Abstract
PURPOSE OF REVIEW:
Male infertility may be secondary to male genital tract infection (MGTI) in an estimated 15% of cases. In the absence of overt clinical signs, evaluation for MGTI beyond semen analysis is not well established. Therefore, we review the literature on the evaluation and management of MGTI in the setting of male infertility.
RECENT FINDINGS:
A set of international guidelines recommends semen culture and polymerase chain reaction testing, but the significance of positive results remains unclear. Clinical trials evaluating anti-inflammatory or antibiotic treatment report improvements in sperm parameters and leukocytospermia, but data on the effect on conception rates are lacking. Human papillomavirus (HPV) and the novel coronavirus (SARS-CoV-2) have been associated with poor semen parameters and decreased conception rates.
SUMMARY:
The finding of leukocytospermia on semen analysis prompts further evaluation for MGTI including focused physical examination. The role of routine semen culture is controversial. Treatment options include anti-inflammatories; frequent ejaculation; and antibiotics, which should not be used in the absence of symptoms or microbiological infection. SARS-CoV-2 represents a subacute threat to fertility that should be screened for in the reproductive history along with HPV and other viruses.
Keywords: male, infertility, genital, infection
INTRODUCTION
Infertility, defined as inability to achieve pregnancy within 1 year of regular and unprotected sexual intercourse, is estimated to affect 8–30% of reproductive-aged couples worldwide [1–3]. Male factor infertility alone accounts for 20–30% of cases but contributes to 50% of cases overall.4 It is attributable to male genital tract infection (MGTI) in 15% of cases [5–7], making MGTI the third most common cause of male factor infertility after idiopathic infertility (28.4%) and varicocele (18.1%) [8]. Despite this prevalence, there is a lack of consensus among male reproductive specialists regarding how and when to investigate infectious etiologies in the absence of overt clinical symptoms. Here, we review the evaluation and management of MGTI in the setting of male infertility.
INITIAL EVALUATION
The initial evaluation of male infertility consists of a comprehensive history and physical examination followed by semen analysis. Men presenting for fertility evaluation rarely have overt clinical symptoms (e.g., dysuria, pelvic pain, scrotal pain) suggesting presence of MGTI [9]. Therefore, the concern for infection as the cause of male infertility typically begins with the finding of round cells on semen analysis (SA). The American Urological Association (AUA) states that increased round cells on SA, defined as >1 x 106 round cells/mL, should be further evaluated to differentiate white blood cells (WBCs) from native cells of the urogenital tract [10**]. WBCs are a marker of inflammation and may be secondary to infection. Alternatively, round cells may represent germ cells, epithelial cells, and/or degenerated spermatozoa, which are not directly associated with inflammation or infection [11*,12].
The differentiation of round cells may be performed with peroxidase staining [13], Papanicolaou staining [14,15], flow cytometry [16,17], or leukocyte antigen (e.g., CD45) immunohistochemistry [18,19*]. Both the AUA and the American Society for Reproductive Medicine (ASRM) recommend immunohistochemistry as the gold standard for identifying seminal leukocytes given the high sensitivity and specificity of this technique [20]. However, immunohistochemistry is relatively expensive and time-consuming. The World Health Organization (WHO) thus recommends peroxidase staining as an initial test, with follow-up flow cytometry or immunohistochemistry if the resources are available and further differentiation is needed [19*].
The finding of >1 x 106 WBCs/mL is defined as leukocytospermia [20], which may represent a threat to fertility since WBCs generate reactive oxygen species that damage sperm [21,22]. Leukocytospermia is found in 10–20% of infertile men [23]. Elevated seminal leukocytes are associated with abnormal sperm morphology, increased DNA fragmentation index, and significant reductions in sperm concentration and sperm motility [24–26]. Although elevated seminal leukocytes are not statistically correlated with bacteriospermia [26], the association between WBCs and inflammation has led to WHO acknowledging in their manual for human semen analysis that leukocytospermia may be associated with infection and poor sperm quality [19*]. However, there is no direct association between seminal leukocyte concentration and conception rate [27], which is reflected in the AUA’s assertion that seminal leukocyte concentration is not a test of fertility [10**].
Many non-infectious causes of leukocytospermia, such as environmental toxins, substance use, varicocele, autoimmune disorders, and nonbacterial chronic prostatitis have been proposed [26]. If non-infectious etiologies are absent, patients with confirmed leukocytospermia should be evaluated for underlying infection [10**]. A major issue is that there is a lack of formal guidelines specifying the components of this evaluation [20]. For starters, the provider may consider a repeat physical examination, employing focused techniques such as stripping the urethra, palpation of the epididymis, and digital rectal exam to evaluate for urethritis, epididymitis and prostatitis, respectively. Positive exam findings would indicate confirmatory laboratory tests (e.g., urinalysis, urine culture) and appropriate antibiotic treatment [28].
