Abstract
The integrative immune markers neutrophil-lymphocyte-ratio (NLR) , platelet-lymphocyte-ratio (PLR) and systemic immune inflammation index (SII) are established markers in clinical patient care. Adoption of these markers in elite athletics might prove beneficial for monitoring training and health. Blood samples of 195 healthy national Olympic squad athletes were collected before a graded bicycle-ergometric exercise test until complete exhaustion. Measurements included white blood cells, lymphocytes and platelets, allowing for the calculation of the integrative immune markers. Correlations between athlete characteristics (sex, age, sporting discipline, training experience, training volume) and integrative immune marker-values were assessed. In a subgroup analysis a second blood sample was collected from 25 athletes at 1 minute after exercise test to assess its effect on the immune marker levels.
An inverse correlation between peak power output and SII-level (Pearson correlation coefficient=−.270, p<.001) and NLR-level (Pearson correlation coefficient=−.249, p<.001) was found. Athletes with higher aerobic fitness had significantly lower values of SII and PLR compared to athletes with lower aerobic fitness. An elevated SII (p=.003) and a reduced PLR (p=.001) was documented as acute response to the exercise test. The integrative immune markers might be a promising tool for monitoring training and health in elite athletes.
Key words: SII, NLR, PLR, olympic athletes, integrative immune markers, immune system
Introduction
In order to maximize professional athletes’ performance and avoid injury and disease, it is crucial to optimize the balance between training load and recovery. While training aims to provoke physiological and psychological adaptions leading to improved performance, overtraining may reduce it and lead to pathological conditions 1 .
An integrative approach to monitor training load and to detect overtraining generally includes psychological and self-reported measures (e. g. pain, fatigue, sleep quality, depressive symptoms) as well as bio-physiological outcomes. The latter consist of various measures detecting the function or state of different organ systems and tissues to quantify internal load upon acute or chronic exercise stimuli. These measures include blood-borne markers like creatine kinase (CK) 2 , lactate levels 3 , testosterone/cortisol ratios (FTCR) 4 , cardio-vascular parameters like resting and training heart rates and heart rate variability 5 , sleep quality and quantity 6 as well as immunological inflammation markers 7 .
As a general hallmark of physiological and psychological stress, as well as of systemic and tissue specific adaptions (damage and recovery) inflammatory processes have been highlighted 8 . In brief, acute exercise provokes local and systemic inflammatory responses in the short term, whereas training leads to an increased anti-inflammatory capacity in the medium to long-term. In contrast, many pathological conditions, as well as overtraining, are characterized by chronic local or systemic inflammation. The complex immunological process cannot be assessed using any one single marker. The mediators of immune response are humoral (cytokines, acute-phase proteins) as well as cellular (leukocytes) 9 . In elite athletes several inflammation biomarkers are used – although inconsistently between training centers – to screen for disease, monitor training and recovery, and detect overtraining. The most frequently used inflammation markers in the field of exercise physiology are white blood cell (WBC) count 10 , Interleukin-6 (IL-6), Interleukin-10 (IL-10) 11 and C-reactive protein (CRP) 12 .
Recently, an additional array of composite laboratory markers of inflammation has emerged in clinical medicine, especially oncology: The neutrophil-lymphocyte-ratio (NLR), platelet-lymphocyte-ratio (PLR) and the systemic immune inflammation index (SII). In the field of oncology, the three integrative immune markers (IIM) are now well-established markers of cancer-related inflammation and valid indicators of prognosis of solid tumors 13 . NLR and SII cut-off values are also used to determine therapeutic strategies in several oncological settings 14 . Different studies showed an increased NLR in patients with multiple sclerosis 15 or increased SII in association with psoriasis and psoriatic arthritis 16 . Even more recently NLR was calculated to assess prognosis and patient risk stratification in patients with Covid-19 17 and has been demonstrated as a strong predictor of the degree of coronary calcification and stenosis in patients with coronary artery disease 18 .
Inexpensive and easily obtained inflammation markers such as NLR, PLR, and SII are potentially useful in the day-to-day work with elite athletes, regarding both training-rest algorithms and medical care. Thus far, the effects of exercise on these integrative immune markers have been assessed by only a few studies 19 20 21 . High-intensity-interval training in form of a three-week intervention was shown to reduce NLR and SII in patients with multiple sclerosis 19 . In healthy individuals endurance exercise led to greater NLR and SII increase than strength exercise as a result of stronger immune cell mobilization in an acute exercise setting 21 . The influence of active recovery in the form of aqua cycling on NLR- and SII-values compared to passive recovery showed interesting results in a recent study: In contrast to passive recovery the actively recovering athletes reached higher peak values of NLR and SII, but return to base values was not prolonged. This may mean that the time of return to baseline values is independent of the recovery modus; an aspect that would help to implement the markers as a tool in the day to day care of athletes with often very different training and recovery protocols 22 . Investigations in elite athletes have not been conducted so far. Based on their benefits in clinical medicine and the results from exercise trials, a transfer of these IIM into elite athleticism and professional sports has recently been discussed 7 . However, before being implemented in the day-to-day athlete care, the IIM have to be better understood in this unique population. Prospectively, norm-values would need to be derived from large trials. However, a marker that is influenced by arbitrary athlete characteristics would not yield adequate norm-values to be implemented as a screening tool in large athlete cohorts. Also, due to the daily training regimens in this cohort – often multiple times per day – the effects of different intensities and durations of different forms of exercise on the integrative immune markers will have to be assessed in larger trials. Most likely a certain resting period before blood-sampling will have to be established to realistically implement the IIM in screening-protocols of elite athletes.
Therefore, this pilot study had the two followings objectives:
To investigate associations of athlete characteristics such as sex, age, sporting discipline, training volume or relative peak power output (PPO) with the NLR, PLR, and SII.
