Abstract
Chenopodium quinoa Willd. is a crop species domesticated over 5000 years ago. This species is highly diverse, with a geographical distribution that covers more than 5000 km from Colombia to Chile, going through a variety of edaphoclimatic conditions. Quinoa grains have great nutritional quality, raising interest at a worldwide level. In this work, by using shotgun proteomics and in silico analysis, we present an overview of mature quinoa seed proteins from a physiological context and considering the process of seed maturation and future seed germination. For this purpose, we selected grains from four contrasting quinoa cultivars (Amarilla de Maranganí, Chadmo, Sajama and Nariño) with different edaphoclimatic and geographical origins. The results give insight on the most important metabolic pathways for mature quinoa seeds including: starch synthesis, protein bodies and lipid bodies composition, reserves and their mobilization, redox homeostasis, and stress related proteins like heat-shock proteins (HSPs) and late embryogenesis abundant proteins (LEAs), as well as evidence for capped and uncapped mRNA translation. LEAs present in our analysis show a specific pattern of expression matching that of other species. Overall, this work presents a complete snapshot of quinoa seeds physiological context, providing a reference point for further studies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-023-01295-8.
Keywords: Proteomics, Quinoa, Seed maturation, Seed physiology
Introduction
Quinoa (Chenopodium quinoa Willd.) is a crop that has been domesticated over 5000 years ago around the Titicaca Lake region, spreading north and south following migrations and cultural trade. It can be found all along the Andean region from the center of Colombia to the Chilean Patagonia (Planella et al. 2015). The species belong to the Amaranthaceae s.l. family, which in turn belongs to the Caryophyllales order (Chase et al. 2016). It is considered a pseudo-cereal because, despite having similar uses to that of cereals, it belongs to the dicots class, while the cereals are monocots.
The nutritional quality of its seeds and the remarkable tolerance to abiotic stress presented by the crop are characteristics of great interest at world-wide level, resulting in the extension of its use beyond the American continent (Kumar et al. 2006; Präger et al. 2018; Pulvento et al. 2010). The great qualities of this grain include a high essential aminoacid and high essential fatty acid content (Vega-Gálvez et al. 2010). Quinoa seed storage proteins have a high lysine content, which is usually not the case for cereals. The amounts of methionine and cysteine are higher than those in other cereals and legumes (Burrieza et al. 2019; Ruales and Nair 1992). Finally, quinoa has been considered a gluten-free food, yet the absence of prolamins has been confirmed recently (Burrieza et al. 2019), validating quinoa as a suitable food for coeliacs.
Given the geographical distribution and the variety of edaphoclimatic conditions that this species is exposed to, there is a vast genetic diversity for tolerance to abiotic stress factors (i.e. drought, salinity and extreme temperatures) (Bertero et al. 2004). A great variety of grain characteristics can be found among quinoa genotypes, with changes in saponin content, pericarp and tegument color and perisperm appearance among other characteristics.
Quinoa grain is an achene that can present different color tones on its pericarp. The tegument of this perispermic seed also presents a range of colors from white to dark brown. Inside the seeds, the embryo surrounds the periphery of the perisperm in a ring-like structure. The storage of proteins and lipids happen mainly in the protein bodies (PBs) and lipid bodies (LBs), respectively, and both of them are found in embryo cells. The perisperm, on the other hand, functions as a storage tissue for carbohydrates, mainly in the form of starch (Burrieza et al. 2014; Prego et al. 1998).
As the seed develops, storage structures differentiate sequentially and accumulate different reserves. Amyloplasts, present in the perisperm which is dead by the time the seeds mature, hold the starch. Synthesis of starch is associated with several proteins, some of which remain trapped inside the starch structure after it is synthesized (e.g. granule-bound starch synthetases (GBSSs), soluble starch synthases (SSSs), starch branching and debranching enzymes) (Baldwin 2001). PBs, present in the embryo cells, hold storage proteins (i.e., legumins, vicilins), while LBs, also present in embryo cells, hold lipid reserves in the form of triacylglycerides (TAGs), and their membranes hold proteins which are fundamental for their structure, maintenance and future metabolism (i.e., oil-body associated proteins, oleosins, caleosins).
Quinoa seeds belong to the group of orthodox seeds. These dehydrate, reaching a water content of around 10% (Causin et al. 2020; Stikic et al. 2012) with no evident or irreversible damage. There are several molecular mechanisms associated with the maturation of orthodox seeds. They can involve regulator genes as Abscisic acid insensitive 3 (ABI3) or Leafy cotyledon 1 (LEC1). These genes can affect the expression of chaperones, late embryogenesis abundant proteins (LEAs) and genes involved in antioxidant activity, among others (Kotak et al. 2007; Bies-Ethève et al. 2008; Mönke et al. 2012). All of those related not only with embryo development, but also with embryo protection when dealing with the stress caused by dehydration during the late stages of seed maturation and later repairs when rehydration and germination start (Delahaie et al. 2013).
Quinoa seeds have three kind of storage nutrients (proteins, lipids and starch) and all of them are used during germination. The germination process is fast (6–48 h), so identifying present enzymes related to reserves metabolism at the mature seed stage is of interest, as they could be involved in the first stages of germination (e.g., α-amylases, β-amylases, α-glycosidases and branching and debranching enzymes, proteases, β-oxidation enzymes). Some enzymes might be present and active before seed imbibition, while others are synthesized de novo during this process (Hager et al. 2014; Mäkinen et al. 2014; Suárez-Estrella et al. 2020).
There are previous reports showing proteins present in mature quinoa seeds. Capriotti et al. (2015) compared extraction methodologies exhaustively, but used proteins belonging to the Caryophyllales order to identify their peptides. This makes for a broader set of results, but the focus of the work is mainly related to the search for optimal extraction methods and not in the resulting protein data analysis. Aloisi et al. (2016) analyzed a set of proteins in quinoa’s proteome and, although the scope is of great interest, highlighting differences in seeds harvested from salt stressed and non-stressed plants, the number of identified proteins does not allow to build metabolic pathways, as they come from selected spots on 2D protein gels. Burrieza et al. (2019) used shotgun proteomics to identify storage proteins, and limited the analysis to this set of proteins. Lastly, an interesting work on quinoa seed proteomes has been published recently (Galindo-Luján et al. 2021). In this work, authors performed LC–MS/MS shotgun proteomics to identify quinoa protein accessions using the published quinoa genome (Jarvis et al. 2017; Yasui et al. 2016; Zou et al. 2017), nonetheless their analysis is limited to the main categories of protein classification regarding molecular function, biological process, cellular component and protein class, and despite having a larger coverage, they focus on the commercial varieties of quinoa obtained from their local supermarket with no origin identification. Our objective is to present a descriptive and comparative analysis of mature C. quinoa seed proteome from four different genotypes with contrasting edaphoclimatic origins (coastal, interandean valleys, high plateau), which cover a span of over 5000 km comprising parallels 1°N to 42°S. This area has been classified and used frequently by researchers (FAO and CIRAD 2015) to compare different genotypes of quinoa, their origins and adaptations. Also, the genotypes selected for this study present differences in grain characteristics such as pericarp and tegument color and type of perisperm (farinaceous or crystalline); Nariño and Sajama have white pericarp and tegument, Amarilla de Maranganí has a yellow pericarp and white tegument, and Chadmo has a translucent pericarp and dark brown tegument. Chadmo is the only genotype that present a crystalline perisperm. Genotypes were studied using a shotgun proteomics approach and identifying the proteins using the available quinoa genome (Jarvis et al. 2017). In order to study intrinsic differences between the seeds of the selected genotypes, the plants were grown in controlled conditions. We use the resulting information to describe the mature seed stage and to gain insight on the processes of seed maturation and germination. We propose this work as an initial reference for the interpretation of future results in studies that use close developmental stages either previous or following mature seed.
