Abstract
Background
In this systematic review and meta-analysis, we evaluated ultrasound (US)-guided injections of platelet-rich plasma (PRP) as conservative treatment of tendinopathies.
Materials and methods
We searched MEDLINE, EMBASE, SCOPUS, OVID, and the Cochrane Library to identify randomized controlled trials (RCT) on the use of US-guided PRP for tendinopathies.
Results
We found 33 RCT (2,025 subjects) that met our inclusion criteria: 8 in lateral epicondylitis, 5 in plantar fasciitis, 5 in Achilles tendinopathy, 7 in rotator cuff tendinopathy, 3 in patellar tendinopathy and 5 in carpal tunnel syndrome. PRP, given as a single injection (20 trials) or multiple injections (13 trials), was compared to US-guided injection of steroids, saline, autologous whole blood, local anesthetic, dry needling, prolotherapy, bone marrow mesenchymal stem cells, or with non-injective interventions. The outcomes more commonly reported included pain and functional measures, subgrouped as in the short-term (<3 months from the intervention), medium-term (3 to 6 months) or long-term (≥12 months). No clear between-group differences in these outcomes were observed in patients with lateral epicondylitis, plantar fasciitis, or Achilles, rotator cuff or patellar tendinopathy. In patients with carpal tunnel syndrome, visual analog scale scores for pain at 3 and 6 months and Boston Carpal Tunnel Questionnaire severity scores at 1, 3 and 6 months were significantly lower in PRP recipients than in controls. The certainty of evidence of all these comparisons was graded as low or very low due to risk of bias, imprecision and/or inconsistency. Pain at the injection site was more common among PRP recipients than among controls receiving other US-guided injections.
Discussion
In patients with tendinopathies, a trend towards pain reduction and functional improvement from baseline was observed after US-guided PRP injection, but in the majority of the comparisons, the effect size was comparable to that observed in control groups.
Keywords: platelet-rich plasma, ultrasound-guided injection, tendinopathies, systematic review, meta-analysis
INTRODUCTION
Platelet-rich plasma (PRP) is a biological product obtained from autologous peripheral blood after centrifugation. PRP contains a high concentration of platelets, growth factors, and cytokines, which basic science studies have shown may improve tendon healing by promoting angiogenesis, cellular migration, proliferation, and matrix synthesis1,2. Infiltrative treatment with PRP to accelerate the healing of injured tendons, ligaments, muscles and joints has increased exponentially during the last years, although the evidence of its efficacy has been highly variable depending on the specific indication3–7.
A number of randomized controlled clinical trials (RCT) have evaluated the use of PRP in the orthopedic setting, particularly for tendon and ligament injuries, and several systematic reviews and meta-analyses have been published, although with contrasting results5,8–15. The heterogeneity of PRP preparations and methods of administration have made it difficult to interpret the existing literature and limit our ability to make definitive treatment recommendations5,9. One option would be to use allogeneic PRP, as the latter offers better opportunities for product standardization than autologous PRP.
Another reason for discrepancies in the results obtained seems to depend on the accuracy in the execution of the injection. Compared to anatomical guidance, ultrasound (US) guidance notably improves injection accuracy in the target intra-articular joint space16–18. Intra-articular and periarticular interventional procedures can be easily performed under continuous US monitoring to ensure correct needle positioning and medication delivery across every anatomical site, more accurately compared with blind/anatomically guided methods19.
For this reason, we undertook a new systematic review of US-guided PRP injections for the treatment of tendinopathies, including new primary studies, analyzing outcomes, and grading the quality of the available evidence following the Cochrane guidance for methodology.
MATERIALS AND METHODS
This systematic review was conducted according to recommended PRISMA checklist guidelines20. The protocol is registered on PROSPERO (registration number CRD42021289419). The protocol was aimed to evaluate US-guided PRP injections in non-surgical orthopedic procedures, including tendon and ligament injuries, and knee and hip osteoarthritis. However, due to the heterogeneity of the clinical conditions considered, the current study presents only the results of the review conducted on tendon and ligament injuries.
Search strategy
A computer-assisted literature search of the MEDLINE (through PUBMED), EMBASE, SCOPUS, OVID, and Cochrane Library databases was performed (latest search November 30, 2021) to identify RCT on the conservative non-surgical use of PRP for tendinopathies. A combination of the following text words was used to maximize search specificity and sensitivity: “platelet rich plasma”, “ultrasound guided injection/ US injection/ US guided”, “orthopedics”, “tendon”, “tendinopathy”, “tendinitis”, “ligament”, “randomized clinical controlled trials”, “Achilles tendinopathy”, “plantar fasciitis”, “lateral epicondylitis”, “tennis elbow”, “patellar tendinopathy”, “carpal tunnel syndrome” and “rotator cuff tendinopathy”. In addition, we checked the reference lists of the most relevant items (original studies and reviews) in order to identify potentially eligible studies not captured by the initial literature search. No restriction on language was applied for the search.
Data collection and analysis
For each RCT included in the systematic review, two reviewers (MC and FM) extracted the following data independently: first author, year of publication, study design, condition, details of interventions in the study and control groups, sample size and years range, outcome measurements, follow-up period and main results. Measures of treatment effect were mean differences (MD) and, when different scales were available, standardized MD together with 95% confidence intervals (CI) for continuous outcome measures (e.g., pain and functional scores), and MD with 95% CI for dichotomous outcomes (e.g., occurrence of adverse events, patients’ satisfaction rate). For continuous outcomes, the score had to be reported as the mean and standard deviation (SD); when studies reported other dispersion measures such as median and range, or standard error (SE) of the mean or 95% CI of the mean, we calculated the mean and SD from these measures in order to perform the relevant meta-analytical pooling21,22. For studies with multiple intervention groups (e.g., PRP as the intervention group and two or more control groups such as steroids and saline, to overcome a unit-of-analysis error due to over-counting the participants in the shared intervention group, we split the “shared” group into two or more groups with smaller sample sizes, and include two or more comparisons22.
We used scores over time and/or change in scores from baseline. Disagreement was resolved by consensus and by the opinion of a third reviewer (IP), if necessary.
The study weight was calculated using the Mantel-Haenszel method. We assessed statistical heterogeneity using T2, Cochran’s Q and I2 statistics. The I2 statistic describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error. In the case of not important heterogeneity (I2 <40), studies were pooled using a fixed-effects model. When values of I2 were >40, a random-effects analysis was undertaken. All calculations were done using Excel and REVMAN 5.4. For the purpose of this systematic review, trials evaluating the role of PRP in surgical orthopedic procedures or after surgical procedures were excluded. We selected six groups of disorders: (i) lateral epicondylitis; (ii) Achilles tendinopathy; (iii) plantar fasciitis; (iv) patellar tendinopathy; (v) rotator cuff tendinopathy; and (vi) carpal tunnel syndrome.
Outcomes
Primary outcomes included pain and functional measures, measured by standard validated scales23–24, and adverse events. For pain, the most commonly reported measures were visual analog scale (VAS) scores (from 0 to 10, with 0=no pain and 10 the worst imaginable pain) or other numeric pain rating scales. Functional measures reported were condition-specific tendinopathy measures and generic measures, and included the following: (i) Disabilities of the Arm, Shoulder and Hand (DASH); Patient-Rated Tennis Elbow Evaluation (PRTEE); Liverpool Elbow Score; Victorian Institute of Sports Assessments (VISA) for Achilles tendinopathy (VISA-A) and for patellar tendinopathy (VISA-P): these are measures used to assess the severity of tendinopathy (higher scores corresponding with less pain and increased activity for VISA and Liverpool Elbow Score, and vice-versa for PRTEE and DASH); (ii) American Shoulder and Elbow Surgeons (ASES), and Shoulder Pain And Disability Index (SPADI): lower score best; (iii) the Boston Carpal Tunnel Questionnaire (BCTQ), a scale developed specifically for carpal tunnel syndrome, which consists of a Functional Status Scale (FSS) and a Severity Scale (SSS); (iv) American Orthopedic Foot and Ankle Society Score (AOFAS), and the Mayo Clinic Performance Index for Elbow (MMCPIE) that combines clinician-reported and patient-reported parts, with higher score indicating no symptoms or impairments; (v) Foot Function Index (FFI), a patient-reported questionnaire, with higher score indicating worst pain and disability; and (vi) the West Ontario Rotator Cuff Index (WORC), ranging from 0 (lowest quality of life) to 100 (highest quality of life). We also evaluated the occurrence of serious adverse events (e.g., infection at the injection site, inflammatory reaction), and the occurrence of any adverse events, particularly injection-related pain.
Measures of effect
The outcome measures were sub-grouped into different time periods: short-term (<3 months from the intervention) and medium-term (from 3 to 6 months); where available, we also evaluated long-term (≥12 months) outcomes.
Subgroup analyses
We undertook subgroup analyses according to type of tendinopathy evaluated, duration of follow-up (short-term, medium-term and long-term, as defined above) and, where possible, for number of PRP injections and type of control intervention (e.g., PRP vs local steroids injection, PRP vs saline injections).
Assessment of risk of bias in included studies
Two review authors (MC, FM) independently assessed the risk of bias of each included study following the domain-based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions22. They discussed any discrepancies and achieved consensus on the final assessment. The Cochrane “Risk of bias” tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting, and other issues relating to bias. For the selective reporting domain, we added an item for the outcome “adverse events” because reporting was inadequate for almost all the trials included for this outcome, but not for the other outcomes. We have presented our assessment of risk of bias using two “Risk of bias “ summary figures: (i) a summary of bias for each item across all studies and (ii) a cross-tabulation of each trial by all of the “Risk of bias “ items.
“SUMMARY OF FINDINGS” TABLES
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes, and constructed a “Summary of findings” table using REVMAN 525. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of available data for the main outcomes26. The “Summary of findings” tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach, which defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within-trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias27.
