Abstract
Biofilms pose a great challenge for wound management. Herein, this study describes a near-infrared (NIR) light-responsive microneedle patch for on-demand release of antimicrobial peptide for treatment of wound biofilms. IR780 iodide as a photothermal conversion agent and molecularly engineered peptide W379 as an antimicrobial agent are loaded in dissolvable poly(vinylpyrrolidone) (PVP) microneedle patches followed by coating with a phase change material 1-tetradecanol (TD). After placing in an aqueous solution or biofilm containing wounds ex vivo and in vivo, upon exposure to NIR light, the incorporated IR780 induces light-to-heat conversion, causing the melting of TD. This leads to the dissolution of PVP microneedles, enabling the release of loaded W379 peptide from the microneedles into surrounding regions (e.g., solution, biofilm, wound bed). Compared with traditional microneedle patches, NIR light responsive microneedle patches can program the release of antimicrobial peptide and show high antibacterial efficacy in vitro. Meanwhile, this work indicates that NIR light responsive TD-coated, W379-loaded PVP microneedle patches show excellent antibiofilm activities ex vivo and in vivo. Additionally, this microneedle system could be a promising platform for delivering other antimicrobial agents.
Keywords: NIR triggered release, antimicrobial peptide, phase changing material coating, microneedle patch, wound biofilm
Graphic Abstract

This work demonstrates the fabrication of TD-coated, W379 and IR780 co-loaded PVP microneedle patches through the molding and spray coating. These microneedle patches displayed an excellent photothermal responsive antibacterial effect without significant cytotoxicity in vitro. In addition, these patches showed high efficacy in combating wound biofilms ex vivo and in vivo.
1. Introduction
Biofilm is formed by pathogens buried in its own extracellular matrix (ECM) consisting of polysaccharides, DNA, proteins, and lipids [1]. Biofilms are found in 60% - 80 % of chronic wounds and constitute a pivotal threat to public health as they potentially cause delayed wound healing, amputation, sepsis, or even death [2]. Due to the dense structure of extracellular polymeric substance, antibiotics exhibit a slow speed to penetrate biofilms, often resulting in an insufficient dose to kill pathogens within biofilms [3]. Meanwhile, bacteria inside biofilm could form a resistance to drugs through a barrier function of the biofilm, a quorum sensing system, special growth characteristics, and other mechanisms, leading to the emergence of multi-drug-resistant bacteria [4]. The conventional anti-infective approaches such as bleach wash, surgical debridement, and use of antibiotics etc., employed to destroy biofilms are associated with various problems and some cases could lead to unwanted severe systemic side effects [5]. Therefore, there is an urgent need to develop new approaches to combat wound biofilms, especially to eliminate established biofilms.
Various methods have been developed to treat biofilms [2]. Chemical methods include the development of bacteriophages [6], nanomaterials [7], and antimicrobial peptides [8], etc. Physical methods consist of non-thermal plasma [9], photodynamic and/or photothermal therapy [10], ultrasound [11], magnetic manipulation [12], and electrical stimulation [13]. Studies have also been attempted to combine physical and chemical methods for combating biofilms. Among them, microneedle patches have been established to assist antimicrobial agents to penetrate the dense structure of biofilms [14]. One study demonstrated the dissolvable microneedle patches for delivery of chloramphenicol for the treatment of Vibrio vulnificus biofilms in vitro [15]. Another study showed microneedle patches for the delivery of oxygen to effectively combat biofilms in porcine skin wounds ex vivo [16]. Similarly, dissolvable microneedle patches were used to deliver doxycycline for reducing infection in an ex vivo porcine skin biofilm model [17]. In addition, our recent study further demonstrated the efficacy of antimicrobial peptide or other clinically used antimicrobial agents incorporated dissolvable microneedle patches in combination with nanofiber membranes for eliminating biofilms in both human skin wounds ex vivo and type Ⅱ diabetic wounds [18, 19]. Despite of the efficacy in eradicating biofilms, all these microneedle patches dissolve rapidly and lack precise control of release of antimicrobial agents, which may potentially cause recurrence of infection and biofilm formation and the generation of drug-resistant bacteria. The application of microneedle patches in vivo requires multiple changes to completely remove biofilms, which might cause unavoidable discomfort and pain to the patient [20]. Thus, we speculate that precise control of release of antimicrobial agents from microneedle patches could reduce the times of dressing changes and achieve high antimicrobial and anti-biofilm efficacy.
Light has beneficial features such as being non-invasive with high spatial resolution and temporal control [21]. Light-responsive materials can respond to light and are mostly employed for chemotherapy and photothermal therapy (PTT) [22]. The most applied electromagnetic wave for light activated microneedles is near-infrared (NIR) radiation [23]. NIR responsive PTT is an attractive alternative to combine with chemotherapy as it can cause membrane damage, cell injury, and protein denaturation. Importantly, the wavelength of NIR is within the “biological window”, minimally absorbed by the blood and soft tissues and leads to deep penetration [24]. Biofilms are closely associated with difficult-to-heal and dysfunctional inflammation in chronic wounds. PTT has emerged as a suitable alternative to disrupt the structure of biofilms with localized physical heat [25]. However, PTT alone also has limitations in the management of biofilms, such as the difficulty of storage and delivery of photothermal agents [10]. Thus, the combinatory approach of using PTT with other technologies can endow a multifunctional system to exhibit synergistic effect as well as remarkable therapeutic effect.
In this study, we aimed to develop a microneedle patch with photothermally triggered release of antimicrobial agents. Such a microneedle patch could physically penetrate biofilms and release antimicrobial agents upon NIR light irradiation. The antimicrobial efficacy could be further enhanced by photothermal therapy. It was hypothesized that the localized heat and heat-triggered release of antimicrobial agents can effectively combine photothermal therapy and chemotherapy into a single system to efficiently destroy bacterial biofilms and avoid frequent change of dressings. To demonstrate the proof-of-concept, molecularly engineered antimicrobial peptide (e.g., W379) and photothermal conversion agent (e.g., IR780 iodide) were loaded into dissolvable poly (vinylpyrrolidone) (PVP) microneedle patches. The phase change material 1-tetradecanol (TD) was then coated on the surface of microneedle patches by a spray-coating method. When the TD-coated microneedle patches were irradiated by NIR light, light-to-heat transduction mediated by IR780 caused the phase change material to melt and enable W379 release from the microneedles. The stimuli responsive microneedle was physico-chemically characterized and evaluated for drug release, cell toxicity and antibacterial activity. The NIR triggered release of encapsulated payload was further examined to combat the biofilms ex vivo and in vivo.
2. Materials and Methods
2.1. Materials
TD, IR780, PVP (Mw=130 kDa), ethyl acetate, and other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) and used directly without further purification. The polydimethylsiloxane (PDMS) mold was purchased from Blueacre Technology (Dundalk, Co Louth, Ireland). W379 peptides (95% pure) were bought from GeneMed Synthesis (San Antonio, TX, USA). MRSA USA300 LAC was obtained from the University of Nebraska Medical Center (UNMC). Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM) and RPMI 1640 medium were obtained from Thermo Fisher Scientific Gibco (Waltham, MA, USA).