Materials & methods
Seed material
The seeds were obtained from the CIP germplasm bank (Lima, Peru) and from the INIA-Vicuña germplasm bank (La Serena, Chile). Four quinoa genotypes were selected: Amarilla de Maranganí and Nariño (from the Andean valleys of Perú and Colombia, respectively), Chadmo (from coastal area of Chiloé, Chile), Sajama (from the Bolivian high-plateau), (Online resource 1). These are broadly distributed between latitudes 1°N and 42°S and were selected taking into account the edaphoclimatic conditions to which they are adapted and their differences. The environmental ecoregions mentioned for each genotype are usually used to classify different genotypes of quinoa (FAO and CIRAD 2015). To avoid genotype x environment interaction, the seeds received from CIP and INIA-Vicuña were handled as described in the next section. The selected seeds present differences in their pericarp, tegument and perisperm (Online Resource 1). All samples and materials are identical to those used for seed storage protein analysis in Burrieza et al., 2019.
Growth conditions
Seeds were germinated separately and pre-selected for homogeneity before being transplanted to a bigger pot filled with GrowMix substrate (Terrafertil, Buenos Aires). Plants were watered every two days with double-distilled water, and fertilized with 0,7 g L−1 HAKAPHOS (Compo Expert, Argentina) once a week. All genotypes were grown in controlled conditions. A light cycle of 16 h light/8 h darkness and a temperature of 22/18 °C (light/darkness, respectively) was used throughout their development. Seed were harvested at physiological maturity, cleaned of debri and stored at 4 °C until used.
Protein extraction and digestion
Protein triplicates were extracted using the TCA/acetone precipitation method developed by Damerval et al. (1986) using 100 mg of lyophilized initial mass. Seeds were pulverized using a mortar and pestle in liquid nitrogen. Samples from each genotype were resuspended in 1 ml of chilled extraction buffer containing 10% (w/v) TCA in acetone with 20 mM DTT and vortexed for 5 min at 8 °C; the mixture was kept at –20 °C for 1 h, then centrifuged at 16,000 g for 30 min at 4 °C. Resulting pellets were washed three times with cold acetone and 20 mM DTT, then centrifuged for 5 min each wash. Pellets were air dried, resuspended in buffer containing 7 M urea, 2 M thiourea, 2% Triton X-100, 1% DTT, 1 mM PMSF and complete protease inhibitor cocktail, and incubated for 30 min on ice. The samples were then vortexed at 8 °C for 30 min and centrifuged at 4 °C for 20 min at 16,000 g. The supernatants were collected, and the protein concentrations were determined using the 2-D Quant Kit.
Protein digestion was performed using the filter-aided sample preparation (FASP) method with trypsin (V5111; Promega, Madison, WI, USA; final ratio 1:100 enzyme:protein) according to Reis et al. (2021). The resulting peptides were quantified by the A205 nm protein and peptide methodology using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, USA).
Mass spectrometry analysis
A nanoAcquity UPLC connected to a Synapt G2-Si HDMS mass spectrometer (Waters, Manchester, UK) was used for ESI-LC–MS/MS analysis. Runs consisted of three biological replicates of 1 µg of digested proteins. During separation, samples were loaded onto the nanoAcquity UPLC 5 μm C18 trap column (180 μm × 20 mm) at 5 μl min− 1 for 3 min and then onto the nanoAcquity HSS T3 1.8 μm analytical reversed phase column (75 μm × 150 mm) at 400 nL min− 1, with a column temperature of 45 °C. For peptide elution, a binary gradient was used, with mobile phase A (water and 0.1% formic acid) and mobile phase B (acetonitrile and 0.1% formic acid). The gradient elution started at 7% B, then ramped from 7 B to 40% B until 91.12 min, then ramped again from 40 B to 99.9% B until 92.72 min, then remained at 99.9% until 106.00 min, then decreased to 7% B until 106.1 min, and finally remained at 7% B until the end of experiment at 120 min. Mass spectrometry was performed in positive and resolution mode (V mode), 35,000 FWHM, with ion mobility, and in data-independent acquisition mode (HDMSE). The ion mobility wave was set to a velocity of 600 m s− 1; the transfer collision energy ramped from 19 to 55 V in high-energy mode; the cone and capillary voltages were 30 and 2750 V, respectively; and the source temperature was 70 °C. In TOF parameters, the scan time was set to 0.5 s in continuum mode with a mass range of 50 to 2000 Da. The human [Glu1]-fibrinopeptide B at 100 fmol μl− 1 was used as an external calibrant and lock mass acquisition was performed every 30 s. Mass spectra were acquired by the MassLynx v4.1 software.
Proteomics data analysis
Spectral processing and database searching were performed using ProteinLynx Global Server (PLGS; version 3.0.2) (Waters) and the ISOQuant workflow software (Distler et al. 2014, 2016). The PLGS was processed using a low-energy threshold of 150 (counts), an elevated energy threshold of 50, and an intensity threshold of 750. In addition, the analysis was performed using the following parameters: two missed cleavages, a minimum fragment ion per peptide equal to 3, a minimum fragment ion per protein equal to 7, a minimum peptide per protein equal to 2, fixed modifications of carbamidomethyl and variable modifications of oxidation and phosphorylation. The false discovery rate was set to a maximum of 1%.
The comparative label-free quantification analysis was performed using the ISOQuant software by using previously described settings and algorithms (Distler et al. 2014, 2016). Briefly, the analysis included retention time alignment, exact mass retention time and ion mobility spectrometry clustering as well as data normalization and protein homology filtering. ISOQuant annotates the resulting feature clusters by evaluating consensus peptide identifications and identification probabilities. Protein identification parameters in ISOQuant were set to a false discovery rate of 1%, a peptide score greater than six, a minimum peptide length of six amino acids, and at least two peptides per protein. Label-free quantification was estimated using the TOP3 quantification approach, followed by the multidimensional normalization process implemented within ISOQuant (Distler et al. 2014).
After ISOQuant data analyses, only the proteins that were either present or absent (for unique proteins) in all three biological replicates were considered for differential abundance analysis. Data were analyzed using Student’s t-test (two-tailed). Proteins with P-values of < 0.05 were considered upregulated if the fold change was greater than 1.5 and downregulated if the fold change was lower than 0.667. Finally, proteins were blasted against the non-redundant (nr) Plants/Viridiplantae_Protein_Sequences database by using the Blast2GO software (www.blast2go.com) (Conesa et al. 2005; Götz et al. 2008) for protein description and cellular component term annotation. All resulting accessions and descriptions were from C. quinoa (taxid: 63,459).
Sequence analysis
Resulting data was used to generate sub-groups focused on different biological processes. Associated data obtained from Blast2GO (Biological process categories) were used for this purpose (Conesa et al. 2005; Götz et al. 2008). Proteins were separated in the following categories: carbohydrate and starch metabolism and Krebs cycle, protein storage and processing, lipid storage and processing, oxidative stress, late embryogenesis abundant proteins, HSPs and other processes according to GO annotation. Proteins that were not assigned one of these biological process by Blast2GO software were manually assigned a category by performing a BLASTp (Johnson et al. 2008) search using default parameters and searching for the best candidate species in Uniprot protein database (Bateman et al. 2021). If no candidate was found in Uniprot, Arabidopsis thaliana data was used to accommodate a given protein inside one of the previously indicated categories using the TAIR database (Rhee et al. 2003).
Protein accessions were used to download FASTA sequence data for both coding sequences and protein sequences. Sequences were obtained from NCBIs Nucleotide and Protein databases (taxid: 63,459) (Sayers et al. 2022) and any alignment needed was performed using NCBIs BLASTp alignment feature using default parameters against C. quinoa. FASTA sequences were analyzed using GRAVY (Fuchs 2020) to obtain the Grand average of hydropathicity index, and SMS2 (Stothard 2000) was used to obtain Isoelectric point information. Molecular weight for each protein was calculated using ProtParam tool by Expasy (Gasteiger et al. 2005).
Venn Diagram was made using Venny 2.1 online tool (Oliveros 2015) by providing accession lists for each genotype. Heat maps were made using ggplot’s heatmap.2 function in R software (R Core Team 2016).
