Abstract
Monocytes are a major population of circulating immune cells that play a crucial role in producing pro-inflammatory cytokines in the body. The actions of monocytes are known to be influenced by the combinations and concentrations of certain fatty acids (FAs) in blood and dietary fats. However, systemic comparisons of the effects of FAs on cytokine secretion by monocytes have not be performed. In this study, we compared how six saturated FAs (SFAs), two monounsaturated FAs (MUFAs), and seven polyunsaturated FAs (PUFAs) modulate human THP-1 monocyte secretion of TNF, IL-1β, and IL-6 in the absence or presence of lipopolysaccharide. SFAs generally stimulated resting THP-1 cells to secrete pro-inflammatory cytokines, with stearic acid being the most potent species. In contrast, MUFAs and PUFAs inhibited lipopolysaccharide-induced secretion of pro-inflammatory cytokines. Interestingly, the inhibitory potentials of MUFAs and PUFAs followed U-shaped (TNF and IL-1β) or inverted U-shaped (IL-6) dose–response curves. Among the MUFAs and PUFAs that were analyzed, docosahexaenoic acid (C22:6 n-3) exhibited the largest number of double bonds and was found to be the most potent anti-inflammatory compound. Together, our findings reveal that the chemical compositions and concentrations of dietary FAs are key factors in the intricate regulation of monocyte-mediated inflammation.
Subject terms: Cytokines, Lipids
Introduction
Inflammation is an essential protective response that helps an organism to resolve infections and injuries1,2. However, in certain circumstances, the acute inflammatory response may progress to a persistent non-resolving response that becomes harmful to the host3. Chronic systemic inflammation can lead to a breakdown in immune tolerance4–6 and increase the risks of various non-communicable diseases, including cancer, cardiovascular disease, metabolic disorders and neurodegenerative diseases7–12. Prolonged inflammation can also weaken the immune system, leading to increased risk of infections and decreased response to vaccination13–15. Additionally, early-life chronic inflammation can have serious developmental consequences that raise an individual’s lifetime risk of developing non-communicable diseases16–19. Notably, chronic inflammation-related diseases now contribute to over 50% of all deaths20,21. Nevertheless, inflammation is necessary for survival, and maintaining a balance of cellular and molecular inflammation mediators is crucial for many essential homeostatic processes, including tissue remodeling, metabolism, and nervous system function22. Thus, there is an urgent need for new clinical strategies to finely control the inflammation state.
The compositions of dietary fats greatly affect the profiles and concentrations of fatty acids (FAs) in the blood23–27, and these circulating FAs are known to modulate inflammatory status in humans and animals28. FAs are generally categorized into three classes according to their chemical structures (i.e., number of double bonds): saturated FAs (SFAs), monounsaturated FAs (MUFAs), and polyunsaturated FAS (PUFAs). In general, SFAs are regarded as pro-inflammatory factors29–31, whereas MUFAs and PUFAs appear to function as anti-inflammatory mediators32–35. As such, it is widely assumed that dietary intake of different classes of fats will alter the circulating FA profiles and differentially affect the activation status of circulating immune cells. Yet, systematic explorations of how different dietary fatty acids affect immune cells are still lacking.
Monocytes are a major population of circulating immune cells that can be recruited from the bloodstream to peripheral tissues, where they differentiate into either macrophages or dendritic cells to support both innate and adaptive immune responses36. Upon activation, macrophages in different tissues respond to certain microenvironmental stimuli by taking on M1 or M2 polarizations37–39. In infected or injured tissues, macrophages first polarize to an M1 state in order to eliminate pathogens; then, the cells take on an M2 polarization and function to repair tissue damage39. Three main categories of stimuli have been identified as inducers of M1 macrophages, including interferon-γ, pathogens, and granulocyte macrophage colony-stimulating factor37. Meanwhile, M2 macrophages can be induced by interleukin (IL)-4, IL-10, glucocorticoids, and macrophage colony-stimulating factor37. The polarization of macrophages is most often characterized by detection of cell surface or secreted markers. For instance, M1 macrophages express high levels of cluster of differentiation (CD)16/32, CD80, and CD86 and are capable of secreting pro-inflammatory cytokines such as tumor necrosis factor (TNF), IL-1β, IL-6, IL-12, and IL-1837,39,40. In contrast, M2 macrophages express high levels of arginase-1, CD206, and anti-inflammatory cytokines and chemokines such as IL-10 and CCL17 and CCL2237,39. Pro-inflammatory cytokines produced by monocytes and M1 macrophages (e.g., TNF, IL-1β and IL-6) may contribute to the development of non-resolving inflammation and play important roles in the pathophysiology of various non-communicable inflammation-related diseases41. Importantly, the production of TNF, IL-1β, and IL-6 by monocytes is known to be substantially influenced by certain FAs42–44.
The goal of this study was to investigate how different classes of dietary FAs might influence the production of TNF, IL-1β, and IL-6 in THP-1 cells, a widely used human monocyte cell line. The concentrations of FAs used in our experiments (0–500 µM) reflected typical levels of circulating FAs in humans45,46. Initially, we compared the impact of 15 different FAs (comprising of six SFAs, two MUFAs, and seven PUFAs as listed in Table 1) on the secretion of targeted cytokines by THP-1 cells. We selected the 15 FAs for our experiments based on their richness in diet, capacity to be detected in blood, and known roles in the pathogenesis of metabolic disorders47–50. In further experiments, THP-1 cells were treated with FAs in the presence of lipopolysaccharide (LPS) to examine the potential anti-inflammatory functions of the FAs.
Table 1.
List of selected dietary fatty acids used in this study.
