Abstract
The conventional approach to investigating functional connectivity in the block-designed study usually concatenates task blocks or employs residuals of task activation. While providing many insights into brain functions, the block design adds more manipulation in functional network analysis that may reduce the purity of the blood oxygenation level-dependent signal. Recent studies utilized one single long run for task trials of the same condition, the so-called continuous design, to investigate functional connectivity based on task functional magnetic resonance imaging. Continuous brain activities associated with the single-task condition can be directly utilized for task-related functional connectivity assessment, which has been examined for working memory, sensory, motor, and semantic task experiments in previous research. But it remains unclear how the block and continuous design influence the assessment of task-related functional connectivity networks. This study aimed to disentangle the separable effects of block/continuous design and working memory load on task-related functional connectivity networks, by using repeated-measures analysis of variance. Across 50 young healthy adults, behavioral results of accuracy and reaction time showed a significant main effect of design as well as interaction between design and load. Imaging results revealed that the cingulo-opercular, fronto-parietal, and default model networks were associated with not only task activation, but significant main effects of design and load as well as their interaction on intra- and inter-network functional connectivity and global network topology. Moreover, a significant behavior-brain association was identified for the continuous design. This work has extended the evidence that continuous design can be used to study task-related functional connectivity and subtle brain-behavioral relationships.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-022-00982-y.
Keywords: Working memory, Functional connectivity, Network topology, Block design, Continuous design, Memory load
Introduction
Most experimental tasks combined with functional magnetic resonance imaging (fMRI) have been designed to optimize the estimation of task-related activation [1]. Thus, the block design for cognitive tasks enables comparisons between specific task conditions that are grouped into smaller “blocks” with a typical duration of 20–60 s and separated by baseline (or rest) periods. With such a design, task-related brain activation can be inferred by contrasts between baseline and task periods [2]. This experimental design has a number of advantages. First, block designs demonstrate efficiency because many trials of similar conditions can be collapsed to attain adequate signal-to-noise ratios [3, 4]. Second, block designs evince superior statistical power [5] and may be more appropriate if the experimental goal is to detect differences in blood oxygenation level-dependent (BOLD) signals across different task conditions [6]. Therefore, block designs are well suited for detecting functional activity during the performance of particular tasks [7]. Third, block designs are simpler to implement because they do not need careful randomization and spacing between different stimulus categories (as would be needed in event-related designs; [8]). Despite the benefits that block designs confer, they are limited insofar as they cannot accommodate tasks that require a trial-by-trial framework [9–12]. Another potential limitation of the block design may be that task-state functional connectivity (FC) must be estimated by using concatenated task data [13], or a task regression approach [14]. Such additional processing and the resulting FC measures might be affected by the non-stationarity (e.g., spike at the time series splicing point) in the time series of block design [15–17].
Recently, more direct and convenient FC identification has been proposed by using ultra-long blocks of trials of the same task condition that are not interleaved by rest periods. In these continuous task fMRI designs, participants engage in the same task condition trial by trial continuously in one run, without rest periods of jittered gaps or long pauses [18]. Such a continuous-designed task is easier to perform, especially with patients who are sensitive to task difficulty, because there is no task switch during each run and the demand for attention switching is reduced. While the task-evoked time series obtained in block designs are influenced by the initial task-induced transient and history effects of the preceding condition [15], the continuous design maintains a more stable task state than the block design, which is suitable for FC assessment [18, 19]. Although fewer studies used the continuous design than block design in the past, accumulating evidence has shown that the continuous task design is suitable to investigate whole-brain functional connectivity patterns related to task states, from passive fixation to working memory, sensory, motor, and semantic task studies [18–20]. However, the effect of experimental design on the task-related FC pattern remains unclear. Therefore, in this study we investigated whether the FC assessment differs between the continuous and block design in the same participants.
Working memory, which is associated with distributed brain regions, is frequently examined by using a block design. For example, the dorsolateral prefrontal cortex, anterior prefrontal cortex, medial frontal cortex, inferior parietal sulcus, and cingulate cortex have been implicated in accommodating increasing memory loads [21–23]. Recent studies have reported that brain regions involved in working memory function partly overlap with two large-scale brain networks, the cingulo-opercular network (CON) and the fronto-parietal network (FPN) [2, 24–29]. As shown in previous studies, the FPN mediates sensory processing during both preparatory attention and orienting within memory. The CON plays a more downstream role in cognitive control, like visuo-spatial attention [30, 31]. In addition, the default mode network (DMN) is associated with self-referential functioning [32, 33], which is often deactivated during working memory task performance [34–36]. Therefore, the FPN, CON, and DMN are deeply involved in the functioning of working memory, which has been shown to vary with the working memory load [29, 37]. The block-designed N-back task, a typical task for working memory research [38], requires the participant to decide whether the current stimulus matches the one N-steps back in the stimulus sequence [39]. Previous studies have reported that brain connectivity and networks are associated with behavioral performance such as reaction time and accuracy [24, 40, 41] as well as that the DMN, FPN, and CON are the substrates for the working memory load effect on FC [37, 42]. Greater network reconfiguration from rest to working memory task is correlated with longer reaction time for working memory tasks [42]. Krienen and colleagues first used the continuous design to reveal task state FC [18]. However, the potential influence of continuous/block design on the load-related FC, as well as their association with behavior performance, are yet to be disentangled.
In this work, we aimed to investigate the experimental (block/continuous) design effect on the task-related FC between brain regions underlying the task activation at different memory loads. We further hypothesized that both the block design and continuous design can reveal working memory load effects on FC patterns, so that both of them can be used to analyze task-related FC. Therefore, we collected fMRI data from 50 healthy young adults while they performed N-back working memory tasks with block and continuous designs. Then, Pearson’s correlation was applied to investigate FC within and between the brain networks, as well as the global network topology, to disentangle the effects of block/continuous design and working memory load, as well as their interaction. Next, we assessed the correlation between individual task-related FC and behavioral performance.
Materials and Methods
Participants
Healthy right-handed volunteers (n = 50; 24 males) were recruited from the University of Electronic Science and Technology of China via an advertisement on campus. The age range was from 19 to 24 years (mean = 22.34 years, SD = 1.303). All participants reported normal auditory and normal or corrected-to-normal visual acuity. They were all suitable for MRI scanning and free of physiological, neurological, or psychiatric problems that might affect brain functions. Written informed consent was given by each participant before all procedures in this study. Participants practiced before they were in the scanner to make sure they fully understood the experiment task. Each participant received an honorarium of 200 Chinese Yuan for participation. The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Review Board of the Intramural Research Program at the University of Electronic Science and Technology of China.
Study Procedure
N-back Task
The N-back task tested working memory for the locations of letter stimuli. A white fixation cross on a dark background was presented in the center of the screen. In each trial, a random letter was presented in 1 of the 4 visual field quadrants around the fixation point. In an N-back condition (N = 1 or 2), participants were asked to press the left button with the left thumb when the location of the current letter matched the location of the letter presented “N” items back, and to press the right button with the right thumb when the locations did not match (Fig. 1). One-third of the total trials were “matches”. The orders of trials were counterbalanced across participants. The participants were instructed to focus only on the location of the letter, not on the letter itself, and to classify the stimuli as accurately and quickly as possible. Visual stimuli were presented and responses were collected using E-Prime (Psychology Software Tools, Inc., Sharpsburg, USA).