Results
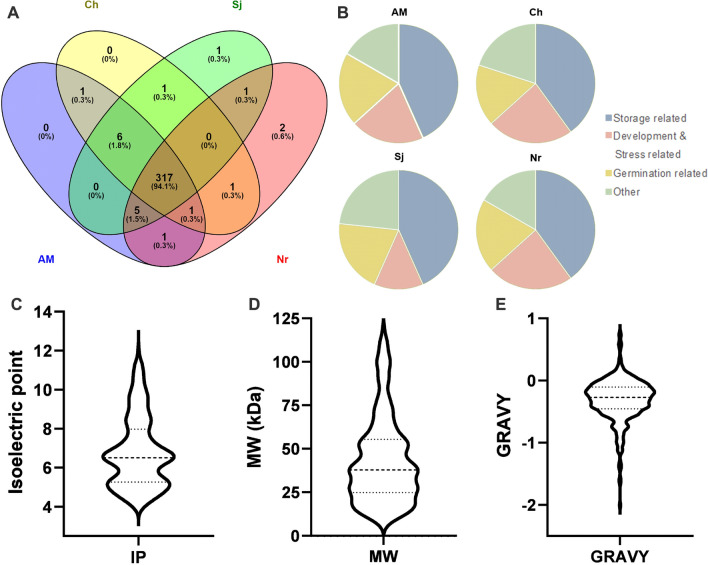
In this work, we identified 321 proteins from Amarilla de Maranganí, 317 proteins from Chadmo, 319 proteins from Nariño and 321 proteins from Sajama, with a total of 337 identified proteins (Online resource 2). For their study, identified proteins were analyzed with focus in their involvement in biological processes important to seed maturation, maintenance and germination. An overview of the results and the comparison between genotypes is presented in Fig. 1. Overall, 308 of the identified proteins are present in all four genotypes (Fig. 1A).
Fig. 1.
Proteomic results overview. A Venn diagram showing identified protein accession matches for all four quinoa genotypes used in the study. B Graphs showing the category distribution of the 30 most abundant proteins identified in each genotype. Bean-plots for C Isoelectric point, D Molecular weight (kDa) and (E) GRAVY index. AM, Amarilla de Maranganí; Ch, Chadmo; Sj, Sajama; Nr, Nariño
Isoelectric point (IPs) analysis shows that the identified proteins have IPs ranging from 4.05 to 12.01, with over 65% of the accessions in the acidic range, with a major frequency of IPs close to 5 and 6.5 (Fig. 1c). Theoretical protein weights range from 7.5 to 119.4 kDa with almost 80% of the accessions have weights under 60 kDa (Fig. 1D). The GRAVY index among all proteins range from − 2.005 to 0.757 (Fig. 1E). Over 88% of the identified proteins have negative GRAVY values, suggesting an hydrophilic behavior, while the rest are hydrophobic. When studying which proteins hold high GRAVY values, we found oleosins, which are involved in lipid bodies (LBs) and membrane integrity during the dehydration of seeds, and lipid-transfer proteins, which serve in non-specific lipid transfer. Also, while negative GRAVY index generally accounts for globular proteins (i.e., seed storage proteins), non-globular proteins, such as late embryogenesis abundant proteins (LEAs), which are widely considered intrinsically disordered proteins (IDPs) and share an important role during seed maturation, can also hold negative GRAVY indexes.
Comparing the results from the accessions and focusing on the proteins differentially identified in each of them, we found: two heat shock proteins only detected in Nariño; a storage protein present in Amarilla de Maranganí and Chadmo, but absent in Sajama and Nariño; three proteins related to LBs absent in at least one of the genotypes studied, and six carbohydrate related proteins absent in at least one of the genotypes studied. Identified proteins that are not present in all genotypes can be found in Online resource 3.
A comparison of the 30 most abundant proteins in each genotype (around 10% of the total identified proteins) were classified according to their relation to seed development, storage structures (amyloplast, PBs and LBs) and seed germination. The proteins found using this abundance cutoff are not identical between genotypes (Fig. 1B), for example, in Chadmo an aquaporin, an oil body-associated protein and a late embryogenesis protein (all related to development and stress) are present and abundant, while these proteins are not included among other genotypes top 30, in their place we can find ribosomal proteins, or proteins related to glycolysis and starch synthesis. The amount of development and stress related proteins are the most variable between genotypes (Fig. 1B). The only protein present in this top that is absent in at least a genotype is the oleosin 18.2 kDa (XP_021769148.1) which was not found in Amarilla de Maranganí or Sajama (Online Resource 3).
When analyzing whole proteomes, only around 6.5% of all the identified proteins were absent in at least one genotype. This includes the previously mentioned oleosin 18.2 kDa-like (XP_021769148.1) along other 19 proteins (Online Resource 3).
A heat map with normalized (Z-score) row data was constructed using Euclidean distance to cluster both rows and columns (Fig. 2). Data clustering suggests Chadmo as the most dissimilar genotype of the study, followed by Nariño and then by Amarilla de Maranganí and Sajama.
Fig. 2.
Heatmap for full proteomic results. Data is scaled to display row Z-score. Dendrogram according to Euclidean distance. Ch, Chadmo; Nr, Nariño; AM, Amarilla de Maranganí; Sj, Sajama
Carbohydrate metabolism
Around 18% of the identified proteins were related to carbohydrate metabolism (Online Resource 4). Almost the totality of the glycolysis/gluconeogenesis pathway related enzymes were identified among these proteins (hexokinase and phosphofructokinase were not found). This was not the case for the tricarboxylic acid (TCA) cycle, where findings were limited to malate dehydrogenase (MDH) and isocitrate dehydrogenase (IDH). A key enzyme in the glyoxylate cycle, a glyoxysomal malate dehydrogenase, was identified (Fig. 3).
Fig. 3.
The main metabolic pathway for glucose metabolism. Glycolysis and Krebs cycle. Products are written in black font. Enzymes written in blue font were found in proteomic data from mature seed protein extract, while the ones in red were not present (FDR 1%). Below the pathway, bar graphs depicting mean abundance and SEM of each present enzyme that presented significant differences among genotypes can be found. Significant differences (p < 0.05 and 0.667 < FC < 1.5) have been indicated with brackets and an asterisk (*). HK, hexokinase; PGI, glucose 6-phosphate isomerase; PFK, phosphofructokinase; ALDO, aldolase; TPI, triosephosphate isomerase; GAPDH, glyceraldehyde phosphate dehydrogenase; PGK, Phosphoglycerate kinase; PGM, phosphoglycerate mutase; ENO, enolase; PK, pyruvate kinase; PDH, Pyruvate dehydrogenase; PC, phosphoenolpyruvate carboxylase; CS, citrate synthase; ACO, aconitase; IDH, isocitrate dehydrogenase; A-KGDH, α-ketoglutarate dehydrogenase; SCS, succinate synthase; SDH, Succinic dehydrogenase; FH, fumarase; MDH, malate dehydrogenase. AM, Amarilla de Maranganí; Ch, Chadmo; Sj, Sajama; Nr, Nariño
Starch is the main seed storage substance in quinoa. Identified proteins involved in its synthesis and degradation are presented in Fig. 4 and Online Resource 4. A pair of the most abundant enzymes present in the analysis are the granule-bound starch synthases (GBSSa, GBSSb), key for starch synthesis. We identified three separate accessions (ALB36800.1, ALB36789.1, XP_021757776.1) corresponding to this enzyme. Starch branching and debranching enzymes were also identified (PUL1; 1,4-α-glucan-branching enzyme 1 and 2–2) along proteins involved in starch synthesis in chloroplasts/amyloplasts (soluble starch synthase 1).
Fig. 4.
Resumed pathway for starch biosynthesis including cytosolic and plastidic processing. Enzymes found among the results of the proteomic analysis are written in blue font, while enzymes not found are written in red. Below the pathway, bar graphs depicting mean abundance and SEM of each present enzyme that presented significant differences among genotypes can be found. Significant differences (p < 0.05 and 0.667 < FC < 1.5) have been indicated with brackets and an asterisk (*). FBP1, Fructose 1,6-biphosphatase 1; PGI, Glucose 6-phosphate isomerase; PGM, Phosphoglucomutase; UGP, UTP–glucose-1-phosphate uridylyltransferase; SS, Sucrose synthase; HK, Hexokinase; PHI, phosphohexose isomerase; GBSSa/1, Granule bound starch synthase a/1; SSSs, Soluble starch synthases; SBEs, Starch bound enzymes; PUL, pullulanase; ISA, isoamylase-type starch debranching enzymes. AM, Amarilla de Maranganí; Ch, Chadmo; Sj, Sajama; Nr, Nariño
No α-amylases, enzymes involved in fast starch degradation during seed germination, were identified. On the other hand, two isoforms of β-amylases (XP_021724363.1, XP_021724736.1) were found, one of them being more abundant. They produce maltose that can be later processed by α-glucosidases (XP_021764998.1, XP_021765002.1, XP_021726757.1, XP_021726756.1) present in this study.
