Abstract
Background:
Low-density lipoprotein (LDL) cholesterol is an important causal risk factor for atherosclerotic cardiovascular disease (ASCVD). However, a sizable proportion of middle-age individuals have not developed coronary atherosclerosis as assessed by coronary artery calcification (CAC). Whether presence of CAC modifies the association of LDL-C with ASCVD risk is unknown. We therefore evaluated the association of LDL-C with future ASCVD events in patients with and without CAC.
Methods:
The study included 23,132 consecutive symptomatic patients evaluated for coronary artery disease using coronary computed tomography angiography (CTA) from the Western Denmark Heart Registry - a semi-national, multi-center based registry with longitudinal registration of patient and procedure data. We assessed the association of LDL-C obtained before CTA with ASCVD (MI and ischemic stroke) events occurring during follow-up stratified by CAC>0 versus CAC=0 using Cox-regression models adjusted for baseline characteristics. Outcomes were identified through linkage among national registries covering all hospitals in Denmark. We replicated our results in the NHLBI-funded Multi-Ethnic Study of Atherosclerosis study.
Results:
During a median follow-up of 4.3 years, 552 patients experienced a first ASCVD event. In the overall population, LDL-C (per 38.7mg/dL increase) was associated with ASCVD events occurring during follow-up (adjusted hazard ratio (aHR) 1.14 (95% CI 1.04–1.24). When stratified by the presence or absence of baseline CAC, LDL-C was only associated with ASCVD in the 10,792/23,132 (47%) patients with CAC>0 (aHR 1.18 (95% CI 1.06–1.31)) while no association was observed among the 12,340/23,132 (53%) patients with CAC=0 (aHR=1.02 (95% CI 0.87–1.18)). Similarly, very high LDL-C (≥193 mg/dL) versus LDL-C <116 mg/dL was associated with ASCVD in patients with CAC>0 (aHR 2.42 (95% CI 1.59–3.67)) but not in those without CAC (aHR 0.92 (0.48–1.79)). In patients with CAC=0, diabetes, current smoking and low HDL-cholesterol levels were associated with future ASCVD events in patients. The principal findings were replicated in the Multi-Ethnic Study of Atherosclerosis.
Conclusion
LDL-C appears almost exclusively associated with ASCVD events over ≈5-years of follow up in middle-aged patients with versus without evidence of coronary atherosclerosis. This information is valuable for individualized risk assessment among middle-aged patients with or without coronary atherosclerosis.
Keywords: computed tomography angiography, coronary artery calcium, coronary artery disease, cardiovascular disease risk, low-density lipoprotein, epidemiology, cohort study
Introduction
An abundance of evidence has demonstrated the causal role of low-density lipoprotein cholesterol (LDL-C) in the development and progression of atherosclerotic cardiovascular disease (ASCVD) (1–4), and measuring LDL-C therefore plays a central role in risk assessment in all international guidelines (5, 6).
Despite the unequivocally causal role of LDL-cholesterol in development of atherosclerosis, a significant proportion of patients with elevated LDL-cholesterol have neither evidence of atherosclerosis (ie. no coronary artery calcification (CAC)) at middle-age(7–10) nor develop ASCVD at long-term follow-up into old age (11–15). This indicates that some patients may display resilience to the detrimental effects of LDL-C.
In the present study, we used data from the Western Denmark Heart Registry (WDHR) to evaluate the association of LDL-C with MI and ASCVD events according to presence versus absence of coronary atherosclerosis defined by presence of CAC among a cohort of symptomatic patients undergoing coronary computed tomography angiography (CTA). We validated our findings in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
Study cohort
The Danish National Health Service provides universal tax-supported health care, as well as partial reimbursement for costs of prescribed medications. Data for this study were provided by the WDHR, a semi-national, multicenter registry(16, 17). This data source has been audited and validated(18). Covariates (i.e hypertension and diabetes) are entered into the registry by the treating clinicians at the time of a procedure (CTA) (18). In Western Denmark, CTA is the first-line diagnostic test for non-emergency patients with symptoms suggesting CAD (19). Our study included all patients >18 years of age who underwent CTA between 1 January 2008 and 31 December 2017 in Western Denmark for the indication of symptoms suggestive of CAD. Exclusion criteria were known CAD [prior myocardial infarction (MI) or coronary revascularization] or a missing LDL cholesterol or CTA measurement. A Civil Personal Registration (CPR) number is assigned to each Danish citizen at birth, allowing accurate linkage of information among the medical and administrative registries used in this study (20).
The study was approved by the Danish Data Protection Agency (record numbers: 2008-58-0035 and 2012-41-0914) with a waiver for individual informed consent by the regional ethics committee.
Data availability
Because data stem from national registries containing data at an individual level, the authors are not permitted to share or grant access to data.
Angiography acquisition and coronary atherosclerosis definition
Different CT scanner platforms were used to perform CTA, including Siemens Healthineers (Erlangen, Germany), Toshiba (Otawara, Tochigi, Japan), and Philips Healthcare (Best, the Netherlands), using ≥64-slice detector rows. All tests were completed according to the Society of Cardiovascular Computed Tomography guidelines for CTA image acquisition (21). Before CTA, all patients underwent non-contrast enhanced ECG-gated CAC scan. We defined the presence of coronary atherosclerosis as CAC>0 and absence of coronary atherosclerosis as CAC=0. In sensitivity analyses, we defined absence of coronary atherosclerosis as CAC=0 combined with no non-calcified plaque and presence of coronary atherosclerosis as any detectable atherosclerosis on CTA (CAC>0 and/or presence of non-calcified plaque).
Comorbidity assessment and ASCVD events
Based on diagnosis codes, a Charlson Comorbidity Index (CCI) score was computed for each patient (Table S1) and comorbidity was categorized as low (CCI score = 0), moderate (CCI score = 1) or severe (CCI score ≥2) as previously done (16)
The two outcomes used for this study were (a) myocardial infarction (MI) and (b) a composite ASCVD outcome consisting of fatal and non-fatal MI or ischemic stroke. The study outcomes were identified through linkage among national registries covering all Danish hospitals (22). Data on prescribed medications were retrieved from the Danish National Health Service Prescription Database (23), with baseline medication use defined as redeemed prescriptions during the 6 months prior to the CTA.
