Abstract
Since ancient times, Korean chefs have fermented foods in an onggi, a traditional earthenware vessel. The porous structure of the onggi mimics the loose soil where lactic acid bacteria is naturally found. This permeability has been purported to facilitate the growth of lactic acid bacteria, but the details of the process remain poorly understood. In this combined experimental and theoretical study, we ferment salted napa cabbage in onggi and hermetic glassware and measure the time course of carbon dioxide concentration, which is a signature of fermentation. We present a mathematical model for carbon dioxide generation rate during fermentation using the onggi’s gas permeability as a free parameter. Our model provides a good fit for the data, and we conclude that porous walls help the onggi to ‘exhale’ carbon dioxide, lowering internal levels to those favoured by lactic acid bacteria. The positive pressure inside the onggi and the constant outflow through its walls act as a safety valve for bacteria growth by blocking the entry of external contaminants without mechanical components. We hope this study draws attention to the work of traditional artisans and inspires energy-efficient methods for fermenting and storing food products.
Keywords: permeability, permeance, onggi, kimchi, lactic acid bacteria, fermentation
1. Introduction
According to ancient texts, Koreans have enjoyed fermented foods since the Goguryeo Kingdom (37 BC–AD 668) [1]. Before the advent of refrigeration, Korean chefs used handmade clay vessels called onggi to ferment their foods. The onggi was designed without modern knowledge of chemistry, microbiology or fluid mechanics. Onggi are depicted in numerous historical records, such as the tomb mural in figure 1a from Anak Tomb No. 3, dating back to AD 357 [2]. Many recent studies purport that fermentation in onggi can help preserve and increase the nutrition of fermented foods [3,4]. while modern mass fermentation is performed in controlled conditions such as metal vessels, there remain a number of artisans who continue to make onggi by hand. The goal of this study is to present a mechanical explanation for the ability of onggi to aid in fermentation. We hope that this study will increase the visibility of onggi makers and inspire energy-efficient ways to preserve and ferment foods.
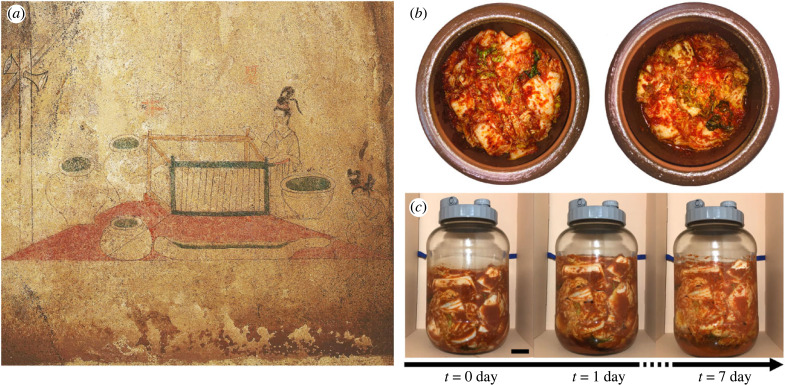
Figure 1.
(a) Onggi depicted in a mural at Anak Tomb No. 3 from Goguryeo Kingdom (37 BC–AD 668). Image courtesy of the Northeast Asian History Foundation. (b) Top view of kimchi inside an onggi before (left) and after (right) fermentation. (c) Side view of kimchi fermented in a glass jar for 7 days at 30°C. Scale bar 30 mm. The process of interest is the lactic acid fermentation driven by two species of anaerobic bacteria, Lactobacillus and Leuconostoc. A dramatic volume increase and the formation of carbon dioxide bubbles were observed within 1 day.
Onggi have traditionally been used to make a variety of fermented foods such as kimchi (fermented spicy cabbage, figure 1b), ganjang (soy sauce), gochujang (red pepper paste) and doenjang (soy bean paste), all of which have become essential parts of Korean cuisine. The addition of salt to these vegetable products, which already contain natural bacteria, prevents spoilage while promoting the proliferation of salt-loving lactic acid bacteria [5,6]. In fact, the signature sour taste of these foods is due to the lactic acid produced by probiotic bacteria including the rod-shaped Lactobacillus species (10 μm in length) and oval-shaped Leuconostoc species (1 μm in length) [7]. Research on onggi has primarily focused on accelerating the growth of lactic acid bacteria while suppressing the growth of other bacterial strains. Kimchi fermented in onggi over four weeks had 100 times higher lactic acid bacteria counts than kimchi fermented in plastic and steel containers [3]. Moreover, onggi fermentation slowed the growth of foul-tasting aerobic bacteria by a factor of 100. Onggi also increases the acidity and antioxidant activity of kimchi [8,9].
While the improved lactic acid bacteria growth in onggi is exciting, a missing link in this field is a connection between the material properties of the onggi and the growth of bacteria. In this study, we apply the methods of fluid mechanics to determine this connection. We hypothesize that onggi influences fermentation by the transmission of gas through its porous walls. Onggi has numerous gas-permeable micropores ranging from 1 to 100 μm in size [10,11]. These pores increase the transmission of oxygen and carbon dioxide with respect to most modern food containers made from polyethylene and glass [3,12]. Many researchers assume that the gas permeability of onggi causes the high bacterial count in fermented food like kimchi, but a clear mechanism has yet to be given. Previous studies only measured the carbon dioxide of an initially gas-filled vessel without living bacteria, or only considered the growth of bacteria without measuring gas generation [3]. In our work, we examined the interaction of fermenting cabbage, the carbon dioxide it generates, and the permeability of the onggi walls. We linked these three subjects using a mathematical model that accounts for the onggi’s permeability to carbon dioxide.
Critical to the onggi’s function is its permeability, the capacity of a porous material to allow fluids to flow through it. By virtue of its liquid permeability k, onggi generates ‘salt flowers’, salt crystals that appear on its outside when fermenting salty foods. As we will show, the onggi’s gas permeability kg enables it to transmit the carbon dioxide generated by fermentation. In general, permeability depends on the shape and size of interconnected pores in the material. It is an intrinsic property of porous materials and has traditionally been used to show how water travels through filters, gravel and aquifers. Permeability is defined in units of area and refers to the area of open space in a cross-section that is perpendicular to the direction of flowing fluid [13,14].