SEMEN MICROBIOLOGICAL ANALYSIS
If there are no positive physical examination findings, the clinician may consider further testing of the semen. While the AUA does not specify such tests, the European Association of Urology (EAU) states that leukocytospermia should be further investigated with semen culture or polymerase chain reaction (PCR) for common genital tract pathogens [29**]. Peroxidase staining with reflexive semen culture may be more cost-effective compared to peroxidase staining alone, given the potential health and cost expenses associated with infection transmission during assisted reproductive technology (ART) procedures [30].
Incidence of positive semen cultures ranges from 6% to 68% based on contemporary studies [31–33*]. This is likely due to differences in laboratory methods (e.g., incubation time, incubation temperature, culture media) and possible contamination by urethral, meatal, and skin flora. The organisms most commonly found on semen culture are gram-positive cocci, including Staphylococcus spp., Streptococcus spp., and Enterococcus spp.; and Enterobacteriaceae, including Escherichia coli [28,31,34,35]. It should be noted that the common genital tract pathogens Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, and Neisseria gonorrhoeae have fastidious growth requirements and are best identified with semen PCR [28,31,36]. Thus, combined microbiological analysis with semen culture and PCR detects a wider range of organisms than either technique alone. A summary of pathogenic male genital tract bacteria and their associated syndromes, diagnostic tests, and treatments is provided in Table 1.
Table 1.
Pathogenic bacteria of the male genital tract
| Organism | Associated clinical syndromes | Diagnostic test of choice | Antibiotic treatment |
|---|---|---|---|
| Chlamydia trachomatis | Urethritis Prostatitis Epididymitis |
NAAT (e.g., PCR) | Doxycycline 100 mg PO BID x 7 days |
| Ureaplasma urealyticum | Urethritis | NAAT (e.g., PCR) | Doxycycline 100 mg PO BID x 7 days |
| Mycoplasma hominis | - | NAAT (e.g., PCR) | Doxycycline 100 mg PO BID x 7 days |
| Neisseria gonorrhoeae | Urethritis Epididymitis |
NAAT (e.g., PCR) | Ceftriaxone 500-1000 mg IM once |
| Enterobacteriaceae (e.g., Escherichia coli) | Prostatitis Epididymitis |
Culture | Levofloxacin 500 mg PO qday x 10 days OR TMP-SMX 160mg/800mg PO BID x 10 days |
| Gram-positive cocci (e.g., Enterococcus spp.) | Prostatitis | Culture | Amoxicillin 500 mg PO TID x 5 days OR Nitrofurantoin 100 mg PO BID x 5 days OR Fosfomycin 3 g PO once |
NAAT: nucleic acid amplification test, PCR: polymerase chain reaction, spp.: species, PO: per os, qday: every day, BID: twice a day, IM: intramuscular.
However, data supporting the clinical utility of semen cultures are lacking. A study using both semen culture and PCR demonstrated that there are no significant differences in the prevalence of Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis between infertile and fertile men, though the presence of Ureaplasma urealyticum was associated with lower mean sperm concentration and lower sperm vitality [37]. Despite only three organisms being investigated, this finding suggests that there are minimal differences in microbiology between fertile and infertile men. In another study, semen cultures performed for men attending an infertility clinic (n = 85) revealed that asymptomatic bacteriospermia did not correlate with abnormal semen parameters [34].
Eini et al. performed semen cultures for men presenting to an infertility clinic (n = 172) and found that leukocytospermia and sperm DNA fragmentation were significantly higher in men with infected samples, relative to those with uninfected samples. There was also deterioration in sperm concentration and motility of infected samples [35].35 Ricci et al. performed a microbiological analysis, including both culture and PCR, of both vaginal/endocervical swabs and semen samples from infertile couples [36].36 This revealed that seminal Enterococcus faecalis was associated with deterioration in sperm motility and morphology. Additionally, Enterococcus faecalis, Ureaplasma urealyticum, and/or Mycoplasma hominis, when found in either partner’s sample, was associated with failed in vitro fertilization [36].36 A systematic review of semen microbiological studies published between 1992 and 2019 found an increased prevalence of Ureaplasma urealyticum in infertile men [33*].33 In addition, Enterococcus faecalis, Chlamydia trachomatis, Ureaplasma urealyticum, and Mycoplasma hominis have been associated with deterioration of semen parameters [33*,38,39].33,38,39
Overall, the clinical relevance of semen culture remains controversial. There is a dearth of literature associating the semen microbiome with natural conception outcomes, and it remains to be seen whether semen culture-guided antibiotics are superior to empiric antibiotics.
TREATMENT
In the absence of consensus guidelines, there have been multiple algorithms proposed for the treatment of male infertility with leukocytospermia and concern for MGTI [11*,28,40].11,28,40 Localizing infectious symptoms (e.g., dysuria, pelvic pain, scrotal pain) or positive physical exam findings (e.g., urethral discharge, prostatic tenderness, epididymal tenderness) may indicate an infectious syndrome such as urethritis, prostatitis, or epididymitis, each of which has its own established treatment guidelines [28]. However, as previously mentioned, MGTIs are often clinically asymptomatic in the context of male infertility workup [9].
