Abstract
Extracellular vesicles (EVs) are released from all cells in the body, forming an important intercellular communication network that contributes to health and disease. The contents of EVs are cell source-specific, inducing distinct signaling responses in recipient cells. The specificity of EVs and their accumulation in fluid spaces that are accessible for liquid biopsies make them highly attractive as potential biomarkers and therapies for disease. The duality of EVs as favorable (therapeutic) or unfavorable (pathological) messengers is context dependent and remains to be fully determined in homeostasis and various disease states. This review describes the use of EVs as biomarkers, drug delivery vehicles, and regenerative therapeutics, highlighting examples involving viral infections, cancer, and neurological diseases. There is growing interest to provide personalized therapy based on individual patient and disease characteristics. Increasing evidence suggests that EV biomarkers and therapeutic approaches are ideal for personalized medicine due to the diversity and multifunctionality of EVs.
Keywords: Nanomedicine, Regenerative medicine, Cancer, Neurological disease, Viruses, Immune response
1. Introduction
Extracellular vesicles (EVs) are released from all cells and form a critical intercellular communication mechanism (Couch et al., 2021). The rich diversity of EVs supports a growing list of functions in maintaining health and promoting disease. EVs are defined and characterized by their size (nanoparticles), the presence of a phospholipid bilayer that contains certain distinguishing markers (e.g., tetraspanins CD9, CD63, CD81), and functional ability (e.g., anti-inflammatory), as described in the most recent guidelines from the International Society for Extracellular Vesicles (ISEV) (Thery et al., 2018). The EV membrane contains bioactive lipids, carbohydrates, and proteins, while nucleic acids, such as DNA and RNA, and proteins (for example, cytokines) can be present in the EV interior. These EV-associated biomolecules reflect the cell of origin, enabling diagnostic and therapeutic applications (Thery et al., 2018). Cells continuously release EVs using both intracellular endocytic pathways and direct budding from the plasma membrane. EVs circulate in the blood and extracellular space, where they act in a paracrine or long-distance manner on recipient cells (Rodrigues et al., 2018). Depending on the context, EVs can have favorable (therapeutic) or unfavorable (pathological) effects, and much remains unknown regarding the role of EVs in homeostasis and various disease states (Yates et al., 2022b). Understanding the contribution of EVs to disease is complicated by the heterogeneity of EVs in biological samples.
A variety of terms and definitions have been used over time for EVs, leading to some confusion in the field (Bazzan et al., 2021; Couch et al., 2021; Thery et al., 2018). Here, we use the term EVs to refer to all extracellular, lipid bilayer, sub-cellular particles and their functional contents with sizes ranging from 30 nm to 1 μm. This definition includes the widely recognized major subgroups termed exosomes, microvesicles, and apoptotic bodies (Fig. 1). Distinctions between these groups are based primarily on their origin (Rodrigues et al., 2018). Exosomes are released by endocytic pathways within cells and range from approximately 30 to 150 nm (DeLeo and Ikezu, 2018). Microvesicles or ectosomes are shed from the plasma membrane into the extracellular space and range from approximately 100 to 1000 nm (Colombo et al., 2014; Janas et al., 2016). Apoptotic bodies arise from degrading cells and range from approximately 100 nm to several micrometers, sometimes large enough to contain entire cellular organelles (Buzas et al., 2014; Gyorgy et al., 2011). The collective term EV is used in this article, as subtypes have overlapping size ranges and biomolecular content, and current technology is unable to accurately separate or distinguish exosomes from microvesicles (Bazzan et al., 2021; Crescitelli et al., 2013; Gyorgy et al., 2011; Khalaj et al., 2019; Rodrigues et al., 2018; Thery et al., 2018; Yates et al., 2022a).
Fig. 1. Biogenic Nanoparticles.
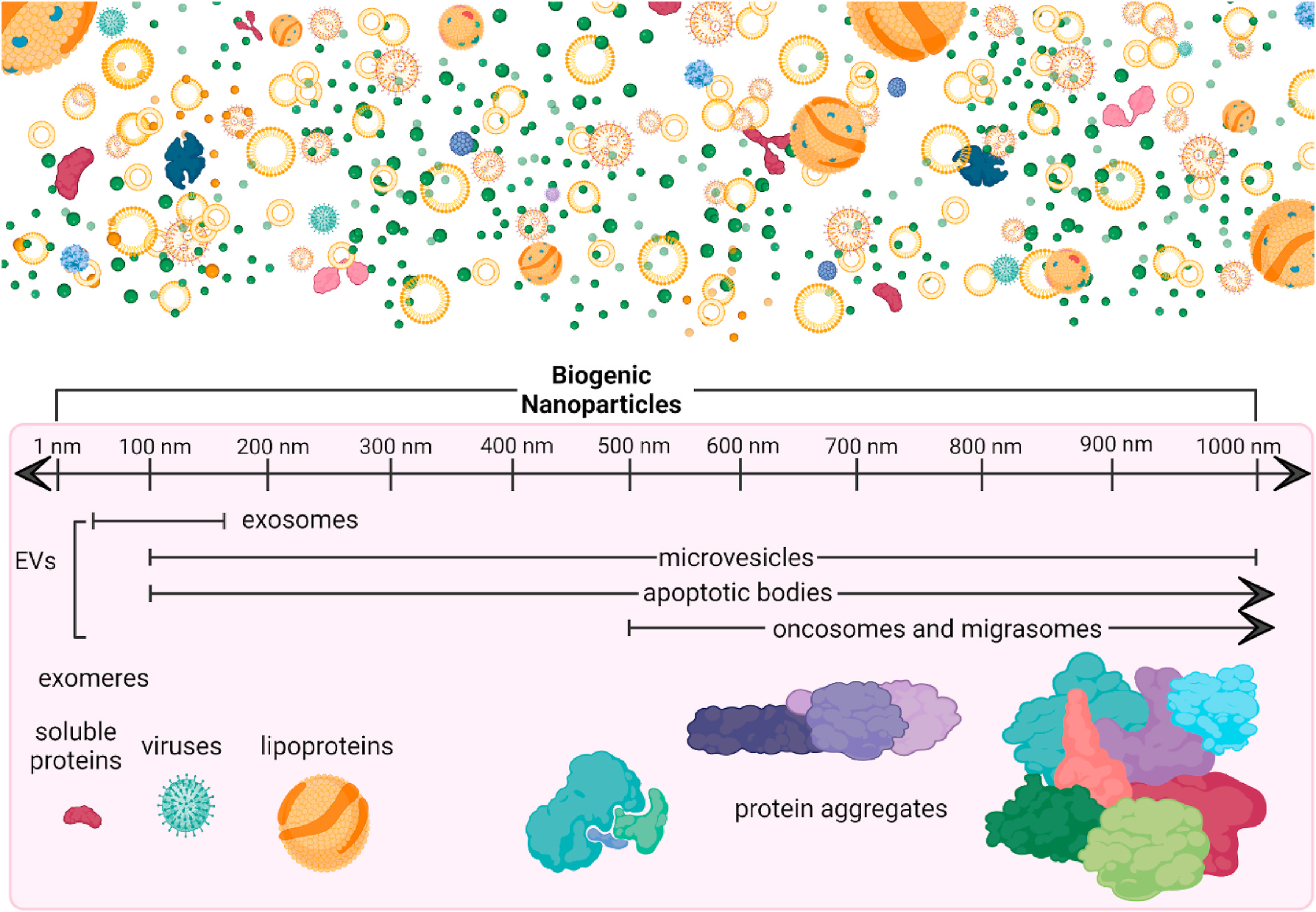
Biogenic nanoparticles include extracellular vesicles (EVs) and other nanosized extracellular particles. Newer inclusions are exomeres: non-membranous nanoparticles smaller than 50 nm, with functional contents secreted by cells (H. Zhang et al., 2018), oncosomes: EVs released from cancer cells ranging between 100 nm and 4 μm (Meehan et al., 2016), and migrasomes: large nano- and microvesicles used for cell migration and intercellular signaling (da Rocha-Azevedo and Schmid, 2015; Ma et al., 2015). More well-established nanoparticles include lipoproteins (Feingold, 2000), viruses (Louten, 2016), and protein aggregates (Goodsell and Olson, 1993). This figure was created in ©BioRender-biorender.com.
Historically, the main role of EVs was thought to be as nano-sized “trash bags”, eliminating unwanted waste from the cell (Couch et al., 2021; Szwedowicz et al., 2022). Today, they are known to play vital roles in cellular communication (Yanez-Mo et al., 2015). Regardless of the origin of EVs (i.e., plasma membrane, intraluminal vesicle), the membranous and inner contents of EVs are influenced by the parent cell. Factors that may affect EV characteristics include donor sex, age, and cellular stress. All components of the lipid membrane and internal compartment of EVs can impact recipient cells (Thery et al., 2018). Larger EVs may contain sub-cellular contents such as mitochondria or other functional macromolecules that can affect recipient cells (Dean et al., 2009; Gasecka et al., 2019). EV-mediated transfer of functional products enables a signaling axis between donor and recipient cells that can be useful in a variety of situations to promote physiological homeostasis or pathology (e.g., stress responses, host cell responses to pathogens, and tumor microenvironment modulation) (Couch et al., 2021; Yanez-Mo et al., 2015; Yates et al., 2022a, 2022b). Once EVs are internalized by recipient cells, intracellular signaling cascades can be initiated by EV-associated biomolecules, such as microRNAs (miRNAs) and proteins, that are released into the cytoplasm (Pant et al., 2012; Rodrigues et al., 2018). In addition to intracellular uptake, EVs may also activate signaling cascades in recipient cells through surface interactions without subsequent uptake, that is, a “kiss-and-run” approach (Morris and Witwer, 2022).
All cells release EVs, which are present in biological secretions, excretions, and tissues (Fig. 1), (Robbins and Morelli, 2014) including ejaculate (Hoog and Lotvall, 2015), lipoaspirate (Tian et al., 2020; Wang et al., 2021), synovial fluid (B. Yin et al., 2022), breast milk (Zhong et al., 2021), amniotic fluid (Costa et al., 2022), saliva (Li et al., 2022; Yuana et al., 2015), urine (Barreiro and Holthofer, 2017; Minkler et al., 2021; Yuana et al., 2015), cerebrospinal fluid (Welton et al., 2017), blood/plasma (Yuana et al., 2015), lymph (Milasan et al., 2016), and mucus (Pastor et al., 2021). Other types of nanoparticles are also found in biofluids, such as lipoproteins (Feingold, 2000) and exomeres (H. Zhang et al., 2018). New sources of EVs continue to be characterized as technology limitations surrounding isolation and authentication improve; however, a comprehensive understanding of the functional properties of EVs in health and disease remains to be determined.
Difficulties in assigning a particular function to EVs stems from their heterogeneity. Without the technical ability to independently isolate subpopulations, it is challenging to accurately assess individual contributions of EVs to homeostasis and disease (Ramirez et al., 2018; Thery et al., 2018; Veerman et al., 2021; Yates et al., 2022a). Limitations in EV separation and subsequent characterization stem from overlapping biomolecular content and size ranges, which can also be affected by isolation methods, storage conditions, and measurement parameters (Ramirez et al., 2018; Thery et al., 2018; Veerman et al., 2021; Yates et al., 2022a). Although there are reports of EVs containing distinct cargo, leading to a specific response in a disease model, such findings are often difficult to replicate due to differences in characterization methods and/or isolation techniques (see Challenges below) (Raposo and Stoorvogel, 2013; Tans et al., 1991; Yates et al., 2022a).
Many of the qualities that make EVs difficult to characterize also imbue them with incredible versatility and applicability as promising candidates for personalized medicine. Because of their specificity for certain tissues and disease states, EVs can be used as personalized diagnostic biomarkers and allogenic or autologous biotherapeutics and/or biogenic drug delivery vehicles. EVs offer promise for a personalized precision medicine approach to healthcare, where therapy can be tailored to the sex, age, and condition/disease of the individual.
2. EVs and personalized medicine
Personalized or precision medicine is based on using patient-specific information like genetic background or environmental and lifestyle factors to make decisions about the best course of treatment (Administration U. S. F. a. D., 2016; Piffoux et al., 2019; Shang et al., 2017). Many biologic patient-specific factors can be traced to blood or tissue biomarkers and many of those biomarkers can be found on/in EVs. Thus, EVs can be used to diagnose disease and ideally, if biomarkers can be identified early, for preventative medicine (Shang et al., 2017). Patient-specific biomarkers and early biomarkers using EVs allow tailored treatment decisions and individualized treatment regimens (Piffoux et al., 2019).
An accessible biopsy, termed the “liquid biopsy,” has long been awaited in various fields of medicine. This tissue-free approach to obtaining a ‘biopsy’ allows minimally-invasive screening, assessment, monitoring, and diagnosis, which is of high interest to all stakeholders (Lone et al., 2022; Shang et al., 2017). Because EVs accumulate in all body fluids and are known to contain biomarkers of cellular states, they are an important component of liquid biopsies (Shang et al., 2017). Use of EVs in liquid biopsies could be especially useful for conditions affecting vulnerable populations where tissue biopsies induce greater risk, such as pregnancy, and for diseases that are difficult to diagnose in early asymptomatic stages, like pancreatic cancer (Costa et al., 2022; Lone et al., 2022; Weingrill et al., 2021; Yates et al., 2022a). Aside from diagnosis, identifying EV-specific biomarkers that promote disease may provide opportunities to interrupt disease progression in patients via early treatment (Yates et al., 2022a).
As the field moves forward to utilize EVs as biomarkers and therapies for personalized medicine, it is important to realize that EVs play a role in normal homeostatic processes that protect against disease (Table 1) as well as promoting disease (Fig. 2) (Focosi et al., 2021; Yates et al., 2022b). Additionally, viruses and bacteria highjack EVs to promote infection. These dual roles of EVs need to be kept in mind in order to understand the communication context of EVs as biomarkers or therapeutic agents. The section below highlights three focus areas of EVs as personalized medicine: viral infection, cancer, and neurological disease, and briefly touches on other areas of growing research.
Table 1.
