Abstract
We present the rationale for testing ketamine as an add-on therapy for treating benzodiazepine refractory (established) status epilepticus. In animal studies, ketamine terminates benzodiazepine refractory status epilepticus by interfering with the pathophysiological mechanisms and is a neuroprotectant. Ketamine does not suppress respiration when used for sedation and anesthesia. A Series of reports suggest that ketamine can help terminate refractory and super refractory status epilepticus. We propose to use 1 or 3 mg/Kg ketamine intravenously based on animal-to-human conversion and pharmacokinetic studies. This paper was presented at the 8th London-Innsbruck Colloquium on Status Epilepticus and Acute Seizures held in September 2022
We need better therapies for status epilepticus (SE). Benzodiazepines are an effective first-line therapy [1,2], but approximately one-third of children and 40% of adults do not respond. Patients who fail benzodiazepines are in established SE (ESE), a condition which is not well treated without anesthesia and mechanical ventilation. ESE is terminated in only half of patients treated with second-line anti-seizure medications, such as levetiracetam, fosphenytoin, or valproate [3]. As reported by the Established Status Epilepticus Treatment Trial (ESETT), these medications are equally effective but have a successful response in only 47% of patients, with rates approaching only 52% in children [4]. Open-label pediatric trials in the United Kingdom and Australasia have reported similarly poor success rates [5][6]. When treatment fails, ongoing convulsive SE may cause neurologic injury unless SE is controlled by inducing anesthetic coma, itself an undesirable outcome.
We propose that adding ketamine to a 2nd line regimen will lead to greater success in treating ESE. Ketamine effectively terminates ESE in a preclinical setting, clinicians use it effectively for treating refractory and super-refractory SE[7,8], and emergency physicians have used it extensively and safely for a variety of indications for decades in nonintubated patients. Unlike general anesthetic agents, which would also be effective at stopping ESE, ketamine maintains respiratory drive and airway protective reflexes. An NMDA receptor antagonist, ketamine attenuates self-reinforcing seizure mechanisms underlying SE. It terminates benzodiazepine refractory SE and reduces inhibitory plasticity, prevents excitotoxicity and neuronal death, and prevents epileptogenesis that accompanies SE in experimental animals. Many experts recommend its clinical use early on in SE treatment in the emergency department (ED)[9]. The FDA approved ketamine as an anesthetic in 1970, and it has since been used extensively in emergency settings for sedation, intubation, agitation, and pain management. Ketamine is used as an anesthetic agent in low-resource settings because of its wide safety margin and maintenance of protective airway reflexes.
SE causes respiratory compromise, but ketamine does not[10]:
Generalized convulsive SE is a life-threatening emergency because it causes cardiopulmonary compromise. In the prehospital treatment of status epilepticus (PHTSE) study, 20% of those treated with a placebo and 9% of those treated with benzodiazepines had cardiorespiratory compromise[11]. The Rapid Anticonvulsant Medications Prior to Arrival Trial (RAMPART) treated patients in generalized convulsive SE with midazolam or lorazepam, and 14% required intubation[12]. In ESETT, approximately 25% of adults required intubation within 1 hour of initiating treatment (Chamberlain et al., 2020). Ketamine does not cause respiratory compromise and maintains protective airway reflexes in contrast to other drugs used for treating SE.