Anti-inflammatories
In the setting of male infertility with leukocytospermia and absence of infectious symptoms, the algorithm proposed by Velez et al. eschews semen microbiological analysis, recommending direct treatment with an anti-inflammatory such as ketotifen, valdecoxib, or rofecoxib [11*]. Insofar as leukocytospermia represents inflammation of the male genital tract, the aforementioned medications may be useful because they target non-specific inflammatory processes regardless of whether an infection is present. Use of anti-inflammatories is backed by clinical trials in which treatment resulted in significant improvement of sperm parameters and reduction of seminal leukocytes [41–43]. In the trial evaluating rofecoxib, a 15.8% natural pregnancy rate was achieved after treatment, although no control group was available for comparison [43]. These trials are limited by small sample size and lack of follow-up studies. Nonetheless, given their demonstrated efficacy and lack of adverse effects, anti-inflammatories represent a reasonable treatment option for leukocytospermia when an infectious organism has not been identified (Figure 1).
Figure 1.
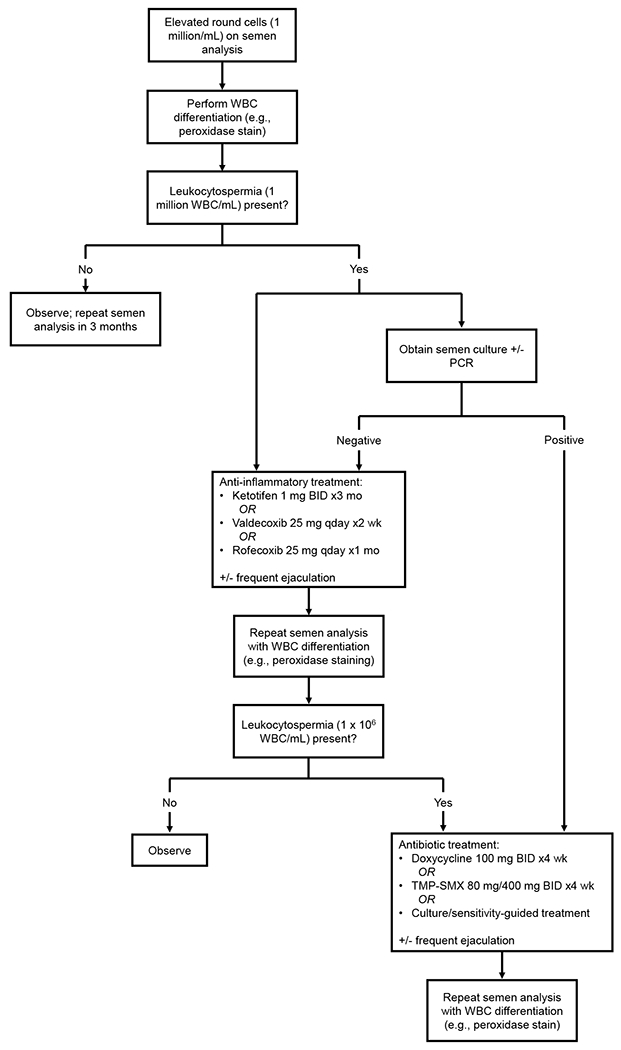
Diagnosis and treatment of leukocytospermia in the setting of male infertility
WBC: white blood cell(s), PCR: polymerase chain reaction, BID: twice a day, qday: every day, TMP-SMX: trimethoprim-sulfamethoxazole.
Antibiotics
In the aforementioned algorithm, anti-inflammatory treatment is followed by repeat semen analysis with peroxidase staining (or other WBC differentiation technique). If leukocytospermia is persistent, antibiotic therapy—doxycycline or trimethoprim-sulfamethoxazole—may be considered [11*]. Antibiotic therapy for male infertility with concern for asymptomatic MGTI is controversial. Many of the clinical trials evaluating such treatments (e.g., doxycycline, TMP-SMX) are from the twentieth century and found no improvement in sperm parameters [44–47]. Though, two of these trials demonstrated reduction of leukocytospermia, with an enhanced effect observed when antibiotic treatment was accompanied by frequent ejaculation [45,46]. A subsequent meta-analysis concluded that antibiotic treatment of male infertility results in increased semen volume, sperm concentration, sperm motility, and normal-form sperm [48]. A more recent clinical trial evaluating antibiotic treatment of asymptomatic MGTI caused by CT or UU also demonstrated significant improvement in all sperm parameters [49], but there remains insufficient evidence that antibiotic treatment increases the rate of conception [44–46,49,50]. Thus, in the absence of clinical infectious symptoms or an isolated pathogen, antibiotic treatment is generally not recommended, but can be attempted if leukocytospermia is refractory to anti-inflammatory treatment (Figure 1).
VIRUSES
The strategies discussed above recognize bacteria as pathogens. Viruses represent another major concern, especially in light of the recent global coronavirus disease 2019 (COVID-19) pandemic, which has been found to affect the male genital tract [51,52]. There are multiple genital tract viruses associated with male infertility including mumps virus, human immunodeficiency virus, hepatitis B and C viruses, and zika virus [53,54]. In the remaining sections, we review human papillomavirus (HPV) and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to their current prevalence and recent evidence of impact on male fertility.