Physiological relevance of EVs in major organ systems.
| Immune System | initiation and resolution of inflammation (Buzas et al., 2014) innate immune system participants (Hong, 2018; Zhou et al., 2020) immune homeostasis (H. P. Chen et al., 2020; Rossaint et al., 2016) directly target antigen (Yates et al., 2022a) anti-microbial (Timar et al., 2013) enhance the immunological role of their parent cell (Yates et al., 2022a) antigen-presentation (Raposo et al., 1996) immune system inflammatory control (Karlsson et al., 2001) promote allergen tolerance (Karlsson et al., 2001) help activation or suppress immune functions when needed (Yates et al., 2022a) |
| Cardiovascular System | maintaining blood pressure (Good et al., 2020; Pironti et al., 2015) |
| Urinary System | fluid balance (Hiemstra et al., 2014) antimicrobial content carriers (Hiemstra et al., 2014) intra-nephron communication (Yates et al., 2022a) |
| Reproductive System | gamete development (Simon et al., 2018) sperm motility (Park et al., 2011) facilitate fertilization (Palmerini et al., 2003; Schuh et al., 2004) assist implantation (Greening et al., 2016; Nguyen et al., 2016; Simon et al., 2018) amniotic fluid signalling (Yates et al., 2022a) maternal-foetal communication and maternal tolerance of pregnancy (Knight et al., 1998; Simon et al., 2018; Tong and Chamley, 2015) early trophoblast development (Yates et al., 2022a) protect sperm from innate immune system of vaginal canal (Rooney et al., 1993) antibacterial (Carlsson et al., 2000) |
| Neurological System | assist intercellular communication for neurovascular unit (Yates et al., 2022a) regional development of the CNS(Yates et al., 2022a) contribute to the lateralization of the CNS(Yates et al., 2022a) communication across blood brain barrier (Dickens et al., 2017; Morales-Prieto et al., 2022) synapse maintenance and pruning (Lachenal et al., 2011; Paolicelli and Ferretti, 2017) |
| Musculoskeletal System | muscle homeostasis and myogenesis (Choi et al., 2016; Coenen-Stass et al., 2016; Forterre et al., 2014; Le Bihan et al., 2012; Romancino et al., 2013) metabolic regulation in muscles (Jalabert et al., 2016) muscle regeneration and repair (Choi et al., 2016; Fry et al., 2017) regulate fibrosis (Fry et al., 2017) bone cell communication (Deng et al., 2015) participate in osteogenesis (Weilner et al., 2016) |
| GI System | facilitate immune tolerance or activation towards gut microbiota (Nahui Palomino, Vanpouille, Costantini and Margolis, 2021) gut balance between microorganisms and between microorganisms and host (Yates et al., 2022a) facilitate symbiotic effects of gut microbiota (Yates et al., 2022a) |
Abbreviations: CNS, central nervous system; GI, gastrointestinal.
Fig. 2. EVs in Pathology.
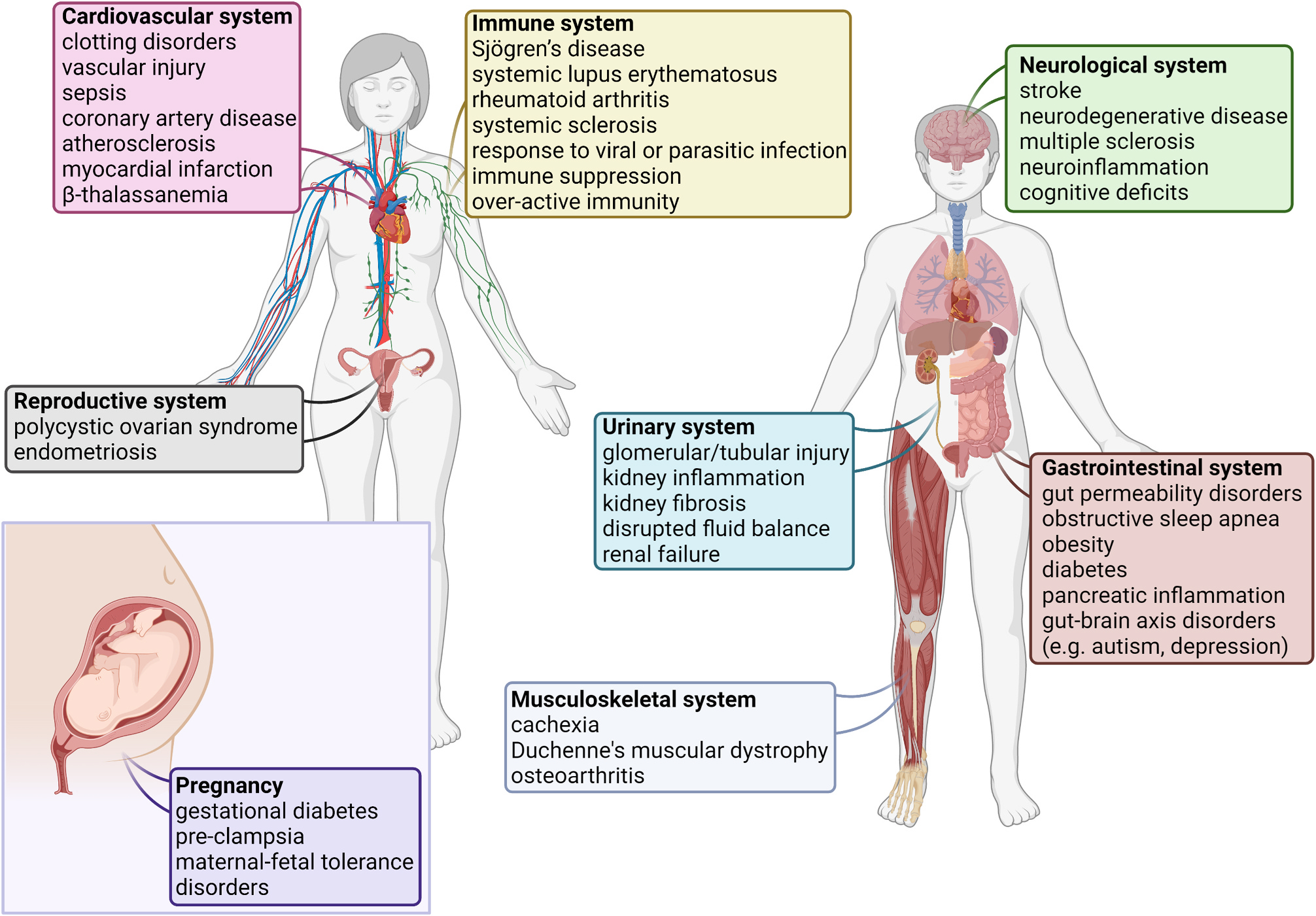
Many studies have linked EVs to the pathogenesis of various diseases, references are listed in Supplement Table 1. The figure depicts a variety of these diseases, grouped by major organ system. This list is constantly growing. This figure was created in ©BioRender-biorender.com.
3. EVs in health and disease
3.1. EVs, viral infection, and immune activation
Launching a protective immune response against viruses is critical to host survival. In response to infection, cells of the immune system use EVs to enhance immunity (Nogueira et al., 2015; Rodrigues et al., 2018; Wen et al., 2017). EVs released from antigen presenting cells (APCs) including dendritic cells, macrophages, and B cells have been found to express MHC class I or II-antigen complexes that are able to directly activate CD8+ or CD4+ T cells, respectively (Admyre et al., 2007; Hwang et al., 2003; Raposo et al., 1996; Thery et al., 2002). EVs released from APCs are also able to indirectly stimulate an immune response by transferring MHC I or II-antigen complexes to other APCs (Hwang et al., 2003). Another factor that is important for the ability of EVs to enhance immunity is binding to immune cells via expression of tetraspanins, including CD9, CD63 and CD81, and integrins (for example, CD11b) on the EV surface. However, many studies on the role of EVs in the immune response have been conducted on cultured cells, and much less is known about the capacity of immune cell-derived or other cell-derived EVs to activate the immune system in vivo. Recently, it was shown that different cell culture media supplements (i.e., presence or absence of lipoproteins) have opposing effects in terms of EV interactions with immune cells, highlighting the importance of mimicking physiological conditions (Busatto et al., 2020, 2022).
It is well established that many viruses such at HIV, coxsackievirus, hepatitis B and C, influenza, Epstein-Barr virus, and even SARS-CoV-2 highjack cellular (exosome ESCRT) and mitochondrial programs to enhance viral replication, package virus into EVs (capsid proteins, virions), and use EVs containing virus or viral products to subvert the immune response to obtain a replicative advantage in the host (Clough et al., 2021; Marcilla et al., 2014; Nogueira et al., 2015; Rodrigues et al., 2018; Sin et al., 2017; Yang et al., 2022). This includes SARS-CoV-2 RNA which has been detected inside EVs (Kwon et al., 2020). The mechanisms involved in exosome development and virus budding for enveloped viruses are very similar, and studies show that many viruses use the same cellular exocytosis machinery that produces EVs (ESCRT) to package viral capsid proteins and other pathogen-derived factors (Rodrigues et al., 2018). Viruses are able to hide from anti-viral antibody responses within EVs and also can use EV receptors for entry to host cells rather than their own viral receptors (Raab-Traub and Dittmer, 2017). Additionally, some viral receptor cargo co-localize with tetraspanins like CD81 on EVs and enhance or are required for viral infection of host cells (Pileri et al., 1998; J. Zhang et al., 2004). Some viruses, such as HIV, have been found to produce viral factors (Nef) that alter host cells causing them to create more EVs to enhance viral replication (Raymond et al., 2011). In contrast, EVs that contain ACE2 can block SARS-CoV-2 spike protein-dependent infection, indicating a protective anti-viral function of EVs (Cocozza et al., 2020; El-Shennawy et al., 2022), although ACE2+ EVs that enhance SARS-CoV-2 replication have also been reported (Tey et al., 2022). Overall, viruses employ multiple EV-based mechanisms to suppress the immune response and to promote viral shedding and persistence that may often overwhelm the body’s ability to defend against them (Rodrigues et al., 2018).
3.2. EVs and cancer
The role of EVs in promoting cancer is well established. EVs are a key mechanism that tumors use to influence the surrounding healthy microenvironment, allowing the tumor to create a niche for invasion and metastasis where they promote immunosuppression, which furthers cancer progression (Martellucci et al., 2020; Massaro et al., 2021). Tumor cells, with their constant growth and division, shed and release more soluble factors than healthy cells, and the EVs that they release have abnormal contents (Martellucci et al., 2020; Sun et al., 2018; Thind and Wilson, 2016). These EVs are involved with cellular communication and disease promotion at every stage of cancer pathogenesis, from development and invasion, to progression and metastasis (Fig. 3) (Sun et al., 2018). The paracrine and long-distance effects of EV-based communication allow early signaling between cancerous and healthy cells, facilitating organotropic metastasis from the primary tumor (An et al., 2015; Urabe et al., 2021).
Fig. 3. EVs Impact the Hallmarks of Cancer.
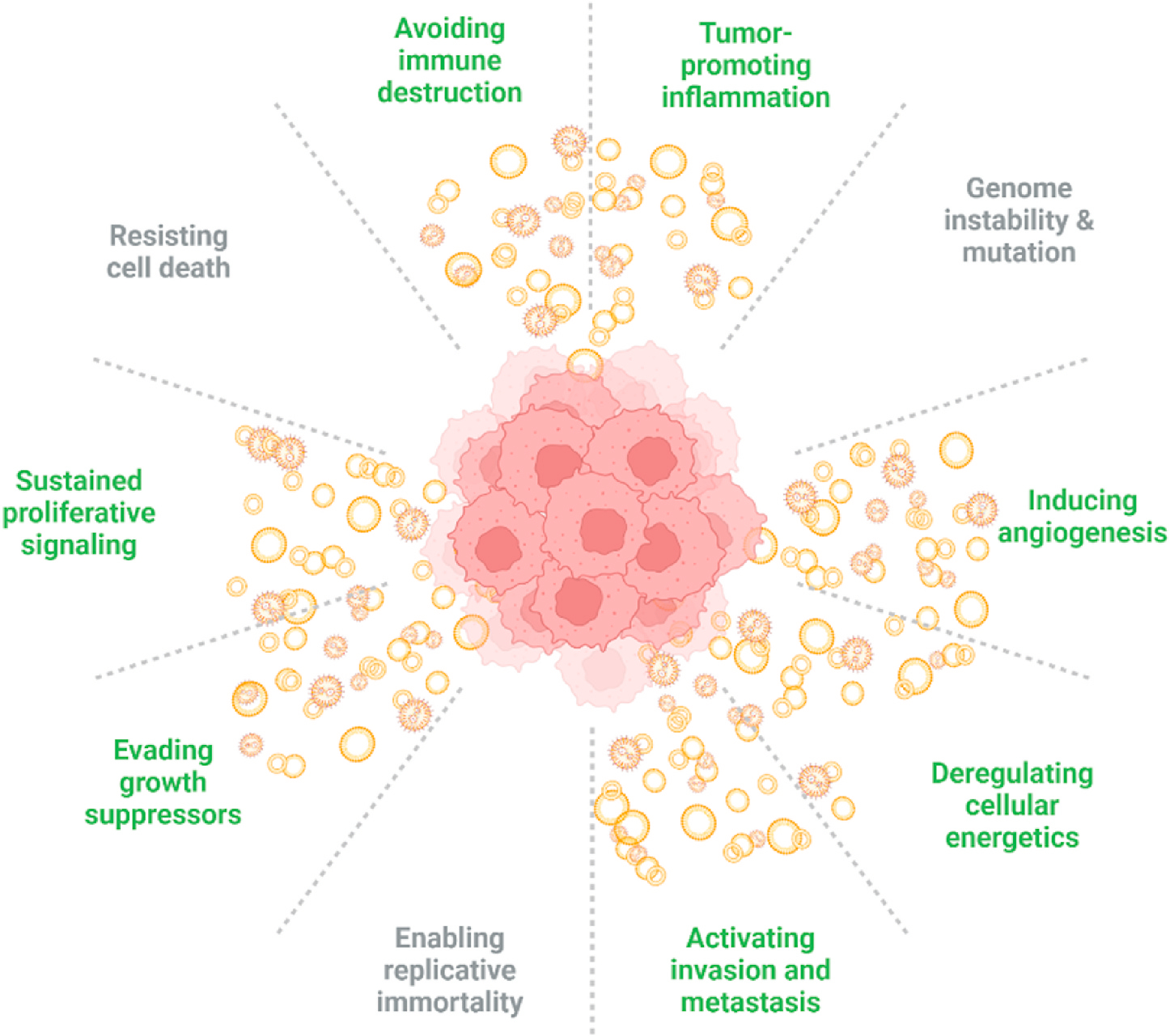
Hallmarks of cancer promoted by EVs depicted in green, other cancer promoters in grey. Adapted from “Hallmarks of cancer (2011 update), including emerging hallmarks and enabling factors”, by ©BioRender-biorender.com. Retrieved from https://app.biorender.com/biorender-templates.
