Abstract
The field of regenerative medicine, encompassing several disciplines including stem cell biology and tissue engineering, continues to advance with the accumulating research on cell manipulation technologies, gene therapy and new materials. Recent progress in preclinical and clinical studies may transcend the boundaries of regenerative medicine from laboratory research towards clinical reality. However, for the ultimate goal to construct bioengineered transplantable organs, a number of issues still need to be addressed. In particular, engineering of elaborate tissues and organs requires a fine combination of different relevant aspects; not only the repopulation of multiple cell phenotypes in an appropriate distribution but also the adjustment of the host environmental factors such as vascularisation, innervation and immunomodulation. The aim of this review article is to provide an overview of the recent discoveries and development in stem cells and tissue engineering, which are inseparably interconnected. The current status of research on tissue stem cells and bioengineering, and the possibilities for application in specific organs relevant to paediatric surgery have been specifically focused and outlined.
Keywords: Stem cell, ES cell, iPS cell, Regenerative medicine, Paediatric surgery, Tissue engineering, Organoid
Introduction
Regenerative medicine encompasses numerous areas of biology and engineering, including stem cell biology, biomaterial engineering and gene therapy [1]. The ultimate aim is to eventually creating functional “artificial” organs and tissues. Its enormous potential to engineer biologic organ substitutes, which can replace damaged organ and tissues of the patients, may fulfil the unmet clinical needs and overcome some of the unacceptable consequences of current therapeutic approaches in paediatric surgery, especially in the severe forms of congenital malformations and end-stage organ failures. Inspired by initial success in both preclinical and clinical studies using relatively simple organ systems and techniques, the research field has further expanded in an attempt to create more complex organs with sophisticated technologies.
This review will first briefly overview technological platforms available for regenerative medicine both after birth and prenatally [2]. Tissue stem and progenitor cells of each organ will be discussed in the later section on clinical application. Various strategies to engineer translatable organs utilising stem cell biology, including novel cell culture methodology, gene editing technology, tissue engineering and biomaterial technologies, will then be reviewed. It is beyond the scope of this review to discuss the entire body of research on biomaterials. Instead, we will describe the surgically-relevant part of these technologies of biomaterials in relation to stem cell biology and tissue engineering approaches, as biomaterials are now an indispensable part to tissue engineering. Next, relevant paediatric surgical preclinical and early studies aiming to alter underlying pathophysiology and to replace damaged organs will be reviewed by organ system. Finally, potential future outlooks in the field and major hurdles for clinical translation will be discussed.
Overview of technological platforms in regenerative medicine
Regenerative medicine aimed to overcome three overarching themes: (1) to facilitate the repair of damaged tissues, (2) to replace damaged cells with healthy functional cells, and (3) to replace the damaged organ system with engineered tissues. There have been many technological advancements to this aim, including tissue engineering, stem cells and organoid biology, and gene editing. These advancements have allowed overcome significant technical hurdles and led to a more profound knowledge of how resident cells communicate with their microenvironment, issues of immunogenicity, and the role of stem or progenitor cells in tissue regeneration.
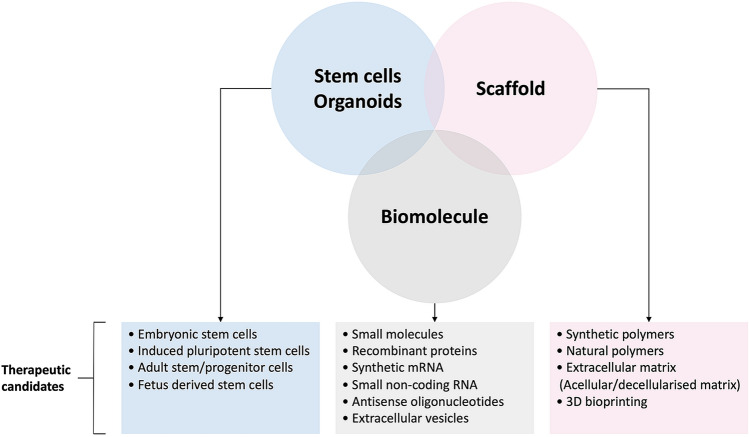
The main components of successful bioengineered tissues include cell sources, scaffolds, and biomolecules (Fig. 1). Although primary cells are ideal cell sources for regenerative medicine, they are usually in short supply, particularly in damaged organs, and do not proliferate enough to provide sufficient cell yield for clinical application. Stem cells can proliferate, differentiate, and have a self-renewal capacity, all of which are ideal for therapeutic application. Stem cells can be derived from native stem and progenitor populations, embryonic stem cells (ESC), or induced pluripotent stem cells (iPSC) from healthy or damaged tissues [3–6]. Furthermore, these stem cells can be genetically modified or corrected using gene editing technologies (such as the CRISPER-Cas system) [7]. The second component is the biomaterials, which are pivotal in providing an optimal microenvironment for cell growth and functional differentiation. These biomaterials include extracellular matrix (ECM) derived from decellularised tissue, natural polymers, and 3D bioprinting [8–10]. The final component is biomolecules, such as growth factors and morphogens. These molecules can be introduced as small molecules, recombinant proteins, synthetic mRNA, small non-coding RNA, and extracellular vesicles (e.g., exosomes) [11]. Some of these molecules can be incorporated into the scaffold while fabricating tissues, and the release can be controlled.
Fig. 1.
Schematic representation of different aspects of tissue engineering and therapeutic candidates
Recently, in vitro human organ models with physiological functions similar to native organs have become feasible through engineering research, such as micro-physiological systems represented by organ-on-chip. Among in vitro models, organoid-based platforms have a wide range of applications, from basic research in embryology and regenerative medicine to disease modeling and drug discovery research. Furthermore, the application of organoids to regenerative medicine using organoids for transplantation is also beginning to be explored.
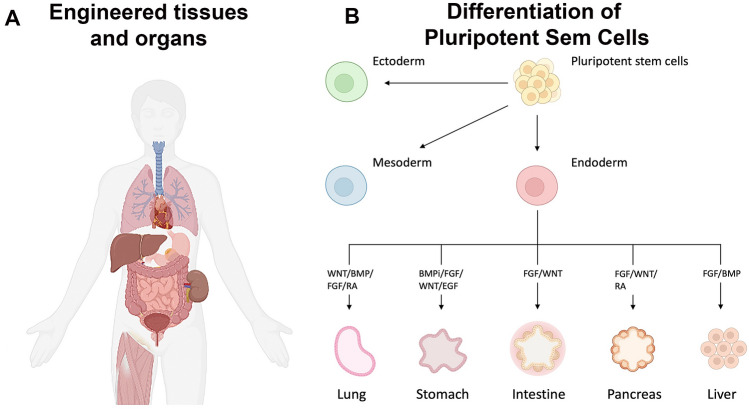
Organoids have three-dimensional structural characteristics similar to those of organs in vivo. Since organoids are composed of multiple differentiated cell types, they are expected to have physiological responses similar to native tissues compared to the “classic” cell culture [12]. Organoids can primarily be derived from adult stem cells or pluripotent stem cells such as ESCs or PSCs [12]. By artificially reconstituting embryological processes in vitro, organoids of various organs of ectodermal, mesodermal, and endodermal lineages have been created (Fig. 2) [13–21].
Fig. 2.
Regenerative medicine approach using somatic cells or pluripotent stem cells: schematic of various organs which can have been engineered A and organoids that can be grown from stem cells and the tissue-specific developmental signals that are employed B
Adapted from Clevers, 2016 [12] and created with BioRender.com
An ultimate application of organoids in tissue engineering is the regeneration of deficient tissues through organoid transplantation. Improved survival of liver failure models through liver organoid transplantation and regeneration of intestinal epithelium through intestinal organoid transplantation have been reported [21–23]. Organoid transplantation is hoped to advance the treatments for critically ill paediatric patients with limited therapeutic options, such as biliary atresia and intestinal failure.
Therapeutic application relevant to paediatric surgery
Trachea and lung
Airway systems encounter two main fields of interests with substantial unmet clinical needs: conduit system and parenchymal disease. Severe forms of congenital tracheal stenosis/agenesis, laryngotracheal clefts, trauma and aggressive forms of cancer may require the replacement of main airways [24]. In paediatric population, cystic fibrosis and lung hypoplasia caused by bronchopulmonary dysplasia or congenital diaphragmatic hernia (CDH) are instead the leading causes of untreatable parenchymal disease. Allogenic transplantation is the ultimate treatment option for end-stage lung diseases, but it is still associated with a significant burden of morbidity and mortality and relies on donated organs [25].
Stem and progenitor cells
The human respiratory system is composed of a highly complex and hierarchical structure from proximal conducting region to distal alveolar region with numerous different types of cells along [26], varying according to the needs and functions each region fulfils. Epithelial cells in the airway are usually quiescent, but progenitor cells retain the potential to propagate and restore the epithelial integrity following injury [27]; these are classified as endogenous lung stem cells. Exogenous stem cell sources originating from other adult tissue or pluripotent stem cells may also be used for airways regeneration.
