Abstract
Meningioma is the most common central nervous system (CNS) tumor. In recent decades, several efforts have been made to eradicate this disease. Surgery and radiotherapy remain the standard treatment options for these tumors. Drug therapy comes to play its role when both surgery and radiotherapy fail to treat the tumor. This mostly happens when the tumors are close to vital brain structures and are nonbenign. Although a wide variety of chemotherapeutic drugs and molecular targeted drugs such as tyrosine kinase inhibitors, alkylating agents, endocrine drugs, interferon, and targeted molecular pathway inhibitors have been studied, the roles of numerous drugs remain unexplored. Recent interest is growing toward studying and engineering exosomes for the treatment of different types of cancer including meningioma. The latest studies have shown the involvement of exosomes in the theragnostic of various cancers such as the lung and pancreas in the form of biomarkers, drug delivery vehicles, and vaccines. Proper attention to this new emerging technology can be a boon in finding the consistent treatment of meningioma.
Keywords: Meningioma, Targeted therapy, Therapeutic drugs, Exosome-based targeted therapy, Exosome drug delivery system, Exosome-based vaccines
Introduction
Meningiomas are tumors arising from the outer membrane of the brain and spinal cord. Primarily, these tumors are formed from meningothelial arachnoid cells, but their presence has also been reported in the ventricles of the CNS and extracranial organs like lungs. Currently, the approximate incidence of meningioma is 7.86 cases per 100,000 people per year confirming it as a dangerous disease. As per the current WHO categorization, around 80% of meningiomas are benign (grade I), while 20% are atypical (grade II) or anaplastic (grade III). Almost 90% of tumors are intracranial while 10% are detected in the spinal area [1]. These tumors are primarily observed in people of the elderly age group (having an age more than 65 years), but the incidence is also increasing in adults [2]. The incidence of meningioma in adults (aged 15–39 years) is approximately 16% of all intracranial tumors. Meningioma is rare in children that account for 0.4–4.1% of all pediatric tumors [3]. Pediatric meningioma occurs equally in males and females; however, in adulthood, meningioma is more prevalent in females than males, with a ratio of 3.5:1 [4]. Radiation [5], diabetes mellitus, arterial hypertension, and smoking are other risk factors for meningioma, the last risk factor being contradictory [6, 7]. The tumor can be identified using magnetic resonance imaging (MRI). When the tumor is small, highly calcified, and asymptomatic, patients may not need any treatment in contrast to patients having large symptomatic tumors causing epilepsy or neurologic deficit. Although surgical excision can cure 70–80% of meningiomas, grade II and grade III meningiomas are not completely removed and can recur [8–10]. As a result, following resection, radiation therapy or stereotactic radiation surgery is done to treat meningioma, either acting as a monotherapy or as an adjuvant therapy [11]. When surgery and radiation therapy fail to give the desired results and the tumor continues to grow, this leads to recurrent meningiomas, which are candidates for systemic therapy. Radiosurgery is also disadvantageous as it causes neurotoxicity and injury to the adjacent vascular and cranial nerves, again increasing the dependency on systemic therapy. Over the last decade, many drugs have been tested for meningioma. Systemic therapy includes chemotherapy (conventional or cytotoxic therapy), hormonal therapy, targeted therapy, and immune therapy in which numerous small-molecule drugs are intended to target cancerous cells without harming normal cells.
Tumor mass is mostly occupied by the TME (tumor microenvironment), which constitutes the stroma of the tumor [12]. Exosomes are small extracellular vesicles having 30 to 150 nm diameter. They are involved in cell-to-cell signaling [13]. They can transfer a cargo of proteins, nucleic acids, carbohydrates, and lipids from donor cells to recipient cells. Exosomes can be produced by all kinds of cells, i.e., diseased and normal cell types, but their increased production has been reported in diseased conditions. They are also found as good diagnostic markers for diseases, especially cancers like meningioma. Exosomes influence the TME component cells, which leads to the progression of cancers (meningioma). Since meningioma is highly vascularized cancer, angiogenesis plays an important role in their growth. Exosomes also affect angiogenesis in oral squamous cell carcinoma [14], nasopharyngeal carcinoma [15], lung cancer [16], and hepatocellular carcinoma [17]. Exosomes and tumor growth are also correlated. Tumor growth involves three main elements: cell-cycle progression, inhibition of apoptosis, and glycolysis [18–22]. Exosomes control growth rate, as has been seen in lung cancer [23], pancreatic cancer [24], colorectal cancer [25], and nonsmall-cell lung cancer [26]. Studies have also shown the involvement of exosomes in metastasis. Metastasis denotes cancer migration and invasion. Both these processes are affected by EMT (epithelial-to-mesenchymal transition) [27]. During EMT-induced metastasis, E-cadherin decreases while N-cadherin increases inside the cancerous cells [28, 29]. Reports on prostate [30], ovarian [31], and breast cancer [32] have shown the role of exosomes in cancer metastasis. In addition to EMT, MMPs (matrix metalloproteinases) are also related to cancer metastasis, but this field is still in its infancy [33–37]. In addition, these nanovesicles are also involved in drug resistance and immune escape. Exosomes genotypically and phenotypically resemble their parent cells, can protect themselves from their surroundings, and are present in all body liquids; accordingly, they are used in liquid biopsies. Although in the past few years, different research groups have published papers emphasizing on the role of engineered exosomes in treating various type of cancers yet their use in detection and treatment of meningioma is new. Undoubtedly, now also continuous work is going on in this area. When it particularly comes to brain tumors, exosomes have also been observed as good therapeutic delivery agents. They can cross the blood–brain barrier, allowing them to deliver biological molecules or pharmaceutical medications to brain tumors. Exosomes are nonviable and, hence, better than transplanted cells. They are good because of biosafety reasons. Exosomes are carriers which deliver therapeutic molecules, while their administration also elicits intrinsic therapeutic effects. Exosomes, derived from dendritic cells, carry machinery including antigenic material and major histocompatibility complex peptide complexes for the antigen presentation process of the immune response; hence, they can be used as noncellular antigens for developing vaccines against infectious diseases or tumors. After antigen presentation, they induce T-cell activation, thereby killing the tumor cells. The present review sheds light on developing new, promising systemic therapies, targeted drug delivery by exosomes, and cell-free vaccine development using exosomes against meningioma.
Targeting therapies
Current knowledge of meningioma-associated growth factors, as well as their receptors and signaling pathways, is not sufficient [38–42]. Deregulation of the signaling pathways is considered one of the major causes of the neoplastic transformation of meningioma. There are reports on meningioma cells showing abnormal expression of critical signaling molecules, resulting in uncontrolled cell division, differentiation, migration, survival, and angiogenesis [43, 44]. Recently, efforts have been made to develop potential inhibitors of several targeted agents. Today, the identification of therapeutic targets and the selection of such agents are major challenges. Most anti-growth factor receptor strategies involve small-molecule tyrosine kinase inhibitors and monoclonal antibodies against EGFR and VEGFR. Other potential inhibitors are PDGFR inhibitors, mTOR inhibitors, integrin path inhibitors, etc. Drugs used in target therapy are listed in the tables below.
Some common cytotoxic agents
Common cytotoxic agents include temozolomide, irinotecan, hydroxyl urea, trabectedin, cyclophosphamide doxorubicin, curcumin, AKBA, and vincristine (Table 1).
Table 1.
Details of some common cytotoxic agents used in target therapy for the treatment of meningioma
| Drug name | Drug composition/molecular formula administration | Mechanism of action | Type of study | References |
|---|---|---|---|---|
| Cytotoxic agents | ||||
| Temozolomide (today) | Imidazotetrazine, orally administered | Alkylated agent | Temozolomide for treatment-resistant recurrent meningioma; phase II trial: no clinical efficacy shown | [45] |
| Irinotecan (Camptosar) | C33H38N4O6 plant alkaloid, intravenously administered | Topoisomerase 1 inhibitor | Irinotecan has growth-inhibitory effects in meningiomas both in vitro and in vivo; phase II trial: no clinical efficacy shown | [46] |
| Hydroxyurea | CH4N2O2, orally administered | Ribonucleotide reductase inhibitor | Hydroxyurea for treatment of unresectable and recurrent meningiomas; phase I trials: inhibition of primary human meningioma cells in culture and meningioma transplants by induction of the apoptotic pathway; phase II trial: mixed results | [47] |
| Trabectedin (Yondalis) | C39H43N3O11S, intravenous infusion | Mechanism unclear; believed to make conformational changes in DNA strands, causing inhibition of transcription factor binding | Trabectedin has promising antineoplastic activity in high-grade meningioma | [48] |
| Cyclophosphamide | C7H15Cl2N2O2P, orally administered | Synthetic nitrogen mustard alkylating agent | Medical management of meningioma in the era of precision medicine | [49] |
| Bacteria-derived agents | ||||
| Doxorubicin | C27H29NO11, anthracycline antibiotic, intravenous | Topoisomerase 2 inhibitor | Recurrent meningioma of the cervical spine, successfully treated with liposomal doxorubicin | [50] |
| Plant-derived agents | ||||
| Curcumin | C21H20O6, orally administered | Interaction with multiple cell signaling proteins | Curcumin has antiproliferative and proapoptotic activity in human meningiomas; preclinical trial: cell culture/in vitro | [51] |
| AKBA | Pentacyclic triterpene | Induction of apoptosis; anti-inflammatory | Cytotoxic action of acetyl-11-keto-beta-boswellic acid (AKBA) on meningioma cells; preclinical trial: cell culture/in vitro | [52] |
| Vincristine | C46H56N4O10, intravenously administered at weekly intervals | Binds to tubulin, thus stopping the polymerization of tubulin dimers; microtubules make cells unable to replicate during metaphase, inducing apoptosis | Adjuvant combined modality therapy for malignant meningiomas | [53] |
Pathway inhibitors
EGFR inhibitors
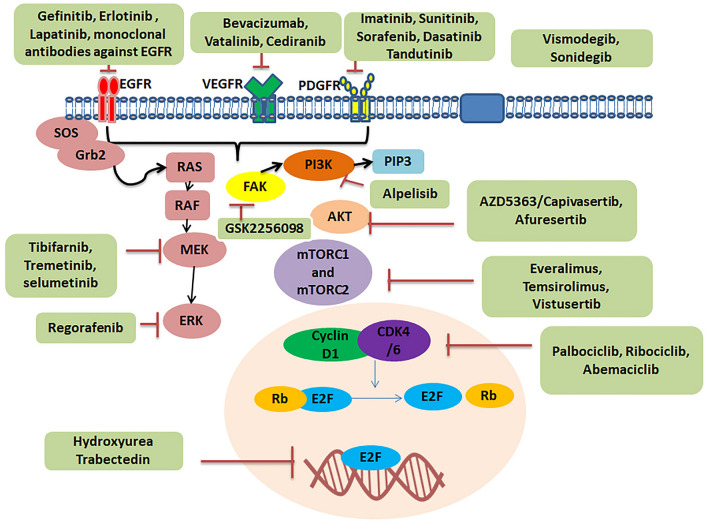
EGFR is a transmembrane receptor tyrosine kinase also called HER1 and ERBB1 [54]. It belongs to the ERBB family. Extracellular ligands such as epidermal growth factor, heparin-binding EGF, and transforming growth factor-α bind to EGFR [55]. On binding, EGFR dimerizes either with itself or with ERBB family receptors. Dimerization causes transphosphorylation of the C-terminal domain, activating the downstream signaling cascades and various physiological processes. The downstream signaling pathways include PI3K/AKT/mTOR, RAS/RAF/ERK, and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways. Expression of EGFR is seen in 60% of meningiomas [56]. According to a study, EGF and TGFα, by inducing meningioma cell growth [57, 58], activate the EGFR pathway. Gefitinib, erlotinib, and lapatinib are important examples of these types of inhibitors (Fig. 1).
Fig. 1.
Schematic diagram of drugs used in target therapy of meningioma including EGFR Inhibitors, Platelet-derived growth factor inhibitors, Anti-angiogenesis drugs, Pi3K/AKT/mTOR Pathway inhibitor, Rb signaling pathway inhibitor, Protein kinase C inhibitors, RAF/MEK/ERK Inhibitors and Hedgehog pathway inhibitors, FAK inhibitors, and Integrin PI3K/Akt pathway
Platelet-derived growth factor receptor inhibitors
There are four members in the PDGF family, namely, PDGFA, PDGFB, PDGFC, and PDGFD. This family has two types of receptors, i.e., alpha-receptor and beta-receptor. When PDGF binds to the receptor, it activates and cross-phosphorylates tyrosine residues in the intracellular domain. This leads to the activation of the PI3K, Jak family kinase, MAPK, Src family kinase, and phospholipase C-gamma signal transduction pathways. These ligands, along with their receptors, have long been connected with tumorigenesis and may play a significant role in meningioma formation and progression. PDGF and its receptors are expressed in meningioma [59]. Studies revealed that PDGF was more highly expressed in atypical and anaplastic meningiomas than in benign meningiomas [60]. It was seen that supplementation of PDGF-BB antibody increased the proliferation of meningioma cells, while the addition of anti-PDGF-BB produced the opposite effect [61]. Examples include imatinib, sunitinib, sorafenib, dasatinib, and tandutinib.
Anti-angiogenesis
Angiogenesis contributes to tumorigenesis, tumor progression, and metastasis. VEGF is involved in the angiogenetic process and responsible for cell migration, endothelial cell proliferation, extracellular matrix degradation, and expression of proangiogenic factors (matrix metalloproteinase-1, plasminogen activator inhibitor-1, urokinase plasminogen activator, and its receptor). Hypoxia, acidosis, and a variety of growth factors such as EGF, PDGF, HGF, c-kit, and their downstream signaling pathways (PI3K/Akt and Ras/MAPK) enhance the expression of VEGF. VEGFR-1 and VEGFR-2 are two types of VEGF-A receptors. Inhibition of cancer-related blood arteries is an essential therapeutic approach. Cancer cells, including meningioma cells, have the property of high vascularization. These blood vessels supply nutrition to the tumor cells, thereby promoting their growth. If this supply is hampered, it could be therapeutically beneficial. Studies found that an antiangiogenic fumagillin analog suppressed the growth of benign and malignant meningioma in xenograft models (TNP 470). Meningioma expresses VGFR, and its expression is higher in atypical and malignant meningiomas than in benign meningioma [61]. Bevacizumab, vatalinib, and cediranib are examples.
Pi3K/AKT/mTOR pathway inhibitors
Phosphorylation of phosphatidylinositol and active downstream components is catalyzed by PI3Ks, which are lipid kinases found inside cells. The main functions of PI3Ks include cell survival, cell cycle, protein translation, and metabolism. There are three types of PI3K: PI3K I, PI3K II, and PI3K III, which function to produce phosphatidylinositol 3,4,5-trisphosphates from phosphatidylinositol 4,5-bisphosphates. According to the membrane receptors that activate it, RTK, and G protein-coupled receptor class, PI3K I is separated into two subfamilies, IA and IB [62]. Class IA PI3Ks consists of two subunits: subunit p85 and subunit p110, whereby p85 is the regulatory subunit, while subunit p110 is the catalytic subunit. There are three isoforms of p85, namely, p85α, p85β, and p55γ, encoded by PIK3R1, PIK3R2, and PIK3R3 genes, respectively. Similarly, p110 has three isoforms p110α, p110β, and p110γ, encoded by genes PIK3CA, PIK3CB, and PIK3CD, respectively. When specific ligands bind to RTK, conformation changes in p85 of class IA occur, which activates the catalytic subunit p110 leading to the transformation of PIP2 to PIP3. This activates Akt and the mTORC1 signaling pathway, which ultimately induces protein translation. Everolimus, temsirolimus, vistusertib, alpelisib, afuresertib, and AZD5363 are important examples.
Rb signaling pathway inhibitors
G1- to S-phase cell-cycle transition is controlled by the Rb signaling pathway which leads to the regulation of DNA replication and cell division [63, 64]. CDK4 and CDK6 are kinases with similar amino-acid sequences and roles. They both interact with cyclin D and influence Rb protein phosphorylation [65]. The mitogen-activated protein kinase signaling pathways PI3K/AKT/mTOR, nuclear factor κb, Wnt, JAK/STAT, and JAK/STAT stimulate cyclin D, leading it to interact with CDK4/6. As a result, CDK4/6 phosphorylates Rb. On phosphorylation, the E2F transcription factor separates from Rb, activating E2F and G1 target genes [66, 67]. In the case of higher-grade meningiomas, genetic alterations of genes encoding these proteins are found [68].
Protein kinase C, RAF/MEK/ERK inhibitors
When ligands such as EGF and PDGF bind to their receptors, tyrosine kinases become autophosphorylated on the cytoplasmic side of receptors. Subsequently, grb2 and sos proteins come close to the plasma membrane. Ras proteins, which are small GTPases, become attenuated, which further activates downstream elements of signal transduction pathways such as Raf, MEK 1, and ERK. These factors phosphorylate various transcription factors. Studies have shown that MEK1 inhibitors suppress MAPK activity in meningioma cell culture; hence, there is less inhibition, leading to changes in growth, differentiation, and apoptosis [69]. Tipifarnib, trametinib, and selumetinib are important such inhibitors.
Hedgehog pathway inhibitors
When the hedgehog ligand binds to the protein patched homolog-1, it causes PTCH1 to be internalized and degraded. It causes SMO protein to be suppressed by PTCH1. SMO then interacts with the fused homologous suppressor (SUFU). This interaction elicits the translocation of the zinc finger protein GL1 to the nucleus, which activates target genes. Examples are vismodegib and sonidegib.
FAK inhibition
In vivo and in vitro studies showed sensitivity for FAK inhibition in cells in the case of NF2-mutant tumors such as serous ovarian carcinoma and malignant pleural mesothelioma. Its widespread inhibitor is GSK2256098 (Fig. 1) [70, 71].
Integrin PI3K/Akt pathway inhibitors
The PI3K/Akt pathway is activated when integrin proteins are activated. The downstream effectors of PI3K/Akt are associated with various cellular processes such as growth, differentiation, and proliferation of cells. Integrins are also associated with FAK and ILK at the downstream signaling cascade level.
Integrin inhibitor
Cilengitide is a pentapeptide and an integrin inhibitor. Cilengitide mimics the Arg–Gly–Asp (RGD) binding site and inhibits the proliferation and differentiation of endothelial progenitor cells, which are critical in tumor neoangiogenesis. Studies have shown higher expression of integrins in brain tumors, thus indicating that cilengitide inhibition of integrins prevents tumor growth (Table 2) [72].
Table 2.