2. Material and methods
2.1. Onggi construction and characterization
In the summer of 2021, we purchased a large onggi from Jeju Onggi Village on Jeju Island, Korea. The onggi had a volume of 4600 ml with a mouth radius of 10 cm and a height of 20 cm. One constraint was that the vessel had to be tall and wide enough to fit our airborne carbon dioxide sensors without touching the cabbage. The onggi was manufactured by a traditional process which we describe here [11]. Raw mud containing water, silt and clay was pressed and slapped by hand to homogenize it. Pebbles were picked out and the material was first formed into long clay rods. Then, the pottery was shaped on a spinning wheel and left for about a day under ambient conditions to dry out. Finally, onggi slowly sintered inside a kiln at around 1200°C for a day then and slowly cooled down. While some onggi are glazed, ours was not.
We characterized the pore structure with both a scanning electron microscope (Hitachi SU-8010 SEM) and computed tomography (Scanco CT-50). The sample for SEM imaging was prepared using a rotary saw with a diamond wafering blade (PELCO Precision Low-Speed Saw) to obtain a smooth surface. The sample for the CT scan was prepared by breaking it with a hammer. A small piece was mounted on a 6 mm diameter sample holder and imaged under high-resolution settings (55 kV of energy, 145 μA of intensity, 1 s of sample time and 2 μm voxel size). A threshold masking was set to lower and upper limits of 5183 HU (Hounsfield units) and 10 000 HU, respectively. The porosity was computed by sampling multiple cylinders (diameter 600 μm and length 1000 μm) within the boundary of the outer surface.
2.2. Kimchi fermentation time-lapse
For the time-lapse video of fermentation (figure 1c; electronic supplementary material, movie S1), we relied on a traditional kimchi recipe. Throughout the study, napa cabbage (Brassica rapa subsp. pekinensis) was purchased from local grocery stores, either on Jeju Island or in Atlanta. We used 1 kg of the crispy layers of cabbage for the time-lapse. Each leaf of cabbage was cut into two or three pieces perpendicular to the midrib so that each piece consisted of both the wrinkled lamina and the surrounding veins. The napa cabbage was immersed in 2 wt% salty water for 6 h and then mixed thoroughly with traditional kimchi seasoning, which for each kg of cabbage consisted of 160 g of powdered red pepper, 200 g of minced onion, 20 g of anchovy fish sauce, 15 g of minced garlic, and 5 g of minced ginger.
Kimchi fermentation could not be easily observed in onggi because of the dark conditions inside the vessel. Thus, we conducted the time-lapse video of kimchi fermentation in a hermetically sealed glass jar. The fermentation process was filmed by a USB camera (Logitech Brio 4K Webcam) for seven days at 3°C, illuminated by a single LED source.
2.3. Salt flower formation
Two time-lapse videos of salt crystal formation were performed: one in a macroscopic view and another in a microscopic view. In the macroscopic view (figure 2a,b; electronic supplementary material, movie S2), the onggi was filled with 2 l of 15 wt% salty water and observed for 24 h using a USB camera (Logitech Brio 4K Webcam).
Figure 2.
(a) Time-lapse of salt flower formation on the outer surface of an onggi for 24 h. Scale bar 50 mm. (b) Onggi surface covered with salt crystals after 3 days. Scale bar 50 mm. (c) The microscopic time-lapse of salt flower at the outer onggi surface for 8 h. Scale bar 500 μm. Salt crystals form as the evaporation dries out the water contents and fluid is imbibed to the surface.
For the microscopic film (figure 2c; electronic supplementary material, movies S3 and S4), a rectangular onggi piece was observed for 2 days while the bottom of the sample was immersed in 15 wt% salty water. This per cent salt water is comparable to the salt concentration in soy sauce (approx. ) which may be fermented in onggi [15]. We used a stereo optical microscope (Leica DVM6 Digital Microscope) in the multi-focus setting.
2.4. Water evaporation measurement
To measure the onggi permeability to water, we measured how quickly water evaporated from its walls. The onggi was initially filled with 2000 g of water and the top was sealed with plastic stretch wrap and a rubber ring. This set-up ensured that evaporation mostly occurred through the porous walls of the onggi. The time course of the mass was measured by a portable balance (Mettler Toledo PL-E).
2.5. Carbon dioxide measurement
For the carbon dioxide measurements, we applied only salt water to the cabbage because the kimchi recipe includes ingredients for flavour but salt is the necessary component to stimulate fermentation. We measured the carbon dioxide concentration and estimated the gas production in two containers, a hermetically sealed glass jar and closed-top onggi (figure 4a). For the onggi, we designed and built a custom-fit lid for three types of gas sensors: a pressure sensor, a carbon dioxide sensor and an oxygen sensor (Vernier Software & Technology). These sensors fit vertically into the onggi mouth using a custom-designed three-dimensional-printed lid (Formlabs Form 3). All purpose sealing film (Bemis, Parafilm) filled any remaining gaps between the devices and the lid. Despite our efforts, our ‘hermetically sealed’ glass jar leaked, but less so than the permeable onggi, as shown by the factor of two differences in their gas permeability kg presented in the results section.
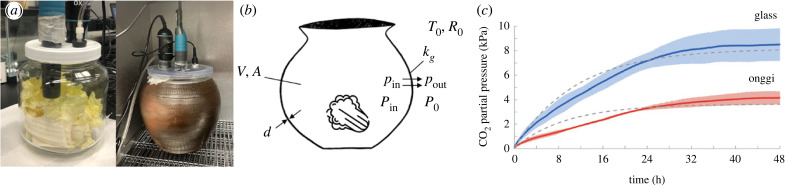
Figure 4.
(a) Headspace gas set-up for measuring carbon dioxide, oxygen and pressure in a hermetic glass container (left, 1900 ml of volume) and onggi (right, 4600 ml of volume). (b) Schematic of carbon dioxide generation. Cabbage and jar icons from Flaticon and Clipart Library, respectively. (c) Time course of the carbon dioxide partial pressure for 200 g of salted cabbage stored in glass (in blue) and onggi (in red). Solid lines are from experiments and the dashed lines are from the theoretical model. Raw data are given in electronic supplementary material, figure S2.
