To The Editor:
Alveolar echinococcosis caused by Echinococcus multilocularis, a small cyclophyllid tapeworm, is a severe parasitic disease in humans. Two years after the diagnosis of a European haplotype of E. multilocularis in a patient in Vermont (Patient VT1) who did not have a history of exposure to alveolar echinococcosis abroad,1 the disease was diagnosed in a second patient in Vermont (Patient VT2), who also did not have such exposure history. Details regarding the two cases are provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
Patient VT2 was an 82-year-old man with memory decline who presented with painless jaundice. The laboratory evaluation revealed a total bilirubin level of 15.4 mg per deciliter (263 μmol per liter), an alkaline phosphatase level of 573 U per liter, an aspartate aminotransferase level of 156 U per liter, and an alanine aminotransferase level of 190 U per liter. Abdominal computed tomography revealed a mass-like hypodensity in the right lobe of the liver with intrahepatic biliary ductal dilatation and mass effect on the inferior vena cava. Biopsy of the mass revealed histologic features consistent with E. multilocularis. Immunohistochemical analysis of a liver-biopsy specimen was positive on testing with both EmG3 and Em2G11 monoclonal antibodies.2
With the diagnosis of two alveolar echinococcosis cases in humans, we sought to investigate whether an animal reservoir could be genetically linked to the human isolates. We screened stool samples obtained from 434 wild canids (296 foxes and 138 coyotes) from Virginia for E. multilocularis DNA, and two red foxes (fox146 and fox197) tested positive. We then sequenced DNA obtained from Patient VT1, fox146, and fox197 for NAD2, COB, and COX1, since the presence of COX1 had previously been identified in the sample obtained from Patient VT1.1 We had samples of formalin-fixed, paraffin-embedded tissue obtained from Patient VT2, which permitted only directed amplification of single-nucleotide polymorphisms (SNPs) in COB and COX1. Details regarding methods and GenBank accession numbers are provided in the Supplementary Appendix.
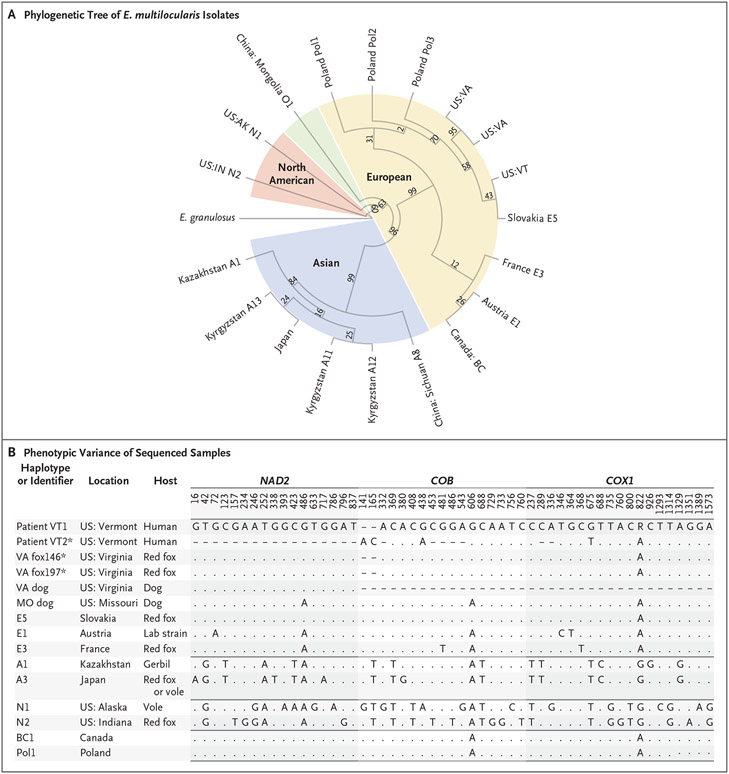
Mitochondrial sequences of E. multilocularis in samples from Patient VT1, fox146, and fox197 showed close identity with the Slovakian E5 European haplotype (Fig. 1A). E. multilocularis DNA in the sample obtained from Patient VT2 was fragmented, but we determined that 27 of the 29 discriminatory COX1 and COB SNPs were identical to the E5 haplotype. Previous studies have suggested the involvement of U.S. domestic dogs in the transmission of E. multilocularis in Virginia3 and Missouri.4 In addition, published sequencing results have shown considerable SNP similarities to the E5 haplotype (Fig. 1B).
