Abstract
Gender medicine deals with differences in approach to diagnostic work-up and management according to gender. Although the issue is relevant in every field of medicine, it is often neglected. However, the recent SARS-CoV-2 pandemic has made consideration of gender even more urgent. In fact, available literature has suggested a higher number of deaths among infected men than in women and more side effects in women than in male recipients of certain anti-COVID-19 vaccines. This review examines sex-disaggregated data on thrombotic and bleeding events associated with vaccination against COVID-19. Thrombotic complications are by far more frequently reported than bleeding events after vaccination and are mostly observed in young women receiving viral-vectored vaccines. However, detailed data that could help better stratify the risk according to sex/gender are generally lacking. Likewise, overall bleeding complications and those associated with a specific vaccine are mainly reported as aggregated data, including thrombocytopenia that is reported to occur in the presence or absence of thrombotic complications. Such information is important as it underlines the need to differentiate between thrombocytopenia with and without thrombosis because management and prognosis differ according to the association of thrombotic events. Here, we highlight how the lack of disaggregated data has led to the publication of conflicting information about adverse events by sex in recipients of viral-vectored vaccines. Lastly, we examine the possible mechanisms underlying vaccine-associated thrombotic and bleeding complications according to sex/gender.
Keywords: thrombosis, hemorrhage, vaccination, COVID-19, sex/gender-disaggregated data
INTRODUCTION
As of 11 February 2022, there had been 404,910,528 confirmed cases and 5,783,776 deaths due to the novel coronavirus disease 2019 (COVID-19) reported worldwide. COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The advent of vaccines against the infection has saved a significant number of lives. By 7 February 2022, a total of 10,095,615,243 vaccine doses had been administered1.
COVID-19 is now considered a systemic disease, as it may affect different organs. Signs and symptoms range from mild to severe acute respiratory distress syndrome to a severe multisystem inflammatory syndrome2.
Among other manifestations, thrombotic events, both arterial and venous, as well as hemorrhagic complications, have been reported3. Thrombocytopenia has been described as being potentially associated with COVID-19 disease and/or vaccination. In recipients of vaccines against COVID-19, venous thromboses at unusual sites, such as cerebral sinus vein thrombosis (CVST) or splanchnic vein thrombosis (SVT), have been reported, sometimes in association with thrombocytopenia (thrombosis thrombocytopenia syndrome [TTS]). They seem to be more frequent in women under 55 years of age4 and are observed approximately 5–30 days (d) after vaccination with virus-vectored vaccines (i.e., ChAdOx1 nCoV-19, Astra-Zeneca [Cambridge, UK] and Ad26.COV2.S Janssen [Beerse, Belgium])5.
Greinacher et al. described the physio-pathological mechanisms underlying TTS that are similar to those described in patients developing heparin-induced thrombocytopenia (HIT)6. Indeed, in both situations, immunoglobulin G class platelet-activating antibodies are directed against the platelet chemokine, platelet factor 4 (PF4).
Sarah Hawkes, co-director of Global Health 50/50, observed that “The COVID-19 pandemic has shone a light on the importance of sex and gender in a way that few other conditions have managed to do”. However, in spite of this, the largest COVID-19 trials sometimes did not include any analysis according to sex7. Likewise, few sex-disaggregated data are available in literature on thrombotic events or bleeding complications after administration of vaccines against COVID-19. This analysis is important because, for example, vaccine studies showed that cisgender females develop a higher antibody response and, in turn, higher efficacy and more side-effects, suggesting the need for sex-differentiated dosing regimens8.
We reviewed the available literature to investigate the presence of sex/gender-disaggregated data with regard to thrombotic and bleeding events following vaccination against COVID-19.
MATERIALS AND METHODS
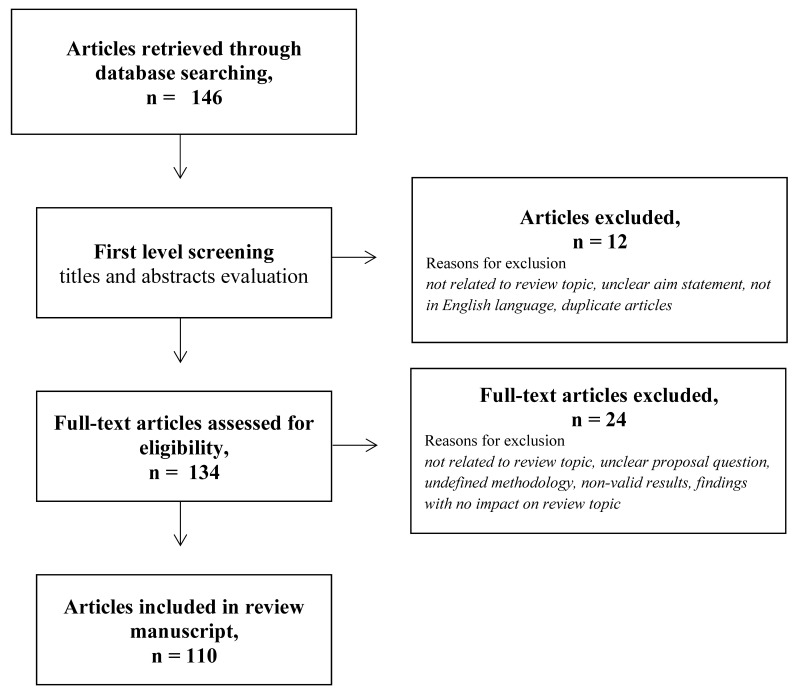
We reviewed data published before 31 December 2021 focusing on adverse events in recipients of vaccines against SARS-CoV-2. Articles were searched on the PubMed and Embase electronic bibliographic databases. We identified articles using a combination of the following keywords or MeSH terms: COVID-19 vaccines; adenovirus vaccines; women; gender; sex; thrombosis; venous thromboembolism; pulmonary embolism; hemorrhage; hemophilia; antibodies; thrombocytopenia; risk factors; cardiovascular; outcomes; hormones; oral contraceptives; hormone replacement therapy; infertility; assisted reproduction; estrogens; progestins; pregnancy; postmenopausal. Our search identified a total of 146 citations (search strategies and results are available upon request). Articles were preliminarily defined as eligible if they: 1) were directly related to the review topic; 2) had a clear statement of aim; 3) were published in peer-reviewed journals; and 4) were in English. No geographical restrictions were applied. A first level screening of articles extracted from electronic databases was carried out by reading titles and abstracts. Subsequent critical assessment of eligible full-text articles was based on an appraisal of relevance of topic, defined clinical questions, study methodology, description of findings, and significance and possible impact of the findings on the review topic. Care was taken to exclude duplicate articles. Two co-authors (EG and RN) dealt with the literature extractions from chosen databases, while the other authors independently reviewed and appraised the extracted literature. Out of the initial selection of 134 articles, 110 were included in the final analysis (Figure 1).