In early development the digestive and respiratory systems share a common precursor from the anterior foregut endoderm (marked by the expression of the transcription factor Nkx2.1). These progenitors are later specified by transcription factors Sox2 and Sox9 in the proximal and distal regions respectively. Despite the mesenchymal instructive paracrine signals are crucial to regulate proliferation and maturation of endodermal progenitors, there is a paucity of evidence concerning the airways mesodermal-derived progenitors, which are nevertheless thought to share a common origin with cardiopulmonary mesoderm progenitors [28].
In the mature proximal conducting region of the human lung, TRP63 + KRT5 + basal cells have been identified as a progenitor cell population [29]. However, to-date little is understood about the molecular mechanisms for basal cell self-renewal and differentiation, yet recent evidence in murine models indicated Notch signalling plays an important role [30]. Various protocols have been described for the isolation and culture of these human basal cells, with the most efficient methods being co-cultures of fibroblast with feeder layer and/or specific signalling pathway inhibitors [31–33].
In the alveolar region, alveolar type 2 (AT2) surfactant-producing cells are widely recognised as the best progenitor candidate [34–36]. In addition, several recent studies have suggested another stem/progenitor cell candidate in the mouse, such as Trp63 + Krt14 + basal cells or alveolar epithelial cells expressing the laminin receptor α6β4 [31, 37].
As exogenous stem cell sources promising candidates are bone marrow-derived stem cells [38], ESCs [39–41], human iPSCs through combination of FGF, BMP and Wnt signalling pathways [18, 42, 43] and human amniotic fluid stem cells (AFSCs) [44]. For instance, administration of hAFSCs into hypoplastic lungs in a nitrofen-induced CDH rat model rescued lung growth, bronchial motility and innervation [45].
Three-dimensional culture system
Most evidence on respiratory stem cell biology had been using 2D cell culture models, clearly too simplistic to reflect the complex in vivo interconnections and microarchitectures. Development of lung organoids has been reported from the different epithelial cell populations, including basal cells, club cells and AT2 cells, in addition to hPSCs [46]. A variety of names have been given to these organoids including tracheosperes, bronchospheres, alveospheres, alveolar spheroids, depending on the site of origin of the cells [46–49]. Recently, SFTPC + AT2 cell-like alveolar stem cells induced from hPSCs in organoids showed long-term stable expansion while maintaining their stem cell properties by utilising a co-culture system with fibroblasts [50] and smooth muscle cells [51].
Air–liquid interface (ALI) culture systems provide cultured cells with a more physiological environment and allow better differentiation for induced pluripotent stem cells [52]. Since the first model described in 1988, ALI has enabled the development of disease models such as asthma and cystic fibrosis [53, 54]. Further studies combining the advantages of 3D-organoid system with ALI culture may lead to the development of a more sophisticated in vitro miniature lung model.
Tissue engineering
Tracheal engineering
The upper airway has been thought to be relatively amenable to bioengineering due to its simple structure and function, and has already been engineered and translated clinically on a compassionate basis [55, 56]. Most successfully engineered upper airways involve co-culturing of different cell lines such as autologous bone marrow-derived mesenchymal stem cells (MSCs) and airway epithelial cells on a decellularised trachea scaffold [56, 57]. Alternatives for decellularised trachea include allogeneic trachea [58], aortic grafts [59] and composite reconstruction from autologous tissue.
Whole lung engineering
Whole lung engineering was obtained in rodent lungs by repopulating decellularised lung ECM with epithelial and endothelial cells and allowed gas exchange [60–62]. More recently, a scaling up in size was obtained successfully engineering human-sized lung by reseeding porcine decellularised lung with human airway epithelial progenitor cells and human umbilical vein endothelial cells in a bioreactor system and achieving a surgical implantation into porcine recipients [63]. An additional promising technique is bioprinting, the deposition of layers of different cell types and matrix material, addressing the need for creation of complicated tubular airflow and vascularity paths [64].
Oesophagus
In paediatrics, many conditions may require the presence of a complete or partial substitute of the oesophagus, including long-gap oesophageal atresia (LGOA), caustic ingestion and traumatic injuries. Despite great advances in clinical care, reconstruction of oesophageal continuity is still associated with substantial morbidity and severe consequences on quality of life [65].
Research on tissue engineering of oesophagus was preceded by advancement of biomaterials, including decellularised, natural derived and synthetic materials. The successful in vivo implantation of these materials, using the small patch implantation model, has demonstrated their biocompatibility [66]. However, when aiming to generate longer oesophageal constructs, spontaneous migration of adjacent cells is not sufficient and identification of suitable oesophageal cell populations for bioengineering and establishment of their in vitro isolation and expansion method is an essential prerequisite for successful clinical translation [67]. Other obstacles for functional oesophageal replacement include the need for vascular supply, crucial for reseeded cells survival, and innervation of the implanted tissue, to enable efficient peristalsis of food intake.
Stem cells of oesophagus
Development of oesophagus from early foregut
While ventral foregut endoderm expresses transcription factor Nkx2.1 giving rise to the respiratory system, transcription factor Sox2 and Pdx1 are vital signalling pathway molecules for specification towards oesophagus, stomach and pancreas [68].
Stem and progenitor cells
Epithelial cells: The continuous turnover of the epithelial layer of the oesophagus relies on the so-called basal layer. These cells mature flattening into a stratified squamous epithelium consisting of 20–30 layers [69, 70]. Recent advances in lineage-tracing technology have revealed the stem/progenitor function of KRT5 positive basal cells in mice [71]. Currently, prevailing models for basal cell homeostasis in mature oesophagus are two: the heterogenous model, with multiple populations of basal cells (low integrin b1 and high laminin b2 [72] and CD34 + [73] subpopulations being the most validated), versus the homogenous model, with a single progenitor population maintaining self-renewal and differentiation capacity [74–76]. More recently, other various markers which indicate basal cell subpopulations have been advocated, although their overlapping has not been determined [77–79]. Further analysis to clarify relationships between these markers and the genuine indicators for stem cell populations is required. As an alternative approach, some researchers successfully used oral epithelial cells as an alternative cell source readily obtained during a minimally invasive procedure, with a high similarity in phenotype to oesophageal epithelial cells [80, 81].
Muscle cells: The submucosa and muscularis externa comprise mesenchymal elements of oesophageal wall; nerve fibres and ganglion cells are responsible for the generation of coordinated peristalsis [82, 83]. However, the origin of cells forming oesophageal muscular layer also remains an unanswered question. Initially, the unique structure of the human oesophageal muscle layer, a mixture of skeletal and smooth muscle, was considered to be the result of transdifferentiation of smooth muscle into skeletal muscle [84, 85]. However, lineage-tracing studies provided this assumption wrong [86]. Alternatively, it has been suggested that a skeletal muscle population originating from cranial mesoderm may migrate into the smooth muscle layers [87–89].
Tissue engineering
Much interest in tissue engineering of the oesophagus has focused on using acellular scaffolds derived from animal or human tissues and with or without cell-seeding. In particular, difficulty mimicking the complex native structure using synthetic materials whilst satisfying all the crucial features required for transplantable tissue constructs has provoked tissue engineering research to shift towards decellularised scaffolds [90, 91]. Badylak et al. demonstrated promising regenerative advantage of small intestinal submucosa (SIS) laid onto the surface of dissected submucosal layer following endoscopic submucosal resection in a human clinical trial [92]. In an attempt to generate a cell-based construct which more closely resembles the native structure of the oesophagus, researchers have attempted combination of synthetic and cells such as polyglycolic acid (PGA) and human amniotic membrane loaded with oral keratinocytes, fibroblasts, and smooth muscle cells in the canine model [93], acellular matrix recellularised with skeletal myoblasts covered by human amniotic membrane seeded with oral keratinocytes in a minipig model [94]. Urbani et al. developed recellularised oesophageal grafts using mesangioblasts and fibroblasts that formed a muscle layer after dynamic bioreactor culture [95–97]. Epithelial progenitor cells and enteric neural crest cells were functionally engrafted, and the graft was successfully prevascularised in vivo [95]. More recently, tubular synthetic scaffolds were seeded with autologous mesenchymal stem cells in preclinical models [98] and clinical application [99].
Stomach
Gastrectomy is commonly performed in adults and children for a variety of conditions, ranging from the need of an oesophageal substitute as in LGOA or caustic ingestion [100] till gastric cancer [101]. After gastrectomy, patients suffer from various symptoms due to a loss of reservoir, digestive and exocrine glandular functions [102]. To compensate for the loss of reservoir, various techniques have been developed but the lack of gastric mucosa, which plays a crucial role for the functionality of stomach, remains an unsolved aspect [103].
Stem cells and progenitors
Epithelial cells: Organogenesis of the stomach originates from a highly coordinated interaction between foregut endoderm and splanchnic mesoderm due to interplay of various factors and signals, such as HHEX, SOX2, retinoic acid, FGF and Wnt pathways and gradients of BMP [104].
The adult stomach is lined with a glandular epithelium in which self-renewal is driven by gastric stem cells. However, their exact location among the glandular functional unit is not clear. Using incorporation of labelled nucleotides in animal models, historical studies suggested that renewal of antral gastric cells was driven by one or a few cells localised in the isthmus [105]. More recently, by adapting lineage-tracing research from the previous intestinal studies, Clevers et al. identified a small number of cells at the bottom of pyloric gastric niches with active Wnt signalling marked with Leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) [106, 107]. These cells were able to form and maintain fully differentiated gastric organoids. Subsequently, Sigal et al. identified the presence of a second stem cells population, by targeting the classic Wnt gene Axin2 [108].