Storage proteins and protein metabolism
Sixteen seed storage proteins were identified: seven vicilin-like proteins family, and the rest legumins. Here we report the findings of proteolytic enzymes including: four aspartic proteinases involved in the breakdown of propeptides in protein-storage vacuoles; a puromycin-sensitive aminopeptidase (XP_021754187.1), with broad substrate specificity and proven to be key for cell division, growth and viability; serine carboxypeptidase (XP_021724360.1), involved in brassinosteroid signaling pathway (BR), regulating plant development and physiology; four vignain-like proteases, though to be involved in seed storage protein mobility; subtilisin-like proteases, with serine-type peptidase activity; a non-catalytic component of the proteasome, the subunit β type-4; two subtilisin-proteases, which are involved in embryogenesis, seed development and germination (Online Resource 4). We detected three different accessions acting as cysteine protease (vignain) activity inhibitors.
Lipid storage & metabolism
We detected 22 proteins related to LBs, related to storage and metabolism (Online Resource 4). While this work did not study it, Burrieza et al. (2016) has previously reported the fatty acid profile for quinoa’s genotypes Utusaya, PRJ and archaeological 2300-year-old seeds, where linoleic, oleic, α-linoleic and palmitic acid are the most abundant.
Fatty acid synthesis involves malonyl-CoA, and although no protein associated with TAG synthesis was identified, biotin carboxylase, a protein that is part of the acetyl-CoA carboxylase complex which takes part in malonyl-CoA synthesis, was identified.
Different groups of important proteins were found: Three oleosins, responsible of membrane stability during seed dehydration stages are present in the analysis. Oleosin 18.2 kDa-like (XP_021762684.1) is particularly abundant. Three oil-body associated proteins which are found in LBs membranes and affect their volume and prevent membrane fusion. Seven proteins related to lipid transfer and vesicle transport, three of which are non-specific lipid-transfer protein-like and three annexins. Two peroxygenases, related to the calcium-dependent degradation of TAGs stored in LBs (a.k.a. caleosins). Their levels tend to be higher in mature seeds and their function almost exclusively reserved to LB metabolism.
Oxidative stress
A set of 19 proteins related to redox homeostasis were detected (Online Resource 4).
Glutathione S-transferases (GSTs), which scavenges ROS under oxidative stress; a glutathione reductase (GR), which is, in this case, associated with plastids and maintains the pool of reduced glutathione; peroxidases (PX), which depending on the isoform can be involved in a variety of responses related or not to redox homeostasis; peroxiredoxins (PRX), specifically related to redox homeostasis mediating the reduction of hydrogen peroxide and other organic peroxides; a single catalase (CAT), which acts against the toxic effects of hydrogen peroxide; and also two superoxide dismutases (SOD), a mitochondrial SOD with Mn-dependent activity and a cytosolic one which is Cu–Zn-dependent, but in both cases associated with detoxification (Fig. 5).
Fig. 5.
Main detoxification pathways for ROS. The enzymes involved and present in this study can be found in blue. Besides the pathway, bar graphs depicting mean abundance and SEM of each present enzyme that presented significant differences among genotypes can be found. Significant differences (p < 0.05 and 0.667 < FC < 1.5) have been indicated with brackets and an asterisk (*). SOD, superoxide-dismutase; CAT, catalase; PX, peroxidase; POX, peroxygenase; GPX, glutathione peroxidase; GST, glutathione S-transferase; GR, glutathione reductase; GSSG/GSH, glutathione oxidized/reduced, respectively. AM, Amarilla de Maranganí; Ch, Chadmo; Sj, Sajama; Nr, Nariño
An enzyme involved in key steps to synthesize tocopherol (vitamin E) and plastoquinone: a chloroplastic 2-methyl-6-phytyl-1,4-hydroquinone methyltransferase (in A. thaliana VTE3, XP_021764333.1), was identified.
LEA proteins, HSP y chaperones
LEA proteins are related to seed development, but their correlation with abiotic stress tolerance at different developmental stages was also studied. We identified a total of 16 LEA proteins (Table 1), 13 of them presenting clear consensus sequence for different LEA groups. The three remaining sequences (XP_021738541.1, XP_021731883.1, XP_021740382.1) do not meet statistical requirements used by conserved domain databases to assign them a group, yet they seem to share similarities.
Table 1.
All LEA proteins identified in all genotypes used in this study. Grouped by Pfam category and classification according to Battaglia et al. (2008). Asterisk (*) was placed beside accessions in cases where automatic classification via Pfam and/or manual classification via BLASTp were not fruitful and manual alignments were interpreted. Accessions, descriptions, group according to Battaglia et al. 2008, grouped according to Pfam, theoretical isoelectric points (IEP), theoretical molecular weights (MW in Da), alignment score, number of peptide hits (#pep), sequence coverage and average values are provided (in order)
| Accession | Description | Battaglia Group | Pfam Group | IEP | MW | Score | #pep | Seq. cov | AVG. amarilla | AVG. chadmo | AVG. sajama | AVG. nariño |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XP_021717847.1 | Dehydrin DHN1-like | LEA 2 | DHN | 6.86 | 23,428.48 | 12,822.10 | 6 | 62.95 | 300,875 | 352,533 | 254,100 | 349,143 |
| XP_021714577.1 | Dehydrin Rab18-like | LEA 2 | DHN | 6.80 | 23,447.48 | 10,288.24 | 7 | 51.79 | 258,986 | 249,107 | 255,176 | 340,213 |
| XP_021731544.1 | Embryogenic cell protein 40-like | LEA 2 | DHN | 6.48 | 38,937.41 | 19,824.40 | 12 | 64.99 | 260,464 | 413,035 | 283,177 | 297,364 |
| XP_021738657.1 | Embryogenic cell protein 40-like | LEA 2 | DHN | 6.38 | 36,855.15 | 5639.67 | 9 | 49.16 | 168,897 | 264,043 | 189,368 | 203,906 |
| XP_021770377.1 | 11 kDa late embryogenesis abundant protein-like | LEA 4 | LEA_1 | 9.68 | 18,873.73 | 3825.91 | 5 | 33.51 | 156,249 | 179,037 | 137,109 | 134,668 |
| XP_021743171.1 | Late embryogenesis abundant protein ECP63-like | LEA 3A | LEA_4 | 8.95 | 52,530.20 | 9743.31 | 24 | 55.12 | 219,330 | 281,174 | 232,651 | 287,456 |
| XP_021737796.1 | Late embryogenesis abundant protein ECP63-like isoform X2 | LEA 3A | LEA_4 | 8.44 | 52,430.99 | 8791.42 | 22 | 56.26 | 196,129 | 125,841 | 243,781 | 205,472 |
| XP_021768105.1 | Late seed maturation protein P8B6-like | LEA 1 | LEA_5 | 5.89 | 9075.86 | 19,888.59 | 6 | 46.43 | 268,582 | 167,824 | 162,854 | 335,688 |
| XP_021744729.1 | Protein SLE1-like | LEA 1 | LEA_5 | 5.68 | 12,430.51 | 35,277.84 | 8 | 73.68 | 270,369 | 294,292 | 273,735 | 401,789 |
| XP_021769542.1 | Protein SLE1-like | LEA 1 | LEA_5 | 6.18 | 12,433.54 | 35,197.29 | 9 | 81.58 | 238,754 | 234,227 | 225,926 | 219,458 |
| XP_021764400.1 | Late embryogenesis abundant protein 32-like | LEA 5A | SMP | 4.55 | 30,752.39 | 517.22 | 3 | 16.90 | 19,459 | 20,061 | 25,440 | 36,255 |
| XP_021716898.1 | Late embryogenesis abundant protein D-34-like | LEA 5A | SMP | 4.70 | 27,661.77 | 6252.17 | 10 | 65.68 | 115,942 | 143,396 | 154,936 | 151,543 |
| XP_021762676.1 | Late embryogenesis abundant protein D-34-like | LEA 5A | SMP | 4.58 | 26,931.17 | 1727.55 | 6 | 50.58 | 46,602 | 26,345 | 39,975 | 33,334 |
| XP_021738541.1* | Late embryogenesis abundant protein 76-like | LEA 3B | N/A | 4.44 | 25,317.49 | 1482.82 | 6 | 46.47 | 73,005 | 51,157 | 47,832 | 49,486 |
| XP_021731883.1* | Late embryogenesis abundant protein Dc3-like | LEA 3A | LEA_4 | 5.26 | 19,923.78 | 5888.16 | 10 | 69.31 | 116,334 | 85,084 | 163,575 | 158,863 |
| XP_021740382.1* | Desiccation-related protein PCC3-06-like isoform X2 | LEA 4 | LEA_1 | 8.39 | 30,750.05 | 5324.52 | 7 | 33.58 | 182,398 | 161,402 | 149,249 | 176,699 |
*Accessions presenting an asterisk were not automatically assigned a group in the LEA family by Pfam of CD-search, yet held similarities when performing BLASTp against other LEA proteins (data not shown). The resulting manually assigned group is presented in columns “Battaglia group” and “Pfam group”. N/A, This accession did not hit/match any conserved domains for LEA
The 13 assigned LEA proteins belong to groups: LEA_1, Dehydrin (DHN), LEA_4, LEA_5 y SMP according to Pfam sequence analysis (Table 1).