When evaluating the “Risk of bias” domain, we downgraded the GRADE assessment when we classified a study as being at high risk of bias for one or more of the following domains: selection, attrition, performance, detection, reporting, and other bias; or when the “Risk of bias” assessment for selection bias was unclear (this was classified as unclear for either the generation of the randomization sequence or the allocation concealment domain). For pain and functional outcomes (e.g., VAS, AOFAS and DASH) we downgraded for high risk of bias in performance and detection domains, since we judged that these outcomes, self-reported by patients or collected by physicians to help standardize the assessments of patients with these disorders, are likely to be influenced by lack of blinding.
We have presented the following outcomes in the "Summary of findings" table: pain and functional scores for the six clinical conditions, and overall adverse events.
RESULTS
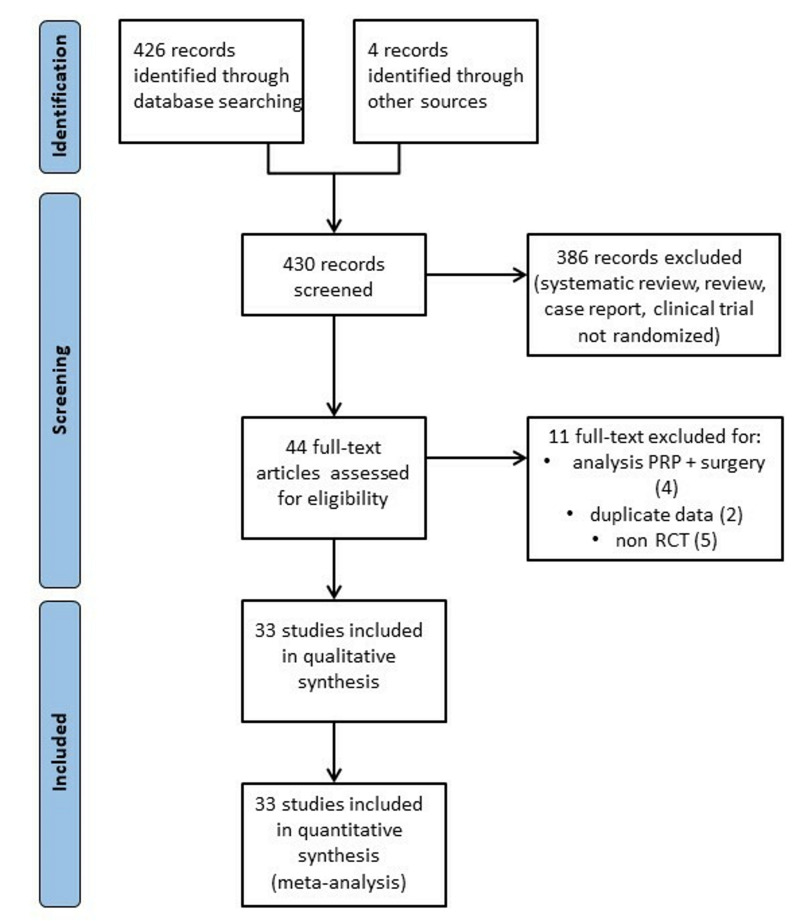
In total, 430 articles were identified after the initial electronic and manual search (Figure 1). Three hundred and eighty-six of them were excluded because they focused on other topics (reviews, RCT protocols, non-randomized studies, duplicates studies, and studies containing no informative data). Thus, 44 potentially relevant articles were selected and the next screening led to the exclusion of 11 more studies. Among RCT reporting different follow-ups of the same trial, we included only the last published update. Thirty-three randomized studies28–60 were included in the systematic review (see Table I for the main characteristics and results of the included studies). Overall, 2,025 patients were enrolled in the 33 RCT selected for the review.
Figure 1.
Flow chart
PRP: platelet-rich plasma; RCT: randomized controlled trial.
Table I.
Characteristics and main results of the included studies on the use of US-guided injection of platelet-rich plasma for tendinopathies
| Study (year) ref | Study design | Condition | Patients & years (range) | Test group (N) | Control group (N) | Other interventions (N) | Outcomes | Follow-up (months) | Main results |
|---|---|---|---|---|---|---|---|---|---|
| Boesen28 (2017) | RCT, DB | AT | 60 (18–59) | PRP (20) | Placebo (20) | Steroid (20) | VAS-pain VISA-A score Tendon thickness DC activity HR test Pts satisfaction |
6 | Steroid may be more effective than PRP in the short term |
| De Jonge29 (2011) | RCT, DB | AT | 54 (18–70) | PRP (27) | Placebo (27) | - | VISA-A score UTC Pts satisfaction Return to sport |
12 | No clinical improvement after PRP injections |
| De Vos30 (2010) | RCT, DB | AT | 54 (18–70) | PRP (27) | Placebo (27) | - | VISA-A score Pts satisfaction Return to sport |
6 | No clinical improvement after PRP injections |
| Krogh31 (2016) | RCT, DB | AT | 24 (37–62) | PRP (12) | Placebo (12) | - | VISA-A score Pain scores at rest Pain score while walking Pain when tendon was squeezed DC activity Tendon thickness Adverse events |
3 | PRP injections only increase tendon thickness |
| Boesen32 (2020) | RCT, DB | ATR | 40 (18–60) | PRP (20) | Placebo (20) | - | ATRS HR test ROM-ankle dorsiflexion ATL Calf circumference Return to sport |
12 | PRP injections do not stimulate tendon healing or improve patients outcomes |
| Chen33 (2021) | RCT, DB | CTS | 26 (31–74) | PRP (13) | Placebo (13) | - | BCTQ-SSS/FSS CSA of the MN DML SNCV |
12 | PRP injections provide a therapeutic effect for 1 year postinjection |
| Malahias34 (2017) | RCT, DB | CTS | 50 (40–75) | PRP (26) | Placebo (24) | - | VAS-pain Q-DASH CSA of the MN |
3 | PRP injections may have positive effects |
| Senna35 (2019) | RCT | CTS | 98 (30–51) | PRP (49) | Steroid (49) | - | VAS-pain BCTQ-SSS/FSS Paresthesia Phalen’s test Tinel’s test DML CMAP SNAP CSA of the MN |
3 | PRP injections are superior to steroid for treatment of mild to moderate CTS |
| Shen36 (2019) | RCT | CTS | 52 (31–77) | PRP (26) | DP (26) | - | BCTQ-SSS/FSS CSA of the MN DML SNCV |
6 | PRP injections are beneficial for the treatment of moderate CTS |
| Wu37 (2017) | RCT | CTS | 60 | PRP (30) | Night splint (30) | - | VAS-pain BCTQ-SSS/FSS DML SNCV CSA of the MN Palmar force |
6 | PRP injections improve pain and disability in patients with CTS |
| Behera38 (2015) | RCT | LET | 25 (27–50) | PRP (15) | Bupivacaine (10) | - | VAS-pain MMCPIE Nirschl score (activity) |
12 | PRP injections enabled good improvement in pain and function |
| Creaney39 (2011) | RCT | LET | 150 | PRP (80) | WB (70) | - | PRTEE | 6 | PRP and WB injections are useful second-line therapies to improve clinical outcomes |
| Krogh40 (2013) | RCT, DB | LET | 60 (35–54) | PRP (20) | Placebo (20) | Steroid (20) | PRTEE-pain PRTEE-disability Tendon thickness Adverse events |
3 | Only steroid injections reduce pain after 1 month |
| Lim41 (2017) | RCT | LET | 120 | PRP (61) | Physiotherapy (59) | - | VAS-pain MMCPIE MRI |
6 | PRP injections improve pain and function |
| Martin42 (2019) | RCT, DB | LET | 71 (40–58) | PRP (36) | Lidocaine (35) | - | VAS-pain DASH-E Tendon thickness Calcification |
12 | PRP results in similar improvements to those obtained with lidocaine |
| Merolla43 (2017) | RCT | LET | 101 | PRP (51) | Arthroscopic Release (50) | - | VAS-pain PRTEE Grip strength Muscle tenderness Adverse events |
24 | PRP injections or arthroscopic release are both effective in the short and medium term |
| Montalvan44 (2016) | RCT, DB | LET | 50 (38–56) | PRP (25) | Placebo (25) | - | VAS-pain Roles Maudsley score ECRB contraction EDC contraction Adverse events |
12 | No clinical improvement after PRP injections |
| Thanasas45 (2011) | RCT | LET | 28 (29–55) | PRP (14) | WB (14) | - | VAS-pain Liverpool Elbow score |
6 | PRP injections seems to be superior to WB in the short term |
| Gogna46 (2016) | RCT | PF | 40 | PRP (20) | Low Dose Radiation (20) | - | VAS-pain AOFAS score Plantar fascia Thickness |
6 | PRP is as good as Low Dose Radiation in patients not responding to physical therapy |
| Kim47 (2013) | RCT | PF | 21 (19–57) | PRP (10) | DP (11) | - | FFI score FFI pain subscale scores FFI disability subscale scores |
6 | PRP and DP injections are both effective |
| Malahias48 (2019) | RCT, DB | PF | 36 | PRP (18) | PPP (18) | - | VAS-pain VAS-function VAS-satisfaction Adverse events |
6 | PRP and PPP injections are both effective |
| Monto49 (2014) | RCT | PF | 40 (24–74) | PRP (20) | Steroid (20) | - | AOFAS score | 24 | PRP was more effective and durable than steroid injection |
| Ugurlar50 (2018) | RCT | PF | 158 (19–62) | PRP (39) | Steroid (40) | ESWT (39); DP (40) | VAS-pain FFI score |
36 | No differences were found among the 4 treatments after 36 months |
| Dragoo51 (2014) | RCT, DB | PT | 23 (20–54) | PRP (10) | Dry needling (13) | - | VISA score VAS-pain Tegner score (activity) Lysholm knee score SF-12 |
6 | PRP injections improve pain, function and stability |
| Rodas52 (2021) | RCT, DB | PT | 20 (18–48) | PRP (10) | BM-MSCs (10) | - | VISA score VAS-pain Tendon thickness DC activity UTC MRI |
6 | No significant differences were found in the 2 groups |
| Scott53 (2019) | RCT | PT | 57 (18–50) | PRP (19) | Placebo (19) | LP-PRP (19) | VISA score NPRS GROC Adverse events |
12 | No significant differences were found among the 3 treatments |
| Dadgostar54 (2021) | RCT, DB | RC | 58 | PRP (30) | Steroid (28) | - | VAS-pain WORC score ROM DASH Muscle thickness |
3 | PRP and steroid injections are both effective |
| Kesikburun55 (2013) | RCT, DB | RC | 40 (18–70) | PRP (20) | Placebo (20) | - | VAS-pain WORC score SPADI ROM |
12 | PRP injection was found to be no more effective than placebo |