We performed three fermentation trials of salted cabbage in a glass jar and an onggi. The glass jar was less than half the volume of the onggi: the glass jar had a volume of 1900 ml, a height of 15 cm and a radius of 10 cm. In each trial, 200 g of cabbage was observed for 2 days. To prepare the salted cabbage for observation, it was first immersed in brine with 2 wt% of salt concentration for 6 h. For all trials, cabbage was drained of water before being placed in the containers for measurement. Unlike traditional kimchi fermentation, there were no extra seasonings in the jars, so the salted cabbage sat without any surrounding liquid, as shown in figure 4a.
The onggi and glass jar were placed in a laboratory oven (Quincy Lab 30GC Convection Lab Oven), controlled by a voltage regulator (Variac TDGC-0.5KM) to maintain darkness and a temperature of 25°C. While the typical temperature for kimchi fermentation is about 10°C [16], we used a higher temperature to expedite fermentation. Since the containers’ pores harbour bacteria [11,17], the containers were sterilized in an autoclave (Steris Amsco Century SV-136H Prevac Steam Sterilizer) before each trial.
3. Mathematical models
We present mathematical models on the permeability of the onggi to liquids and gases. The former was used to estimate the liquid permeability k and the latter to infer the carbon dioxide generation rate of the lactic acid bacteria. At the end of the section, we enumerate the values of the dimensionless groups used in these models. The dimensionless groups justify our modelling decisions.
3.1. Liquid permeability model
Our model infers the liquid permeability k of the onggi wall based on the evaporation rate of water from the onggi. This model follows our experiment in which a capped water-filled onggi loses mass at a steady rate. Based on our observations of salt formation on the outside of the onggi (figure 2), we assume this mass loss is due to water that has imbibed through the porous walls and evaporated from the outside.
Based on the flow speeds of water presented in the Dimensional analysis section, the flow through the onggi is characterized by incompressibility and low Reynolds number. We apply Darcy’s Law, which is valid for creeping laminar flow at low Reynolds numbers [18,19]. Darcy’s Law states that the volumetric flow rate per unit area q is proportional to the gradient of pressure, , where d is the onggi wall thickness. In all,
| 3.1 |
where the constant of proportionality involves the liquid permeability k of onggi and the water’s dynamic viscosity μ. The outside wall of the onggi is at atmospheric pressure. The two driving pressures of the fluid are the water’s hydrostatic pressure and the capillary pressure which tends to imbibe fluid into the hydrophilic wall. The sum of these two pressures may be written
| 3.2 |
where ρ is the density of water, g is gravitational acceleration, z is the height from the water surface, σ is the surface tension of water, θ is the contact angle of water on the onggi and dp is the pore diameter, which we show using microscopy to be of the order of 10 μm. The first term on the right-hand side is the hydrostatic pressure, and the second term is the capillary pressure, or Laplace pressure. The ratio of the hydrostatic term to the capillary term is related to the modified Bond number, which we calculate to be less than 1, indicating the dominance of capillary pressure. We proceed by considering only capillary pressure.
We assume a cylindrical container with an effective radius R, which is the average of the onggi mouth radius, maximum radius and bottom radius. The evaporation rate, or the total flow rate integrated across the height of the liquid zr may be written (figure 3c)
| 3.3 |
where q can be substituted using equations (3.1) and (3.2). Simplifying equation (3.3), we write the liquid permeability k as
| 3.4 |
We may solve equation (3.4) by measuring Qe from the rate of mass loss of the onggi. The rate of mass loss was converted to a volumetric flow rate using water density. If the evaporation rate is measured over a sufficiently small time window, the water height zr can be considered constant. The model presented applies to liquids passing through the onggi walls. In the next section, we consider the passage of gases.
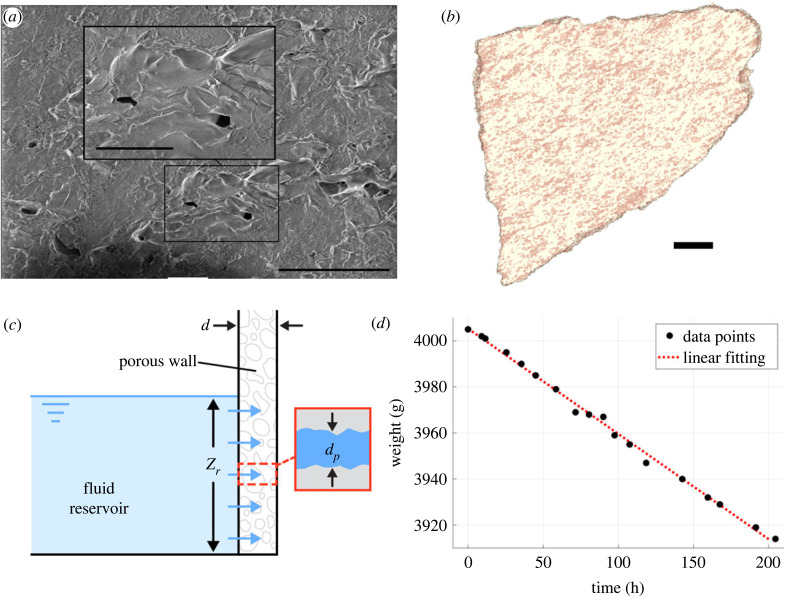
Figure 3.
(a) Scanning electron microscope view of micropores in onggi. Scale bar, 50 μm. Inset scale bar, 20 μm. Pore size varies from 1 to 100 μm diameter with an average of 5 μm. Additional SEM images can be found in electronic supplementary material, figure S1. (b) Three-dimensional model of a 40 μm thick onggi slice using computed tomography (CT) scan. Scale bar, 200 μm. Pores are pink, and onggi clay is transparent. (c) Schematic of water flow through the porous wall. (d) Time course of the weight of the onggi due to evaporation at the onggi surface.
3.2. Gas permeability model
The goal of this model is to infer the carbon dioxide generation rate by the bacteria. This model is necessary because the onggi is permeable to carbon dioxide. Figure 4b depicts the variables used in this model. The primary input to our model is the concentration of carbon dioxide in the vessel as measured by our sensor. In order to determine the carbon dioxide generation rate , we converted to the partial pressure of carbon dioxide in the vessel pin. We used p to denote partial pressure of carbon dioxide and P to denote the total pressure, which according to Dalton’s Law, is the sum of all the partial pressures of the gases (, , ). With this notation, pin and pout are the partial pressures of carbon dioxide inside and outside the vessel. Similarly, Pin and P0 denote the total pressures inside and outside the vessel, respectively. The total pressure in the vessel Pin was measured using our pressure sensor. Pressure P0 and temperature T0 outside the vessel were given by ambient conditions, which in this case corresponded to a temperature-controlled oven at atmospheric pressure.