SE injures neurons:
The understanding of SE has evolved in the last 20 years. We now recognize the concepts of time t1 (when seizures have progressed to SE) and time t2 (when there is a risk of neuronal damage) [2]. Based on animal and clinical research, t2 begins for convulsive SE between 30–60 minutes. NMDA receptor-mediated excitotoxicity increases neuronal metabolic demand, and respiratory compromise contributes to neurotoxicity [13,14]. SE’s underlying cause and duration determine its morbidity and mortality [15,16]. The Febrile Status Epilepticus Study (FEBSTAT) suggests that SE of 30 minutes, but not individual seizures, injures the hippocampus [17–19]. Early termination of SE can limit the development of refractory SE, neurological injury, and mortality in experimental animals[14,20]. The primary goal of treatment is prompt termination of seizures because adverse consequences of SE increase with seizure duration [14,21,22]
Adding ketamine to the second-line agent shortens SE treatment:
More aggressive second-line therapies are needed to accelerate SE termination. Current evidence-based guidelines for SE guide treatment through a stepwise approach[1]: a benzodiazepine within 5 minutes of SE (or on arrival in the ambulance or the ED); a repeat dose after 5 minutes of continued SE; then a second-line agent such as fosphenytoin (FOS), levetiracetam(LEV), or valproate (VPA) after an additional 5 minutes. Second-line agents are infused over 5–10 minutes (FOS requires 10 minutes) and allowed an additional 10 minutes to take effect. If 2nd line therapy fails, third-line agents include another second-line agent or a general anesthetic. This stepwise approach delays the termination of SE by introducing serial latencies that affect half of all patients with ESE. This timeline also assumes the optimal situation in which medical care (e.g. advanced life support EMS) is available within 5 minutes of the onset of seizures, which is rarely the case. Retrospective analyses of data from academic medical centers also find prolonged delays in treatment and poor outcomes associated with such delays[22]. Failure of second-line agents means that SE will exceed 30–60 minutes, the t2 time point at which the patient is already at risk for permanent neuronal injury. More aggressive second-line therapies are needed. Adding ketamine as an adjuvant, second-line agent should accelerate the termination of SE.
Ketamine terminates both seizures and SE.
Ketamine is a non-competitive NMDA receptor antagonist that produces an open-channel block of NMDA receptors. The anticonvulsant effects of ketamine in experimental animals were first described in 1965. A 2011 review notes more than 40 animal studies in which ketamine has demonstrated effectiveness against convulsions caused by more than 15 different inciting stimuli [23]. Rapidly repeated seizures get progressively longer in a kindling-like phenomenon, which is blocked by ketamine and MK 801, another non-competitive receptor antagonist [24]. In electrical stimulation models of SE, NMDA receptor antagonists can effectively terminate SE [25,26]. In these models, NMDA antagonists are more effective in SE’s prolonged, self-sustaining phase. In electrical stimulation models, non-competitive NMDA receptor antagonists are superior to competitive and allosteric modulators[27]. Ketamine acts synergistically with benzodiazepines in other models of SE [28,29]
NMDA receptor activation triggers pathological neuroplasticity of SE, reduced GABAergic inhibition, and enhanced glutamatergic transmission.
SE is a vicious cycle in which seizures activate NMDA receptors that trigger changes (neuroplasticity) at the neuronal and receptor level, facilitating further seizures and neuronal injury[30,31]. Unique properties of the NMDA receptor make it the master regulator; glutamate-induced depolarization activates and allows the passage of calcium ions. NMDA receptor-mediated calcium entry then triggers dephosphorylation of GABA-A receptors, which accelerates their endocytosis, reducing synaptic inhibition [32] and responsiveness to benzodiazepines[33–35]. During ESE, when benzodiazepines have failed, glutamate-mediated excitatory transmission is enhanced. NMDA receptors and AMPA receptors mediate excitatory transmission in the brain.
SE enhances AMPA receptor-mediated receptor (AMPAR) transmission[36], associated with increased surface expression of the GluA1 subunit and diminished surface expression of the GluA2 subunit of AMPARs[37]. An NMDA receptor antagonist, ketamine blocks the enhancement of AMPAR-mediated transmission during ESE[37].
NMDA receptor-mediated excitatory currents and extrasynaptic receptor-mediated tonic currents are enhanced during SE in experimental animals, accompanied by an accumulation of NMDA receptors on the postsynaptic membrane[31]. These studies suggest trafficking the receptor from the intracellular compartment to the cell membrane, which causes enhancement of synaptic and extrasynaptic NMDA receptor-mediated transmission[31].