Figure 1.
Flowchart
RESULTS
Thrombotic risk by type of vaccine and sex-gender disaggregated data
We first analyzed data on thrombotic events according to type of vaccine used, and searched for details on sex- and gender-related data. Three systematic reviews reported data on CVST and/or thrombosis and TTS, defined as the occurrence of CVST or thromboses at unusual sites associated to thrombocytopenia (Table I). Palaiodimou et al. included 69 studies and found 370 CVST out of 4,182 patients with any thrombotic event in association with SARS-CoV-2 vector-based vaccine9. Seventy-two additional cases of TTS were also reported; among them, the pooled proportion of CVST was 51% (95% confidence interval [CI] 36–66%; I2=61%). TTS was independently associated with a higher likelihood of CVST when compared to patients without TTS with thrombotic events after vaccination (odds ratio [OR] 13.8; 95% CI: 2.0–97.3; I2=78%). The pooled mortality rates of TTS and TTS-associated CVST were 28% (95% CI: 21–36%) and 38% (95% CI: 27–49%), respectively.
Table I.
Studies on thrombotic risk in recipients of COVID-19 vaccines
| First author, JournalRef Type of study | Date of publication | Vaccine | CSVT | TTS | Other information |
|---|---|---|---|---|---|
|
Pottegard A, BMJ
12
Population-based |
May 2021 | Viral vectored | Morbidity ratio 20.25 (8.14–41.73) | Morbidity ratio for any thrombocytopenia/coagulation disorders 1.52 (0.97 to 2.25) | Morbidity ratio for arterial events: 0.97 (95% CI 0.77–1.20) Morbidity ratio for VTE: 1.97 (1.50–2.54) |
|
Bikdeli B, 2021, JAMA
11
Surveillance |
July 2021 | Viral vectored | 3.6 (99% CI: 2.7–4.8) per million recipients | n.a. | MHRA No sex-disaggregated data |
|
Simpson CR Nat Med
16
Population-based (with nested-incident matched case-control and self-controlled case-series analysis) |
July 2021 | Viral vectored and m-RNA | Not informative data | n.a. | No association with VTE for both types of vaccines Arterial events in viral vectored vaccine recipients aRR 1.48 (95% CI 1.12–1.96) |
|
Cari L J Autoimm
13
Eudra Vigilance European database |
August 2021 | Viral vectored and m-RNA | Increase of SAE rate in subjects 18–64-years vs >64 years: mean (95% CI) Viral vector recipients 5.7 (5.1–6.2) mRNA recipients 2.0 (1.7–2.3) |
Increase of SAE rate in subjects 18–64 years vs >64 years: mean (95% CI) Viral vectored recipients 2.5 (2.3–2.7) mRNA recipients 1.9 (1.6–2.2) |
Splanchnic vein thromboses increase of SAE rate in subjects 18–64 years vs >64 years: mean (95% CI) Viral vectored recipients 4.0 (3.7–4.3) mRNA recipients 2.1 (1.8–2.4) Sex-disaggregated raw data (see Figures 2, 3 and 4) |
|
Palaiodimou L, Neurology
9
Systematic review |
October 2021 | Viral vectored | 370 patients (69 studies) Women proportion pooled estimate 75% (95% CI 69–80%) 28% of those with post-vaccination CVST died during hospitalization (pooled rate 28%; 95% CI 21–36%; 14 studies) |
72 patients (12 studies) TTS was independently associated with a higher likelihood of CVST when compared to patients without TTS with thrombotic events after vaccination (OR 13.8; 95% CI 2.0–97.3) Women with TTS-CSVT proportion pooled estimate 75% (95% CI 69–81) Women with any TTS 71% (95% CI 62–80%) |
All venous and arterial thromboses were eligible. Women among patients with any thrombotic event, 69% (95% CI 60–77%) |
|
Hafeez MU, Clin Appl Thromb Hemost
10
Systematic review |
October 2021 | Viral vectored and m-RNA | n.a. | 59 studies F/M: 51/18 63 out of 69 (91.3%) occurred after viral vector vaccine |
|
|
Klein NP, JAMA
14
Surveillance |
October 2021 | m-RNA | Excess cases in risk interval 0.2 (−1.1 to 0.5) per million doses | Excess cases in risk interval 1.0 (−4.6 to 1.4) per million doses | MHRA No sex-disaggregated data |
|
Uaprasert N, Thromb J
28
Systematic review and meta-analysis |
November 2021 | Viral vector ed and m-RNA | n.a. | n.a. | 8 RCTs included No increased risk of thromboembolism, and death from thromboembolism. No sex-disaggregated data |
CI: confidence interval; MHRA: Medicines and Healthcare Products Regulatory Agency; aRR: adjusted relative risk; VTE: venous thromboembolism; RCT: randomized controlled trials; TTS: thrombosis and thrombocytopenia syndrome; n.a.: not applicable.