DHNs has been recently classified in three orthologues groups associated to specific protein domains named Y-, F- and H-segments (Melgar and Zelada, 2021). In all quinoa varieties analyzed, only Y-DHN proteins were identified, belonging to the structural types Y2SK2 (XP_021717847.1, XP_021714577.1) and Y4SK2 (XP_021731544.1, XP_021738657.1). Interestingly, Y4SK2-DHNs are more abundant in Chadmo compared to the other accessions (Online Resource 5).
The same two LEA_1 proteins were present in all quinoa accessions with no significant differences in their abundance between varieties. Concerning LEA_4, LEA_5 and SMP groups, three different proteins were present for each quinoa accession seeds. It is worth noting that XP_021737796.1 (LEA_4), XP_021768105.1 (LEA_5) and XP_021764400.1 and XP_021762676.1 (SMPs) are significantly less abundant in Chadmo (Online resource 2 and 5) showing differences in the accumulation between proteins codified by orthologous genes.
In total, 21 chaperones and heat-shock proteins were identified (Online Resource 4). We found ten HSP70s, two HSP80s, a single HSP90 and six HPSP20s. Although every identified accession takes part in protein folding, they do so under different circumstances: some of them operate on de novo synthesized proteins, while others are important when dealing with protein aggregation and misfolding or even work as positive regulators of enzymatical activity.
Expression mechanisms
A total of 50 proteins related to expression mechanisms were identified in all genotypes (Online Resource 4). Most of them ribonucleoproteins: 13 40 S, two 50 S and 13 60 S. We identified seven elongation factors including EF1-α, EF1-β, EF1-δ, EF1-γ, EF2 and EF1Tu (one plastidic and one mitochondrial); two initiation factors IF-4A-9 and IF-5A, a polyadenylate-binding protein and two ERBB-3 binding proteins, among others.
Discussion
When comparing our proteomics results to those obtained in other species, we found that proteomic results from barley have a similar GRAVY distribution (Mahalingam 2017), but their isoelectric point does not follow the pattern presented in quinoa (Fig. 1C). In barley, nearly 250 proteins were in the 5.5–6 pI range, with a second cluster between 8.5 and 9. Quinoa holds two clusters containing the majority of proteins, the first one and most abundant between 6.5 and 7, and a second one on the acidic range between 5.5 and 6.
The heat map from the totality of results presents the genotype Chadmo as the most dissimilar among the selected genotypes. This is particularly curious, as it is thought that Chadmo, along other quinoa genotypes could be product of an independent domestication event. This idea will be discussed in the sections below.LEA proteins, HSP and chaperones" and "Concluding remarks".
Carbohydrate metabolism
Besides proteins related to glycolysis and gluconeogenesis, an accession of PC, two accessions of IDH and six accessions of plastidic MDHs, one of them glyoxysomal, were identified. In plants the glyoxylate cycle happens in the glyoxysomes and is a variation of the TCA cycle presenting a by-pass of the decarboxylation stages, allowing seeds to use lipids as an efficient energy source for germination and seedling establishment. Lipids are mainly used for later carbohydrate synthesis. This pathway has been previously highlighted by Catusse et al. (2008) in beet seeds, another member of the amaranthaceae family, as they found every enzyme of the cycle. Even though we did not identify every enzyme from the TCA cycle, those that were identified are key for its function. Both, PC and MDH are responsible for the presence of oxaloacetate. It is possible that their presence facilitates the activation of the cycle during seed germination, when metabolic activity is triggered.
The presence of Granule-bound starch synthase (GBSS) is associated with the fact that this enzyme is trapped inside the amyloplasts present in the perisperm of the seed, dead in a mature seed. Once dead, the perisperm can have a range of appearances from crystalline to farinaceous. The internal structure acquired by starch is a combination of amylose and amylopectin, which have higher proportion of α-1,4 or α-1,6 bonds between sugars, respectively, and is related to the presence of starch branching/debranching enzymes. Previous studies prove that mutating some of the related genes to a SNP scale can significantly alter starch structure in wheat grains (Li et al. 2019; Sestili et al. 2010; Slade et al. 2012). The presence of branching enzymes as 1,4-α-glucan branching enzymes, can be linked to higher amylopectin presence, visible as a crystalline perisperm in the seed of some quinoa genotypes. In our case, Chadmo is usually a genotype that presents a crystalline perisperm (Online Resource 1). In our study we identified a 1,4-alpha-glucan-braching enzyme (XP_021732199.1) which is present at around half the abundance compared to the other three genotypes. Also, a debranching enzyme (pullulanase, XP_021723192.1), is around 60% more abundant in Chadmo than in Amarilla de Maranganí or Sajama, with a level comparable to Nariño. This could explain the crystalline perisperm in Chadmo (Online Resource 1) (Li et al. 2019; Seung 2020).
Absence of α-amylases, involved in starch degradation, does not conflict with published literature. Hager et al. (2014), studied α-amylase activity in quinoa seeds and were unable to detect it until 24 h after seed imbibition. This suggests that the presence of α-amylases for starch degradation is a product of de novo synthesis. The identification of β-amylases along α-glucosidases is also related to starch degradation. This enzyme work together with α-amylases during the first stages of starch metabolism (Stanley et al. 2011; Sun and Henson 1990).
Protein storage & metabolism
Mature quinoa seeds present PBs that hold densely packed storage protein. This protein is used as an energy source during germination and seedling establishment, for which protease and proteinase activity are needed.
Altogether we found: three aspartic proteases, two subtilisin-like proteins, a serine carboxypeptidase, four vignain-like proteins (cysteine-proteases) and a puromycin-sensitive aminopeptidase. Proteolytic activity in germinating quinoa seeds has been studied previously by Mäkinen, Hager, & Arendt (2014), showing high activity in germinating quinoa seeds and that different proteases hold tissue specific activity. Cysteine protease activity is focused in the micropylar endosperm and the perisperm, while metallo-, serine- and aspartic-proteases hold their activity mainly in the embryo until 72 hai. We can therefore estimate that most of the identified proteases will develop their functions after imbibition (Joshi 2018), as PBs have are degraded along LBs during germination.
The presence of cysteine protease inhibitory proteins might be related to the dehydrated state of the seed, as we found previous reports of their induction during abiotic stress conditions (Zhang et al. 2008).
Lipid storage & metabolism
The LBs are simple structural organelles with the main function to store fatty acids in the form of TAGs. In this work we found both caleosins and oleosins. The former can help LBs docking to glyoxysomes facilitating lipid reserve mobilization. There is evidence showing that defective caleosin expression can have negative effects on oil body degradation during germination (Fortune et al. 2005).
Even though we could not identify proteins directly related to TAG synthesis, we did identify a protein involved in the acetyl-CoA complex, which synthesizes malonyl-CoA, a key metabolite for fatty acid synthesis (J. Sun et al. 1997).