To examine acute changes in the IIM in response to a graded exercise test until exhaustion in a cohort of elite athletes.
Ultimately, the results obtained herein will serve as a basis for larger follow-up trials to establish norm-values and time-frames for testing.
Materials and Methods
The study was approved by the ethics committee of the German Sport University Cologne (104/2020) and conducted in accordance with the Declaration of Helsinki. Every participant or their legal representative signed a written informed consent.
Inclusion criteria were: Status as athlete of the German Olympic team or German Olympic prospective team; minimum of 6 training hours per week; minimum of 2 years active training in the respective discipline; written informed consent. Exclusion criteria were:<12 or>33 years of age; infection within the last four weeks; pause from training for>2 weeks during the 3 months prior to eligibility assessment.
Participants
As part of their medical check-up, 195 healthy national Olympic squad athletes were recruited over a 4-month period. Participant characteristics are displayed in Table 1 . All subjects were healthy and had abstained from alcohol consumption or intense physical activity for at least 16 hours.
Table 1 Athlete characteristics.
| Overall sample | Subgroup of acute effect study | |||||
|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | |
| N | 195 | 112 | 83 | 25 | 11 | 14 |
| Age (years) | 19.8±7.1 | 20.9±8.0 | 18.3±5.2 | 16.1±3.9 | 15.8±2.8 | 16.3±3.8 |
| Height (cm) | 173.8±12.2 | 178.3±11.9 | 167.6±9.7 | 172.6±7.9 | 180.4±6.4 | 167.7±3.2 |
| Weight (kg) | 67.1±16.5 | 72.2±16.7 | 60.1±13.6 | 60.9±9.0 | 67.7±9.1 | 56.9±6.0 |
| BMI (kg/m 2 ) | 22.2±3.5 | 22.4±3.3 | 21.3±3.7 | 20.3±2.3 | 20.3±2.26 | 20.3±2.4 |
| Training years | 8.9±4.3 | 9.2±4.5 | 8.7±4.1 | 8.0±4.1 | 6.4±2.7 | 8.5±4.1 |
| Sessions per week | 6.7±2.9 | 7.0±3.0 | 6.3±2.8 | 4.8±1.4 | 4.7±1.4 | 4.9±1.4 |
| Training hours per week | 13.7±5.8 | 13.6±5.5 | 13.8±6.2 | 9.4±4.7 | 8.0±3.0 | 10.5±5.8 |
Values are presented as mean±SD. BMI, body-mass-index.
Study design
A cross-sectional study design was applied. Upon arrival of the athlete in the testing institution, a venous blood sample was obtained between 8:30 and 9:00 a.m., using a vacutainer blood withdrawal system (Becton, Dickinson and Company, Heidelberg, Germany). Athlete information about sex, age, sporting discipline, training experience (total training years in their specific discipline) and training volume (average training hours per week) was collected in a standard sports-medical questionnaire. Weight was measured using a body composition analyzer (Seca GmbH, Germany). Participants then performed a standardized bicycle ergometer (Ergoselect Ergoline & ECGpro, Amedtec, Germany) protocol (Hollman-Venrath: start at 30 W, increase by 40 W every 3 minutes) until exhaustion. Heart rate was obtained from a resting electrocardiogram (FX8322, Fukuda Denshi Co., Japan) and blood pressure was averaged from two upper-arm measurements (one left and one right arm) at rest (Omron MM500, Omron Healthcare Co., Japan). Since PPO, calculated as Watts per kilogram (W/kg), has a well-documented reliability as a measure of aerobic fitness 23 , it was used to assess the participants’ aerobic fitness-level. In a subgroup of 25 athletes a second venous blood sample was drawn 1 minute after termination of the graded exercise test.
Blood sampling and analysis
EDTA blood samples were analyzed with a hematology analyzer (Sysmex XN350, Sysmex Deutschland GmbH). The cellular immune inflammation markers were calculated with the following equations:
NLR [A.U.]=neutrophil count [×10 3 /μL]/lymphocyte count [×10 3 /μL]
PLR [A.U.]=platelet count [×10 3 /μL]/lymphocyte count [×10 3 /μL]
SII [×10 3 /μL]=neutrophil count [×10 3 /μL]×platelet count [×10 3 /μL]/lymphocyte count [×10 3 /μL]
Data accumulation and statistical analysis
Raw data was documented in Excel (Microsoft Corporation, 2018). Statistical analyses were conducted using SPSS statistics 28 (IBM). All parameters were tested for normality using Shapiro-Wilk test before further statistical analyses were conducted.
Pearson correlation coefficients were calculated to determine potential correlations between metric athlete characteristics (age, training volume, PPO) and values of white blood cells (WBC), NLR, PLR and SII, respectively. To investigate differences in NLR, PLR and SII values in dependence on participant characteristics, athletes were divided into subgroups according to sex, age, training volume, sporting disciplines and PPO. The categories were formed as follows: Sex (male vs. female), age (youth:<18 y/o; adult:≥18 y/o), training volume (low:<11 h/week; moderate: 11–15 h/week; high:>15 h/week), sporting disciplines (endurance, combat, technique-based, athletics and ball sports), PPO (low:≤3.03 W/kg; intermediate: 3.04–3.66 W/kg; high:≥3.67 W/kg). Due to a large number of different sporting disciplines the above sub-group-differentiation into five cohorts was chosen based on key characteristics of the disciplines. Significant differences between subgroups were assessed via one-way analysis of variance (ANOVA). In case of significant differences, Bonferroni-corrected post-hoc tests were conducted. The effects of acute exercise on NLR, PLR and SII were investigated by paired t-tests (pre and post exercise). The level of significance was set to p<.05 for all statistical analyses.
