Abstract
Tuberculosis has remained a global concern for public health affecting the lives of people for ages. Approximately 10 million people are affected by the disease and 1.5 million succumb to the disease worldwide annually. The COVID-19 pandemic has highlighted the role of early diagnosis to win the battle against such infectious diseases. Thus, advancement in the diagnostic approaches to provide early detection forms the foundation to eradicate and manage contagious diseases like tuberculosis. The conventional diagnostic strategies include microscopic examination, chest X-ray and tuberculin skin test. The limitations associated with sensitivity and specificity of these tests demands for exploring new techniques like probe-based assays, CRISPR-Cas and microRNA detection. The aim of the current review is to envisage the correlation between both the conventional and the newer approaches to enhance the specificity and sensitivity. A significant emphasis has been placed upon nanodiagnostic approaches manipulating quantum dots, magnetic nanoparticles, and biosensors for accurate diagnosis of latent, active and drug-resistant TB. Additionally, we would like to ponder upon a reliable method that is cost-effective, reproducible, require minimal infrastructure and provide point-of-care to the patients.
Keywords: Mycobacterium tuberculosis, Multi-drug resistant TB, Pathogenesis, TB, Diagnostics, Biosensors
Graphical abstract
1. Introduction
Tuberculosis (TB) is an extremely contagious disease caused by the virulent pathogen Mycobacterium tuberculosis (Mtb) which predominantly infects the lungs. However, it can damage other vital organs often leading to extra-pulmonary TB. The global tuberculosis statistics by the WHO 2022 suggests that approximately 10.6 million people fell ill with TB and the global TB mortality is around 1.6 million including HIV-positive patients. The number of individuals newly diagnosed with TB fell from 7.1 million in 2019 to 5.8 million in 2020. This drastic fall in the diagnosis is due to the COVID-19 pandemic. The pandemic shifted the access to services and resources from TB to COVID-19, limiting the availability of requisite amenities to overcome TB. Thus, advanced TB diagnostic methods are needed that are easily accessible to the population and are not hampered due to other pandemics in the future [1].
An accurate diagnosis of TB is necessary to provide the correct treatment for drug-sensitive as well as drug-resistant TB. The medication for drug-sensitive TB comprises of isoniazid (H), rifampicin (R), pyrazinamide (Z), and ethambutol (E) for the duration of 6 months. Resistance against any of these drugs causes drug-resistant TB which can be treated by a combination of second-line drugs such as bedaquiline, fluoroquinolones (levofloxacin or moxifloxacin), delamanid, cycloserine, clofazimine and levofloxacin [2,3]. In 2021, around 450,000 people developed Multi-drug resistant TB (MDR-TB) or Rifampicin-resistant TB (RR-TB). Countries accounting for 42% of global cases in 2021 are India (26%), the Russian Federation (8.5%) and Pakistan (7.9%). Correct diagnosis corresponds to an accurate treatment regimen allowing for an increased treatment success rate. This addition facilitates accomplishing the treatment target of the UN-high-level meeting on TB, i.e., treating 40 million drug-sensitive patients and 1.5 million drug-resistant infected individuals by 2022 [1].
Diagnosis of TB is underpinned by a combination of immunological assays, molecular methods, or observing the microbial growth using direct culture methods (smear microscopy). The molecular methods of nucleic acid amplification test whereas the serological detection has its own sets of limitations such as being inaccurate and imprecise for both pulmonary and extra-pulmonary TB. They also require a well-trained lab module and steady electricity supply which are unavailable in economically developing countries [4]. Hence, we need to focus on a diagnostic regimen that is both cost-effective and versatile enough to detect the pathogen. Additionally, the modern arena of nanotechnology improved the efficacy of the pre-existing techniques in combination with next-generation sequencing which helps in targeting gene amplification for detection.
The review focuses on elucidating the pathogenesis of the pathogen and makes a comparative analysis of the pre-existing and available diagnostic strategies. It also highlights the improvements incorporated in the conventional approaches and the new methods devised to fight TB. Additionally, it emphasizes the future of these strategies and it can help to expand the potential of these approaches. Hence, the review presents an overall scenario in the field of TB diagnosis.
2. Pathogenesis
Mtb spreads from an infected person to a healthy person by suspended aerosolized air droplets. The size of these droplets ranges from 0.65 to 7.0 μm. Upon inhalation of these droplets by the healthy individual, the bacteria transits past the trachea or the nasopharyngeal region followed by the adhesion on the surface of resident alveolar macrophages in the lungs [5]. The macrophages engulf the microbes by a process called phagocytosis resulting in the formation of the phagosome [6]. The interaction between the mycobacterial conserved molecular patterns with the pathogen recognition receptors like Toll-like receptors (TLRs), nucleotide-binding oligomerization domain-like receptors and C-type lectins on macrophages, causes stimulation of the innate immune response against Mtb like phagocytosis and activation proinflammatory cytokines production [7,8].
Macrophages act as a primary defense cell combating foreign pathogens; hence the entrapped microbes experience acidification, reactive oxygen species (ROS), and hydrolytic enzymes [9]. Acidification is mediated by the maturation of the phagosome, followed by its fusion with the lysosome forming a phagolysosome with a pH ranging between 4.5 and 5 [10]. As soon as the process of acidification commences, the ROS and reactive nitrogen species (RNS) suppress the metabolism of the microbe simultaneously [11]. Further, macrophages deliver copper and zinc which are toxic to Mtb at a higher concentration. This hostile environment stimulates the lysis of the bacterial cell wall components, killing the phagocytosed bacteria. As soon as the process of acidification is initiated by the matured phagosome, ROS and RNS suppress the metabolism of the microbe simultaneously [11]. Further, the lysed phagosomal components progress towards the antigen presentation pathway.
The bacilli that survive these harsh conditions replicate and divide in the phagosomes, increasing the bacterial load in the host and inducing the release of cytokines like TNF-α, IL-6, IL-12p80, IL-1α and IL-1β. Mtb infection of macrophages results in the production of several small molecules to regulate the infection, known as the chemokines such as CCL2, CCL3, CCL7, CCL12, CXCL2 and CXCL10 [7]. All these inflammatory molecules regulate the immune cell trafficking to initiate T cell response and recruitment of other immune cell types including monocytes, neutrophils and dendritic cells to the primary infection site. The secretory proteins produced by Mtb are further processed by the dendritic cells (DC). They have a vital role in antigen presentation [12].
With the onset of the adaptive immune response, immune cells like the lymphocytes, monocytes, etc. infiltrate the site of infection which forms a structure known as the granuloma (which undergoes calcification to encapsulate the bacilli from the immune system called the Gohn complex). Granuloma formation is often regarded as the hallmark of TB infection, a stage where the immune system arrests the progress of the disease (latent TB infection) [13]. A typical feature of latent TB infection is the transformation of the bacteria to a non-replicating stage with a very low metabolic activity often termed as the dormant stage [14]. The regrowth of the dormant bacteria is due to the re-establishment of the replicative and metabolic processes – resuscitation. Research suggests that in addition to the presence of nonreplicating bacteria, a few metabolically active bacteria also exist known as scouts [6]. Under favorable conditions, the scouts signal the other dormant bacteria to resuscitate. The active bacteria then replicate and increase in number, causing caseation of the granuloma and spill of active Mtb to a new site such as the adipocytes or the lymph nodes where it can evade the immune response. This causes coughing in the host which leads to the release of Mtb bacilli into the environment in the form of aerosols (active TB infection) (Fig. 1 ) [15].
Fig. 1.
Pathogenesis of Mycobacterium tuberculosis. Infection in a healthy individual begins with inhalation of air droplets containing Mtb bacilli (1). The bacteria are phagocytosed by the alveolar macrophages (2). Formation of phagolysosomal complex occurs in the macrophages to kill the bacteria (3). Few bacteria escape the host innate immune response and replicate in the macrophages (4). This induces adaptive immune response causing release of pro-inflammatory cytokines and recruitment of other immune cells (5). The immune cells form granuloma to contain the infection at primary site (latent TB infection) (6). Under immune compromised condition the granuloma ruptures causing release of Mtb to other sites (active TB infection) (7–8).
The mycobacterial infection established in an individual can range from latent to active TB infection, thus it is essential to understand the impact of bacterial growth on Mtb detection that will lead to precise diagnosis and treatment. Fig. 2 summarises the effect of different TB infection state on diagnosis. The conventional approach like sputum culture and radiology are effective in detection of active TB infection, similarly the immunological based assays are effective in identification of latent as well as active TB infection. But the above mentioned assays are not effective in discriminating between the TB infection states, additionally does not identify multi-drug resistant TB [16]. Thus, to fill up the gap newer approaches like mi-RNA, whole genome sequencing, mass spectrometry and diagnostic kits based on nanoparticles are explored to detect different mycobacterial stages that will result into reduced number of missed TB cases and correct treatment regimen.
Fig. 2.
Diagnostic tools during TB pathogenesis. Tuberculosis infection in host can be categorized into latent, sub-clinical and active states. Latent TB infection is an asymptomatic, non-transmissible state with strong host immune response. The sub-clinical stage is asymptomatic but transmissible infection. Patients with active TB infection are symptomatic with persistent cough and high disease transmission rate. Differentiation between the various stages of TB is essential in order to understand the pathogenic state of Mtb. This aids in providing accurate medications to the diseased individual and reduced community transmission. Diagnostic tools like microbiological observation and radiology are efficient in diagnosis of active TB but are inefficient in diagnosis of latent and sub-clinical stages. Immunological based methods like ELISA and TST rely on immune response of individual against mycobacterial antigens. These approaches can identify all pathogenic stage of Mtb yet cannot discriminate between them. New methods for diagnosis like CRISPR, Lipoarabinomannan detection, miRNA, digital PCR, nanoparticles (NPs) based diagnosis and mass spectrometry provides increased sensitivity and specificity along with precise diagnosis of latent and active TB infection. The diagram has been adapted from Pai et al., 2016 [16] with some modification including the recent advanced approaches.
3. TB diagnosis
An accurate and timely diagnosis of TB can reduce mortality and untimely death. The emerging cases of drug resistance are a threat to the goal of TB eradication. Major advances have taken place in laboratory diagnostic strategies for infection detection. These strategies either complement or replace the already existing conventional approaches. Some of the conventional methods are Acid-fast bacillus smear microscopy, microbial culture, etc. whereas the advanced strategies include CRISPR Cas, Gene Xpert and LAMP. The conventional approaches are slow, time-consuming but intriguingly predominant in high TB burden countries. The lack of cutting-edge advanced techniques to detect Mycobacterial infection can be attributed to below optimal funding, low sensitivity, specificity and lack of accessibility to the peripheral health care system where patients seek treatment, thus posing a threat to eradication and control. Fig. 3 compiles diagnostic methods used to detect active, latent, and multi-drug resistant TB. Table 1 describes sensitivity, specificity of conventional methods used for TB diagnosis.
Fig. 3.
Diagnostic methods for detection of tuberculosis infection. Early diagnosis of TB is performed using microscopy in which the presence of active bacilli are visualized under microscope and radiology where the chest X-ray is read to detect lesions (1). The Latent TB infection is diagnosed using tuberculin skin test, ELISA and IGRA. The tests are based upon the inflammatory reaction initiated by the host in the presence of Mtb pathogen (2). For the diagnosis of multi-drug resistant TB more specific and sensitive assays need to be performed like RT-PCR, DNA microarray and LAMP. These methods specifically determine the mutations in the genes allowing for identification of drug resistance (3). Advancement in diagnosis with the exploitation of nanoparticles, miRNA and CRISPR-Cas brings revolution in TB diagnosis (4).
Table 1.
Conventional methods of TB diagnostics.
| S.No. | Diagnostic method | Principle | Sensitivity (%) | Specificity (%) | Turnaround duration | Description | References |
|---|---|---|---|---|---|---|---|
| 1. | Microbiological observation | AFB staining and light microscopy | 32–94 | 50–99 | Same day |
|
[17,18] |
| Auramine staining and fluorescence Microscopy | 52–97 | 94–100 | Same day |
|
|||
| |||||||
| Limitations | |||||||
| |||||||
| 2. | Radiology | Imaging | 98 | 75 | Same day |
|
[19] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| 3. | Tuberculin skin test | Immune response against TB antigens | 94 | 88 | 2–3 days |
|
[20] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| 4. | IGRA | Immune response against Mtb in blood | 98.9 | 98.1 | 2–3 days |
|
[21] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| 5. | ELISA | Antigen-antibody interaction | 98.06 | 98.67 | 2–3 h |
|
[22] |
| |||||||
| |||||||
| |||||||
| |||||||
| 6. | Drug susceptibility testing | Liquid culture media | 89 | >99 | 10–21 days |
|
[23] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| 7. | Line probe assay | PCR | 95.6–97.5%8 | 98.7– 99.5% |
Same day |
|
[24] |
| |||||||
| |||||||
| 8. | LAMP | NAAT (Nucleic Acid Amplification Test) | 76–80 | 97–98 | Same day |
|
[25,26] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
|
4. Diagnostic methods for active TB infection
Due to the complex pathogenesis of the disease, the dearth of an effective vaccine and the unique ability of the pathogen to escape the immune system; the bacteria still remain a threat to the public health. The WHO data suggest that one out of every three people is infected with the bacterium yet remains asymptomatic. However, there is a high chance of relapse in these individuals. Geographically, India (26%), Indonesia (8.5%), China (8.4%), the Philippines (6.0%), Pakistan (5.7%), Nigeria (4.4%), Bangladesh (3.6%), and South Africa (3.6%) contributed to two-third of global TB infection [27]. In 2019, 10 million people developed TB infection whereas only 7.1 million were reported through the National Tuberculosis program launched by the Government of India [28]. The WHO reports that a drop in 50% of identified TB diagnostics can add up to 40,000 additional deaths globally [29]. In order to combat this peril, the gap between active TB cases and diagnostics should be abridged with rapid rollout detection methods for TB diagnosis. This section deals with certain diagnostic approaches that are rapidly used for the identification of TB infection.
4.1. Microbiological observation
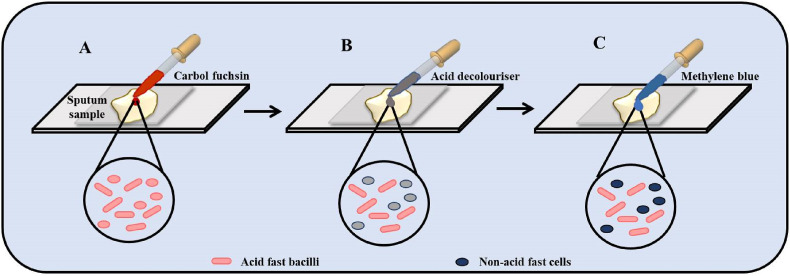
Culturing Mtb in a suitable growth media followed by microscopic examination is regarded as the gold standard of TB diagnostics [30]. Sputum samples or laryngeal swabs are collected from the suspected patients to isolate the bacterium [31]. This is followed by the decontamination of the specimen using a mucolytic agent of 7% saline and 4% NaOH [32]. Some of the other media often used are the Middlebrook 7H10 medium, and Middlebrook 7H9 broth supplemented with 10% OADC (oleic acid, albumin, dextrose, and catalase) [33]. Supplementation with inorganic salts and albumin prevents the toxic fatty acids from binding with the mycobacterial cell wall. In order to detect the bacillus quickly, sputum can be concentrated by centrifugation in sodium hypochlorite, and ammonium hydroxide solution which further improves the sensitivity [34,35]. The staining of Mtb is dependent on the acid-resistant property of its high lipid content cell wall. The most common staining technique is the Ziehl-Neelsen method [36]. Other techniques include the cold staining method. Both these staining procedures use basic dyes such as Fuschia (primary dye), methylene blue (counterstain), and phenol compounds which penetrate the bacterial cell wall. The acid-fast bacilli take up the red color of the primary dye however it is unable to pick up the blue color of the counter stain hence appearing red under a microscope (Fig. 4 ). In order to improve the detection of light-emitting diode fluorescent microscopy is often recommended [[37], [38], [39]].
Fig. 4.
Microbiological observation of Mtb by acid-fast staining. (A) The collected sputum sample is initially stained by carbol fuchsin dye which stains all the cells pink. (B) Decolourisation of cells by acid decoloriser causing discoloration of non-acid fast bacilli. (C) Counter-staining of cells by methylene blue leads to staining the non-acid fast bacilli as blue. Thus, the acid-fast bacilli (pink in color) can be easily distinguished from the non-acid fast bacilli (blue in colour) under a microscope. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4.2. Radiology for pulmonary TB
Mtb damages multiple organs of the body although the clinical manifestation involves the lungs. Hence, the initial site of involvement in the lungs, followed by the lymph node, heart as well as skeletal muscles. In order to comprehend the involvement of these organs, radiological imaging is imperative. It can help in assessing the complexity of the disease followed by its prognosis such as the response of the patient to drugs. Some of the common radiological methods for TB diagnosis are Computed Tomography (CT) scan, Positron emission tomography-CT scan (PET-CT), Chest X-ray, and MRI.