When combined with Lgr5 labelling, AXIN2 + LGR5 + and AXIN2 + LGR5- subpopulations located at the base and lower isthmus of the gastric glands were identified. Importantly, the two cell types can replenish each other, being alternatively activated and silenced in case of specific depletion [109]. Moreover, among all Wnt signalling, R-spondin 3 secreted by a-SMA positive mesenchymal cells beneath the gland has been pinpointed as an essential guide to epithelial stem cell renewal, in a reciprocal interaction [108].
Finally, in 2014 McCracken et al. firstly described the de novo generation of gastric mucosa from human iPSCs by temporal manipulation of the abovementioned signalling pathway [19] and subsequently obtained to recreate a functional epithelium [110].
Smooth muscle cells: The smooth muscle of the stomach is thicker than that of other digestive organs, but the mechanisms of stomach-specific myogenesis from a pool of progenitors are not fully understood [111]. Moreover, enteric neural crest cells also have a role in stomach smooth muscle development [112]. For example, specialised muscle cells in the pyloric sphincter integrate neuronal and hormonal signals to control the progression of food into the intestine [113].
Tissue engineering
Tissue engineering of the stomach for translational medicine has two main aims: regeneration of epithelial lineage itself and physiological reinforcement of gastric wall [114]. In the literature, different approaches have so far been applied: historically, scaffold biomaterials, such as collagen sponges or nonwoven poly glycolic acid (PGA) fibres, with or without cells loaded on the inner surface have been commonly used [115]. However, the regeneration achieved was just partial with a disturbed epithelialisation, a non-functional muscular layer and a significant contraction over time [116]. Recently, Tanaka et al. demonstrated that a myoblast cell sheet without scaffold could prevent leakage of the enteral contents in the mice model and obtained a rapid recovery of the discontinuation [117].
In addition to the reinforcement of the gastric wall defect, improvements in the functional regeneration of the mucosa have been seen once the scaffold has been loaded with organoids. Vacanti's group seeded organoids of a mesenchymal cores surrounded by epithelia onto a PGA tubular construct and then implanted both small (2-step replacement of native stomach in the murine model [118]) and large animal models (in the porcine model the constructs were implanted intraperitoneally [119]). To our knowledge, present literature is lacking naturally derived scaffolds, which may potentially facilitate regeneration, as composition of ECM in each tissue is unique and it could influence cell migration and differentiation.
Intestine
Various paediatric gastrointestinal diseases, including necrotising enterocolitis, gastroschisis, midgut malrotation and volvulus, intestinal atresia, and inflammatory bowel diseases, may result in short bowel syndrome (SBS), in which limited length of the intestine cannot perform the required functions such as absorption and barrier function, commonly caused [120]. Patients with SBS eventually develop intestinal failure when the residual bowel can no longer have sufficient absorptive capacity to meet their nutritional needs and become dependent on total parenteral nutrition associated with poor quality of life, risk of sepsis and liver dysfunction, and mortality [121]. Although small bowel transplantation is the ultimate cure and has already been performed in more than 2000 cases, surgical outcomes still remain unsatisfactory, with the survival rate at five years around 50% [122].
Intestinal stem cells and progenitor cells
The intestine is a highly complex organ with several essential functions; nutrient absorption, host immunity, and mucin secretion [123]. The intestinal epithelium has an integrated crypt-villus structure comprised of different cell types with specialised functions. The characteristic high turnover rate of the intestinal epithelium is mediated by intestinal stem cells located in the crypt base [124]. The aligned musculature and innervation are crucial to achieving synchronised and coordinated contraction throughout the enormous gastrointestinal tract. Smooth muscle cells and the enteric nervous system, together with the interstitial cells of Cajal (ICCs) as pace-making cells, mediate this function [125]. The vascular and lymphatic systems play a role in both absorption of nutrients and the maintenance of vascular supply [126].
Organogenesis and maturation of the intestine were recently reviewed in [127], emphasising the role of signalling pathways on intestinal specification, gut tube patterning, integration of smooth muscle and enteric nervous system (ENS), crypt-villus formation and postnatal maturation and maintenance. For example, the foregut and hindgut are specified by the expression of Sox2 and Cdx2 [128, 129]. Wnt and Notch signalling are two major signalling pathways regulating intestinal stem cells [130, 131]. This knowledge of molecular pathways, such as Wnt and Notch signalling, has been adapted into successful epithelial cell culture protocols.
Stem and progenitor cells
Generating functional intestinal epithelium is crucial to restoring a patient's enteral autonomy. Barker et al. first identified a widely accepted intestinal stem cell marker, Leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5), expressed in crypt base columnar cells, and demonstrated that a single Lgr5 + cell has the capacity to regenerate crypt-villus structures [124, 132]. Mesenchymal-free 3D culture systems allow intestinal stem cells to form enteroids or organoids with intestine-specific crypt-villus architecture by combining niche factors [132]. These mouse organoids were successfully repopulated onto a damaged colonic epithelial layer in vivo when delivered via a simple colonic enema, indicating their potential for clinical application for intestinal regeneration [23]. Pluripotent stem cells are alternative cell sources for regenerating human intestinal epithelium. Human iPSCs were successfully differentiated into definitive endodermal cells, followed by hindgut specification [20]. When transplanted under the mouse kidney capsule, enhancing vascularisation, these cells formed mature organoids with terminally differentiated intestinal cell lineages and adult-type crypt-villus architecture [133].
The mesenchymal and smooth muscle components of the intestine represent another challenge for intestinal regeneration, as they have a crucial role in maintaining the epithelial niche. Recently, human iPSC-derived intestinal organoids, in combination with human-PSC-derived neural crest cells, were reported to generate a smooth muscle layer that is positive for the smooth muscle marker desmin and can functionally respond to electrical and pharmacological stimuli [134].
Large-scale vascularisation capable of enabling successful surgical anastomosis into isolated engineered intestinal segments remains a challenge. Kitano et al. successfully repopulated human endothelial cells and human iPSCs-derived intestinal progenitors on a decellularised intestinal scaffold in a rat model. They restored perfusability and patency following in vivo isolated transplantation, as well as in ex vivo perfusion testing [135].
Tissue engineering
To create a sizable intestinal equivalent that can be successfully anastomosed to the native intestine, tissue engineering of the intestine has attempted to bring some or all cellular components and systems together into one platform. Given the complex structure and highly organised function of the intestine, the creation of synthetic biomaterials which recapitulate intestinal ultrastructure, such as the crypt-villus structure and stem cell niche, is challenging. Decellularised scaffolds of the intestine have attracted attention with advantages over synthetic materials. Our group has established a detergent-based decellularisation protocol that retains essential ECM proteins, angiogenic properties, and mechanical strength [90]. Recent studies focus on the integration of intestinal organoids into tissue-engineered intestines. Both human and mouse organoids replicated the structure and absorptive function of the intestine when implanted in a mouse model [136]. Implantation of human intestinal organoid-seeded scaffolds results in a tissue-engineered intestine resembling a native structure [137, 138].
The enteric nervous system (ENS)
Neurological dysfunction within the enteric nervous system (ENS) is responsible for a wide range of gastrointestinal motility disorders, such as oesophageal atresia [139], oesophageal achalasia [140, 141], and most commonly, Hirschsprung's disease (HD) and intestinal aganglionosis in children [142, 143]. Specifically, HD is known to be the result of impaired migration of enteric neural crest cells (NCCs) during intestinal development, with the primary symptom being intestinal obstruction caused by the lack of peristalsis [144]. Surgical resection of the aganglionic segment is the standard therapy; however, it is often associated with persistent symptoms, such as repeated enterocolitis and dysmotility [145, 146]. The restoration of the ENS through stem cell transplantation is attracting attention as a potentially curative therapeutic method [147].
Recently, substantial progress has been made in ENS research through the identification and isolation of enteric neural stem cells (ENSCs): multipotent cells which can be isolated from human GI tracts. McCann et al. reported the restoration of the ENS and colonic motility following the implantation of ENSCs into a mouse model of human enteric neuropathy (neuronal nitric oxide synthase deficient mouse), which highlighted the significant possibility of clinical ENSC therapy [148]. PSCs are alternative sources to circumvent the above issue, with abundant proliferative capacity. Fattahi et al. successfully isolated ENS progenitors from human PSCs and demonstrated their maturation into functional ENS [149]. They also reported restoring ENS components on human PSC-derived intestinal organoids by combining human PSC-derived NCCs in 3D growth conditions. Co-implanted NCCs migrated into intestinal mesenchyme and functionally integrated into the intestinal smooth muscle of the intestinal organoids [134, 150].
Pancreas
The intense interest in understanding human pancreas development is related to the treatment of diabetes, a growing health problem worldwide. Type 2 diabetes, the most prevalent form of this disorder, is typically characterised by a progressive failure of β-cells to meet the body’s demands for insulin; in contrast, β-cells are lost by autoimmune destruction in type 1 diabetes mellitus. Both mechanisms leading to diabetes are related to genome sequence variants in adult β-cells [151] and during developmental events [152]. In both cases, cell therapy and tissue engineering of the whole or partial organ are of great interest for potential transplantation or drug development.
