Abstract
Background
Airway oedema (swelling) and mucus plugging are the principal pathological features in infants with acute viral bronchiolitis. Nebulised hypertonic saline solution (≥ 3%) may reduce these pathological changes and decrease airway obstruction. This is an update of a review first published in 2008, and updated in 2010, 2013, and 2017.
Objectives
To assess the effects of nebulised hypertonic (≥ 3%) saline solution in infants with acute bronchiolitis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily, Embase, CINAHL, LILACS, and Web of Science on 13 January 2022. We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov on 13 January 2022.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs using nebulised hypertonic saline alone or in conjunction with bronchodilators as an active intervention and nebulised 0.9% saline or standard treatment as a comparator in children under 24 months with acute bronchiolitis. The primary outcome for inpatient trials was length of hospital stay, and the primary outcome for outpatients or emergency department (ED) trials was rate of hospitalisation.
Data collection and analysis
Two review authors independently performed study selection, data extraction, and assessment of risk of bias in included studies. We conducted random‐effects model meta‐analyses using Review Manager 5. We used mean difference (MD), risk ratio (RR), and their 95% confidence intervals (CI) as effect size metrics.
Main results
We included six new trials (N = 1010) in this update, bringing the total number of included trials to 34, involving 5205 infants with acute bronchiolitis, of whom 2727 infants received hypertonic saline. Eleven trials await classification due to insufficient data for eligibility assessment. All included trials were randomised, parallel‐group, controlled trials, of which 30 were double‐blinded. Twelve trials were conducted in Asia, five in North America, one in South America, seven in Europe, and nine in Mediterranean and Middle East regions. The concentration of hypertonic saline was defined as 3% in all but six trials, in which 5% to 7% saline was used. Nine trials had no funding, and five trials were funded by sources from government or academic agencies. The remaining 20 trials did not provide funding sources.
Hospitalised infants treated with nebulised hypertonic saline may have a shorter mean length of hospital stay compared to those treated with nebulised normal (0.9%) saline or standard care (mean difference (MD) −0.40 days, 95% confidence interval (CI) −0.69 to −0.11; 21 trials, 2479 infants; low‐certainty evidence). Infants who received hypertonic saline may also have lower postinhalation clinical scores than infants who received normal saline in the first three days of treatment (day 1: MD −0.64, 95% CI −1.08 to −0.21; 10 trials (1 outpatient, 1 ED, 8 inpatient trials), 893 infants; day 2: MD −1.07, 95% CI −1.60 to −0.53; 10 trials (1 outpatient, 1 ED, 8 inpatient trials), 907 infants; day 3: MD −0.89, 95% CI −1.44 to −0.34; 10 trials (1 outpatient, 9 inpatient trials), 785 infants; low‐certainty evidence). Nebulised hypertonic saline may reduce the risk of hospitalisation by 13% compared with nebulised normal saline amongst infants who were outpatients and those treated in the ED (risk ratio (RR) 0.87, 95% CI 0.78 to 0.97; 8 trials, 1760 infants; low‐certainty evidence). However, hypertonic saline may not reduce the risk of readmission to hospital up to 28 days after discharge (RR 0.83, 95% CI 0.55 to 1.25; 6 trials, 1084 infants; low‐certainty evidence). We are uncertain whether infants who received hypertonic saline have a lower number of days to resolution of wheezing compared to those who received normal saline (MD −1.16 days, 95% CI −1.43 to −0.89; 2 trials, 205 infants; very low‐certainty evidence), cough (MD −0.87 days, 95% CI −1.31 to −0.44; 3 trials, 363 infants; very low‐certainty evidence), and pulmonary moist crackles (MD −1.30 days, 95% CI −2.28 to −0.32; 2 trials, 205 infants; very low‐certainty evidence).
Twenty‐seven trials presented safety data: 14 trials (1624 infants; 767 treated with hypertonic saline, of which 735 (96%) co‐administered with bronchodilators) did not report any adverse events, and 13 trials (2792 infants; 1479 treated with hypertonic saline, of which 416 (28%) co‐administered with bronchodilators and 1063 (72%) hypertonic saline alone) reported at least one adverse event such as worsening cough, agitation, bronchospasm, bradycardia, desaturation, vomiting and diarrhoea, most of which were mild and resolved spontaneously (low‐certainty evidence).
Authors' conclusions
Nebulised hypertonic saline may modestly reduce length of stay amongst infants hospitalised with acute bronchiolitis and may slightly improve clinical severity score. Treatment with nebulised hypertonic saline may also reduce the risk of hospitalisation amongst outpatients and ED patients. Nebulised hypertonic saline seems to be a safe treatment in infants with bronchiolitis with only minor and spontaneously resolved adverse events, especially when administered in conjunction with a bronchodilator. The certainty of the evidence was low to very low for all outcomes, mainly due to inconsistency and risk of bias.
Plain language summary
What are the benefits and risks of hypertonic saline solution via nebuliser for treating infants with acute bronchiolitis, compared to normal saline solution?
Key messages
Compared to nebulised normal saline, nebulised hypertonic saline may reduce hospital stay by almost 10 hours for infants admitted with acute bronchiolitis; may improve 'clinical severity scores', which are used by doctors to assess disease severity; and may reduce the risk of hospitalisation by 13% amongst children treated as outpatients or in the emergency department.
We found only minor and spontaneously resolved adverse events (such as worsening cough, agitation, bronchospasm, bradycardia, desaturation, vomiting and diarrhoea) from the use of nebulised hypertonic saline when given with treatment to relax airways (bronchodilators).
Our confidence in the evidence is low to very low; future large studies are needed to confirm the benefits of nebulised hypertonic saline for children with acute bronchiolitis.
What is acute bronchiolitis?
Acute bronchiolitis is the most common lower respiratory tract infection in children aged up to two years. Bronchiolitis occurs when small structures (bronchioles) leading to the lungs become infected, causing inflammation, swelling, and mucus production. This makes breathing difficult, especially in very young children, who develop coughs and wheezing.
Because bronchiolitis is usually caused by a virus, drug treatment is generally not effective. Hypertonic saline (a strong, or highly concentrated, sterile salt water solution) breathed in as a fine mist using a nebuliser may help relieve wheezing and breathing difficulty.
What did we want to find out?
We wanted to find out if hypertonic saline solution via nebuliser is more effective and safe for the treatment of infants with acute bronchiolitis compared to normal saline solution.
What did we do?
We searched for studies that compared nebulised hypertonic (≥ 3%) saline solution alone or combined with bronchodilators versus nebulised normal (0.9%) saline or standard treatment for infants with acute bronchiolitis. We combined the results across the included studies.
What did we find?
We included 34 trials involving 5205 infants with acute bronchiolitis. Eleven trials await assessment. Nine trials had no funding, and five trials were funded by government sources or academic agencies. The remaining 20 trials did not provide funding sources. Nebulised hypertonic saline may reduce hospital stay by 9.6 hours in comparison to normal saline or standard treatment for infants admitted with acute bronchiolitis. Clinical severity scores of infants improved slightly when administered nebulised hypertonic saline compared to normal saline. It remains unclear whether nebulised hypertonic saline can reduce the number of days to resolution of symptoms. Treatment with nebulised hypertonic saline may also reduce the risk of hospitalisation by 13% amongst children treated as outpatients or in the emergency department. However, hypertonic saline may not reduce the risk of readmission to hospital after discharge. We found only minor and spontaneously resolved adverse events (such as worsening cough, agitation, bronchospasm, bradycardia, desaturation, vomiting and diarrhoea) from the use of nebulised hypertonic saline when given with bronchodilators.
What are the limitations of the evidence?
Our confidence in the evidence is low to very low, and further research is likely to change the results of this review. Two main factors reduced our confidence in the evidence. Firstly, in some trials children were not randomly placed into different treatment groups, which means that any differences between groups could be due to differences between people rather than treatments. Secondly, there were inconsistencies in results across trials.
How up‐to‐date is the evidence?
The evidence is current to 13 January 2022.
Summary of findings
Summary of findings 1. Nebulised hypertonic saline compared with nebulised 0.9% saline for acute bronchiolitis in infants.
| Nebulised hypertonic saline compared with nebulised 0.9% saline for acute bronchiolitis in infants | ||||||
|
Patient or population: infants up to 24 months of age with acute bronchiolitis Settings: outpatient, emergency department, or inpatient Intervention: nebulised hypertonic saline (≥ 3%) Comparison: nebulised 0.9% saline or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk** | Corresponding risk | |||||
| Nebulised normal saline | Nebulised hypertonic saline | |||||
|
Length of hospital stay (days) |
The mean length of hospital stay ranged across control groups from 1.8 to 7.4 days. | The mean length of hospital stay in the intervention groups was on average 0.40 days shorter (95% CI −0.69 to −0.11). |
‐ | 2479 (21 trials) |
⊕⊕⊝⊝ Lowa | The effect size of nebulised hypertonic saline shown in this 2022 updated review, as well as in the 2017 review, was only approximately one‐third of that shown in the 2013 review, which included 6 inpatient trials involving 500 infants (MD −1.15 days, 95% CI −1.49 to −0.82 days). All but 2 trials published in 2013 and after, including 2 European multicentre studies, did not find significant effects of hypertonic saline on length of stay amongst inpatients with acute bronchiolitis. Despite the effects of nebulised hypertonic saline on reduction in length of hospital stay being smaller than were estimated previously, a reduction of almost 10 hours in length of hospital stay in infants with bronchiolitis may still be considered clinically relevant given the relatively short disease course, high prevalence, and huge burden of illness on healthcare systems around the world. |
|
Clinical severity score (post‐treatment) at day 1 Assessed with: Wang clinical severity score Scale from 0 to 12 (lower = better) |
The mean clinical severity score ranged across control groups from 1.9 to 8.8. | The mean clinical severity score in the intervention groups was on average 0.64 lower (95% CI −1.08 to −0.21). |
‐ | 893 (10 trials: 1 outpatient, 1 ED, 8 inpatients) |
⊕⊕⊝⊝
Lowa |
The meta‐analysis was based on data from only 10 trials, with reduced number of participants. The reduction of 0.64 in clinical score represents 11% of the mean score in the control group. |
|
Clinical severity score (post‐treatment) at day 2 Assessed with: Wang clinical severity score Scale from 0 to 12 (lower = better) |
The mean clinical severity score ranged across control groups from 0.8 to 8.2. |
The mean clinical severity score in the intervention groups was on average 1.07 lower (95% CI −1.60 to −0.53). |
‐ | 907 (10 trials: 1 outpatient, 1 ED, 8 inpatient) |
⊕⊕⊝⊝
Lowa |
The meta‐analysis was based on data from only 10 trials, with reduced number of participants. The reduction of 1.07 in clinical score represents 21% of the mean score in the control group. |
|
Clinical severity score (post‐treatment) at day 3 Assessed with: Wang clinical severity score Scale from 0 to 12 (lower = better) |
The mean clinical severity score ranged across control groups from 0.1 to 7.6. |
The mean clinical severity score in the intervention groups was on average 0.89 lower (95% CI −1.44 to −0.34). |
‐ | 785 (10 trials: 1 outpatient, 9 inpatient) |
⊕⊕⊝⊝
Lowa |
The meta‐analysis was based on data from only 10 trials, with reduced number of participants. The reduction of 0.89 in clinical score represents 22% of the mean score in the control group. |
|
Rate of hospitalisation Follow‐up: range 1 to 72 hours after enrolment |
34 per 100 (15 to 52) |
28 per 100 (10 to 46) |
RR 0.87 (0.78 to 0.97) |
1760 (8 trials: 1 outpatient, 7 ED) |
⊕⊕⊝⊝ Lowb | 2 trials contributed 73% of weight to the overall summary estimate of effects (Angoulvant 2017; Wu 2014). |
|
Rate of readmission to hospital Follow‐up: up to 28 days after discharge |
15 per 100 (4 to 25) |
13 per 100 (7 to 19) |
RR 0.83 (0.55 to 1.25) |
1084 (6 trials: 1 inpatient, 5 ED) |
⊕⊕⊝⊝ Lowc | The meta‐analysis was based on data from only 6 trials, with reduced number of participants. |
|
Number of days to resolution of symptoms and signs (wheezing) Follow‐up: during hospitalisation |
The mean time to resolution ranged across control groups from 3.8 to 4.8 days. | The mean time to resolution in the intervention groups was on average 1.16 days shorter (95% CI −1.43 to −0.89). |
‐ | 205 (2 trials) | ⊕⊝⊝⊝ Very lowd | The meta‐analysis was based on data from only 2 trials (of the same research group), with reduced number of participants. |
|
Number of days to resolution of symptoms and signs (cough) Follow‐up: during hospitalisation |
The mean time to resolution ranged across control groups from 5.5 to 6.3 days. | The mean time to resolution in the intervention groups was on average 0.87 days shorter (95% CI −1.31 to −0.44). | ‐ | 363 (3 trials) | ⊕⊝⊝⊝ Very lowd | The meta‐analysis was based on data from only 3 trials, with reduced number of participants. |
|
Number of days to resolution of symptoms and signs(pulmonary moist crackles) Follow‐up: during hospitalisation |
The mean time to resolution ranged across control groups from
6.2 to 6.2 days. |
The mean time to resolution in the intervention groups was on average 1.30 days shorter (95% CI −2.28 to −0.32). | ‐ | 205 (2 trials) | ⊕⊝⊝⊝ Very lowd | The meta‐analysis was based on data from only 2 trials (of the same research group), with reduced number of participants. |
|
Adverse events Assessed by investigators or reported by parents Follow‐up: during and immediately after nebulisation |
See comment | See comment | Not estimable | 4416 (2246 received hypertonic saline) (27 trials) |
⊕⊕⊝⊝ Lowe | 14 trials (1624 infants, 767 treated with hypertonic saline) did not report any adverse events, and 13 trials (2792 infants, 1479 treated with hypertonic saline) reported at least 1 adverse event; most adverse events were mild and resolved spontaneously. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**The assumed risk was based on data from the included trials. CI: confidence interval; ED: emergency department; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded the certainty of the evidence to low due to inconsistent results between studies (high heterogeneity) and risk of bias. bWe downgraded the certainty of the evidence to low due to high clinical heterogeneity between studies and publication bias. cWe downgraded the certainty of the evidence to low due to imprecision and risk of bias. dWe downgraded the certainty of the evidence to very low due to high clinical heterogeneity between studies, imprecision, and risk of bias. eWe downgraded the certainty of the evidence to low due to high clinical heterogeneity between studies (lack of standard collection and reporting) and risk of bias.
Background
Description of the condition
Acute bronchiolitis is the most frequent lower respiratory tract infection in infants (Klassen 1997a), and the most common causative organism is respiratory syncytial virus (RSV). Other less common pathogens include parainfluenza viruses, adenovirus, influenza A and B, rhinovirus, human metapneumovirus, and Mycoplasma pneumoniae (García‐García 2006; Henderson 1979; Jacques 2006; Rose 1987; Shay 2001). Virtually all infants are infected by RSV by the age of two years; around 40% to 50% develop involvement of the lower respiratory tract; and 1% to 2% develop severe disease leading to hospitalisation (Meissner 2003; Rakshi 1994; Shay 1999). It is estimated that globally in 2015, 1.4 million (uncertainty range (UR) 1.2 to 1.7) hospital admissions, and 27,300 (UR 20,700 to 36,200) in‐hospital deaths were due to RSV‐acute lower respiratory infection in infants younger than six months of age (Shi 2017).
The principal pathological findings in acute bronchiolitis include a peribronchial infiltrate of inflammatory cells, mucosal and submucosal oedema, necrosis and desquamation of ciliated epithelial cells, proliferation of cuboidal cells, and excess mucus secretion (Panitch 1993; Wohl 1978). The combination of airway wall swelling, sloughing of necrotic debris, increased mucus production, and impaired secretion clearance eventually leads to airway obstruction, gas trapping, atelectasis, and impaired gas exchange.
The diagnosis of acute bronchiolitis is usually based on clinical grounds. Despite differences in defining bronchiolitis, it is generally accepted that acute bronchiolitis refers to the first episode of acute wheezing in children aged less than two years, starting as a viral upper respiratory infection (coryza, cough, or fever) (Panitch 1993). These criteria for diagnosis of acute bronchiolitis have also been widely used in clinical trials (Bertrand 2001; Klassen 1997b; Schuh 1992; Wainwright 2003; Zhang 2003). Direct fluorescent antibody tests, enzyme immuno‐assay techniques, and cultures of the nasopharyngeal aspirate may be used to identify the causative pathogen.
Description of the intervention
The standard treatment for acute bronchiolitis remains supportive care and includes ensuring adequate oxygen exchange, fluid intake, and feeding of the infant (Panitch 2003; Wohl 2003). Convincing evidence for any other therapy is lacking. Because airway oedema and mucus plugging are the predominant pathological features in acute bronchiolitis, any therapy that can reduce these changes and improve the clearance of airway secretions may be beneficial.
Epinephrine has a theoretical effect on acute bronchiolitis because it contains alpha adrenergic properties which lead to vasoconstriction and reduction of airway oedema (Wohl 1978). However, a Cochrane Review showed that nebulised epinephrine for acute bronchiolitis results in a modest short‐term improvement in outpatients, but not amongst inpatients (Hartling 2011). Inhaled recombinant deoxyribonuclease (rhDNase), a mucolytic agent, has also been tested in hospitalised infants with acute bronchiolitis (Nasr 2001). This drug is thought to exert its major effect by enhancing airway secretion clearance. However, no significant effect was observed on clinical severity scores or length of hospital stay (Enriquez 2012). Another widely used approach is chest physiotherapy, which is thought to assist infants by enhancing the clearance of secretions and reducing ventilatory effort. However, current evidence has shown that chest physiotherapy (vibration and percussion or passive expiratory techniques) does not reduce length of hospital stay or oxygen requirements or improve the severity of the disease respiratory parameters in hospitalised infants with acute bronchiolitis (Roqué i Figuls 2016).
Hypertonic saline has been used as a treatment for infants with acute bronchiolitis. Earlier randomised trials have demonstrated that nebulised 3% saline may significantly reduce length of hospital stay and improve the clinical severity score in infants with acute viral bronchiolitis (Luo 2010; Mandelberg 2003; Sarrell 2002; Tal 2006). However, several later trials did not show significant benefits of nebulised hypertonic saline in infants with acute bronchiolitis (Everard 2014; Sharma 2013; Teunissen 2014).
How the intervention might work
Hypertonic saline solution has been shown to increase mucociliary clearance in disease‐free people and people with asthma, bronchiectasis, cystic fibrosis, and sinonasal diseases (Daviskas 1996; Kellett 2005; Shoseyov 1998; Wark 2018). Such benefits would also be expected in infants with acute bronchiolitis (Mandelberg 2010). The postulated mechanisms of benefit of hypertonic saline are:
induces an osmotic flow of water into the mucus layer, rehydrating the airway surface liquid and improving mucus clearance (Mandelberg 2010; Robinson 1997);
breaks the ionic bonds within the mucus gel, thereby reducing the degree of cross‐linking and entanglements and lowering the viscosity and elasticity of the mucus secretion (Ziment 1978); and
stimulates cilial beat via the release of prostaglandin E2 (Assouline 1977).
Moreover, by absorbing water from the mucosa and submucosa, hypertonic saline solution can theoretically reduce oedema of the airway wall in infants with acute bronchiolitis (Mandelberg 2003; Mandelberg 2010; Sarrell 2002). Hypertonic saline inhalation also causes sputum induction and cough, which can help to clear the sputum outside of the bronchi and thus improve airway obstruction (Mandelberg 2003).
These theoretical benefits provide the rationale for the treatment of acute bronchiolitis with nebulised hypertonic saline solution. To obtain optimal therapeutic effects, saline solution should be effectively delivered to the target, that is patient's airway surface liquid. However, trying to deliver aerosols to the lower respiratory tract of a crying baby is frequently futile (Iles 1999). It is very possible that some 'negative' studies have not demonstrated a hypertonic saline 'drug failure', but rather a drug delivery failure.
Why it is important to do this review
The hypothesis of this review is that nebulised hypertonic saline solution is beneficial in the management of acute bronchiolitis as assessed by clinically relevant outcomes, both in inpatients and outpatients. The establishment of a therapeutic role for hypertonic saline solution in acute bronchiolitis has relevant clinical implications. This modality may provide a cheap and effective therapy for children with acute bronchiolitis.
Objectives
To assess the effects of nebulised hypertonic (≥ 3%) saline solution in infants with acute bronchiolitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs (in which there is alternate allocation to treatment and control groups) in the review. We excluded studies including participants who had recurrent wheezing or who were intubated and ventilated, and studies that assessed pulmonary function alone. We also excluded abstract‐only citations for which we were unable to obtain additional data.
Types of participants
Children up to 24 months of age diagnosed with acute bronchiolitis. We defined acute bronchiolitis as the first episode of acute wheezing associated with clinical evidence of a viral infection (cough, coryza, or fever). Confirmation of viral aetiology was not necessary for study inclusion. We included studies of inpatients, emergency department patients, or outpatients.
Types of interventions
Nebulised hypertonic saline alone versus nebulised normal (0.9%) saline.
Nebulised hypertonic saline plus bronchodilator versus nebulised normal saline.
Nebulised hypertonic saline plus bronchodilator versus nebulised normal saline plus same bronchodilator.
Nebulised hypertonic saline alone or plus bronchodilator versus standard treatment.
Given that we identified few studies initially in 2007, we subsequently added comparisons of nebulised hypertonic saline alone versus nebulised normal saline or standard treatment (Zhang 2008). We defined hypertonic saline as a concentration of saline greater than or equal to 3%.
Types of outcome measures
Primary outcomes
Length of hospital stay or time taken to be ready for discharge (inpatients).
Rate of hospitalisation (outpatients or emergency department patients).
Secondary outcomes
Clinical severity score, measured at any time point after treatment.
Rate of readmission to hospital up to 28 days after discharge.
Haemoglobin saturation (oximetry), measured at any time point after treatment.
Respiratory rate, measured at any time point after treatment.
Heart rate, measured at any time point after treatment.
Number of days to resolution of symptoms or signs, measured as wheezing, cough, and pulmonary moist crackles.
Duration of in‐hospital oxygen supplementation.
Need for add‐on treatment (bronchodilator, systemic corticosteroids, antibiotics, and oxygen supplementation) at any time point after treatment.
Results of pulmonary function tests, measured at any time point after treatment.
Radiological findings, measured at any time point after treatment.
Adverse events (tachycardia, hypertension, pallor, tremor, nausea, vomiting, diarrhoea, and acute urinary retention)
When available, we used the following time points and intervals for combining the secondary outcomes (clinical severity scores, haemoglobin saturation, respiratory rate, heart rate, need for add‐on treatment, pulmonary function tests, and radiological findings): 60 and 120 minutes; 3 to 6, > 6 to 12, > 12 to 24, > 24 to 72 hours; and 3 to 10 days.
Search methods for identification of studies
Electronic searches
On 13 January 2022 we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 5), which includes the Cochrane Acute Respiratory Infections Group Specialised Register; MEDLINE ALL (Ovid), which includes Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, and Daily Updates (August 2017 to 13 January 2022); Embase (Ovid) (August 2017 to 13 January 2022); CINAHL (Cumulative Index to Nursing and Allied Health Literature; EBSCO, August 2017 to 13 January 2022); LILACS (Latin American and Caribbean Health Science Information Database, August 2017 to 13 January 2022); and Web of Science (August 2017 to 13 January 2022).
We used the search strategy in Appendix 1 for Ovid MEDLINE and Embase and Appendix 2 for CENTRAL. We adapted the searches previously adapted for LILACS (Appendix 3), CINAHL (Appendix 4), and Web of Science (Appendix 5). We used the search strategy in Appendix 6 for ClinicalTrials.gov and Appendix 7 for the WHO ICTRP. We did not apply any language or publication restrictions.
We searched the US National Institutes of Health Ongoing Trial Register ClinicalTrials.gov (clinicaltrials.gov/) to identity any new or ongoing trials on 13 January 2022. We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (trialsearch.who.int/) on 13 January 2022.
Details of searches conducted for the previous versions of this review are provided in Appendix 8.
Searching other resources
We checked the reference lists of included studies and other systematic reviews for additional relevant articles or trials.
Data collection and analysis
We conducted the review update according to the published protocol and reported any deviations from it in the Differences between protocol and review section.
Selection of studies
Two review authors (LZ, RAM) independently screened the titles and abstracts of all studies identified as a result of the search for potential relevance. We retrieved the full‐text study reports of all potentially eligible studies, and two review authors (LZ, RAM) independently screened the full‐text reports to identify studies for inclusion and identify and record reasons for exclusion of ineligible studies. Any disagreements were resolved through discussion or by consulting a third review author (CW) if required. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies table.
1.
Study flow diagram.
Data extraction and management
One review author (LZ) extracted study details from the included trials using a standardised data extraction form, and another review author (RAM) checked the data extraction. Any disagreements were resolved by discussion. We entered the extracted the following data into Review Manager 5 (RevMan 2020).
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, parental smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
We used Engauge digitising software to extract the first and third quartile values of length of hospital stay from a figure in Teunissen 2014 (Mitchell 2017). For this trial, we estimated mean and standard deviation from median and interquartile range of length of hospital stay using methods described by Wan 2014.