Patients suffering from tuberculosis experience severe cough with blood in the sputum, and develop severe complications such as emphysema, distortion of the bronchioles, thickening of the pleural membrane, etc. In order to investigate the unexplained cough or study the involvement of the extra-thoracic cavity, a chest X-ray is often the primary test. Chest X-ray helps in a comprehensive assessment of the lungs and the pleural membrane (Fig. 5 ) [40,41]. Computed Tomography is a significant tool to assess the complexity of the disease by evaluating the formation of lesions, pleural effusion, and transient thickening of the lobular septa. It aids to differentiate the etiologies of TB from pneumonia [40,42,43]. The lesions are detected in patients with active TB infection; they disappear within 5–9 months after treatment. In case of reactivation of the disease, these lesions were observed followed by fibrosis. Hence CT test can provide valuable evidence of the progression of the disease especially if there is a chance of relapse [40,44].
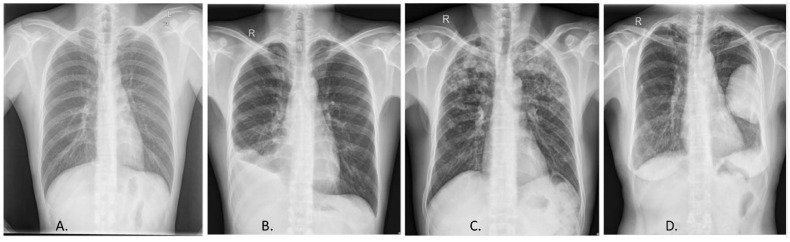
Fig. 5.
Radiological diagnosis of TB, Chest X-ray. (A) Normal chest X-ray. X-ray displaying different manifestations of TB, (B) pleural effusion, (C) infiltrates, (D) cavity lung lesion. Reprinted from (Ayaz et al., 2021) (open access).
MRI is used to detect abnormalities in the lungs with a significant advantage over CT scans in terms of resolution and lack of exposure to radiation. It can detect thoracic lymphadenopathies, necrosis, and inflammation in the pleural membrane as well as hypersensitivity of the thoracic wall because of TB infection [40,45]. MRI is clearly superior for detecting the changes in the morphology of the pleural membrane as well as for the characterization of the lung tissue (fibrosis of lungs) [40]. Positron emission tomography-CT is a holistic approach to associating with the immunological, pathological aspects of TB. Often PET SCAN is used to detect the granulomas formed during TB infection (both pulmonary and extrapulmonary) [46,47]. PET emission is often the targeted approach to assess specific organs of the body such as tuberculosis infection of the skeleton. It also offers a unique opportunity to characterize the lesions of TB infection thus elucidating the molecular basis of TB infection [40].
5. Diagnostic methods for latent TB infection
Latent TB infection (LTBI) is an infection form that is at a subclinical level and may be transformed into an active TB infection at later stages of life. Patients infected with latent TB serve as an active source of infection and several risk factors are involved with it [28]. The latest data indicates that China has the heaviest load of latent TB infection; about 350 million people are infected with latent Mtb [48]. There is a lack of differential diagnosis between latent and active TB, hence diagnosing latent TB is of vital importance for the overall control of the disease and for mitigating mortality and morbidity. If these patients are administered anti-tuberculosis treatment, the chance of developing active TB reduces significantly hence timely detection is essential [49]. Therefore, countries with the burden of latent TB infection should accelerate LTBI research and improvise diagnostics for an accurate diagnosis to reduce the global TB burden by 2025 [50]. This section elaborates on the diagnostic approaches for the detection of latent TB infection.
5.1. Tuberculin skin test (TST)
Tuberculin skin test (TST) is a classic example of the hypersensitive cellular T cell response upon inoculation with the mycobacterial antigen [51]. It is based on the fact that a person exposed to the antigen is bound to show an immune response due to the presence of bacterial protein. The patient is injected intradermally with Purified Protein Derivative (PDD), the most common antigen of Mtb. Within 48–72 h post-injection the person develops edema, fibrin deposition and redness due to the migration of the lymphokine producing T cells, neutrophils, and monocytes at the site of injection [52]. PPD was first developed by Florence B. Seibert in 1934. It was produced by steaming cultures of Mtb in an Arnold sterilizer. Purification of proteins was done by repeating precipitation with ammonium sulfate. PPD comprises of approximately 92.9% protein, 5.9% polysaccharide and 1.2% nucleic acid. Potency of PPD is expressed as either international unit (IU) or tuberculin unit (TU). One IU is equal to the biological activity contained in 0.028 μg of PPD. Five IU is the standard dose for intradermal TB diagnosis [53].
In order to deduce the results, the diameter of the induration is measured perpendicular to the axis of the forearm. Based on the diameter of the induration the risk of developing a TB infection is classified by the American Thoracic Society in 2000. If the diameter of the induration is 10 mm and the patient has a history of previous exposure to Mtb then the test result is positive whereas a diameter of 5 mm is considered positive only in case of recent contact with TB infection (Fig. 6 ) [51]. However, there is no correlation between the size of the induration and the future risk of TB. TST being the most conventional method for TB diagnosis faces some fundamental drawbacks. The basic concern with TST is lack of specificity. The test often gives false-positive results because of the inability to differentiate between Mtb infection with that of non-tuberculosis mycobacteria or vaccination with Bacille Calmett-Guérin (BCG). Both cases of false positive responses are generally due to cross reactivity where an immune response is elicited by homologous antigens either from vaccination with BCG or from environmental non-pathogenic mycobacteria. Despite these snags, TST remains the most commonly used platform to detect Mtb infection [53].
Fig. 6.
Detection of Latent tuberculosis infection. (A) Tuberculin skin test. Intradermal injection of protein derived derivative (PDD) is administered to the patient's forearm. After 48–72 h post incubation, patient develops edema and redness due to antigen processing and migration of immune cells like lymphokines producing T cells, at the site of infection. Diameter of induration developed in the forearm is measured for detection of TB infection. (B) Blood test. Blood sample from the patient is taken and whole blood cells are incubated along with Mtb antigens. Antigen processing and presentation leads to the release of IFN-γ from T-cells. Detection of the antibodies producing cells is done by ELI-SPOT orthe quantitative analysis IFN-γ can be done by QuantiFERON-TB Gold assay. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
5.2. Interferon-gamma Release Assays (IGRAs)
The assay is based on the immune response of the patient to Mtb in an immune-competent individual [54]. After exposure to the pathogen, the memory T cells remain active and upon relapse or reinfection, the antigen-presenting cells process the antigenic peptides to the memory T cells. These T cells produce lymphokines, particularly interferon-gamma (IFN-γ) due to the stimulation of antigens specific to the Mtb complex i.e., the Early secretory antigen targeting 6 (ESAT-6) and the Culture Filtrate Protein (CFP-10) circulating in blood during Mtb infection. Both of these induce a strong T-cell response [55,56]. Hence these two are the most common targets for Interferon-gamma Release Assay. In case the patient doesn't respond to the above antigens, ESX-1 substrate protein C (EspC) can be a suitable alternative. IFN-γ production can be directly correlated to the bacillary burden or antigenic load since it is an essential biomarker for TB infection as well as for detecting the treatment response in the patients [57]. The IFN-γ level in the sample is quantified at an absorbance of 450 nm (Fig. 6). However, the performance of IGRA can be affected negatively by immune-mediated inflammatory diseases (IMIDs) due to impaired immune response in Crohn's disease where the function of immune cells is suppressed as well as in patients with immune-modulatory drugs such as teriflunomide having an inhibitory effect on T cell response [58].
Identifying the temporal changes is crucial for evaluating the dynamics of cellular immunity, hence can be used to evaluate the chance of developing active TB [57]. Studies suggest that the risk of TB is 2.90 folds higher if the IFN-γ levels in the blood are 1IU/ml, 10.38 folds higher at 5IU/ml, and 21.82 folds higher at 15IU/ml (International Units) [59]. Other additional comorbid conditions such as alcohol, drug, and malnutrition will also determine the risk of developing active TB infection. The two different types of Interferon Gamma Release Assays are the QuantiFERON-TB Gold test and the ELISPOT assay.
QuantiFERON-TB Gold is a quantitative assay to estimate the production of IFN-γ in a blood sample when incubated with Mycobacterial antigens such as ESAT-6 and CFP-10 [60,61]. Both these antigens are epitopes of CD4+ and CD8+ T cells but mainly stimulate the CD4+ T cells to release interferons [62]. Blood from the suspected patient is collected and incubated overnight with the above antigens at 37 °C [63]. The lymphocytes become sensitized upon exposure to mycobacterial antigens and release IFN-γ which can be quantified to assist diagnosis of TB. It is cost-effective with high specificity in the age group 1–15 years i.e., 97.8%. However, in a study performed by Hossein et al., 2015, it was concluded that after 3 months 2/7th of QFT GIT negative initially and 66% of QFT GIT positive reverted. Thus it is not reliable for the diagnosis of latent TB in unexposed BCG vaccinated children [64].
T spot TB/ELISPOT ASSAY test determines if a person is infected with Mtb by quantifying the level of cytokine-producing T cells in the body [65,66]. Blood of the suspected patient is collected and the mononuclear T cells are separated. The cell suspension is seeded with antigenic peptides such as the ESAT-6 and CFP-10 and placed in a microwell. The microwells are washed in a phosphate buffer and incubated for 1 h at 37 °C followed by observation in a light source. Each spot on the well represents a cytokine-producing T cell [67]. The spot counts are directly correlated to TB infection. A high spot number indicates an active TB infection whereas a low spot number suggests a latent TB infection [21]. Despite several advantages such as reducing the number of false-negative results and high suitability for the high-risk groups, this test fails to be sensitive to different subtypes of TB infection.
5.3. Enzyme-linked immuno-sorbent assay (ELISA)
The cellular immunity in humans produces a wide paradigm of antibody-mediated responses against M tuberculosis [68]. The paradigm can be validated by quantifying the level of antibodies in the sera [69]. The level of antibody shows a wide difference in both active and latent TB infection, often used to distinguish between the infections [70]. More recently the role of antibodies to mycobacterial antigens has evolved; hence a suitable antibody profiling can be done to detect the type of TB infection. For example, the IgG directed against the mycobacterial transmembrane protein (Rv2626c) is higher in latent TB infection than in active TB infection [68]. Additionally, IgM directed against membrane-associated antigens, and IgA directed against alpha-crystallin (Acr) are at a higher level in latent TB infection [71]. However, the most common antigen for ELISA is 6, 27, 30 and 38 kDa mycobacterial proteins [22]. Sun et al. developed a universal ELISA for serological detection of Mtb complex in multiple hosts. Infection can be detected by using fusion protein MMEC (MPB70, MPB83, ESAT6, and CFP10 i.e., common in different species of mycobacteria) as coating antigen. The sensitivity and specificity of this ELISA is 100% and 94.85% respectively for Mtb detection in humans, making it a reliable procedure for TB detection [72].
5.4. Urinary lipoarabinomannan test
The assay discussed above pose challenges in accurate detection of TB in children and extremely-ill patients like HIV-infected individuals. Detecting tuberculosis in children is exceedingly difficult due to non-specific clinical presentation, low accuracy of Chest X-ray and the collection of sputum sample; hence antigen detection in urine sample remains the gold standard [73]. Lipoarabinomannan (LAM) is an envelope component of mycobacteria which is excreted via urine in children due to weaker T cell and IL-12 response; providing a high chance of detecting mycobacterial antigen in urine. Often LAM can be detected in HIV co-infected TB patients and WHO recommends it for its application in critically ill HIV patients and children with an extremely low CD4+T cell count (<100 cells/mm3) [74]. Urine LAM detection technology by ELISA or lateral flow are commercially available by Fujifilm SILVAMP TB LAM (Fuji LAM) test and the Alere Determine TB LAM Ag (Alere LAM) test with short turnaround time and point-of-care to patients [75,76]. Hence, lateral flow LAM is the preferred approach in which unprocessed patient urine samples are applied on the sample pad of TB LAM Ag, followed by incubation at room temperature. The strip is visualized for the intensity of the bands and graded by comparing it with the manufacture's standard (Seid et al., 2022). Overall the sensitivity and specificity of the above tests were higher in children with HIV. This can be attributed to the excretion of LAM in HIV positive active pulmonary TB patients due to renal dysfunction. Additionally in HIV negative TB patients, formation of immune complexes with non LAM proteins masks the concentration of LAM in urine. Thus, urine LAM assay is easy to perform and are highly recommended in malnourished children, yet elaborative evidence is required for its usage in diagnosis of TB in children independent of HIV status. Another alternative strategy is to test the liquid phase of exhaled air termed as exhaled breath condensate (EBC). TB patients have a higher range of LAM (15–120 μg/mL) as well as specific lipids and proteins not detected in healthy or community acquired pneumonia patients [77]. These allowed us to distinguish between TB patients at baseline, including smear-negative and culture-negative adults and children. Overall, the collection of sample is extremely easy, non-invasive, and may reflect lung disease specific markers from infected patients. However there is a lack of standardization of collection techniques which hampers their introduction to clinical practice [78].
6. Diagnostic approaches for multi-drug resistant TB
Multidrug-resistant TB (MDR-TB) can be described as TB infection resistance to drugs such as isoniazid and rifampicin. The WHO 2020 report suggests that Bangladesh, Pakistan, India South Africa, etc. would contribute to 84% of TB cases from 2021 to 2025 whereas the remaining 5% would be contributed by Congo, Zambia, Liberia, etc. The increasing cases of drug resistance are because of a lack of drug susceptibility testing [79]. Thus, the use of advanced and newer technology is preferred to diagnose patients for MDR-TB, so that the proper treatment is provided to the individual before the condition worsens.
6.1. Phenotypic drug susceptibility testing
Phenotypic drug susceptibility testing (DST) is regarded as the definitive diagnostic test for MDR-TB. The increasing expansion of multidrug-resistance TB enhances the demand for drug susceptibility tests, often regarded as the gold standard to detect MDR TB [80,81]. It can be done by observing the viability or metabolic inhibition in a medium containing anti-tuberculosis drugs [82]. The susceptibility can be determined from various technical standpoints such as macroscopic observation in drug-free or in the media-containing drug [83]. The metabolic activity can be another parameter to test their susceptibility. However, it takes a long time to give a confirmatory test (2–3 months). In order to avoid delay, use of liquid culture media is advisable than solid media [84]. In addition to the above, the method is expensive and increases the chance of contamination with non-tuberculous mycobacteria.
6.2. Molecular drug susceptibility testing
To reduce the turnaround time and the chances of contamination, molecular DSTs have been developed. This technique relies on the amplification of nucleic acid and targets the genes responsible for drug resistance [85]. Some of the molecular DSTs are LATE PCR with Lights-On/Lights off Probe and Ligase chain reaction.
LATE PCR with Lights-On/Lights off Probe is an advanced technique to generate double-stranded DNA followed by the production of single-stranded amplicons. It uses different types of fluorescent dyes to analyze nucleotide substitution. The number of thermal cycles used to amplify the DNA depends on the number of target molecules being amplified. Usually, the temperature is lowered to such an extent that the complementary dsDNA molecules are unavailable to interact with the probes (light on/off probes are low-temperature probes) as their melting temperature is usually 5° Celsius less than the primer. Thus, this probe is labeled with a quencher dye which quenches off the fluorescent signal after it binds to the target probe. Although each probe binds to a specific region of the DNA, these fluorescent signals can be integrated to analyze the entire nucleotide. The melting curve for the entire probe during annealing can be analyzed to detect the mutation. In the case of mutation, there is a change in the melting point. The use of multiple probes allows the detection of multiple targets in a single reaction mixture. For example, the rifampicin resistance in Mtb (rpoB gene) is analyzed using this method. This is a very sensitive test with a high specificity of 88–100% [86,87].
Ligase chain reaction is an amplification process that amplifies the genes responsible for multidrug-resistant TB. It can detect Single Nucleotide Polymorphisms (SNPs) responsible for isoniazid and rifampicin mutations in Mtb [88]. The initial step involves the identification of the mutation responsible for the drug resistance [88,89]. After the initial step is completed, probes specific to the targeted regions are designed followed by the ligation of the probes using ligase. The ligated probes serve as a template for the next cycle. This is followed by the amplification of these segments by the thermostable Taq polymerase. This method display average sensitivity of 75%. It is an expensive test to detect drug resistance often giving false-positive results [90].
6.3. Probe-based assays
Probe-based assays have revolutionized the diagnosis of MDR-TB since these methods use PCR to amplify the gene mutations responsible for drug resistance. Molecular DST can be performed only when there is clear evidence of bacillus load in the sample or if the recent tests suggest that the patient has stopped responding to the treatment. Some of the commercial assays approved by the WHO are line probe assays (LPA), Gene Xpert, and GenoType MTBDR plus for detecting mutation to isoniazid and rifampicin [91].
