Postoperative cognitive dysfunction (POCD) occurs frequently after both cardiac and non-cardiac surgery, affecting up to 50% of patients [1,2]. The mechanisms of POCD remains elusive, although several contributing factors have been proposed including disruption of the blood brain barrier (BBB) [3]. BBB disruption has been reported in patients undergoing cardiac surgery with one small study demonstrating a correlation between BBB disruption and greater global cognitive decline from before surgery to 5 days after surgery [4,5]. However, the longer-term effects of BBB disruption on cognitive function is unknown. In this pilot study, we assessed BBB permeability changes in the early post-operative period in patients who underwent either cardiac or non-cardiac surgery under general anesthesia, and then examined the relationship between permeability changes and neurocognitive scores measured at 6 weeks post-operatively. We hypothesized that global and regional increases in permeability would be associated with cognitive performance at 6 weeks after surgery.
Following approval by the Duke University Health Systems Institutional Review Board and informed consent, 10 patients undergoing cardiac surgery (coronary artery bypass graft (CABG), valve, CABG + valve) with cardiopulmonary bypass (CPB) and 8 patients undergoing non-cardiac surgery with general anesthesia were enrolled between September 2013 and January 2016 (Supplementary Methods S1 and S2). Cognitive testing was performed at baseline (preoperatively) and at 6 weeks after surgery (Supplementary Methods S3) in accordance with the consensus statement on assessment of neurobehavioral outcomes after cardiac surgery [6]. Magnetic resonance imaging (MRI) was performed post-operatively (on days 1–5, based on clinical stability) on a single 3 T Siemens Magnetom Trio Trim scanner. We used a variant of the delayed contrast extravasation subtraction method for determining BBB permeability by comparing variant flip angle 3D FLASH T1-maps rather than T1-weighted signal intensity images over time following contrast administration (Supplementary Methods S4). To characterize cognitive function over time while minimizing potential redundancy in the cognitive measures, a factor analysis with orthogonal rotation was performed on the 14 cognitive test scores. Since this was a pilot study, only the strength of association between cognitive score change at 6 weeks and the quantitative measures of BBB disruption (globally and by region) was measured using Pearson or Spearman correlations, as appropriate. Differences in cognitive change for patients with and without BBB disruption were assessed using t-tests or Wilcoxon rank sum tests (Supplementary Methods S5).
