS) and transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guidelines (Supplementary Tables 1S and 2S) [16, 17]. The data are in accordance with the findable, accessible, interoperable, reusable (FAIR) principles [18].
Operative technique
When a portion of the gallbladder, regardless of size, was left in its anatomical site during cholecystectomy, this was described as STC. The variants (STC-1, STC-2, STC-3, and STC-4) and subvariants (STC-1A, STC-1B, STC-1C, STC-2A, and STC-2B) of STC adopted by the operating surgeon in this series were described previously [19], and an overview is included in the Supplementary Material (Section 2.2). Briefly, STC-1 describes circumferential resection of both the visceral and hepatic walls of the gallbladder (Supplementary Figures 1S and 2S). With STC-2, only the visceral wall was resected, either without (STC-2A) or with closing/clipping of the cystic duct (STC-2B) (Supplementary Figure 3S). STC-3 is involved in excision of the fundus of the gallbladder. None of the patients in this case series underwent STC-4.
STC was also broadly characterised into two types–closed-tract and open-tract STC. When the gallbladder remnant or cystic duct was closed, irrespective of the technique used, we described this type as a closed-tract STC. If the gallbladder remnant and cystic duct were left open, then we described this type as an open-tract STC.
Study data and outcomes
Data deduced from the database were subdivided into preoperative factors, intraoperative factors, postoperative outcomes, and long-term outcomes. Preoperative factors included demographic characteristics, medical history, clinical presentation, biochemistry, preoperative diagnosis, and disease severity according to the Tokyo classification [20]. The intraoperative factors included the surgical approach, technical variant, main surgical finding, type of STC, and methods of closing the gallbladder remnant or cystic duct. Data of 152 preoperative and intraoperative variables were available; however, we only considered the most clinically relevant variables in the data analyses for this series.
The primary postoperative outcome was bile leakage. Postoperative bile leaks were classified into grades A, B, and C according to the International Study Group for Liver Surgery [21]. Grade A bile leaks required little to no change in clinical management. Grade B bile leaks required additional interventional procedures such as ERCP or percutaneous drainage, but they were manageable without reoperation. The grade C bile leak required reoperation. In this study, grade A leaks that persisted longer than 1 week were not classified as grade B leaks. The secondary postoperative outcomes included 30-day complications, admission to the intensive therapy unit, secondary surgical procedures, length of hospitalisation, and readmission. The Clavien–Dindo classification was used to categorise postoperative complications [22]. Long-term outcomes included acute biliary events, completion of cholecystectomy, and overall survival.
Statistical analysis
The data collected were entered using Microsoft Excel, validated, and edited before being analysed using GraphPad Prism version 9.3.1 (350) for macOS (GraphPad Software, Inc., San Diego, CA, USA). The computing packages for conventional and advanced statistics were used. To assess whether the relationship between the independent risk factor and the occurrence of bile leak after STC was more than expected by chance, a contingency table was used to compute the odds ratio (OR) with the 95% confidence interval (CI) via the Baptista–Pike method. Fisher’s two-sided test was used to determine the strength of each univariate relationship. Statistically significant variables associated with bile leak (P ≤ 0.05) were further considered for multivariable analyses via multiple logistic regression. To evaluate postoperative outcomes, continuous data were compared using the Kolmogorov–Smirnov test.
To evaluate the discriminatory performance of the model, particularly how well the model separates those with post-STC bile leak development from those without, the area under the receiver-operating characteristic curve, which is a trade-off between sensitivity and specificity and ranges from 0.5 (a model no better than chance) to 1.0 (a model with perfect accuracy), was reported [23]. The positive and negative predictive values of the model were also reported. Tjur’s coefficient of discrimination (pseudo-R2) statistic was calculated to describe the ability of the model to predict post-STC bile leak with a range from 0 (model with no predictive ability) to 1 (model with perfect predictive ability) [24]. The model calibration, specifically the goodness-of-fit, was evaluated using the Hosmer–Lemeshow test, and P > 0.05 indicated a robust model that cannot be rejected [25].
Results
General characterisation
Between 2013 and 2021, 538 cholecystectomies were performed; 81 (15.1%) were STC. A complete report of the characteristics and clinical outcomes of the 81 patients who underwent STC is provided in Supplementary Table 3S (Section 3). The key characteristics of this cohort are described below.
Baseline demographic and preoperative characteristics
The median age was 56 years (interquartile range [IQR], 22.5 years), and most patients were female (69.1%). Forty-four patients (54.3%) had a body mass index ≥ 30 kg/m2. Twenty-eight patients (34.6%) had a Charlson’s age-comorbidity index ≥ 3. Fifty-six patients (69.1%) had a history of unplanned hospitalisation with an acute complication of gallstone disease, and 30% of these patients had been hospitalised at least twice. Forty-six patients (56.8%) underwent emergent STC during the index admission, and the rest of the patients underwent elective surgery. One in four patients (27.2%) of the STC surgical cohort had a history of either ERCP (17 patients) or a tube cholecystostomy (5 patients).