| Common name | IUPAC name | Lipid numbers |
|---|---|---|
| caprylic acid | octanoic acid | C8:0 |
| capric acid | decanoic acid | C10:0 |
| undecylic acid | undecanoic acid | C11:0 |
| lauric acid | dodecanoic acid | C12:0 |
| palmitic acid | hexadecanoic acid | C16:0 |
| stearic acid | octadecanoic acid | C18:0 |
| palmitoleic acid | (9Z)-hexadec-9-enoic acid | C16:1 (n-7) |
| oleic acid | (9Z)-octadec-9-enoic acid | C18:1 (n-9) |
| linoleic acid | (9Z,12Z)-octadeca-9,12-dienoic acid | C18:2 (n-6) |
| α-linolenic acid | (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid | C18:3 (n-3) |
| γ-linolenic acid | (6Z,9Z,12Z)-octadeca-6,9,12-trienoic acid | C18:3 (n-6) |
| arachidonic acid | (6Z,9Z,12Z)-octadeca-6,9,12-trienoic acid | C20:4 (n-6) |
| eicosapentaenoic acid (EPA) | (5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentenoic acid | C20:5 (n-3) |
| docosapentaenoic acid (DPA) | (7Z,10Z,13Z,16Z,19Z)-docosapentaenoic acid | C22:5 (n-3) |
| docosahexaenoic acid (DHA) | (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid | C22:6 (n-3) |
Results
Effects of FAs on secretion of TNF by human THP-1 monocytes
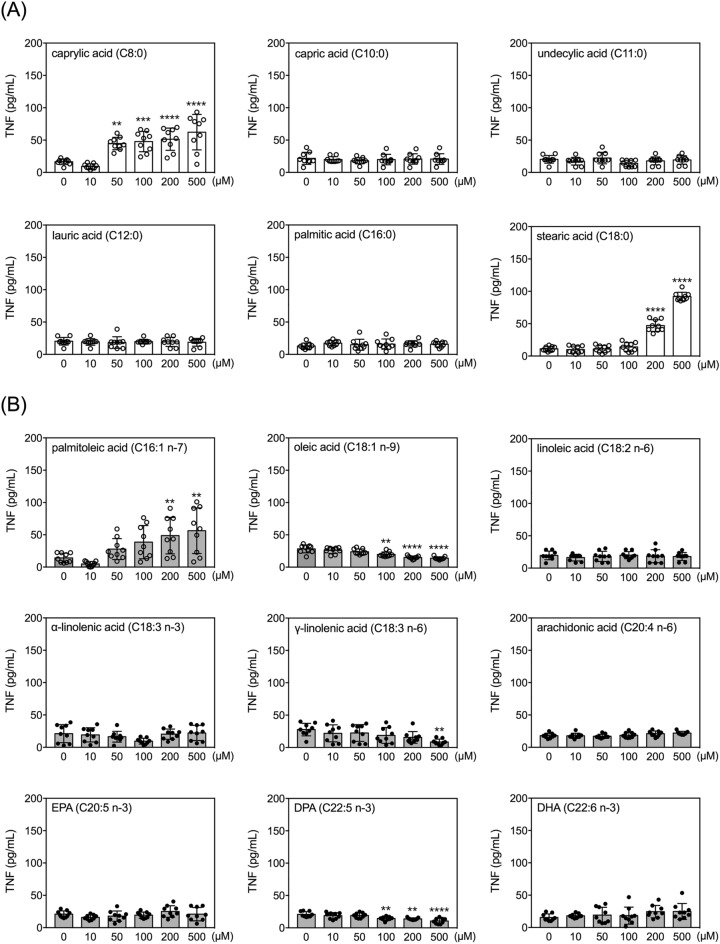
The viability of THP-1 cells was not significantly affected by incubation with any of the 15 FAs up to 24 h at any of the tested concentrations (up to 500 µM) (Supplementary Table 1). However, six of the 15 FAs significantly and dose-dependently changed the levels of TNF in the conditioned media of THP-1 cells (Fig. 1). Among these six FAs, caprylic acid, stearic acid and palmitoleic acid increased TNF, while oleic acid, γ-linolenic acid, and DPA decreased levels of secreted TNF (Fig. 1). Among the three FAs that increased TNF levels, caprylic acid had the lowest effective dose (50 µM). Oleic acid and DPA treatments decreased TNF levels at concentrations of 100 µM and higher (Fig. 1).
Figure 1.
Effects of fatty acids on TNF secretion by human THP-1 monocytes. Quantitative results of levels of TNF in the conditioned media of THP-1 cells treated with selected FAs at different doses for 24 h. Data are presented as mean ± standard deviation. **p < 0.01, ***p < 0.001, ****p < 0.0001, versus 0 µM vehicle group, Dunnett’s multiple comparisons after one-way ANOVAs. n = 9.
Fold-changes in TNF levels were calculated from cultures treated with 500 µM and 0 µM (vehicle control) of each of the 15 FAs (Fig. 2). Stearic acid induced the largest fold-change, followed by palmitoleic acid and caprylic acid. Among the three FAs that decreased TNF levels, γ-linolenic acid most potently decreased the level of TNF (to about 30% of the vehicle control group), followed by oleic acid and DPA (Fig. 2).
Figure 2.
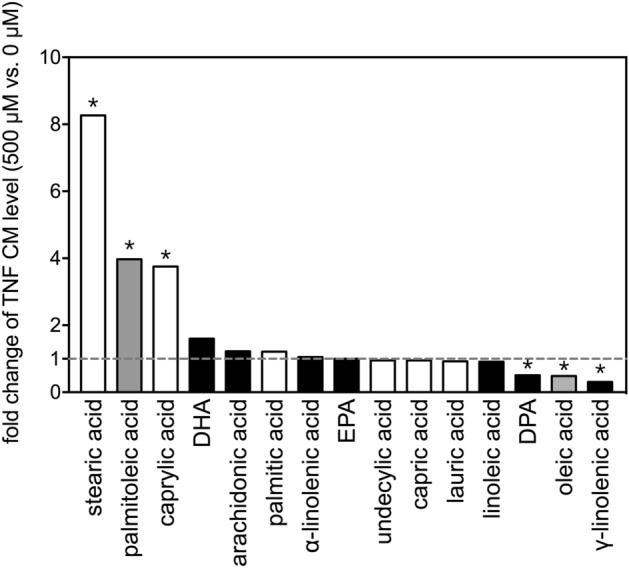
Fold-changes of TNF levels after treatment with selected fatty acids. Fold changes in levels of TNF in the conditioned media between 500 µM and 0 µM (vehicle control) of the 15 FAs after incubation with THP-1 cells for 24 h. The asterisks indicate significance in post-hoc comparison (500 µM vs. 0 µM) shown in Fig. 1.