Thrombotic complications developed within two weeks of exposure to vector-based SARS-CoV-2 vaccines (mean interval 10 d; 95% CI: 8–12).
A systematic review with post-hoc analysis reports similar findings with a significant association between TTS (during arterial or venous thrombosis) and death10. Platelet nadir and D-dimer peak were strong predictors of death (Area Under Curve: 0.646 and 0.604, respectively). Regarding sex data, the pool estimate in women was 75% (95% CI: 69–80%) for CSVT and 75% (95% CI: 69–81%) for TTS. TTS was shown to mostly affect young women (i.e., under 45 years of age) (69%; 95% CI: 60%–77%), even in the absence of prothrombotic risk factors9.
Notably, authors report that diagnosed thrombophilia or use of the contraceptive pill were not associated with an increased incidence of TTS9,10; however, detailed results were not shown.
A surveillance study reports the rate of CVST in US associated with viral-vector-based vaccines according to publicly reported data11. This study summarizes findings published by the Medicine and Healthcare Regulatory Agency (MHRA) but does not show sex/gender disaggregated data.
A sample largely represented by women was described in a population-based study carried out in Norway and Denmark. In this analysis, authors compared 28-d rates of hospital contacts for incident arterial events, venous thromboembolism, thrombocytopenia/ coagulation disorders and bleeding among recipients of viral-vectored vaccines with those expected based on sex- and age-specific background rates. The study found a higher rate of CVST with a standardized morbidity ratio of 20.25 (8.14–41.73); the reported excess in vaccinated people was 2.5 (0.9–5.2) events per 100,000 vaccinations.
As stated, this sample was largely represented by women (77.6 in Norway and 80.1 in Denmark) in whom no excess rate of thrombocytopenia/coagulation disorders or other outcomes were observed. Notably, the analysis carried out in men highlighted no excess rate of venous thromboembolism, with a standardized morbidity ratio of 0.67 (0.22–1.56)12.
Post-hoc analyses showed that the proportion of women who redeemed a prescription for hormone therapy (oral contraceptives or oestradiol) during the year before cohort entry was slightly lower than that observed in the background population12. However, authors do not give any additional information on adverse events in this sub-group or any details on the type of hormone therapy administered.
Cari et al.13 performed an interesting analysis that aimed to take into account gender data. They used the data of the Eudra Vigilance European database registered up to 16 April 2021 to calculate the incidence of thrombocytopenia, bleeding, and thromboses in recipients of ChA (i.e., virus-vectored vaccine) and of BNT162b2 (i.e., m-RNA vectored vaccine). Of note, they defined adverse events as those with “blood clots and/orthrombocytopenia”, whereas the European Medicines Agency (EMA) investigated adverse events due to “blood clots and thrombocytopenia”. CSVT, SVT and/or thrombocytopenia were categorized as thrombo-hemorrhagic events.
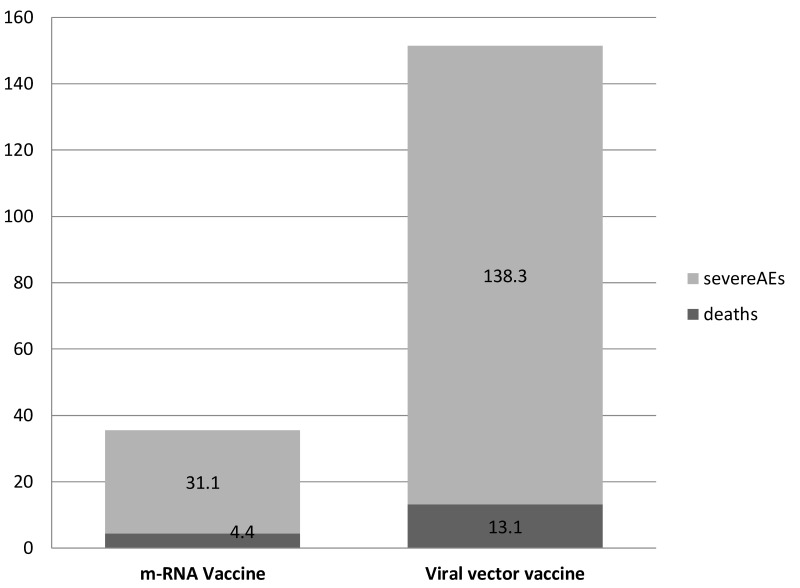
The most relevant finding was that all these symptoms were found with the virus-vectored vaccine and not with the m-RNA vaccine13. Although the European Center for Disease Prevention and Control database did not report separate numbers of vaccine doses administered in women and in men, authors calculated the woman: man ratio based on data on the sex of recipients reported by some European countries. Therefore, in a scenario where the women: men ratio is 3 : 2, the risk of Serious Adverse Event (SAE) was highest in women and men in the age range 18–24 years for recipients of adeno-virus vaccine (approx. 50 events per million doses). Figure 2 shows SAE and deaths per million doses by type of vaccine.