Intraoperative characteristics
Cholecystitis was classified as acute in 50 patients (61.7%), and 31 patients (38.3%) had chronic cholecystitis. The three most common forms of acute gallbladder disease were suppurative (23 patients, 28.4%), gangrenous (16 patients, 19.8%), and perforated (10 patients, 12.1%) cholecystitis. Contracted gallbladder and Mirizzi syndrome type 1 were detected in 13 (16%) and 2 (2.5%) patients, respectively. STC was performed laparoscopically in the majority of patients (97.5%). Two patients (2.5%) underwent open conversion. Regarding the extent of gallbladder resection, STC-1 was performed in 61 patients (75.3%), STC-2 in 19 patients (23.5%), and STC-3 in 1 patient (1.2%). A closed-tract STC was performed in 43 patients (53.1%), and of these, ENDOLOOP ligature was used in 35 patients (81.4%). There were no bile duct, vascular, or visceral injuries. The perihepatic space was drained in 76 patients (93.8%).
All gallbladder specimens were sent for histopathological assessment. There were no malignancies detected. Based on the histology, acute and chronic cholecystitis were confirmed in 46 (56.8%) and 34 (42.0%) patients, respectively (one patient had missing data). Of those with acute cholecystitis, 45.7% had suppurative or gangrenous cholecystitis.
Postoperative characteristics
Twenty-eight patients (34.6%) had bile leaks. There were 10 (12.3%) grade A, 14 (17.3%) grade B, and 4 (4.9%) grade C bile leaks. The median maximum volume of the bile leak was 230 mL (range, 50–1200 mL; IQR, 290 mL). The median duration of the bile leak was 5 days (range, 2–26 days; IQR, 7.5 days).
Univariable analyses of preoperative factors
As highlighted in Table 1, there were no significant differences in the age, sex, body mass index, comorbidities, surgical history, history of gallstone disease, duration of acute symptoms, preoperative laboratory values, preoperative diagnosis of acute cholecystitis, Tokyo classification of acute cholecystitis, American Society of Anaesthesiologists grade, and nature of admission of the bile leak and no bile leak groups.
Table 1.
Summary of the univariable analysis to detect risk factors for bile leak associated with subtotal cholecystectomy
| Variable | Bile leak (%) | No bile leak (%) | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|
| Number of patients | 28 | 53 | – | – |
| Preoperative factors | ||||
| Age | ||||
| Median (IQR) | 58 (25.25) | 55 (23) | – | – |
| ≥ 60 years | 14 (50.0) | 24 (45.3) | 1.208 (0.4988–2.941) | 0.8155 |
| Sex | ||||
| Female | 17 (60.7) | 39 (73.6) | 0.5548 (0.2046–1.554) | 0.3123 |
| Male | 11 (39.3) | 14 (26.4) | 1.803 (0.6434–4.887) | |
| BMI, median (IQR) | 30.7 (6.5) | 29.8 (7.8) | – | – |
| BMI, by category | ||||
| < 25 kg/m2 | 4 (14.3) | 7 (13.2) | 1.095 (0.3319–4.069) | > 0.9999 |
| ≥ 25–29.9 kg/m2 | 6 (21.4) | 20 (37.7) | 0.4500 (0.1529–1.278) | 0.2104 |
| ≥ 30–39.9 kg/m2 | 16 (57.1) | 19 (35.8) | 2.386 (0.9778–6.247) | 0.0983 |
| ≥ 40 kg/m2 | 2 (7.1) | 7 (13.2) | 0.5055 (0.1010–2.659) | 0.4865 |
| Comorbidities excluding obesity | 20 (71.4) | 29 (54.7) | 2.069 (0.8106–5.664) | 0.1604 |
| Past surgical history | ||||
| Midline laparotomy | 0 | 2 (3.8) | 0.000 (0.000–4.092) | 0.5420 |
| Laparoscopic RYGB | 1 (3.6) | 0 | + ∞ (0.2103 to + ∞) | 0.3457 |
| Previous hospitalisation for gallstone disease | 19 (67.9) | 37 (69.8) | 0.9129 (0.3417–2.382) | > 0.9999 |
| Duration of acute symptoms before admission, median (IQR) | 6.5 (5) | 6 (5) | – | – |
| Common bile duct dilatation (≥ 7 mm) | 12 (42.9%) | 14 (26.4) | 2.089 (0.7745–5.641) | 0.1431 |
| WBC count per mm3, median (IQR) | 9 (9.1) | 8.9 (5.7) | – | – |
| CRP per mg/L, median (IQR) | 230 (280) | 121 (260) | – | – |