Oleosins are thought to be responsible for avoiding LBs fusion, by presenting a negatively charged section on the outside of the LBs membrane (Frandsen et al. 2001). Studies developed on A. thaliana show that the gene OBAP1 is expressed and accumulated during seed development until its germination. Mutants carrying a OBAP1 loss of function mutation present LBs with bigger volumes and up to 40% less TAGs stored. The seed from these mutants lost 98% of their germination rate (López-Ribera et al. 2014). Species that have seeds that do not undergo desiccation do not present oleosins in their LBs. It’s hypothesized that these proteins are necessary for long term storage of fatty acids in LBs (Murphy and Vance 1999).
Oxidative stress
We detected several enzymes directly operating over ROS, as well as enzymes belonging to detoxification cycles as the ascorbate–glutathione cycle. These species have to be strictly regulated, as they take part in mechanisms involving both stress tolerance, regulation of physiological signals and programmed cell death (PCD) (Cai-Hong Pang and Wang 2010; Van Breusegem and Dat 2006). The identified proteins related to redox homeostasis and their relative abundance pictures the importance of their presence in mature quinoa seeds. Their presence in mature seeds can be of great influence in later seed viability (Goel et al. 2003).
During germination, metabolic rates go up substantially. The use of the stored reserves of nutrients generates free radicals which must be reduced to avoid toxicity, especially in fatty acid metabolism, such as β-oxidation (Eastmond 2007). The importance of a protein pool dedicated to remediate or substantially diminish potential damage is clear. We hypothesize that more proteins related to these tasks are already available in the embryo, while others are probably synthesized de novo when the seed is rehydrated and germination begins.
LEA proteins, HSP and chaperones
Characterization of the LEA proteome in quinoa seeds revealed the presence of a similar subset of LEA proteins that are critical to desiccation tolerance. We identified sixteen LEA proteins belonging to LEA_1, dehydrin, LEA_4, LEA_5 and SMP groups. In accordance with previous studies in seeds of other species, no LEA_3 proteins were detected (Chatelain et al. 2012; Ginsawaeng et al. 2021). Another work in Amaranth species (Bojórquez-Velázquez et al. 2019), closely related to quinoa, studies differential LEA accumulation in seeds. This study found the presence of SMPs, LEA_5, and links their abundance and presence with seed longevity. Moreover, the authors find that SMPs are accumulated in cultivated species, while wild species have less abundance.
Interestingly, only dehydrins belonging to Y-orthologuous group are present in the proteome of mature quinoa seeds. Analysis of DHN domain structures allowed us to determine the exclusive presence of Y-type dehydrins in the seed proteome of A. thaliana; M. truncatula and Zea mays (Chatelain et al. 2012; Ginsawaeng et al. 2021; Verdier et al. 2013) suggesting a functional specialization of the different orthologous groups recently proposed (Melgar and Zelada 2021).
The B3 domain-containing transcription factor ABSCISIC ACID INSENSITIVE3 (ABI3) is an essential component of the regulatory network controlling the development and maturation of orthodox seeds (Mönke et al. 2012). A comparative analysis of heat-stable proteome of orthodox (M. truncatula) and recalcitrant (Castanospermum australe) seeds showed a down regulation of LEA_4, LEA_5 and SMP genes. A similar result was obtained with desiccation-sensitive abi3 mutants of M. truncatula and the presence of ABI responsive elements in the promotor of these genes was demonstrated (Delahaie et al. 2013). Interestingly, Chadmo accession presented a lower accumulation of 3 proteins belonging to the LEA_4, LEA_5 and SMP groups suggesting this accession could be insensitive to abscisic acid regulation of LEAs genes implicated in desiccation tolerance during maturation.
The germination of Chadmo seeds is negatively affected under salt stress (Causin et al. 2020), which could be contradictory with the greater accumulation of dehydrin Y4SK2 observed in this accession. However, the profile of dehydrins produced during seed germination in A. thaliana (Ginsawaeng et al. 2021) and M. truncatula (Chatelain et al. 2012) reveal a very specific pattern of LEA proteins during the different stages of germination whose disturbance could be deleterious for this process.
The ubiquitous heat-shock proteins are related to higher tolerance to diverse abiotic stress factors. From their subgroups the HSP70s are well studied and related to seed thermotolerance and to proper establishment and development of A. thaliana seedlings (Kotak et al. 2007; Su and Li 2008). Some were found to express in a constitutive fashion, while others respond to stress. Proteins belonging to small HSPs were also linked to embryo development and protection during seed dehydration stages (Wehmeyer and Vierling 2000).
Expression mechanisms
Among the identified proteins, 50 are related to expression mechanism. Those related to translation are more abundant. Thirteen 40 s, two 50 s (chloroplastic) and thirteen 60 s ribosomal proteins were identified and seven elongation factors and two initiation factors are present. These factors are found along a protein that, to our knowledge, has not been reported numerous times (Catusse et al. 2008): the EBB-3 binding protein. This protein is responsible for ribosomal protein recruiting in cap-independent translation via independent ribosome entry sites (IRESs) and has been found to be specially active during stress. These appear along the polyadenylate-binding protein, which binds to the poly-A end of mRNA, affecting its translation and decay. The results indicate that the seed presents an active expression system comprising the translation of both capped and uncapped mRNAs.
Concluding remarks
It’s worth highlighting that Chadmo is the most dissimilar genotype of the study. This agrees with Jarvis et al. (2017), where two independent and isolated domestication events of quinoa are proposed: one of them giving origin to the genotypes of the coastal region of Chile, where Chadmo originates, and another to highland areas, where the rest of the genotypes selected for this study probably derived. This double-event hypothesis is also supported by the result presented by Patiranage et al. (2022).
Proteins related to seed development and maturation are present in the study (e.g., GBSSs, oil-body-associated proteins, storage proteins, LEAs) along a significant set of proteins related to stress. Some of them are important during and after seed development and dehydration stage, such as LEAs, HSPs and proteinase inhibitors; while others can also operate during the seed rehydration, when storage nutrients (specially TAGs) are processed and high amounts of H2O2 are produced, like those involved in redox homeostasis, proteases and glycolytic pathways.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Axel J. Rizzo and María B. Palacios thank the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Argentina, for their fellowships.
Funding
This research was possible thanks to the grants provided by Universidad de Buenos Aires (UBACyT 20020170200265BA) to Hernan Burrieza and Agencia Nacional de Investigaciones Científicas y Tecnológicas (FONCYT, PICT-2015–3527) to Sara Maldonado and Hernan P. Burrieza.