Figure 2.
Severe adverse events (SAEs) and deaths by type of vaccine
Absolute number of SAEs and death per million of administrated doses.
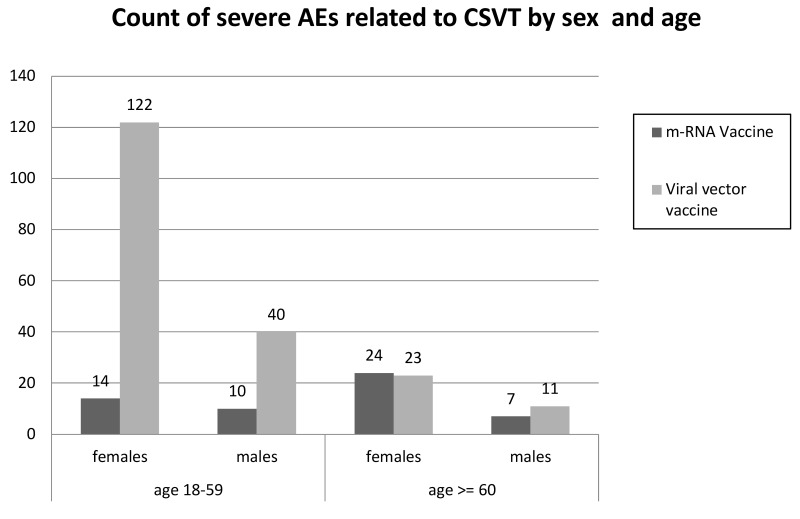
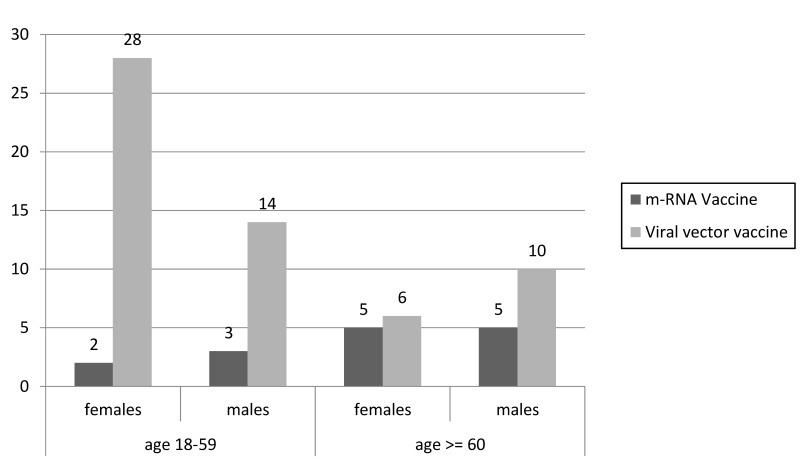
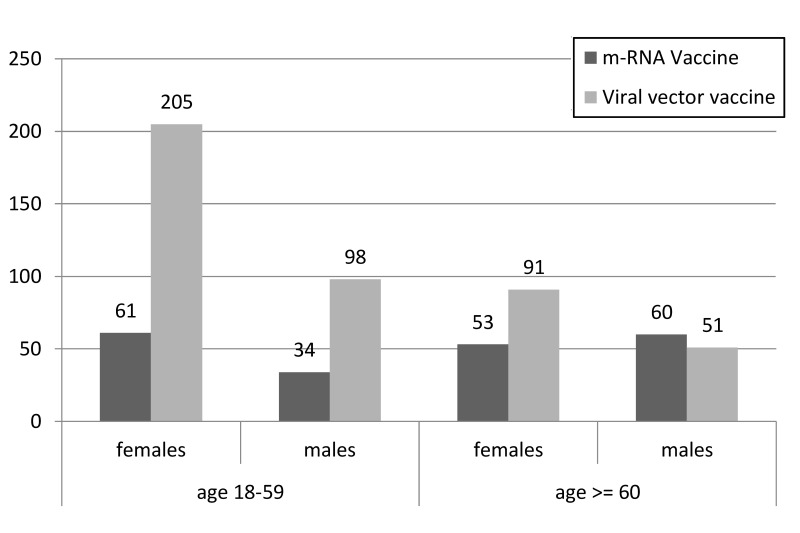
In the age ranges 25–49 years and 50–59 years, the SAE risk in women remained high, whereas that in men of the same age decreased. Similar SAE risk was observed in women and men aged >59 years who received adeno-virus vaccine (approx. 15–25 SAEs per million doses). Figures 3, 4 and 5 show SAEs related to CSVT, SVT and thrombocytopenia by sex and age, as reported by Cari et al.
Figure 3.
Severe adverse events (SAEs) related to cerebral sinus vein thrombosis (CSVT) by sex and age (adapted from Cari et al.13)
Figure shows the absolute number of SAEs related to CSVT in males and females divided into two age groups: 18–59 years vs ≥60 years.