| Preoperative diagnosis of acute cholecystitis | 18 (64.3) | 28 (52.8) | 1.607 (0.6594–3.930) | 0.3549 |
| Acute cholecystitis, by Tokyo classification | ||||
| Grade 1 | 3 (10.7) | 8 (15.1) | 0.6750 (0.1815–2.595) | 0.7397 |
| Grade 2 | 7 (25.0) | 15 (28.3) | 0.8444 (0.3245–2.446) | 0.7991 |
| Grade 3 | 8 (28.6) | 5 (9.4) | 3.657 (1.166–11.27) | 0.0546 |
| ASA, by class | ||||
| I | 4 (14.3) | 8 (15.1) | 0.9375 (0.2902–3.164) | > 0.9999 |
| II | 22 (78.6) | 40 (75.5) | 1.192 (0.4299–3.723) | > 0.9999 |
| III | 1 (3.6) | 5 (9.4) | 0.3556 (0.02921–2.923) | 0.6589 |
| IV | 1 (3.6) | 0 | + ∞ (0.2103 to + ∞) | 0.3457 |
| Emergent/urgent STC | 18 (64.3) | 28 (52.8) | 1.607 (0.6594–3.930) | 0.3549 |
| Intraoperative factors | ||||
| Surgical approach | ||||
| Laparoscopic | 27 (96.4) | 52 (98.1) | 0.5192 (0.02688–10.20) | > 0.9999 |
| Conversion to open | 1 (3.6) | 1 (1.9) | 1.926 (0.09801–37.20) | > 0.9999 |
| Main surgical finding | ||||
| Acute cholecystitis | 22 (78.6) | 28 (52.8) | 3.274 (1.182–9.503) | 0.0308 |
| Mild | 1 (3.6) | 0 | + ∞ (0.2103 to + ∞) | 0.3457 |
| Severe | 21 (75.0) | 28 (52.8) | 2.679 (1.015–6.773) | 0.0601 |
| Suppurative | 9 (32.1) | 14 (26.4) | 1.320 (0.4946–3.681) | 0.6121 |
| Gangrenous | 6 (21.4) | 10 (18.9) | 1.173 (0.3556–3.634) | 0.7770 |
| Perforated | 6 (21.4) | 4 (7.5) | 3.341 (0.9209–11.15) | 0.0864 |
| Chronic cholecystitis | 6 (21.4) | 25 (47.2) | 0.3055 (0.1052–0.8461) | 0.0308 |
| STC, by variant | ||||
| STC-1 (A, B and C) | 16 (57.1) | 45 (84.9) | 0.2370 (0.0872–0.6641) | 0.0132 |
| STC-1A | 6 (21.4) | 28 (52.8) | 0.2435 (0.0839–0.6727) | 0.0089 |
| STC-1B | 9 (32.1) | 14 (26.4) | 1.320 (0.4946–3.681) | 0.6121 |
| STC-1C | 1 (3.6) | 3 (5.7) | 0.6173 (0.0460–4.334) | > 0.9999 |
| STC-2 (A and B) | 11 (39.3) | 8 (15.1) | 3.640 (1.313–9.991) | 0.0257 |
| STC-2A | 11 (39.3) | 5 (9.4) | 6.212 (1.898–18.25) | 0.0026 |
| STC-2B | 0 | 3 (5.7) | 0.000 (0.000–2.166) | 0.5478 |
| STC-3 | 1 (3.6) | 0 | + ∞ (0.2103 to + ∞) | 0.3457 |
| STC, by intervention to hepatic wall | ||||
| Removal of hepatic wall | 16 (57.1) | 45 (84.9) | 0.2370 (0.0872–0.6641) | 0.0132 |
| Non-removal of hepatic wall | 12 (42.9) | 8 (15.1) | 4.219 (1.506–11.46) | |
| STC, by type of completion | ||||
| Open-tract STC | 22 (78.6) | 16 (30.2) | 8.479 (2.941–25.68) | < 0.0001 |
| Closed-tract STC | 6 (21.4) | 37 (69.8) | 0.1179 (0.0389–0.3401) | |
| Method of closing the biliary tract | ||||
| Endoloop ligature | 4 (14.3) | 31 (58.5) | 0.1183 (0.0408–0.3866) | 0.0001 |
| Continuous suture | 2 (7.1) | 3 (5.7) | 1.282 (0.2163–6.578) | > 0.9999 |
| Endo GIA stapler | 0 | 1 (1.9) | 0.000 (0.000–17.04) | > 0.9999 |
| Hem-o-lock system | 0 | 2 (3.8) | 0.000 (0.000–4.092) | 0.5420 |
OR, odds ratio; CI, confidence interval; ICR, interquartile range; SD, standard deviation; BMI, body mass index; RYGB, Roux-en-Y gastric bypass; STC, subtotal cholecystectomy; WBC, white blood cell; ASA, American Society of Anaesthesiologists; ∞, infinity; GIA, gastrointestinal anastomosis
Univariable analyses of intraoperative factors
Table 1 shows the five factors significantly associated with post-STC bile leak. Bile leaks were more likely to occur in patients whose surgical findings were consistent with those of acute cholecystitis (OR, 3.274; 95% CI, 1.182–9.503; P = 0.0308). Furthermore, undergoing STC-2 (OR, 3.640; 95% CI, 1.313–9.991; P = 0.0257) and STC-2A (OR, 6.212; 95% CI, 1.898–18.25; P = 0.0026), leaving the hepatic wall intact (OR, 4.219; 95% CI 1.506–11.46; P = 0.0132), and leaving the gallbladder tract open (OR, 8.479; 95% CI, 2.941–25.68; P < 0.0001) significantly increased the likelihood of post-STC bile leak.