Line probe assay technology involves extraction of DNA from the isolates of patients followed by amplification of the resistance determining region using suitable primers [80]. The PCR products are then hybridized with suitable oligonucleotide probes. These hybridized labels enable the detection of the Mtb complex in the sample. Line probe assays not only identify Mtb in the samples but also determine the drug resistance pattern with an optimal sensitivity of 71% [92]. If mutations are present, the probe will not bind to the target region. The post-hybridization reaction will lead to the formation of colored bands on the line strips at the site of probe binding. It has a high specificity and the results can be obtained within 48 h [93]. This assay is specific to a particular mutation and only focuses on the hotspot of mutations. For example, the INNO-LiPA Rif TB LPA developed in Belgium is sensitive only to rpoB gene mutations from codon 509 to codon 534 (Asp516Val, His526Tyr, His526Asp, and Ser531Leu mutations). Thus, it can be used for an initial screening to confirm drug susceptibility and suggest suitable antibiotics [94]. Xpert MTB/RIF a commercial form of line probe assay is used to diagnose pulmonary and extra-pulmonary TB as well as MDR-TB. It is an automated real-time PCR for detecting drug resistance within a span of 2 h. In this method, the sputum sample is collected from the patient and mixed with suitable reagents such as liquid buffers and lyophilized reagent beads for sample processing and nucleic acid extraction. Simultaneously, the sputum samples are treated with sodium hydroxide and isopropanol-containing sample reagent at room temperature for 15 min. The mixture is then placed in the cartridge inside the Gene Xpert machine which is fully automated to purify, concentrate and identify the targeted nucleic acid sequence. The result of this test is classified as detected, undetected or indeterminate. Results that are positive for RIF resistance mean the bacteria are resistant to rifampicin. Further, molecular tests should be performed to select an effective drug for treatment. On the other hand, negative RIF indicates the susceptibility of the bacteria towards the drug whereas results indeterminate means that the test could not determine accurately if the bacteria are susceptible or not [93,95,96].
Gene drive MTB/RIF ID KIT is an innovative diagnostic test assembled to detect Mtb complex directly from the raw spectrum at a faster rate [97]. The kit is portable and operates at a low voltage supply (12V) and consumes less power [98]. It uses real-time PCR technology and a simple paper-based DNA extraction procedure [99]. The paper on which the DNA is collected is decontaminated followed by its extraction from the bacteria without the need of a vortex or centrifuge. The targeted genes which are amplified for PCR include a short repetitive region (REP13E12) and the hotspot region which harbors 81% of mutations responsible for drug resistance (rpoB at positions 516, 526, and 531). It has an overall sensitivity of 93.5% and the results can be obtained within 75 min. It is user-friendly and affordable for low-income countries [98].
6.4. DNA microarray
DNA microarray helps in the accurate and rapid identification of 17 mycobacterial species simultaneously by designing an oligonucleotide sequence complementary to the 16sRNA (most common). Recently few more oligonucleotide probes have been designed that target the 13 most common mutation sites from the rpoB gene, katG gene as well as mutations in the promoter region of the inhA gene [100]. This array can identify the exact nucleic acid substitution unlike conventional assays. In order to identify the mutations, Mtb strains are isolated from sputum samples and cultured in Lowenstein-Jensen at 37 °C for 3–10 weeks [101]. Suitable oligonucleotide probes (10–40bp) are designed against the validated target genes (labeled with fluorescent dyes) which hybridize with the complementary probe. The non-specific and non-bonding regions are washed off-post hybridization. The hybridized probes form a spot and give signals on the immobilized microarray chip. If no spots are obtained then the mutation is confirmed [102]. DNA microarray chips are rapid, accurate, and highly efficient and can address the shortcomings of traditional methods of nucleic acid amplification like low automation and low detection efficiency. Hence, DNA microarrays can be used to comprehend drug resistance-associated gene mutation.
6.5. Lucifer reporter assay
Lucifer reporter assay is a test used to detect drug resistance in Mtb which uses luciferin as a reporter to identify the expression of drug resistance genes [103]. There are two strategies that are employed for this test. The first strategy involves the creation of shuttle phages by integrating mycobacterial phages into the cosmid vectors followed by propagation in E. coli. The second strategy involves the homologous recombination of the DNA fragment to a bacteriophage which is expressed upon infecting Mtb. A luciferase-expressing mycobacteriophage is designed to infect Mtb. The genome of the bacteriophage carries the gene for antibiotic resistance as well as the firefly luciferase gene (fflux). These phages are propagated and allowed to infect Mtb. Antibiotic resistance is determined by analyzing the light output from the luciferase-expressing mycobacterial population [104]. The antibiotics will disrupt the mycobacterial cell wall, thus preventing the infection of bacteriophage and luciferin expression. The luciferase expression requires ATP; hence only viable cells will produce ATP. Thus, the light intensity will reduce significantly after antibiotic treatment. In contrast, antibiotic-resistant cells will be unaffected and will continue to produce light. There are several advantages of this assay as it is easier to perform and it can be applicable to a wide variety of mycobacterial strains. Despite several advantages, this assay is only limited to the unpredictable phage infectious rate of the Mtb strain [105].
6.6. Loop-mediated isothermal amplification (LAMP)
LAMP is a technique to detect the genes responsible for multidrug resistance in Mtb. It is a novel colorimetric method that amplifies mycobacterial DNA. This technique detects the genes responsible for resistance against several anti-TB drugs like isoniazid, rifampicin, amikacin, and ciprofloxacin [106]. Hence the targeted genes for amplification are katG, inhA, rpoB, rrs, gyrA, and gyrB [107]. Often the major reason behind multidrug resistance is the point mutation in these genes, so this method targets the hotspot regions of the genes responsible for the mutation. In the first step primers (allele-specific primers) known as the forward (FIP) and backward primers (BIP) are designed specifically for these genes which bind to the antibiotic resistance determining region [108,109]. The DNA strands will only be amplified if the primers anneal completely to the target sequences. The product obtained is then displaced by the loop primer elongation. This result in the replacement product getting amplified with biotinylated LAMP attached. The allele-specific primers elongate and displace the biotinylated LAMP product [110]. Subsequently, allele-specific primers elongate the biotinylated product. Additionally, several other mutations can be detected simultaneously by synthesizing LAMP primers and adding primers specific to the mutated allele with a specificity of 60%. It is inexpensive and does not require a sophisticated platform, hence available to the peripheral health care system [106].
7. Limitations of conventional diagnostics methods
The age old methods used for TB detection, like sputum microscopy, culture test, radiology, drug susceptibility testing have been able to detect the acid fast bacilli, provide point-of-care diagnosis and are cost effective. However, the major shortcomings of these diagnostic methods are low sensitivity, time-consuming, false negative results, poor efficacy, lack of differentiation between different strains and bacterial viability detection [111,112]. These drawbacks lead to delayed TB diagnosis. Delayed diagnosis accelerates infection severity, increases the risk of mortality, and enables the transmission of bacilli within the healthy community. Further, incorrect diagnosis will lead to imprecise treatment therapy that will eventually lead to development of drug-resistant in the infected individual [113]. Thus, rapid, highly sensitive and specific diagnostic tests and approaches need to be explored for detection of Mtb.
Recent technologies using new andemerging tools and methods prove to be essential for development of better diagnostic assays against TB. The advance diagnostic tests such as digital PCR, CRISPR-based detection, whole genome sequencing, mass spectrometry and nanodiagnostics display excellent sensitivity and specificity. These tests are efficient in detection and differentiation of drug sensitive as well as drug resistant strains in the suspected individuals so as to start with the accurate and appropriate therapy. MicroRNA detection allows in understanding of pathogenic states of Mtb in the diseased person based on the expression level of mi-RNAs. Application of nanoparticles with the conventional methods like ELISA provides rapid point-of-care diagnostic tool. Raman spectroscopy can be used in rural areas as well for rapid screening of vast number of patients and mass spectrometry that can be performed in a routine clinical lab within short time [112,114]. A detailed description of these methods has been elaborated in the following section to understand the current TB diagnostic status.
8. Advance TB diagnostics
One peculiar trend of TB is that 90% of TB infected individuals live in low resource areas, where adequate health care facilities are not available and the labs are not well equipped to give results within a short span [115]. Hence, the disease is detected when it is beyond prophylaxis. Globally the cases of drug-resistant TB infections are rising tremendously; hence efficient user-friendly, low-cost diagnostic tests are required. Despite the introduction of advanced techniques such as the MTB/RIF assay, the global burden of TB infection is still alarming. Apart from that, no suitable test is available to detect TB in HIV-infected patients or in pregnant women and children [116]. Hence, in order to reduce the annual burden of infections, diagnostic tests should be designed to meet the minimal specifications in case of urgency in low-resource settings. The diagnostic tests should identify a new point of TB control such as identification of a new biomarker, simplifying the technological platforms, and developing new reference standards for children and HIV patients. It should also aim to reduce the cost of diagnosis along with the accessibility to develop a new horizon towards delivering the best patient care [116]. Table 2 describes the recent advances for TB diagnosis.
Table 2.
Recent techniques for TB diagnosis.
| S.No. | Test | Type | Sensitivity (%) | Specificity (%) | Turnaround duration | Description | References |
|---|---|---|---|---|---|---|---|
| 1. | CRISPR/Cas12a | Gene editing | 90% | 98% | 7 and 14 days |
|
[117,118] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| 2. | AI Processing | Artificial intelligence | 68–96% | 72–85% | NA |
|
[119] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| 3. | MinIONNanopore-sequencing (OxfordNanpore technologies, U.K.) | Detection of Rifampicin resistance supersedes | 94.8% | 98% | 6 h |
|
[120] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| 4. | Whole-genome sequencing | Next-generation sequencing (NGS) | 95% | 95% | up to 72 h |
|
[121] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| 5. | miRNA | PCR based assay | 24.7–39.9% | >90% | 24 h |
|
[122,123,124] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| 6. | Raman spectroscopy | Raman scattering | Active TB: 84.62% LTBI:86.84% | Active TB: 89.47% LTBI: 65% | 2 h |
|
[125] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| 7. | Aeonose (eNose BV,Netherlands | Detection of VOC(breath analyzer) | 75–92% | 44–65% | 10 min |
|
[126] |
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
| |||||||
|
8.1. Digital PCR
Droplet Polymerized Chain Reaction is the third generation PCR that estimates the exact quantity of nucleic acid in a sample [127,128]. This method was first conceptualized in 1990 and is based on the endpoint method of nucleic acid quantification; hence no standard graph is needed to interpret the result [129]. The sample is collected and distributed into different chambers containing more than one target, whereas the other chambers may have no target at all. This is followed by thermal amplification where the targets are amplified to several copies. This is followed by the step where the sample is fractionated to generate droplets based on fluorescent probes such as FAM (Fluorescein amidite) and HEX (Hexachloro-Fluorescein)or Eva Green [129]. After the generation of approximately 20,000 droplets, it is suspended in a water-oil emulsion [130,131]. A single droplet can have more than one target gene while others may not have any. Amplification of the sample droplets is carried out in an endpoint thermal cycler. Post amplification the droplets are placed in an analyser to detect target genes based on the color produced by the fluorescent dyes. The signals produced by these dyes are quantified and are then classified into the positive and the negative fractions [129]. The positive fraction is the one with the desired gene of interest whereas the negative fraction lacks any. The reading process is usually done in a software analyzer that uses the software i.e., Quanta soft. Data analysis is done after the droplet reading is accomplished. The data can be visualized, analyzed, and exported. In recent years, Digital PCR is exclusively used to study the expression of mutant genes and often to understand the mechanism behind resistance to 5′FU (Fluorouracil) [132].Usually, it has been reported that the resistance to fluorouracil is due to mutations in upp or pyrR genes [133]. Hence the gene expression can be understood. Digital PCR can also be coupled with several other diagnostic platforms such as the Xpert MTB/RIF assay to improve the quantitative estimation and in turn elevate reproducibility [130]. In addition to this, it can also be used for the diagnosis of extra-pulmonary TB even with low levels of DNA [134]. Even if the target genes are present in a low copy number, it amplifies and produces an accurate result. Conventional DST tests used to last for around 4 days, but with this technology, the result is obtained within 5 h. This means that a more complicated TB infection with a low copy number of genes in the sample can be detected with precision that eventually leads to reduced transmission. Hence the use of these technologies should be incorporated to navigate the problems that are deemed unsolvable [135].
8.2. Next gene sequencing
Next gene sequencing (NGS), also known as metagenomic sequencing is an advanced technique to generate billions of DNA molecules and can be used to detect antibiotic resistance in TB infection [136]. Using this technique, the genes responsible for drug resistance such as rpoB, embB, pncA, rpsA, gyrA, gyrB, rrs, eis can be amplified. This method is highly sensitive for detecting mutations associated with resistance to isoniazid, rifampicin, streptomycin, ofloxacin, levofloxacin, and moxifloxacin [137].The overall process can be categorized into several stages such as sample preparation, construction of DNA library, and sequencing followed by data interpretation. Initially, a sample from the patient is collected and a nucleic acid extraction kit is used to extract the nucleic acid. NGS uses a flow cell with several small microbeads to conduct the sequencing [138]. The extracted nucleic acid is ligated with an adapter sequence complementary to several oligonucleotides present on the microbeads. The adaptor sequence will bind to the oligonucleotides on the flow cell. In order to amplify the extracted DNA primers dNTPs, DNA polymerase is added to the reaction mixture. Hence a double-stranded DNA is formed due to amplification. The amplified DNA sequence is converted to a ssDNA through denaturation and circularization. Rolling circle amplification can be used to generate DNA nanoballs to identify the reads (long or short) and to identify single nucleotide polymorphism [139,140]. Hence, NGS is an attractive option to characterize drug resistance to TB due to its high sensitivity. It can also provide detailed information to identify multiple genes simultaneously. As DNA sequence is multiplied numerous times the assessment generates accurate data and can detect the probability of occurrence of rare mutations, unlike the conventional molecular techniques. Despite the advantages, it is still unavailable in low-income countries which are a great hindrance to accurate detection. Recent advances in NGS such as massive parallel gene sequencing techniques have reduced the cost and time. Also, the availability of these platforms in developing countries can reduce the occurrence of drug resistance [141].
8.3. MicroRNA detection (miRNA) for TB detection
MicroRNAs are non-coding single-stranded RNA molecules that are highly conserved and have a length of 18–25 nucleotides [142]. They play an important role in cellular differentiation, also for initiating innate and adaptive immunity. Thus, can serve as a biomarker for diagnostic purpose [143]. Gene expression data in macrophages and NK cells suggested the production of these small molecules of RNA for guiding the immune response by either activating or promoting the function of macrophages, dendritic cells, T cell function, and differentiation [144,145]. Meanwhile, these miRNAs have an invaluable role in regulating autophagy and apoptosis induced by Mtb infection by upregulating the expression of miR-33 and miR-33* which in turn target key autophagy effectors such as ATG5, LC3B, LAMP, miR-155, miR-223 and miR-17-92 cluster. Another study revealed that cytokine and chemokine production is also dependent on miRNA-regulated pathways. For example, miR-223 promotes the differentiation and proliferation of the neutrophils during mycobacterial infection [146]. Thus, the distinct miRNA profiles in TB suggest the regulatory function of miRNA in orchestrating protective immune responses. Pattnaik et al., critically reviewed the role of miRNAs in pathogenesis. Understanding the differential expression of various mi-RNA allows in accurate diagnosis of latent and active TB infection. For instance, let-7e-5p, let-7d-5p, miR-450a-5p, miR-140-5p were up regulated in latent TB infection and miR-1246, miR-2110, miR-370-3p, miR-28-3p, and miR-193b-5p were up regulated in active TB infection [147,122].
There are several advantages of using miRNA as a biomarker as they are not easily degraded by RNases, and hence can be used for early diagnosis preventing the community transmission of TB. Signature miRNA molecules such as miR-196b and miR-376c miRNAs act as potential diagnostic markers for TB. Differential gene expression suggested that miRNA expression can modulate the expression of target genes and is crucial for studying disease pathogenesis. Simultaneously RNA expression analysis showed the downregulation of 29 miRNAs, miR-1, miR-155, miR-31, miR-146a, miR-10a, miR-125b, and miR-150, in paediatric patients with the increasing incidence of drug-resistant. Hence the expression level of the miRNAs can be used as a suitable diagnostic biomarker as well as to comprehend the process of gene regulation in drug resistance. However advance studies are needed to explore the role of miRNA in drug resistance. An integrative approach (miRNA and proteome) is needed to develop a diagnostic approach to quantify differential expression correlated with TB [148].
8.4. Whole genome sequencing
Whole-genome sequencing (WGS) involves several steps such as a collection of a sputum sample from patients followed by its culture on specialized media such as Lowenstein–Jensen or Mycobacteria Growth Indicator Tube [93,149]. The next step involves DNA isolation from the patient sample and creating a DNA library by cloning the targeted gene into a suitable vector-like Bacterial Artificial. This is followed by sequencing using a suitable platform such as Illumina for accurate sequencing [150]. After the key steps are accomplished, the data obtained is analyzed using bioinformatics-based approaches [151]. Using WGS, single nucleotide polymorphisms can be easily detected including deletion or insertion. Based on the variants detected, several different tasks can be achieved such as prediction of drug resistance, stain typing, etc. [152,153]. WGS uses mycobacterial interspersed repeats or a variable number of tandem repeats to identify their transmission potential as the data can be applied at several levels of epidemiological complexity. This approach is highly specific as well as sensitive and the results can be obtained quickly hence suitable for endemic areas.The implementation of whole-genome sequencing techniques in clinical labs requires strategic planning with proper operating procedures as well as data interpretation. Scale-up of WGS needs adequate infrastructure such as sample preparation, PCR power supply, and other favorable conditions. Hence a lot of efforts are needed to validate and standardize WGS approaches in high burden countries.
