Abstract
Glioblastoma (GBM), is the most malignant form of gliomas and the most common and lethal primary brain tumor in adults. Conventional cancer treatments have limited to no efficacy on GBM. GBM cells respond and adapt to the surrounding brain parenchyma known as tumor microenvironment (TME) to promote tumor preservation. Among specific TME, there are 3 of particular interest for GBM biology: the perivascular niche, the subventricular zone neurogenic niche, and the immune microenvironment. GBM cells and TME cells present a reciprocal feedback which results in tumor maintenance. One way that these cells can communicate is through extracellular vesicles. These vesicles include exosomes and microvesicles that have the ability to carry both cancerous and non-cancerous cargo, such as miRNA, RNA, proteins, lipids, and DNA. In this review we will discuss the booming topic that is extracellular vesicles, and how they have the novelty to be a diagnostic and targetable vehicle for GBM.
Keywords: glioblastoma multiforme, tumor microenvironment, perivascular niche, subventricular zone/neurogenic niche, exosomes, microvesicles
1. Introduction
Glioblastoma multiforme, or GBM, is the most malignant form of gliomas and is considered the most common and lethal primary brain tumor in adults. Despite current standard of care, the median survival of GBM patients remains at 14–16 months after diagnosis, 70% of patients will present disease progression after one year of diagnosis and less than 5% of patients survive past five years (Davis, 2016). Under current brain tumor classification, GBM arise de novo (previously called primary GBM) and present a wild type form of IDH1 gene. Grade 4, IDH1 mutant tumors, which can progress from lower grade gliomas (previously known as secondary GBM) are currently known as Grade 4 astrocytoma (Louis et al., 2021). The median age of diagnosis is 65 years old, with an incidence of 3 cases in 100,000 people with a slight increased incidence in men than women (Ostrom et al., 2020). Patients with suspected high-grade glioma are initially subject to a physical and clinical evaluation, followed by a contrasted MRI. Definite diagnosis of GBM is done by histological analysis of a tissue biopsy or tumor resection. Current glioma classification considers histopathology findings and key molecular tests (Ostrom et al., 2020). The current standard of care for GBM was largely established almost 2 decades ago, with little modifications (Stupp et al. 2005). This protocol includes maximal safe surgical resection followed by concurrent postoperative radiation therapy with oral alkylating chemotherapy agent, Temozolomide (TMZ). This treatment protocol has shown to extend median patient survival from 12.1 months to 14.6. However, this multidisciplinary approach is not curative for GBM patients (Louis et al., 2021; Stupp et al., 2005).
Histologically, GBM presents as a diffuse glioma (D’Alessio et al., 2019) with microvascular proliferation and pseudopalisading necrosis as pathognomonic histological features. Additional aspects can be observed, such as hypercellularity, nuclear atypia (D’Alessio et al., 2019), and multinucleated giant cells (Jaiswal et al., 2012). Histological analyses remain an important and regularly used tool for clinical diagnosis. Moreover, technological advancements and discoveries particularly in “-omics” related fields continually improve scientific understanding of these tumors at a molecular level.
While GBM is considered a singular diagnosis, there is considerable intertumoral heterogeneity as observed in transcriptome, mutation, and copy number variations (Brennan et al., 2013; Verhaak et al., 2010). Four GBM subtypes were identified from these analyses: Classical, mesenchymal, proneural, and proliferative. However, the impact of this classification on patient survival and treatment response has been marginal and continues to be evaluated (Wang et al., 2020). Nevertheless, the concept of additional GBM molecular stratification continues to be enhanced as scientists pursue more effective classification methods beyond DNAm (DNA methylation) and gene-expression based classifiers only (Ensenyat-Mendez et al., 2021). Collectively, this further categorization is critical for patient prognosis as these different molecular subtypes could give rise to subtype-specific treatments that address these molecular variations, potentially increasing treatment efficacy for patients. Furthermore, single cell sequencing technology has allowed to demonstrate GBM heterogeneity is also present intratumorally (Patel et al., 2014). This characteristic functions as a major determining factor of treatment efficacy, potential recurrence, and overall prognosis (Bedard et al., 2013; Patel et al., 2014). The prevalent intra- and inter-tumoral heterogeneity in GBM calls for more studies focused on its regulatory mechanisms.
The source of intratumoral heterogeneity hints at the idea of a common cellular source with pluripotent capabilities. Stem cells in cancer were initially described in liquid malignancies and eventually in GBM (Gimple et al., 2019; Singh et al., 2004). Glioblastoma stem cells (GSC) are believed to be major contributors to tumor growth, recurrence, and heterogeneity (Gimple et al., 2019). These are cells with slower proliferation rate (quiescent) and the ability to self-renew, give rise to differentiated progeny, and generate a tumor with GBM features, upon secondary transplantation. GSCs are strongly influenced by the surrounding environment, including cellular components, oxygen tension, genetic factors, and soluble proteins (Lathia et al., 2015; Mohyeldin et al., 2010). The elimination of GSCs has been the focus of multiple research studies with unsatisfactory results. GSC display resistance towards radiotherapy (Bao et al., 2006) as well as TMZ chemotherapy (Chen et al., 2012; Liu et al., 2006).
2. Glioblastoma Multiforme tumor microenvironment
The tumor microenvironment (TME) is the ecosystem of cells and extracellular components surrounding tumor cells. The cell populations within this environment coordinate to adapt in ways that collectively favor and support survival of cancer cells as well as facilitating local invasion, making the TME highly dynamic (Anderson and Simon, 2020). TMEs generally consist of tumor cells, stroma, blood vessels and vascular components, and infiltrating inflammatory cells (Whiteside, 2008). The GBM TME includes these same components while displaying particular heterogeneity. GSC and differentiated GBM cells (DGCs) are the major proliferating tumor cell populations. The non-tumor cells that are typically present include endothelial and vascular pericytes (Charles and Holland, 2010) (Anderson and Simon 2020). GBMs also have an immunosuppressive TME. In this environment, immune cells that would carry out tumor-suppressing activities transition towards a state promoting inflammation and/or tumor escape. These altered immune cells include innate and recruited/infiltrative cells such as microglia, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and glioblastoma associated myeloid cells (GAMs) (Darmanis et al., 2017; Gabrusiewicz et al., 2016; Shi et al., 2015). This environment is complex as the tumor mass itself can actively change the TME, while the TME also affects the growth of the tumor. The interactions taking place between the neighboring cancer and non-transformed cells make the TME a key component in tumor progression (Balkwill et al., 2012). The diffusion of cytokines, chemokine, growth factors, and matrix remodeling enzymes drives this bidirectional intercellular communication shared between the tumor cells and their TME (Balkwill et al., 2012) as the dynamic activities of these molecules trigger responsive alterations to local/surrounding tissue.
In the brain, some areas present tumor promoting conditions as they interact with GBM tumors. Of particular interest in this review are the Subventricular Zone (SVZ) neurogenic niche in the lateral wall of the lateral ventricles (LV), the immune microenvironment, and the Perivascular Niche (PVN).
2.1. The neurogenic niche
Postnatally, in mammalian brains, the largest region that maintains its capacity of producing neural stem cells (NSCs) is the SVZ (Alvarez-Buylla et al., 1998; Luskin, 1993; Reznikov, 1991; Sanai et al., 2004). The SVZ anatomy of adult humans consists of 4 layers. The first being comprised of an ependymal cell monolayer facing towards the lateral ventricle cavity. Behind the ependymal layer, there is a hypocellular gap, consisting of intertwined processes of Glial Fibrillary Acidic Protein (GFAP)-positive astrocytes. The third layer contains an astrocytic ribbon along (Quiñones-Hinojosa et al., 2006; Sanai et al., 2004)
GBM patients with tumors in direct contact with the LVs have worse prognosis with lower survival rates than GBMs not contacting the lateral ventricles (Chaichana et al., 2008; Mistry et al., 2017; Mistry et al., 2019). Additionally, GBM in contact with the lateral ventricles show higher incidence of distal recurrence, compared to GBM distant from lateral ventricles vicinity (Lim et al., 2007). The mechanisms behind this increased malignancy of GBM tumors proximal to the lateral ventricles are not well understood. Interactions between SVZ-NSCs, GBM cells, and the lateral ventricles alter this specific region in ways that influence tumor progression. In animal models of LV-proximal GBM, tumor proximity causes a decrease in NSC proliferation and decreases SVZ-derived neuroblasts migration. This animal model replicates important clinical observations present in patients, like lowered survival and increased tumor cell proliferation (Ripari et al., 2021). In bulk tumor samples from patients, LV-proximal GBM show similar transcriptome profiles, when compared to LV-distal samples (Mistry et al., 2019; Steed et al., 2016; Steed et al., 2020). However, this phenomenon should be studied at a single cell level due to the intratumoral heterogeneity mentioned above.
2.2. Immune microenvironment
A collection of non-neoplastic immune cells, such as macrophages, microglia, and reactive astrocytes also take residency in the TME forming an immune microenvironment inside and around the tumor (Chen and Hambardzumyan, 2018; Henrik Heiland et al., 2019). TAMs originate from microglia found in the brain and are considered the dominant infiltrating immune population as they make up approximately 30–40% of the cell population in GBM (Engler et al., 2012). These cells infiltrate the tumor guided by chemoattractants, such as osteopontin (OPN) and glial cell-derived neurotrophic factor (GDNF), released into the extracellular environment by tumor cells (Andersen et al., 2021).
The amount of tumor-associated microglia and TAM is positively associated with immunoregulation in glioma, giving this recruitment of cells clinical relevance(Chen et al., 2020; Zhang et al., 2021a). The impact of GBM on immune cells in the brain induces a strong inhibition of anti-tumor T cell response and bone marrow entrapment of T cells (Chongsathidkiet et al., 2018). Additionally, tumor cells release ligands like PD-L1 which upon binding to the PD1 receptor induces an immune evasion response. Importantly, tumor-infiltrating lymphocytes show a higher expression of checkpoint molecules like PD-1, LAG3 and TIM-3 which impairs their activity against tumor cells (Davidson et al., 2019; Pombo Antunes et al., 2020). Overall, GBM cells and immune cells present constant intercommunication that results in a defective anti-tumor immune response.