Figure 4.
Severe adverse events (SAEs) related to splanchnic vein thrombosis (SVT) by sex and age (adapted from Cari et al.13)
Figure shows the number of SAEs related to SVT in males and females divided into two age groups: 18–59 years vs ≥60 years.
Figure 5.
Severe adverse events (SAEs) related to thrombocytopenia by sex and age (adapted from Cari et al.13)
Figure shows the number of SAEs related to thrombocytopenia in males and females divided in two age groups: 18–59 years vs ≥60 years.
Consistent with these findings, an interim analysis of surveillance data carried out in the USA for mRNA-only vaccines14 found that the incidence of thrombosis (arterial and venous, including pulmonary embolism) or TTS were not significantly higher 1–21 d post vaccination compared with 22–42 d post vaccination. Unfortunately, these authors did not show sex/gender-disaggregated data.
Arterial thromboses are reported in some studies (Table II). A Scottish national population-based analysis among 2.53 million people who received their first doses of SARS-CoV-2 vaccines found an increased risk of arterial thromboembolic events in viral-vector recipients. This risk was associated with increasing age (especially over 60 years), male sex, presence of certain comorbidities (such as heart failure, coronary heart disease, peripheral vascular disease, severe mental illness, sickle cell disease, prior stroke, type 1 and 2 diabetes and chronic kidney disease)1.
Table II.
Systematic reviews, surveillance and population-based studies on hemorrhagic risk in recipients of COVID-19 vaccines
| First author, JournalRef Type of study | Date of publication | Vaccine | Risk of bleeding events | Type of bleeding | Other information |
|---|---|---|---|---|---|
|
Pottegard A, BMJ
12
Population-based |
May 2021 | Viral-vectored | Morbidity ratio for any bleeding 1.23 (0.97–1.55). | / | All venous and arterial thromboses and bleedings were eligible |
|
Simpson CR Nat Med
15
Population-based |
July 2021 | Viral-vectored and m-RNA | Adjusted RR 1.48 (95% CI 1.12–1.96) in viral-vector vaccine recipients | (ITP) in viral-vector vaccine recipients: adjusted RR 5.77 (95% CI 2.41–13.83) | Nested-incident-matched case-control Self-controlled case-series analysis Males are predicted to develop thrombotic and hemorrhagic events (included ITP) with adjusted RR 1.31 (95% CI 1.23–1.39) |
|
Cari L J Autoimm
13
Eudra Vigilance European database |
August 2021 | Viral-vectored and m-RNA | 33 and 151 SAEs/1 million doses in mRNA-and viral-vector vaccine recipients, respectively | Increase in SAE rate in subjects 18–64 years vs >64 years Viral vector recipients mRNA recipients Gastrointestinal bleeding Viral vector recipients 1.6 (1.5–1.8) mRNA recipients 1.6 (1.3–1.8) Cerebral bleeding Viral vector recipients 1.4 (1.3–1.5) mRNA recipients 0.6 (0.6–0.7) |
|
|
Trogstad L Vaccine
17
Population-based (self-reported side-effects) |
September 2021 | Viral-vectored and m-RNA | 0.2% recipients of single dose mRNA vaccine reported skin bleeding vs 3.2% recipients of a single-dose viral-vectored vaccine. OR 16.0, 95% CI 7.5–34.1) | ORs for nose and gingival bleeding 8.0 (4.0–15.8) and 9.3 (4.3–20.0), respectively | Among recipients of viral-vectored vaccine, 3.5% (152/4,365) women reported skin bleeding as opposed to 1.4% (11/767) among men, OR 2.5 (95% CI 1.3–4.6) |
|
Alghamdi AN, Frontiers in Med
18
Questionnaire-based |
October 2021 | Viral-vectored and m-RNA | Some details on hormonal status | ||
|
Uaprasert N, Thromb J
28
Systematic review and meta-analysis |
November 2021 | Viral-vectored and m-RNA | n.a. | n.a. | 8 RCTs included No increased risk of hemorrhage, and death from hemorrhage No sex-disaggregated data |
CI: confidence interval; VTE: venous thromboembolism; ITP: idiopathic thrombocytopenic purpura; RCT: randomized controlled trials, n.a.: not applicable.
Hemorrhagic risk by type of vaccines and sex-gender disaggregated
In a population-based cohort study involving more than 80,000 participants, the overall risk of hemorrhagic events in recipients of virus-vectored vaccine 0–27 d after vaccination was 1.48 (95% CI: 1.12–1.9). Recipients of the first dose of adenovirus vaccines were at higher risk of skin bleeding events (3.2 vs 0.2% of mRNA vaccine recipients; OR 16.0, 95% CI: 7.5–34.1). Similarly, nose and gingival bleedings were significantly more frequent in recipients of viral-vectored vaccines with an adjusted OR of 7.0 (95% CI: 3.5–13.9) and 8.1 (3.7–17.6), respectively15.
When we focused on occurrence of thrombocytopenia or idiopathic thrombocytopenic purpura (ITP), we found that a first dose of virus-vectored vaccine was associated with a small, although significant, increase of ITP16. The Scottish national population-based study revealed a potential association between recipients of a first dose ChAdOx1 vaccination and occurrence of ITP. The adjusted risk ratio for ITP was 5.77 (95% CI: 2.41–13.83) with an estimated incidence of 1.13 (0.62–1.63) cases per 100,000 doses.