Fig. 1.
An example of a block-design run. The right examples are N-back working memory trials. In the 1-back condition, compare whether the letters in the screens highlighted in green and blue are in the same location. In the 2-back condition, compare whether the letters in the screens highlighted in red and blue are in the same location. The line chart below represents block design. Block-design N-back tasks took 3 min 46 s each. In addition, continuous N-back tasks took 5 min 10 s each without task switching. Four types of experimental design (block 1221-back, block 2112-back, continuous 1-back, and continuous 2-back) were established
In total, four runs were collected for the N-back task: two runs of block design and two runs of continuous design. For both designs of the N-back tasks, each trial started with an interstimulus interval of 2500 ms, after which a 500-ms letter stimulus was presented in 1 of the 4 quadrants around the fixation point (Fig. 1). In the block design, each run began with a 10-s fixation and had four blocks [two 1-back and two 2-back blocks, and each block had 8 trials of 3 s (in total, 24 s)]. The blocks were separated by the baseline with a fixation (rest period) and ordered in the counter-balanced manner of ABBA and BAAB for two runs. In the continuous design, each run had 100 trials of purely 1-back or 2-back stimuli without a rest period (in total, 300 s), beginning with a 10-s fixation. To reduce the bias of learning experience, all participants had been trained to ensure they were proficient with the tasks before the fMRI scan. We sought to hold the participants’ experience with the task constant (i.e., block design first, followed by continuous design) since the experience of block design hardly transferred to continuous design. For instance, those participants receiving the block design would receive additional training in task-switching (between 1-back and 2-back), and be trained into a block task rhythm. But neither experience was related to the continuous task. So the training could negatively transfer to their performance once they switched to the continuous design.
We evaluated task performance by accuracy (ACC) and reaction time (RT). The number of discontinuously wrong trials (the first trial’s response was right and the second trial’s response was wrong) was counted and normalized by dividing the total number of trials.
MRI Acquisition
The MRI data were acquired on a 3T GE Signa scanner (General Electric Co., Milwaukee, USA), using an 8-channel head coil, at the University of Electronic Science and Technology of China. The fMRI data were collected using a gradient echo planar imaging sequence, and the scanning parameters were: repetition time (TR)/echo time (TE) = 2000 ms/30 ms; flip angle = 90°; field of view (FOV) = 240 × 240 mm2; matrix size = 64 × 64; axial slice number = 42 with slice thickness = 3 mm and gap = 0. In total, the block design had 226 images and the continuous design had 310 images. High-resolution anatomical T1-weighted MRI data were then collected using a spoiled gradient recalled (SPGR) pulse sequence. The scanning parameters were: TR/TE/inversion time (TI) = 6 ms/Minimum/450 ms; FOV = 256 × 256 mm2; matrix size = 256 × 256; sagittal slice number = 156 with a slice thickness of 1 mm.
fMRI Data Preprocessing
The preprocessing was performed for each participant and each run by using SPM12 (University College London, London, UK) in the MatLab (MathWorks, Natick, USA) 2014a environment. Briefly, the first 5 volumes were discarded. The remaining images were realigned to the first image of each run and then normalized into the standard MNI (Montreal Neurological Institute) space by using the echo-planar imaging (EPI) template. All the functional images were spatially smoothed by using a Gaussian kernel of 8 mm full width at half maximum. The preprocessed block-design data were used to detect task activation. None of the participants’ data exceeded the excessive head motion criteria (maximal head motion of 2 mm or 2°; and mean frame-wise displacement of 0.2 mm). For task-related FC analysis, we further pre-processed the fMRI data of both block and continuous designs by nuisance variable regression (including average signals of cerebrospinal fluid and white matter, 24 head motion time courses), linear detrending, and 0.01–0.15 Hz band-pass filtering [19, 40, 43–45].
Block Design and Working Memory Task Activation
The general linear model was created for two block-design runs to detect N-back task activations at the individual level. The 2-back and 1-back conditions were defined as two box-car functions convolved with the canonical hemodynamic response function. Six realignment parameters were included as regressors of no interest to account for head motion effects. After the model estimation, two contrast maps (2-back vs rest and 1-back vs rest) were generated. The second-level analysis was performed for each contrast map across 50 participants by using one-sample t-tests. Significant clusters for task activation were identified by using the initial threshold of P < 0.001 and the cluster level false discovery rate (FDR) corrected P < 0.05 for multiple comparison correction. To confirm that CON, FPN, and DMN overlapped with the task activation patterns, regions of interest (ROIs) of the three networks according to Dosenbach’s atlas (radius of 8 mm) were used [46, 47]. The group level one sample t-tests were performed on the contrast values from each ROI to detect significant activations or deactivations (FDR corrected P < 0.05).
Network Construction, Intra-/Inter-network FC, and Graph-Based Analysis
The ROIs associated with working memory task activation were defined as the nodes for task-related FC network construction (in total, 66 ROIs, 24 for CON, 15 for FPN, and 27 for DMN; see details in Table S1). First, in the block design, block splicing was applied in the following manner: (1) the first 3 volumes (6 s) of each task block were discarded, and the first 2 volumes (4 s) of the next rest block were added, in order to control for the effect of hemodynamic delay [48]; (2) all blocks of the 1- or 2-back condition were concatenated respectively to form the time series for the FC of the single condition. In the continuous design, the time series of each run corresponding to a single condition was used directly. Second, the prepared time series were extracted from each ROI and averaged to compute pairwise Pearson’s correlation, which defined the edges for task-related FC networks. Therefore, each participant had four FC matrices for 1/2-back and block/continuous design. Fisher’s r-to-z transformation was applied to ensure approximate normal distribution. Because negative connections have no clear biological substrate [49–52], negative values were set to zero [37, 43]. Finally, edge weights were normalized to the range between 0 and 1 for inter-participant comparability [53].
The intra- and inter-network FCs were computed for CON, FPN, and DMN separately in line with the prior study [37]. The global network topology was investigated by calculating global efficiency (efficiency_wei.m), participation coefficient (PC; participation_coef.m), and transitivity (transitivity_wu.m) across the three networks with the Brain Connectivity Toolbox (http://www.brain-connectivity-toolbox.net/) [53]. Briefly, the transitivity is similar to the clustering coefficient but computed for the whole network instead of each node [54]. High transitivity suggests strong functional segregation. The global efficiency is the average of the inverse shortest path length. The PC characterizes the extent of a node’s communications between different networks compared to the total number of communications. A higher PC or global efficiency indicates higher functional integration.