| Kwong56 (2021) | RCT, DB | RC | 99 | PRP (47) | Steroid (52) | - | VAS-pain WORC score ASES |
12 | No sustained benefit of PRP over steroid at 12 months |
| Nejati57 (2017) | RCT | RC | 62 | PRP (22) | Physiotherapy (20) | - | VAS-pain ROM DASH Muscle strength WORC score MRI |
6 | Both PRP injection and exercise therapy were effective in reducing pain and disability |
| Rha58 (2013) | RCT, DB | RC | 39 (36–79) | PRP (20) | Dry needling (19) | - | SPADI ROM Adverse events |
6 | PRP injections reduce pain and disability when compared to dry needling |
| Sari59 (2020) | RCT | RC | 129 (27–75) | PRP (33) | Steroid (33) | DP (32); Lidocaine (31) | VAS-pain WORC score ASES |
6 | The 4 interventions are both effective |
| Schwitzguebel60 (2019) | RCT, DB | RC | 80 (18–70) | PRP (41) | Placebo (39) | - | MRI SANE Constant score ASES VAS-pain Adverse events |
12 | No significant differences were found in the 2 groups |
AOFAS: American Orthopaedic Foot and Ankle Society; ASES: American Shoulder and Elbow Surgeons; AT: achilles tendinopathy; ATL: achilles tendon length; ATR: achilles tendinopathy rupture; ATRS: achilles tendon total rupture score; BCTQ: Boston Carpal Tunnel Syndrome Questionnaire; BM-MSCs: bone marrow mesenchymal stem cells; CMAP: compound muscle action potential; CSA: cross-sectional area; CTS: carpal tunnel syndrome; DASH-E: Spanish version of the Disabilities of the Arm, Shoulder and Hand questionnaires; DB: double blind; DC: Dopper Colour (vascolarity); DP: dextrose prolotherapy; DML: distal motor latency; ECRB: extensor carpi radialis brevis; EDC: extensor digitorum communis; ESWT: extracorporeal shock wave therapy; FFI: foot function index; FSS: functional status scale; GROC: global rating of change; HR test: Heel-Rise Test (muscle functional evaluation); LET: lateral epicondyle tendinopathy; LP-PRP: leukocyte-poor PRP; MMCPIE: modified Mayo clinic performance index for elbow; MN: median nerve; MRI: magnetic resonance imaging; NPRS: numeric pain rating scale; OSS: Oxford Shoulder Score; PF: plantar fasciitis; PPP: platelet poor plasma; PRP: platelet rich plasma; PRTEE: Patient-Rated Tennis Elbow Evaluation; Pts: patients; PT: patellar tendinopathy; Q-DASH: disabilities of the Arm, Shoulder and Hand questionnaire; RC: rotator cuff; RCT: randomized controlled trial; ROM: range of motion; SAIS: subacromial impingement syndrome; SANE: single assessment numeric evaluation; SF-12: Short Form 12-Item Health Survey; SNAP: sensory nerve action potential; SNCV: sensory nerve conduction velocity; SPADI: Shoulder Pain and Disability Index;SSS: symptom severity scale; US: ultrasound; UTC: ultrasound tissue characterization; VAS: visual analog scale; VISA: Victorian Institute of Sport Assessment; VISA-A: Victorian Institute of Sports Assessment-Achilles; WB: whole blood; WORC: Western Ontario Rotator Cuff.
Of the 33 studies included in the systematic review, eight were conducted in patients with lateral epicondylitis, five in patients with plantar fasciitis, five in patients with Achilles tendinopathy, seven in patients with rotator cuff tendinopathy, three in patients with patellar tendinopathy, and five in patients with carpal tunnel syndrome. In the 33 studies, PRP was compared to local steroids injection (9 studies or subsets of patients), saline injection (9 studies/subsets of patients), autologous whole blood (2), local anesthetic injection (5), dry needling injection (3), prolotherapy (4), bone marrow-mesenchymal stem cells (1), extracorporeal shock wave therapy (1), radiation (1), and non-injective interventions (e.g., exercise) (5 studies). PRP was given as a single injection in 20 trials, two injections in ten trials, and three or four injections in three trials.
The outcomes more commonly reported included pain and functional measures. For pain, the most commonly reported measure was a VAS score. Functional measures reported were condition-specific tendinopathy measures and generic measures (DASH, PRTEE, Liverpool Elbow Score, VISA-A and VISA-P, ASES, SPADI, BCTQ, AOFAS, MMCPIE, FFI, WORC). Adverse events were recorded and reported in seven out of 33 trials (21 %).
Risk of bias in included studies
Thirty-two out of 33 studies were at high risk of bias for one or more domains, and all were at unclear risk of bias for one or more domains.
Allocation
We assessed two studies as being at high risk of selection bias, as randomization was by alternation of the two treatments45,47 so the intervention allocations could have been foreseen in advance. Two studies47,52 were judged at high risk of selection bias since allocation was not concealed. The reports of the other 22 studies were unclear for random sequence generation and/or allocation concealment, while eight studies were at low risk of selection biases.
Blinding
Ten studies were reported as open label, and they were graded as being at high risk of performance bias (blinding of participants and personnel). Eight studies were graded as at unclear risk of detection bias due to the fact that they did not provide information to enable judgment about “high” or “low” risk of bias related to the blinding of participants and personnel. Fifteen studies were reported as double blind. Twenty-two studies were graded at low risk of detection bias due to the fact that the assessor was blinded to treatment allocation; nine studies were graded at unclear risk of detection bias due to the fact that information was not provided to enable judgment about “high” or “low” risk of bias related to the blinding of outcome assessors; two studies31,50 were graded at “high risk” of bias because the outcome assessors were not blinded to treatment allocation.
Incomplete outcome data
Four studies31,40,42,58 were judged at high risk of attrition bias because a large proportion of enrolled subjects left the study, due to an unsatisfactory effect of the initial treatment or for other reasons. Another three studies35,46,49 were judged at unclear risk of bias. The remaining studies were judged at low risk of bias.
Selective reporting
Selective reporting was low in all included studies for all the outcomes except adverse events. For the outcome adverse events, 26 out of 33 trials (78 %) were judged at high risk of bias. The reporting of adverse events was generally inadequate, and 12 trials did not mention complications of treatment at all. Where there were reports on adverse events, these often comprised short statements on the absence of adverse events in the study results or discussion without indication of systematic recording.
Other potential sources of bias
We judged two studies49,56 to be at high risk of other sources of bias because of unbalance between groups at baseline, and six studies at unclear risk of bias based on limitations acknowledged by the authors (e.g., small size study, short duration of follow-up).
Effects of interventions
For the six clinical conditions considered, we extracted data related to pain and functional scales for 19 comparisons and 67 different follow-up times (Table II).Statistically significant differences in the effect size between groups were found in 12 out of 67 comparisons, favoring PRP in ten instances (rotator cuff: change of VAS score for pain from baseline at 3 months and in the overall analysis; plantar fasciitis: AOFAS at 12 months; carpal tunnel syndrome: BCTQ severity score at 1, 3, and 6 months and in the overall analysis; VAS score for pain at 3 and 6 months, and in the overall analysis), and favoring controls in two instances (patellar tendinopathy: VAS for pain in the overall analysis; carpal tunnel syndrome: BCTQ functional score at 3 months).
Table II.