2.2. Fabrication of TD-coated, IR780 and W379 co-loaded PVP microneedle patches
The PDMS mold was used to prepare an inverse microneedle patch (diameter = 6 mm, density = 150 needles, needle height = 300 μm, diameter of needle base = 300 μm). The TD-coated, IR780 and W379 co-loaded PVP microneedle patches were fabricated as follows (Figure 1). In brief, 50 μL of a 20% (w/w) PVP aqueous solution containing 25 mg/g W379 antimicrobial peptide and different concentrations of IR780 (0.05 %, 0.1 %, 0.2 %, 0.4 %, 0.5 % and 1 %) was deposited into the cavities of the PDMS mold and kept under vacuum for 10 min. Subsequently, the mold was allowed to dry at room temperature. After complete desiccation, the IR780 and W379 co-loaded PVP microneedle patch was carefully separated from the mold. Finally, TD-coated, IR780 and W379 co-loaded PVP microneedle patch was obtained by spraying TD (0.25 g/mL in ethyl acetate) onto the surface of IR780 and W379 co-loaded PVP microneedle patch using a commercial Nano spray water compensator (MH-DM2101, Babel of Eden, China) for 5 s at room temperature with 10 cm of distance between spray nozzle and patch.
Fig. 1.

Schematic illustrating 1-tetradecanol (TD)-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches for treatment of biofilms in chronic wounds. (A): IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches were coated with TD. (B): Upon exposure to NIR light, the coated TD layer melts, PVP dissolves, and W379 peptide releases to kill bacteria within biofilms.
2.3. Morphology characterization of TD-coated, IR780 and W379 co-loaded PVP microneedle patches
The morphologies of TD-coated, IR780 and W379 co-loaded PVP microneedle patches were characterized by a scanning electron microscope (SEM) (FEI, Quanta 200, OR, USA). To avoid charging, samples were fixed on a metallic stud with a double-sided conductive tape and coated with platinum for 4 min under vacuum at a current intensity of 10 mA using a sputter coater. SEM images were acquired at an accelerating voltage of 30 kV. To further illustrate the TD coating, 0.25 g/mL TD ethyl acetate solution mixed with fluorescein isothiocyanate isomer I (FITC, 0.1 wt %) was used to obtain the FITC-TD coated microneedles using the same method mentioned above. The fluorescent images of FITC-TD coated, IR780 and W379 co-loaded PVP microneedle patches were obtained by a laser scanning confocal microscope (LSM880, Zeiss, Germany).
2.4. NIR light responsive properties
To evaluate the NIR light responsive property of TD-coated, IR780 and W379 co-loaded PVP microneedle patches, the patches loaded with different concentrations of IR780 were exposed to the 808 nm NIR laser light (0.4 W) continuously at a power density of 0.4 W/cm2 until the temperature was steady. The maximal temperatures of the patches were recorded every second and plotted as a function of the exposure time. The thermographic images and temperatures of patches were captured using an advanced thermal camera (E53, FLIR, Sweden).
2.5. NIR-triggered drug release in vitro
To investigate the NIR light-triggered drug release in vitro, TD-coated, IR780 and W379 co-loaded PVP microneedle patches were placed into agar medium and exposed to NIR irradiation for 5 min each cycle with an interval of 2 h. Three times of laser on/off cycles were repeated to analyze the triggered release kinetics. Then, the exposed microneedle patches were pulled out and immersed into an ethyl acetate solution to wipe off the TD coating followed by being dissolved in 10 mL of deionized water. The drug concentrations of the above solutions were analyzed by a UV-vis spectrophotometer (U3900H, Hitachi, Japan) according to a previous standard calibration curve to determine the residual W379 peptide. The released peptide was calculated by deduction of residual content from the initial loading.
2.6. Antibacterial test of TD-coated, IR780 and W379 co-loaded PVP microneedle patches in vitro upon NIR light irradiation
To test the antibacterial activity of TD-coated, IR780 and W379 co-loaded PVP microneedle patches, MRSA USA300 colonies of were picked up by inoculating loops and cultured at 37 °C and 200 rpm in liquid LB overnight. Ten microliters of bacterial culture were added into 2 mL of fresh LB and incubated for additional 2 h. Then, the cultures were centrifuged and washed with PBS twice. Bacteria were resuspended and then diluted into 1.0 × 107 CFU/mL in PBS. Subsequently, 100 μL bacteria suspension was dropped onto a sterilized PDMS slide, and the TD-coated, IR780 and W379 co-loaded PVP microneedle patch was placed onto the bacterial suspension for 2 h at 37 °C. Different NIR irritation times were applied, and total living bacteria were determined by culturing on agar plates. Negative control used in the in vitro antibacterial test was IR780 PVP microneedle without W379. The log reduction of bacteria was calculated by the following equation:
2.7. In vitro cytotoxicity test
The in vitro cytotoxicity of TD-coated, IR780 and W379 co-loaded PVP microneedle patches to skin cells and monocytes was investigated by determining the cell viability of co-incubated HaCaT cells, U937 cells and NIH3T3 cells as described in the previous work [26]. The microneedle patches were first sterilized by ethylene oxide (Gas Sterilizer, AN74i, Anprolene, Andersen Sterilizers, Inc., NC, USA). HaCaT cells and NIH3T3 cells were cultured in DMEM with 10% FBS, and U937 cells were cultured in RMPI1640 with 10% FBS. Cells were seeded in 24-well plates. Each well contained 2.5 × 104 cells and 1mL of culture media. The presterilized TD-coated, IR780 and W379 co-loaded PVP microneedle patches were placed into the wells with the surface coatings contacting the cells with or without NIR irradiation. The negative control was TCPS plate. The plate containing cells and slides was cultured for 5 days, and the culture medium was refreshed every 2 days. On days 1, 3, and 5, the cell viability was investigated by the Alamar Blue assay.
2.8. Antibiofilm efficacy test TD-coated, IR780 and W379 co-loaded PVP microneedle patches ex vivo upon NIR light irradiation
To determine the antibiofilm efficacy ex vivo, biofilm-containing wounds were created on human skin explants. Briefly, the human skin tissues were collected from patients who underwent plastic surgery, as per the IRB protocol approved by the University of Nebraska Medical Center. After collection, skin tissue was kept on ice. Fat tissue was removed, and the skin tissue was rinsed in PBS thrice in order to remove blood. Then, the skin tissue was cut into pieces (2 cm × 2 cm). A wound was generated by an 8-mm punch in the center of each skin fragment. The wound depth was around 1 mm. MRSA was prepared by the same method as described above. Twenty μL of bacterial solution at a concentration of 1 × 108 CFU/mL was added to the wound. All the cultures were maintained at 37 °C for 48 h. After the establishment of the biofilm, different treatments were conducted to the wounds. No treatment, free W379 peptide, W379 loaded PVP microneedle patches, TD-coated, IR780-loaded PVP microneedle patches, and TD-coated, IR780 and W379 co-loaded PVP microneedle patches were applied to the biofilm-containing wounds with 4 times of NIR irradiation in 48 h (NIR light wavelength = 808 nm, 5 min NIR treatment per 12 h). After treatment, the wound and surrounding tissues were collected by a 10-mm-diameter punch and put into sterilized tubes. Then, 1 mL of sterilized PBS was added to each tube, which was blended by a homogenizer (TH115, Fisher Scientific, Hampton, NH, USA). Subsequently, the mixed liquid was diluted and plated on agar dishes. All the dishes were inoculated in a 37 °C microbial incubator for 18 h, and the CFU numbers were counted.