HPV
HPV is the most common virus of the reproductive tract and the most commonly sexually transmitted infection in the United States. More than 200 types have been identified [55]. Low-risk HPV strains cause oral and anogenital warts, while high-risk strains such cause cervical cancer in women and oropharyngeal, anal, and penile cancer in men [56,57]. However, most HPV infections are asymptomatic [57,58].
With regards to male infertility, HPV-positive semen has been significantly associated with deterioration in sperm count, sperm motility, sperm morphology, and DNA fragmentation index compared to HPV-negative semen [59–61,62*,63–65]. HPV-positive semen has also been associated with decreased pregnancy rate and increased miscarriage rate in couples undergoing assisted reproductive technology (ART) procedures such as in vitro fertilization and intrauterine insemination [61,66,67].
The clear association of HPV-infected semen with poor semen parameters and poor ART outcomes calls for effective prevention and management of HPV infection in infertile couples. One strategy is sexual hygiene counseling (e.g., hygiene of the reproductive tract, avoiding oral and anal sex), which has been shown to significantly reduce HPV persistence in infected couples [68]. Another strategy is post-exposure HPV vaccination; in a retrospective study, administering the vaccine to men with HPV detected in semen who were in infertile relationships resulted in significantly increased pregnancy rates and decreased miscarriage rates compared to non-vaccinated HPV-positive men. The vaccinated men were also shown to have increased sperm motility and decreased anti-sperm antibody levels [69]. More data is needed before altering management guidelines for HPV-infected patients undergoing ART procedures.
SARS-CoV-2
In addition to causing the respiratory disease COVID-19, SARS-CoV-2 has been found to affect the male reproductive system. Since SARS-CoV-2 infects cells by binding the angiotensin-converting enzyme 2 (ACE2) receptor [70], cells that are high in ACE2 expression, such as testicular spermatogonia, Leydig cells, and Sertoli cells, are highly susceptible to SARS-CoV-2 [71,72].
In semen samples collected from patients 30 days after testing positive for SARS-CoV-2, the sperm concentration was significantly reduced compared to an age-matched, uninfected control group [73]. In a Belgian cohort study, sperm concentration and progressive motility were signific8antly reduced relative to baseline within the first month after COVID-19 infection, with gradual improvement of sperm parameters over 6 months [74*]. Additionally, male SARS-CoV2 infection was found to have a mildly negative impact on fecundability, but with increased fecundability observed sixty days after infection [75]. These findings suggest that COVID-19 has a negative but short-lived impact of male infertility, with improvement in sperm parameters and conception rate multiple months after infection.
While there are currently no treatments for COVID-19-related infertility, vaccines have become widely available for the prevention of COVID-19. In a recent cohort analyzing the impact of the Pfizer-BioNTech vaccine on sperm parameters, patients who received two doses of the vaccine did not experience any change in semen parameters from before to 14 months after vaccination [76*]. This finding has been supported by recent meta-analyses [77,78], indicating that COVID-19 vaccines do not affect male fertility. More data on the effect of male COVID-19 infection on conception rates are needed to inform further management guidelines.
CONCLUSION
MGTI is a major cause of male infertility that should be accounted for in the reproductive history and physical examination. In the absence of clinical signs or symptoms, the concern for MGTI begins with the finding of leukocytospermia, which may be further investigated with semen culture and PCR based on the clinician’s discretion. However, further research is needed to verify the utility of semen microbiological analysis. Treatment for MGTI in the setting of male infertility includes anti-inflammatory agents or antibiotics, the former being preferred if no pathogenic organism has been detected. Finally, the physician should be aware of not only bacteria, but also common viruses such as HPV and SARS-CoV-2 as causes of MGTI and associated infertility.
KEY POINTS.
The finding of leukocytospermia on semen analysis can be further evaluated with semen culture and polymerase chain reaction, but the clinical relevance of positive results is not fully established.
Leukocytospermia can be treated with anti-inflammatory agents, though antibiotics are preferred if there is clinical or microbiological evidence of infection
In addition to bacteria, common viruses such as human papillomavirus (HPV) and the novel coronavirus (SARS-CoV-2) can infect the male genital tract and cause infertility.
Financial support and sponsorship
This work was supported by NIH Grant R01 DK130991 and the ACS Clinician Scientist Development Grant.
Footnotes
Conflicts of interest
None.