Results
Of the 195 included Olympic team athletes, all datasets were complete and included in the analysis. The general characteristics of the study population are displayed in Table 1 . Baseline values of NLR, PLR and SII for the whole cohort as well as separated by athlete characteristics are presented in Table 2 . The average duration of the bicycle ergometer test for the whole cohort was 17 minutes and 12 seconds, resulting in an average of 224.10 Watt maximum and 3.40 Watts per kilogram (PPO).
Table 2 Baseline values of NLR, PLR, and SII in elite athletes.
| n | NLR | PLR | SII [×10 3 /μL] | |
|---|---|---|---|---|
| Total | 195 | 1.49±0.63 | 121.3±35.1 | 343.9±161.9 |
| Sex | ||||
| Male | 112 | 1.48±0.60 | 122.9±39.6 | 337.3±162.3 |
| Female | 83 | 1.50±0.66 | 119.2±28.0 | 352.9±162.9 |
| Age | ||||
| Youth (<18 y) | 90 | 1.38±0.66 | 122.8±36.2 | 326.5±162.0 |
| Adult (≥18 y) | 105 | 1.58±0.58# | 120.0±34.2 | 358.9±161.1 |
| Training volume | ||||
| High (>15 h/week) | 65 | 1.57±0.67 | 120.0±29.0 | 368.6±184.1 |
| Moderate (11–15 h/week) | 61 | 1.42±0.57 | 119.0±35.0 | 329.5±157.9 |
| Low (<11 h/week) | 69 | 1.47±0.63 | 124.5±40.3 | 333.6±141.4 |
| PPO | ||||
| Low (≤3.03 W/kg) | 65 | 1.31±0.46 | 117.7±30.9 | 283 .9±111.2 |
| Moderate (3.04–3.66 W/kg) | 66 | 1.45±0.68 | 126.3±44.1* | 354.2±189.1 |
| High (≥3.67 W/kg) | 64 | 1.71±0.66* | 119.9±27.8*§ | 394.4±157.6 |
Table 3 depicts the spectrum of sporting disciplines of the 195 included athletes. Fig. 1 shows the SII, NLR and PLR for five subgroups (endurance disciplines, combat disciplines, technique-based disciplines, athletics and ball sports). Regarding the whole cohort correlation analyses revealed a significant inverse correlation between PPO and SII-levels (Pearson correlation coefficient=−.270, p<.001) and NLR-levels (Pearson correlation coefficient=−.249, p<.001) ( Fig. 2 ). No differences whatsoever were documented for the five different subgroups of sporting disciplines. NLR revealed a significant correlation to athlete age (Pearson correlation coefficient=.167, p=.019); no age association was found for the PLR or the SII. Furthermore, the WBC showed a strong positive correlation to NLR (Pearson correlation coefficient=.433, p<.001) and SII (Pearson correlation coefficient=.526, p<.001). Considering training volume, maximum heart rate or blood pressure under exercise no significant correlations were found for NLR, PLR, and SII.
Table 3 Spectrum of sporting disciplines of the 195 included athletes.
| n | |
|---|---|
| Archery | 1 |
| Badminton | 1 |
| Basketball | 6 |
| BMX | 1 |
| Boxing | 2 |
| Climbing | 2 |
| Cycling | 1 |
| Dancing | 2 |
| Diving | 13 |
| Fencing | 14 |
| Football | 4 |
| Figure skating | 1 |
| Gymnastics | 2 |
| Ice hockey | 6 |
| Handball | 3 |
| Judo | 31 |
| Javelin throw | 2 |
| Kick boxing | 6 |
| Rowing | 5 |
| Skating | 2 |
| Surfing | 9 |
| Swimming | 15 |
| Table Tennis | 5 |
| Tennis | 7 |
| Track and Field | 38 |
| Triathlon | 3 |
| Volleyball | 8 |
| Water polo | 2 |
| Wakeboard | 1 |
| Wrestle | 2 |
| Total | 195 |
Fig. 1.
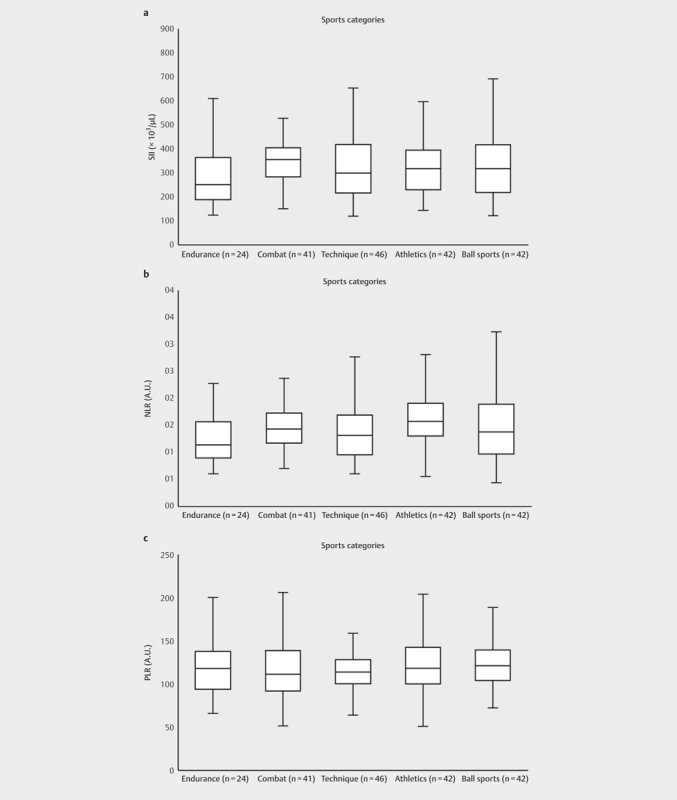
Five subgroups of the 195 athletes and their size showing their median a : SII-levels b : NLR-levels c : PLR-levels. SII: systemic immune-inflammation index. NLR: neutrophil-lymphocyte ratio. The whiskers indicate the minimum and maximum values.