Figure 1. Phylogenetic Tree of Echinococcus multilocularis Isolates and Single-Nucleotide Polymorphisms (SNPs) That Distinguish among International Haplotypes.
Panel A shows the unscaled phylogenetic tree of the sequencing of E. multilocularis samples for NAD2, COB, and COX1, as reported previously and in the current study. The tree was constructed with the use of the maximum likelihood method. The isolate from a liver-biopsy sample obtained from Patient VT1 is indicated as US:VT, and two isolates from stool samples obtained from red foxes in Virginia are indicated as US:VA. Values were obtained by means of bootstrapping techniques performed with 1000 iterations. E. granulosus is shown on the left of the tree as an outgrouping. Panel B shows SNPs in NAD2, COB, and COX1 that most discriminate among Asian, European, and North American E. multilocularis haplotypes. Listed are most of the isolates that are shown in Panel A for comparison alongside all reported isolates from the United States that are consistent with a European strain. Dashes indicate that no data were available, and periods indicate that the nucleotide was identical to that in the lead sequence. Asterisks indicate that the sequences are reported in this study. Only NAD2 and COB sequences are reported in this study for Patient VT1.
Before the diagnosis in Patient VT1, reports of E. multilocularis in humans in the United States have been limited to North American haplotypes that have been detected only in Alaska and the North Central area of the country. European strains have been regarded as more virulent than the lower-risk North American haplotypes, as illustrated by findings after the emergence of a European-like strain in wildlife in western Canada in 2012. By 2016, the human incidence of this strain was mirroring the incidence in Europe.5
Our data suggest the presence of changing epidemiologic features of E. multilocularis in the United States and provide evidence of the possible establishment of a reservoir and active transmission of a European strain. The emergence of this more virulent strain necessitates a growing diagnostic awareness among physicians, particularly in patients with no history of travel outside the United States who present with liver masses.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Louis B. Polish, University of Vermont Larner College of Medicine, Burlington, VT
Elise M. O’Connell, National Institute of Allergy and Infectious Diseases, Bethesda, MD
Thomas F.E. Barth, University of Ulm, Ulm, Germany
Bruno Gottstein, University of Bern, Bern, Switzerland
Anne Zajac, Virginia–Maryland College of Veterinary Medicine, Blacksburg, VA
Pamela C. Gibson, University of Vermont Larner College of Medicine, Burlington, VT
Aissatou Bah, National Institute of Allergy and Infectious Diseases, Bethesda, MD
Megan Kirchgessner, Virginia Department of Wildlife Resources, Blacksburg, VA
Marko Estrada, IDEXX Reference Laboratories, Westbrook, ME
M. Alexis Seguin, IDEXX Reference Laboratories, Westbrook, ME
Roger Ramirez-Barrios, Virginia–Maryland College of Veterinary Medicine, Blacksburg, VA
References
- 1.Polish LB, Pritt B, Barth TFE, Gottstein B, O’Connell EM, Gibson PC. First European haplotype of Echinococcus multilocularis identified in the United States: an emerging disease? Clin Infect Dis 2021;72:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth TF, Herrmann TS, Tappe D, et al. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl Trop Dis 2012;6(10):e1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zajac A, Fairman D, McGee E, et al. Alveolar echinococcosis in a dog in the eastern United States. J Vet Diagn Invest 2020;32:742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroki K, Morishima Y, Neil J, Beerntsen BT, Matsumoto J, Stich RW. Intestinal echinococcosis in a dog from Missouri. J Am Vet Med Assoc 2020;256:1041–6. [DOI] [PubMed] [Google Scholar]
- 5.Massolo A, Klein C, Kowalewska-Grochowska K, et al. European Echinococcus multilocularis identified in patients in Canada. N Engl J Med 2019;381:384–5. DOI: 10.1056/NEJMc2210000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.