However, the many roles EVs play in the pathogenesis of cancer may provide equally important, novel therapeutic targets to prevent disease. This communication network can be targeted to reduce tumor burden, reduce or prevent metastasis or relapse, and circumvent drug resistance (Lopez de Andres, Grinan-Lison, Jimenez and Marchal, 2020; Sun et al., 2018). If EV-mediated immune suppression within tumors is blocked, natural antitumor immunity could help decrease progression (Massaro et al., 2021). EV inhibitors are being studied for their potential to change the effects that tumor EVs have on the microenvironment, making them more amenable to traditional therapies (Catalano and O’Driscoll, 2020; W. Sun et al., 2018). Moreover, EVs can be isolated from immune cells with natural antitumor capabilities and used as anticancer agents (Del Vecchio et al., 2021), or they can be used as drug vehicles for nanodelivery of chemotherapies (Sun et al., 2018).
As a step toward developing therapies, identification of altered EVs linked to specific tumor subtypes are being developed as biomarkers (Avendano-Vazquez and Flores-Jasso, 2020; Bondhopadhyay et al., 2021; Del Bene et al., 2022; Li et al., 2022; Martellucci et al., 2020; Ramirez-Garrastacho et al., 2022; Sun et al., 2018). These biomarkers come from various accessible liquid biopsy sources, including saliva for esophageal cancer (Li et al., 2022), urine for prostate cancer (Ramirez-Garrastacho et al., 2022), and blood (Belov et al., 2016; Hu et al., 2021). Early detection of cancers that are typically found in later stages is vital to improve survival rates and treatment responses (Thery et al., 2018). Additionally, metastatic monitoring via liquid biopsies could increase access to care and reduce disease burden for patients that often must travel to specialized centers annually to monitor cancer progression (Lone et al., 2022; Thery et al., 2018). Diagnosis of cancer with a liquid biopsy instead of a tissue biopsy would provide a less invasive alternative that may be more accurate than a tissue biopsy, which can miss focal, abnormal cells (Lone et al., 2022; Thery et al., 2018). Advances in sequencing have allowed robust molecular profiling, essential for these carriers of diverse functional macromolecules (Gonzalez-Kozlova, 2022). These trends towards easier, continuous monitoring and biomarker-driven prognosis and treatment decisions demonstrate the many ways EVs can be used in personalized medicine for cancer patients.
3.3. EVs and neurological disease
EVs have also been found to promote the pathogenesis of disease in many neurological diseases such as Alzheimer’s dementia, Parkinson’s disease, and prion diseases. These conditions have a pattern of aberrant protein accumulation (Soto and Pritzkow, 2018) that spreads throughout the brain over time as the disease progresses (Braak et al., 2003; DeLeo and Ikezu, 2018; Takeuchi, 2021; You and Ikezu, 2019). EVs have been identified that promote disease by carrying misfolded proteins or their coding material between neurons (Hill, 2019; Khalaj et al., 2019; Takeuchi, 2021; You et al., 2022). Specifically, EVs have been found to spread RNA repeats in Huntington’s disease (Khalaj et al., 2019), α-synuclein in Parkinson’s disease (Khalaj et al., 2019; Takeuchi, 2021), prions in Prion disease (Fevrier et al., 2004; Khalaj et al., 2019), TDP-43 in amyotrophic lateral sclerosis (Feiler et al., 2015; Takeuchi, 2021), and phosphorylated tau in Alzheimer’s dementia (Ruan et al., 2021; Takeuchi, 2021). In a process that is similar to cancer metastasis, transfer of this cargo transforms healthy cells into dysfunctional, diseased cells. Research has shown that EV-packaged, pathogenic macromolecules like TDP-43 are preferentially taken up by cells compared to non-packaged, free TDP-43 (Feiler et al., 2015). Additionally, EVs contribute to physical deposits within the brain, either by actively catalyzing aggregation driven by lipid membrane properties, as observed with α-synuclein (Grey et al., 2015; Khalaj et al., 2019), or by providing a “sticky surface” for accumulation due to outer membrane surface molecules, as observed with amyloid beta-containing plaques in Alzheimer’s disease (Takeuchi, 2021). The severity of some neurodegenerative diseases have also been linked to levels of certain EVs in cerebrospinal fluid (Agosta et al., 2014; Hill, 2019; Muraoka et al., 2019, 2020), further indicating the association of EVs with the disease process.
Although new discoveries of the roles of EVs in neurological diseases are continuously being made, a similar number of studies are being published on the physiological and neuroprotective roles of EVs. For example, EVs have been found to increase synaptic activity, maintain and form myelin sheaths, and promote inflammatory regulation (Antonucci et al., 2012; Bakhti et al., 2011; Bianco et al., 2005). In addition to contributing to protein aggregates in the brain, there is also evidence that EVs actively prevent aggregate formation (Khalaj et al., 2019). EVs have also been found to actively scavenge and remove pathogenic molecules like amyloid beta, TDP-43, and α-synuclein from the intracellular space (Takeuchi, 2021). This is one of the reasons EVs were originally thought to be cellular ‘garbage bags’, which remains one of their most essential homeostatic roles (Takeuchi, 2021). Extracellularly, EVs also insulate aberrant proteins from interfering with synapse function (An et al., 2013). This selective externalization of pathogenic and/or misfolded proteins could help slow or suppress progression of neurodegenerative disease, revealing the dual role of EVs in the brain (Takeuchi, 2021). Furthermore, healthy neural cells can release protective molecules to other cells in the extracellular space via EVs, for example, transferring heat shock proteins in acute settings (Takeuchi, 2021). Therapeutically administered EVs can simulate neuroprotection, with in vitro models showing that EVs from healthy, non-CNS sources can reduce neurodegenerative disease and even promote restoration of proteostasis (Bonafede and Mariotti, 2017; Bonafede et al., 2016).
With the discovery of the role of EVs in the pathogenesis of many neurological diseases, a large number of therapeutic targets have arisen. Importantly, neural communication facilitated by EVs is not likely to be the sole means of propagation of disease, therefore, targeting or inhibiting pathogenic EVs has to be precise. For example, pharmacologic suppression of EV release or uptake has been suggested as a method to reduce progression of diseases linked to EV transmission of pathogenic molecules (Asai et al., 2015; Nath et al., 2012; Ruan et al., 2020; Sardar Sinha et al., 2018), although such approaches may have side effects due to the critical role of EVs in homeostasis.
Similar to the cancer field, there is interest in the neuroscience community to use liquid biopsies (Picca et al., 2022). Perhaps surprisingly, EVs in peripheral body fluids have been found to reflect cellular messaging from the brain, as EVs are capable of crossing the blood brain barrier to send peripheral signals (Thery et al., 2018). In disease states, this barrier is more compromised, facilitating greater transfer of EVs to the periphery (Tominaga et al., 2015). A number of studies are assessing whether EVs found in the blood of elderly individuals with neurodegenerative conditions, such as Alzheimer’s disease (Eren et al., 2022; Picca et al., 2022) or Parkinson’s disease (Calvani et al., 2020; Picca et al., 2022), can be used as biomarkers. EVs originating from the brain are isolated/detected based on neuron-specific markers, and higher Ab42 and synaptic molecule levels in neuron-derived EVs have been associated with improved cognitive function (Eren et al., 2022).
As communication tools, EVs provide both diagnostic and therapeutic opportunities for neurogenerative disease. Similar to viral infections and cancer, studies have primarily been conducted in vitro and it is yet unclear if EVs are direct drivers of neurodegenerative disease in vivo (Hill, 2019). More research is needed to determine the role of EVs in maintaining healthy neurons versus promoting disease (Hill, 2019). Additionally, the ability to identify EV subpopulations from specific neural cell types will be important for early diagnosis of neurological diseases and for tailoring personalized therapies (You et al., 2022).
3.4. EVs and other conditions
EVs have been found to contribute to health and disease in many other conditions. One area of focus of EV research has been on the urinary system, in part due to the ease of accessing urine. Urine is a source of early biomarkers of kidney diseases (Barreiro and Holthofer, 2017; Minkler et al., 2021) and EVs in urine have even been suggested as an alternative to kidney biopsy (Cricri et al., 2021). One major stumbling block to the potential use of urine EVs is the unknown effect of kidney filtration on EV populations. Specifically, it is unknown to what extent EV subpopulations from circulation change pre- and post-renal filtration and the ability to use urine-derived EVs to detect pathologies beyond kidney disease.
EVs have been linked to the development of a number of chronic diseases, especially those where the immune system is involved. In autoimmune diseases, EV signatures are altered, demonstrating their association with the pathogenesis of inflammatory disease (Grieco et al., 2021). In particular, autoimmune diseases with EVs as autoantigens or as contributors to pathology have been identified (Yates et al., 2022b). In joints, as in most tissues, EVs are important for maintaining the microenvironment; however, they can also mediate transport of proinflammatory molecules that contribute to cartilage degradation and osteoarthritis (Yin et al., 2022; Zhou et al., 2020). Conversely, EVs have been proposed as ideal therapies and diagnostic tools for osteoarthritis due to their regenerative capabilities in cartilage repair (Zhou et al., 2020) as well as diagnostic tools to track osteoarthritis progression, respectively (Yin et al., 2022). In type I and type II diabetes, EVs have been found to promote disease (Jayaseelan and Arumugam, 2019; Xiao et al., 2019). In inflammatory bowel disease, EV communication between gut microbiota and the host is affected (Gul et al., 2022). EVs are also implicated in pro-thrombotic diseases and conditions, where elevated EV levels increase the risk of dysregulated coagulation cascades (Curry et al., 2014). Similarly, in pregnancy, EVs have been correlated with pre-eclampsia and pregnancy-induced hypercoagulability in patients, and found to be a direct cause of these conditions in mice (Chen et al., 2021). Taken together, the role of EVs is currently being studied in many different conditions.
4. EVs as personalized therapies
The past two decades have seen major advancements in technologies that enable improved understanding and characterization of EVs. Before EVs are released from cells, they acquire intracellular cargo that is often comprised of a mixture of proteins, metabolites, and nucleic acids (usually RNA). A variety of ‘omic’ analyses of EV populations isolated from patient blood in a number of disease conditions have revealed that EVs carry unique molecular signatures during disease (de Miguel Perez et al., 2020; Hendrix, 2021; Hood, 2019; Kinoshita et al., 2017; Marleau et al., 2012; Szabo and Momen-Heravi, 2017; Thompson et al., 2016; Wu et al., 2020; Xu et al., 2020). This has accelerated the scientific community’s understanding of the significance of EVs in the context of disease and how these biogenic nanoparticles can be used to phenotype various disease conditions, treat degenerative conditions, or deliver drugs.
4.1. EVs as biomarkers
The contents of EVs change during the pathogenesis of disease, and technological advancements in the analysis of RNA, proteins, glycans, and lipid profiles allows more comprehensive signature detection than previously possible (Cheng et al., 2015; Saugstad et al., 2017; Walker et al., 2020). A biomarker is optimal if it is specific and sensitive to a particular disease, and EVs offer this opportunity in stable packaging (Bei et al., 2017; Rodrigues et al., 2018). Another advantage is that EVs are highly abundant in many bioavailable fluids, offering several sources from which to diagnose or track disease (El-Shennawy et al., 2022; Zhong et al., 2021). The heterogeneity of these populations present opportunities for truly tailored personalized medicine approaches, but are also extremely challenging to fully characterize (Zhong et al., 2021).
Emerging methods for more detailed characterization of EVs include multiplexed super resolution microscopy via directed stochastic reconstruction microscopy (dSTORM) (Fig. 4) and flow-cytometry that allow single EV analysis. These methods are not yet available for use clinically, but demonstrate progress in approaches to understand and characterize specific EV populations in the context of disease (Thery et al., 2018). Currently, some EV biomarker panels have been developed and approved by the Food and Drug Administration (FDA) for use in patients-the first for cancer reached the market in 2016 (Sheridan, 2016). While these panels show great potential, their use in the clinic is hindered by poor reproducibility, which is affected by the lack of widely accepted guidelines or standardized protocols for isolation, handling, and storage of EVs (Khalaj et al., 2019).
Fig. 4. Representative image of dSTORM tetraspanin profiling of an EV.
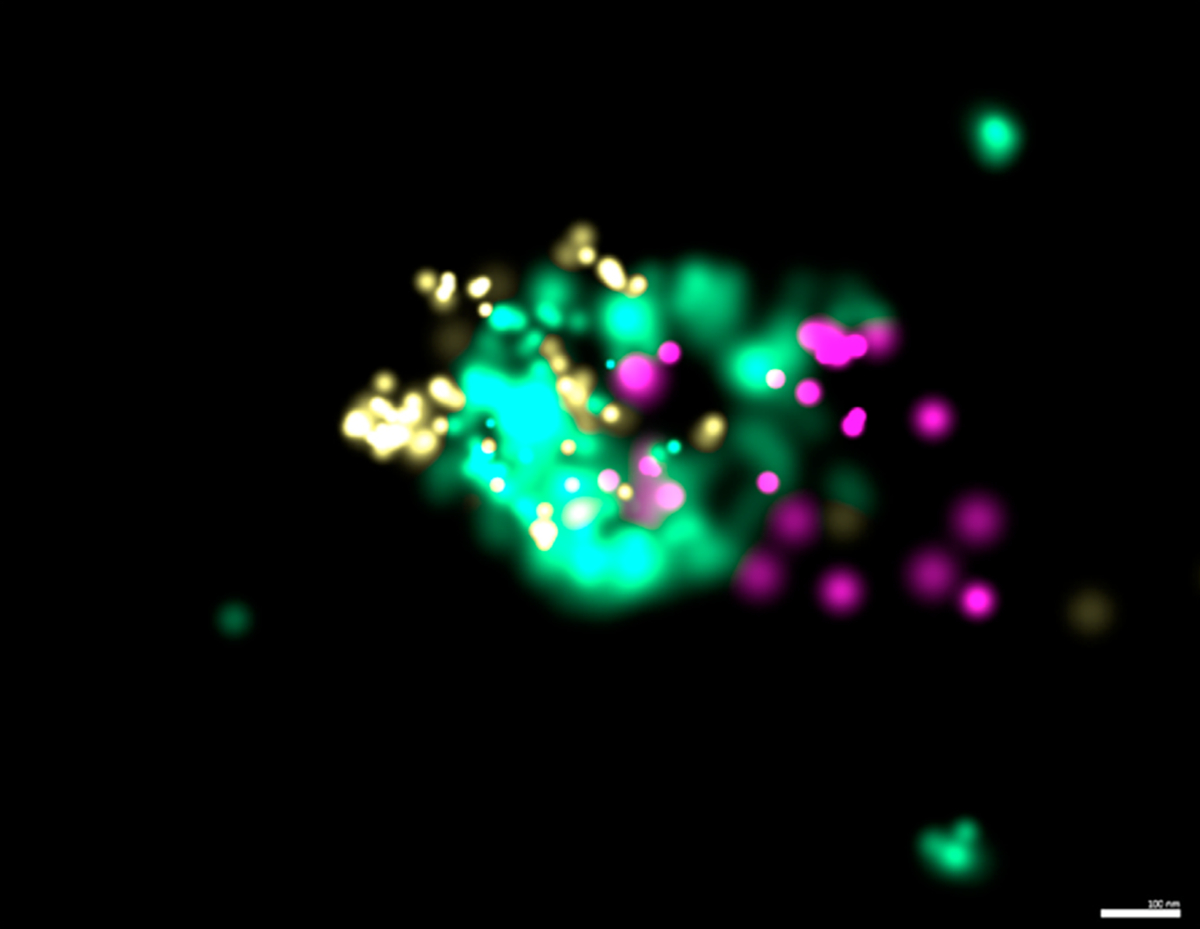
CD9 (yellow), CD81 (teal), CD63 (purple) using ONI EV profiling kit and precision depiction. Scale bar: 100 nm.