Ketamine is neuroprotective:
SE results in neuronal injury and death, which ketamine blocks. Both necrotic and programmed cell death occurs due to SE. NMDA receptors play an essential role in the induction of cell loss during status epilepticus[38,39]. Competitive NMDA receptor antagonists can also offer neuroprotection against SE-induced cell loss. Furthermore, NMDA receptor antagonists prevent the delayed development of epilepsy in animal models [40–43].
Extrapolation of Animal Data to Human Equivalent Doses:
Rat studies, in which animals were not artificially ventilated used doses of ketamine ranging from 30–100 mg/kg intraperitoneally (IP). Using FDA guidance for conversion from rat doses, the corresponding human doses are 4.8 to 16 mg/kg IP, or approximately 2.4 to 8 mg/kg IV (assuming a bioavailability of 50%). serum
PK simulations:
An alternate approach to the use of the FDA guidance is use of PK simulations based on PK parameters reported for rats and humans. PK simulations of 1 mg/kg or 3 mg/kg infusions of ketamine over 5 minutes, or ketamine 4 mg/kg IM (a dose commonly used to achieve dissociative anesthesia for procedural sedation in the ED setting) are shown in Figure 1[44]. 1 mg/kg IV over 5 minutes will attain serum levels for approximately 6–8 minutes that are associated with anticonvulsant effectiveness in rodents (administered 30 mg/kg IP). 3 mg/kg infused over 5 minutes will achieve serum levels associated with dissociative anesthesia for 8–10 minutes. Serum levels following 4 mg/kg IM are predicted to demonstrate a later peak, while achieving serum concentrations that may result in dissociative anesthesia for 15–30 minutes. Thus, clinical effects seen by infusing 3 mg/kg IV over 5 minutes are of shorter duration than the effects of 4 mg/kg IM, a dose commonly used for procedural sedation.
Figure 1.
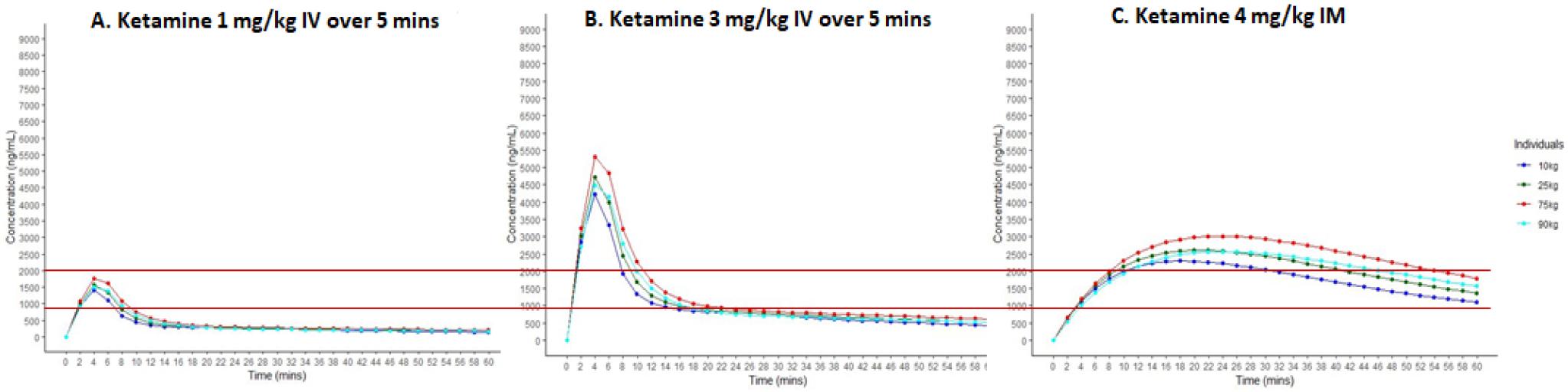
Pharmacokinetic simulations of ketamine 1 mg/kg IV (Panel A), 3 mg/kg IV (Panel B), and 4 mg/kg IM, a common dose for procedural sedation (Panel C). The lower red line is at 900 ng/mL and represents the minimum serum level associated with anti-seizure activity in rodents (Naidoo et al., 2019). PK simulations used PK parameters reported by Sherwin et al. (2015) for IV dosing and Hornik et al. (2018) for IM dosing and fixed dosing at 75 kg and greater. The upper red line at 2000 ng/mL represents serum levels associated with dissociative anesthesia.