2.9. Antibiofilm efficacy test TD-coated, IR780 and W379 co-loaded PVP microneedle patches in vivo upon NIR light irradiation
We first established a biofilm-containing chronic wound model following previous studies [26]. Briefly, MRSA was grown in LB overnight. Subsequently, 100 μL of bacterial strain was pipetted into 4 mL of fresh LB medium and cultivated for 3 h followed by PBS washing for three times. Then, the bacterial concentration was adjusted to 1 × 108 CFU/mL and stored in the ice box before use. Nine female 000697-B6.BKS(D)-Leprdb/J diabetic defective mice (8–9 weeks, 35–45 g, GLU > 200 mg/dL) fed with standard pellet diet and water were used in this study. Two 6-mm-diameter full-thickness wounds were created on the back of a mouse using a disposable biopsy punch (Integra Miltex, Kai Medical) and fixed with a wound splint (Grace Biolabs, Inc., Bend, OR, USA). The biofilms were established in the mice excisional wounds. The wounds were all inoculated with 10 μL of 1 × 108 CFU/mL MRSA instantly after surgery, and 2% mupirocin was applied to treat the wounds at day 2 (24 h after surgery). After the establishment of the biofilm-containing wound model, W379 loaded PVP microneedle patches, TD-coated, IR780-loaded PVP microneedle patches, or TD-coated, IR780 and W379 co-loaded PVP microneedle patches were placed on the wounds upon NIR irradiation 4 times in 48 h each time 5 min. At different time points, the wound and surrounding tissue was collected by a 10-mm-diameter punch into sterilized tubes. Then, 1 mL of sterilized PBS was added to each tube, which was blended by a homogenizer. Subsequently, the mixed liquid was diluted and plated on agar dishes. All the dishes were inoculated in a 37 °C microbial incubator for 18 h, and the CFU numbers were counted. The animal study was approved by the Institutional Animal Care and Use Committee of University of Nebraska Medical Center (protocol #19–069-07-FC).
2.10. Statistical analysis
Statistical analysis was performed using GraphPadPrism (version 8.2.0, GraphPad Software Inc., San Diego, CA, USA). The data obtained were normalized and evaluated for statistical significance using an ordinary two-way ANOVA followed by a post-hoc Tukey’s multiple comparison test. The results are reported as mean ± SD from at least 6 independent experiments. The differences observed between samples were considered significant at *p < 0.05.
3. Results
3.1. Preparation and characterization of TD-coated, IR780 and W379 co-loaded PVP microneedle patches
Fig. 1 illustrates the preparation of NIR light responsive TD-coated, IR780 and W379 co-loaded PVP microneedle patches and their potential applications for treatment of wound biofilms. Briefly, PVP microneedle patches composed of engineered antimicrobial peptide W379 and IR780 with different mass ratios were prepared using a customized PDMS mold. In order to control the dissolution of microneedles and release of antimicrobial peptide, after demolding, the TD solution was sprayed onto the W379 and IR780 co-loaded PVP microneedle patches to obtain TD-coated, W379 and IR780 co-loaded PVP microneedle patches (Fig. 1A). As shown in Fig. 1B, when the TD-coated, W379 and IR780 co-loaded PVP microneedle patches are placed onto a biofilm chronic wound and exposed to 808 nm NIR light, the loaded IR780 would convert light to heat, resulting in an increase in temperature surpassing the phase change temperature of TD. Such an increase in temperature causes melting of the coated TD layer, leading to the exposure of PVP microneedles to external environment and subsequent dissolution of PVP microneedles and W379 peptide release to kill bacteria within biofilms.
We then performed the morphological characterization of TD-coated, W379 and IR780 co-loaded PVP microneedle patches. Fig. 2A shows a photograph of a TD-coated, W379 and IR780 co-loaded PVP microneedle patch. Fig. 2B–D shows the SEM images of W379 and IR780 co-loaded PVP microneedle patches with and without TD coatings in different magnifications revealing the surface appeared slightly different between the TD coated and non-coated patches (Fig.2E–F). To further illustrate the TD coating, we performed laser scanning confocal microscopy imaging of a FITC-TD coated, W379 and IR780 co-loaded PVP microneedle patch. Fig. 2, G and H show fluorescent images of IR780 and W379 co-loaded PVP microneedles in red and FITC-TD coating in green. Figure 2I shows the merged image of Figure 2G and Figure 2H. These results clearly revealed the TD coating on the microneedle surface.
Fig. 2.
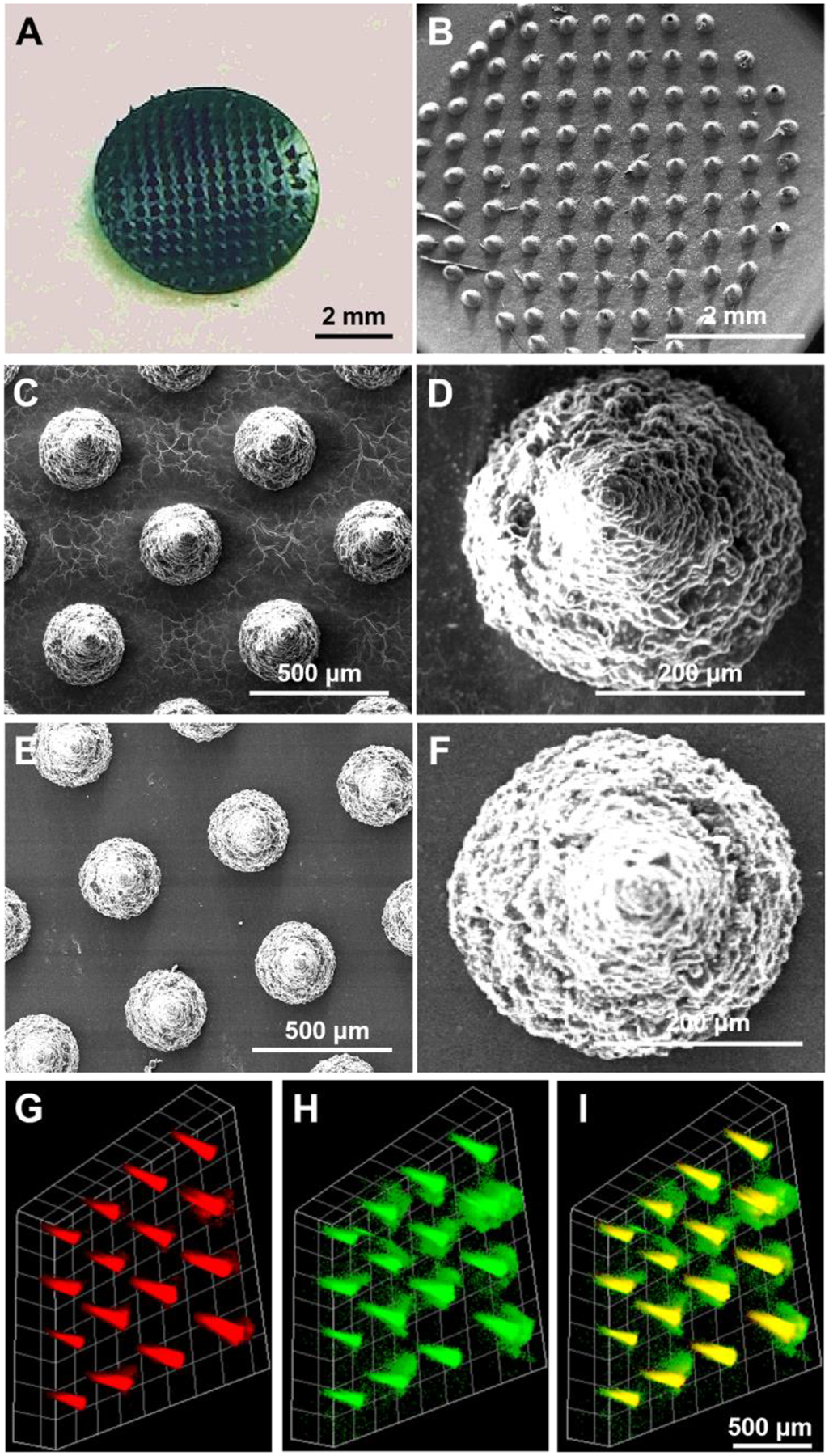
Morphology of TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches. (A): Photograph showing a TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patch. (B)-(D): SEM images of a TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patch with different magnifications. (E)-(F): SEM images of an uncoated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patch with different magnifications. (G)-(I): Fluorescent images of an FITC-TD coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patch. (G) IR780 iodide dye and W379 peptide co-loaded PVP microneedle in red. (H): FITC-TD coating in green. (I): merged (G) and (H).