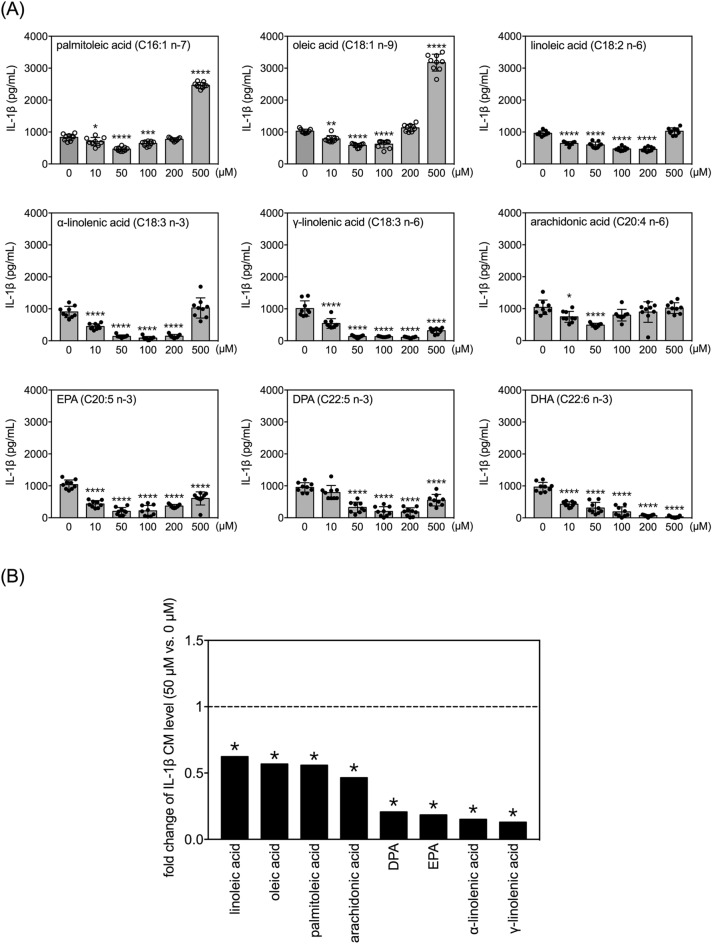
Effects of FAs on secretion of IL-1β by human THP-1 monocytes
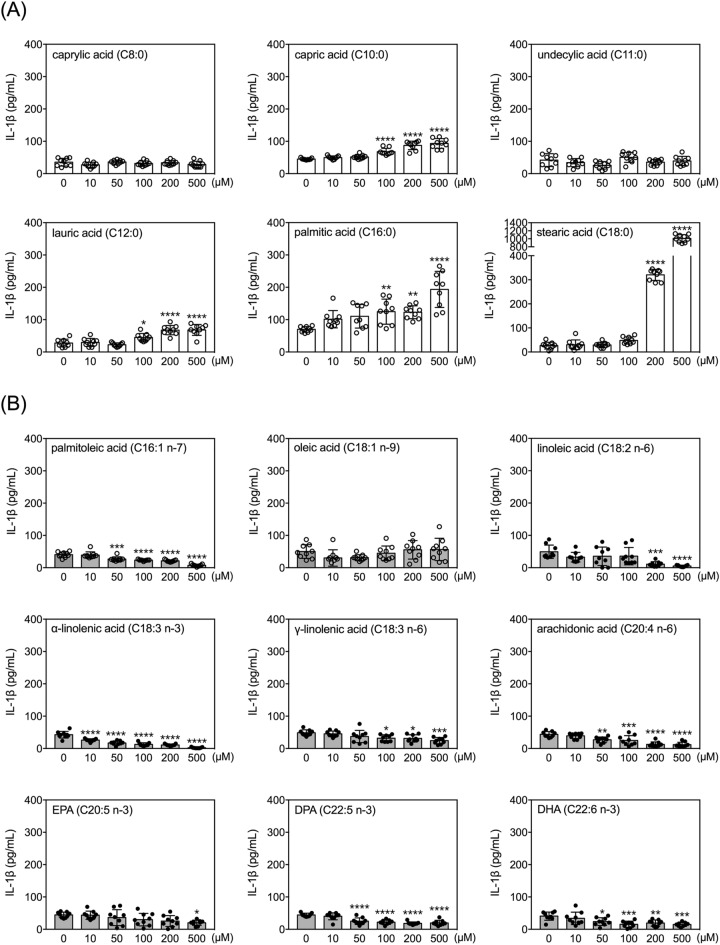
Within the concentration range tested, capric acid, lauric acid, palmitic acid, and stearic acid increased IL-1β in conditioned media. Palmitoleic acid, linoleic acid, α-linolenic acid, γ-linolenic acid, arachidonic acid, EPA, DPA, and DHA decreased the levels of IL-1β in the conditioned media (Fig. 3). All of these effects were dose dependent. Three FAs (caprylic acid, undecylic acid, and oleic acid) did not significantly affect IL-1β levels in THP-1 conditioned media. Among the four FAs that increased IL-1β levels, caprylic acid, lauric acid, and palmitic acid were effective at concentrations of 100 µM or higher. Among the eight FAs that decreased IL-1β levels, α-linolenic acid had the lowest effective dose (10 µM), followed by palmitoleic acid, arachidonic acid, DPA and DHA (effective dose, 50 µM) (Fig. 3).
Figure 3.
Effects of fatty acids on IL-1β secretion by human THP-1 monocytes. Quantitative results of levels of IL-1β in the conditioned media of THP-1 cells treated with selected FAs at different doses for 24 h. Data are presented as mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, versus 0 µM vehicle group, Dunnett’s multiple comparisons after one-way ANOVAs. n = 9.
The fold-changes in IL-1β levels (comparing 500 µM to 0 µM) for the 15 FAs are shown in Fig. 4. Stearic acid dramatically increased levels of IL-1β in the conditioned medium to more than 30-fold the level seen in the vehicle control group. Meanwhile, α-linolenic acid and linoleic acid potently decreased levels of IL-1β to about 10% of that in the vehicle control group.
Figure 4.
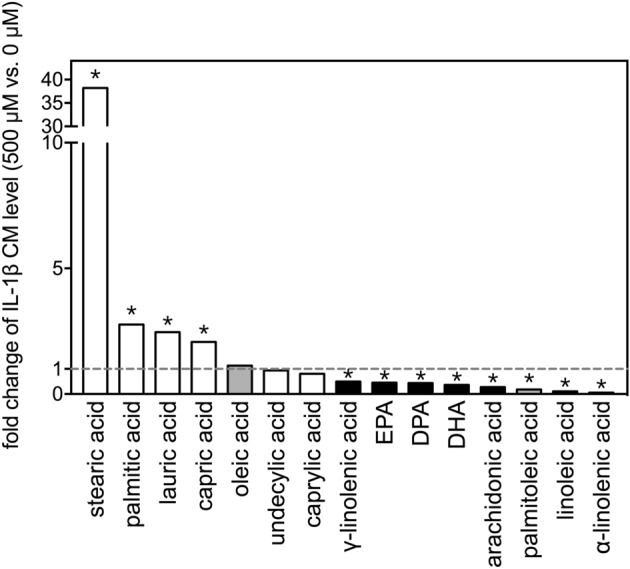
Fold-changes of IL-1β levels after treatment with selected fatty acids. Fold changes in levels of IL-1β in the conditioned media between 500 µM and 0 µM (vehicle control) of the 15 FAs after incubation with THP-1 cells for 24 h. The asterisks indicate significance in post-hoc comparison (500 µM vs. 0 µM) shown in Fig. 3.
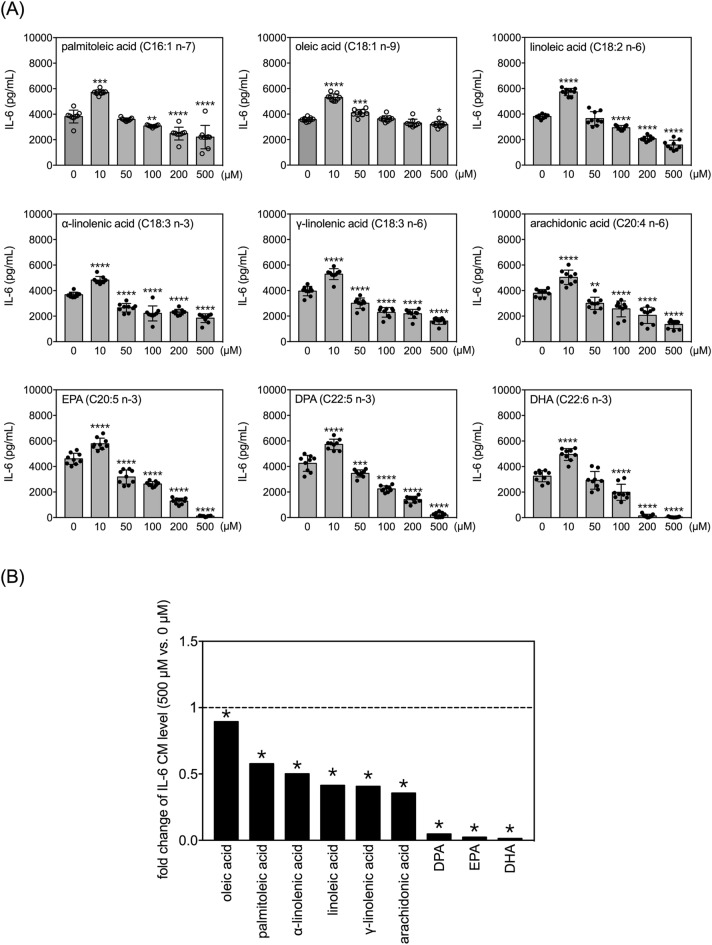
Effects of FAs on secretion of IL-6 by human THP-1 monocytes
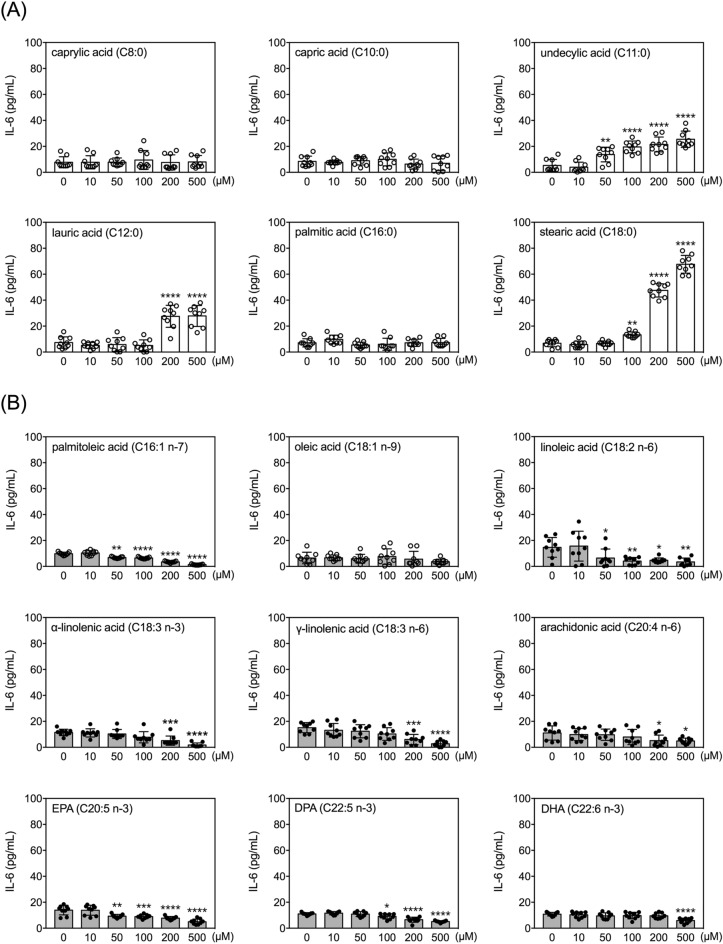
Within the tested concentration range, undecylic acid, lauric acid, and stearic acid dose-dependently increased levels of IL-6 in conditioned media, while palmitoleic acid, linoleic acid, α-linolenic acid, γ-linolenic acid, arachidonic acid, EPA, DPA and DHA all decreased the levels of IL-6 (Fig. 5). Among the three FAs that increased IL-6 levels, undecylic acid had the lowest effective dose (50 µM). Regarding the FAs that decreased IL-6 levels, palmitoleic acid, linoleic acid and EPA were effective at 50 µM and higher concentrations (Fig. 5).