Choosing the appropriate biofluid to study is important in determining the mechanistic contribution of EVs to regeneration or disease; for example, urine may not be a good biofluid to identify EVs related to lung injury. Understanding the nuances of EV derivation is essential for appropriate biomarker characterization and development of clinical tests. Blood is the most common source of biofluid used for biomarker studies due in part to accessibility. Studies characterizing pathogenic RNA and protein content of EVs from blood have been reviewed for several diseases such as hepatitis (Szabo and Momen-Heravi, 2017), cancer (de Miguel Perez et al., 2020; Hood, 2019; Kinoshita et al., 2017; Marleau et al., 2012; Nawaz et al., 2014), neurodegeneration (Thompson et al., 2016; You and Ikezu, 2019), autoimmune disease (Wu et al., 2020; Xu et al., 2020), and cardiovascular disease (Bei et al., 2017; Chong et al., 2019; Dickhout and Koenen, 2018; Fu et al., 2020). These studies represent the next generation of biomarker development.
Developing early and rapid detection of diseases using EVs has led to the concept of EV depletion as a potential therapeutic option (Marleau et al., 2012). Aethlon Medical Incorporated is in recruitment stages for an early feasibility study to use their Hemopurifier™ for EV depletion for patients with head and neck squamous cell cancers. The study is expected to be completed by 2023 (Clinical Trials.gov website). Side effects from a reduction in total EV numbers may impact underlying physiological processes that rely on them to maintain health or allow other disease conditions to emerge (Hill, 2019). Simple depletion may not be the answer; instead, targeting EVs from specific cell types or pathways has been suggested (Hill, 2019; Khalaj et al., 2019). This specific targeting strategy is limited by current technical ability/knowledge, as it is challenging to differentiate pathological and physiological EVs (Rodrigues et al., 2018). This issue is further complicated in early timepoints or stages of disease, when EV disease signatures can only be found in relatively rare EV populations compared to other physiological populations, which range in the trillions (Hill, 2019; Rodrigues et al., 2018). A potential solution to this rarity is being assessed in neurological disease settings where antibody-meditated pull down for non-pathogenic EVs is being performed on blood in the periphery, allowing more efficient isolation of rarer populations for biomarker discovery, a technique that could also be applied to pathogenic EVs for specific depletion of pathogenic messengers (Hill, 2019).
4.2. EVs and regenerative medicine
While stem cell therapy has traditionally dominated the regenerative space, there is increasing evidence that the restorative functions once assumed to be due to stem cells are mediated by released products from stem cells termed the ‘secretome’, which includes EVs (Ding et al., 2021; Tao et al., 2018; Whittaker et al., 2020). Some advantages to using the secretome or EVs instead of cells include easier handling and storage, for example, freeze-drying can be performed to produce an “off the shelf” product that is readily available when needed. Additionally, EVs are unable to form cancerous growths, which is a risk with cell therapy (Feng et al., 2020; Qi et al., 2020; Szwedowicz et al., 2022; Tao et al., 2018; Wan et al., 2022; Wellings et al., 2021; Willis et al., 2020). The small size of EVs compared to cells also makes vascular obstructions less likely following intravenous administration and results in different biodistribution profiles (Ali et al., 2020). Finally, cells are more responsive to environmental conditions than EVs, and can change characteristics, which may be advantageous or disadvantageous depending on the context. EVs can also be modified by altering surface and/or internal components, allowing modular component design (Tao et al., 2018), and several EV-based drug delivery applications have been developed (S. Walker et al., 2019; Witwer, 2021).
The reparative functions of EVs are numerous, for example, EVs are able to reduce the effects of aging on cells (Feng et al., 2020; Mensa et al., 2020; Prattichizzo et al., 2019; Y. Yin et al., 2021). EVs obtained from the plasma of young mice have been found to reduce aging when administered to old mice (Iannotta et al., 2021; Prattichizzo et al., 2019; Sahu et al., 2021; Yoshida et al., 2019). Cellular senescence that occurs with age has been linked to EV signaling, providing new targets to mitigate the deteriorative effects of aging (Yin et al., 2021).
Altering inflammatory responses (Grieco et al., 2021), tissue/wound healing (Bray et al., 2021; Costa et al., 2022), and brain remodeling (Gualerzi et al., 2021) are all active areas of research with EV products for both tracking and inducing regenerative processes (Gualerzi et al., 2021). Inhibiting inflammation is an avid area of EV research. EVs are known to participate in the cross-talk between the immune system and other cells of the body, and are altered in a variety of disease states (Grieco et al., 2021). Fibrosis often leaves scar tissue which is an endpoint of tissue damage that has been irreparable with medication or surgery. Thus, great interest has been placed on determining whether EVs are able to prevent or return scar tissue to a healthy state (Qi et al., 2020; Wan et al., 2022; Wellings et al., 2021). Complete tissue regeneration via EV therapy is being actively tested in bone, skin, and cardiac muscle (Kost et al., 2022; Pishavar et al., 2021; Thankam and Agrawal, 2020; Yin et al., 2021).
4.3. EVs as drug delivery vehicles
Aside from their capacity as biomarkers and endogenous therapeutics, EVs can also be used for delivery of exogenous therapeutic agents, including small molecules, peptides/proteins, and RNA. Nanodelivery improves the site-specific accumulation of free drugs, resulting in increased therapeutic efficacy and less side effects (Khalid et al., 2017; Shen et al., 2017; Wolfram et al., 2015). Additionally, nanoparticles enable protection of RNA and protein therapeutics that are sensitive to degradation by extracellular and intracellular enzymes (Shen et al., 2015). Nanoformulations also have advantages over micro-formulations, including larger surface area to volume ratio, which can improve interactions with targets, and reduced risk of vascular obstructions (Martin et al., 2005).
There has been considerable interest in the use of EVs as medication carriers, especially for chemotherapeutics (Busatto et al., 2019; S. Walker et al., 2019). Although laboratory-created simple nanocarriers, such as liposomes, can be easily made through well-established methods and have been in clinical use for decades (Gentile et al., 2013), biologically-derived nanoparticles like EVs have the potential to outperform conventional delivery systems. Currently, the recognition and clearance of intravenously injected EVs by the innate immune system is much faster than that of synthetic nanoparticles (Couch et al., 2021). However, studies in reporter mice indicate that endogenous EVs can avoid immunological clearance and reach target tissues over long distances (Luo et al., 2020). Therefore, selecting the optimal EV subtype, preserving endogenous characteristics (i.e., minimizing damage from isolation, drug loading, and labeling), and lowering the infusion rate could overcome rapid immunological recognition. Clinically approved nanoparticles have simple surfaces that lack protein and glycan decorations, and attempts to develop targeted delivery systems have repeatedly failed in clinical trials (Wolfram and Ferrari, 2019). It is likely that the aforementioned failures are partially due to overly simplistic strategies (one surface ligand) to target highly complex biological surfaces with thousands of biomolecules. EVs demonstrate specificity for recipient cells through complex surface interactions that involve multiple molecules in optimal orientations, spatial arrangements, and ratios, and may therefore, be more equipped than synthetic nanoparticles to mediate site-specific delivery. Additionally, EVs have been shown to cross the blood brain barrier, a major roadblock for many neuro-therapies (Hill, 2019). EVs can also be harnessed in infectious diseases for their specificity and ability to target pathogens (Schorey and Harding, 2016). Many EV-based drug delivery systems are currently in the translational pipeline (Lener et al., 2015; Rodrigues et al., 2018), and the upcoming decade is likely to reveal the utility of these intercellular messengers as drug carriers.
4.4. Challenges
Many challenges need to be overcome before EVs can successfully be used as biomarkers or therapies for diseases in the clinical setting. A critical challenge is the multitude of isolation methods that vary widely in EV enrichment capabilities. Specifically, over 190 different isolation methods and over 1000 unique protocols have been reported for EV isolation (EV-TRACK Consortium et al., 2017). Isolation and storage methods substantially impact structure and function of EVs, which remains a fundamental issue in the field. Additionally, there is a lack of controls that can be used to standardize conditions between laboratories. There are a number of technical challenges in isolating EVs from biological samples, in particular, separation from similar sized contaminants. Improved tools, such as those based on flow cytometry and super resolution microscopy, are needed for an improved understanding of EV heterogeneity, which is also a limiting factor for clinical translation. Data derived from super-resolution microscopy, for example, can be altered based on the chemistry of the imaging mediums used (Arsic et al., 2020).
Although EVs have been studied for almost 60 years, many aspects of EV biology remain largely unknown (Couch et al., 2021; De Tkaczevski, 1968; Feller and Chopra, 1968; Sun, 1966; Wolf, 1967). The study of EVs is further complicated by host factors that alter the phenotype of EVs, including donor age, biological sex, current or previous pregnancy, menopause, pre/postprandial status (fasting/non-fasting), time of day of collection (circadian variations), exercise level and time of last exercise, diet, body mass index, specific infectious and noninfectious diseases, medications, and other factors (Thery et al., 2018). EV characteristics are also affected by sample collection conditions, such as collection volume, first tube discard, type of container(s), time to processing, choice of anticoagulant (for blood plasma), mixing or agitation, temperature (of both storage and processing), type of transport (if any), whether the tube remained upright before processing, exact centrifugation or filtration procedures, degree of hemolysis, possible confirmation of platelet and lipoprotein depletion prior to storage, and so on (Thery et al., 2018). Overall, there are a vast number of factors contributing to variation in EV characteristics and functions that need to be resolved in order to create a consistent product that can be used for personalized medicine.
5. Summary
EVs are promising biological nanoparticles for use as biomarkers to diagnose disease, to monitor progression of disease (or pre-disease) and to use as therapies to prevent or reverse disease. The ability of EVs to communicate directly with recipient cells in a cell/tissue, sex, age, and disease-specific manner places them at the forefront of candidates for personalized medicine. EV research over the past 60 years has laid the foundation and resulted in critical insights, yet a great deal remains to be understood. EV research holds the promise of changing how we understand, monitor, and treat disease, and researchers are racing forward to make those discoveries.
Acknowledgements
All figures were created with ©BioRender-biorender.com.
Appendix
Supplemental Table 1.
EVs in Pathology References from Fig. 2.
Footnotes
Declaration of competing interest
The authors declare that there are no conflicts of interest. The work has been performed with partial support from National Institutes of Health (NIH) grant TL1 TR002380 (to DJB, DND), U54 TR002377 (to DJB, DND, DF), National Institute of Allergy and Infectious Disease (NIAID) grants R21 AI145356, R21 AI154927 (to DF), R21 AI152318 (to JW and DF), and R21 AI163302 (to KAB), National Institute on Aging (NIA) grants R01 AG072719, R01 AG054199, R01 AG067763, R01 AG066429, R01 AG054672 (to TI), National Heart, Lung and Blood Institute (NHLBI) grant R01 HL164520 (to DF), American Heart Association grant 20TPA35490415 (to DF), a Mayo Clinic Team Science Award (to DF), the Mayo Clinic Center for Regenerative Medicine in Florida (to JW and DF), the University of Queensland grant (to JW), and Ionis Pharmaceuticals Ion-ARPA Operation Payload Delivery grant (to JW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
References
- Administration U. S. F. a. D., 2016. Personalized medicine: a biological approach to patient treatment. https://www.fda.gov/drugs/news-events-human-drugs/personalized-medicine-biological-approach-patient-treatment.
- Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, Gabrielsson S, 2007. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J. Allergy Clin. Immunol. 120 (6), 1418–1424. 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Agosta F, Dalla Libera D, Spinelli EG, Finardi A, Canu E, Bergami A, Furlan R, 2014. Myeloid microvesicles in cerebrospinal fluid are associated with myelin damage and neuronal loss in mild cognitive impairment and Alzheimer disease. Ann. Neurol. 76 (6), 813–825. 10.1002/ana.24235. [DOI] [PubMed] [Google Scholar]
- Agouni A, Parray AS, Akhtar N, Mir FA, Bourke PJ, Joseph S, Shuaib A, 2019. There is selective increase in pro-thrombotic circulating extracellular vesicles in acute ischemic stroke and transient ischemic attack: a study of patients from the Middle East and southeast asia. Front. Neurol. 10, 251. 10.3389/fneur.2019.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, Pham A, Wang X, Wolfram J, Pham S, 2020. Extracellular vesicles for treatment of solid organ ischemia-reperfusion injury. Am. J. Transplant. 20 (12), 3294–3307. 10.1111/ajt.16164. [DOI] [PubMed] [Google Scholar]
- Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, Boulanger CM, 2005. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J. Am. Soc. Nephrol. 16 (11), 3381–3388. 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- An K, Klyubin I, Kim Y, Jung JH, Mably AJ, O’Dowd ST, Kim JH, 2013. Exosomes neutralize synaptic-plasticity-disrupting activity of Abeta assemblies in vivo. Mol. Brain 6, 47. 10.1186/1756-6606-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An T, Qin S, Xu Y, Tang Y, Huang Y, Situ B, Zheng L, 2015. Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. J. Extracell. Vesicles 4, 27522. 10.3402/jev.v4.27522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, Verderio C, 2012. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 31 (5), 1231–1240. 10.1038/emboj.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsic A, Stajkovic N, Spiegel R, Nikic-Spiegel I, 2020. Effect of Vectashield-induced fluorescence quenching on conventional and super-resolution microscopy. Sci. Rep. 10 (1), 6441. 10.1038/s41598-020-63418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Ikezu T, 2015. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 18 (11), 1584–1593. 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avendano-Vazquez SE, Flores-Jasso CF, 2020. Stumbling on elusive cargo: how isomiRs challenge microRNA detection and quantification, the case of extracellular vesicles. J. Extracell. Vesicles 9 (1), 1784617. 10.1080/20013078.2020.1784617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon L, Suades R, Fuentes E, Palomo I, Padro T, 2016. Role of platelet-derived microvesicles as crosstalk mediators in atherothrombosis and future pharmacology targets: a link between inflammation, atherosclerosis, and thrombosis. Front. Pharmacol. 7, 293. 10.3389/fphar.2016.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhti M, Winter C, Simons M, 2011. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 286 (1), 787–796. 10.1074/jbc.M110.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro K, Holthofer H, 2017. Urinary extracellular vesicles. A promising shortcut to novel biomarker discoveries. Cell Tissue Res. 369 (1), 217–227. 10.1007/s00441-017-2621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashratyan R, Sheng H, Regn D, Rahman MJ, Dai YD, 2013. Insulinoma-released exosomes activate autoreactive marginal zone-like B cells that expand endogenously in prediabetic NOD mice. Eur. J. Immunol. 43 (10), 2588–2597. 10.1002/eji.201343376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzan E, Tine M, Casara A, Biondini D, Semenzato U, Cocconcelli E, Cosio MG, 2021. Critical review of the evolution of extracellular vesicles’ knowledge: from 1946 to Today. Int. J. Mol. Sci. 22 (12) 10.3390/ijms22126417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei Y, Yu P, Cretoiu D, Cretoiu SM, Xiao J, 2017. Exosomes-based biomarkers for the prognosis of cardiovascular diseases. Adv. Exp. Med. Biol. 998, 71–88. 10.1007/978-981-10-4397-0_5. [DOI] [PubMed] [Google Scholar]
- Belov L, Matic KJ, Hallal S, Best OG, Mulligan SP, Christopherson RI, 2016. Extensive surface protein profiles of extracellular vesicles from cancer cells may provide diagnostic signatures from blood samples. J. Extracell. Vesicles 5, 25355. 10.3402/jev.v5.25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C, 2005. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J. Immunol. 174 (11), 7268–7277. 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- Blonda M, Amoruso A, Martino T, Avolio C, 2018. New insights into immune cell-derived extracellular vesicles in multiple sclerosis. Front. Neurol. 9, 604. 10.3389/fneur.2018.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Lee DM, 2010. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327 (5965), 580–583. 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafede R, Mariotti R, 2017. ALS pathogenesis and therapeutic approaches: the role of mesenchymal stem cells and extracellular vesicles. Front. Cell. Neurosci. 11, 80. 10.3389/fncel.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafede R, Scambi I, Peroni D, Potrich V, Boschi F, Benati D, Mariotti R, 2016. Exosome derived from murine adipose-derived stromal cells: neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp. Cell Res. 340 (1), 150–158. 10.1016/j.yexcr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Bondhopadhyay B, Sisodiya S, Alzahrani FA, Bakhrebah MA, Chikara A, Kasherwal V, Hussain S, 2021. Exosomes: a forthcoming era of breast cancer therapeutics. Cancers 13 (18). 10.3390/cancers13184672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E, 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24 (2), 197–211. 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Bray ER, Oropallo AR, Grande DA, Kirsner RS, Badiavas EV, 2021. Extracellular vesicles as therapeutic tools for the treatment of chronic wounds. Pharmaceutics 13 (10). 10.3390/pharmaceutics13101543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busatto S, Pham A, Suh A, Shapiro S, Wolfram J, 2019. Organotropic drug delivery: synthetic nanoparticles and extracellular vesicles. Biomed. Microdevices 21 (2), 46. 10.1007/s10544-019-0396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busatto S, Yang Y, Iannotta D, Davidovich I, Talmon Y, Wolfram J, 2022. Considerations for extracellular vesicle and lipoprotein interactions in cell culture assays. J. Extracell. Vesicles 11 (4), e12202. 10.1002/jev2.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busatto S, Yang Y, Walker SA, Davidovich I, Lin WH, Lewis-Tuffin L, Wolfram J, 2020. Brain metastases-derived extracellular vesicles induce binding and aggregation of low-density lipoprotein. J. Nanobiotechnol. 18 (1), 162. 10.1186/s12951-020-00722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S, 2014. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 10 (6), 356–364. 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- Calvani R, Picca A, Landi G, Marini F, Biancolillo A, Coelho-Junior HJ, Marzetti E, 2020. A novel multi-marker discovery approach identifies new serum biomarkers for Parkinson’s disease in older people: an EXosomes in Parkinson Disease (EXPAND) ancillary study. Geroscience 42 (5), 1323–1334. 10.1007/s11357-020-00192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L, Pahlson C, Bergquist M, Ronquist G, Stridsberg M, 2000. Antibacterial activity of human prostasomes. Prostate 44 (4), 279–286. . [DOI] [PubMed] [Google Scholar]
- Casella G, Colombo F, Finardi A, Descamps H, Ill-Raga G, Spinelli A, Furlan R, 2018. Extracellular vesicles containing IL-4 modulate neuroinflammation in a mouse model of multiple sclerosis. Mol. Ther. 26 (9), 2107–2118. 10.1016/j.ymthe.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano M, O’Driscoll L, 2020. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J. Extracell. Vesicles 9 (1), 1703244. 10.1080/20013078.2019.1703244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HP, Wang XY, Pan XY, Hu WW, Cai ST, Joshi K, Ma D, 2020. Circulating neutrophil-derived microparticles associated with the prognosis of patients with sepsis. J. Inflamm. Res. 13, 1113–1124. 10.2147/JIR.S287256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Huang P, Han C, Li J, Liu L, Zhao Z, Xue F, 2021. Association of placenta-derived extracellular vesicles with pre-eclampsia and associated hypercoagulability: a clinical observational study. BJOG 128 (6), 1037–1046. 10.1111/1471-0528.16552. [DOI] [PubMed] [Google Scholar]
- Cheng L, Doecke JD, Sharples RA, Villemagne VL, Fowler CJ, Rembach A, Lifestyle Research G, 2015. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol. Psychiatr. 20 (10), 1188–1196. 10.1038/mp.2014.127. [DOI] [PubMed] [Google Scholar]
- Choi JS, Yoon HI, Lee KS, Choi YC, Yang SH, Kim IS, Cho YW, 2016. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J. Contr. Release 222, 107–115. 10.1016/j.jconrel.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Chong SY, Lee CK, Huang C, Ou YH, Charles CJ, Richards AM, Wang JW, 2019. Extracellular vesicles in cardiovascular diseases: alternative biomarker sources, therapeutic agents, and drug delivery carriers. Int. J. Mol. Sci. 20 (13) 10.3390/ijms20133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough E, Inigo J, Chandra D, Chaves L, Reynolds JL, Aalinkeel R, Mahajan SD, 2021. Mitochondrial dynamics in SARS-COV2 spike protein treated human microglia: implications for neuro-COVID. J. Neuroimmune Pharmacol. 16 (4), 770–784. 10.1007/s11481-021-10015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocozza F, Nevo N, Piovesana E, Lahaye X, Buchrieser J, Schwartz O, Martin-Jaular L, 2020. Extracellular vesicles containing ACE2 efficiently prevent infection by SARS-CoV-2 Spike protein-containing virus. J. Extracell. Vesicles 10 (13), 3272. 10.1002/jev2.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen-Stass AM, Betts CA, Lee YF, Mager I, Turunen MP, El Andaloussi S, Roberts TC, 2016. Selective release of muscle-specific, extracellular microRNAs during myogenic differentiation. Hum. Mol. Genet. 25 (18), 3960–3974. 10.1093/hmg/ddw237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C, 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289. 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Costa A, Quarto R, Bollini S, 2022. Small extracellular vesicles from human amniotic fluid samples as promising theranostics. Int. J. Mol. Sci. 23 (2), 590. 10.3390/ijms23020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch Y, Buzas EI, Vizio DD, Gho YS, Harrison P, Hill AF, Carter DRF, 2021. A brief history of nearly EV-erything - the rise and rise of extracellular vesicles. J. Extracell. Vesicles 10 (14), e12144. 10.1002/jev2.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, Lotvall J, 2013. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2. 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cricri G, Bellucci L, Montini G, Collino F, 2021. Urinary extracellular vesicles: uncovering the basis of the pathological processes in kidney-related diseases. Int. J. Mol. Sci. 22 (12), 6507. 10.3390/ijms22126507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry N, Raja A, Beavis J, Stanworth S, Harrison P, 2014. Levels of procoagulant microvesicles are elevated after traumatic injury and platelet microvesicles are negatively correlated with mortality. J. Extracell. Vesicles 3, 25625. 10.3402/jev.v3.25625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha-Azevedo B, Schmid SL, 2015. Migrasomes: a new organelle of migrating cells. Cell Res. 25 (1), 1–2. 10.1038/cr.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel Perez D, Rodriguez Martinez A, Ortigosa Palomo A, Delgado Urena M, Garcia Puche JL, Robles Remacho A, Serrano MJ, 2020. Extracellular vesicle-miRNAs as liquid biopsy biomarkers for disease identification and prognosis in metastatic colorectal cancer patients. Sci. Rep. 10 (1), 3974. 10.1038/s41598-020-60212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tkaczevski LZ, 1968. [“Small particles” found in various animal sera (electron microscopy study)]. Rev. Soc. Argent. Biol. 44 (1), 19–27. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/4902747. [PubMed] [Google Scholar]
- Dean WL, Lee MJ, Cummins TD, Schultz DJ, Powell DW, 2009. Proteomic and functional characterisation of platelet microparticle size classes. Thromb. Haemostasis 102 (4), 711–718. 10.1160/TH09-04-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene M, Osti D, Faletti S, Beznoussenko GV, DiMeco F, Pelicci G, 2022. Extracellular vesicles: the key for precision medicine in glioblastoma. Neuro Oncol. 24 (2), 184–196. 10.1093/neuonc/noab229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio F, Martinez-Rodriguez V, Schukking M, Cocks A, Broseghini E, Fabbri M, 2021. Professional killers: the role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T-cells and tumour cells. J. Extracell. Vesicles 10 (6), e12075. 10.1002/jev2.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo AM, Ikezu T, 2018. Extracellular vesicle biology in alzheimer’s disease and related tauopathy. J. Neuroimmune Pharmacol. 13 (3), 292–308. 10.1007/s11481-017-9768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Wang Y, Peng Y, Wu Y, Ding Y, Jiang Y, Fu Q, 2015. Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone 79, 37–42. 10.1016/j.bone.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Dias MVS, Costa CS, daSilva LLP, 2018. The ambiguous roles of extracellular vesicles in HIV replication and pathogenesis. Front. Microbiol. 9, 2411. 10.3389/fmicb.2018.02411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens AM, Tovar YRLB, Yoo SW, Trout AL, Bae M, Kanmogne M, Haughey NJ, 2017. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci. Signal. 10 (473), eaai7696. 10.1126/scisignal.aai7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickhout A, Koenen RR, 2018. Extracellular vesicles as biomarkers in cardiovascular disease; chances and risks. Front Cardiovasc Med 5, 113. 10.3389/fcvm.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Li Y, Sun Z, Han X, Chen Y, Ge Y, Wang W, 2021. Cell-derived extracellular vesicles and membranes for tissue repair. J. Nanobiotechnol. 19 (1), 368. 10.1186/s12951-021-01113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shennawy L, Hoffmann AD, Dashzeveg NK, McAndrews KM, Mehl PJ, Cornish D, Liu H, 2022. Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2. Nat. Commun. 13 (1), 405. 10.1038/s41467-021-27893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren E, Leoutsakos JM, Troncoso J, Lyketsos CG, Oh ES, Kapogiannis D, 2022. Neuronal-derived EV biomarkers track cognitive decline in alzheimer’s disease. Cells 11 (3). 10.3390/cells11030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Ciotola M, Schisano B, Gualdiero R, Sardelli L, Misso L, Giugliano D, 2006. Endothelial microparticles correlate with endothelial dysfunction in obese women. J. Clin. Endocrinol. Metab. 91 (9), 3676–3679. 10.1210/jc.2006-0851. [DOI] [PubMed] [Google Scholar]