Development of pancreas
Human pancreas formation starts with dorsal bud formation deriving from endoderm, and it continues with the appearance of the ventral bud. After the posterior migration, the ventral bud fuses with the dorsal pancreatic bud, giving rise to most of the pancreas. Although knowledge of events in human pancreas organogenesis is limited, Jennings et al. recently reported that contact between the dorsal pancreatic endoderm and the notochord leads to the exclusion of sonic hedgehog (SHH) expression from this endodermal region [153]. As a result, expression of the pancreatic and duodenal homeobox 1 (PDX) gene, a master gene for pancreatic development, is upregulated. Although all of the downstream effectors of pancreas development have not been determined yet, it appears that expression of the specific homeobox genes of the PAX family guides differentiation towards endocrine cell lineages that are known as α (glucagon), β (insulin), δ (somatostatin) and γ (pancreatic polypeptide) cells. By contrast, little is known about exocrine pancreas development [154].
Stem cells in the pancreas
Pancreatic progenitor cells arise from a cluster of cells originating from the developing foregut. These clusters have been characterised to show multipotent properties [155] and the ability to differentiate into endocrine, exocrine, and ductal lineage precursors [156]. More recently, adult pancreatic stem cells have been found as a subpopulation of biliary tree stem cells, precursors of both hepatic and pancreatic stem cells in the hepato-pancreatic common duct [157, 158]. MSCs can differentiate into immature islet-like cells with such low efficiency that they are not a practical source for clinical products [159].
Tissue engineering
Since the first experiments pursuing pancreatic differentiation [160], there has been a number of differentiation protocols using hESCs [161] or hiPSCs [162, 163], focusing on the generation of mature, single hormone-expressing, glucose-responsive human β-cells [164]. However, some transcriptional similarities have been identified between PSC-derived β-cells and human foetal β-cells, which may explain a relative immaturity of PSC-derived β-cells [165]. This immaturity may also reflect the lack of an appropriate developmental niche containing the necessary signalling factors for pancreatic cell differentiation [164], consistent with the better maturity achieved by in vivo incubation in mouse [157, 161] or culture on synthetic scaffolds [166]. In this context, some preliminary results are highlighting niche-derived influence on cell differentiation [167], although the whole mechanism remains to be clarified [168]. Some latest protocols have significantly improved in generating a higher proportion of monohormonal cells [169]. However, it is unclear whether these cells possess the ability of glucose-responsive insulin secretion and this remains a vital requirement for clinical transplantation [170].
Diaphragm and skeletal muscle
Several different approaches have been attempted to repair large defect in congenital diaphragmatic hernia (CDH), including synthetic mesh materials, autologous thoracic or abdominal muscle flap, with PTFE/Gore-tex® being the current standard practice. Because synthetic materials are related to various long-term complications, recent studies described using naturally derived materials, such as small intestine submucosa (SIS) and acellular dermis, with a failure of regeneration and a resultant re-herniation [171, 172]. A new strategy for a more effective biological substitute applicable for diaphragmatic defect repair or other muscular-related diseases (such as abdominal wall defect, trauma, volumetric muscle loss, or genetic disorders) represents a significant challenge.
Skeletal muscle regeneration
Skeletal muscle retains high regenerative capacity upon mechanical damage or diseases. This regenerative ability is mediated by a resident stem cell population for skeletal muscle, named muscle satellite cell (SC), and identified by the transcription factor Pax7 [173, 174]. The repair and regeneration of skeletal muscle comprise three phases: (1) host inflammatory cells are recruited to a damaged site in the inflammatory phase. (2) In the repair phase, pro-inflammatory macrophage (M1) is gradually replaced by anti-inflammatory macrophage (M2), which contributes to the proliferation and differentiation of SCs and their progeny; myoblasts [175]. (3) In the remodelling phase, newly formed muscle fibres are joined into the existing muscles [176].
Stem cells for skeletal muscle therapy and bioengineering
SCs and myoblasts are physiological skeletal muscle precursors, so it is rational that most studies on muscle cell therapy have been performed on these cell populations. Although investigations on SCs have demonstrated their proliferation and multipotent differentiation capabilities, utilising SCs for human cell therapy and bioengineered skeletal muscle has faced severe challenges and limitations [177]. Early-phase clinical studies showed that insufficient cells could be obtained from human biopsies, poor expansion potential of SCs cultured in vitro, poor survival in vivo, and low contribution of implanted SCs on muscle regeneration [178, 179].
Notably, in the setting of congenital disorders, SCs and myoblasts are exhausted, hindering efficient cell isolation and expansion in vitro. These drawbacks of SCs and myoblasts have led to the identification of alternative stem cell candidates for skeletal muscle regeneration, including mesangioblasts/pericytes [180], CD133-positive cells [181], Aldehyde dehydrogenase 1A1 (ALDH)-positive cells [182], MuStem cells [183] and hPSC [184].
Regarding the microenvironment of stem cell niche, the importance of mechanical rigidity of substrate used during in vitro culture has been noted to preserve satellite cell capacity. Appropriate elasticity or geometric micropatterning of a substrate where cells cultured on enhanced survival, self-renewal, and engraftment abilities of satellite cells in vivo [185, 186], proposing the requirement of investigation on optimal bioengineered stem cell niche [187].
Tissue engineering for skeletal muscle regeneration
In the setting of congenital muscular defects such as CDH or abdominal wall defects, a purely cell-based (scaffold-free) strategy is not applicable due to the massive skeletal muscle defects seen in many cases, which overwhelms the inherent regenerative potential. A bioengineered construct comprising exogenous myogenic stem/progenitor cells combined with scaffold biomaterials would be an attractive option for reconstructing these large defects. Growing evidence has suggested appropriate biomaterials can enhance the potential of stem/progenitor cells (see comprehensive review in [188]).
Most of these biomaterials are broadly divided into synthetic and naturally derived materials. Among various synthetic biodegradable materials, polycaprolactone (PCL), PGA, and poly-lactide-co-glycolic acid (PLGA) have been the most frequently tested materials in vitro and in vivo animal model, with relatively disappointing outcomes due to a pro-inflammatory response of the host immune system against these materials [189–192]. In contrast, an acellular ECM scaffold obtained by decellularisation of native muscle tissues is advantageous in mimicking native extracellular environments preferable for precursor cells, such as mechanical strength, microarchitecture, and growth factors.
Two approaches
Researchers have investigated an acellular approach, where only decellularised scaffold was used without cells. Sicari and Badylak et al. utilised an acellular scaffold derived from a porcine small intestinal submucosa-ECM and urinary bladder matrix for rodent volumetric muscle loss (VML) model and human patients. They showed constructive regeneration of skeletal muscle tissue [193, 194]. Piccoli et al. performed orthotopic transplantation of an acellular ECM scaffold derived from diaphragmatic muscle and demonstrated improvement of diaphragmatic function in an atrophic mouse model [195]. Based on results from preclinical trials, the first human clinical study implanting acellular ECM into severe VML cases have conducted, demonstrating early functional improvements and site-appropriate remodelling [194, 196].
In contrast, a cell-based tissue construct combining stem/progenitor cells and acellular ECM could be a better therapeutic solution, especially when the recipient's muscle is severely compromised and a scarcity of muscle progenitor pool [197, 198]. The combination of bone marrow-derived MSCs and acellular ECM demonstrated increased regeneration of muscle fibres and blood vessels [199].
Future perspectives
In the last decade, regenerative medicine has expanded dramatically, combining numerous relevant fields and technologies, including stem cell biology, biomaterial engineering, immunology, and genetics, indicating the interdisciplinary nature of this emerging discipline. Many novel therapeutic strategies have been proposed to treat adult and paediatric pathologies and realise finely tuned transplantable "artificial" organs. However, regarding applying this innovative paradigm to the paediatric surgical clinical practice, significant practical and ethical limitations still need to be addressed, including optimisation, scalability, and manufacturing standardisation [200].
Recent breakthroughs in this field might provide various alternative platforms and therapeutic options. Since 2017, researchers have developed an artificial placenta system comprising an extracorporeal circuit, oxygenator, and fluid-filled womb-like environment, and they proved the concept using premature animal models [201, 202]. Another promising alternative is xenotransplantation of genetically modified organs. In January 2022, the first pig-to-human heart transplantation was conducted at University of Maryland School of Medicine and the University of Maryland Medical Center. The transplanted xenogeneic heart was obtained from genetically modified pig [203]. Although there are still significant obstacles to overcome before the eventual clinical application of these platforms, they could facilitate the development of novel therapeutic approaches or a deeper understanding of the underlying mechanism in regenerative medicine.