2.3. Perivascular niche
One of the GBM TME areas particularly enriched for GSCs is the regions surrounding small blood vessels and capillaries, known as the PVN. (Calabrese et al., 2007; Charles et al., 2010; Hambardzumyan et al., 2008). In the PVN, GSC are maintained by signals from endothelial cells like Notch, OPN, and nitric oxide (Calabrese et al., 2007; Charles et al., 2010; Stroeher et al., 1988). Additionally, GBM cells utilize blood vessels (and myelin tracts) as roads to invade the brain parenchyma (Farin et al., 2006; Riquelme et al., 2008; Watkins et al., 2014; Winkler et al., 2009). The interaction of GSCs with the PVN microenvironment contributes to tumor expansion cellular heterogeneity. There are endothelial and stromal cells, such as fibroblasts and pericytes, that make up most of this niche and drive the progression of GBM (Charles and Holland, 2010; Charles et al., 2012). Pericytes surround, stabilize, and help maintain the integrity of the newly developed vasculature walls (Gerhardt and Betsholtz, 2003). Recruitment of these cells is driven by overexpression of Platelet Derived Growth Factor Receptor Beta/Platelet Derived Growth Factor B (PDGFRβ/PDGFB) to newly developing vessels (Bergers and Song, 2005; Dunn et al., 2000), and contributes to stabilization of tumor vessels in many different types of cancers (Guo et al., 2003; Rajantie et al., 2004; Song et al., 2005). In addition to the major cell types found in the PVN, one can also find immune cells including lymphocytes, macrophages, microglia, astrocytes (Charles and Holland, 2010). There are additional similar signaling pathways and genes upregulated in the PVN that are implicated in cancers, such as O6-methylguanine-DNA methyltransferase (MGMT), Epidermal Growth Factor Receptor (EGFR), and PI3k-Akt and Ras/MAPK signaling (Ngo and Harley, 2019).
3. Characterization of extracellular vesicles
Intercellular communication in the GBM TME includes soluble factors diffusing through the extracellular matrix, direct cell-cell contact, like Notch activation, and signaling molecules encapsulated in extracellular vesicles (EVs)(Fig. 2). EVs are a heterogenous group of cell-derived membranous structures that provide a mechanism for intercellular communication, allowing for cells to exchange proteins, lipids, RNA, and other genetic material (van Niel et al., 2018). The term EVs encompasses a variety of different membrane secreted vesicles, which makes characterization of these vesicles an ongoing field of study. Due to the similar morphology and overlapping size of different EV, complete characterization and distinction remain onerous. Based on what has been discovered, the two defined categories of EVs are exosomes and microvesicles.
Figure 2. Extracellular vesicles biogenesis occurs by invagination or blebbing on the cell membrane originating different vesicle subtypes.
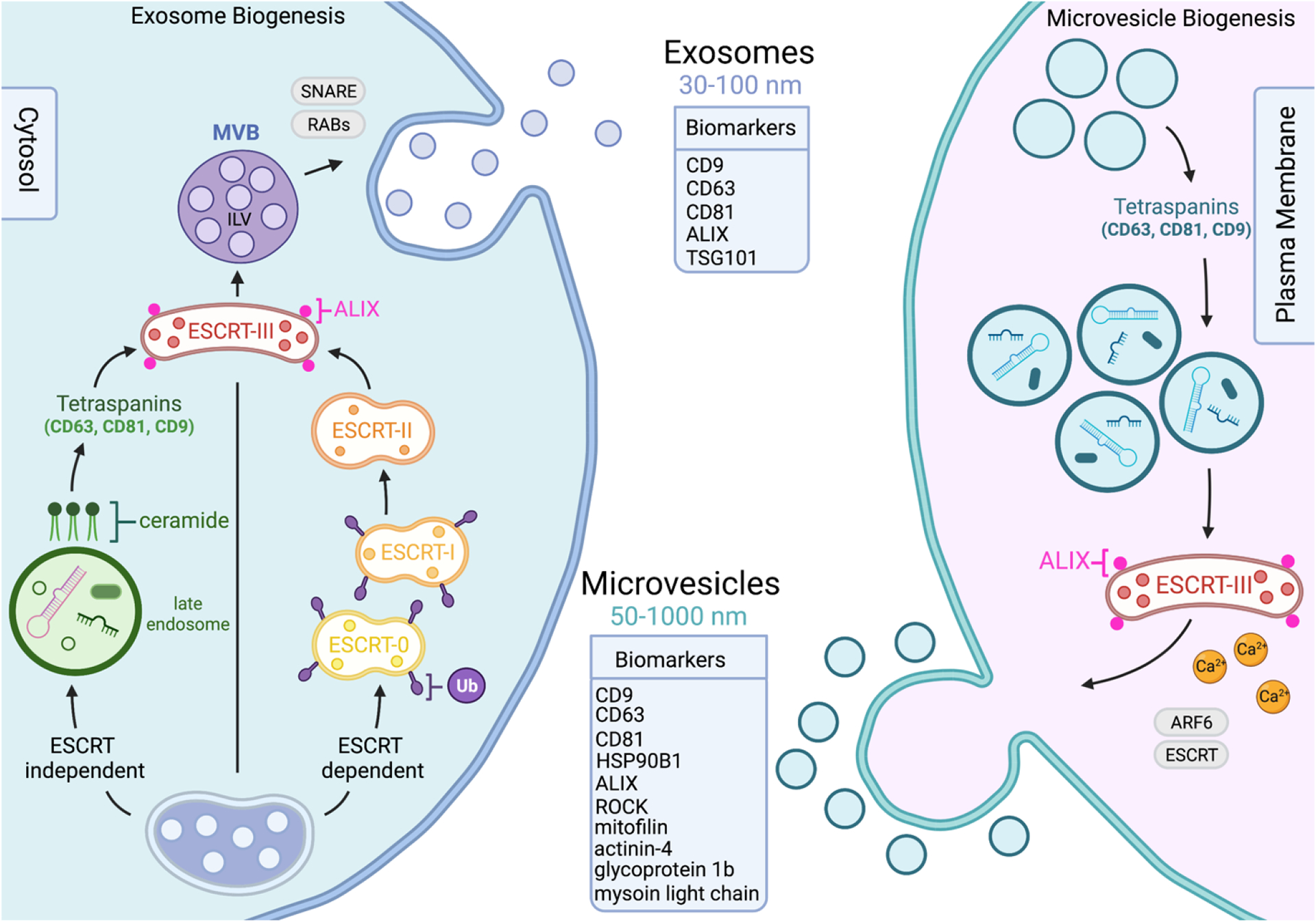
Extracellular vesicle biogenesis differs depending on the sub-population of vesicles in question. Exosomes range from 30–100nm in size, formed by plasma membrane invagination utilizing ESCRT-independent, shown to include ceramides and tetraspanin driven sorting, and ESCRT-dependent pathways, which gathers ubiquitylated cargo using ESCRT protein complexes. The release of exosomes derived from both pathways are influenced by SNARE and Ras-related proteins. Microvesicles are generally larger, ranging from 50–1000nm in size, and form through blebbing and budding on the plasma membrane. Microvesicles share components with exosome formation, including tetraspanins, however cargo in this case is determined by a component’s lipid raft affinity and anchorage to plasma membrane. MVBs fuse with the plasma membrane and release the ILVs into the extracellular space. The budding and consequential release of microvesicles is influenced by Ca2+ levels, ESCRT pathway associated proteins, and ADP-ribosylation factor 6. Exosomes and microvesicles have distinct surface markers to help differentiate the two populations from each other.
Exosomes are typically 30–100nm in diameter (Johnstone et al., 1987), and form due to an invagination of the endosomal membrane as intraluminal vesicles (ILVs), which are then secreted during fusion of multivesicular bodies (MVBs) to the cell surface (Harding et al., 1984; van Niel et al., 2018). There are two known machineries involved in the biogenesis of exosomes: endosomal sorting complex required for transport (ESCRT)-dependent and ESCRT-independent. The ESCRT machinery pathway modulates the production of exosomes by regulating the formation of ILVs and MVBs (Hurley, 2008; van Niel et al., 2018). This pathway begins with ESCRT0 and ESCRTI gathering ubiquitylated cargoes from MVBs, which then recruits the ESCRTIII subunit, with the help of ESCRTII, to cause exosome budding (van Niel et al., 2018). The ESCRT-independent pathway operates through the generation of ceramide to impose negative curvature on the membranes to help with the formation of exosomes, or by the metabolism of ceramide to activate G-protein-coupled sphingosine 1-phosphate receptor, an important mechanism for sorting exosomal cargo into ILVs (Goñi and Alonso, 2009; Kajimoto et al., 2013; Trajkovic et al., 2008). In addition to the ceramide driven ESCRT-independent mechanisms, proteins in the tetraspanin family are also involved in endosomal sorting, such as CD63, CD81, CD9, ALIX, and TSG101 which are enriched on the surface of exosomes and used as characteristic markers (Buschow et al., 2009; Chairoungdua et al., 2010; van Niel et al., 2011) (Fig. 2). The release of exosomes is mediated by multiple protein families. For example, Ras-related proteins (RAB11, RAB35, RAB27) help to promote secretion, along with SNARE proteins (SNAP25, SNAP23) that help mediate membrane fusion and cell navigation (Colombo et al., 2014; Zylbersztejn and Galli, 2011).
The second characterized group of EVs are microvesicles, which are typically 50–1000nm in diameter (van Niel et al., 2018). They form via an outward budding of the plasma membrane, resulting in a release of these vesicles into extracellular space (Tricarico et al., 2017). The biogenesis of microvesicles requires changes in lipid and protein composition within the plasma membrane, although specific mechanisms are still being investigated (Theos et al., 2006). Lipids such as cholesterol (Del Conde et al., 2005) and the activity of RHO family of GTPases and the RHO-associated protein 30kinase (ROCK) are involved in the formation of microvesicles in certain tumor cells (Li et al., 2012). Additionally, the Ca2+ levels and the enzymatic machineries driven by Ca2+ promote physical tension on the plasma membrane, which helps to restructure the actin cytoskeleton and favor budding of microvesicles (Al-Nedawi et al., 2008; Piccin et al., 2007). Similar to the mechanisms involved in retroviral budding, microvesicle-targeted cargo is regulated through their affinity to lipid rafts or their anchoring to plasma membrane lipids (Shen et al., 2011; Yang and Gould, 2013). The budding of microvesicles from the plasma membrane is mediated by ADP-ribosylation factor 6 (ARF6) and other components of the ESCRT pathway (Colombo et al., 2014). In comparison to the known exosome markers, the known markers for microvesicles also include heat shock protein (HSP) 90B1, glycoprotein 1b, actinin-4, mitofilin, and myosin light chain (Bruschi et al., 2019; Haraszti et al., 2016; Kowal et al., 2016; Zhang et al., 2018) (Fig. 2).