Conservation of mass states that the rate of change of carbon dioxide in the vessel is the sum of the rates of carbon dioxide outflow , and carbon dioxide generation . Note that is negative, corresponding to carbon dioxide leaving the vessel.
| 3.5 |
The rate of outflow is dictated by the gas permeance equation, which is equivalent to Darcy’s Law and states that the molar flow rate is proportional to the pressure gradient. In a porous vessel (figure 5b), the gas permeance equation may be written [3,11]
| 3.6 |
where is the gas permeability coefficient, A is the vessel surface area, d is the vessel wall thickness and pout and pin are the partial pressures of carbon dioxide outside and inside the vessel, respectively. The gas permeance is defined as . The partial pressure of carbon dioxide outside the vessel is dictated by atmospheric conditions and is given in table S1 in the electronic supplementary material. Carbon dioxide flows out of the vessel if the outflow of carbon dioxide is negative. This is the case for an onggi fermenting kimchi where the pressure building up inside the vessel exceeds the pressure outside: pin is higher than pout.
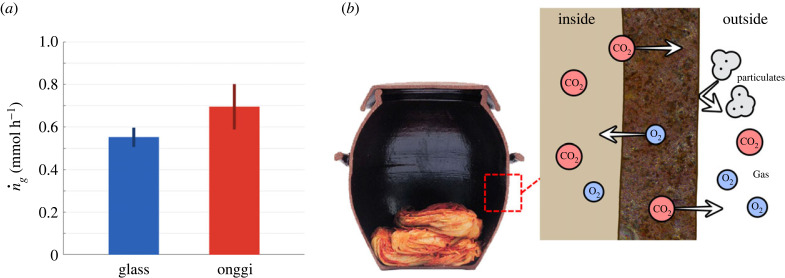
Figure 5.
(a) Carbon dioxide generation rates of salted cabbage in glass and onggi, as calculated with our theoretical model. Salted cabbage in onggi generated 26% more carbon dioxide than in glass. (b) Schematic of selective permeability of onggi. The porous onggi wall allows carbon dioxide to exit and oxygen to enter. It also blocks the passage of unwanted particulates larger than the pore size of 5 μm. Onggi cross-section image from Korean Ministry of Culture, Sports and Tourism.
The gas permeability coefficient of the vessel is defined in terms of the gas permeability and conditions outside the vessel
| 3.7 |
where kg is the gas permeability of the vessel, P0 is the ambient pressure, T0 is ambient temperature, R0 is the gas constant and is the dynamic viscosity of carbon dioxide at the prescribed pressure and temperature. Note that equation (3.7) is arbitrary in that it neglects the conditions inside the vessel. However, this assumption does not affect our final solution since we will be using kg as a free parameter.
Before we proceed, we provide a brief cautionary note on the use of gas permeability kg and gas permeance . In the kimchi fermentation literature, authors may inadvertently interchange the terms permeability, permeance and permeation [3,11,20]. We define gas permeance in terms of gas molecules per hour divided by a cross-sectional area and a pressure difference. Thus, permeance has units of mol kPa−1 m−2 h−1, but it is dependent on the intrinsic gas permeability of the material, which has a unit of m2 [22–24]. We set the gas permeability kg as a free parameter then proceed by presenting the relationships between gas permeability and partial pressures of carbon dioxide.
The partial pressure of carbon dioxide pin inside the vessel is given by the ideal gas law, which states that pin is proportional to ntotal the number of moles of carbon dioxide inside the vessel
| 3.8 |
where V is the vessel volume, T0 is ambient temperature, and R0 is the ideal gas constant. Note, here we assume that the temperature inside the vessel is the same as that outside, which is supported by our temperature measurements. The partial pressure of carbon dioxide (in kPa) is simply the product of carbon dioxide concentration (in ppm), the total pressure (in kPa), and a constant to convert ppm to a proportion
| 3.9 |
Further constants are given in table S1 in the electronic supplementary material.
By assuming pout and are constant, we may rewrite equation (3.8) using equations (3.5)–(3.7) in terms of the variable pin
| 3.10 |
The initial condition of pin is the partial pressure of carbon dioxide in the ambient. This differential equation may be solved to find the time course of the partial pressure of carbon dioxide inside the vessel
| 3.11 |
where the time constant τ is
| 3.12 |
and the plateau value of pin is influenced by the constant
| 3.13 |
Thus, the carbon dioxide generation rate influences only the steady-state plateau without influencing the time constant. Equation (3.11) is the primary tool that we will use to measure the carbon dioxide generation rate from the time course of carbon dioxide data in the onggi headspace.
We collected six total experimental curves for two separate conditions: salted cabbage in each of the onggi and glass containers. Figure 4c shows the average of three trials per condition. The fitting was conducted in Matlab and OriginPro based on the least-square method. There exist two free parameters: kg and . Optimal kg values for each of the glass and onggi containers were obtained using a Matlab code that minimizes the mean of the R2 values for the datasets. Then, OriginPro produced the associated values with uncertainties.
3.3. Dimensional analysis
Our mathematical modelling relied on dimensionless groups, which we calculate using our experimental results below. These dimensionless groups justify our choices in mathematical modelling. A small Reynolds number supports Darcy’s Law, and a small Bond number reinforces the capillary force dominance, simplifying the calculations for liquid permeability in equation (3.4).
We begin with characteristic velocities and length scales that are relevant for liquid and air flow through the pores of onggi. Using microscopy, we found the pores in the onggi wall had a characteristic diameter of dp = 10 μm. The characteristic velocity Uw for water flow through onggi was obtained by dividing the volumetric flow rate per unit area by the porosity: using equation (3.1), , where we converted porosity from percentage to proportion.