Conclusion
The last decade has witnessed remarkable advance in both the basic knowledge and technologies in stem cell biology and bioengineering, which has made it possible to create a variety of engineered tissues and organs with the clinical translational potential. Due to the complexity of reconstruction in paediatrics, many paediatric surgeons contributed to the development of the field. In 1954 Dr Joe Murray, a paediatric plastic surgeon from Children's Hospital Boston, who subsequently received the Nobel prize in 1990, and his team, performed the first successful kidney transplant where the donor and the recipient were two identical twins [204]. Dr Judah Folkman, the father of angiogenesis research, was the director of the Vascular Biology Program and a former surgeon-in-chief at the Children's Hospital Boston. He had the ability to bring together both a scientist's and a surgeon's perspectives to finding solutions to medical problems. Dr Folkman published his ideas about angiogenesis in 1971 [205]. Working together in his laboratory at that time were Dr Jay Vacanti and Dr Robert Langer who started collaborating to create new matrices and scaffolds that could allow cells to be expanded and to differentiate. They defined the concept of tissue engineering as a new field that applies the principles of biology and engineering to the development of functional substitutes for damaged tissue [9]. Dr Folkman’s mentorship influenced many working in Boston at that time and just a few floors below in the same building Dr Anthony Atala, engineered the first human bladder which was later implanted in children requiring bladder augmentation [206]. Dr Prem Puri contributed as well to the field by investigating many of the diseases which will ultimately benefitting of a regenerative medicine approach like Hirschsprung’s Disease, congenital diaphragmatic hernia and abdominal wall defect. Moreover, we can identify Prof Puri as one of the contributors to the field by introducing and popularised the correction of vesicoureteric reflux by endoscopic injection of polytef paste. The bulging mechanism created by the polymer can be considered a first step towards an engineering approach which is safe, simple, and effective in correcting all grades of reflux [207].
Despite the enormous progress, the field of regenerative medicine is still in its relative infancy phase: however, with present momentum on research progress and interdisciplinary collaboration, it is reasonable to expect that shortly, regenerative medicine will lead to realising unprecedented surgical therapeutic strategies where impaired organs are replaced by novel biological substitutes.
Acknowledgements
P.D.C. is supported by National Institute for Health Research (NIHR-RP-2014-04-046). All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Author contributions
KD and EZ did the literature review. KD, EZ and PDC wrote the main manuscript text and prepared the figures.
Data availability
Due to the nature of the paper, no original data are presented.
Declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tam PKH, Wong KKY, Atala A, et al. Regenerative medicine: postnatal approaches. Lancet Child Adolesc Heal. 2022;6:654–666. doi: 10.1016/s2352-4642(22)00193-6. [DOI] [PubMed] [Google Scholar]
- 2.de Coppi P, Loukogeorgakis S, Götherström C, et al. Regenerative medicine: prenatal approaches. Lancet Child Adolesc Heal. 2022;6:643–653. doi: 10.1016/s2352-4642(22)00192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damdimopoulou P, Rodin S, Stenfelt S, et al. Human embryonic stem cells. Best Pract Res Cl Ob. 2016;31:2–12. doi: 10.1016/j.bpobgyn.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 5.Klimanskaya I, Chung Y, Becker S, et al. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444:481–485. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 7.Frangoul H, Altshuler D, Cappellini MD, et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. New Engl J Med. 2020;384:252–260. doi: 10.1056/nejmoa2031054. [DOI] [PubMed] [Google Scholar]
- 8.Hussey GS, Keane TJ, Badylak SF. The extracellular matrix of the gastrointestinal tract: a regenerative medicine platform. Nat Rev Gastroentero. 2017;14:540–552. doi: 10.1038/nrgastro.2017.76. [DOI] [PubMed] [Google Scholar]
- 9.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 10.MacArthur BD, Oreffo ROC. Bridging the gap. Nature. 2005;433:19–19. doi: 10.1038/433019a. [DOI] [PubMed] [Google Scholar]
- 11.Witman N, Zhou C, Beverborg NG, et al. Cardiac progenitors and paracrine mediators in cardiogenesis and heart regeneration. Semin Cell Dev Biol. 2020;100:29–51. doi: 10.1016/j.semcdb.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 13.Lancaster MA, Renner M, Martin C-A, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 15.Eiraku M, Sasai Y. Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr Opin Neurobiol. 2012;22:768–777. doi: 10.1016/j.conb.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi H, Kadoshima T, Soen M, et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun. 2015;6:8896. doi: 10.1038/ncomms9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taguchi A, Kaku Y, Ohmori T, et al. Redefining the In Vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Longmire TA, Ikonomou L, Hawkins F, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCracken KW, Catá EM, Crawford CM, et al. Modeling human development and disease in pluripotent stem cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 22.Takebe T, Enomura M, Yoshizawa E, et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 2015;16:556–565. doi: 10.1016/j.stem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Yui S, Nakamura T, Sato T, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 24.Fishman JM, Wiles K, Lowdell MW, et al. Airway tissue engineering: an update. Expert Opin Biol Th. 2014;14:1477–1491. doi: 10.1517/14712598.2014.938631. [DOI] [PubMed] [Google Scholar]
- 25.Sueblinvong V, Weiss DJ. Stem cells and cell therapy approaches in lung biology and diseases. Transl Res. 2010;156:188–205. doi: 10.1016/j.trsl.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breeze RG, Wheeldon EB. The cells of the pulmonary airways 1, 2. Am Rev Respir Dis. 1977;116:705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- 27.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol-lung C. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng T, Tian Y, Boogerd CJ, et al. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500:589–592. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc National Acad Sci. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rock JR, Gao X, Xue Y, et al. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar PA, Hu Y, Yamamoto Y, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suprynowicz FA, Upadhyay G, Krawczyk E, et al. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc National Acad Sci. 2012;109:20035–20040. doi: 10.1073/pnas.1213241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler CR, Hynds RE, Gowers KHC, et al. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Resp Crit Care. 2016;194:156–168. doi: 10.1164/rccm.201507-1414oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mason RJ, Williams MC. Type II alveolar cell. Am Rev Respir Dis. 2015;115:81–91. doi: 10.1164/arrd.1977.115.s.81. [DOI] [PubMed] [Google Scholar]
- 35.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/jci68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapman HA, Li X, Alexander JP, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/jci57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albera C, Polak JM, Janes S, et al. Repopulation of human pulmonary epithelium by bone marrow cells: a potential means to promote repair. Tissue Eng. 2005;11:1115–1121. doi: 10.1089/ten.2005.11.1115. [DOI] [PubMed] [Google Scholar]
- 39.Kubo A, Shinozaki K, Shannon JM, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 40.Samadikuchaksaraei A, Cohen S, Isaac K, et al. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12:867–875. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- 41.Wong AP, Bear CE, Chin S, et al. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol. 2012;30:876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green MD, Chen A, Nostro M-C, et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mou H, Zhao R, Sherwood R, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carraro G, Perin L, Sedrakyan S, et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008;26:2902–2911. doi: 10.1634/stemcells.2008-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pederiva F, Ghionzoli M, Pierro A, et al. Amniotic fluid stem cells rescue both in vitro and in vivo growth, innervation, and motility in nitrofen-exposed hypoplastic rat lungs through paracrine effects. Cell Transplant. 2013;22:1683–1694. doi: 10.3727/096368912x657756. [DOI] [PubMed] [Google Scholar]
- 46.Dye BR, Hill DR, Ferguson MA, et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4:e05098. doi: 10.7554/elife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gotoh S, Ito I, Nagasaki T, et al. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Rep. 2014;3:394–403. doi: 10.1016/j.stemcr.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaisani A, Delgado O, Fasciani G, et al. Branching morphogenesis of immortalized human bronchial epithelial cells in three-dimensional culture. Differentiation. 2014;87:119–126. doi: 10.1016/j.diff.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danahay H, Pessotti AD, Coote J, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 2015;10:239–252. doi: 10.1016/j.celrep.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto Y, Gotoh S, Korogi Y, et al. Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat Methods. 2017;14:1097–1106. doi: 10.1038/nmeth.4448. [DOI] [PubMed] [Google Scholar]
- 51.Knaneh-Monem H, Thornton ME, Grubbs BH, et al. Differential epithelial growth in tissue-engineered larynx and trachea generated from postnatal and fetal progenitor cells. Biochem Bioph Res Co. 2019;510:205–210. doi: 10.1016/j.bbrc.2019.01.060. [DOI] [PubMed] [Google Scholar]