Based on pharmacokinetic modeling, animal-to-human dose conversion as proposed by the FDA, data on the safety of ketamine, anesthesia literature, and prior studies of ketamine for super-refractory SE, we propose to test two ketamine doses, 1 and 3 mg/kg, each infused over 5 minutes..
Ketamine has a good safety profile within the dose range for the proposed study:
Ketamine has a good safety profile within the dose range for the proposed study: anesthetic ketamine serum concentrations may be required to interrupt SE. Other general anesthetics are used as third-line or fourth-line agents for SE. Still, these invariably require endotracheal intubation and in current clinical practice, are given too late in the course of SE after neurons are injured. Ketamine, a dissociative anesthetic of short duration, generally maintains airway reflexes and respiratory drive; thus, it may interrupt SE without needing endotracheal intubation[45,46]. The major adverse events caused by ketamine are respiratory, but they are rarely severe or persistent. A meta-analysis of 70,000 adult subjects receiving ketamine found only one adverse cardiorespiratory event with lasting significance [10]. Green (1998) reported on 1,022 consecutive cases of patients treated with ketamine 4 mg/kg IM in children. Apnea occurred in 2 patients, and laryngospasm occurred in 4; none required intubation [44]. A meta-analysis of more than 8,000 patients found laryngospasm in 0.3%, and apnea occurred in 0.8%. No patients required paralytic agents or intubation[47]. Even in those patients receiving an initial IV dose of 3 mg/kg or higher, apnea occurred in 3% and laryngospasm in < 0.5%. It is important to note that ketamine is usually administered as an IV push over 1 minute; we propose a slower infusion over 5 minutes intended to provide a more sustained effect but a lower peak target concentration. In a large prospective registry of pediatric use of ketamine outside the operating room setting, ketamine was administered to 22,645 patients, including 28% who had an American Society of Anesthesiologists (ASA) risk score of 3 or higher (i.e. severe active systemic disease or worse) and thus were at higher risk of adverse outcomes[48]. Fifty-eight percent received concomitant benzodiazepines, and 35% received concomitant propofol. Airway obstruction occurred in 1% and laryngospasm in 0.4%, requiring emergency airway intervention in 0.8%.
Typical doses for ketamine for dissociative anesthesia are 1 to 4.5 mg/kg IV and 6.5 to 13 mg/kg IM [49]. Historically, higher doses of up to 15 mg/kg IM were used[46]. In low-resource settings without mechanical ventilators, ketamine is used as a sole general anesthetic because of its safety profile [50]. Mankikian administered 3 mg/kg IV, followed by a continuous infusion, to adults breathing spontaneously through an endotracheal tube[51]. Functional residual capacity, minute ventilation, and tidal volume were all maintained.
Ketamine is used routinely in the ED setting:
Ketamine is used daily for procedural sedation for painful procedures such as fracture reduction, treating agitation, and pain control in dissociative anesthesia doses. Therefore nurses and physicians are familiar with its clinical effects. Laryngospasm, generally of short duration, may occur in 0.3% of patients. Apnea, also of short duration, may occur in 0.8%, related to the total dose and rate of infusion. Upper airway obstruction responds to re-positioning. Endotracheal intubation is rarely required (upper limits of 95% confidence interval 0.04%,[47].