Summary data and analyses
| Outcome or subgroup | No. studies (participants) | Statistical methods | Effect estimate | p-value | Direction of the effect* |
|---|---|---|---|---|---|
| Achillean tendinopathy | |||||
| VISA-A (increase from baseline)-total | 4 (373) | Mean Difference (IV, Random, 95% CI) | −0.17 [−4.25, 3.90] | 0.93 | No difference between groups |
| At 6 wks | 2 (92) | Mean Difference (IV, Random, 95% CI) | −4.46 [−20.59, 11.67] | 0.59 | No difference between groups |
| At 12 wks | 3 (116) | Mean Difference (IV, Random, 95% CI) | −2.23 [−12.04, 7.58] | 0.66 | No difference between groups |
| At 24 wks | 3 (103) | Mean Difference (IV, Random, 95% CI) | −0.70 [−7.66, 6.25] | 0.84 | No difference between groups |
| At 52 wks | 2 (62) | Mean Difference (IV, Random, 95% CI) | 6.34 [−2.42, 15.09] | 0.12 | No difference between groups |
| VISA-A (values over time)-total | 2 (368) | Mean Difference (IV, Random, 95% CI) | 1.74 [−2.40, 5.87] | 0.41 | No difference between groups |
| At 6 wks | 2 (92) | Mean Difference (IV, Random, 95% CI) | 0.48 [−6.92, 7.88] | 0.90 | No difference between groups |
| At 12 wks | 2 (92) | Mean Difference (IV, Random, 95% CI) | 2.98 [−5.86, 11.82] | 0.51 | No difference between groups |
| At 24 wks | 2 (92) | Mean Difference (IV, Random, 95% CI) | 2.88 [−5.08, 10.83] | 0.48 | No difference between groups |
| At 52 wks | 2 (92) | Mean Difference (IV, Random, 95% CI) | 0.79 [−8.51, 10.10] | 0.87 | No difference between groups |
| Patient satisfaction-total | 3 (299) | Risk Difference (M-H, Fixed, 95% CI) | 0.03 [−0.08, 0.13] | 0.64 | No difference between groups |
| At 12 wks | 2 (84) | Risk Difference (M-H, Fixed, 95% CI) | −0.14 [−0.34, 0.06] | 0.17 | No difference between groups |
| At 24–52 wks | 3 (139) | Risk Difference (M-H, Fixed, 95% CI) | 0.02 [−0.13, 0.18] | 0.76 | No difference between groups |
| PRP vs placebo: 12 wks | 1 (38) | Risk Difference (M-H, Fixed, 95% CI) | 0.26 [−0.04, 0.57] | 0.09 | No difference between groups |
| PRP vs placebo: 24 wks | 1 (38) | Risk Difference (M-H, Fixed, 95% CI) | 0.16 [−0.16, 0.47] | 0.32 | No difference between groups |
| Return to sport activity | 4 (174) | Risk Difference (M-H, Fixed, 95% CI) | −0.05 [−0.19, 0.10] | 0.54 | No difference between groups |
| Rotator cuff tendinopathy | |||||
| VAS for pain (values over time)-total | 8 (1046) | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [−0.18, 0.50] | 0.35 | No difference between groups |
| At 1 mo | 7 (324) Std. | Mean Difference (IV, Random, 95% CI) | 0.68 [−0.09, 1.45] | 0.08 | No difference between groups |
| At 3 mos | 7 (290) | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [−0.53, 1.02] | 0.53 | No difference between groups |
| At 6 mos | 7 (312) | Std. Mean Difference (IV, Random, 95% CI) | −0.25 [−0.70, 0.20] | 0.27 | No difference between groups |
| At 12 mos | 2 (120) | Std. Mean Difference (IV, Random, 95% CI) | −0.21 [−0.56, 0.15] | 0.24 | No difference between groups |
| VAS for pain (change from baseline)-total | 1 (281) | Mean Difference (IV, Fixed, 95% CI) | −8.59 [−14.69, −2.49] | 0.006 | Favours PRP |
| At 1 mo | 1 (99) | Mean Difference (IV, Fixed, 95% CI) | −1.90 [−11.76, 7.96] | 0.71 | No difference between groups |
| At 3 mos | 1 (98) | Mean Difference (IV, Fixed, 95% CI) | −14.00 [−24.36, −3.64] | 0.008 | Favours PRP |
| At 12 mos | 1 (94) | Mean Difference (IV, Fixed, 95% CI) | −11.10 [−22.82, 0.62] | 0.06 | No difference between groups |
| Shoulder functional questionnaires (SPADI, DASH, ASES)-total | 8 (966) | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [−0.12, 0.64] | 0.53 | No difference between groups |
| At 1 mo | 7 (299) | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [−0.31, 1.01] | 0.29 | No difference between groups |
| At 3 mos | 7 (315) | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [−0.39, 1.51] | 0.25 | No difference between groups |
| At 6 mos | 7 (312) | Std. Mean Difference (IV, Random, 95% CI) | −0.05 [−0.59, 0.49] | 0.86 | No difference between groups |
| At 12 mos | 1 (40) | Std. Mean Difference (IV, Random, 95% CI) | −0.11 [−0.73, 0.51] | 0.73 | No difference between groups |
| WORC-total | 6 (762) | Std. Mean Difference (IV, Random, 95% CI) | −3.37 [−8.53, 1.79] | 0.20 | No difference between groups |
| At 1 mo | 6 (260) | Mean Difference (IV, Random, 95% CI) | −0.62 [−9.36, 8.13] | 0.89 | No difference between groups |
| At 3 mos | 6 (260) | Mean Difference (IV, Random, 95% CI) | −3.95 [−14.70, 6.80] | 0.47 | No difference between groups |
| At 6 mos | 5 (202) | Mean Difference (IV, Random, 95% CI) | −8.29 [−19.66, 3.08] | 0.15 | No difference between groups |
| At 12 mos | 1 (40) | Mean Difference (IV, Random, 95% CI) | 9.30 [−6.28, 24.88] | 0.24 | No difference between groups |
| Plantar fasciitis | |||||
| VAS for pain (values over time)-total | 6 (626) | Mean Difference (IV, Random, 95% CI) | −3.62 [−8.16, 0.91] | 0.6 | No difference between groups |
| At 3 mos | 5 (214) | Mean Difference (IV, Random, 95% CI) | −3.22 [−10.56, 4.12] | 0.4 | No difference between groups |
| At 6 mos | 6 (254) | Mean Difference (IV, Random, 95% CI) | −3.55 [−10.92, 3.83] | 0.35 | No difference between groups |
| At 12 mos | 3 (158) | Mean Difference (IV, Random, 95% CI) | −5.48 [−16.76, 5.79] | 0.09 | No difference between groups |
| Functional index (AOFAS, FFI)-total | 6 (674) | Mean Difference (IV, Random, 95% CI) | 5.93 [−5.81, 17.67] | 0.32 | No difference between groups |
| At 3 mos | 5 (218) | Mean Difference (IV, Random, 95% CI) | 6.00 [−4.25, 16.25] | 0.25 | No difference between groups |
| At 6 mos | 6 (258) | Mean Difference (IV, Random, 95% CI) | 3.95 [−11.46, 19.36] | 0.62 | No difference between groups |
| At 12 mos | 4 (198) | Mean Difference (IV, Random, 95% CI) | 28.34 [9.08, 47.60] | 0.004 | Favours PRP |
| Lateral epicondilitis | |||||
| VAS for pain (over time)-total | 4 (785) | Mean Difference (IV, Random, 95% CI) | −0.24 [−0.73, 0.25] | 0.34 | No difference between groups |
| At 1 mo | 4 (203) | Mean Difference (IV, Random, 95% CI) | 0.53 [−0.20, 1.26] | 0.15 | No difference between groups |
| At 3 mos | 4 (203) | Mean Difference (IV, Random, 95% CI) | −0.15 [−1.19, 0.88] | 0.77 | No difference between groups |
| At 6 mos | 4 (203) | Mean Difference (IV, Random, 95% CI) | −0.49 [−1.78, 0.81] | 0.46 | No difference between groups |
| At 12 mos | 3 (176) | Mean Difference (IV, Random, 95% CI) | −1.23 [−2.83, 0.37] | 0.13 | No difference between groups |
| Pain change from baseline (VAS, PRTEE)-total | 4 (501) | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [−0.23, 0.35] | 0.69 | No difference between groups |
| At 1 mo | 4 (157) | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [−0.63, 0.89] | 0.74 | No difference between groups |
| At 3 mos | 4 (157) | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [−0.24, 0.47] | 0.52 | No difference between groups |
| At 6 mos | 4 (113) | Std. Mean Difference (IV, Random, 95% CI) | −0.02 [−0.73, 0.69] | 0.96 | No difference between groups |
| At 12 mos | 3 (74) | Std. Mean Difference (IV, Random, 95% CI) | −0.30 [−1.25, 0.65] | 0.53 | No difference between groups |
| Functional measures over time (PRTEE, Liverpool s., MMCPI)-total | 4 (914) | Std. Mean Difference (IV, Random, 95% CI) | −0.05 [−0.34, 0.23] | 0.71 | No difference between groups |
| At 1 mo | 4 (263) | Std. Mean Difference (IV, Random, 95% CI) | −0.22 [−0.47, 0.02] | 0.08 | No difference between groups |