Women have a significantly higher risk of bleeding episodes. Indeed, Trogstad et al. found that 3.5% (152/4,365) women receiving adeno-viral vectored vaccine experienced skin bleeding as opposed to 1.4% (11/767) among men, with an OR of 2.5 (95% CI: 1.3–4.6). Notably, 37% reported prolonged duration of skin bleeds (10% lasting 3–4 weeks), 14% nose bleeds (3.7% lasting 3–4 weeks), and 26.5% gingival bleeds (10.2% lasting 3–4 weeks)15.
On the other hand, Simpson et al. refer to male sex as a predictor of ITP and hemorrhagic events (not specified) within 28 d post vaccination with an adjusted rate ratio of 1.31 (1.23–1.39). These findings are intriguing because, similar to other autoimmune diseases, ITP has an overall ratio of women to men of 3–4 to 1 in other clinical contexts17. A single study reported abnormal menstrual cycle (delayed/increased hemorrhages or pain) in 0.98% (18/1,846) m-RNA and 0.68% (7/1,028) viral-vector vaccine recipients18.
Why are women more exposed to thrombocytopenia and CSVT than men?
It is well known that women are more prone than men to several autoimmune diseases, and it has been hypothesized that exposure to hormonal changes during pubertal development are responsible for the gender bias in autoimmune diseases19–21. Gene-gene and gene-environment interactions are responsible for the susceptibility to develop autoimmune disease. Furthermore, environmental factors, exposure to infectious agents, chemicals or dietary components confer susceptibility to autoimmune disease, whereas other factors, such as sunlight, can protect from the development of these conditions22. Sex hormones, and particularly estrogens, modulate immune response in humans through sex hormone receptors found on immune system cells such as T cells, B cells, and monocytes. Depending on the context, estrogens can either promote inflammation by enhancing Th1 or increase the regulatory arm of the adaptive immune response by promoting anti-inflammatory/ regulatory Th2-type23. Therefore, it is not surprising that estrogens show an ability to increase antibody responses to vaccines, infections and autoantigens by activating B cells, while androgens have the opposite effect24. Megakaryocytes contain mRNA for estrogen receptors and this suggests a potential mechanism through which sex hormones may mediate gender differences in platelet function and thrombotic diseases25. Taken together, all these findings indicate that estrogen can favor an exaggerated response of platelets or their precursors to vaccines, with ITP as a possible consequence. These data are consistent with the knowledge that ITP is secondary to autoantibodies targeting multiple platelet glycoproteins. Changes in hormonal status through different stages of life can explain different platelet susceptibility to vaccine and, in turn, the higher prevalence of young women among those who develop thrombocytopenia and vein thrombosis after vaccination against COVID-19. Interestingly, in the animal model (sheep) the expression of alfa and beta receptors in the brain and pituitary gland is influenced by circulating estrogen concentrations, and is acutely regulated in response to cerebral hypoperfusion26. A lower blood flow in cerebral veins, especially in certain menstrual phases27, together with estrogen-mediated hyper-response to vaccine, might be responsible for the higher prevalence of thrombocytopenia associated to CSVT in women after COVID-19 vaccination.
CONCLUSIONS
This review highlights the much more frequent reports of thrombotic complications compared to bleeds after vaccinations against SARS-CoV-2. These adverse events are mostly observed in young women who are administered with viral-vectored vaccines. In young women, sex steroids and the immune system likely affect the increased risk of thrombotic events. As for several autoimmune diseases, we need to collect additional data on specific side-effects of vaccines, especially those with adeno viral vectors. The immune reaction promoted by virus-vectored vaccine may lead, not only to thrombocytopenia and cerebral/splanchnic venous thrombosis, but also to other thrombotic and thromboembolic SAEs.
Bleeding events are mainly reported as aggregated data, with the exception of ITP and thrombocytopenia. This latter can occur in the presence or absence of thrombotic complications; therefore, we must be careful when differentiating between thrombocytopenia occurring with thrombosis and that occurring without, because we may have to adopt different approaches to management, follow-up and prognosis. There are conflicting data on the prevalence of bleeding events according to sex in recipients of viral-vectored vaccines. Indeed, one study showed a higher risk of bleeding episodes in women, while other investigators found that males are predicted to develop hemorrhagic events.
Future reporting of sex/gender-disaggregated data and a discussion of how sex factors influence outcomes would benefit the decision-making process for both regulatory agencies and the general public, and help in the design of mass vaccination programs8.
RESEARCH AGENDA
Out of 45 COVID-19 randomized controlled trials whose results were published in December 2020, only eight reported the impact of sex or gender7. All trials reported numbers of men and women participants, but only eight examined whether results differed among women and men. Likewise, sex/gender-disaggregated data in vaccine recipients are lacking, although preliminary information suggests that such an analysis is essential.
It is unknown why women frequently show cerebral or splanchnic vein thrombosis in association with thrombocytopenia. In this context, there are no sex/gender-disaggregated data on the development of anti-PF4 antibodies after virus-vectored vaccine exposure or on the underlying pathophysiological mechanism(s). We urgently need data on the possible role of hormonal status in those who develop thrombosis or bleeds.
It is important to consider that platelets are probably the most important player in the pathogenesis mechanism of both thrombotic and bleeding complications. Therefore, future studies should focus especially on platelets and on global tests, such as thrombin generation assays.