Alternatives to ketamine exist, including other anesthetic infusions, such as midazolam, propofol, and pentobarbital. While highly effective in terminating seizures, these necessarily produce a loss of respiratory drive and airway protection reflexes, necessitating intubation, an undesirable event that successful treatment is intended to prevent. As above, ketamine is a medication that can potentially overcome the current inability to simultaneously provide seizure control while preventing unnecessary complications from either prolonged seizure activity or from utilizing anesthetic agents that require intubation. Its mechanism-specific, safety, and efficacy profile suggest that ketamine may enable shortening the duration to effective control of SE while minimizing both neurologic and respiratory complications of the existing treatment paradigm.
Declarations of interest:
JK and TBP serve on a Data Safety Monitoring Board of a status epilepticus treatment clinical trial sponsored by Marinus Pharmaceuticals. LC, ESR, TPB JE, JC, JC, SS, RS, and JK receive grant support from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: Treatment of convulsive status epilepticus in children and adults: Report of the guideline committee of the American epilepsy society. Epilepsy Curr 2016;16. 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus - Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56:1515–23. 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- [3].Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D, et al. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N Engl J Med 2019;381:2103–13. 10.1056/NEJMoa1905795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chamberlain JM, Kapur J, Shinnar S, Elm J, Holsti M, Babcock L, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet 2020;0. 10.1016/S0140-6736(20)30611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dalziel SR, Borland ML, Furyk J, Bonisch M, Neutze J, Donath S, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet (London, England) 2019;393:2135–45. 10.1016/S0140-6736(19)30722-6. [DOI] [PubMed] [Google Scholar]
- [6].Lyttle MD, Rainford NEA, Gamble C, Messahel S, Humphreys A, Hickey H, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet 2019;393:2125–34. 10.1016/S0140-6736(19)30724-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alkhachroum A, Der-Nigoghossian CA, Mathews E, Massad N, Letchinger R, Doyle K, et al. Ketamine to treat super-refractory status epilepticus. Neurology 2020;95:e2286–94. 10.1212/WNL.0000000000010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gaspard N, Foreman B, Judd LM, Brenton JN, Nathan BR, McCoy BM, et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia 2013;54:1498–503. 10.1111/EPI.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fujikawa DG. Starting ketamine for neuroprotection earlier than its current use as an anesthetic/antiepileptic drug late in refractory status epilepticus. Epilepsia 2019;60:373–80. 10.1111/EPI.14676. [DOI] [PubMed] [Google Scholar]
- [10].Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med 2008;26:985–1028. 10.1016/J.AJEM.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [11].Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, et al. A Comparison of Lorazepam, Diazepam, and Placebo for the Treatment of Out-of-Hospital Status Epilepticus. 10.1056/NEJMoa002141 2009;345:631–7. . [DOI] [PubMed] [Google Scholar]
- [12].Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. Intramuscular versus Intravenous Therapy for Prehospital Status Epilepticus. N Engl J Med 2012;366:591–600. 10.1056/nejmoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fujikawa DG. Neuroprotective Effect of Ketamine Administered After Status Epilepticus Onset. Epilepsia 1995;36:186–95. 10.1111/j.1528-1157.1995.tb00979.x. [DOI] [PubMed] [Google Scholar]