REFERENCES
- 1.Ombelet W, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008;14(6):605–621. doi: 10.1093/humupd/dmn042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Human Reproduction Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016 [DOI] [PubMed] [Google Scholar]
- 3.Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clinical Biochemistry. 2018;62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 4.Sharlip ID, Jarow JP, Belker AM, et al. Best practice policies for male infertility. Fertil Steril. 2002;77(5):873–882. doi: 10.1016/s0015-0282(02)03105-9 [DOI] [PubMed] [Google Scholar]
- 5.Schaeffer AJ. Aetiopathology and pathogenesis of urogenital infections. Andrologia. 1998;30 Suppl 1:3–6. doi: 10.1111/j.1439-0272.1998.tb02819.x [DOI] [PubMed] [Google Scholar]
- 6.Pellati D, Mylonakis I, Bertoloni G, et al. Genital tract infections and infertility. Eur J Obstet Gynecol Reprod Biol. 2008;140(1):3–11. doi: 10.1016/j.ejogrb.2008.03.009 [DOI] [PubMed] [Google Scholar]
- 7.Sandoval JS, Raburn D, Muasher S. Leukocytospermia: Overview of diagnosis, implications, and management of a controversial finding. Middle East Fertility Society Journal. 2013;18(3):129–134. doi: 10.1016/j.mefs.2013.02.004 [DOI] [Google Scholar]
- 8.Nieschlag SMA, Nieschlag E, Behre H. Andrology: Male Reproductive Health and Dysfunction. Springer Science & Business Media; 2013. [Google Scholar]
- 9.Haidl G, Allam JP, Schuppe HC. Chronic epididymitis: impact on semen parameters and therapeutic options. Andrologia. 2008;40(2):92–96. doi: 10.1111/j.1439-0272.2007.00819.x [DOI] [PubMed] [Google Scholar]
- 10**.Schlegel PN, Sigman M, Collura B, et al. Diagnosis and Treatment of Infertility in Men: AUA/ASRM Guideline Part I. J Urol. 2021;205(1):36–43. doi: 10.1097/JU.0000000000001521 [DOI] [PubMed] [Google Scholar]; Most recent American Urological Association expert guideline stating that the finding of leukocytospermia should be further investigated for presence of infection.
- 11*.Velez D, Ohlander S, Niederberger C. Pyospermia: background and controversies. F S Rep. 2021;2(1):2–6. doi: 10.1016/j.xfre.2021.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; An evidence-based diagnosis and treatment algorithm for leukocytospermia.
- 12.Sharma R, Gupta S, Agarwal A, et al. Relevance of Leukocytospermia and Semen Culture and Its True Place in Diagnosing and Treating Male Infertility. World J Mens Health. 2022;40(2):191–207. doi: 10.5534/wjmh.210063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff H. Methods for the detection of male genital tract inflammation. Andrologia. 1998;30 Suppl 1:35–39. doi: 10.1111/j.1439-0272.1998.tb02824.x [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Sharma S, Jain M, Chauhan R. Importance of papanicolaou staining for sperm morphologic analysis: comparison with an automated sperm quality analyzer. Am J Clin Pathol. 2011;136(2):247–251. doi: 10.1309/AJCPCLCSPP24QPHR [DOI] [PubMed] [Google Scholar]
- 15.Puri V, Gaur K, Hooda S, Shukla S, Sharma S. Papanicolaou-Stained Cytosmear Preparations in the Evaluation of Leucocytospermia: A Tertiary Centre Experience and Assessment of Utility. J Lab Physicians. 2021;13(3):208–213. doi: 10.1055/s-0041-1730846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricci G, Presani G, Guaschino S, Simeone R, Perticarari S. Leukocyte detection in human semen using flow cytometry. Hum Reprod. 2000;15(6):1329–1337. doi: 10.1093/humrep/15.6.1329 [DOI] [PubMed] [Google Scholar]
- 17.Fathy A, Chen SJ, Novak N, Schuppe HC, Haidl G, Allam JP. Differential leucocyte detection by flow cytometry improves the diagnosis of genital tract inflammation and identifies macrophages as proinflammatory cytokine-producing cells in human semen. Andrologia. 2014;46(9):1004–1012. doi: 10.1111/and.12188 [DOI] [PubMed] [Google Scholar]
- 18.Wolff H, Anderson DJ. Immunohistologic characterization and quantitation of leukocyte subpopulations in human semen. Fertil Steril. 1988;49(3):497–504. [PubMed] [Google Scholar]
- 19*.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 6th ed. World Health Organization; 2021. Accessed November 27, 2022. https://apps.who.int/iris/handle/10665/343208 [Google Scholar]; Description of peroxidase staining as an initial screening test for leukocytes in the setting of elevated round cells on semen analysis.
- 20.Brunner RJ, Demeter JH, Sindhwani P. Review of Guidelines for the Evaluation and Treatment of Leukocytospermia in Male Infertility. World J Mens Health. 2019;37(2):128–137. doi: 10.5534/wjmh.180078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal A, Mulgund A, Alshahrani S, et al. Reactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermia. Reprod Biol Endocrinol. 2014;12:126. doi: 10.1186/1477-7827-12-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018;50(11):e13126. doi: 10.1111/and.13126 [DOI] [PubMed] [Google Scholar]
- 23.Wolff H. The biologic significance of white blood cells in semen. Fertil Steril. 1995;63(6):1143–1157. doi: 10.1016/s0015-0282(16)57588-8 [DOI] [PubMed] [Google Scholar]
- 24.Wolff H, Politch JA, Martinez A, Haimovici F, Hill JA, Anderson DJ. Leukocytospermia is associated with poor semen quality. Fertil Steril. 1990;53(3):528–536. [PubMed] [Google Scholar]
- 25.Yanushpolsky EH, Politch JA, Hill JA, Anderson DJ. Is leukocytospermia clinically relevant? Fertil Steril. 1996;66(5):822–825. [PubMed] [Google Scholar]
- 26.Domes T, Lo KC, Grober ED, Mullen JBM, Mazzulli T, Jarvi K. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil Steril. 2012;97(5):1050–1055. doi: 10.1016/j.fertnstert.2012.01.124 [DOI] [PubMed] [Google Scholar]
- 27.Tomlinson MJ, Barratt CL, Cooke ID. Prospective study of leukocytes and leukocyte subpopulations in semen suggests they are not a cause of male infertility. Fertil Steril. 1993;60(6):1069–1075. doi: 10.1016/s0015-0282(16)56412-7 [DOI] [PubMed] [Google Scholar]
- 28.Jue JS, Ramasamy R. Significance of positive semen culture in relation to male infertility and the assisted reproductive technology process. Transl Androl Urol. 2017;6(5):916–922. doi: 10.21037/tau.2017.06.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Minhas S, Bettocchi C, Boeri L, et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. European Urology. 2021;80(5):603–620. doi: 10.1016/j.eururo.2021.08.014 [DOI] [PubMed] [Google Scholar]; Expert guideline states that the finding of leukocytospermia should trigger a semen culture or polymerase chain reaction test for common genital tract pathogens.