Statistical Analyses
Study population characteristics are presented as means and standard deviations for continuous variables, while percentages are used for categorical variables.
The association between LDL-C and the presence of CAC was assessed using logistic regression adjusted for age, sex, diabetes, hypertension, CCI, smoking and pre-CTA statin use.
The association between LDL-C and myocardial infarction and ASCVD was examined using Cox proportional hazards regression with 95% confidence intervals (CI) and age as the underlying time scale with delayed entry (left truncation). In all analyses, the association was adjusted for age, sex, smoking status, diabetes, hypertension, level of comorbidity based on CCI score (0 = low, 1= moderate and ≥2 =severe) and statin use. The association between LDL-C and MI and ASCVD was examined on a continuous scale using restricted cubic splines incorporated in Cox proportional hazards models. To balance best fit and overfitting in the main splines for MI and ASCVD, the number of knots was chosen as the lowest value for the Akaike information criterion.
The association between LDL-C and MI was further assessed on a continuous scale per 38.7 mg/dL higher LDL-C in the overall population and stratified by presence versus absence of CAC. This was done because LDL-C is included in most risk prediction models on a continuous scale. The proportional hazard assumption was assessed using Schoenfeld residuals and was met for the models.
Further, given that very high LDL-C ≥193 mg/dL is considered very high risk with an accompanying class Ia recommendation for statin use in both European and American guidelines irrespectively of other known risk factors or clinical disease, we assessed the association of LDL-C ≥193 mg/dL using the generally recommended LDL-C <116 mg/dL as a reference. These analyses were performed in the overall population and stratified by presence versus absence of CAC.
Incidence event rates for MI and ASCVD were assessed in the overall population and stratified by LDL-C levels and by the presence versus absence of CAC.
The association of other traditional risk factors including age, sex diabetes, smoking status and HDL-cholesterol was also assessed stratified by presence versus absence of CAC.
Validation in MESA
Finally, the association of LDL-C with ASCVD events ((myocardial infarction, coronary death, resuscitated cardiac arrest, revascularization, and stroke) was evaluated in MESA. MESA is an NHLBI-funded prospective cohort that enrolled participants free of known ASCVD that included baseline CAC scans and long-term follow-up for clinical ASCVD events. Exact definition of endpoints in MESA can be found at https://www.mesa-nhlbi.org/. In brief, trained personnel abstracted all relevant hospital records, and two paired physicians provided independent endpoint adjudication. Analyses were approached identically to those in the WDHR, and Cox proportional hazards models were adjusted for age, sex, MESA site, ethnicity, smoking status, diabetes, hypertension, HDL-cholesterol and lipid-lowering therapy.
All statistical analyses were performed using STATA 16 (Stata Corp., College Station, Texas). Statistical significance was defined as a p-value <0.05 on a 2-tailed test.
Results
Baseline characteristics of the 23,132 patients included in this study is shown in Table 1. Median age was 57 years (IQR 50–62 years) and 55% were women. Age was lower in patients without CAC compared to patients with CAC>0 (53 versus 62 years). Median LDL-C was 3.1 (IQR 2.4–3.7) and the median time from LDL-C measurements to time of CTA was 24 days (IQR 7–74 days), and in 95% of patients it was measured <365 days before the CTA. Overall, 12,340 (53%) had CAC=0 and 10,792 (47%) had CAC>0.
Table 1.
Baseline characteristics of patients from the Western Denmark Heart Registry.
| Characteristics | All | CAC >0 | CAC=0 |
|---|---|---|---|
|
| |||
| Participants, n | 23,132 | 10,792 | 12,340 |
| Age, median (IQR) | 57 (50–65) | 62 (55–68) | 53 (46–61) |
| Current smoking, % | 21 | 22 | 20 |
| Male (%) | 45 | 53 | 37 |
| Plasma parameters, median | |||
| Total cholesterol (mmol/L) | 5.2 (4.5–5.9) | 5.2 (4.5–6.0) | 5.2 (4.5–5.9) |
| (mg/dL) | 201 (174–228) | 201 (174–232) | 201 (174–228) |
| LDL cholesterol (mmol/L) | 3.1 (2.4–3.7) | 3.1 (2.4–3.8) | 3.1 (2.5–3.7) |
| (mg/dL) | 120 (93–143) | 120 (93–147) | 120 (266–143) |
| HDL cholesterol (mmol/L) | 1.4 (1.2–1.8) | 1.4 (1.2–1.8) | 1.4 (1.2–1.8) |
| (mg/dL) | 54 (46–70) | 54 (46–70) | 54 (46–70) |
| Triglycerides. (mmol/L) | 1.2 (0.9–1.8) | 1.2 (0.9–1.8) | 1.2 (0.9–1.8) |
| (mg/dL) | 106 (71–160) | 106 (71–160) | 106 (71–1160) |
| Systolic blood pressure (mmHg) | 132 (113–148) | 137 (119–151) | 130 (110–144) |
| Hypertension (%) | 46 | 56 | 38 |
| Diabetes Mellitus (%) | 8 | 11 | 6 |
| Body Mass Index, median (IQR) | 26.2 (23.7–29.4) | 26.3 (23.8–29–4) | 26.1 (23.4–29.4) |
| Baseline statin use (%)* | 22 | 30 | 15 |
| Agatston calcium score (median, IQR) | 0 (0–57) | 69 (18–211) | 0 (0–0) |
Data are n (%), median (IQR), or n.
Defined as more than ≥2 statin prescriptions before CTA
Over a median follow-up of 4.3 (IQR 2.4 to 6.1) years of follow-up, 275 (1.2%) patients experienced a first MI and 552 (2.4%) experienced a first ASCVD event (271 MIs and 281 strokes). The number of MI and ASCVD events were 35 and 94 in patients with CAC=0 compared to 96 and 192 event in those with CAC>0.