3.2. NIR responsiveness of TD-coated, W379 and IR780 co-loaded PVP microneedle patches in vitro
The IR780 have a strong absorption in the NIR region with high photothermal conversion efficiency [27]. For the NIR light responsive microneedles, the loading amount of IR780 iodide is an important parameter. The optimized IR780 loading should efficiently induce the conversion of heat from NIR light to melt the coated TD layer, causing the release of loaded W379 antimicrobial peptide from microneedles. Simultaneously, the produced heat should cause minimal damage to the surrounding tissues. Therefore, in order to optimize the IR780 loading, different amounts of IR780 were loaded into microneedles for investigating the photothermal conversion under a continuous 808 nm NIR-light irradiation with a power of 0.4 W/cm2. Fig. 3A–F shows thermal infrared camera photographs of microneedle patches with and without containing 0.4 wt% IR780 iodide dye upon NIR light irradiation, indicating the microneedle patches with incorporation of 0.4 wt% IR780 exhibited a rapid increase in temperature to ~47.8 °C within 180 s while the temperature of the microneedle patches without incorporation of IR780 maintained at ~25.0 °C within the same period. Fig. 3G shows the time-temperature curves of microneedle patches loaded with different amounts of IR780. It is seen that with increasing the IR780 loading, the temperatures of microneedle patches elevated more rapidly under a NIR light irradiation. For the microneedle patches loaded with 1 wt% of IR780, the temperature increased to more than 50 °C within 60 s of NIR light exposure. The microneedle patches containing 0.4 wt% of IR780 were able to reach 40 °C after exposure to NIR irradiation for 100 s, which is still larger than the melting point of TD (38~39 °C) [28]. In contrast, the temperature change of microneedle patches without containing IR780 was negligible. Because the temperature of the normal human body is about 37 °C, the temperature of microneedle patches with a high loading of IR780 could quickly elevate over 50 °C after NIR light irradiation, which can melt the TD coating layer but potentially cause skin tissue damage [29]. If the microneedle patches contain too low amount of IR780 (<0.1 wt%), NIR light irradiation can only induce a small increase in temperature, which is not sufficient to melt the TD coating layer. Hence, microneedle patches could achieve controlled drug release without having any detrimental effects on the skin tissue by optimizing the loading of IR780 and regulating the melting kinetics of TD coating layer.
Fig. 3.

TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches are thermally responsive to NIR irradiation. (A)-(F): Thermal infrared camera photographs of microneedle patches with (left) and without (right) containing 0.4 wt% IR780 iodide dye upon continuous exposure to 0.4 W/cm2 NIR irradiation for 180 s. (G): Temperature-time curves of microneedle patches incorporated with different amounts of IR780 in microneedles.
3.3. NIR light controlled release properties and antibacterial effects in vitro
The design and development of stimuli responsive microneedle patches for controlled drug delivery is highly important for exhibiting potential therapeutic efficacy especially in the treatment of wound biofilms [30]. In this study, the designed microneedle patch was coated with NIR light responsive TD to modulate the release kinetics. Several cycles of NIR light on/off were investigated to demonstrate the NIR light-triggered drug release in vitro. To quantify the peptide release, an ethyl acetate solution was first used to dissolve the TD coating layer of microneedle patches after NIR light exposure for a fixed duration (5 min/every 2 h). Then, the treated microneedle patches were dissolved in DI water and the residual amount of W379 peptide was determine by a UV-vis spectrophotometer at 280 nm. PVP possesses only one peak (207 nm) in UV-vis region which is due to the presence of carbonyl functional group in ring structure [31]. IR780 shows a characteristic UV-vis absorbance peak at 780 nm [32]. Thus, PVP and IR780 would not affect the quantification of W379 using the UV-vis spectroscopy. The amount of released peptide was calculated by deducting the residual amount from the initial loading. Fig. 4A shows a switchable on/off release pattern of W379 peptide. During a NIR light-on state, the microneedle patches displayed a consistent and rapid release of W379, while there was no obvious drug release from the patches when the NIR light was off. It is seen that approximately 90.32% of loaded W379 can be released from microneedle patches after three cycles of NIR light on/off (first cycle, ~40.12%; second cycle, ~29.24%; third cycle, ~20.29%). These results indicate the TD-coated, W379 and IR780 co-loaded PVP microneedle patches can be triggered to release drugs in a controlled manner with the assistance of NIR light irradiation.
Fig. 4.

NIR light controlled release of W379 antimicrobial peptide from microneedle patches and their antimicrobial activity in vitro. (A): Release profiles of W379 peptide from TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches with and without NIR irradiation. At each time point, the patch is exposed to NIR light for 5 min. (B): In vitro antibacterial activities against MRSA USA300 of TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches with NIR irradiation for different periods of time and incubation for 2 h.