Currently,the most accessible platforms used for TB diagnostics are Roche 454 Illumina HiSeq/MiSeq, Life Technologies SOLiD, and Ion Torrent [154]. These platforms were introduced recently and only require 10 h to get the complete sequence. Illumina HiSeq/MiSeq is the cheapest of all and the cost of sequencing is $0.02 per million bases which makes it the most preferred one [155]. The Ion torrent was introduced in 2010 and is the most advanced gene sequencing approach. It is based on monitoring the change in pH during DNA polymerization and is coupled with a silicon detector [156]. It is relatively fast and is only applicable to smaller data sets. The average reading capacity of this approach range between 50 and 250 bp and utilizes a reversible sequencing chemistry approach. WGS often targets the sequence of a particular gene and its constitutional variants and the coverage may be expanded to facilitate more delicate gene assessments [155].
9. Nanomaterial for TB diagnostics
For better detection of Mtb and in order to overcome the limitations of current diagnostic methods such as high cost, insufficient resources and instruments, there is requirement of a better detection system. Nanotechnology based diagnostic approaches would provide rapid and efficient point-of-care diagnostics leading to effective treatment and elimination of TB [157,158]. Since the last decade, significant development has been seen in nanodiagnostics of TB, due to the unique features of nanoparticles for TB biomarkers detection [[159], [160]].
Nanoparticles are minute particles that are less than 100 nm in diameter and have unique properties unlike other atoms and materials [161,162]. Some of the most exceptional properties are a large surface-to-volume ratio (gold nanoparticle), high thermal and electrical conductivity, potent catalytic property on the surface, Surface-Enhanced Raman Scattering (SERS), and enhanced photoluminescence. All these characteristics when combined with biology aided enhanced drug discovery and therapy especially the development of novel diagnostics [88,163]. Nanomaterial can be exploited for the diagnosis of infectious diseases both bacterial and viral which can spread out rapidly with a high mortality rate [164]. With the advent of nanotechnology, ferrofluid and nanoparticles coated with ceramics can aid in pathogen quantification within a short span of time (20 min). Interestingly this unprecedented advancement of nano-technology demonstrates its advantage in the field of diagnosis and can be incorporated in a wide range of analytical tools for developing bioassays.
Nanoparticle-based diagnosis depends on the interaction of nanoparticles often termed as probes with target biomolecules such as proteins (often serving as the biomarker). Upon interaction of the nanoparticles with the desired biomolecule, it produces a quantifiable signal [165]. Some of the most promising ones are the gold nanoparticles, quantum dots, nanotubes, and nano-shells [[166], [167], [168], [169]]. Nanoparticle-based diagnostics have been widely explored due to their high sensitivity and accuracy owing to certain unique optical and magnetic properties. For example, QD (Quantum Dots) are often fluorescently labeled and have a strong absorbance and thus are most promising due to the characteristics of photostability, size-tunable emission, and single wavelength excitation [170]. Due to the distinctive property of a large surface area of volume ratio they are being exploited to develop nanodiagnostic approaches with the ability of rapid detection using a minute volume of clinical sample [171]. The last two decades have witnessed significant progress in the field of nanotechnology and have numerous potential applications in biomedicine, biotechnology, and the development of diagnostics, especially for infectious diseases such as TB [172,173].
The revolutionary diagnostics started with the development of a droplet-based system (nano-femtoliter range) which has the potential to encapsulate biological and biomedical specimens for diagnostic purposes [165]. Other developments include the fabrication of tailored gold nanoparticles that invokes specific functionalization of ligand to the targeted biomarker. For example, this approach is most commonly applied by ligating the thiol group to DNA or functionalization of a specific protein or antibody binding [174]. A recent tool using gold nanoparticles for diagnosis of TB using sputum was developed by Samadony et al. with a short turnaround time of 2 h and 11.2 ng/μl detection limit of the bacterial DNA. The assay was evaluated for its accuracy in active TB diagnosis in reference with BACTEC MGIT culture test, in addition with SSM with CXR. The assay performed better than these tests with excellent sensitivity (95%) and specificity (100%) [175].
The latest technology in the field of nanomedicine is the advancement of microfluidic or lab on a chip system [176]. The device is fabricated in such a way that it incorporates a series of DNA analysis systems on a silicon and glass base. Other components incorporated in the device include detectors for fluorescence, compartments for electrophoresis, heaters, and temperature sensors [177]. The integration of lithography and semiconductor has paved the way for nanoscale cantilevers that can detect DNA or protein [39]. In countries with a heavy TB burden, nanocantilevers can be used which absorb TB antigens on their surface and quantifies the piezoresistive change based on the principle of nanomechanical deflection. For example, the BioMEMS (bio micro electromechanical system) is a micro diagnostic kit that can estimate the interaction between TB antigen and antibodies which are immobilized on the surface of the microcantilever in picogram concentration [178]. Thus the improvement in the field of nano-diagnostics has transformed the conventional method of diagnosis and made it affordable, easily accessible and rapid. The following section will explain all of the principles in detail.
9.1. Quantum dots-based detection system
Quantum dots, also termed as semiconductor-based nanocrystals, are widely used for the development of diagnostic platforms due to their broad spectra of absorption, narrow emission spectra, and slow decay rates [167,179,180]. They possess the unique ability to identify multiple targets simultaneously from a patient's sample in a clinical setting [167]. Hence, they are most widely sought over conventional fluorescence-based methods. They are nowadays finding huge applications in detecting TB infection for which a hybrid detection system is used i.e., a combination of magnetic beads and quantum dots. These in conjugation to molecular probes specific to the 23S RNA gene are used precisely. A second molecular probe specific to the IS900 conserved sequence in Mtb can be used as well, followed by treatment with sulfurous acid chromium quantum dots. Once the molecular probes combine with the specific target sequences, a sandwich-like structure is obtained which further emits red fluorescence when irradiated with UV light (observable to the naked eye) [172]. These hybrid detection systems are highly flexible and can be easily upgraded for detection purposes [[181], [182], [183]]. They can also detect unamplified DNA from suspected patients with Mtb infection. Nowadays quantum dots are coupled with CdSeO3 streptavidin and molecular probes specific to a species. This is followed by the technique of sandwich hybridization (biotinylated probes) to detect a specific DNA hence they can be used to detect surface antigens of Mtb. However, the detection limit is only 104 cells/ml of the sample [184]. Yang et al. identified a novel method based on a sandwich complex where magnetic microspheres are coupled with quantum dots and conjugated with antibodies and phage display derived peptides. The quantum dots provide a fluorescent signal whereas the magnetic microspheres allow magnetic separation. For Mtb detection peptide ligand H8 derived from the phage display library is used. The combinations of magnetic microspheres-polyclonal antibody + quantum dots-H8 and magnetic microspheres-H8+ quantum dots-H8 give a strong signal of 103 colony-forming units/mL H37Rv. This method displays enhanced detection limit and specificity of Mtb from the sputum samples [185]. Recently, a quantum dot based nanobeacon along with multicomponent nucleic acid enzyme system (QD-NB MNAzyme) was developed as a colorimetric TB detection system, providing good sensitivity and specificity. The assay was highly reproducible, convenient, rapid and showed good accuracy. LOD of the assay was as low as 2 copies/μl). In the study, the assay was successful in TB detection from over 36 clinical patients [186]. In another study, hydrophilic CsPbBr3 quantum dots were functionalized using carboxyl groups and used as a biosensor for DNA detection of Mtb. The study showed low LOD of this biosensing system (51.9 pM) and was also efficient in the detection of drug resistant strains [187].
9.2. Magnetic nanoparticles based diagnosis
The recent advancement in nanomedicine and nano-diagnostics has gained huge popularity and finds multiple applications in biotechnology, diagnostics, and tissue engineering due to their optical properties [188,189]. Magnetic nanoparticles are small-scale particles that can be easily identified and have been extensively used to detect Mtb in an unprocessed clinical sample. Iron oxides, γ-Fe2O3 (maghemite), Fe3O4 (magnetite), and other ferrites are used for the large surface area to volume ratio and the biocompatibility makes them ideal for diagnostic application [190]. Leon et al. employed magnetic nanoparticles and developed a sandwich immunoassay to detect Mtb protein antigens. The antibodies of the secretory proteins like CFP10, ESAT6, and Ag85B were elicited in animal models. These were immobilized on amine-silanized nanoparticles (MNP@Si). The functionalized MNP@Si@ab was tested in a colorimetric sandwich enzyme-linked immunosorbent assay (sELISA-MNP@Si@ab) to identify specific antigens in sputum samples. This method is rapid as compared to the conventional ELISA and also exhibits higher sensitivity in the clinical samples [191]. Engstrom and his co-workers developed streptavidin-tagged magnetic nanobead labeled with biotin to detect mutation in the rpoB gene of Mtb responsible for antibiotic resistance. This method is highly specific and often gives promising output within 2.5 h [192]. Nanobeads were also exploited to particularly identify Mtb antigens for enhanced TB diagnosis [193].
Other developments in the field of nano diagnostics for the detection of Mtb include the cell phone dongle platform, diagnostic magnetic resonance platform (DMR), paper-based POCT platform, and cell phone-based microscopy polarized light microscopy platform [194]. The DMR technology is based on the strong powers of microprocessors for rapid quantitative detection of Mtb [195]. The DMR system is unique due to the ability of testing unprocessed biological samples with nanoparticles in close proximity [196]. The functionalization of the DMR sensor enables the formation of soluble nanoclusters between magnetic nanoparticles and target proteins. The interaction can be recorded electrically through the NMR technique [195,197]. The need for rapid computing and wireless data processing led to the advent of cell-based dongle platforms. In this system, a small dongle can be modified in the form of an ELISA microplate [198]. Unlike the ELISA system, the amplification is carried out using enzymes and substrates and it can be operated with the power of mobile phones. Another advantageous feature is that the test results can be obtained in 15 min with a specificity ranging from 79 to 100% and a sensitivity of 92–100%. Patients prefer to opt for the cell-based platform over the conventional ELISA test due to its ultra-high sensitivity and specificity [199]. A gold nano-dropped dual-channel immuno-chromatography strip detects the antibody in the patient sample [200]. In this approach, the strips are labeled with gold nanoparticles forming detection conjugates. The conjugate is further labeled with Mtb antigen which captures the desired antibody from the patient sample. An ESEQuant reader is used to analyze the concentration [201]. However, the detection limit is only 5 ng/ml and the intensity of the signal increases further with the increased concentration of Mtb antibody [202].
Recently the unique properties of nanoparticles have been harnessed for the development of paper-based point-of-care testing (POCT) platform based on multiplexed lateral flow system in one individual strip. Usually, the POCT plat comprises a wick (an adsorbent pad), a nitrocellulose membrane in conjugation with a conjugation pad (CP), and a sample pad. The pads are labeled with different colored antibodies in conjugation with silver nanoparticles loading the CP which produce distinguishable absorption spectra. More importantly, they can function without excitation from an external source [194]. The integration of cell phone transmission polarized light microscope is both promising and revolutionary. The 3D printed accessories are conjugated with a high-resolution mobile phone camera and it could have the same field view as a laboratory microscope. This system has indeed a very high fidelity and an optical resolution which potentiates it as a user-friendly and low-cost diagnostic platform [203].
9.3. Nanofabricated devices
Nanofabricated devices show a promising approach because of their potential low cost and exhibit a promising trend in bioanalytical and several biochemical methods. The electro immunosensorsare based on the application of nanofabricated biosystems. It is a non-invasive procedure and uses a miniaturized biosystem to detect ESTAT6 antibody in the serum of patients (the most common antibody detected in case of Mtb infection) [204]. Electro immunosensors are graphene-based nanocomposite biosensors designed to detect DNA ranging in femtomolar concentration.It utilizes two different varieties of the probe which are conjugated with the genomic DNA and gold nanoparticles which are further immobilized on a SAM/ITO electrode [205]. This facilitates a high sensitivity coupled with specificity which can be used as a tool to monitor Mycobacterial strains in a clinical sample. There are several advantages associated with using gold fabricated nanodevices as it has a unique physiochemical property such as being inert and nontoxic. Thus, they are most appropriate for interdisciplinary research in a clinical setting [206,207]. The antigens are immobilized on the surface of gold nanoparticles and the antigen-antibody interaction is enhanced due to the functionalization of gold thus intensifying the immunoassay signal [208]. The first utilization of gold fabricated nanoparticles employed DNA probes (mycobacterial genomic DNA) and enabled the colorimetric detection of DNA at 526 nm [209]. Another novel electrochemical impedance was developed to screen the concentration of a pro-inflammatory cytokine IFN-γ in which the electrode was coated with ZnO. Further, the IFN-γ monoclonal antibody was coated on the ZnO modified electrode to measure the change in impedance due to the reaction between IFN-γ and IFN-γ antibodies. It is clearly evident that the sensitivity of the prepared immunosensors can be enhanced by nanofabrication, in addition to being economical, highly sensitive, and superior to the conventional assays [194].
9.4. Biosensor based nano diagnosis
Biosensors are analytical devices that can be used to deduce interaction between proteins, antibodies, receptor-enzymes, whole cells, tissues, and an isolated enzyme into an optical or thermal image signal which can be quantified for further analysis. They offer a large number of technical advantages such as being user-friendly and can be connected to advanced instrumentation [210]. Optical biosensors employ two principles to detect mycobacterial DNA in a patient sample, the surface plasmon resonance, and the surface-enhanced Raman scattering. Surface plasmon-based diagnostics involve the visualization of mycobacterial specific antigens (CFP 10) isolated from tissue samples by binding to nanoparticles (gold) immobilized on the surface of the optical sensor surface [211]. In this method, anti-CFP10 is confined on the surface of an immunosensor cheaply integrated with an SPR-based optical immunosensor system. A linear relation is obtained between the SPR and the concentration of CFP10 most commonly used as a TB marker [139,212].This technique is relatively advantageous, with low sample requirement, high reusability, and no pre-treatment is needed. Also, it is highly disposable and cost-effective and in addition to the above advantages, it integrates the utility of light sources, photodetectors, and electronic equipment for a promising output. It can detect up to 3 μl of DNA sample, hence superior to other approaches, and can be applied for early diagnosis of TB. Ma et al. constructed a silicon nanowire field-effect transistor (SiNW-FET) biosensor for rapid detection of Mtb. The functionalized biosensor displays a limit of detection of 0.01 pg/ml and the test can be performed within 30 s. The sputum sample can be analyzed for the presence of Mtb within 2–3 min [213].The SiNW-FET biosensors are too sensitive and environmental factors like light, temperature and pH interfere in the assay performance. Therefore, Xie et al. constructed a self-contained microfluidic nano-detection system comprising SiNW-FET biosensors for bio-detection and analysis. The samples are loaded into the system via automatic injection mode. The system bio-detection and analysis were tested by detecting Mtb. The limit of detection was found to be 1 pg/ml. The constructed system provides compactness, portability along with good stability and robustness (Fig. 7 ) [214].
Fig. 7.
Detection of tuberculosis infection by Biosensors.Mtb sample is taken from the tuberculosis patient. Silicon nanowire present on the biosensor, coated with antibodies of Mtb, detect the respective antigens present in the patient's sample. The signal is further amplified and analyzed. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
With the recent advancement, electrically conductive hybridization probes have been developed such as the Piezoresistive-based nano diagnosis which is based on the change in the electrical resistivity of the semiconductor when mechanical stress is applied. In this diagnostic technique, a nano cantilever device is incorporated to detect Mtb in clinical samples. Antibodies specific to the Mtb antigens are designed and immobilized on the upper surface of the cantilever. It facilitates the detection of TB based on the capacitance and resonant frequency attached to biosensors. In this technique, Quartz Crystal Microbalance (QCM) is used to detect Mtb. Anti Mtb antibodies are immobilized on the surface of the QCM sensing surface. The sensing surface traps the pathogen from the clinical sample and the frequency shift because of the loading of bacterial mass [210,215]. Interestingly, this can be used to detect the immunological biomarkers of TB such as the MPT64 which is indeed a step forward in TB diagnostics due to its easy detection by the immune system [216].
Conventional methods of MPT64 detection are difficult owing to laboratory and environmental constraints. In order to resolve the issue, electrochemical aptamers are being designed with a high-performance coefficient and a suitable detection strategy [217,218]. The fullerene C60 is an extremely sensitive electrochemical immunosensor developed based on the redox activity of this carbon compound. Several other nanocomposites such as MOF (metal-organic framework) have been developed due to high porosity, and large surface to volume ratio and are labeled with antigen MPT64 aptamers (single-stranded oligonucleotides with specificity for target protein). Hence this can be used as a suitable strategy for the detection of Mtb antigen [101]. Despite being rapid and efficient several other factors such as density, viscosity, and electrical conductivity hinder its usage [211,213].
9.5. Nucleic acid hybridization nano diagnosis
The development of the nucleic acid hybridization technique uses both modified and unmodified nanoprobe to detect mycobacterial DNA. In unmodified nanoprobe-based detection, the gold nanoparticles can be utilized to detect the Mtb by using specific nanoprobes such as (Au, Ag, and Zr) capable of isolating the bacteria. For example, for detecting 16s rRNA, specific oligonucleotides can be designed complementary to the gene of interest. It is extremely advantageous as it can detect unamplified DNA from clinical samples. Additionally, they are sensitive which makes them the most preferred choice for diagnosis [168].
The modified technique, on the other hand, utilizes DNA probes specific to the gene of interest which are immobilized on nanostructured metal oxides such as zirconia (ZrO2). ZrO2 is an inorganic metal oxide with certain excellent features such as high thermal stability, chemically inert, nontoxicity, and an exceptional affinity for groups containing oxygen [219]. The DNA ZrO2/Au complex is highly stable for a longer duration (over 4 months) with a detection limit of 100 pM of genomic DNA. Nowadays, this method is coupled with LAMP to enhance efficiency. The results can be obtained in less than 1 h. Also, this method is cost-effective and doesn't require extra reagents. Hence, LAMP coupled with nanoparticle (AuNP) is considered a safe and suitable alternative to traditional approaches [220,221].