To calculate the characteristic velocity of carbon dioxide, we use a combination of equation (3.6), and the time derivative of the ideal gas law applied to the outgoing carbon dioxide , which is calculated from the experiment
| 3.14 |
where is the volumetric flow rate of carbon dioxide. This equation, along with the surface area A of the onggi yields the volumetric carbon dioxide flow rate per unit area . Consequently, the characteristic velocity of the carbon dioxide was .
The Reynolds number is defined as
| 3.15 |
where U is the velocity of the fluid (either Uw or Ugas) and ν is the kinematic viscosity of the fluid. The Reynolds numbers for the water and carbon dioxide flow are 5 × 10−3 and 3 × 10−6, respectively. Both liquid and gas flow are in the creeping regime, which indicates fluid flow is laminar and inertia-free.
The driving force of water flow through the onggi walls is capillarity. Capillary pressure in a pore may be estimated as Pc = 4σcosθ/dp ∼ 24.9 kPa, which is much higher than the measured steady-state gas pressure for fermenting salted cabbage in the onggi, 4.1 kPa. The modified Bond number may be defined as the ratio of the hydrostatic pressure ρg zr and the capillary pressure at the pores σ/dp. Note that there are two length scales at play here, which lead to
| 3.16 |
The Bo is calculated to be 0.14, which implies that the water imbibition at the onggi pores is dominated by capillarity.
4. Results
We used several cabbage preparations in this study. The first was ‘kimchi cabbage’ which involved napa cabbage (Brassica rapa subsp. pekinensis) and traditional kimchi seasonings. The fermentation of kimchi cabbage was captured by time-lapse video. The second preparation was ‘salted cabbage’ which only involved cabbage, salt and no other seasonings. The salted cabbage was used in fermentation experiments in which carbon dioxide is measured. Since the salted preparation was performed over six times, for convenience, salt alone was used as the minimal ingredient to trigger fermentation.
We filmed kimchi fermentation for 7 days (figure 1c; electronic supplementary material, movie S1). After 24 h of fermentation, the solution increased by 500 ml, which was approximately of the initial liquid and comparable to half of the volume of the original cabbage (which weighed 1 kg). Throughout the next few days, the cabbage generated bubbles, presumably carbon dioxide due to the metabolism of lactic acid bacteria which appears naturally on the cabbage. The changing carbon dioxide in the vessel may in turn influence the growth of the bacteria. We proceed by characterizing the porosity and liquid permeability of the onggi wall, before turning to its gas permeability.
Onggi releases gas through its permeable wall, which we characterized using SEM microscope and CT scan. Figure 3a shows the SEM image of a cross-section of the onggi wall. Pore diameters ranged from 1 to 100 μm, which is comparable to the range of 20−150 μm (accounting for 93.04% of the pores) reported by Kim et al. [25]. Onggi pore formation is driven by variables in the manufacturing process such as the composition of ingredients, sintering temperature and sintering time. In comparison, porcelain’s ingredients and processing cause its uniformly fine particles to be impermeable to gas [25].
We used a CT scanner to measure the porosity of the sample. Since the CT scan resolution was 2 μm voxel size, it could not resolve pores smaller than this size. Threshold masking produced a solid three-dimensional model of an onggi piece (figure 3b; electronic supplementary material, movie S5). We found a porosity of , which is comparable to , measured using mercury porosimetry [11], and four times smaller than , the average value reported in Seo et al. [20]. We note that these other workers used indirect measurements of porosity through chemical absorption, while ours is a visualization of the porous structure using X-rays.
We next performed water evaporation experiments to measure onggi’s permeability to liquids. When Korean chefs ferment ganjang (soy sauce) in onggi, salt crystals precipitate on the outside of the container, a phenomenon known as ‘salt flower’. We demonstrated this process by filming an onggi halfway full of salty water (figure 2a,b; electronic supplementary material, movie S2). Within 8 h, a white layer of salt crystals appeared on the outside, intensifying over time. Salt was initially limited to the water line but climbs above it after 24 h. Historians believe the appearance of the salt flower indicates a good quality fermentation vessel: 'ancient ancestors who valued the taste of sauce say, “If [structures resembling] buckwheat flowers bloom in the vessel, the taste of the sauce is good.” meaning the salt components pass through the pores and the salt flowers form on the surface of onggi. Also, it implies that a vessel without salt flowers cannot have the best taste of the sauces. We say “the pottery breathes” since the salt can emerge from inside to outside through the pores' [21].
To better visualize the salt flower formation, we performed time-lapse videography of a small piece of onggi atop salt water using a microscope. Figure 2c and electronic supplementary material, movies S3,4 show a dry outer wall covered by a thin film of water and then by salt crystals. Based on these events, we surmise that salt water flows through the wall and evaporates at the outer surface, leaving salt crystals behind. Evaporation of water at the outer surface is replenished by water wicked through the wall.
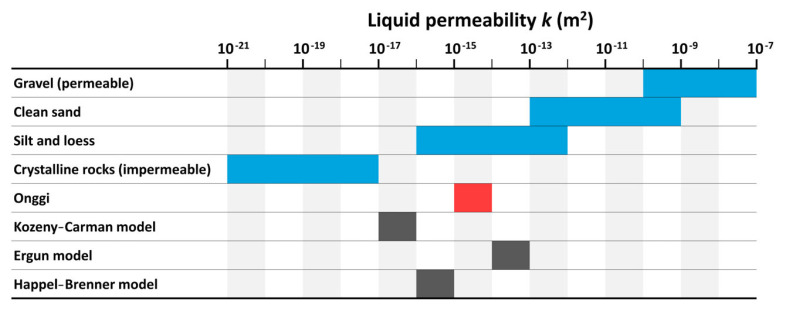
Now that we see onggi is permeable to water, we measure the flow rate. We filled the onggi with 2000 g of water and sealed the top. The water flowed through the walls and evaporated at a constant rate of 0.75 ± 0.18 g h−1 (R2 = 0.989) as shown in figure 3d, implying a volumetric flow rate of Qe = 2.1 ± 0.5 × 10−10 m3 s−1. Using equation (3.4), we estimate the liquid permeability of the onggi sample to be k = (4.58 ± 1.10) × 10−15 m2. Converting liquid permeability to hydraulic conductivity K = kρg/μ yields K = (4.58 ± 1.10) × 10−8 m s−1, where ρ is the density of water, g is the acceleration of gravity and μ is the dynamic viscosity of water [26]. Table 1 compares the liquid permeability of onggi with other well-characterized geological materials that are not tested here, but simply included for comparison. Onggi is comparable to silt and loess (a layer of windblown dust and silt covering 10 per cent of the Earth). This makes sense since onggi is made mainly from natural mud, which has silt as a large component. We classify onggi as semi-permeable because it lies between permeable materials such as clean sand and gravel (k ∼ 10−7−10−10 m2) and impermeable materials such as crystalline rocks like granite (k ∼ 10−17−10−21 m2).