Declarations
Conflict of interest
Authors declare no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aloisi I, Parrotta L, Ruiz KB, Landi C, Bini L, Cai G, Biondi S, Del Duca S. New insight into quinoa seed quality under salinity: changes in proteomic and amino acid profiles, phenolic content, and antioxidant activity of protein extracts. Front Plant Sci. 2016;7:1–21. doi: 10.3389/fpls.2016.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin PM. Starch granule-associated proteins and polypeptides: a review. Starch/staerke. 2001;53(10):475–503. doi: 10.1002/1521-379X(200110)53:10<475::AID-STAR475>3.0.CO;2-E. [DOI] [Google Scholar]
- Bateman A, Martin MJ, Orchard S, Magrane M, Agivetova R, Ahmad S, Alpi E, Bowler-Barnett EH, Britto R, Bursteinas B, Bye-A-Jee H, Coetzee R, Cukura A, da Silva A, Denny P, Dogan T, Ebenezer TG, Fan J, Castro LG, Teodoro D. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49(D1):D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008;148(1):6–24. doi: 10.1104/pp.108.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertero HD, De La Vega AJ, Correa G, Jacobsen SE, Mujica A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field Crops Res. 2004;89(2–3):299–318. doi: 10.1016/j.fcr.2004.02.006. [DOI] [Google Scholar]
- Bies-Ethève N, Gaubier-Comella P, Debures A, et al. Inventory, evolution and expression profiling diversity of the LEA (late embryogenesis abundant) protein gene family in Arabidopsis thaliana. Plant Mol Biol. 2008;67:107–124. doi: 10.1007/s11103-008-9304-x. [DOI] [PubMed] [Google Scholar]
- Bojórquez-Velázquez E, Barrera-Pacheco A, Espitia-Rangel E, Herrera-Estrella A, De La Rosa APB. Protein analysis reveals differential accumulation of late embryogenesis abundant and storage proteins in seeds of wild and cultivated amaranth species. BMC Plant Biol. 2019;19(1):1–17. doi: 10.1186/s12870-019-1656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrieza HP, López-Fernández MP, Maldonado S. Analogous reserve distribution and tissue characteristics in quinoa and grass seeds suggest convergent evolution. Front Plant Sci. 2014;5:1–11. doi: 10.3389/fpls.2014.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrieza HP, Rizzo AJ, Moura Vale E, Silveira V, Maldonado S. Shotgun proteomic analysis of quinoa seeds reveals novel lysine-rich seed storage globulins. Food Chem. 2019;293:299–306. doi: 10.1016/j.foodchem.2019.04.098. [DOI] [PubMed] [Google Scholar]
- Burrieza HP, Sanguinetti A, Michieli CT, Bertero HD, Maldonado S. Death of embryos from 2300-year-old quinoa seeds found in an archaeological site. Plant Science. Plant Science. 2016;253:107–117. doi: 10.1016/j.plantsci.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Capriotti AL, Cavaliere C, Piovesana S, Stampachiacchiere S, Ventura S, ZeneziniChiozzi R, Laganà A. Characterization of quinoa seed proteome combining different protein precipitation techniques: improvement of knowledge of nonmodel plant proteomics. J Sep Sci. 2015;38(6):1017–1025. doi: 10.1002/jssc.201401319. [DOI] [PubMed] [Google Scholar]
- Catusse J, Strub JM, Job C, Van Dorsselaer A, Job D. Proteome-wide characterization of sugarbeet seed vigor and its tissue specific expression. Proc Natl Acad Sci USA. 2008;105(29):10262–10267. doi: 10.1073/pnas.0800585105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causin HF, Bordón DAE, Burrieza H. Salinity tolerance mechanisms during germination and early seedling growth in chenopodium quinoa Wild. Genotypes with different sensitivity to saline stress. Environ Exp Botany. 2020;172:103995. doi: 10.1016/j.envexpbot.2020.103995. [DOI] [Google Scholar]
- Chase MW, Christenhusz MJM, Fay MF, Byng JW, Judd WS, Soltis DE, Mabberley DJ, Sennikov AN, Soltis PS, Stevens PF, Briggs B, Brockington S, Chautems A, Clark JC, Conran J, Haston E, Möller M, Moore M, Olmstead R, Weber A. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Botan J Linn Soc. 2016;181(1):1–20. doi: 10.1111/boj.12385. [DOI] [Google Scholar]
- Chatelain E, Hundertmark M, Leprince O, Gall SL, Satour P, Deligny-Penninck S, Rogniaux H, Buitink J. Temporal profiling of the heat-stable proteome during late maturation of medicago truncatula seeds identifies a restricted subset of late embryogenesis abundant proteins associated with longevity. Plant Cell Environ. 2012;35(8):1440–1455. doi: 10.1111/j.1365-3040.2012.02501.x. [DOI] [PubMed] [Google Scholar]
- Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Damerval C, De Vienne D, Zivy M, Thiellement H. Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis. 1986;7(1):52–54. doi: 10.1002/elps.1150070108. [DOI] [Google Scholar]
- Delahaie J, Hundertmark M, Bove J, Leprince O, Rogniaux H, Buitink J. LEA polypeptide profiling of recalcitrant and orthodox legume seeds reveals ABI3-regulated LEA protein abundance linked to desiccation tolerance. J Exp Bot. 2013;64(14):4559–4573. doi: 10.1093/jxb/ert274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler U, Kuharev J, Navarro P, Levin Y, Schild H, Tenzer S. Drift time-specific collision energies enable deep-coverage data-independent acquisition proteomics. Nat Methods. 2014;11(2):167–170. doi: 10.1038/nmeth.2767. [DOI] [PubMed] [Google Scholar]
- Distler U, Kuharev J, Navarro P, Tenzer S. Label-free quantification in ion mobility-enhanced data-independent acquisition proteomics. Nat Protoc. 2016;11(4):795–812. doi: 10.1038/nprot.2016.042. [DOI] [PubMed] [Google Scholar]
- Eastmond PJ. Monodehyroascorbate reductase4 is required for seed storage oil hydrolysis and postgerminative growth in arabidopsis. Plant Cell. 2007;19(4):1376–1387. doi: 10.1105/tpc.106.043992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, CIRAD (2015) State of the art report of Quinoa in the World in 2013 In: Bazile D, Bertero D, Nieto C (eds)
- Fortune SM, Jaeger A, Sarracino DA, Chase MR, Sassetti CM, Sherman DR, Bloom BR, Rubin EJ (2005) Sciences of the USA 10676–10681 PNAS (Vol 102, 30) [DOI] [PMC free article] [PubMed]
- Frandsen GI, Mundy J, Tzen JTC. Oil bodies and their associated proteins, oleosin and caleosin. Physiol Plant. 2001;112(3):301–307. doi: 10.1034/j.1399-3054.2001.1120301.x. [DOI] [PubMed] [Google Scholar]
- Fuchs S (2020) GRAVY calculator. http://www.gravy-calculator.de/
- Galindo-Luján R, Pont L, Minic Z, Berezovski MV, Sanz-Nebot V, Benavente F. Characterization and differentiation of quinoa seed proteomes by label-free mass spectrometry-based shotgun proteomics. Food Chem. 2021 doi: 10.1016/j.foodchem.2021.130250. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. Protein identification and analysis tools on the ExPASy server. Totowa, New Jersey, United States: Humana Press; 2005. pp. 571–607. [Google Scholar]
- Ginsawaeng O, Gorka M, Erban A, Heise C, Brueckner F, Hoefgen R, Kopka J, Skirycz A, Hincha DK, Zuther E. Characterization of the heat-stable proteome during seed germination in arabidopsis with special focus on LEA proteins. Int J Molecular Sci. 2021 doi: 10.3390/ijms22158172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Goel AK, Sheoran IS. Changes in oxidative stress enzymes during artificial ageing in cotton (Gossypium hirsutum L.) seeds. J Plant Phys. 2003;160(9):1093–1100. doi: 10.1078/0176-1617-00881. [DOI] [PubMed] [Google Scholar]
- Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J, Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager AS, Mäkinen OE, Arendt EK. Amylolytic activities and starch reserve mobilization during the germination of quinoa. Eur Food Res Technol. 2014;239(4):621–627. doi: 10.1007/s00217-014-2258-0. [DOI] [Google Scholar]
- Jarvis DE, Ho YS, Lightfoot DJ, Schmöckel SM, Li B, Borm TJA, Ohyanagi H, Mineta K, Michell CT, Saber N, Kharbatia NM, Rupper RR, Sharp AR, Dally N, Boughton BA, Woo YH, Gao G, Schijlen EGWM, Guo X, Tester M. The genome of chenopodium quinoa. Nature. 2017;542(7641):307–312. doi: 10.1038/nature21370. [DOI] [PubMed] [Google Scholar]
- Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36:5–9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R. Role of enzymes in seed germination. Int J Creat Res Thoughts. 2018;6(2):1481–1485. [Google Scholar]
- Kotak S, Vierling E, Bäumlein H, Von Koskull-Dörlng P. A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis. Plant Cell. 2007;19(1):182–195. doi: 10.1105/tpc.106.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bhargava A, Shukla S, Singh HB, Ohri D. Screening of exotic chenopodium quinoa accessions for downy mildew resistance under mid-eastern conditions of India. Crop Prot. 2006;25(8):879–889. doi: 10.1016/j.cropro.2005.11.012. [DOI] [Google Scholar]