Multivariable analyses of bile leak predictors
Table 2 presents the model derived from multiple logistic regression using four variables significantly associated with postoperative bile leak in the univariate analyses. STC-2 was excluded from the regression model because of multicollinearity (i.e., it was less specific and highly correlated with STC-2A) because this could diminish the statistical significance of the model. The multivariate model was built by entering the factors using a single step. The multivariate analysis identified two independent factors as predictors of post-STC bile leak. Open-tract STC was the strongest predictor of post-STC bile leak (OR, 7.07; 95% CI, 2.191–25.89). Acute cholecystitis was another predictor. Its presence increased the odds of developing post-STC bile leak by fivefold (95% CI, 1.584–23.48).
Table 2.
Multiple logistic regression model to predict bile leak associated with subtotal cholecystectomy
| Variables in model | Regression coefficient β (standard error) | Odds ratio (95% CI) | Wald test | P-value |
|---|---|---|---|---|
| Intercept, β0 | −3.196 (0.7684) | 0.0409 (0.0074–0.1563) | 4.151 | < 0.0001 |
| Acute cholecystitis, β1 | 1.695 (0.6755) | 5.449 (1.584–23.48) | 2.510 | 0.0121 |
| STC-2A, β2 | 1.148 (1.361) | 3.151 (0.2580–81.69) | 0.8432 | 0.3991 |
| Non-removal of hepatic wall, β3 | 0.3339 (1.219) | 1.396 (0.0645–12.72) | 0.2738 | 0.7842 |
| Open-tract STC, β4 | 1.956 (0.6223) | 7.070 (2.191–25.89) | 3.143 | 0.0017 |
β, relating coefficient to probability, either β0 (intercept) or β1, β2, β3, β4, β5 (slopes) for each variable
CI, confidence interval; STC, subtotal cholecystectomy; STC-2A, subtotal cholecystectomy, subvariant 2A
Overall performance of the prediction model
Figure 1 depicts the highly accurate discriminatory performance of the multiple logistic regression model [26–29]. An area under the receiver-operating characteristic curve of 82.11% was observed. The positive and negative predictive values of the model were 66.67% and 84.31%, respectively. Tjur’s pseudo-R2 statistic was estimated to be 0.3189, and the Hosmer–Lemeshow statistic was 4.916 (P = 0.7665), thus demonstrating a reliable model with highly accurate calibration of the predicted and actual bile leak rates.
Fig. 1.
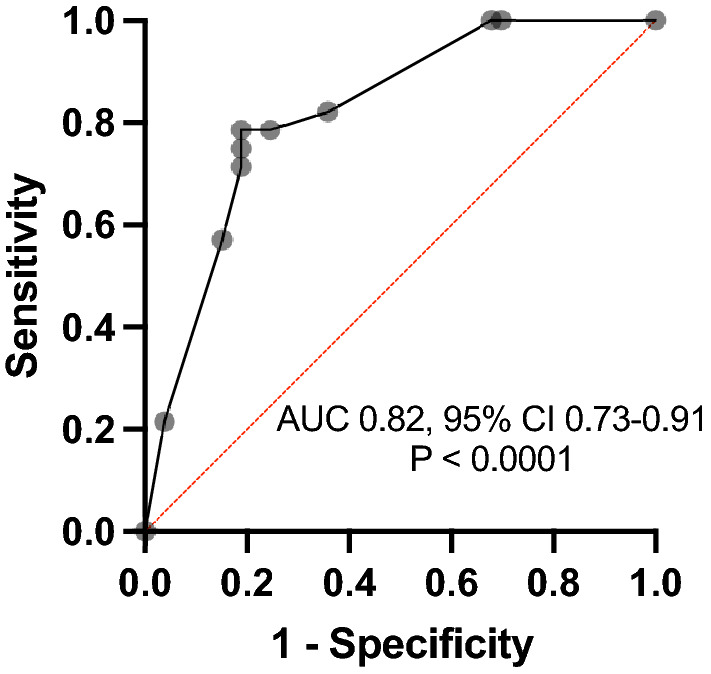
Discriminatory performance of the multivariable model to predict bile leak associated with subtotal cholecystectomy. AUC, area under the receiver-operating characteristic curve; CI, confidence interval
Associations between bile leak and short-term postoperative outcomes
As shown in Table 3, post-STC bile leaks were significantly associated with Clavien–Dindo grade III and IV complications (OR, 93.6; 95% CI, 13.74–990.5; P < 0.0001). Post-STC bile leaks were significantly associated with admission to the intensive care unit (10.5% vs. 0%; P < 0.0001) and longer postoperative hospitalisation (9 days vs. 4 days; P < 0.0001). Patients with bile leaks were more likely to undergo secondary surgical procedures during the index admission (OR, 60.0; 95% CI, 9.014–642.2; P < 0.0001).
Table 3.