Figure 5.
Effects of fatty acids on IL-6 secretion by human THP-1 monocytes. Quantitative results of levels of IL-6 in the conditioned media of THP-1 cells treated with selected FAs at different doses for 24 h. Data are presented as mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, versus 0 µM vehicle group, Dunnett’s multiple comparisons after one-way ANOVAs. n = 9.
Comparing the 500 µM dose to respective vehicle controls (0 µM), stearic acid induced the largest increase in IL-6 level (~ tenfold) (Fig. 6), whereas palmitoleic acid, α-linolenic acid, and γ-linolenic acid all reduced the levels of IL-6 to less than 20% of those in the respective vehicle control groups (Fig. 6).
Figure 6.
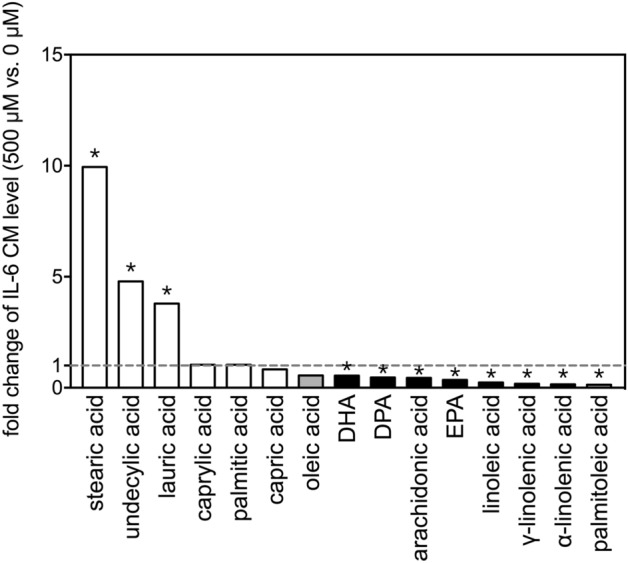
Fold-changes of IL-6 levels after treatment with selected fatty acids. Fold changes in levels of IL-6 in the conditioned media between 500 µM and 0 µM (vehicle control) of the 15 FAs after incubation with THP-1 cells for 24 h. The asterisks indicate significance in post-hoc comparison (500 µM vs. 0 µM) shown in Fig. 5.
Effects of FAs on secretion of TNF by LPS-treated human THP-1 monocytes
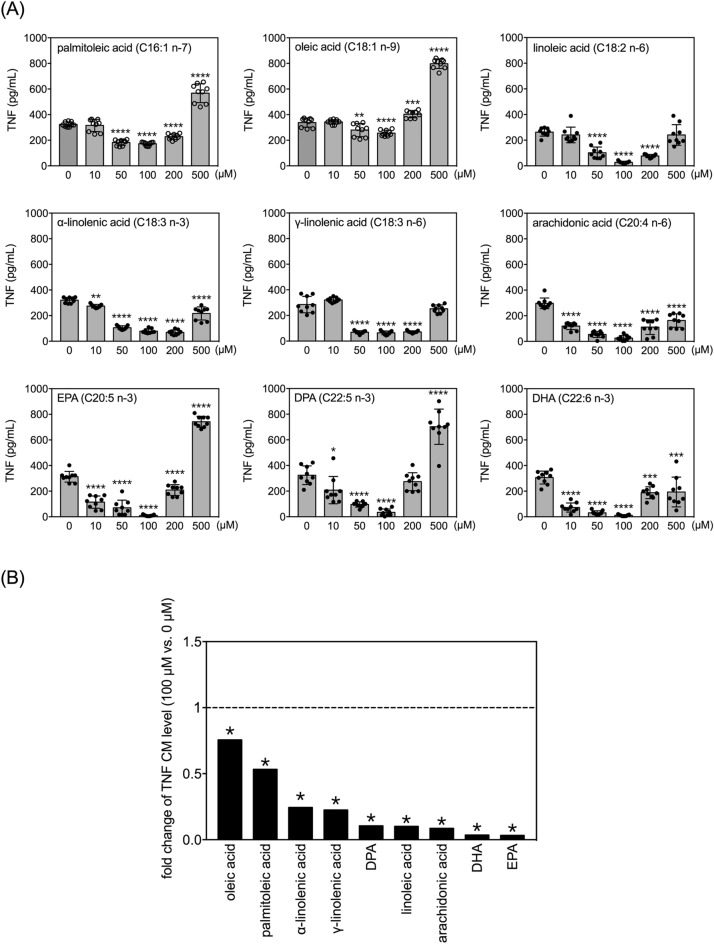
Since two MUFAs and seven PUFAs were capable of diminishing the levels of TNF, IL-1β and IL-6 in conditioned media of resting THP-1 monocytes (Figs. 1, 3, and 5), we next wanted to examine how FAs affect the three pro-inflammatory cytokines after LPS treatment. THP-1 cells were treated with LPS at 100 ng/mL, which significantly increased the level of TNF in the conditioned medium (Fig. 7A compared to Fig. 1). Interestingly, both MUFAs and all seven PUFAs affected LPS-induced TNF production with a U-shaped dose–response curve (Fig. 7A). All nine FAs strongly inhibited LPS-induced TNF production when treated at 100 µM (Fig. 7A). However, these inhibitory effects became either less pronounced (i.e., α-linolenic acid, arachidonic acid, and DHA), insignificant (i.e., linoleic acid and γ-linolenic acid), or even reversed (i.e., palmitoleic acid, oleic acid, EPA, and DPA) at concentrations of 500 µM (Fig. 7A).
Figure 7.