Details of some common signaling pathway inhibitors used in target therapy for the treatment of meningioma
| Drug name | Drug composition/molecular formula administration | Mechanism of action | Type of study | References | ||||
|---|---|---|---|---|---|---|---|---|
| EGFR antagonists | ||||||||
| Gefitinib (Iressa) | It is an anilinoquinazoline with anticancer activity, orally administered | It competes with ATP for binding to the EGFR’s tyrosine kinase domain, inhibiting receptor autophosphorylation and signal transmission; it is also in charge of stopping the cell cycle and preventing angiogenesis | The study showed its involvement in the treatment of meningioma as an EGFR antagonist; phase: no survival benefit shown | [73] | ||||
| Erlotin ib (Tarceva) | C22H23N3O4 is a quinazoline derivative with anticancer activity, orally given | Similar to gefitinib, it competes with ATP binding to the tyrosine kinase domain of EGFR, thereby inhibiting autophosphorylation of EGFR and blocking signal transduction | Its use in meningioma treatment is underway; phase II: no survival benefit shown | [73] | ||||
| Lapatinib | C43H42ClFN4O10S3 is a small molecule and a dual EGFR/ErbB2 inhibitor, as shown by preclinical and clinical data, orally given | EGFR antagonist | Effect of lapatinib on meningioma growth in adults with neurofibromatosis type 2 revealed anticancer activity against schwannomas; it is also hypothesized to have growth-inhibitory effects on meningiomas (Fig. 1) | [74] | ||||
| Monoclonal antibodies, humanized monoclonal antibodies of EGFR | ||||||||
| Cetuximab | Fv region of a murine anti-EGFR antibody with human IgG1 heavy and kappa light chain constant regions | By binding to EGFR on a cancer cell, cetuximab blocks EGF from binding (activation); this stops the cell from continuing the pathway that promotes cell division and growth, effectively stopping cancer by stopping the cancerous cells from growing and multiplying | Assessment of epidermal growth factor receptor (EGFR) expression in human meningioma | [75] | ||||
| Panitumumab | ABX-EGF is a fully human monoclonal antibody specific to the epidermal growth factor receptor | Panitumumab works by binding to the extracellular domain of the EGFR preventing its activation; this results in halting the cascade of intracellular signals dependent on this receptor | Is there effective systemic therapy for recurrent surgery- and radiation-refractory meningioma? | [76] | ||||
| Matuzumab (EMD72000) | A fully humanized ErbB-1-specific monoclonal antibody | Matuzumab binds to the epidermal growth factor receptor (EGFR) on the outer membrane of normal and tumor cells; the matuzumab epitope has been mapped to domain III of the extracellular domain of the EGFR | Innovative therapeutic strategies in the treatment of meningioma | [77] | ||||
| mAb 806 | mAb 806 is a second-generation antibody | Depatuxizumab selectively targets a unique epitope on the epidermal growth factor receptor (EGFR), which is only expressed on cancer cells (and not on normal cells) | Targeting a unique EGFR epitope with monoclonal antibody 806 activates NF-κB and initiates tumor vascular normalization | [78] | ||||
| Nimotuzumab | Nimotuzumab (also known by the lab code h-R3) is a humanized IgG1 isotype monoclonal antibody | Nimotuzumab binds with optimal affinity and high specificity to the extracellular region of EGFR (epidermal growth factor receptor). This results in a blockade of ligand binding and receptor activation; epidermal growth factor receptor (EGFR) is a key target in the development of cancer therapeutics | Nimotuzumab is a promising therapeutic monoclonal for the treatment of tumors of epithelial origin | [79] | ||||
| PDGFR antagonists | ||||||||
| Imatinib (Gleevec) | C30H35N7O4S is a 2-phenyl amino pyrimidine derivative, orally given | Imatinib mesylate (Gleevec) is an inhibitor of PDGFR-a and b, Bcr –Abl, c-Fms, and c-Kit tyrosine kinases, which are responsible for abnormal cell growth through its constitutive expression; it has the power to inhibit PDGFR-a or b, which makes it a superior meningioma therapeutic alternative | The North American Brain Tumor Consortium (NABTC) evaluated the efficacy of imatinib in meningioma patients PDGFR antagonist; phase II: some stabilization of disease | [80] | ||||
| Nilotinib | C28H22F3N7O, orally given | Second-generation PDGFR inhibitor | Nilotinib alone or in combination with selumetinib is a drug candidate for neurofibromatosis type 2; no studies | [81] | ||||
| Dasatinib | C22H26ClN7O2S, orally given | Dasatinib inhibits the growth-promoting actions of SRC-family protein tyrosine kinases by binding to them; PDGFR antagonist | Combination therapy with mTOR kinase inhibitor and dasatinib as a novel therapeutic strategy for vestibular schwannoma; dasatinib is a multikinase inhibitor targeting SFKs, several EPH receptors, and c-Kit23 dasatinib and AZD2014 combination therapy was performed on NF2-deficient meningioma cells; in vivo and in vitro, this combination therapy efficiently inhibited the development of NF2-deficient meningioma cells; dasatinib and AZD2014 combined therapy was more effective than either monotherapy | [82] | ||||
| Tandutinib | C31H42N6O4 is a piperazinyl quinazoline, a potent anticancer agent,that specifically acts on the angiogenic pathways, orally given | This is a tyrosine kinase inhibitor that targets PDGFRb, C-Kit, and Fms-like tyrosine kinase 3 (FLT3), thereby inhibiting cellular proliferation and inducing apoptosis (Fig. 1); PDGFR B antagonist | Vascular endothelial growth factor signals through platelet-derived growth factor receptor β in meningiomas in vitro | [61] | ||||
| VEGFR antagonists | ||||||||
| Bevacizumab (Avastin) | Bevacizumab is a full-length IgG1κ isotype antibody composed of two identical light chains (214 amino-acid residues) and two heavy chains (453 residues), intravenous infusion | Bevacizumab was engineered as a humanized monoclonal antibody to VEGF receptors that inhibits the binding and signaling cascade required for vascularization, as it leads to diminished tumor blood supply | Recently, numerous studies on anti-angiogenesis have been conducted on meningioma humanized monoclonal antibodies of EGFR; SSCTs: mixed results | [83] | ||||
| Cediranib (recentin) | C25H27FN4O3, also called AZD2171, is a potent anticancer agent, orally given | It is a small-molecule tumor kinase inhibitor of VEGFRs that also targets c-KIT and PDGFR-a | The effect of cediranib has been studied in various types of cancers, and 81 clinical trials have been conducted; it is currently being analyzed in juvenile recurrent CNS malignancies in phase I clinical trials (Fig. 1);VEGFR antagonist; no studies | [84] | ||||
| Combination antagonists | ||||||||
| Sorafenib (Nexavar) | C21H16ClF3N4O3 is a multi-tyrosine kinase synthetic inhibitor, orally administered | It is a potent anticancer agent with antiangiogenetic and cytostatic effects; it was the first approved angiogenetic multikinase inhibitor; this compound targets RAF kinase of the RAF/MEK/ERK signaling pathway and inhibits the VEGFR-2/PDGFR beta signaling cascade, thereby preventing tumor angiogenesis | Receptor tyrosine kinase inhibition by regorafenib/sorafenib inhibits the growth and invasion of meningioma cells | [85] | ||||
| Sunitinib (Sutent) | C22H27FN4O2 is a pyrrole and a monocarboxylic acid amide with potent anticancer activity, orally given | Inhibitor of VEGFR, PDGFR, and KIT tyrosine kinase; sunitinib blocks the tyrosine kinase activity of VEGFR and PDGFR; it inhibits angiogenesis and cell proliferation | Sunitinib is widely used in cancer treatment; association between meningioma and sunitinib was seen in studies; dual VGFER and PDGFR antagonist; phase II: some stabilization of disease | [86] | ||||
| Vatalanib (PTK-787) | C20H15ClN4 is an anilinophthalazine with potential anticancer activity, orally administered | Vatalanib, also called PTK787, is an inhibitor of VEGFR-1 (Flt-1), VEGFR-2 (KDR), and VEGFR3 (Flt4), by binding to the protein kinase domain of VEGFRs; dual VGFER and PDGFR antagonist; vatalanib induces a dose-dependent inhibition of VEGF-induced angiogenesis, as well as tumor-derived angiogenesis; it also binds to the tyrosine kinase domain of other receptors such as PDGFR, C-Fms, and c-Kit | Preclinical studies showed that, when given orally to orthotopic models, some stabilization of disease occurred | [69] | ||||
| Farnesyl transferase inhibitors | ||||||||
| Tipifarnib (Zrnestra) | C27H22Cl2N4O is a nonpeptidomimetic quinolinone and is a potent anticancer agent, orally administered | Farnesyl protein transferase is an enzyme that causes protein farnesylation and is involved in signal transduction; tipifarnib binds to and inhibits this enzyme, thereby inhibiting protein processing; it stops Ras oncogenes from being activated, resulting in the induction of apoptosis, as well as the prevention of cell proliferation and angiogenesis | Research on the use of tipifarnib against meningioma is underway | [69] | ||||
| mTOR inhibitors | ||||||||
| Temsirolimus (Torisel) | C56H87NO16 is an ester analog of rapamycin and a kinase inhibitor, intravenous infusion | Temsirolimus inhibits mTOR after attaching to it, leading to the downregulation of mRNAs required for cell-cycle progression; the cell cycle is halted in the G1 phase; mTOR is a serine/threonine kinase that is involved in the PI3K/AKT signaling cascade; mTOR has been discovered to be elevated in certain malignancies; as it is like a protein kinase inhibitor, inhibition of mTOR activation is a kind of therapy for treating various types of cancers | Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04–02 | [87] | ||||
| Everolimus (Afinitor) | C53H83NO14 is derived from the macrocyclic lactone sirolimus in nature; it is an immunosuppressant, antiangiogenic, and anti-cell proliferative agent, orally given | Everolimus is an inhibitor that forms an immunosuppressive complex with the immunophilin FK binding protein-12 (FKBP-12), which then binds to and inhibits mTOR (mammalian target of Rapamycin); because mTOR is a regulatory kinase, it causes the production of mRNA that codes for cell-cycle proteins and hinders the glycolysis process, thus inhibiting tumor growth; as already explained, mTOR is upregulated in cancer and, hence, inhibition of mTOR activation may help in cancer treatment | Antitumor effect of everolimus in the case of grade III meningioma was reported; mTOR inhibitor; phase I | [88] | ||||
| Vistusertib | C25H30N6O3 is an antineoplastic agent and is administered orally | It is an inhibitor of the mammalian target of rapamycin (mTOR); it inhibits mTORC1 and mTORC2, leading to tumor cell death and a reduction in tumor cell growth; it is a serine/threonine kinase that is involved in the PI3K/Akt/mTOR signaling cascade; its suppression is the cornerstone of anticancer therapy because it is elevated in a variety of malignancies | Vistusertib (AZD2014) is under investigation for the treatment of advanced gastric adenocarcinoma; mTOR C1 and C2 inhibitor; phase II | [69] | ||||
| Akt inhibitors | ||||||||
| AZD5363/capivasertib | It is a novel pyrrolopyrimidine derivative with a potency of 10 nM or less in inhibiting the AKT isoform, orally administered | Capivarsertib inhibited the phosphorylation of AKT (Iat, Ser, and Thr) in BT474c cells in three cell lines; in vivo investigations revealed that, when given orally to nude mice, it reduced the phosphorylation of PRAS40, GSK3, and S6 in BT474c xenografts in a dose- and time-dependent manner; it inhibited the growth of xenografts in a dose-dependent manner in a variety of malignancies (Fig. 1) | Durable control of metastatic AKT1-mutant WHO grade 1 meningothelial meningioma was shown using the Akt inhibitor AZD5363 | [89] | ||||
| Afuresurtib | C18H17Cl2FN4OS is an orally administered drug | Afuresurtib is an inhibitor of Akt, a protein kinase inhibitor, and thus, a potent anticancer agent; afuresurtib inhibits Akt, which is increased in several types of cancer and is implicated in the PI3K/Akt signaling pathway, resulting in tumor growth inhibition and induction of apoptosis | Emerging medical treatments for meningioma in the molecular era | [90] | ||||
| PI3K inhibitor | ||||||||
| Alpelisib | C19H22F3N5O2S is a well-tolerated drug given orally | Phosphoinositide 3-kinase α (PI3Kα)-specific inhibitor; cell development and survival may be inhibited as a result | Phase I trial of alpelisib in combination with concurrent cisplatin-based chemoradiotherapy in patients with locoregionally advanced squamous cell carcinoma of the head and neck showed that alpelisib in combination with trametinib can be used to treat aggressive and recurrent meningioma | [91] | ||||
| CDK4/6 inhibitors | ||||||||
| Palbociclib | C24H29N7O2 is an orally available cyclin-dependent kinase (CDK) inhibitor with potential anticancer properties, orally administered | CDK4 and 6 are serine/threonine kinases that regulate cell-cycle progression and are overexpressed in several malignancies; palbociclib inhibits cyclin-dependent kinases 4 (CDK4) and 6 (CDK6), which leads to cell-cycle arrest by inhibiting retinoblastoma (Rb) protein phosphorylation during the early G1 phase of the cell cycle; DNA replication is inhibited, and tumor cell proliferation is reduced | Palbociclib had effects in combination with radiation in preclinical models of aggressive meningioma; CDK4/6 inhibitor | [92] | ||||
| Ribociclib | C23H30N8O is orally given | CDK4/6 inhibitor | Ribociclib (LEE011) in preoperative glioma and meningioma patients; phase I | [69] | ||||
| Abemaciclib | C27H32F2N8 is orally given | CDK4/6 inhibitor | Meningioma is not always a benign tumor: a review of advances in the treatment of meningiomas | [93] | ||||
| MEK1/2 inhibitors | ||||||||
| Trametinib | C26H23FIN5O4 is an inhibitor of MAP2K/ERK kinase (MEK) 1 and 2 and a potent anticancer agent, orally given | Trametinib inhibits growth factor-mediated cell signaling and cellular proliferation in a variety of malignancies by targeting MEK 1 and 2, which are two types of MEK; MEK 1 and 2 are serine/threonine and tyrosine kinases with dual specificity that are commonly overexpressed in cancer; it affects cell proliferation by activating the RAS/RAF/MEK/ERK signaling pathway | Trametinib is used in combination with dabrafenib for the treatment of advanced malignant melanoma | [94] | ||||
| Selumetinib | C17H15BrClFN4O3 is a small molecule that has potential anticancer activity, used to treat symptomatic, refractory fibroma in neurofibromatosis type 1, orally given | The RAS/RAF/MEK/ERK pathway regulates a variety of cellular activities, including proliferation; this pathway is abnormally regulated in cancer, and MEK1 and 2, which are dual-specificity kinases, are overexpressed; selumetinib inhibits mitogen-activated protein kinases (MEK or MAPK/ERK kinases) 1 and 2, resulting in cellular proliferation suppression in a variety of malignancies | Trial of selumetinib in patients with neurofibromatosis type II-related tumors (SEL-TH-1601) | [69] | ||||
| ERK inhibitor | ||||||||
| Regorafenib | C21H15ClF4N4O3 is orally given | ERK inhibitor | Receptor tyrosine kinase inhibition by regorafenib/sorafenib inhibits the growth and invasion of meningioma cells | [85] | ||||
| Hedgehog pathway inhibitors | ||||||||
| Vismodegib | C19H14Cl2N2O3S, also called aDC-0449, is a small molecule and a potent anticancer agent. SMO inhibitor, orally given | Vismodegib is a Hedgehog antagonist that works by inhibiting Hedgehog signaling; it suppresses Hedgehog signaling by blocking the activity of the Hedgehog ligand cell surface receptors PTCH and/or SMO; the Hedgehog signaling system is so important for tissue growth and repair that its improper and constitutive activation leads to uncontrolled cellular proliferation | Vismodegib, FAK inhibitor GSK2256098, capivasertib, and abemaciclib in treating patients with progressive meningiomas; phase II | [69] | ||||
| Sonidegib | C26H26F3N3O3 is a small molecule that is administered orally for cellular growth, differentiation, and repair | It is an antagonist of smoothened (SMO), which shows potential antineoplastic activity; in cancer cells where the hedgehog pathway is abnormally activated, sonidegib inhibits the Hh signaling cascade by binding to the hedgehog (Hh) ligand cell surface receptor SMO; SMO inhibitor | Selective vulnerability of the primitive meningeal layer to prenatal SMO activation for skull base meningothelial meningioma formation; the Hh signaling pathway is responsible for this abnormal activation of the hedgehog path and way may be associated with a mutation in SMO | [95] | ||||
| FAK inhibitors | ||||||||
| GSK2256098 | C20H23ClN6O2 | FAK inhibitor | Vismodegib, FAK inhibitor GSK2256098, capivasertib, and abemaciclib in treating patients with progressive meningiomas; phase II | [69] | ||||
Hormonal therapy
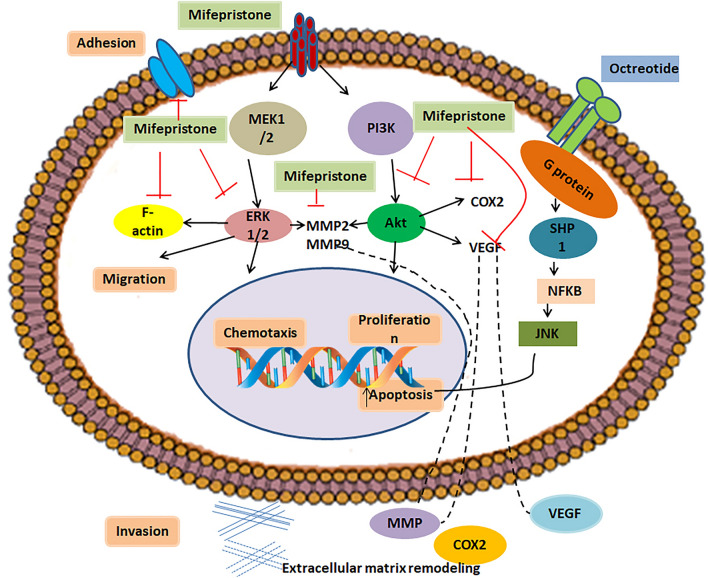
Females are more likely to have meningiomas. They are more common after puberty and during the reproductive years. A study found a direct link between the number of pregnancies and the occurrence of meningioma. Increased risk of meningioma in women over the age of 50 has been seen [5, 96]. Breast cancer patients showed a higher tendency of meningioma [97]. Furthermore, 10% of meningiomas express estrogen receptors with a higher percentage of progesterone receptors [98, 99]. The greater degree of progesterone expression in meningioma has sparked extensive interest for research purposes. Somatostatin is a hormone majorly produced by the hypothalamus. This neuropeptide is released into the systemic circulation and reaches its primary sites of action, which are the pituitary, pancreas, and gastrointestinal tract. It is responsible for inhibiting the endocrine and exocrine secretions, as well as the motility of the gastrointestinal tract. Somatostatin prevents cancer by acting as an anti-angiogenesis agent. It also inhibits invasion and apoptosis. Because somatostatin has a limited half-life, various analogs with extended half-lives have been created. Octreotide is a well-known somatostatin receptor agonist. One of the most successful treatments for pituitary adenomas and gastroenteropancreatic endocrine tumors is somatostatin analog therapy. Biochemical analysis or scintigraphy detected that the majority of meningiomas express somatostatin receptors. There are five subtypes of somatostatin receptors (sstr1–sstr5), with nearly 90% of meningiomas expressing somatostatin receptors. In one study, somatostatin analog octreotide showed an antitumor effect on progressive meningioma grade II to grade I. Mifepristone and octreotide are examples of hormone inhibitors. According to various studies, mifepristone can be used in cancer therapy alone or in combination with other drugs to treat different types of cancers; examples include nonsmall-cell lung cancer [100, 101], renal cancer [102], and pancreatic cancer [103]. Documented studies have shown that mifepristone also has an impact on brain tumors. Mifepristone acts as an antagonist to progesterone receptors. Reports have shown that progesterone is capable of inducing infiltration and migration in the rat cortex [104]. Glucocorticoid and progesterone receptors were found to be highly expressed in high-grade glioma patients, and they play role in cell proliferation; therefore, as an antagonist of progesterone and glucocorticoids, mifepristone blocks the capacity of progesterone to induce the growth, migration, and invasion of human astrocytoma cell lines [105, 106] (Fig. 2).
Fig. 2.
Schematic diagram of drugs used in hormonal therapy of meningioma
Retinoids
Retinoids are derivatives of vitamin A that are potent anticancer agents. Their inhibitory effects include inhibition of growth, enhancement of differentiation, and anti-angiogenesis. Some cancers (e.g., acute promyelocytic leukemia) have been treated with synthetic retinoids. A study showed that retinoids induce noninvasive phenotypes in meningioma cells and, hence, can be used to treat meningioma [107] (Table 3).
Table 3.
Drugs used in hormonal therapy of meningioma
| Drug name | Drug composition/molecular formula administration | Mechanism of action | Type of study | References | ||||
|---|---|---|---|---|---|---|---|---|
| Hormonal agents | ||||||||
| Progesterone receptor-binding agents | ||||||||
| Megestrol (Megace) | C22H30O3, orally administered | Progesterone receptor partial agonist | SSCT: no survival benefit shown | [108] | ||||
| Mifepristone (RU- 486) | C29H35NO2 is a substituted 19-nor steroid compound and the first clinically available progesterone receptor antagonist; it is a derivative of the synthetic progestin norethindrone, orally given | It has antiprogesterone activity; it competes with progesterone for binding to its receptor, which leads to inhibition of the effects of endogenous or exogenous progesterone; progesterone receptor competitive antagonist | Mifepristone is useful in the treatment of vestibular schwannoma because of its anti-glucocorticoid effect; it also binds to progesterone receptors in meningiomas | [109] | ||||
| Estrogen antagonists | ||||||||
| Tamoxifen | C26H29NO is a tertiary amino compound and a stilbenoid, orally given | Estrogen receptor competent antagonist | A population-based study in Sweden found an association of tamoxifen with meningioma | [110] | ||||
| Somatostatin | ||||||||
| Octreotide (Sandostatin) | C49H66N10O10S2 is a synthetic long-acting cyclic octapeptide with anticancer properties, subcutaneous (s.c.) injection or intravenous (i.v.) infusion after dilution | It mimics somatostatin but is a more potent inhibitor of growth hormone, glucagon, and insulin; similar to somatostatin, this agent also suppresses the luteinizing hormone response to the gonadotropin-releasing hormone, decreases splanchnic blood flow, and inhibits the release of gastrin, serotonin, secretin, motilin, pancreatic polypeptide, and thyroid-stimulating hormone | Octreotide was administered with low toxicity in a small series of recurrent meningioma cases; somatostatin was found to decrease meningioma cell proliferation in vivo (Fig. 2) | [111] | ||||
| Pasireotide (Signifor) | C58H66N10O9, subcutaneous injection | Somatostatin mimetic | Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma | [112] | ||||
| GH receptor antagonists | ||||||||
| Pegvisomant (somavert) | PEGylated form of mutant growth hormone, subcutaneously | PEGylated GH receptor antagonist | Pegvisomant was investigated in the treatment of acromegaly | [113] | ||||
| IGF-I and IGF-II Antagonists | ||||||||
| Fenretinide | C26H33NO2 is a synthetic retinoid, orally given | It shows inhibition of growth via apoptosis in various tumor cell lines | Studies were aimed at finding the role and mechanism of fenretinide in controlling the growth of cells in meningioma | [114] | ||||
| Retinoids | [107] | |||||||
Interferons, immune checkpoint inhibitors, and immunotherapy
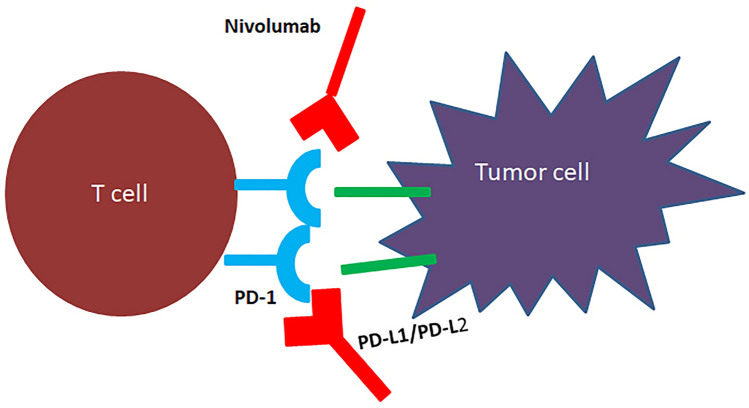
Our body is protected from foreign substances or antigens such as transplanted grafts, bacteria, and viruses by natural mechanisms through the immune system. Our immune system selectively eliminates foreign substances and prevents them from entering our bodies. It is also responsible for the removal of cancerous cells from our bodies. Some cancer cells, however, manage to evade the immune system detection. Immunotherapy has emerged as a viable cancer treatment option in recent years. The immune system has stimulatory or inhibitory regulators called immune checkpoints. They control how the antigen is presented to T-cell receptors. Immune checkpoints that restrict over activation of the immune system, such as cytotoxic T lymphocyte-associated antigen 4 and programmed cell death protein 1 protect normal cells from being mistakenly killed by the immune system. By deregulating the CTLA4/PD-1 immune checkpoint pathway, certain cancer cells can evade the immune response. Immunotherapy reactivates the T-cell-mediated immune response to destroy tumor cells by targeting inhibitory immunological checkpoints like CTLA4/PD-1. There is evidence showing that checkpoint inhibition via PD-1/PD-L1 blockade has the potential to treat meningioma. There is a need to explore other new promising targets. Several studies in the past years confirmed the identification of previously unrecognized immunomodulatory proteins such as PD-L2, B7-H3, and NY-ESO1 [115–117]. Examples are nivolumab, pembrolizumab, and avelumab (Fig. 3) (Table 4).
Fig. 3.
Diagram of antibodies used in the treatment of meningioma
Table 4.