Subgroup analysis of the short-term and long-term postoperative clinical outcomes
| Variable | Bile leak (%) | No bile leak (%) | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|
| Total number of patients | 28 | 53 | ||
| Short-term outcomes | ||||
| Complication, by Clavien–Dindo classification | ||||
| Grades I and II | 10 (35.7) | 44 (83.0) | 0.1136 (0.0433–0.329) | < 0.0001 |
| Grades III and IV | 18 (64.3) | 1 (1.9) | 93.60 (13.74–990.5) | < 0.0001 |
| Admission to intensive care unit | 3 (10.7) | 0 | + ∞ (1.714 to + ∞) | 0.0384 |
| Drain removed (days), median (range) | 9 (3 – 36) | 2.5 (1 – 25) | – | < 0.0001 |
| Hospital length of stay (days), median (range) | 9 (4 – 38) | 4 (1 – 42) | – | < 0.0001 |
| Post-STC surgical procedures, index admission | 15 (53.6) | 1 (1.2) | 60.00 (9.014–642.2) | < 0.0001 |
| 30-day readmission, unplanned | 3 (10.7) | 6 (11.3) | 0.9400 (0.2416–3.510) | > 0.9999 |
| 30-day mortality | 0 | 0 | – | – |
| Long-term outcomes | ||||
| Patients with acute biliary events | 3 (10.7) | 3 (5.7) | 2.000 (0.4379–8.973) | 0.4114 |
| Acute biliary events | 5 (17.9) | 5 (9.4) | 2.087 (0.5861–7.353) | 0.3026 |
| Cholecystitis | 2 (7.1) | 0 | + ∞ (0.8909 to + ∞) | 0.1167 |
| Liver abscess | 1 (3.6) | 1 (1.2) | 1.926 (0.09801–37.20) | > 0.9999 |
| Symptomatic choledocholithiasis | 1 (3.6) | 4 (7.5) | 0.4537 (0.0359–3.022) | 0.6544 |
| Common bile duct stricture | 1 (3.6) | 0 | + ∞ (0.2103 to + ∞) | 0.3457 |
| Completion cholecystectomy | 2 (7.1) | 0 | + ∞ (0.8909 to + ∞) | 0.1167 |
| Overall survival, by 13/05/2022 | 27 (96.4) | 48 (90.6) | 2.813 (0.3421–34.23) | 0.6589 |
STC, subtotal cholecystectomy; ∞, infinity
Associations between bile leak and long-term postoperative outcomes
None of the patients were lost to follow-up. The median follow-up duration for all 81 patients was 3.9 years (range, 0.4–9 years). Acute biliary events, completion of cholecystectomy, and all-cause mortality did not appear to be significantly altered by post-STC bile leaks (Table 3). By 13 May 2022, 6 of the 81 patients (7.4%) died because of malignant oncological disease (pancreas, breast, lung, and kidney) and COVID-19.
Discussion
Bile leak is one of the most common adverse outcomes after STC [6–8]. Our findings indicate that patients who experience bile leaks after STC are more likely to require secondary surgical interventions, admissions to the intensive care unit, and longer hospitalisation. Despite a growing body of high-quality evidence surrounding the outcomes of STC, the factors associated with post-STC bile leakage have not been sufficiently investigated. Identifying the predictors of post-STC bile leak would enable surgeons to optimise perioperative care, improve communication with patients, and mitigate the burden of the disease.
This study identified open-tract STC as the most significant independent predictor of bile leakage after STC. This was not surprising because of the similarities between open-tract STC and the classic fenestrating STC, which has been associated with a higher incidence of bile leak [7, 12]. Fenestrating STC was described as the excision of the gallbladder wall with no attempt to close the gallbladder remnant other than an internal purse-string suture of the cystic duct [10, 30]. However, Strasberg and colleagues stressed that it is not always possible to close the cystic duct [30].
In our series, we deviated from using the words ‘fenestrating’ and ‘reconstituting’ [10, 12, 30, 31] because they do not directly emphasize whether the cystic duct is closed or left open. Because of the morbidity associated with a patent biliary tract, we believe that the terms ‘subtotal open-tract cholecystectomy’ and ‘subtotal closed-tract cholecystectomy’ more accurately capture the complexity and differences in the technical execution of STC.
Whether open-tract STC as an independent predictor is a modifiable risk factor remains debatable. However, open-tract STC should be the last resort when neither total cholecystectomy nor closed-tract STC can be performed. Intraoperative ultrasonography [32] and intraoperative cholangiography [33] can be utilised in difficult gallbladder cases necessitating STC, even though their role in these settings is debated [34]. A national database study revealed that, in 2019, intraoperative cholangiography and bile duct exploration were performed in conjunction with only 5% of STCs (88 of 1772) in England [2, 35]. We did not attempt to perform intraoperative ultrasonography or intraoperative cholangiography. This was in accordance with our institutional policy, which stresses that postoperative patients with significant postoperative bile leakage and/or suspected retained gallstones in the common bile duct should be referred to the hepatology team to discuss the indications for an endoscopic ultrasound scan of the extrahepatic biliary tract and ERCP.