Assessment of risk of bias in included studies
Two review authors (LZ, RAM) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or by involving a third review author (CW). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as low, high, or unclear, and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the risk of bias table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
We entered outcome data for each study into data tables in Review Manager 5 to calculate the treatment effects (RevMan 2020). We used risk ratio (RR) for dichotomous outcomes, and mean differences (MDs) or standardised mean differences (SMDs) for continuous outcomes.
We conducted meta‐analyses only where this was meaningful, that is if the treatments, participants, and underlying clinical question were sufficiently similar for pooling to make sense.
Unit of analysis issues
In studies with a single parallel‐group design, the participants in each intervention arm were the unit of analysis. When trials recruited multiple groups, we combined data to create hypertonic saline and normal saline groups. We used the Review Manager 5 calculator to combine groups (RevMan 2020). We combined data for the 5% and 3% saline groups into the hypertonic saline group for Al‐Ansari 2010, and 7% and 3% saline groups into the hypertonic saline group for Köse 2016.
Dealing with missing data
We planned to contact investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing sensitivity analyses.
We planned that if numerical outcome data such as standard deviations or correlation coefficients were missing and could not be obtained from the authors, we would calculate these from other available statistics such as P values according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We contacted the authors of seven studies for additional trial data (Köse 2016; Kuzik 2007; Luo 2010; Mandelberg 2003; Sharma 2013; Teunissen 2014; Wu 2014), of whom five responded and provided data (Köse 2016; Kuzik 2007; Luo 2010; Mandelberg 2003; Wu 2014).
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity amongst the trials in each analysis. If we identified substantial heterogeneity, we reported this and explored possible causes in subgroup analysis. We used I² values of 25%, 50%, and 75% corresponding to low, moderate, and high heterogeneity, respectively (Higgins 2003).
Assessment of reporting biases
We planned that if we were able to pool more than 10 trials, we would create and examine a funnel plot to explore possible small‐study effects and publication bias. We created the funnel plot using Stata (StataCorp 2009).
Data synthesis
We pooled data from studies judged to be clinically homogeneous using Review Manager 5 software (RevMan 2020). If more than one study provided usable data in any single comparison, we performed meta‐analysis using the random‐effects model. We used intention‐to‐treat data where reported.
Subgroup analysis and investigation of heterogeneity
We performed pre‐planned subgroup analysis on clinical scores according to patient status (outpatient, emergency department patient, and inpatient). For length of hospital stay, we conducted post hoc subgroup analyses according to availability of virological investigation (available versus not available), upper age limits for participants (12 months versus > 12 to 24 months), hypertonic saline concentration (3% versus > 3%), administration interval (every 4 to 6 hours versus every 8 hours), co‐administration with bronchodilators (β₂ agonist, epinephrine versus no), and length of hospital stay in the control group (< 3 days versus ≥ 3 days). We believe that these patient and intervention factors may affect the effect size of nebulised hypertonic saline and may contribute to heterogeneity across studies. In the 2017 update, we conducted a post hoc subgroup analysis to assess the impact of risk of selection bias on the results of the meta‐analysis. In this 2022 update, we conducted such post hoc subgroup analysis based on risk of bias in any domain (low versus unclear/high) rather than selection bias alone. We conducted another post hoc subgroup analysis according to year of publication (before 2013 versus 2013 and thereafter). We defined use of year 2013 for subgroup classification in the 2017 update after observing that all 10 trials published in 2013 and thereafter failed to find significant effects of nebulised hypertonic saline on length of stay amongst inpatients with bronchiolitis. These results are quite different from those reported by earlier trials. For hospitalisation rate, we conducted the same subgroup analyses, except for length of stay in the control group.
We performed post hoc random‐effects meta‐regression using restricted maximum likelihood (REML) estimation to investigate the potential modifiers of effects of hypertonic saline on length of hospital stay and clinical severity score. We conducted meta‐regression using Stata (StataCorp 2009).
Sensitivity analysis
For length of hospital stay, we performed four post hoc sensitivity analyses in the 2017 update, excluding open trials, trials in which mean and standard deviation were estimated from median and interquartile range, trials with withdrawal rate over 15%, and trials with very short (< two days) or very long (> six days) length of stay in the control group. In this 2022 update, we conducted only two post hoc sensitivity analyses, excluding trials with mean and standard deviation estimated from median and interquartile range, and outlier trials with very short or very long length of stay in the control group. The impact of unblinding and high withdrawal rate had already been assessed by subgroup analysis according to risk of bias in any domain.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table using the following outcomes: length of hospital stay; clinical severity score post‐treatment at days 1, 2, and 3; rate of readmission to hospital; number of days to resolution of symptoms and signs (wheezing, cough, crackles); and adverse events. We used the five factors of the GRADE approach (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence relating to the studies that contributed data to meta‐analyses for outcomes (Atkins 2004). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We justified all decisions to down‐ or upgrade the certainty of the evidence using footnotes, and made comments to aid readers' understanding of the review where necessary.
Results
Description of studies
Results of the search
In this 2022 update, we identified 253 unique records from our searches of databases and trials registers. After title and abstract screening, we identified nine potentially relevant papers, which we reviewed in full text. We included six trials involving 1010 participants in this update (Awang 2020; Bashir 2018; Hmar 2021; Jaquet‐Pilloud 2020; Morikawa 2017; Uysalol 2017), bringing the total number of included trials to 34 involving 5205 infants with acute bronchiolitis. Eleven records await classification due to insufficient data for eligibility assessment. See Figure 1.
Included studies
All 34 included studies were parallel‐group RCTs. All but four trials were double‐blinded (Everard 2014; Hmar 2021; Morikawa 2017; NCT01238848). Six studies were multicentre: a hospital in the United Arab Emirates, and two hospitals in Canada (Kuzik 2007); 10 centres in England and Wales (Everard 2014); two centres in the USA (Wu 2014); 24 centres in France (Angoulvant 2017); two hospitals in Switzerland (Jaquet‐Pilloud 2020); and five hospitals in Japan (Morikawa 2017). Three trials were conducted by the same group of investigators in Israel (Mandelberg 2003; Sarrell 2002; Tal 2006), and two trials were conducted by one group of investigators in China (Luo 2010; Luo 2011). The remaining 29 studies were conducted in Argentina (NCT01238848), Canada (Grewal 2009; Kuzik 2007), China (Li 2014), France (Angoulvant 2017), India (Bashir 2018; Hmar 2021; Mahesh Kumar 2013; Pandit 2013; Sharma 2013), Italy (Miraglia Del Giudice 2012), Japan (Morikawa 2017), Malaysia (Awang 2020), Nepal (Khanal 2015; Ojha 2014), the Netherlands (Teunissen 2014), Poland (Ratajczyk‐Pekrul 2016), Portugal (Flores 2016), Qatar (Al‐Ansari 2010), Switzerland (Jaquet‐Pilloud 2020), Tunisia (Tinsa 2014), Turkey (Anil 2010; Ipek 2011; Köse 2016; Uysalol 2017), the UK (Everard 2014), and the USA (Florin 2014; Jacobs 2014; Wu 2014). For details, see Characteristics of included studies table.
Participants
Two trials recruited outpatient participants (N = 194) (Li 2014; Sarrell 2002); 10 trials recruited emergency department participants (N = 2307) (Al‐Ansari 2010; Angoulvant 2017; Anil 2010; Florin 2014; Grewal 2009; Ipek 2011; Jacobs 2014; Jaquet‐Pilloud 2020; Uysalol 2017; Wu 2014); 21 trials recruited inpatients (N = 2604) (Awang 2020; Bashir 2018; Everard 2014; Flores 2016; Hmar 2021; Köse 2016; Kuzik 2007; Luo 2010; Luo 2011; Mahesh Kumar 2013; Mandelberg 2003; Miraglia Del Giudice 2012; Morikawa 2017; NCT01238848; Ojha 2014; Pandit 2013; Ratajczyk‐Pekrul 2016; Sharma 2013; Tal 2006; Teunissen 2014; Tinsa 2014); and one trial recruited both outpatients and emergency department participants (N = 100) (Khanal 2015). The mean age of participants was 2.6 to 12.5 months (range: 9 days to 24 months).
The criteria for diagnosis of viral bronchiolitis were clearly defined in all but nine trials (Hmar 2021; Jaquet‐Pilloud 2020; Luo 2010; Mandelberg 2003; Miraglia Del Giudice 2012; Morikawa 2017; NCT01238848; Sarrell 2002; Tal 2006).
Virological investigation was reported in 20 trials (Angoulvant 2017; Awang 2020; Bashir 2018; Everard 2014; Flores 2016; Grewal 2009; Hmar 2021; Jacobs 2014; Jaquet‐Pilloud 2020; Kuzik 2007; Luo 2010; Luo 2011; Mandelberg 2003; Miraglia Del Giudice 2012; Ratajczyk‐Pekrul 2016; Sarrell 2002; Tal 2006; Teunissen 2014; Uysalol 2017; Wu 2014). The positive rate for respiratory syncytial virus (RSV) varied from 42% to 88%.
All 34 trials excluded infants with previous wheezing episodes. Infants hospitalised with severe bronchiolitis (requiring mechanical ventilation or intensive care, or oxygen saturation < 85% on room air) were excluded from all but four trials (Awang 2020; Teunissen 2014; Uysalol 2017; Wu 2014).
Interventions
The concentration of hypertonic saline was defined as 3% in all but six trials (Al‐Ansari 2010; Jacobs 2014; Li 2014; Köse 2016; Teunissen 2014; Tinsa 2014). Two concentrations were used by Al‐Ansari 2010 and Li 2014 (3% and 5%), Teunissen 2014 (3% and 6%), and Köse 2016 (3% and 7%). The concentration of hypertonic saline was defined as 5% in Tinsa 2014 and 7% in Jacobs 2014.
Treatment regimens of nebulised hypertonic saline (volume, interval of administration, addition of bronchodilator, and treatment duration) varied across studies, especially in emergency department‐based trials (Table 2). Oxygen or compressed air‐driven jet nebulisers were used for drug deliveries in all trials but Tal 2006, which used ultrasonic nebulisers.
1. Treatment regimens of nebulised hypertonic saline.
| Study ID | Saline concentration | Saline volume | Bronchodilator administered | Administration interval | Treatment duration |
| Outpatient trials | |||||
| Li 2014 | 3%, 5% | 3 mL | None | Twice daily | 3 days |
| Sarrell 2002 | 3% | 2 mL | Terbutaline 5 mg | Every 8 hours | 5 days |
| Emergency department trials | |||||
| Al‐Ansari 2010 | 3%, 5% | 5 mL | Epinephrine 1.5 mL | Every 4 hours | Until discharge |
| Angoulvant 2017 | 3% | 4 mL | None | Study solution was given at 0 and 30 minutes. | Until 2 doses had been administered |
| Anil 2010 | 3% | 4 mL | Epinephrine 1.5 mL or salbutamol 2.5 mg | Every 30 minutes | Until 2 doses had been administered |
| Florin 2014 | 3% | 4 mL | None | Within 90 minutes after albuterol administration | Single dose |
| Grewal 2009 | 3% | 2.5 mL | 2.25% racaemic epinephrine 0.5 mL | If needed, the second dose was given during the 120‐minute study period. | Up to 2 doses |
| Ipek 2011 | 3% | 4 mL | Salbutamol 0.15 mg/kg | Every 20 minutes | Until 3 doses had been administered |
| Jacobs 2014 | 7% | 3 mL | Racaemic epinephrine 0.5 mL | Study solution was given after initial screening and assessment. | Single dose (if the infant was admitted, the same solution was given every 6 h until discharge or 24 h after the admission) |
| Khanal 2015 | 3% | 4 mL | Epinephrine 1.5 mg | Study solution was given at 0 and 30 minutes. | Until 2 doses had been administered |
| Uysalol 2017 | 3% | 4 mL | None | 4 times daily | Until discharge criteria were fulfilled |
| Wu 2014 | 3% | 4 mL | None | Emergency department physicians could order 2 additional doses every 20 minutes. | Up to 3 doses |
| Inpatient trials | |||||
| Awang 2020 | 3% | 3.5 mL | Salbutamol 2.5 mg (0.5 mL) | Every 6 hours | Until clinical severity score ≤ 4 |
| Bashir 2018 | 3% | 4 mL | None | Every 2 hours for 3 doses, followed by every 4 hours for 6 doses, then every 6 hours | Until discharge |
| Everard 2014 | 3% | 4 mL | None | Every 6 hours | Until fit for discharge |
| Flores 2016 | 3% | 3 mL | Salbutamol 0.25 mL (1.25 mg) | Every 6 hours | Until discharge |
| Hmar 2021 | 3% | 3 mL | Salbutamol (?) mL | Every 6 hours | Until discharge |
| Jaquet‐Pilloud 2020 | 3% | 4 mL | None | Every 6 hours | Until discharge |
| Köse 2016 | 3%, 7% | 2.5 mL | Salbutamol 0.15 mg/kg | 2 doses were given at 30‐minute interval, followed by every 6 hours. | Until discharge |
| Kuzik 2007 | 3% | 4 mL | Albuterol was added in 37% of the treatments, and racaemic epinephrine was added in 23% of the treatments by attending physicians. | Every 2 hours for 3 doses, followed by every 4 hours for 5 doses, then every 6 hours | Until discharge |
| Luo 2010 | 3% | 4 mL | Salbutamol 2.5 mg | Every 8 hours | Until discharge |
| Luo 2011 | 3% | 4 mL | None | Every 2 hours for 3 doses, followed by every 4 hours for 5 doses, then every 6 hours | Until discharge |
| Mahesh Kumar 2013 | 3% | 3 mL | Salbutamol 0.15 mg/kg | Every 6 hours | Until ready for discharge |
| Mandelberg 2003 | 3% | 4 mL | Epinephrine 1.5 mg | Every 8 hours | Until discharge |
| Miraglia Del Giudice 2012 | 3% | ? mL | Epinephrine 1.5 mg | Every 6 hours | Until discharge |
| Morikawa 2017 | 3% | 2 mL | 0.5% salbutamol 0.1 mL | 4 times daily | Until discharge criteria were fulfilled |
| NCT01238848 | 3% | 3 mL | Albuterol 0.25 mg/kg/day | 4 times a day | 5 days |
| Ojha 2014 | 3% | 4 mL | None | Every 8 hours | Until discharge |
| Pandit 2013 | 3% | 4 mL | Epinephrine 1.0 mL | 3 doses were given at 1‐hour intervals, followed by every 6 hours. | Until discharge |
| Ratajczyk‐Pekrul 2016 | 3% | 3 mL | Salbutamol 0.15 mg/kg | Every 4 hours | Until discharge |
| Sharma 2013 | 3% | 4 mL | Salbutamol 2.5 mg | Every 4 hours | Until ready for discharge |
| Tal 2006 | 3% | 4 mL | Epinephrine 1.5 mg | Every 8 hours | Until discharge |
| Teunissen 2014 | 3%, 6% | 4 mL | Salbutamol 2.5 mg | Every 8 hours | Until discharge |
| Tinsa 2014 | 5% | 4 mL | Epinephrine 2 mL | Every 4 hours | Until discharge |
Outcome measures
All 21 inpatient trials except Tinsa 2014 used length of hospital stay as the primary outcome measure. Length of hospital stay was defined as time from hospital admission to discharge in all but two trials, which reported both time until fit for discharge and time until discharge (Everard 2014; Flores 2016). We used time until fit for discharge as length of hospital stay for Everard 2014 and Flores 2016.
The same clinical severity score was used by 17 inpatient trials as a secondary outcome measure (Awang 2020; Bashir 2018; Flores 2016; Hmar 2021; Jaquet‐Pilloud 2020; Köse 2016; Luo 2010; Luo 2011; Mahesh Kumar 2013; Mandelberg 2003; Miraglia Del Giudice 2012; Morikawa 2017; Ratajczyk‐Pekrul 2016; Sharma 2013; Tal 2006; Tinsa 2014; Uysalol 2017). This clinical score was initially described by Wang 1992, grading respiratory rate, wheezing, retraction, and general condition on a scale from 0 to 3, with a higher score indicating increased severity. Other clinical scoring systems were used by two inpatient trials (Kuzik 2007; Ojha 2014).
For outpatient or emergency department participants, outcome measures used were rate of hospitalisation (1 to 72 hours after enrolment) (Angoulvant 2017; Anil 2010; Florin 2014; Grewal 2009; Ipek 2011; Jacobs 2014; Sarrell 2002; Wu 2014), and rate of readmission (up to 28 days after discharge) (Al‐Ansari 2010; Anil 2010; Everard 2014; Florin 2014; Grewal 2009; Khanal 2015; Uysalol 2017). All outpatient or emergency department trials measured clinical severity score.
Other outcome measures were haemoglobin saturation (oximetry) (Al‐Ansari 2010; Anil 2010; Florin 2014; Grewal 2009; Ipek 2011; Khanal 2015; Mandelberg 2003; Pandit 2013; Tinsa 2014), heart rate (Anil 2010; Florin 2014; Ipek 2011; Khanal 2015; Mandelberg 2003; Pandit 2013; Sarrell 2002), respiratory rate (Florin 2014; Ipek 2011; Khanal 2015; Pandit 2013), number of days to resolution of signs and symptoms (Hmar 2021; Luo 2010; Luo 2011), and need for add‐on treatment (Flores 2016; Ipek 2011; Mahesh Kumar 2013; Mandelberg 2003; Pandit 2013; Teunissen 2014; Wu 2014).
The radiological assessment score initially described by Nasr 2001 was used in two trials (Mandelberg 2003; Sarrell 2002).
Side effects associated with inhaled therapies were reported in all but seven trials (Awang 2020; Bashir 2018; Hmar 2021; Ipek 2011; Mahesh Kumar 2013; Miraglia Del Giudice 2012; Ojha 2014).
Funding sources and declarations of interest
Four trials did not provide funding sources (Bashir 2018; Khanal 2015; Luo 2011; Miraglia Del Giudice 2012); 12 trials did not provide either funding sources or declarations of interest (Anil 2010; Ipek 2011; Li 2014; Luo 2010; Mahesh Kumar 2013; Mandelberg 2003; NCT01238848; Sarrell 2002; Tal 2006; Teunissen 2014; Tinsa 2014; Uysalol 2017); and four trials did not provide declarations of interest (Awang 2020; Kuzik 2007; Ojha 2014; Wu 2014). The remaining 14 studies provided both funding sources and declarations of interest. Nine trials had no funding, and five trials were funded by government sources or academic agencies.
Excluded studies
We excluded two new studies in this 2022 update (Sapkota 2021; Teijeiro 2018), for a total of 12 excluded studies. Reasons for exclusion were: other comparisons (Amirav 2005; Bueno Campaña 2014; Flores‐González 2016; Nenna 2014); inclusion of infants with previous history of wheezing (Kuzik 2010; Silver 2015); not an RCT (Al‐bahadily 2017; Sapkota 2021; Teijeiro 2018; Tribastone 2003); and abstract only (Bagus 2012; Guomo 2007).
We recategorised two records excluded in the 2017 update due to suspected plagiarism as 'studies awaiting classification' (Gupta 2016; Malik 2015). The two papers presented identical results. We contacted the first authors of both papers and the editors of the journals in which the papers were published, but were unable to obtain clarification from either authors or editors.
Risk of bias in included studies
A summary assessment of six key risk of bias domains is presented below and in the risk of bias tables (Characteristics of included studies) and risk of bias graph (Figure 2).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Five trials used an online randomisation tool (Grewal 2009; Mandelberg 2003; Morikawa 2017; Sarrell 2002; Tal 2006); 21 trials used a computer‐based random number program (Al‐Ansari 2010; Angoulvant 2017; Anil 2010; Awang 2020; Bashir 2018; Everard 2014; Flores 2016; Florin 2014; Jaquet‐Pilloud 2020; Khanal 2015; Köse 2016; Kuzik 2007; Luo 2011; Mahesh Kumar 2013; Miraglia Del Giudice 2012; Ojha 2014; Pandit 2013; Ratajczyk‐Pekrul 2016; Sharma 2013; Tinsa 2014; Wu 2014); two trials used a random numbers table to generate the random sequence (Hmar 2021; Li 2014), and one trial used a lottery randomisation method (Uysalol 2017). Two trials used block randomisation, but it was unclear how blocks were chosen at random to create the allocation sequence (Jacobs 2014; Teunissen 2014). Ipek 2011 assigned infants to treatment groups according to the consecutive order of their admission to the emergency department. Three trials did not provide information regarding random sequence generation (Luo 2010; Mandelberg 2003; NCT01238848).
Fourteen trials used sequentially numbered or coded drug containers of identical appearance for allocation concealment (Angoulvant 2017; Anil 2010; Flores 2016; Grewal 2009; Kuzik 2007; Luo 2010; Mandelberg 2003; Ojha 2014; Sarrell 2002; Sharma 2013; Tal 2006; Teunissen 2014; Tinsa 2014; Wu 2014). Seven trials used sequentially numbered, sealed envelopes for allocation concealment (Al‐Ansari 2010; Awang 2020; Florin 2014; Jacobs 2014; Khanal 2015; Luo 2011; Pandit 2013). Everard 2014 used a centralised web‐based randomisation system. In Miraglia Del Giudice 2012, study solutions were prepared by the local hospital pharmacy, but the method of allocation concealment was not described. Twelve trials did not provide information regarding allocation concealment (Anil 2010; Bashir 2018; Hmar 2021; Ipek 2011; Jaquet‐Pilloud 2020; Köse 2016; Li 2014; Mahesh Kumar 2013; Morikawa 2017; NCT01238848; Ratajczyk‐Pekrul 2016; Uysalol 2017).
Blinding
In all but 11 trials, infants, investigators, and care providers were blinded to group assignment (Everard 2014; Hmar 2021; Ipek 2011; Jaquet‐Pilloud 2020; Köse 2016; Li 2014; Mahesh Kumar 2013; Morikawa 2017; NCT01238848; Pandit 2013; Uysalol 2017). Four trials were open‐label (Everard 2014; Morikawa 2017; NCT01238848; Pandit 2013). Another four trials were reported as being double‐blinded but with no details provided (Ipek 2011; Köse 2016; Mahesh Kumar 2013; Uysalol 2017). Three trials did not provide information regarding blinding (Hmar 2021; Jaquet‐Pilloud 2020; Li 2014).
Incomplete outcome data
The number of withdrawals after randomisation was small in all but two trials (NCT01238848; Ojha 2014), in which the withdrawal rate was 18%. We assessed these two trials as having high risk of attrition bias, not only due to the relatively high withdrawal rate, but also to unbalanced attrition between treatment groups. We assessed another two trials as having unclear risk of attrition bias because the reasons for withdrawals, Everard 2014, or the distribution of withdrawals between study arms, Sarrell 2002, was not reported. Incomplete outcome data may not be a source of bias in the remaining trials.
Selective reporting
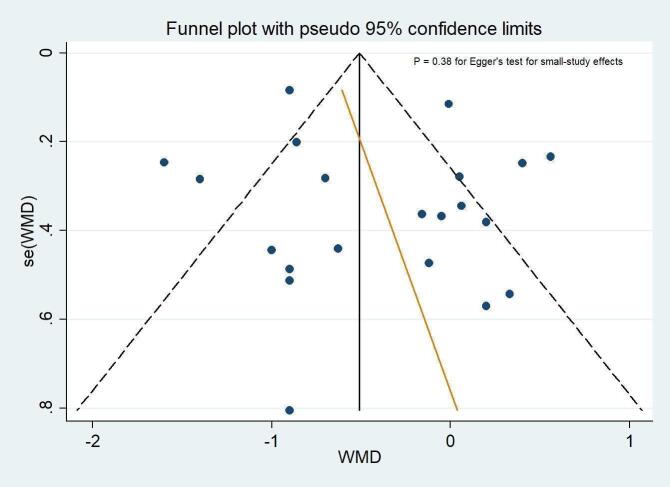
There appeared to be no evidence of selective reporting of outcomes in the included studies. All outcomes proposed in the methods or study protocols were reported in the results. The funnel plots did not suggest small‐study effects and publication bias for length of hospital stay amongst inpatient trials (Figure 3; P = 0.38 for Egger's test).
3.
Funnel plot of the weighted mean difference (WMD) of length of hospital stay (days) against its standard error. The circles represent risk estimates of each study, and the black vertical line represents the pooled effect estimate. Dashed lines represent pseudo‐95% confidence limits. Egger test (P = 0.38) suggests no small‐study effects.
Other potential sources of bias
We observed no other potential sources of bias in the included trials.