Details of some common immunomodulators used in target therapy for the treatment of meningioma
| Drug name | Drug composition/molecular formula administration | Mechanism of Action | Type of study | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Immunomodulators | ||||||||
| IFN Α2b | C16H17Cl3I2N3NaO5S | Antiproliferative and antiangiogenic properties | Phase II: mixed results | [118] | ||||
| Nivolumab | It is a human IgG4 monoclonal antibody, intravenously | It works as a checkpoint inhibitor; T cells kill cancer cells and protect our body from cancer; in turn, cancer cells develop proteins to defend themselves against T lymphocytes; nivolumab inhibits the protective proteins in T lymphocytes, causing them to attack cancer cells; the protein PD-1 is found on the surface of activated T lymphocytes. T cells become inactive when another molecule termed programmed cell death 1 ligand 1 or programmed cell death 1 ligand 2 binds to PD-1; this anticancer drug works by preventing PD-L1/2 from attaching to PD-1, allowing T lymphocytes to destroy cancer cells | Outside of the respiratory epithelium and placental tissue, PD-L1 is expressed in 40–50% of melanomas and has low expression in most visceral organs; PD-1 receptor and ligand inhibitor; phase II | [69] | ||||
| Pembrolizumab | It is an immunoglobulin G4, intravenous injection | It is an immune checkpoint inhibitor like nivolumab; its mechanism of action is similar to nivolumab, which treats melanoma, head and neck cancer, lung cancer, Hodgkin lymphoma, and stomach cancer; under normal conditions, T cells interact with other cells through PD-1 and PD-L1 or PD-L2; PD-1 receptors present on the activated T-cell bind to ligands PD-L1 or PD-L2 on other cells and deactivate the T-cell-mediated immune response; in this way, the body prevents the immune system from acting on normal cells; in many cancers, cells make protein PD-L1 that binds PD-1, thus shutting down the ability of the body to kill cancer cells; pembrolizumab inhibits the binding of PD-1 on lymphocytes to PD-L1 and PD-L2 on cancer cells, thereby allowing lymphocytes to destroy cancer cells | Pembrolizumab affects meningioma cell growth; PD-1 receptor and ligand, Inhibitor; phase II | [69] | ||||
| Avelumab | It is a human monoclonal antibody of isotype IgG1, intravenous infusion | Its working mechanism is the same as that of pembrolizumab and nivolumab | Avelumab is used for the treatment of Merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma (Fig. 3); PD-1 receptor and ligand inhibitor; phase II | [119] | ||||
MicroRNAs
MiRNAs are short RNAs of 21–23 nucleotides that regulate the expression of several target genes after transcription. They may promote cancer or may suppress cancer. Recently, downregulation of miRNA-29c-3p and miR-219-5p has been linked to meningioma, with a similar expression of miRNA 145, miR200a, and miR355 affecting meningioma (Table 5).
Table 5.
Details of some common miRNAs used in target therapy for the treatment of meningioma
| Drug name | Drug Composition/molecular formula administration | Mechanism of action | Type of study | References | ||||
|---|---|---|---|---|---|---|---|---|
| MicroRNA | ||||||||
| miR-29c-3p | miRNA | A study revealed that miR-29c-3p promoted the malignant development of HCC by regulating the methylation of LATS1 caused by DNMT3B and inhibiting the anticancer function of the Hippo signaling pathway | Simultaneous analysis of miRNA–mRNA in human meningiomas by integrating transcriptome, investigating the relationship between PTX3 and miR-29c | [120, 121], | ||||
| miR-219-5p | miRNA | Overexpression of miR-219-5p can inhibit gastric cancer cell malignancy by targeting the liver receptor homolog-1/Wnt/β-catenin signaling pathways | This microRNA expression signature predicts meningioma recurrence | [120, 122] | ||||
| miR-190A | miRNA | A recent study showed that miR-190a is involved in estrogen receptor (ERα) signaling, causing inhibition of breast tumor metastasis | While miR-190a is downregulated in aggressive neuroblastoma (NBL), its overexpression leads to the inhibition of tumor growth and prolonged dormancy periods in fast-growing tumors8 | [120] | ||||
| miR-145 | miRNA | miR-145 may serve as a tumor suppressor which can downregulate E-cad expression by targeting MUC1, leading to the inhibition of tumor invasion and metastasis | MiRNA-145 is downregulated in atypical and anaplastic meningiomas and negatively regulates the motility and proliferation of meningioma cells | [120, 122] | ||||
| miR-335 | miRNA | miR-335 promotes cell proliferation by directly targeting Rb1 in meningiomas | miR-335 promotes cell proliferation by directly targeting Rb1 in meningiomas | [120, 123] | ||||
Tumor suppressor proteins
There are certain proteins whose loss is widely distributed among meningioma grades such as protein 4.1B. Drugs targeting periostin reduce meningioma. Variation in the genes coding for proteins NF2, MN1, ARID1, MUC 5, and SEMA4D leads to meningioma. The pan histone deacetylase inhibitor AR42 increased the expression of p16, p21, and p27, but reduced the expression of cyclins D1, E, and A, as well as proliferating cell nuclear antigen, in the meningeal cell, which reduced the expression of cyclin B required for progression through the G2 phase. The differential effects of AR42 on cell-cycle progression of normal meningeal cells and meningioma cells can be of therapeutic use [124] (Table 6).
Table 6.
Details of some common tumor suppressor proteins used in target therapy for the treatment of meningioma
| Drug name | Drug composition/molecular formula administration | Mechanism of action | Type of study | References | ||||
|---|---|---|---|---|---|---|---|---|
| Tumor suppressor protein inhibitors | ||||||||
| AR42 | Protein, orally given | Histone deacetylase inhibitor | AR42, a novel histone deacetylase inhibitor, is a potential therapy for vestibular schwannomas and meningiomas; phase I | [124] | ||||
| Protein 4.1B | Protein, orally given | Growth suppressor | Protein 4.1B is differentially expressed in adenocarcinoma of the lung and functions as a growth suppressor in meningioma cells by activating rac1-dependent c-Jun-NH2-kinase signaling | [125] | ||||
| NF2, MN1, ARID1B, SEMA4D, MUC5 | Proteins | The NF2 gene provides instructions for the production of a protein called merlin, also known as schwannoma; this protein is made in the nervous system, particularly in specialized cells called Schwann cells that wrap around and insulate nerves, MN1 function is unclear, The ARID1B gene provides instructions for making a protein that forms one piece (subunit) of several different SWI/SNF protein complexes; SWI/SNF complexes regulate gene activity (expression) via a process known as chromatin remodelling, Semaphorin 4D (SEMA4D) is an axon guidance molecule that is secreted by oligodendrocytes and induces growth cone collapse in the central nervous system; by binding plexin B1 receptor, it functions as an R-Ras GTPase-activating protein (GAP) and repels axon growth cones in the mature central nervous system, Mucin 5AC (MUC5AC) is a protein with unknown function that is encoded by the MUC5AC gene in humans; Muc5AC is a large gel-forming glycoprotein; in the respiratory tract, it protects against infection by binding to inhaled pathogens that are subsequently removed by mucociliary clearance | Exome sequencing on malignant meningiomas identified mutations in neurofibromatosis type 2 (NF2) and meningioma 1 (MN1) genes | [126, 127] | ||||
| Periostin and Ki-67 | Protein | Periostin has essential roles in wound healing, ECM deposition, mesenchymal cell proliferation, and tissue fibrosis, During mitosis, Ki-67 is essential for the formation of the perichromosomal layer (PCL), a ribonucleoprotein sheath coating the condensed chromosomes; in this structure, Ki-67 acts to prevent the aggregation of mitotic chromosomes | Periostin is a novel prognostic predictor for meningiomas, Ki-67/MIB-1 has a prognostic role in meningioma | [128, 129] | ||||
Other important drugs used in targeted therapy of meningioma
Epigenetic modifier inhibitor
Changes in epigenetic modifiers such as KDM5C are found in 8% of meningiomas. KDM5 inhibitor KDOAM-25 is being tested for meningioma.
BAP1 inhibition
Breast cancer 1-associated protein 1 inhibition is associated with early tumor occurrence.
Tissue factor pathway inhibitor 2
When the malignant meningioma cell line IOMM-Lee was transfected with tissue factor pathway inhibitor 2 (TFPI-2) and tumor growth was evaluated in vitro and in vivo, studies indicated that it could have therapeutic potential in malignant meningioma (Table 7). Table 7 Details of some common drugs used in target therapy for the treatment of meningioma.
Table 7.
Details of some other common drugs used in target therapy for the treatment of meningioma
| Drug name | Drug composition/molecular formula administration | Mechanism of action | Type of study | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Antiretrovirals survivin protein downregulation, preclinical: cell culture/in vitro | ||||||||
| Nelfinavir | C32H45N3O4S, orally administered | The anticancer mechanism of nelfinavir includes modulation of different cellular conditions, such as unfolded protein response, cell cycle, apoptosis, autophagy, the proteasome pathway, oxidative stress, the tumor microenvironment, and multidrug efflux pumps | The HIV protease inhibitor nelfinavir has anticancer properties | [130] | ||||
| Lopinavir | C37H48N4O5, orally administered | Cell cycle arrest | Lopinavir inhibits meningioma cell proliferation via Akt-independent mechanism | [131] | ||||
| Epigenetic inhibitor | ||||||||
| KDOAM-25 | Histone lysine demethylase 5 (KDM5) inhibitor | KDOAM-25 is a KDM5 enzyme inhibitor that increases H3K4me3 levels at lower concentrations in MCF-7 cells | Advances in multidisciplinary therapy for meningiomas | [132] | ||||
| Possible adjunctive agents | ||||||||
| Calcium channel blockers | Benzothiazepines, phenylalkylamines, and dihydropyridines | Reduction of intracellular calcium concentrations | Calcium channel antagonists have effects on in vitro meningioma signal transduction pathways after growth factor stimulation; preclinical: animal models | [133] | ||||
| Statins | Pharmacophores, which are dihydroxyheptanoic acid segments, such as simvastatin (SIM), fluvastatin (FLU), atorvastatin (ATO), rosuvastatin (ROS), lovastatin (LOV), pravastatin (PRA), and pitavastatin (PIT) | Inhibition of MAPK pathway | Statins and thiazolidinediones have cytotoxic effects on meningioma cells; preclinical: cell culture/in vitro | [134] | ||||
| TL4 inhibitors | ||||||||
| Ipilimumab | Antibody | Binding to TL4, preventing the escape of immune surveillance mechanisms, | Toll-like receptors are therapeutic targets in central nervous system tumors; preclinical: cell cultures/in vitro | [135] | ||||
| Integrin inhibitor | ||||||||
| Cilengitide | Cyclic Arg–Gly–Asp peptide with antiangiogenic activity | Cilengitide acts as a highly potent inhibitor of angiogenesis and induces apoptosis of growing endothelial cells via the inhibition of the interaction between integrins with their ECM ligands | The integrin inhibitor cilengitide affects meningioma cell motility and invasion | [72] | ||||
| BAPI inhibitor | ||||||||
| Tazamatostat | C34H44N4O4 | Tazemetostat is a cancer drug that acts as a potent selective EZH2 inhibitor. Tazemetostat blocks the activity of the EZH2 methyltransferase, which may help keep the cancer cells from growing | Distant metastases in meningioma: an underestimated problem | [136] | ||||
| Tissue factor pathway inhibitor 2 | ||||||||
| Tissue factor pathway inhibitor 2 | TFPI-2 functions in the maintenance of the stability of the tumor environment and inhibits invasiveness and growth of neoplasms, as well as metastasis formation; TFPI-2 has also been shown to induce apoptosis and inhibit angiogenesis, which may contribute significantly to tumor growth inhibition | Restoration of tissue factor pathway inhibitor inhibited invasion and tumor growth in vitro and in vivo in a malignant meningioma cell line | [137] | |||||
| RNAi | Antisense abrogation of mRNA strands | Preclinical: cell culture/in vitro | ||||||
Completed and ongoing clinical trial
Hydroxyurea has been found to have many hematological and dermatological side effects [138–146]. In a phase III placebo-controlled clinical trial, mifepristone which is an antiprogesterone drug showed no changes in radiographic response, six-month progression-free survival, time to tumor progression, and overall survival from the placebo [147, 148]. Two single-armed studies, showing no comparison of drug’s effectiveness with the proper comparator group, was done on tamoxifen limiting its use as an effective drug. Recently, some new pharmacotherapy targets and their targeted agents have been identified. Wide varieties of these agents are under investigation for treatment of meningioma. Ongoing studies are testing the Mitogen-activated protein kinase (MEK)/mitogen-activated protein kinase (MAPK) pathway inhibitors (trametinib, selumetinib) as drugs for meningioma, phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/the mammalian target of rapamycin (mTOR) pathway inhibitors, and AKT inhibitor (alpelisib, Vistusertib, Capivasertib) are currently under investigation for meningioma treatment [149, 150]. Despite putting efforts in treating meningioma using the drugs like epidermal growth factor receptor (EGFR) inhibitors (brigatinib, afatinib), vascular endothelial growth factor receptor (VEGFR) inhibitors (cabozantinib, apatinib), no success could be achieved and work using this category drugs are still ongoing. VEGF inhibitors (bevacizumab, sunitinib, vatalanib) may offer some benefit in antiangiogenic treatments in meningiomas [86, 90, 150–154]. Findings suggest some role of c-MET and AXL inhibitor (cabozantinib) against meningioma by suppressing these proteins which are generally found elevated in meningioma [155, 156], similarly, smoothened (SMO) are potent targets for meningioma therapy [149, 157] and inhibitors of smoothened (SMO) are sonidegib and vismodegib. Focal adhesion kinase (FAK) inhibitor (GSK2256098) has shown some response toward meningioma treatment [117, 158], ongoing research on ribociclib and abemaciclib, cyclin-dependent kinase (CDK) inhibitors (Ribociclib, abemaciclib) individually are on the way. Histone deacetylase inhibitor (OSU-HDAC42) is recently used in first clinical trial for the treatment of meningioma, Glycogen synthase kinase 3-beta (GSK-3β) inhibitor (9-ING-41). This inhibitor enhances NF-_B’s the transcriptional activity [159–161]. GSK-3β can be used to get control over multiple malignancy including meningiomas [162]. Dopamine receptor D2 (DRD2) inhibitor (ONC206), this is an imipridone small molecule which produces cytotoxicity to tumor cells by increasing TNF-related apoptosis-inducing ligand’s activity [162]. PD-1 inhibitors (nivolumab, pembrolizumab sintilimab), PD-L1 inhibitors (avelumab) can also be potential drugs against meningioma. PD-1 and PD-L1 expression increases with tumor grades so anti-PD-1 and anti-PD-L1 therapy can be given to tumor patients [163, 164]. Currently, seven trials are going on to explore the effect of anti-PD-1 or anti-PD-L1 therapy on patients with meningioma. A current phase I trial study is being carried out on a newly modified drug, 177 Lu-DOTA-JR11, to investigate its safety and efficacy. While treating advanced and recurrent meningiomas, it is postulated to have better clinical efficacy and therapeutic index [165]. Two current phase II trials are evaluating the efficacy of LUTATHERA for the treatment of high-grade meningioma.
Limitations of conventional chemotherapy
Chemotherapy destroys normal cells, such as cells in the bone marrow, digestive tract, macrophages, and hair follicles, along with the cancerous cells [161].
Chemotherapeutic drugs cannot kill solid tumors as they fail to reach their core [162].
Traditional drugs are engulfed by macrophages and come out of circulation, remaining in circulation for a very short duration.
Some conventional chemotherapy drugs are unable to cross the plasma membrane and, hence, prove to be inefficient treatment options [163].
P-glycoprotein is a multidrug resistance protein that is overexpressed on the surface of cancerous cells due to which drugs do not accumulate inside the tumor, ultimately leading to resistance to anticancer drugs [164–167].
Exosomes and their role in cancer
Exosomes are a type of extracellular vesicles originating from the endosome system, which play role in cell-to-cell communication inside the tumor microenvironment (TME) [168]. The tumor microenvironment (TME) consists of tumor blood vessels, the extracellular matrix, and nonmalignant cells such as stromal cells, fibroblasts, and inflammatory cells [169–172]. Therefore, there are interactions among cancer cells, mesenchymal cells, and endothelial cells for the proper development of cancer [171]. Exosomes transfer proteins, lipids, and nucleic acids from parent cells to recipient cells. The various steps involved in exosome formation are as follows:
Extracellular components are captured via endocytosis leading to the formation of early endosomes.
Some specific endocytic bodies carrying proteins and nucleic acids sprout to form intraluminal vesicles (ILVs).
Late endosomes are formed from multivesicular bodies which carry multiple ILVs [172].
Many MVBs are digested by lysosomes while some vesicles with CD63, LAMP1, and LAMP2 on their surface fuse with the membrane, which are then secreted extracellularly [173].
ILVs and MVBs are formed with the help of endosomal sorting complexes required for transport (ESCRT). There are four types, including vacuolar protein sorting-associated protein 4 (VPS4), apoptosis linked gene 2-interacting protein X (ALIX), and vacuolar protein sorting-associated protein 1 (VTA1). ESCRT is mainly involved in sorting specific substances in ILVs. Exosomes act as a double-edged sword as they promote cancer formation on one hand, while they can kill the targeted cancer cells by encapsulating anticancer drugs, on the other hand.
Exosomes and tumor microenvironment
The TME was defined in the previous section. The TME has important features such as low oxygen levels, extracellular acidosis, poor nutrient content, and high lactate levels [174, 175]. As described earlier, the TME is composed of various cells including fibroblasts, endothelial cells, mesenchymal stem cells, and immune cells which secrete cytokines and growth factors [176]. Tumor progression is predicted by interactions between the TME and activation/inhibition pathways, which help in developing therapies targeting the TME [177–179]. Macrophages are found in excess in the TME and exhibit two phenotypes (M1 and M2) [180]. When there is shift toward the M2 phenotype, the chances of tumor progression are increased, mediating therapy resistance [181, 182]. One related signaling pathway with an oncogenic role is the signal transducer and activator of transcription factor 3 (STAT3) pathway [183–187]. Recently, a relationship was revealed among STAT3, exosomes, macrophage polarization, and glioma. In glioma, which is a very disastrous type of brain tumor, there is an increase in the secretion of exosomes along with an increase in hypoxic conditions. Exosomes release interleukin 6 and miRNA-155-3p, which activate STAT3, thus leading to autophagy. Autophagy then elevates the shift toward M2 polarization, which further increases glioma progression [188]. This study provided a big clue of how similar mechanisms can be found in meningioma, facilitating the development of a new targeted therapy.
Role of exosomes in tumor progression, tumor metastasis, and angiogenesis
Exosomes are an important component of the TME that can affect the proliferation and differentiation of cells. Therefore, an option for the treatment of cancer is to target the TME. Exosomes carrying CD171 from their parent cells can cause glioma cell invasiveness, proliferation, and motility [189]. Four important steps for cancer metastasis are (1) local infiltration of cancer cells, (2) entry of cells into circulation through the lymphatic system or blood vessels, and (3) entry or exit of cancer cells from remote organs. All steps are carried out by exosomes. Another important characteristic of cancer cells is metabolic reprogramming [190] which involves various cellular events such as the Warburg effect, changes in metabolites of the Krebs cycle, and the rate of oxidative phosphorylation, all of which fulfill the energy and structural requirements for growth and invasiveness of cancer cells [191].
The role of exosomes in the transition of grades and subtypes of meningiomas
As mentioned earlier, meningiomas are classified on the basis of their histological features. However, this system of grading remains unsatisfactory due to poor reproducibility and considerable variability within grades. According to the WHO 2021 classification, there are total of 15 subtypes. Grade I meningiomas have nine histological subtypes: meningothelial, fibrous, transitional, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, and metaplastic. Grade II menangiomas have three subtypes: chordoid, clear cell, and atypical. Grade III menangiomas have three subtypes: papillary, rhabdoid, and anaplastic (malignant) [192]. The most common subtype is meningothelial (57.7% of all meningiomas) originating more commonly from the parasagittal area, followed by transitional (19%) and fibrous (13%) meningiomas, while metaplastic and lymphoplasmacyte-rich meningiomas are exceptionally rare [193]. Several new markers are now being discovered, thanks to the availability of genomic and epigenomic profiling. These markers indicate the location, histological subtype, and clinical behavior of meningiomas. These discoveries enable us to develop new targeted therapies, as well as new adjuvant methods. Some of the latest techniques used for this purpose include copy number alterations, specific genetic abnormalities (germline or sporadic), and genome-wide methylation profiles. Since exosomes are also the source of different types of molecular markers, they can help in categorizing the various grades and subtypes of meningiomas. This may also provide information related to the detection of the grade or the subtype, resulting in the creation of new, advanced targeted therapies. According to recent studies, circulating miRNAs such as miRNA 497 and 219 inside the exosomes have the potential to be used as cancer biomarkers in various tumors including meningiomas [194]. In another study, M2 macrophage-derived exosomes were found to stimulate meningioma progression through the TGF-β signaling pathway [195]. Fibulin-2 was observed as another marker for differentiating grade II and grade I meningiomas [196]. Similar studies showed that the serum levels of miR-106a-5p, miR-219-5p, miR-375, and miR-409-3p were significantly increased, whereas the serum levels of miR-197 and miR224 were markedly decreased in the case of meningiomas; hence these six miRNAs act as noninvasive markers for meningioma [197]. Therefore, the abovementioned examples show how exosomes can be exploited as potent biomarkers for the detection and treatment of meningiomas.
The role of exosomes in immune escape
To develop properly, tumors adopt various strategies and manipulate the surrounding microenvironment. Evading the immune system is a powerful strategy opted by tumor cells to proliferate and metastasize. Studies have shown that several mechanisms render cancerous cells tolerant to the immune system. Cancer cells can cause the death of immune cells by following the FasL/Fas and PD-L1/PD-1 pathways, which lead to a decrease in the number of T cells and NK cells. Furthermore, cancer cells also recruit myeloid-derived suppressor cells (MDSCs) and immunosuppressive regulatory T cells (Tregs) that halt CD8 + T-cell functioning, resulting in tumor immune escape. Recently, extensive efforts have been made to understand the role of cancer cell-derived extracellular vesicles in activating the immune escape mechanism [198, 199]. EVs are involved in tumor microenvironment remodeling through angiogenesis [200–202], invasion [203, 204], metastasis [205–207], and resistance to therapies [208, 209].
Exploring the mechanisms of tumor-derived exosomes-mediated immune escape
Previous studies showed that extracellular vesicles inhibit the immune response against cancer at the innate and adapted levels [210]. Tumor-derived exosomes act on different components of the immune system through three mechanisms: functional activation, functional inhibition, and functional polarization.
Functional activation: As the tumor develops, it promotes the production of myeloid-derived suppressor cells (MDSCs), which enhance the immunosuppressive activity within the tumor microenvironment [211]. Regulatory T cells (Tregs) are upregulated in cancer patients and perform an immunosuppressive function within the tumor microenvironment, leading to tumor progression [212–214]. In a study by Szajnik and colleagues, tumor-derived extracellular vesicles induced the expansion of human Tregs, whereas normal cell-derived EVs did not. This study was the first to show that TEVs (Tumor-derived extracellular vesicles/tumor-derived exosomes) can cause the expansion of human Tregs [215].
Functional inhibition: The tumor microenvironment refers to the environment around the tumor and consists of surrounding blood vessels, immune cells, fibroblasts, signaling molecules, and the extracellular matrix, as described earlier. Dendritic cells are antigen-presenting cells that are involved in the innate and adaptive immune response. DCs are part of the tumor microenvironment, which act by capturing antigens and presenting them to T cells, subsequently activating the antitumor immune response. A mechanism that facilitates tumor cells escaping immune surveillance is the inhibition of DC maturation [216, 217]. Tumor-derived extracellular vesicles inhibit the dendritic cell maturation process. For example, in the case of lung carcinoma and breast cancer, the TEV-mediated inhibition of DCs has been studied [218]. NK cells are an important component of the immune system as they cause the lysis of target cells. Some studies have shown that TEVs cause the inhibition of NK cells, thus facilitating escape from the immune system. TEVs have also been reported to inhibit T lymphocyte cells, particularly CD8 + T cells [219]. This inhibition helps the tumor to grow and metastasize, leading to a tumor-friendly environment.