- 30.Cumming JA, Carrell DT. Utility of reflexive semen cultures for detecting bacterial infections in patients with infertility and leukocytospermia. Fertil Steril. 2009;91(4 Suppl):1486–1488. doi: 10.1016/j.fertnstert.2008.07.1756 [DOI] [PubMed] [Google Scholar]
- 31.Gdoura R, Kchaou W, Znazen A, et al. Screening for bacterial pathogens in semen samples from infertile men with and without leukocytospermia. Andrologia. 2008;40(4):209–218. doi: 10.1111/j.1439-0272.2008.00845.x [DOI] [PubMed] [Google Scholar]
- 32.Isaiah IN, Nche BT, Nwagu IG, Nnanna II. Current studies on bacterospermia the leading cause of male infertility: a protégé and potential threat towards mans extinction. N Am J Med Sci. 2011;3(12):562–564. doi: 10.4297/najms.2011.3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Farahani L, Tharakan T, Yap T, Ramsay JW, Jayasena CN, Minhas S. The semen microbiome and its impact on sperm function and male fertility: A systematic review and meta-analysis. Andrology. 2021;9(1):115–144. doi: 10.1111/andr.12886 [DOI] [PubMed] [Google Scholar]; Systematic review of semen microbiome studies finds that Enterococcus faecalis, Ureaplasma urealyticum, and Mycoplasma hominis are associated with deterioration of sperm parameters.
- 34.Vilvanathan S, Kandasamy B, Jayachandran AL, et al. Bacteriospermia and Its Impact on Basic Semen Parameters among Infertile Men. Interdiscip Perspect Infect Dis. 2016;2016:2614692. doi: 10.1155/2016/2614692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eini F, Kutenaei MA, Zareei F, Dastjerdi ZS, Shirzeyli MH, Salehi E. Effect of bacterial infection on sperm quality and DNA fragmentation in subfertile men with Leukocytospermia. BMC Mol Cell Biol. 2021;22(1):42. doi: 10.1186/s12860-021-00380-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci S, De Giorgi S, Lazzeri E, et al. Impact of asymptomatic genital tract infections on in vitro Fertilization (IVF) outcome. PLoS One. 2018;13(11):e0207684. doi: 10.1371/journal.pone.0207684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Wang Q, Ji X, et al. Prevalence of Ureaplasma urealyticum, Mycoplasma hominis, Chlamydia trachomatis infections, and semen quality in infertile and fertile men in China. Urology. 2014;83(4):795–799. doi: 10.1016/j.urology.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 38.Ahmadi MH, Mirsalehian A, Sadighi Gilani MA, Bahador A, Talebi M. Asymptomatic Infection With Mycoplasma hominis Negatively Affects Semen Parameters and Leads to Male Infertility as Confirmed by Improved Semen Parameters After Antibiotic Treatment. Urology. 2017;100:97–102. doi: 10.1016/j.urology.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 39.Dehghan A, Pourmand MR, Salimi V, et al. The effects of Chlamydia trachomatis, Mycoplasma hominis, and Ureaplasma urealyticum loads on semen quality: Detection and quantitative analysis. Microbial Pathogenesis. 2022;169:105676. doi: 10.1016/j.micpath.2022.105676 [DOI] [PubMed] [Google Scholar]
- 40.Haidl G, Haidl F, Allam JP, Schuppe HC. Therapeutic options in male genital tract inflammation. Andrologia. 2019;51(3):e13207. doi: 10.1111/and.13207 [DOI] [PubMed] [Google Scholar]
- 41.Oliva A, Multigner L. Ketotifen improves sperm motility and sperm morphology in male patients with leukocytospermia and unexplained infertility. Fertil Steril. 2006;85(1):240–243. doi: 10.1016/j.fertnstert.2005.06.047 [DOI] [PubMed] [Google Scholar]
- 42.Lackner JE, Herwig R, Schmidbauer J, Schatzl G, Kratzik C, Marberger M. Correlation of leukocytospermia with clinical infection and the positive effect of antiinflammatory treatment on semen quality. Fertil Steril. 2006;86(3):601–605. doi: 10.1016/j.fertnstert.2006.01.032 [DOI] [PubMed] [Google Scholar]
- 43.Gambera L, Serafini F, Morgante G, Focarelli R, De Leo V, Piomboni P. Sperm quality and pregnancy rate after COX-2 inhibitor therapy of infertile males with abacterial leukocytospermia. Hum Reprod. 2007;22(4):1047–1051. doi: 10.1093/humrep/del490 [DOI] [PubMed] [Google Scholar]
- 44.Comhaire FH, Rowe PJ, Farley TM. The effect of doxycycline in infertile couples with male accessory gland infection: a double blind prospective study. Int J Androl. 1986;9(2):91–98. doi: 10.1111/j.1365-2605.1986.tb00871.x [DOI] [PubMed] [Google Scholar]