- 52.Firth AL, Menon T, Parker GS, et al. Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Rep. 2015;12:1385–1390. doi: 10.1016/j.celrep.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wark PAB, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Medicine. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herr C, Beisswenger C, Hess C, et al. Suppression of pulmonary innate host defence in smokers. Thorax. 2009;64:144. doi: 10.1136/thx.2008.102681. [DOI] [PubMed] [Google Scholar]
- 55.Elliott MJ, de Coppi P, Speggiorin S, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994–1000. doi: 10.1016/s0140-6736(12)60737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elliott MJ, Butler CR, Varanou-Jenkins A, et al. Tracheal replacement therapy with a stem cell-seeded graft: lessons from compassionate use application of a GMP-compliant tissue-engineered medicine. Stem Cell Transl Med. 2017;6:1458–1464. doi: 10.1002/sctm.16-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dhasmana A, Singh A, Rawal S. Biomedical grafts for tracheal tissue repairing and regeneration “Tracheal tissue engineering: an overview”. J Tissue Eng Regen M. 2020;14:653–672. doi: 10.1002/term.3019. [DOI] [PubMed] [Google Scholar]
- 58.Delaere P, Vranckx J, Verleden G, et al. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. New Engl J Med. 2010;362:138–145. doi: 10.1056/nejmoa0810653. [DOI] [PubMed] [Google Scholar]
- 59.Wurtz A, Porte H, Conti M, et al. Tracheal replacement with aortic allografts. New Engl J Medicine. 2006;355:1938–1940. doi: 10.1056/nejmc066336. [DOI] [PubMed] [Google Scholar]
- 60.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 61.Song JJ, Kim SS, Liu Z, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998–1006. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 62.Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou H, Kitano K, Ren X, et al. Bioengineering human lung grafts on porcine matrix. Ann Surg. 2018;267:590–598. doi: 10.1097/sla.0000000000002129. [DOI] [PubMed] [Google Scholar]
- 64.Galliger Z, Vogt CD, Panoskaltsis-Mortari A. 3D bioprinting for lungs and hollow organs. Transl Res. 2019;211:19–34. doi: 10.1016/j.trsl.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan JY, Chua CK, Leong KF, et al. Esophageal tissue engineering: an in-depth review on scaffold design. Biotechnol Bioeng. 2012;109:1–15. doi: 10.1002/bit.23323. [DOI] [PubMed] [Google Scholar]
- 66.Lee E, Milan A, Urbani L, et al. Decellularized material as scaffolds for tissue engineering studies in long gap esophageal atresia. Expert Opin Biol Th. 2017;17:573–584. doi: 10.1080/14712598.2017.1308482. [DOI] [PubMed] [Google Scholar]
- 67.Kuppan P, Sethuraman S, Krishnan UM. Tissue engineering interventions for esophageal disorders—Promises and challenges. Biotechnol Adv. 2012;30:1481–1492. doi: 10.1016/j.biotechadv.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Perin S, McCann CJ, Borrelli O, et al. Update on foregut molecular embryology and role of regenerative medicine therapies. Front Pediatr. 2017;5:91. doi: 10.3389/fped.2017.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marques-Pereira JP, Leblond CP. Mitosis and differentiation in the stratified squamous epithelium of the rat esophagus. Am J Anat. 1965;117:73–87. doi: 10.1002/aja.1001170106. [DOI] [PubMed] [Google Scholar]
- 70.Treuting PM, Dintzis SM, Montine KS (2018) Comparative anatomy and histology (Second Edition). 10.1016/b978-0-12-802900-8.00001-4
- 71.Jovov B, Que J, Tobey NA, et al. Role of E-cadherin in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1039–1047. doi: 10.1038/ajg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seery JP, Watt FM. Asymmetric stem-cell divisions define the architecture of human oesophageal epithelium. Curr Biol. 2000;10:1447–1450. doi: 10.1016/s0960-9822(00)00803-4. [DOI] [PubMed] [Google Scholar]
- 73.Kalabis J, Oyama K, Okawa T, et al. A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification. J Clin Invest. 2008;118:3860–3869. doi: 10.1172/jci35012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doupé DP, Alcolea MP, Roshan A, et al. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science. 2012;337:1091–1093. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alcolea MP, Greulich P, Wabik A, et al. Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nat Cell Biol. 2014;16:612–619. doi: 10.1038/ncb2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frede J, Greulich P, Nagy T, et al. A single dividing cell population with imbalanced fate drives oesophageal tumour growth. Nat Cell Biol. 2016;18:967–978. doi: 10.1038/ncb3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Croagh D, Phillips WA, Redvers R, et al. Identification of candidate murine esophageal stem cells using a combination of cell kinetic studies and cell surface markers. Stem Cells. 2007;25:313–318. doi: 10.1634/stemcells.2006-0421. [DOI] [PubMed] [Google Scholar]
- 78.Liu K, Jiang M, Lu Y, et al. Sox2 cooperates with inflammation-mediated stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 2013;12:304–315. doi: 10.1016/j.stem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeWard AD, Cramer J, Lagasse E. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep. 2014;9:701–711. doi: 10.1016/j.celrep.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohki T, Yamato M, Murakami D, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704. doi: 10.1136/gut.2005.088518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lopes MF, Cabrita A, Ilharco J, et al. Esophageal replacement in rat using porcine intestinal submucosa as a patch or a tube-shaped graft. Dis Esophagus. 2006;19:254–259. doi: 10.1111/j.1442-2050.2006.00574.x. [DOI] [PubMed] [Google Scholar]
- 82.Kablar B, Tajbakhsh S, Rudnicki MA. Transdifferentiation of esophageal smooth to skeletal muscle is myogenic bHLH factor-dependent. Dev Camb Engl. 2000;127:1627–1639. doi: 10.1242/dev.127.8.1627. [DOI] [PubMed] [Google Scholar]
- 83.Luc G, Durand M, Collet D, et al. Esophageal tissue engineering. Expert Rev Med Devic. 2014;11:225–241. doi: 10.1586/17434440.2014.870470. [DOI] [PubMed] [Google Scholar]
- 84.Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol. 1995;191:381–396. doi: 10.1007/bf00304424. [DOI] [PubMed] [Google Scholar]
- 85.Patapoutian A, Wold BJ, Wagner RA. Evidence for developmentally programmed transdifferentiation in mouse esophageal muscle. Science. 1995;270:1818–1821. doi: 10.1126/science.270.5243.1818. [DOI] [PubMed] [Google Scholar]
- 86.Rishniw M, Xin H, Deng K, Kotlikoff MI. Skeletal myogenesis in the mouse esophagus does not occur through transdifferentiation. Genesis. 2003;36:81–82. doi: 10.1002/gene.10198. [DOI] [PubMed] [Google Scholar]
- 87.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 88.Nathan E, Monovich A, Tirosh-Finkel L, et al. The contribution of Islet1-expressing splanchnic mesoderm cells to distinct branchiomeric muscles reveals significant heterogeneity in head muscle development. Development. 2008;135:647–657. doi: 10.1242/dev.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gopalakrishnan S, Comai G, Sambasivan R, et al. A cranial mesoderm origin for esophagus striated muscles. Dev Cell. 2015;34:694–704. doi: 10.1016/j.devcel.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 90.Totonelli G, Maghsoudlou P, Garriboli M, et al. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials. 2012;33:3401–3410. doi: 10.1016/j.biomaterials.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hannon E, Pellegrini M, Scottoni F, et al. Lessons learned from pre-clinical testing of xenogeneic decellularized esophagi in a rabbit model. Iscience. 2022;25:105174. doi: 10.1016/j.isci.2022.105174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Badylak SF, Hoppo T, Nieponice A, et al. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic Scaffold. Tissue Eng Pt A. 2011;17:1643–1650. doi: 10.1089/ten.tea.2010.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakase Y, Nakamura T, Kin S, et al. Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J Thorac Cardiovasc Surg. 2008;136:850–859. doi: 10.1016/j.jtcvs.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 94.Poghosyan T, Sfeir R, Michaud L, et al. Circumferential esophageal replacement using a tube-shaped tissue-engineered substitute: an experimental study in minipigs. Surgery. 2015;158:266–277. doi: 10.1016/j.surg.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 95.Urbani L, Camilli C, Phylactopoulos D-E, et al. Multi-stage bioengineering of a layered oesophagus with in vitro expanded muscle and epithelial adult progenitors. Nat Commun. 2018;9:4286. doi: 10.1038/s41467-018-06385-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giobbe GG, Zambon A, Vetralla M, et al. Preservation over time of dried acellular esophageal matrix. Biomed Phys Eng Express. 2018;4:065021. doi: 10.1088/2057-1976/aae4ff. [DOI] [Google Scholar]
- 97.Urbani L, Maghsoudlou P, Milan A, et al. Long-term cryopreservation of decellularised oesophagi for tissue engineering clinical application. PLoS ONE. 2017;12:e0179341. doi: 10.1371/journal.pone.0179341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sundaram S, Jensen T, Roffidal T, et al. Esophageal regeneration following surgical implantation of a tissue engineered esophageal implant in a pediatric model. Npj Regen Medicine. 2022;7:1. doi: 10.1038/s41536-021-00200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aho JM, Francesca SL, Olson SD, et al. First-in-human segmental esophageal reconstruction using a bioengineered mesenchymal stromal cell-seeded implant. Jto Clin Res Rep. 2021;2:100216. doi: 10.1016/j.jtocrr.2021.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharma S, Gupta DK. Surgical techniques for esophageal replacement in children. Pediatr Surg Int. 2017;33:527–550. doi: 10.1007/s00383-016-4048-1. [DOI] [PubMed] [Google Scholar]
- 101.Mantoan S, Cavallin F, Pinto E, et al. Long-term quality of life after esophagectomy with gastric pull-up. Jso. 2018;117:970–976. doi: 10.1002/jso.24995. [DOI] [PubMed] [Google Scholar]