Previous studies showed W379 peptide has remarkable antimicrobial properties [33]. To demonstrate NIR light controlled antimicrobial activity, we performed an in vitro antibacterial test of TD-coated, W379 and IR780 co-loaded PVP microneedle patches upon exposure to NIR light. The inset in upper left of Fig. 4B shows a schematic diagram to illustrate the in vitro antibacterial test. The bacterial suspension was placed on a PDMS substrate. The patch was then placed onto the bacterial suspension and let the microneedle arrays face the bacterial suspension for 2 h and expose to NIR light for different periods of time. It is observed that Log reduction (CFU/mL) increased with increasing the exposure time (within 5 min) (Fig. 4B). It was found that after irradiation for 5 min or longer, the amount of W379 antimicrobial peptide released by microneedle patches was sufficient to kill almost all the MRSA in the bacterial suspension on the PDMS substrate (6 Log (CFU/mL) reduction). The results indicate that the patches with NIR light exposure time less than 4 min was not able to release enough amount of W379 peptide for killing all the bacteria in the solution. These results reveal that the NIR light exposure time plays a critical role in controlling the antibacterial efficacy of TD-coated, W379 and IR780 co-loaded PVP microneedle patches in vitro. This in vitro test provided a good guidance for the following studies.
3.4. In vitro cytotoxicity test
Before we pursued the further tests, we examined the cytotoxicity of the TD-coated, W379 and IR780 co-loaded PVP microneedle patches in vitro. The cytotoxicity was evaluated using the Alamar Blue assay with three different types of cells (keratinocytes HaCat cells, monocytes U937 cells, and fibroblasts NIH3T3 cells) as they are mainly related to wound healing. Fig. 5 shows that on day 1, 3, and 5, there was no significant difference in the cell viability among all the tested groups (TD-coated, W379 and IR780 co-loaded PVP microneedle patches without and with NIR light irradiation for 5 min and the TCPS control). The results revel that there was no significant cytotoxicity of TD-coated, W379 and IR780 co-loaded PVP microneedle patches without or with exposure to NIR light for 5 min when they were in direct contact with cells, supporting their potential applications in the treatment of wound biofilms.
Fig. 5.

In vitro cytotoxicity of TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches with and without NIR irradiation for 5 min against keratinocyte (HaCat), fibroblasts (NIIH3T3), and monocytes (U937).
3.5. Efficacy of TD-coated, W379 and IR780 co-loaded PVP microneedle patches against biofilms ex vivo
To further assess the efficacy and controlled release of peptides against biofilms, we established an ex vivo MRSA USA300 biofilm-containing human skin wound model. We first examined the effect of NIR light on the microneedle patches inserted on human skin explants by continuous irradiation of 0.4 W/cm2 NIR light for 5 min. Fig. 6 shows thermal infrared camera photographs of TD-coated, W379 peptide loaded PVP microneedle patches without (Fig. 6A) and with (Fig. 6B) containing IR780 iodide dye placed on partial-thickness wounds (not penetrate below dermis) created on human skin explants. These photographs show that after 5 min of NIR light exposure, the temperature of TD-coated, W379 peptide and IR780 co-loaded PVP microneedle patches increased to ~49.4 °C higher than the melting point of TD, while the temperature of patches without containing IR780 after the same period of NIR light exposure increased to ~27 °C much lower than the melting point of TD. Hence, the microneedle patches after insertion into excisional wounds created in human skin explants could display suitable photo-thermal conversion through regulating the exposure time when the power of NIR light is constant.
Fig. 6.

Thermal infrared camera photographs of TD-coated, W379 peptide loaded PVP microneedle patches without (A) and with (B) containing IR780 iodide dye placed on partial-thickness biofilm containing wounds created on human skin explants upon continuous irradiation of 0.4 W/cm2 NIR light for 5 mins.
Next, we quantified the ex vivo release of W379 peptide from patches after NIR light exposure (5 min at each time point) for different times within 48 h using the same method as described in the in vitro test. Fig. 7A–C shows that the percentages of released W379 peptide from TD-coated, W379 peptide and IR780 co-loaded PVP microneedle patches after NIR light exposure for 2–4 times were 60.29 ±3.33%, 77.32 ±5.21 %, and 95.23±2.33 %. It appears that almost all the W379 peptide could be released from microneedle patches after 4 times of NIR irradiation. As expected, almost no W379 peptide was released from the microneedle patches without NIR light exposure due to the presence of TD coating layer which could prevent the dissolution of microneedles. In comparison, the microneedle patches after exposing to NIR light, the TD coating layer could start melting, resulting in the exposure of the underlying PVP layer to the surrounding tissue, causing dissolution and subsequent release of W379 peptide. By simply controlling the NIR light exposure time, drug release kinetics of microneedle patches can be precisely regulated after insertion into excisional wounds created in human skin tissues ex vivo.
Fig. 7.

Efficacy of TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches against MRSA USA300 biofilms ex vivo upon NIR irradiation. Release profiles of W379 peptide from microneedle patches into human skin tissues without and with (A) 2 times of NIR triggering in 48 h (exposure for 5 min at each time point: 0 and 24 h), (B) 3 times of NIR triggering in 48 h (exposure for 5 min at each time point: 0, 16 h and 32 h), and (C) 4 times of NIR triggering in 48 h (exposure for 5 min at each time point: 0, 12 h, 24 h, and 48 h). (D): Antibiofilm efficacy of different treatments in an ex vivo biofilm-containing human skin wound model. Control: without treatment. Free W379: equivalent free W379 peptide. Uncoated W379 MN 48h: R780 iodide dye and W379 peptide co-loaded PVP microneedle patches were applied for 48 h. Coated W379 MN x min/y h in 48 h: TD-coated, R780 iodide dye and W379 peptide co-loaded PVP microneedle patches were applied and irradiated by NIR light for x min every y h in 48 h. Coated MN x min/y h in 48 h: TD-coated, R780 iodide dye loaded PVP microneedle patches were applied and irradiated by NIR light for x min every y h in 48 h. (E): SEM image showing morphology of IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches after applying to biofilm containing wounds ex vivo for 5 min. (F) SEM image showing morphology of TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches after applying to biofilm containing wounds ex vivo for 5 min. *p < 0.05
We then tested the efficacy of different treatments in combating MRSA biofilms in the excisional wounds created in human skin explants (Fig.7D). We applied no treatment, free W379 peptide, and different microneedle patches to the biofilm-containing wounds for 48 h and conducted CFU counting. It is clearly seen that W379 in the form of free drug reduced the CFU by only ~2.26 Log reduction, and the W379-loaded PVP microneedle patches without TD coating can further reduce the CFU counting to 2.14 × 104 CFU/g, resulting in ~3.79 Log reduction of CFU. Regarding the TD-coated, IR780 and W379 co-loaded PVP microneedle patch treatment groups, it is found that higher frequency of NIR irradiations generated higher antibacterial and antibiofilm efficacy, which could be attributed to more W379 released. It was found that after 4 times of NIR irradiation in 48 h, no bacteria colonies could be detected. In contrast, 1.29 × 103 CFU/g and 1.95 × 102 CFU/g of bacteria were detected after 2 and 3 times of NIR irradiation. Additionally, we also tested the TD-coated, IR780-loaded PVP microneedle patches without containing W379 peptide. It is seen that with increasing the times and frequencies of NIR irradiation, the number of CFU of bacteria decreased accordingly but the reduction was not significant after 2 times of NIR light exposure (5 min each time, 2 times within 48 h). Studies showed that local hyperthermia damages the bacterial structure, disrupts cell membrane permeability, and ultimately results in bacterial death [34, 35]. We speculated the reduction of the number of CFU could be attributed to the heat generated by IR780 iodide dye induced photo-thermal conversion. The generated heat may elevate the temperature to ~49 °C, which is unfavorable to the survival of MRSA, leading to the death of some bacteria and a slight decrease in the bacteria load. Overall, the TD-coated, IR780 and W379 co-loaded PVP microneedle patch showed significantly higher antibiofilm efficacy than the uncoated W379 loaded PVP microneedle patch. Our previous studies demonstrated that three changes of the uncoated W379 loaded PVP microneedle-based dressings can eradicate wound biofilms. NIR light controlled release could alleviate the patient’s discomfort caused by the dressing changes. In addition, the increase of local temperature and the controlled release of W379 could work together to achieve the high antibiofilm efficacy of the TD-coated, IR780 and W379 co-loaded PVP microneedle patch. To further illustrate the effect of TD coating on the release, we also performed SEM imaging of IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches without (Fig. 7E) and with (Fig. 7F) TD coating after applying to biofilm containing wounds ex vivo for 5 min. It is observed that the microneedles almost dissolved completely for IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches without TD coating, while the microneedles still maintained their integrity for the coated ones. This result indicates TD coating served as a barrier for prevention of dissolution of PVP microneedles and release of W379 peptide.