10. CRISPR cas for TB diagnosis
The rapid and urgent need to develop a suitable diagnostic method that would detect Mtb in patient samples gave rise to the popularity of the CRISPR Cas system. CRISPR is a short interspersed repeat of a palindromic sequence of DNA that encodes a protein called the Cas proteins. CRISPR Cas is the component of the bacterial adaptive immune system [[222], [223], [224]]. The application of CRISPR in the diagnosis of Mtb was incorporated very recently [225,226]. Different varieties of Cas protein share a common guide RNA probe, enzyme domain, and nucleotide activators. For example, the Cas12 protein recognizes the double-stranded DNA as an activator and often cleaves single-stranded DNA. Cas13 recognizes ss-RNA, whereas Cas 14 cleaves act on ss-DNA [225]. On the other hand, the protospacer adjacent motif (PAM) is a 2 to 6 bp nucleotide sequence responsible for the specificity of the Cas proteins. Hence, they can detect a minute quantity of nucleic acid in a patient's sample [223,227]. A highly sensitive fluorescence-based detection method was proposed by Xu et al. The method combined CRISPR Cas 12a with recombinase polymerase amplification (RPA) for specific and sensitive identification of Mtb DNA. When compared with conventional culture method, the assay showed excellent sensitivity (99.29%) and specificity (100%) [228].
CRISPR showed great diagnostic efficiency in various specimens collected from sputum and cerebrospinal fluid. CRISPR Cas has proved to be an ultrasensitive promising approach for both pulmonary and extrapulmonary TB. Firstly the complexity associated with this technique is extremely low and can be integrated with the current approaches to increasing efficiency. Due to the ultrasensitive nature of CRISPR, it can detect multiple sites responsible for drug response simultaneously, mainly rifampicin and isoniazid. It can also provide genetic insight into drug resistance for both first and second-line drugs. To conclude it has proved to be better over culture and Xpert MTB/RIF not only in terms of sensitivity but also in terms of turnover period [119].
11. Mass spectrometry/matrix associated laser desorption time of flight (MS/MALDI TOF)
TB in humans is caused by a diverse array of organism from different genetic lineages, known as Mycobacterium tuberculosis complex (MTBC), some of which are poorly understood and investigated. Despite their close genetic association with each other they often differ in epidemiology, geographical prevalence, pathogenicity, host preferences, immunogenicity, and the degree of response to antibiotic treatment. Hence it is essential to characterize each mycobacterial species at the proteome level on the basis of protein expression at different stages of the disease [229,230]. The conventional methods are based on phenotypic, biochemical, and molecular characterization but fail to distinguish MTBC species thus affecting the line of treatment and diagnosis. With the recent advances, discrimination of mycobacterial strains at the species level can be achieved by mass spectrometry which is of paramount clinical relevance to classify MTBC complex rapidly. It is based on protein profiling to differentiate clinical relevant mycobacterial entities with high accuracy [231]. Hettick et al. subtyped six mycobacterial species based on protein profiles and mass/charge ratio of small molecules such as lipids and polypeptides which could serve as biomarker [232]. Robinne et al., recently published a first report where MALDI TOF-MS method is used for differentiating between MTBC with specificity of 88% that could be easily employed in laboratory settings [233]. Mass spectrometry combined with liquid chromatography (LC-MS) was applied by Bajaj et al., for identification of MTBC. This approach is based on protein profiling used as markers for differentiation. This assay is faster and displayed accuracy of 95% with successful identification of closely related mycobacterial strains [234]. Application of High-resolution mass spectrometry for detection of TB by the analysis of Exhaled Breath Particles was explored by Chen et al. Identification of specific lipids in peripheral lung fluid samples is the main objective of the study. This study promotes exploring machine learning and mass spectrometry for accurate detection of positive TB patients [235]. MS is a robust technique involved in characterizing antigenic epitope in infectious agents with high accuracy. Although MS is relatively accurate and inexpensive there are some technical challenges associated. Firstly clinical isolates will provide a high array of proteome depending on the stage of infection, replication rate and rate of protein expression that are difficult to analyze. The direct quantification of mycobacterial protein will be challenging in the background of complex host proteome, hence enrichment of bacterial proteome is recommended before MS [236].
12. Conclusion
TB diagnostics has evolved from basic microbiological observations to advanced immunological and molecular techniques. The global TB report lists diagnostic tests such as Xpert MTB/RIF, Line probe assays, Truenat MTB, QuantiFERON-TB Gold Plus (QFT-Plus), culture-based phenotypic DST, Light and light-emitting diode microscopy for detection of TB. However, these tests faces drawback in desirable sensitivity, are time consuming, requires extensive laboratory infrastructure and trained technical personnel. Thus, exploring diagnostic platforms that are both sensitive and specific may result in improved clinical outcomes and reduced transmission of the disease.
Recent progress in comprehending the molecular basis of Mtb pathogenesis has led to the advancement of tests that are rapid and highly specific. Methods like ELISA with sensitivity and specificity of 87% and 93% respectively and molecular drug susceptibility testing that specifically detect drug resistant TB facilitates accurate diagnosis. Gene drive MTB/RIF ID KIT based on real-time PCR technology is user-friendly with 93.5% sensitivity and are affordable in resource limited settings. The endorsement of these assays will help in reducing and analysing the impact of multidrug-resistant TB infection (gene mutations) allowing doctors to design better TB diagnostics.
In the future, it can be assumed that the implementation of advanced TB diagnostics such as the digital PCR, CRISPR-Cas and nano diagnostics in combination with the conventional methods would enable a timely diagnosis and impactful treatment regimen for TB patients. A QIAreach® QuantiFERON-TB assay developed in Germany integrates lateral flow immunoassay with nanotechnology to measure the level of IFN-γ in plasma. This method is capable of providing rapid and portable platform for TB diagnosis using single use cartridges. The sensitivity and specificity for the assay were 93.7% and 97.7% respectively [237]. Progression in radiological modalities in combination with artificial intelligence, allows computer aided detection (CAD) for digital chest radiography. The CAD4TB, Delft Imaging, Netherlands; Lunit INSIGHT CXR, Lunit, South Korea; qXR, qure.ai, India; DxTB, Deeptek, USA are some of the CAD that are under evaluation by WHO [27,238]. Radiology or optical imaging combined with chemical probes offers real-time quantification of bacteria providing a substitute to culture detection [114,239]. Another method for screening the population for TB is electronic nose (eNose). The eNose is a diagnostic method that uses the pattern of volatile organic compounds produced by either Mtb or the host metabolism due to TB infection. The method allows screening population without the exposure of radiation with significant sensitivity (95%) and specificity (82%) [126].
Pillar 1 of End TB strategy is early diagnosis of TB, including universal drug susceptibility testing and systematic screening of contacts and high-risk groups. In the absence of gold standard test for Mtb detection, the End TB strategy target i.e., 80% reduction in the TB incidence rate and 90% reduction in the annual number of TB deaths by 2030, remains unachievable. The above explained approaches provide a way forward in TB diagnosis. Extensive research in this field will allow for identification of rapid, sensitive and specific method which requires minimal lab personnel, accessible in resource limited settings, accurately diagnose different group of individual including children and immuno-compromised patients.
Funding
The work is supported by the Department of Science and Technology-Science and Engineering Research Board (DST SERB) for Startup Research grant (SRG/2022/001567). Summaya Perveen (UGC-JRF) and Anjali Negi (UGC-JRF) are highly thankful to University Grants Commission (UGC), New Delhi for fellowship assistance.
Authorship contribution statement
SM reviewed the literature and compiled the first draft of the manuscript. SP and AN contributed to reviewing and editing. RS contributed to the conception of the manuscript and finalized the sections of the manuscript. All authors have read and approved the final version of the manuscript.
Declaration of competing interest
Authors declares no conflict of interest.
References
- 1.Global Tb report WHO . World Health Organization; 2022. Global TB report. 2022. [Google Scholar]
- 2.Jang J.G., Chung J.H. Diagnosis and treatment of multidrug-resistant tuberculosis. Yeungnam Univ J Med. 2020;37:277–285. doi: 10.12701/yujm.2020.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perveen S., Kumari D., Singh K., Sharma R. Tuberculosis drug discovery: progression and future interventions in the wake of emerging resistance. Eur J Med Chem. 2022;229 doi: 10.1016/j.ejmech.2021.114066. [DOI] [PubMed] [Google Scholar]
- 4.Nurwidya F., Handayani D., Burhan E., Yunus F. Molecular diagnosis of tuberculosis. Chonnam Med J. 2018;54:1. doi: 10.4068/cmj.2018.54.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev. 2003;16:463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gengenbacher M., Kaufmann S.H.E. Mycobacterium tuberculosis : success through dormancy. FEMS Microbiol Rev. 2012;36:514–532. doi: 10.1111/j.1574-6976.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuñiga J., Torres-García D., Santos-Mendoza T., Rodriguez-Reyna T.S., Granados J., Yunis E.J. Cellular and humoral mechanisms involved in the control of tuberculosis. Clin Dev Immunol. 2012;2012:1–18. doi: 10.1155/2012/193923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perveen S., Sharma R. Screening approaches and therapeutic targets: the two driving wheels of tuberculosis drug discovery. Biochem Pharmacol. 2022;197 doi: 10.1016/j.bcp.2021.114906. [DOI] [PubMed] [Google Scholar]
- 9.Pasula R., Downing J.F., Wright J.R., Kachel D.L., Davis T.E., Martin W.J. Surfactant protein A (SP-A) mediates attachment of Mycobacterium tuberculosis to murine alveolar macrophages. Am J Respir Cell Mol Biol. 1997;17:209–217. doi: 10.1165/ajrcmb.17.2.2469. [DOI] [PubMed] [Google Scholar]
- 10.Flannagan R.S., Cosío G., Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 11.Fang F.C. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 12.Cambi A., Gijzen K., de Vries I.J.M., Torensma R., Joosten B., Adema G.J., et al. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol. 2003;33:532–538. doi: 10.1002/immu.200310029. [DOI] [PubMed] [Google Scholar]
- 13.Delogu G., Sali M., Fadda G. The biology of MYCOBACTERIUM tuberculosis infection. Mediterr J Hematol Infect Dis. 2013;5 doi: 10.4084/mjhid.2013.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan H., Yan B.-S., Rojas M., Shebzukhov Y.V., Zhou H., Kobzik L., et al. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2006 doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parbhoo T., Sampson S.L., Mouton J.M. Recent developments in the application of flow cytometry to advance our understanding of Mycobacterium tuberculosis physiology and pathogenesis. Cytometry. 2020;97:683–693. doi: 10.1002/cyto.a.24030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pai M., Behr M.A., Dowdy D., Dheda K., Divangahi M., Boehme C.C., et al. Tuberculosis. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 17.Davis J.L., Cattamanchi A., Cuevas L.E., Hopewell P.C., Steingart K.R. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:147–154. doi: 10.1016/S1473-3099(12)70232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singhal R., Myneedu V.P. Microscopy as a diagnostic tool in pulmonary tuberculosis. Int J Mycobacteriol. 2015;4:1–6. doi: 10.1016/j.ijmyco.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Pande T., Pai M., Khan F.A., Denkinger C.M. Use of chest radiography in the 22 highest tuberculosis burden countries. Eur Respir J. 2015;46:1816–1819. doi: 10.1183/13993003.01064-2015. [DOI] [PubMed] [Google Scholar]
- 20.Lee J.E., Kim H.-J., Lee S.W. The clinical utility of tuberculin skin test and interferon-γ release assay in the diagnosis of active tuberculosis among young adults: a prospective observational study. BMC Infect Dis. 2011;11:96. doi: 10.1186/1471-2334-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takasaki J., Manabe T., Morino E., Muto Y., Hashimoto M., Iikura M., et al. Sensitivity and specificity of QuantiFERON-TB gold plus compared with QuantiFERON-TB gold in-tube and T-SPOT.TB on active tuberculosis in Japan. J Infect Chemother. 2018;24:188–192. doi: 10.1016/j.jiac.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Tiwari D., Tiwari R.P., Chandra R., Bisen P.S., Haque S. Efficient ELISA for diagnosis of active tuberculosis employing a cocktail of secretory proteins of Mycobacterium tuberculosis. Folia Biol (Praha) 2014:60. doi: 10.14712/fb2014060010010. [DOI] [PubMed] [Google Scholar]
- 23.Acharya B., Acharya A., Gautam S., Ghimire S.P., Mishra G., Parajuli N., et al. Advances in diagnosis of Tuberculosis: an update into molecular diagnosis of Mycobacterium tuberculosis. Mol Biol Rep. 2020;47:4065–4075. doi: 10.1007/s11033-020-05413-7. [DOI] [PubMed] [Google Scholar]
- 24.Nathavitharana R.R., Cudahy P.G.T., Schumacher S.G., Steingart K.R., Pai M., Denkinger C.M. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2017;49 doi: 10.1183/13993003.01075-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boehme C.C., Nabeta P., Henostroza G., Raqib R., Rahim Z., Gerhardt M., et al. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J Clin Microbiol. 2007;45:1936–1940. doi: 10.1128/JCM.02352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey B.D., Poudel A., Yoda T., Tamaru A., Oda N., Fukushima Y., et al. Development of an in-house loop-mediated isothermal amplification (LAMP) assay for detection of Mycobacterium tuberculosis and evaluation in sputum samples of Nepalese patients. J Med Microbiol. 2008;57:439–443. doi: 10.1099/jmm.0.47499-0. [DOI] [PubMed] [Google Scholar]
- 27.Global TB report . World Health Organization; 2020. Global TB report. 2020. [Google Scholar]
- 28.Chakaya J., Khan M., Ntoumi F., Aklillu E., Fatima R., Mwaba P., et al. Global tuberculosis report 2020 – reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021;113 doi: 10.1016/j.ijid.2021.02.107. S7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Global TB report . World Health Organization; 2021. Global TB report (2021) [Google Scholar]
- 30.Asmar S., Drancourt M. Rapid culture-based diagnosis of pulmonary tuberculosis in developed and developing countries. Front Microbiol. 2015;6 doi: 10.3389/fmicb.2015.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habtamu M., van den Boogaard J., Ndaro A., Buretta R., Irongo C.F., Lega D.A., et al. Light-emitting diode with various sputum smear preparation techniques to diagnose tuberculosis. Int J Tubercul Lung Dis. 2012;16:402–407. doi: 10.5588/ijtld.10.0762. [DOI] [PubMed] [Google Scholar]
- 32.Martinez L., Cords O., Horsburgh C.R., Andrews J.R., Acuna-Villaorduna C., Desai Ahuja S., et al. The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet. 2020;395:973–984. doi: 10.1016/S0140-6736(20)30166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drancourt M., Carrieri P., Gévaudan M.-J., Raoult D. Blood agar and Mycobacterium tuberculosis : the End of a dogma. J Clin Microbiol. 2003;41:1710–1711. doi: 10.1128/JCM.41.4.1710-1711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bobadilla-del-Valle M., Ponce-de-León A., Kato-Maeda M., Hernández-Cruz A., Calva-Mercado J.J., Chávez-Mazari B., et al. Comparison of sodium carbonate, cetyl-pyridinium chloride, and sodium borate for preservation of sputa for culture of Mycobacterium tuberculosis. J Clin Microbiol. 2003;41:4487–4488. doi: 10.1128/JCM.41.9.4487-4488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardini M., Varaine F., Iona E., Arzumanian E., Checchi F., Oggioni M.R., et al. Cetyl-pyridinium chloride is useful for isolation of Mycobacterium tuberculosis from sputa subjected to long-term storage. J Clin Microbiol. 2005;43:442–444. doi: 10.1128/JCM.43.1.442-444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabic E., Cabic A.G., Esposo S.M., Dizon F., Quinones G.J., Guia A. Alternative method for histopathological detection of Mycobacterium tuberculosis and Mycobacterium leprae using a modified acid-fast technique. PJP. 2018;3:29–33. doi: 10.21141/PJP.2018.007. [DOI] [Google Scholar]