- EV-TRACK Consortium, Van Deun J, Mestdagh P, Agostinis P, Akay O, Anand S, Hendrix A, 2017. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 14 (3), 228–232. 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- Feiler MS, Strobel B, Freischmidt A, Helferich AM, Kappel J, Brewer BM, Weishaupt JH, 2015. TDP-43 is intercellularly transmitted across axon terminals. J. Cell Biol. 211 (4), 897–911. 10.1083/jcb.201504057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold KR, 2000. Introduction to lipids and lipoproteins. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP (Eds.), Endotext. South Dartmouth (MA). [PubMed] [Google Scholar]
- Feller WF, Chopra HC, 1968. A small virus-like particle observed in human breast cancer by means of electron microscopy. J. Natl. Cancer Inst. 40 (6), 1359–1373. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/5660272. [PubMed] [Google Scholar]
- Feng R, Ullah M, Chen K, Ali Q, Lin Y, Sun Z, 2020. Stem cell-derived extracellular vesicles mitigate ageing-associated arterial stiffness and hypertension. J. Extracell. Vesicles 9 (1), 1783869. 10.1080/20013078.2020.1783869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Raposo G, 2004. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. U. S. A. 101 (26), 9683–9688. 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focosi D, Franchini M, Pirofski LA, Burnouf T, Fairweather D, Joyner MJ, Casadevall A, 2021. COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental Co-factors. Viruses 13 (8), 1594. 10.3390/v13081594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forterre A, Jalabert A, Chikh K, Pesenti S, Euthine V, Granjon A, Rome S, 2014. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle 13 (1), 78–89. 10.4161/cc.26808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlie G, Cohen N, Ming X, 2018. The perturbance of microbiome and gut-brain Axis in autism spectrum disorders. Int. J. Mol. Sci. 19 (8), 2251. 10.3390/ijms19082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA, 2017. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20 (1), 56–69. 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Zhang Y, Li Y, Luo L, Zhao Y, Yao Y, 2020. Extracellular vesicles in cardiovascular diseases. Cell Death Dis. 6, 68. 10.1038/s41420-020-00305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasecka A, Nieuwland R, van der Pol E, Hajji N, Cwiek A, Pluta K, Filipiak KJ, 2019. P2Y12 antagonist ticagrelor inhibits the release of procoagulant extracellular vesicles from activated platelets. Cardiol. J. 26 (6), 782–789. 10.5603/CJ.a2018.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile E, Cilurzo F, Di Marzio L, Carafa M, Ventura CA, Wolfram J, Celia C, 2013. Liposomal chemotherapeutics. Future Oncol. 9 (12), 1849–1859. 10.2217/fon.13.146. [DOI] [PubMed] [Google Scholar]
- Gildea JJ, Seaton JE, Victor KG, Reyes CM, Bigler Wang D, Pettigrew AC, Felder RA, 2014. Exosomal transfer from human renal proximal tubule cells to distal tubule and collecting duct cells. Clin. Biochem. 47 (15), 89–94. 10.1016/j.clinbiochem.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Kozlova EE, 2022. Molecular profiling of liquid biopsies for precision oncology. Adv. Exp. Med. Biol. 1361, 235–247. 10.1007/978-3-030-91836-1_13. [DOI] [PubMed] [Google Scholar]
- Good ME, Musante L, La Salvia S, Howell NL, Carey RM, Le TH, Erdbrugger U, 2020. Circulating extracellular vesicles in normotension restrain vasodilation in resistance arteries. Hypertension 75 (1), 218–228. 10.1161/HYPERTENSIONAHA.119.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodsell DS, Olson AJ, 1993. Soluble proteins: size, shape and function. Trends Biochem. Sci. 18 (3), 65–68. 10.1016/0968-0004(93)90153-e. [DOI] [PubMed] [Google Scholar]
- Greening DW, Nguyen HP, Elgass K, Simpson RJ, Salamonsen LA, 2016. Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: insights into endometrial-embryo interactions. Biol. Reprod. 94 (2), 38. 10.1095/biolreprod.115.134890. [DOI] [PubMed] [Google Scholar]
- Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S, 2015. Acceleration of alpha-synuclein aggregation by exosomes. J. Biol. Chem. 290 (5), 2969–2982. 10.1074/jbc.M114.585703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco GE, Fignani D, Formichi C, Nigi L, Licata G, Maccora C, Dotta F, 2021. Extracellular vesicles in immune system regulation and type 1 diabetes: cell-to-cell communication mediators, disease biomarkers, and promising therapeutic tools. Front. Immunol. 12, 682948 10.3389/fimmu.2021.682948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualerzi A, Picciolini S, Roda F, Bedoni M, 2021. Extracellular vesicles in regeneration and rehabilitation recovery after stroke. Biology 10 (9), 843. 10.3390/biology10090843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul L, Modos D, Fonseca S, Madgwick M, Thomas JP, Sudhakar P, Korcsmaros T, 2022. Extracellular vesicles produced by the human commensal gut bacterium Bacteroides thetaiotaomicron affect host immune pathways in a cell-type specific manner that are altered in inflammatory bowel disease. J. Extracell. Vesicles 11 (1), e12189. 10.1002/jev2.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorgy B, Modos K, Pallinger E, Paloczi K, Pasztoi M, Misjak P, Buzas EI, 2011. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood 117 (4), e39–e48. 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- Han C, Wang C, Chen Y, Wang J, Xu X, Hilton T, Zhang J, 2020. Placenta-derived extracellular vesicles induce preeclampsia in mouse models. Haematologica 105 (6), 1686–1694. 10.3324/haematol.2019.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix A, 2021. The nature of blood(y) extracellular vesicles. Nat. Rev. Mol. Cell Biol. 22 (4), 243. 10.1038/s41580-021-00348-8. [DOI] [PubMed] [Google Scholar]
- Hiemstra TF, Charles PD, Gracia T, Hester SS, Gatto L, Al-Lamki R, Karet Frankl FE, 2014. Human urinary exosomes as innate immune effectors. J. Am. Soc. Nephrol. 25 (9), 2017–2027. 10.1681/ASN.2013101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AF, 2019. Extracellular vesicles and neurodegenerative diseases. J. Neurosci. 39 (47), 9269–9273. 10.1523/JNEUROSCI.0147-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CW, 2018. Extracellular vesicles of neutrophils. Immune Netw 18 (6), e43. 10.4110/in.2018.18.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JL, 2019. Natural melanoma-derived extracellular vesicles. Semin. Cancer Biol. 59, 251–265. 10.1016/j.semcancer.2019.06.020. [DOI] [PubMed] [Google Scholar]
- Hoog JL, Lotvall J, 2015. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J. Extracell. Vesicles 4, 28680. 10.3402/jev.v4.28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Wolfram J, Srivastava S, 2021. Extracellular vesicles in cancer detection: hopes and hypes. Trends Cancer 7 (2), 122–133. 10.1016/j.trecan.2020.09.003. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li R, Ye S, Lin S, Yin G, Xie Q, 2020. Recent Advances in the use of exosomes in sjogren’s syndrome. Front. Immunol. 11, 1509. 10.3389/fimmu.2020.01509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Shen X, Sprent J, 2003. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc. Natl. Acad. Sci. U. S. A. 100 (11), 6670–6675. 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotta D, Yang M, Celia C, Di Marzio L, Wolfram J, 2021. Extracellular vesicle therapeutics from plasma and adipose tissue. Nano Today 39, 101159. 10.1016/j.nantod.2021.101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabert A, Vial G, Guay C, Wiklander OP, Nordin JZ, Aswad H, Rome S, 2016. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia 59 (5), 1049–1058. 10.1007/s00125-016-3882-y. [DOI] [PubMed] [Google Scholar]
- James-Allan LB, Rosario FJ, Barner K, Lai A, Guanzon D, McIntyre HD, Jansson T, 2020. Regulation of glucose homeostasis by small extracellular vesicles in normal pregnancy and in gestational diabetes. Faseb. J. 34 (4), 5724–5739. 10.1096/fj.201902522RR. [DOI] [PubMed] [Google Scholar]
- Janas AM, Sapon K, Janas T, Stowell MH, Janas T, 2016. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim. Biophys. Acta 1858 (6), 1139–1151. 10.1016/j.bbamem.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Jayaseelan VP, Arumugam P, 2019. Dissecting the theranostic potential of exosomes in autoimmune disorders. Cell. Mol. Immunol. 16 (12), 935–936. 10.1038/s41423-019-0310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jella KK, Yu L, Yue Q, Friedman D, Duke BJ, Alli AA, 2016. Exosomal GAPDH from proximal tubule cells regulate ENaC activity. PLoS One 11 (11), e0165763. 10.1371/journal.pone.0165763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Fan J, Cui W, Liu S, Li N, Lau WB, Wei Y, 2017. Endothelial cell-derived microparticles from patients with obstructive sleep apnea hypoxia syndrome and coronary artery disease increase aortic endothelial cell dysfunction. Cell. Physiol. Biochem. 43 (6), 2562–2570. 10.1159/000484508. [DOI] [PubMed] [Google Scholar]
- Kahn R, Mossberg M, Stahl AL, Johansson K, Lopatko Lindman I, Heijl C, Karpman D, 2017. Microvesicle transfer of kinin B1-receptors is a novel inflammatory mechanism in vasculitis. Kidney Int. 91 (1), 96–105. 10.1016/j.kint.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E, 2001. “Tolerosomes” are produced by intestinal epithelial cells. Eur. J. Immunol. 31 (10), 2892–2900. . [DOI] [PubMed] [Google Scholar]
- Kerris EWJ, Hoptay C, Calderon T, Freishtat RJ, 2020. Platelets and platelet extracellular vesicles in hemostasis and sepsis. J. Invest. Med. 68 (4), 813–820. 10.1136/jim-2019-001195. [DOI] [PubMed] [Google Scholar]
- Khalaj K, Miller JE, Lingegowda H, Fazleabas AT, Young SL, Lessey BA, Tayade C, 2019. Extracellular vesicles from endometriosis patients are characterized by a unique miRNA-lncRNA signature. JCI Insight 4 (18), e128846. 10.1172/jci.insight.128846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid A, Persano S, Shen H, Zhao Y, Blanco E, Ferrari M, Wolfram J, 2017. Strategies for improving drug delivery: nanocarriers and microenvironmental priming. Expet Opin. Drug Deliv. 14 (7), 865–877. 10.1080/17425247.2017.1243527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheansaard W, Phongpao K, Paiboonsukwong K, Pattanapanyasat K, Chaichompoo P, Svasti S, 2018. Microparticles from beta-thalassaemia/HbE patients induce endothelial cell dysfunction. Sci. Rep. 8 (1), 13033 10.1038/s41598-018-31386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Hohjoh H, Yamamura T, 2018. The role for exosomal microRNAs in disruption of regulatory T cell homeostasis in multiple sclerosis. J. Exp. Neurosci. 12, 1179069518764892 10.1177/1179069518764892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Yip KW, Spence T, Liu FF, 2017. MicroRNAs in extracellular vesicles: potential cancer biomarkers. J. Hum. Genet. 62 (1), 67–74. 10.1038/jhg.2016.87. [DOI] [PubMed] [Google Scholar]
- Knight M, Redman CW, Linton EA, Sargent IL, 1998. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br. J. Obstet. Gynaecol. 105 (6), 632–640. 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, Fulzele S, 2017. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 7 (1), 2029. 10.1038/s41598-017-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost Y, Muskat A, Mhaimeed N, Nazarian RS, Kobets K, 2022. Exosome therapy in hair regeneration: a literature review of the evidence, challenges, and future opportunities. J. Cosmet. Dermatol. 21 (8), 3226–3231. 10.1111/jocd.15008. [DOI] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Faden AI, 2017. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J. Neuroinflammation 14 (1), 47. 10.1186/s12974-017-0819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Nukala SB, Srivastava S, Miyamoto H, Ismail NI, Jousma J, Ong SG, 2020. Detection of viral RNA fragments in human iPSC cardiomyocytes following treatment with extracellular vesicles from SARS-CoV-2 coding sequence overexpressing lung epithelial cells. Stem Cell Res. Ther. 11 (1), 514. 10.1186/s13287-020-02033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Sadoul R, 2011. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell. Neurosci. 46 (2), 409–418. 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Le Bihan MC, Bigot A, Jensen SS, Dennis JL, Rogowska-Wrzesinska A, Laine J, Butler-Browne G, 2012. In-depth analysis of the secretome identifies three major independent secretory pathways in differentiating human myoblasts. J. Proteonomics 77, 344–356. 10.1016/j.jprot.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Lee EE, Winston-Gray C, Barlow JW, Rissman RA, Jeste DV, 2020. Plasma levels of neuron- and astrocyte-derived exosomal amyloid beta1–42, amyloid beta1–40, and phosphorylated tau levels in schizophrenia patients and non-psychiatric comparison subjects: relationships with cognitive functioning and psychopathology. Front. Psychiatr. 11, 532624 10.3389/fpsyt.2020.532624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Giebel B, 2015. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles 4, 30087. 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Lin Y, Luo Y, Xiong X, Wang L, Durante K, Zhang H, 2022. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: a multicenter prospective study. Mol. Cancer 21 (1), 21. 10.1186/s12943-022-01499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Zhang HG, 2006. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 176 (3), 1375–1385. 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang SZ, Wang QL, Du JG, Wang BB, 2018. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placental exosomes in the maternal circulation across gestation. Eur. Rev. Med. Pharmacol. Sci. 22 (7), 2036–2043. 10.26355/eurrev_201804_14733. [DOI] [PubMed] [Google Scholar]
- Liu X, Miao J, Wang C, Zhou S, Chen S, Ren Q, Liu Y, 2020. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int. 97 (6), 1181–1195. 10.1016/j.kint.2019.11.026. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li Q, Hosen MR, Zietzer A, Flender A, Levermann P, Jansen F, 2019. Atherosclerotic conditions promote the packaging of functional MicroRNA-92a-3p into endothelial microvesicles. Circ. Res. 124 (4), 575–587. 10.1161/CIRCRESAHA.118.314010. [DOI] [PubMed] [Google Scholar]
- Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, Macha MA, 2022. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 21 (1), 79. 10.1186/s12943-022-01543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Andres J, Grinan-Lison C, Jimenez G, Marchal JA, 2020. Cancer stem cell secretome in the tumor microenvironment: a key point for an effective personalized cancer treatment. J. Hematol. Oncol. 13 (1), 136. 10.1186/s13045-020-00966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louten J, 2016. Virus structure and classification. In: Louten J (Ed.), Essential Human Virology, pp. 19–29. [Google Scholar]