Effects of selected MUFAs and PUFAs on TNF secretion by LPS-treated human THP-1 monocytes. (A) Quantitative results of levels of TNF in the conditioned media of THP-1 cells treated with selected FAs at different doses and 100 ng/mL of LPS for 24 h. Data are presented as mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 verses 0 µM vehicle group, Dunnett’s multiple comparisons after one-way ANOVAs. n = 9. (B) Fold changes in levels of TNF in the conditioned media between 100 µM and 0 µM (vehicle control) among the selected fatty acids with LPS for 24 h. The asterisks indicate significance in post-hoc comparison (100 µM vs. 0 µM) shown in panel (A).
Fold-changes in TNF levels between the most effective dose (100 µM) and vehicle control were compared across the nine FAs (Fig. 7B). EPA and DHA elicited the strongest inhibition of LPS-induced TNF production (< 5% of the vehicle control group), followed by arachidonic acid, linoleic acid and DPA (~ 10% of the vehicle control group).
Effects of FAs on secretion of IL-1β by LPS-treated human THP-1 monocytes
Treatment with 100 ng/mL LPS also significantly increased levels of IL-1β in the conditioned medium of THP-1 monocytes (Fig. 8A vs. Figure 3). Similar to the results of TNF measurements, all of the examined MUFAs and PUFAs except DHA regulated LPS-induced IL-1β production with U-shaped dose–response curves (Fig. 8A). Within the tested concentration range, DHA dose-dependently repressed LPS-induced elevation of IL-1β to less than 5% of the vehicle control group (Fig. 8A). The other eight FAs inhibited LPS-induced IL-1β production most potently at either 50 or 100 µM (Fig. 8A).
Figure 8.
Effects of selected MUFAs and PUFAs on IL-1β secretion by LPS-treated human THP-1 monocytes. (A) Quantitative results of levels of IL-1β in the conditioned media of THP-1 cells treated with selected FAs at different doses and 100 ng/mL of LPS for 24 h. Data are presented as mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 verses 0 µM vehicle group, Dunnett’s multiple comparisons after one-way ANOVAs. n = 9. (B) Fold changes in levels of IL-1β in the conditioned media between 50 µM and 0 µM (vehicle control) among the selected fatty acids with LPS for 24 h. The asterisks indicate significance in post-hoc comparison (50 µM vs. 0 µM) shown in panel (A).
For comparison of fold-changes in IL-1β levels, we selected the 50 µM dose for normalization to the respective vehicle control groups (Fig. 8B). Among the two MUFAs and six PUFAs (excluding DHA) that exhibited effects, γ-linolenic acid, α-linolenic acid, EPA and DPA most strongly inhibited LPS-induced IL-1β secretion to approximately 20% or less of the level seen in the respective vehicle control group.
Effects of FAs on secretion of IL-6 by LPS-treated human THP-1 monocytes
LPS treatment (100 ng/mL) strongly increased levels of IL-6 in the conditioned media of THP-1 monocytes (Fig. 9A vs. Fig. 5). Unlike the U-shaped dose–response curves observed for TNF and IL-1β, the nine FAs generally regulated LPS-induced IL-6 secretion according to inverted U-shaped dose–response curves (Fig. 9A). All nine FAs potentiated LPS-induced IL-6 production at 10 µM (Fig. 9A). However, at concentrations higher than 100 µM, eight of the nine FAs (except oleic acid) dose-dependently inhibited LPS-induced IL-6 production (Fig. 9A).
Figure 9.
Effects of selected MUFAs and PUFAs on IL-6 secretion by LPS-treated human THP-1 monocytes. (A) Quantitative results of levels of IL-6 in the conditioned media of THP-1 cells treated with selected FAs at different doses and 100 ng/mL of LPS for 24 h. Data are presented as mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 verses 0 µM vehicle group, Dunnett’s multiple comparisons after one-way ANOVAs. n = 9. (B) Fold changes in levels of IL-6 in the conditioned media between 50 µM and 0 µM (vehicle control) among the selected fatty acids with LPS for 24 h. The asterisks indicate significance in post-hoc comparison (50 µM vs. 0 µM) shown in panel (A).
We selected the most potent effective dose within the selected concentration range (500 µM) to compare fold changes in IL-6 levels to respective vehicle controls (Fig. 9B). DHA, EPA and DPA strongly inhibited the LPS-induced IL-6 levels to less than 5% of their respective vehicle control groups.
Discussion
Several studies have explored the differences between various FAs in terms of their impacts on innate immune cell responses. However, to the best of our knowledge, there has been no systematic comparison of these effects across all three types of dietary FAs (SFAs, MUFAs, and PUFAs) in human monocytes. In this study, we evaluated the effects of 15 of the most common dietary FAs at a range of concentrations. In particular, we measured production of three key pro-inflammatory cytokines (TNF, IL-1β, and IL-6) in human THP-1 monocytes under both normal and inflammation-stimulated conditions. Our results indicate that in the basal condition, SFAs generally act as pro-inflammatory factors, whereas, PUFAs mostly have anti-inflammatory actions (Table 2). The effects of MUFAs vary according to the type of cytokine. Among the six SFAs selected for study, stearic acid was the most potent activator and the only one that could stimulate the release of all three pro-inflammatory cytokines from the resting THP-1 monocytes. Under the LPS treatment condition, all nine selected MUFAs and PUFAs exhibited anti-inflammatory effects within some concentration range. Among these FAs, DHA was the most powerful anti-inflammatory mediator, effectively inhibiting LPS-induced secretion of all three pro-inflammatory cytokines. Furthermore, DPA exerted a global anti-inflammatory effect in both basal and LPS-stimulated conditions. Taken together, these results delineate important composition- and concentration-specific effects of dietary FAs in controlling inflammation.
Table 2.
Summary of the effects of selected fatty acids on the productions of TNF, IL-1β, and IL-6 in the THP-1 monocytes cultured with and without LPS treatment.
| Fatty acid | without LPS | with LPS | ||||
|---|---|---|---|---|---|---|
| TNF | IL-1β | IL-6 | TNF | IL-1β | IL-6 | |
| caprylic acid | ↑ | ― | ― | N/A | N/A | N/A |
| capric acid | ― | ↑ | ― | N/A | N/A | N/A |
| undecylic acid | ― | ― | ↑ | N/A | N/A | N/A |
| lauric acid | ― | ↑ | ↑ | N/A | N/A | N/A |
| palmitic acid | ― | ↑ | ― | N/A | N/A | N/A |
| stearic acid | ↑ | ↑ | ↑ | N/A | N/A | N/A |
| palmitoleic acid | ↑ | ↓ | ↓ | ↓/↑a | ↓/↑ | ↑/↓ |
| oleic acid | ↑ | ― | ― | ↓/↑ | ↓/↑ | ↑/↓ |
| linoleic acid | ― | ↓ | ↓ | ↓/↓ | ↓/― | ↑/↓ |
| α-linolenic acid | ― | ↓ | ↓ | ↓/↓ | ↓/― | ↑/↓ |
| γ-linolenic acid | ↓ | ↓ | ↓ | ↓/― | ↓/↓ | ↑/↓ |
| arachidonic acid | ― | ↓ | ↓ | ↓/↓ | ↓/― | ↑/↓ |
| EPA | ― | ↓ | ↓ | ↓/↑ | ↓/↓ | ↑/↓ |
| DPA | ↓ | ↓ | ↓ | ↓/↑ | ↓/↓ | ↑/↓ |
| DHA | ― | ↓ | ↓ | ↓/ ↓ | ↓/↓ | ↑/↓ |
↑: increase; ↓: decrease; ―: unchanged; a: trend at low concentration/trend at high concentration of FAs.