We may also compare the measured liquid permeability with predictions from theoretical models [28–30] including the Kozeny–Carman, Ergun and Happel–Brenner models reprinted below
| 4.1 |
where is the porosity, dpar is the particle diameter, dch is the channel diameter and other coefficients are empirically found. Table 1 also shows the calculated permeability based on these models, assuming dpar and dch equal to the pore size of 10 μm, and equals 4.72%. These models all give values within three orders of magnitude of our measurement, which was of the order of 10−15. Ergun and Happel–Brenner models appear the most accurate, differing by only a factor of 100 from our measurement. We proceed by studying the effect of this permeability to carbon dioxide on fermentation.
Table 1.
Onggi permeability compared with other geological materials and predictions from mathematical models. Onggi permeability is in the middle of the conventional range of permeable and impermeable materials. Data on rocks and sediments from [26,27].
 |
We investigated the carbon dioxide generated by salted napa cabbage placed in an onggi and a glass jar. We performed three experiments for each container and report the average time course of carbon dioxide. We assumed that the salted cabbage had negligible cellular respiration and generated carbon dioxide mainly through bacterial fermentation. Using equation (3.9), we converted carbon dioxide concentration to partial pressure. Figure 4c shows the time course of the partial pressure of carbon dioxide for salted cabbage, where the red line denotes onggi experiments and the blue line denotes glass jar experiments. The onggi’s permeable wall clearly permits carbon dioxide to escape, resulting in a slower increase in carbon dioxide and a lower plateau level. Through the lens of our mathematical modelling, we will see that the onggi involves greater carbon dioxide generation than the glass container.
The main challenge with interpreting these results is that the carbon dioxide generation rate is not explicitly known since carbon dioxide is escaping the onggi’s permeable walls. To proceed further, we apply our mathematical model, equation (3.11), to calculate the carbon dioxide generation rate corresponding to each experiment. We constrain our model with two conditions (i) to have a constant gas permeability for each container, and (ii) to minimize the error with respect to the time course of experimental carbon dioxide. The modelling shows a good fit to our experimental results, with R2 values of 0.954 and 0.850, respectively, for glass and onggi. In the electronic supplementary material, we also show tests of cellular respiration for unsalted cabbage.
Our calculation showed the glass container had a gas permeability kg of 0.796 × 10−18 m2, while the onggi had more than double the value at 1.701 × 10−18 m2. We surmise that the permeability of the glass container was due to leaks from the three-dimensional-printed top. Since the onggi had a similarly manufactured top, its increased permeability was probably due to its porous walls. Previous researchers showed that the gas permeability of onggi depends on the glazing treatment, raw ingredients and the kind of gas [3,20]. They presented gas permeance values only so we convert our permeability to permeability coefficient using equation (3.7) then divide it by the wall thickness. Previously measured gas permeance values for unglazed onggi lie between , which are of the same order of magnitude compared with our results, 3.4 × 10−3 mol kPa−1 m−2 h−1.
Figure 5a shows the calculated carbon dioxide generation rates (mmol h−1) for onggi and the glass container. Salted cabbage carbon dioxide generation rates were 0.552 mmol h−1, for glass and 0.695 mmol h−1 for onggi. Thus, salted cabbage in onggi generated 26% more carbon dioxide than in the glass container, indicative of 26% more bacterial proliferation in the onggi. One-tailed t-test shows this difference was significant (p = 0.0498). Moreover, our carbon dioxide generation rates are also comparable to previous measurements. Given the carbon dioxide molecular mass of 44.01 g mol−1, carbon dioxide generation rates per unit mass of cabbage for the glass and onggi were 2915 and 3670 mg kg−1 day−1, respectively, which were similar to that of fermenting kimchi in glass containers, 2531 mg kg−1 day−1 under 25°C and 2 wt% salt contents [31].
In conclusion, permeable onggi permits carbon dioxide to escape the container, which in turn accelerates the rate of fermentation. Porosity thus acts as a safety valve for carbon dioxide, maintaining its levels at less than half the values of hermetically sealed containers. Unlike a mechanical valve, there are no moving parts to break. The many pores permit a continuous outflow of gas, which helps reduce the entry of external contaminants. This one-way flow is shown schematically in figure 5b. In comparison with the passive gas control of the onggi, modern fermentation vessels use manual valves or other mechanical means to allow gas to escape.
5. Discussion
We discuss a few assumptions and caveats to be taken with our salted cabbage experiments. We assumed that the addition of salt to the cabbage triggered fermentation while impeding cellular respiration. Plants exposed to saline conditions experience physiological stress, disruptions to major biochemical processes, and inhibited mitochondrial activity [32,33]. While salt-tolerant plant species can maintain cellular respiration in saline conditions, we assume in this study napa cabbage has inhibited cellular respiration under high salinity.
Given the high division rate of bacteria compared with plant cells, we infer that increases in carbon dioxide generation are due to bacterial proliferation. Since we cannot distinguish lactic acid bacteria from other bacteria, we assume that the increase in carbon dioxide is due to increased lactic acid bacteria alone. Our assumption is helped by the fact that salt supports lactic acid bacteria growth over other bacteria strains.
In our evaporation experiments, we found that onggi’s permeability can maintain most of the liquid inside while releasing the gases generated from fermentation. This ability to be semi-porous is a unique feature compared with other potteries. In comparison, terracotta, a common pottery for houseplants, quickly leaks any water poured into it. For plants, this property may be favourable by preventing the roots from sitting in stagnant water, but it is clearly poorly suited for fermentation, a process where fermented vegetables should be kept moist.