Fig. 2.
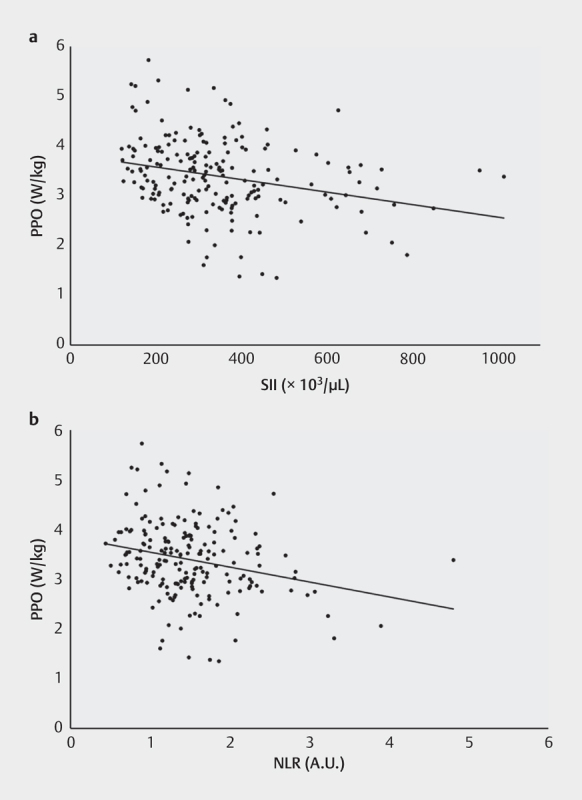
Linear correlation plots between peak power output (PPO) and a : SII-levels b : NLR-levels for all 195 athletes. SII: systemic immune-inflammation index. NLR: neutrophil-lymphocyte ratio.
Comparing the different PPO subgroups, a significant difference in SII values was found regarding PPO (p<.001) both when considering all the athletes and when dividing them by sex (p=.0127 for males; p=.035 for females). In detail, athletes with high PPO showed significantly lower SII values when compared to athletes with moderate (p=.032) or low PPO (p<.001) ( Fig. 3 ). A significant difference was also observed for the NLR-values regarding PPO-subgroups. Athletes with high PPO showed significantly lower NLR values when compared to athletes with low PPO (p<.001). These differences applied to both sex-subgroups similar to the SII (p=.045 for males; p=.035 for females) In contrast to the SII-findings, no significant differences between the high PPO and moderate PPO groups were found for NLR ( Fig. 4 ). Additionally, significantly lower NLR-values for the subgroup “youth” in comparison to the subgroup “adult” were found (p=.029) (see Table 2 ). Considering the PPO subgroups PLR showed no differences between the high, moderate and low W/kg-groups ( Fig. 5 ). Considering all other subgroups (sex, age, BMI, training volume), no further significant differences were found for NLR, PLR, and SII.
Fig. 3.
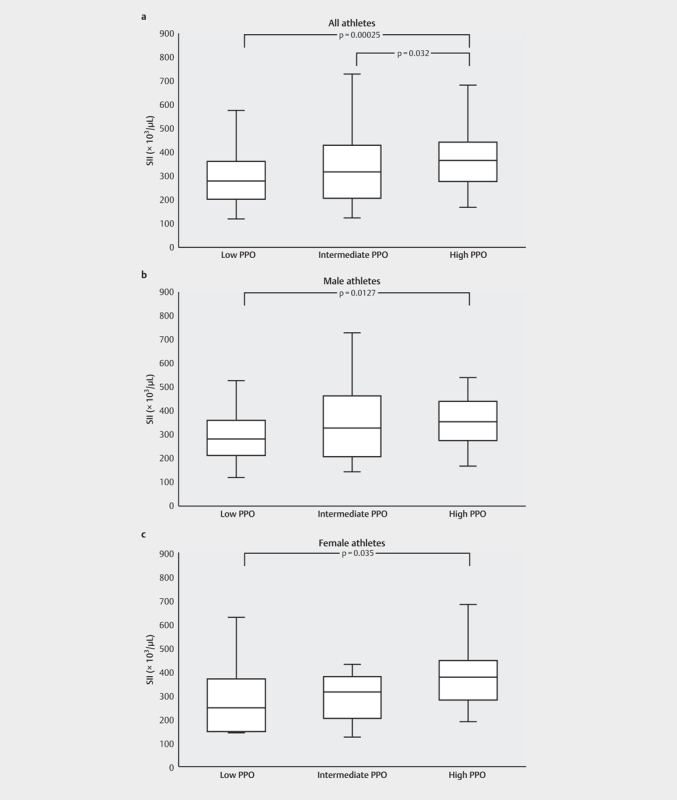
Comparison of the SII between the different relative peak power outputs (PPO) subgroups divided by sex considering: a : All athletes (n=195). b : All male athletes (n=112). c : All female athletes (n=83). Significant differences between the groups are indicated by p-values. The whiskers indicate the minimum and maximum values. SII: systemic immune-inflammation index.
Fig. 4.