Statistical Analysis
In this study, task performance (ACC and RT) and FC characteristics (intra- and inter-network FC as well as global network topology) were obtained for each participant for 1/2-back and block/continuous respectively. We calculated ACC by the number of trials of correct responses divided by all trials. The RT was defined by averaged RT of trials of correct responses. We used a 2 (design: block/continuous) × 2 (load: 1-back/2-back) repeated-measures analysis of variance (ANOVA) to assess the significant effects of memory load and design as well as their interaction, on the behavioral and imaging measures. The threshold for significant results was set at P < 0.05. Post hoc t-tests were applied following significant interactions. The statistical analyses were conducted in SPSS 21 (IBM, Armonk, USA). The brain-behavior relationships between task performance and task-related FC were explored using Spearman’s correlation.
Validation Analysis
To determine if the network topology results were stable, we used not only the three networks, but also whole brain ROIs (all the nodes from Dosenbach’s atlas, including FPN, CON, and DMN, as well as sensorimotor, visual, and cerebellar networks) to calculate PC, global efficiency, and transitivity. To determine if the results were sensitive to the length of time series in the continuous design, we further cut the whole time series in the continuous design into three time series (from the first, middle, and last parts of time series), each of which had the same length as the spliced time series in the block design, and then re-ran following analyses.
Results
Task Performance
The ANOVA of behavioral performance in the working memory task demonstrated significant results (Table 1), including the significant main effect of memory load and interaction (memory load × block/continuous design) on the ACC and RT. Post hoc results showed significantly higher ACC and lower RT for the 1-back condition than the 2-back condition, for both block and continuous design (Fig. 2). Although the main effect of design was not significant, the interaction between design and memory load was significant, showing that ACC of the 2-back condition was significantly higher for the block design than the continuous design, while RT of the 1-back condition was significantly higher for the block design than the continuous design.
Table 1.
Repeated-measures ANOVA results of behavior performance, functional network connectivity, and global network topology
| Main effect of design | Main effect of memory load | Interaction of design × memory load | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F1, 49 | P-value | F1, 49 | P-value | F1, 49 | P-value | ||||
| Behavioral performance | |||||||||
| ACC | 0.540 | 0.466 | 0.011 | 23.935 | < 0.001*** | 0.328 | 7.899 | < 0.01** | 0.139 |
| RT | 1.920 | 0.172 | 0.038 | 46.140 | < 0.001*** | 0.485 | 6.171 | < 0.05* | 0.112 |
| Intra-network connectivity | |||||||||
| CON | 3.823 | 0.056 | 0.072 | 1.414 | 0.240 | 0.028 | 0.010 | 0.923 | < 0.001 |
| FPN | 1.772 | 0.189 | 0.035 | 7.518 | < 0.01** | 0.133 | 8.199 | < 0.01** | 0.143 |
| DMN | 15.166 | <0.001*** | 0.236 | 5.485 | < 0.05* | 0.101 | 0.002 | 0.966 | < 0.001 |
| Inter-network connectivity | |||||||||
| CON-FPN | 3.414 | 0.071 | 0.065 | 0.136 | 0.714 | 0.003 | 0.767 | 0.385 | 0.015 |
| CON-DMN | 4.137 | < 0.05* | 0.078 | 35.906 | < 0.001*** | 0.423 | 11.235 | < 0.01** | 0.187 |
| DMN-FPN | 2.295 | 0.136 | 0.045 | 8.325 | < 0.01** | 0.145 | 10.076 | < 0.01** | 0.171 |
| Global network topology | |||||||||
| Efficiency | 4.409 | < 0.05* | 0.083 | 17.437 | < 0.001*** | 0.262 | 8.286 | < 0.01** | 0.145 |
| Participation coefficient | 0.023 | 0.881 | < 0.001 | 42.594 | < 0.001*** | 0.465 | 23.512 | < 0.001*** | 0.324 |
| Transitivity | 3.066 | 0.086 | 0.059 | 12.559 | < 0.001*** | 0.204 | 7.160 | < 0.05* | 0.127 |
ACC, accuracy; RT, reaction time; CON, cingulo-opercular network; FPN, fronto-parietal network; DMN, default mode network
*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001
Fig. 2.
Behavior performance is associated with memory load and design (left: ACC, right: RT, n = 50 per condition). Significant main effects and interactions were tested by ANOVA. For significant interactions, post hoc tests were examined by paired t-tests. Post hoc testing showed higher ACCs and shorter RTs for the 1-back condition than the 2-back condition in both the continuous and the block design. *P ≤ 0.05; ***P ≤ 0.001. Error bars, SD. ACC, Accuracy; RT, reaction time
The results of discontinuously wrong trials showed that the continuous 2-back condition had the largest mean number compared to the other conditions (block 1-back: 1.96; block 2-back: 2.43; continuous 1-back: 1.5; continuous 2-back: 3.94). Similarly, high ACC was linked with a low number of discontinuously wrong trials. Therefore, it is likely that the participants focused on the current task during the experiment to some extent. For more details see Supplementary Materials.
Task-Related Brain Activation Associated with the Block Design
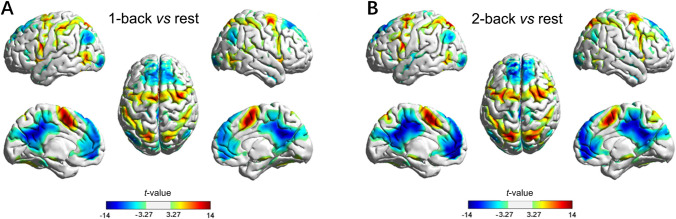
The contrast for activation detection was constructed between N-back and rest condition in the first-level analysis only for the block-designed data. In the second-level analysis, one-sample t-test was applied to identify significant task-related brain activation for the 1-back and 2-back conditions (P < 0.05, FDR corrected, Fig. 3). For the N-back conditions compared with the rest condition, the results showed task activations in CON and FPN including the dorsolateral prefrontal cortex, inferior prefrontal gyrus, bilateral superior, middle, and inferior frontal gyrus, bilateral superior and inferior parietal gyrus, bilateral insula, anterior cingulate cortex, supplementary motor area, and bilateral medial cingulate cortex. In contrast, deactivation was found in the DMN including the medial prefrontal cortex, bilateral precuneus, and angular gyrus (Fig. 3). In the current study, a task-related FC network was constructed based on brain regions of both activation and deactivation, resulting in 66 network nodes for further analysis.
Fig. 3.