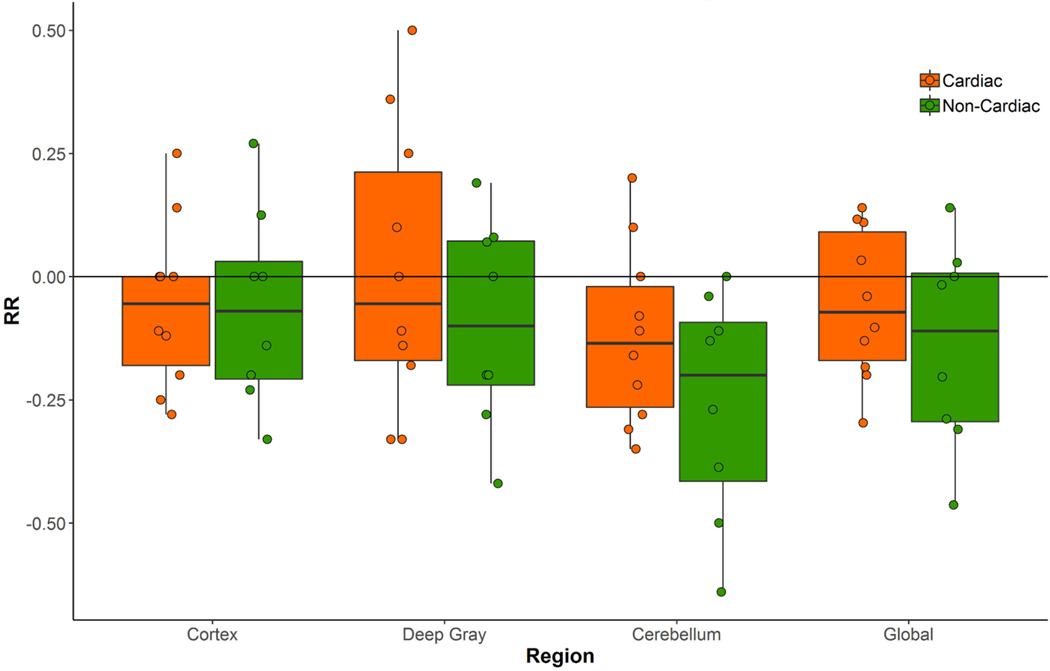
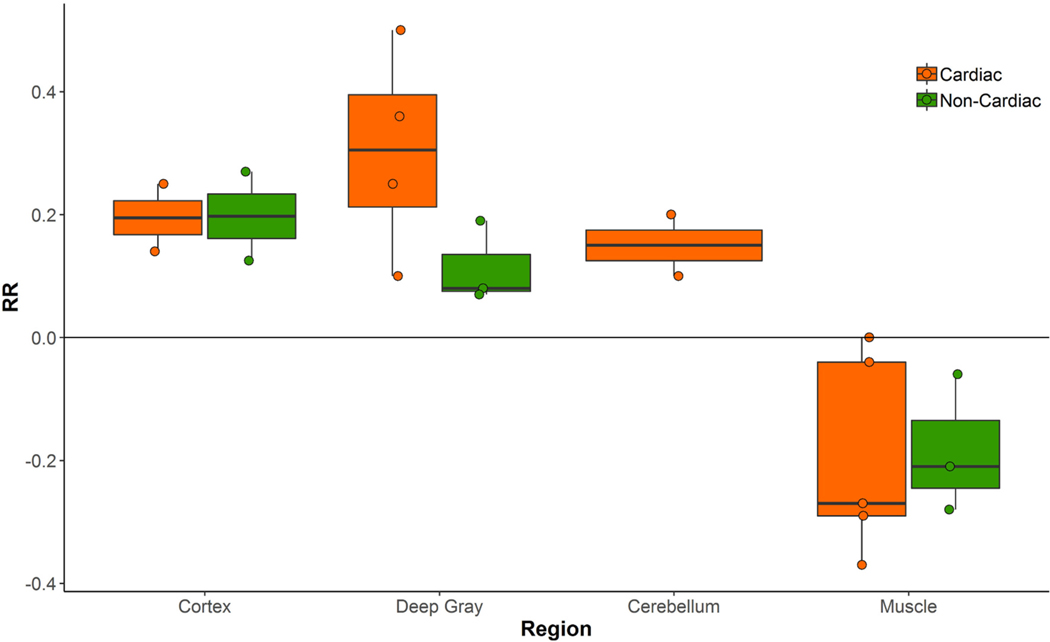
There were no statistical differences in mean age, co-morbid illness, years of education, or baseline cognitive scores (Supplementary Table 1). The day for MRI acquisition post-operatively was slightly but significantly higher in cardiac patients (Median 3, interquartile range = 3–4) versus non-cardiac patients (Median 2, interquartile range = 1–2). Overall, BBB disruption (positive value relative ratio (RR) = T1 map3 –T1 map1 / T1 map1 of retained contrast material in one or more brain regions (cortex, deep gray matter, or cerebellum)) was detected in 8 of 18 (44%) post-operative patients, with evidence of retained brain enhancement in 5 of 10 (50%) cardiac surgery patients and 3 of 8 (38%) non-cardiac patients. While the cardiac group had equal or higher values of RR across regions compared to the non-cardiac group, there were no statistically significant differences in RR values between surgical groups in this small sample (Fig. 1). The largest difference between surgical groups was in the cerebellum (mean difference 0.14 ± 0.20; p = 0.17). When considering different brain regions, the majority of the patients in both cardiac and non-cardiac groups with some BBB permeability had evidence of BBB leakage in the deep gray matter (Fig. 2). Although relative BBB permeability trended higher in deep gray structures, cortical permeability changes were most strongly associated with a lower composite cognitive change (cognitive index) score at 6 weeks after surgery (effect size = 0.65; Pearson rho = −0.21 [−0.61, 0.29], p = 0.40, Supplementary Table 2). There was also evidence of a trend, toward lower cognitive change scores in patients with a greater number of regions involved (cortex, deep gray, and cerebellum) (Spearman rho (95% CI) = −0.39 [−0.72, 0.10], p = 0.11), and this correlation was strongest in cardiac surgical patients (Spearman rho = −0.61 [−0.89, 0.07], p = 0.06). With regards to specific cognitive domains, consistent inverse correlations were detected between the degree of permeability in all regions and executive function, with the strongest inverse correlation for the deep gray structures (effect size = 0.28; Pearson rho = −0.36 [−0.70, 0.11], p = 0.14). There was also an inverse correlation between the global permeability measure and executive function (effect size = 0.52; Pearson rho = −0.39 [−0.72, 0.11], p = 0.29).