Association of LDL-C with presence of CAC
LDL-C prior to CTA was associated with the presence of CAD defined as CAC>0 (Table S2). Per 38.7 mg/dL higher LDL-C, the OR for CAC>0 versus CAC=0 was 1.17 (95% CI: 1.13–1.20) adjusted for age, sex, diabetes, hypertension, CCI, smoking and pre-CTA statin use. Very high LDL-C ≥193 mg/dL versus <116 mg/dL increased the odds of having CAC>0 versus CAC=0 to 1.80 (95% CI: 1.55–2.10). For comparison, the OR for having CAC>0 versus CAC=0 was 1.90 (95%CI 1.71–2.20) for diabetes, 1.69 (95%CI 1.52–1.87) for current smoking, and 1.49 (95%CI 1.34–1.63) for hypertension.
Association of LDL-C with MI and ASCVD events in the overall population
The association of LDL-C on a continuous scale with MI and ASCVD events occurring after CTA are shown Figure 1 (upper panels). For each 38.7 mg/dL higher LDL-C, the multivariable adjusted hazard ratio (HR) for MI and ASCVD events was 1.28 (95% CI: 1.13–1.44) and 1.14 (95% CI 1.04–1.24), respectively (Figure 2). For very high LDL-C ≥193 mg/dL versus <116 mg/dL, the multivariable adjusted HR was 2.34 (95% CI 1.51–3.62) for MI events and 1.54 (95% CI: 1.10–2.15) for ASCVD events (Table 2).
Figure 1. Multivariable-adjusted hazard ratios for myocardial infarction and atherosclerotic cardiovascular disease according to low-density lipoprotein cholesterol level.
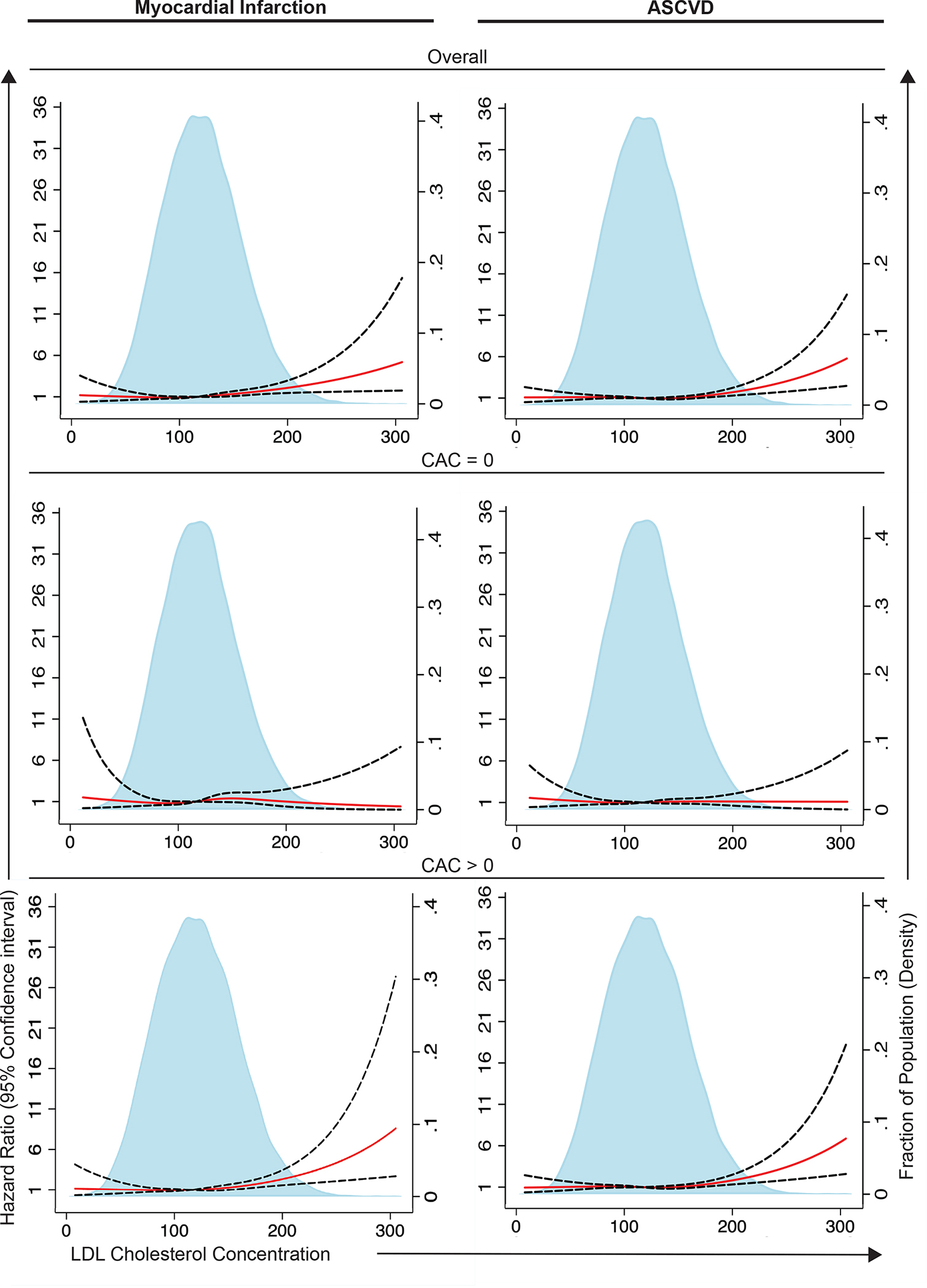
Analyses are shown in the overall population and stratified by coronary artery calcium (CAC) groups in the Western Denmark Heart Registry. Solid red lines are multivariable-adjusted hazard ratios, and dashed lines show 95% confidence intervals on the basis of restricted cubic spline regressions. Analyses were adjusted for age, sex, smoking status, diabetes, Charlson Comorbidity Index score, hypertension, and statin use at baseline. ASCVD indicates atherosclerotic cardiovascular disease.