- 102.Konishi H, Nakada K, Kawamura M, et al. Impaired gastrointestinal function affects symptoms and alimentary status in patients after gastrectomy. World J Surg. 2016;40:2713–2718. doi: 10.1007/s00268-016-3613-z. [DOI] [PubMed] [Google Scholar]
- 103.Jun W, Wei W, Weibing W, et al. Clinical outcome of using gastric remnant or jejunum or colon conduit in surgery for esophageal carcinoma with previous gastrectomy. Jso. 2017;115:729–737. doi: 10.1002/jso.24564. [DOI] [PubMed] [Google Scholar]
- 104.Tiso N, Filippi A, Pauls S, et al. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Develop. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- 105.Leblond CP, Stevens CE, Bogoroch R. Histological localization of newly-formed desoxyribonucleic acid. Science. 1948;108:531–533. doi: 10.1126/science.108.2811.531. [DOI] [PubMed] [Google Scholar]
- 106.Barker N, Huch M, Kujala P, et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 107.Bartfeld S, Clevers H. Organoids as model for infectious diseases: culture of human and murine stomach organoids and microinjection of helicobacter pylori. J Vis Exp. 2015 doi: 10.3791/53359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sigal M, Logan CY, Kapalczynska M, et al. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature. 2017;548:451–455. doi: 10.1038/nature23642. [DOI] [PubMed] [Google Scholar]
- 109.Thomas H. R-spondin 3 is a critical regulator of gastric antral stem cell homeostasis. Nat Rev Gastroentero. 2017;14:565–565. doi: 10.1038/nrgastro.2017.128. [DOI] [PubMed] [Google Scholar]
- 110.McCracken KW, Aihara E, Martin B, et al. Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541:182–187. doi: 10.1038/nature21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mao Z, Ma X, Rong Y, et al. Connective tissue growth factor enhances the migration of gastric cancer through downregulation of E-cadherin via the NF-κB pathway. Cancer Sci. 2011;102:104–110. doi: 10.1111/j.1349-7006.2010.01746.x. [DOI] [PubMed] [Google Scholar]
- 112.Faure S, McKey J, Sagnol S, de Barbara Santa P. Enteric neural crest cells regulate vertebrate stomach patterning and differentiation. Development. 2014;142:331–342. doi: 10.1242/dev.118422. [DOI] [PubMed] [Google Scholar]
- 113.Ramkumar D, Schulze KS. The pylorus. Neurogastroenterol Motil. 2005;17:22–30. doi: 10.1111/j.1365-2982.2005.00664.x. [DOI] [PubMed] [Google Scholar]
- 114.Kanetaka K, Eguchi S. Regenerative medicine for the upper gastrointestinal tract. Regen Ther. 2020;15:129–137. doi: 10.1016/j.reth.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hori Y, Nakamura T, Matsumoto K, et al. Experimental study on in situ tissue engineering of the stomach by an acellular collagen sponge scaffold graft. Asaio J. 2001;47:206–210. doi: 10.1097/00002480-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 116.Hori Y, Nakamura T, Kimura D, et al. Functional analysis of the tissue-engineered stomach wall. Artif Organs. 2002;26:868–872. doi: 10.1046/j.1525-1594.2002.07006.x. [DOI] [PubMed] [Google Scholar]
- 117.Tanaka S, Kanetaka K, Fujii M, et al. Cell sheet technology for the regeneration of gastrointestinal tissue using a novel gastric perforation rat model. Surg Today. 2017;47:114–121. doi: 10.1007/s00595-016-1360-2. [DOI] [PubMed] [Google Scholar]
- 118.Maemura T, Shin M, Sato M, et al. A tissue-engineered stomach as a replacement of the native stomach. Transplantation. 2003;76:61–65. doi: 10.1097/01.tp.0000068903.63554.1b. [DOI] [PubMed] [Google Scholar]
- 119.Sala FG, Kunisaki SM, Ochoa ER, et al. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J Surg Res. 2009;156:205–212. doi: 10.1016/j.jss.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 120.Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403–412. doi: 10.1097/01.sla.0000179647.24046.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goulet O, Ruemmele F, Lacaille F, Colomb V. Irreversible intestinal failure. J Pediatr Gastr Nutr. 2004;38:250–269. doi: 10.1097/00005176-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 122.Grant D, Abu-Elmagd K, Reyes J, et al. 2003 report of the intestine transplant registry: a new era has dawned. Ann Surg. 2005;241:607–613. doi: 10.1097/01.sla.0000157265.85388.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kiela PR, Ghishan FK. Physiology of Intestinal Absorption and Secretion. Best Pract Res Clin Gastroenterology. 2016;30:145–159. doi: 10.1016/j.bpg.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 125.Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility—insights from smooth muscle biology. Nat Rev Gastroentero. 2012;9:633–645. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alexander JS, Ganta VC, Jordan PA, Witte MH. Gastrointestinal lymphatics in health and disease. Pathophysiol. 2010;17:315–335. doi: 10.1016/j.pathophys.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chin AM, Hill DR, Aurora M, Spence JR. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin Cell Dev Biol. 2017;66:81–93. doi: 10.1016/j.semcdb.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Que J, Okubo T, Goldenring JR, et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell. 2009;16:588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mah AT, Yan KS, Kuo CJ. Wnt pathway regulation of intestinal stem cells. J Physiology. 2016;594:4837–4847. doi: 10.1113/jp271754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiology. 2016;594:4791–4803. doi: 10.1113/jp271667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 133.Watson CL, Mahe MM, Múnera J, et al. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20:1310–1314. doi: 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Workman MJ, Mahe MM, Trisno S, et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23:49–59. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kitano K, Schwartz DM, Zhou H, et al. Bioengineering of functional human induced pluripotent stem cell-derived intestinal grafts. Nat Commun. 2017;8:765. doi: 10.1038/s41467-017-00779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grant CN, Mojica SG, Sala FG, et al. Human and mouse tissue-engineered small intestine both demonstrate digestive and absorptive function. Am J Physiol-gastr L. 2015;308:G664–G677. doi: 10.1152/ajpgi.00111.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Finkbeiner SR, Freeman JJ, Wieck MM, et al. Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol Open. 2015;4:1462–1472. doi: 10.1242/bio.013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meran L, Massie I, Campinoti S, et al. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nat Med. 2020;26:1593–1601. doi: 10.1038/s41591-020-1024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boleken M, Demirbilek S, Kirimiloglu H, et al. Reduced neuronal innervation in the distal end of the proximal esophageal atretic segment in cases of esophageal atresia with distal tracheoesophageal fistula. World J Surg. 2007;31:1512–1517. doi: 10.1007/s00268-007-9070-y. [DOI] [PubMed] [Google Scholar]
- 140.Goldblum J, Rice T, Richter J. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology. 1996;111:648–654. doi: 10.1053/gast.1996.v111.pm8780569. [DOI] [PubMed] [Google Scholar]
- 141.Knowles CH, Lindberg G, Panza E, Giorgio RD. New perspectives in the diagnosis and management of enteric neuropathies. Nat Rev Gastroentero. 2013;10:206–218. doi: 10.1038/nrgastro.2013.18. [DOI] [PubMed] [Google Scholar]
- 142.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 143.Amiel J, Lyonnet S. Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet. 2001;38:729. doi: 10.1136/jmg.38.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroentero. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 145.Sulkowski JP, Cooper JN, Congeni A, et al. Single-stage versus multi-stage pull-through for Hirschsprung’s disease: Practice trends and outcomes in infants. J Pediatr Surg. 2014;49:1619–1625. doi: 10.1016/j.jpedsurg.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Laughlin DM, Friedmacher F, Puri P. Total colonic aganglionosis: a systematic review and meta-analysis of long-term clinical outcome. Pediatr Surg Int. 2012;28:773–779. doi: 10.1007/s00383-012-3117-3. [DOI] [PubMed] [Google Scholar]
- 147.Burns AJ, Roberts RR, Bornstein JC, Young HM. Development of the enteric nervous system and its role in intestinal motility during fetal and early postnatal stages. Semin Pediatr Surg. 2009;18:196–205. doi: 10.1053/j.sempedsurg.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 148.McCann CJ, Cooper JE, Natarajan D, et al. Transplantation of enteric nervous system stem cells rescues nitric oxide synthase deficient mouse colon. Nat Commun. 2017;8:15937. doi: 10.1038/ncomms15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fattahi F, Steinbeck JA, Kriks S, et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531:105–109. doi: 10.1038/nature16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schlieve CR, Fowler KL, Thornton M, et al. Neural crest cell implantation restores enteric nervous system function and alters the gastrointestinal transcriptome in human tissue-engineered small intestine. Stem Cell Rep. 2017;9:883–896. doi: 10.1016/j.stemcr.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pasquali L, Gaulton KJ, Rodríguez-Seguí SA, et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet. 2014;46:136–143. doi: 10.1038/ng.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Travers ME, Mackay DJG, Nitert MD, et al. Insights Into the molecular mechanism for type 2 diabetes susceptibility at the KCNQ1 locus from temporal changes in imprinting status in human islets. Diabetes. 2013;62:987–992. doi: 10.2337/db12-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jennings RE, Berry AA, Kirkwood-Wilson R, et al. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013;62:3514–3522. doi: 10.2337/db12-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pan FC, Brissova M. Pancreas development in humans. Curr Opin Endocrinol Diabetes Obes. 2014;21:77–82. doi: 10.1097/med.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ku YP, Jin M, Kim KH, et al. Immunolocalization of 8-OHdG and OGG1 in pancreatic islets of streptozotocin-induced diabetic rats. Acta Histochem. 2009;111:138–144. doi: 10.1016/j.acthis.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 156.Kahan BW, Jacobson LM, Hullett DA, et al. Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells. Diabetes. 2003;52:2016–2024. doi: 10.2337/diabetes.52.8.2016. [DOI] [PubMed] [Google Scholar]