| At 3 mos | 4 (263) | Std. Mean Difference (IV, Random, 95% CI) | −0.21 [−0.62, 0.20] | 0.99 | No difference between groups |
| At 6 mos | 4 (263) | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [−0.69, 0.76] | 0.92 | No difference between groups |
| At 12 mos | 2 (125) | Std. Mean Difference (IV, Random, 95% CI) | 1.11 [−1.80, 4.03] | 0.45 | No difference between groups |
| Functional measures, change from baseline (PRTEE, Liverpool s., MMCPIE)-total | 3 (241) | Std. Mean Difference (IV, Random, 95% CI) | −0.09 [−0.43, 0.25] | 0.61 | No difference between groups |
| At 1 mo | 3 (107) | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [−0.49, 0.86] | 0.60 | No difference between groups |
| At 3 mos | 3 (107) | Std. Mean Difference (IV, Random, 95% CI) | −0.25 [−0.63, 0.13] | 0.20 | No difference between groups |
| At 6 mos | 1 (27) | Std. Mean Difference (IV, Random, 95% CI) | −0.51 [−1.28, 0.26] | 0.19 | No difference between groups |
| Patellar tendinopathy | |||||
| VAS for pain (over time)-total | 3 (212) | Mean Difference (IV, Random, 95% CI) | 0.17 [−0.64, 0.98] | 0.68 | No difference between groups |
| At 1 mo | 1 (38) | Mean Difference (IV, Random, 95% CI) | 0.60 [−0.86, 2.06] | 0.42 | No difference between groups |
| At 3 mos | 2 (61) | Mean Difference (IV, Random, 95% CI) | 0.39 [−0.88, 1.65] | 0.55 | No difference between groups |
| At 6 mos | 3 (81) | Mean Difference (IV, Random, 95% CI) | −0.26 [−2.12, 1.60] | 0.78 | No difference between groups |
| At 12 mos | 1 (32) | Mean Difference (IV, Random, 95% CI) | 0.40 [−1.06, 1.86] | 0.59 | No difference between groups |
| VISA-P (over time)-total | 3 (212) | Mean Difference (IV, Random, 95% CI) | −5.39 [−10.53, −0.25] | 0.04 | Favours control |
| At 1 mo | 1 (38) | Mean Difference (IV, Random, 95% CI) | −6.00 [−19.76, 7.76] | 0.39 | No difference between groups |
| At 3 mos | 2 (61) | Mean Difference (IV, Random, 95% CI) | −4.07 [−13.12, 4.98] | 0.38 | No difference between groups |
| At 6 mos | 3 (81) | Mean Difference (IV, Random, 95% CI) | −3.93 [−17.45, 9.58] | 0.57 | No difference between groups |
| At 12 mos | 1 (32) | Mean Difference (IV, Random, 95% CI) | −9.00 [−22.18, 4.18] | 0.18 | No difference between groups |
| Carpal tunnel syndrome | |||||
| BCTQ-severity score-total | 4 (724) | Mean Difference (IV, Fixed, 95% CI) | −0.24 [−0.32, −0.16] | <0.00001 | Favours PRP |
| At 1 mo | 4 (258) | Mean Difference (IV, Fixed, 95% CI) | −0.17 [−0.30, −0.03] | 0.02 | Favours PRP |
| At 3 mos | 4 (258) | Mean Difference (IV, Fixed, 95% CI) | −0.31 [−0.46, −0.16] | <0.00001 | Favours PRP |
| At 6 mos | 3 (160) | Mean Difference (IV, Fixed, 95% CI) | −0.29 [−0.47, −0.12] | 0.0009 | Favours PRP |
| At 12 mos | 1 (48) | Mean Difference (IV, Fixed, 95% CI) | −0.20 [−0.43, 0.03] | 0.08 | No difference between groups |
| BCTQ-functional score | 4 (724) | Mean Difference (IV, Random, 95% CI) | −0.22 [−0.40, −0.03] | 0.02 | Favours control |
| At 1 mo | 4 (258) | Mean Difference (IV, Random, 95% CI) | 0.00 [−0.23, 0.24] | 0.98 | No difference between groups |
| At 3 mos | 4 (258) | Mean Difference (IV, Random, 95% CI) | −0.43 [−0.85, −0.01] | 0.05 | Favours control |
| At 6 mos | 3 (160) | Mean Difference (IV, Random, 95% CI) | −0.40 [−0.87, 0.08] | 0.10 | No difference between groups |
| At 12 mos | 1 (48) | Mean Difference (IV, Random, 95% CI) | −0.10 [−0.33, 0.13] | 0.39 | No difference between groups |
| VAS for pain (over time)-total | 2 (376) | Mean Difference (IV, Fixed, 95% CI) | −5.29 [−5.67, −4.91] | <0.0001 | Favours PRP |
| At 1 mo | 2 8158) | Mean Difference (IV, Fixed, 95% CI) | 0.01 [−0.73, 0.75] | 0.98 | No difference between groups |
| At 3 mos | 2 (158) | Mean Difference (IV, Fixed, 95% CI) | −3.97 [−4.61, −3.33] | <0.00001 | Favours PRP |
| At 6 mos | 1 (60) | Mean Difference (IV, Fixed, 95% CI) | −10.20 [−10.82, −9.58] | <0.0001 | Favours PRP |
| Adverse events | |||||
| Pain at injection site (% pts) | 7 (359) | Risk Difference (M-H, Random, 95% CI) | 0.10 [0.01, 0.20] | 0.04 | Favours control |
| VAS for pain | 2 (80) | Mean Difference (IV, Fixed, 95% CI) | 2.50 [1.51, 3.49] | <0.0001 | Favours control |
VAS: visual analogue score; DASH: disabilities of the arm, shoulder and hand; PRTEE: patient-rated tennis elbow evaluation; VISA-A: Victorian Institute of Sports Assessments for achillean tendinopathy and for patellar tendinopathy (VISA-P); ASES: American Shoulder and Elbow Surgeons; SPADI: shoulder pain and disability index; BCTQ: Boston Carpal Tunnel Questionnaire, FSS: Functional Status Scale (FSS); SS Severity status Scale; AOFAS: American Orthopedic Foot and Ankle Society Score ; MMCPIE: Mayo Clinic Performance Index for Elbow; FFI: foot function index; WORC: West Ontario rotator cuff index; Std.: standardized; IV: inverse variance method; M-H: Mantel-Haenszel method; Fixed: fixed effect model; random: random effect model.
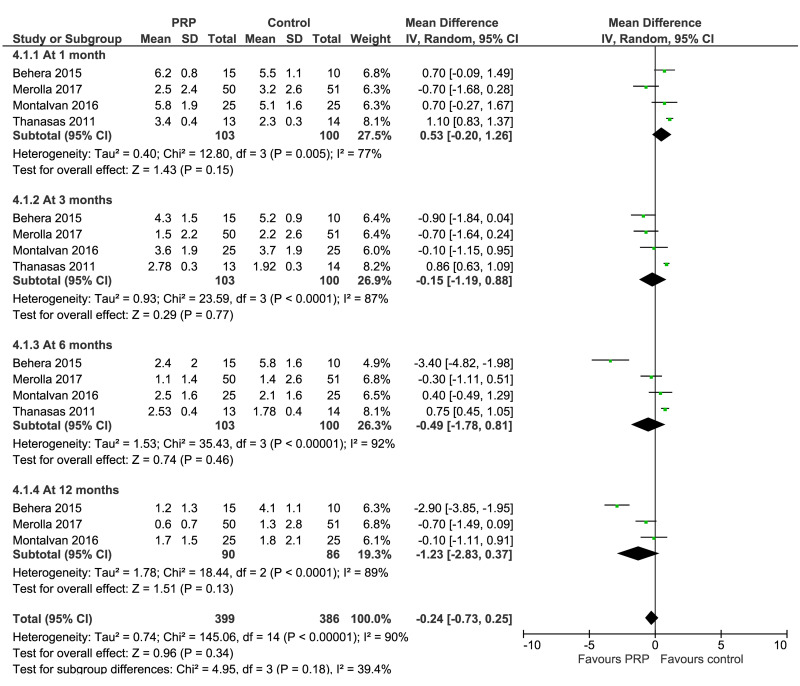
Lateral epicondylitis
Data from eight studies investigating PRP for lateral epicondylitis reported VAS or PRTEE (1 study) for pain over time and/or change from baseline at 1, 3, 6, and 12 months after treatment; functional measure scales reported were PRTEE, the Liverpool Elbow Scale, and MMCPIE. (Figure 2). The VAS scores for pain at 1 month (4 trials, 203 patients), 3 months (4 trials, 203 patients), 6 months (4 trials, 203 patients), and 12 months (3 trials, 176 patients) showed no significant between-group differences, although a trend towards a progressive reduction of pain score over time was observed in PRP recipients compared to controls (very low quality of evidence, downgraded for imprecision, inconsistency, and risk of bias); likewise, change of pain from baseline was not significantly different between PRP recipients and controls during the study period.
Figure 2.
Forest plot. Lateral epicondylitis. Outcome: pain score over time
PRP: platelet-rich plasma; SD: standard deviation; 95% CI: 95% confidence interval.
Functional measures over time (263 patients and 4 trials at 1, 3 and 6 months; 125 patients, 2 trials at 12 months) showed no clear between-group differences in PRP recipients and controls, although a trend towards a progressive increase of functional score over time was observed in PRP recipients compared to controls; very low quality of evidence, downgraded for imprecision, inconsistency, and risk of bias (Summary of findings table, Table SI).
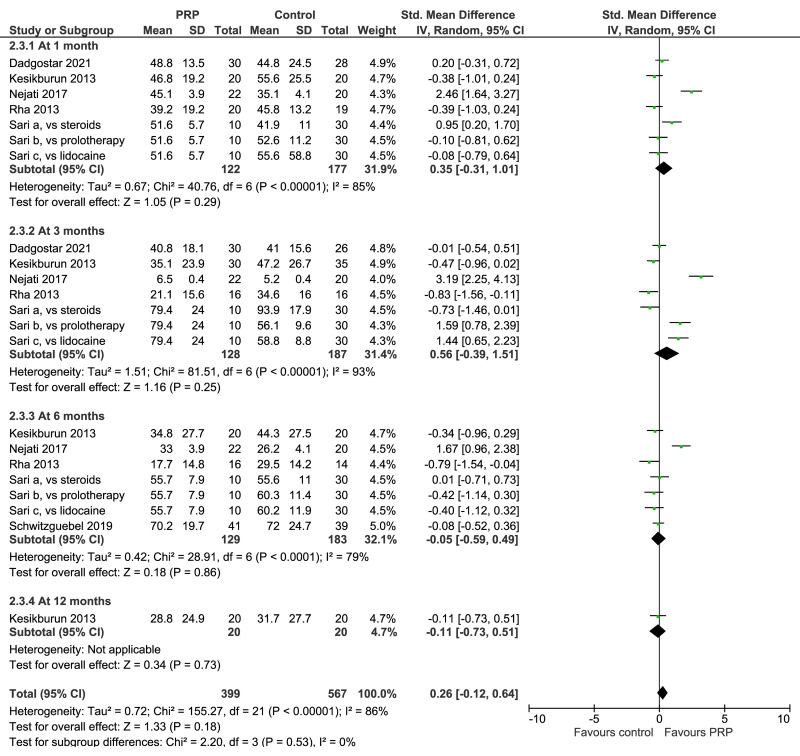
Rotator cuff tendinopathy
Seven trials investigating PRP for rotator cuff tendinopathy reported VAS scores for pain over time and/or change from baseline, and shoulder functional questionnaires (WORC, and/or SPADI, DASH, ASES). (Figure 3). VAS scores for pain at 1 month were lower in controls than in PRP recipients (5 trials, 324 patients), while at 3 months (5 trials, 290 patients), at 6 months (5 trials, 312 patients), and 12 months (2 studies, 120 patients) no clear between-group differences were observed (Figure 3, left). Likewise, shoulder functional questionnaires showed comparable scores over time for PRP recipients and controls (Figure 3, right). All these comparisons were graded as very low quality evidence due to risk of bias, inconsistency and imprecision.
Figure 3.
Forest plot. Rotator cuff tendinopathy. Outcomes: visual analog scale score for pain (left), and shoulder functional questionnaires (right)
PRP: platelet-rich plasma; SD: standard deviation; 95% CI: 95% confidence interval.