Figure 2. Association of 38.7 mg/dL higher low-density lipoprotein cholesterol level with development of myocardial infarction and atherosclerotic cardiovascular disease.

Analyses are shown in overall population and stratified by coronary artery calcium (CAC) groups in the Western Denmark Heart Registry. Analyses were adjusted for age, sex, smoking status, diabetes, hypertension, Charlson Comorbidity Index score, and statin use. ASCVD indicates atherosclerotic cardiovascular disease; HR, hazard ratio; and LDL, low-density lipoprotein.
Table 2.
Association of LDL cholesterol ≥193 mg/dL vs <116 mg/dL with development of myocardial infarction and ASCVD, stratified by CAC groups in the Western Denmark Heart Registry
| Myocardial infarction |
ASCVD |
||||
|---|---|---|---|---|---|
| Atherosclerosis measurement | N (%) | N (%) | Adjusted* Hazard ratio | N (%) | Adjusted* Hazard ratio |
|
|
|
||||
| Overall | 11,805 | 131 | 3.12 (1.96–4.98) | 286 | 2.10 (1.45–3.03) |
| CAC = 0 | 6,366 | 35 (0.5) | 0.95 (0.33–2.73) | 94 | 0.92 (0.48–1.79) |
| CAC > 0 | 5,439 | 96 (1.8) | 3.53 (2.11–5.92) | 192 | 2.42 (1.59–3.67) |
Adjusted for age, sex, smoking status, diabetes, hypertension, level of comorbidity based on Charlson Comorbidity Index score (0 = low, 1= moderate and ≥2severe), post-CTA statin use, HDL-cholesterol and LDL-C. ASCVD=atherosclerotic cardiovascular disease, CAC=coronary artery calcium, LDL=low-density lipoprotein.
Association of LDL-C with MI and ASCVD events stratified by CAC
The association of LDL-C on a continuous scale with MI and ASCVD events in patients with CAC>0 and CAC=0, respectively, are shown Figure 1 (middle and lower panels). Notably, there was a significant interaction effect of CAC and LDL-C on MI and ASCVD events (p interaction <0.05 in fully adjusted model). The higher the LDL-C, the higher the risk for MI and ASCVD in patients with CAC>0 but not in those with CAC=0. In the 10,792 patients with CAC>0, the multivariable adjusted HR per 38.7 mm/L higher LDL-C was 1.30 (95% CI 1.14–1.49) for MI events and 1.18 (95% CI 1.06–1.31) for ASCVD events (Figure 2). In the 12,340 patients with CAC=0, the multivariable adjusted HR per (38.7 mg/dL higher LDL-C was 1.13 (95% CI: 0.90–1.40) for MI events and 1.02 (95% CI 0.87–1.18) for ASCVD events.
The multivariable adjusted HR for MI in patients with very high LDL-C ≥ 193 mg/dL versus <116 mg/dL was 3.53 (2.11–5.92) among patients with CAC>0 compared to 0.95 (0.33–2.73) among patients with CAC=0. For ASCVD events the corresponding HRs were 2.42 (1.59–3.07) and 0.92 (0.48–1.79), respectively.
Incidence of ASCVD stratified by CAC and LDL-C
For MI events, the overall incidence per 1000 person-years was 2.77 (95% CI: 2.46–3.11) compared to 4.40 (95% CI 3.79–5.01) in patients with CAC>0 and 1.46 (95% CI 1.18–1.83) in patients with CAC=0 (Table S3). The incidence event-rate in patients with LDL-C ≥193 mg/dL versus <116 mg/dL was 12.36 (95% CI: 8.06–19.00) and 3.69 (95% CI 2.93–4.64), respectively, for those with CAC>0 compared with 1.30 (95%CI 0.31–5.12) and 1.27 (95% CI 0.90–1.80) for those with CAC=0 (Figure S1). For each 38.7 mg/dL higher LDL-C, MI incidence was 1.30 per 1000 person-year higher for those with CAC>0 compared with 0.19 for those with CAC=0 (Figure 3).
Figure 3. Higher myocardial infarction and atherosclerotic cardiovascular disease incidence per 38.7 mg/dL higher low-density lipoprotein cholesterol level in the population.

Analyses are shown in the overall population and stratified by coronary artery calcium (CAC) groups in the Western Denmark Heart Registry. Analyses are on the basis of the linear association of low-density lipoprotein (LDL) cholesterol level with risk of myocardial infarction and atherosclerotic cardiovascular disease (ASCVD) in the different CAC groups.
For ASCVD events, the overall incidence per 1000 person-years was 5.60 (95% CI: 5.16–6.10) compared with 8.23 (95%CI 7.43–9.12) in patients with CAC>0 and 3.45 (95% CI 2.99–3.98) in patients with CAC=0. The incidence event-rate in patients with LDL-C ≥193 mg/dL versus <116 mg/dL was 15.40 (95% CI: 10.48–22.60) and 8.10 (95% CI 7.00–9.54), respectively, for those with CAC>0 compared to 3.92 (95%CI 1.77–8.76) and 3.44 (95% CI 2.78–4.27) for those with CAC=0. Per 38 mg/dL higher LDL-C, ASCVD event incidence per 1000 person-years was 1.41 higher for those with CAC>0 compared to 0.07 for those with CAC=0 (Figure 3).
Association of traditional risk factors with MI and ASCVD events stratified by CAC
Table 3 shows the association of other traditional risk factors with MI and ASCVD events. All traditional risk factors were associated with events in the overall population and in patients with CAC>0. In those with CAC=0, age, diabetes, current smoking and HDL-cholesterol was significantly associated with ASCVD events.
Table 3.