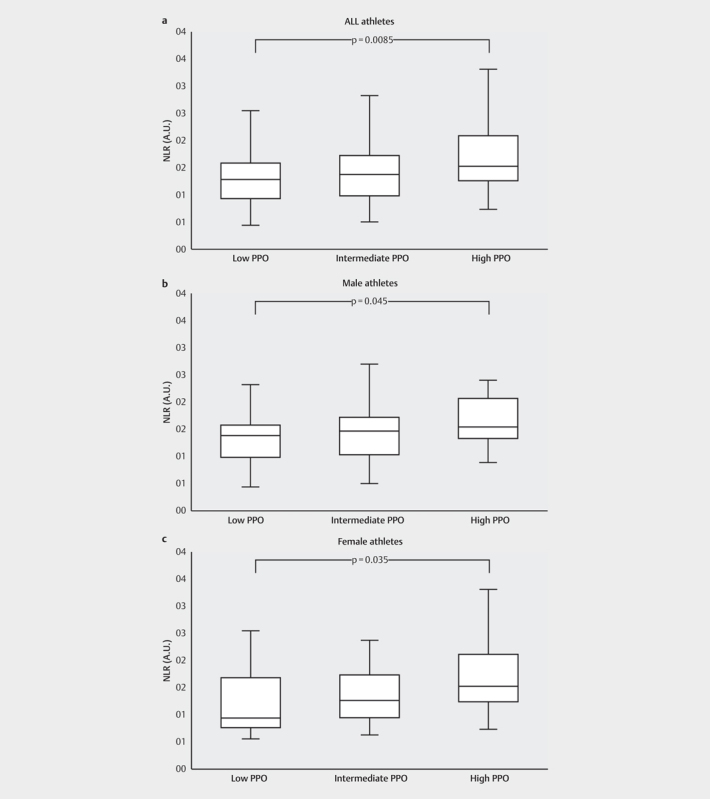
Comparison of the NLR between the different relative peak power outputs (PPO) subgroups divided by sex considering: a: All athletes (n=195). b : All male athletes (n=112). c : All female athletes (n=83). Significant differences between the groups are indicated by p-values. The whiskers indicate the minimum and maximum values. NLR: neutrophil-lymphocyte ratio.
Fig. 5.
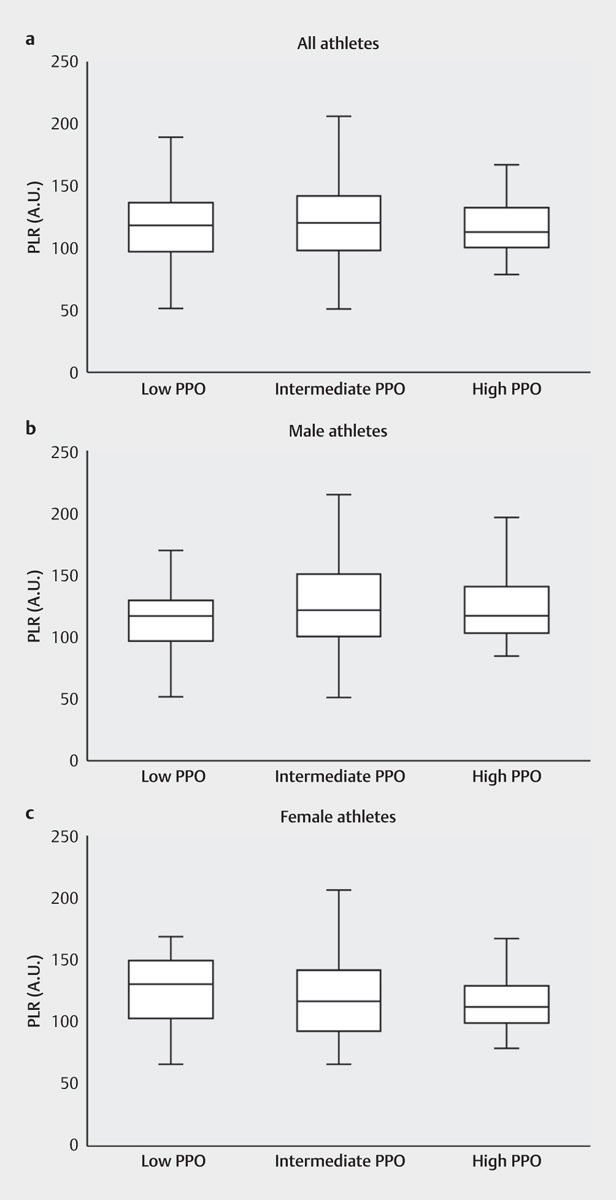
Comparison of the PLR between the different relative peak power outputs (PPO) subgroups divided by sex considering: a: All athletes (n=195). b : All male athletes (n=112). C : All female athletes (n=83). Significant differences between the groups are indicated by p-values. The whiskers indicate the minimum and maximum values. PLR: platelet-lymphocyte ratio.
The duration of the bicycle ergometer test to exhaustion in the subgroup of 25 athletes, from whom a second blood-sample was obtained afterwards, was 16 minutes and 48 seconds. The average peak resistance was 210.10 Watts and 3.32 W/kg. The effect of acute exercise on the integrative immune markers revealed a significant increase of the SII (p=.003) and decrease of the PLR (p=.001), while NLR did not change significantly (see Fig. 6 ).
Fig. 6.
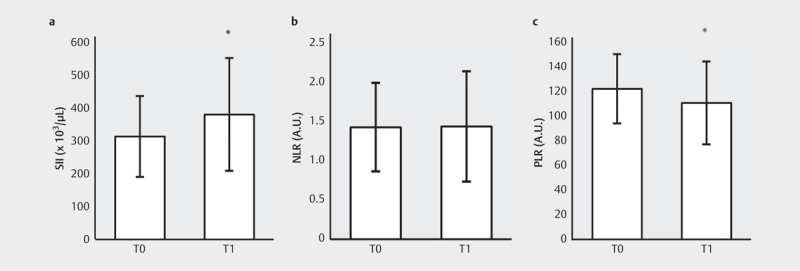
Alterations of NLR, PLR, and SII after acute exercise.; Values are presented as mean±SD. * significant changes (p<0.05). a : SII, systemic immune-inflammation index. b : NLR, neutrophil-lymphocyte ratio. c : PLR, platelet-lymphocyte ratio.