- 45.Branigan EF, Muller CH. Efficacy of treatment and recurrence rate of leukocytospermia in infertile men with prostatitis. Fertil Steril. 1994;62(3):580–584. [PubMed] [Google Scholar]
- 46.Yamamoto M, Hibi H, Katsuno S, Miyake K. Antibiotic and ejaculation treatments improve resolution rate of leukocytospermia in infertile men with prostatitis. Nagoya J Med Sci. 1995;58(1-2):41–45. [PubMed] [Google Scholar]
- 47.Yanushpolsky EH, Politch JA, Hill JA, Anderson DJ. Antibiotic therapy and leukocytospermia: a prospective, randomized, controlled study. Fertil Steril. 1995;63(1):142–147. [PubMed] [Google Scholar]
- 48.Skau PA, Folstad I. Do bacterial infections cause reduced ejaculate quality? A meta-analysis of antibiotic treatment of male infertility. Behavioral Ecology. 2003;14(1):40–47. doi: 10.1093/beheco/14.1.40 [DOI] [Google Scholar]
- 49.Pajovic B, Radojevic N, Vukovic M, Stjepcevic A. Semen analysis before and after antibiotic treatment of asymptomatic Chlamydia- and Ureaplasma-related pyospermia. Andrologia. 2013;45(4):266–271. doi: 10.1111/and.12004 [DOI] [PubMed] [Google Scholar]
- 50.Vicari E. Effectiveness and limits of antimicrobial treatment on seminal leukocyte concentration and related reactive oxygen species production in patients with male accessory gland infection. Hum Reprod. 2000;15(12):2536–2544. doi: 10.1093/humrep/15.12.2536 [DOI] [PubMed] [Google Scholar]
- 51.Sheikhzadeh Hesari F, Hosseinzadeh SS, Asl Monadi Sardroud MA. Review of COVID-19 and male genital tract. Andrologia. 2021;53(1):e13914. doi: 10.1111/and.13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seymen CM. The other side of COVID-19 pandemic: Effects on male fertility. J Med Virol. 2021;93(3):1396–1402. doi: 10.1002/jmv.26667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W, Han R, Wu H, Han D. Viral threat to male fertility. Andrologia. 2018;50(11):e13140. doi: 10.1111/and.13140 [DOI] [PubMed] [Google Scholar]
- 54.Batiha O, Al-Deeb T, Al-Zoubi E, Alsharu E. Impact of COVID-19 and other viruses on reproductive health. Andrologia. 2020;52(9):e13791. doi: 10.1111/and.13791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manini I, Montomoli E. Epidemiology and prevention of Human Papillomavirus. Ann Ig. 2018;30(4 Supple 1):28–32. doi: 10.7416/ai.2018.2231 [DOI] [PubMed] [Google Scholar]
- 56.Bansal A, Singh MP, Rai B. Human papillomavirus-associated cancers: A growing global problem. Int J Appl Basic Med Res. 2016;6(2):84–89. doi: 10.4103/2229-516X.179027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suhaila K, Mukherjee A, Maharjan B, et al. Human Papillomavirus, Related Diseases, and Vaccination: Knowledge and Awareness Among Health Care Students and Professionals in Nepal. J Cancer Educ. 2022;37(6):1727–1735. doi: 10.1007/s13187-021-02018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pandey D, Solleti V, Jain G, et al. Human Papillomavirus (HPV) Infection in Early Pregnancy: Prevalence and Implications. Infect Dis Obstet Gynecol. 2019;2019:4376902. doi: 10.1155/2019/4376902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Jia CW, Ma YM, Zhou LY, Wang SY. Correlation between HPV sperm infection and male infertility. Asian J Androl. 2013;15(4):529–532. doi: 10.1038/aja.2013.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeršovienė V, Gudlevičienė Ž, Rimienė J, Butkauskas D. Human Papillomavirus and Infertility. Medicina (Kaunas). 2019;55(7):377. doi: 10.3390/medicina55070377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinberg M, Sar-Shalom Nahshon C, Feferkorn I, Bornstein J. Evaluation of human papilloma virus in semen as a risk factor for low sperm quality and poor in vitro fertilization outcomes: a systematic review and meta-analysis. Fertil Steril. 2020;113(5):955–969.e4. doi: 10.1016/j.fertnstert.2020.01.010 [DOI] [PubMed] [Google Scholar]
- 62*.Moreno-Sepulveda J, Rajmil O. Seminal human papillomavirus infection and reproduction: a systematic review and meta-analysis. Andrology. 2021;9(2):478–502. doi: 10.1111/andr.12948 [DOI] [PubMed] [Google Scholar]; Systematic review associated seminal human papillomavirus infection with poor semen parameters, increased risk of miscarriage, and reduced chance of ongoing pregnancy.