The onggi in this study was unglazed. The traditional way to make onggi glaze is using natural ingredients such as water, tree ash and humus (leaf mould and soil), which come from rotten grass and fallen leaves [3]. While the glaze is aesthetically pleasing, we surmise that it would reduce permeability and in turn reduce its fermentation ability.
Our goal with designing the cover of the glass container was to make it hermetically sealed, but from our modelling, we find the glass container still had half the gas permeability of the onggi. We did not further improve the design because our focus in this study was on the onggi. For simplicity, we modelled both glass and onggi containers as having constant permeability. However, the permeability might be a function of pressure difference. For example, the glass container may be hermetic below a critical pressure, but then permeable as pressure increases. In fact, such nonlinear behaviour is how mechanical relief valves work: they have an energy storage mechanism in the form of a spring or gravity so that gas escapes when a critical pressure is reached.
The glass container only had half the volume of the onggi, and this difference may have influenced the bacteria’s activity. Our mathematical model uses the container volume as a parameter to calculate the carbon dioxide partial pressure. However, the volume of the glass container could affect the interaction between the lactic acid bacteria and carbon dioxide. For example, if the container is large, the carbon dioxide may be diluted to a point where bacteria cannot detect any change. Such behaviour may provide an alternative explanation for the results observed.
Another assumption was a constant carbon dioxide generation rate , which reflects the average carbon dioxide rate generated by the lactic acid bacteria in the experimental interval of 48 h. In reality, the bacteria population will increase with time, and so will the carbon dioxide generation rate. However, we did not explore this scenario because it would require knowledge of the doubling time of the bacteria as well as their carbon dioxide generation.
The onggi is just one of many types of fermentation vessels [34,35] and cooking techniques [36,37] with ancient origins. Traditional fermentation vessels like oak barrels or amphora are mainly used for yeast-fermentation of alcoholic beverages, and especially the wooden barrels add flavour by ageing [38,39]. Onggi were designed to promote lactic acid fermentation, which provides the unique taste of kimchi.
6. Conclusion
We used fluid mechanics techniques to show that the onggi continuously ‘breathes’ carbon dioxide during fermentation, an idea that has been hypothesized for centuries, but had not been directly measured. We find that the onggi’s porous walls are permeable to carbon dioxide, which reduces the carbon dioxide level inside the vessel. We surmise that this low carbon dioxide level is favoured by lactic acid bacteria, leading to their greater proliferation, and the increased carbon dioxide generation observed relative to hermetic glass containers. Our mathematical model predicts gas permeability that matches the range of previously reported values for onggi. We hope that this work draws interest to ancient cooking and food preparation strategies.
Acknowledgements
We appreciate Christopher Zhang (Brian Hammer Lab) and Pablo Bravo (Peter Yunker Lab) for their initial work on growing lactic acid bacteria. We also thank Laxminarayanan Krishnan in Georgia Tech for CT scan support. Finally, we thank Deok-Saeng Kang for teaching how to make kimchi.
Data accessibility
The data and code supporting the findings of this study are available from the Github repository: https://github.com/Delicate-Kim/2023-JRSI-onggi.
The data are also provided in electronic supplementary material [40].
Authors' contributions
S.K.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, visualization, writing—original draft; D.L.H.: funding acquisition, resources, supervision, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This material was supported by the Woodruff Faculty fellowship and the NSF Physics of Living Systems student network.
References
- 1.Lee CH, Ahn BS. 1995. Literature review on kimchi, Korean fermented vegetable foods-I. History of kimchi making. J. Korean Soc. Food Cult. 10, 311-319. [Google Scholar]
- 2.Lena K. 2010. Koguryo Tomb murals: world cultural heritage. Seoul, Korea: ICOMOS – Korea Cultural Heritage. [Google Scholar]
- 3.Jeong JK, Kim YW, Choi HS, Lee DS, Kang SA, Park KY. 2011. Increased quality and functionality of kimchi when fermented in Korean earthenware (onggi). Int. J. Food Sci. Technol. 46, 2015-2021. ( 10.1111/j.1365-2621.2011.02710.x) [DOI] [Google Scholar]
- 4.Florou-Paneri P, Christaki E, Bonos E. 2013. Lactic acid bacteria as source of functional ingredients. In Lactic acid bacteria-R&D for food, health and livestock purposes. IntechOpen.
- 5.Chao SH, Wu RJ, Watanabe K, Tsai YC. 2009. Diversity of lactic acid bacteria in suan-tsai and fu-tsai, traditional fermented mustard products of Taiwan. Int. J. Food Microbiol. 135, 203-210. ( 10.1016/j.ijfoodmicro.2009.07.032) [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Hu W, Xiu Z, Jiang A, Yang X, Saren G, Ji Y, Guan Y, Feng K. 2020. Effect of salt concentration on microbial communities, physicochemical properties and metabolite profile during spontaneous fermentation of Chinese northeast sauerkraut. J. Appl. Microbiol. 129, 1458-1471. ( 10.1111/jam.14786) [DOI] [PubMed] [Google Scholar]
- 7.Dharaneedharan S, Heo MS. 2016. Korean traditional fermented foods – a potential resource of beneficial microorganisms and their applications. J. Life Sci. 26, 496-502. ( 10.5352/JLS.2016.26.4.496) [DOI] [Google Scholar]
- 8.Park S, Lee S, Park S, Kim I, Jeong Y, Yu S, Shin SC, Kim M. 2015. Antioxidant activity of Korean traditional soy sauce fermented in Korean earthenware, onggi, from different regions. J. Korean Soc. Food Sci. Nutr. 44, 847-853. ( 10.3746/jkfn.2015.44.6.847) [DOI] [Google Scholar]
- 9.Lee KS, Lee YB, Lee DS, Chung SK. 2006. Quality evaluation of Korean soy sauce fermented in Korean earthenware (onggi) with different glazes. Int. J. Food Sci. Technol. 41, 1158-1163. ( 10.1111/j.1365-2621.2006.01161.x) [DOI] [Google Scholar]
- 10.Seo GH, Song BS, An DS, Chung SK, Lee DS. 2006. Physical properties of Korean earthenware (onggi) as food container. Korean J. Packaging Sci. Technol. 12, 87-90. [Google Scholar]
- 11.Seo GH, Yun JH, Chung SK, Park WP, Lee DS. 2006. Physical properties of Korean earthenware containers affected by soy sauce fermentation use. Food Sci. Biotechnol. 15, 168-172. [Google Scholar]
- 12.Yam KL, Lee D. 1995. Design of modified atmosphere packaging for fresh produce. In Active food packaging, pp. 55–73. New York, NY: Springer.