Brain regions associated with the task activation of working memory load (n = 50 per condition). A t-map of task activation for 1-back vs rest. B t-map of task activation for 2-back vs rest. The significance threshold is set as t > 3.27, PFDR < 0.05. The paired t-test shows higher activation in the FPN and lower activation in the DMN during the 1-back condition and 2-back condition than in the rest condition. Red, positive activation; blue, negative activation
FC and Network Results
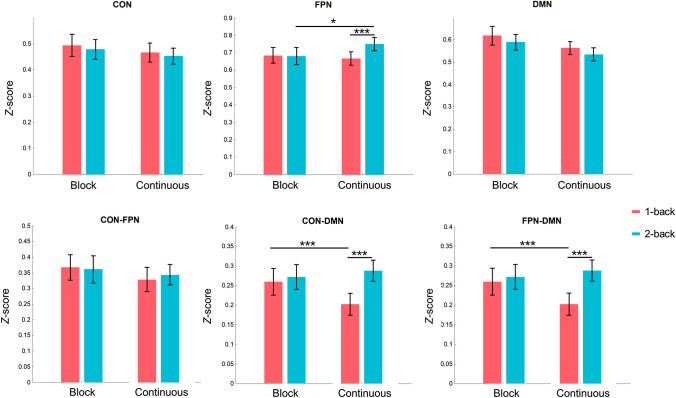
Four weighted networks were constructed for each participant, to investigate intra- and inter-network connectivity for CON, FPN, and DMN, as well as global network topology (global efficiency, PC, and transitivity). The results of ANOVA are summarized in Table 1 and Figs 4 and 5.
Fig. 4.
Memory load and continuous/block design differences in intra- (upper row) and inter-network connectivity (lower row, n = 50 per condition). Significant main effects and interactions were tested by ANOVA. For significant interaction, post hoc tests were examined by the paired t-test. Post hoc testing showed significant connectivity changes in the FPN, CON-FPN, and DMN-FPN. Error bars, SD. *P ≤ 0.05; ***P ≤ 0.001. CON, cingulo-opercular network; FPN, fronto-parietal network; DMN, default mode network
Fig. 5.
Global network topology is associated with memory load and design (n = 50 per condition). Significant main effects and interactions were tested by ANOVA. For significant interaction, post hoc tests were examined by paired t-tests. Post hoc testing showed significant connectivity changes in global efficiency, PC, and transitivity. Error bars, SD. **P ≤ 0.01, ***P ≤ 0.001
Intra-network Connectivity
ANOVA of intra-network connectivity indicated a significant interaction between design and memory load in the FC within the FPN (F1, 49 = 8.199, P < 0.01), meaning that the effect of memory load on connectivity is affected by the experimental design in the FPN. Post hoc tests showed increased connectivity within the FPN in the continuous 2-back condition compared to the continuous 1-back condition. Also, for the 2-back condition, there were more communications in the continuous condition than the block condition within the FPN.
A significant main effect of design on FC within the DMN (F1, 49 = 15.166, P < 0.001), indicated that the block condition had higher network communications within the DMN than the continuous condition. A significant main effect of memory load on FC within the FPN (F1, 49 = 7.518, P < 0.01) and the DMN (F1, 49 = 5.485, P < 0.05). Post hoc tests showed that the 1-back condition had higher network communications within the networks than the 2-back condition within the DMN, and lower network communications within the networks than the 2-back condition within the FPN.
Inter-network Connectivity
ANOVA results showed significant interactions between design and memory load, for inter-network connectivity between CON and DMN (F1, 49 = 11.235, P < 0.01) and between FPN and DMN (F1, 49 = 10.076, P < 0.01), indicating that the effect of memory load was affected by experimental design for connectivity between the DMN and the CON/FPN. Post hoc tests showed higher connectivity in the continuous 2-back condition than in the continuous 1-back condition. For the 1-back condition, the continuous condition showed fewer communications between the DMN and CON/FPN than the block condition.
There was also a significant main effect of design on inter-network connectivity between CON and DMN (F1, 49 = 4.137, P < 0.05), indicating higher network communications between CON and DMN in the block than in the continuous condition. A significant main effect of memory load on inter-network connectivity between CON and DMN (F1, 49 = 35.906, P < 0.001), and between FPN and DMN (F1, 49 = 8.325, P < 0.01), indicated that the 1-back condition had lower network communications between CON as well as DMN and between FPN and DMN than the 2-back condition.
Global Network Topology
We then calculated graph-based global measures for these 4 networks with 66 nodes, including commonly-used network metrics, PC, global efficiency, and transitivity. ANOVA was used to determine how they were modulated by different experimental designs and memory loads.
Results of global efficiency revealed a significant interaction between design and memory load (F1, 49 = 8.286, P < 0.01) indicating that the load effects upon efficiency are affected by experimental design. Post hoc tests showed increased efficiency from the 1-back to 2-back, and from the continuous 1-back to block 1-back. The main effect of design was significant (F1, 49 = 4.409, P < 0.05), indicating that inter-network communications in the block condition had higher efficiency in CON, FPN, and DMN than in the continuous condition. There was a significant main effect of memory load (F1, 49 = 17.437, P < 0.001), indicating that FC networks of 2-back had higher efficiency than 1-back.
PC results revealed a significant interaction between design and memory load (F1, 49 = 25.512, P < 0.001), indicating that the effect of memory load on PC is affected by experimental design. Post hoc tests showed increased PC from continuous 1-back to continuous 2-back. The continuous 1-back had a lower PC than the block 1-back, and the continuous 2-back had a higher PC than the block 2-back. A significant main effect of memory load (F1, 49 = 42.594, P < 0.001) indicated that the nodal communications in the 2-back condition are distributed more widely than in the 1-back condition.
Transitivity results revealed a significant interaction between design and memory load (F1, 49 = 7.160, P < 0.05), indicating that the effect of memory load upon transitivity is affected by experimental design. Post hoc tests showed increased transitivity from continuous 1-back to continuous 2-back, and the continuous 1-back had lower transitivity than the block 1-back. There was a significant main effect of memory load (F1, 49 = 12.559, P < 0.001) indicating that, in the 2-back condition, there was a higher prevalence of clustered connectivity than in the 1-back condition.
Correlations with Behavioral Results
We then explored the relationships between behavioral performance and task-related FC networks. The positive correlation between ACC and global network topology (PC; r = 0.473, PFDR = 0.019) for the continuous 2-back condition was significant (Fig. 6). The correlation pattern between behavior performance and functional network of the block as well as continuous design can be seen in Fig. S5.
Fig. 6.

Correlation between ACC and PC in the continuous 2-back condition (P < 0.05, FDR corrected, n = 50). ACC, accuracy, PC, participation coefficient. The shaded area indicates the 95% confidence interval
Validation Analysis Results
The results of the whole brain network topology were similar (Table S2 and Fig. S1). The ANOVA and post hoc results showed that for whole brain network topology results, the main effects of memory load were significant (but weaker than topology results of three memory-related networks), while the interaction results were not significant but showed consistent trends. They indicated that whole brain networks are not as sensitive as the three memory-related networks in finding effects of interest in the current study. Additionally, results based on time series in continuous design cut to the same length as those in block design, were similar to results based on the whole length of time series. (ACC and RT results; Tables S3 and S4; Fig. S2; network connectivity and graph-based analysis results; Tables S3–S5; Figs S3 and S4).