- [14].Chen JWY, Wasterlain CG. Status epilepticus: Pathophysiology and management in adults. Lancet Neurol 2006;5:246–56. 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- [15].Neligan A, Shorvon SD. Frequency and Prognosis of Convulsive Status Epilepticus of Different Causes: A Systematic Review. Arch Neurol 2010;67:931–40. 10.1001/ARCHNEUROL.2010.169. [DOI] [PubMed] [Google Scholar]
- [16].Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of Mortality in Status Epilepticus. Epilepsia 1994. 10.1111/j.1528-1157.1994.tb02908.x. [DOI] [PubMed] [Google Scholar]
- [17].Nordli DR, Moshé SL, Shinnar S, Hesdorffer DC, Sogawa Y, Pellock JM, et al. Acute EEG findings in children with febrile status epilepticus: Results of the FEBSTAT study. Neurology 2012;79:2180–6. 10.1212/WNL.0b013e3182759766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shinnar S, Bello JA, Chan S, Hesdorffer DC, Lewis DV, Macfall J, et al. MRI abnormalities following febrile status epilepticus in children: the FEBSTAT study. Neurology 2012;79:871–7. 10.1212/WNL.0b013e318266fcc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lewis DV, Shinnar S, Hesdorffer DC, Bagiella E, Bello JA, Chan S, et al. Hippocampal sclerosis after febrile status epilepticus: The FEBSTAT study. Ann Neurol 2014;75:178–85. 10.1002/ana.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+sensitivity of hippocampal dentate granule cell GABA(A) receptors. J Neurosci 1997;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fountain NB, Lothman EW. Pathophysiology of Status Epilepticus. J Clin Neurophysiol 1995;12:326–42. [PubMed] [Google Scholar]
- [22].Gaínza-Lein M, Fernández IS, Jackson M, Abend NS, Arya R, Nicholas Brenton J, et al. Association of time to treatment with short-term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol 2018;75:410–8. 10.1001/jamaneurol.2017.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sivakumar S, Ghasemi M, Schachter SC. Targeting NMDA Receptor Complex in Management of Epilepsy. Pharmaceuticals (Basel) 2022;15. 10.3390/PH15101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kapur JW Lothman E. NMDA receptor activation mediates the loss of GABAergic inhibition induced by recurrent seizures. Epilepsy Res 1990;5. 10.1016/0920-1211(90)90025-Q. [DOI] [PubMed] [Google Scholar]
- [25].Borris DJ, Bertram EH, Kapur J. Ketamine controls prolonged status epilepticus. Epilepsy Res 2000;42. 10.1016/S0920-1211(00)00175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mazarati AM, Wasterlain CG. N-Methyl-D-asparate receptor antagonists abolish the maintenance phase of self-sustaining status epilepticus in rat. Neurosci Lett 1999;265:187–90. 10.1016/S0304-3940(99)00238-4. [DOI] [PubMed] [Google Scholar]
- [27].Yen W, Williamson J, Bertram EH, Kapur J. A comparison of three NMDA receptor antagonists in the treatment of prolonged status epilepticus. Epilepsy Res 2004;59. 10.1016/j.eplepsyres.2004.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia 2008;49:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Niquet J, Baldwin R, Norman K, Suchomelova L, Lumley L, Wasterlain CG. Midazolam–ketamine dual therapy stops cholinergic status epilepticus and reduces Morris water maze deficits. Epilepsia 2016;57:1406–15. 10.1111/epi.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kapur J Role of NMDA receptors in the pathophysiology and treatment of status epilepticus. Epilepsia Open 2018;3:165–8. 10.1002/epi4.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Naylor DE, Liu H, Niquet J, Wasterlain CG. Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobiol Dis 2013;54:225–38. 10.1016/j.nbd.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, et al. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci 2008;28:376–84. 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Goodkin HP, Sun C, Yeh J-L, Mangan PS, Kapur J. GABAA receptor internalization during seizures. Epilepsia 2007;48. 10.1111/j.1528-1167.2007.01297.x. [DOI] [PubMed] [Google Scholar]