3.1. Current extracellular vesicle isolation methods
EV extraction can be done from a variety of different samples, such as human brain tissue, cerebrospinal fluid (CSF), blood, as well as from mouse tissue and primary and secondary cell culture (Muraoka et al., 2020; Théry et al., 2018). Due to not having a complete understanding of the characterization of these EVs, the extraction processes can be variable. The proper extraction methods for the different kinds of EVs are still debatable, causing there to be a myriad of protocols available. There is no current gold standard for EV isolation, especially one that is applicable to extraction from all biofluids. In a recently updated guideline published in 2018 titled Minimal Information for Studies of Extracellular Vesicles (MISEV), the authors describe the different methods of separating EVs and create guidelines for verifying that the isolated sample effectively yields EVs (Théry et al., 2018).
EV separation from frozen unfixed human and mouse brain tissue has been done successfully. The methods begin with digestion of the brain tissue, followed by differential centrifugation, and completed with either a sucrose gradient ultracentrifugation (SG-UC) or size exclusion chromatography (qEV) (Muraoka et al., 2020). When using both SG-UC and qEV, although qEV yielded a higher EV particle number compared to SG-UC, both methods showed the EVs to be enriched in the necessary EV identification markers, such as CD9, CD81, annexins, and other specific lysosomal markers (Muraoka et al., 2020). Additionally, EVs can also be isolated from cells grown in vitro, using methods such as differential centrifugation and ultracentrifugation (Mathivanan et al., 2010b), precipitation kits (ExoQuick) (Kaur et al., 2014) or the use of total exosome isolation reagent (Wen et al., 2020). Interestingly, there is also an EV capture method that uses a synthetic peptide (Vn96) that has a high binding affinity to heat shock proteins, that proved to be superior to ultracentrifugation (Ghosh et al., 2014). This method captures EVs from small samples, and is available mainly for cells in culture, but can also be applied to biofluids such as plasma and urine.
Isolating EVs from fixed frozen brain tissues or cells grown in vitro is helpful for identifying potential biomarkers, but it is the use of biofluids which takes extraction of EVs from the bench to the clinic. There are many available biofluids to separate EVs from, such as plasma, serum, cerebrospinal fluid, and saliva. In blood, there is the choice to isolate sera or plasma. EVs are more easily recovered from and are more abundant in sera than plasma (George et al., 1982). Additionally, a study published in 2012, analyzing micro-RNA expression in early non-small cell lung cancer, found that expression levels in serum did not correlate with the levels in plasma (Heegaard et al., 2012). The available methods for isolating EVs from plasma are size exclusion chromatography, ultracentrifugation, and precipitation kits (ExoQuick and ExoQuick ULTRA) (Ter-Ovanesyan et al., 2021). These methods are still being optimized and compared for contamination level, relative EV recovery, and known surface markers such as CD81, CD63, and CD9 (Ter-Ovanesyan et al., 2021). Overall, additional studies need to be run in order to identify the most reliable option for serum and plasma EV isolation. Another available biofluid that contains EVs is CSF, although it has been reported to be difficult to retrieve a large amount from realistic samples of CSF (Street et al., 2012). The available methods for isolation of EVs from CSF range from differential centrifugation and affinity capture (MagCapture) (Muraoka et al., 2020), to precipitation kits (ExoQuick and ExoQuick ULTRA), qEV and ultracentrifugation (Ter-Ovanesyan et al., 2021). Saliva, a more readily available biofluid, is also an explored source to isolate EVs. In this case, differential centrifugation, ultracentrifugation (Lässer et al., 2011; Sharma et al., 2011) and gel filtration (Ogawa et al., 2008) are the few studied methods for saliva EVs. Overall, over the past decade, the methods for separation of EVs have continued to evolve which is why it is imperative to establish reliable and non-variable methods for this field of study.
4. Extracellular vesicle role in glioblastoma multiforme
EVs derived from gliomas or non-glioma cells in the tumor microenvironment are involved in tumor cell proliferation, invasion, malignancy, and drug resistance (Balakrishnan et al., 2020; Matarredona and Pastor, 2019; Mathieu et al., 2019; Xia et al., 2019). Cancer cells are shown to release higher amounts of EVs compared to non-malignant cells, and these cancer EVs are what help communicate with other nearby cells, leading to promotion of tumorigenesis (Bebelman et al., 2018). Additionally, cancer EVs alter the behavior of the local and recruited pericytes or other cells, which results in the generation of a tumor-promoting niche supporting tumor angiogenesis, immunosuppression, and the acquisition of malignant traits by cancer cells (Bebelman et al., 2018).
The development of a perfect method to purify EVs may still be in progress, but there have been explorations in extracting RNA from different biofluid-derived EVs. For example, blood plasma is the most used source of EV-RNAs, as it mainly contains EVs present in circulating blood, compared to using blood serum that contains EVs released by platelets (Antwi-Baffour et al., 2015; Mateescu et al., 2017). Studies have shown EV-contained miRNA 21 (miR-21) and miRNA 128 (miR-128) were upregulated in the blood of GBM patients (Holdhoff et al., 2013; Roth et al., 2011). Furthermore, when comparing levels of miR-21 expression between patients with and without GBM, the levels of miR-21 are significantly greater in microvesicles originating from the CSF of patients with GBM (Akers et al., 2013). The actual process of RNA sorting into EVs of specific cell types is unknown but thought to be specific to parent cell and location of formation of EVs. Most EV isolation methods are not able to distinguish between different vesicle subpopulations (exosomes vs microvesicles), and the exact subpopulations relevant to a particular disease state, making sorting of RNA difficult to detect in heterogeneous EV mixtures (Mateescu et al., 2017). Additionally, the amount of sample available can act as a limiting factor, especially when trying to isolate such a small amount of EVs, and subsequently, a small amount of RNA. There are, however, ways to distinguish the origination of vesicle based on the RNA cargo contained or released from a group of EVs.
In addition to obtaining RNA and DNA from EVs, researchers have been able to identify protein content in EVs. In a recent 2021 publication, Greco et al compare serum-derived EV protein profiles against murine CSF and serum, and conclude there to be significant differences in protein levels contained in EVs compared to these two biofluids during GBM progression (Greco et al., 2021). Additionally, proteins such as GFAP, Vascular Endothelial Growth Factor (VEGF), basic Fibroblast Growth Factor (bFGF), Chitinase-3-Like Protein (CHI3L1 or YKL-40), and Matrix Metalloproteinase 9 (MMP9) (Redzic et al., 2014), have been identified as potential EV biomarkers in GBM.
EVs contain abundant proteins and RNAs that can serve as biomarkers for cancer. There have been several potential cancer markers identified from different biofluids (Li et al., 2017). As mentioned, GBM has shown to have a complicated genetic profile as the disease is considered to be pathologically and clinically heterogeneous (Bedard et al., 2013). The involvement of EV in GBM biology and the increasing availability of technologies to study them allows scientists to explore the roles of EV content in tumor progression, biomarker research, and therapeutic approaches. Here, we will take the time to delve into the three niches described above (SVZ, PVN, and the immune microenvironment), their specific EV cargo, and the impact this interaction can have on GBM diagnosis and prognosis.
4.1. Neurogenic niche cell specific extracellular vesicle cargo
GSCs, neurons, and glia form and release EVs within the neurogenic niche (Losurdo and Grilli, 2020). With EVs being recognized for their contribution to intercellular gene regulation, it is no surprise that they play key roles in the maintenance and homeostasis of the neurogenic niche and its interaction with GBM.
In some cases, the EVs released by GSCs help establish a fundamental crosstalk with other local GSCs. miRNA-21a has been identified in NSC-EV cargo through RNA sequencing and is shown to increase NSC proliferation via targeting the genes SOX2 and Stat3 (Ma et al., 2019a) , both of which contribute to GBM malignancy and are upregulated in GBM (Li et al., 2019). NPC-EVs also contain growth-factor-associated proteins, such as EGF-like domain and the EGF-like calcium-binding domain, which also promote GSC proliferation but instead by means of ERK pathways downstream (Ma et al., 2019b). Along with those that moderate stem cell quiescence/proliferation ratio, other molecules with roles in regulating adult neurogenesis and NSC fate have been identified in the cargo of NSC and NPC-EVs (Losurdo and Grilli, 2020). This would include miR-9 (Xia et al., 2019; Zhao et al., 2009), miR-let7b (Ma et al., 2019a; Morton et al., 2018; Zhao et al., 2010), miR-124, and miR-137 (Bielefeld et al., 2017).
Aside from initiating positive and negative feedback loops amongst themselves (NSCs), many of the miRNAs being carried and released by GSC-derived EVs appear to have immunological relevance as they show to selectively target microglia. For instance, miR-9, miR-let-7, miR-26a, miR-181c have been identified in GSC-EVs and are known to be associated with microglia physiology and general morphology (Kumar et al., 2015; Lehmann et al., 2012; Yao et al., 2014; Zhang et al., 2015). In some studies, SVZ NSC-EVs were shown to function as microglia morphogens as they were observed to activated immune/inflammatory response-related transcriptional programs (Morton et al. 2018). These NSC-EVs show to bridge communication between the immune microenvironment and the neurogenic niche as said EVs have displayed their potential to increase the number of CD11b+ microglia and increase their cytokine release in the SVZ (Morton et al., 2018). What makes this type of immune-associated interaction even more interesting, this communication still takes place between host microglia and grafted NPCs (Cossetti et al., 2014; Matarredona et al., 2018; Pluchino and Cossetti, 2013). Other studies have noted an upregulated expression of CD11b in microglia to be associated with activation (Hoek et al., 2000; Kierdorf and Prinz, 2013).
However, this EV-based cross talk stemming out of the neurogenic niche proves to be bilateral. Specifically, EVs generated by non-GSCs are capable of altering cellular mechanisms of neurogenic niche components, especially in the context/framework of GBM. To regulate its TME, GBM cells can recruit and alter the phenotype of non-tumor cells (Wang et al., 2019). It’s also been observed that NSCs can advance tumorigenesis by migrating towards gliomas to then disperse throughout the tumor bed (Aboody et al., 2000). EVs have the potential to contribute to diverse processes in cell-cell communication. The specific mechanisms affected by EVs depend upon the cellular microenvironment as this determines the contents packaged into EV (Bahram Sangani et al., 2021; Cossetti et al., 2014). In the case of NSC cultures treated with glioblastoma-derived EVs, the cells appeared to increase in proliferation and migration rate resembling that of a transformed cell. In other words, it was confirmed that GBM cells could carry over biological information to local NSCs in a way that transformed them into becoming cancerous (Wang et al., 2019). Considering that recurrent GBMs are often found in white matter bordering regions like the SVZ and the poor prognosis of SVZ-contacting GBM patients (compared to non-contacting SVZ GBM) (Ellingson et al., 2013), EVs may provide an explanation or may even function as an agent that prompts this phenomenon.