When indicated, an intraoperative ERCP can be considered to remove residual calculi in the common bile duct and/or stent the duct, especially in patients with a history of common bile duct dilatation, choledocholithiasis, deranged liver function tests, and/or fresh bile visible in the gallbladder remnant. Although no reports support these suggestions for STC, few authors have shown that more favourable outcomes are achieved with simultaneous laparoscopic cholecystectomy and intraoperative ERCP than with two-stage preoperative ERCP and laparoscopic cholecystectomy [36–38]. Ultimately, the decision to utilise these intraoperative diagnostic procedures, especially in laparoscopic surgery, is multidisciplinary and should be made in planned clinical governance meetings involving surgeons, hepatologists, and radiologists in specialised centres. It is worth noting that a closed-tract STC is not completely free of bile leaks. We found that 14% (6/43) of patients who underwent this operation developed bile leaks, and it is likely that the genesis of bile leak after closed-tract STC is multifactorial.
Furthermore, our analyses revealed that acute cholecystitis was an independent predictor of bile leak for patients who underwent STC. Our findings are synchronous with those of a recently published study that demonstrated that the incidence of post-STC bile leak was higher for patients with acute cholecystitis compared to chronic cholecystitis [13]. Based on these results, a plausible course of action would be to delay surgery for patients with acute cholecystitis. However, Kohga and colleagues [13] highlighted the benefit of pursuing early surgery for patients with acute cholecystitis, particularly within the first 10 days of disease onset, because this was linked to a significantly decreased incidence of bile leak after STC compared to surgery performed between 10 days and 10 weeks (7.7% vs. 47.1%) after onset. Additionally, attempts to delay surgery contradict the World Society of Emergency Surgery guidelines, which advocate early laparoscopic cholecystectomy, especially within 10 days of the onset of acute symptoms, in the absence of absolute contraindications [39].
This series improves our awareness of the factors associated with bile leak development after undergoing STC for benign gallbladder diseases. The main strengths of this study are the variety of potential risk factors obtained from a prospectively managed database of patients treated at a busy regional academic centre for emergency and hepatobiliary surgery that were analysed. Additionally, we performed relatively long, rigorous, and systematic surveillance to assess short-term and long-term postoperative outcomes, and no patient was lost to follow-up. To the best of our knowledge, this is the most comprehensive analysis of predictors of post-STC bile leak. By performing a multivariable logistic regression analysis, we determined some relevant risk factors.
However, this study had several limitations. Although our sample size was relatively larger than that of most published STC case series, it was still small. Consequently, the multivariable model could not be validated, and the decreased statistical power of the study could have resulted in the loss of certain associations or potential risk factors. Furthermore, the study cohort comprised patients who were exclusively treated by a single surgeon at a single institution, thus diminishing the generalisability of the study to a wider population.
Conclusions
As STC rates steadily increase, it is crucial to generate precise predictive models. The overall performance of our multiple logistic regression-based prediction model is accurate. Therefore, open-tract STC and acute cholecystitis are the most reliable predictors of bile leakage after STC. Our results will enable surgeons to identify patients at risk for bile leaks. Furthermore, they highlight the need for further multicentre studies with large sample sizes to evaluate the risk of post-STC bile leaks prospectively. Although we appreciate that the risk of bile leak development after STC cannot be eliminated completely, further research would foster the development of externally validated predictive models and risk assessment tools, as well as guidelines for mitigating such risks and optimising the management of post-STC bile leaks. Ultimately, this would be another step in the right direction toward developing clinical pathways for patients requiring complex care for advanced gallbladder disease of inflammatory origin.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
RL: Conceptualisation, methodology, data curation, formal analysis, supervision, writing – original draft, writing – review, and editing. ICN: data validation, methodology, formal analysis, writing – review, and editing.
Funding
No funding was received.