Achilles tendinopathy
VISA-A was reported in four trials as values over time or change from baseline values at 1, 3, 6 and 12 months. Scores ranged from 0 – 100, with 0 being the worst. In both groups, VISA-A increased over time, but the differences between PRP recipients and controls were not statistically significant: low quality of evidence, downgraded for imprecision and risk of bias. Others outcomes reported were patients’ satisfaction (3 studies) and return to sport activity (4 studies), and both showed no significant between-group differences: low quality of evidence due to serious imprecision.
Plantar fasciitis
Five trials reported VAS scores for pain and/or functional scores (AOFAS, FFI). Pain at 1, 3, 6, and 12 months after treatment did not differ significantly in PRP recipients and controls. Likewise, functional indices were comparable between groups at 1, 3 and 6 months, but at 12 months were significantly higher (better) in PRP recipients (MD: 28.3; 95% CI: 9.0/47.6; p=0.04). In both cases, the quality of evidence was judged as very low due to imprecision, inconsistency and risk of bias.
Patellar tendinopathy
The VAS score for pain was reported in three trials; there was a trend towards decreased pain over time in both groups, but the differences between PRP and controls were not statistically significant. As far as functional scores are concerned, three trials reported VISA-P as a measure of severity of the tendinopathy; there was a trend towards an increase of VISA-P over time in both arms, but the differences between PRP recipients and control were not statistically significant (low quality of evidence due to imprecision and risk of bias).
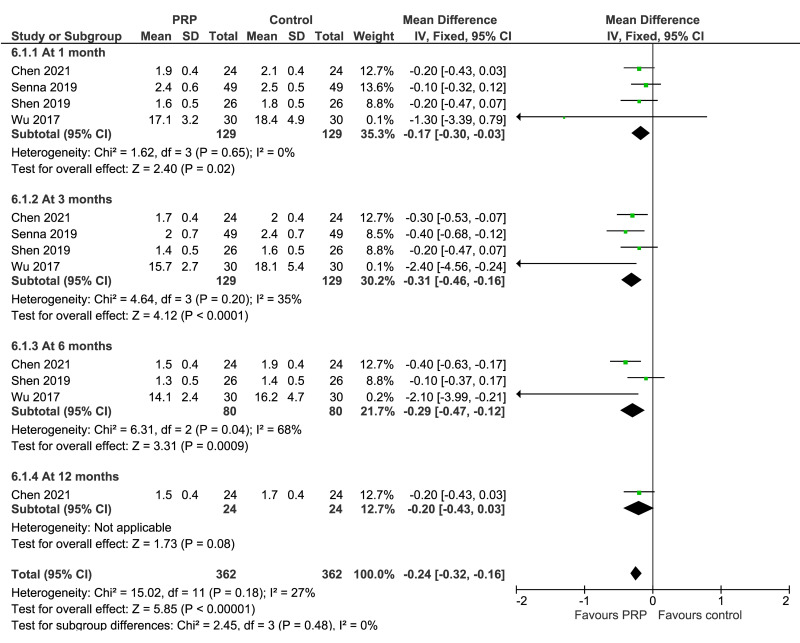
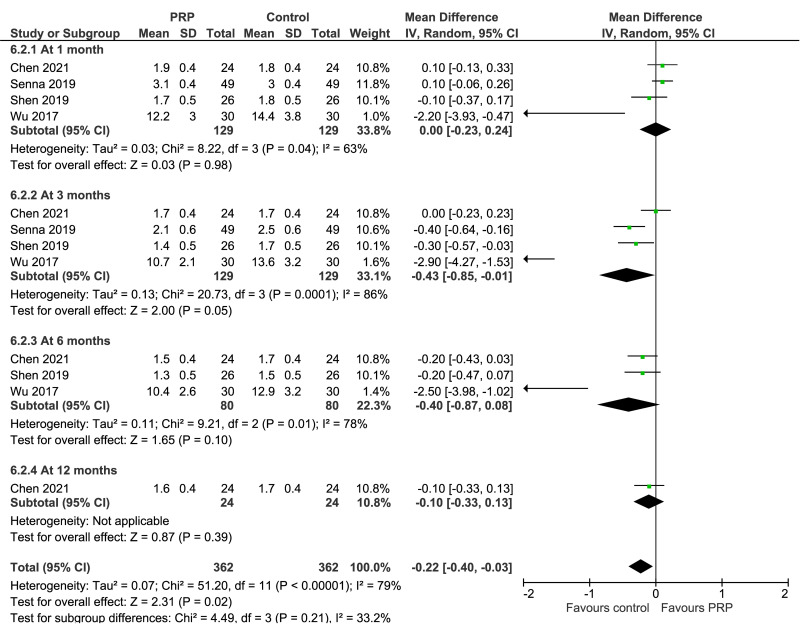
Carpal tunnel syndrome
VAS scores for pain at 3 months (2 trials, 158 patients) and 6 months (1 trial, 60 patients) were significantly lower in PRP recipients (MD: −3.97; 95% CI: −4.51 to −3.33; and MD: −10.2; 95% CI: −10.8 to −9.58, respectively): low certainty of evidence due to imprecision and risk of bias; at 1 month (2 studies, 158 patients) no difference between groups were observed. BCTQ severity score at 1 month (4 trials, 258 patients), 3 months (4 trials, 258 patients) and 6 months (3 trials, 160 patients) showed significantly lower (better) results in PRP recipients compared to controls (MD: −0.17; 95% CI: −0.30 to −0.03; MD: −0.31; 95% CI: −0.46 to −0.16, and MD: −0.29; 95% CI: −0.47 to −0.12, respectively) (low certainty of evidence due to inconsistency and risk of bias). BCTQ severity score at 12 months (1 trial, 48 patients), and BCTQ functional score at 1 month (4 trials, 158 patients), 6 months (3 trials, 160 patients), and 12 months (1 trial, 48 patients) were not significantly different between groups, and of borderline significance (MD: −0.04; 95% CI: −0.86 to −0.01; p=0.05) favoring controls at 3 months (4 trials, 158 patients): level of certainty from low to very-low (Figure 4).
Figure 4.
Forest plot. Carpal tunnel syndrome. Outcomes: Boston Carpal Tunnel Questionnaire-severity score (left), and Boston Carpal Tunnel Questionnaire-functional score (right)
PRP: platelet-rich plasma; SD: standard deviation; 95% CI: 95% confidence interval.
Subgroup analyses
The results were much the same in subgroup analyses of studies evaluating a single injection (20 studies) or multiple injections (13 studies) of PRP: the differences in the effect size between PRP recipients and controls did not differ significantly regardless of the number of injections administered (data not shown).
Adverse events
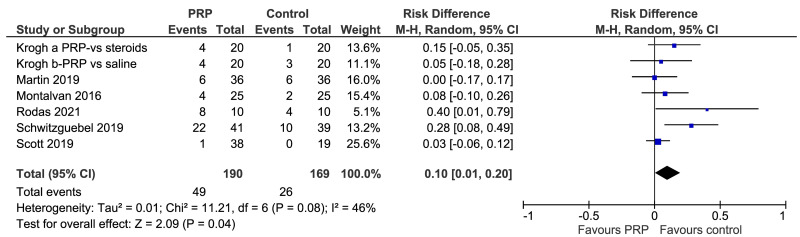
No participant reported having developed any serious events (e.g., application site infections, inflammatory reaction) in the follow-up period (from 1 to 24 months) in either the PRP or control group. This comparison was graded as moderate quality evidence and downgraded once due to a risk of reporting bias because most trials did not describe monitoring processes for identifying or recording complications and usually limited reporting to a single statement of their absence.
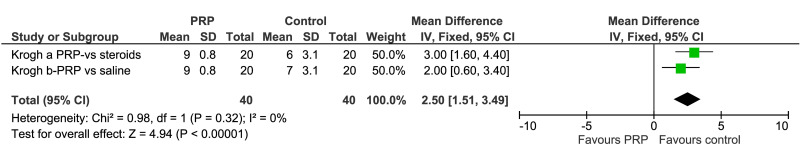
Other less serious, short-term adverse events, mostly post-injection pain, were recorded and reported in seven trials (Figure 5). Pain at the injection site was more common among PRP recipients than among controls receiving other US-guided injections (saline in 4 trials, and steroid, lidocaine, or bone marrow-mesenchymal stem cells in 1 trial each): risk difference: 0.10 (95% CI: 0.01 to 0.20, p=0.04) (Figure 5, top). However, as for serious adverse events, the quality of the evidence was graded moderate since most trials did not describe monitoring processes for identifying or recording overall side effects, including pain at the injection site, or limited reporting to a single statement of their absence. The VAS score for pain associated with injection was reported in one trial (Krogh) (mean difference favoring controls: 2.50; 95% CI: 1.51 to 3.49) (Figure 5, bottom).
Figure 5.
Forest plot. Outcome: Adverse events. Pain at the injection site (top), and VAS for pain associated with injection (bottom)
PRP: platelet-rich plasma; M-H: Mantel-Haenszel; 95% CI: 95% confidence interval.
DISCUSSION
PRP has been used in clinical practice to promote healing in a wide array of musculoskeletal disorders61. Infiltrative treatment with PRP to accelerate the healing of injured tendons, ligaments, muscles and joints has increased exponentially during the last years, although evidence of its efficacy has been highly variable5,9,12–15. The procedure used to inject PRP into an affected area is crucial when assessing the effectiveness of PRP in the treatment of tendinopathies, and available evidence suggests that using US improves the accuracy and effectiveness of injection therapy16,19,62–64. For this reason, we have updated our previous systematic review5, focusing only on RCT comparing US-guided PRP injections to controls for the conservative treatment of tendinopathies.