4.2. Immune microenvironment cell specific extracellular vesicle cargo
Microglia and astrocytes are two important components of an intracranial tumor immune microenvironment, where both display plasticity in their nature/behavior to either induce or hinder neurogenic processes, eliciting appropriate responses to changes in the neurogenic niche. Microglia activity can tip the scale of adult neurogenesis by either inducing or hindering the proliferation of NSCs, as well as the differentiation and survival of adult-borne neurons (Losurdo and Grilli 2020). Cytokines generated and released by microglia have a significant impact on adult neurogenesis given their ability to prompt different inflammatory processes in the brain. EVs released by M1-phase, or active microglia have been found to carry cytokines as well, such as interleukin-6 (IL-6), that promote NSC proliferation as well as neuronal maturation (Bowen et al., 2011). Similarly, reactive astrocytes (or RAs) also play a role in controlling neurogenesis bidirectionally. EVs released by these astrocytes contain enzymes such as EAAT-1 (Espósito et al., 2005; Ge et al., 2006) and NTPDases (Gampe et al., 2015) which can increase GSC differentiation and decrease NSC proliferation respectively (Losurdo and Grilli, 2020). Astrocyte-derived EVs were also found to carry neuroglobin, which functions as a neuroprotectant while simultaneously carrying out increased GSC proliferation associated with Wnt signaling in numerous brain injury models (Yu et al., 2018b).
The reciprocal aspect of this relationship shows EVs generated and released by neighboring GBM cells can manipulate these processes and their resulting products to favor or hinder GSC-related mechanisms maintaining the TME. Similar to what was described with gliomas cells inducing NSCs towards a tumor-promoting phenotype, glioma cells show a similar ability to induce the transition of an astrocyte in an active-phase or reactive astrocyte (Yu et al., 2018a). Consequentially, this transformed type of astrocyte has been seen to carry and release O6-alkylguanine DNA alkyltransferase (AGT) to other local glioma cells (Yu et al., 2018a). This cargo holds clinical relevance as it is associated with TMZ resistance in tumor treatments like that for GBM (Fig. 1).
Figure 1. Extracellular vesicles participation in the communication between glioblastoma cells and non-cancer cells in the neurogenic, immune, and perivascular tumor microenvironment.
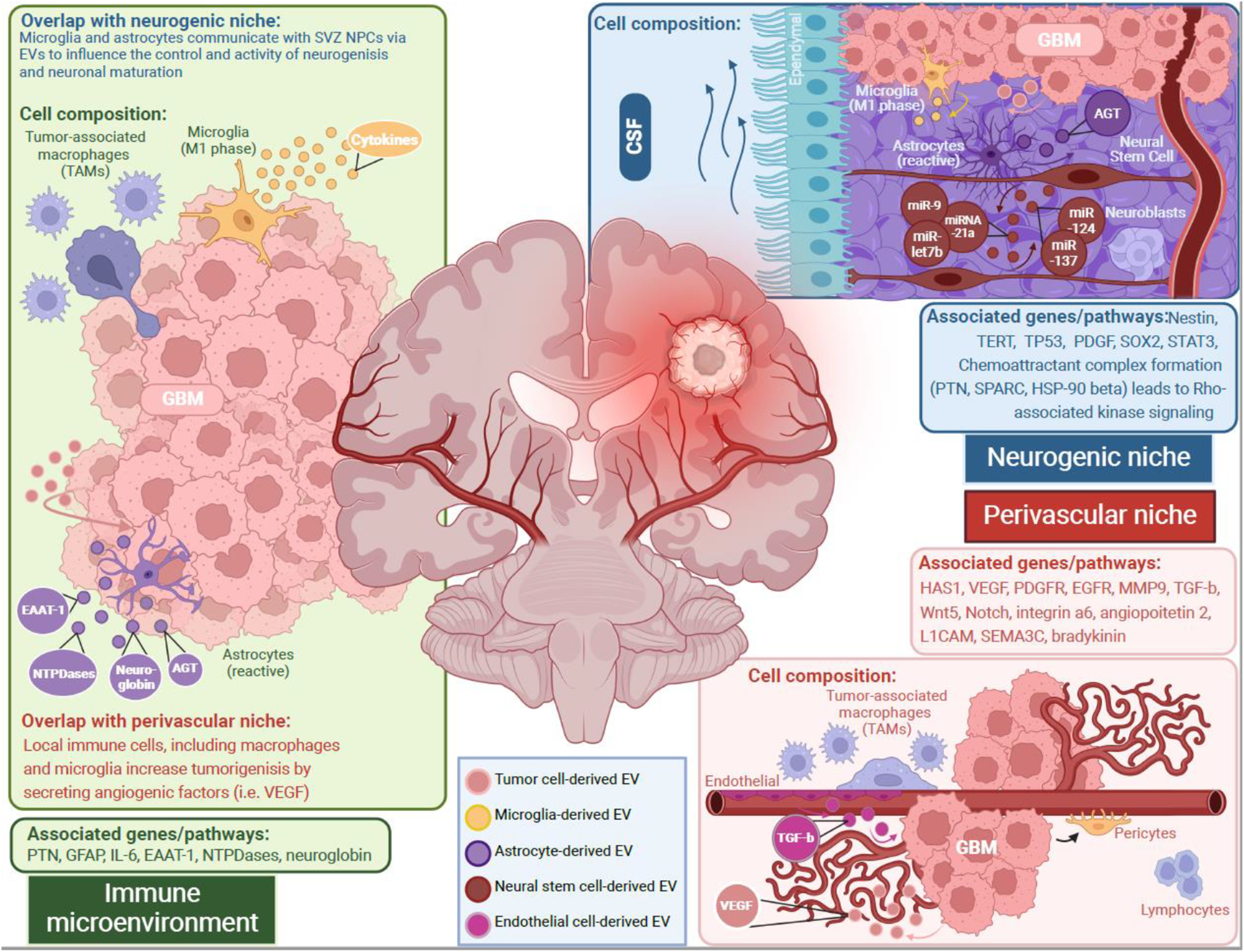
Each microenvironment presents niche-specific cell types and pathways that regulate tumor cell behavior. In each tumor microenvironment, there is reciprocal intercellular transport of proteins and microRNAs. Immune cells can release extracellular vesicles with cargo that impacts neural stem cell proliferation, such as cytokines from microglia extracellular vesicles which increase neural stem cell proliferation or EAAT-1 from activated astrocyte extracellular vesicles that decrease neural stem cell proliferation. These astrocytes can also increase tumor cell proliferation and survival with extracellular vesicles containing neuroglobin and alkylguanine DNA alkyltransferase respectively. Neural stem cells of the neurogenic niche can release extracellular that increase activated microglia and their cytokine release. Said neural stem cells also establish a crosstalk between themselves moderating quiescence and adult neurogenesis (miR-9, miRNA-21a, miR-let7b, miR-124, miR-137). Glioblastoma stem cells can manipulate this regulation by releasing extracellular vesicles that transform neural stem cells towards being cancerous. In the perivascular niche, endothelial cells have been seen to release extracellular vesicles containing TGF-β which prompts local glioblastoma stem cells towards differentiating into pericytes. Glioblastoma stem cells are also seen to recruit tumor-associated macrophages to increase tumor growth as well as supporting angiogenesis by increased VEGF.
4.3. Perivascular niche cell specific extracellular vesicle cargo
Due to its proximity to blood vessels, the PVN is characterized by being involved in angiogenesis, but also notably responsible for tumor invasion, NSC survival, and drug resistance. In vitro, genes related to angiogenesis, such as HAS1, VEGF, PDGFR, EGFR, MMP9, TNC, were upregulated in GBM (Ngo and Harley, 2019). Most of these observed upregulated genes are members of signaling pathways that are known to be altered in GBM. We know that proteins from a few of the markers listed above can be successfully isolated from EVs, and therefore used as potential specific biomarkers for the PVN in GBM. Severity of the tumor progression can be measured using these biomarkers extracted from EVs. There are many different cell types that interact in the PVN, and therefore provide several potential cell specific EV markers. For example, GSCs are differentiated into pericytes by TGF-b (Cheng et al., 2013), an exosomal surface marker (Shelke et al., 2019), and therefore a possible biomarker of PVN derived EVs. Furthermore, GSCs in the PVN recruit TAMs that promote tumor growth, while also inducing overexpression of MMP9 (Ye et al., 2012; Zhou et al., 2015), making this protein a potential candidate biomarker in the PVN. There also have been many other pathways that have been identified to be crucial in GBM-PVN interactions and serve as prospective biomarkers and targetable axes for therapy. These include Wnt5 (Hu et al., 2016), Notch (Zhu et al., 2011), integrin a6 (Lathia et al., 2010), angiopoietin 2 (Bentolila et al., 2016), L1CAM (Burgett et al., 2016), SEMA3C (Man et al., 2014), and bradykinin (Montana and Sontheimer, 2011), all of which are involved in GSC survival, progression, and angiogenesis. To date, there are no miRNA markers specific to the GBM-PVN model (Fig. 1).
5. Translational Uses of EVs
As previously discussed, EVs contain many proteins and types of RNAs that can be isolated and traced to specific brain regions. Both exosomes and microvesicles have specific surface markers, and therefore allow EVs to be purified from many different biological samples by checking for the presence of these markers. Moreover, we also discussed certain miRNAs (Holdhoff et al., 2013; Roth et al., 2011) and proteins (Redzic et al., 2014) that are being explored as possible biomarkers for GBM. Different cellular components released by tumor cells or antigen presenting cells (dendritic cells, macrophages, B cells) can be efficiently packaged into exosomes and serve as cargo transporters in cancers (Tran et al., 2015). By combining what is known about EVs and GBM, there is potential to use GBM-EVs and the cells involved as both a diagnostic and therapeutic tool.