- Loyer X, Zlatanova I, Devue C, Yin M, Howangyin KY, Klaihmon P, Boulanger CM, 2018. Intra-cardiac release of extracellular vesicles shapes inflammation following myocardial infarction. Circ. Res. 123 (1), 100–106. 10.1161/CIRCRESAHA.117.311326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Dai Y, Chen Z, Yue X, Andrade-Powell KC, Chang J, 2020. Spatial and temporal tracking of cardiac exosomes in mouse using a nano-luciferase-CD63 fusion protein. Commun Biol 3 (1), 114. 10.1038/s42003-020-0830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL, Liu BC, 2018. Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J. Am. Soc. Nephrol. 29 (3), 919–935. 10.1681/ASN.2017050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, Yu L, 2015. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 25 (1), 24–38. 10.1038/cr.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallardi A, Nuzziello N, Liguori M, Avolio C, Palazzo G, 2018. Counting of peripheral extracellular vesicles in Multiple Sclerosis patients by an improved nanoplasmonic assay and dynamic light scattering. Colloids Surf. B Biointerfaces 168, 134–142. 10.1016/j.colsurfb.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Marcilla A, Martin-Jaular L, Trelis M, de Menezes-Neto A, Osuna A, Bernal D, Del Portillo HA, 2014. Extracellular vesicles in parasitic diseases. J. Extracell. Vesicles 3, 25040. 10.3402/jev.v3.25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marleau AM, Chen CS, Joyce JA, Tullis RH, 2012. Exosome removal as a therapeutic adjuvant in cancer. J. Transl. Med. 10, 134. 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martellucci S, Orefice NS, Angelucci A, Luce A, Caraglia M, Zappavigna S, 2020. Extracellular vesicles: new endogenous shuttles for miRNAs in cancer diagnosis and therapy? Int. J. Mol. Sci. 21 (18) 10.3390/ijms21186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FJ, Melnik K, West T, Shapiro J, Cohen M, Boiarski AA, Ferrari M, 2005. Acute toxicity of intravenously administered microfabricated silicon dioxide drug delivery particles in mice: preliminary findings. Drugs R 6 (2), 71–81. 10.2165/00126839-200506020-00002. [DOI] [PubMed] [Google Scholar]
- Massaro C, Min W, Pegtel DM, Baglio SR, 2021. Harnessing EV communication to restore antitumor immunity. Adv. Drug Deliv. Rev. 176, 113838 10.1016/j.addr.2021.113838. [DOI] [PubMed] [Google Scholar]
- Maugeri N, Capobianco A, Rovere-Querini P, Ramirez GA, Tombetti E, Valle PD, Manfredi AA, 2018. Platelet microparticles sustain autophagy-associated activation of neutrophils in systemic sclerosis. Sci. Transl. Med. 10 (451), eaao3089. 10.1126/scitranslmed.aao3089. [DOI] [PubMed] [Google Scholar]
- Medicine U. S. N. L. o. Retrieved from. https://www.clinicaltrials.gov/. https://www.clinicaltrials.gov/. from U.S. Department of Health and Human Services. [Google Scholar]
- Meehan B, Rak J, Di Vizio D, 2016. Oncosomes - large and small: what are they, where they came from? J. Extracell. Vesicles 5, 33109. 10.3402/jev.v5.33109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensa E, Guescini M, Giuliani A, Bacalini MG, Ramini D, Corleone G, Olivieri F, 2020. Small extracellular vesicles deliver miR-21 and miR-217 as pro-senescence effectors to endothelial cells. J. Extracell. Vesicles 9 (1), 1725285. 10.1080/20013078.2020.1725285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midura EF, Jernigan PL, Kuethe JW, Friend LA, Veile R, Makley AT, Goodman MD, 2015. Microparticles impact coagulation after traumatic brain injury. J. Surg. Res. 197 (1), 25–31. 10.1016/j.jss.2015.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milasan A, Tessandier N, Tan S, Brisson A, Boilard E, Martel C, 2016. Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis. J. Extracell. Vesicles 5, 31427. 10.3402/jev.v5.31427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagar A, Jy W, Jimenez JJ, Sheremata WA, Mauro LM, Mao WW, Ahn YS, 2001. Elevated plasma endothelial microparticles in multiple sclerosis. Neurology 56 (10), 1319–1324. 10.1212/wnl.56.10.1319. [DOI] [PubMed] [Google Scholar]
- Minkler S, Lucien F, Kimber MJ, Sahoo DK, Bourgois-Mochel A, Musser M, Mochel JP, 2021. Emerging roles of urine-derived components for the management of bladder cancer: one man’s trash is another man’s treasure. Cancers 13 (3), 422. 10.3390/cancers13030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S, Feldbrugge L, Csizmadia E, Mitsuhashi M, Robson SC, Moss AC, 2016. Luminal extracellular vesicles (EVs) in inflammatory bowel disease (IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm. Bowel Dis. 22 (7), 1587–1595. 10.1097/MIB.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Prieto DM, Murrieta-Coxca JM, Stojiljkovic M, Diezel C, Streicher PE, Henao-Restrepo JA, Marz M, 2022. Small extracellular vesicles from peripheral blood of aged mice pass the blood-brain barrier and induce glial cell activation. Cells 11 (4), 625. 10.3390/cells11040625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KV, Witwer KW, 2022. The evolving paradigm of extracellular vesicles in intercellular signaling and delivery of therapeutic RNAs. Mol. Ther. 30 (7), 2393–2394. 10.1016/j.ymthe.2022.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortberg J, Lundwall K, Mobarrez F, Wallen H, Jacobson SH, Spaak J, 2019. Increased concentrations of platelet- and endothelial-derived microparticles in patients with myocardial infarction and reduced renal function- a descriptive study. BMC Nephrol. 20 (1), 71. 10.1186/s12882-019-1261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka S, Jedrychowski MP, Tatebe H, DeLeo AM, Ikezu S, Tokuda T, Ikezu T, 2019. Proteomic profiling of extracellular vesicles isolated from cerebrospinal fluid of former national football league players at risk for chronic traumatic encephalopathy. Front. Neurosci. 13, 1059. 10.3389/fnins.2019.01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka S, Jedrychowski MP, Yanamandra K, Ikezu S, Gygi SP, Ikezu T, 2020. Proteomic profiling of extracellular vesicles derived from cerebrospinal fluid of Alzheimer’s disease patients: a pilot study. Cells 9 (9), 1959. 10.3390/cells9091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahui Palomino RA, Vanpouille C, Costantini PE, Margolis L, 2021. Microbiota-host communications: bacterial extracellular vesicles as a common language. PLoS Pathog. 17 (5), e1009508 10.1371/journal.ppat.1009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S, Agholme L, Kurudenkandy FR, Granseth B, Marcusson J, Hallbeck M, 2012. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of beta-amyloid. J. Neurosci. 32 (26), 8767–8777. 10.1523/JNEUROSCI.0615-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz M, Camussi G, Valadi H, Nazarenko I, Ekstrom K, Wang X, Kislinger T, 2014. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat. Rev. Urol. 11 (12), 688–701. 10.1038/nrurol.2014.301. [DOI] [PubMed] [Google Scholar]
- Nguyen HP, Simpson RJ, Salamonsen LA, Greening DW, 2016. Extracellular vesicles in the intrauterine environment: challenges and potential functions. Biol. Reprod. 95 (5), 109. 10.1095/biolreprod.116.143503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira PM, Ribeiro K, Silveira AC, Campos JH, Martins-Filho OA, Bela SR, Torrecilhas AC, 2015. Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune responses. J. Extracell. Vesicles 4, 28734. 10.3402/jev.v4.28734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmerini CA, Saccardi C, Carlini E, Fabiani R, Arienti G, 2003. Fusion of prostasomes to human spermatozoa stimulates the acrosome reaction. Fertil. Steril. 80 (5), 1181–1184. 10.1016/s0015-0282(03)02160-5. [DOI] [PubMed] [Google Scholar]
- Pant S, Hilton H, Burczynski ME, 2012. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem. Pharmacol. 83 (11), 1484–1494. 10.1016/j.bcp.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Ferretti MT, 2017. Function and dysfunction of microglia during brain development: consequences for synapses and neural circuits. Front. Synaptic Neurosci. 9, 9. 10.3389/fnsyn.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Kim BJ, Kang J, Nam TS, Lim JM, Kim HT, Kim UH, 2011. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 4 (173), ra31. 10.1126/scisignal.2001595. [DOI] [PubMed] [Google Scholar]
- Pastor L, Vera E, Marin JM, Sanz-Rubio D, 2021. Extracellular vesicles from airway secretions: new insights in lung diseases. Int. J. Mol. Sci. 22 (2), 583. 10.3390/ijms22020583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picca A, Guerra F, Calvani R, Coelho-Junior HJ, Bucci C, Marzetti E, 2022. Circulating extracellular vesicles: friends and foes in neurodegeneration. Neural Regen Res 17 (3), 534–542. 10.4103/1673-5374.320972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piffoux M, Nicolas-Boluda A, Mulens-Arias V, Richard S, Rahmi G, Gazeau F, Silva AKA, 2019. Extracellular vesicles for personalized medicine: the input of physically triggered production, loading and theranostic properties. Adv. Drug Deliv. Rev. 138, 247–258. 10.1016/j.addr.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Abrignani S, 1998. Binding of hepatitis C virus to CD81. Science 282 (5390), 938–941. 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Rockman HA, 2015. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131 (24), 2120–2130. 10.1161/CIRCULATIONAHA.115.015687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishavar E, Luo H, Naserifar M, Hashemi M, Toosi S, Atala A, Behravan J, 2021. Advanced hydrogels as exosome delivery systems for osteogenic differentiation of MSCs: application in bone regeneration. Int. J. Mol. Sci. 22 (12), 6203. 10.3390/ijms22126203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prattichizzo F, Giuliani A, Sabbatinelli J, Mensa E, De Nigris V, La Sala L, Ceriello A, 2019. Extracellular vesicles circulating in young organisms promote healthy longevity. J. Extracell. Vesicles 8 (1), 1656044. 10.1080/20013078.2019.1656044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Liu Q, Reisdorf RL, Boroumand S, Behfar A, Moran SL, Zhao C, 2020. Characterization of a purified exosome product and its effects on canine flexor tenocyte biology. J. Orthop. Res. 38 (8), 1845–1855. 10.1002/jor.24587. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N, Dittmer DP, 2017. Viral effects on the content and function of extracellular vesicles. Nat. Rev. Microbiol. 15 (9), 559–572. 10.1038/nrmicro.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MJ, Regn D, Bashratyan R, Dai YD, 2014. Exosomes released by islet-derived mesenchymal stem cells trigger autoimmune responses in NOD mice. Diabetes 63 (3), 1008–1020. 10.2337/db13-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Garrastacho M, Bajo-Santos C, Line A, Martens-Uzunova ES, de la Fuente JM, Moros M, Llorente A, 2022. Extracellular vesicles as a source of prostate cancer biomarkers in liquid biopsies: a decade of research. Br. J. Cancer 126 (3), 331–350. 10.1038/s41416-021-01610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MI, Amorim MG, Gadelha C, Milic I, Welsh JA, Freitas VM, Dias-Neto E, 2018. Technical challenges of working with extracellular vesicles. Nanoscale 10 (3), 881–906. 10.1039/c7nr08360b. [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ, 1996. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183 (3), 1161–1172. 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W, 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200 (4), 373–383. 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AD, Campbell-Sims TC, Khan M, Lang M, Huang MB, Bond VC, Powell MD, 2011. HIV Type 1 Nef is released from infected cells in CD45(+) microvesicles and is present in the plasma of HIV-infected individuals. AIDS Res. Hum. Retrovir. 27 (2), 167–178. 10.1089/aid.2009.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PD, Morelli AE, 2014. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14 (3), 195–208. 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M, Fan J, Lyon C, Wan M, Hu Y, 2018. Role of extracellular vesicles in viral and bacterial infections: pathogenesis, diagnostics, and therapeutics. Theranostics 8 (10), 2709–2721. 10.7150/thno.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romancino DP, Paterniti G, Campos Y, De Luca A, Di Felice V, d’Azzo A, Bongiovanni A, 2013. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Lett. 587 (9), 1379–1384. 10.1016/j.febslet.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong H, Fan Y, Yang M, Zhang B, Sun D, Zhao Z, Zhang J, 2018. Brain-derived microparticles activate microglia/macrophages and induce neuroinflammation. Brain Res. 1694, 104–110. 10.1016/j.brainres.2018.05.015. [DOI] [PubMed] [Google Scholar]
- Rooney IA, Atkinson JP, Krul ES, Schonfeld G, Polakoski K, Saffitz JE, Morgan BP, 1993. Physiologic relevance of the membrane attack complex inhibitory protein CD59 in human seminal plasma: CD59 is present on extracellular organelles (prostasomes), binds cell membranes, and inhibits complement-mediated lysis. J. Exp. Med. 177 (5), 1409–1420. 10.1084/jem.177.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossaint J, Kuhne K, Skupski J, Van Aken H, Looney MR, Hidalgo A, Zarbock A, 2016. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat. Commun. 7, 13464 10.1038/ncomms13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Z, Delpech JC, Venkatesan Kalavai S, Van Enoo AA, Hu J, Ikezu S, Ikezu T, 2020. P2RX7 inhibitor suppresses exosome secretion and disease phenotype in P301S tau transgenic mice. Mol. Neurodegener. 15 (1), 47. 10.1186/s13024-020-00396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Z, Pathak D, Venkatesan Kalavai S, Yoshii-Kitahara A, Muraoka S, Bhatt N, Ikezu T, 2021. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain 144 (1), 288–309. 10.1093/brain/awaa376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz-Cuesta M, Irizar H, Castillo-Trivino T, Munoz-Culla M, Osorio-Querejeta I, Prada A, Otaegui D, 2014. Circulating microparticles reflect treatment effects and clinical status in multiple sclerosis. Biomarkers Med. 8 (5), 653–661. 10.2217/bmm.14.9. [DOI] [PubMed] [Google Scholar]
- Sahu A, Clemens ZJ, Shinde SN, Sivakumar S, Pius A, Bhatia A, Ambrosio F, 2021. Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles. Nat Aging 1 (12), 1148–1161. 10.1038/s43587-021-00143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, Illanes SE, 2016. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes 65 (3), 598–609. 10.2337/db15-0966. [DOI] [PubMed] [Google Scholar]
- Sang Q, Yao Z, Wang H, Feng R, Wang H, Zhao X, Wang L, 2013. Identification of microRNAs in human follicular fluid: characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 98 (7), 3068–3079. 10.1210/jc.2013-1715. [DOI] [PubMed] [Google Scholar]
- Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjo C, Larsson M, Lannfelt L, Hallbeck M, 2018. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 136 (1), 41–56. 10.1007/s00401-018-1868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad JA, Lusardi TA, Van Keuren-Jensen KR, Phillips JI, Lind B, Harrington CA, Hochberg FH, 2017. Analysis of extracellular RNA in cerebrospinal fluid. J. Extracell. Vesicles 6 (1), 1317577. 10.1080/20013078.2017.1317577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorey JS, Harding CV, 2016. Extracellular vesicles and infectious diseases: new complexity to an old story. J. Clin. Invest. 126 (4), 1181–1189. 10.1172/JCI81132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, Williams JC, Neyses L, 2004. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem. 279 (27), 28220–28226. 10.1074/jbc.M312599200. [DOI] [PubMed] [Google Scholar]
- Shang M, Ji JS, Song C, Gao BJ, Jin JG, Kuo WP, Kang H, 2017. Extracellular vesicles: a brief overview and its role in precision medicine. Methods Mol. Biol. 1660, 1–14. 10.1007/978-1-4939-7253-1_1. [DOI] [PubMed] [Google Scholar]