Functional polarization: The tumor microenvironment also contains tumor-associated macrophages (TAMs), which have the potential to infiltrate tumors and suppress the function of cytotoxic T lymphocytes, thereby enhancing cancer progression. A recent study showed that TEVs contribute to TAM polarization, whereby TV-treated macrophages were seen to promote in vivo tumor growth [220].
Immune checkpoints and cancer
Cancer cells escape the immune system [221] through various mechanisms:
Tumor cells can express corrupted versions of self-molecules;
Tumor cells can release immunosuppressive substances;
Aberrant changes in lymphocyte expression can lead to the antitumor immune response of tumor cells. Tumor cells can modulate macrophage function through this antitumor immune response [222].
The immune checkpoint inhibitor PD-1
Cancer cells survive inside the human body by generating an immunosuppressive tumor microenvironment. These cells express higher amounts of inhibitory ligands such as PD-L1 and PD-L2. These ligands inhibit the responses of T lymphocytes by binding to PD-L1 inhibitor receptors, which are expressed by T lymphocytes. They resist the apoptosis of cancer cells by T lymphocytes. PD-L1 expression was found to be higher in various types of cancer, including pancreatic cancer [223], renal cell carcinoma [224], lung cancer [225, 226], breast cancer [227, 228], gastric cancer [229, 230], and colorectal cancer [231].
Tumor-derived exosomes are carriers of PD-L1
It is known that PD-L1 plays a role as a tumor biomarker. Currently, interest is being garnered in the role of PD-L1-carrying tumor-derived EVs in regulating tumor progression. Several in vivo and in vitro tumor models such as melanoma, breast, glioblastoma, and prostate cancer have revealed the biological effects of ExoPD-L1 in causing cancer [232]. There is evidence showing that TEVs carrying PD-L1 are crucial players in inhibiting the proliferation and activation of CD4 + and CD8 + T cells, which infiltrate the tumor microenvironment. Hence, PD-L1 is responsible for inhibiting immune surveillance and promoting tumor progression. Recently, substantial focus has been given toward blocking the PD-1/PD-L1 pathways to resist cancer progression. Immune checkpoint protein inhibitors such as antibodies against PD-L1 and PD-1 can be used for curing numerous cancers [227–244]. In the future, characterization of the biological activities of ExoPD-L1 can contribute to understanding the mechanisms of immune escape.
Tumor-derived exosomes as modulators of PD-L1 expression in target cells
Recent studies have shown that tumor-derived extracellular vesicles carry PD-L1 and induce its expression in myeloid cells.
Role of exosomes in chemoresistance
Exosomes mediate tumorigenesis, metastasis, angiogenesis, and drug resistance, and they play role in physiological processes and pathological conditions [245, 246]. Chemoresistance can be classified as primary drug resistance and multidrug resistance. In primary drug resistance, cancer cells are resistant to induced drugs, whereas, in multidrug resistance, cancer cells become resistant to induced drugs, as well as other drugs to which cancer cells were not exposed [247]. Chemoresistant cancer cells show several properties and follow some mechanisms for their production. These include induced DNA repair, downregulation of apoptosis, increased drug efflux, alterations of drug targets, and overexpression of MDR proteins [248, 249]. Several proteins and nucleic acids have been identified as responsible for making cells resistant to drugs.
Role of exosomes in theranostics for the management of meningioma
Exosomes as diagnostic tools
Exosomes are present in body fluids such as breast milk, plasma, saliva, serum, malignant ascites fluids, and urine, which can be easily isolated [250–252]. Exosomes have clinical applications as diagnostic biomarkers, as well as therapeutic tools. The bodily fluids of both healthy individuals and diseased patients contain different proteins, mRNAs, and microRNAs inside exosomes, which can be potential biomarkers. Exosomes can be biomarkers in both cancerous and noncancerous diseases. Abundant nucleic acids, proteins, etc., in tumor-derived exosomes can serve as tumor markers; for example, EGFRVIII mRNAs are found in increased amounts in the circulating exosomes of patients with glioblastoma multiforme and lung cancer [253, 254]. Similarly, higher levels of proteoglycan glypican-1-positive exosomes have been detected in pancreatic cancer patients [255–258]. Specific mRNA/miRNA profiles in exosomes isolated from serum are seen in patients with ovarian cancer and lung cancer. miR-21, miR-16, miR-93, miR-100, miR-126, miR-200, and miR-223 were found upregulated in ovary cancer patients as compared to normal [259]. Breast cancer cells showed increased level of EpCAM-positive exosome, Del-1 exosome level, and HER2 expression [260–262]. Exosomal proteins present in urine are potential biomarkers for bladder and prostate cancer [263, 264]. Enrichment of lncRNA that sponges miRNA in the exosomes of prostate cancer patients showed their involvement. In this case, 26 lncRNAs observed downregulated and 19 lncRNAs got upregulated [265]. A recent comparative analysis of exosomal proteins from patients with different types of cancers showed that the level of CD63 was higher in exosomes isolated from cancer cells than the exosomes of normal cell lines [260].
Exosome-based cancer therapy by engineering of exosomes, extracellular vesicles using different types of nucleic acids, proteins and drugs
Several methods have been developed for exosome-based cancer therapy [261, 265–268], including the use of immune cell-derived natural exosomes to suppress cancer [269], preventing the production of cancer cell-derived exosomes, using exosomes as gene carriers [266], and using exosomes as anticancer drug carriers [267]. Extracellular vesicles are good drug carriers for tumor therapy due to their excellent properties such as biosafety, stability, and target specificity. In recent years, substantial efforts have been directed toward specifically engineering EVs to improve their tumor-targeting ability and drug delivery efficiency. These modifications can be applied directly or indirectly.
Engineering EVs in tumor therapy
-
Modification methods
Direct modifications: Direct modifications involve altering the surface proteins and the contents of purified EVs. Such modifications can be introduced physically or chemically. Specific peptide sequences and target proteins are inserted into the membrane of EVs. There are several physical modification methods, including simple incubation, electroporation, sonication, extrusion, freeze–thaw cycles, and saponin. Chemical modifications can be covalent or noncovalent, electrostatic interactions, ligand–receptor interaction, etc.
Indirect modifications: In the case of indirect modifications, parent cells are incubated so that specific types of EVs can be produced. Parent cells of EVs can be genetically and metabolically engineered to enhance their tumor-targeting capabilities and drug delivery efficiency. Indirect modifications can be applied via genetic engineering, metabolic engineering, membrane engineering, and loading components in parent cells.
-
Sources
Various cell types including mesenchymal stem cells, immune cells, and cancer cells are suitable choices for EV-based drug delivery [270]. EVs from other types of immune cells such as M1 EVs, DC-EVs, and NK-EVs are capable of affecting the tumor microenvironment, and hence, are used for inhibiting tumor progression.
-
Route of administration
The routes of administration of various drugs via exosomes include intravenous injection [271, 272], intraperitoneal injection [273, 274], subcutaneous injection [275], intratumoral injection [276], oral administration [277], and nasal administration [278, 279]. EVs have several advantages for being used in engineering and drug loading like biological stability, long-term storage, low immunogenicity, good biocompatibility, and a lack of differentiation activity [280–284].
EVs are suitable for storing water-soluble drugs and hydrophobic drugs and protecting them from degrading. EVs have an enhanced permeability and retention effect which lead to targeted aggregation. They can easily fuse with the cell membrane and unload the drug to target cells without problems of drug release and cytotoxicity linked to the phagocytosis/lysosome pathway.
-
Mechanism of loading drugs
Pre-loading: In the preloading technique, cells are incorporated with cargo that is encapsulated in exosomes during their production. Cells can feature biologically synthesized material such as proteins, nucleic acid, and synthetic compounds.
Post-loading: After the production of EVs, they are mixed with therapeutic drugs leading to drug-containing EVs. The post-loading of exosomes has a limitation that only hydrophobic drugs can be introduced not the hydrophilic drugs as they do not pass through the hydrophobic exosomal membrane.
Exosomes as nucleic acid carriers
DNA as therapeutic agent
Recent reported studies show that cancer can be treated with the help of exosome-based delivery of various types of functionalized DNA into cells via exosome-endocytosis process [285–289]. Disadvantage here is that exosomes cannot package large DNA. Using relatively small ASO, plasmid DNA or engineered modified exosomes can be the solution to this problem. The very first preclinical studies showing delivery of ASO by engineered exosomes for tumor suppression was done by Codiac Biosciences [290]. These data revealed how exoASO causes immunosuppression of transcription factors STAT6 and C/EBP by targeting their expression. Application of exoASO-STAT6 as a noval drug has recently been approved by FDA. Since CRISPER/Cas9-mediated genome editing being the emerging technology, exosomes having Cas9 encoding plasmids has also been tried for the treatment. A study on ovarian cancer demonstrated the efficient electroporation of CRISPER/Cas9 plasmids inside the ovarian cancer-derived exosomes to inhibit the expression of PARP-1[Poly (ADP-ribose) polymerase-1], a pro-cancer protein, which finally inhibited the increase in tumor volume [291] this technique can correct or degrade cancer causing genes by regulating mRNA translation or therapeutic genome editing including gene insert, gene deletion, gene correction, or gene degradation, respectively [292, 293].
mRNA as therapeutic agent
Again, another exciting work proved for the first time, the exosome-mediated exogenous mRNA delivery as a therapeutic approach. An mRNA was generated by transfecting cells with XPort/HChrR6 encoding plasmid which are subsequently loaded in the exosomes. In this study by Wang et al., these exosomes were delivered to HER2 + ve human breast cancer cells and showed the desired results [294]. An oncogenic miRNA namely miR-125b-2 found in leukemia was also targeted by using the similar approach. Human blood cells (RBCs) were loaded with Cas9 mRNA and gRNA for targeting the human miR-125b-2 locus. Leukemia cells when treated with these human blood cells-derived exosomes, downregulation of miR-125a and miR-125b was seen [295]. A new technology for producing large number of therapeutic mRNAs called the cellular nano perforation technology was introduced by Yang et al. [296]. In this technique, cells from various sources were transfected with plasmid DNA followed by stimulation with focal and transient electrical stimulation to enhance the release of transcribed mRNA carrying exosomes. This method was used to treat glioma cells by keeping in mind that as PTEN is an anti-tumor protein and its expression is less in tumor cells, its expression should be restored which can inhibit tumor growth and prolong survival. To fulfill this, large numbers of targeted functional exosomes were deliberately produced by transferring PTEN and CDX plasmids into glioma cell [296].
MiRNA as therapeutic agent
Studies have shown that miRNAs are very useful to treat cancer as they are endogenous, small, noncoding and they can regulate gene expression by binding to target mRNA. In miRNA-based therapy, either the exogenous tumor suppressing miRNAs are introduced to promote the inhibition of tumor growth called miRNA replacement or the specific miRNA inhibitor is introduced to inhibit the action of oncogenic miRNAs called miRNA inhibitor. Tumor-derived exosomes were used to deliver miR-375 mimic and inhibit migration and invasion of colon cancer cells [297]. Cisplatin-resistance OSCC was treated by introducing miR-155 inhibitor (an inhibitor of oncogenic miRNA-155), a therapeutic agent using exosome as a carrier. The results showed increase in FOXO3α expression and induction of EMT transition which could reverse the chemoresistance in oral cancer [298]. Genetically modified exosomes (exosomes with functional ligands modified on exosomal surface) bind to overexpressed receptors on the tumor surface hence targeting them and transferring more and more miRNA to tumor cells finally enhancing the therapeutic effect. Inhibition of cell migration was achieved by using Apo-A1-modified exosomes-loaded miR-26a (Apo-Exo/miR-26a) that target HepG2 cells via the SR-B1 receptor-mediated endocytosis [299]. GE-11 peptide-modified exosomes carrying let-7a miRNA targeted xenograft breast cancer tissue in RAG2–/– mice that stopped tumor development. Another example of this type targeting tumor cells with target peptide transcriptional transactivator (TAT) protein and T7 modified exosomes to deliver different miRNAs that inhibited various tumor’s development including glioma [300]. Recent findings revealed co-encapsulation of miRNAs and chemotherapeutics within engineered exosomes is a better idea to inhibit tumor growth. In a study by Liang et al., Firstly, Fusion protein Her2-LAMP2 was incorporated in the surface of exosomes and then engineered by encapsulating miR-21i and chemotherapeutics 5-Fluorouracil (5-FU). These were targeted through EGFR mediated endocytosis to 5-FU-resistant HCT-116 colorectal cancer cell. The results showed significant increase in apoptosis and proliferation inhibition [301]. Gioblastoma multiforme (GBM) cells have been seen to have chemoresistance and radioresistance [302]. GBM exhibited chemotherapeutic drug resistance for the drug temozolomide by elevating the expression of miR-9 and hence P-glycoprotein, a drug efflux transporter in cells. A study showed that introduction of mesenchymal stem cell-derived exosomes carrying anti-miR-9 can reverse the expression pattern of multidrug transporter protein in GBM cells and made them sensitive to temozolomide [303]. A study on model zebrafish for the treatment of brain tumor involved the use of brain endothelium-derived exosomes carrying anticancer drug doxorubicin [304]. Similarly, exosome-mediated delivery of miR-138 decreased the angiogenesis in glioma [305]. However, they have certain disadvantages; they are easily degraded, and their delivery to the target cells is difficult. Exosomes are suitable as a carrier of miRNAs as they are stable and highly specific [301–305]. Recently, researchers have taken great interest in the exosome-based delivery of miRNA and miRNA inhibitors [306–311]. There are several examples revealing the potential of this strategy for treating cancer. Anti-glioma miRNA-containing exosomes were shown to have tumor-fighting properties [308]. In another case, exosomes enriched with anti-osteosarcoma microRNA were proven as a remedy for cancer [310–316].
SiRNA as therapeutic agent
Under cancerous conditions, anti-apoptotic proteins, cell mitosis causing proteins, and cell growth factors such as BCL-2, PLK1, KRAS survivin get upregulated. These oncogenes are downregulated by siRNA-based exosomal therapy to get control over growing tumor. Natural killer (NK) cell-derived exosomes loaded with BCL-2 were seen to have anti-tumor effect on ER + breast cancer cells [317]. Apoptosis of bladder cancer cells got increased when treated with PLK1 SiRNA carrying exosomes [318]. Delivery of Grp78 SiRNA using bone marrow mesenchymal stem cells-derived exosomes was seen to provide drug sensitivity to sorafenib in hepatocellular carcinoma [319]. Si–c-Met delivered by exosomes silenced c-Met which increased the drug sensitivity [320]. In another similar study, iRGD-modified exosomes were used to deliver Carnitine palmitoyltransferase (CPT1A) in drug-resistant colon cancer cells and the sensitivity to oxaliplatin was found to be increased [321]. Fibrinogen-like protein 1 (FGL1), an important immune checkpoint, siRNA and TGF-β, an immunosuppressive chemokine in TME, siRNAs were delivered simultaneously in cRGD-modified exosomes and silenced these two proteins leading to tumor inhibition. Genes can be silenced by using siRNA-carrying exosomes. Nucleic acids can be loaded either endogenously or externally, resulting in a variety of functions. To regulate gene expression and maintain physiological equilibrium in the cell, exosomes can carry siRNAs with specialized functionality to designated cells.
CirRNA as therapeutic agent
CirRNAs are naturally present in exosomes and are currently being tried for its use in cancer treatment. Exosomal circRNA_100284 sponged the effect of miR-217 which inhibited the G2/M phase transition and hence the cell proliferation in various cancers [322]. POU3F3 cirRNA treatment was given to decrease the angiogenesis for the treatment of glioma [323].
lncRNA as therapeutic agent
Malignant behavior of bladder cancer was treated by getting the secreted exosomes of lncRNA PTENP1 lentiviral vector-transfected HEK293A cells. This exosomal PTENP1 sponged miR-17 and protected PTEN and thereby inhibited tumor development [324].
Exosomes as protein carriers
Exosome-based cancer vaccines represent a recent technique used to remove cancer [320–325]. Exosomes enriched with TRAIL, a cytokine, showed anticancer effects [323, 324]. Another example is represented by IL-18-enriched exosomes, which induced Th1 cytokine release and were responsible for the proliferation of peripheral blood mononuclear cells [325]. Exosomes can deliver various tumor antigens [326], nanobodies [327], apoptosis-inducing proteins [328], tumor or tissue-specific peptides [329], deficient or mutant antiapoptosis proteins [330], transferrins and lactoferrins [331], or proteasomes [332–336] into tumor cells for targeted therapy. Major histocompatibility complexes, heat-shock proteins, cytoskeletal proteins, signal transduction proteins, membrane transport proteins, and other essential proteins can also be delivered via exosomes. Catalase, Cre, recombinase, BDNF, and other enzymes are some examples that fall into this category [337–342]. Exosomes obtained from peptide-pulsed DCs are used to present antigens to T cells and induce their immune response. Exosomes derived from DCs contain MHC peptide complexes and costimulatory molecules on their membranes, which help them to present antigens efficiently and boost immunization. These engineered exosomes are better than antigen-presenting DC cells [260]. As an anti-apoptotic protein, survivin, suppresses apoptosis activation. An inactive mutation of survivin, T34A, hampers its pro-survival activity and induces caspase activation [338], subsequently inducing apoptosis in cancer cells. The utilization of dendritic cell-derived exosomes (DEXs), tumor cell-derived exosomes (TEXs), and ascetic cell-derived exosomes (AEXs) in the production of cancer vaccines is currently a hotspot for cancer research, particularly against brain tumors such as meningiomas. Dendritic cell-derived exosomes (DEXs) are promising candidates for the development of anticancer vaccines, as they are antigen-presenting cells. DEXs feature major histocompatibility complex (MHC)-I, MHC-II, and costimulatory proteins including CD86 on their surface, contributing to their selection as vaccine candidates [339, 340]. The basic concept behind this is, when DEXs are incubated with cancer antigens and administered in the body, they can induce a cancer-specific T-cell response. Mature DEXs induce a strong T-cell response in comparison to immature DEXs [341]. Natural killer cells are immune cells activated by the surface proteins of DEXs such as NKG2D ligand and IL-15Rα [342]. Cancer cell-derived exosomes can also be used as potential anticancer vaccines because they contain antigens. There are two ways to produce an anticancer vaccine. In the first method, DEXs are directly treated with antigens and transferred to the human body, while, in the other method, the tumor cells are modified, and their exosomes are pulsed with dendritic cells, thus finally producing DEXs for transfer to the human body. The use of exosomes for vaccine development against numerous infectious diseases such as tuberculosis is not new. Ongoing research indicates that vaccines of DEXs, TEXs, and AEXs using the above-described methods are being developed to treat various kinds of cancers such as lung and pancreatic cancer [343]. Since exosomes play an important role in cell-to-cell signaling in the brain, these vaccines deserve vigorous exploration in the case of brain tumors for its eradication.
Exosomes as drug delivery tools
Drugs have several disadvantages such as poor biocompatibility, low water solubility, difficulty to be observed in diseased cells, inability to penetrate cells, easy accumulation in normal tissue, fast metabolic rate inside the body, and many other side effects. Therefore, there is a need to develop an efficient drug delivery system. To date, several drug delivery systems have been developed, which are based on lipids, inorganic nanoparticles, viruses, and polymers. These nanotechnology-based drug delivery systems (DDSs) such as liposomes, dendrimers, magnetic nanoparticles, and polymeric nanoparticles are used to deliver chemotherapeutics, miRNAs, and anti-inflammatory drugs [344–346]. Among the various DDSs, exosomes are the best because of their very low immunogenicity, high level of blood stability, high protection of loaded drugs, easy infiltration through the biofilm barrier because of their unique membrane structure, similar structure to recipient cells, allowing them to be taken up by a large variety of cells, efficiency in targeting drugs to recipient cells, ability to load numerous therapeutic drugs, and direct migration to diseased organs or tissues after entering the body.
Chemotherapy is the first line of cancer treatment, but it has several disadvantages. EVs can nullify such side effects, establishing good targeting and delivery efficiency. EVs can be loaded with different types of drugs such as chemotherapeutic drugs, RNAs, proteins, and viruses. Studies have shown that small-molecule drugs such as doxorubicin, paclitaxel, curcumin, celastrol, and anthocyanidins can be loaded in modified exosomes and used to treat different types of cancers. Doxorubicin is an amphiphilic drug used for therapeutic purposes, as it can control cancer growth and inhibit angiogenesis [346]. Exosomes loaded with this drug can cross the blood–brain barrier. Other drugs with antitumor effects include tirapazamine, docetaxel, and porphyrin. Curcumin is a lipophilic polyphenolic compound that can act as an antitumor drug [347]. Exosomes loaded with berry anthocyanins have shown the ability to inhibit ovarian cancer [348].
PTX is used for treating different kinds of tumors as it induces mitotic arrest [349]. Tumor-derived EVs loaded with PTX have been used to target prostate cancer [350]. Doxorubicin-loaded EVs were encapsulated with A33 antibody and used for the treatment of colon cancer [351]. Another example is glioma treatment in which apolipoprotein-containing fibroblast-derived EVs loaded with methotrexate were used for tumor-targeting and killing effects [352]. Similarly, gentamicin and cisplatin-loaded EVs have been used for tumor-targeting therapy [353]. EVs can be loaded with a specific type of RNA to perform similar functions. In one study, EVs were used to deliver fluorouracil and miR-21 inhibitor oligonucleotides, which resulted in the treatment of colon cancer expressing human epidermal growth factor 2 (HER2) [296]. Engineered EVs can be loaded with oncolytic viruses which specifically kill cancer cells and do not affect normal cells [354]. In a related study, lung cancer cell-derived EVs were loaded with oncolytic virus and PTX, and the desired effect was obtained. This type of treatment using oncolytic viruses was more potent and efficient than treatment with PTX alone [355]. Another drug named Withaferin A was used in exosome-mediated delivery therapy as a potent inhibitor of angiogenesis and cancer growth. It was revealed that, upon administration, the drug showed an antitumor effect in a human lung cancer xenograft mouse model [356].