- 157.Wang Y, Lanzoni G, Carpino G, et al. Biliary tree stem cells, precursors to pancreatic committed progenitors: evidence for possible life-long pancreatic organogenesis. Stem Cells. 2013;31:1966–1979. doi: 10.1002/stem.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bonner-Weir S, Toschi E, Inada A, et al. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2004;5:16–22. doi: 10.1111/j.1399-543x.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 159.Talebi S, Aleyasin A, Soleimani M, Massumi M. Derivation of islet-like cells from mesenchymal stem cells using PDX1-transducing lentiviruses. Biotechnol Appl Bioc. 2001;59:205–212. doi: 10.1002/bab.1013. [DOI] [PubMed] [Google Scholar]
- 160.Assady S, Maor G, Amit M, et al. Insulin Production by Human Embryonic Stem Cells. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 161.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 162.Tateishi K, He J, Taranova O, et al. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601–31607. doi: 10.1074/jbc.m806597200. [DOI] [PubMed] [Google Scholar]
- 163.Amer MG, Embaby AS, Karam RA, Amer MG. Role of adipose tissue derived stem cells differentiated into insulin producing cells in the treatment of type I diabetes mellitus. Gene. 2018;654:87–94. doi: 10.1016/j.gene.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 164.Pagliuca FW, Melton DA. How to make a functional β-cell. Development. 2013;140:2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hrvatin S, O’Donnell CW, Deng F, et al. Differentiated human stem cells resemble fetal, not adult, β cells. Proc National Acad Sci. 2014;111:3038–3043. doi: 10.1073/pnas.1400709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Enderami SE, Kehtari M, Abazari MF, et al. Generation of insulin-producing cells from human induced pluripotent stem cells on PLLA/PVA nanofiber scaffold. Artif Cells Nanomed Biotechnology. 2018;46:1062–1069. doi: 10.1080/21691401.2018.1443466. [DOI] [PubMed] [Google Scholar]
- 167.Takahashi K, Ehata S, Koinuma D, et al. Pancreatic tumor microenvironment confers highly malignant properties on pancreatic cancer cells. Oncogene. 2018;37:2757–2772. doi: 10.1038/s41388-018-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Damodaran RG, Vermette P. Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture. J Tissue Eng Regen M. 2018;12:1230–1237. doi: 10.1002/term.2655. [DOI] [PubMed] [Google Scholar]
- 169.Pagliuca FW, Millman JR, Gürtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hanley N. Closing in on pancreatic beta cells. Nat Biotechnol. 2014;32:1100–1102. doi: 10.1038/nbt.3064. [DOI] [PubMed] [Google Scholar]
- 171.Vecchia LD, Engum S, Kogon B, et al. Evaluation of small intestine submucosa and acellular dermis as diaphragmatic prostheses. J Pediatr Surg. 1999;34:167–171. doi: 10.1016/s0022-3468(99)90250-6. [DOI] [PubMed] [Google Scholar]
- 172.Fauza DO. Tissue engineering in congenital diaphragmatic hernia. Semin Pediatr Surg. 2014;23:135–140. doi: 10.1053/j.sempedsurg.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 173.Mauro A. Satellite cell of skeletal muscle fibers. J Cell Biology. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Seale P, Asakura A, Rudnicki MA. The Potential of Muscle Stem Cells. Dev Cell. 2001;1:333–342. doi: 10.1016/s1534-5807(01)00049-1. [DOI] [PubMed] [Google Scholar]
- 175.Malerba A, Pasut A, Frigo M, et al. Macrophage-secreted factors enhance the in vitro expansion of DMD muscle precursor cells while preserving their myogenic potential. Neurol Res. 2010;32:55–62. doi: 10.1179/174313209x380865. [DOI] [PubMed] [Google Scholar]
- 176.Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347:759–774. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- 177.Asakura A, Rudnicki MA, Komaki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 178.Péault B, Rudnicki M, Torrente Y, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 179.Negroni E, Gidaro T, Bigot A, et al. Advances in cell therapy strategies. Neuropath Appl Neuro. 2015;41:270–287. doi: 10.1111/nan.12198. [DOI] [PubMed] [Google Scholar]
- 180.Cossu G, Previtali SC, Napolitano S, et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. Embo Mol Med. 2015;7:1513–1528. doi: 10.15252/emmm.201505636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meng J, Chun S, Asfahani R, et al. Human skeletal muscle–derived CD133+ cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol Ther. 2014;22:1008–1017. doi: 10.1038/mt.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vauchez K, Marolleau J-P, Schmid M, et al. Aldehyde dehydrogenase activity identifies a population of human skeletal muscle cells with high myogenic capacities. Mol Ther. 2009;17:1948–1958. doi: 10.1038/mt.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lorant J, Saury C, Schleder C, et al. Skeletal muscle regenerative potential of human mustem cells following transplantation into injured mice muscle. Mol Ther. 2018;26:618–633. doi: 10.1016/j.ymthe.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chal J, Pourquié O. Making muscle: skeletal myogenesis in vivo and in vitro. Development. 2017;144:2104–2122. doi: 10.1242/dev.151035. [DOI] [PubMed] [Google Scholar]
- 185.Gilbert PM, Havenstrite KL, Magnusson KEG, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yennek S, Burute M, Théry M, Tajbakhsh S. Cell adhesion geometry regulates non-random dna segregation and asymmetric cell fates in mouse skeletal muscle stem cells. Cell Rep. 2014;7:961–970. doi: 10.1016/j.celrep.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 187.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 188.Qazi TH, Mooney DJ, Pumberger M, et al. Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials. 2015;53:502–521. doi: 10.1016/j.biomaterials.2015.02.110. [DOI] [PubMed] [Google Scholar]
- 189.Thorrez L, Shansky J, Wang L, et al. Growth, differentiation, transplantation and survival of human skeletal myofibers on biodegradable scaffolds. Biomaterials. 2008;29:75–84. doi: 10.1016/j.biomaterials.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Smith MK, Peters MC, Richardson TP, et al. Locally enhanced angiogenesis promotes transplanted cell survival. Tissue Eng. 2004;10:63–71. doi: 10.1089/107632704322791709. [DOI] [PubMed] [Google Scholar]
- 191.Saxena AK, Willital GH, Vacanti JP. Vascularized three-dimensional skeletal muscle tissue-engineering. Bio-med Mater Eng. 2001;11:275–281. [PubMed] [Google Scholar]
- 192.Choi JS, Lee SJ, Christ GJ, et al. The influence of electrospun aligned poly(ɛ-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29:2899–2906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 193.Sicari BM, Agrawal V, Siu BF, et al. A murine model of volumetric muscle loss and a regenerative medicine approach for tissue replacement. Tissue Eng Pt A. 2012;18:1941–1948. doi: 10.1089/ten.tea.2012.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sicari BM, Rubin JP, Dearth CL, et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 2014;6:234ra58. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Piccoli M, Urbani L, Alvarez-Fallas ME, et al. Improvement of diaphragmatic performance through orthotopic application of decellularized extracellular matrix patch. Biomaterials. 2016;74:245–255. doi: 10.1016/j.biomaterials.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 196.Dziki J, Badylak S, Yabroudi M, et al. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. Npj Regen Medicine. 2016;1:16008. doi: 10.1038/npjregenmed.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Corona BT, Wu X, Ward CL, et al. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials. 2013;34:3324–3335. doi: 10.1016/j.biomaterials.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 198.Aurora A, Roe JL, Corona BT, Walters TJ. An acellular biologic scaffold does not regenerate appreciable de novo muscle tissue in rat models of volumetric muscle loss injury. Biomaterials. 2015;67:393–407. doi: 10.1016/j.biomaterials.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 199.Merritt EK, Cannon MV, Hammers DW, et al. Repair of traumatic skeletal muscle injury with bone-marrow-derived mesenchymal stem cells seeded on extracellular matrix. Tissue Eng Pt A. 2010;16:2871–2881. doi: 10.1089/ten.tea.2009.0826. [DOI] [PubMed] [Google Scholar]
- 200.Terzic A, Pfenning MA, Gores GJ, Harper CM. Regenerative medicine build-out. Stem Cell Transl Med. 2015;4:1373–1379. doi: 10.5966/sctm.2015-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Partridge EA, Davey MG, Hornick MA, et al. An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun. 2017;8:15112. doi: 10.1038/ncomms15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Usuda H, Watanabe S, Saito M, et al. Successful use of an artificial placenta–based life support system to treat extremely preterm ovine fetuses compromised by intrauterine inflammation. Am J Obstet Gynecol. 2020;223:755.e1–755.e20. doi: 10.1016/j.ajog.2020.04.036. [DOI] [PubMed] [Google Scholar]
- 203.Reardon S. First pig-to-human heart transplant: what can scientists learn? Nature. 2022;601:305–306. doi: 10.1038/d41586-022-00111-9. [DOI] [PubMed] [Google Scholar]
- 204.de Coppi P. Regenerative medicine for congenital malformations. J Pediatr Surg. 2013;48:273–280. doi: 10.1016/j.jpedsurg.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 205.Sherwood LM, Parris EE, Folkman J. Tumor angiogenesis: therapeutic implications. New Engl J Med. 1971;285:1182–1186. doi: 10.1056/nejm197111182852108. [DOI] [PubMed] [Google Scholar]
- 206.Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/s0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 207.Puri P. Endoscopic correction of primary vesicoureteric reflux by subureteric injection of polytetrafluoroethylene. Lancet. 1990;335:1320–1322. doi: 10.1016/0140-6736(90)91197-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the nature of the paper, no original data are presented.