Discussion
In the current study, participants performed block- and continuous-design N-back working memory tasks during fMRI scanning. Network analyses were applied to investigate how task design (block vs continuous design) and task demand (1- vs 2-back) affected the underlying properties of FC networks associated with task activation. These properties included the intra- and inter-network connectivity as well as the global network topology. To our knowledge, this is the first study to reveal that different task designs modulate functional interactions within and between DMN, CON, and FPN, along with task performance. The global network topology showed a significant interaction between the memory load and task design. In addition, we replicated previous results by showing that elevated activation in the FPN and attenuated activation in the DMN were linked with higher memory load. This work provides new insight into the effect of a continuous working memory task paradigm on task-related FC and networks, which might be useful in normal and abnormal brain research because of less demand for task manipulation during each run.
Our findings demonstrated that three intrinsic functional networks play important roles and vary with memory load during the working memory task, as reflected by task-related activation of working memory, changes of FC and network topology, and associations with behavioral performance. Moreover, the CON and the FPN regions overlapped in their working memory function [26, 29, 55]. Fronto-parietal regions showed a higher BOLD signal during the high memory-load condition than the low memory-load condition (Fig. 3). Also, there were more communications within fronto-parietal regions in the 2-back condition than 1-back in the continuous condition. However, no such difference was found in the block condition (Table 1, Fig. 4). The fronto-parietal regions are critical in the working memory process [56–58]. Many studies have shown that the prefrontal cortex plays an important role in working-memory processes both in animal studies [59–61] and human studies [62–64]. Longitudinal studies have also shown activity in the frontal and parietal lobes associated with the capacity of working memory [65, 66]. Barch and colleagues used Human Connectome Project data to calculate brain activation during 2-back versus rest and found that the bilateral dorsal and ventral prefrontal cortex, dorsal parietal cortex, and dorsal anterior cingulate are activated [67]. However, in the process of preparing attention and memory orientation, the FPN is associated with regulating more advanced sensory cortical activity than the CON [30, 31]. Working memory processes require attentional control, possibly mediated by dorsolateral prefrontal regions [46, 68, 69]. The dorsolateral prefrontal cortex is a central region in the FPN. Thus, the FPN is a very important network in the working memory process because it is associated with many memory-related processes. Further, the DMN showed deactivations during the high memory-load condition (Fig. 3) and the intra- and inter-network FCs showed that a higher memory load reduced connectivity within the DMN in the N-back task (Table 1, Fig. 4; [51]).
Higher memory loads were associated with increased communications between nodes in the three networks. Our behavioral results replicated others, indicating higher ACC and shorter RTs in the 1-back than the 2-back condition in both the continuous and block designs. Whole-brain activation results also showed higher activation for the N-back condition than the rest condition in the FPN during the block design. The connectivity results showed increased connectivity within memory-related regions and decreased connectivity within the DMN in the 2-back condition compared to the 1-back condition. These are consistent with previous behavioral studies [51, 70] and neuroimaging studies [21, 71, 72]. We also found significant memory load main effects in the results of graph-based global measures. A higher memory load (2-back) increased PC, global efficiency, and transitivity compared with a lower memory load (1-back). These results suggested that, as memory load increases, more resources are required [73], and the FC tends to be more globally organized [74].
Here, we tested the effects of task design and working memory load on behavioral performance, fMRI-derived task-state FC, and network topology. There were significant interactions between task design and memory load (Table 1, Figs. 2, 4, 5), suggesting that between two working memory task designs, significant differences in task-related FC and graph-based measures were related to memory load. Interestingly, as revealed by post hoc analyses, memory-load effects were higher in the continuous than the block design regarding intra- and inter-network FC as well as global network topology. Specifically, in the continuous design task, 1-back performance was better, and 2-back performance was worse than in the block design. One reason may be because the 1-back task was easy as the ACC of 1-back was very high (mean ACC >95% for both block and continuous design). ACC may also not be sensitive enough to detect a design effect. By contrast, for a difficult task (high memory load)—2-back task, participants need more time to respond. The RT of 2-back was very similar (mean RT ~ 960 ms for both block and continuous design). RT may not have been sensitive enough to detect the design effect. Previous studies have found that, as memory load increases, the difference between the ACCs becomes larger, and the RTs become smaller [75–77]. On the other hand, this result might be attributable to the long duration and thus more trials using the same N-back stimulus in the single block for the continuous design compared with the classic block design. The improved behavioral performance in the 1-back condition may be explained as practice or learning effects. Earlier studies have suggested that practice can shorten RT [78–80]. Recent meta-analyses have shown that training can increase working memory ACC much like a greater number of trials [81–83]. Moreover, in block design experiments, adjacent blocks often have different conditions, so that participants must switch tasks at the beginning of each block. Task-switching is known to incur performance costs [16, 84, 85]. For the easy condition (1-back), task-switching may lead to poorer behavioral performance than in other conditions. By contrast, using the continuous design the trials of N-back tasks are continuously presented (one by one), requiring the participant to sustain attention during the whole run. For the more difficult condition (2-back), the longer task duration may lead to poorer behavioral performance than other conditions. The longer duration of difficult tasks increases RT [79, 86, 87] and reduces ACC [87, 88], possibly reflecting fatigue effects [89, 90]. In fact, the mean number of discontinuously wrong trials in the continuous 2-back condition was much higher than in other conditions, which is consistent with the finding of the lowest accuracy.
Longer task-duration enhanced graph-based global measures. Higher memory load (2-back) increased PC compared with lower memory load (1-back) significantly only in the continuous condition. Similar results were obtained for global efficiency and transitivity. Significant memory load effects only occurred in the continuous-design condition. Previous studies have reported spatial distributions between transient and sustained responses [91–93]. Fox and colleagues, for instance, had participants perform a block-design 2-back task and found that block transient and sustained components had different BOLD signal changes and spatial distributions [94]. In addition to behavioral performance, changes associated with the three networks might be related to the different methods by which FC is calculated for continuous and block designs. Despite both using Pearson’s correlation for time series between network nodes to characterize network edges, (i.e., FC), longer and continuous time series characterized the continuous design while a block-concatenated time series was applied for the block design consistent with the literature [46, 51]. These results suggested that splice-and-concatenate of time series operation may add noise to data. The block-concatenated time series cannot be as smooth or as stable as the continuous time series.
Significant brain-behavior association between PC and task ACC in the continuous 2-back condition was identified, suggesting that higher PC corresponds to better working memory performance (Fig. 6). The functional network tends to be more globally organized as memory load increases [51, 74]. The PC characterizes the extent of a node’s communications between different networks to the total number of communications. And high PC suggests that the communications of the node tend to facilitate global intermodular integration [53]. These results suggested that the higher mean value of the diversity of intermodular interconnections of nodes reflects better behavioral performance. Previous research has shown that PC is significantly correlated with working memory performance [95]. Moreover, task training significantly increases the relationship between PC and behavioral performance [96, 97]. Therefore, the involvement of PC in network integration is related to behavioral performance. Cohen and D’Esposito showed significant correlations between global efficiency and ACC in the 3-back condition [41]. However, it is possible that more similar trends artificially inflate higher correlations in these data. In block designs, participants must switch tasks in different blocks. In the continuous design, however, no such switching is required. Continuous designs have more trials and longer task durations than block designs. These features of continuous designs yield time series that are more stable and smoother, yielding higher network correlation. The continuous 2-back condition had a more stable BOLD signal with higher memory loads, yielding a stronger correlation between behavioral and brain data. It is possible that these FC differences could be attributed to differences in task performance rather than design differences. There was a significant correlation between PC and ACC in the continuous 2-back condition; however, other FCs showed no association with task performance. Importantly, the main effects of design were not significant in ACC and RT. Moreover, interactions between design and memory load on task performance and FC differences were not consistent. We believe these results support our hypothesis that these differences are due to the effects of task design and not task performance.