- 37.Chen P., Shi M., Feng G.-D., Liu J.-Y., Wang B.-J., Shi X.-D., et al. A highly efficient ziehl-neelsen stain: identifying de novo intracellular Mycobacterium tuberculosis and improving detection of extracellular M. tuberculosis in cerebrospinal fluid. J Clin Microbiol. 2012;50 doi: 10.1128/JCM.05756-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weldu Y., Asrat D., Woldeamanuel Y., Hailesilasie A. Comparative evaluation of a two-reagent cold stain method with Ziehl-Nelseen method for pulmonary tuberculosis diagnosis. BMC Res Notes. 2013;6:323. doi: 10.1186/1756-0500-6-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta A.K., Singh A., Singh S. In: Diagnosis of tuberculosis: nanodiagnostics approaches. Saxena S.K., Khurana S.M.P., editors. Springer Singapore; NanoBioMedicine, Singapore: 2020. pp. 261–283. [DOI] [Google Scholar]
- 40.Bhalla A.S., Goyal A., Guleria R., Gupta A.K. Chest tuberculosis: radiological review and imaging recommendations. Indian J Radiol Imag. 2015;25:213–225. doi: 10.4103/0971-3026.161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayaz M., Shaukat F., Raja G. Ensemble learning based automatic detection of tuberculosis in chest X-ray images using hybrid feature descriptors. Phys Eng Sci Med. 2021;44:183–194. doi: 10.1007/s13246-020-00966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heussel C.P., Kauczor H.U., Heussel G., Fischer B., Mildenberger P., Thelen M. Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. AJR Am J Roentgenol. 1997;169:1347–1353. doi: 10.2214/ajr.169.5.9353456. [DOI] [PubMed] [Google Scholar]
- 43.Ito I., Ishida T., Togashi K., Niimi A., Koyama H., Ishimori T., et al. Differentiation of bacterial and non-bacterial community-acquired pneumonia by thin-section computed tomography. Eur J Radiol. 2009;72:388–395. doi: 10.1016/j.ejrad.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raniga S., Parikh N., Arora A., Vaghani M., Vora P.A., Vaidya V. Is HRCT reliable in determining disease activity in pulmonary tuberculosis? Indian J Radiol Imag. 2006;16:221–228. doi: 10.4103/0971-3026.29096. [DOI] [Google Scholar]
- 45.Busi Rizzi E., Schinina’ V., Cristofaro M., Goletti D., Palmieri F., Bevilacqua N., et al. Detection of Pulmonary tuberculosis: comparing MR imaging with HRCT. BMC Infect Dis. 2011;11:243. doi: 10.1186/1471-2334-11-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang C.-M., Hsu C.-H., Lee C.-M., Wang F.-C. Intense uptake of [F-18]-fluoro-2 deoxy-d-glucose in active pulmonary tuberculosis. Ann Nucl Med. 2003;17:407–410. doi: 10.1007/BF03006610. [DOI] [PubMed] [Google Scholar]
- 47.Harkirat S., Anand S., Indrajit I., Dash A. Pictorial essay: PET/CT in tuberculosis. Indian J Radiol Imag. 2008;18:141. doi: 10.4103/0971-3026.40299. [DOI] [Google Scholar]
- 48.Cui X., Gao L., Cao B. Management of latent tuberculosis infection in China: exploring solutions suitable for high-burden countries. Int J Infect Dis. 2020;92:S37–S40. doi: 10.1016/j.ijid.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 49.Blumberg H.M., Ernst J.D. The challenge of latent TB infection. JAMA. 2016;316:931. doi: 10.1001/jama.2016.11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Floyd K., Glaziou P., Zumla A., Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018;6:299–314. doi: 10.1016/S2213-2600(18)30057-2. [DOI] [PubMed] [Google Scholar]
- 51.Nayak S., Acharjya B. Mantoux test and its interpretation. Indian Dermatol Online J. 2012;3:2. doi: 10.4103/2229-5178.93479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zahrani K.A., Jahdali H.A., Menzies D. Does size matter? Utility of size of tuberculin reactions for the diagnosis of mycobacterial disease. Am J Respir Crit Care Med. 2000;162:1419–1422. doi: 10.1164/ajrccm.162.4.9912048. [DOI] [PubMed] [Google Scholar]
- 53.Yang H., Kruh-Garcia N.A., Dobos K.M. Purified protein derivatives of tuberculin — past, present, and future. FEMS Immunol Med Microbiol. 2012;66:273–280. doi: 10.1111/j.1574-695X.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kampmann B., Whittaker E., Williams A., Walters S., Gordon A., Martinez-Alier N., et al. Interferon- release assays do not identify more children with active tuberculosis than the tuberculin skin test. Eur Respir J. 2009;33:1374–1382. doi: 10.1183/09031936.00153408. [DOI] [PubMed] [Google Scholar]
- 55.Lalvani A., Pareek M. Interferon gamma release assays: principles and practice. Enferm Infecc Microbiol Clín. 2010;28:245–252. doi: 10.1016/j.eimc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Pai M., Denkinger C.M., Kik S.V., Rangaka M.X., Zwerling A., Oxlade O., et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sester M., Sotgiu G., Lange C., Giehl C., Girardi E., Migliori G.B., et al. Interferon- release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37:100–111. doi: 10.1183/09031936.00114810. [DOI] [PubMed] [Google Scholar]
- 58.Latorre I., Mínguez S., Carrascosa J.-M., Naves J., Villar-Hernández R., Muriel B., et al. Immune-mediated inflammatory diseases differently affect IGRAs' accuracy for latent tuberculosis infection diagnosis in clinical practice. PLoS One. 2017;12 doi: 10.1371/journal.pone.0189202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aichelburg M.C., Rieger A., Breitenecker F., Pfistershammer K., Tittes J., Eltz S., et al. Detection and prediction of active tuberculosis disease by a whole‐blood interferon‐γ release assay in HIV‐1–Infected individuals. Clin Infect Dis. 2009;48:954–962. doi: 10.1086/597351. [DOI] [PubMed] [Google Scholar]
- 60.Veldsman C., Kock M.M., Rossouw T., Nieuwoudt M., Maeurer M., Hoosen A.A., et al. QuantiFERON-TB GOLD ELISA assay for the detection of Mycobacterium tuberculosis -specific antigens in blood specimens of HIV-positive patients in a high-burden country. FEMS Immunol Med Microbiol. 2009;57:269–273. doi: 10.1111/j.1574-695X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 61.Andrews J.R., Nemes E., Tameris M., Landry B.S., Mahomed H., McClain J.B., et al. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med. 2017;5:282–290. doi: 10.1016/S2213-2600(17)30060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaur R.L., Pai M., Banaei N. Impact of blood volume, Tube shaking, and incubation time on reproducibility of QuantiFERON-TB gold in-tube assay. J Clin Microbiol. 2013;51:3521–3526. doi: 10.1128/JCM.01627-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katiyar S.K., Sampath A., Bihari S., Mamtani M., Kulkarni H. Use of the QuantiFERON-TB Gold In-Tube® test to monitor treatment efficacy in active pulmonary tuberculosis. Int J Tubercul Lung Dis. 2008 [PubMed] [Google Scholar]
- 64.Hossain S., Zaman K., Quaiyum A., Banu S., Husain A., Islam A., et al. Factors associated with poor knowledge among adults on tuberculosis in Bangladesh: results from a nationwide survey. J Health Popul Nutr. 2015;34:2. doi: 10.1186/s41043-015-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du F., Xie L., Zhang Y., Gao F., Zhang H., Chen W., et al. Prospective comparison of QFT-GIT and T-SPOT.TB assays for diagnosis of. Active Tuberculosis. Sci Rep. 2018;8:5882. doi: 10.1038/s41598-018-24285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma Y., Li R., Shen J., He L., Li Y., Zhang N., et al. Clinical effect of T-SPOT.TB test for the diagnosis of tuberculosis. BMC Infect Dis. 2019;19:993. doi: 10.1186/s12879-019-4597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandalakas A.M., Highsmith H.Y., Harris N.M., Pawlicka A., Kirchner H.L. T-SPOT.TB performance in routine pediatric practice in a low TB burden setting. J Pediatr Infect Dis. 2018;37:292–297. doi: 10.1097/INF.0000000000001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Attallah A., Malak C.A.A., Ismail H., El-Saggan A., Omran M., Tabll A. Rapid and simple detection of a Mycobacterium tuberculosis circulating antigen in serum using dot-ELISA for field diagnosis of pulmonary tuberculosis. J Immunoassay Immunochem. 2003;24:73–87. doi: 10.1081/IAS-120018470. [DOI] [PubMed] [Google Scholar]
- 69.Kweon O.J., Lim Y.K., Kim H.R., Kim T.-H., Lee M.-K. Evaluation of standard E TB-feron enzyme-linked immunosorbent assay for diagnosis of latent tuberculosis infection in health care workers. J Clin Microbiol. 2019;57 doi: 10.1128/JCM.01347-19. e01347-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carranza C., Pedraza-Sanchez S., de Oyarzabal-Mendez E., Torres M. Diagnosis for latent tuberculosis infection: new alternatives. Front Immunol. 2020;11:2006. doi: 10.3389/fimmu.2020.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumar S.K., Arya S., Aggarwal A., Kapoor P., Nath A., Misra R., et al. Immune responses to Mycobacterium tuberculosis membrane-associated antigens including alpha crystallin can potentially discriminate between latent infection and active tuberculosis disease. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun L., Chen Y., Yi P., Yang L., Yan Y., Zhang K., et al. Serological detection of Mycobacterium Tuberculosis complex infection in multiple hosts by One Universal ELISA. PLoS One. 2021;16 doi: 10.1371/journal.pone.0257920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iskandar A. The diagnostic value of urine lipoarabinomannan (L aM) antigen in childhood tuberculosi s. J Clin Diagn Res. 2017 doi: 10.7860/JCDR/2017/20909.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bulterys M.A., Wagner B., Redard-Jacot M., Suresh A., Pollock N.R., Moreau E., et al. Point-of-care urine LAM tests for tuberculosis diagnosis: a status update. JCM. 2019;9:111. doi: 10.3390/jcm9010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z., Tong X., Liu S., Yue J., Fan H. The value of FujiLAM in the diagnosis of tuberculosis: a systematic review and meta-analysis. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.757133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seid G., Alemu A., Tsedalu T., Dagne B. Value of urine-based lipoarabinomannan (LAM) antigen tests for diagnosing tuberculosis in children: systematic review and meta-analysis. IJID Regions. 2022;4:97–104. doi: 10.1016/j.ijregi.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mosquera-Restrepo S.F., Zuberogoïtia S., Gouxette L., Layre E., Gilleron M., Stella A., et al. A Mycobacterium tuberculosis fingerprint in human breath allows tuberculosis detection. Nat Commun. 2022;13:7751. doi: 10.1038/s41467-022-35453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thit S.S., Aung N.M., Htet Z.W., Boyd M.A., Saw H.A., Anstey N.M., et al. The clinical utility of the urine-based lateral flow lipoarabinomannan assay in HIV-infected adults in Myanmar: an observational study. BMC Med. 2017;15:145. doi: 10.1186/s12916-017-0888-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chekesa B., Gumi B., Chanyalew M., Zewude A., Ameni G. Prevalence of latent tuberculosis infection and associated risk factors in prison in East Wollega Zone of western Ethiopia. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singla N., Satyanarayana S., Sachdeva K.S., Van den Bergh R., Reid T., Tayler-Smith K., et al. Impact of introducing the line probe assay on time to treatment initiation of MDR-TB in Delhi, India. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aung W.W., Ei P.W., Nyunt W.W., Swe T.L., Lwin T., Htwe M.M., et al. Phenotypic and genotypic analysis of anti-tuberculosis drug resistance in Mycobacterium tuberculosis isolates in Myanmar. Ann Lab Med. 2015;35:494–499. doi: 10.3343/alm.2015.35.5.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Demers A.-M., Kim S., McCallum S., Eisenach K., Hughes M., Naini L., et al. Drug susceptibility patterns of Mycobacterium tuberculosis from adults with multidrug-resistant tuberculosis and implications for a household contact preventive therapy trial. BMC Infect Dis. 2021;21:205. doi: 10.1186/s12879-021-05884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Butler T.E., Lee A.J., Yang Y., Newton M.D., Kargupta R., Puttaswamy S., et al. Direct-from-sputum rapid phenotypic drug susceptibility test for mycobacteria. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cambau E., Viveiros M., Machado D., Raskine L., Ritter C., Tortoli E., et al. Revisiting susceptibility testing in MDR-TB by a standardized quantitative phenotypic assessment in a European multicentre study. J Antimicrob Chemother. 2015;70:686–696. doi: 10.1093/jac/dku438. [DOI] [PubMed] [Google Scholar]
- 85.Cirillo D.M., Miotto P., Tortoli E. In: Gagneux S., editor. vol. 1019. Springer International Publishing; Cham: 2017. Evolution of phenotypic and molecular drug susceptibility testing; pp. 221–246. (Strain variation in the Mycobacterium tuberculosis complex: its role in biology, epidemiology and control). [DOI] [Google Scholar]
- 86.Hou G., Zhang T., Kang D., Wang W., Hu X., Wang Q., et al. Efficacy of real-time polymerase chain reaction for rapid diagnosis of endobronchial tuberculosis. Int J Infect Dis. 2014;27:13–17. doi: 10.1016/j.ijid.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen T.N.A., Anton-Le Berre V., Bañuls A.-L., Nguyen T.V.A. Molecular diagnosis of drug-resistant tuberculosis; A literature review. Front Microbiol. 2019;10:794. doi: 10.3389/fmicb.2019.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S., Su R., Nie S., Sun M., Zhang J., Wu D., et al. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25:363–376. doi: 10.1016/j.jnutbio.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sethi S., Nanda R., Chakraborty T. Clinical application of volatile organic compound analysis for detecting infectious diseases. Clin Microbiol Rev. 2013;26:462–475. doi: 10.1128/CMR.00020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ribeiro F.K.C., Dettoni V. do V., Peres R.L., Vinhas S.A., Có T.R., Dietze R., et al. Evaluation of a commercial test based on ligase chain reaction for direct detection of Mycobacterium tuberculosis in respiratory specimens. Rev Soc Bras Med Trop. 2004;37:431–435. doi: 10.1590/S0037-86822004000600001. [DOI] [PubMed] [Google Scholar]
- 91.Choi Y.J., Kim H.J., Shin H.B., Nam H.S., Lee S.H., Park J.S., et al. Evaluation of peptide nucleic acid probe-based real-time PCR for detection of Mycobacterium tuberculosis complex and nontuberculous mycobacteria in respiratory specimens. Ann Lab Med. 2012;32:257–263. doi: 10.3343/alm.2012.32.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Global TB report . World Health Organization; 2016. Global TB report. 2016. [Google Scholar]
- 93.Coll F., McNerney R., Guerra-Assunção J.A., Glynn J.R., Perdigão J., Viveiros M., et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat Commun. 2014;5:4812. doi: 10.1038/ncomms5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deggim-Messmer V., Bloemberg G.V., Ritter C., Voit A., Hömke R., Keller P.M., et al. Diagnostic molecular mycobacteriology in regions with low tuberculosis endemicity: combining real-time PCR assays for detection of multiple mycobacterial pathogens with line probe assays for identification of resistance mutations. EBioMedicine. 2016;9:228–237. doi: 10.1016/j.ebiom.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lawn S.D., Nicol M.P. Xpert ® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6:1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mechal Y., Benaissa E., El mrimar N., Benlahlou Y., Bssaibis F., Zegmout A., et al. Evaluation of GeneXpert MTB/RIF system performances in the diagnosis of extrapulmonary tuberculosis. BMC Infect Dis. 2019;19:1069. doi: 10.1186/s12879-019-4687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shenai S., Armstrong D.T., Valli E., Dolinger D.L., Nakiyingi L., Dietze R., et al. Analytical and clinical evaluation of the epistem genedrive assay for detection of Mycobacterium tuberculosis. J Clin Microbiol. 2016;54:1051–1057. doi: 10.1128/JCM.02847-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Niemz A., Ferguson T.M., Boyle D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011;29:240–250. doi: 10.1016/j.tibtech.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Castan P., de Pablo A., Fernández-Romero N., Rubio J.M., Cobb B.D., Mingorance J., et al. Point-of-Care system for detection of Mycobacterium tuberculosis and rifampin resistance in sputum samples. J Clin Microbiol. 2014;52:502–507. doi: 10.1128/JCM.02209-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang P., Wang X., Shen X., Shi M., Zhu X., Yu X., et al. Use of DNA microarray chips for the rapid detection of Mycobacterium tuberculosis resistance to rifampicin and isoniazid. Exp Ther Med. 2017;13:2332–2338. doi: 10.3892/etm.2017.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen C.-Y., Weng J.-Y., Huang H.-H., Yen W.-C., Tsai Y.-H., Cheng T.C., et al. A new oligonucleotide array for the detection of multidrug and extensively drug-resistance tuberculosis. Sci Rep. 2019;9:4425. doi: 10.1038/s41598-019-39339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singpanomchai N., Akeda Y., Tomono K., Tamaru A., Santanirand P., Ratthawongjirakul P. Rapid detection of multidrug-resistant tuberculosis based on allele-specific recombinase polymerase amplification and colorimetric detection. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nilsson L.E., Hoffner S.E., Anséhn S. Rapid susceptibility testing of Mycobacterium tuberculosis by bioluminescence assay of mycobacterial ATP. Antimicrob Agents Chemother. 1988;32:1208–1212. doi: 10.1128/AAC.32.8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cooksey R.C., Crawford J.T., Jacobs W.R., Shinnick T.M. A rapid method for screening antimicrobial agents for activities against a strain of Mycobacterium tuberculosis expressing firefly luciferase. Antimicrob Agents Chemother. 1993;37:1348–1352. doi: 10.1128/AAC.37.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riska P.F., Su Y., Bardarov S., Freundlich L., Sarkis G., Hatfull G., et al. Rapid film-based determination of antibiotic susceptibilities of Mycobacterium tuberculosis strains by using a luciferase reporter phage and the bronx box. J Clin Microbiol. 1999;37:1144–1149. doi: 10.1128/JCM.37.4.1144-1149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Monden Y., Takasaki K., Futo S., Niwa K., Kawase M., Akitake H., et al. A rapid and enhanced DNA detection method for crop cultivar discrimination. J Biotechnol. 2014;185:57–62. doi: 10.1016/j.jbiotec.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 107.Murray J.F., Matthay M.A., Luce J.M., Flick M.R. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 108.Rahim Z., Nakajima C., Raqib R., Zaman K., Endtz H.P., van der Zanden A.G.M., et al. Molecular mechanism of rifampicin and isoniazid resistance in Mycobacterium tuberculosis from Bangladesh. Tuberculosis. 2012;92:529–534. doi: 10.1016/j.tube.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 109.Aye K.S., Nakajima C., Yamaguchi T., Win M.M., Shwe M.M., Win A.A., et al. Genotypic characterization of multi-drug-resistant Mycobacterium tuberculosis isolates in Myanmar. J Infect Chemother. 2016;22:174–179. doi: 10.1016/j.jiac.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 110.Takarada Y., Kodera T., Kobayashi K., Nakajima C., Kawase M., Suzuki Y. Rapid detection of rifampicin-resistant Mycobacterium tuberculosis, based on isothermal DNA amplification and DNA chromatography. J Microbiol Methods. 2020;177 doi: 10.1016/j.mimet.2020.106062. [DOI] [PubMed] [Google Scholar]