Targeting tumors using EVs
Traditional chemotherapeutic drugs are poor in terms of tumor-targeting ability; therefore, the focus is now on engineering EVs for the same purpose. MSCs have the property of crossing the vascular endothelium, allowing them to reach and colonize target cells [357]. MSC-EVs target 5-fluorocytosine and the mRNA of tumor cells; hence, they can be useful in developing new targeting therapy [358]. The retention time of the drug inside the tumor can be increased by fusing tumor-derived chemotherapeutic drug, doxorubicin-loaded EVs, with maternal cancer cells [359]. The homing properties of tumor-derived EVs represent a new method for tumor-targeted therapy. The folate receptor is a glycoprotein found in the cell membrane and is highly expressed in tumor cells. Thus, FR can be a target in tumor cells [360]. Similarly, hyaluronic acid is a glycosaminoglycan found in the extracellular matrix that is highly expressed in tumor cells. Hyaluronidases are β-N-acetylglucosaminidases that degrade HA via hydrolysis of the β (1, 4) glycosidic bond between D-glucuronic acid and N-acetyl-D-glucosamine [361]. Several other substances such matrix metalloproteinases (MMPs) can be used for tumor-targeted therapy [362].
Donor cells can be engineered to produce modified exosomes with specific receptors on their surface, enabling them to recognize particular cells. For this purpose, donor cells can be engineered in such a way to produce candidate proteins fused with exosomal surface proteins such as CD63 and CD9.
Similarly, drug-loaded exosomes can be protected from liver clearance and used for cancer treatment by blocking the scavenger receptor class A family, which is a monocyte macrophage uptake receptor for exosomes, thereby decreasing the exosome liver clearance and increasing accumulation in tumors [363]. Exosome–liposome hybridization is a new method to increase specificity and stability. Cationic lipids were used as the glue to help display pH-sensitive fusogenic peptides on the exosome surface. This type of exosome modification increases cell membrane-binding ability and cell uptake efficiency [364] (Fig. 4). To date, various drugs have been encapsulated in exosomes for the treatment of central nervous system tumors, as listed in Table 8.
Fig. 4.
Schematic diagram representing the use of exosomes for theragonistics in meningioma
Table 8.
The utility of exosomes in brain tumor treatment
| EV source | Therapeutic cargo | Target | Result | References |
|---|---|---|---|---|
| EL4-4 T cells | Curcumin, JSI-124 | Microglial cells | Curcumin and JSI-124 are administered intranasally by encapsulating the drugs in exosomes; no surgery is required; can be used to treat inflammatory diseases | [278] |
| Mouse macrophage cell line | Catalase (antioxidant) | Endothelial cells, neurons, astrocytes | Catalase administered intranasally has neuroprotective effects (in vitro and in vivo); can be used to treat inflammatory and neurodegenerative disease | [365] |
| Blood of kunming mice | Dopamine | Brain epithelial cells | Blood-derived exosomes can be delivered across the BBB via the transferrin–TfR interaction, with a > 15-fold increase in dopamine distribution observed, while toxicity decreased | [366] |
| Bone marrow-derived mesenchymal stem cells modified with rabies virus glycoprotein | MicroRNA-124 | The ischemic cortex of the brain | microRNA-124 was proven to be neuroprotective and promote neurogenesis, leading to neuron remodeling; can be used for the treatment of ischemic injury | [367] |
| Brain endothelial bAND.3 | Vascular endothelial growth factor small interfering RNA | Neuronal glioblastoma, astrocytoma U-87, malignant glioma cells | Vascular endothelial growth factor RNA and protein level were inhibited by siRNA encapsulated in exosomes | [368] |
| Brain neuronal glioblastoma-astrocytoma U-87 MG cells, brain endothelial bAND.3 cells, neuroectodermal tumor PF SK-1 cells, and glioblastoma A-172 cells | Rhodamine 123, Paclitaxel, doxorubicin | Neuronal glioblastoma-astrocytoma U-87, malignant glioma cells | The success of this treatment strategy is attributed to a higher expression level of CD63 | [346] |
| HEK293T cells | MiRNA-21 | Brain tumor | Suppression of tumor growth; can pass through the BBB | [369] |
| The targeting ligand is the ApoA1 mimetic peptide | Methotrexate, KLA (Lys, Leu, Ala) | Glioma | Selective brain tumor treatment | [352] |
| MSCs | MiRNA-146b (In vivo) | Glioma | Intratumoral exosome injection significantly reduced tumor volume | [305] |
| MSCs | Anti-miR-9 (In vitro) | Glioblastoma multiforme | Reverse expression of miR-9 sensitized the GBM cells to TMZ, which increased cell death and caspase activity | [298] |
| HEK293T | CD-UPRT fusion protein | Schwannoma | Treatment of Schwannoma | [276] |
Concluding remarks and future perspectives
Today, the available treatment options for meningioma include surgery, radiotherapy, chemotherapy, and immunotherapy. Surgery occupies the top position as a treatment option for meningioma. Immunotherapy is a relatively new field that requires attention. Although chemotherapeutic drugs have been researched immensely, there remain many drugs to be explored for meningioma treatment. Here, we tried to bring together all information on chemotherapeutic drugs to date that can be used against meningioma. Currently, if we talk specifically of meningioma, several drugs such as monoclonal antibodies, growth factor antagonists, hormonal antagonists, and inhibitors of several associated pathways have been discovered. Some drugs are under trial and many need to be explored.
Identification of novel diagnostic, prognostic, and predictive markers is of utmost importance. Liquid biopsy is a method to diagnose and screen various types of cancers, thus helping in the improvement of treatment efficacy. Exosomes are small vesicles secreted by all types of cells and are responsible for cell-to-cell communication. They can be obtained easily from different types of body fluids such as breast milk, semen, saliva, and urine; therefore, they can be used in the diagnosis of meningiomas. Exosomes are also excellent drug delivery systems. There exist several challenges in the development of exosome-based therapeutics due to the low productivity of exosomes, requiring large-scale production, challenges related to the collection of high-quality and uniform exosomes, the optimization of storage conditions, the improvement of their therapeutic potential and delivery. However, recent studies have shown that exosomes can be used as vaccines, thus helping in eradication of diseases like meningioma. DEXs, TEXs, and AEXs are of extreme importance for vaccine production. In conclusion, this review is based on drug target therapy and exosome-based target therapy for cancer. Nevertheless, there is a need to do more in-depth studies for the identification of effective chemotherapeutic drugs, which include the proper understanding of their mechanism of action and to determine how engineered exosomes can be used for targeted therapy of meningioma.
Acknowledgements
Authors are gratefully acknowledged by Sir Ganga Ram Hospital, Delhi, India for providing necessary support.
Author contributions
R.R. contributed to the study design and concept. R.R., S.S. were involved in the study organization. S.S. and R.R wrote the manuscript. R.R., P.P and N.K.G. – elaboration of the final version of the manuscript.
Funding
No external funding was used in the preparation of this article.
Data availability
Not applicable.
Declarations
Conflict of interest
The author(s) confirm that this article content has no conflicts of interest.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Swati Sharma, Email: swati.shutu@gmail.com.
Rashmi Rana, Email: rashmi.rana@sgrh.com, Email: rana_aiims@yahoo.co.in.
Prem Prakash, Email: prem@jamiahamdard.ac.in.
Nirmal Kumar Ganguly, Email: gangulynk1@gmail.com.
References
- 1.Harter PN, Braun Y, Plate KH. Classification of meningiomas-advances and controversies. Chin Clin Oncol. 2017;6:S2. doi: 10.21037/cco.2017.05.02. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17:iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tufan K, Dogulu F, Kurt G, Emmez H, Ceviker N, Baykaner MK. Intracranial meningiomas of childhood and adolescence. Pediatr Neurosurg. 2005;41(1):1–7. doi: 10.1159/000084858. [DOI] [PubMed] [Google Scholar]
- 4.Ostrom QT, Gittleman H, De Blank PM, Finlay JL, Gurney JG, McKean-Cowdin R, Stearns DS, Wolff JE, Liu M, Wolinsky Y, Kruchko C. American brain tumor association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2016;18:i1–50. doi: 10.1093/neuonc/nov297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klaeboe L, Lonn S, Scheie D, Auvinen A, Christensen HC, Feychting M, Johansen C, Salminen T, Tynes T. Incidence of intracranial meningiomas in Denmark, Finland, Norway and Sweden, 1968–1997. Int J Cancer. 2005;117(6):996–1001. doi: 10.1002/ijc.21255. [DOI] [PubMed] [Google Scholar]
- 6.Schneider B, Pülhorn H, Röhrig B, Rainov NG. Predisposing conditions and risk factors for development of symptomatic meningioma in adults. Cancer Detect Prevent. 2005;29(5):440–447. doi: 10.1016/j.cdp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Flint-Richter P, Mandelzweig L, Oberman B, Sadetzki S. Possible interaction between ionizing radiation, smoking, and gender in the causation of meningioma. Neuro Oncol. 2011;13(3):345–352. doi: 10.1093/neuonc/noq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gousias K, Schramm J, Simon M. The Simpson grading revisited: aggressive surgery and its place in modern meningioma management. J Neurosurg. 2016;125(3):551–560. doi: 10.3171/2015.9.JNS15754. [DOI] [PubMed] [Google Scholar]
- 10.Kaley T, Barani I, Chamberlain M, McDermott M, Panageas K, Raizer J, Rogers L, Schiff D, Vogelbaum M, Weber D, Wen P. Historical benchmarks for medical therapy trials in surgery-and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16(6):829–840. doi: 10.1093/neuonc/not330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magill ST, Dalle Ore CL, Diaz MA, Jalili DD, Raleigh DR, Aghi MK, Theodosopoulos PV, McDermott MW. Surgical outcomes after reoperation for recurrent non–skull base meningiomas. J Neurosurg. 2018;131(4):1179–1187. doi: 10.3171/2018.6.JNS18118. [DOI] [PubMed] [Google Scholar]
- 12.Camuzard O, Santucci-Darmanin S, Carle GF, Pierrefite-Carle V. Autophagy in the crosstalk between tumor and microenvironment. Cancer Lett. 2020;10(490):143–153. doi: 10.1016/j.canlet.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Yáñez-Mó M, Siljander PR, Andreu Z, Bedina Zavec A, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colás E. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Wang L, Zhou X, Luo X, Liu K, Jiang E, Chen Y, Shao Z, Shang Z. OSCC exosomes regulate miR-210–3p targeting EFNA3 to promote oral cancer angiogenesis through the PI3K/AKT pathway. BioMed Res Int. 2020 doi: 10.1155/2020/2125656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao L, You BO, Shi SI, Shan Y, Zhang Q, Yue H, Zhang J, Zhang W, Shi Y, Liu Y, Wang X. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene. 2018;37(21):2873–2889. doi: 10.1038/s41388-018-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Liu Q, Lu J, Adah D, Yu S, Zhao S, Yao Y, Qin L, Chen X. Exosomes from Plasmodium-infected hosts inhibit tumor angiogenesis in a murine Lewis lung cancer model. Oncogenesis. 2017;6(6):e351. doi: 10.1038/oncsis.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie JY, Wei JX, Lv LH, Han QF, Yang WB, Li GL, Wang PX, Wu SB, Duan JX, Zhuo WF, Liu PQ. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun Signal. 2020;18(1):1–3. doi: 10.1186/s12964-020-00535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai X, Wang L, Deivasigamni A, Looi CY, Karthikeyan C, Trivedi P, Chinnathambi A, Alharbi SA, Arfuso F, Dharmarajan A, Goh BC. A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget. 2017;8(8):12831. doi: 10.18632/oncotarget.14606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong PS, Wang LZ, Dai X, Tseng SH, Loo SJ, Sethi G. Judicious toggling of mTOR activity to combat insulin resistance and cancer: current evidence and perspectives. Front Pharmacol. 2016;7:395. doi: 10.3389/fphar.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan CD, Bharathkumar H, Bulusu KC, Pandey V, Rangappa S, Fuchs JE, Shanmugam MK, Dai X, Li F, Deivasigamani A, Hui KM. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J Biol Chem. 2014;289(49):34296–34307. doi: 10.1074/jbc.M114.601104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manu KA, Shanmugam MK, Li F, Chen L, Siveen KS, Ahn KS, Kumar AP, Sethi G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J Mol Med. 2014;92(3):267–276. doi: 10.1007/s00109-013-1095-0. [DOI] [PubMed] [Google Scholar]
- 22.Tan SC. Low penetrance genetic polymorphisms as potential biomarkers for colorectal cancer predisposition. J Gene Med. 2018;20(4):e3010. doi: 10.1002/jgm.3010. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Wang L, Pan H, Wang Y, Shi M, Yu H, Wang C, Pan X, Chen Z. Exosomes derived from macrophages enhance aerobic glycolysis and chemoresistance in lung cancer by stabilizing c-Myc via the inhibition of NEDD4L. Front Cell Develop Biol. 2021;8:620603. doi: 10.3389/fcell.2020.620603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen T, Huang Z, Shi C, Pu X, Xu X, Wu Z, Ding G, Cao L. Pancreatic cancer-derived exosomes induce apoptosis of T lymphocytes through the p38 MAPK-mediated endoplasmic reticulum stress. FASEB J. 2020;34(6):8442–8458. doi: 10.1096/fj.201902186R. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Wang Y, Yan Z, Sun Y, Su C. Colorectal cancer cell-derived exosomes promote proliferation and decrease apoptosis by activating the ERK pathway. Int J Clin Exp Pathol. 2019;12(7):2485. [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Xu P, Xie X, Hu F, Jiang L, Hu R, Ding F, Xiao H, Zhang H. Down regulation of SIRT2 Reduced ASS induced NSCLC apoptosis through the release of autophagy components via exosomes. Front Cell Develop Biol. 2020;8:601953. doi: 10.3389/fcell.2020.601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang ST, Yang MH, Kumar AP, Sethi G, Ahn KS. Corilagin represses epithelial to mesenchymal transition process through modulating Wnt/β-catenin signaling cascade. Biomolecules. 2020;10(10):1406. doi: 10.3390/biom10101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang MH, Lee JH, Ko JH, Jung SH, Sethi G, Ahn KS. Brassinin represses invasive potential of lung carcinoma cells through deactivation of PI3K/Akt/mTOR signaling cascade. Molecules. 2019;24(8):1584. doi: 10.3390/molecules24081584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko JH, Nam D, Um JY, Jung SH, Sethi G, Ahn KS. Bergamottin suppresses metastasis of lung cancer cells through abrogation of diverse oncogenic signaling cascades and epithelial-to-mesenchymal transition. Molecules. 2018;23(7):1601. doi: 10.3390/molecules23071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaballa R, Ali HE, Mahmoud MO, Rhim JS, Ali HI, Salem HF, Saleem M, Kandeil MA, Ambs S, Abd Elmageed ZY. Exosomes-mediated transfer of Itga2 promotes migration and invasion of prostate Cancer cells by inducing epithelial-mesenchymal transition. Cancers. 2020;12(8):2300. doi: 10.3390/cancers12082300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai J, Gong L, Li G, Guo J, Yi X, Wang Z. Exosomes in ovarian cancer ascites promote epithelial–mesenchymal transition of ovarian cancer cells by delivery of miR-6780b-5p. Cell Death Dis. 2021;12(2):1–7. doi: 10.1038/s41419-021-03490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shojaei S, Hashemi SM, Ghanbarian H, Sharifi K, Salehi M, Mohammadi-Yeganeh S. Delivery of miR-381–3p mimic by mesenchymal stem cell-derived exosomes inhibits triple negative breast cancer aggressiveness; an in vitro study. Stem Cell Rev Rep. 2021;17(3):1027–1038. doi: 10.1007/s12015-020-10089-4. [DOI] [PubMed] [Google Scholar]
- 33.Monisha J, Roy NK, Padmavathi G, Banik K, Bordoloi D, Khwairakpam AD, Arfuso F, Chinnathambi A, Alahmadi TA, Alharbi SA, Sethi G. NGAL is downregulated in oral squamous cell carcinoma and leads to increased survival, proliferation, migration and chemoresistance. Cancers. 2018;10(7):228. doi: 10.3390/cancers10070228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko JH, Um JY, Lee SG, Yang WM, Sethi G, Ahn KS. Conditioned media from adipocytes promote proliferation, migration, and invasion in melanoma and colorectal cancer cells. J Cell Physiol. 2019;234(10):18249–18261. doi: 10.1002/jcp.28456. [DOI] [PubMed] [Google Scholar]
- 35.Kothapalli R, Sivaraman Siveen K, Tan TZ, Thiery JP, Kumar AP, Sethi G, Swaminathan K. Functional characterization of selective exosite-binding inhibitors of matrix metalloproteinase-13 (MMP-13)–experimental validation in human breast and colon cancer. Biosci Biotechnol Biochem. 2016;80(11):2122–2131. doi: 10.1080/09168451.2016.1200456. [DOI] [PubMed] [Google Scholar]
- 36.Lee H, Baek SH, Lee JH, Kim C, Ko JH, Lee SG, Chinnathambi A, Alharbi SA, Yang WM, Um JY, Sethi G. Isorhynchophylline, a potent plant alkaloid, induces apoptotic and anti-metastatic effects in human hepatocellular carcinoma cells through the modulation of diverse cell signaling cascades. Int J Mol Sci. 2017;18(5):1095. doi: 10.3390/ijms18051095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung YY, Lee JH, Nam D, Narula AS, Namjoshi OA, Blough BE, Um JY, Sethi G, Ahn KS. Anti-myeloma effects of icariin are mediated through the attenuation of JAK/STAT3-dependent signaling cascade. Front Pharmacol. 2018;9:531. doi: 10.3389/fphar.2018.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMullen KP, Stieber VW. Meningioma: current treatment options and future directions. Curr Treat Options Oncol. 2004;5(6):499–509. doi: 10.1007/s11864-004-0038-y. [DOI] [PubMed] [Google Scholar]
- 39.Johnson M, Toms S. Mitogenic signal transduction pathways in meningiomas: novel targets for meningioma chemotherapy? J Neuropathol Exp Neurol. 2005;64(12):1029–1036. doi: 10.1097/01.jnen.0000189834.63951.81. [DOI] [PubMed] [Google Scholar]
- 40.Jagannathan J, Oskouian RJ, Yeoh HK, Saulle D, Dumont AS. Molecular biology of unreresectable meningiomas: implications for new treatments and review of the literature. Skull Base. 2008;18(3):173. doi: 10.1055/s-2007-1003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5(12):1045–1054. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- 42.Ragel B, Jensen RL. New approaches for the treatment of refractory meningiomas. Cancer Control. 2003;10(2):148–158. doi: 10.1177/107327480301000206. [DOI] [PubMed] [Google Scholar]
- 43.Johnson MD, Sade B, Milano MT, Lee JH, Toms SA. New prospects for management and treatment of inoperable and recurrent skull base meningiomas. J Neuro-Oncol. 2008;86(1):109–122. doi: 10.1007/s11060-007-9434-z. [DOI] [PubMed] [Google Scholar]
- 44.Simon M, Boström JP, Hartmann C. Molecular genetics of meningiomas: from basic research to potential clinical applications. Neurosurgery. 2007;60(5):787–798. doi: 10.1227/01.NEU.0000255421.78431.AE. [DOI] [PubMed] [Google Scholar]
- 45.Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;62(7):1210–1212. doi: 10.1212/01.WNL.0000118300.82017.F4. [DOI] [PubMed] [Google Scholar]
- 46.Gupta V, Su YS, Samuelson CG, Liebes LF, Chamberlain MC, Hofman FM, Schönthal AH, Chen TC. Irinotecan: a potential new chemotherapeutic agent for atypical or malignant meningiomas. J Neurosurg. 2007;106(3):455–462. doi: 10.3171/jns.2007.106.3.455. [DOI] [PubMed] [Google Scholar]
- 47.Schrell UM, Rittig MG, Anders M, Kiesewetter F, Marschalek R, Koch UH, Fahlbusch R. Hydroxyurea for treatment of unresectable and recurrent meningiomas. I. Inhibition of primary human meningioma cells in culture and in meningioma transplants by induction of the apoptotic pathway. Neurosurg Focus. 1997;2(4):E10. doi: 10.3171/foc.1997.2.4.11. [DOI] [PubMed] [Google Scholar]
- 48.Preusser M, Spiegl-Kreinecker S, Lötsch D, Wöhrer A, Schmook M, Dieckmann K, Saringer W, Marosi C, Berger W. Trabectedin has promising antineoplastic activity in high-grade meningioma. Cancer. 2012;118(20):5038–5049. doi: 10.1002/cncr.27460. [DOI] [PubMed] [Google Scholar]
- 49.Gupta S, Bi WL, Dunn IF. Medical management of meningioma in the era of precision medicine. Neurosurg Focus. 2018;44(4):E3. doi: 10.3171/2018.1.FOCUS17754. [DOI] [PubMed] [Google Scholar]
- 50.Read WL, Williams F. Recurrent meningioma of the cervical spine, successfully treated with liposomal doxorubicin. Case Rep Oncol. 2017;10(2):656–659. doi: 10.1159/000477844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curic S, Wu Y, Shan B, Schaaf C, Utpadel D, Lange M, Kuhlen D, Perone MJ, Arzt E, Stalla GK, Renner U. Curcumin acts anti-proliferative and pro-apoptotic in human meningiomas. J Neuro-oncol. 2013;113(3):385–396. doi: 10.1007/s11060-013-1148-9. [DOI] [PubMed] [Google Scholar]
- 52.Park YS, Lee JH, Bondar J, Harwalkar JA, Safayhi H, Golubic M. Cytotoxic action of acetyl-11-keto-β-boswellic acid (AKBA) on meningioma cells. Planta Med. 2002;68(05):397–401. doi: 10.1055/s-2002-32090. [DOI] [PubMed] [Google Scholar]
- 53.Chamberlain MC. Adjuvant combined modality therapy for malignant meningiomas. J Neurosurg. 1996;84(5):733–736. doi: 10.3171/jns.1996.84.5.0733. [DOI] [PubMed] [Google Scholar]
- 54.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 55.Singh B, Coffey RJ. Trafficking of epidermal growth factor receptor ligands in polarized epithelial cells. Annu Rev Physiol. 2014;76:275. doi: 10.1146/annurev-physiol-021113-170406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersson U, Guo D, Malmer B, Bergenheim AT, Brännström T, Hedman H, Henriksson R. Epidermal growth factor receptor family (EGFR, ErbB2–4) in gliomas and meningiomas. Acta Neuropathol. 2004;108(2):135–142. doi: 10.1007/s00401-004-0875-6. [DOI] [PubMed] [Google Scholar]
- 57.Johnson MD, Horiba M, Winnier AR, Arteaga CL. The epidermal growth factor receptor is associated with phospholipase C-γ1 in meningiomas. Human Pathol. 1994;25(2):146–153. doi: 10.1016/0046-8177(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 58.Nagashima G, Asai JI, Suzuki R, Fujimoto T. Different distribution of c-myc and MIB-1 positive cells in malignant meningiomas with reference to TGFs, PDGF, and PgR expression. Brain Tumor Pathol. 2001;18(1):1–5. doi: 10.1007/BF02478918. [DOI] [PubMed] [Google Scholar]