One potential limitation is that the current study used a visual-spatial N-back working memory task, which is known to differ somewhat from other kinds of working memory tasks [98]. Although the N-back paradigm is a classic paradigm for measuring working memory function [70, 99], we must be cautious about generalization and need more research on the effect of continuous design on task-related FC. However, previous research has also shown generalizable FC network properties across different kinds of tasks, such as passive tasks (e.g., imagine task and monitor task), sensory tasks (e.g., auditory detection task and visual discrimination task), and word tasks (e.g., N-back tasks and semantic tasks) [18]. Therefore, to comprehensively explore the design effect, other working memory paradigms like the digit span task or operation span task, as well as other cognitive tasks, should be tested to further our understanding of the task-related FC of continuous design. Besides, the different time series lengths between the block and continuous designs might affect the task-related FC characteristics. Usually, longer scans improve the reliability of derived metrics [100, 101]. However, our validation results showed the results based on the same length of continuous design with block design remained largely unchanged compared to the primary results, suggesting that the influence of different lengths was minimal for the results reported in the current study. Second, we used weighted positive edges to calculate network properties following the previous studies [37, 51, 52]. The results might be affected by the density of the network. Further research should try to control the density.
In conclusion, using both block-design and continuous-design data, our study suggested that the continuous design can be used to characterize task-related FC, which can reveal the effect of working memory load and the brain-behavioral relationship. Given that the task of continuous design is convenient to conduct for participants and to identify task-related FC, current findings support that the continuous design has the potential to study the abnormal task-related FCs of patients and for future task-based fMRI research.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We thank all participants in the study. This work was supported by the National Natural Science Foundation of China (62071109 and 61871420) and the Provincial Natural Science Foundation of Sichuan (2022NSFSC0504).
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Chun Meng, Email: chunmeng@uestc.edu.cn.
Bharat Biswal, Email: bbiswal@gmail.com.
References
- 1.D’Esposito M, Ballard D, Zarahn E, Aguirre GK. The role of prefrontal cortex in sensory memory and motor preparation: An event-related fMRI study. Neuroimage. 2000;11:400–408. doi: 10.1006/nimg.2000.0571. [DOI] [PubMed] [Google Scholar]
- 2.Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- 3.Bandettini PA. MRI studies of brain activation: Dynamic characteristics. Funct MRI Brain 1993, 144–151.
- 4.Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- 6.Petersen SE, Dubis JW. The mixed block/event-related design. Neuroimage. 2012;62:1177–1184. doi: 10.1016/j.neuroimage.2011.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaldson DI. Parsing brain activity with fMRI and mixed designs: What kind of a state is neuroimaging in? Trends Neurosci. 2004;27:442–444. doi: 10.1016/j.tins.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson DI, Buckner RL. Effective paradigm design. In: Functional MRI: An Introduction to Methods. Oxford: Oxford University Press, 2001. 10.1093/acprof:oso/9780192630711.003.0009
- 9.Maccotta L, Zacks JM, Buckner RL. Rapid self-paced event-related functional MRI: Feasibility and implications of stimulus- versus response-locked timing. Neuroimage. 2001;14:1105–1121. doi: 10.1006/nimg.2001.0912. [DOI] [PubMed] [Google Scholar]
- 10.Meltzer JA, Negishi M, Constable RT. Biphasic hemodynamic responses influence deactivation and may mask activation in block-design fMRI paradigms. Hum Brain Mapp. 2008;29:385–399. doi: 10.1002/hbm.20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI I. The Method. NeuroImage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- 12.Yarkoni T, Speer NK, Balota DA, McAvoy MP, Zacks JM. Pictures of a thousand words: Investigating the neural mechanisms of reading with extremely rapid event-related fMRI. Neuroimage. 2008;42:973–987. doi: 10.1016/j.neuroimage.2008.04.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole MW, Ito T, Schultz D, Mill R, Chen R, Cocuzza C. Task activations produce spurious but systematic inflation of task functional connectivity estimates. Neuroimage. 2019;189:1–18. doi: 10.1016/j.neuroimage.2018.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon S, Watanabe M, Fischer E, Bartels A. Attention reorganizes connectivity across networks in a frequency specific manner. Neuroimage. 2017;144:217–226. doi: 10.1016/j.neuroimage.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Qiao L, Zhang L, Chen A, Egner T. Dynamic trial-by-trial recoding of task-set representations in the frontoparietal cortex mediates behavioral flexibility. J Neurosci. 2017;37:11037–11050. doi: 10.1523/JNEUROSCI.0935-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krienen FM, Yeo BTT, Buckner RL. Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130526. doi: 10.1098/rstb.2013.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin SY, Lee CC, Chen YS, Kuo LW. Investigation of functional brain network reconfiguration during vocal emotional processing using graph-theoretical analysis. Soc Cogn Affect Neurosci. 2019;14:529–538. doi: 10.1093/scan/nsz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di X, Zhang H, Biswal BB. Anterior cingulate cortex differently modulates frontoparietal functional connectivity between resting-state and working memory tasks. Hum Brain Mapp. 2020;41:1797–1805. doi: 10.1002/hbm.24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, et al. Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimura K, Chushak MS, Westbrook A, Braver TS. Intertemporal decision-making involves prefrontal control mechanisms associated with working memory. Cereb Cortex. 2018;28:1105–1116. doi: 10.1093/cercor/bhx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamichhane B, Westbrook A, Cole MW, Braver TS. Exploring brain-behavior relationships in the N-back task. NeuroImage. 2020;212:116683. doi: 10.1016/j.neuroimage.2020.116683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biswal BB, Eldreth DA, Motes MA, Rypma B. Task-dependent individual differences in prefrontal connectivity. Cereb Cortex. 2010;20:2188–2197. doi: 10.1093/cercor/bhp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Esposito M, Postle BR. The cognitive neuroscience of working memory. Annu Rev Psychol. 2015;66:115–142. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers NE, Stokes MG, Nobre AC. Prioritizing information during working memory: Beyond sustained internal attention. Trends Cogn Sci. 2017;21:449–461. doi: 10.1016/j.tics.