3.6. Efficacy of TD-coated, W379 and IR780 co-loaded PVP microneedle patches against MRSA biofilms in vivo
To further evaluate the antibiofilm efficacy of TD-coated, W379 and IR780 co-loaded PVP microneedle patches, we established MRSA biofilms in type II diabetic mouse wounds based on our previously established protocols [18] (Fig. 8A). Briefly, wounds were created and fixed with splints (Fig. 8B). Ten μL of ~108 CFU/mL MRSA was inoculated to each wound for 48 h followed by 24 h treatment of 2% mupirocin ointment to remove the planktonic bacteria on the wounds. For the in vivo antibiofilm testing, we applied TD-coated, W379 and IR780 co-loaded PVP microneedle patches plus NIR irradiation (5 min at each time point, 4 times within 48 h) to the biofilm-containing wounds. Other treatments including no treatment, uncoated W379 loaded PVP microneedle patches, and TD-coated, IR780 loaded PVP microneedle patches were used for comparison. After 48 h, there were 3.63 × 1010 CFU/g in the tissues collected from the wounds in the untreated control group. The treatment with the uncoated W379 loaded PVP microneedle patches resulted in a 6.27-Log reduction of CFU/g. Meanwhile, compared with the control group, the CFU number of TD-coated, IR780 loaded PVP microneedle patches slightly declined. It is estimated that 4 times of NIR irradiation would raise the local temperature to a temperature unsuitable for bacterial survival. In the TD-coated, W379 and IR780 co-loaded PVP microneedle patch treatment group, after 5 min NIR irradiation every 12 h within 48 h, the colonies could no longer be detected. Very importantly, this result was consistent with the ex vivo result (Fig. 7D). This result indicates that the TD-coated, W379 and IR780 co-loaded PVP microneedle patch was also effective against biofilms in vivo and able to destroy the biofilm completely after 4 times of NIR light exposure within 48 h with 5 min each time. Due to the precise control of drug release, the number of dressing changes could be reduced to achieve the similar therapeutic effect as previous microneedle-based dressings. In the future we could integrate with bacterial sensing system and feed-back loop to control the release of antimicrobial peptide using NIR light and simultaneously monitor the infection status. By this way, we could minimize the dose of antimicrobial peptide to achieve optimized therapeutic effect. Although the NIR light responsive release of antimicrobial peptide was demonstrated in this study, such microneedle patches could be used to regulate the release of other therapeutic agents (e.g., angiogenic agents and growth factors) as well for promoting wound healing.
Fig. 8.

Efficacy of TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches against MRSA USA300 biofilms in vivo upon NIR irradiation. (A): Schematic illustrating the in vivo experiment. (B): Photograph showing wounds were created and fixed with splints, and MRSA was inoculated for 48 h. (C): Antibiofilm efficacy of different treatments. *p<0.05 Control: without treatment. Uncoated W379 MN 48h: IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches were applied for 48 h. Coated W379 MN 5 min/12 h in 48 h: TD-coated, IR780 iodide dye and W379 peptide co-loaded PVP microneedle patches were applied and irradiated by NIR light for 5 min every 12 h in 48 h. Coated MN 5 min/12 h in 48 h: TD-coated, R780 iodide dye loaded PVP microneedle patches were applied and irradiated by NIR light for 5 min every 12 h in 48 h.
4. Discussion
Chronic wound biofilms have been a big problem in clinical practice. The present study aims to develop an approach for combating chronic wound biofilms which are normally difficult to manage and easy to relapse [36]. The major problem in developing an effective treatment to wound biofilms lies in the extracellular polymeric substance (EPS) rendered by biofilms which serves as a barrier for penetration of various antimicrobial agents. To overcome this problem, we have attempted to use the microneedle-based dressings with a biphasic structure (upper layer: nanofiber scaffold, bottom layer: PVP microneedle array) for the delivery of various antimicrobial agents (e.g., LL-37 derived peptides, silver ions, gallium ions, vancomycin) with distinct acting mechanisms to combat wound biofilms [18–19]. It is worth mentioning that even though our previous microneedle-based dressings showed extremely high efficacy, the fly in the ointment was that it would take 2–3 changes or administrations of microneedle-based dressings to achieve the complete eradication of biofilms [18–19]. Therefore, a dressing or patch loaded with a sufficient dose of antimicrobial agents with precise control of their release kinetics using external stimulation could be ideal for combating wound biofilms and eliminating the multiple administrations or dressing changes.
Herein, we integrated the TD coating to the W379 and IR780 co-loaded PVP microneedle patch. The incorporated IR780 makes microneedle patches NIR responsive, while the TD coating renders the patches the capacity of the tailored release of W379. Unlike conventional wound patches, our microneedle patch ensured the effectiveness, continuity, and stability of treatment with precisely controlled release of antimicrobial peptides and could potentially reduce treatment cost and dressing administration/change times. Benefiting from the excellent NIR properties of this microneedle patch, an effective, controllable, and safe antibiofilm method could become possible. The treatment can be performed within a short period of NIR irradiation. Meanwhile, PVP serving as the base material renders microneedle patches with sufficient mechanical strength which can readily penetrate into biofilms in chronic wounds manually.