Association of traditional risk factors with development of myocardial infarction and ASCVD, stratified by CAC groups in the Western Denmark Heart Registry
|
*Adjusted hazard ratio |
|||
|---|---|---|---|
| Risk factor | Overall | CAC>0 | CAC==0 |
|
|
|||
| ASCVD events | |||
| Age, per 1 year | 1.05 (1.04–1.6) | 1.05 (1.04–1.06) | 1.04 (1.03–1.06) |
| Sex | 1.35 (1.14–1.62) | 1.31 (1.05–1.63) | 1.15 (0.84–1.58) |
| Diabetes | 1.38 (1.06–1.81) | 1.32 (1.07–1.82) | 1.52 (1.01–2.49) |
| Hypertension | 1.23 (1.03–1.48) | 1.26 (1.00–1.60) | 1.10 (0.82–1.49) |
| Current smoking | 1.71 (1.41–2.08) | 1.54 (1.21–1.96) | 1.89 (1.36–2.62) |
| HDL-cholesterol, per 38.7 mg/dL | 0.69 (0.56–0.85) | 0.72 (0.56–0.95) | 0.64 (0.44–0.93) |
| MI events | |||
| Age, per 1 year | 1.05 (1.03–1.06) | 1.04 (1.02–1.06) | 1.01 (0.99–1.04) |
| Sex | 1.34 (1.04–1.73) | 1.37 (1.01–1.87) | 0.81 (0.49–1.33) |
| Diabetes | 1.35 (1.00–1.97) | 1.34 (0.95–2.08) | 1.25 (0.60–2.72) |
| Hypertension | 1.38 (1.07–1.79) | 1.34 (1.02–1.85) | 1.28 (0.81–2.03) |
| Current smoking | 1.96 (1.50–2.55) | 1.84 (1.34–2.53) | 1.90 (1.17–3.09) |
| HDL-cholesterol, per 38.7 mg/dL |
0.60 (0.43–0.82) | 0.68 (0.47–0.98) | 0.45 (0.24–2.30) |
Adjusted for age, sex, smoking status, diabetes, hypertension, level of comorbidity based on Charlson Comorbidity Index score (0 = low, 1= moderate and ≥2severe), post-CTA statin use, HDL-cholesterol and LDL-C.
ASCVD: Atherosclerotic cardiovascular disease. MI: Myocardial infaction. CAC: Coronary artery calcification.
Sensitivity analyses
In sensitivity analyses, we defined absence of coronary atherosclerosis as no detectable atherosclerosis on CTA (CAC=0 including no non-calcified plaque). In total, 10,707 patients had no CTA-defined atherosclerosis while 12,425 patients had detectable atherosclerosis (CAC>0 or presence of non-calcified plaque). The results of these analyses were similar to the main findings with respect to both MI and ASCVD (Table S4 and Figure S2). For example, the association of 38.7 mg/dL higher LDL-C with ASCVD was 1.19 (95% 1.07–1.32) for those with detectable atherosclerosis versus 1.03 (95% CI 0.89–1.17) for patients with no CTA-defined atherosclerosis. The multivariable adjusted HR for very high LDL-C ≥193 mg/dL versus <116 mg/dL was 2.16 (95% CI 1.43–3.27) among patients with presence of CTA-defined coronary atherosclerosis compared to 0.99 (0.54–1.83) among patients with no CTA-defined atherosclerosis.
Finally, excluding patients taking statins at baseline did not change the overall results. For example, the association of 38.7 mg/dL higher LDL-C with ASCVD was 0.90 (95% CI 0.70–1.10) for patients with CAC=0 versus 1.18 (95% 1.01–1.36) for patients with CAC>0. The multivariable adjusted HR for very high LDL-C ≥193 mg/dL versus <116 mg/dL was 2.49 (95% CI 1.37–4.52) among patients with presence of CAC>0 compared to 0.44 (0.05–2.39) among patients with CAC=0.
Validation in MESA
Median age of participants in MESA was 62 years, median LDL-C was 116 mg/dL, and 50% had CAC at baseline. During a median follow-up time of 16.6 years, 1,007 ASCVD events occurred in MESA. In the 3,340 individuals with CAC>0, the multivariable adjusted HR per 38.7 mg/dL higher LDL-C was 1.15 (p=0.004) compared to 0.91 (p=0.26) for the 3,361 individuals with CAC=0 (pinteraction =0.02 in fully adjusted model).
Discussion
Using a large cohort of 23,132 middle-aged symptomatic patients undergoing CTA, we provide important insights into the association of LDL-C with near-term MI and ASCVD events. We observed that LDL-C was strongly associated with both MI and ASCVD events in the overall population. However, there was a significant interaction whereby the presence of coronary atherosclerosis modified the association of LDL-C with future events. Thus, when stratified by presence versus absence of CAC, LDL-C was nearly exclusively associated with MI and ASCVD events in patients with CAC>0 while the effect sizes were much smaller in those with CAC=0. These results were consistent in analyses that 1) assessed the association of LDL-C on a continuous scale, 2) assessed the association per 38.7 mg/dL higher LDL-C, 3) assessed the association of very high LDL-C ≥193 mg/dL versus the generally recommended LDL-C levels <116 mg/dL and 4) checked for external validity in asymptomatic MESA participants with 16 years of follow-up. Taken together, this provides valuable information on the relative importance of LDL-C as a risk factor for future MI and ASCVD in the context of coronary atherosclerosis. Our results challenge the general assumption that LDL-C provide consistent and universal increases in risk throughout different patient populations. Instead, other risk factors such as current smoking, diabetes, and low HDL cholesterol seem to be more important for near-term risk in middle-age patients without coronary atherosclerosis. Our results add to other studies that have revealed that there is marked heterogeneity in risk, as well as the underlying pathophysiology, of events that occur in patients with versus without coronary atherosclerosis. This has important implications for risk assessment as well as the use of preventive therapies.