- 111.Chopra K.K., Singh S. Newer diagnostic tests for tuberculosis, their utility, and their limitations. Current Medicine Research and Practice. 2020;10:8–11. doi: 10.1016/j.cmrp.2020.01.004. [DOI] [Google Scholar]
- 112.MacGregor-Fairlie M., Wilkinson S., Besra G.S., Goldberg Oppenheimer P. Tuberculosis diagnostics: overcoming ancient challenges with modern solutions. Emerging Topics in Life Sciences. 2020;4:435–448. doi: 10.1042/ETLS20200335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Santos J.A., Leite A., Soares P., Duarte R., Nunes C. Delayed diagnosis of active pulmonary tuberculosis - potential risk factors for patient and healthcare delays in Portugal. BMC Publ Health. 2021;21:2178. doi: 10.1186/s12889-021-12245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dong B., He Z., Li Y., Xu X., Wang C., Zeng J. Improved conventional and new approaches in the diagnosis of tuberculosis. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.924410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dye C. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- 116.Walzl G., McNerney R., du Plessis N., Bates M., McHugh T.D., Chegou N.N., et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. 2018;18:e199–e210. doi: 10.1016/S1473-3099(18)30111-7. [DOI] [PubMed] [Google Scholar]
- 117.Ai J.-W., Zhou X., Xu T., Yang M., Chen Y., He G.-Q., et al. CRISPR-based rapid and ultra-sensitive diagnostic test for Mycobacterium tuberculosis. Emerg Microb Infect. 2019;8:1361–1369. doi: 10.1080/22221751.2019.1664939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lyu C., Shi H., Cui Y., Li M., Yan Z., Yan L., et al. CRISPR-based biosensing is prospective for rapid and sensitive diagnosis of pediatric tuberculosis. Int J Infect Dis. 2020;101:183–187. doi: 10.1016/j.ijid.2020.09.1428. [DOI] [PubMed] [Google Scholar]
- 119.Qin Z.Z., Sander M.S., Rai B., Titahong C.N., Sudrungrot S., Laah S.N., et al. Using artificial intelligence to read chest radiographs for tuberculosis detection: a multi-site evaluation of the diagnostic accuracy of three deep learning systems. Sci Rep. 2019;9 doi: 10.1038/s41598-019-51503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tafess K., Ng T.T.L., Lao H.Y., Leung K.S.S., Tam K.K.G., Rajwani R., et al. Targeted-sequencing workflows for comprehensive drug resistance profiling of Mycobacterium tuberculosis cultures using two commercial sequencing platforms: comparison of analytical and diagnostic performance, turnaround time, and cost. Clin Chem. 2020;66:809–820. doi: 10.1093/clinchem/hvaa092. [DOI] [PubMed] [Google Scholar]
- 121.Makhado N.A., Matabane E., Faccin M., Pinçon C., Jouet A., Boutachkourt F., et al. Outbreak of multidrug-resistant tuberculosis in South Africa undetected by WHO-endorsed commercial tests: an observational study. Lancet Infect Dis. 2018;18:1350–1359. doi: 10.1016/S1473-3099(18)30496-1. [DOI] [PubMed] [Google Scholar]
- 122.Pattnaik B., Patnaik N., Mittal S., Mohan A., Agrawal A., Guleria R., et al. Micro RNAs as potential biomarkers in tuberculosis: a systematic review. Non-Coding RNA Research. 2022;7:16–26. doi: 10.1016/j.ncrna.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu Z., Zhou A., Ni J., Zhang Q., Wang Y., Lu J., et al. Differential expression of miRNAs and their relation to active tuberculosis. Tuberculosis. 2015;95:395–403. doi: 10.1016/j.tube.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 124.Gupta R.K., Turner C.T., Venturini C., Esmail H., Rangaka M.X., Copas A., et al. Concise whole blood transcriptional signatures for incipient tuberculosis: a systematic review and patient-level pooled meta-analysis. Lancet Respir Med. 2020;8:395–406. doi: 10.1016/S2213-2600(19)30282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaewseekhao B., Nuntawong N., Eiamchai P., Roytrakul S., Reechaipichitkul W., Faksri K. Diagnosis of active tuberculosis and latent tuberculosis infection based on Raman spectroscopy and surface-enhanced Raman spectroscopy. Tuberculosis. 2020;121 doi: 10.1016/j.tube.2020.101916. [DOI] [PubMed] [Google Scholar]
- 126.Saktiawati A.M.I., Triyana K., Wahyuningtias S.D., Dwihardiani B., Julian T., Hidayat S.N., et al. eNose-TB: a trial study protocol of electronic nose for tuberculosis screening in Indonesia. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baker M. Digital PCR hits its stride. Nat Methods. 2012;9:541–544. doi: 10.1038/nmeth.2027. [DOI] [Google Scholar]
- 128.Kuypers J., Jerome K.R. Applications of digital PCR for clinical microbiology. J Clin Microbiol. 2017;55:1621–1628. doi: 10.1128/JCM.00211-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pinheiro L.B., Coleman V.A., Hindson C.M., Herrmann J., Hindson B.J., Bhat S., et al. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem. 2012;84:1003–1011. doi: 10.1021/ac202578x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang J., Han X., Liu A., Bai X., Xu C., Bao F., et al. Use of digital droplet PCR to detect Mycobacterium tuberculosis DNA in whole blood-derived DNA samples from patients with pulmonary and extrapulmonary tuberculosis. Front Cell Infect Microbiol. 2017;7:369. doi: 10.3389/fcimb.2017.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Košir A.B., Divieto C., Pavšič J., Pavarelli S., Dobnik D., Dreo T., et al. Droplet volume variability as a critical factor for accuracy of absolute quantification using droplet digital PCR. Anal Bioanal Chem. 2017;409:6689–6697. doi: 10.1007/s00216-017-0625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Devonshire A.S., O'Sullivan D.M., Honeyborne I., Jones G., Karczmarczyk M., Pavšič J., et al. The use of digital PCR to improve the application of quantitative molecular diagnostic methods for tuberculosis. BMC Infect Dis. 2016;16:366. doi: 10.1186/s12879-016-1696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ushio R., Yamamoto M., Nakashima K., Watanabe H., Nagai K., Shibata Y., et al. Digital PCR assay detection of circulating Mycobacterium tuberculosis DNA in pulmonary tuberculosis patient plasma. Tuberculosis. 2016;99:47–53. doi: 10.1016/j.tube.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 134.Luo J., Luo M., Li J., Yu J., Yang H., Yi X., et al. Rapid direct drug susceptibility testing of Mycobacterium tuberculosis based on culture droplet digital polymerase chain reaction. Int J Tubercul Lung Dis. 2019;23:219–225. doi: 10.5588/ijtld.18.0182. [DOI] [PubMed] [Google Scholar]
- 135.Nyaruaba R., Mwaliko C., Kering K.K., Wei H. Droplet digital PCR applications in the tuberculosis world. Tuberculosis. 2019;117:85–92. doi: 10.1016/j.tube.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 136.Daum L.T., Rodriguez J.D., Worthy S.A., Ismail N.A., Omar S.V., Dreyer A.W., et al. Next-generation Ion torrent sequencing of drug resistance mutations in Mycobacterium tuberculosis strains. J Clin Microbiol. 2012;50:3831–3837. doi: 10.1128/JCM.01893-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hillemann D., Weizenegger M., Kubica T., Richter E., Niemann S. Use of the genotype MTBDR assay for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2005;43:3699–3703. doi: 10.1128/JCM.43.8.3699-3703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McNerney R., Wondafrash B.A., Amena K., Tesfaye A., McCash E.M., Murray N.J. Field test of a novel detection device for Mycobacterium tuberculosis antigen in cough. BMC Infect Dis. 2010;10 doi: 10.1186/1471-2334-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Masson J.-F. Surface plasmon resonance clinical biosensors for medical diagnostics. ACS Sens. 2017;2:16–30. doi: 10.1021/acssensors.6b00763. [DOI] [PubMed] [Google Scholar]
- 140.Dheda K., Gumbo T., Maartens G., Dooley K.E., McNerney R., Murray M., et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;5:291–360. doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 141.Zignol M., Cabibbe A.M., Dean A.S., Glaziou P., Alikhanova N., Ama C., et al. Genetic sequencing for surveillance of drug resistance in tuberculosis in highly endemic countries: a multi-country population-based surveillance study. Lancet Infect Dis. 2018;18:675–683. doi: 10.1016/S1473-3099(18)30073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cloonan N. Re‐thinking miRNA‐mRNA interactions: intertwining issues confound target discovery. Bioessays. 2015;37:379–388. doi: 10.1002/bies.201400191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maudet C., Mano M., Eulalio A. MicroRNAs in the interaction between host and bacterial pathogens. FEBS (Fed Eur Biochem Soc) Lett. 2014;588:4140–4147. doi: 10.1016/j.febslet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 144.Turner M.L., Schnorfeil F.M., Brocker T. MicroRNAs regulate dendritic cell differentiation and function. J Immunol. 2011;187:3911–3917. doi: 10.4049/jimmunol.1101137. [DOI] [PubMed] [Google Scholar]
- 145.Alivernini S., Gremese E., McSharry C., Tolusso B., Ferraccioli G., McInnes I.B., et al. MicroRNA-155—at the critical interface of innate and adaptive immunity in arthritis. Front Immunol. 2018;8:1932. doi: 10.3389/fimmu.2017.01932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dorhoi A., Iannaccone M., Farinacci M., Faé K.C., Schreiber J., Moura-Alves P., et al. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Invest. 2013;123:4836–4848. doi: 10.1172/JCI67604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lyu L., Zhang X., Li C., Yang T., Wang J., Pan L., et al. Small RNA profiles of serum exosomes derived from individuals with latent and active tuberculosis. Front Microbiol. 2019;10:1174. doi: 10.3389/fmicb.2019.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu J., Lu C., Diao N., Zhang S., Wang S., Wang F., et al. Analysis of microRNA expression profiling identifies miR-155 and miR-155* as potential diagnostic markers for active tuberculosis: a preliminary study. Hum Immunol. 2012;73:31–37. doi: 10.1016/j.humimm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 149.Lipworth S., Hough N., Leach L., Morgan M., Jeffery K., Andersson M., et al. AAC; 2019. Whole-genome sequencing for predicting clarithromycin resistance in Mycobacterium abscessus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oniciuc E., Likotrafiti E., Alvarez-Molina A., Prieto M., Santos J., Alvarez-Ordóñez A. The present and future of whole genome sequencing (WGS) and whole metagenome sequencing (WMS) for surveillance of antimicrobial resistant microorganisms and antimicrobial resistance genes across the food chain. Genes. 2018;9:268. doi: 10.3390/genes9050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hatherell H.-A., Colijn C., Stagg H.R., Jackson C., Winter J.R., Abubakar I. Interpreting whole genome sequencing for investigating tuberculosis transmission: a systematic review. BMC Med. 2016;14:21. doi: 10.1186/s12916-016-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Biek R., Pybus O.G., Lloyd-Smith J.O., Didelot X. Measurably evolving pathogens in the genomic era. Trends Ecol Evol. 2015;30:306–313. doi: 10.1016/j.tree.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stimson J., Gardy J., Mathema B., Crudu V., Cohen T., Colijn C. Beyond the SNP threshold: identifying outbreak clusters using inferred transmissions. Mol Biol Evol. 2019;36:587–603. doi: 10.1093/molbev/msy242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tagliani E., Anthony R., Kohl T.A., de Neeling A., Nikolayevskyy V., Ködmön C., et al. Use of a whole genome sequencing-based approach for Mycobacterium tuberculosis surveillance in europe in 2017–2019: an ECDC pilot study. Eur Respir J. 2020 doi: 10.1183/13993003.02272-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wadapurkar R.M., Vyas R. Computational analysis of next generation sequencing data and its applications in clinical oncology. Inform Med Unlocked. 2018;11:75–82. doi: 10.1016/j.imu.2018.05.003. [DOI] [Google Scholar]
- 156.Vogel M., Utpatel C., Corbett C., Kohl T.A., Iskakova A., Ahmedov S., et al. Implementation of whole genome sequencing for tuberculosis diagnostics in a low-middle income, high MDR-TB burden country. Sci Rep. 2021;11 doi: 10.1038/s41598-021-94297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tu Phan L.M., Tufa L.T., Kim H.-J., Lee J., Park T.J. Trends in diagnosis for active tuberculosis using nanomaterials. Curr Med Chem. 2019;26:1946–1959. doi: 10.2174/0929867325666180912105617. [DOI] [PubMed] [Google Scholar]
- 158.Muthukrishnan L. Multidrug resistant tuberculosis – diagnostic challenges and its conquering by nanotechnology approach – an overview. Chem Biol Interact. 2021;337 doi: 10.1016/j.cbi.2021.109397. [DOI] [PubMed] [Google Scholar]
- 159.El-Samadony H., Althani A., Tageldin M.A., Azzazy H.M.E. Nanodiagnostics for tuberculosis detection. Expert Rev Mol Diagn. 2017;17:427–443. doi: 10.1080/14737159.2017.1308825. [DOI] [PubMed] [Google Scholar]
- 160.Joshi H., kandari D., sundar maitra S., Bhatnagar R. Biosensors for the detection of Mycobacterium tuberculosis: a comprehensive overview. Crit Rev Microbiol. 2022;48:784–812. doi: 10.1080/1040841X.2022.2035314. [DOI] [PubMed] [Google Scholar]
- 161.Gatoo M.A., Naseem S., Arfat M.Y., Mahmood Dar A., Qasim K., Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. BioMed Res Int. 2014;2014:1–8. doi: 10.1155/2014/498420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Khan I., Saeed K., Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 163.Curtis A., Wilkinson C. Nantotechniques and approaches in biotechnology. Trends Biotechnol. 2001;19:97–101. doi: 10.1016/S0167-7799(00)01536-5. [DOI] [PubMed] [Google Scholar]
- 164.Rosi N.L., Mirkin C.A. Nanostructures in biodiagnostics. Chem Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 165.Alharbi K.K., Al-sheikh Y.A. Role and implications of nanodiagnostics in the changing trends of clinical diagnosis. Saudi J Biol Sci. 2014;21:109–117. doi: 10.1016/j.sjbs.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.West J.L., Halas N.J. Engineered nanomaterials for biophotonics applications: improving sensing, imaging, and therapeutics. Annu Rev Biomed Eng. 2003;5:285–292. doi: 10.1146/annurev.bioeng.5.011303.120723. [DOI] [PubMed] [Google Scholar]
- 167.Rizvi S.B., Ghaderi S., Keshtgar M., Seifalian A.M. Semiconductor quantum dots as fluorescent probes for in vitro and in vivo bio-molecular and cellular imaging. Nano Rev. 2010;1:5161. doi: 10.3402/nano.v1i0.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hussain M.M., Samir T.M., Azzazy H.M.E. Unmodified gold nanoparticles for direct and rapid detection of Mycobacterium tuberculosis complex. Clin Biochem. 2013;46:633–637. doi: 10.1016/j.clinbiochem.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 169.Capek I. Polymer decorated gold nanoparticles in nanomedicine conjugates. Adv Colloid Interface Sci. 2017;249:386–399. doi: 10.1016/j.cis.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 170.Azzazy H.M., Mansour M.M., Kazmierczak S.C. Nanodiagnostics: a new frontier for clinical laboratory medicine. Clin Chem. 2006;52:1238–1246. doi: 10.1373/clinchem.2006.066654. [DOI] [PubMed] [Google Scholar]