Discussion
This study focused on the three clinical integrative immune markers (IIM) NLR, PLR and SII in a large cohort of elite athletes. While well-established in some areas of medical disciplines, these potentially promising markers have not yet found their way into the day-to-day management of athletes’ health and training. To lay the foundation for larger follow-up trials potentially elucidating norm-values for the IIM, this trial aimed to assess the independence of the IIM from different athlete characteristics in a broad spectrum of athletes of different ages and disciplines. To this end potential associations between the IIM and several selected athlete characteristics were assessed. Of these characteristics only aerobic fitness defined as peak power output (PPO) showed significant associations with the levels of several IIM: An inverse correlation with the SII- and NLR-values was documented. High levels of aerobic fitness may be the consequence of regular endurance training. This would be consistent with prior discoveries that regular exercise reduces baseline inflammation 7 . Additionally, a strong correlation between the overall white blood-cell count and the IIM underscores their potential as an immunological marker. A correlation between the WBC-count and the IIM is likely, as the latter are in part calculated using subsets of the former, but a correlation is not guaranteed depending on the distribution of the different white blood cells and on the platelet count 24 . As a result, the SII and NLR may be well suited as a measure of immunological alterations in athletes. Also, endurance training was previously shown to have a higher influence on immune markers than resistance training 21 , lending plausibility to a stronger association of anti-inflammatory effects amongst athletes with higher fitness-levels. This finding is also in accordance with the observation of Weinhold et al. 25 , who reported a positive correlation of levels of regulatory T-cells (Treg) with aerobic fitness; as Treg have an anti-inflammatory effect, this elevation is congruent with an SII- and NLR-reduction amongst fitter athletes. Interestingly, the comparison of the IIM in the sports discipline subgroups did not yield any significant results. One may have expected lower values amongst the athletes classified as “endurance”-athletes in accordance with the general fitness-correlation. However, the relatively small sample sizes of the subgroups or large variations in fitness-levels within the subgroups may have led to these insignificant results. While no association with sporting discipline subgroups was demonstrated in this trial, this may be due to the small respective sample sizes of the included disciplines. Larger cohorts in future trials may show lower IIM-levels in cohorts of endurance athletes, but this remains conjecture at this point.
Importantly, of all other assessed athletes’ characteristics (see Table 1 ) only one other association was discovered: a weak correlation between age and NLR. Large studies assessing IIM-values in the general non-athletic community focus on adults 26 27 . To our knowledge no studies focusing on the IIM in healthy children and adolescents have been published. Only smaller trials assessing the IIM in cohorts of ages<18 years with certain diseases are available 28 29 30 , rendering any comparison to the healthy cohort of youths of our trial ineffective. The age-association of the NLR is in accordance with the results of a large population-based study by Meng et al. that documented lower NLR-values in younger adults than older adults; albeit, this investigation did not include participants<18 years of age 31 . A potential age-association of the NLR does not reduce its value as a future marker in day-to-day athlete care; several laboratory markers differentiate between youth- and adult-athletes (i. e. hemoglobin, ferritin, etc.). Future investigations are needed to further elucidate possible age-specific differences in the NLR and its potential impact for athlete care.
Apart from PPO for SII and NLR and age for NLR no other associations between the IIM and athlete characteristics were documented. This hints at the potential of these markers as helpful parameters to detect significant changes in an athlete’s immune status. To enable their use in the day-to-day work with large, often heterogeneous athlete cohorts – a common setting in large sports-medical centers – a certain independence of the inflammatory markers from athlete characteristics is necessary. Otherwise, as for example with the heart rate variability, only intra-individual longitudinal assessments would be feasible.
The high sensitivity and reliability of the IIM has been demonstrated in oncological and cardiac patients 13 18 . Not only are they used for risk stratification, but the SII, for example, can be used to assess treatment efficacy 32 . Based on these observations and the current findings in our study, the SII might be suitable to detect overtraining or early stages of disease or infection in a- or oligosymptomatic athletes; to that end future studies should include athletes at different stages of overtraining and/or disease to further assess the applicability of these novel markers in elite sports.
No sex differences were documented for the IIM in this athlete cohort. Large studies in the general population including tens of thousands of participants present heterogeneous data regarding IIM-values for the two sexes: Meng et al. find no differences between the sexes, while Luo et al. find significant differences for PLR and SII but not NLR [33, 31 34 26 27 . Possibly, the modulation of the immune system as a result of years of high training volumes (see Table 1 ) has lessened the difference between the sexes amongst athletes. We are assessing here a cohort of very fit, highly-trained elite athletes: Considering the modulation possibilities of acute and chronic exercise on the immune system and thus the integrative immune markers, this cohort may as a whole have shifted towards “athlete levels” of IIM, lessening the relative differences between the sexes. Higher numbers of participants and a non-athletic control group in the follow-up trials may shed further light on this issue and potentially yet yield sex differences.