- 59.Yang SY, Xu GM. Expression of PDGF and its receptor as well as their relationship to proliferating activity and apoptosis of meningiomas in human meningiomas. J Clin Neurosci. 2001;8(4):49–53. doi: 10.1054/jocn.2001.0877. [DOI] [PubMed] [Google Scholar]
- 60.Todo T, Adams EF, Fahlbusch R, Dingermann T, Werner H. Autocrine growth stimulation of human meningioma cells by platelet-derived growth factor. J Neurosurg. 1996;84(5):852–858. doi: 10.3171/jns.1996.84.5.0852. [DOI] [PubMed] [Google Scholar]
- 61.Pfister C, Pfrommer H, Tatagiba MS, Roser F. Vascular endothelial growth factor signals through platelet-derived growth factor receptor β in meningiomas in vitro. British J Cancer. 2012;107(10):1702–1713. doi: 10.1038/bjc.2012.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao HF, Wang J, Shao W, Wu CP, Chen ZP, To SS, Li WP. Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: current preclinical and clinical development. Mol Cancer. 2017;16(1):1–6. doi: 10.1186/s12943-017-0670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98(6):859–869. doi: 10.1016/S0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 64.Shapiro WR, Schmid M, Glantz M, Miller JJ. A randomized phase III/IV study to determine benefit and safety of cytarabine liposome injection for treatment of neoplastic meningitis. J Clin Oncol. 2006;24(18):1528. doi: 10.1200/jco.2006.24.18_suppl.1528. [DOI] [Google Scholar]
- 65.Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. Elife. 2014 doi: 10.7554/eLife.02872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baker SJ, Reddy EP. CDK4: a key player in the cell cycle, development, and cancer. Gene Cancer. 2012;3(11–12):658–669. doi: 10.1177/1947601913478972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das A, Alshareef M, Martinez Santos JL, Porto GB, McDonald DG, Infinger LK, Vandergrift WA, Lindhorst SM, Varma AK, Patel SJ, Cachia D. Evaluating anti-tumor activity of palbociclib plus radiation in anaplastic and radiation-induced meningiomas: pre-clinical investigations. Clin Trans Oncol. 2020;22(11):2017–2025. doi: 10.1007/s12094-020-02341-7. [DOI] [PubMed] [Google Scholar]
- 68.Domingues P, González-Tablas M, Otero Á, Pascual D, Ruiz L, Miranda D, Sousa P, Gonçalves JM, Lopes MC, Orfao A, Tabernero MD. Genetic/molecular alterations of meningiomas and the signaling pathways targeted. Oncotarget. 2015;6(13):10671. doi: 10.18632/oncotarget.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Available online: https://clinicaltrials.gov Accessed on 30 Aug 2022
- 70.Shah NR, Tancioni I, Ward KK, Lawson C, Chen XL, Jean C, Sulzmaier FJ, Uryu S, Miller NL, Connolly DC, Schlaepfer DD. Analyses of merlin/NF2 connection to FAK inhibitor responsiveness in serous ovarian cancer. Gynecol Oncol. 2014;134(1):104–111. doi: 10.1016/j.ygyno.2014.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shapiro IM, Kolev VN, Vidal CM, Kadariya Y, Ring JE, Wright Q, Weaver DT, Menges C, Padval M, McClatchey AI, Xu Q. Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci Transl Med. 2014 doi: 10.1126/scitranslmed.3008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilisch-Neumann A, Kliese N, Pachow D, Schneider T, Warnke JP, Braunsdorf WE, Böhmer FD, Hass P, Pasemann D, Helbing C, Kirches E. The integrin inhibitor cilengitide affects meningioma cell motility and invasioncilengitide in meningiomas. Clin Cancer Res. 2013;19(19):5402–5412. doi: 10.1158/1078-0432.CCR-12-0299. [DOI] [PubMed] [Google Scholar]
- 73.Norden AD, Raizer JJ, Abrey LE, Lamborn KR, Lassman AB, Chang SM, Yung WK, Gilbert MR, Fine HA, Mehta M, DeAngelis LM. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neuro-oncol. 2010;96(2):211–217. doi: 10.1007/s11060-009-9948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Osorio DS, Hu J, Mitchell C, Allen JC, Stanek J, Hagiwara M, Karajannis MA. Effect of lapatinib on meningioma growth in adults with neurofibromatosis type 2. J Neuro-oncol. 2018;139(3):749–755. doi: 10.1007/s11060-018-2922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wernicke AG, Dicker AP, Whiton M, Ivanidze J, Hyslop T, Hammond EH, Perry A, Andrews DW, Kenyon L. Assessment of epidermal growth factor receptor (EGFR) expression in human meningioma. Radiation Oncol. 2010;5(1):1–7. doi: 10.1186/1748-717X-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chamberlain MC. Is there effective systemic therapy for recurrent surgery-and radiation-refractory meningioma? CNS Oncol. 2013;2(1):1–5. doi: 10.2217/cns.12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caruso G, Elbabaa SK, Gonzalez-Lopez P, Barresi V, Passalacqua M, Caffo M. Innovative therapeutic strategies in the treatment of meningioma. Anticancer Res. 2015;35(12):6391–6400. [PubMed] [Google Scholar]
- 78.Gan HK, Lappas M, Cao DX, Cvrljevdic A, Scott AM, Johns TG. Targeting a unique EGFR epitope with monoclonal antibody 806 activates NF-κB and initiates tumour vascular normalization. J Cell Mol Med. 2009;13(9b):3993–4001. doi: 10.1111/j.1582-4934.2009.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramakrishnan MS, Eswaraiah A, Crombet T, Piedra P, Saurez G, Iyer H, Arvind A. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs. 2009;1:41–48. doi: 10.4161/mabs.1.1.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wen PY, Yung WA, Lamborn KR, Norden AD, Cloughesy TF, Abrey LE, Fine HA, Chang SM, Robins HI, Fink K, DeAngelis LM. Phase II study of imatinib mesylate for recurrent meningiomas (North American brain tumor consortium study 01–08) Neuro Oncol. 2009;11(6):853–860. doi: 10.1215/15228517-2009-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ammoun S, Schmid MC, Triner J, Manley P, Hanemann CO. Nilotinib alone or in combination with selumetinib is a drug candidate for neurofibromatosis type 2. Neuro Oncol. 2011;13(7):759–766. doi: 10.1093/neuonc/nor056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sagers JE, Beauchamp RL, Zhang Y, Vasilijic S, Wu L, DeSouza P, Seist R, Zhou W, Xu L, Ramesh V, Stankovic KM. Combination therapy with mTOR kinase inhibitor and dasatinib as a novel therapeutic strategy for vestibular schwannoma. Sci Rep. 2020;10(1):1. doi: 10.1038/s41598-020-60156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lou E, Sumrall AL, Turner S, Peters KB, Desjardins A, Vredenburgh JJ, McLendon RE, Herndon JE, McSherry F, Norfleet J, Friedman HS. Bevacizumab therapy for adults with recurrent/progressive meningioma: a retrospective series. Journal of Neuro-oncol. 2012;109(1):63–70. doi: 10.1007/s11060-012-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Available online: https://go.drugbank.com Accessed on 30 Aug 2022.
- 85.Tuchen M, Wilisch-Neumann A, Daniel EA, Baldauf L, Pachow D, Scholz J, Angenstein F, Stork O, Kirches E, Mawrin C. Receptor tyrosine kinase inhibition by regorafenib/sorafenib inhibits growth and invasion of meningioma cells. Euro J Cancer. 2017;73:9–21. doi: 10.1016/j.ejca.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Kaley TJ, Wen P, Schiff D, Ligon K, Haidar S, Karimi S, Lassman AB, Nolan CP, DeAngelis LM, Gavrilovic I, Norden A. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17(1):116–121. doi: 10.1093/neuonc/nou148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wen PY, Chang SM, Lamborn KR, Kuhn JG, Norden AD, Cloughesy TF, Robins HI, Lieberman FS, Gilbert MR, Mehta MP, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American brain tumor consortium trial 04–02. Neuro Oncol. 2014;16:567–578. doi: 10.1093/neuonc/not247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Graillon T, Sanson M, Campello C, Idbaih A, Peyre M, Peyrière H, Basset N, Autran D, Roche C, Kalamarides M, Roche PH. Everolimus and octreotide for patients with recurrent meningioma: results from the phase II CEVOREM trialeverolimus and octreotide in aggressive meningiomas. Clin Cancer Res. 2020;26(3):552–557. doi: 10.1158/1078-0432.CCR-19-2109. [DOI] [PubMed] [Google Scholar]
- 89.Weller M, Roth P, Sahm F, Burghardt I, Schuknecht B, Rushing EJ, Regli L, Lindemann JP, Von Deimling A. Durable control of metastatic AKT1-mutant WHO grade 1 meningothelial meningioma by the AKT inhibitor, AZD5363. J Natl Cancer Inst. 2017 doi: 10.1093/jnci/djw320. [DOI] [PubMed] [Google Scholar]
- 90.Nigim F, Wakimoto H, Kasper EM, Ackermans L, Temel Y. Emerging medical treatments for meningioma in the molecular era. Biomedicines. 2018;6(3):86. doi: 10.3390/biomedicines6030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Day D, Prawira A, Spreafico A, Waldron J, Karithanam R, Giuliani M, Weinreb I, Kim J, Cho J, Hope A, Bayley A. Phase I trial of alpelisib in combination with concurrent cisplatin-based chemoradiotherapy in patients with locoregionally advanced squamous cell carcinoma of the head and neck. Oral Oncol. 2020;108:104753. doi: 10.1016/j.oraloncology.2020.104753. [DOI] [PubMed] [Google Scholar]
- 92.Horbinski C, Xi G, Wang Y, Hashizume R, Gopalakrishnan M, Phillips JJ, Houghton P, James CD, Kalapurakal JA. The effects of palbociclib in combination with radiation in preclinical models of aggressive meningioma. Neuro-oncol Adv. 2021 doi: 10.1093/noajnl/vdab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maggio I, Franceschi E, Tosoni A, Nunno VD, Gatto L, Lodi R, Brandes AA. Meningioma: not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. 2021 doi: 10.2217/cns-2021-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Available online: https://ichgcp.net Accessed on 30 Aug 2022.
- 95.Boetto J, Apra C, Bielle F, Peyre M, Kalamarides M. Selective vulnerability of the primitive meningeal layer to prenatal Smo activation for skull base meningothelial meningioma formation. Oncogene. 2018;37(36):4955–4963. doi: 10.1038/s41388-018-0328-7. [DOI] [PubMed] [Google Scholar]
- 96.Wigertz A, Lönn S, Hall P, Auvinen A, Christensen HC, Johansen C, Klæboe L, Salminen T, Schoemaker MJ, Swerdlow AJ, Tynes T. Reproductive factors and risk of meningioma and glioma. Cancer Epidemiol Biomarkers Prevent. 2008;17(10):2663–2670. doi: 10.1158/1055-9965.EPI-08-0406. [DOI] [PubMed] [Google Scholar]
- 97.Schoenberg BS, Christine BW, Whisnant JP. Nervous system neoplasms and primary malignancies of other sites: the unique association between meningiomas and breast cancer. Neurology. 1975;25(8):705. doi: 10.1212/WNL.25.8.705. [DOI] [PubMed] [Google Scholar]
- 98.McCutcheon IE. The biology of meningiomas. J Neuro-oncol. 1996;29(3):207–216. doi: 10.1007/BF00165650. [DOI] [PubMed] [Google Scholar]
- 99.Hsu DW, Efird JT, Hedley-Whyte ET. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86(1):113–120. doi: 10.3171/jns.1997.86.1.0113. [DOI] [PubMed] [Google Scholar]
- 100.Check JH, Check D, Poretta T. Mifepristone extends both length and quality of life in a patient with advanced non-small cell lung cancer that has progressed despite chemotherapy and a check-point inhibitor. Anticancer Res. 2019;39(4):1923–1926. doi: 10.21873/anticanres.13301. [DOI] [PubMed] [Google Scholar]
- 101.Check DL, Check JH, Poretta T, Aikins J, Wilson CJ. Prolonged high-quality life in patients with non-small cell lung cancer treated with mifepristone who advanced despite osimertinib. Cancer Sci Res. 2020;3(2):1–5. [Google Scholar]
- 102.Check JH, Check D, Wilson C, Lofberg P. Long-term high-quality survival with single-agent mifepristone treatment despite advanced cancer. Anticancer Res. 2016;36(12):6511–6513. doi: 10.21873/anticanres.11251. [DOI] [PubMed] [Google Scholar]
- 103.Check JH, Check D, Srivastava MD, Poretta T, Aikins JK. Treatment with mifepristone allows a patient with end-stage pancreatic cancer in hospice on a morphine drip to restore a decent quality of life. Anticancer Res. 2020;40(12):6997–7001. doi: 10.21873/anticanres.14724. [DOI] [PubMed] [Google Scholar]
- 104.Germán-Castelán L, Manjarrez-Marmolejo J, González-Arenas A, Camacho-Arroyo I. Intracellular progesterone receptor mediates the increase in glioblastoma growth induced by progesterone in the rat brain. Archiv Med Res. 2016;47(6):419–426. doi: 10.1016/j.arcmed.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 105.González-Agüero G, Gutiérrez AA, González-Espinosa D, Solano JD, Morales R, González-Arenas A, Cabrera-Munoz E, Camacho-Arroyo I. Progesterone effects on cell growth of U373 and D54 human astrocytoma cell lines. Endocrine. 2007;32(2):129–135. doi: 10.1007/s12020-007-9023-0. [DOI] [PubMed] [Google Scholar]
- 106.Piña-Medina AG, Hansberg-Pastor V, González-Arenas A, Cerbón M, Camacho-Arroyo I. Progesterone promotes cell migration, invasion and cofilin activation in human astrocytoma cells. Steroids. 2016;105:19–25. doi: 10.1016/j.steroids.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 107.Pereda MP, Hopfner U, Pagotto U, Renner U, Uhl E, Arzt E, Missale C, Stalla GK. Retinoic acid stimulates meningioma cell adhesion to the extracellular matrix and inhibits invasion. Br J Cancer. 1999;81(3):381–386. doi: 10.1038/sj.bjc.6690705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grunberg SM, Weiss MH. Lack of efficacy of megestrol acetate in the treatment of unresectable meningioma. J Neuro-oncol. 1990;8(1):61–65. doi: 10.1007/BF00182088. [DOI] [PubMed] [Google Scholar]
- 109.Lamberts SW, Tanghe HL, Avezaat CJ, Braakman R, Wijngaarde R, Koper JW, De Jong H. Mifepristone (RU 486) treatment of meningiomas. J Neurol Neurosurg Psychiatry. 1992;55(6):486–490. doi: 10.1136/jnnp.55.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ji J, Sundquist J, Sundquist K. Association of tamoxifen with meningioma: a population-based study in Sweden. Euro J Cancer Prevent. 2016;25(1):29. doi: 10.1097/CEJ.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rammo R, Rock A, Transou A, Raghunathan A, Rock J. Anaplastic meningioma: octreotide therapy for a case of recurrent and progressive intracranial disease. J Neurosurg. 2016;124(2):496–500. doi: 10.3171/2015.1.JNS142260. [DOI] [PubMed] [Google Scholar]
- 112.Norden AD, Ligon KL, Hammond SN, Muzikansky A, Reardon DA, Kaley TJ, Batchelor TT, Plotkin SR, Raizer JJ, Wong ET, et al. Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology. 2015;84:280–286. doi: 10.1212/WNL.0000000000001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roelfsema F, Biermasz NR, Pereira AM, Romijn JM. Nanomedicines in the treatment of acromegaly: focus on pegvisomant. Int J Nanomed. 2006;1(4):385. doi: 10.2147/nano.2006.1.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Puduvalli VK, Li JT, Chen L, McCutcheon IE. Induction of apoptosis in primary meningioma cultures by fenretinide. Cancer Res. 2005;65(4):1547–1553. doi: 10.1158/0008-5472.CAN-04-0786. [DOI] [PubMed] [Google Scholar]
- 115.Proctor DT, Patel Z, Lama S, Resch L, Van Marle G, Sutherland GR. Identification of PD-L2, B7–H3 and CTLA-4 immune checkpoint proteins in genetic subtypes of meningioma. Oncoimmunology. 2019;8:e1512943. doi: 10.1080/2162402X.2018.1512943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baia GS, Caballero OL, Ho JS, Zhao Q, Cohen T, Binder ZA, Salmasi V, Gallia GL, Quinones-Hinojosa A, Olivi A, Brem H. NY-ESO-1 expression in meningioma suggests a rationale for new immunotherapeutic approachesNY-ESO-1 expression in meningioma. Cancer Immunol Res. 2013;1(5):296–302. doi: 10.1158/2326-6066.CIR-13-0029. [DOI] [PubMed] [Google Scholar]
- 117.Han SJ, Reis G, Kohanbash G, Shrivastav S, Magill ST, Molinaro AM, McDermott MW, Theodosopoulos PV, Aghi MK, Berger MS, Butowski NA. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neuro-oncol. 2016;130(3):543–552. doi: 10.1007/s11060-016-2256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaba SE, DeMonte F, Bruner JM, Kyritsis AP, Jaeckle KA, Victor L, Yung WA. The treatment of recurrent unresectable and malignant meningiomas with interferon alpha-2B. Neurosurgery. 1997;40(2):271–275. doi: 10.1097/00006123-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 119.Giles AJ, Hao S, Padget M, Song H, Zhang W, Lynes J, Sanchez V, Liu Y, Jung J, Cao X, Fujii R. Efficient ADCC killing of meningioma by avelumab and a high-affinity natural killer cell line, haNK. JCI Insight. 2019 doi: 10.1172/jci.insight.130688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhi F, Zhou G, Wang S, Shi Y, Peng Y, Shao N, Guan W, Qu H, Zhang Y, Wang Q, Yang C. A microRNA expression signature predicts meningioma recurrence. Int J Cancer. 2013;132(1):128–136. doi: 10.1002/ijc.27658. [DOI] [PubMed] [Google Scholar]
- 121.Dalan AB, Gulluoglu S, Tuysuz EC, Kuskucu A, Yaltirik CK, Ozturk O, Ture U, Bayrak OF. Simultaneous analysis of miRNA-mRNA in human meningiomas by integrating transcriptome: A relationship between PTX3 and miR-29c. BMC Cancer. 2017;17(1):1–9. doi: 10.1186/s12885-017-3198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kliese N, Gobrecht P, Pachow D, Andrae N, Wilisch-Neumann A, Kirches E, Riek-Burchardt M, Angenstein F, Reifenberger G, Riemenschneider MJ, Meese E. miRNA-145 is downregulated in atypical and anaplastic meningiomas and negatively regulates motility and proliferation of meningioma cells. Oncogene. 2013;32(39):4712–4720. doi: 10.1038/onc.2012.468. [DOI] [PubMed] [Google Scholar]
- 123.Shi L, Jiang D, Sun G, Wan Y, Zhang S, Zeng Y, Pan T, Wang Z. miR-335 promotes cell proliferation by directly targeting Rb1 in meningiomas. J Neuro-oncol. 2012;110(2):155–162. doi: 10.1007/s11060-012-0951-z. [DOI] [PubMed] [Google Scholar]
- 124.Bush ML, Oblinger J, Brendel V, Santarelli G, Huang J, Akhmametyeva EM, Burns SS, Wheeler J, Davis J, Yates CW, Chaudhury AR. AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro Oncol. 2011;13(9):983–999. doi: 10.1093/neuonc/nor072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gerber MA, Bahr SM, Gutmann DH. Protein 4.1 B/differentially expressed in adenocarcinoma of the lung-1 functions as a growth suppressor in meningioma cells by activating Rac1-dependent c-Jun-NH2-kinase signaling. Cancer Res. 2006;66(10):5295–5303. doi: 10.1158/0008-5472.CAN-05-1628. [DOI] [PubMed] [Google Scholar]
- 126.Bachir S, Shah S, Shapiro S, Koehler A, Mahammedi A, Samy RN, Zuccarello M, Schorry E, Sengupta S. Neurofibromatosis type 2 (NF2) and the implications for vestibular schwannoma and meningioma pathogenesis. Int J Mol Sci. 2021;22(2):690. doi: 10.3390/ijms22020690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang X, Jia H, Lu Y, Dong C, Hou J, Wang Z, Wang F, Zhong H, Wang L, Wang K. Exome sequencing on malignant meningiomas identified mutations in neurofibromatosis type 2 (NF2) and meningioma 1 (MN1) genes. Discov Med. 2014;18(101):301. [PMC free article] [PubMed] [Google Scholar]
- 128.Liu Y, Shi J, Chen M, Cao YF, Liu YW, Pan J, Qi ST. Periostin: a novel prognostic predictor for meningiomas. J Neuro-oncol. 2015;121(3):505–512. doi: 10.1007/s11060-014-1678-9. [DOI] [PubMed] [Google Scholar]
- 129.Liu N, Song SY, Jiang JB, Wang TJ, Yan CX. The prognostic role of Ki-67/MIB-1 in meningioma: A systematic review with meta-analysis. Medicine. 2020 doi: 10.1097/MD.0000000000018644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Subeha MR, Telleria CM. The anti-cancer properties of the hiv protease inhibitor nelfinavir. Cancers. 2020;12(11):3437. doi: 10.3390/cancers12113437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Johnson MD, O’Connell M, Pilcher W. Lopinavir inhibits meningioma cell proliferation by Akt independent mechanism. J Neuro-oncol. 2011;101(3):441–448. doi: 10.1007/s11060-010-0281-y. [DOI] [PubMed] [Google Scholar]
- 132.Brastianos PK, Galanis E, Butowski N, Chan JW, Dunn IF, Goldbrunner R, Herold-Mende C, Ippen FM, Mawrin C, McDermott MW, Sloan A. Advances in multidisciplinary therapy for meningiomas. Neuro Oncol. 2019;21(1):i18–i31. doi: 10.1093/neuonc/noy136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jensen RL, Petr M, Wurster RD. Calcium channel antagonist effect on in vitro meningioma signal transduction pathways after growth factor stimulation. Neurosurgery. 2000;46(3):692–703. doi: 10.1097/00006123-200003000-00032. [DOI] [PubMed] [Google Scholar]
- 134.Gehring S, Tapia-Pérez JH, Kirches E, Firsching R, Keilhoff G, Schneider T, Mawrin C. Cytotoxic effects of statins and thiazolidinediones on meningioma cells. J Neuro-Oncol. 2011;102(3):383–393. doi: 10.1007/s11060-010-0351-1. [DOI] [PubMed] [Google Scholar]
- 135.Abarca-Merlin DM, Maldonado-Bernal C, Alvarez-Arellano L. Toll-like receptors as therapeutic targets in central nervous system tumors. BioMed Res Int. 2019 doi: 10.1155/2019/5286358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shankar GM, Abedalthagafi M, Vaubel RA, Merrill PH, Nayyar N, Gill CM, Brewster R, Bi WL, Agarwalla PK, Thorner AR, Reardon DA. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro Oncol. 2017;19(4):535–545. doi: 10.1093/neuonc/now235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kondraganti S, Gondi CS, Gujrati M, McCutcheon I, Dinh DH, Rao JS, Olivero WC. Restoration of tissue factor pathway inhibitor inhibits invasion and tumor growth in vitro and in vivo in a malignant meningioma cell line. Int J Oncol. 2006;29(1):25–32. doi: 10.3892/ijo.29.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schrell UM, Rittig MG, Anders M, Koch UH, Marschalek R, Kiesewetter F, Fahlbusch R. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. Neurosurg Focus. 1997 doi: 10.3171/foc.1997.2.4.12. [DOI] [PubMed] [Google Scholar]
- 139.Newton HB, Slivka MA, Stevens C. Hydroxyurea chemotherapy for unresectable or residual meningioma. J Neuro-oncol. 2000;49:165–170. doi: 10.1023/A:1026770624783. [DOI] [PubMed] [Google Scholar]
- 140.Mason WP, Gentili F, Macdonald DR, Hariharan S, Cruz CR, Abrey LE. Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningiomas. J Neurosurg. 2002;97(2):341–346. doi: 10.3171/jns.2002.97.2.0341. [DOI] [PubMed] [Google Scholar]
- 141.Rosenthal MA, Ashley DL, Cher L. Treatment of high risk or recurrent meningiomas with hydroxyurea. J Clin Neurosci. 2002;9(2):156–158. doi: 10.1054/jocn.2001.1019. [DOI] [PubMed] [Google Scholar]
- 142.Loven D, Hardoff R, Bar Sever Z, Steinmetz AP, Gornish M, Rappaport ZH, Fenig E, Ram Z, Sulkes A. Non-resectable slow-growing meningiomas treated by hydroxyurea. J Neuro-oncol. 2004;67:221–226. doi: 10.1023/B:NEON.0000021827.85754.8e. [DOI] [PubMed] [Google Scholar]
- 143.Newton HB, Scott SR, Volpi C. Hydroxyurea chemotherapy for meningiomas: enlarged cohort with extended follow-up. Br J Neurosurg. 2004;18(5):495–499. doi: 10.1080/02688690400012392. [DOI] [PubMed] [Google Scholar]
- 144.Hahn BM, Schrell UM, Sauer R, Fahlbusch R, Ganslandt O, Grabenbauer GG. Prolonged oral hydroxyurea and concurrent 3d-conformal radiation in patients with progressive or recurrent meningioma: results of a pilot study. J Neuro-oncol. 2005;74:157–165. doi: 10.1007/s11060-004-2337-3. [DOI] [PubMed] [Google Scholar]