Fig. 1.
Relative relaxivity (RR) comparisons of the two surgical cohorts in the three brain regions and the combined global area.
Fig. 2.
Relative relaxivity (RR) in subjects with blood brain barrier disruption demonstrating greater permeability in the deep gray structures of cardiac surgical patients.
Animal models of surgery-induced neuroinflammation have highlighted a role for BBB dysfunction and infiltration of immune cells into the brain parenchyma [7]. Orthopedic surgery contributes to fibrinogen deposition within 24 h after tibial fracture [8] which is accompanied by disruption of tight junction expression, such as claudin-5, and infiltration of monocytes into the hippocampus [9,10]. In humans, using MRI, Merino et al. [5] reported that of 19 patients studied, approximately 50% demonstrated the hyperintense acute reperfusion marker (HARM - delayed CSF enhancement). Similarly, Abrahamov and colleagues [4] studied 7 patients with dynamic contrast enhancement MRI preoperatively and postoperatively on days 1 and 5 after CABG surgery with CPB. BBB disruption was evident in 71% of patients on POD 1, most commonly in the frontal lobes, but had completely resolved by POD 5. Potential etiologic factors for BBB disruption during surgery include hypoperfusion, hypothermia, microembolic showers, and activation of the systemic inflammatory response resulting in endothelial dysfunction and subsequent neuroinflammation, breakdown of the extracellular matrix, and impaired expression of tight junctions [7].
In conclusion, in the first study to examine both cardiac and noncardiac surgical patients using an MR imaging methodology that evaluates wash out dynamics over time using internal controls, we found that nearly half of surgical patients experience an increase in postoperative BBB permeability. Regional variation in the permeability increases were also detected, and potential relationships with lower cognitive performance were suggested. These findings should be considered preliminary and require replication in a larger study.
Supplementary Material
Funding
This study is supported in part by grants #HL108280, HL109971, and HL130443 from the National Institutes of Health.
Footnotes
Credit author statement
Christopher D. Lascola: Conceptualization, Data curation, Formal analysis, Writing – original draft. Sarah F. Cotter: Data curation, Writing – review & editing. Rebecca Y. Klinger: Supervision, Writing – original draft. Tiffany Bisanar: Project Administration, Supervision, Writing – review & editing. Mary Cooter Wright: Data curation, Formal analysis, Writing – review & editing. Miles Berger: Conceptualization, Writing – review & editing. Gavin Martin: Supervision, Writing – review & editing. Mihai V. Podgoreanu: Supervision, Writing – review & editing. Mark F. Newman: Funding acquisition, Writing – review & editing. Niccolò Terrando: Conceptualization, Writing – original draft. Joseph P. Mathew: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – original draft.
Declaration of Competing Interest
None to report.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jclinane.2023.111059.
References
- [1].McDonagh DL, Mathew JP, White WD, Phillips-Bute B, Laskowitz DT, Podgoreanu MV, et al. Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury. Anesthesiology. 2010;112:852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 2001;344:395–402. [DOI] [PubMed] [Google Scholar]
- [3].He HJ, Wang Y, Le Y, Duan KM, Yan XB, Liao Q, et al. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci Ther 2012;18:994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abrahamov D, Levran O, Naparstek S, Refaeli Y, Kaptson S, Abu Salah M, et al. Blood-brain barrier disruption after cardiopulmonary bypass: diagnosis and correlation to cognition. Ann Thorac Surg 2017;104:161–9. [DOI] [PubMed] [Google Scholar]
- [5].Merino JG, Latour LL, Tso A, Lee KY, Kang DW, Davis LA, et al. Blood-brain barrier disruption after cardiac surgery. AJNR Am J Neuroradiol 2013;34:518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg 1995;59:1289–95. [DOI] [PubMed] [Google Scholar]
- [7].Yang T, Velagapudi R, Terrando N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol 2020;21:1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 2011;70:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Degos V, Vacas S, Han Z, van Rooijen N, Gressens P, Su H, et al. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang T, Xu G, Newton PT, Chagin AS, Mkrtchian S, Carlstrom M, et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br J Anaesth 2019;122:350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.