LDL-C and coronary atherosclerosis
Experimental animal models have clearly demonstrated that LDL-C plays a central pathophysiological role in the development of atherosclerosis(24). This is further supported by human evidence, including the high risk for premature ASCVD in patients with familial hypercholesterolemia(25). In our study, we found that LDL-C values obtained before CTA were strongly associated with the presence of CAC. For example, LDL-C ≥193 mg/dL versus <116 mg/dL was associated with a multivariable adjusted 80% higher risk of having CAC>0. This is in line with data from primary prevention cohorts that have also demonstrated an association of LDL-C and related lipid parameters with prevalent CAC in asymptomatic individuals (26).
LDL-C and risk for MI and ASCVD in patients undergoing CTA
We further found a strong association of LDL-C with the risk for MI and ASCVD. Per 38.7 mg/dL higher LDL-C levels, the risk for MI and ASCVD events increased by 28% and 14%, respectively, in our cohort of primarily middle-aged patients. Importantly, these estimates are similar to the associations observed in the general, primary prevention population (4). For example, in a recent analysis among 91,131 asymptomatic individuals without known ASCVD from the Copenhagen General Population Study, we found that 38.7 mg/dL higher LDL-C was associated with ≈25–28% higher risk for MI and 11–17% higher risk for ASCVD in patients >50 years of age during a median follow-up of 7.7 years(27). Likewise, the multivariable adjusted HR in the Copenhagen General Population study for LDL-C ≥193 mg/dL versus <116 mg/dL was ≈1.82 to 2.99 for MI (3.12 in the current WDHR study) and ≈1.25 to 1.90 for ASCVD (2.10 in the current WDHR study) in patients >50 years of age(27).
Modification by presence versus absence of coronary artery disease
Although the overall association of LDL-C with future events in the WDHR population is similar to that observed in general population cohorts, our study found that the presence versus absence of coronary atherosclerosis modified the association of LDL-C with future events. For example, 38.7 mg/dL higher LDL-C was not significantly associated ASCVD risk in those with CAC=0, while it was associated with an 18% increased risk in those with CAC>0. To further support that the presence of coronary atherosclerosis modifies the LDL-C associated risk for events, very high LDL-C ≥193 mg/dL versus <116 mg/dL was associated with a substantial increased risk of MI and ASCVD in those with CAC>0 while no association was found for those with CAC=0. Instead, other risk factors seem to be more important for determining future risk in those without coronary atherosclerosis such as current smoking, diabetes and low HDL-C that were all independently associated with events in those with and without coronary atherosclerosis. This is in line with prior results from the Multi-Ethnic Study of Atherosclerosis where LDL-C was not associated with future events among asymptomatic individuals with CAC=0 followed for up to 16-years (28) (15). We confirm these results in the present study and extend them by showing that LDL-C is associated with ASCVD events in MESA individuals with CAC>0. Taken together, these results indicate that the strong association of LDL-C with MI and ASCVD events is largely mediated through its impact on atherogenesis. If coronary atherosclerosis is not present in middle-aged patients, then LDL-C is not a strong risk factor for near-term events. It is plausible that patients who have elevated LDL-C and no evidence of plaque could have some “protective factors” that render them to be more resilient. Indeed, given that atherosclerosis is a multifactorial disease with numerous known risk factors as well as genetic determinants(24, 29), not all patients will develop atherosclerosis despite elevated LDL-C levels. Interestingly, heterogeneity in the association of LDL-C with coronary artery disease has also been found in the UK biobank across the polygenetic background with no association between LDL-C and coronary heart disease in those with low polygenic cardiovascular risk (30). In addition, >40% of patients with genetically verified familial hypercholesterolemia have not developed detectable coronary atherosclerosis at middle-age (8). Likewise, many middle-aged individuals with decades long history of hypercholesterolemia do not develop ASCVD over long-term follow-up into old age. In an analysis of 55 years old individuals from the Framingham Offspring Cohort, for example, Navar-Boggan et al. showed cumulative exposure to hypercholesterolemia in young adulthood increased the subsequent 15-year risk for coronary heart disease in a dose-dependent manner (11). However, even among those with 11–20 years of exposure to hypercholesterolemia who were at highest risk, only ≈15% developed coronary heart disease when followed to age 70. Thus, although LDL-C is central to the pathophysiology of atherosclerosis and increases risk for ASCVD, not all patients will develop clinical disease when followed into old age.
Potential clinical implications
Numerous studies have shown that the risk for MI and ASCVD events is low in patients with absence of coronary atherosclerosis (ie. CAC=0) despite having multiple risk factors or advanced age, and it has therefore been suggested that absence of CAC could be used in shared-decision making to not intensify preventive lipid-lowering treatment as the absolute benefit will generally be low(31–33). Our results provide additional support for this concept by highlighting that in the absence of coronary plaque, LDL-C is only weakly associated with events while current smoking, diabetes and low HDL-cholesterol is strongly associated with events. This indicates that the pathophysiology behind the events that occur in patients with and without evidence of coronary atherosclerosis is likely heterogenous, with non-atherosclerotic processes such as emboli, endothelial erosions, type-2 infarctions, or dissections likely representing a greater proportion in those without CAC. The optimal strategy for preventing future events may therefore differ between middle-aged patients with versus without CAC.
Our findings are in line with a recent report by Mitchell et. all who among 13,644 patients with a mean age of 50 years from the Walter Reed Army Medical Center showed that the presence of CAC identified patients most likely to derive benefit from statin therapy (34). In that analysis, statin therapy was not associated with lower risk of ASCVD in patients with CAC=0, while statin therapy was associated with a ≈25 lower risk of ASCVD during follow-up in those with CAC>0. Together, the results from Mitchell et al. combined with the results from the present study, indicate the middle-aged patients without evidence of coronary atherosclerosis derives little absolute as well as potentially less relative benefit from lipid-lowering therapies and support the 2018 ACC/AHA cholesterol guidelines statement that “It is reasonable to use CAC score in the decision to withhold, postpone or initiate statin therapy” (Class IIA recommendation) (6).