Declarations
Disclosure
Raimundas Lunevicius and Ikemsinachi C. Nzenwa have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sabour AF, Matsushima K, Love BE, Alicuben ET, Schellenberg MA, Inaba K, Demetriades D. Nationwide trends in the use of subtotal cholecystectomy for acute cholecystitis. Surgery. 2020;167:569–574. doi: 10.1016/j.surg.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Lunevicius R, Nzenwa IC, Mesri M. A nationwide analysis of gallbladder surgery in England between 2000 and 2019. Surgery. 2022;171:276–284. doi: 10.1016/j.surg.2021.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Lidsky ME, Speicher PJ, Ezekian B, Holt EW, Nussbaum DP, Castleberry AW, Perez A, Pappas TN. Subtotal cholecystectomy for the hostile gallbladder: failure to control the cystic duct results in significant morbidity. HPB (Oxford) 2017;19:547–556. doi: 10.1016/j.hpb.2017.02.441. [DOI] [PubMed] [Google Scholar]
- 4.Lunevicius R, Haagsma JA. Subtotal cholecystectomy: results of a single-center, registry-based retrospective cohort study of 180 adults in 2011–2018. J Laparoendosc Adv Surg Tech A. 2021;31:1019–1033. doi: 10.1089/lap.2020.0713. [DOI] [PubMed] [Google Scholar]
- 5.Chávez-Villa M, Dominguez-Rosado I, Figueroa-Méndez R, De Los S-Pérez A, Mercado MA. Subtotal cholecystectomy after failed critical view of safety is an effective and safe bail out strategy. J Gastrointest Surg. 2021;25:2553–2561. doi: 10.1007/s11605-021-04934-1. [DOI] [PubMed] [Google Scholar]
- 6.Elshaer M, Gravante G, Thomas K, Sorge R, Al-Hamali S, Ebdewi H. Subtotal cholecystectomy for "difficult gallbladders": systematic review and meta-analysis. JAMA Surg. 2015;150:159–168. doi: 10.1001/jamasurg.2014.1219. [DOI] [PubMed] [Google Scholar]
- 7.Nzenwa IC, Mesri M, Lunevicius R. Risks associated with subtotal cholecystectomy and the factors influencing them: a systematic review and meta-analysis of 85 studies published between 1985 and 2020. Surgery. 2021;170:1014–1023. doi: 10.1016/j.surg.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 8.Koo JGA, Chan YH, Shelat VG. Laparoscopic subtotal cholecystectomy: comparison of reconstituting and fenestrating techniques. Surg Endosc. 2021;35:1014–1024. doi: 10.1007/s00464-020-08096-0. [DOI] [PubMed] [Google Scholar]
- 9.Shimoda M, Udo R, Imasato R, Oshiro Y, Suzuki S. What are the risk factors of conversion from total cholecystectomy to bailout surgery? Surg Endosc. 2021;35:2206–2210. doi: 10.1007/s00464-020-07626-0. [DOI] [PubMed] [Google Scholar]
- 10.LeCompte MT, Robbins KJ, Williams GA, Sanford DE, Hammill CW, Fields RC, Hawkins WG, Strasberg SM. Less is more in the difficult gallbladder: recent evolution of subtotal cholecystectomy in a single HPB unit. Surg Endosc. 2021;35:3249–3257. doi: 10.1007/s00464-020-07759-2. [DOI] [PubMed] [Google Scholar]
- 11.Lunevicius R, Haagsma JA (2020). Supplementary Material to ‘Subtotal cholecystectomy: results of a single-centre, registry-based retrospective cohort study of 180 adults in 2011–2018’, Mendeley Data, V1. 10.17632/khv3b7b6wf.1. Accessed at https://data.mendeley.com/datasets/khv3b7b6wf/1 on 25 June 2022
- 12.Purzner RH, Ho KB, Al-Sukhni E, Jayaraman S. Safe laparoscopic subtotal cholecystectomy in the face of severe inflammation in the cystohepatic triangle: a retrospective review and proposed management strategy for the difficult gallbladder. Can J Surg. 2019;62:402–411. doi: 10.1503/cjs.014617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohga A, Suzuki K, Okumura T, Yamashita K, Isogaki J, Kawabe A, Kimura T. Risk factors for postoperative bile leak in patients who underwent subtotal cholecystectomy. Surg Endosc. 2020;34:5092–5097. doi: 10.1007/s00464-019-07309-5. [DOI] [PubMed] [Google Scholar]
- 14.Lunevicius R. Cholecystectomy: advances and issues. J Clin Med. 2022;11:3534. doi: 10.3390/jcm11123534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesri M, Nzenwa IC, Lunevicius R. Evaluating the patient and setting-specific factors that influenced the quality of informed consent in a retrospective cohort of subtotal cholecystectomy patients. J Laparoendosc Adv Surg Tech A. 2021;31:77–84. doi: 10.1089/lap.2020.0376. [DOI] [PubMed] [Google Scholar]
- 16.Agha RA, Sohrabi C, Mathew G, Franchi T, Kerwan A, O'Neill N, PROCESS Group The PROCESS 2020 guideline: updating consensus preferred reporting of case series in surgery (PROCESS) guidelines. Int J Surg. 2020;84:231–235. doi: 10.1016/j.ijsu.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMC Med. 2015;13:1. doi: 10.1186/s12916-014-0241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GO FAIR. FAIR principles. https://www.go-fair.org/fair-principles/. Accessed 1 July 2022