- 13.Nolen-Hoeksema R. 2014. Defining and determining permeability. Oilfield Rev. 26, 1-2. [Google Scholar]
- 14.Al-Doury M. 2010. A discussion about hydraulic permeability and permeability. Pet. Sci. Technol. 28, 1740-1749. ( 10.1080/10916460903261715) [DOI] [Google Scholar]
- 15.Erickson DR. 2015. Practical handbook of soybean processing and utilization. Maryland Heights, MO: Elsevier. [Google Scholar]
- 16.National Research Council (US) Panel on the Applications of Biotechnology to Traditional Fermented Foods. 1992. Applications of biotechnology to fermented foods: report of an ad hoc panel of the Board on Science and Technology for International Development. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 17.De Roos J, Van Der Veken D, De Vuyst L. 2019. The interior surfaces of wooden barrels are an additional microbial inoculation source for lambic beer production. Appl. Environ. Microbiol. 85, e02226-18. ( 10.1128/AEM.02226-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bear J. 1988. Dynamics of fluids in porous media. New York, NY: Courier Corporation. [Google Scholar]
- 19.Woessner WW, Poeter EP. 2020. Hydrogeologic properties of earth materials and principles of groundwater flow. Guelph, Canada: The Groundwater Project. [Google Scholar]
- 20.Seo GH, Chung SK, An DS, Lee DS. 2005. Permeabilities of Korean earthenware containers and their potential for packaging fresh produce. Food Sci. Biotechnol. 14, 82-88. [Google Scholar]
- 21.Seo J, Kim H. 2008. A study of preservation of manufacturing method on Korean traditional pottery 'onggi'. J. Korean Cult. Hist. 30, 53-90. [Google Scholar]
- 22.Hägg M. 2015. Gas permeation: permeability, permeance, and separation factor. In Encyclopedia of membranes (eds Drioli E, Giorno L), pp. 1-4. Berlin, Gemany: Springer. [Google Scholar]
- 23.Zhang C, Wang Z, Cai Y, Yi C, Yang D, Yuan S. 2013. Investigation of gas permeation behavior in facilitated transport membranes: relationship between gas permeance and partial pressure. Chem. Eng. J. 225, 744-751. ( 10.1016/j.cej.2013.03.100) [DOI] [Google Scholar]
- 24.Civan F. 2010. Effective correlation of apparent gas permeability in tight porous media. Transp. Porous Media 82, 375-384. ( 10.1007/s11242-009-9432-z) [DOI] [Google Scholar]
- 25.Kim S, No H, Kim U, Cho WS. 2014. A study on sources of pore formation in onggi via the comparison with porcelains. J. Korean Ceram. Soc. 51, 11-18. ( 10.4191/kcers.2014.51.1.011) [DOI] [Google Scholar]
- 26.Freeze R, Cherry J. 1979. Groundwater. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- 27.Hornberger GM, Wiberg PL, Raffensperger JP, D’Odorico P. 2014. Elements of physical hydrology. Rugby, UK: JHU Press. [Google Scholar]
- 28.Carman PC. 1937. Fluid flow through granular beds. Trans. Inst. Chem. Eng. 15, 150-166. [Google Scholar]
- 29.Happel J, Brenner H. 2012. Low Reynolds number hydrodynamics: with special applications to particulate media, vol. 1. New York, NY: Springer Science & Business Media. [Google Scholar]
- 30.Ergun S. 1952. Fluid flow through packed columns. Chem. Eng. Prog. 48, 89-94. [Google Scholar]
- 31.Lee DS, Kwon HR, Ha JU. 1997. Estimation of pressure and volume changes for packages of kimchi, a Korean fermented vegetable. Packaging Technol. Sci.: An Int. J. 10, 15-32. () [DOI] [Google Scholar]
- 32.Che-Othman MH, Millar AH, Taylor NL. 2017. Connecting salt stress signalling pathways with salinity-induced changes in mitochondrial metabolic processes in C3 plants. Plant Cell Environ. 40, 2875-2905. [DOI] [PubMed] [Google Scholar]
- 33.Jacoby RP, Taylor NL, Millar AH. 2011. The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci. 16, 614-623. ( 10.1016/j.tplants.2011.08.002) [DOI] [PubMed] [Google Scholar]
- 34.McGovern PE, et al. 2004. Fermented beverages of pre- and proto-historic China. Proc. Natl Acad. Sci. USA 101, 17 593-17 598. ( 10.1073/pnas.0407921102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L, Wang J, Levin MJ, Sinnott-Armstrong N, Zhao H, Zhao Y, Shao J, Di N, Zhang TE. 2019. The origins of specialized pottery and diverse alcohol fermentation techniques in Early Neolithic China. Proc. Natl Acad. Sci. USA 116, 12 767-12 774. ( 10.1073/pnas.1902668116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko H, Hu DL. 2020. The physics of tossing fried rice. J. R. Soc. Interface 17, 20190622. ( 10.1098/rsif.2019.0622) [DOI] [Google Scholar]
- 37.Kaufman CK. 2006. Cooking in ancient civilizations. Westport, CT: Greenwood Publishing Group. [Google Scholar]
- 38.Díaz C, Molina AM, Nähring J, Fischer R. 2013. Characterization and dynamic behavior of wild yeast during spontaneous wine fermentation in steel tanks and amphorae. BioMed Res. Int. 2013, 540465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maga JA. 1989. The contribution of wood to the flavor of alcoholic beverages. Food Rev. Int. 5, 39-99. ( 10.1080/87559128909540844) [DOI] [Google Scholar]
- 40.Kim S, Hu DL. 2023. Onggi’s permeability to carbon dioxide accelerates kimchi fermentation. Figshare. Available from: https://figshare.com/articles/media/Onggi_s_Permeability_to_Carbon_Dioxide_Accelerates_Kimchi_Fermentation/22230085.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and code supporting the findings of this study are available from the Github repository: https://github.com/Delicate-Kim/2023-JRSI-onggi.
The data are also provided in electronic supplementary material [40].