For diagnostic purposes, EV cargo can act as the perfect biomarkers for GBM. Previous studies have shown that cancer cell derived EVs are highly enriched in proangiogenic factors miR-9 (Zhuang et al., 2012) and miR210 (Tadokoro et al., 2013), signaling factors involved in cell invasion, such as IL-6 and VEGF (Skog et al., 2008), and promoters of tumor progression such as neutral sphingomyelinase 2 (nSMase 2) (Kosaka et al., 2013). Additionally, differing expression levels of circulating miR-146b, miR-221, miR-let7a, miR-155, miR-17–5p, miR-27a and miR-106a in serum and plasma in small cell lung cancer correlated with different patient mortality stages (Heegaard et al., 2012). In GBM, miR-21 and miR-128 were upregulated in the blood and CSF in microvesicles of GBM patients (Akers et al., 2013; Holdhoff et al., 2013; Roth et al., 2011). Furthermore, proteins such as GFAP, VEGF, bFGF, CHI3L1, MMP9 (Redzic et al., 2014), have been identified as potential biomarkers in GBM. In a proteomic study of serum derived EVs, Greco et al., identified 9 proteins that were present in all serum EV samples (Greco et al., 2021), making these proteins additional potential biomarkers in this specific biofluid. Using EVs and their cargo as a diagnostic tool for GBM is the first step in gaining a better characterization of these highly heterogenous tumors. As discussed above, there are many already identified potential biomarkers for GBM, but much more work needs to be done in this elusive field. Together, the known biomarkers that are upregulated in GBM, need to be investigated further in the context of applying them as GBM-EV biomarkers (Table 1).
EVs could also act as delivery vehicles for tumors. Drug resistance has been a large obstacle to evade when attempting to treat cancers, leaving many tumor cells to have a low drug response rate. Since EVs serve as intercellular communicators (Mathivanan et al., 2010a), they are able to interact with membranes of other cells and deliver cargo (Théry et al., 2006; van Niel et al., 2018). A previous study looking at multiple drug resistance (MDR) in cancer has shown that exosomes released by macrophages carrying paclitaxel (PTX) have a higher cytotoxicity to drug resistant cells (Kim et al., 2016). Additionally, breast cancer cell derived EVs can transfer resistance of docetaxel, an anti-cancer chemotherapy drug, to cells that are sensitive to the drug (Lv et al., 2014). In non-small cell lung carcinoma cells, the transfer of the pro-survival Akt/mTOR complex through EV cargo leads to resistance to gefitinib (Choi et al., 2014), a typical drug used to treat non-small cell lung cancer. In regard to glioblastoma, reactive astrocyte exosomes can deliver AGT to glioma cells, resulting in TMZ resistance in cancer cells (Yu et al., 2018a). An early study conducted in 2011 used exosome mediated delivery of Lamp2b, and exosomal membrane protein, from engineered dendritic cells in mice to silence genes related to Alzheimer’s Disease (Alvarez-Erviti et al., 2011). Additionally, researchers found a crosstalk between cardiac fibroblasts that secrete miR-21 from their exosomes to cardiomyocytes, inducing hypertrophy (Bang et al., 2014). Mechanistic studies have shown that activation of the PI3K-Akt signaling pathway is how glioblastoma cell-derived EVs have their effect on promoting tumorigenesis on recipient NPCs, but when inhibiting this pathway, the effect of GSC-EVs on target cells is reversed (Pan et al., 2022). In ovarian serious cystadenocarcinoma, normal and overexpression of exosome protein miR-940 inhibits proliferation and migration, triggers cell cycle arrest, and reduces downstream signaling of cancer promoting pathways such as PI3K-Akt and FAK (Rashed et al., 2017). The PTEN/AKT cancer signaling pathway can be targeted by MSC-EV delivery of miR-144, in order to alleviate cell apoptotic injury in hypoxic conditions (Wen et al., 2020).
Moreover, EVs derived from the tumor microenvironment are involved in tumor cell proliferation, invasion, and malignancy of the tumor (Mathieu et al., 2019; Xia et al., 2019). Due to the numerous cell types in the tumor microenvironment, crosstalk between cells is common, and even promoted when in the presence of EVs (Mathivanan et al., 2010a). Mesenchymal stem cells (MSCs) are one source of EVs (Pascucci et al., 2014) that were previously discussed as an avenue for drug therapeutics, but there are other cell types that produce EVs. T cell derived EVs expressing CD47, were shown to interact with endothelial cells and alter gene expression of endothelial genes, which in turn affects cell proliferation and other physiological processes (Kaur et al., 2014). MSC-derived microvesicles that are carrying PTX show a strong anti-proliferative activity on human pancreatic cancer cell lines (Pascucci et al., 2014). More recently, bone marrow MSC exosomes show improved tumor targeting and accumulation of drug at the site of the tumor in pancreatic cancer cells (Zhou et al., 2021). Additionally, MSCs that were engineered to express miR-29a-3p successfully delivered this miRNA to cells, causing tumor suppressive effects, such as inhibition of migration and alternative angiogenesis in gliomas (Zhang et al., 2021b). These results are promising because it shows the ability of MSCs to package and deliver active drugs through their microvesicle and exosomal cargo, opening a new door for using MSC-EVs as a potential trojan horse in drug delivery. In brain tumors, the concept of targeting glioblastoma progression with engineered MSCs and using EVs as drug delivery vehicles still needs to be explored despite promising results when examining other cancers.
In addition to using EVs as a therapeutic target, researchers have become more interested in targeting the EV formation pathway with the goal of reducing EV formation in cancer cells. For instance, silencing and inhibiting CD9 to evaluate the production and release of EV resulted in the triggering of a compensatory mechanism to maintain EV production (Suárez et al., 2021). Although this is a promising way to reduce biogenesis of EVs carrying tumor promoting factors, the differing biogenesis pathways of the EV subtypes poses a challenge for researchers trying to develop one drug that can block all EV biogenesis (Catalano and O’Driscoll, 2020). In the field, there are two distinct “categories” for drugs that inhibit EV biogenesis: one that affects EV trafficking (calpeptin, manumycin A, and Y27632) and the other that affects lipid metabolism (pantethine, imipramine, and GW4869). For example, within the category of drugs that affect EV trafficking falls calpeptin, a cysteine proteinase inhibitor. The anti-tumoral effects of calpeptin have been studied in a mouse xenograft model of prostate cancer, in which when mice were administered with calpeptin, they showed a significant reduction in tumor growth, a reduced vascularization, an increase in apoptosis, and reduced proliferation of cancer cells (Jorfi et al., 2015). The effects of the rest of the EV trafficking inhibitor drugs have yet to be studied in the context of GBM and EV interactions. Imipramine, a common anti-depression drug, has also been explored in the context of microvesicle release in glial cells and osteoblasts (Bianco et al., 2009; Deng et al., 2017). GW4869 is the only drug that has been studied in the context of GBM EVs, in which the results show that when GBM cells in vitro are treated with GW4869, their microRNA exosomal profiles change (Ipas et al., 2015). The field of EV biogenesis inhibitors needs to be explored further in the context of GBM but seems promising overall.
Another new method to use EVs as a therapeutic tool is to deliver purified EVs to a patient systemically. There have been promising studies conducted in vivo to look at the effects of systemic delivery of cell derived EVs on different murine disease models. Previous results show the ability of EVs carrying specific siRNAs or mRNAs to not only reach target tumor tissue (Ohno et al., 2013), but silence genes involved in neurodegenerative diseases (Alvarez-Erviti et al., 2011), when EVs are injected intravenously in mice. A more recent study published in 2021 showed that in rats, dendritic cell derived EVs that were administered nasally successfully entered the brain and were taken up mainly by oligodendrocytes, with little clearance by the liver (Pusic et al., 2021). These extremely promising results shown in vivo open the doors to potential systemic delivery of EVs in therapeutic trials in humans. To date, this is still a field in which scientists have only scratched the surface in understanding, so therefore only a few clinical trials have been completed.
6. Conclusion
EV derived from GBM or non-GBM cells in the TME are involved in tumor cell proliferation, invasion, malignancy, and drug resistance (Mathieu et al., 2019; Xia et al., 2019). In the neurogenic niche, reciprocal communication between GSC and NSC occurs through soluble factors, direct cell contact, and EVs, resulting in changes that drive tumorigenesis (Wang et al., 2019). In the immune microenvironment, microglia and astrocytes react to the presence of tumor cells by changing their activation status and overall phenotype. This response occurs through multiple signals, including EVs (Bowen et al., 2011; Losurdo and Grilli, 2020). Finally, in the perivascular niche, the resident endothelial cells release EVs that interact with the tumor cells and contribute to GSC survival, progression, and angiogenesis (Bentolila et al., 2016; Burgett et al., 2016; Hu et al., 2016; Lathia et al., 2010; Man et al., 2014; Zhu et al., 2011).
Cancer cells are shown to release higher amounts of EVs compared to non-malignant cells, and these cancer EVs help communicate with other nearby cells, leading to promotion of tumorigenesis (Bebelman et al., 2018). Protocols for EV purification and the criteria for their characterization are still being optimized, with very promising advances. Novel technologies, like cell-specific labeling of biomolecules, will allow scientists to better understand the role of EVs in highly heterogeneous GBM tumors. Similarly, it will shed light on their use as diagnostic and therapeutic tools for GBM patients.
Acknowledgements
The authors would like to acknowledge the Mayo Clinic Graduate School of Biomedical Sciences and the University of North Florida for their support. MNR and ESN were supported by the Mayo Clinic Graduate School of Biomedical Sciences; ESN received funds from the Mayo Clinic Center for Regenerative Medicine, the Uihlein Professorship Research Grant, and the National Institute of Health (NIH; F31NS120605). LAW, NZ, and HGC were supported by the Uncle Kory Foundation and NINDS (K01NS11093001).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests Statement
The authors declare no competing interests.