APPENDIX 1
List of the Representatives for Gender Medicine of Scientific Hospitalization and Treatment Institutes-Italian Ministry of Health
*Coordinator
Maria Novella Luciani, Ministero della Salute, Direzione Ricerca e Innovazione in Sanità, Rome, Italy
Members
Marta Allena - IRCCS Fondazione Mondino, Pavia, Italy
Marialuisa Appetecchia - IRCCS Istituti Fisioterapici Ospitalieri IFO, Rome, Italy
Irene Aprile -IRCCS Fondazione Don Carlo Gnocchi Onlus, Milan, Italy
Giuseppe Banfi - IRCCS Istituto Ortopedico Galeazzi, Milan, Italy
Stefania Bargagna - IRCCS Fondazione Stella Maris, Calambrone, Italy
Nicola Bergonzi Concesi - IRCCS Ospedale Pediatrico Bambino Gesù, Rome, Italy
Giovanna Borsellino - IRCCS Fondazione Santa Lucia, Rome, Italy
Serenella Castelvecchio - IRCCS Policlinico San Donato, San Donato Milanese, Italy
Annamaria Cattaneo - IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy
Antonio Cherubini - IRCCS INRCA, Ancona, Italy
Susanna Chiocca - IEO, Istituto Europeo di Oncologia IRCCS, Milan, Italy
Paola Cudia - IRCCS Ospedale San Camillo srl, Venice, Italy
Laura Adelaide Dalla Vecchia - IRCCS Istituti Clinici Scientifici Maugeri, Milan, Italy
Lucia Del Mastro - IRCCS Ospedale Policlinico S. Martino, Genoa, Italy
Maria Benedetta Donati - IRCCS Neuromed, Pozzilli, Italy
Cinthia Farina - IRCCS Ospedale San Raffaele, Milan, Italy
Piero Fenu - Istituto di Candiolo, FPO-IRCCS, Candiolo, Italy
Milena Fini - IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy
Laura Folini - IRCCS MultiMedica, Sesto San Giovanni, Italy
Barbara Garavaglia - Fondazione IRCCS Istituto Neurologico “C.Besta” Milan, Italy
Stefania Gori - IRCCS Sacro Cuore Don Calabria, Negrar di Valpolicella, Italy
Elvira Grandone - IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy
Stefano Greggi - IRCCS Istituto Nazionale Tumori Pascale, Naples, Italy
Cecilia Invitti - IRCCS Istituto Auxologico Italiano, Milan, Italy
Maria Paola Landini - IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy
Vittoria Lapadula - IRCCS CROB, Rionero in Vulture, Italy
Giuseppina Liuzzi - IRCCS Istituto Nazionale Malattie Infettive “L. Spallanzani”-Rome, Italy
Alessandra Maestro - IRCCS Materno Infantile Burlo Garofolo, Trieste, Italy
Chiara Mannelli - Istituto di Candiolo, FPO-IRCCS, Candiolo, Italy
Riccardo Masetti - IRCCS Fondazione Policlinico Universitario A. Gemelli, Rome, Italy
Emanuela Mazzon - IRCCS Centro Neurolesi Bonino-Pulejo, Messina, Italy
Rosalba Miceli - Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy
Paola Mosconi - Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milan, Italy
Oriana Nanni - Istituto Scientifico Romagnolo per lo Studio dei Tumori “Dino Amadori” -IRCCS IRST S.r.l., Meldola, Italy
Monica Napolitano - Istituto Dermopatico dell’Immacolata (IDI)-IRCCS, Rome, Italy
Rossella E. Nappi - IRCCS Fondazione Policlinico S. Matteo, Pavia, Italy
Maria Cristina Parravano - IRCCS Fondazione Bietti, Rome, Italy
Federico Pea, IRCCS Azienda Ospedaliero-Universitaria, Bologna, Italy
Federica Provini - IRCCS Istituto delle Scienze Neurologiche, Bologna, Italy
Elena Ravizza - IRCCS Istituto Ortopedico Galeazzi, Milan, Italy
Benedetta Riboldi - IRCCS Istituto in Tecnologie Avanzate e Modelli Assistenziali in Oncologia, Reggio Emilia, Italy
Giuseppe Rosano - IRCCS San Raffaele Pisana, Rome, Italy
Anna Sapino - Istituto di Candiolo, FPO-IRCCS, Candiolo, Italy
Giuseppe Toffoli - Centro di Riferimento Oncologico IRCCS (CRO), Aviano, Italy
Daniela Trabattoni - IRCCS Centro Cardiologico Monzino, Milan, Italy
Pierluigi Viale - IRCCS Azienda Ospedaliero-Universitaria, Bologna, Italy
Tonia Marina Zacheo - IRCCS Istituto Oncologico Veneto (IOV), Padua, Italy
Germana Zollesi - Istituto di Candiolo, FPO-IRCCS, Candiolo, Italy
Footnotes
FUNDING AND RESOURCES
This article was supported in part by the Italian Ministry of Health (Ricerca Corrente).
AUTHORSHIP CONTRIBUTION
Conceptualization: EG, SuCh, SeCa, RN; methodology: EG; formal analysis: EG, RN; original draft preparation: EG, SuCh; writing, review and editing: EG, SuCh, SeCa, RN; All others read, critically revised and approved analysis and final draft.
The Authors declare no conflicts of interest.