In this study, at the beginning of the in vitro experiment, we applied the NIR treatment for 5 min. We first tested whether 5 min of NIR irradiation was enough to release a sufficient dose of antimicrobial peptide W379, and the result was satisfactory. We demonstrated that the microneedle patches could be triggered to release W379 in vitro with the assistance of NIR irradiation. Furthermore, nearly ~90% of loaded W379 was released from the microneedle patches after three NIR irradiation cycles, and the corresponding release ratios for each time were ~40%, 30%, and 20%, respectively. The first release ratio was the largest, and later release ratios gradually decreased. This release model also showed advantages for subsequent biological applications. For example, the first release of a large number of antimicrobial peptides could exert a burst killing effect on bacteria, and the subsequent release of a fewer number of antimicrobial peptides may consolidate the effect and prevent biofilms from reforming. Thus, in the following ex vivo and in vivo experiments, the NIR irradiation time was maintained for 5 min although the frequencies of light exposure were different. In addition, the pattern of 5 min NIR irradiation per cycle with a 2 h interval would be relevant or feasible for clinical applications, as this treatment protocol is easy and convenient to implement, and it would not burden the patients. In addition, NIR light equipment is cheap and easy to use. The NIR irradiation can be performed by patients themselves or their family members at home.
PVP has been used in many pharmaceutical products approved by the U.S. Food and Drug Administration (FDA), and it is generally recognized as safe [37]. PVP is also included in the Inactive Ingredient Database for use in oral, topical, and injectable formulations. IR780 is a common and highly safe cell imaging fluorescent dye, and it is widely used in photothermal therapy [38]. W379 peptide has been examined in vitro and in vivo without showing evident toxicities in previous publications [18, 33, 39]. TD is a non-toxic fatty alcohol which has been used as an ingredient in cosmetics such as cold creams [40, 41]. Moreover, TD is a biocompatible solid-state phase changing material and it is an FDA-approved food additive and therefore considered safe for the use in the human body [42]. Therefore, we chose PVP, IR780, TD and W379 for preparation of NIR-responsive microneedle patches in this study to ensure their biosafety.
5. Conclusion
We have demonstrated the fabrication of TD-coated, W379 and IR780 co-loaded PVP microneedle patches through the molding and spray coating. IR780 in the microneedles served as a NIR light absorbing agent to convert photoenergy to heat, while the TD coating layer served as a switch for controlled releases of W379 peptide due to its phase changing property. Under the NIR irradiation, the TD coating melted, promoting the release of encapsulated W379 peptide from PVP microneedles. The TD-coated, W379 and IR780 co-loaded PVP microneedle patches showed an intermittent release profile corresponding to the NIR light on/off cycle. The prepared microneedle patches displayed an excellent photothermal responsive antibacterial effect without significant cytotoxicity in vitro. In addition, these patches showed high efficacy in combating wound biofilms ex vivo and in vivo. Taken together, such an NIR light responsive microneedle-based drug delivery system could provide a platform technology for controlled release of antimicrobial agents and growth factors for combating biofilms and promoting wound healing.
Acknowledgments
This work was partially supported by startup funds from the University of Nebraska Medical Center (UNMC), and National Institute of General Medical Science (NIGMS) of the National Institutes of Health under Award Numbers R01GM138552 and P30GM127200.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Yajuan Su: Conceptualization, Methodology, Data curation, Writing-original draft. Syed Muntazir Andrabi: Methodology, Data curation. S M Shatil Shahriar: Methodology, Data curation. Shannon L. Wong: Methodology, Research Resources. Guangshun Wang: Conceptualization, Methodology, Research Resources, Writing-review & editing, Funding acquisition. Jingwei Xie: Supervision, Conceptualization, Methodology, Research Resources, Writing-review & editing, Funding acquisition.
References
- [1].Dufrêne YF, Persat A, Mechanomicrobiology: how bacteria sense and respond to forces, Nat. Rev. Microbiol 18 (2020) 227–240. [DOI] [PubMed] [Google Scholar]
- [2].Su Y, Yrastorza JT, Matis M, Cusick J, Zhao S, Wang G, Xie J, Biofilms: formation, research models, potential targets, and methods for prevention and treatment, Adv. Sci (2022) 2203291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lewis K, Riddle of biofilm resistance, Antimicrob. Agents Chemother 45 (2001) 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mah TFC, O’Toole GA, Mechanisms of biofilm resistance to antimicrobial agents, Trends Microbiol. 9 (2001) 34–39. [DOI] [PubMed] [Google Scholar]
- [5].Brooks BD, Brooks AE, Therapeutic strategies to combat antibiotic resistance, Adv. Drug Del. Rev 78 (2014) 14–27. [DOI] [PubMed] [Google Scholar]
- [6].Donlan RM, Preventing biofilms of clinically relevant organisms using bacteriophage, Trends Microbiol. 17 (2009) 66–72. [DOI] [PubMed] [Google Scholar]
- [7].Wang L-S, Gupta A, Rotello VM, Nanomaterials for the treatment of bacterial biofilms, ACS Infect. Dis 2 (2016) 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yasir M, Willcox MDP, Dutta D, Action of antimicrobial peptides against bacterial biofilms, Materials 11 (2018) 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gupta TT, Ayan H, Application of non-thermal plasma on biofilm: a review, Appl. Sci 9 (2019) 3548. [Google Scholar]
- [10].Huo J, Jia Q, Huang H, Zhang J, Li P, Dong X, Huang W, Emerging photothermal-derived multimodal synergistic therapy in combating bacterial infections, Chem. Soc. Rev 50 (2021) 8762–8789. [DOI] [PubMed] [Google Scholar]
- [11].Erriu M, Blus C, Szmukler-Moncler S, Buogo S, Levi R, Barbato G, Madonnaripa D, Denotti G, Piras V, Orrù G, Microbial biofilm modulation by ultrasound: current concepts and controversies, Ultrason. Sonochem 21 (2014) 15–22. [DOI] [PubMed] [Google Scholar]
- [12].Quan K, Zhang Z, Ren Y, Busscher HJ, van der Mei HC, Peterson BW, Possibilities and impossibilities of magnetic nanoparticle use in the control of infectious biofilms, J. Mater. Sci. Technol 69 (2021) 69–78. [Google Scholar]
- [13].Ashrafi M, Novak-Frazer L, Morris J, Baguneid M, Rautemaa-Richardson R, Bayat A, Electrical stimulation disrupts biofilms in a human wound model and reveals the potential for monitoring treatment response with volatile biomarkers, Wound Repair Regen. 27 (2019) 5–18. [DOI] [PubMed] [Google Scholar]