- 19.Lunevicius R. Laparoscopic subtotal cholecystectomy: a classification, which encompasses the variants, technical modalities, and extent of resection of the gallbladder. Ann R Coll Surg Engl. 2020;102:315–317. doi: 10.1308/rcsann.2020.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirota M, Takada T, Kawarada Y, Nimura Y, Miura F, Hirata K, Mayumi T, Yoshida M, Strasberg S, Pitt H, Gadacz TR, de Santibanes E, Gouma DJ, Solomkin JS, Belghiti J, Neuhaus H, Büchler MW, Fan ST, Ker CG, Padbury RT, Liau KH, Hilvano SC, Belli G, Windsor JA, Dervenis C. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14:78–82. doi: 10.1007/s00534-006-1159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Florkowski CM. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: communicating the performance of diagnostic tests. Clin Biochem Rev 29. Suppl. 2008;1:S83–S87. [PMC free article] [PubMed] [Google Scholar]
- 24.Tjur T. Coefficients of determination in logistic regression models—a new proposal: the coefficient of discrimination. Am Stat. 2009;63:366–372. doi: 10.1198/tast.2009.08210. [DOI] [Google Scholar]
- 25.Fagerland MW, Hosmer DW. A generalized Hosmer-Lemeshow goodness-of-fit test for multinomial logistic regression models. Stand Genomic Sci. 2012;3:447–453. [Google Scholar]
- 26.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 27.Fischer JE, Bachmann LM, Jaeschke R. A readers' guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29:1043–1051. doi: 10.1007/s00134-003-1761-8. [DOI] [PubMed] [Google Scholar]
- 28.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton DF, Ghert M, Simpson AH. Interpreting regression models in clinical outcome studies. Bone Joint Res. 2015;4:152–153. doi: 10.1302/2046-3758.49.2000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strasberg SM, Pucci MJ, Brunt LM, Deziel DJ. Subtotal cholecystectomy-"Fenestrating" vs "Reconstituting" subtypes and the prevention of bile duct injury: definition of the optimal procedure in difficult operative conditions. J Am Coll Surg. 2016;222:89–96. doi: 10.1016/j.jamcollsurg.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Deng SX, Greene B, Tsang ME, Jayaraman S. Thinking your way through a difficult laparoscopic cholecystectomy: technique for high-quality subtotal cholecystectomy. J Am Coll Surg. 2022;235:e8–e16. doi: 10.1097/XCS.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 32.Dili A, Bertrand C. Laparoscopic ultrasonography as an alternative to intraoperative cholangiography during laparoscopic cholecystectomy. World J Gastroenterol. 2017;23:5438–5450. doi: 10.3748/wjg.v23.i29.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strasberg SM. A three-step conceptual roadmap for avoiding bile duct injury in laparoscopic cholecystectomy: an invited perspective review. J Hepatobiliary Pancreat Sci. 2019;26:123–127. doi: 10.1002/jhbp.616. [DOI] [PubMed] [Google Scholar]
- 34.Brunt LM, Deziel DJ, Telem DA, Strasberg SM, Aggarwal R, Asbun H, Bonjer J, McDonald M, Alseidi A, Ujiki M, Riall TS, Hammill C, Moulton CA, Pucher PH, Parks RW, Ansari MT, Connor S, Dirks RC, Anderson B, Altieri MS, Tsamalaidze L, Stefanidis D, the Prevention of Bile Duct Injury Consensus Work Group Safe cholecystectomy multi-society practice guideline and state of the art consensus conference on prevention of bile duct injury during cholecystectomy. Ann Surg. 2020;272:3–23. doi: 10.1097/SLA.0000000000003791. [DOI] [PubMed] [Google Scholar]
- 35.Lunevicius R, Nzenwa IC, Mesri M (2021) Supplemental Data Content to ‘A nationwide analysis of gallbladder surgery in England between 2000 and 2019’, Mendeley Data, V1. https://data.mendeley.com/datasets/gp9vfvs76n/2. 10.17632/gp9vfvs76n.2 [DOI] [PubMed]
- 36.Tan C, Ocampo O, Ong R, Tan KS. Comparison of one stage laparoscopic cholecystectomy combined with intra-operative endoscopic sphincterotomy versus two-stage pre-operative endoscopic sphincterotomy followed by laparoscopic cholecystectomy for the management of pre-operatively diagnosed patients with common bile duct stones: a meta-analysis. Surg Endosc. 2018;32:770–778. doi: 10.1007/s00464-017-5739-y. [DOI] [PubMed] [Google Scholar]
- 37.Singh AN, Kilambi R. Single-stage laparoscopic common bile duct exploration and cholecystectomy versus two-stage endoscopic stone extraction followed by laparoscopic cholecystectomy for patients with gallbladder stones with common bile duct stones: systematic review and meta-analysis of randomized trials with trial sequential analysis. Surg Endosc. 2018;32:3763–3776. doi: 10.1007/s00464-018-6170-8. [DOI] [PubMed] [Google Scholar]
- 38.Liao Y, Cai Q, Zhang X, Li F. Single-stage intraoperative ERCP combined with laparoscopic cholecystectomy versus preoperative ERCP followed by laparoscopic cholecystectomy in the management of cholecystocholedocholithiasis: a meta-analysis of randomized trials. Medicine (Baltimore) 2022;101:e29002. doi: 10.1097/MD.0000000000029002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisano M, Allievi N, Gurusamy K, Borzellino G, Cimbanassi S, Boerna D, Coccolini F, Tufo A, Di Martino M, Leung J, Sartelli M, Ceresoli M, Maier RV, Poiasina E, De Angelis N, Magnone S, Fugazzola P, Paolillo C, Coimbra R, Di Saverio S, De Simone B, Weber DG, Sakakushev BE, Lucianetti A, Kirkpatrick AW, Fraga GP, Wani I, Biffl WL, Chiara O, Abu-Zidan F, Moore EE, Leppäniemi A, Kluger Y, Catena F, Ansaloni L. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World J Emerg Surg. 2020;15:61. doi: 10.1186/s13017-020-00336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