Effects of interventions
See: Table 1
Primary outcomes
1. Length of hospital stay or time taken to be ready for discharge (inpatients)
All but one of the 21 inpatient trials investigated length of hospital stay as the primary outcome (Tinsa 2014). Tinsa 2014 investigated clinical severity score as the primary outcome and length of stay as the secondary outcome. Two emergency department trials reported the length of stay in infants who required inpatient admission (Angoulvant 2017; Wu 2014). We did not include data from these two trials in the meta‐analysis because inpatients represented only some of the randomised participants. Twelve trials with 1404 infants compared hypertonic saline plus salbutamol/albuterol versus normal saline plus salbutamol/albuterol (Awang 2020; Flores 2016; Hmar 2021; Köse 2016; Luo 2010; Mahesh Kumar 2013; Morikawa 2017; NCT01238848; Ojha 2014; Ratajczyk‐Pekrul 2016; Sharma 2013; Teunissen 2014); four trials with 299 infants compared hypertonic saline plus epinephrine versus normal saline plus epinephrine (Mandelberg 2003; Miraglia Del Giudice 2012; Pandit 2013; Tal 2006); three trials with 392 infants compared hypertonic saline alone with normal saline (Bashir 2018; Kuzik 2007; Luo 2011); one trial with 94 infants compared both hypertonic saline alone and hypertonic saline plus epinephrine versus normal saline (Tinsa 2014); and one trial with 291 infants compared hypertonic saline alone with standard treatment (Everard 2014). Overall, the meta‐analysis of 21 trials (2479 infants) showed that infants treated with nebulised hypertonic saline may have a shorter mean length of hospital stay compared to those treated with nebulised normal saline or standard treatment (mean difference (MD) −0.40 days, 95% confidence interval (CI) −0.69 to −0.11; low‐certainty evidence; Analysis 1.1). There was significant heterogeneity in results amongst studies (I² = 83%). The pooled MD (95% CI) was −0.13 days (−0.48 to 0.22) for the comparison hypertonic saline plus salbutamol/albuterol versus normal saline plus salbutamol/albuterol (12 trials); −0.65 days (−1.01 to −0.29) for the comparison hypertonic saline plus epinephrine versus normal saline plus epinephrine (5 trials); −1.13 days (−1.60 to −0.66) for the comparison hypertonic saline alone versus normal saline (4 trials); and 0.06 days (−0.62 to 0.74) for the comparison hypertonic saline alone versus standard treatment (1 trial).
1.1. Analysis.

Comparison 1: Hypertonic saline versus normal saline or standard treatment, Outcome 1: Length of hospital stay (days)
The results of eight post hoc subgroup analyses are shown in Table 3. No significant subgroup difference was found in all but one analysis, in which the effect size of hypertonic saline on length of stay appeared to be greater in subgroups of trials published before 2013 (−0.98 days, 95% CI −1.41 to −0.55) compared to those published in 2013 or after (−0.14 days, 95% CI −0.48 to 0.20; P = 0.003 for subgroup difference). Moderate to high levels of heterogeneity persisted in most subgroup analyses.
2. Subgroup analyses on length of hospital stay amongst inpatients.
| Subgroups | Length of hospital stay (days) | ||||
| Trial (n) | Participants (N) | Effect size (MD, 95% CI) | P values for subgroup difference (Chi²) | Heterogeneity (I²) | |
| Virological investigation | |||||
| Available Not available |
11 10 |
1307 1172 |
−0.51 (−1.02 to 0.002) −0.28 (−0.67 to 0.10) |
0.49 | 84% 85% |
| Upper age limits for infants | |||||
| 12 months 18 to 24 months |
7 14 |
773 1706 |
−0.27 (−0.63 to 0.09) −0.43 (−0.79 to −0.07) |
0.55 | 15% 89% |
| Hypertonic saline solution plus bronchodilator | |||||
| β₂ agonist Epinephrine No |
12 5 4 |
1424 404 651 |
−0.19 (−0.57 to 0.17) −0.65 (−1.01 to −0.30) −0.63 (−1.28 to 0.01) |
0.18 | 79% 0% 89% |
| Administration interval* | |||||
| A B |
16 5 |
1987 492 |
−0.38 (−0.69 to −0.06) −0.51 (−1.33 to 0.32) |
0.77 | 82% 88% |
| Hypertonic saline concentration** | |||||
| 3% > 3% |
20 3 |
2037 442 |
−0.40 (−0.70 to −0.09) 0.12 (−0.54 to 0.77) |
0.16 | 86% 33% |
| Length of stay in the control group | |||||
| < 3 days ≥ 3 days |
6 15 |
884 1595 |
−0.08 (−0.64 to 0.47) −0.56 (−0.90 to −0.23) |
0.15 | 93% 68% |
| Risk of bias in any domain | |||||
| Low Unclear/high |
8 13 |
1001 1478 |
−0.35 (−0.93 to 0.23) −0.45 (−0.76 to −0.14) |
0.76 | 87% 73% |
| Year of publication | |||||
| Before 2013 2013 and after |
7 14 |
577 1902 |
−0.98 (−1.41 to −0.55) −0.14 (−0.48 to 0.20) |
0.003 | 59% 84% |
CI: confidence interval MD: mean difference
*Regimen A: every 4 to 6 hours; regimen B: every 8 hours **Köse 2016 used 3% and 7% saline, and Teunissen 2014 used 3% and 6% saline.
The meta‐regression analysis did not reveal an independent effect of availability of virological testing, hypertonic saline concentration, co‐administration with bronchodilators, length of hospital stay in the control group, risk of bias, and year of publication.
The results of two post hoc sensitivity analyses are shown in Table 4. Only the sensitivity analysis excluding two trials, Luo 2010 and Luo 2011, with very long (greater than six days) and one trial, Ojha 2014, with very short (less than two days) length of hospital stay in the control group substantially reduced the effect size of hypertonic saline (MD from −0.40 days (95% CI −0.69 to −0.11) to −0.28 days (95% CI −0.58 to 0.01)), but high heterogeneity remained.
3. Sensitivity analyses: length of hospital stay amongst inpatients.
| Length of hospital stay (days) | |||
| Effect size (RR, 95% CI) | Heterogeneity (I²) | Trials excluded from analysis | Reasons for exclusion |
| −0.46 (−0.74 to −0.18) | 81% | Teunissen 2014 | Mean and standard deviation were estimated from median and interquartile range. |
| −0.28 (−0.58 to 0.01) | 80% | Luo 2010; Luo 2011; Ojha 2014 | Very short (< 2 days) or very long (> 6 days) length of stay in the control group |
CI: confidence interval RR: risk ratio
2. Rate of hospitalisation (outpatients or emergency department patients)
One outpatient trial, Sarrell 2002, and seven emergency department trials with 1760 infants assessed the efficacy of hypertonic saline in reducing the risk of hospitalisation (Angoulvant 2017; Anil 2010; Florin 2014; Grewal 2009; Ipek 2011; Jacobs 2014; Wu 2014). Four trials with 398 infants compared hypertonic saline plus bronchodilator versus normal saline (Anil 2010; Grewal 2009; Jacobs 2014; Sarrell 2002); three trials with 1242 infants compared hypertonic saline alone versus normal saline (Angoulvant 2017; Florin 2014; Wu 2014); and one trial with 120 infants compared both hypertonic saline plus salbutamol versus normal saline plus salbutamol and hypertonic alone versus normal saline (Ipek 2011). Overall, the meta‐analysis of 8 trials (1760 infants) showed that infants treated with nebulised hypertonic saline may have a lower risk of hospitalisation compared to those treated with nebulised normal saline (risk ratio (RR) of 0.87, 95% CI 0.78 to 0.97; low‐certainty evidence; Analysis 1.2). There was no significant heterogeneity amongst studies (I² = 0%). The pooled RR (95% CI) was 0.78 (0.55 to 1.10) for the comparison hypertonic saline plus bronchodilator versus normal saline plus bronchodilator (5 trials) and 0.87 (0.69 to 1.08) for the comparison hypertonic saline alone versus normal saline (4 trials).
1.2. Analysis.

Comparison 1: Hypertonic saline versus normal saline or standard treatment, Outcome 2: Rate of hospitalisation
The results of six post hoc subgroup analyses are shown in Table 5. No significant subgroup difference was found in all subgroup analyses.
4. Subgroup analyses: hospitalisation rate amongst outpatients and emergency department patients.
| Subgroups | Hospitalisation rate (%) | ||||
| Trial (n) | Participants (N) | Effect size (RR, 95% CI) | P values for subgroup difference (Chi²) | Heterogeneity (I²) | |
| Virological investigation | |||||
| Available Not available |
5 3 |
1392 368 |
0.82 (0.69 to 0.97) 1.05 (0.76 to 1·45) |
0.19 | 21% 0% |
| Upper age limits for infants | |||||
| 12 months 18 to 24 months |
2 6 |
818 942 |
0.86 (0.64 to 1.15) 0.82 (0.67 to 1.02) |
0.75 | 26% 0% |
| Hypertonic saline solution plus bronchodilator* | |||||
| β₂ agonist Epinephrine No |
3 3 3 |
276 242 1242 |
0.62 (0.26 to 1.48) 0.80 (0.56 to 1.14) 0.87 (0.69 to 1.11) |
0.72 | 0% 0% 66% |
| Administration interval** | |||||
| A B |
2 6 |
163 1597 |
1.0 (0.78 to 1.31) 0.85 (0.75 to 0.96) |
0.27 | 0% 0% |
| Hypertonic saline concentration | |||||
| 3% > 3% |
7 1 |
1659 101 |
0.86 (0.74 to 0.99) 0.86 (0.56 to 1.32) |
0.94 | 20% ‐ |
| Risk of bias in any domain | |||||
| Low Unclear/high |
4 4 |
1288 472 |
0.85 (0.68 to 1.06) 0.82 (0.56 to 1.21) |
0.91 | 57% 0% |
| Year of publication | |||||
| Before 2013 2013 and after |
4 4 |
417 1343 |
0.64 (0.38 to 1.08) 0.87 (0.72 to 1.05) |
0.28 | 0% 49% |
CI: confidence interval RR: risk ratio
*Anil 2010 used two intervention groups: hypertonic saline plus salbutamol and hypertonic saline plus epinephrine. **Regimen A: single dose; regimen B: multiple doses (≥ 2).
Secondary outcomes
1. Clinical severity scores
One outpatient trial, Sarrell 2002, one emergency department trial, Al‐Ansari 2010, and nine inpatient trials compared postinhalation Wang clinical severity score between infants treated with nebulised hypertonic saline and those treated with nebulised 0.9% saline on the first three days of treatment (Awang 2020; Flores 2016; Hmar 2021; Köse 2016; Luo 2010; Luo 2011; Mandelberg 2003; Miraglia Del Giudice 2012; Tal 2006). The baseline clinical scores were comparable between groups in all 11 trials.
On the first day of treatment, Sarrell 2002 (N = 65 outpatients) showed that the 3% saline group may have a lower clinical severity score compared to the 0.9% saline group (MD −1.28, 95% CI −1.92 to −0.64). Eight inpatient trials (N = 657) also demonstrated that nebulised hypertonic saline may reduce clinical severity score (pooled MD −0.64, 95% CI −1.15 to −0.13; I² = 79%) (Awang 2020; Flores 2016; Köse 2016; Luo 2010; Luo 2011; Mandelberg 2003; Miraglia Del Giudice 2012; Tal 2006). In contrast, Al‐Ansari 2010 (N = 171 emergency department infants) did not show superiority of hypertonic saline over normal saline in reducing clinical score (MD −0.09, 95% CI −0.51 to 0.33). The pooled results of 10 trials showed a lower clinical severity score favouring treatment with nebulised hypertonic saline over nebulised normal saline on the first day of treatment (pooled MD −0.64, 95% CI −1.08 to −0.21; I² = 80%; low‐certainty evidence; Analysis 1.3). The difference of 0.64 in clinical score represents 11% of the mean score in the control group.
1.3. Analysis.

Comparison 1: Hypertonic saline versus normal saline or standard treatment, Outcome 3: Clinical severity score (post‐treatment) at day 1
On the second day of treatment, Sarrell 2002 (N = 65 outpatients) showed a lower clinical severity score in the 3% saline group compared to the 0.9% saline group (MD −2.0, 95% CI −2.93 to −1.07). We also observed a significant difference between treatment and control groups amongst 671 inpatients (pooled MD −1.08, 95% CI −1.68 to −0.47; I² = 89%) favouring the 3% saline group (Awang 2020; Flores 2016; Hmar 2021; Luo 2010; Luo 2011; Mandelberg 2003; Miraglia Del Giudice 2012; Tal 2006). Al‐Ansari 2010 (N = 171 emergency department infants) did not demonstrate a benefit of hypertonic saline in reducing clinical score (MD −0.27, 95% CI −0.63 to 0.09). Meta‐analysis of 10 trials demonstrated superiority of nebulised 3% saline over 0.9% saline in reducing clinical severity score on the second day of treatment (pooled MD −1.07, 95% CI −1.60 to −0.53; I² = 89%; low‐certainty evidence; Analysis 1.4). The difference of 1.07 in clinical score represents 21% of the mean score in the control group.
1.4. Analysis.

Comparison 1: Hypertonic saline versus normal saline or standard treatment, Outcome 4: Clinical severity score (post‐treatment) at day 2
On the third day of treatment, Sarrell 2002 (N = 65 outpatients) showed a lower clinical severity score in the 3% saline group compared to the 0.9% saline group (MD −2.64, 95% CI −3.85 to −1.43). Nine inpatient trials (N = 720) also showed a lower clinical severity score in the 3% saline group (pooled MD −0.74, 95% CI −1.31 to −0.18; I² = 93%) (Awang 2020; Flores 2016; Hmar 2021; Luo 2010; Luo 2011; Mandelberg 2003; Miraglia Del Giudice 2012; Morikawa 2017; Tal 2006). The pooled results from 10 trials demonstrated superiority of nebulised 3% saline over 0.9% saline in reducing clinical severity score on the third day of treatment (pooled MD −0.89, 95% CI −1.44 to −0.34; I² = 92%; low‐certainty evidence; Analysis 1.5). The difference of 0.89 in clinical score represents 22% of the mean score in the control group.
1.5. Analysis.

Comparison 1: Hypertonic saline versus normal saline or standard treatment, Outcome 5: Clinical severity score (post‐treatment) at day 3
We performed post hoc meta‐regression analysis to explore possible causes of heterogeneity amongst studies regarding the effect size of hypertonic saline on clinical score during the first three days of treatment. The small number of studies enabled inclusion of only one relevant covariate in the model, which was the severity of bronchiolitis assessed by baseline clinical score in the 0.9% saline group. The meta‐regression analysis yielded a regression coefficient of −0.19 (95% CI −0.85 to 0.47; P = 0.56), suggesting that disease severity did not significantly influence the effect size of hypertonic saline on clinical score.
Eight emergency department‐based trials assessed short‐term effects (30 minutes to 120 minutes) of up to three doses of nebulised hypertonic saline in improving clinical severity score amongst infants with acute bronchiolitis (Angoulvant 2017; Anil 2010; Florin 2014; Grewal 2009; Ipek 2011; Jacobs 2014; Khanal 2015; Wu 2014). Variation in scoring methods and assessment time points made conducting meta‐analyses inappropriate. Only two trials showed the superiority of hypertonic saline over normal saline in improving clinical severity scores (Angoulvant 2017; Khanal 2015). Khanal 2015 found that infants who received nebulised hypertonic saline had more significant improvement in baseline clinical severity scores at the end of two hours of treatment. Angoulvant 2017 found that the change in Respiratory Distress Assessment Instrument (RDAI) score before and after nebulisation was greater in the hypertonic saline group than in the normal saline group (adjusted difference −0.7, 95% CI −1.2 to −0.2). Al‐Ansari 2010, an emergency department trial, compared nebulised 5% and 3% hypertonic saline with nebulised 0.9% saline, given at enrolment and every four hours thereafter until the child was ready for discharge. There was a small but statistically significant lower clinical score favouring treatment with nebulised 5% saline over nebulised 0.9% saline at 48 hours after randomisation (3.69 ± 1.09 versus 4.12 ± 1.11; P = 0.04), but not at 24 hours after randomisation (3.75 ± 1.27 versus 3.97 ± 1.40; P = 0.38). Al‐Ansari 2010 did not find a significant difference in clinical score between 3% saline and 0.9% saline at 24 and 48 hours after randomisation.
2. Rate of readmission to hospital
Five emergency department trials, Al‐Ansari 2010; Anil 2010; Florin 2014; Khanal 2015; Uysalol 2017, and one inpatient trial included rate of readmission after discharge as an outcome (Everard 2014). The pooled results of these trials did not demonstrate significant benefits of nebulised hypertonic saline in reducing the risk of readmission (pooled RR 0.83, 95% CI 0.55 to 1.25; I² = 31%; low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Hypertonic saline versus normal saline or standard treatment, Outcome 6: Rate of readmission to hospital
3. Haemoglobin saturation (oximetry)
Eight trials reported the results of haemoglobin saturation measured at different time points (Anil 2010; Florin 2014; Grewal 2009; Ipek 2011; Khanal 2015; Mandelberg 2003; Pandit 2013; Tinsa 2014). Only Khanal 2015 revealed a significant difference between the hypertonic saline group and the 0.9% saline group in terms of room air saturation of oxyhaemoglobin throughout the study period, showing a significantly higher haemoglobin saturation in the hypertonic saline group than in the 0.9% saline group at 60 and 120 minutes after treatment.
4. Respiratory rate
Five trials reported no difference in respiratory rate, measured at different time points, between the hypertonic saline group and the 0.9% saline group (Flores 2016; Ipek 2011; Khanal 2015; Pandit 2013; Tinsa 2014).
5. Heart rate
Seven trials reported no difference in heart rate, measured at different time points, between the hypertonic saline group and the 0.9% saline group (Anil 2010; Florin 2014; Ipek 2011; Khanal 2015; Mandelberg 2003; Pandit 2013; Sarrell 2002).
6. Number of days to resolution of symptoms and signs
Luo 2010, Luo 2011, and Hmar 2021 reported number of days to resolution of at least one symptom or sign (wheezing, cough, or pulmonary moist crackles). The pooled results showed that infants treated with nebulised 3% saline had a shorter duration of wheezing (−1.16 days, 95% CI −1.43 to −0.89; I² = 0%; 2 trials, 205 infants; very low‐certainty evidence), cough (−0.87 days, 95% CI −1.31 to −0.44; I² = 12%; 3 trials, 363 infants; very low‐certainty evidence), and pulmonary moist crackles (−1.30 days, 95% CI −2.28 to −0.32; I² = 95%; 2 trials, 205 infants; very low‐certainty evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1: Hypertonic saline versus normal saline or standard treatment, Outcome 7: Number of days to resolution of symptoms and signs (days)
7. Duration of in‐hospital oxygen supplementation
Ojha 2014, Teunissen 2014, Morikawa 2017, and Jaquet‐Pilloud 2020 reported no difference in duration of in‐hospital oxygen supplementation between the hypertonic saline group and the 0.9% saline group. The pooled results of three trials did not demonstrate significant benefits of nebulised hypertonic saline in reducing the duration of in‐hospital oxygen supplementation (pooled MD −0.25 hours, 95% CI −9.36 to 8.86; I² = 0%; very low‐certainty evidence; Analysis 1.8) (Jaquet‐Pilloud 2020; Morikawa 2017; Ojha 2014).
1.8. Analysis.

Comparison 1: Hypertonic saline versus normal saline or standard treatment, Outcome 8: Duration of in‐hospital oxygen supplementation (hours)
8. Need for add‐on treatment (bronchodilator, systemic corticosteroids, antibiotics, and oxygen supplementation)
Eight trials compared the need for add‐on treatment between treatment groups (Al‐Ansari 2010; Flores 2016; Kuzik 2007; Mahesh Kumar 2013; Pandit 2013; Tal 2006; Teunissen 2014; Wu 2014). None of the trials revealed a significant difference between hypertonic saline and 0.9% saline groups.
9. Results of pulmonary function tests
No included studies reported pulmonary function test results.
10. Radiological findings
In Mandelberg 2003 and Sarrell 2002, the second chest radiograph was obtained on the third day after hospital admission. The pooled results did not show a significant difference in radiological score between the hypertonic saline and 0.9% saline groups (pooled MD −0.08, 95% CI −0.90 to 0.75; very low‐certainty evidence; Analysis 1.9).
1.9. Analysis.

Comparison 1: Hypertonic saline versus normal saline or standard treatment, Outcome 9: Radiological assessment score
11. Adverse events (tachycardia, hypertension, pallor, tremor, nausea, vomiting, diarrhoea, and acute urinary retention)
Seven trials did not report safety data (Awang 2020; Bashir 2018; Hmar 2021; Ipek 2011; Mahesh Kumar 2013; Miraglia Del Giudice 2012; Ojha 2014). The remaining 27 trials (4416 infants) reported safety data in treatment groups. Amongst the 4416 infants, 2246 received nebulised hypertonic saline (3% saline: N = 1912; 5% saline: N = 165; 6% saline: N = 83; 7% saline: N = 86). Fourteen trials did not find any significant adverse events amongst a total of 1624 infants, of whom 767 received nebulised hypertonic saline (co‐administered with bronchodilators: N = 735, 96%; hypertonic saline alone: N = 32, 4%). In the remaining 13 trials involving 2792 infants, of whom 1479 received nebulised hypertonic saline (co‐administered with bronchodilators: N = 416, 28%; hypertonic saline alone: N = 1063, 72%), at least one adverse event was reported. Variations in reporting and outcomes precluded the possibility of conducting meta‐analysis of safety data. We narratively summarised the safety data of 13 trials (Table 6). Various adverse events were reported in both hypertonic saline and control groups; in most cases, these were mild and resolved spontaneously. Only one inpatient trial involving 142 infants who received 3% saline alone without bronchodilator reported one serious adverse event (bradycardia and desaturation), possibly related to hypertonic saline inhalation, but it resolved the following day.
5. Narrative summary: adverse events of treatment reported in 13 trials.
| Trials | Comparisons | Narrative summary |
| Kuzik 2007 | 3% saline (N = 47) vs 0.9% saline (N = 49) | No infants were withdrawn by the medical staff due to AEs, although 5 infants were withdrawn at parents’ request due to perceived AEs, only 2 of which were in the hypertonic saline group (1 presented with vigorous crying and another with agitation). |
| Grewal 2009 | 3% saline + epinephrine (N = 23) vs 0.9% saline + epinephrine (N = 23) | Adverse events were noted in 4 infants (vomiting 3; diarrhoea 1) in the hypertonic saline group. No additional bronchodilators were given to any enrolled infant during the study period. |
| Luo 2011 | 3% saline (N = 57) vs 0.9% saline (N = 55) | No infants were withdrawn by the medical staff due to AEs. Coughing and wheezing did not worsen during saline inhalation. Although 5 infants had hoarse voices, only 2 of these were in the hypertonic saline group, and the symptom disappeared after 3 to 4 days. |
| Pandit 2013 | 3% saline + epinephrine (N = 51) vs 0.9% saline + epinephrine (N = 49) | No AEs were observed in the 3% saline group. In the 0.9% saline group, 3 infants had vomiting, and 1 infant had diarrhoea. |
| Everard 2014 | 3% saline (N = 142) vs standard care (N = 143) | 6 AEs were possibly related to saline treatment, including 1 SAE, bradycardia and desaturation, which resolved the following day. The remaining 5 non‐SAEs were: bradycardia (self‐correcting), desaturation, coughing fit, and increased respiratory rate (all of which resolved within 1 day), and a chest infection, which resolved after 6 days. |
| Li 2014 | 5% saline (N = 40), 3% saline (N = 42) vs 0.9% saline (N = 42) | No AEs were observed in the 3% and 0.9% saline groups. 4 infants in the 5% saline group presented with paroxysmal cough during saline inhalation. |
| Teunissen 2014 | 3%, 6% saline + salbutamol (N = 167) vs 0.9% saline + salbutamol (N = 80) | A substantial number of AEs (cough, bronchospasm, agitation, desaturation, etc.) were noted in all treatment groups. Except for cough, which occurred more significantly in the hypertonic saline groups (P = 0.03), no differences were found between groups. Withdrawals due to AEs did not differ between groups (4.3%, 6.1%, and 7.9% in the 3%, 6%, and 0.9% saline groups, respectively; P = 0.59). |
| Wu 2014 | 3% saline (N = 211) vs 0.9% saline (N = 197) | 3 infants in the normal saline group and 4 infants in the hypertonic saline group withdrew due to parent request. Of these parent requests, 1 in the normal saline group and 2 in the hypertonic saline group were attributed to worsening cough. For these 3 infants, pre‐treatment and post‐treatment vital signs and Respiratory Distress Assessment Instrument score were the same or improved, and no intervention or additional treatment was necessary. |
| Flores 2016 | 3% saline + salbutamol (N = 33) vs 0.9% saline + salbutamol (N = 35) | Exacerbation of coughing and excessive rhinorrhoea were more common in the 3% saline group (45.5% and 57.6%) than in the 0.9% saline group (20% and 31.4%). There was no significant difference in bronchial constriction and agitation between groups. Apnoea, cyanosis, saturation dips, tachycardia, and vomiting were not observed. |
| Köse 2016 | 3% saline + salbutamol (N = 35), 7% saline + salbutamol (N = 32) vs 0.9% saline + salbutamol (N = 35) | No AEs were reported in the 3% and 0.9% saline groups. In the 7% saline group, bronchospasm was observed in 2 infants, and exacerbation of coughing was observed in another 2 infants. Both bronchospasm and cough were observed during nebulisation in 1 infant. |
| Angoulvant 2017 | 3% saline (N = 385) vs 0.9% saline (N = 387) | No SAEs were reported. Mild AEs occurred 57 times amongst 50 infants, in 35 of 392 infants (8.9%) in the HS group versus 15 of 384 infants (3.9%) in the NS group (risk difference 5.0%, 95% confidence interval 1.6% to 8.4%; P = 0.005). Worsening of cough without respiratory distress was the most frequent AE, occurring in 26 infants (6.6%) in the HS group and 3 infants (0.8%) in the NS group. Bronchospasm occurred in 3 infants (0.8%) in the NS group. |
| Uysalol 2017 | 3% saline (N = 79) and 3% saline + epinephrine (N = 75) vs 0.9% saline (N = 82) and 0.9% saline + epinephrine (N = 76) | Adverse events (tachycardia, pallor, tremor, nausea or vomiting) were reported in 5 infants in the hypertonic saline group and 9 infants in the normal saline group. |
| Jaquet‐Pilloud 2020 | 3% saline + standard care (N = 60) vs standard care only (N = 60) | No SAEs (bronchospasm, excessive coughing, infection, apnoea and cyanosis) were observed during the study. However, HS was discontinued in 10 infants at parents’ request (sleep preservation, N = 5; agitation with the inhalation face mask, N = 5). |
AE: adverse event HS: hypertonic saline NS: normal saline SAE: serious adverse event
We downgraded the certainty of the evidence to low due to high clinical heterogeneity between studies (lack of standard collection and reporting) and risk of bias.