In the present systematic review and meta-analysis, we found low/very low-quality evidence that PRP administered under US-guided injection may not result in lower pain and function scores in the short-, medium-, or long-term follow-up, compared to control treatments (mostly US-guided injections of steroids, saline, and/or anesthetic). In this review we included 33 trials evaluating patients with lateral epicondylitis, Achilles tendinopathy, plantar fasciitis, patellar tendinopathy, rotator cuff tendinopathy, and carpal tunnel syndrome. Although a trend towards pain reduction and functional improvement from baseline was observed after US-guided PRP injection, in the majority of the comparisons the effect size observed in PRP recipients was comparable to that observed in control groups. In most of the comparisons, the 95% CI for the effect size crossed the line of no benefit, and at best indicates the possibility of a very marginal clinical benefit. Of note, in trials conducted in patients with carpal tunnel syndrome there was evidence that VAS scores for pain at 3 and 6 months, and the BCTQ severity score at 1, 3 and 6 months were significantly lower (better) in PRP recipients than in controls. However, the level of evidence was graded as low due to inconsistency (low number of small-size trials included) and risk of bias. Overall, most of the differences observed for the comparisons between groups at different time points were below the minimal clinically important difference, and at best indicate the possibility of a very marginal clinical benefit65–67.
Pain at the injection site was more common among PRP recipients than in controls receiving other US-guided injections (moderate certainty of evidence). According to our subgroup analyses, a single injection was as effective as multiple PRP injections for both pain and functional scores. Likewise, in a systematic review evaluating PRP injection in the treatment of knee osteoarthritis, a single injection was as effective as multiple PRP injections at improving pain; however, for these clinical indications multiple injections seemed more effective in joint functionality than a single injection at 6 months68.
The quantitative analysis conducted in this systematic review has several limitations that do not allow us to draw definite conclusions on the efficacy of PRP in this setting. The first limitation is certainly related to the lack of standardization of PRP production among the different studies, which made the PRP products heterogeneous and qualitatively different from each other, which limits the validity of inter-study comparisons. Compared to the previously published systematic reviews, however, the current review includes only trials in which the PRP was injected under US guidance, thus reducing inconsistency related to anatomically-guided injection techniques62. Actually, ultrasonography guidance is increasingly recommended for several tendinopathy procedures to ensure the precision and efficacy of these treatments63,64. Another limitation of this meta-analysis is that only 13 of the 33 trials included in this review reported the long-term (≥12 months) effect of PRP. Therefore, the comparison at 12 months for each clinical condition was often limited to one or two trials. It is possible, as claimed by some investigators, that the best clinical benefit of PRP application in orthopedics may occur in the long-term period69. Nevertheless, in the current analysis no clear benefits of PRP over control were observed in long-term follow-up, with the exception of a significantly higher increase of functional index in patients with plantar fasciitis treated with PRP compared to controls. At present, specific contributions supporting the use of PRP in tendinopathies lack the methodological features essential to define the clinical efficacy of the treatment. Furthermore, there is no defined consensus on the type and standards of the product, or on the mode of application. There is wide variability in PRP production methods depending on the various instruments and concentration techniques used70. Not all PRP treatments are the same, with the most important differences being related to the initial volume of blood, the centrifugation system used, the concentration of platelets per volume of PRP, and the type of activation. It is important that the amount of PRP per injection is standardized. The same holds true for the depth of the injection and the distance between injected sites.
CONCLUSIONS
We conclude that there is insufficient evidence to routinely recommend US-guided PRP injections. Further well-designed, large randomized trials are needed to better define potential indications for, long-term benefits of, and optimal treatment protocols for PRP as a conservative treatment in orthopedics. These studies should also perform an adequate cost-benefit analysis of US-guided PRP therapy compared to other US-guided interventions.
Supplementary Information
Footnotes
AUTHORSHIP CONTRIBUTIONS
MC, FM, IP, EV, and SP made substantial contributions to the conception and design of the work. MC, FM, and IP drafted the protocol. MC and FM extracted data and performed the statistical analysis. All the Authors contributed to interpreting the data of the work, revised the work and approved the final version.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Sheean AJ, Anz AW, Bradley JP. Platelet-rich plasma: fundamentals and clinical applications. Arthroscopy. 2021;37:2732–2734. doi: 10.1016/j.arthro.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 3.Hurley ET. Editorial commentary: Platelet-rich plasma for rotator cuff repairs: no evidence for improved long-term outcomes … yet! Arthroscopy. 2022;38:62–64. doi: 10.1016/j.arthro.2021.06.035. [DOI] [PubMed] [Google Scholar]
- 4.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987–996. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 5.Franchini M, Cruciani M, Mengoli C, Marano G, Pupella S, Veropalumbo E, et al. Efficacy of platelet-rich plasma as conservative treatment in orthopaedics: a systematic review and meta-analysis. Blood Transfus. 2018;16:502–513. doi: 10.2450/2018.0111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field LD. Editorial commentary: Elbow lateral epicondylitis treatment using platelet-rich plasma. Arthroscopy. 2021;37:3368–3370. doi: 10.1016/j.arthro.2021.05.048. [DOI] [PubMed] [Google Scholar]
- 7.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624–634. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi LA, Piuzzi N, Giunta D, Tanoira I, Brandariz R, Pasqualini I, et al. Subacromial platelet-rich plasma injections decrease pain and improve functional outcomes in patients with refractory rotator cuff tendinopathy. Arthroscopy. 2021;37:2745–2753. doi: 10.1016/j.arthro.2021.03.079. Epub 2021 Apr 20. [DOI] [PubMed] [Google Scholar]
- 9.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;29:2014CD010071. doi: 10.1002/14651858.CD010071.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen PC, Wu KT, Chou WY, Huang YC, Wang LY, Yang TH, et al. Comparative effectiveness of different nonsurgical treatments for patellar tendinopathy: a systematic review and network meta-analysis. Arthroscopy. 2019;35:3117–3131e2. doi: 10.1016/j.arthro.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Baksh N, Hannon CP, Murawski CD, Smyth NA, Kennedy JG. Platelet-rich plasma in tendon models: a systematic review of basic science literature. Arthroscopy. 2013;29:596–607. doi: 10.1016/j.arthro.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 12.de Vos RJ, van Veldhoven PL, Moen MH, Weir A, Tol JL, Maffulli N. Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull. 2010;95:63–67. doi: 10.1093/bmb/ldq006. [DOI] [PubMed] [Google Scholar]
- 13.de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48:952–956. doi: 10.1136/bjsports-2013-093281. [DOI] [PubMed] [Google Scholar]
- 14.Krogh TP, Bartels EM, Ellingsen T, Stengaard-Pedersen K, Buchbinder R, Fredberg U, et al. Comparative effectiveness of injection therapies in lateral epicondylitis: a systematic review and network meta-analysis of randomized controlled trials. Am J Sports Med. 2013;41:1435–1446. doi: 10.1177/0363546512458237. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick J, Bulsara M, Zheng MH. The effectiveness of platelet-rich plasma in the treatment of tendinopathy: a meta-analysis of randomized controlled clinical trials. Am J Sports Med. 2017;45:226–233. doi: 10.1177/0363546516643716. [DOI] [PubMed] [Google Scholar]
- 16.Guerini H, Ayral X, Vuillemin V, Morvan G, Thévenin F, Campagna R, et al. Ultrasound-guided injection in osteoarticular pathologies: General principles and precautions. Diagnostic and Interventional Imaging. 2012;93:674–679. doi: 10.1016/j.diii.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Barile A, La Marra A, Arrigoni F, Mariani S, Zugaro L, Splendiani A, et al. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol. 2016;89:20150355. doi: 10.1259/bjr.20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tortora S, Messina C, Albano D, Serpi F, Corazza A, Carrafiello G, et al. Ultrasound-guided musculoskeletal interventional procedures around the elbow, hand and wrist excluding carpal tunnel procedures. J Ultrason. 2021;21:e169–e176. doi: 10.15557/JoU.2021.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang WH, Chen XT, Vangsness CT., Jr Ultrasound-Guided Knee Injections Are More Accurate Than Blind Injections: A Systematic Review of Randomized Controlled Trials. Arthrosc Sports Med Rehabil. 2021;3:e1177–e1187. doi: 10.1016/j.asmr.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. [Accessed on 01/04/2022]. Available from: www.handbook.cochrane.org. [Google Scholar]
- 23.Macdermid JC, Silbernagel KG. Outcome evaluation in tendinopathy: foundations of assessment and a summary of selected measures. J Orthop Sports Phys Ther. 2015;45:950–964. doi: 10.2519/jospt.2015.6054. [DOI] [PubMed] [Google Scholar]
- 24.Angst F, Schwyzer HK, Aeschlimann A, Simmen BR, Goldhahn J. Measures of adult shoulder function: Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and its short version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, Constant (Murley) Score (CS), Simple Shoulder Test (SST), Oxford Shoulder Score (OSS), Shoulder Disability Questionnaire (SDQ), and Western Ontario Shoulder Instability Index (WOSI) Arthritis Care Res (Hoboken) 2011;63:S174–188. doi: 10.1002/acr.20630. [DOI] [PubMed] [Google Scholar]
- 25.Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and “Summary of findings” tables. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Accessed on 01/04/2022]. Available from: handbook.cochrane.org. [Google Scholar]