In our results, stearic acid readily stimulated human THP-1 monocytes to release pro-inflammatory cytokines. Stearic acid is naturally enriched in various dietary sources of fat, such as beef tallow, butterfat, lard, cocoa butter, shea nut oil, and others. The main sources of dietary stearic acid include meat, poultry, fish, eggs, milk products, and oils51. Furthermore, pro-inflammatory effects of stearic acid have been demonstrated in the other types of cells. For example, stearic acid upregulates gene expression of TNF, IL-1β, and IL-6 and elicits endoplasmic reticulum stress and apoptosis in triacsin C (long-chain acyl coenzyme A synthetase inhibitor)-treated mouse peritoneal macrophages52. In bone marrow-derived macrophages stimulated with macrophage colony-stimulating factor (M-CSF), stearic acid promotes the expression of CD11c, which mediates the production of inflammatory cytokines53 and contributes to obesity-associated chronic inflammation and insulin resistance54–56. Furthermore, the levels of CD11c induced by stearic acid are higher than those after cells are treated with palmitic acid56. Stearic acid also enhances the expression levels of pro-inflammatory markers in microglia, a population of macrophage-like cells in the central nervous system57. The stearic acid-induced activation of both macrophages and microglia has been linked to the activation of the toll-like receptor 4/NF-κB signaling pathway57,58. Although stearic acid induces pronounced inflammatory responses in macrophages and microglia56, it has been reported that hypercholesterolemic postmenopausal women who consume a diet enriched with stearic acid have lower fasting low-density lipoprotein-cholesterol concentrations than those who consume a diet enriched with palmitic acid59. It was further postulated that the hypocholesterolemic effect of stearic acid may be due to the reduced overall synthesis of intestinal hydrophobic secondary bile acids. These findings also suggested that dietary SFAs could differentially regulate multiple physiological functions.
DPA and DHA are omega-3 PUFAs that are enriched in fish oil and may potentially be used for treating cardiovascular diseases60–62 and metabolic syndrome63,64. In our experiments, DPA and DHA exerted anti-inflammatory effects in both resting and LPS-treated THP-1 cells. This result is not surprising because anti-inflammatory effects of omega-3 PUFAs are widely recognized. Reported actions of omega-3 PUFAs include inhibitory effects on T cell reactivity, leukocyte chemotaxis, and inflammatory cytokine production65. These effects are at least partly regulated by different omega-3 PUFAs-derived specific pro-resolving mediators, which are bioactive metabolites produced by fatty acid oxygenases66. In the group of four tested omega-3 PUFAs (α-linolenic acid, EPA, DPA, DHA), we noticed a positive relationship between the number of unsaturated bonds and the anti-inflammatory effect size. DHA (C22:6) exhibited the strongest effect, followed by DPA (C22:5) and EPA (C20:5), and then α-linolenic acid (C18:3). Stronger anti-inflammatory effects of DHA compared to other omega-3 PUFAs has also been reported in previous studies using THP-1-derived macrophages67. Furthermore, a recently published randomized controlled trial study revealed that DHA has a broader suppressive effect on pro-inflammatory cytokines than EPA in humans with chronic inflammation68. Interestingly, we found that almost all of the MUFAs and PUFAs we tested increased the LPS-induced production of TNF and IL-1β when treated at 500 µM, while the 10 µM dose increased production of IL-6 in the THP-1 monocytes. These results suggest that the inhibitory effects of MUFAs and PUFAs on LPS-induced cytokine secretion follow either U-shaped (TNF and IL-1β) or inverted U-shaped (IL-6) dose–response curves. However, these types of dose–response curves were not observed in THP-1 cells in a resting state. Although the underlying mechanisms remain unclear, these findings suggest that there is a particular dosage window in which MUFAs/PUFAs act on monocytes in an anti-inflammatory manner. Similar non-linear dose responses have also been observed for vitamin and mineral supplements on health-related outcomes. While it is widely known that vitamins and minerals are essential for maintaining normal physiological functions, excessive supplementation can lead to toxicity69. Omega-3 FAs are also frequently consumed as dietary supplements due to their beneficial effects in a broad range of health conditions70. As such, it will be crucial to determine the correlation between circulating levels of dietary omega-3 FAs and pro-inflammatory cytokines in humans in order to recommend optimal doses for supplements to reduce systemic inflammation. These findings also highlight the need for individuals to be mindful of systemic inflammation effects when consuming high doses of omega-3 FAs for other purposes.
In conclusion, we found that SFAs generally stimulated secretion of pro-inflammatory cytokines in resting THP-1 cells, with stearic acid being the most potent species. Meanwhile, MUFAs and PUFAs inhibited LPS-induced secretion of pro-inflammatory cytokines. These inhibitory effects followed either U-shaped (TNF and IL-1β) or inverted U-shaped (IL-6) dose–response curves, implying that distinct dose windows exist for anti-inflammatory effects of dietary MUFAs/PUFAs. Among the MUFAs and PUFAs that we tested, DHA exhibited the largest number of double bonds and was found to be the most potent anti-inflammatory compound. Together, our findings reveal that dietary FA chemical compositions (double bond number) and concentrations (nonlinear effects) are key factors in the intricate regulation of monocyte-mediated inflammation.
Materials and methods
THP-1 cell cultures
THP-1 cells were obtained from the Bioresource Collection and Research Center of Taiwan (Cat. #: 60430, Hsinchu, Taiwan) and cultured in ATCC-modified RPMI 1640 medium (Cat. #: A1049101, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum with endotoxin less than 0.05 EU/mL (Lot# VP2002200, Cat. #: TMS-013-BKR, Merck-Millipore, Burlington, MA, USA), 0.05 mM β-mercaptoethanol (Cat. #: 31350010, Thermo Fisher Scientific), and penicillin–streptomycin (Cat. #: 15140122, Thermo Fisher Scientific). Cultures were kept in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Subcultures were carried out when cell concentration reached 8 × 105 cells/mL by adding fresh medium. Cell density was kept under 1 × 106 cells/mL during this study.
Preparation of FAs
The 15 selected FAs are listed in Table 1. Caprylic acid (Cat. #: C2875, Sigma-Aldrich, St. Louis, MO, USA), capric acid (Cat. #: C1875, Sigma-Aldrich), undecylic acid (Cat. #: U0004, Tokyo Chemical Industry, Chuo-ku, Tokyo, Japan), lauric acid (Cat. #: W261408, Sigma-Aldrich), myristic acid (Cat. #: M3128, Sigma-Aldrich), palmitoleic acid (Cat. #: P9417, Sigma-Aldrich), stearic acid (Cat. #: S4751, Sigma-Aldrich), oleic acid (Cat. #: O1008, Sigma-Aldrich), linoleic acid (Cat. #: L1376, Sigma-Aldrich), α-linolenic acid (Cat. #: L2376, Sigma-Aldrich), γ-linolenic acid (Cat. #: L2378, Sigma-Aldrich), arachidonic acid (Cat. #: A0781, Tokyo Chemical Industry), eicosapentaenoic acid (EPA, Cat. #: E0441, Tokyo Chemical Industry), docosapentaenoic acid (DPA, Cat. #: D1797, Sigma-Aldrich), and docosahexaenoic acid (DHA, Cat. #: D2534, Sigma-Aldrich) were dissolved in ethanol (Cat. #: 1.00983, Sigma-Aldrich) to make a working solution of 200 mM. The working solution was diluted ten times in PBS (Cat. #: 10010023, Thermo Fisher Scientific) containing 20% FA-free bovine serum albumin (BSA, Cat. #: A8806, Sigma-Aldrich) and incubated at 55 °C for one hour to make a 20 mM BSA-conjugated FA solution. However, when the palmitic acid–ethanol solution was mixed with bovine serum albumin, a noticeable precipitate formed. To resolve this issue, palmitic acid (Cat. #: P0500, Sigma-Aldrich) was instead dissolved in 0.01 N NaOH (Cat. #: S8045, Sigma-Aldrich; 20 mM), combined with PBS (Cat. #: 10010023, Thermo Fisher Scientific) containing 5% FA-free bovine serum albumin (Cat. #: A8806, Sigma-Aldrich) in a 2:3 ratio, and incubated at 55 °C for an hour to produce an eight mM BSA-conjugated palmitic acid stock solution. The vehicle solution for each FA was prepared using the same process.