References
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY, 2000. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A 97 (23), 12846–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers JC, Ramakrishnan V, Kim R, Skog J, Nakano I, Pingle S, Kalinina J, Hua W, Kesari S, Mao Y, Breakefield XO, Hochberg FH, Van Meir EG, Carter BS, Chen CC, 2013. MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS One 8 (10), e78115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J, 2008. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol 10 (5), 619–624. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Mateo AS, Merchant-Larios H, 1998. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci 18 (3), 1020–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ, 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29 (4), 341–345. [DOI] [PubMed] [Google Scholar]
- Andersen RS, Anand A, Harwood DSL, Kristensen BW, 2021. Tumor-Associated Microglia and Macrophages in the Glioblastoma Microenvironment and Their Implications for Therapy. Cancers (Basel) 13 (17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NM, Simon MC, 2020. The tumor microenvironment. Curr Biol 30 (16), R921–r925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antwi-Baffour S, Adjei J, Aryeh C, Kyeremeh R, Kyei F, Seidu MA, 2015. Understanding the biosynthesis of platelets-derived extracellular vesicles. Immun Inflamm Dis 3 (3), 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahram Sangani N, Gomes AR, Curfs LMG, Reutelingsperger CP, 2021. The role of Extracellular Vesicles during CNS development. Prog Neurobiol 205, 102124. [DOI] [PubMed] [Google Scholar]
- Balakrishnan A, Roy S, Fleming T, Leong HS, Schuurmans C, 2020. The Emerging Role of Extracellular Vesicles in the Glioma Microenvironment: Biogenesis and Clinical Relevance. Cancers (Basel) 12 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill FR, Capasso M, Hagemann T, 2012. The tumor microenvironment at a glance. J Cell Sci 125 (Pt 23), 5591–5596. [DOI] [PubMed] [Google Scholar]
- Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T, 2014. Cardiac fibroblast–derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. The Journal of Clinical Investigation 124 (5), 2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN, 2006. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444 (7120), 756–760. [DOI] [PubMed] [Google Scholar]
- Bebelman MP, Smit MJ, Pegtel DM, Baglio SR, 2018. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther 188, 1–11. [DOI] [PubMed] [Google Scholar]
- Bedard PL, Hansen AR, Ratain MJ, Siu LL, 2013. Tumour heterogeneity in the clinic. Nature 501 (7467), 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila LA, Prakash R, Mihic-Probst D, Wadehra M, Kleinman HK, Carmichael TS, Péault B, Barnhill RL, Lugassy C, 2016. Imaging of Angiotropism/Vascular Co-Option in a Murine Model of Brain Melanoma: Implications for Melanoma Progression along Extravascular Pathways. Sci Rep 6, 23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, 2005. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 7 (4), 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C, 2009. Acid sphingomyelinase activity triggers microparticle release from glial cells. Embo j 28 (8), 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld P, Mooney C, Henshall DC, Fitzsimons CP, 2017. miRNA-Mediated Regulation of Adult Hippocampal Neurogenesis; Implications for Epilepsy. Brain Plast 3 (1), 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen KK, Dempsey RJ, Vemuganti R, 2011. Adult interleukin-6 knockout mice show compromised neurogenesis. Neuroreport 22 (3), 126–130. [DOI] [PubMed] [Google Scholar]
- Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, 2013. The somatic genomic landscape of glioblastoma. Cell 155 (2), 462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruschi M, Granata S, Santucci L, Candiano G, Fabris A, Antonucci N, Petretto A, Bartolucci M, Del Zotto G, Antonini F, Ghiggeri GM, Lupo A, Gambaro G, Zaza G, 2019. Proteomic Analysis of Urinary Microvesicles and Exosomes in Medullary Sponge Kidney Disease and Autosomal Dominant Polycystic Kidney Disease. Clin J Am Soc Nephrol 14 (6), 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgett ME, Lathia JD, Roth P, Nowacki AS, Galileo DS, Pugacheva E, Huang P, Vasanji A, Li M, Byzova T, Mikkelsen T, Bao S, Rich JN, Weller M, Gladson CL, 2016. Direct contact with perivascular tumor cells enhances integrin αvβ3 signaling and migration of endothelial cells. Oncotarget 7 (28), 43852–43867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschow SI, Nolte-’t Hoen ENM, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, Melief CJM, Raposo G, Wubbolts R, Wauben MHM, Stoorvogel W, 2009. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 10 (10), 1528–1542. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ, 2007. A perivascular niche for brain tumor stem cells. Cancer Cell 11 (1), 69–82. [DOI] [PubMed] [Google Scholar]
- Catalano M, O’Driscoll L, 2020. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles 9 (1), 1703244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A, 2008. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol 89 (2), 219–224. [DOI] [PubMed] [Google Scholar]
- Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ, 2010. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J. Cell Biol 190 (6), 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles N, Holland EC, 2010. The perivascular niche microenvironment in brain tumor progression. Cell Cycle 9 (15), 3012–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC, 2010. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 6 (2), 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H, 2012. The brain tumor microenvironment. Glia 60 (3), 502–514. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF, 2012. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488 (7412), 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Hsu WH, Chang A, Tan Z, Lan Z, Zhou A, Spring DJ, Lang FF, Wang YA, DePinho RA, 2020. Circadian Regulator CLOCK Recruits Immune-Suppressive Microglia into the GBM Tumor Microenvironment. Cancer Discov 10 (3), 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Hambardzumyan D, 2018. Immune Microenvironment in Glioblastoma Subtypes. Front Immunol 9, 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, Bao S, 2013. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153 (1), 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DY, You S, Jung JH, Lee JC, Rho JK, Lee KY, Freeman MR, Kim KP, Kim J, 2014. Extracellular vesicles shed from gefitinib-resistant nonsmall cell lung cancer regulate the tumor microenvironment. Proteomics 14 (16), 1845–1856. [DOI] [PubMed] [Google Scholar]
- Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, Woroniecka K, Elsamadicy AA, Dechant CA, Kemeny HR, Sanchez-Perez L, Cheema TA, Souders NC, Herndon JE, Coumans JV, Everitt JI, Nahed BV, Sampson JH, Gunn MD, Martuza RL, Dranoff G, Curry WT, Fecci PE, 2018. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med 24 (9), 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Théry C, 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30, 255–289. [DOI] [PubMed] [Google Scholar]
- Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D, Alfaro-Cervello C, Saini HK, Davis MP, Schaeffer J, Vega B, Stefanini M, Zhao C, Muller W, Garcia-Verdugo JM, Mathivanan S, Bachi A, Enright AJ, Mattick JS, Pluchino S, 2014. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell 56 (2), 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessio A, Proietti G, Sica G, Scicchitano BM, 2019. Pathological and Molecular Features of Glioblastoma and Its Peritumoral Tissue. Cancers (Basel) 11 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmanis S, Sloan SA, Croote D, Mignardi M, Chernikova S, Samghababi P, Zhang Y, Neff N, Kowarsky M, Caneda C, Li G, Chang SD, Connolly ID, Li Y, Barres BA, Gephart MH, Quake SR, 2017. Single-Cell RNA-Seq Analysis of Infiltrating Neoplastic Cells at the Migrating Front of Human Glioblastoma. Cell Rep 21 (5), 1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TB, Lee A, Hsu M, Sedighim S, Orpilla J, Treger J, Mastall M, Roesch S, Rapp C, Galvez M, Mochizuki A, Antonios J, Garcia A, Kotecha N, Bayless N, Nathanson D, Wang A, Everson R, Yong WH, Cloughesy TF, Liau LM, Herold-Mende C, Prins RM, 2019. Expression of PD-1 by T Cells in Malignant Glioma Patients Reflects Exhaustion and Activation. Clin Cancer Res 25 (6), 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, 2016. Glioblastoma: Overview of Disease and Treatment. Clin J Oncol Nurs 20 (5 Suppl), S2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Conde I, Shrimpton CN, Thiagarajan P, López JA, 2005. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 106 (5), 1604–1611. [DOI] [PubMed] [Google Scholar]
- Deng L, Peng Y, Jiang Y, Wu Y, Ding Y, Wang Y, Xu D, Fu Q, 2017. Imipramine Protects against Bone Loss by Inhibition of Osteoblast-Derived Microvesicles. Int J Mol Sci 18 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn IF, Heese O, Black PM, 2000. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol 50 (1–2), 121–137. [DOI] [PubMed] [Google Scholar]
- Ellingson BM, Lai A, Harris RJ, Selfridge JM, Yong WH, Das K, Pope WB, Nghiemphu PL, Vinters HV, Liau LM, Mischel PS, Cloughesy TF, 2013. Probabilistic radiographic atlas of glioblastoma phenotypes. AJNR Am J Neuroradiol 34 (3), 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler JR, Robinson AE, Smirnov I, Hodgson JG, Berger MS, Gupta N, James CD, Molinaro A, Phillips JJ, 2012. Increased microglia/macrophage gene expression in a subset of adult and pediatric astrocytomas. PLoS One 7 (8), e43339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensenyat-Mendez M, Íñiguez-Muñoz S, Sesé B, Marzese DM, 2021. iGlioSub: an integrative transcriptomic and epigenomic classifier for glioblastoma molecular subtypes. BioData Min 14 (1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF, 2005. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 25 (44), 10074–10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P, 2006. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia 53 (8), 799–808. [DOI] [PubMed] [Google Scholar]
- Gabrusiewicz K, Rodriguez B, Wei J, Hashimoto Y, Healy LM, Maiti SN, Thomas G, Zhou S, Wang Q, Elakkad A, Liebelt BD, Yaghi NK, Ezhilarasan R, Huang N, Weinberg JS, Prabhu SS, Rao G, Sawaya R, Langford LA, Bruner JM, Fuller GN, Bar-Or A, Li W, Colen RR, Curran MA, Bhat KP, Antel JP, Cooper LJ, Sulman EP, Heimberger AB, 2016. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight 1 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampe K, Stefani J, Hammer K, Brendel P, Pötzsch A, Enikolopov G, Enjyoji K, Acker-Palmer A, Robson SC, Zimmermann H, 2015. NTPDase2 and purinergic signaling control progenitor cell proliferation in neurogenic niches of the adult mouse brain. Stem Cells 33 (1), 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh ELK, Sailor KA, Kitabatake Y, Ming G. l., Song H, 2006. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439 (7076), 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JN, Thoi LL, McManus LM, Reimann TA, 1982. Isolation of human platelet membrane microparticles from plasma and serum. Blood 60 (4), 834–840. [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C, 2003. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 314 (1), 15–23. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Davey M, Chute IC, Griffiths SG, Lewis S, Chacko S, Barnett D, Crapoulet N, Fournier S, Joy A, Caissie MC, Ferguson AD, Daigle M, Meli MV, Lewis SM, Ouellette RJ, 2014. Rapid isolation of extracellular vesicles from cell culture and biological fluids using a synthetic peptide with specific affinity for heat shock proteins. PLoS One 9 (10), e110443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimple RC, Bhargava S, Dixit D, Rich JN, 2019. Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev 33 (11–12), 591–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goñi FM, Alonso A, 2009. Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim Biophys Acta 1788 (1), 169–177. [DOI] [PubMed] [Google Scholar]