- Shen J, Wolfram J, Ferrari M, Shen H, 2017. Taking the vehicle out of drug delivery. Mater. Today 20 (3), 95–97. 10.1016/j.mattod.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Wu X, Lee Y, Wolfram J, Yang Z, Mao ZW, Shen H, 2015. Porous silicon microparticles for delivery of siRNA therapeutics. JoVE 95, 52075. 10.3791/52075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Hassanali S, Nugent C, Wen L, Hamilton-Williams E, Dias P, Dai YD, 2011. Insulinoma-released exosomes or microparticles are immunostimulatory and can activate autoreactive T cells spontaneously developed in nonobese diabetic mice. J. Immunol. 187 (4), 1591–1600. 10.4049/jimmunol.1100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C, 2016. Exosome cancer diagnostic reaches market. Nat. Biotechnol. 34 (4), 359–360. 10.1038/nbt0416-359. [DOI] [PubMed] [Google Scholar]
- Siljander P, Carpen O, Lassila R, 1996. Platelet-derived microparticles associate with fibrin during thrombosis. Blood 87 (11), 4651–4663. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/8639834. [PubMed] [Google Scholar]
- Simon C, Greening DW, Bolumar D, Balaguer N, Salamonsen LA, Vilella F, 2018. Extracellular vesicles in human reproduction in health and disease. Endocr. Rev. 39 (3), 292–332. 10.1210/er.2017-00229. [DOI] [PubMed] [Google Scholar]
- Sin J, McIntyre L, Stotland A, Feuer R, Gottlieb RA, 2017. Coxsackievirus B escapes the infected cell in ejected mitophagosomes. J. Virol. 91 (24), e01347–17. 10.1128/JVI.01347-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skonieczna-Zydecka K, Marlicz W, Misera A, Koulaouzidis A, Loniewski I, 2018. Microbiome-the missing link in the gut-brain Axis: focus on its role in gastrointestinal and mental health. J. Clin. Med. 7 (12), 521. 10.3390/jcm7120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto C, Pritzkow S, 2018. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21 (10), 1332–1340. 10.1038/s41593-018-0235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CN, 1966. Lattice structures and osmiophilic bodies in the developing respiratory tissue of rats. J Ultrastruct Res 15 (3), 380–388. 10.1016/s0022-5320(66)80114-4. [DOI] [PubMed] [Google Scholar]
- Sun W, Luo JD, Jiang H, Duan DD, 2018. Tumor exosomes: a double-edged sword in cancer therapy. Acta Pharmacol. Sin. 39 (4), 534–541. 10.1038/aps.2018.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Momen-Heravi F, 2017. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 14 (8), 455–466. 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwedowicz U, Lapinska Z, Gajewska-Naryniecka A, Choromanska A, 2022. Exosomes and other extracellular vesicles with high therapeutic potential: their applications in oncology, neurology, and dermatology. Molecules 27 (4), 1303. 10.3390/molecules27041303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, 2021. Pathogenic and protective roles of extracellular vesicles in neurodegenerative diseases. J. Biochem. 169 (2), 181–186. 10.1093/jb/mvaa131. [DOI] [PubMed] [Google Scholar]
- Tans G, Rosing J, Thomassen MC, Heeb MJ, Zwaal RF, Griffin JH, 1991. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood 77 (12), 2641–2648. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/2043766. [PubMed] [Google Scholar]
- Tao SC, Guo SC, Zhang CQ, 2018. Modularized extracellular vesicles: the dawn of prospective personalized and precision medicine. Adv. Sci. 5 (2), 1700449 10.1002/advs.201700449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessandier N, Melki I, Cloutier N, Allaeys I, Miszta A, Tan S, Boilard E, 2020. Platelets disseminate extracellular vesicles in lymph in rheumatoid arthritis. Arterioscler. Thromb. Vasc. Biol. 40 (4), 929–942. 10.1161/ATVBAHA.119.313698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tey SK, Lam H, Wong SWK, Zhao H, To KK, Yam JWP, 2022. ACE2-enriched extracellular vesicles enhance infectivity of live SARS-CoV-2 virus. J. Extracell. Vesicles 11 (5), e12231. 10.1002/jev2.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankam FG, Agrawal DK, 2020. Infarct zone: a novel platform for exosome trade in cardiac tissue regeneration. J Cardiovasc Transl Res 13 (5), 686–701. 10.1007/s12265-019-09952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S, 2002. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 3 (12), 1156–1162. 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Zuba-Surma EK, 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7 (1), 1535750. 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind A, Wilson C, 2016. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J. Extracell. Vesicles 5, 31292. 10.3402/jev.v5.31292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AG, Gray E, Heman-Ackah SM, Mager I, Talbot K, Andaloussi SE, Turner MR, 2016. Extracellular vesicles in neurodegenerative disease - pathogenesis to biomarkers. Nat. Rev. Neurol. 12 (6), 346–357. 10.1038/nrneurol.2016.68. [DOI] [PubMed] [Google Scholar]
- Tian M, Ticer T, Wang Q, Walker S, Pham A, Suh A, Wolfram J, 2020. Adipose-derived biogenic nanoparticles for suppression of inflammation. Small 16 (10), e1904064. 10.1002/smll.201904064. [DOI] [PubMed] [Google Scholar]
- Timar CI, Lorincz AM, Csepanyi-Komi R, Valyi-Nagy A, Nagy G, Buzas EI, Ligeti E, 2013. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood 121 (3), 510–518. 10.1182/blood-2012-05-431114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Ochiya T, 2015. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 6, 6716. 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Chamley LW, 2015. Placental extracellular vesicles and feto-maternal communication. Cold Spring Harb Perspect Med 5 (3), a023028. 10.1101/cshperspect.a023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullal AJ, Reich CF 3rd, Clowse M, Criscione-Schreiber LG, Tochacek M, Monestier M, Pisetsky DS, 2011. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J. Autoimmun. 36 (3–4), 173–180. 10.1016/j.jaut.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Urabe F, Patil K, Ramm GA, Ochiya T, Soekmadji C, 2021. Extracellular vesicles in the development of organ-specific metastasis. J. Extracell. Vesicles 10 (9), e12125. 10.1002/jev2.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Rivoltini L, 2006. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 66 (18), 9290–9298. 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- Veerman RE, Teeuwen L, Czarnewski P, Gucluler Akpinar G, Sandberg A, Cao X, Eldh M, 2021. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J. Extracell. Vesicles 10 (9), e12128. 10.1002/jev2.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Muzio L, Turola E, Bergami A, Novellino L, Ruffini F, Furlan R, 2012. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 72 (4), 610–624. 10.1002/ana.23627. [DOI] [PubMed] [Google Scholar]
- Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, Wolfram J, 2019. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 9 (26), 8001–8017. 10.7150/thno.37097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SA, Aguilar Diaz De Leon JS, Busatto S, Wurtz GA, Zubair AC, Borges CR, Wolfram J, 2020. Glycan node analysis of plasma-derived extracellular vesicles. Cells 9 (9), 1946. 10.3390/cells9091946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Hussain A, Behfar A, Moran SL, Zhao C, 2022. The therapeutic potential of exosomes in soft tissue repair and regeneration. Int. J. Mol. Sci. 23 (7), 3869. 10.3390/ijms23073869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pham A, Kang L, Walker SA, Davidovich I, Iannotta D, Wolfram J, 2021. Effects of adipose-derived biogenic nanoparticle-associated microRNA-451a on Toll-like receptor 4-induced cytokines. Pharmaceutics 14 (1), 16. 10.3390/pharmaceutics14010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilner S, Keider V, Winter M, Harreither E, Salzer B, Weiss F, Grillari J, 2016. Vesicular Galectin-3 levels decrease with donor age and contribute to the reduced osteo-inductive potential of human plasma derived extracellular vesicles. Aging (Albany NY) 8 (1), 16–33. 10.18632/aging.100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingrill RB, Paladino SL, Souza MLR, Pereira EM, Marques ALX, Silva ECO, Borbely AU, 2021. Exosome-enriched plasma analysis as a tool for the early detection of hypertensive gestations. Front. Physiol. 12, 767112 10.3389/fphys.2021.767112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings EP, Huang TC, Li J, Peterson TE, Hooke AW, Rosenbaum A, Houdek MT, 2021. Intrinsic tendon regeneration after application of purified exosome product: an in vivo study. Orthop J Sports Med 9 (12), 23259671211062929. 10.1177/23259671211062929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton JL, Loveless S, Stone T, von Ruhland C, Robertson NP, Clayton A, 2017. Cerebrospinal fluid extracellular vesicle enrichment for protein biomarker discovery in neurological disease; multiple sclerosis. J. Extracell. Vesicles 6 (1), 1369805. 10.1080/20013078.2017.1369805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C, Seeger RC, Fabbri M, Wang L, Wayne AS, Jong AY, 2017. Biological roles and potential applications of immune cell-derived extracellular vesicles. J. Extracell. Vesicles 6 (1), 1400370. 10.1080/20013078.2017.1400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker TE, Nagelkerke A, Nele V, Kauscher U, Stevens MM, 2020. Experimental artefacts can lead to misattribution of bioactivity from soluble mesenchymal stem cell paracrine factors to extracellular vesicles. J. Extracell. Vesicles 9 (1), 1807674. 10.1080/20013078.2020.1807674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis GR, Fernandez-Gonzalez A, Reis M, Yeung V, Liu X, Ericsson M, Kourembanas S, 2020. Mesenchymal stromal cell-derived small extracellular vesicles restore lung architecture and improve exercise capacity in a model of neonatal hyperoxia-induced lung injury. J. Extracell. Vesicles 9 (1), 1790874. 10.1080/20013078.2020.1790874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer KWW, Joy, 2021. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat. Rev. Mater. 6 (2), 4. 10.1038/s41578-020-00277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P, 1967. The nature and significance of platelet products in human plasma. Br. J. Haematol. 13 (3), 269–288. 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- Wolfram J, Ferrari M, 2019. Clinical cancer nanomedicine. Nano Today 25, 85–98. 10.1016/j.nantod.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, Zhao Y, 2015. Safety of nanoparticles in medicine. Curr. Drug Targets 16 (14), 1671–1681. 10.2174/1389450115666140804124808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Song SJ, Zhang Y, Li X, 2020. Role of extracellular vesicles in autoimmune pathogenesis. Front. Immunol. 11, 579043 10.3389/fimmu.2020.579043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Zheng L, Zou X, Wang J, Zhong J, Zhong T, 2019. Extracellular vesicles in type 2 diabetes mellitus: key roles in pathogenesis, complications, and therapy. J. Extracell. Vesicles 8 (1), 1625677. 10.1080/20013078.2019.1625677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Liu Q, Wu K, Liu L, Zhao M, Yang H, Wang W, 2020. Extracellular vesicles as potential biomarkers and therapeutic approaches in autoimmune diseases. J. Transl. Med. 18 (1), 432. 10.1186/s12967-020-02609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, De Wever O, 2015. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066. 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wu Y, Meng X, Wang Z, Younis M, Liu Y, Huang X, 2022. SARS-CoV-2 membrane protein causes the mitochondrial apoptosis and pulmonary edema via targeting BOK. Cell Death Differ. 29 (7), 1395–1408. 10.1038/s41418-022-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates AG, Pink RC, Erdbrugger U, Siljander PR, Dellar ER, Pantazi P, Couch Y, 2022a. In sickness and in health: the functional role of extracellular vesicles in physiology and pathology in vivo: Part I: health and Normal Physiology: Part I: health and Normal Physiology. J. Extracell. Vesicles 11 (1), e12151. 10.1002/jev2.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates AG, Pink RC, Erdbrugger U, Siljander PR, Dellar ER, Pantazi P, Couch Y, 2022b. In sickness and in health: the functional role of extracellular vesicles in physiology and pathology in vivo: Part II: pathology: Part II: Pathology. J. Extracell. Vesicles 11 (1), e12190. 10.1002/jev2.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B, Ma Q, Song C, Zhao L, Yu F, Wang C, Ye L, 2021. Exosome-derived noncoding RNAs as a promising treatment of bone regeneration. Stem Cell. Int. 2021, 6696894 10.1155/2021/6696894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B, Ni J, Witherel CE, Yang M, Burdick JA, Wen C, Wong SHD, 2022. Harnessing tissue-derived extracellular vesicles for osteoarthritis theranostics. Theranostics 12 (1), 207–231. 10.7150/thno.62708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Chen H, Wang Y, Zhang L, Wang X, 2021. Roles of extracellular vesicles in the aging microenvironment and age-related diseases. J. Extracell. Vesicles 10 (12), e12154. 10.1002/jev2.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N, Imai SI, 2019. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metabol. 30 (2), 329–342 e325. 10.1016/j.cmet.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Ikezu T, 2019. Emerging roles of extracellular vesicles in neurodegenerative disorders. Neurobiol. Dis. 130, 104512 10.1016/j.nbd.2019.104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Muraoka S, Jedrychowski MP, Hu J, McQuade AK, Young-Pearse T, Ikezu T, 2022. Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer’s disease brain. J. Extracell. Vesicles 11 (1), e12183. 10.1002/jev2.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuana Y, Boing AN, Grootemaat AE, van der Pol E, Hau CM, Cizmar P, Nieuwland R, 2015. Handling and storage of human body fluids for analysis of extracellular vesicles. J. Extracell. Vesicles 4, 29260. 10.3402/jev.v4.29260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeri A, Whitehead BJ, Stensballe A, de Korne C, Williams AR, Everts B, Nejsum P, 2021. Parasite worm antigens instruct macrophages to release immunoregulatory extracellular vesicles. J. Extracell. Vesicles 10 (10), e12131. 10.1002/jev2.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Lyden D, 2018. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20 (3), 332–343. 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Randall G, Higginbottom A, Monk P, Rice CM, McKeating JA, 2004. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J. Virol. 78 (3), 1448–1455. 10.1128/jvi.78.3.1448-1455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Xia B, Shan S, Zheng A, Zhang S, Chen J, Liang XJ, 2021. High-quality milk exosomes as oral drug delivery system. Biomaterials 277, 121126. 10.1016/j.biomaterials.2021.121126. [DOI] [PubMed] [Google Scholar]
- Zhou X, Xie F, Wang L, Zhang L, Zhang S, Fang M, Zhou F, 2020. The function and clinical application of extracellular vesicles in innate immune regulation. Cell. Mol. Immunol. 17 (4), 323–334. 10.1038/s41423-020-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubairova LD, Nabiullina RM, Nagaswami C, Zuev YF, Mustafin IG, Litvinov RI, Weisel JW, 2015. Circulating microparticles alter formation, structure, and properties of fibrin clots. Sci. Rep. 5, 17611 10.1038/srep17611. [DOI] [PMC free article] [PubMed] [Google Scholar]