In order to implement the investigated immune inflammation markers into every-day athlete care, fluctuations at different time-points after several types of exercise need to be analyzed. Naturally, athletes train often, usually daily, potentially multiple times per day. The athletes of this cohort had an average weekly training volume of 13 hours. Thus, blood sampling in a medical or regulatory setting will often take place in chronologically close proximity to the last exercise session. To our knowledge no data regarding the changes in IIM-levels in response to exercise are available for elite athletes. In a first step towards analyzing the effects of a strenuous exertion on the IIM, these were assessed in a sub-group of 25 athletes immediately after a maximal exercise test. We observed a significant elevation of SII- and reduction of PLR-values (see Fig. 6 ). This coincides only partly with the findings of Wahl et al., who observed a PLR-reduction immediately after 30-second sprint-intervals, but not after 4×4 minute high-intensity-interval tests in non-athlete subjects. No change was documented in SII immediately after the interventions by Wahl et al. 20 . However, significant fluctuations vs. baseline values for PLR and SII in a 3-hour observational follow-up period after the two different intervention types were noted 20 . This underscores the need for the documentation of the levels of the IIM in large athlete cohorts at several time points after different exercise interventions, ideally up to 24 hours. Thus, Joisten et. al demonstrated a sharp increase in NLR- and SII-values in the first hours after a bout of exercise in a non-athlete cohort, with a gradual decline until reaching the resting-values roughly 24 hours post-exercise 22 .
The current investigation does not suffice for the establishment of norm-values and reference intervals for athletes 35 . Besides understanding associations between athlete characteristics and the IIM, reference intervals are the second prerequisite for applying the IIM in the day-to-day athlete care. In this pilot-like trial we were able to demonstrate that the IIM seem to be largely independent of most athlete characteristics. Follow-up trials with larger elite athlete cohorts are planned to verify these results and to determine reference ranges.
Strengths and Limitations
To our knowledge, this is the first and largest documentation of base-line values of the integrative immune markers (IIM) NLR, PLR and SII in elite athletes. It is the first assessment of potential associations between athlete characteristics and IIM in elite athletes. The athlete inclusion from one of Germany’s largest sports-medical centers ensures a selection of elite athletes: All participants were part of the German Olympic team or the German Olympic prospective team at the time of assessment. Training history and weekly training volume represent levels of elite athletes. Many disciplines and age-groups were included. However, the inclusion of many disciplines and age-groups limits the size of the relative subgroups. The measurement of fitness via W/kgs may over- or underscore an athlete’s fitness-level based on body stature and composition. The sub-group used to analyze acute effects of exertion on the IIM was small with only 25 athletes. The overall sample size was too small to calculate reference ranges of IIM for elite athletes.
Conclusion
In this study we analyzed possible associations between the clinical integrative immune markers (IIM) NLR, PLR and SII and athlete characteristics in a large cohort of elite athletes. We observed an association of aerobic fitness with the base-value of the SII and NLR, while no other interactions of relevance with any other athlete characteristics (including sex) were documented; a correlation between age and NLR needs to be investigated further in future analyses. The IIM, especially the SII and the NLR, may be promising markers in the training regulation and medical care of elite athletes in the future. To this end, follow-up trials of larger elite-athlete cohorts are planned to further strengthen the validity of the associations established here and to deduce reference ranges for the IIM in elite athletes.
The exact kinetics of the IIM at different post-exercise time points remain to be characterized in order to prospectively implement the use of NLR, SII, and PLR in day-to-day athlete care.
Acknowledgements
The authors Jonas Zacher and Fabian Wesemann are contributed equally as first authors. The authors thank Anke Schmitz and Christopher Spang for their support during testing.
Conflict of Interest The authors declare that they have no conflict of interest.
Authors contributed equally as first authors
References
- 1.Halson S L, Jeukendrup A E. Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med. 2004;34:967–981. doi: 10.2165/00007256-200434140-00003. [DOI] [PubMed] [Google Scholar]
- 2.Cadegiani F A, Kater C E. Basal hormones and biochemical markers as predictors of overtraining syndrome in male athletes: the EROS-BASAL study. J Athl Train. 2019;54:906–914. doi: 10.4085/1062-6050-148-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urhausen A, Kindermann W. Diagnosis of overtraining: what tools do we have? Sports Med. 2002;32:95–102. doi: 10.2165/00007256-200232020-00002. [DOI] [PubMed] [Google Scholar]
- 4.Chicharro J L, López-Mojares L M, Lucía A et al. Overtraining parameters in special military units. Aviat Space Environ Med. 1998;69:562–568. [PubMed] [Google Scholar]
- 5.Hinde K, White G, Armstrong N. Wearable devices suitable for monitoring twenty four hour heart rate variability in military populations. Sensors (Basel) 2021;21:1061. doi: 10.3390/s21041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lastella M, Vincent G E, Duffield R et al. Can sleep be used as an indicator of overreaching and overtraining in athletes? Front Physiol. 2018;9:436. doi: 10.3389/fphys.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walzik D, Joisten N, Zacher J et al. Transferring clinically established immune inflammation markers into exercise physiology: focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index. Eur J Appl Physiol. 2021;121:1803–1814. doi: 10.1007/s00421-021-04668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eming S A, Wynn T A, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356:1026–1030. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 9.Germolec D R, Shipkowski K A, Frawley R P et al. Markers of inflammation. Methods Mol Biol. 2018;1803:57–79. doi: 10.1007/978-1-4939-8549-4_5. [DOI] [PubMed] [Google Scholar]