- Greco F, Anastasi F, Pardini LF, Dilillo M, Vannini E, Baroncelli L, Caleo M, McDonnell LA, 2021. Longitudinal Bottom-Up Proteomics of Serum, Serum Extracellular Vesicles, and Cerebrospinal Fluid Reveals Candidate Biomarkers for Early Detection of Glioblastoma in a Murine Model. Molecules 26 (19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Hu B, Gu W, Xu L, Wang D, Huang H-JS, Cavenee WK, Cheng S-Y, 2003. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am. J. Pathol 162 (4), 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC, 2008. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev 22 (4), 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA, Aronin N, Khvorova A, 2016. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles 5, 32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P, 1984. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur. J. Cell Biol 35 (2), 256–263. [PubMed] [Google Scholar]
- Heegaard NH, Schetter AJ, Welsh JA, Yoneda M, Bowman ED, Harris CC, 2012. Circulating micro-RNA expression profiles in early stage nonsmall cell lung cancer. Int J Cancer 130 (6), 1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrik Heiland D, Ravi VM, Behringer SP, Frenking JH, Wurm J, Joseph K, Garrelfs NWC, Strähle J, Heynckes S, Grauvogel J, Franco P, Mader I, Schneider M, Potthoff AL, Delev D, Hofmann UG, Fung C, Beck J, Sankowski R, Prinz M, Schnell O, 2019. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun 10 (1), 2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD, 2000. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290 (5497), 1768–1771. [DOI] [PubMed] [Google Scholar]
- Holdhoff M, Yovino SG, Boadu O, Grossman SA, 2013. Blood-based biomarkers for malignant gliomas. J Neurooncol 113 (3), 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Wang Q, Wang YA, Hua S, Sauvé CG, Ong D, Lan ZD, Chang Q, Ho YW, Monasterio MM, Lu X, Zhong Y, Zhang J, Deng P, Tan Z, Wang G, Liao WT, Corley LJ, Yan H, Zhang J, You Y, Liu N, Cai L, Finocchiaro G, Phillips JJ, Berger MS, Spring DJ, Hu J, Sulman EP, Fuller GN, Chin L, Verhaak RGW, DePinho RA, 2016. Epigenetic Activation of WNT5A Drives Glioblastoma Stem Cell Differentiation and Invasive Growth. Cell 167 (5), 1281–1295.e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, 2008. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol 20 (1), 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipas H, Guttin A, Issartel JP, 2015. Exosomal MicroRNAs in Tumoral U87 MG Versus Normal Astrocyte Cells. Microrna 4 (2), 131–145. [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Vij M, Jaiswal AK, Srivastava AK, Behari S, Pandey R, 2012. Cytomorphology of giant cell glioblastoma: Report of a case and brief review of literature. Diagn Cytopathol 40 (5), 440–443. [DOI] [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C, 1987. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem 262 (19), 9412–9420. [PubMed] [Google Scholar]
- Jorfi S, Ansa-Addo EA, Kholia S, Stratton D, Valley S, Lange S, Inal J, 2015. Inhibition of microvesiculation sensitizes prostate cancer cells to chemotherapy and reduces docetaxel dose required to limit tumor growth in vivo. Sci Rep 5, 13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S, 2013. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun 4, 2712. [DOI] [PubMed] [Google Scholar]
- Kaur S, Singh SP, Elkahloun AG, Wu W, Abu-Asab MS, Roberts DD, 2014. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol 37, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, Prinz M, 2013. Factors regulating microglia activation. Front Cell Neurosci 7, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV, 2016. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine: Nanotechnology, Biology and Medicine 12 (3), 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T, 2013. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem 288 (15), 10849–10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C, 2016. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 113 (8), E968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bhatia HS, de Oliveira AC, Fiebich BL, 2015. microRNA-26a modulates inflammatory response induced by toll-like receptor 4 stimulation in microglia. J Neurochem 135 (6), 1189–1202. [DOI] [PubMed] [Google Scholar]
- Lässer C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A, Sjöstrand M, Gabrielsson S, Lötvall J, Valadi H, 2011. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN, 2010. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 6 (5), 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN, 2015. Cancer stem cells in glioblastoma. Genes Dev 29 (12), 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann SM, Krüger C, Park B, Derkow K, Rosenberger K, Baumgart J, Trimbuch T, Eom G, Hinz M, Kaul D, Habbel P, Kälin R, Franzoni E, Rybak A, Nguyen D, Veh R, Ninnemann O, Peters O, Nitsch R, Heppner FL, Golenbock D, Schott E, Ploegh HL, Wulczyn FG, Lehnardt S, 2012. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nature Neuroscience 15 (6), 827–835. [DOI] [PubMed] [Google Scholar]
- Li B, Antonyak MA, Zhang J, Cerione RA, 2012. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31 (45), 4740–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li C, Zhou T, Liu X, Liu X, Li X, Chen D, 2017. Role of exosomal proteins in cancer diagnosis. Mol Cancer 16 (1), 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Chen Y, An T, Liu P, Zhu J, Yang H, Zhang W, Dong T, Jiang J, Zhang Y, Jiang M, Yang X, 2019. Nuciferine inhibits the progression of glioblastoma by suppressing the SOX2-AKT/STAT3-Slug signaling pathway. J Exp Clin Cancer Res 38 (1), 139. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS, 2007. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol 9 (4), 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS, 2006. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 5, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losurdo M, Grilli M, 2020. Extracellular Vesicles, Influential Players of Intercellular Communication within Adult Neurogenic Niches. Int J Mol Sci 21 (22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW, 2021. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23 (8), 1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, 1993. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11 (1), 173–189. [DOI] [PubMed] [Google Scholar]
- Lv MM, Zhu XY, Chen WX, Zhong SL, Hu Q, Ma TF, Zhang J, Chen L, Tang JH, Zhao JH, 2014. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol 35 (11), 10773–10779. [DOI] [PubMed] [Google Scholar]
- Ma Y, Li C, Huang Y, Wang Y, Xia X, Zheng JC, 2019a. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun Signal 17 (1), 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Wang K, Pan J, Fan Z, Tian C, Deng X, Ma K, Xia X, Huang Y, Zheng JC, 2019b. Induced neural progenitor cells abundantly secrete extracellular vesicles and promote the proliferation of neural progenitors via extracellular signal-regulated kinase pathways. Neurobiol Dis 124, 322–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man J, Shoemake J, Zhou W, Fang X, Wu Q, Rizzo A, Prayson R, Bao S, Rich JN, Yu JS, 2014. Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation. Cell Rep 9 (5), 1812–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarredona ER, Pastor AM, 2019. Extracellular Vesicle-Mediated Communication between the Glioblastoma and Its Microenvironment. Cells 9 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarredona ER, Talaveron R, Pastor AM, 2018. Interactions Between Neural Progenitor Cells and Microglia in the Subventricular Zone: Physiological Implications in the Neurogenic Niche and After Implantation in the Injured Brain. Front Cell Neurosci 12, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateescu B, Kowal EJ, van Balkom BW, Bartel S, Bhattacharyya SN, Buzás EI, Buck AH, de Candia P, Chow FW, Das S, Driedonks TA, Fernández-Messina L, Haderk F, Hill AF, Jones JC, Van Keuren-Jensen KR, Lai CP, Lässer C, Liegro ID, Lunavat TR, Lorenowicz MJ, Maas SL, Mäger I, Mittelbrunn M, Momma S, Mukherjee K, Nawaz M, Pegtel DM, Pfaffl MW, Schiffelers RM, Tahara H, Théry C, Tosar JP, Wauben MH, Witwer KW, Nolte-’t Hoen EN, 2017. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J Extracell Vesicles 6 (1), 1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M, Martin-Jaular L, Lavieu G, Théry C, 2019. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21 (1), 9–17. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Ji H, Simpson RJ, 2010a. Exosomes: Extracellular organelles important in intercellular communication. Journal of Proteomics 73 (10), 1907–1920. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, Simpson RJ, 2010b. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics 9 (2), 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry AM, Dewan MC, White-Dzuro GA, Brinson PR, Weaver KD, Thompson RC, Ihrie RA, Chambless LB, 2017. Decreased survival in glioblastomas is specific to contact with the ventricular-subventricular zone, not subgranular zone or corpus callosum. J Neurooncol 132 (2), 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry AM, Wooten DJ, Davis LT, Mobley BC, Quaranta V, Ihrie RA, 2019. Ventricular-Subventricular Zone Contact by Glioblastoma is Not Associated with Molecular Signatures in Bulk Tumor Data. Sci Rep 9 (1), 1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A, 2010. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7 (2), 150–161. [DOI] [PubMed] [Google Scholar]
- Montana V, Sontheimer H, 2011. Bradykinin promotes the chemotactic invasion of primary brain tumors. J Neurosci 31 (13), 4858–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton MC, Neckles VN, Seluzicki CM, Holmberg JC, Feliciano DM, 2018. Neonatal Subventricular Zone Neural Stem Cells Release Extracellular Vesicles that Act as a Microglial Morphogen. Cell Rep. 23 (1), 78–89. [DOI] [PubMed] [Google Scholar]
- Muraoka S, Lin W, Chen M, Hersh SW, Emili A, Xia W, Ikezu T, 2020. Assessment of separation methods for extracellular vesicles from human and mouse brain tissues and human cerebrospinal fluids. Methods 177, 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo MT, Harley BAC, 2019. Perivascular signals alter global gene expression profile of glioblastoma and response to temozolomide in a gelatin hydrogel. Biomaterials 198, 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R, 2008. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull 31 (6), 1059–1062. [DOI] [PubMed] [Google Scholar]
- Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M, 2013. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21 (1), 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS, 2020. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol 22 (12 Suppl 2), iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Sheng S, Ye L, Xu X, Ma Y, Feng X, Qiu L, Fan Z, Wang Y, Xia X, Zheng JC, 2022. Extracellular vesicles derived from glioblastoma promote proliferation and migration of neural progenitor cells via PI3K-Akt pathway. Cell Commun Signal 20 (1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Viganò L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G, Pessina A, 2014. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. Journal of Controlled Release 192, 262–270. [DOI] [PubMed] [Google Scholar]
- Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suvà ML, Regev A, Bernstein BE, 2014. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344 (6190), 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccin A, Murphy WG, Smith OP, 2007. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 21 (3), 157–171. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Cossetti C, 2013. How stem cells speak with host immune cells in inflammatory brain diseases. Glia 61 (9), 1379–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombo Antunes AR, Scheyltjens I, Duerinck J, Neyns B, Movahedi K, Van Ginderachter JA, 2020. Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic KM, Kraig RP, Pusic AD, 2021. IFNγ-stimulated dendritic cell extracellular vesicles can be nasally administered to the brain and enter oligodendrocytes. PLoS One 16 (8), e0255778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A, 2006. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol 494 (3), 415–434. [DOI] [PubMed] [Google Scholar]
- Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P, 2004. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood 104 (7), 2084–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed MH, Kanlikilicer P, Rodriguez-Aguayo C, Pichler M, Bayraktar R, Bayraktar E, Ivan C, Filant J, Silva A, Aslan B, Denizli M, Mitra R, Ozpolat B, Calin GA, Sood AK, Abd-Ellah MF, Helal GK, Berestein GL, 2017. Exosomal miR-940 maintains SRC-mediated oncogenic activity in cancer cells: a possible role for exosomal disposal of tumor suppressor miRNAs. Oncotarget 8 (12), 20145–20164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redzic JS, Ung TH, Graner MW, 2014. Glioblastoma extracellular vesicles: reservoirs of potential biomarkers. Pharmgenomics Pers Med 7, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov KY, 1991. Cell proliferation and cytogenesis in the mouse hippocampus. Adv Anat Embryol Cell Biol 122, 1–74. [DOI] [PubMed] [Google Scholar]
- Ripari LB, Norton ES, Bodoque-Villar R, Jeanneret S, Lara-Velazquez M, Carrano A, Zarco N, Vazquez-Ramos CA, Quiñones-Hinojosa A, de la Rosa-Prieto C, Guerrero-Cázares H, 2021. Glioblastoma Proximity to the Lateral Ventricle Alters Neurogenic Cell Populations of the Subventricular Zone. Front Oncol 11, 650316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme PA, Drapeau E, Doetsch F, 2008. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos. Trans. R. Soc. Lond. B Biol. Sci 363 (1489), 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth P, Wischhusen J, Happold C, Chandran PA, Hofer S, Eisele G, Weller M, Keller A, 2011. A specific miRNA signature in the peripheral blood of glioblastoma patients. J Neurochem 118 (3), 449–457. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A, 2004. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427 (6976), 740–744. [DOI] [PubMed] [Google Scholar]
- Sharma S, Gillespie BM, Palanisamy V, Gimzewski JK, 2011. Quantitative nanostructural and single-molecule force spectroscopy biomolecular analysis of human-saliva-derived exosomes. Langmuir 27 (23), 14394–14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelke GV, Yin Y, Jang SC, Lässer C, Wennmalm S, Hoffmann HJ, Li L, Gho YS, Nilsson JA, Lötvall J, 2019. Endosomal signalling via exosome surface TGFβ−1. J Extracell Vesicles 8 (1), 1650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Fang Y, Wu N, Gould SJ, 2011. Biogenesis of the posterior pole is mediated by the exosome/microvesicle protein-sorting pathway. J. Biol. Chem 286 (51), 44162–44176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ping YF, Zhang X, Bian XW, 2015. Hostile takeover: glioma stem cells recruit TAMs to support tumor progression. Cell Stem Cell 16 (3), 219–220. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB, 2004. Identification of human brain tumour initiating cells. Nature 432 (7015), 396–401. [DOI] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr., Carter BS, Krichevsky AM, Breakefield XO, 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10 (12), 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G, 2005. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat. Cell Biol 7 (9), 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed TC, Treiber JM, Patel K, Ramakrishnan V, Merk A, Smith AR, Carter BS, Dale AM, Chow LM, Chen CC, 2016. Differential localization of glioblastoma subtype: implications on glioblastoma pathogenesis. Oncotarget 7 (18), 24899–24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed TC, Treiber JM, Taha B, Engin HB, Carter H, Patel KS, Dale AM, Carter BS, Chen CC, 2020. Glioblastomas located in proximity to the subventricular zone (SVZ) exhibited enrichment of gene expression profiles associated with the cancer stem cell state. J Neurooncol 148 (3), 455–462. [DOI] [PubMed] [Google Scholar]
- Street JM, Barran PE, Mackay CL, Weidt S, Balmforth C, Walsh TS, Chalmers RT, Webb DJ, Dear JW, 2012. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeher VL, Gaiser JC, Garber RL, 1988. Alternative RNA splicing that is spatially regulated: generation of transcripts from the Antennapedia gene of Drosophila melanogaster with different protein-coding regions. Mol Cell Biol 8 (10), 4143–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for, R., Treatment of Cancer Brain, T., Radiotherapy, G., National Cancer Institute of Canada Clinical Trials, G., 2005. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352 (10), 987–996. [DOI] [PubMed] [Google Scholar]
- Suárez H, Andreu Z, Mazzeo C, Toribio V, Pérez-Rivera AE, López-Martín S, García-Silva S, Hurtado B, Morato E, Peláez L, Arribas EA, Tolentino-Cortez T, Barreda-Gómez G, Marina AI, Peinado H, Yáñez-Mó M, 2021. CD9 inhibition reveals a functional connection of extracellular vesicle secretion with mitophagy in melanoma cells. J Extracell Vesicles 10 (7), e12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH, 2013. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem 288 (48), 34343–34351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Ovanesyan D, Norman M, Lazarovits R, Trieu W, Lee JH, Church GM, Walt DR, 2021. Framework for rapid comparison of extracellular vesicle isolation methods. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS, 2006. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev. Cell 10 (3), 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Amigorena S, Raposo G, Clayton A, 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3, Unit 3.22. [DOI] [PubMed] [Google Scholar]
- Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach J-M, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang Y-T, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG-E, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano S-I, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers E-M, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee M-S, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr., Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PRM, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr., Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang J-Y, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK, 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7 (1), 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M, 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319 (5867), 1244–1247. [DOI] [PubMed] [Google Scholar]
- Tran T-H, Mattheolabakis G, Aldawsari H, Amiji M, 2015. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clinical Immunology 160 (1), 46–58. [DOI] [PubMed] [Google Scholar]
- Tricarico C, Clancy J, D’Souza-Schorey C, 2017. Biology and biogenesis of shed microvesicles. Small GTPases 8 (4), 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G, 2011. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell 21 (4), 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, Raposo G, 2018. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol 19 (4), 213–228. [DOI] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, 2010. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17 (1), 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu J, Sun G, Meng H, Wang J, Guan Y, Yin Y, Zhao Z, Dong X, Yin S, Li H, Cheng Y, Wu H, Wu A, Yu X, Chen L, 2019. Glioblastoma extracellular vesicles induce the tumour-promoting transformation of neural stem cells. Cancer Lett 466, 1–12. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sun D, Chen YJ, Xie X, Shi Y, Tabar V, Brennan CW, Bale TA, Jayewickreme CD, Laks DR, Alcantara Llaguno S, Parada LF, 2020. Cell Lineage-Based Stratification for Glioblastoma. Cancer Cell 38 (3), 366–379.e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H, 2014. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun 5, 4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Mai Z, Zhu X, Wu T, Chen Y, Geng D, Wang J, 2020. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther 11 (1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL, 2008. The tumor microenvironment and its role in promoting tumor growth. Oncogene 27 (45), 5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler F, Kienast Y, Fuhrmann M, Von Baumgarten L, Burgold S, Mitteregger G, Kretzschmar H, Herms J, 2009. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia 57 (12), 1306–1315. [DOI] [PubMed] [Google Scholar]
- Xia X, Wang Y, Huang Y, Zhang H, Lu H, Zheng JC, 2019. Exosomal miRNAs in central nervous system diseases: biomarkers, pathological mediators, protective factors and therapeutic agents. Prog Neurobiol 183, 101694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Gould SJ, 2013. The cis-acting signals that target proteins to exosomes and microvesicles. Biochem Soc Trans 41 (1), 277–282. [DOI] [PubMed] [Google Scholar]
- Yao H, Ma R, Yang L, Hu G, Chen X, Duan M, Kook Y, Niu F, Liao K, Fu M, Hu G, Kolattukudy P, Buch S, 2014. MiR-9 promotes microglial activation by targeting MCPIP1. Nat Commun 5, 4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, Xiao HL, Wang B, Yi L, Wang QL, Jiang XF, Yang L, Zhang P, Qian C, Cui YH, Zhang X, Bian XW, 2012. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol 189 (1), 444–453. [DOI] [PubMed] [Google Scholar]
- Yu T, Wang X, Zhi T, Zhang J, Wang Y, Nie E, Zhou F, You Y, Liu N, 2018a. Delivery of MGMT mRNA to glioma cells by reactive astrocyte-derived exosomes confers a temozolomide resistance phenotype. Cancer Lett 433, 210–220. [DOI] [PubMed] [Google Scholar]
- Yu Z, Cheng C, Liu Y, Liu N, Lo EH, Wang X, 2018b. Neuroglobin promotes neurogenesis through Wnt signaling pathway. Cell Death Dis 9 (10), 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GL, Wang CF, Qian C, Ji YX, Wang YZ, 2021a. Role and mechanism of neural stem cells of the subventricular zone in glioblastoma. World J Stem Cells 13 (7), 877–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, Fang J, Rampersaud S, Hoshino A, Matei I, Kenific CM, Nakajima M, Mutvei AP, Sansone P, Buehring W, Wang H, Jimenez JP, Cohen-Gould L, Paknejad N, Brendel M, Manova-Todorova K, Magalhães A, Ferreira JA, Osório H, Silva AM, Massey A, Cubillos-Ruiz JR, Galletti G, Giannakakou P, Cuervo AM, Blenis J, Schwartz R, Brady MS, Peinado H, Bromberg J, Matsui H, Reis CA, Lyden D, 2018. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20 (3), 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li YJ, Wu XY, Hong Z, Wei WS, 2015. MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4. J Neurochem 132 (6), 713–723. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Guo X, Guo X, Yu R, Qian M, Wang S, Gao X, Qiu W, Guo Q, Xu J, Chen Z, Wang H, Qi Y, Zhao R, Xue H, Li G, 2021b. MicroRNA-29a-3p delivery via exosomes derived from engineered human mesenchymal stem cells exerts tumour suppressive effects by inhibiting migration and vasculogenic mimicry in glioma. Aging (Albany NY) 13 (4), 5055–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Sun G, Li S, Lang MF, Yang S, Li W, Shi Y, 2010. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci U S A 107 (5), 1876–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Sun G, Li S, Shi Y, 2009. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol 16 (4), 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN, Bao S, 2015. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol 17 (2), 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, Zhang Y, Liu P, Zhang Y, Li C, Chu Y, Sun T, Jiang C, 2021. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 268, 120546. [DOI] [PubMed] [Google Scholar]
- Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, He X, Hervey-Jumper SL, Heth JA, Muraszko KM, DiMeco F, Vescovi AL, Fan X, 2011. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res 71 (18), 6061–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, Ferrara N, 2012. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. Embo j 31 (17), 3513–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylbersztejn K, Galli T, 2011. Vesicular traffic in cell navigation. Febs j 278 (23), 4497–4505. [DOI] [PubMed] [Google Scholar]