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44:2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Taghia J, Cai W, Ryali S, Kochalka J, Nicholas J, Chen T, et al. Uncovering hidden brain state dynamics that regulate performance and decision-making during cognition. Nat Commun. 2018;9:2505. doi: 10.1038/s41467-018-04723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallis G, Stokes M, Cousijn H, Woolrich M, Nobre AC. Frontoparietal and cingulo-opercular networks play dissociable roles in control of working memory. J Cogn Neurosci. 2015;27:2019–2034. doi: 10.1162/jocn_a_00838. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Wassermann D, Abrams DA, Kochalka J, Gallardo-Diez G, Menon V. The visual word form area (VWFA) is part of both language and attention circuitry. Nat Commun. 2019;10:5601. doi: 10.1038/s41467-019-13634-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 35.Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA. Default mode network connectivity during task execution. Neuroimage. 2015;122:96–104. doi: 10.1016/j.neuroimage.2015.07.053. [DOI] [PubMed] [Google Scholar]
- 36.Vatansever D, Menon DK, Stamatakis EA. Default mode contributions to automated information processing. Proc Natl Acad Sci U S A. 2017;114:12821–12826. doi: 10.1073/pnas.1710521114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang X, Zou Q, He Y, Yang Y. Topologically reorganized connectivity architecture of default-mode, executive-control, and salience networks across working memory task loads. Cereb Cortex. 2016;26:1501–1511. doi: 10.1093/cercor/bhu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaple Z, Arsalidou M. N-back working memory task: Meta-analysis of normative fMRI studies with children. Child Dev. 2018;89:2010–2022. doi: 10.1111/cdev.13080. [DOI] [PubMed] [Google Scholar]
- 39.Vilà-Balló A, Salmi J, Soveri A, Rodríguez-Fornells A, Lehtonen M, Laine M. Neural signatures for active maintenance and interference during working memory updating. Biol Psychol. 2018;132:233–243. doi: 10.1016/j.biopsycho.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen JR, D’Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. J Neurosci. 2016;36:12083–12094. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuo N, Yang Z, Liu Y, Li J, Jiang T. Core networks and their reconfiguration patterns across cognitive loads. Hum Brain Mapp. 2018;39:3546–3557. doi: 10.1002/hbm.24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolt T, Nomi JS, Rubinov M, Uddin LQ. Correspondence between evoked and intrinsic functional brain network configurations. Hum Brain Mapp. 2017;38:1992–2007. doi: 10.1002/hbm.23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A. 2011;108:7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betzel RF, Bertolero MA, Bassett DS. Non-assortative community structure in resting and task-evoked functional brain networks. bioRxiv 2018. 10.1101/355016.
- 46.Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di X, Biswal BB. Toward task connectomics: Examining whole-brain task modulated connectivity in different task domains. Cereb Cortex. 2019;29:1572–1583. doi: 10.1093/cercor/bhy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp. 2011;32:919–934. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Douw L, Quaak M, Fitzsimmons SMDD, de Wit SJ, van der Werf YD, van den Heuvel OA, et al. Static and dynamic network properties of the repetitive transcranial magnetic stimulation target predict changes in emotion regulation in obsessive-compulsive disorder. Brain Stimul. 2020;13:318–326. doi: 10.1016/j.brs.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Newman MEJ. The structure and function of complex networks. SIAM Rev. 2003;45:167–256. doi: 10.1137/S003614450342480. [DOI] [Google Scholar]
- 55.Finn ES, Huber L, Jangraw DC, Molfese PJ, Bandettini PA. Layer-dependent activity in human prefrontal cortex during working memory. Nat Neurosci. 2019;22:1687–1695. doi: 10.1038/s41593-019-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, et al. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, et al. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon EM, Devaney JM, Bean S, Vaidya CJ. Resting-state striato-frontal functional connectivity is sensitive to DAT1 genotype and predicts executive function. Cereb Cortex. 2015;25:336–345. doi: 10.1093/cercor/bht229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci U S A. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Owen AM, Herrod NJ, Menon DK, Clark JC, Downey SP, Carpenter TA, et al. Redefining the functional organization of working memory processes within human lateral prefrontal cortex. Eur J Neurosci. 1999;11:567–574. doi: 10.1046/j.1460-9568.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- 61.Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: Evidence for mnemonic “scotomas”. J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ackerman CM, Courtney SM. Spatial relations and spatial locations are dissociated within prefrontal and parietal cortex. J Neurophysiol. 2012;108:2419–2429. doi: 10.1152/jn.01024.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zarahn E, Aguirre GK, D’Esposito M. Temporal isolation of the neural correlates of spatial mnemonic processing with fMRI. Brain Res Cogn Brain Res. 1999;7:255–268. doi: 10.1016/S0926-6410(98)00029-9. [DOI] [PubMed] [Google Scholar]
- 64.Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11:145–156. doi: 10.1006/nimg.1999.0527. [DOI] [PubMed] [Google Scholar]
- 65.Ullman H, Almeida R, Klingberg T. Structural maturation and brain activity predict future working memory capacity during childhood development. J Neurosci. 2014;34:1592–1598. doi: 10.1523/JNEUROSCI.0842-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Darki F, Klingberg T. The role of fronto-parietal and fronto-striatal networks in the development of working memory: A longitudinal study. Cereb Cortex. 2015;25:1587–1595. doi: 10.1093/cercor/bht352. [DOI] [PubMed] [Google Scholar]
- 67.Barch DM, Sheline YI, Csernansky JG, Snyder AZ. Working memory and prefrontal cortex dysfunction: Specificity to schizophrenia compared with major depression. Biol Psychiatry. 2003;53:376–384. doi: 10.1016/S0006-3223(02)01674-8. [DOI] [PubMed] [Google Scholar]
- 68.Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: An event-related fMRI study. Brain Res Cogn Brain Res. 2000;9:103–109. doi: 10.1016/S0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- 69.Sherwood MS, Kane JH, Weisend MP, Parker JG. Enhanced control of dorsolateral prefrontal cortex neurophysiology with real-time functional magnetic resonance imaging (rt-fMRI) neurofeedback training and working memory practice. Neuroimage. 2016;124:214–223. doi: 10.1016/j.neuroimage.2015.08.074. [DOI] [PubMed] [Google Scholar]