- 145.Weston GJ, Martin AJ, Mufti GJ, Strong AJ, Gleeson MJ. Hydroxyurea treatment of meningiomas: a pilot study. Skull Base. 2006;16(03):157–160. doi: 10.1055/s-2006-949518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karsy M, Hoang N, Barth T, Burt L, Dunson W, Gillespie DL, Jensen RL. Combined hydroxyurea and verapamil in the clinical treatment of refractory meningioma: human and orthotopic xenograft studies. World Neurosurg. 2016;86:210–219. doi: 10.1016/j.wneu.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 147.Grunberg SM, Weiss MH, Spitz IM, Ahmadi J, Sadun A, Russell CA, Lucci L, Stevenson LL. Treatment of unresectable meningiomas with the antiprogesterone agent mifepristone. J Neurosurg. 1991;74(6):861–866. doi: 10.3171/jns.1991.74.6.0861. [DOI] [PubMed] [Google Scholar]
- 148.Grunberg SM, Weiss MH, Russell CA, Spitz IM, Ahmadi J, Sadun A, Sitruk-Ware R. Long-term administration of mifepristone (RU486): clinical tolerance during extended treatment of meningioma. Cancer Investig. 2006;24(8):727–733. doi: 10.1080/07357900601062339. [DOI] [PubMed] [Google Scholar]
- 149.Houshmandi SS, Emnett RJ, Giovannini M, Gutmann DH. The neurofibromatosis 2 protein, merlin, regulates glial cell growth in an ErbB2-and Src-dependent manner. Mol Cell Biol. 2009;29(6):1472–1486. doi: 10.1128/MCB.01392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Raizer JJ, Grimm SA, Rademaker A, Chandler JP, Muro K, Helenowski I, Rice L, McCarthy K, Johnston SK, Mrugala MM, Chamberlain M. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J Neuro-oncol. 2014;117:93–101. doi: 10.1007/s11060-014-1358-9. [DOI] [PubMed] [Google Scholar]
- 151.Karsy M, Guan J, Cohen A, Colman H, Jensen RL. Medical management of meningiomas: current status, failed treatments, and promising horizons. Neurosurg Clin. 2016;27(2):249–260. doi: 10.1016/j.nec.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 152.Dasanu CA, Samara Y, Codreanu I, Limonadi FM, Hamid O, Alvarez-Argote J. Systemic therapy for relapsed/refractory meningioma: Is there potential for antiangiogenic agents? J Oncol Pharm Pract. 2019;25(3):638–647. doi: 10.1177/1078155218799850. [DOI] [PubMed] [Google Scholar]
- 153.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yun S, Koh JM, Lee KS, Seo AN, Nam KH, Choe G. Expression of c-MET in invasive meningioma. J Pathol Transl Med. 2015;49(1):44–51. doi: 10.4132/jptm.2014.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Preusser M, Brastianos PK, Mawrin C. Advances in meningioma genetics: novel therapeutic opportunities. Nat Rev Neurol. 2018;14(2):106–115. doi: 10.1038/nrneurol.2017.168. [DOI] [PubMed] [Google Scholar]
- 156.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 157.Carneiro BA, Cavalcante L, Bastos BR, Powell SF, Ma WW, Sahebjam S, Harvey D, De Souza AL, Dhawan MS, Safran H, Giles FJ. Phase I study of 9-ing-41, a small molecule selective glycogen synthase kinase-3 beta (GSK-3β) inhibitor, as a single agent and combined with chemotherapy, in patients with refractory tumors. J Clin Oncol. 2020 doi: 10.1200/JCO.2020.38.15_suppl.3507. [DOI] [Google Scholar]
- 158.Theeler BJ, Jung J, Burton E, Leeper H, Wu J, Zaghloul K, Ray-Chaudhury A, Quezado M, Raffeld M, Yuan Y, Aldape KD. First-in-human dose escalation and food effect study of oral ONC206 in adults with recurrent primary CNS neoplasms. J Clin Oncol. 2021 doi: 10.1200/JCO.2021.39.15_suppl.TPS2072. [DOI] [Google Scholar]
- 159.Du Z, Abedalthagafi M, Aizer AA, McHenry AR, Sun HH, Bray MA, Viramontes O, Machaidze R, Brastianos PK, Reardon DA, Dunn IF. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget. 2015;6(7):4704. doi: 10.18632/oncotarget.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wild D, Fani M, Fischer R, Del Pozzo L, Kaul F, Krebs S, Rivier JE, Reubi JC, Maecke HR, Weber WA. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J Nuclear Med. 2014;55(8):1248–1252. doi: 10.2967/jnumed.114.138834. [DOI] [PubMed] [Google Scholar]
- 161.Zhao G, Rodriguez BL. Molecular targeting of liposomal nanoparticles to tumor microenvironment. Int J Nanomed. 2012;28:61–71. doi: 10.2147/IJN.S37859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tannock IF, Lee CM, Tunggal JK, Cowan DS, Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res. 2002;8(3):878–884. [PubMed] [Google Scholar]
- 163.Mousa SA, Bharali DJ. Nanotechnology-based detection and targeted therapy in cancer: nano-bio paradigms and applications. Cancers. 2011;3(3):2888–2903. doi: 10.3390/cancers3032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer: mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Euro J Pharm Sci. 2000;11(4):265–283. doi: 10.1016/S0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 165.Brown R, Links M. Clinical relevance of the molecular mechanisms of resistance to anti-cancer drugs. Exp Rev Mol Med. 1999;1(15):1–21. doi: 10.1017/S1462399499001099X. [DOI] [PubMed] [Google Scholar]
- 166.Gottesman MM, Hrycyna CA, Schoenlein PV, Germann UA, Pastan I. Genetic analysis of the multidrug transporter. Ann Rev Gene. 1995;29(1):607–649. doi: 10.1146/annurev.ge.29.120195.003135. [DOI] [PubMed] [Google Scholar]
- 167.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 168.Jadli AS, Ballasy N, Edalat P, Patel VB. Inside (sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem. 2020;467(1):77–94. doi: 10.1007/s11010-020-03703-z. [DOI] [PubMed] [Google Scholar]
- 169.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–294. doi: 10.1016/j.jconrel.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 170.Junttila MR, De Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 171.Ruivo CF, Adem B, Silva M, Melo SA. The biology of cancer exosomes: insights and new perspectivesbiology of cancer exosomes. Cancer Res. 2017;77(23):6480–6488. doi: 10.1158/0008-5472.CAN-17-0994. [DOI] [PubMed] [Google Scholar]
- 172.Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 173.Fujita Y, Kosaka N, Araya J, Kuwano K, Ochiya T. Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol Med. 2015;21(9):533–542. doi: 10.1016/j.molmed.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 174.Ma Z, Wang LZ, Cheng JT, Lam WS, Ma X, Xiang X, Wong AL, Goh BC, Gong Q, Sethi G, Wang L. Targeting hypoxia-inducible factor-1-mediated metastasis for cancer therapy. Antioxid Redox Signal. 2021;34(18):1484–1497. doi: 10.1089/ars.2019.7935. [DOI] [PubMed] [Google Scholar]
- 175.Shanmugam MK, Warrier S, Kumar AP, Sethi G, Arfuso F. Potential role of natural compounds as anti-angiogenic agents in cancer. Curr Vascular Pharmacol. 2017;15(6):503–519. doi: 10.2174/1570161115666170713094319. [DOI] [PubMed] [Google Scholar]
- 176.Ye J, Wu D, Wu P, Chen Z, Huang J. The cancer stem cell niche: cross talk between cancer stem cells and their microenvironment. Tumor Biol. 2014;35(5):3945–3951. doi: 10.1007/s13277-013-1561-x. [DOI] [PubMed] [Google Scholar]
- 177.Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Sig Transduct Targeted Ther. 2020;5(1):1–6. doi: 10.1038/s41392-020-00280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Datta A, Deng S, Gopal V, Yap KC, Halim CE, Lye ML, Ong MS, Tan TZ, Sethi G, Hooi SC, Kumar AP. Cytoskeletal dynamics in epithelial-mesenchymal transition: insights into therapeutic targets for cancer metastasis. Cancers. 2021;13(8):1882. doi: 10.3390/cancers13081882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cheng JT, Wang L, Wang H, Tang FR, Cai WQ, Sethi G, Xin HW, Ma Z. Insights into biological role of LncRNAs in epithelial-mesenchymal transition. Cells. 2019;8(10):1178. doi: 10.3390/cells8101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Moraes LA, Kar S, Foo SL, Gu T, Toh YQ, Ampomah PB, Sachaphibulkij K, Yap G, Zharkova O, Lukman HM, Fairhurst AM. Annexin-A1 enhances breast cancer growth and migration by promoting alternative macrophage polarization in the tumour microenvironment. Sci Rep. 2017;7(1):1–2. doi: 10.1038/s41598-017-17622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang Y, Guo Z, Chen W, Wang X, Cao M, Han X, Zhang K, Teng B, Cao J, Wu W, Cao P. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic ductal adenocarcinoma by targeting E2F2. Mol Ther. 2021;29(3):1226–1238. doi: 10.1016/j.ymthe.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dong F, Ruan S, Wang J, Xia Y, Le K, Xiao X, Hu T, Wang Q. M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. 2020;11(9):1–4. doi: 10.1038/s41419-020-02926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jung YY, Um JY, Nasif O, Alharbi SA, Sethi G, Ahn KS. Blockage of the JAK/STAT3 signaling pathway in multiple myeloma by leelamine. Phytomedicine. 2021;87:153574. doi: 10.1016/j.phymed.2021.153574. [DOI] [PubMed] [Google Scholar]
- 184.Mohan CD, Rangappa S, Nayak SC, Sethi G, Rangappa KS. Paradoxical functions of long noncoding RNAs in modulating STAT3 signaling pathway in hepatocellular carcinoma. Biochim Biophys Acta Rev Cancer. 2021;1876:188574. doi: 10.1016/j.bbcan.2021.188574. [DOI] [PubMed] [Google Scholar]
- 185.Garg M, Shanmugam MK, Bhardwaj V, Goel A, Gupta R, Sharma A, Baligar P, Kumar AP, Goh BC, Wang L, Sethi G. The pleiotropic role of transcription factor STAT3 in oncogenesis and its targeting through natural products for cancer prevention and therapy. Med Res Rev. 2021;41(3):1291–1336. doi: 10.1002/med.21761. [DOI] [PubMed] [Google Scholar]
- 186.Jung YY, Ha IJ, Um JY, Sethi G, Ahn KS. Fangchinoline diminishes STAT3 activation by stimulating oxidative stress and targeting SHP-1 protein in multiple myeloma model. J Adv Res. 2022;35:245–257. doi: 10.1016/j.jare.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee JH, Mohan CD, Deivasigamani A, Jung YY, Rangappa S, Basappa S, Chinnathambi A, Alahmadi TA, Alharbi SA, Garg M, Lin ZX. Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J Adv Res. 2020;26:83–94. doi: 10.1016/j.jare.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu J, Zhang J, Zhang Z, Gao Z, Qi Y, Qiu W, Pan Z, Guo Q, Li B, Zhao S, Guo X. Hypoxic glioma-derived exosomes promote M2-like macrophage polarization by enhancing autophagy induction. Cell Death Dis. 2021;12(4):1–6. doi: 10.1038/s41419-021-03664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pace KR, Dutt R, Galileo DS. Exosomal L1CAM stimulates glioblastoma cell motility, proliferation, and invasiveness. Int J Mol Sci. 2019;20(16):3982. doi: 10.3390/ijms20163982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lane AN, Higashi RM, Fan TW. Metabolic reprogramming in tumors: contributions of the tumor microenvironment. Gene Dis. 2020;7(2):185–198. doi: 10.1016/j.gendis.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ji K, Mayernik L, Moin K, Sloane BF. Acidosis and proteolysis in the tumor microenvironment. Cancer Metastasis Rev. 2019;38(1):103–112. doi: 10.1007/s10555-019-09796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bhat AR, Wani MA, Kirmani AR, Ramzan AU. Histological-subtypes and anatomical location correlated in meningeal brain tumors (meningiomas) J Neurosci Rural Pract. 2014;5(03):244–249. doi: 10.1055/s-0039-1700321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Abdelrahman A, Negroni C, Sahm F, Adams CL, Urbanic-Purkart T, Khalil M, Vergura R, Morelli C, Hanemann CO. miR-497 and 219 in blood aid meningioma classification. J Neuro-Oncol. 2022;8:1–1. doi: 10.1007/s11060-022-04126-0. [DOI] [PubMed] [Google Scholar]
- 195.Fu XH, Li JP, Li XY, Tan Y, Zhao M, Zhang SF, Wu XD, Xu JG. M2-macrophage-derived exosomes promote meningioma progression through TGF-β signaling pathway. J Immunol Res. 2022 doi: 10.1155/2022/8326591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sofela AA, Hilton DA, Ammoun S, Baiz D, Adams CL, Ercolano E, Jenkinson MD, Kurian KM, Teo M, Whitfield PC, Sahm F. Fibulin-2: a novel biomarker for differentiating grade II from grade I meningiomas. Int J Mol Sci. 2021;22(2):560. doi: 10.3390/ijms22020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhi F, Shao N, Li B, Xue L, Deng D, Xu Y, Lan Q, Peng Y, Yang Y. A serum 6-miRNA panel as a novel non-invasive biomarker for meningioma. Sci Rep. 2016;6(1):1. doi: 10.1038/srep32067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126(4):1216–1223. doi: 10.1172/JCI81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xie F, Zhou X, Fang M, Li H, Su P, Tu Y, Zhang L, Zhou F. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci. 2019;6(24):1901779. doi: 10.1002/advs.201901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, Vishwanatha JK. Exosomal annexin II promotes angiogenesis and breast cancer metastasisexosomal Anx II in angiogenesis and metastasis. Mol Cancer Res. 2017;15(1):93–105. doi: 10.1158/1541-7786.MCR-16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chiba M, Kubota S, Sato K, Monzen S. Exosomes released from pancreatic cancer cells enhance angiogenic activities via dynamin-dependent endocytosis in endothelial cells in vitro. Sci Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-30446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lang HL, Hu GW, Zhang B, Kuang W, Chen Y, Wu L, Xu GH. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol Rep. 2017;38(2):785–798. doi: 10.3892/or.2017.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Emmanouilidi A, Paladin D, Greening DW, Falasca M. Oncogenic and non-malignant pancreatic exosome cargo reveal distinct expression of oncogenic and prognostic factors involved in tumor invasion and metastasis. Proteomics. 2019;19(8):1800158. doi: 10.1002/pmic.201800158. [DOI] [PubMed] [Google Scholar]
- 204.Li M, Lu Y, Xu Y, Wang J, Zhang C, Du Y, Wang L, Li L, Wang B, Shen J, Tang J. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene. 2018;676:101–109. doi: 10.1016/j.gene.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 205.Chen Y, Zeng C, Zhan Y, Wang H, Jiang X, Li W. Aberrant low expression of p85α in stromal fibroblasts promotes breast cancer cell metastasis through exosome-mediated paracrine Wnt10b. Oncogene. 2017;36(33):4692–4705. doi: 10.1038/onc.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Develop Cell. 2019;49(3):347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 208.Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, Schmandt R. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7(1):1–4. doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, Ter Brugge PJ. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Barros FM, Carneiro F, Machado JC, Melo SA. Exosomes and immune response in cancer: friends or foes? Front Immunol. 2018;9:730. doi: 10.3389/fimmu.2018.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13(10):739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 213.Elkord E, Alcantar-Orozco EM, Dovedi SJ, Tran DQ, Hawkins RE, Gilham DE. T regulatory cells in cancer: recent advances and therapeutic potential. Exp Opinion Biol Therapy. 2010;10(11):1573–1586. doi: 10.1517/14712598.2010.529126. [DOI] [PubMed] [Google Scholar]
- 214.Paluskievicz CM, Cao X, Abdi R, Zheng P, Liu Y, Bromberg JS. T regulatory cells and priming the suppressive tumor microenvironment. Front Immunol. 2019;10:2453. doi: 10.3389/fimmu.2019.02453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PLoS ONE. 2010;5(7):e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ma Y, Shurin GV, Peiyuan Z, Shurin MR. Dendritic cells in the cancer microenvironment. J Cancer. 2013;4(1):36. doi: 10.7150/jca.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:14–29. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 218.Huang SH, Li Y, Zhang J, Rong J, Ye S. Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Investig. 2013;31(5):330. doi: 10.3109/07357907.2013.789905. [DOI] [PubMed] [Google Scholar]
- 219.Maimela NR, Liu S, Zhang Y. Fates of CD8+ T cells in tumor microenvironment. Comput Struct Biotechnol J. 2019;17:1–3. doi: 10.1016/j.csbj.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T, Taniguchi K, Ito Y, Akao Y. Regulated polarization of tumor-associated macrophages by mir-145 via colorectal cancer–derived extracellular vesicles. J Immunol. 2017;199(4):1505–1515. doi: 10.4049/jimmunol.1700167. [DOI] [PubMed] [Google Scholar]
- 221.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 222.Bercovici N, Guérin MV, Trautmann A, Donnadieu E. The remarkable plasticity of macrophages: a chance to fight cancer. Front Immunol. 2019;10:1563. doi: 10.3389/fimmu.2019.01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Geng L, Huang D, Liu J, Qian Y, Deng J, Li D, Hu Z, Zhang J, Jiang G, Zheng S. B7–H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol. 2008;134(9):1021–1027. doi: 10.1007/s00432-008-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Choueiri TK, Figueroa DJ, Fay AP, Signoretti S, Liu Y, Gagnon R, Deen K, Carpenter C, Benson P, Ho TH, Pandite L. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled TrialPD-L1 correlation with outcome in RCC patients in COMPARZ. Clin Cancer Res. 2015;21(5):1071–1077. doi: 10.1158/1078-0432.CCR-14-1993. [DOI] [PubMed] [Google Scholar]
- 225.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28(3):682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 226.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, Hoshino T. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25(10):1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 227.Ghebeh H, Mohammed S, Al-Omair A, Qattant A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Amer SB, Tulbah A, Ajarim D. The B7–H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108(1):19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 230.Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu GL, Luo H, Yang YX, Dai XY, Zhou SF, Wang D. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Design Develop Therapy. 2015;9:901. doi: 10.2147/DDDT.S75152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hou J, Yu Z, Xiang R, Li C, Wang L, Chen S, Li Q, Chen M, Wang L. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7–H1 in the tumor tissues of gastric cancer. Exp Mol Pathol. 2014;96(3):284–291. doi: 10.1016/j.yexmp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 232.Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Modern Pathol. 2016;29(9):1104–1112. doi: 10.1038/modpathol.2016.95. [DOI] [PubMed] [Google Scholar]
- 233.Xie F, Xu M, Lu J, Mao L, Wang S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer. 2019 doi: 10.1186/s12943-019-1074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Brahmer JR, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18(12):1600–1609. doi: 10.1016/S1470-2045(17)30690-3. [DOI] [PubMed] [Google Scholar]
- 235.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, Britten CD, Dirix L, Lee KW, Taylor M, Schöffski P. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51–64. doi: 10.1016/S1470-2045(17)30900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, Lee JL, Ong M, Sridhar SS, Vogelzang NJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3:e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 239.Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R, Manzuk L, Piha-Paul SA, Xu L, Zeigenfuss S, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37:1470–1478. doi: 10.1158/1078-0432.CCR-19-3014. [DOI] [PubMed] [Google Scholar]
- 240.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 242.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1271. doi: 10.1056/NEJMoa1504030. [DOI] [PubMed] [Google Scholar]
- 243.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, Grob JJ. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 245.Namee NM, O’Driscoll L. Extracellular vesicles and anti-cancer drug resistance. Biochim Biophys Acta Rev Cancer. 2018;1870:123–136. doi: 10.1016/j.bbcan.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 246.Yi M, Xu L, Jiao Y, Luo S, Li A, Wu K. The role of cancer-derived microRNAs in cancer immune escape. J Hematol Oncol. 2020;13(1):1–4. doi: 10.1186/s13045-020-00848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gacche RN, Assaraf YG. Redundant angiogenic signaling and tumor drug resistance. Drug Res Updates. 2018;36:47–76. doi: 10.1016/j.drup.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 248.Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: an overview. Cancers. 2014;6(3):1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Alharbi M, Zuñiga F, Elfeky O, Guanzon D, Lai A, Rice GE, Perrin L, Hooper J, Salomon C. The potential role of miRNAs and exosomes in chemotherapy in ovarian cancer. Endocr Relat Cancer. 2018;25(12):R663–R685. doi: 10.1530/ERC-18-0019. [DOI] [PubMed] [Google Scholar]
- 250.Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, Mustea A, Sehouli J, Kristiansen G, Altevogt P. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007;107(3):563–571. doi: 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 251.Lässer C, Seyed Alikhani V, Ekström K, Eldh M, Torregrosa Paredes P, Bossios A, Sjöstrand M, Gabrielsson S, Lötvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9(1):1–8. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, Hager HD, Abdel-Bakky MS, Gutwein P, Altevogt P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72(9):1095–1102. doi: 10.1038/sj.ki.5002486. [DOI] [PubMed] [Google Scholar]
- 253.Skog J, Würdinger T, Van Rijn S, Meijer DH, Gainche L, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yamashita T, Kamada H, Kanasaki S, Maeda Y, Nagano K, Abe Y, Inoue M, Yoshioka Y, Tsutsumi Y, Katayama S, Tsunoda S. Epidermal growth factor receptor localized to exosome membranes as a possible biomarker for lung cancer diagnosis. Pharm Int J Pharm Sci. 2013;68(12):969–973. doi: 10.1691/ph.2013.3599. [DOI] [PubMed] [Google Scholar]