- [34].Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABAA receptors during status epilepticus. J Neurosci 2008;28. 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 2005;25:7724–33. 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rajasekaran K, Todorovic M, Kapur J. Calcium-permeable AMPA receptors are expressed in a rodent model of status epilepticus. Ann Neurol 2012;72. 10.1002/ana.23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Joshi S, Rajasekaran K, Sun H, Williamson J, Kapur J. Enhanced AMPA receptor-mediated neurotransmission on CA1 pyramidal neurons during status epilepticus. Neurobiol Dis 2017;103. 10.1016/j.nbd.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fujikawa DG. Neuroprotective Effect of Ketamine Administered After Status Epilepticus Onset. Epilepsia 1995;36:186–95. 10.1111/j.1528-1157.1995.tb00979.x. [DOI] [PubMed] [Google Scholar]
- [39].DG F Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav 2005;7 Suppl 3:3–11. 10.1016/J.YEBEH.2005.08.003. [DOI] [PubMed] [Google Scholar]
- [40].Prasad A, Williamson JM, Bertram EH. Phenobarbital and MK-801, but not phenytoin, improve the long-term outcome of status epilepticus. Ann Neurol 2002;51:175–81. 10.1002/ana.10085. [DOI] [PubMed] [Google Scholar]
- [41].Rice AC, DeLorenzo RJ. NMDA receptor activation during status epilepticus is required for the development of epilepsy. Brain Res 1998;782:240–7. 10.1016/S0006-8993(97)01285-7. [DOI] [PubMed] [Google Scholar]
- [42].Joshi S, Kapur J. N-Methyl-D-Aspartic acid receptor activation downregulates expression of δ subunit-containing GABAA receptors in cultured hippocampal neuronss. Mol Pharmacol 2013;84. 10.1124/mol.112.084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Joshi S, Rajasekaran K, Williamson J, Kapur J. Neurosteroid-sensitive δ-GABAA receptors: A role in epileptogenesis? Epilepsia 2017;58:494–504. 10.1111/epi.13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Green SM, Rothrock SG, Harris T, Hopkins GA, Garrett W, Sherwin T. Intravenous ketamine for pediatric sedation in the emergency department: safety profile with 156 cases. Acad Emerg Med 1998;5:971–6. 10.1111/J.1553-2712.1998.TB02773.X. [DOI] [PubMed] [Google Scholar]
- [45].Green SM, Li J. Ketamine in Adults What Emergency Physicians Need to Know about Patient Selection and Emergence Reactions. Acad Emerg Med 2000;7:278–81. 10.1111/J.1553-2712.2000.TB01076.X. [DOI] [PubMed] [Google Scholar]
- [46].Green SM, Roback MG, Kennedy RM, Krauss B. Clinical practice guideline for emergency department ketamine dissociative sedation: 2011 update. Ann Emerg Med 2011;57:449–61. 10.1016/J.ANNEMERGMED.2010.11.030. [DOI] [PubMed] [Google Scholar]
- [47].Green SM, Roback MG, Krauss B, Brown L, McGlone RG, Agrawal D, et al. Predictors of emesis and recovery agitation with emergency department ketamine sedation: an individual-patient data meta-analysis of 8,282 children. Ann Emerg Med 2009;54. 10.1016/J.ANNEMERGMED.2009.04.004. [DOI] [PubMed] [Google Scholar]
- [48].Grunwell JR, Travers C, McCracken CE, Scherrer PD, Stormorken AG, Chumpitazi CE, et al. Procedural Sedation Outside of the Operating Room Using Ketamine in 22,645 Children: A Report From the Pediatric Sedation Research Consortium. Pediatr Crit Care Med 2016;17:1109–16. 10.1097/PCC.0000000000000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Elkomy MH, Alruwaili N, Elmowafy M, Shalaby K, Drover DR, Ramamoorthy C. Assessment of Ketamine Adult Anesthetic Doses in Pediatrics Using Pharmacokinetic Modeling and Simulations. Pharmacotherapy 2019;39:454–62. 10.1002/PHAR.2243. [DOI] [PubMed] [Google Scholar]
- [50].Makin J, Suarez-Rebling D, Suarez S, Leone A, Burke TF. Operations supported by ketamine anesthesia in resource-limited settings: Surgeons’ perceptions and recommendations – Qualitative Study. Int J Surg Open 2021;29:1–8. 10.1016/J.IJSO.2020.12.009. [DOI] [Google Scholar]
- [51].Mankikian B, Cantineau JP, Sartene R, Clergue F, Viars P. Ventilatory pattern and chest wall mechanics during ketamine anesthesia in humans. Anesthesiology 1986;65:492–9. 10.1097/00000542-198611000-00007. [DOI] [PubMed] [Google Scholar]