- 10.Malm C, Nyberg P, Engstrom M et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. 2000;529:243–262. doi: 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizza F X, Mitchell J B, Davis B H et al. Exercise-induced muscle damage: effect on circulating leukocyte and lymphocyte subsets. Med Sci Sports Exerc. 1995;27:363–370. [PubMed] [Google Scholar]
- 12.Ostrowski K, Rohde T, Asp S et al. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515:287–291. doi: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122:474–488. doi: 10.4149/BLL_2021_078. [DOI] [PubMed] [Google Scholar]
- 14.Feng J-F, Chen S, Yang X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine (Baltimore) 2017;96:e5886. doi: 10.1097/MD.0000000000005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasselbalch I C, Søndergaard H B, Koch-Henriksen N et al. The neutrophil-to-lymphocyte ratio is associated with multiple sclerosis. Mult Scler J Exp Transl Clin. 2018;4:2.055217318813183E15. doi: 10.1177/2055217318813183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yorulmaz A, Hayran Y, Akpinar U et al. Systemic immune-inflammation index (SII) predicts increased severity in psoriasis and psoriatic arthritis. Curr Health Sci J. 2020;46:352–357. doi: 10.12865/CHSJ.46.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai J, Li H, Zhang C et al. The neutrophil-to-lymphocyte ratio determines clinical efficacy of corticosteroid therapy in patients with COVID-19. Cell Metab. 2021;33:258–269000. doi: 10.1016/j.cmet.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Candemir M, Kiziltunç E, Nurkoç S et al. Relationship between systemic immune-inflammation index (SII) and the severity of stable coronary artery disease. Angiology. 2021;72:575–581. doi: 10.1177/0003319720987743. [DOI] [PubMed] [Google Scholar]
- 19.Joisten N, Proschinger S, Rademacher A et al. High-intensity interval training reduces neutrophil-to-lymphocyte ratio in persons with multiple sclerosis during inpatient rehabilitation. Mult Scler. 2021;27:1136–1139. doi: 10.1177/1352458520951382. [DOI] [PubMed] [Google Scholar]
- 20.Wahl P, Mathes S, Bloch W et al. Acute impact of recovery on the restoration of cellular immunological homeostasis. Int J Sports Med. 2020;41:12–20. doi: 10.1055/a-1015-0453. [DOI] [PubMed] [Google Scholar]
- 21.Schlagheck M L, Walzik D, Joisten N et al. Cellular immune response to acute exercise: Comparison of endurance and resistance exercise. Eur J Haematol. 2020;105:75–84. doi: 10.1111/ejh.13412. [DOI] [PubMed] [Google Scholar]
- 22.Joisten N, Walzik D, Schenk A et al. Aqua cycling for immunological recovery after intensive, eccentric exercise. Eur J Appl Physiol. 2019;119:1369–1375. doi: 10.1007/s00421-019-04127-4. [DOI] [PubMed] [Google Scholar]
- 23.Sammito S, Gundlach N, Böckelmann I. Correlation between the results of three physical fitness tests (endurance, strength, speed) and the output measured during a bicycle ergometer test in a cohort of military servicemen. Mil Med Res. 2016;3:12. doi: 10.1186/s40779-016-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozmen S, Timur O, Calik I et al. Neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) may be superior to C-reactive protein (CRP) for predicting the occurrence of differentiated thyroid cancer. Endocr Regul. 2017;51:131–136. doi: 10.1515/enr-2017-0013. [DOI] [PubMed] [Google Scholar]
- 25.Weinhold M, Shimabukuro-Vornhagen A, Franke A et al. Physical exercise modulates the homeostasis of human regulatory T cells. J Allergy Clin Immunol. 2016;137:1607–1.61E11. doi: 10.1016/j.jaci.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 26.Luo H, He L, Zhang G et al. Normal reference intervals of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and systemic immune inflammation index in healthy adults: a large multi-center study from western China. Clin Lab. 2019;65:255–265. doi: 10.7754/Clin.Lab.2018.180715. [DOI] [PubMed] [Google Scholar]
- 27.Fest J, Ruiter R, Ikram M A et al. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8:10566. doi: 10.1038/s41598-018-28646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.INCIR S. Can immature granulocyte count and hemogram indices be good predictors of urinary tract infections in children? Int J Med Biochem. 2021;4:178–184. [Google Scholar]
- 29.Amitai M, Kaffman S, Kroizer E et al. Neutrophil to-lymphocyte and platelet-to-lymphocyte ratios as biomarkers for suicidal behavior in children and adolescents with depression or anxiety treated with selective serotonin reuptake inhibitors. Brain Behav Immun. 2022;104:31–38. doi: 10.1016/j.bbi.2022.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Güngör T, Özdel S, Çakici E K et al. An assessment on the effectiveness of the immature granulocyte percentage in predicting internal organ involvement among children with Henoch-Schönlein purpura. J Pediatr Hematol Oncol. 2022;44:e413–e417. doi: 10.1097/MPH.0000000000002288. [DOI] [PubMed] [Google Scholar]
- 31.Meng X, Chang Q, Liu Y et al. Determinant roles of gender and age on SII, PLR, NLR, LMR and MLR and their reference intervals defining in Henan, China: A posteriori and big-data-based. J Clin Lab Anal. 2018;32:e22228. doi: 10.1002/jcla.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer J P, Cao Y, Ibrahim S et al. Baseline systemic inflammatory immune index may predict overall survival and progression-free survival in patients with non-small cell lung cancer patients on immune checkpoint inhibitors. J Clin Oncol. 2021;39:e21202. [Google Scholar]
- 33.Lee J S, Kim N Y, Na S H et al. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine (Baltimore) 2018;97:e11138. doi: 10.1097/MD.0000000000011138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fei Y, Wang X, Zhang H et al. Reference intervals of systemic immune-inflammation index, neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume to platelet ratio, mean platelet volume and red blood cell distribution width-standard deviation in healthy Han adults in Wuhan region in central China. Scand J Clin Lab Invest. 2020;80:500–507. doi: 10.1080/00365513.2020.1793220. [DOI] [PubMed] [Google Scholar]
- 35.Ichihara K, Ozarda Y, Barth J H et al. A global multicenter study on reference values: 1. Assessment of methods for derivation and comparison of reference intervals. Clin Chim Acta. 2017;467:70–82. doi: 10.1016/j.cca.2016.09.016. [DOI] [PubMed] [Google Scholar]