- 171.Jackson T.C., Patani B.O., Ekpa D.E. Nanotechnology in diagnosis: a review. ANP. 2017;6:93–102. doi: 10.4236/anp.2017.63008. [DOI] [Google Scholar]
- 172.Kumar A., Mazinder Boruah B., Liang X.-J. Gold nanoparticles: promising nanomaterials for the diagnosis of cancer and HIV/AIDS. J Nanomater. 2011;2011:1–17. doi: 10.1155/2011/202187. [DOI] [Google Scholar]
- 173.Valdemar-Aguilar C.M., Manisekaran R., Acosta-Torres L.S., López-Marín L.M. Spotlight on mycobacterial lipid exploitation using nanotechnology for diagnosis, vaccines, and treatments. Nanomed Nanotechnol Biol Med. 2023;48 doi: 10.1016/j.nano.2023.102653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chandra P., Das D., Abdelwahab A.A. Gold nanoparticles in molecular diagnostics and therapeutics. DJNB. 2010;5 [Google Scholar]
- 175.El-Samadony H., Azzazy H.M.E., Tageldin M.A., Ashour M.E., Deraz I.M., Elmaghraby T. Nanogold assay improves accuracy of conventional TB diagnostics. Lung. 2019;197:241–247. doi: 10.1007/s00408-018-00194-0. [DOI] [PubMed] [Google Scholar]
- 176.Burns M.A., Johnson B.N., Brahmasandra S.N., Handique K., Webster J.R., Krishnan M., et al. An integrated nanoliter DNA analysis device. Science. 1998;282:484–487. doi: 10.1126/science.282.5388.484. [DOI] [PubMed] [Google Scholar]
- 177.Wu J., Dong M., Rigatto C., Liu Y., Lin F. Lab-on-chip technology for chronic disease diagnosis. Npj Digital Med. 2018;1:7. doi: 10.1038/s41746-017-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nelson B.P., Grimsrud T.E., Liles M.R., Goodman R.M., Corn R.M. Surface plasmon resonance imaging measurements of DNA and RNA hybridization adsorption onto DNA microarrays. Anal Chem. 2001;73:1–7. doi: 10.1021/ac0010431. [DOI] [PubMed] [Google Scholar]
- 179.Smith A.M., Nie S. Semiconductor nanocrystals: structure, properties, and band gap engineering. Acc Chem Res. 2010;43:190–200. doi: 10.1021/ar9001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kairdolf B.A., Smith A.M., Stokes T.H., Wang M.D., Young A.N., Nie S. Semiconductor quantum dots for bioimaging and biodiagnostic applications. Annu Rev Anal Chem. 2013;6:143–162. doi: 10.1146/annurev-anchem-060908-155136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gazouli M., Liandris E., Andreadou M., Sechi L.A., Masala S., Paccagnini D., et al. Specific detection of unamplified mycobacterial DNA by use of fluorescent semiconductor quantum dots and magnetic beads. J Clin Microbiol. 2010;48:2830–2835. doi: 10.1128/JCM.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nasiruddin M., Neyaz MdK., Das S. Nanotechnology-based approach in tuberculosis treatment. Tuberc Res Treat. 2017;2017:1–12. doi: 10.1155/2017/4920209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xu K., Liang Z.C., Ding X., Hu H., Liu S., Nurmik M., et al. Nanomaterials in the prevention, diagnosis, and treatment of Mycobacterium tuberculosis infections. Adv Healthcare Mater. 2018;7 doi: 10.1002/adhm.201700509. [DOI] [PubMed] [Google Scholar]
- 184.Liandris E., Gazouli M., Andreadou M., Sechi L.A., Rosu V., Ikonomopoulos J. Detection of pathogenic mycobacteria based on functionalized quantum dots coupled with immunomagnetic separation. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang Q., Xu Q., Chen Q., Li J., Zhang M., Cai Y., et al. Discriminating active tuberculosis from latent tuberculosis infection by flow cytometric measurement of cd161-expressing T cells. Sci Rep. 2015;5 doi: 10.1038/srep17918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hu O., Li Z., Wu J., Tan Y., Chen Z., Tong Y. A multicomponent nucleic acid enzyme-cleavable quantum dot nanobeacon for highly sensitive diagnosis of tuberculosis with the naked eye. ACS Sens. 2023;8:254–262. doi: 10.1021/acssensors.2c02114. [DOI] [PubMed] [Google Scholar]
- 187.Jiang X., Zeng H., Duan C., Hu Q., Wu Q., Yu Y., et al. One-pot synthesis of stable and functional hydrophilic CsPbBr 3 perovskite quantum dots for “turn-on” fluorescence detection of Mycobacterium tuberculosis. Dalton Trans. 2022;51:3581–3589. doi: 10.1039/D1DT03624F. [DOI] [PubMed] [Google Scholar]
- 188.Unsoy G., Gunduz U., Oprea O., Ficai D., Sonmez M., Radulescu M., et al. Magnetite: from synthesis to applications. CTM. 2015;15:1622–1640. doi: 10.2174/1568026615666150414153928. [DOI] [PubMed] [Google Scholar]
- 189.Ali A., Zafar H., Zia M., ul Haq I., Phull A.R., Ali J.S., et al. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. NSA. 2016;ume 9:49–67. doi: 10.2147/NSA.S99986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.He H., Zhong Y., Liang X., Tan W., Zhu J., Yan Wang C. Natural Magnetite: an efficient catalyst for the degradation of organic contaminant. Sci Rep. 2015;5 doi: 10.1038/srep10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.León-Janampa N., Shinkaruk S., Gilmanc R H., Kirwand D E., Fouquet E., Szlosek M., et al. Biorecognition and detection of antigens from Mycobacterium tuberculosis using a sandwich ELISA associated with magnetic nanoparticles. J Pharm Biomed Anal. 2022;215 doi: 10.1016/j.jpba.2022.114749. [DOI] [PubMed] [Google Scholar]
- 192.Engström A., Zardán Gómez de la Torre T., Strømme M., Nilsson M., Herthnek D. Detection of rifampicin resistance in Mycobacterium tuberculosis by padlock probes and magnetic nanobead-based readout. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sheffee N.S., Rubio-Reyes P., Mirabal M., Calero R., Carrillo-Calvet H., Chen S., et al. Engineered Mycobacterium tuberculosis antigen assembly into core-shell nanobeads for diagnosis of tuberculosis. Nanomed Nanotechnol Biol Med. 2021;34 doi: 10.1016/j.nano.2021.102374. [DOI] [PubMed] [Google Scholar]
- 194.Wang Y., Yu L., Kong X., Sun L. Application of nanodiagnostics in point-of-care tests for infectious diseases. IJN. 2017;12:4789–4803. doi: 10.2147/IJN.S137338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee H., Sun E., Ham D., Weissleder R. Chip–NMR biosensor for detection and molecular analysis of cells. Nat Med. 2008;14:869–874. doi: 10.1038/nm.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Perez J.M., Josephson L., O'Loughlin T., Högemann D., Weissleder R. Magnetic relaxation switches capable of sensing molecular interactions. Nat Biotechnol. 2002;20:816–820. doi: 10.1038/nbt720. [DOI] [PubMed] [Google Scholar]
- 197.Wang L., Leng C., Tang S., Lei J., Ju H. Enzyme-free signal amplification for electrochemical detection of Mycobacterium lipoarabinomannan antibody on a disposable chip. Biosens Bioelectron. 2012;38:421–424. doi: 10.1016/j.bios.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 198.Zurovac D., Talisuna A.O., Snow R.W. Mobile phone text messaging: tool for malaria control in Africa. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chin C.D., Laksanasopin T., Cheung Y.K., Steinmiller D., Linder V., Parsa H., et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat Med. 2011;17:1015–1019. doi: 10.1038/nm.2408. [DOI] [PubMed] [Google Scholar]
- 200.Mdluli K., Kaneko T., Upton A. Tuberculosis drug discovery and emerging targets: tuberculosis drug discovery. Ann NY Acad Sci. 2014;1323:56–75. doi: 10.1111/nyas.12459. [DOI] [PubMed] [Google Scholar]
- 201.Banerjee R., Jaiswal A. Recent advances in nanoparticle-based lateral flow immunoassay as a point-of-care diagnostic tool for infectious agents and diseases. Analyst. 2018;143:1970–1996. doi: 10.1039/C8AN00307F. [DOI] [PubMed] [Google Scholar]
- 202.Cimaglia F., Aliverti A., Chiesa M., Poltronieri P., De Lorenzis E., Santino A., et al. Quantum dots nanoparticle-based lateral flow assay for rapid detection of Mycobacterium species using anti-FprA antibodies. Nanotechnol Dev. 2012;2:5. doi: 10.4081/nd.2012.e5. [DOI] [Google Scholar]
- 203.Geißler D., Charbonnière L.J., Ziessel R.F., Butlin N.G., Löhmannsröben H.-G., Hildebrandt N. Quantum dot biosensors for ultrasensitive multiplexed diagnostics. Angew Chem, Int Ed. 2010;49:1396–1401. doi: 10.1002/anie.200906399. [DOI] [PubMed] [Google Scholar]
- 204.Sepulveda D., Aroca M., Varela A., Del Portillo P., Osma J. Bioelectrochemical detection of Mycobacterium tuberculosis ESAT-6 in an antibody-based biomicrosystem. Sensors. 2017;17:2178. doi: 10.3390/s17102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Thiruppathiraja C., Kamatchiammal S., Adaikkappan P., Santhosh D.J., Alagar M. Specific detection of Mycobacterium sp. genomic DNA using dual labeled gold nanoparticle based electrochemical biosensor. Anal Biochem. 2011;417:73–79. doi: 10.1016/j.ab.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 206.Choi H.S., Frangioni J.V. Nanoparticles for biomedical imaging: fundamentals of clinical translation. Mol Imag. 2010;9 doi: 10.2310/7290.2010.00031. 7290.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sonawane M.D., Nimse S.B. Surface modification chemistries of materials used in diagnostic platforms with biomolecules. J Chem. 2016;2016:1–19. doi: 10.1155/2016/9241378. [DOI] [Google Scholar]
- 208.Kim J., Oh S.Y., Shukla S., Hong S.B., Heo N.S., VivekK Bajpai, et al. Heteroassembled gold nanoparticles with sandwich-immunoassay LSPR chip format for rapid and sensitive detection of hepatitis B virus surface antigen (HBsAg) Biosens Bioelectron. 2018;107:118–122. doi: 10.1016/j.bios.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 209.Baptista P., Pereira E., Eaton P., Doria G., Miranda A., Gomes I., et al. Gold nanoparticles for the development of clinical diagnosis methods. Anal Bioanal Chem. 2008;391:943–950. doi: 10.1007/s00216-007-1768-z. [DOI] [PubMed] [Google Scholar]
- 210.Srivastava S.K., van Rijn C.J.M., Jongsma M.A. Biosensor-based detection of tuberculosis. RSC Adv. 2016;6:17759–17771. doi: 10.1039/C5RA15269K. [DOI] [Google Scholar]
- 211.Bai C., Lu Z., Jiang H., Yang Z., Liu X., Ding H., et al. Aptamer selection and application in multivalent binding-based electrical impedance detection of inactivated H1N1 virus. Biosens Bioelectron. 2018;110:162–167. doi: 10.1016/j.bios.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 212.Nguyen H., Park J., Kang S., Kim M. Surface plasmon resonance: a versatile technique for biosensor applications. Sensors. 2015;15:10481–10510. doi: 10.3390/s150510481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ma J., Du M., Wang C., Xie X., Wang H., Li T., et al. Rapid and sensitive detection of Mycobacterium tuberculosis by an enhanced nanobiosensor. ACS Sens. 2021;6:3367–3376. doi: 10.1021/acssensors.1c01227. [DOI] [PubMed] [Google Scholar]
- 214.Xie X., Ma J., Wang H., Cheng Z., Li T., Chen S., et al. A self-contained and integrated microfluidic nano-detection system for the biosensing and analysis of molecular interactions. Lab Chip. 2022;22:1702–1713. doi: 10.1039/D1LC01056E. [DOI] [PubMed] [Google Scholar]
- 215.Hiatt L.A., Cliffel D.E. Real-time recognition of Mycobacterium tuberculosis and lipoarabinomannan using the quartz crystal microbalance. Sens Actuators B Chem. 2012;174:245–252. doi: 10.1016/j.snb.2012.06.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yin X., Zheng L., Lin L., Hu Y., Zheng F., Hu Y., et al. Commercial MPT64-based tests for rapid identification of Mycobacterium tuberculosis complex: a meta-analysis. J Infect. 2013;67:369–377. doi: 10.1016/j.jinf.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 217.Ellington A.D., Szostak J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 218.Deng B., Lin Y., Wang C., Li F., Wang Z., Zhang H., et al. Aptamer binding assays for proteins: the thrombin example—a review. Anal Chim Acta. 2014;837:1–15. doi: 10.1016/j.aca.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 219.Das M., Dhand C., Sumana G., Srivastava A.K., Nagarajan R., Nain L., et al. Electrophoretic fabrication of Chitosan−Zirconium-oxide nanobiocomposite platform for nucleic acid detection. Biomacromolecules. 2011;12:540–547. doi: 10.1021/bm1013074. [DOI] [PubMed] [Google Scholar]
- 220.Kaewphinit T., Santiwatanakul S., Promptmas C., Chansiri K. Detection of non-amplified Mycobacterium tuberculosis genomic DNA using piezoelectric DNA-based biosensors. Sensors. 2010;10:1846–1858. doi: 10.3390/s100301846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Veigas B., Doria G., P V. In: Understanding tuberculosis - global experiences and innovative approaches to the diagnosis. Cardona P.-J., editor. InTech; 2012. Nanodiagnostics for tuberculosis. [DOI] [Google Scholar]
- 222.Marraffini L.A., Sontheimer E.J. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat Rev Genet. 2010;11:181–190. doi: 10.1038/nrg2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Barrangou R., Marraffini L.A. CRISPR-cas systems: prokaryotes upgrade to adaptive immunity. Mol Cell. 2014;54:234–244. doi: 10.1016/j.molcel.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Abudayyeh O.O., Gootenberg J.S., Konermann S., Joung J., Slaymaker I.M., Cox D.B.T., et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353:aaf5573. doi: 10.1126/science.aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen Y., Liu X., Guo S., Cao J., Zhou J., Zuo J., et al. A sandwich-type electrochemical aptasensor for Mycobacterium tuberculosis MPT64 antigen detection using C60NPs decorated N-CNTs/GO nanocomposite coupled with conductive PEI-functionalized metal-organic framework. Biomaterials. 2019;216 doi: 10.1016/j.biomaterials.2019.119253. [DOI] [PubMed] [Google Scholar]
- 227.Boyle D.S., McNerney R., Teng Low H., Leader B.T., Pérez-Osorio A.C., Meyer J.C., et al. Rapid detection of Mycobacterium tuberculosis by recombinase polymerase amplification. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xu H., Zhang X., Cai Z., Dong X., Chen G., Li Z., et al. An isothermal method for sensitive detection of Mycobacterium tuberculosis complex using clustered regularly interspaced short palindromic repeats/cas12a cis and trans cleavage. J Mol Diagn. 2020;22:1020–1029. doi: 10.1016/j.jmoldx.2020.04.212. [DOI] [PubMed] [Google Scholar]
- 229.Gagneux S., Small P.M. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis. 2007;7:328–337. doi: 10.1016/S1473-3099(07)70108-1. [DOI] [PubMed] [Google Scholar]
- 230.Chen H., Li S., Zhao W., Deng J., Yan Z., Zhang T., et al. A peptidomic approach to identify novel antigen biomarkers for the diagnosis of tuberculosis. IDR. 2022;15:4617–4626. doi: 10.2147/IDR.S373652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Huang Y., Ai L., Wang X., Sun Z., Wang F. Review and updates on the diagnosis of tuberculosis. JCM. 2022;11:5826. doi: 10.3390/jcm11195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hettick J.M., Kashon M.L., Simpson J.P., Siegel P.D., Mazurek G.H., Weissman D.N. Proteomic profiling of intact mycobacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Chem. 2004;76:5769–5776. doi: 10.1021/ac049410m. [DOI] [PubMed] [Google Scholar]
- 233.Robinne S., Saad J., Morsli M., Hamidou Z.H., Tazerart F., Drancourt M., et al. Rapid identification of Mycobacterium tuberculosis complex using mass spectrometry: a proof of concept. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.753969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bajaj A.O., Saraswat S., Knuuttila J.E.A., Freeke J., Stielow J.B., Barker A.P. Accurate identification of closely related Mycobacterium tuberculosis complex species by high resolution tandem mass spectrometry. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.656880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chen D., Bryden W.A., Wood R. Detection of tuberculosis by the analysis of exhaled breath particles with high-resolution mass spectrometry. Sci Rep. 2020;10:7647. doi: 10.1038/s41598-020-64637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.López-Hernández Y., Patiño-Rodríguez O., García-Orta S.T., Pinos-Rodríguez J.M. Mass spectrometry applied to the identification of Mycobacterium tuberculosis and biomarker discovery. J Appl Microbiol. 2016;121:1485–1497. doi: 10.1111/jam.13323. [DOI] [PubMed] [Google Scholar]
- 237.Saluzzo F., Mantegani P., Poletti de Chaurand V., Cirillo D.M. QIAreach QuantiFERON-TB for the diagnosis of Mycobacterium tuberculosis infection. Eur Respir J. 2022;59 doi: 10.1183/13993003.02563-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tavaziva G., Harris M., Abidi S.K., Geric C., Breuninger M., Dheda K., et al. Chest X-ray analysis with deep learning-based software as a triage test for pulmonary tuberculosis: an individual patient data meta-analysis of diagnostic accuracy. Clin Infect Dis. 2022;74:1390–1400. doi: 10.1093/cid/ciab639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hira J., Uddin MdJ., Haugland M.M., Lentz C.S. From differential stains to next generation physiology: chemical probes to visualize bacterial cell structure and physiology. Molecules. 2020;25:4949. doi: 10.3390/molecules25214949. [DOI] [PMC free article] [PubMed] [Google Scholar]