Our analyses were performed among middle-aged individuals and should not be extended to young individuals. Further, the results should not be extended to long-term follow-up and should not be used to withhold well-indicated statins from high-risk patients without CAC, including those with familial hypercholesterolemia. In younger patients, coronary plaque is often non-calcified and calcifies over time. Thus, although the risk for absolute event is low over 10–15 years in such younger patients with CAC=0 (35), extensive coronary plaque can develop over the next decades if risk factors, including high LDL-C, are left uncontrolled. Further, a previous study has shown that statin-treated individuals with CAC>0 have higher risk than individuals with CAC=0, indicating that baseline risk cannot be restored once disease is present which can be used as an argument against delaying preventive treatment until a positive CAC is recorded (36). As such, the decision to initiate or withhold statin therapy should also be guided by shared decision making, considering all other risk factors and the fact that the risks of therapy are so low and the potential benefits so large, that waiting until detectable disease is present, as evidenced by CAC>0, may not be the right choice for the individual patient.
Limitations
Some limitations should be considered. First, our study was observational. Although we adjusted our Cox analyses for baseline characteristics, LDL-C could potentially still play a causal role in the events despite not being associated with them. However, this does not change the fact that LDL-C is not a strong risk factor for near-term events in those without coronary atherosclerotic disease. Second, although we adjusted our analyses for post-CTA statin use, this is methodologically challenging as the benefit is related to a number of factors, including to the duration of therapy and the extent to which LDL-C was lowered. However, given that many more patients with CAC>0 were treated with statin after CTA than patients with CAC=0, this would most likely bias our results towards the null. Third, we did not have access to other lipid biomarkers (ie. Apolipoprotein(B) or LDL-particle size) or metabolic factors (i.e HbA1c or c-reactive protein) that could potentially be associated with events in those with CAC=0. Fourth, follow-up time in the WDHR was limited to a median of 4.3 years. LDL-C may be associated with events occurring after longer follow-up time. However, among MESA individuals with 16 years of follow-up, we found similar results. Fifth, no association between LDL-C and events, such as in the CAC=0 group, could mean no relation exists or that the study is underpowered to demonstrate one, that is, not enough events over the period of time encompassed by the study. Sixth, we defined plaque based on CTA which may not be able to detect the earliest stages of atherosclerosis thereby misclassifying patients who have started to develop atherosclerosis. Indeed, some middle-aged patients had purely non-calcified plaque. This is a very interesting subgroup that needs to be studied in detail in the future. Unfortunately, only 1633 patients had CAC=0 but evidence of atherosclerosis on CTA. The number of events in this subgroup was too little to provide any reliable estimates.
A major strength of this study is that the WDHR data stemmed from a large all-comer real-world practice cohort of middle-aged patients. We were able to adjust for major risk factors, comorbidity, and use of statin medication. Further, no patients were lost to follow-up.
Conclusion
In this large cohort of more than 23,000 middle-aged symptomatic patients undergoing CTA, LDL-C levels were strongly associated with ≈5-year MI and ASCVD events in those with evidence of coronary atherosclerosis (i.e.CAC>0) while no significant association was found among those without CAC. These results were similar among MESA individuals with 16-years of follow-up. Thus, both the relative and absolute significance of LDL-C as a risk factor for future events in middle-aged patients may be dependent on whether they have developed coronary atherosclerosis or not which have important implications for future risk assessment.
Supplementary Material
Clinical perspective.
What is new?
The risk for atherosclerotic cardiovascular disease events in middle-aged patients associated with LDL-C is modulated by whether or not they have developed coronary atherosclerosis as assessed by computed tomography
LDL-C is associated with near-term atherosclerotic cardiovascular disease events in middle-aged patients with presence of coronary atherosclerosis while no association was found for those without coronary atherosclerosis
In middle-aged patients without coronary atherosclerosis as assessed by computed tomography, other risk factors such as current smoking, diabetes and low HDL-cholesterol remain associated with near-term atherosclerotic cardiovascular disease events
What are the clinical implications?
This information is valuable for individualized risk assessment and risk-based preventive treatment plans among middle-aged patients with or without coronary atherosclerosis
In middle-aged patients without coronary atherosclerosis, current smoking, diabetes and low HDL-cholesterol signals increased risk for near- and long term atherosclerotic cardiovascular disease and may warrant intensified prevention
Approval:
The study was approved by the Danish Data Protection Agency (record numbers: 2008-58-0035 and 2012-41-0914) and the regional ethics committee
Funding:
This study was funded by Aarhus University Hospital.