Discussion
Summary of main results
We included 34 trials involving 5205 infants (2727 infants received nebulised hypertonic saline) with acute viral bronchiolitis (21 inpatient trials (N = 2604); 2 outpatient trials (N = 194); 10 emergency department trials (N = 2307); 1 outpatient and emergency department trial (N = 100)) (Table 1).
Hospitalised infants treated with nebulised hypertonic saline may have a modest shorter mean length of hospital stay compared to those treated with nebulised normal saline or standard treatment (MD −0.40 days, 95% CI −0.69 to −0.11; low‐certainty evidence). Children treated with hypertonic saline may have a lower postinhalation clinical score than the normal saline group in the first three days of treatment (day 1: MD −0.64, 95% CI −1.08 to −0.21; day 2: MD −1.07, 95% CI −1.60 to −0.53; day 3: MD −0.89, 95% CI −1.44 to −0.34; low‐certainty evidence). These differences in clinical score represent 11% to 22% of the mean clinical score in the control group. Nebulised hypertonic saline may also reduce the risk of hospitalisation by 13% compared with nebulised 0.9% saline amongst children treated as outpatients and in emergency departments (RR 0.87, 95% CI 0.78 to 0.97; low‐certainty evidence). However, hypertonic saline may not reduce the risk of readmission to hospital up to 28 days after discharge (RR 0.83, 95% CI 0.55 to 1.25; low‐certainty evidence). We do not know if infants who received hypertonic saline had a lower number of days to resolution of wheezing (MD −1.16 days, 95% CI −1.43 to −0.89; 2 trials, 205 infants; very low‐certainty evidence), cough (MD −0.87 days, 95% CI −1.31 to −0.44; 3 trials, 363 infants; very low‐certainty evidence), or pulmonary moist crackles (MD −1.30 days, 95% CI −2.28 to −0.32; 2 trials, 205 infants; very low‐certainty evidence) compared to those who received normal saline.
Twenty‐seven trials involving 4416 infants (of which 2246 received nebulised hypertonic saline) reported treatment safety data. Fourteen of these trials did not report any significant adverse events amongst a total of 1624 infants (of which 767 received nebulised hypertonic saline, mixed with bronchodilators in 96% of infants). Thirteen trials involving 2792 infants (of which 1479 received nebulised hypertonic saline, alone in 72% of infants) reported at least one adverse event. In most cases, adverse events were mild and resolved spontaneously.
Overall completeness and applicability of evidence
In this 2022 update and in the 2017 update, we found that the effect size of nebulised hypertonic saline on reducing length of stay in hospitalised infants was approximately a third of what was found in the 2013 update of this review, which included six inpatient trials involving 500 infants (MD −1.15 days, 95% CI −1.49 to −0.82 days) (Zhang 2013). Moreover, all but two trials published in 2013 or later, including two European multicentre studies with relatively large sample sizes, did not find significant effects of nebulised hypertonic saline on length of hospital stay amongst inpatients with bronchiolitis (Bashir 2018; Hmar 2021). We found two main differences between recently published trials and those published before 2013. Virological investigation was available in 86% of trials published before 2013, whereas such testing was available in only 35% of trials published in 2013 and later. Another difference was that none of the seven older trials had a mean length of stay in the control group of less than three days, whilst 43% of the recently published trials had a mean length of stay in the control group of less than three days. These two factors may partially explain the inconsistency in results between older trials and trials published in 2013 and thereafter. However, the subgroup analyses failed to find a significant subgroup difference, and the meta‐regression analysis did not confirm an independent effect of these factors on the effect size of hypertonic saline.
For outpatients and emergency department infants, we found a 13% (RR 0.87, 95% CI 0.78 to 0.97) reduction in the risk of hospitalisation associated with nebulised hypertonic saline, in contrast to a 37% non‐statistically significant reduction shown in the 2013 review, which included four outpatient and emergency department trials involving 380 infants (RR 0.63, 95% CI 0.37 to 1.07).
Clinical score is generally considered a relatively objective measure to assess the severity of illness. In this review, 12 trials used the clinical severity score system proposed by Wang 1992, which assesses respiratory rate, wheezing, retraction, and general condition. The benefits of nebulised hypertonic saline in improving clinical score were observed in the first three days of treatment in both outpatients and inpatients. However, most emergency department trials failed to demonstrate significant effects of hypertonic saline in improving clinical score over a short time period (30 to 120 minutes). The validity of the Wang 1992 score system has not yet been assessed. Another commonly used approach for grading clinical severity was the Respiratory Distress Assessment Instrument (RDAI). However, RDAI may have poor to moderate construct validity, considerable test‐retest measurement error, and does not encompass all determinants of bronchiolitis severity (Fernandes 2015).
Potential side effects, principally acute bronchospasm, remain a concern with nebulised hypertonic saline. No significant adverse events were observed in 14 trials involving 1624 infants (767 treated with nebulised hypertonic saline). Saline solutions were co‐administered with bronchodilators in 96% of these infants. The majority of infants (74%) received saline solution alone. Most adverse events were mild and resolved spontaneously. These results suggest that nebulised hypertonic saline is a safe treatment in infants with bronchiolitis, especially when administered in conjunction with a bronchodilator.
Inhalation therapy was administrated via jet nebulisers in all the included studies except Tal 2006, which used ultrasonic nebulisers. There are some theoretical differences in the physical properties of aerosols produced by jet nebulisers and ultrasonic nebulisers, which may affect their therapeutic efficacy. On the one hand, ultrasonic nebulisers induce sputum more efficiently than jet nebulisers; on the other hand, jet nebulisers generate aerosols with smaller aerodynamic mass median diameter, which may more easily reach smaller bronchi and bronchioles. We could not provide direct evidence regarding the impact of the physical properties of aerosols generated by different types of nebulisers on the efficacy of inhaled hypertonic saline in infants with viral bronchiolitis. However, at least one trial demonstrated that both jet nebulisers and ultrasonic nebulisers are efficient methods of delivery of hypertonic saline for infants with bronchiolitis (Tal 2006).
The optimal treatment regimen for nebulised hypertonic saline in acute bronchiolitis remains unclear. Amongst inpatients, study solutions were given more frequently in 16 trials (every four to six hours) and less frequently in five trials (every eight hours). Subgroup analysis did not reveal a significant difference in reduction of length of hospital stay between regimens. Amongst outpatients and emergency department infants, effect size of nebulised hypertonic saline appeared to be greater when multiple doses (≥ two) of saline solutions were administered compared to a single dose.
The concentration of nebulised hypertonic saline was 3% in all but five trials (Al‐Ansari 2010; Köse 2016; Li 2014; Teunissen 2014; Tinsa 2014). We did not observe superiority of higher concentration (5%, 6%, and 7%) of hypertonic saline over 3% saline in improving clinical outcomes.
We included trials conducted in high‐ and low‐income countries, and in different settings (inpatient, outpatient, and emergency department). The evidence derived from this review may thus have wide applicability. However, as all but four included trials only recruited infants with mild to moderate bronchiolitis, care should be taken when extrapolating the findings of this review to infants with more severe bronchiolitis, such as those requiring mechanical ventilation, intensive care, or with oxygen saturation readings below 85% on room air (Awang 2020; Teunissen 2014; Uysalol 2017; Wu 2014). The underlying airway pathological changes may differ between severe and mild to moderate bronchiolitis, so different responses to treatments with hypertonic saline may be expected in children with more severe illness. Further trials are needed to assess the potential effects of nebulised hypertonic saline in infants hospitalised with severe acute bronchiolitis.
Despite our findings that the effects of nebulised hypertonic saline on reduction in length of hospital stay are smaller than were previously estimated, a reduction of almost 10 hours in length of hospital stay in infants with bronchiolitis may still be considered clinically relevant given the relatively short disease course, high prevalence, and huge burden of illness on healthcare systems around the world. Moreover, nebulised hypertonic saline may have benefits on other outcomes such as rate of hospitalisation and clinical severity score in infants with acute bronchiolitis, providing a good safety profile and low cost.
Certainty of the evidence
We had no serious concerns regarding three domains of the GRADE approach (indirectness, imprecision, and publication bias) for length of hospital stay. We downgraded the certainty of the evidence to low for length of hospital stay due to high levels of statistical heterogeneity and potential risk of selection bias in one‐third of the included trials. High heterogeneity could be expected given variations amongst trials in definitions of acute bronchiolitis, disease severity, standard care, intervention regimen, criteria for discharge, and risk of potential bias. We conducted several subgroup analyses to investigate the potential sources of heterogeneity, but moderate to high levels of heterogeneity persisted in most subgroup analyses.
We downgraded the certainty of the evidence to low for clinical severity score due to high heterogeneity and risk of bias, as mentioned above.
We downgraded the certainty of evidence to low for rate of hospitalisation. Despite the absence of statistical heterogeneity, there was substantial clinical heterogeneity between studies, especially in treatment regimens. The rate of hospitalisation in the control group also varied greatly between studies, from 1.3%, Anil 2010, to 52.2%, Angoulvant 2017. Publication bias was another concern because at least seven emergency department or outpatient trials were completed more than five years ago, but the results were neither published nor posted on clinical trials registries (NCT00677729; NCT01777347; NCT01834820; NCT02029040; NCT02045238; NCT02233985; NCT02834819).
Imprecision and risk of bias were the main reasons for downgrading the certainty of the evidence to low for rate of readmission to hospital after discharge. We downgraded the certainty of the evidence to very low for number of days to resolution of respiratory symptoms and signs, duration of in‐hospital oxygen supplementation, and radiological assessment score, due to high clinical heterogeneity between studies, imprecision, and risk of bias.
Potential biases in the review process
We searched for both published and unpublished trials to identify all relevant studies. We obtained additional trial data from five principal investigators. All included inpatient trials contributed data for meta‐analysis of length of hospital stay. However, the results of meta‐analyses of some secondary outcomes, such as clinical severity score and rate of readmission, may be biased because only some included trials contributed data for analysis. The number of trials and participants in outpatient and emergency department settings was limited: Wu 2014 and Angoulvant 2017 contributed 73% of weight to the overall summary estimate of effects of hypertonic saline on reduction of risk of hospitalisation. All studies except Everard 2014 used 0.9% saline as the comparison. The use of normal saline enables the trial to be double‐blind; however, normal saline is not technically a placebo, as high‐volume normal saline inhalation could potentially have physiological effects by improving airway mucociliary clearance, which may have beneficial effects on acute bronchiolitis (Wohl 2003). Use of normal saline as the control may tend to minimise the effect size of hypertonic saline.
Agreements and disagreements with other studies or reviews
Four published systematic reviews addressed the efficacy and safety of nebulised hypertonic saline in children with acute bronchiolitis (Badgett 2015; Chen 2014; Maguire 2015; Zhang 2017). We comparatively summarised the main findings of these four reviews in Table 7. The number of included trials varied from 11, in Chen 2014, to 28, in Zhang 2017. All four reviews used length of hospital stay as the efficacy outcome. The reduction in length of stay varied from −0.36 days (95% CI −0.50 to −0.22; 15 trials), Maguire 2015, to −0.96 days (95% CI −1.38 to −0.54; 6 trials), Chen 2014. Two reviews assessed the effects of hypertonic saline on rate of hospitalisation, and the reduction varied from 41% (RR 0.59, 95% CI 0.37 to 0.93; 5 trials), Chen 2014, to 14% (RR 0.86, 95% CI 0.76 to 0.98; 8 trials), Zhang 2017.
6. Comparative summary: main findings of 4 systematic reviews addressing efficacy and safety of nebulised hypertonic saline for infants with acute bronchiolitis.
| Review | Trials included (n) | Participants (N) | Hospital length‐of‐stay reduction (MD, 95% CI) | Clinical score reduction (MD, 95% CI) | Days to resolution of symptoms and signs reduction (MD, 95% CI) | Hospitalisation rate reduction (RR, 95% CI) | Readmission rate reduction (RR, 95% CI) | Other findings |
| Chen 2014 | 11 | 1070 (infants with previous wheeze excluded) |
−0.96 days (−1.38 to −0.54) (6 trials) |
Day 1: −0.77 (−1.30 to −0.24) Day 2: −0.85 (−1.30 to −0.39) Day 3: −1.14 (−1.69 to −0.58) (6 trials) |
‐ | 0.59 (0.37 to 0.93) (5 trials) |
1.08 (0.68 to 1.73) (3 trials) |
None |
| Badgett 2015 | 19 | 2441 | −0.42 days (−0.72 to −0.11) (19 trials) |
‐ | ‐ | ‐ | ‐ | ‐ |
| Maguire 2015 | 15 | 1922 | −0.36 days (−0.50 to −0.22) (15 trials) |
−1.36 (−1.52 to −1.20) (5 trials) |
‐ | ‐ | ‐ | ‐ |
| Zhang 2017 | 28 | 4195 (infants with previous wheeze excluded) |
−0.41 days (−0.75 to −0.07) (17 trials) |
Day 1: −0.77 (−1.18 to −0.36) (9 trials) Day 2: −1.28 (−1.91 to −0.65) (8 trials) Day 3: −1.43 (−1.82 to −1.04) (7 trials) |
Wheezing: −1.16 days (−1.43 to −0.89) (2 trials) Cough: −1.01 days (−1.35 to −0.66) (2 trials) Pulmonary moist crackles: −1.30 days (−2.28 to −0.32) (2 trials) |
0.86 (0.76 to 0.98) (8 trials) |
Readmission to
hospital 0.77 (0.48 to 1.25) (6 trials) |
No significant difference between the hypertonic saline group and the control group in terms of oxygen saturation, duration of oxygen supplementation, respiratory rate, heart rate, and radiograph scores |
CI: confidence interval MD: mean difference RR: risk ratio
Authors' conclusions
Implications for practice.
Current evidence suggests that nebulised hypertonic saline may modestly reduce the length of hospital stay amongst infants hospitalised with acute viral bronchiolitis, and may slightly improve clinical severity scores. Treatment with nebulised hypertonic saline may also reduce the risk of hospitalisation amongst outpatients and emergency department infants. We do not know if nebulised hypertonic saline reduces the number of days to resolution of wheezing, cough, and pulmonary moist crackles. Nebulised hypertonic saline seems to be a safe treatment in infants with bronchiolitis with only minor and spontaneously resolved adverse events, especially when administered in conjunction with a bronchodilator. The certainty of the evidence was low to very low for all outcomes.
Implications for research.
Further multicentre randomised trials are required to evaluate the efficacy and safety of nebulised hypertonic saline in infants with acute bronchiolitis, in inpatient, outpatient, and emergency department settings. There are some common challenges for all clinical trials in infants with acute bronchiolitis. The currently used definition of 'bronchiolitis' may include a heterogeneous group of patients with different underlying aetiologies and pathologies. The development of valid diagnostic criteria for acute bronchiolitis in infants is urgently needed. There is a lack of robust and well‐accepted efficacy outcome measures. Length of hospital stay and rate of hospitalisation are the most clinically important endpoints, but they are usually more susceptible to bias. Well‐defined valid admission and discharge criteria should thus be used. Further trials should have sufficient statistical power to detect modest but clinically relevant effects of the intervention. The optimal treatment regimen of nebulised hypertonic saline for infants with acute bronchiolitis remains to be determined. The mechanism of action of nebulised hypertonic saline in infants with viral bronchiolitis also needs to be addressed in future studies.
What's new
| Date | Event | Description |
|---|---|---|
| 4 April 2023 | New search has been performed | We updated our searches and added new analyses. We included six new trials in this updated review (Awang 2020; Bashir 2018; Hmar 2021; Jaquet‐Pilloud 2020; Morikawa 2017; Uysalol 2017), and excluded two new trials (Teijeiro 2018; Sapkota 2021). We updated all figures and tables, and revised the review text. |
| 4 April 2023 | New citation required but conclusions have not changed | Conclusions remain unchanged. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 11 August 2017 | New citation required and conclusions have changed | Nebulised hypertonic saline may modestly reduce the length of hospital stay among infants hospitalised with acute bronchiolitis and improve clinical severity score. Nebulised hypertonic saline may also reduce the risk of hospitalisation amongst outpatients and emergency department patients. |
| 11 August 2017 | New search has been performed | We updated the searches and conducted new analyses. We created and examined a funnel plot to explore possible small‐study effects and publication bias. We revised the review text. We included 17 new trials in this updated review (Angoulvant 2017; NCT01238848; Everard 2014; Flores 2016; Florin 2014; Jacobs 2014; Khanal 2015; Köse 2016; Li 2014; Mahesh Kumar 2013; Ojha 2014; Pandit 2013; Ratajczyk‐Pekrul 2016; Sharma 2013; Teunissen 2014; Tinsa 2014; Wu 2014). We excluded nine new trials (Al‐bahadily 2017; Bagus 2012; Bueno Campaña 2014; Flores‐González 2016; Flores‐González 2016; Gupta 2016; Malik 2015; Nenna 2014; Silver 2015). Nine studies await classification (CTRI/2010/091/003065a; EudraCT2009‐014758‐14a; NCT00677729a; NCT01777347a; NCT01834820a; NCT02029040a; NCT02045238a; NCT02233985a; NCT02834819a). We conducted additional post hoc subgroup, sensitivity, and meta‐regression analyses. |
| 8 May 2013 | New search has been performed | Searches conducted. We included four new trials and performed new analyses (Al‐Ansari 2010; Miraglia Del Giudice 2012; Ipek 2011; Luo 2011). Our conclusions remain unchanged. |
| 7 June 2010 | New search has been performed | Searches conducted. We included three new trials and conducted new analyses (Anil 2010; Grewal 2009; Luo 2010). The conclusions remain unchanged. |
| 10 May 2010 | New search has been performed | Searches conducted. We included three new trials and performed new analyses (Anil 2010; Grewal 2009; Luo 2010). Our conclusions remain unchanged. |
| 13 May 2009 | Amended | No changes; republished to fix technical problem |
| 18 February 2008 | Amended | Converted to new review format |
| 13 November 2007 | New search has been performed | Searches conducted. |
Acknowledgements
For previous versions of this review, we thank Ruth Foxlee, Sarah Thorning, Justin Clark, and David Honeyman for help in defining the search strategy and running the literature searches. Thanks also to Libby Lissiman for assistance in the early stages of this review and to the Cochrane Acute Respiratory Infections Group, especially Liz Dooley and Ann Jones, for editorial assistance. The authors wish to thank the following people for commenting on previous drafts of this review: Hayley Edmonds, Avigdor Mandelberg, Federico Martinón Torres, Sree Nair, Amy Zelmer, Karen Lee, Anne Lyddiatt, Federico Martinón Torres, and Meenu Singh.
The following people conducted the editorial process for this review update:
Sign‐off Editors (final editorial decision): Mark Jones (Bond University, Australia); Mieke van Driel (The University of Queensland, Australia).
Managing Editors (provided editorial guidance to authors, edited the review, selected peer reviewers, collated peer‐reviewer comments): Liz Dooley (Bond University, Australia); Fiona Russell (Bond University, Australia).
Contact Editor (provided comments and recommended an editorial decision): Meenu Singh (Post Graduate Institute of Medical Education and Research, India).
Statistical Editor (provided comments): Mark Jones (Bond University, Australia).
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Copy Edit Support.
Peer reviewers (provided comments and recommended an editorial decision):
Clinical/content review: Avigdor Mandelberg MD (Pediatric Pulmonary Unit, The E. Wolfson Medical Center, Sackler Faculty of Medicine, Tel Aviv University).
Consumer review: Juliana Ester Martin‐Lopez, PhD (Medical Researcher at Andalusian Public Foundation Progress and Health, Spain).
Methods review: Rachel Richardson (Associate Editor, Cochrane).
Search review: Justin Clark (Institute for Evidence‐Based Healthcare, Bond University, Australia); Anne Littlewood, Cochrane Oral Health.
Appendices
Appendix 1. MEDLINE and Embase (Ovid) search strategy
1 exp Bronchiolitis/ 2 (bronchiolit* or wheez*).tw. 3 respiratory syncytial viruses/ or respiratory syncytial virus, human/ 4 Respiratory Syncytial Virus Infections/ 5 (respiratory syncytial virus* or rsv).tw. 6 parainfluenza virus 1, human/ or parainfluenza virus 3, human/ 7 Parainfluenza Virus 2, Human/ 8 Respirovirus Infections/ 9 Adenovirus Infections, Human/ 10 Rhinovirus/ 11 Influenza, Human/ 12 exp influenzavirus a/ or exp influenzavirus b/ 13 (parainfluenza* or respirovirus* or adenovirus* or rhinovirus* or influenza*).tw. 14 or/1‐13 15 Saline Solution, Hypertonic/ 16 (hypertonic adj3 (saline or solution*)).tw. 17 Sodium Chloride/ 18 (sodium chloride or saline).tw. 19 or/15‐18 20 exp "Nebulizers and Vaporizers"/ 21 (nebuli* or vapor* or vapour* or atomi*).tw. 22 Administration, Inhalation/ 23 inhal*.tw. 24 Aerosols/ 25 aerosol*.tw. 26 or/20‐25 27 14 and 19 and 26
Appendix 2. CENTRAL search strategy
24. #12 AND #16 AND #23 23. #17 OR #18 OR #19 OR #20 OR #21 OR #22 22. aerosol*:ab,ti 21. 'aerosol'/de 20. inhal*:ab,ti 19. 'inhalational drug administration'/de 18. nebuli*:ab,ti OR vapour*:ab,ti OR vapour*:ab,ti OR atomi*:ab,ti 17. 'nebulizer'/exp 16. #13 OR #14 OR #15 15. 'sodium chloride':ab,ti OR saline:ab,ti 14. (hypertonic NEAR/3 (saline OR solution*)):ab,ti 13. 'hypertonic solution'/de OR 'sodium chloride'/de 12. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 11. parainfluenza*:ab,ti OR respirovirus*:ab,ti OR adenovirus*:ab,ti OR rhinovirus*:ab,ti OR influenza*:ab,ti 10. 'influenza virus'/de OR 'influenza virus a'/exp OR 'influenza virus b'/de OR 'influenza'/exp 9. 'rhinovirus infection'/de 8. 'human adenovirus infection'/de 7. 'respirovirus infection'/de 6. 'parainfluenza virus 1'/de OR 'parainfluenza virus 2'/de OR 'parainfluenza virus 3'/de 5. 'respiratory syncytial virus':ab,ti OR 'respiratory syncytial viruses':ab,ti OR rsv:ab,ti 4. 'respiratory syncytial virus infection'/de 3. 'respiratory syncytial pneumovirus'/de 2. bronchiolit*:ab,ti 1. 'bronchiolitis'/exp
Appendix 3. LILACS search strategy
> Search > (MH:Bronchiolitis OR bronchiolit$ OR Bronquiolitis OR Bronquiolite OR MH:C08.127.446.135$ OR MH:C08.381.495.146.135$ OR MH:C08.730.099.135$ OR wheez$ OR MH:"Respiratory Syncytial Viruses" OR "Virus Sincitiales Respiratorios" OR "Vírus Sinciciais Respiratórios" OR "Respiratory Syncytial Virus, Human" OR "Respiratory Syncytial Virus Infections" OR "Infecciones por Virus Sincitial Respiratorio" OR "Infecções por Vírus Respiratório Sincicial" OR rsv "respiratory syncytial virus" OR "respiratory syncytial virus infection" OR "respiratory syncytial virus infections") AND (MH:"Saline Solution, Hypertonic" OR "Solución Salina Hipertónica" OR "Solução Salina Hipertônica" OR "Hypertonic Saline Solution" OR "Solución Hipertónica de Cloruro de Sodio" OR "Solução Salina Hipertônica" OR "Solução Hipertônica de Cloreto de Sódio" OR MH:"Sodium Chloride" OR "sodium chloride" OR "Cloruro de Sodio" OR "Cloreto de Sódio" OR salin$) AND (MH:"Nebulizers and Vaporizers" OR MH:E07.605$ OR atomi$ OR inhal$ OR vapor$ OR vapour$ OR nebuli$ OR Inala$ OR MH:Aerosols OR aerosol$ OR Aerossóis OR MH:"Administration, Inhalation" OR "Administración por Inhalación" OR "Administração por Inalação")
Appendix 4. CINAHL (EBSCO) search strategy
S22 S10 and S15 and S21 S21 S16 or S17 or S18 or S19 or S20 S20 TI (inhal* or aerosol*) OR AB (inhal* or aerosol*) S19 (MH "Aerosols") S18 (MH "Administration, Inhalation") S17 TI (nebuli* or vapor* or vapour* or atomi*) OR AB (nebuli* or vapor* or vapour* or atomi*) S16 (MH "Nebulizers and Vaporizers") S15 S11 or S12 or S13 or S14 S14 TI (sodium chloride or saline) OR AB (sodium chloride or saline) S13 (MH "Sodium Chloride") S12 TI (hypertonic N3 (salin* or solut*)) OR AB (hypertonic N3 (salin* or solut*)) S11 (MH "Saline Solution, Hypertonic") S10 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 S9 TI (influenza* or flu) OR AB (influenza* or flu) S8 (MH "Influenzavirus A+") OR (MH "Influenzavirus B+") S7 (MH "Influenza") OR (MH "Influenza, Human") OR (MH "Influenza A H5N1") OR (MH "Influenza, Pandemic (H1N1) 2009") OR (MH "Influenza, Seasonal") S6 TI (parainfluenza* or respirovirus* or adenovirus* or rhinovirus*) OR AB (parainfluenza* or respirovirus* or adenovirus* or rhinovirus*) S5 TI (respiratory syncytial virus* or rsv) OR AB (respiratory syncytial virus* or rsv) S4 (MH "Respiratory Syncytial Virus Infections") S3 (MH "Respiratory Syncytial Viruses") S2 TI (bronchiolit* or wheez*) OR AB (bronchiolit* or wheez*) S1 (MH "Bronchiolitis+")
Appendix 5. Web of Science (Thomson Reuters) search strategy
| # 3 | 93 | #2 AND #1 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 2 | 1,322,438 | Topic=(random* or placebo* or ((single or double) NEAR/1 blind*) or allocat* or (clinical NEAR/1 trial*)) OR Title=(trial) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 1 | 173 | Topic=(bronchiolit* or wheez* or "respiratory syncytial virus" or "respiratory syncytial viruses" or rsv or parainfluenza* or "respirovirus infection" or "respirovirus infections" or rhinovirus* or adenovirus* or influenza*) AND Topic=((hypertonic NEAR/3 (salin* or solut*)) or "sodium chloride" or saline) AND Topic=(nebuli* or vapor* or vapour* or atomi* or inhal* or aerosol*) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
Appendix 6. ClinicalTrials.gov search strategy
(saline OR sodium chloride) | acute bronchiolitis OR RSV or respiratory syncytial virus* OR Respirovirus* OR Rhinoviurs OR influenza | Child
Applied Filters: Child (birth–17)
Appendix 7. WHO ICTRP search strategy
(saline OR sodium chloride) | acute bronchiolitis OR RSV or respiratory syncytial virus* OR Respirovirus* OR Rhinoviurs OR influenza | Child |
Search for clinical trials in children
Appendix 8. Previous searches
For the 2017 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4), part of the Cochrane Library (searched 11 August 2017), which contains the Cochrane Acute Respiratory Infections Group Specialised Register, MEDLINE Ovid, MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily (April 2013 to 11 August 2017), Embase.com (April 2013 to 11 August 2017), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCO; May 2013 to 11 August 2017), LILACS (Latin American and Caribbean Health Science Information Database) (May 2013 to 11 August 2017), and Web of Science (May 2013 to 11 August 2017). The strategies are as below for MEDLINE (Appendix 1), Embase (Appendix 2), LILACS (Appendix 3), CINAHL (Appendix 4), and Web of Science (Appendix 5). The previous searches for Embase and Cochrane were adapted for different interfaces and shown. ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) were also searched to identify new or ongoing trials.