Cell treatments
THP-1 cells were centrifuged at 200 × g for 5 min and the pellet was suspended in FBS-free culture media to make a concentration of 9.5 × 105 cells/mL before being transferred into 6-well plates (one mL/well). After a 16-h incubation period, the THP-1 cultures were treated with different doses of FAs (final concentrations: 0, 10, 50, 100, 200, and 500 µM) either alone or in combination with 100 ng/mL of LPS (from Escherichia coli O55:B5, Cat. #: L2880, Sigma-Aldrich) for 24 h. Vehicle controls contained the same amounts of BSA and ethanol/NaOH used in different doses of FA experiments. At the end of the incubation, cells were collected by gently flushing. Twenty µL of the cell mixture was used to assess the cell viability through trypan blue dye exclusion staining and counting using a hemocytometer as previously described71,72. The rest of the mixtures were centrifuged at 200 × g for five min and the conditioned media were harvested for the determination of cytokine concentrations. Sample size = nine in each experiment.
Measurements of pro-inflammatory cytokines
The concentrations of TNF, IL-1β, and IL-6 in the conditioned media were determined by commercial human TNF (Cat. #: 550610, Becton, Dickinson and Company, Franklin Lakes, NJ, USA), IL-1β (Cat. #: 557966, Becton, Dickinson and Company), and IL-6 (Cat. #: 550799, Becton, Dickinson and Company) ELISA kits following the manufacturer’s instructions.
Statistical analysis
All numerical data are expressed as mean ± standard deviation. Statistical analyses and graph plotting were performed using the Prism software (v. 7.0a, GraphPad Software Inc., San Diego, CA, USA). Significance was set at p < 0.05. One-way ANOVA followed by Dunnett’s multiple comparisons was used to analyze differences between independent groups.
Supplementary Information
Acknowledgements
The authors are grateful for the financial support from Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan (Grant #: KSVGH111‐177, H.C.H.), National Cheng Kung University Hospital, Tainan, Taiwan (Grant #: NCKUH-11104022, H.W.C.), and Ministry of Science and Technology, Taiwan (Grant #: 109-2320-B-006-043-MY3 and 110-2811-B-006-546, Y.M.K.; Grant #: 109-2326-B-037-002-MY3, P.L.H.).
Author contributions
H.C.H., S.F.T., H.W.C., P.L.H., and Y.M.K. conceived this study and designed the experiments. S.F.T. carried out the experiments. S.F.T., P.L.H., and Y.M.K. analyzed and interpreted the results. S.F.T., P.L.H., and Y.M.K. prepared the manuscript. M.J.T. commented on the results and manuscript. H.C.H., P.L.H., and Y.M.K. supervised the conduction and progress of this study. H.C.H., H.W.C., P.L.H., and Y.M.K. acquired funding.
Data availability
All datasets generated or analyzed in this study were included in the published article. Detailed datasets supporting the current study are available from the Corresponding Authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pei-Ling Hsu, Email: plhsu@kmu.edu.tw.
Yu-Min Kuo, Email: kuoym@mail.ncku.edu.tw.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-32710-5.
References
- 1.Henson PM. Dampening inflammation. Nat. Immunol. 2005;6:1179–1181. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Furman D, et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fullerton JN, Gilroy DW. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016;15:551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 7.Jaiswal S, Libby P. Clonal haematopoiesis: Connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 2020;17:137–144. doi: 10.1038/s41569-019-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 11.Gistera A, Hansson GK. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017;13:368–380. doi: 10.1038/nrneph.2017.51. [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci L, Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fourati S, et al. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat. Commun. 2016;7:10369. doi: 10.1038/ncomms10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDade TW, Adair L, Feranil AB, Kuzawa C. Positive antibody response to vaccination in adolescence predicts lower C-reactive protein concentration in young adulthood in the Philippines. Am. J. Hum. Biol. 2011;23:313–318. doi: 10.1002/ajhb.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verschoor CP, et al. Serum C-reactive protein and congestive heart failure as significant predictors of herpes zoster vaccine response in elderly nursing home residents. J. Infect. Dis. 2017;216:191–197. doi: 10.1093/infdis/jix257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming TP, et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet. 2018;391:1842–1852. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renz H, et al. An exposome perspective: Early-life events and immune development in a changing world. J. Allergy Clin. Immunol. 2017;140:24–40. doi: 10.1016/j.jaci.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Singer K, Lumeng CN. The initiation of metabolic inflammation in childhood obesity. J. Clin. Invest. 2017;127:65–73. doi: 10.1172/JCI88882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olvera Alvarez HA, Kubzansky LD, Campen MJ, Slavich GM. Early life stress, air pollution, inflammation, and disease: An integrative review and immunologic model of social-environmental adversity and lifespan health. Neurosci. Biobehav. Rev. 2018;92:226–242. doi: 10.1016/j.neubiorev.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan C. Nonresolving inflammation redux. Immunity. 2022;55:592–605. doi: 10.1016/j.immuni.2022.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collaborators GBDCOD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374:1070–1075. doi: 10.1126/science.abi5200. [DOI] [PubMed] [Google Scholar]
- 23.DiNicolantonio JJ, Oeefe JH. Effects of dietary fats on blood lipids: A review of direct comparison trials. Open Heart. 2018;5:e000871. doi: 10.1136/openhrt-2018-000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raatz SK, Bibus D, Thomas W, Kris-Etherton P. Total fat intake modifies plasma fatty acid composition in humans. J. Nutr. 2001;131:231–234. doi: 10.1093/jn/131.2.231. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The atherosclerosis risk in communities (ARIC) study investigators. Am. J. Clin. Nutr. 1995;62:564–571. doi: 10.1093/ajcn/62.3.564. [DOI] [PubMed] [Google Scholar]
- 26.Judd JT, Marshall MW, Dupont J. Relationship of dietary fat to plasma fatty acids, blood pressure, and urinary eicosanoids in adult men. J. Am. Coll. Nutr. 1989;8:386–399. doi: 10.1080/07315724.1989.10720313. [DOI] [PubMed] [Google Scholar]
- 27.Lopes SM, Trimbo SL, Mascioli EA, Blackburn GL. Human plasma fatty acid variations and how they are related to dietary intake. Am. J. Clin. Nutr. 1991;53:628–637. doi: 10.1093/ajcn/53.3.628. [DOI] [PubMed] [Google Scholar]
- 28.Fritsche KL. The science of fatty acids and inflammation. Adv Nutr. 2015;6:293S–301S. doi: 10.3945/an.114.006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung TT, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am. J. Clin. Nutr. 2001;73:61–67. doi: 10.1093/ajcn/73.1.61. [DOI] [PubMed] [Google Scholar]
- 30.King DE, Egan BM, Geesey ME. Relation of dietary fat and fiber to elevation of C-reactive protein. Am. J. Cardiol. 2003;92:1335–1339. doi: 10.1016/j.amjcard.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Garcia E, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004;80:1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 32.Esposito K, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 33.Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc. Soc. Exp. Biol. Med. 1992;200:177–182. doi: 10.3181/00379727-200-43413. [DOI] [PubMed] [Google Scholar]