- 26.Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Accessed on 01/04/2022]. Available from handbook.cochrane.org. [Google Scholar]
- 27.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boesen AP, Hansen R, Boesen MI, Malliaras P, Langberg H. Effect of high-volume injection, platelet-rich plasma, and sham treatment in chronic midportion Achilles tendinopathy: a randomized double-blinded prospective study. Am J Sports Med. 2017;45:2034–2043. doi: 10.1177/0363546517702862. [DOI] [PubMed] [Google Scholar]
- 29.De Jonge S, de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double blind randomized placebo-controlled trial. Am J Sports Med. 2011;39:1623–1629. doi: 10.1177/0363546511404877. [DOI] [PubMed] [Google Scholar]
- 30.De Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 31.Krogh TP, Ellingsen T, Christensen R, Jensen P, Fredberg U. Ultrasound-guided injection therapy of Achilles tendinopathy with platelet-rich plasma or saline: a randomized, blinded, placebo-controlled trial. Am J Sports Med. 2016;44:1990–1997. doi: 10.1177/0363546516647958. [DOI] [PubMed] [Google Scholar]
- 32.Boesen AP, Boesen MI, Hansen R, Barfod KW, Lenskjold A, Malliaras P, et al. Effect of platelet-rich plasma on nonsurgically treated acute Achilles tendon ruptures: a randomized, double-blinded prospective study. Am J Sports Med. 2020;48:2268–2276. doi: 10.1177/0363546520922541. [DOI] [PubMed] [Google Scholar]
- 33.Chen SR, Shen YP, Ho TY, Li TY, Su YC, Chou YC, et al. One-year efficacy of platelet-rich plasma for moderate-to-severe carpal tunnel syndrome: a prospective, randomized, double blind, controlled trial. Arch Phys Med Rehabil. 2021;102:951–958. doi: 10.1016/j.apmr.2020.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Malahias MA, Nikolaou VS, Johnson EO, Kaseta MK, Kazas ST, Babis GC. Platelet-rich plasma ultrasound-guided injection in the treatment of carpal tunnel syndrome: A placebo-controlled clinical study. J Tissue Eng Regen Med. 2018;12:e1480–e1488. doi: 10.1002/term.2566. [DOI] [PubMed] [Google Scholar]
- 35.Senna MK, Shaat RM, Ali AAA. Platelet-rich plasma in treatment of patients with idiopathic carpal tunnel syndrome. Clin Rheumatol. 2019;38:3643–3654. doi: 10.1007/s10067-019-04719-7. [DOI] [PubMed] [Google Scholar]
- 36.Shen YP, Li TY, Chou YC, Ho TY, Ke MJ, Chen LC, et al. Comparison of perineural platelet-rich plasma and dextrose injections for moderate carpal tunnel syndrome: A prospective randomized, single-blind, head-to-head comparative trial. J Tissue Eng Regen Med. 2019;13:2009–2017. doi: 10.1002/term.2950. [DOI] [PubMed] [Google Scholar]
- 37.Wu YT, Ho TY, Chou YC, Ke MJ, Li TY, Huang GS, et al. Six-month efficacy of platelet-rich plasma for carpal tunnel syndrome: a prospective randomized, single-blind controlled trial. Sci Rep. 2017;7:94. doi: 10.1038/s41598-017-00224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg. 2015;23:6–10. doi: 10.1177/230949901502300102. [DOI] [PubMed] [Google Scholar]
- 39.Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966–971. doi: 10.1136/bjsm.2010.082503. [DOI] [PubMed] [Google Scholar]
- 40.Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625–635. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- 41.Lim W, Park SH, Kim B, Kang SW, Lee JW, Moon YL. Relationship of cytokine levels and clinical effect on platelet-rich plasma-treated lateral epicondylitis. J Orthop Res. 2018;36:913–920. doi: 10.1002/jor.23714. [DOI] [PubMed] [Google Scholar]
- 42.Martin JI, Atilano L, Merino J, Gonzalez I, Iglesias G, Areizaga L, et al. Platelet-rich plasma versus lidocaine as tenotomy adjuvants in people with elbow epicondylopathy: a randomized controlled trial. J Orthop Surg Res. 2019;14:109. doi: 10.1186/s13018-019-1153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merolla G, Dellabiancia F, Ricci A, Mussoni MP, Nucci S, Zanoli G, et al. Arthroscopic debridement versus platelet-rich plasma injection: a prospective, randomized, comparative study of chronic lateral epicondylitis with a nearly 2-year follow-up. Arthroscopy. 2017;33:1320–1329. doi: 10.1016/j.arthro.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Montalvan B, Le Goux P, Klouche S, Borgel D, Hardy P, Breban M. Inefficacy of ultrasound-guided local injections of autologous conditioned plasma for recent epicondylitis: results of a double-blind placebo-controlled randomized clinical trial with one-year follow-up. Rheumatology. 2016;55:279–285. doi: 10.1093/rheumatology/kev326. [DOI] [PubMed] [Google Scholar]
- 45.Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130–2134. doi: 10.1177/0363546511417113. [DOI] [PubMed] [Google Scholar]
- 46.Gogna P, Gaba S, Mukhopadhyay R, Gupta R, Rohilla R, Yadav L. Plantar fasciitis: A randomized comparative study of platelet rich plasma and low dose radiation in sports persons. Foot (Edinb) 2016;28:16–19. doi: 10.1016/j.foot.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PM R. 2014;6:152–158. doi: 10.1016/j.pmrj.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 48.Malahias MA, Mavrogenis AF, Nikolaou VS, Megaloikonomos PD, Kazas ST, Chronopoulos E, et al. Similar effect of ultrasound-guided platelet-rich plasma versus platelet-poor plasma injections for chronic plantar fasciitis. Foot (Edinb) 2019;38:30–33. doi: 10.1016/j.foot.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Monto RR. Platelet-rich plasma efficacy versus corticosteroid injection treatment for chronic severe plantar fasciitis. Foot Ankle Int. 2014;35:313–318. doi: 10.1177/1071100713519778. [DOI] [PubMed] [Google Scholar]
- 50.Uğurlar M, Sönmez MM, Uğurlar ÖY, Adıyeke L, Yıldırım H, Eren OT. Effectiveness of four different treatment modalities in the treatment of chronic plantar fasciitis during a 36-month follow-up period: a randomized controlled trial. J Foot Ankle Surg. 2018;57:913–918. doi: 10.1053/j.jfas.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Dragoo JL, Wasterlain AS, Braun HJ, Nead KT. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610–618. doi: 10.1177/0363546513518416. [DOI] [PubMed] [Google Scholar]
- 52.Rodas G, Soler-Rich R, Rius-Tarruella J, Alomar X, Balius R, Orozco L, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492–1504. doi: 10.1177/0363546521998725. [DOI] [PubMed] [Google Scholar]
- 53.Scott A, LaPrade RF, Harmon KG, Filardo G, Kon E, Della Villa S, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654–1661. doi: 10.1177/0363546519837954. [DOI] [PubMed] [Google Scholar]
- 54.Dadgostar H, Fahimipour F, Pahlevan Sabagh A, Arasteh P, Razi M. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013;41:2609–2616. doi: 10.1177/0363546513496542. [DOI] [PubMed] [Google Scholar]
- 56.Kwong CA, Woodmass JM, Gusnowski EM, Bois AJ, Leblanc J, More KD, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510–517. doi: 10.1016/j.arthro.2020.10.037. [DOI] [PubMed] [Google Scholar]
- 57.Nejati P, Ghahremaninia A, Naderi F, Gharibzadeh S, Mazaherinezhad A. Treatment of subacromial impingement syndrome: platelet-rich plasma or exercise therapy? A randomized controlled trial. Orthop J Sports Med. 2017;5:2325967117702366. doi: 10.1177/2325967117702366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rha DW, Park GY, Kim YK, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013;27:113–122. doi: 10.1177/0269215512448388. [DOI] [PubMed] [Google Scholar]
- 59.Sari A, Eroglu A. Comparison of ultrasound-guided platelet-rich plasma, prolotherapy, and corticosteroid injections in rotator cuff lesions. J Back Musculoskelet Rehabil. 2020;33:387–396. doi: 10.3233/BMR-191519. [DOI] [PubMed] [Google Scholar]
- 60.Schwitzguebel AJ, Kolo FC, Tirefort J, Kourhani A, Nowak A, Gremeaux V, et al. Efficacy of platelet-rich plasma for the treatment of interstitial supraspinatus tears: a double-blinded, randomized controlled trial. Am J Sports Med. 2019;47:1885–1892. doi: 10.1177/0363546519851097. [DOI] [PubMed] [Google Scholar]
- 61.Cohn CS, Lockhart E, McCullough JJ. The use of autologous platelet-rich plasma in the orthopaedic setting. Transfusion. 2015;55:1812–1820. doi: 10.1111/trf.13005. [DOI] [PubMed] [Google Scholar]
- 62.Daley EL, Bajaj S, Bisson LJ, Cole BJ. Improving injection accuracy of the elbow, knee, and shoulder: does injection site and imaging make a difference? A systematic review. Am J Sports Med. 2011;39:656–662. doi: 10.1177/0363546510390610. [DOI] [PubMed] [Google Scholar]
- 63.Sconfienza LM, Adriaensen M, Albano D, Alcala-Galiano A, Allen G, Aparisi Gómez MP, et al. Clinical indications for image-guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)-part V, knee. Eur Radiol. 2021 doi: 10.1007/s00330-021-08258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sconfienza LM, Adriaensen M, Albano D, Alcala-Galiano A, Allen G, Aparisi Gómez MP, et al. Clinical indications for image-guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)-part VI, foot and ankle. Eur Radiol. 2022;32:1384–1394. doi: 10.1007/s00330-021-08125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landorf KB, Radford JA, Hudson S. Minimal important difference (MID) of two commonly used outcome measures for foot problems. J Foot Ankle Res. 2010;3:7. doi: 10.1186/1757-1146-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCormack J, Underwood F, Slaven E, Cappaert T. The minimum clinically important difference on the VISA-A and LEFS for patients with insertional Achilles tendinopathy. Int J Sports Phys Ther. 2015;10:639–644. [PMC free article] [PubMed] [Google Scholar]
- 67.Louwerens JKG, van den Bekerom MPJ, van Royen BJ, Eygendaal D, van Noort A, Sierevelt IN. Quantifying the minimal and substantial clinical benefit of the Constant-Murley score and the Disabilities of the Arm, Shoulder and Hand score in patients with calcific tendinitis of the rotator cuff. JSES Int. 2020;4:606–611. doi: 10.1016/j.jseint.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vilchez-Cavazos F, Millán-Alanís JM, Blázquez-Saldaña J, Álvarez-Villalobos N, Peña-Martínez VM, Acosta-Olivo CA, et al. Comparison of the clinical effectiveness of single versus multiple injections of platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Orthop J Sports Med. 2019;7:2325967119887116. doi: 10.1177/2325967119887116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller LE, Parrish WR, Roides B, Bhattacharyya S. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017-000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author›s perspective. J Cutan Aesthet Surg. 2014;7:189–197. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.