For the 2013 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 4, part of The Cochrane Library, www.thecochranelibrary.com (accessed 8 May 2013), which contains the Cochrane Acute Respiratory Infections Group Specialised Register, MEDLINE (May 2010 to April week 4, 2013), EMBASE (June 2010 to April 2013) and LILACS (June 2010 to May 2013). We broadened our search to include two further databases and searched CINAHL (1981 to May 2013) and Web of Science (1955 to May 2013). We used the search strategy detailed in Appendix 2 to search MEDLINE and CENTRAL. As there were so few search results we used no filter to identify randomised trials in MEDLINE. We adapted the search terms to search EMBASE (Appendix 2), LILACS (Appendix 3), CINAHL (Appendix 4) and Web of Science (Appendix 5).
For the 2010 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 2), which contains the Cochrane Acute Respiratory Infections Group Specialised Register, OLDMEDLINE (1951 to 1965), MEDLINE (1966 to May Week 4, 2010), EMBASE (1974 to June 2010) and LILACS (1985 to June 2010).
For the original search we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 4), which contains the Cochrane Acute Respiratory Infections Group Specialised Register, OLDMEDLINE (1951 to 1965), MEDLINE (1966 to November 2007), EMBASE (1974 to November 2007) and LILACS (November 2007).
For the original search and the 2010 update the following search terms were combined with the highly sensitive search strategy as recommended by The Cochrane Collaboration (Dickersin 1994) to search MEDLINE. These terms were adapted to search CENTRAL, EMBASE and LILACS as required.
Data and analyses
Comparison 1. Hypertonic saline versus normal saline or standard treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Length of hospital stay (days) | 21 | 2479 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.69, ‐0.11] |
| 1.1.1 Hypertonic saline plus salbutamol/albuterol versus normal saline plus salbutamol/albuterol | 12 | 1404 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.48, 0.22] |
| 1.1.2 Hypertonic saline plus epinephrine versus normal saline plus epinephrine or normal saline alone | 5 | 348 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.01, ‐0.29] |
| 1.1.3 Hypertonic saline alone versus normal saline alone | 4 | 436 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.60, ‐0.66] |
| 1.1.4 Hypertonic saline versus standard treatment | 1 | 291 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.62, 0.74] |
| 1.2 Rate of hospitalisation | 8 | 1760 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.78, 0.97] |
| 1.2.1 Hypertonic saline plus bronchodilator versus normal saline plus bronchodilator | 5 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.55, 1.10] |
| 1.2.2 Hypertonic saline alone versus normal saline alone | 4 | 1302 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.08] |
| 1.3 Clinical severity score (post‐treatment) at day 1 | 10 | 893 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.08, ‐0.21] |
| 1.3.1 Outpatients | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐1.92, ‐0.64] |
| 1.3.2 Emergency department patients | 1 | 171 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.51, 0.33] |
| 1.3.3 Inpatients | 8 | 657 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.15, ‐0.13] |
| 1.4 Clinical severity score (post‐treatment) at day 2 | 10 | 907 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.60, ‐0.53] |
| 1.4.1 Outpatients | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐2.93, ‐1.07] |
| 1.4.2 Emergency department patients | 1 | 171 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.63, 0.09] |
| 1.4.3 Inpatients | 8 | 671 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.68, ‐0.47] |
| 1.5 Clinical severity score (post‐treatment) at day 3 | 10 | 785 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.44, ‐0.34] |
| 1.5.1 Outpatients | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐2.64 [‐3.85, ‐1.43] |
| 1.5.2 Inpatients | 9 | 720 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.31, ‐0.18] |
| 1.6 Rate of readmission to hospital | 6 | 1084 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.55, 1.25] |
| 1.7 Number of days to resolution of symptoms and signs (days) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.7.1 Wheezing | 2 | 205 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.43, ‐0.89] |
| 1.7.2 Cough | 3 | 363 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.31, ‐0.44] |
| 1.7.3 Pulmonary moist crackles | 2 | 205 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.28, ‐0.32] |
| 1.8 Duration of in‐hospital oxygen supplementation (hours) | 3 | 269 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐9.36, 8.86] |
| 1.9 Radiological assessment score | 2 | 117 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.90, 0.75] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Ansari 2010.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: paediatric emergency facility in Qatar
Assessed for eligibility: 187
Randomised: 115 hypertonic saline group (5% saline: 57; 3% saline: 58); 56 normal saline group
Completed: 115 hypertonic saline group; 56 normal saline group
Gender (male): 59.1%
Age (mean ± SD): 3.8 ± 2.8 months in 3% saline group; 4.0 ± 2.5 months in 5% saline group; 3.3 ± 2.4 months in normal saline group Inclusion criteria: infants aged ≤ 18 months, with a prodromal history of viral upper respiratory tract infection, followed by wheezing or crackles, or both on auscultation and Wang clinical severity score ≥ 4 Exclusion criteria: born at ≤ 34 weeks’ gestation, previous history of wheezing, steroid use within 48 h of presentation, obtundation and progressive respiratory failure requiring ICU admission, history of apnoea within 24 hours before presentation, SaO₂ ≤ 85% on room air at the time of recruitment, history of a diagnosis of chronic lung disease, congenital heart disease, or immunodeficiency |
|
| Interventions | Intervention groups:
Group 1: nebulised 5% saline (5 mL) plus 1.5 mL of epinephrine
Group 2: nebulised 3% saline (5 mL) plus 1.5 mL of epinephrine Control group: nebulised 0.9% saline (5 mL) plus 1.5 mL of epinephrine Treatment was given every 4 hours, until the infant was ready for discharge. Nebulised medications were delivered through a tight‐fitting face mask by pressurised oxygen with the flow meter set at 10 L/min. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Supported by Hamad Medical Corporation, which employs all but 1 author who previously worked at Hamad. The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation program |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered and sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals after randomisation reported. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Angoulvant 2017.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: 24 paediatric emergency departments in France
Assessed for eligibility: 2445
Randomised: 387 hypertonic saline group; 390 normal saline group
Completed: 385 hypertonic saline group; 387 normal saline group
Gender (male): 60.2%
Age: median (interquartile range): 3 (2 to 5) months in hypertonic saline group; 3 (2 to 5) months in normal saline group Inclusion criteria: infants aged 6 weeks to 12 months with first episode of moderate to severe bronchiolitis defined as viral upper respiratory tract infection plus wheezing or crackles, or both on chest auscultation with respiratory distress Exclusion criteria: premature birth (birth before 37 weeks of gestation), immunologic, cardiac, or chronic pulmonary disease, bone malformation of the chest, previous use of nebulised hypertonic saline, inability to communicate with the family (a language barrier or lack of telephone for contact), need of admission to a paediatric ICU |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) Control group: nebulised 0.9% saline (4 mL) Study medication was given at 0 and 30 min using a jet nebuliser through a firmly applied face mask with an oxygen flow rate of 6 L/min. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 84.5% in hypertonic saline group; 88.2% in control group Supported by grant P110143/IDRCB2012‐A00228‐35 from the French Hospital Program for Clinical Research/French Ministry of Health. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation sequence using a 1:1 ratio and permutation blocks with a block size of 4, stratified according to centre |
| Allocation concealment (selection bias) | Low risk | The investigational pharmacy prepared the study drugs in sequentially numbered and visually identical packets. Randomisation codes were kept secure until data entry was complete. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Hospitalisation data were not available for 5 infants (2 in hypertonic saline group and 3 in normal saline group). |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Anil 2010.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: emergency department of a teaching hospital in Turkey
Assessed for eligibility: 190
Randomised: 75 hypertonic saline group; 111 normal saline group
Completed: 75 hypertonic saline group; 111 normal saline group
Gender (male): 64.5%
Age (mean ± SD): 9.5 ± 5.3 months (range 1.5 to 24 months) Inclusion criteria: infants with diagnosis of bronchiolitis requiring a history of upper respiratory infection and the presence of bilateral wheezing or crackles, or both on chest auscultation, plus clinical severity score between 1 and 9 Exclusion criteria: prematurity, any underlying disease (e.g. cystic fibrosis, bronchopulmonary dysplasia, and cardiac or renal disease), prior history of wheezing, atopic dermatitis, allergic rhinitis or asthma, SaO₂ < 85% on room air, clinical severity score > 9, obtunded consciousness, progressive respiratory failure requiring mechanical ventilation, previous treatment with bronchodilators, and any steroid therapy within 2 weeks |
|
| Interventions | Intervention groups:
Group 1: nebulised 3% saline (4 mL) plus 1.5 mg epinephrine
Group 2: nebulised 3% saline (4 mL) plus 2.5 mg salbutamol Control groups: Group 3: nebulised 0.9% saline (4 mL) plus 1.5 mg epinephrine Group 4: nebulised 0.9% saline (4 mL) plus 2.5 mg salbutamol Group 5: nebulised 0.9% saline (4 mL) alone The study drug was administered at 0 and 30 min by Medic‐Aid Sidestream nebuliser (Medic‐Aid Ltd, West Sussex, UK) using a face mask with continuous flow of 100% oxygen at 6 L/min. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation program |
| Allocation concealment (selection bias) | Unclear risk | Study medications were identical in appearance and odour, but no other details were provided regarding allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals after randomisation reported. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Awang 2020.
| Study characteristics | ||
| Methods | Design: double‐blind, randomised controlled trial | |
| Participants | Setting: inpatient ward of a university tertiary hospital in Malaysia
Assessed for eligibility: 277
Randomised: 52 hypertonic saline group; 49 normal saline group
Completed: 52 hypertonic saline group; 48 normal saline group
Gender (male): 48.5%
Age (mean ± SD): 7.2 ± 3.78 months in hypertonic saline group; 8.0 ± 3.71 months in normal saline group Inclusion criteria: previously healthy infants younger than 18 months, having first hospitalisation with mild to moderate acute bronchiolitis, defined as strong clinical suspicion of viral lower respiratory tract infection associated with airways obstruction as manifested by hyperinflation, tachypnoea and subcostal recession with widespread crepitations, prolonged expiratory phase and rhonchi on auscultation Exclusion criteria: other lower airway infection such as pneumonia, chronic respiratory disorders, i.e. chronic lung disease, congenital lung or airway malformation, bronchial asthma, previous hospitalisation for wheezing episode, and other underlying chronic illnesses such as gastro‐oesophageal reflux and congenital heart disease |
|
| Interventions | Intervention group: nebulised 3% saline (3.5 mL) plus salbutamol (2.5 mg, 0.5 mL) Control group: nebulised 0.9% normal saline (3.5 mL) plus salbutamol (2.5 mg, 0.5 mL) The study solutions were given every 6 hours, via a nebuliser with mask and oxygen (5 L/min), until clinical severity score ≤ 4. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funded by a Short Term grant (304/PPSP/61313039) from the Universiti Sains Malaysia. Declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation program |
| Allocation concealment (selection bias) | Low risk | Sealed and opaque envelopes containing the choice of therapy, which were numbered accordingly |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parents, care providers, investigators, and outcome assessor were blinded. Preparation of the nebulised solutions was done by an independent pharmacist. The solutions were indistinguishable by colour, appearance, or smell. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 withdrawal after randomisation in the control group |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Bashir 2018.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind controlled trial | |
| Participants | Setting: inpatient ward of a tertiary teaching hospital, India
Assessed for eligibility: 214
Randomised: 96 hypertonic saline group; 93 normal saline group
Completed: 95 hypertonic saline group; 89 normal saline group
Gender (male): 67%
Age (mean, 95% CI): 4.0 (2.63 to 8.0) months in hypertonic saline group; 4.0 (2.0 to 7.0) months in normal saline group Inclusion criteria: previously healthy infants, aged 2 to 18 months, hospitalised with first episode of respiratory tract infection with wheeze, starting as a viral upper respiratory infection (coryza, cough, or fever), and with a clinical score between 4 and 8 Exclusion criteria: previous episode of wheezing, chronic cardiopulmonary disease or immunodeficiency; critical illness at presentation requiring admission to intensive care; the use of nebulised hypertonic saline within the previous 12 hours; or premature birth (gestational age 34 weeks) |
|
| Interventions | Intervention group: nebulised 3% saline solution (4 mL) Control group: nebulised 0.9% saline solution (4 mL) The study solutions were given every 2 hours for 3 doses, followed by every 4 hours for 6 doses, then by every 6 hours until discharge. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding sources not provided. The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation program |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study solutions were prepared to be identical appearance. Codes of solutions were blinded to all participants, care providers, and investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 (2.6%) withdrawals after randomisation, 1 in the hypertonic saline group and 4 in the normal saline group. All infants included in the final intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Everard 2014.
| Study characteristics | ||
| Methods | Design: multicentre, parallel‐group, open, randomised controlled trial | |
| Participants | Setting: assessment units and paediatric wards of 10 participating centres in England and Wales
Assessed for eligibility: 772
Randomised: 158 hypertonic saline group (3% saline); 159 standard care group
Completed: 141 hypertonic saline group; 149 standard care group
Gender (male): 54.5%
Age (mean ± SD): 3.3 ± 2.6 months in hypertonic saline group; 3.4 ± 2.8 months in standard care group Inclusion criteria: infants < 12 months with diagnosis of bronchiolitis defined as an apparent viral respiratory tract infection associated with airways obstruction manifest by hyperinflation, tachypnoea and subcostal recession with widespread crepitations on auscultation, needing supplementary oxygen for SaO₂ of < 92% in air Exclusion criteria: history of wheezy bronchitis or asthma, gastro‐oesophageal reflux, previous lower respiratory tract infections, risk factors for severe disease, carers lacking fluent English in the absence of translator service, and requiring admission to high‐dependency or intensive care units at presentation |
|
| Interventions | Intervention group: 4 mL 3% saline + standard care Control group: standard care Hypertonic saline given every 6 h, administered via PARI Sprint nebuliser with appropriate face mask, until primary outcome achieved. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 58.5% in hypertonic saline group; 64.4% in control group Funded by the National Institute for Health Research Health Technology Assessment (HTA) Programme (project number 09/91/22). All but 1 author declare no conflicts of interest. PSMN received personal fees/honoraria from Paul Alios Biopharma and Janssen Pharmaceuticals for consultancy and advisory board membership. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation in blocks of size 2, 4, and 6, stratified by hospital |
| Allocation concealment (selection bias) | Low risk | Centralised web‐based randomisation system |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27 (8.5%) withdrawals after randomisation (17 hypertonic saline group, 10 control group). Reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Flores 2016.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: paediatric ward of a general urban hospital in Portugal
Assessed for eligibility: not stated
Randomised: 38 hypertonic saline group (3% saline); 40 normal saline group
Completed: 33 hypertonic saline group; 35 normal saline group
Gender (male): 52.9%
Age (mean ± SD): 3.3 ± 2.4 months hypertonic saline group; 3.8 ± 2.5 months normal saline group Inclusion criteria: infants aged < 12 months with acute bronchiolitis, defined as an apparent viral respiratory tract infection manifested by nasal discharge and wheezy cough, with presence of fine inspiratory crackles and/or high‐pitched expiratory wheeze, even apnoea Exclusion criteria: previous episodes of wheezing, personal history of prematurity (gestational age < 34 weeks), physician diagnosis of eczema, food allergy, or chronic (cardiac, respiratory, immunological, neurological, or metabolic) disease, and high severity criteria (coma, respiratory rate > 80 breaths/minute, SaO₂ < 88% on room air or need for assisted ventilation) |
|
| Interventions | Intervention group: nebulised 3% saline (3 mL) plus 0.25 mL (1.25 mg) salbutamol Control group: nebulised 0.9% saline (3 mL) plus 0.25 mL (1.25 mg) salbutamol Treatment was given every 6 h until discharge. All inhaled therapies were delivered through a tight‐fitting face mask from an oxygen‐driven nebuliser (Cirrus 2 Nebuliser, Wokingham, Berkshire, UK), connected to a source of pressurised oxygen from the wall, set to a flow rate of 6 L/min. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 87.9% in hypertonic saline group; 82.9% in normal saline group Funding source: none The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Both solutions were similar in appearance and smell, stored in identical syringes, and labelled only by a code number. Randomisation list was concealed by the pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 (12.8%) withdrawals after randomisation (5 hypertonic saline group, 5 control group) because of clinical deterioration with need for ICU |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Florin 2014.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: urban paediatric emergency department in the USA
Assessed for eligibility: 2256
Randomised: 31 hypertonic saline group (3% saline); 31 normal saline group
Completed: 31 hypertonic saline group; 31 normal saline group
Gender (male): 45.2%
Age (mean ± SD): 7.2 ± 5.1 months in hypertonic saline group; 6.1 ± 3.6 months in normal saline group Inclusion criteria: children aged 2 months up to 24 months presenting to the emergency department with acute bronchiolitis, defined as a first episode of wheezing associated with signs and symptoms of respiratory distress and upper respiratory infection, with RDAI score of 4 to 15 (moderate to severe) Exclusion criteria: infants with a history of wheezing or asthma, bronchodilator therapy prior to the current illness, chronic lung or heart disease, critical illness, inability to receive nebulised medications, and infants with non–English‐speaking guardians |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) Control group: nebulised 0.9% saline (4 mL) Treatment delivered using a jet nebuliser with an oxygen flow rate of 8 L/min. Study medication was given within 90 minutes after albuterol administration. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Supported by a Young Investigator Award from the Academic Pediatric Association. The Academic Pediatric Association had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted block randomisation |
| Allocation concealment (selection bias) | Low risk | The investigational pharmacy prepared the study medications, which were stored in sequentially numbered envelopes with blinded syringes labelled only with the study number. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Grewal 2009.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: emergency department of a children's hospital in Canada
Assessed for eligibility: not stated
Randomised: 24 hypertonic saline group; 24 normal saline group
Completed: 23 hypertonic saline group; 23 normal saline group
Gender (male): 60.9%
Age (mean ± SD): 5.6 ± 4.0 months in hypertonic saline group; 4.4 ± 3.4 months in normal saline group Inclusion criteria: infants aged 6 weeks to 12 months presenting with a first episode of wheezing and clinical symptoms of a viral respiratory infection, plus an initial SaO₂ of 85% or more but 96% or less, and RDAI score ≥ 4 Exclusion criteria: pre‐existing cardiac or pulmonary disease, previous diagnosis of asthma by a physician, any previous use of bronchodilators (except for treatment of the current illness), severe disease requiring resuscitation room care, inability to take medication using a nebuliser, inability to obtain informed consent secondary to a language barrier, or no phone access for follow‐up |
|
| Interventions | Intervention group: nebulised 3% saline (2.5 mL) plus 0.5 mL 2.25% racaemic epinephrine Control group: nebulised 0.9% saline (2.5 mL) plus 0.5 mL 2.25% racaemic epinephrine Both groups received inhalation solutions at 0 minutes. Each treatment was given by nebuliser with continuous flow of oxygen at 6 L/min. 2 doses of the study drug were available for each infant such that, if the physician felt that a second dose of racaemic epinephrine was needed during the 120‐minute study period, the infant received the same drug combination again. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 82.6% in hypertonic saline group; 81.8% in normal saline group Supported by the Department of Pediatrics, University of Alberta; and the Alberta Research Centre for Child Health Evidence. Personnel for data collection were funded by the Department of Pediatrics, University of Alberta. Statistical analysis and interpretation of data were graciously provided by the Alberta Research Centre for Child Health Evidence. The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Website randomisation scheme |
| Allocation concealment (selection bias) | Low risk | The solutions prepared by the hospital pharmacy were similar in appearance and smell, stored in identical syringes, labelled only by a code number, and placed in the research cupboard within the emergency department. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (4.1%) withdrawals (1 hypertonic saline group; 1 normal saline group) |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Hmar 2021.
| Study characteristics | ||
| Methods | Design: randomised clinical trial | |
| Participants | Setting: inpatient ward of a hospital in India
Assessed for eligibility (n): not stated
Randomised: 79 hypertonic saline group; 79 normal saline group
Completed: 79 hypertonic saline group; 79 normal saline group
Gender (male, %): 56.9%
Age (months, mean ± SD): 10.02 ± 5.45 months in hypertonic saline group; 8.45 ± 4.88 months in normal saline group Inclusion criteria: children aged 3 months to 2 years admitted with features of acute bronchiolitis Exclusion criteria: bacterial or aspiration pneumonia, previous wheezing episodes, oxygen saturation < 92% in room air, cyanosis, obtunded consciousness, progressive respiratory failure requiring mechanical ventilation, foreign body inhalation, cardiac disease, congenital malformations, and parents refusing consent |
|
| Interventions | Intervention group: nebulised 3% saline (3 mL) plus salbutamol (? mL) Control group: nebulised 0.9% saline (3 mL) plus salbutamol (? mL) The medication was given every 6 hours until discharge, via an Apex Eco‑Plus nebuliser (Apex Medical Corp, France). |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Bronchiolitis diagnosis criteria not provided. Financial support and sponsorship: nil The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Standard randomisation table |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No report of withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Ipek 2011.