- 34.Hu FB, et al. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am. J. Clin. Nutr. 1999;69:890–897. doi: 10.1093/ajcn/69.5.890. [DOI] [PubMed] [Google Scholar]
- 35.Rallidis LS, et al. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis. 2003;167:237–242. doi: 10.1016/S0021-9150(02)00427-6. [DOI] [PubMed] [Google Scholar]
- 36.Kantari C, Pederzoli-Ribeil M, Witko-Sarsat V. The role of neutrophils and monocytes in innate immunity. Contrib. Microbiol. 2008;15:118–146. doi: 10.1159/000136335. [DOI] [PubMed] [Google Scholar]
- 37.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray PJ. Macrophage polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 39.Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020;877:173090. doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 40.Chavez-Galan L, Olleros ML, Vesin D, Garcia I. Much More than M1 and M2 Macrophages, there are also CD169(+) and TCR(+) macrophages. Front. Immunol. 2015;6:263. doi: 10.3389/fimmu.2015.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Netea MG, et al. A guiding map for inflammation. Nat. Immunol. 2017;18:826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Lima-Salgado TM, Alba-Loureiro TC, do Nascimento CS, Nunes MT, Curi R. Molecular mechanisms by which saturated fatty acids modulate TNF-alpha expression in mouse macrophage lineage. Cell Biochem. Biophys. 2011;59:89–97. doi: 10.1007/s12013-010-9117-9. [DOI] [PubMed] [Google Scholar]
- 43.Weigert C, et al. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J. Biol. Chem. 2004;279:23942–23952. doi: 10.1074/jbc.M312692200. [DOI] [PubMed] [Google Scholar]
- 44.Song C, Li X, Leonard BE, Horrobin DF. Effects of dietary n-3 or n-6 fatty acids on interleukin-1beta-induced anxiety, stress, and inflammatory responses in rats. J. Lipid. Res. 2003;44:1984–1991. doi: 10.1194/jlr.M300217-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Abdelmagid SA, et al. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS One. 2015;10:e0116195. doi: 10.1371/journal.pone.0116195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lust CAC, Bi X, Henry CJ, Ma DWL. Development of fatty acid reference ranges and relationship with lipid biomarkers in middle-aged healthy Singaporean men and women. Nutrients. 2021 doi: 10.3390/nu13020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas DD, et al. Effects of medium chain triglycerides supplementation on insulin sensitivity and beta cell function: A feasibility study. PLoS One. 2019;14:e0226200. doi: 10.1371/journal.pone.0226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerendiain M, et al. Changes in plasma fatty acid composition are associated with improvements in obesity and related metabolic disorders: A therapeutic approach to overweight adolescents. Clin. Nutr. 2018;37:149–156. doi: 10.1016/j.clnu.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Bakan E, et al. Effects of type 2 diabetes mellitus on plasma fatty acid composition and cholesterol content of erythrocyte and leukocyte membranes. Acta. Diabetol. 2006;43:109–113. doi: 10.1007/s00592-007-0224-4. [DOI] [PubMed] [Google Scholar]
- 50.Gil-Campos M, et al. Metabolic syndrome affects fatty acid composition of plasma lipids in obese prepubertal children. Lipids. 2008;43:723–732. doi: 10.1007/s11745-008-3203-4. [DOI] [PubMed] [Google Scholar]
- 51.Pendleton RG, Gessner G, Horner E. Comparative effects of angiotensin II and angiotensin III in rabbit adrenal and aortic tissues. J. Pharmacol. Exp. Ther. 1991;256:614–620. [PubMed] [Google Scholar]
- 52.Anderson EK, Hill AA, Hasty AH. Stearic acid accumulation in macrophages induces toll-like receptor 4/2-independent inflammation leading to endoplasmic reticulum stress-mediated apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:1687–1695. doi: 10.1161/ATVBAHA.112.250142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patsouris D, et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakajima S, et al. Accumulation of CD11c+CD163+ adipose tissue macrophages through upregulation of intracellular 11beta-HSD1 in human obesity. J. Immunol. 2016;197:3735–3745. doi: 10.4049/jimmunol.1600895. [DOI] [PubMed] [Google Scholar]
- 55.Wentworth JM, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng J, et al. Stearic acid induces CD11c expression in proinflammatory macrophages via epidermal fatty acid binding protein. J. Immunol. 2018;200:3407–3419. doi: 10.4049/jimmunol.1701416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, et al. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-kappaB signalling. Br. J. Nutr. 2012;107:229–241. doi: 10.1017/S0007114511002868. [DOI] [PubMed] [Google Scholar]
- 58.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 59.Meng H, et al. Comparison of diets enriched in stearic, oleic, and palmitic acids on inflammation, immune response, cardiometabolic risk factors, and fecal bile acid concentrations in mildly hypercholesterolemic postmenopausal women-randomized crossover trial. Am. J. Clin. Nutr. 2019;110:305–315. doi: 10.1093/ajcn/nqz095. [DOI] [PubMed] [Google Scholar]
- 60.Yaqoob P, Calder PC. N-3 polyunsaturated fatty acids and inflammation in the arterial wall. Eur. J. Med. Res. 2003;8:337–354. [PubMed] [Google Scholar]
- 61.Thies F, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 62.Abeywardena MY, Head RJ. Dietary polyunsaturated fatty acid and antioxidant modulation of vascular dysfunction in the spontaneously hypertensive rat. Prostaglandins Leukot. Essent. Fatty Acids. 2001;65:91–97. doi: 10.1054/plef.2001.0294. [DOI] [PubMed] [Google Scholar]
- 63.Storlien LH, et al. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885–888. doi: 10.1126/science.3303333. [DOI] [PubMed] [Google Scholar]
- 64.Harris WS, Connor WE, Alam N, Illingworth DR. Reduction of postprandial triglyceridemia in humans by dietary n-3 fatty acids. J. Lipid. Res. 1988;29:1451–1460. doi: 10.1016/S0022-2275(20)38424-8. [DOI] [PubMed] [Google Scholar]
- 65.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishihara T, Yoshida M, Arita M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 2019;31:559–567. doi: 10.1093/intimm/dxz001. [DOI] [PubMed] [Google Scholar]
- 67.Mullen A, Loscher CE, Roche HM. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010;21:444–450. doi: 10.1016/j.jnutbio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 68.So J, et al. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: A randomized, double-blind, crossover study. Atherosclerosis. 2021;316:90–98. doi: 10.1016/j.atherosclerosis.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 69.Wooltorton E. Too much of a good thing? Toxic effects of vitamin and mineral supplements. CMAJ. 2003;169:47–48. [PMC free article] [PubMed] [Google Scholar]
- 70.Yashodhara BM, et al. Omega-3 fatty acids: A comprehensive review of their role in health and disease. Postgrad. Med. J. 2009;85:84–90. doi: 10.1136/pgmj.2008.073338. [DOI] [PubMed] [Google Scholar]
- 71.Louis KS, Siegel AC. Cell viability analysis using trypan blue: Manual and automated methods. Method Mol. Biol. 2011;740:7–12. doi: 10.1007/978-1-61779-108-6_2. [DOI] [PubMed] [Google Scholar]
- 72.Hu PS, et al. Devising hyperthermia dose of NIR-irradiated Cs0.33WO3 nanoparticles for HepG2 hepatic cancer cells. Nanoscale. Res. Lett. 2021;16:108. doi: 10.1186/s11671-021-03565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated or analyzed in this study were included in the published article. Detailed datasets supporting the current study are available from the Corresponding Authors upon request.