- 63.Wang S, Liu L, Zhang A, Song Y, Kang J, Liu X. Association between human papillomavirus infection and sperm quality: A systematic review and a meta-analysis. Andrologia. 2021;53(5):e14034. doi: 10.1111/and.14034 [DOI] [PubMed] [Google Scholar]
- 64.Jaworek H, Koudelakova V, Oborna I, et al. Impact of human papillomavirus infection on semen parameters and reproductive outcomes. Reprod Biol Endocrinol. 2021;19(1):156. doi: 10.1186/s12958-021-00840-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciavattini A, Marconi C, Giannella L, Delli Carpini G, Sopracordevole F, Di Giuseppe J. The Impact of 9-Valent HPV Vaccination on Couple Infertility Prevention: A Comprehensive Review. Front Med (Lausanne). 2021;8:700792. doi: 10.3389/fmed.2021.700792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perino A, Giovannelli L, Schillaci R, et al. Human papillomavirus infection in couples undergoing in vitro fertilization procedures: impact on reproductive outcomes. Fertil Steril. 2011;95(5):1845–1848. doi: 10.1016/j.fertnstert.2010.11.047 [DOI] [PubMed] [Google Scholar]
- 67.Garolla A, Engl B, Pizzol D, et al. Spontaneous fertility and in vitro fertilization outcome: new evidence of human papillomavirus sperm infection. Fertil Steril. 2016;105(1):65–72.e1. doi: 10.1016/j.fertnstert.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 68.Garolla A, Pizzol D, Vasoin F, Barzon L, Bertoldo A, Foresta C. Counseling reduces HPV persistence in coinfected couples. J Sex Med. 2014;11(1):127–135. doi: 10.1111/jsm.12358 [DOI] [PubMed] [Google Scholar]
- 69.Garolla A, De Toni L, Bottacin A, et al. Human Papillomavirus Prophylactic Vaccination improves reproductive outcome in infertile patients with HPV semen infection: a retrospective study. Sci Rep. 2018;8(1):912. doi: 10.1038/s41598-018-19369-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malki MI. COVID-19 and male infertility: An overview of the disease. Medicine (Baltimore). 2022;101(27):e29401. doi: 10.1097/MD.0000000000029401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li R, Yin T, Fang F, et al. Potential risks of SARS-CoV-2 infection on reproductive health. Reprod Biomed Online. 2020;41(1):89–95. doi: 10.1016/j.rbmo.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan C, Lu W, Li K, Ding Y, Wang J. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Infection in COVID-19 Patients. Front Med (Lausanne). 2020;7:563893. doi: 10.3389/fmed.2020.563893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Best JC, Kuchakulla M, Khodamoradi K, et al. Evaluation of SARS-CoV-2 in Human Semen and Effect on Total Sperm Number: A Prospective Observational Study. World J Mens Health. 2021;39(3):489–495. doi: 10.5534/wjmh.200192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74*.Donders GGG, Bosmans E, Reumers J, et al. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: a prospective, observational study and validation of the SpermCOVID test. Fertil Steril. 2022;117(2):287–296. doi: 10.1016/j.fertnstert.2021.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]; Prospective observational study reveals poor sperm quality in first month after COVID infection, though with significant improvement over 3 months.
- 75.Wesselink AK, Hatch EE, Rothman KJ, et al. A Prospective Cohort Study of COVID-19 Vaccination, SARS-CoV-2 Infection, and Fertility. Am J Epidemiol. 2022;191(8):1383–1395. doi: 10.1093/aje/kwac011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76*.Karavani G, Chill HH, Meirman C, et al. Sperm quality is not affected by the BNT162b2 mRNA SARS-CoV-2 vaccine: results of a 6-14 months follow-up. J Assist Reprod Genet. 2022;39(10):2249–2254. doi: 10.1007/s10815-022-02621-x [DOI] [PMC free article] [PubMed] [Google Scholar]; No change in semen parameters from before to 6 to 14 months after SARS-CoV-2 vaccination.
- 77.Zaçe D, La Gatta E, Petrella L, Di Pietro ML. The impact of COVID-19 vaccines on fertility-A systematic review and meta-analysis. Vaccine. 2022;40(42):6023–6034. doi: 10.1016/j.vaccine.2022.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang J, Fang Z, Huang L, et al. Effect of COVID-19 vaccination on semen parameters: A systematic review and meta-analysis. J Med Virol. Published online October 30, 2022:e28263. doi: 10.1002/jmv.28263 [DOI] [PMC free article] [PubMed] [Google Scholar]