| Study characteristics | ||
| Methods | Design: quasi‐randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: paediatric emergency department of a training and research hospital in Turkey
Eligible: not stated
Randomised: 60 hypertonic saline group; 60 normal saline group
Completed: 60 hypertonic saline group; 60 normal saline group
Gender (male): 59.2%
Age (mean ± SD): 7.9 ± 3.9 months Inclusion criteria: age < 2 years, a history of preceding viral upper respiratory infection followed by wheezing and crackles on auscultation, and a clinical severity score of 4 to 8 on admission Exclusion criteria: infants with clinical severity score < 4 or > 8, SaO₂ < 85% on room air, chronic cardiac illness, premature birth, birthweight < 2500 g, history of recurrent wheezing episodes, proven immune deficiency, severe neurological disease, age < 1 month or > 2 years, consolidation or atelectasis on a chest roentgenogram |
|
| Interventions | Intervention groups:
Group 1: nebulised 3% saline (4 mL) plus salbutamol 0.15 mg/kg
Group 2: nebulised 3% saline (4 mL) alone Control groups: Group 1: nebulised 0.9% saline (4 mL) plus salbutamol 0.15 mg/kg Group 2: nebulised 0.9% saline (4 mL) alone Treatment was given every 20 min until 3 doses had been administered (0, 20, and 40 min). All inhaled therapies were delivered via a compressor nebuliser through a face mask with continued flow of oxygen at 4 to 5 L/min (Mini Compressor Nebulizer, CN‐02WD, Ace‐Tec Co, Ltd, Guangdong, China). |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Infants were assigned to 1 of 4 groups according to the consecutive order of their admission to the short‐stay unit. |
| Allocation concealment (selection bias) | High risk | As stated above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was stated as double‐blind, but no details were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Jacobs 2014.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: emergency department of an urban tertiary care centre in the USA
Assessed for eligibility: 128
Randomised: 52 hypertonic saline group; 49 normal saline group
Completed: 52 hypertonic saline group; 49 normal saline group
Gender (male): 63.3%
Age (mean ± SD): 6.0 ± 3.9 months in hypertonic saline group; 5.6 ± 3.3 months in normal saline group Inclusion criteria: infants aged 6 weeks to 18 months presenting to the emergency department with acute bronchiolitis, defined as viral respiratory illness and first episode of wheeze, and a modified Wang clinical severity score of ≥ 4 Exclusion criteria: previous history of wheezing, any use of bronchodilators within 2 hours of presentation, gestational age ≤ 34 weeks, history of congenital heart disease or chronic pulmonary or chronic renal disease, SaO₂ ≤ 85% at the time of recruitment, severe disease requiring ICU admission, or inability to obtain informed consent |
|
| Interventions | Intervention group: nebulised 7% saline (3 mL) plus 2.25% racaemic epinephrine (0.5 mL) Control group: nebulised 0.9% saline (3 mL) plus 2.25% racaemic epinephrine (0.5 mL) The medication was given via a nebuliser driven by oxygen flow at 6 L/min after initial screening and assessment. If admitted, the infant continued to receive the same designated medication every 6 h until discharge or 24 h after admission. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 68% in hypertonic saline group; 50% in control group Funding: no external funding The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation in blocks of 10, but it is unclear how to choose blocks at random to create the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, concealed envelopes containing either 7% or 0.9% saline solution |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Jaquet‐Pilloud 2020.
| Study characteristics | ||
| Methods | Design: randomised multicentre clinical trial | |
| Participants | Setting: emergency department of 2 hospitals in Switzerland
Assessed for eligibility (n): 768
Randomised: 61 hypertonic saline group; 61 standard care group
Completed: 60 hypertonic saline group; 60 standard care group
Gender (male, %): 63.9%
Age (months, mean (95% CI)): 7.7 (6.4 to 9.1) months in hypertonic saline group; 7.5 (6.2 to 8.9) months in standard care group Inclusion criteria: children aged 6 weeks up to 24 months with a first episode of acute bronchiolitis, defined as symptoms of upper respiratory tract infection in addition to tachypnoea, wheezing, and widespread crackles at auscultation, and with a Wang score of 5 to 12 (moderate to severe) on arrival Exclusion criteria: mild bronchiolitis (Wang score < 5), previous episodes of wheezing, cardiac or chronic respiratory disease, immunocompromised, gestational age < 34 weeks, requiring immediate admission to ICU, RSV immunoglobulin therapy, corticotherapy in the preceding 2 weeks, bronchodilators within 24 hours prior to presentation |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) plus standard care Control group: standard care only The medication was given every 6 hours until discharge, via Pari LC sprint nebulisers with an oxygen flow at 6 L/min. Nebulised 4 mg epinephrine could be administered up to 3 times within the hour if child showed signs of respiratory failure (either persistent major respiratory distress, signs of exhaustion with a partial pressure of carbon dioxide above > 50 mmHg on the capillary blood gas) |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Bronchiolitis diagnosis criteria not provided. Funding: none The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation program in blocks of 10 |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No report of withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Khanal 2015.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: emergency and outpatient departments of a children's hospital in Nepal
Assessed for eligibility: 146
Randomised: 50 hypertonic saline group (3% saline); 50 normal saline group
Completed: 49 hypertonic saline group; 50 normal saline group
Gender (male): 48%
Age (mean ± SD): 9.8 ± 5.0 months in hypertonic saline group; 9.5 ± 4.2 months in normal saline group Inclusion criteria: infants aged 6 weeks to 2 years with acute bronchiolitis defined as the first episode of acute wheezing, starting as a viral upper respiratory infection (coryza, cough, or fever), with Wang clinical severity score between 1 and 9 Exclusion criteria: any underlying disease (e.g. cystic fibrosis, bronchopulmonary dysplasia, and cardiac or renal disease), prior history of wheezing, diagnosed case of asthma, SaO₂ < 85% on room air, clinical severity score > 9, progressive respiratory distress requiring mechanical ventilation, previous treatment with bronchodilators within last 4 h, and any steroid therapy within 48 h |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) plus L‐epinephrine (1.5 mg) Control group: nebulised 0.9% saline (4 mL) plus L‐epinephrine (1.5 mg) The study drug was administered at 0 and 30 min by a jet nebuliser using a face mask. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding sources not provided. The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation (in blocks of 10) |
| Allocation concealment (selection bias) | Low risk | Study solutions were labelled with the codes and wrapped in an envelope bearing the respective codes. Study solutions were identical in appearance and odour. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 (1%) withdrawal after randomisation in hypertonic saline group |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Kuzik 2007.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: inpatient wards of 3 regional tertiary care hospitals, 1 in United Arab Emirates and 2 in Canada
Eligible: not stated
Randomised: 47 hypertonic saline group; 49 normal saline group
Completed: 45 hypertonic saline group; 46 normal saline group
Gender (male): 59.4%
Age (mean ± SD): 4.7 ± 4.2 months (range 10 days to 18 months) Inclusion criteria: infants with diagnosis of moderately severe bronchiolitis requiring a history of a preceding viral upper respiratory infection, the presence of wheezing or crackles on chest auscultation, plus either an SaO₂ < 94% in room air or RDAI score ≥ 4 Exclusion criteria: previous episode of wheezing, chronic cardiopulmonary disease or immunodeficiency, critical illness at presentation requiring admission to intensive care, the use of nebulised hypertonic saline within the previous 12 h, or premature birth (gestational age ≤ 34 weeks) |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) Control group: nebulised 0.9% saline (4 mL) Treatment was given every 2 h for 3 doses, followed by every 4 h for 5 doses, followed by every 6 h until discharge. All inhaled therapies were delivered to a settled infant from a standard oxygen‐driven hospital nebuliser through a tight‐fitting face mask or head box, whichever the infant tolerated better. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 62% in hypertonic saline group; 75% in normal saline group Supported by the Queen Alexandra Foundation for Children, British Columbia, Canada; Vancouver Island Health Authority, Youth and Maternal Programme, British Columbia, Canada; and an Ontario Thoracic Society block term grant. Declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation program |
| Allocation concealment (selection bias) | Low risk | Study solutions were prepared by a research pharmacist and were identical in appearance and odour. The identity of the study solutions was blinded to all participants, care providers, and investigators. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 (5.2%) withdrawals after randomisation (2 hypertonic saline group; 3 normal saline group); intention‐to‐treat analysis used |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Köse 2016.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: inpatient wards of a children's hospital in Turkey
Assessed for eligibility: not stated
Randomised: 35 (3% saline group); 34 (7% saline group); 35 (normal saline group)
Completed: 35 (3% saline group); 32 (7% saline group); 35 (normal saline group)
Gender (male): 40.3%
Age: median (min‐max): 7.6 (2 to 23) months in 3% saline group; 7.7 (1 to 24) months in 7% saline group; 7.6 (1 to 18) months in normal saline group Inclusion criteria: infants aged 1 to 24 months with clinical diagnosis of bronchiolitis, defined as the first wheezing episode followed by a viral upper respiratory infection, with crackles on auscultation, and Wang clinical severity score ≥ 4 Exclusion criteria: infants with clinical severity score < 4, SaO₂ < 80% in room air, chronic cardiopulmonary or neurological disease, premature birth, birthweight < 2500 g, history of recurrent wheezing episodes, proven immune deficiency, age < 1 month or > 2 years, proven or suspected acute bacterial infection, previous treatment with bronchodilators or corticosteroids, the presence of symptoms > 7 days, consolidation or atelectasis on a chest roentgenogram |
|
| Interventions | Intervention groups:
Nebulised 3% saline (2.5 mL) plus salbutamol (0.15 mg/kg)
Nebulised 7% saline (2.5 mL) plus salbutamol (0.15 mg/kg) Control group: nebulised 0.9% saline (2.5 mL) plus salbutamol (0.15 mg/kg) 2 doses were given at 30‐minute interval, followed by every 6 h until discharge. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding: none The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated stratified randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was stated as double‐blind, but no details were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (1.9%) withdrawals after randomisation in 7% saline group |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Li 2014.
| Study characteristics | ||
| Methods | Design: randomised, parallel‐group, controlled trial | |
| Participants | Setting: outpatient department of a children's hospital in China
Assessed for eligibility: not stated
Randomised: 85 hypertonic saline groups (5% saline: 41; 3% saline: 44); 44 normal saline group
Completed: 82 hypertonic saline groups (5% saline: 40; 3% saline: 42); 42 normal saline group
Gender (male): 73.3%
Age: median (quartiles): 6.7 (3.1) months in 3% saline group; 6.7 (3.6) months in 5% saline group; 7.6 (3.9) months in normal saline group Inclusion criteria: infants aged 2 months to 18 months with clinical diagnosis of acute bronchiolitis and Wang clinical severity score ≥ 4 Exclusion criteria: severe bronchiolitis (respiratory rate > 80 breaths per minute, SaO₂ < 85% on room air or need for mechanical ventilation), immunological deficiency diseases, cardiac diseases, neurological or metabolic diseases, chronic respiratory diseases, prematurity, and previous history of wheezing |
|
| Interventions | Intervention groups:
Nebulised 3% saline (3 mL)
Nebulised 5% saline (3 mL) Control group: nebulised 0.9% saline (3 mL) The study drug was administered by a jet nebuliser, twice daily for 3 days. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated using a random number table |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 (3.8%) withdrawals after randomisation (1 in 5% saline group, 2 in 3% saline group, 2 in normal saline group) |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Luo 2010.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: inpatient wards of a teaching hospital for children in China
Eligible: not stated
Randomised: 50 hypertonic saline group; 43 normal saline group
Completed: 50 hypertonic saline group; 43 normal saline group
Gender (male): 60.2%
Age (mean ± SD): 6.0 ± 4.3 months in hypertonic saline group; 5.6 ± 4.5 months in normal saline group Inclusion criteria: infants with a diagnosis of mild to moderately severe bronchiolitis Exclusion criteria: age > 24 months, previous episode of wheezing, chronic cardiac and pulmonary disease, immunodeficiency, accompanying respiratory failure, requiring mechanical ventilation, inhaling the nebulised 3% saline solution and salbutamol 12 h before treatment, and premature infants born at less than 34 weeks gestation |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) plus 2.5 mg salbutamol Control group: nebulised 0.9% saline (4 mL) plus 2.5 mg salbutamol Infants in each group received 3 treatments every day, delivered at intervals of 8 h until discharge using air‐compressed nebulisers. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 70% in hypertonic saline group; 69.7% in normal saline group Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were reported. |
| Allocation concealment (selection bias) | Low risk | No detectable difference in colour, smell, or other physical properties between the therapeutic packages containing 0.9% saline solution or 3% saline solution. The codes of the therapeutic packages were not available to the investigators, nurses, or parents. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Luo 2011.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: inpatient ward of a teaching hospital for children in China
Assessed for eligibility: not stated
Randomised: 64 hypertonic saline group; 62 normal saline group
Completed: 57 hypertonic saline group; 55 normal saline group
Gender (male): 56.3%
Age (mean ± SD): 5.9 ± 4.1 months in hypertonic saline group; 5.8 ± 4.3 months in normal saline group Inclusion criteria: infants aged < 24 months with a first episode of wheezing, hospitalised for treatment of moderate to severe bronchiolitis Exclusion criteria: age > 24 months, previous episode of wheezing, chronic cardiac and pulmonary disease, immunodeficiency, accompanying respiratory failure, requiring mechanical ventilation, inhaling the nebulised 3% saline solution 12 h before treatment, and prematurity with birth at < 34 weeks of gestation |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) Control group: nebulised 0.9% saline (4 mL) Treatment was given every 2 h for 3 doses, followed by every 4 h for 5 doses, followed by every 6 h until discharge. All inhaled treatments were delivered to infants from standard air‐compressed nebulisers (PARI Corporation, Stanford, Germany). |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 73.7% in hypertonic saline group; 72.7% in normal saline group Funding sources not provided. The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation program |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 (11.1%, 7 infants from each group) discharged within 12 hours after enrolment and data were not collected. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Mahesh Kumar 2013.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: inpatient ward of a teaching hospital in India
Assessed for eligibility: 78
Randomised: 20 hypertonic saline group; 20 normal saline group
Completed: 20 hypertonic saline group; 20 normal saline group
Gender (male): 62.5%
Age (mean ± SD): 5.9 ± 3.8 months Inclusion criteria: children aged < 2 years, hospitalised with acute bronchiolitis defined as the first episode of lower respiratory tract infection with wheeze and having a moderate respiratory distress with clinical severity score between 4 and 8 Exclusion criteria: children with pre‐existing cardiac disease, previous wheezing episodes, severe disease (clinical severity score > 8) requiring mechanical ventilation (SaO₂ < 85% on room air, cyanosis, obtunded consciousness, and/or progressive respiratory failure) |
|
| Interventions | Intervention group: nebulised 3% saline (3 mL) plus salbutamol (0.15 mg/kg) Control group: nebulised 0.9% saline (3 mL) plus salbutamol (0.15 mg/kg) The medication was given via a nebuliser driven by oxygen flow at 5 to 6 L/min, every 6 h until the infant was ready for discharge. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was stated as double‐blind, but no details were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Mandelberg 2003.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: paediatric inpatient ward, Edith Wolfson Medical Center, Israel
Eligible: not stated
Randomised: 27 hypertonic saline group; 26 normal saline group
Completed: 27 hypertonic saline group; 25 normal saline group
Gender (male): 57.7%
Age (mean ± SD): 2.9 ± 2.1 months (range 0.5 to 12 months) Inclusion criteria: infants with clinical presentation of viral bronchiolitis with temperature > 38 °C that led to hospitalisation Exclusion criteria: cardiac disease, chronic respiratory disease, previous wheezing episode, age > 12 months, SaO₂ < 85% in room air, changes in consciousness and/or progressive respiratory failure requiring mechanical ventilation |
|
| Interventions | Intervention group: nebulised 3% saline solution (4 mL) plus 1.5 mg epinephrine Control group: nebulised 0.9% saline solution (4 mL) plus 1.5 mg epinephrine Treatment was given 3 times/day at intervals of 8 h, until the infant was ready for discharge. All inhaled treatments were delivered using a nebuliser (Aeromist Nebulizer Set 61400; B&F Medical by Allied; Toledo, OH) connected to a source of pressurised oxygen at a flow rate of 5 L/min. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 85% in hypertonic saline group; 88% in normal saline group Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Low risk | Study solutions were similar in colour, smell, and other physical properties. The code of the therapeutic package (hypertonic saline versus normal saline solution) was deposited with the statistician. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 (1.8%) withdrawal after randomisation |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Miraglia Del Giudice 2012.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: division of paediatrics of a general hospital in Italy
Assessed for eligibility: 136
Randomised: 53 hypertonic saline group; 56 normal saline group
Completed: 52 hypertonic saline group; 54 normal saline group
Gender (male): 65.1%
Age (mean ± SD): 4.8 ± 2.3 months in hypertonic saline group; 4.2 ± 1.6 months in normal saline group Inclusion criteria: children aged under 2 years with a diagnosis of bronchiolitis, defined as the first episode of wheezing and clinical symptoms of a viral respiratory infection and SaO₂ < 94% in room air and significant respiratory distress Exclusion criteria: pre‐existing cardiac or pulmonary diseases, premature birth < 36 weeks of gestational age, previous diagnosis of asthma, initial SaO₂ ≤ 85% or respiratory distress severe enough to require resuscitation |
|
| Interventions | Intervention group: nebulised 3.0% hypertonic saline (volume not reported) plus 1.5 mg epinephrine Control group: nebulised 0.9% saline (volume not reported) plus 1.5 mg epinephrine Study solutions were given at intervals of 6 h until discharge. Each treatment was delivered by a nebuliser with continuous flow of oxygen at 6 L/min through a tight‐fitting face mask. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 80.7% in hypertonic saline group; 83.3% in normal saline group Funding sources not provided. The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation program |
| Allocation concealment (selection bias) | Unclear risk | Study solutions were prepared by the local hospital pharmacy, but the method of allocation concealment was not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 withdrawals (1 hypertonic saline group; 2 normal saline group) |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Morikawa 2017.
| Study characteristics | ||
| Methods | Design: open‐label, multicentre, randomised controlled trial | |
| Participants | Setting: 2 tertiary children's hospitals and 3 general hospitals in Tokyo, Japan
Assessed for eligibility: 398
Randomised: 63 hypertonic saline group; 65 normal saline group
Completed: 63 hypertonic saline group; 65 normal saline group
Gender (male, %): 61%
Age (months, mean ± SD): 4.4 ± 3.1 months in hypertonic saline group; 4.2 ± 3.0 months in normal saline group Inclusion criteria: hospitalised infants less than 12 months of age with acute moderate bronchiolitis due to RSV Exclusion criteria: pCO₂ > 60 mmHg, saturation < 95% on oxygen administration, episodes of apnoea, previous episodes of wheezing, cerebral palsy, congenital heart disease, lung disease, muscular disorder, malformation syndrome, immune deficiency disorder, a history of preterm birth defined as a gestational age of less than 36 weeks, and progressive respiratory failure requiring mechanical ventilation or previous administration of palivizumab |
|
| Interventions | Intervention group: nebulised 3% saline (2 mL) plus 0.5% salbutamol (0.1 mL) Control group: nebulised 0.9% saline (2 mL) plus 0.5% salbutamol (0.1 mL) All nebulisation therapies were delivered via standard oxygen‐driven hospital nebulisers, 4 times daily, until discharge criteria were fulfilled. |
|
| Outcomes |
|
|
| Notes | RSV identification was an inclusion criterion. Funding: none The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation system |
| Allocation concealment (selection bias) | High risk | Dynamic (minimisation) allocation was used, but participants and treating physicians were not masked to assignment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Only biostatisticians were blinded to the allocation during the trial and analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No report of withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
NCT01238848.
| Study characteristics | ||
| Methods | Design: randomised, open‐label, parallel‐group, controlled trial | |
| Participants | Setting: inpatient ward of a children’s hospital in Buenos Aires, Argentina
Assessed for eligibility: not stated
Randomised: 50 hypertonic saline group; 50 normal saline group
Completed: 37 hypertonic saline group; 45 normal saline group
Gender (male): 50.0%
Age (mean ± SD): 4.5 ± 3.8 months Inclusion criteria: infants aged 1 to 24 months, hospitalised for first episode of bronchiolitis, with severity score ≥ 5 and oxygen saturation ≥ 97% Exclusion criteria: chronic respiratory or cardiovascular disease, respiratory failure |
|
| Interventions | Intervention group: nebulised 3.0% hypertonic saline (3 mL) plus albuterol (0.25 mg/kg/day) Control group: nebulised 0.9% saline (3 mL) plus albuterol (0.25 mg/kg/day) Study solutions were given 4 times a day for 5 days. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was stated as randomised, but no details were provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18 (18%) withdrawals (13 hypertonic saline group, 5 normal saline group); unbalanced attrition between treatment groups |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Ojha 2014.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: inpatient ward of a teaching hospital in Nepal
Assessed for eligibility: 104
Randomised: 36 hypertonic saline group; 36 normal saline group
Completed: 28 hypertonic saline group; 31 normal saline group
Gender (male): 74%
Age (mean ± SD): 8.5 ± 5.0 months Inclusion criteria: children aged over 6 weeks up to 24 months, hospitalised with acute bronchiolitis, defined as the first episode of wheezing associated with tachypnoea, increased respiratory effort, and an upper respiratory tract infection Exclusion criteria: previous episode of wheezing, chronic cardiac and pulmonary disease, immunodeficiency, accompanying respiratory failure, requiring mechanical ventilation, inhaling the nebulised 3% saline solution and salbutamol 12 h before treatment, premature infants born at less than 34 weeks' gestation, SaO₂ < 85% on room air |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) Control group: nebulised 0.9% saline (4 mL) Treatment was given every 8 h until discharge. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Supported by University Grant Commission (UGC). Declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | The random numbers were kept in a sealed envelope. The solutions were similar in appearance and smell and were kept in 2 identical containers, labelled only by a code number. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 (18%) withdrawals after randomisation (8 hypertonic saline group; 5 normal saline group). Different reasons for withdrawals between study groups |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Pandit 2013.