REFERENCES
- 1.WHO World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. 2020. [Accessed on 27/06/2021]. Available at: https://covid19.who.int.
- 2.Ramos-Casals M, Brito-Zerón P, Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol. 2021;17:315–332. doi: 10.1038/s41584-021-00608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller SC, Porterfield L, Davis J, Wilkinson GS, Chen L, Baillargeon J. Incidence of venous thrombotic events and events of special interest in a retrospective cohort of commercially insured US patients. BMJ Open. 2022;12:e054669. doi: 10.1136/bmjopen-2021-054669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiele T, Weisser K, Schönborn L, Funk MB, Weber G, Greinacher A, et al. Laboratory confirmed vaccine-induced immune thrombotic thrombocytopenia: retrospective analysis of reported cases after vaccination with ChAdOx-1 nCoV-19 in Germany. Lancet Reg Health Eur. 2022;12:100270. doi: 10.1016/j.lanepe.2021.100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady E, Nielsen MW, Andersen JP, Oertelt-Prigione S. Lack of consideration of sex and gender in COVID-19 clinical studies. Nat Commun. 2021;12:4015. doi: 10.1038/s41467-021-24265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijayasingham L, Bischof E, Wolfe J. Gender and COVID-19 Research Agenda-setting Initiative. Sex-disaggregated data in COVID-19 vaccine trials. Lancet. 2021;397:966–967. doi: 10.1016/S0140-6736(21)00384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palaiodimou L, Stefanou MI, Katsanos AH, Aguiar de Sousa D, Coutinho JM, Lagiou P, et al. Cerebral venous sinus thrombosis and thrombotic events after vector-based COVID-19 vaccines: a systematic review and meta-analysis. Neurology. 2021;97:e2136–e2147. doi: 10.1212/WNL.0000000000012896. [DOI] [PubMed] [Google Scholar]
- 10.Hafeez MU, Ikram M, Shafiq Z, Sarfraz A, Sarfraz Z, Jaiswal V, et al. COVID-19 vaccine-associated thrombosis with thrombocytopenia syndrome (TTS): a systematic review and post hoc analysis. Clin Appl Thromb Hemost. 2021;27:10760296211048815. doi: 10.1177/10760296211048815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikdeli B, Chatterjee S, Arora S, et al. Cerebral Venous Sinus Thrombosis in the U.S. Population, After Adenovirus-Based SARS-CoV-2 Vaccination, and After COVID-19. J Am Coll Cardiol. 2021;78:408–411. doi: 10.1016/j.jacc.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pottegård A, Lund LC, Karlstad Ø, Dahl J, Andersen M, Hallas J, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cari L, Fiore P, Naghavi Alhosseini M, Sava G, Nocentini G. Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: an analysis of European data. J Autoimmun. 2021;122:102685. doi: 10.1016/j.jaut.2021.102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trogstad L, Robertson AH, Mjaaland S, Magnus P. Association between ChAdOx1 nCoV-19 vaccination and bleeding episodes: large population-based cohort study. Vaccine. 2021;39:5854–5857. doi: 10.1016/j.vaccine.2021.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27:1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrès E. What impact for sex difference on immune thrombocytopenic purpura? Women Health Open J. 2016;2:e1–e3. doi: 10.17140/WHOJ-2-e004. [DOI] [Google Scholar]
- 18.Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel-Moneim AS. BNT162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front Med (Lausanne) 2021;8:760047. doi: 10.3389/fmed.2021.760047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Florio DN, Sin J, Coronado MJ, Atwal PS, Fairweather D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020;31:101482. doi: 10.1016/j.redox.2020.101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moulton VR. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol. 2018;9:2279. doi: 10.3389/fimmu.2018.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347–369. doi: 10.1016/j.yfrne.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases-multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267–1275. doi: 10.1562/2005-02-15-IR-441. [DOI] [PubMed] [Google Scholar]
- 23.Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. 2004;3:97–104. doi: 10.2174/1568010043483944. [DOI] [PubMed] [Google Scholar]
- 24.Aguilar-Pimentel JA, Cho YL, Gerlini R, Calzada-Wack J, Wimmer M, Mayer-Kuckuk P, et al. Increased estrogen to androgen ratio enhances immunoglobulin levels and impairs B cell function in male mice. Sci Rep. 2020;10:18334. doi: 10.1038/s41598-020-75059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khetawat G, Faraday N, Nealen ML, Vijayan KV, Bolton E, Noga SJ, et al. Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR): testosterone regulates AR expression. Blood. 2000;95:2289–2296. [PubMed] [Google Scholar]
- 26.Wood CE. Cerebral hypoperfusion increases estrogen receptor abundance in the ovine fetal brain and pituitary. Neuroendocrinology. 2008;87:216–22. doi: 10.1159/000112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peltonen GL, Harrell JW, Aleckson BP, LaPlante KM, Crain MK, Schrage WG. Cerebral blood flow regulation in women across menstrual phase: differential contribution of cyclooxygenase to basal, hypoxic, and hypercapnic vascular tone. Am J Physiol Regul Integr Comp Physiol. 2016;311:R222–3. doi: 10.1152/ajpregu.00106.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uaprasert N, Panrong K, Rojnuckarin P, Chiasakul T. Thromboembolic and hemorrhagic risks after vaccination against SARS-CoV-2: a systematic review and meta-analysis of randomized controlled trials. Thromb J. 2021;19:86. doi: 10.1186/s12959-021-00340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]