- [14].Jamaledin R, Yiu CK, Zare EN, Niu LN, Vecchione R, Chen G, Gu Z, Tay FR, Makvandi P, Advances in antimicrobial microneedle patches for combating infections, Adv. Mater 32 (2020) 2002129. [DOI] [PubMed] [Google Scholar]
- [15].Xu J, Danehy R, Cai H, Ao Z, Pu M, Nusawardhana A, Rowe-Magnus D, Guo F, Microneedle patch-mediated treatment of bacterial biofilms, ACS Appl. Mater. Interfaces 11 (2019) 14640–14646. [DOI] [PubMed] [Google Scholar]
- [16].Woodhouse I, Nejati S, Selvamani V, Jiang H, Chittiboyina S, Grant J, Mutlu Z, Waimin J, Abutaleb NS, Seleem MN, Flexible microneedle array patch for chronic wound oxygenation and biofilm eradication, ACS Appl. Bio Mater 4 (2021) 5405–5415. [DOI] [PubMed] [Google Scholar]
- [17].Permana AD, Mir M, Utomo E, Donnelly RF, Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study, Int. J. Pharm.: X 2 (2020) 100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Su Y, Mainardi VL, Wang H, McCarthy A, Zhang YS, Chen S, John JV, Wong SL, Hollins RR, Wang G, Dissolvable microneedles coupled with nanofiber dressings eradicate biofilms via effectively delivering a database-designed antimicrobial peptide, ACS Nano 14 (2020) 11775–11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Su Y, McCarthy A, Wong SL, Hollins RR, Wang G, Xie J, Simultaneous delivery of multiple antimicrobial agents by biphasic scaffolds for effective treatment of wound biofilms, Adv. Healthcare Mater 10 (2021) 2100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Farahani M, Shafiee A, Wound healing: From passive to smart dressings, Adv. Healthcare Mater 10 (2021) 2100477. [DOI] [PubMed] [Google Scholar]
- [21].Li L, Zhang X, Zhou J, Zhang L, Xue J, Tao W, Non-invasive thermal therapy for tissue engineering and regenerative medicine, Small 18 (2022) 2107705. [DOI] [PubMed] [Google Scholar]
- [22].Raza A, Hayat U, Rasheed T, Bilal M, Iqbal HM, “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: a review, J. Mater. Res. Technol 8 (2019) 1497–1509. [Google Scholar]
- [23].Makvandi P, Jamaledin R, Chen G, Baghbantaraghdari Z, Zare EN, Di Natale C, Onesto V, Vecchione R, Lee J, Tay FR, Stimuli-responsive transdermal microneedle patches, Mater. Today 47 (2021) 206–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].He S, Song J, Qu J, Cheng Z, Crucial breakthrough of second near-infrared biological window fluorophores: design and synthesis toward multimodal imaging and theranostics, Chem. Soc. Rev 47 (2018) 4258–4278. [DOI] [PubMed] [Google Scholar]
- [25].Yuan Z, Lin C, He Y, Tao B, Chen M, Zhang J, Liu P, Cai K, Near-infrared light-triggered nitric-oxide-enhanced photodynamic therapy and low-temperature photothermal therapy for biofilm elimination, ACS Nano 14 (2020) 3546–3562. [DOI] [PubMed] [Google Scholar]
- [26].Su Y, Wang H, Mishra B, Narayana JL, Jiang J, Reilly DA, Hollins RR, Carlson MA, Wang G, Xie J, Nanofiber dressings topically delivering molecularly engineered human cathelicidin peptides for the treatment of biofilms in chronic wounds, Mol. Pharm 16 (2019) 2011–2020. [DOI] [PubMed] [Google Scholar]
- [27].Li S, Zhou S, Li Y, Li X, Zhu J, Fan L, Yang S, Exceptionally high payload of the IR780 iodide on folic acid-functionalized graphene quantum dots for targeted photothermal therapy, ACS Appl. Mater. Interfaces 9 (2017) 22332–22341. [DOI] [PubMed] [Google Scholar]
- [28].Rajan JT, Jayapal VS, Krishna M, Firose KM, Vaisakh S, John AK, Suryan A, Analysis of battery thermal management system for electric vehicles using 1-Tetradecanol phase change material, Sustain. Energy Technol. Assess 51 (2022) 101943. [Google Scholar]
- [29].Garcia PA, Rossmeisl JH, Neal RE, Ellis TL, Davalos RV, A parametric study delineating irreversible electroporation from thermal damage based on a minimally invasive intracranial procedure, Biomed. Eng. Online 10 (2011) 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gowda BJ, Ahmed MG, Sahebkar A, Riadi Y, Shukla R, Kesharwani P, Stimuli-responsive microneedles as a transdermal drug delivery system: a demand-supply strategy, Biomacromolecules 23 (2022) 1519–1544. [DOI] [PubMed] [Google Scholar]
- [31].Zaman AC, Kaya C, Determination of quantity of materials in suspensions and in electrophoretic coatings by UV-visible absorption spectroscopy, J. Electrochem. Soc 162 (2015) D3109. [Google Scholar]
- [32].Wang K, Zhang Y, Wang J, Yuan A, Sun M, Wu J, Hu Y, Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy, Sci. Rep 6 (2016) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Narayana JL, Mishra B, Lushnikova T, Wu Q, Chhonker YS, Zhang Y, Zarena D, Salnikov ES, Dang X, Wang G, Two distinct amphipathic peptide antibiotics with systemic efficacy, Proc. Natl. Acad. Sci. USA 117 (2020) 19446–19454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ren Y, Liu H, Liu X, Zheng Y, Li Z, Li C, Yeung KWK, Zhu S, Liang Y, Cui Z, Wu S, Photoresponsive materials for antibacterial applications, Cell Rep. Phys. Sci 1 (2020) 100245. [Google Scholar]
- [35].Li J, Liu X, Zhou Z, Tan L, Wang X, Zheng Y, Han Y, Chen D-F, Yeung KWK, Cui Z, Yang X, Liang Y, Li Z, Zhu S, Wu S, Lysozyme-assisted photothermal eradication of methicillin-resistant Staphylococcus aureus infection and accelerated tissue repair with natural melanosome nanostructures, ACS Nano 13 (2019) 11153–11167. [DOI] [PubMed] [Google Scholar]
- [36].Bjarnsholt T, The role of bacterial biofilms in chronic infections, APMIS, 121 (2013) 1–58. [DOI] [PubMed] [Google Scholar]
- [37].Ronnander P, Simon L, Pilgies H, Koch A, Scherr S, Dissolving polyvinylpyrrolidone-based microneedle systems for in-vitro delivery of sumatriptan succinate, Eur. J. Pharm. Sci 114 (2018) 84–92. [DOI] [PubMed] [Google Scholar]
- [38].Yi X, Yan F, Wang F, Qin W, Wu G, Yang X, Shao C, Chung LWK, Yuan J, IR-780 dye for near-infrared fluorescence imaging in prostate cancer. Med. Sci. Monit 21 (2015) 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].John JV, Sharma NS, Tang G, Luo Z, Su Y, Weihs S, Shahriar SMS, Wang G, McCarthy A, Dyke J, Zhang YS, Khademhosseini A, Xie J, Nanofiber aerogels with precision macrochannels and LL-37-mimic peptides synergistically promote diabetic wound healing, Adv. Funct. Mater (2022) 2206936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mitran RA, Matei C, Berger D, Băjenaru L, Moisescu MG, Controlling drug release from mesoporous silica through an amorphous, nanoconfined 1-tetradecanol layer, Eur. J. Pharm. Biopharm 127 (2018) 318–325. [DOI] [PubMed] [Google Scholar]
- [41].Elder RL, Final report on the safety assessment of cetearyl alcohol, cetyl alcohol, isostearyl alcohol, myristyl alcohol, and behenyl alcohol, J. Am. Coll. Toxicol 7 (1988) 359–413. [Google Scholar]
- [42].Lee J, Jeong C, Kim WJ,Facile fabrication and application of near-IR light-responsive drug release system based on gold nanorods and phase change material, J. Mater. Chem. B 2(2014) 8338–8345. [DOI] [PubMed] [Google Scholar]