| Study characteristics | ||
| Methods | Design: randomised, non‐blind, parallel‐group, controlled trial | |
| Participants | Setting: paediatric department of a government multispeciality hospital in India
Assessed for eligibility: not stated
Randomised: 51 hypertonic saline group; 49 normal saline group
Completed: 51 hypertonic saline group; 49 normal saline group
Gender (male): not reported
Age: mean age: not reported Inclusion criteria: infants aged 2 months to 12 months, admitted with clinical diagnosis of acute bronchiolitis, defined as the first attack of wheezing after a short history of cough with or without fever of less than 7 days duration Exclusion criteria: recurrent episodes of wheezing, 1 or more episodes of respiratory distress in past, family history of asthma, atopy, congenital heart disease, history of prematurity or mechanical ventilation in newborn period, very sick patients with shock, seizures, heart rate > 180/min, respiratory rate > 100/min and adjudged to be in incipient respiratory failure, severe malnutrition, consolidation lung on chest X‐ray |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) plus 1:1000 adrenaline (1 mL) Control group: nebulised 0.9% saline (4 mL) plus 1:1000 adrenaline (1 mL) The medication was given 3 times with an interval of 1 hour, via a nebuliser driven by oxygen flow at 6 to 8 L/min. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding: none The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Group allocation was concealed in an opaque envelope. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Non‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Ratajczyk‐Pekrul 2016.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: inpatient ward of a general hospital in Poland
Assessed for eligibility: 80
Randomised: 41 hypertonic saline group; 37 normal saline group
Completed: 41 hypertonic saline group; 36 normal saline group
Gender (male): 58.9%
Age (mean): 5.34 months in hypertonic saline group; 4.43 months in normal saline group Inclusion criteria: children aged 0 to 18 months, hospitalised with acute bronchiolitis defined as prolonged expiration, wheezes, and crepitations, with a history of a preceding viral upper respiratory infection, and with SaO₂ ≤ 95% or Wang score ≥ 5 Exclusion criteria: preterm babies < 34 weeks, chronic cardiac or respiratory disease, immunological deficiencies, 2 or more episodes of bronchial obstruction, treatment with systemic glucocorticosteroids, received a hypertonic saline nebulisation in 24 hours prior to admission, or with SaO₂ < 85% |
|
| Interventions | Intervention group: nebulised 3% saline (3 mL) plus salbutamol (0.15 mg/kg, max 1.5 mg) Control group: nebulised 0.9% saline (3 mL) plus salbutamol (0.15 mg/kg, max 1.5 mg) The medication was given 6 times daily until discharge, via a nebuliser driven by oxygen flow at 6 to 8 L/min. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 51% in hypertonic saline group; 56% in normal saline group Funding: none The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 (5.1%) withdrawals (2 infants from each group) |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Sarrell 2002.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: Pediatric and Adolescent Ambulatory Community Clinic of General Health Services of Petach‐Tikva, Israel
Eligible: not stated
Randomised: 70
Completed: 33 (hypertonic saline group); 32 (normal saline group)
Gender (male): 59%
Age (mean ± SD): 12.5 ± 6.0 months (range 3 to 24 months) Inclusion criteria: infants with clinical presentation of mild to moderate viral bronchiolitis Exclusion criteria: cardiac disease, chronic respiratory disease, previous wheezing episode, age ≥ 24 months, SaO₂ < 96% on room air, and need for hospitalisation |
|
| Interventions | Intervention group: nebulised 3% saline solution (2 mL) plus 5 mg (0.5 mL) terbutaline Control group: nebulised 0.9% saline solution (2 mL) plus 5 mg (0.5 mL) terbutaline Treatment was given 3 times/day at intervals of 8 h for 5 days. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 82% in hypertonic saline group; 78% in normal saline group Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in blocks of 4, using an online randomiser |
| Allocation concealment (selection bias) | Low risk | Study solutions were similar in colour, smell, and other physical properties. The code of the therapeutic package (hypertonic saline versus normal saline solution) was deposited with the statistician. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 (7.1%) withdrawals after randomisation; distribution of withdrawals between study groups not reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Sharma 2013.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: inpatient ward of a tertiary care teaching hospital in India
Assessed for eligibility: 277
Randomised: 125 hypertonic saline group; 125 normal saline group
Completed: 125 hypertonic saline group; 123 normal saline group
Gender (male): 76.2%
Age (mean ± SD): 8.5 ± 5.0 months Inclusion criteria: infants aged 1 to 24 months, hospitalised with moderate (clinical severity score 3 to 6) acute bronchiolitis, defined as the first episode of wheezing along with prodrome of upper respiratory tract infection Exclusion criteria: children with obtunded consciousness, cardiac disease, chronic respiratory disease, previous wheezing episode, progressive respiratory distress requiring respiratory support other than supplemental oxygen, use of nebulised hypertonic saline within the previous 12 h |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) plus salbutamol (2.5 mg) Control group: nebulised 0.9% saline (4 mL) plus salbutamol (2.5 mg) The medication was given via a jet nebuliser with tight‐fitting face mask, driven by oxygen flow at 7 L/min, every 4 h until the infant was ready for discharge. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding: none The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Study solutions were similar in colour, smell, and other physical properties. The code of the therapeutic package (hypertonic saline versus normal saline solution) was deposited with the statistician. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (0.8%) withdrawals after randomisation in normal saline group |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Tal 2006.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: paediatric inpatient ward, Wolfson Medical Center, Israel
Eligible: not stated
Randomised: 22 hypertonic saline group; 22 normal saline group
Completed: 21 hypertonic saline group; 20 normal saline group
Gender (male): 56.1%
Age (mean ± SD): 2.6 ± 1.0 months (range 1 to 5 months) Inclusion criteria: infants with clinical presentation of viral bronchiolitis leading to hospitalisation Exclusion criteria: cardiac disease, chronic respiratory disease, previous wheezing episode, age > 12 months, SaO₂ < 85% on room air, obtunded consciousness and/or progressive respiratory failure requiring mechanical ventilation |
|
| Interventions | Intervention group: nebulised 3% saline solution (4 mL) plus 1.5 mg epinephrine Control group: nebulised 0.9% saline solution (4 mL) plus 1.5 mg epinephrine Treatment was given 3 times/day at 8‐hour intervals until the infant was ready for discharge. All inhaled treatments were delivered using an ultrasonic nebuliser (Omron UI, OMRON Matsusaka Co Ltd, Japan). |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 86% in hypertonic saline group; 75% in normal saline group Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in blocks of 4, using an online randomiser |
| Allocation concealment (selection bias) | Low risk | Study solutions were similar in colour, smell, and other physical properties. The code of the therapeutic package (hypertonic saline versus normal saline solution) was deposited with the statistician. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 (6.8%) withdrawals after randomisation (1 hypertonic saline group; 2 normal saline group) |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Teunissen 2014.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: inpatient wards of 11 general hospitals and 1 tertiary medical centre in the Netherlands
Assessed for eligibility: not stated
Randomised: 97 (3% saline group); 102 (6% saline group); 93 (normal saline group)
Completed: 84 (3% saline group); 83 (6% saline group); 80 (normal saline group)
Gender (male): 57.1%
Age: median 3.4 months (range 10 days to 23 months) Inclusion criteria: children aged birth to 24 months, hospitalised with mild to severe (Wang clinical severity score ≥ 3) viral bronchiolitis, defined as symptoms of an upper respiratory tract infection with wheezing, tachypnoea, and dyspnoea Exclusion criteria: Wang clinical severity score improved at least 2 points after inhalation of 2.5 mg salbutamol, haemodynamically important congenital heart disease, chronic pre‐existent lung disease, T‐cell immunodeficiency, treatment with corticosteroids, and previous wheezing, (food) allergy, or eczema |
|
| Interventions | Intervention groups:
Nebulised 3% saline (4 mL) plus salbutamol (2.5 mg)
Nebulised 6% saline (4 mL) plus salbutamol (2.5 mg) Control group: nebulised 0.9% saline (4 mL) plus salbutamol (2.5 mg) The solutions were given via a HOT Top Plus Nebuliser (Intersurgical, Uden, Netherlands) with a tight‐fitting face mask, driven by oxygen flow at 6 to 8 L/min, every 8 h until discharge. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 83.7% in 3% saline group; 91.4% in 6% saline group; 88.6% in control group Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (in blocks of 6) |
| Allocation concealment (selection bias) | Low risk | Study solutions were identical in vial packaging, colour, smell, and other physical characteristics. The trial codes were kept by the pharmacist. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43 (14.7%) withdrawals after randomisation (13 (3% saline group); 18 (6% saline group); 12 (normal saline group)). The reasons for withdrawals were similar between study groups. Intention‐to‐treat and per‐protocol analyses yielded similar results. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Tinsa 2014.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: inpatient ward of a children’s hospital in Tunisia
Assessed for eligibility: not stated
Randomised: 32 (5% saline group); 37 (5% saline + epinephrine group); 28 (normal saline group)
Completed: 31 (5% saline group); 37 (5% saline + epinephrine group); 26 (normal saline group)
Gender (male): 61.7%
Age (mean ± SD): 3.7 ± 2.8 months in 5% saline group; 3.2 ± 2.5 months in 5% saline + epinephrine group; 3.0 ± 2.4 months in normal saline group Inclusion criteria: children aged 1 to 12 months, hospitalised with moderate (Wang clinical severity score of 3) bronchiolitis, defined as an acute infection of the lower respiratory tract, preceded by or accompanied by fever or rhinitis, or both, and characterised by expiratory wheezing and increased respiratory effort Exclusion criteria: prematurity (gestational age at birth < 34 weeks), underlying chronic cardiac or pulmonary disease (e.g. bronchopulmonary dysplasia, cystic fibrosis), recurrent wheezing, severe respiratory distress (apnoeas, heart rate > 200 beats/minute, respiratory rate > 80 breaths/minute, profound lethargy, duration of illness exceeding 15 days) |
|
| Interventions | Intervention groups:
Nebulised 5% saline (4 mL)
Nebulised 5% saline (2 mL) plus standard epinephrine (2 mL) Control group: nebulised 0.9% saline (4 mL) The solutions were given via a jet nebuliser with a tight‐fitting face mask, driven by oxygen flow at 6 to 7 L/min, every 4 h until discharge. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Study solutions were similar in appearance and smell and were stored in identical syringes, labelled only by a code number. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 (3.1%) withdrawals after randomisation (2 normal saline group, 1 hypertonic saline group) |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Uysalol 2017.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, controlled trial | |
| Participants | Setting: paediatric emergency department of Istanbul University, Turkey
Assessed for eligibility: 450
Randomised: 79 (hypertonic saline group); 75 (hypertonic saline plus adrenaline group); 76 (normal saline plus adrenaline group); 82 (normal saline group). 74 infants allocated to the normal saline plus salbutamol group were excluded for analysis due to lack of appropriate control group.
Completed: 77 (hypertonic saline group); 75 (hypertonic saline plus adrenaline group); 75 (normal saline plus adrenaline group); 79 (normal saline group)
Gender (male, %): 54.9%
Age (months, median (25th to 75th percentile)): 7 (4 to 10) months in all groups Inclusion criteria: infants aged 2 to 24 months with acute moderate (Wang clinical severity score of 4 to 8) bronchiolitis, defined as viral respiratory tract infections (coryza, cough, fever) with tachypnoea, respiratory distress with chest recession, wheezing and/or crackles Exclusion criteria: younger than 2 months old, prematurity (less than 36th gestational week), low birthweight (less than 2500 g), history of admission to neonatal ICU due to respiratory distress, history of intubation in the ICU, congenital heart/lung/neurologic or immunologic disease, history of atopic disease or recurrent wheezing, clinical or radiologic findings of bacterial infections, atelectasis or consolidations on X‐ray, and refusal to consent by parents |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL); nebulised 3% saline (4 mL) plus adrenaline (0.1 mg/kg) Control group: nebulised 0.9% saline (5 mL); nebulised 0.9% saline (4 mL) plus adrenaline (0.1 mg/kg) All nebulisation therapies were delivered via standard oxygen (6 L/min)‐driven hospital nebulisers, 4 times daily until discharge criteria fulfilled. |
|
| Outcomes |
|
|
| Notes | Virological identification not available. Funding sources/declarations of interest not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lottery method for simple randomisation |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was stated as double‐blind, but no details were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 (1.9%) withdrawals after randomisation (4 normal saline group, 2 hypertonic saline group) |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
Wu 2014.
| Study characteristics | ||
| Methods | Design: randomised, double‐blind, parallel‐group, controlled trial | |
| Participants | Setting: emergency departments of 2 urban freestanding tertiary children’s hospitals in the USA
Assessed for eligibility: 1254
Randomised: 211 hypertonic saline group; 197 normal saline group
Completed: 211 hypertonic saline group; 197 normal saline group
Gender (male): 56.8%
Age (mean ± SD): 6.5 ± 5.1 months in hypertonic saline group; 6.4 ± 5.3 months in normal saline group Inclusion criteria: children younger than 24 months with a primary diagnosis of viral bronchiolitis during bronchiolitis season Exclusion criteria: children with a prior illness with wheezing or bronchodilator use, premature (gestational age < 34 weeks), cyanotic congenital heart disease, chronic lung disease, or tracheostomy |
|
| Interventions | Intervention group: nebulised 3% saline (4 mL) Control group: nebulised 0.9% saline (4 mL) The solutions were given via a small‐volume wall nebuliser at study entry. Emergency department physicians could order 2 additional treatments every 20 minutes to a maximum of 3 inhaled doses. Admitted infants continued receiving study medication every 8 h until discharge. |
|
| Outcomes |
|
|
| Notes | RSV‐positive: 65.6% hypertonic saline group; 59.2% normal saline group Supported by grant 02826‐3 from the Thrasher Research Fund and by a Mentored Junior Faculty Career Development Award from the Department of Pediatrics, University of Southern California Keck School of Medicine. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors declare no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Saline solutions were prepared by the investigational pharmacy and stored in sequentially numbered identical vials. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported at emergency department setting. |
| Selective reporting (reporting bias) | Low risk | All outcome measures and analyses listed in the methods section reported. |
| Other bias | Low risk | No other bias found. |
CI: confidence interval ICU: intensive care unit pCO₂: partial pressure of carbon dioxide RDAI: Respiratory Distress Assessment Instrument RSV: respiratory syncytial virus SaO₂: oxygen saturation SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐bahadily 2017 | Not an RCT. The authors classified the study design as "prospective case second multicenter study" in the Abstract and "Prospective comparison study" in the Methods. We contacted the first author for more details about study design, but did not receive a reply. |
| Amirav 2005 | Study of drug delivery (hood versus face mask) |
| Bagus 2012 | Abstract available only |
| Bueno Campaña 2014 | Other comparison (hypertonic saline versus high‐flow therapy) |
| Flores‐González 2016 | Other comparison (epinephrine versus placebo) |
| Guomo 2007 | Abstract available only |
| Kuzik 2010 | Inclusion of infants with previous history of wheezing |
| Nenna 2014 | Other comparison (hypertonic saline + 0.1% hyaluronic acid versus 0.9% saline) |
| Sapkota 2021 | Not an RCT |
| Silver 2015 | Inclusion of infants with previous history of wheezing |
| Teijeiro 2018 | Not an RCT (comment on Morikawa 2017) |
| Tribastone 2003 | Not an RCT (comment on Sarrell 2002) |
RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
CTRI /2010/091/003065.
| Methods | Randomised, double‐blind, parallel‐group, controlled trial |
| Participants | Consecutive patients with moderate to severe bronchiolitis, aged 2 months to 2 years, of either sex, admitted to the hospital during the study period |
| Interventions | Nebulised hypertonic saline versus nebulised normal saline |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Notes | Starting date: July 2009 Completion of data collection: February 2013 Last updated: 3 April 2017 Contact information: Lopamudra Mishra, 27A South Sinthee Road Kolkata 700050 Kolkata, West Bengal, India; email: lopamudra83.cmc@gmail.com |
Eudra CT2009‐014758‐14.
| Methods | Randomised, double‐blind, parallel‐group, controlled trial |
| Participants | Infants under the age of 12 months with a clinical diagnosis of bronchiolitis |
| Interventions | Nebulised 3% saline plus salbutamol |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Notes | Starting date: May 2010 Last updated: 19 March 2012 |
Gupta 2016.
| Methods | Randomised, parallel‐group, controlled trial |
| Participants | Children aged 2 months to 24 months, admitted to a teaching hospital, with history of preceding viral upper respiratory infection (fever > 38 °C or coryza), first episode of respiratory distress associated with wheezing, clinical severity score > 3, and no evidence of bacterial infection |
| Interventions | Nebulised 3% saline (4 mL) Nebulised 0.9% saline (4 mL) Nebulised 0.9% saline (4 mL) plus salbutamol (0.15 mg/kg, minimum dose 1 mg) Study solutions were given via a nebuliser driven by oxygen flow at 8 L/min, every 6 h until discharge. |
| Outcomes |
|
| Notes | Suspected plagiarism. This trial presented results identical to those of the Malik 2015 trial. We contacted the first authors of both trials and the editors of the journals in which the trials were published, but neither authors nor editors provided clarification. |
Malik 2015.
| Methods | Randomised, parallel‐group, controlled trial |
| Participants | Children aged 1 to 24 months, admitted to a teaching hospital, with clinical diagnosis of acute bronchiolitis and clinical severity score > 3 |
| Interventions | Nebulised 3% saline (4 mL) Nebulised 0.9% saline (4 mL) Nebulised salbutamol (0.15 mg/kg) Study solutions were given via a nebuliser driven by oxygen flow at 8 L/min, every 6 h until discharge. |
| Outcomes |
|
| Notes | Suspected plagiarism. This trial presented results identical to those of the Gupta 2016 trial. We contacted the first authors of both trials and the editors of the journals in which the trials were published, but neither authors nor editors provided clarification. |
NCT00677729.
| Methods | Randomised, double‐blind, parallel‐group, controlled trial |
| Participants | Infants aged up to 24 months, presenting to ED or outpatient department with moderately severe viral bronchiolitis defined as history of viral upper respiratory tract infection within previous 7 days, presence of wheezing or crackles, or both, on chest auscultation, and RDAI score > 4 (of 17) or transcutaneous oxygen saturation < 94% in room air |
| Interventions | Nebulised 3% saline (4 mL) plus 1.0 mg salbutamol Nebulised 0.9% saline (4 mL) plus 1.0 mg salbutamol Study solutions were given every 20 minutes for a total of 3 doses. |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Notes | Starting date: June 2008 Completion of data collection: April 2009 Last updated: November 2015 Contact information: Brian Kuzik, MD, The Royal Victoria Hospital of Barrie, Ontario, Canada L4M6M2 |
NCT01777347.
| Methods | Randomised, double‐blind, parallel‐group, controlled trial |
| Participants | Children aged 6 weeks to 12 months with first moderate to severe episode of acute viral bronchiolitis (history of viral upper respiratory tract infection plus wheezing or crackles, or both, on chest auscultation with respiratory distress), admitted in ED |
| Interventions | Nebulised 3% saline (4 mL) Nebulised 0.9% saline (4 mL) 2 doses of study solutions were given every 20 minutes. |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Notes | Starting date: October 2012 Completion of data collection: April 2014 Last updated: 25 July 2014 Contact information: Vincent Gajdos, MD, PhD, Assistance Publique Hôpitaux de Paris ‐ Paris Sud Medical School |
NCT01834820.
| Methods | Randomised, double‐blind, parallel‐group, controlled trial |
| Participants | Children under 2 years of age diagnosed with mild to moderate bronchiolitis, presenting to outpatient Department of Hospital General Naval de Alta Especialidad, Mexico |
| Interventions | Intervention 1: epinephrine and dexamethasone
1 dose of nebulised dexamethasone (4 mg) was given, followed by 2 doses of nebulised 1:1000 epinephrine (3 mL) at an interval of 20 minutes on the first day. Nebulised dexamethasone (4 mg) was given every 24 hours for 3 days. Intervention 2: 3% saline 3 doses of nebulised 3% saline (4 mL) were given every 20 minutes on the first day of treatment, followed by nebulised 3% saline (4 mL) every 24 hours for 3 days. Active comparator: 0.9% saline 3 doses of nebulised 0.9% saline (4 mL) were given at an interval of 20 minutes on the first day of treatment, followed by nebulised 0.9% saline (4 mL) every 24 hours for 3 days. |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Notes | Starting date: January 2013 Completion of data collection: June 2015 Last updated: 4 July 2015 Contact information: José Luis Rodríguez Cuevas, Hospital General Naval de Alta Especialidad, México, Distrito Federal, Distrito Federal, Mexico 04480 |
NCT02029040.
| Methods | Randomised, double‐blind, parallel‐group, controlled trial |
| Participants | Children aged 2 to 12 months, presenting to ED with a diagnosis of bronchiolitis (RDAI score = 6), defined as the first episode of wheezing or crackles, or both, in a child younger than 12 months who has physical findings of a viral respiratory infection and there is no other explanation for the wheezing and/or crackles |
| Interventions | Nebulised 3% saline (3 mL) Nebulised 0.9% saline (3 mL) A single dose of study solution was given. |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Notes | Starting date: December 2013 Completion of data collection: December 2014 Last updated: 3 May 2016 Contact information: Mohamed Badawy, MD, University of Texas Southwestern Medical Center |
NCT02045238.
| Methods | Randomised, double‐blind, parallel‐group, controlled trial |
| Participants | Children aged up to 12 months with clinical diagnosis of bronchiolitis (viral respiratory disease and first episode of wheezing) and with moderate respiratory distress, defined as having at least 2 of the following criteria: SaO₂ < 93%, respiratory rate > 60, and/or RDAI score > 4 |
| Interventions | Nebulised 3% saline (5 mL) Nebulised 0.9% saline (5 mL) Study solutions were initially given every 2 hours, then every 4 hours if the following criteria were met: SaO₂ > 94%, respiratory rate < 60, and RDAI score < 4. |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Notes | Starting date: July 2013 Completion of data collection: December 2014 Last updated: 5 January 2015 Contact information: Mateus D Leme, MD, Sao Paulo University, Brazil |
NCT02233985.
| Methods | Randomised, double‐blind, parallel‐group, controlled trial |
| Participants | Children aged 2 to 24 months attending the paediatric emergency service with moderate to severe bronchiolitis, defined as first episode of wheezing associated with respiratory distress and a history of upper respiratory tract infection |
| Interventions | Nebulised 3% saline (4 mL) plus salbutamol (100 μg/kg) Nebulised 0.9% saline (4 mL) plus salbutamol (100 μg/kg) 3 doses of study solutions were initially given at an interval of 20 minutes, then every 4 hours during the entire hospital stay. |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Notes | Starting date: August 2013 Completion of data collection: April 2015 Last updated: 25 January 2017 Contact information: Gloria P Sosa‐Bustamante, MD, Unidad Medica de Alta Especialidad Bajio 48. Hospital de Gineco ‐ Pediatria, Instituto Mexicano del Seguro Social, Mexico |
NCT02834819.
| Methods | Randomised, single‐blind (investigator), parallel‐group, controlled trial |
| Participants | Children aged 3 to 18 months, presenting to Children's Hospital Colorado Emergency Department with diagnosis of bronchiolitis and persistent hypoxia following initial supportive care |
| Interventions | Nebulised 3% saline (4 mL) plus standard care Standard care alone A single dose of study solution was given. |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Notes | Starting date: September 2013 Completion of data collection: September 2015 Last updated: 14 July 2016 Contact information: Cortney Braund, MD, University of Colorado, Denver |
CBSS: Clinical Bronchiolitis Severity Score ED: emergency department RDAI: Respiratory Distress Assessment Instrument SaO₂: oxygen saturation
Differences between protocol and review
Given the very limited number of studies that were identified initially, we added the comparison of nebulised hypertonic saline alone versus nebulised 0.9% saline since the 2011 version of the review (Zhang 2011). We added one exclusion criterion: abstract‐only citations and additional data unavailable. We also clarified the population according to age and changed the title to specify infants. We performed post hoc subgroup analyses, post hoc meta‐regression, and post hoc sensitivity analyses in the updated reviews.
In the 2022 update, we stratified the meta‐analysis of length of hospital stay by four comparisons: hypertonic saline plus salbutamol/albuterol versus normal saline plus salbutamol/albuterol, hypertonic saline plus epinephrine versus normal saline plus epinephrine, hypertonic saline alone versus normal saline, and hypertonic saline alone versus standard treatment.
We also stratified the meta‐analysis of rate of hospitalisation by two comparisons: hypertonic saline plus bronchodilator versus normal saline plus bronchodilator and hypertonic saline alone versus normal saline alone.
For the outcome 'number of days to resolution of symptoms and signs', we presented the results of meta‐analysis for each symptom and sign (i.e. wheezing, cough, and pulmonary moist crackles) without pooling for overall estimates.
We also created and examined a funnel plot to explore possible small‐study effects and publication bias.
Cochrane Review methods have evolved over time, and these have been applied.
Contributions of authors
Linjie Zhang conceived the idea and wrote the draft protocol, primary review, and review updates. Linjie Zhang and Raúl A Mendoza‐Sassi were responsible for study selection, quality assessment, data collection, and data analysis. Raúl A Mendoza‐Sassi, Claire Wainwright, and Terry P Klassen provided input in the writing of the protocol and primary and updated reviews. Alex Aregbesola was responsible for the 2022 updated literature searches and provided input in the writing of the 2022 update review. All authors approved the final version of this review update.
Sources of support
Internal sources
-
Faculty of Medicine, Universidade Federal do Rio Grande, Brazil
LZ and RAM are employees of the Universidade Federal do Rio Grande.
External sources
-
National Council for Scientific and Technological Development (CNPq), Brazil
Fellowship of research productivity (PQ) (2013 to 2022)
Declarations of interest
Linjie Zhang: declared that he has no conflict of interest. Raúl A Mendoza‐Sassi: declared that he has no conflict of interest. Claire Wainwright: declared that her institution has received funding to support participation in multiple clinical trials sponsored by Vertex Pharmaceuticals since 2009, but she has received no direct funding for this. Alex Aregbesola: declared that he has no conflict of interest. Terry P Klassen: declared that he was contracted by Alberta Research Centre for Child Health Evidence between 14 February 2004 and 3 March 2005 to conduct a clinical trial that was included in the 2022 update review (Grewal 2009).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Al‐Ansari 2010 {published data only}
- Al-Ansari K, Sakran M, Davidson BL, Sayyed RE, Mahjoub H, Ibrahim K. Nebulized 5% or 3% hypertonic or 0.9% saline for treating acute bronchiolitis in infants. Journal of Pediatrics 2010;157(4):630-4. [DOI] [PubMed] [Google Scholar]
Angoulvant 2017 {published data only}
- Angoulvant F, Bellêttre X, Milcent K, Teglas JP, Claudet I, Le Guen CG, et al. Effect of nebulized hypertonic saline treatment in emergency departments on the hospitalization rate for acute bronchiolitis: a randomized clinical trial. JAMA Pediatrics 2017;171(8):e171333. [DOI: 10.1001/jamapediatrics.2017.1333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anil 2010 {published data only}
- Anil AB, Anil M, Saglam AB, Cetin N, Bal A, Aksu N. High volume normal saline alone is as effective as nebulized salbutamol-normal saline, epinephrine-normal saline, and 3% saline in mild bronchiolitis. Pediatric Pulmonology 2010;45(1):41-7. [DOI] [PubMed] [Google Scholar]
Awang 2020 {published data only}
- Awang N, Nasir A, Rawi RM, Taib F. A double blind randomized controlled trial comparing treatment with nebulized 3% hypertonic saline plus salbutamol versus nebulized 0.9% saline plus salbutamol in patients with acute bronchiolitis. International Medical Journal 2020;27(3):304-7. [Google Scholar]
Bashir 2018 {published data only}
- Bashir T, Reddy KV, Ahmed K, Shafi S. Comparative study of 3% hypertonic saline nebulisation versus 0.9% normal saline nebulisation for treating acute bronchiolitis. Journal of Clinical and Diagnostic Research 2018;12(6):SC05-8. [DOI: 10.7860/JCDR/2018/34766.11594] [DOI] [Google Scholar]
Everard 2014 {published data only}
- Everard ML, Hind D, Ugonna K, Freeman J, Bradburn M, Cooper CL, et al. SABRE: a multicentre randomised control trial of nebulised hypertonic saline in infants hospitalised with acute bronchiolitis. Thorax 2014;69(12):1105-12. [DOI: 10.1136/thoraxjnl-2014-205953] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flores 2016 {published data only}
- Flores P, Mendes AL, Neto AS. A randomized trial of nebulized 3% hypertonic saline with salbutamol in the treatment of acute bronchiolitis in hospitalized infants. Pediatric Pulmonology 2016;51(4):418-25. [DOI: 10.1002/ppul.23306] [DOI] [PubMed] [Google Scholar]
Florin 2014 {published data only}
- Florin TA, Shaw KN, Kittick M, Yakscoe S, Zorc JJ. Nebulized hypertonic saline for bronchiolitis in the emergency department: a randomized clinical trial. JAMA Pediatrics 2014;168(7):664–70. [DOI] [PubMed] [Google Scholar]
Grewal 2009 {published data only}
- Grewal S, Ali S, McConnell DW, Vandermeer B, Klassen TP. A randomized trial of nebulized 3% hypertonic saline with epinephrine in the treatment of acute bronchiolitis in the emergency department. Archives of Pediatrics & Adolescent Medicine 2009;163(11):1007-12. [DOI] [PubMed] [Google Scholar]
Hmar 2021 {published data only}
- Hmar L, Brahmacharimayum S, Golmei N, Moirangthem M, Chongtham S. Comparison of 3% saline versus normal saline as a diluent for nebulization in hospitalized children with acute bronchiolitis: a randomized clinical trial. Journal of Medical Society 2021;34:86-90. [Google Scholar]
Ipek 2011 {published data only}
- Ipek IO, Yalcin EU, Sezer RG, Bozaykut A. The efficacy of nebulized salbutamol, hypertonic saline and salbutamol/hypertonic saline combination in moderate bronchiolitis. Pulmonary Pharmacology & Therapeutics 2011;24(6):633-7. [DOI] [PubMed] [Google Scholar]
Jacobs 2014 {published data only}
- Jacobs JD, Foster M, Wan J, Pershad J. 7% hypertonic saline in acute bronchiolitis: a randomized controlled trial. Pediatrics 2014;133(1):e8–13. [DOI] [PubMed] [Google Scholar]
Jaquet‐Pilloud 2020 {published data only}
- Jaquet-Pilloud R, Verga M-E, Gehri M, Russo M, Gehri M, Pauchard J-Y, Russo M. Nebulised hypertonic saline therapy compared to supportive care in moderate to severe bronchiolitis: A randomized controlled trial. Swiss Medical Weekly 2017;147(Suppl 222):10-11. [CRSSTD: 20816351] [Google Scholar]
- Jaquet-Pilloud R, Verga M-E, Russo M, Gehri M, Pauchard J-Y. Nebulised hypertonic saline in moderate-to-severe bronchiolitis: a randomised clinical trial. Archives of Disease in Childhood 2020;105(3):236-40. [DOI: 10.1136/archdischild-2019-317160] [DOI] [PubMed] [Google Scholar]
Khanal 2015 {published data only}
- Khanal A, Sharma A, Basnet S, Sharma PR, Gami FC. Nebulised hypertonic saline (3%) among children with mild to moderately severe bronchiolitis - a double blind randomized controlled trial. BMC Pediatrics 2015;15:115. [DOI: 10.1186/s12887-015-0434-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Köse 2016 {published data only}
- Köse S, Şehriyaroğlu A, Esen F, Özdemir A, Kardaş Z, Altuğ U, et al. Comparing the efficacy of 7%, 3% and 0.9% saline in moderate to severe bronchiolitis in infants. Balkan Medical Journal 2016;33(2):193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuzik 2007 {published data only}
- Kuzik BA, Al Qaghi SA, Kent S, Flavin MP, Hopman W, Hotte S, et al. Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants. Journal of Pediatrics 2007;151(3):266-70. [DOI] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li G, Zhao J. Effectiveness of inhaled hypertonic saline in children with bronchiolitis. Zhonghua Er Ke Za Zh 2014;25(8):607-10. [PubMed] [Google Scholar]
Luo 2010 {published data only}
- Luo Z, Liu E, Luo J, Li S, Zeng F, Yang X, et al. Nebulized hypertonic saline/salbutamol solution treatment in hospitalized children with mild to moderate bronchiolitis. Pediatrics International 2010;52(2):199-202. [DOI] [PubMed] [Google Scholar]
Luo 2011 {published data only}
- Luo Z, Fu Z, Liu E, Xu X, Fu X, Peng D, et al. Nebulized hypertonic saline treatment in hospitalized children with moderate to severe viral bronchiolitis. Clinical Microbiology and Infection 2011;17(12):1829-33. [DOI] [PubMed] [Google Scholar]
Mahesh Kumar 2013 {published data only}
- Mahesh Kumar KB, Karunakara BP, Manjunath MN, Mallikarjuna HB. Aerosolised hypertonic saline in hospitalized young children with acute bronchiolitis: a randomized controlled clinical trial. Journal of Pediatric Sciences 2013;5(1):e174. [Google Scholar]
Mandelberg 2003 {published data only}
- Mandelberg A, Tal G, Witzling M, Someck E, Houri S, Balin A, et al. Nebulized 3% hypertonic saline solution treatment in hospitalized infants with viral bronchiolitis. Chest 2003;123(2):481-7. [DOI] [PubMed] [Google Scholar]
Miraglia Del Giudice 2012 {published data only}
- Miraglia Del Giudice M, Saitta F, Leonardi S, Capasso M, Niglio B, Chinellato I, et al. Effectiveness of nebulized hypertonic saline and epinephrine in hospitalized infants with bronchiolitis. International Journal of Immunopathology and Pharmacology 2012;25(2):485-91. [DOI] [PubMed] [Google Scholar]
Morikawa 2017 {published data only}
- Morikawa Y, Miura M, Furuhata MY, Morino S, Omori T, Otsuka M, et al. Nebulized hypertonic saline in infants hospitalized with moderately severe bronchiolitis due to RSV infection: A multicenter randomized controlled trial. Pediatric Pulmonology 2018;53(3):358-65. [DOI: 10.1002/ppul.23945] [DOI] [PubMed] [Google Scholar]
NCT01238848 {unpublished data only}
- NCT01238848. Efficacy of nebulized hypertonic saline in the treatment of acute bronchiolitis [A randomized controlled trial to evaluate efficacy of nebulized hypertonic saline vs. normal saline in the treatment of hospitalized children with bronchiolitis]. clinicaltrials.gov/ct2/show/NCT01238848 (first received 11 November 2010).