- 70.Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, et al. Working memory for complex figures: An fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–379. doi: 10.1037/0894-4105.16.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piccoli T, Valente G, Linden DEJ, Re M, Esposito F, Sack AT, et al. The default mode network and the working memory network are not anti-correlated during all phases of a working memory task. PLoS One. 2015;10:e0123354. doi: 10.1371/journal.pone.0123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J, Xia M, Dai Z, Wang X, Liao X, Bi Y, et al. Intrinsic brain hub connectivity underlies individual differences in spatial working memory. Cereb Cortex. 2017;27:5496–5508. doi: 10.1093/cercor/bhw317. [DOI] [PubMed] [Google Scholar]
- 73.Zhang H, Zhao R, Hu X, Guan S, Margulies DS, Meng C, Biswal BB. Cortical connectivity gradients and local timescales during cognitive states are modulated by cognitive loads. Brain Struct Funct. 2022 doi: 10.1007/s00429-022-02564-0. [DOI] [PubMed] [Google Scholar]
- 74.Kitzbichler MG, Henson RNA, Smith ML, Nathan PJ, Bullmore ET. Cognitive effort drives workspace configuration of human brain functional networks. J Neurosci. 2011;31:8259–8270. doi: 10.1523/JNEUROSCI.0440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magen H, Emmanouil TA. Working memory for self-initiated and provided spatial configurations. Q J Exp Psychol (Hove) 2018;71:2186–2206. doi: 10.1177/1747021817739808. [DOI] [PubMed] [Google Scholar]
- 76.Milchgrub G, Magen H. Self-initiated spatial working memory in young and older adults. Memory. 2018;26:712–726. doi: 10.1080/09658211.2017.1402938. [DOI] [PubMed] [Google Scholar]
- 77.Pergher V, Wittevrongel B, Tournoy J, Schoenmakers B, van Hulle MM. N-back training and transfer effects revealed by behavioral responses and EEG. Brain Behav. 2018;8:e01136. doi: 10.1002/brb3.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Church RM, Camp DS. Change in reaction-time as a function of knowledge of results. Am J Psychol. 1965;78:102–106. doi: 10.2307/1421087. [DOI] [PubMed] [Google Scholar]
- 79.Maylor EA, Rabbitt PM, James GH, Kerr SA. Effects of alcohol, practice, and task complexity on reaction time distributions. Q J Exp Psychol A. 1992;44:119–139. doi: 10.1080/14640749208401286. [DOI] [PubMed] [Google Scholar]
- 80.Shanks DR, Cameron A. The effect of mental practice on performance in a sequential reaction time task. J Mot Behav. 2000;32:305–313. doi: 10.1080/00222890009601381. [DOI] [PubMed] [Google Scholar]
- 81.Constantinidis C, Klingberg T. The neuroscience of working memory capacity and training. Nat Rev Neurosci. 2016;17:438–449. doi: 10.1038/nrn.2016.43. [DOI] [PubMed] [Google Scholar]
- 82.Peijnenborgh JCAW, Hurks PM, Aldenkamp AP, Vles JSH, Hendriksen JGM. Efficacy of working memory training in children and adolescents with learning disabilities: A review study and meta-analysis. Neuropsychol Rehabil. 2016;26:645–672. doi: 10.1080/09602011.2015.1026356. [DOI] [PubMed] [Google Scholar]
- 83.Schwaighofer M, Fischer F, Bühner M. Does working memory training transfer? A meta-analysis including training conditions as moderators. Educ Psychol. 2015;50:138–166. doi: 10.1080/00461520.2015.1036274. [DOI] [Google Scholar]
- 84.de Baene W, Brass M. Cue-switch effects do not rely on the same neural systems as task-switch effects. Cogn Affect Behav Neurosci. 2011;11:600–607. doi: 10.3758/s13415-011-0055-9. [DOI] [PubMed] [Google Scholar]
- 85.Loose LS, Wisniewski D, Rusconi M, Goschke T, Haynes JD. Switch-independent task representations in frontal and parietal cortex. J Neurosci. 2017;37:8033–8042. doi: 10.1523/JNEUROSCI.3656-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Benton AL, Blackburn HL. Practice effects in reaction-time tasks in brain-injured patients. J Abnorm Psychol. 1957;54:109–113. doi: 10.1037/h0047176. [DOI] [PubMed] [Google Scholar]
- 87.Küper M, Kaschani P, Thürling M, Stefanescu MR, Burciu RG, Göricke S, et al. Cerebellar fMRI activation increases with increasing working memory demands. Cerebellum. 2016;15:322–335. doi: 10.1007/s12311-015-0703-7. [DOI] [PubMed] [Google Scholar]
- 88.Kübler A, Murphy K, Kaufman J, Stein EA, Garavan H. Co-ordination within and between verbal and visuospatial working memory: Network modulation and anterior frontal recruitment. Neuroimage. 2003;20:1298–1308. doi: 10.1016/S1053-8119(03)00400-2. [DOI] [PubMed] [Google Scholar]
- 89.Klaassen EB, Evers EAT, de Groot RHM, Backes WH, Veltman DJ, Jolles J. Working memory in middle-aged males: Age-related brain activation changes and cognitive fatigue effects. Biol Psychol. 2014;96:134–143. doi: 10.1016/j.biopsycho.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 90.Jain S, Nataraja NP. The effect of fatigue on working memory and auditory perceptual abilities in trained musicians. Am J Audiol. 2019;28:483–494. doi: 10.1044/2019_AJA-IND50-18-0102. [DOI] [PubMed] [Google Scholar]
- 91.Konishi S, Donaldson DI, Buckner RL. Transient activation during block transition. Neuroimage. 2001;13:364–374. doi: 10.1006/nimg.2000.0691. [DOI] [PubMed] [Google Scholar]
- 92.Paret C, Kluetsch R, Ruf M, Demirakca T, Kalisch R, Schmahl C, et al. Transient and sustained BOLD signal time courses affect the detection of emotion-related brain activation in fMRI. Neuroimage. 2014;103:522–532. doi: 10.1016/j.neuroimage.2014.08.054. [DOI] [PubMed] [Google Scholar]
- 93.Song XW, Bhinge S, Quiton RL, Adalı T. An ICA based approach for steady-state and transient analysis of task fMRI data: Application to study of thermal pain response. J Neurosci Methods. 2019;326:108356. doi: 10.1016/j.jneumeth.2019.108356. [DOI] [PubMed] [Google Scholar]
- 94.Fox MD, Snyder AZ, Barch DM, Gusnard DA, Raichle ME. Transient BOLD responses at block transitions. Neuroimage. 2005;28:956–966. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 95.Pedersen M, Omidvarnia A, Shine JM, Jackson GD, Zalesky A. Reducing the influence of intramodular connectivity in participation coefficient. Netw Neurosci. 2020;4:416–431. doi: 10.1162/netn_a_00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Q, Turnbull A, Cole M, Zhang Z, Lin FV. Enhancing cortical network-level participation coefficient as a potential mechanism for transfer in cognitive training in aMCI. Neuroimage. 2022;254:119124. doi: 10.1016/j.neuroimage.2022.119124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han K, Chapman SB, Krawczyk DC. Cognitive training reorganizes network modularity in traumatic brain injury. Neurorehabil Neural Repair. 2020;34:26–38. doi: 10.1177/1545968319868710. [DOI] [PubMed] [Google Scholar]
- 98.Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/S0140525X01003922. [DOI] [PubMed] [Google Scholar]
- 99.Blokland GAM, McMahon KL, Hoffman J, Zhu G, Meredith M, Martin NG, et al. Quantifying the heritability of task-related brain activation and performance during the N-back working memory task: A twin fMRI study. Biol Psychol. 2008;79:70–79. doi: 10.1016/j.biopsycho.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Termenon M, Jaillard A, Delon-Martin C, Achard S. Reliability of graph analysis of resting state fMRI using test-retest dataset from the Human Connectome Project. Neuroimage. 2016;142:172–187. doi: 10.1016/j.neuroimage.2016.05.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.