- 255.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;7559:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pan C, Stevic I, Meuller V, Ni Q, Oliveira-Ferrer L, Pantel K, Schwarzenbach H. Exosomal micro RNA s as tumor markers in epithelial ovarian cancer. Mol Oncol. 2018 doi: 10.1002/1878-0261.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fang S, Tian H, Li X, Jin D, Li X, Kong J, Yang C, Yang X, Lu Y, Luo Y. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS ONE. 2017;12:e0175050. doi: 10.1371/journal.pone.0175050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lee SJ, Lee J, Jung JH, Park HY, Moon PG, Chae YS, et al. Exosomal del-1 as a potent diagnostic marker for breast cancer: prospective cohort study. Clin Breast Cancer. 2021 doi: 10.1016/j.clbc.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 259.Ahadi A, Khoury S, Losseva M, Tran N. A comparative analysis of lncRNAs in prostate cancer exosomes and their parental cell lines. Genom Data. 2016;9:7e9. doi: 10.1016/j.gdata.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. 2013;2(1):20424. doi: 10.3402/jev.v2i0.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Liu H, Chen L, Peng Y, Yu S, Liu J, Wu L, Zhang L, Wu Q, Chang X, Yu X, Liu T. Dendritic cells loaded with tumor derived exosomes for cancer immunotherapy. Oncotarget. 2018;9(2):2887. doi: 10.18632/oncotarget.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gomari H, Moghadam MF, Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. Onco Targets Ther. 2018;11:5753. doi: 10.2147/OTT.S173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Smalley DM, Sheman NE, Nelson K, Theodorescu D. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J Proteome Res. 2008;7(5):2088–2096. doi: 10.1021/pr700775x. [DOI] [PubMed] [Google Scholar]
- 264.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang J, Zheng Y, Zhao M. Exosome-based cancer therapy: implication for targeting cancer stem cells. Front Pharmacol. 2017;7:533. doi: 10.3389/fphar.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lin Q, Qu M, Zhou B, Patra HK, Sun Z, Luo Q, Yang W, Wu Y, Zhang Y, Li L, Deng L. Exosome-like nanoplatform modified with targeting ligand improves anti-cancer and anti-inflammation effects of imperialine. J Control Release. 2019;311:104–116. doi: 10.1016/j.jconrel.2019.08.037. [DOI] [PubMed] [Google Scholar]
- 267.Nie W, Wu G, Zhang J, Huang LL, Ding J, Jiang A, Zhang Y, Liu Y, Li J, Pu K, Xie HY. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angewandte Chem. 2020;132(5):2034–2038. doi: 10.1002/ange.201912524. [DOI] [PubMed] [Google Scholar]
- 268.Pullan JE, Confeld MI, Osborn JK, Kim J, Sarkar K, Mallik S. Exosomes as drug carriers for cancer therapy. Mol Pharm. 2019;16(5):1789–1798. doi: 10.1021/acs.molpharmaceut.9b00104. [DOI] [PubMed] [Google Scholar]
- 269.Gilligan KE, Dwyer RM. Engineering exosomes for cancer therapy. Int J Mol Sci. 2017;18(6):1122. doi: 10.3390/ijms18061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chulpanova DS, Kitaeva KV, James V, Rizvanov AA, Solovyeva VV. Therapeutic prospects of extracellular vesicles in cancer treatment. Front Immunol. 2018;9:1534. doi: 10.3389/fimmu.2018.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Marcus ME, Leonard JN. FedExosomes: engineering therapeutic biological nanoparticles that truly deliver. Pharmaceuticals. 2013;6(5):659–680. doi: 10.3390/ph6050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 273.Yang G, Chen Q, Wen D, Chen Z, Wang J, Chen G, Wang Z, Zhang X, Zhang Y, Hu Q, Zhang L. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano. 2019;13(4):4354–4360. doi: 10.1021/acsnano.8b09573. [DOI] [PubMed] [Google Scholar]
- 274.Maremanda KP, Sundar IK, Rahman I. Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. Toxicol Appl Pharmacol. 2019;385:114788. doi: 10.1016/j.taap.2019.114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sheller-Miller S, Choi K, Choi C, Menon R. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am J Obstet Gynecol. 2019 doi: 10.1016/j.ajog.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 276.Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Ströbel T, Breakefield XO, Saydam O. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Therapy. 2013;21(1):101–108. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S, Gupta RC. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed Nanotechnol Biol Med. 2017;13(5):1627–1636. doi: 10.1016/j.nano.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 278.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Daßler-Plenker J, Küttner V, Egeblad M. Communication in tiny packages: exosomes as means of tumor-stroma communication. Biochim Biophys Acta Rev Cancer. 2020;1873:188340. doi: 10.1016/j.bbcan.2020.188340. [DOI] [PubMed] [Google Scholar]
- 280.Guescini M, Guidolin D, Vallorani L, Casadei L, Gioacchini AM, Tibollo P, Battistelli M, Falcieri E, Battistin L, Agnati LF, Stocchi V. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res. 2010;316(12):1977–1984. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 281.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2(1):180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289(7):3869–3675. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yokoi A, Villar-Prados A, Oliphint PA, Zhang J, Song X, De Hoff P, Morey R, Liu J, Roszik J, Clise-Dwyer K, Burks JK. Mechanisms of nuclear content loading to exosomes. Sci Adv. 2019 doi: 10.1126/sciadv.aax8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Andreeva OE, Shchegolev YY, Scherbakov AM, et al. Secretion of mutant DNA and mRNA by the exosomes of breast cancer cells. Molecules. 2021;26:2499. doi: 10.3390/molecules26092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kamerkar S, Burzyn D, Leng C, Burenkova O, Jang SC, Yang R, Boutin A, Kirwin K, Zi T, Dahlberg W, Zhang E. Reprogramming of tumor associated M2 macrophages with antisense oligonucleotide-loaded exosomes results in potent single-agent antitumor activity. Cancer Immunol Res. 2020;8(3):56. [Google Scholar]
- 286.Kim SM, Yang Y, Oh SJ, Hong Y, Seo M, Jang M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J Control Release. 2017;266:8–16. doi: 10.1016/j.jconrel.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 287.Wang Y, Shahi PK, Xie R, Zhang H, Abdeen AA, Yodsanit N, Ma Z, Saha K, Pattnaik BR, Gong S. A pH-responsive silica–metal–organic framework hybrid nanoparticle for the delivery of hydrophilic drugs, nucleic acids, and CRISPR-Cas9 genome-editing machineries. J Control Release. 2020;324:194–203. doi: 10.1016/j.jconrel.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tong S, Moyo B, Lee CM, Leong K, Bao G. Engineered materials for in vivo delivery of genome-editing machinery. Nat Rev Mater. 2019;4(11):726–737. doi: 10.1038/s41578-019-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wang JH, Forterre AV, Zhao J, Frimannsson DO, Delcayre A, Antes TJ, Efron B, Jeffrey SS, Pegram MD, Matin AC. Anti-HER2 scFv-directed extracellular vesicle-mediated mRNA-based gene delivery inhibits growth of HER2-positive human breast tumor xenografts by prodrug activation. Mol Cancer Ther. 2018;17(5):1133–1142. doi: 10.1158/1535-7163.MCT-17-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Usman WM, Pham TC, Kwok YY, Vu LT, Ma V, Peng B, Chan YS, Wei L, Chin SM, Azad A, He AB. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun. 2018;9(1):2359. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, Zhao Y, Zhao X, Wang X, Ma Y, Malkoc V. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4(1):69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Rezaei R, Baghaei K, Amani D, Piccin A, Hashemi SM, Aghdaei HA, Zali MR. Exosome-mediated delivery of functionally active miRNA-375–3p mimic regulate epithelial mesenchymal transition (EMT) of colon cancer cells. Life Sci. 2021;269:119035. doi: 10.1016/j.lfs.2021.119035. [DOI] [PubMed] [Google Scholar]
- 293.Kirave P, Gondaliya P, Kulkarni B, Rawal R, Garg R, Jain A, Kalia K. Exosome mediated miR-155 delivery confers cisplatin chemoresistance in oral cancer cells via epithelial-mesenchymal transition. Oncotarget. 2020;11(13):1157. doi: 10.18632/oncotarget.27531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Liang G, Kan S, Zhu Y, Feng S, Feng W, Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HepG2 cells. Int J Nanomed. 2018;13:585. doi: 10.2147/IJN.S154458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhou W, Xu M, Wang Z, Yang M. Engineered exosomes loaded with miR-449a selectively inhibit the growth of homologous non-small cell lung cancer. Cancer Cell Int. 2021;21(1):1–1. doi: 10.1186/s12935-021-02157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, Sun B, Chen B, Xiao Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnol. 2020;18(1):1–5. doi: 10.1186/s12951-019-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yamada R, Nakano I. Glioma stem cells: their role in chemoresistance. World Neurosurg. 2012;77(2):237–240. doi: 10.1016/j.wneu.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 298.Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of functional anti-miR-9 by mesenchymal stem cell–derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ren Y, Ji N, Kang X, Wang R, Ma W, Hu Z, Liu X, Wang Y. Aberrant ceRNA-mediated regulation of KNG1 contributes to glioblastoma-induced angiogenesis. Oncotarget. 2016;5:1–22. [Google Scholar]
- 301.Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. 2013;14(7):14240–14269. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Deng F, Miller J. A review on protein markers of exosome from different bio-resources and the antibodies used for characterization. J Histotechnol. 2019;42(4):226–239. doi: 10.1080/01478885.2019.1646984. [DOI] [PubMed] [Google Scholar]
- 303.Von Schulze A, Deng F. A review on exosome-based cancer therapy. J Cancer Metastasis Treat. 2020;6:42. doi: 10.20517/2394-4722.2020.79. [DOI] [Google Scholar]
- 304.Bruschi M, Ravera S, Santucci L, Candiano G, Bartolucci M, Calzia D, Lavarello C, Inglese E, Petretto A, Ghiggeri G, Panfoli I. The human urinary exosome as a potential metabolic effector cargo. Exp Rev Proteomics. 2015;12(4):425–432. doi: 10.1586/14789450.2015.1055324. [DOI] [PubMed] [Google Scholar]
- 305.Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F, Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhang K, Dong C, Chen M, Yang T, Wang X, Gao Y, Wang L, Wen Y, Chen G, Wang X, Yu X. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10(1):411. doi: 10.7150/thno.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wang F, Li L, Piontek K, Sakaguchi M, Selaru FM. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology. 2018;67(3):940–954. doi: 10.1002/hep.29586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.O’brien KP, Khan S, Gilligan KE, Zafar H, Lalor P, Glynn C, O’Flatharta C, Ingoldsby H, Dockery P, De Bhulbh A, et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37:2137–2149. doi: 10.1038/s41388-017-0116-9. [DOI] [PubMed] [Google Scholar]
- 309.Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y, Cui Q, Mei W, Li F. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145–5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2019;442:351–361. doi: 10.1016/j.canlet.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 310.Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, Hu J. Cancer-derived exosomal miR-25–3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1):1–4. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Rountree RB, Mandl SJ, Nachtwey JM, Dalpozzo K, Do L, Lombardo JR, Schoonmaker PL, Brinkmann K, Dirmeier U, Laus R, Delcayre A. Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacyexosome targeting of tumor antigens improves efficacy. Cancer Res. 2011;71(15):5235–5244. doi: 10.1158/0008-5472.CAN-10-4076. [DOI] [PubMed] [Google Scholar]
- 312.Kaban K, Hinterleitner C, Zhou Y, Salva E, Kantarci AG, Salih HR, Märklin M. Therapeutic silencing of BCL-2 using NK cell-derived exosomes as a novel therapeutic approach in breast cancer. Cancers. 2021;13(10):2397. doi: 10.3390/cancers13102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Greco KA, Franzen CA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN. PLK-1 silencing in bladder cancer by siRNA delivered with exosomes. Urology. 2016;91:241.e1. doi: 10.1016/j.urology.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 314.Li H, Yang C, Shi Y, Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J Nanobiotechnol. 2018;16:1–3. doi: 10.1186/s12951-018-0429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zhang Q, Zhang H, Ning T, Liu D, Deng T, Liu R, Bai M, Zhu K, Li J, Fan Q, Ying G. Exosome-delivered c-Met siRNA could reverse chemoresistance to cisplatin in gastric cancer. Int J Nanomed. 2020;1:2323–2335. doi: 10.2147/IJN.S231214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Lin D, Zhang H, Liu R, Deng T, Ning T, Bai M, Yang Y, Zhu K, Wang J, Duan J, Ge S. iRGD-modified exosomes effectively deliver CPT1A siRNA to colon cancer cells, reversing oxaliplatin resistance by regulating fatty acid oxidation. Mol Oncol. 2021;15(12):3430–3446. doi: 10.1002/1878-0261.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Xue J, Liu Y, Luo F, Lu X, Xu H, Liu X, Lu L, Yang Q, Chen C, Fan W. Liu Q (2017) Circ100284, via miR-217 regulation of EZH2, is involved in the arsenite-accelerated cell cycle of human keratinocytes in carcinogenesis. Biochim Biophys Acta Mol Basis Dis. 1863;3:753–763. doi: 10.1016/j.bbadis.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 318.Lang HL, Hu GW, Chen Y, Liu Y, Tu W, Lu YM, Wu L, Xu GH. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur Rev Med Pharmacol Sci. 2017;21(5):959–972. [PubMed] [Google Scholar]
- 319.Zheng R, Du M, Wang X, Xu W, Liang J, Wang W, Lv Q, Qin C, Chu H, Wang M, Yuan L. Exosome–transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol Cancer. 2018;17:1–3. doi: 10.1186/s12943-018-0880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.André F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, Tursz T. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172(4):2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 321.Chaput N, Schartz NE, André F, Taïeb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, Adema G. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172(4):2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 322.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 323.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271(22):12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 324.Dai S, Zhou X, Wang B, Wang Q, Fu Y, Chen T, Wan T, Yu Y, Cao X. Enhanced induction of dendritic cell maturation and HLA-A* 0201-restricted CEA-specific CD8+ CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells. J Mol Med. 2006;84(12):1067–1076. doi: 10.1007/s00109-006-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, Koh JS, Kim YN, Kim CW. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J cancer. 2005;114(4):613–622. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- 326.Kooijmans SA, Aleza CG, Roffler SR, van Solinge WW, Vader P, Schiffelers RM. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;5(1):31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Hall J, Prabhakar S, Balaj L, Lai CP, Cerione RA, Breakefield XO. Delivery of therapeutic proteins via extracellular vesicles: review and potential treatments for Parkinson’s disease, glioma, and schwannoma. Cell Mol Neurobiol. 2016;36(3):417–427. doi: 10.1007/s10571-015-0309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hung ME, Leonard JN. Stabilization of exosome-targeting peptides via engineered glycosylation. J Biol Chem. 2015;290(13):8166–8172. doi: 10.1074/jbc.M114.621383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3(1):23743. doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Malhotra H, Sheokand N, Kumar S, Chauhan AS, Kumar M, Jakhar P, Boradia VM, Raje CI, Raje M. Exosomes: tunable nano vehicles for macromolecular delivery of transferrin and lactoferrin to specific intracellular compartment. J Biomed Nanotechnol. 2016;12(5):1101–1114. doi: 10.1166/jbn.2016.2229. [DOI] [PubMed] [Google Scholar]
- 331.Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, De Kleijn DP, Choo A, Lim SK. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics. 2012 doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Satake T, Suetsugu A, Nakamura M, Kunisada T, Saji S, Moriwaki H, Shimizu M, Hoffman RM. Color-coded imaging of the fate of cancer-cell-derived exosomes during pancreatic cancer metastases in a nude-mouse model. Anticancer Res. 2019;39(8):4055–4060. doi: 10.21873/anticanres.13561. [DOI] [PubMed] [Google Scholar]
- 333.Wahlgren J, Karlson TD, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17):e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, Kabanov AV. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–2. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Zarovni N, Corrado A, Guazzi P, Zocco D, Lari E, Radano G, Muhhina J, Fondelli C, Gavrilova J, Chiesi A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46–58. doi: 10.1016/j.ymeth.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 336.Tang P, Tao L, Yuan C, Zhang L, Xiu D. Serum derived exosomes from pancreatic cancer patients promoted metastasis: an iTRAQ-based proteomic analysis. Onco Targets Ther. 2019;12:9329. doi: 10.2147/OTT.S229494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Han S, Huo Z, Nguyen K, Zhu F, Underwood PW, Basso KB, George TJ, Hughes SJ. The proteome of pancreatic cancer-derived exosomes reveals signatures rich in key signaling pathways. Proteomics. 2019;19(13):1800394. doi: 10.1002/pmic.201800394. [DOI] [PubMed] [Google Scholar]
- 338.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat Med. 1998;5(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 339.Kim OY, Lee J, Gho YS. Extracellular vesicle mimetics: novel alternatives to extracellular vesicle-based theranostics, drug delivery, and vaccines. Semin Cell Develop Biol. 2017;67:74–82. doi: 10.1016/j.semcdb.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 340.De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed. 2008;3(2):133. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64(5):1020–1037. doi: 10.1016/S1734-1140(12)70901-5. [DOI] [PubMed] [Google Scholar]
- 342.Li D, Yao S, Zhou Z, Shi J, Huang Z, Wu Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydrate Res. 2020;493:108032. doi: 10.1016/j.carres.2020.108032. [DOI] [PubMed] [Google Scholar]
- 343.Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, Kabanov AV, Batrakova EV. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomed Nanotechnol Biol Med. 2018;14(1):195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 344.Aqil F, Kausar H, Agrawal AK, Jeyabalan J, Kyakulaga AH, Munagala R, Gupta R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp Mol Pathol. 2016;101(1):12–21. doi: 10.1016/j.yexmp.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 345.Munagala R, Aqil F, Jeyabalan J, Agrawal AK, Mudd AM, Kyakulaga AH, Singh IP, Vadhanam MV, Gupta RC. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017;393:94–102. doi: 10.1016/j.canlet.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Aqil F, Jeyabalan J, Agrawal AK, Kyakulaga AH, Munagala R, Parker L, Gupta RC. Exosomal delivery of berry anthocyanidins for the management of ovarian cancer. Food Funct. 2017;8(11):4100–4107. doi: 10.1039/C7FO00882A. [DOI] [PubMed] [Google Scholar]
- 349.Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677–2681. doi: 10.1091/mbc.e14-04-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle-and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;28(220):727–737. doi: 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 351.Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F, Hu C, Zhang L, Guo H, Gao S. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomed Nanotechnol Biol Med. 2018;14(7):1973–1985. doi: 10.1016/j.nano.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 352.Ye Z, Zhang T, He W, Jin H, Liu C, Yang Z, Ren J. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl Mater Interfaces. 2018;10(15):12341–12350. doi: 10.1021/acsami.7b18135. [DOI] [PubMed] [Google Scholar]
- 353.Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, Xiang DX. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020;1(101):519–530. doi: 10.1016/j.actbio.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 354.Antonio CE. Oncolytic viruses. Nat Rev Cancer. 2002;2(12):938–950. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 355.Garofalo M, Saari H, Somersalo P, Crescenti D, Kuryk L, Aksela L, Capasso C, Madetoja M, Koskinen K, Oksanen T, Mäkitie A. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J Control Release. 2018;10(283):223–234. doi: 10.1016/j.jconrel.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 356.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ullah M, Liu DD, Thakor AS. Mesenchymal stromal cell homing: mechanisms and strategies for improvement. Iscience. 2019;31(15):421–438. doi: 10.1016/j.isci.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Altanerova U, Jakubechova J, Benejova K, Priscakova P, Pesta M, Pitule P, Topolcan O, Kausitz J, Zduriencikova M, Repiska V, Altaner C. Prodrug suicide gene therapy for cancer targeted intracellular by mesenchymal stem cell exosomes. Int J Cancer. 2019;144(4):897–908. doi: 10.1002/ijc.31792. [DOI] [PubMed] [Google Scholar]
- 359.Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 360.Niu W, Xiao Q, Wang X, Zhu J, Li J, Liang X, Peng Y, Wu C, Lu R, Pan Y, Luo J. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. doi: 10.1021/acs.nanolett.0c04753. [DOI] [PubMed] [Google Scholar]
- 361.Shen J, Hu Y, Putt KS, Singhal S, Han H, Visscher DW, Murphy LM, Low PS. Assessment of folate receptor alpha and beta expression in selection of lung and pancreatic cancer patients for receptor targeted therapies. Oncotarget. 2018;9(4):4485. doi: 10.18632/oncotarget.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106(3):818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, Jones JC. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;1(105):195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, Shiku H, Akiyoshi K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6(1):1–1. doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;10(207):18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, Fu Y, Yang S, Zhang Z, Zhang L, Sun X. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson's disease. J Control Release. 2018;10(287):156–166. doi: 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 367.Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Molr Ther Nucleic Acids. 2017;16(7):278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Yang T, Fogarty B, LaForge B, Aziz S, Pham T, Lai L, Bai S. Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J. 2017;19(2):475–486. doi: 10.1208/s12248-016-0015-y. [DOI] [PubMed] [Google Scholar]
- 369.Kim G, Kim M, Lee Y, Byun JW, Lee M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J Control Release. 2020;10(317):273–281. doi: 10.1016/j.jconrel.2019.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.