Ojha 2014 {published data only}
- Ojha AR, Mathema S, Sah S, Aryal UR. A comparative study on use of 3% saline versus 0.9% saline nebulization in children with bronchiolitis. Journal of Nepal Health Research Council 2014;12(26):39–43. [PubMed] [Google Scholar]
Pandit 2013 {published data only}
- Pandit S, Dhawan N, Thakur D. Utility of hypertonic saline in the management of acute bronchiolitis in infants: a randomized controlled study. International Journal of Clinical Pediatrics 2013;2(1):24–9. [Google Scholar]
Ratajczyk‐Pekrul 2016 {published data only}
- Ratajczyk-Pekrul K, Gonerko P, Peregud-Pogorzelski J. The clinical use of hypertonic saline/salbutamol in treatment of bronchiolitis. Pediatria Polska 2016;91(4):301-7. [Google Scholar]
Sarrell 2002 {published data only}
- Sarrell EM, Tal G, Witzling M, Someck E, Houri S, Cohen HA, et al. Nebulized 3% hypertonic saline solution treatment in ambulatory children with viral bronchiolitis decreases symptoms. Chest 2002;122(6):2015-20. [DOI] [PubMed] [Google Scholar]
Sharma 2013 {published data only}
- Sharma BS, Gupta MK, Rafik SP. Hypertonic (3%) saline for acute viral bronchiolitis: a randomized trial. Indian Pediatrics 2013;50(8):743-7. [DOI] [PubMed] [Google Scholar]
Tal 2006 {published data only}
- Tal G, Cesar K, Oron A, Houri S, Ballin A, Mandelberg A. Hypertonic saline/epinephrine treatment in hospitalized infants with viral bronchiolitis reduces hospitalization stay: 2 years experience. Israel Medical Association Journal 2006;8(3):169-73. [PubMed] [Google Scholar]
Teunissen 2014 {published data only}
- Teunissen J, Hochs AH, Vaessen-Verberne A, Boehmer AL, Smeets CC, Brackel H, et al. The effect of 3% and 6% hypertonic saline in viral bronchiolitis: a randomised controlled trial. European Respiratory Journal 2014;44(4):913–21. [DOI] [PubMed] [Google Scholar]
Tinsa 2014 {published data only}
- Tinsa F, Abdelkafi S, Bel Haj I, Hamouda S, Brini I, Zouari B, et al. A randomized, controlled trial of nebulized 5% hypertonic saline and mixed 5% hypertonic saline with epinephrine in bronchiolitis. La Tunisie Medicale 2014;92(11):674-7. [PubMed] [Google Scholar]
Uysalol 2017 {published data only}
- Uysalol M, Haşlak F, Özünal ZG, Vehid H, Uzel N. Rational drug use for acute bronchiolitis in emergency care. Turkish Journal of Pediatrics 2017;59:155-61. [DOI] [PubMed] [Google Scholar]
Wu 2014 {published data only}
- Wu S, Baker C, Lang ME, Schrager SM, Liley FF, Papa C, et al. Nebulized hypertonic saline for bronchiolitis: a randomized clinical trial. JAMA Pediatrics 2014;168(7):657-63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐bahadily 2017 {published data only}
- Al-bahadily AJM, Al-Omrani AAM, Atiya AA. Hypertonic 3% saline in comparison with 0.9% (normal) saline in treatment of acute bronchiolitis. International Journal of Pediatrics 2017;5(1):4209-16. [Google Scholar]
Amirav 2005 {published data only}
- Amirav I, Oron A, Tal G, Cesar K, Ballin A, Houri S, et al. Aerosol delivery in RSV bronchiolitis: hood or face-mask? Journal of Pediatrics 2005;147(5):627-31. [DOI] [PubMed] [Google Scholar]
Bagus 2012 {published data only}
- Bagus SI, Putu SP. Efficacy of nebulized hypertonic saline and albuterol combination for the management of acute bronchiolitis: a randomized, double-blind controlled trial. Paediatric Respiratory Reviews 2012;13(Suppl 1):S76. [Google Scholar]
Bueno Campaña 2014 {published data only}
- Bueno Campaña M, Olivares Ortiz J, Notario Muñoz C, Rupérez Lucas M, Fernández Rincón A, Patiño Hernández O, et al. High flow therapy versus hypertonic saline in bronchiolitis: randomised controlled trial. Archives of Disease in Childhood 2014;99(6):511-5. [DOI] [PubMed] [Google Scholar]
Flores‐González 2016 {published data only}
- Flores-González JC, Dominguez-Coronel MT, Matamala Morillo MA, Aragón Ramírez M, García Ortega RM, Dávila Corrales FJ, et al. Does nebulized epinephrine improve the efficacy of hypertonic saline solution in the treatment of hospitalized moderate acute bronchiolitis? A double blind, randomized clinical trial. Minerva Pediatrica 2016;68(2):81-8. [CRSREF 7221692] [PubMed] [Google Scholar]
- Flores-González JC, Matamala-Morillo MA, Rodríguez-Campoy P, Pérez-Guerrero JJ, Serrano-Moyano B, Comino-Vazquez P, et al. Epinephrine improves the efficacy of nebulized hypertonic saline in moderate bronchiolitis: a randomised clinical trial. PLoS ONE 2015;10(11):e0142847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guomo 2007 {published data only}
- Guomo R, Cossettini M, Saretta F, Fasoli L, Guerrera T, Canciani M. Efficacy of hypertonic saline solution in infants with acute bronchiolitis. European Respiratory Journal 2007;30(Suppl):E3016. [Google Scholar]
Kuzik 2010 {published data only}
- Kuzik BA, Flavin MP, Kent S, Zielinski D, Kwan CW, Adeleye A, et al. Effect of inhaled hypertonic saline on hospital admission rate in children with viral bronchiolitis: a randomized trial. Canadian Journal of Emergency Medicine 2010;12(6):477-84. [DOI] [PubMed] [Google Scholar]
Nenna 2014 {published data only}
- Nenna R, Papoff P, Moretti C, De Angelis D, Battaglia M, Papasso S, et al. Seven percent hypertonic saline - 0.1% hyaluronic acid in infants with mild-to-moderate bronchiolitis. Pediatric Pulmonology 2014;49(9):919-25. [DOI] [PubMed] [Google Scholar]
Sapkota 2021 {published data only}
- Sapkota S, Kaleem A, Huma S, Aleem Ud Din M, Ahmad S, ShahAlam S. Comparison of 3% saline and 0.9% normal saline nebulization as diluent in children with bronchiolitis. Journal of Pakistan Medical Association 2021;71:822. [DOI: 10.47391/JPMA.569] [DOI] [PubMed] [Google Scholar]
Silver 2015 {published data only}
- Silver AH, Esteban-Cruciani N, Azzarone G, Douglas LC, Lee DS, Liewehr S, et al. 3% hypertonic saline versus normal saline in inpatient bronchiolitis: a randomized controlled trial. Pediatrics 2015;136(6):1036-43. [DOI] [PubMed] [Google Scholar]
Teijeiro 2018 {published data only}
- Teijeiro A. Nebulized hypertonic saline in infants hospitalized with moderately severe bronchiolitis due to RSV infection: A multicenter randomized controlled trial. Archivos Argentinos de Pediatria 2018;116(3):e483-4. [DOI] [PubMed] [Google Scholar]
Tribastone 2003 {published data only}
- Tribastone AD. Nebulized 3% saline effective for viral bronchiolitis. Journal of Family Practice 2003;52(5):359-60. [PubMed] [Google Scholar]
References to studies awaiting assessment
CTRI /2010/091/003065 {unpublished data only}
- CTRI/2010/091/003065. A clinical trial to study the effect of nebulized hypertonic saline as compared to normal saline in infants and children with bronchiolitis [Study of nebulized hypertonic saline in the treatment of bronchiolitis in infants and children]. tri.nic.in/Clinicaltrials/pmaindet2.php?trialid=2459 (first received 1 November 2011).
Eudra CT2009‐014758‐14 {unpublished data only}
- EudraCT2009-014758-14. Nebulised hypertonic (3%) saline in the treatment of bronchiolitis [Does nebulised hypertonic (3%) saline reduce the duration of hospital admission in infants with bronchiolitis?]. https://www.clinicaltrialsregister.eu/ctr-search/trial/2009-014758-14/GB (first received 17 March 2010).
Gupta 2016 {published data only}
- Gupta HV, Gupta VV, Kaur G, Baidwan AS, George PP, Shah JC, et al. Effectiveness of 3% hypertonic saline nebulization in acute bronchiolitis among Indian children: a quasi experimental study. Perspectives in Clinical Research 2016;7(2):88-93. [CRSREF: 7221694] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malik 2015 {published data only}
- Malik G, Singh A, Singh K, Pannu MS, Singh P, Banga S, et al. A comparative study to assess the effects of nebulised 3% hypertonic saline, 0.9% normal saline and salbutamol in management of acute bronchiolitis among Indian children. Journal of Evolution of Medical and Dental Sciences 2015;4(21):3662-8. [CRSREF: 7221696] [Google Scholar]
NCT00677729 {unpublished data only}
- NCT00677729. Hypertonic saline to reduce hospital admissions in bronchiolitis [Inhaled hypertonic saline to reduce hospital admissions in infants with viral bronchiolitis (HS in ER study)]. clinicaltrials.gov/ct2/show/NCT00677729 (first received 2 May 2008).
NCT01777347 {unpublished data only}
- NCT01777347. Efficacy of 3% hypertonic saline in acute viral bronchiolitis (GUERANDE) [3% hypertonic saline to reduce hospitalization rate in acute viral bronchiolitis: a randomized double blind clinical trial]. clinicaltrials.gov/ct2/show/NCT01777347 (first received 14 November 2012).
NCT01834820 {unpublished data only}
- NCT01834820. Epinephrine, dexamethasone, and hypertonic saline in bronchiolitis, randomised clinical trial of efficacy and safety [Pilot study: epinephrine, dexamethasone, and hypertonic saline in children with bronchiolitis, randomised clinical trial of efficacy and safety]. clinicaltrials.gov/ct2/show/NCT01834820 (first received 15 January 2013).
NCT02029040 {unpublished data only}
- NCT02029040. Nebulized 3% hypertonic saline in the treatment of acute bronchiolitis [A randomized trial of nebulized 3% hypertonic saline in the treatment of acute bronchiolitis in the emergency department]. clinicaltrials.gov/ct2/show/NCT02029040. (first received 3 January 2014).
NCT02045238 {unpublished data only}
- NCT02045238. Inhaled hypertonic saline use in the emergency department to treat acute viral bronchiolitis [Study of the effect of inhaled 3% hypertonic saline compared with normal saline (0.9%) for the treatment of acute viral bronchiolitis in a short stay ward]. linicaltrials.gov/ct2/show/NCT02045238 (first received 22 January 2014).
NCT02233985 {unpublished data only}
- NCT02233985. Nebulized 3% hypertonic saline solution treatment of bronchiolitis in infants [A randomized trial of nebulized 3% hypertonic saline with salbutamol in the treatment of acute bronchiolitis in pediatric hospital]. clinicaltrials.gov/ct2/show/NCT02233985 (first received 7 May 2014).
NCT02834819 {unpublished data only}
- NCT02834819. Nebulized 3% hypertonic saline vs. standard of care in patients with bronchiolitis [A randomized controlled trial of nebulized 3% hypertonic saline vs. standard of care in patients with bronchiolitis]. clinicaltrials.gov/ct2/show/NCT02834819 (first received 15 July 2016).
Additional references
Assouline 1977
- Assouline G, Leibson V, Danon A. Stimulation of prostaglandin output from rat stomach by hypertonic solution. European Journal of Pharmacology 1977;44(3):271-3. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badgett 2015
- Badgett RG, Vindhyal M, Stirnaman JT, Gibson CM, Halaby R. A living systematic review of nebulized hypertonic saline for acute bronchiolitis in infants. JAMA Pediatrics 2015;169(8):788-9. [DOI] [PubMed] [Google Scholar]
Bertrand 2001
- Bertrand P, Aranibar H, Castro E, Sanchez I. Efficacy of nebulized epinephrine versus salbutamol in hospitalized infants with bronchiolitis. Pediatric Pulmonology 2001;31(4):284-8. [DOI] [PubMed] [Google Scholar]
Chen 2014
- Chen YJ, Lee WL, Wang CM, Chou HH. Nebulized hypertonic saline treatment reduces both rate and duration of hospitalization for acute bronchiolitis in infants: an updated meta-analysis. Pediatrics and Neonatology 2014;55(6):431-8. [DOI] [PubMed] [Google Scholar]
Daviskas 1996
- Daviskas E, Anderson SD, Gonda I, Eberl S, Meikle S, Seale JP, et al. Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects. European Respiratory Journal 1996;9(4):725-32. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Enriquez 2012
- Enriquez A, Chu IW, Mellis C, Lin WY. Nebulised deoxyribonuclease for viral bronchiolitis in children younger than 24 months. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD008395. [DOI: 10.1002/14651858.CD008395.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandes 2015
- Fernandes RM, Plint AC, Terwee CB, Sampaio C, Klassen TP, Offringa M, et al. Validity of bronchiolitis outcome measures. Pediatrics 2015;135(6):e1399-408. [DOI] [PubMed] [Google Scholar]
García‐García 2006
- García-García ML, Calvo C, Pérez-Breña P, De Cea JM, Acosta B, Casas I. Prevalence and clinical characteristics of human metapneumovirus infections in hospitalized infants in Spain. Pediatric Pulmonology 2006;41(9):863-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartling 2011
- Hartling L, Bialy LM, Vandermeer B, Tjosvold L, Johnson DW, Plint AC, et al. Epinephrine for bronchiolitis. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No: CD003123. [DOI: 10.1002/14651858.CD003123.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 1979
- Henderson FW, Clyde WA Jr, Collier AM, Denny FW, Senior RJ, Sheaffer CI, et al. The etiologic and epidemiologic spectrum of bronchiolitis in pediatric practice. Journal of Pediatrics 1979;95(2):183-90. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Iles 1999
- Iles R, Lister P, Edmunds AT. Crying significantly reduces absorption of aerosolised drug in infants. Archives of Disease in Childhood 1999;81:81:163–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacques 2006
- Jacques J, Bouscambert-Duchamp M, Moret H, Carquin J, Brodard V, Lina B, et al. Association of respiratory picornaviruses with acute bronchiolitis in French infants. Journal of Clinical Virology 2006;25(4):463-6. [DOI] [PubMed] [Google Scholar]
Kellett 2005
- Kellett F, Redfern J, Niven RM. Evaluation of nebulised hypertonic saline (7%) as an adjunct to physiotherapy in patients with stable bronchiectasis. Respiratory Medicine 2005;99(1):27-31. [DOI] [PubMed] [Google Scholar]
Klassen 1997a
- Klassen TP. Recent advances in the treatment of bronchiolitis and laryngitis. Pediatric Clinics of North America 1997;44(1):249-61. [DOI] [PubMed] [Google Scholar]
Klassen 1997b
- Klassen TP, Sutcliffe T, Watters LK, Wells GA, Allen UD, Li MM. Dexamethasone in salbutamol-treated inpatients with acute bronchiolitis: a randomized, controlled trial. Journal of Pediatrics 1997;130(2):191-6. [DOI] [PubMed] [Google Scholar]
Maguire 2015
- Maguire C, Cantrill H, Hind D, Bradburn M, Everard M. Hypertonic saline (HS) for acute bronchiolitis: systematic review and meta-analysis. BMC Pulmonary Medicine 2015;15:148. [DOI: 10.1186/s12890-015-0140-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandelberg 2010
- Mandelberg A, Amirav I. Hypertonic saline or high volume normal saline for viral bronchiolitis: mechanisms and rationale. Pediatric Pulmonology 2010;45(1):36-40. [DOI] [PubMed] [Google Scholar]
Meissner 2003
- Meissner HC. Selected populations at increased risk from respiratory syncytial virus infection. Pediatric Infectious Disease Journal 2003;22(Suppl 2):40-4. [DOI] [PubMed] [Google Scholar]
Mitchell 2017
- Mitchell M, Muftakhidinov B, Winchen T. Engauge Digitizer Software. http://markummitchell.github.io/engauge-digitizer.
Nasr 2001
- Nasr SZ, Strouse PJ, Soskoline E, Maxvold NJ, Garver KA, Rubin BK, et al. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest 2001;120(1):203-8. [DOI] [PubMed] [Google Scholar]
Panitch 1993
- Panitch HB, Callahan CW, Schidlow DV. Bronchiolitis in children. Clinics in Chest Medicine 1993;14(4):715-31. [PubMed] [Google Scholar]
Panitch 2003
- Panitch HB. Respiratory syncytial virus bronchiolitis: supportive care and therapies designed to overcome airway obstruction. Pediatric Infectious Disease Journal 2003;22(Suppl):83-8. [DOI] [PubMed] [Google Scholar]
Rakshi 1994
- Rakshi K, Couriel JM. Management of acute bronchiolitis. Archives of Disease in Childhood 1994;71(5):463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Robinson 1997
- Robinson M, Hemming A, Regnis J, Wong A, Bailey D, Bautotvich GJ, et al. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax 1997;52(10):900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roqué i Figuls 2016
- Roqué i Figuls M, Giné-Garriga M, Granados Rugeles C, Perrotta C, Vilaró J. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD004873. [DOI: 10.1002/14651858.CD004873.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 1987
- Rose RM, Pinkston P, O'Donnell C, Jensen WA. Viral infection of the lower respiratory tract. Clinical Chest Medicine 1987;8:405-18. [PubMed] [Google Scholar]
Schuh 1992
- Schuh S, Johnson D, Canny G, Reisman J, Shields M, Kovesi T, et al. Efficacy of adding nebulized ipratropium bromide to nebulized albuterol therapy in acute bronchiolitis. Pediatrics 1992;90(6):920-3. [PubMed] [Google Scholar]
Shay 1999
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis hospitalizations. JAMA 1999;282(15):1440-6. [DOI] [PubMed] [Google Scholar]
Shay 2001
- Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children 1979-1997. Journal of Infectious Diseases 2001;183(1):16-22. [DOI] [PubMed] [Google Scholar]
Shi 2017
- Shi T, McAllister DA, O'Brien KL, Simoes EA, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390(10098):946-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shoseyov 1998
- Shoseyov D, Bibi H, Shai P, Ahoseyov N, Shazberg G, Hurvitz H. Treatment with hypertonic saline versus normal saline nasal wash of pediatric chronic sinusitis. Journal of Allergy and Clinical Immunology 1998;101(5):602-5. [DOI] [PubMed] [Google Scholar]
StataCorp 2009 [Computer program]
- Stata Statistical Software. Version 11. College Station, TX: StataCorp LP, 2009.
Wainwright 2003
- Wainwright C, Altamirano L, Cheney M, Cheney J, Barber S, Price D, et al. A multicenter, randomized, double-blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. New England Journal of Medicine 2003;349(1):27-35. [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [DOI: 10.1186/1471-2288-14-135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1992
- Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. American Review of Respiratory Diseases 1992;145(1):106-9. [DOI] [PubMed] [Google Scholar]
Wark 2018
- Wark PA, McDonald V. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD001506. [DOI: 10.1002/14651858.CD001506.pub4] [DOI] [PubMed] [Google Scholar]
Wohl 1978
- Wohl ME, Chernick V. State of the art: bronchiolitis. American Review of Respiratory Diseases 1978;118(4):759-81. [DOI] [PubMed] [Google Scholar]
Wohl 2003
- Wohl ME, Chernick VC. Treatment of acute bronchiolitis. New England Journal of Medicine 2003;349(1):82-3. [DOI] [PubMed] [Google Scholar]
Zhang 2003
- Zhang L, Ferruzzi E, Bonfanti T, Auler MI, Davila NE, Faria CS, et al. Long and short-term effect of prednisolone in hospitalized infants with acute bronchiolitis. Journal of Paediatrics and Child Health 2003;39(7):548-51. [DOI] [PubMed] [Google Scholar]
Ziment 1978
- Ziment I. Respiratory Pharmacology and Therapeutics. Philadelphia: WB Saunders, 1978. [Google Scholar]
References to other published versions of this review
Zhang 2008
- Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD006458. [DOI: 10.1002/14651858.CD006458.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2011
- Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD006458. [DOI: 10.1002/14651858.CD006458.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013
- Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD006458. [DOI: 10.1002/14651858.CD006458.pub3] [DOI] [PubMed] [Google Scholar]
Zhang 2015
- Zhang L, Mendoza-Sassi RA, Klassen TP, Wainwright C. Nebulized hypertonic saline for acute bronchiolitis: a systematic review. Pediatrics 2015;136(4):687-701. [DOI] [PubMed] [Google Scholar]
Zhang 2016
- Zhang L, Mendoza-Sassi RA, Klassen TP, Wainwright C. Nebulized hypertonic saline for acute bronchiolitis: a systematic review [erratum]. Pediatrics 2016;137(4):e20160017. [DOI: 10.1542/peds.2016-0017] [DOI] [PubMed] [Google Scholar]
Zhang 2017
- Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD006458. [DOI: 10.1002/14651858.CD006458.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]