Abbreviations:
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CTA
computed tomography angiography
- LDL
low-density lipoprotein
- MI
myocardial infarction
- WDHR
Western Denmark Heart Registry
Footnotes
Conflicts of interests:
The authors report no conflicts of interest in this work. Martin Bødtker Mortensen recieved lecture fees from Sanofi, Amgen, Amarin and AstraZeneca. Michael Blaha reports grants from the National Institutes of Health, US Food and Drug Administration, American Heart Association, and Aetna Foundation; grants and personal fees from Amgen; and personal fees from Sanofi, Regeneron, Novartis, Bayer, and Novo Nordisk outside the submitted work. Dr Nasir reported serving on the advisory board of Amgen Inc, Esperion Therapeutics Inc, Novo Nordisk A/S, and Novartis International AG outside the submitted work and receiving research support from Katz Academy of Translational Research, Novartis International AG, and Esperion Therapeutics Inc. The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Disclosures: Martin Bødtker Mortensen received lecture fees from Sanofi, Amgen, Amarin and AstraZeneca. Michael Blaha reports grants from the National Institutes of Health, US Food and Drug Administration, American Heart Association, and Aetna Foundation; grants and personal fees from Amgen; and personal fees from Sanofi, Regeneron, Novartis, Bayer, and Novo Nordisk outside the submitted work. Dr Nasir reported serving on the advisory board of Amgen Inc, Esperion Therapeutics Inc, Novo Nordisk A/S, and Novartis International AG outside the submitted work and receiving research support from Katz Academy of Translational Research, Novartis International AG, and Esperion Therapeutics Inc. The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Borén J, Chapman MJ, Krauss RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2020;41:2313–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannel WB DAWBER TR, KAGAN A, REVOTSKIE N, STOKES J. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann. Intern. Med. 1961;55:33–50. [DOI] [PubMed] [Google Scholar]
- 3.Prospective Studies Collaboration, Lewington S, Whitlock G, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007;370:1829–1839. [DOI] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors Collaboration, Di Angelantonio E, Gao P, et al. Lipid-related markers and cardiovascular disease prediction. JAMA 2012;307:2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–3337. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019. Jun 18;139(25):e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin SS, Blaha MJ, Blankstein R, et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multi-ethnic study of atherosclerosis. Circulation 2014;129:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miname MH, Bittencourt MS, Moraes SR, et al. Coronary Artery Calcium and Cardiovascular Events in Patients With Familial Hypercholesterolemia Receiving Standard Lipid-Lowering Therapy. JACC Cardiovasc Imaging 2019;12:1797–1804. [DOI] [PubMed] [Google Scholar]
- 9.Sandesara PB, Mehta A, O’Neal WT, et al. Clinical significance of zero coronary artery calcium in individuals with LDL cholesterol ≥190 mg/dL: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2020;292:224–229. [DOI] [PubMed] [Google Scholar]
- 10.Gallo A, Pérez de Isla L, Charrière S, et al. The Added Value of Coronary Calcium Score in Predicting Cardiovascular Events in Familial Hypercholesterolemia. JACC Cardiovasc Imaging 2021;14:2414–2424. [DOI] [PubMed] [Google Scholar]
- 11.Navar-Boggan AM, Peterson ED, D’Agostino RB, Neely B, Sniderman AD, Pencina MJ. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation 2015;131:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Pletcher MJ, Vittinghoff E, et al. Association Between Cumulative Low-Density Lipoprotein Cholesterol Exposure During Young Adulthood and Middle Age and Risk of Cardiovascular Events. JAMA Cardiol 2021;6:1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging 2009;2:692–700. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen MB, Fuster V, Muntendam P, et al. Negative Risk Markers for Cardiovascular Events in the Elderly. Journal of the American College of Cardiology 2019;74:1–11. [DOI] [PubMed] [Google Scholar]
- 15.Rifai Al M, Blaha MJ, Nambi V, et al. Determinants of Incident Atherosclerotic Cardiovascular Disease Events Among Those With Absent Coronary Artery Calcium: Multi-Ethnic Study of Atherosclerosis. Circulation 2022;145:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortensen MB, Dzaye O, Steffensen FH, et al. Impact of Plaque Burden Versus Stenosis on Ischemic Events in Patients With Coronary Atherosclerosis. Journal of the American College of Cardiology 2020;76:2803–2813. [DOI] [PubMed] [Google Scholar]
- 17.Mortensen MB, Steffensen FH, Bøtker HE, et al. Heterogenous Distribution of Risk for Cardiovascular Disease Events in Patients With Stable Ischemic Heart Disease. JACC Cardiovasc Imaging 2020. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M, Mæng M, Madsen M, Sørensen HT, Jensen LO, Jakobsen C-J. The Western Denmark Heart Registry: Its Influence on Cardiovascular Patient Care. Journal of the American College of Cardiology 2018;71:1259–1272. [DOI] [PubMed] [Google Scholar]
- 19.Nørgaard BL, Terkelsen CJ, Mathiassen ON, et al. Coronary CT Angiographic and Flow Reserve-Guided Management of Patients With Stable Ischemic Heart Disease. Journal of the American College of Cardiology 2018;72:2123–2134. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014;29:541–549. [DOI] [PubMed] [Google Scholar]
- 21.Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016;10:435–449. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johannesdottir SA, Horváth-Puhó E, Ehrenstein V, Schmidt M, Pedersen L, Sørensen HT. Existing data sources for clinical epidemiology: The Danish National Database of Reimbursed Prescriptions. Clin Epidemiol 2012;4:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circulation Research 2014;114:1852–1866. [DOI] [PubMed] [Google Scholar]
- 25.Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. In: Vol 34. 2013:3478–90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsao CW, Preis SR, Peloso GM, et al. Relations of long-term and contemporary lipid levels and lipid genetic risk scores with coronary artery calcium in the framingham heart study. Journal of the American College of Cardiology 2012;60:2364–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortensen MB, Nordestgaard BG. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: a contemporary primary prevention cohort. The Lancet 2020;0. [DOI] [PubMed] [Google Scholar]
- 28.Budoff MJ, McClelland RL, Nasir K, et al. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Am. Heart J. 2009;158:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 30.Bolli A, Di Domenico P, Pastorino R, Busby GB, Bottà G. Risk of Coronary Artery Disease Conferred by Low-Density Lipoprotein Cholesterol Depends on Polygenic Background. Circulation. 2021. Apr 6;143(14):1452–1454. doi: 10.1161/CIRCULATIONAHA.120.051843. Epub 2021 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet 2011;378:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen MB, Fuster V, Muntendam P, et al. A Simple Disease-Guided Approach to Personalize ACC/AHA-Recommended Statin Allocation in Elderly People: The BioImage Study. Journal of the American College of Cardiology 2016;68:881–891. [DOI] [PubMed] [Google Scholar]
- 33.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). Journal of the American College of Cardiology 2015;66:1657–1668. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell JD, Fergestrom N, Gage BF, et al. Impact of Statins on Cardiovascular Outcomes Following Coronary Artery Calcium Scoring. Journal of the American College of Cardiology 2018;72:3233–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr JJ, Jacobs DR, Terry JG, et al. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and Death. JAMA Cardiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu K, Wilkins JT, Colangelo LA, Lloyd-Jones DM. Does Lowering Low-Density Lipoprotein Cholesterol With Statin Restore Low Risk in Middle-Aged Adults? Analysis of the Observational MESA Study. J Am Heart Assoc. 2021. Jun;10(11):e019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Because data stem from national registries containing data at an individual level, the authors are not permitted to share or grant access to data.