- Li H, Yu W, Dhital S, Gidley MJ, Gilbert RG. Starch branching enzymes contributing to amylose and amylopectin fine structure in wheat. Carbohyd Polym. 2019;224:115185. doi: 10.1016/j.carbpol.2019.115185. [DOI] [PubMed] [Google Scholar]
- López-Ribera I, La Paz JL, Repiso C, García N, Miquel M, Hernández ML, Martínez-Rivas JM, Vicient CM. The evolutionary conserved oil body associated protein OBAP1 participates in the regulation of oil body size. Plant Physiol. 2014;164(3):1237–1249. doi: 10.1104/pp.113.233221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R. Shotgun proteomics of the barley seed proteome. BMC Genomics. 2017;18(1):1–11. doi: 10.1186/s12864-016-3408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen OE, Hager AS, Arendt EK. Localisation and development of proteolytic activities in quinoa (Chenopodium quinoa) seeds during germination and early seedling growth. J Cereal Sci. 2014;60(3):484–489. doi: 10.1016/j.jcs.2014.08.009. [DOI] [Google Scholar]
- Melgar AE, Zelada AM. Evolutionary analysis of angiosperm dehydrin gene family reveals three orthologues groups associated to specific protein domains. Sci Rep. 2021;11(1):1–12. doi: 10.1038/s41598-021-03066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mönke G, Seifert M, Keilwagen J, Mohr M, Grosse I, Hähnel U, Junker A, Weisshaar B, Conrad U, Bäumlein H, Altschmied L. Toward the identification and regulation of the Arabidopsis thaliana ABI3 regulon. Nucleic Acids Res. 2012;40(17):8240–8254. doi: 10.1093/nar/gks594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ, Vance J. Mechanisms of lipid-body formation. Trends Biochem Sci. 1999;24(3):109–115. doi: 10.1016/S0968-0004(98)01349-8. [DOI] [PubMed] [Google Scholar]
- Oliveros JC (2015) An interactive tool for comparing lists with Venn’s diagrams. Csic. https://bioinfogp.cnb.csic.es/tools/venny/index.html
- Pang C-H, Wang B-S (2010) Role of ascorbate peroxidase and glutathione reductase in ascorbate–glutathione cycle and stress tolerance in plants. In: ascorbate-glutathione pathway and stress tolerance in plants (pp 91–113). 10.1007/978-90-481-9404-9
- Patiranage DSR, Rey E, Emrani N, Wellman G, Schmid K, Schmöckel SM, Tester M, Jung C. Genome-wide association study in the pseudocereal quinoa reveals selection pattern typical for crops with a short breeding history. ELife. 2022 doi: 10.1101/2020.12.03.410050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planella MT, Lopez ML, Bruno MC (2015) Domestication and prehistoric distribution. In: State of the art report on Quinoa around the world in 2013. https://scholar.dickinson.edu/faculty_publications/1027
- Präger A, Munz S, Nkebiwe PM, Mast B, Graeff-Hönninger S. Yield and quality characteristics of different quinoa (Chenopodium quinoa willd.) cultivars grown under field conditions in southwestern Germany. Agronomy. 2018;8(10):197. doi: 10.3390/agronomy8100197. [DOI] [Google Scholar]
- Prego I, Maldonado S, Otegui M. Seed structure and localization of reserves in Chenopodium quinoa. Ann Bot. 1998;82(4):481–488. doi: 10.1006/anbo.1998.0704. [DOI] [Google Scholar]
- Pulvento C, Riccardi M, Lavini A, D’Andria R, Iafelice G, Marconi E. Field trial evaluation of two Chenopodium quinoa genotypes grown under rain-fed conditions in a typical mediterranean environment in South Italy. J Agron Crop Sci. 2010;196(6):407–411. doi: 10.1111/j.1439-037X.2010.00431.x. [DOI] [Google Scholar]
- R Core Team (2016) R: a language and environment for statistical computing. 2 1–12
- Reis RS, Vale EM, Sousa KR, Santa-Catarina C, Silveira V. Pretreatment free of 2,4-dichlorophenoxyacetic acid improves the differentiation of sugarcane somatic embryos by affecting the hormonal balance and the accumulation of reserves. Plant Cell Tissue Organ Cult. 2021;145(1):101–115. doi: 10.1007/s11240-020-01995-z. [DOI] [Google Scholar]
- Rhee SY, Beavis W, Berardini TZ, Chen G, Dixon D, Doyle A, Garcia-Hernandez M, Huala E, Lander G, Montoya M, Miller N (2003) The arabidopsis information resource (TAIR): a model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. In: Nucleic acids research (Vol 31, Issue 1, pp 224–228). Doi: 10.1093/nar/gkg076 [DOI] [PMC free article] [PubMed]
- Ruales J, Nair BM. Nutritional quality of the protein in quinoa (Chenopodium quinoa, Willd) seeds. Plant Foods Hum Nutr. 1992;42(1):1–11. doi: 10.1007/BF02196067. [DOI] [PubMed] [Google Scholar]
- Sayers EW, Bolton EE, Brister JR, Canese K, Chan J, Comeau DC, Connor R, Funk K, Kelly C, Kim S, Madej T, Marchler-Bauer A, Lanczycki C, Lathrop S, Lu Z, Thibaud-Nissen F, Murphy T, Phan L, Skripchenko Y, Sherry ST. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022;50(1):20–26. doi: 10.1093/nar/gkab1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestili F, Janni M, Doherty A, Botticella E, D’Ovidio R, Masci S, Jones HD, Lafiandra D. Increasing the amylose content of durum wheat through silencing of the SBEIIa genes. BMC Plant Biol. 2010 doi: 10.1186/1471-2229-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung D. Amylose in starch: towards an understanding of biosynthesis, structure and function. New Phytol. 2020;228(5):1490–1504. doi: 10.1111/nph.16858. [DOI] [PubMed] [Google Scholar]
- Slade AJ, McGuire C, Loeffler D, Mullenberg J, Skinner W, Fazio G, Holm A, Brandt KM, Steine MN, Goodstal JF, Knauf VC. Development of high amylose wheat through TILLING. BMC Plant Biol. 2012 doi: 10.1186/1471-2229-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D, Rejzek M, Naested H, Smedley M, Otero S, Fahy B, Thorpe F, Nash RJ, Harwood W, Svensson B, Denyer K, Field RA, Smith AM. The role of α-glucosidase in germinating barley grains. Plant Physiol. 2011;155(2):932–943. doi: 10.1104/pp.110.168328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stikic R, Glamoclija D, Demin M, Vucelic-Radovic B, Jovanovic Z, Milojkovic-Opsenica D, Jacobsen SE, Milovanovic M. Agronomical and nutritional evaluation of quinoa seeds (Chenopodium quinoa Willd) as an ingredient in bread formulations. J Cereal Sci. 2012;55(2):132–138. doi: 10.1016/j.jcs.2011.10.010. [DOI] [Google Scholar]
- Stothard P. Internet on-ramp internet on-ramp. Biotechniques. 2000;28(6):1102–1104. doi: 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
- Su PH, Li HM. Arabidopsis stromal 70-kD heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol. 2008;146(3):1231–1241. doi: 10.1104/pp.107.114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Estrella D, Bresciani A, Iametti S, Marengo M, Pagani MA, Marti A. Effect of sprouting on proteins and starch in quinoa (Chenopodium quinoa Willd) Plant Foods Human Nutrition. 2020;75(4):635–641. doi: 10.1007/s11130-020-00864-6. [DOI] [PubMed] [Google Scholar]
- Sun Z, Henson CA. Degradation of native starch granules by barley α-glucosidases. Plant Physiol. 1990;94(1):320–327. doi: 10.1104/pp.94.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ke J, Johnson JL, Nikolau BJ, Wurtele ES. Biochemical and molecular biological characterization of CAC2, the Arabidopsis thaliana gene coding for the biotin carboxylase subunit of the plastidic acetyi-coenzyme a carboxylase. Plant Physiol. 1997;115(4):1371–1383. doi: 10.1104/pp.115.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141(2):384–390. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Gálvez A, Miranda M, Vergara J, Uribe E, Puente L, Martínez EA. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.) an ancient Andean grain: a review. J Sci Food Agricult. 2010;90(15):2541–2547. doi: 10.1002/jsfa.4158. [DOI] [PubMed] [Google Scholar]
- Verdier J, Lalanne D, Pelletier S, Torres-Jerez I, Righetti K, Bandyopadhyay K, Leprince O, Chatelain E, Vu BL, Gouzy J, Gamas P, Udvardi MK, Buitink J. A regulatory network-based approach dissects late maturation processes related to the acquisition of desiccation tolerance and longevity of medicago truncatula seeds. Plant Physiol. 2013;163(2):757–774. doi: 10.1104/pp.113.222380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer N, Vierling E. The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiol. 2000;122(4):1099–1108. doi: 10.1104/pp.122.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Hirakawa H, Oikawa T, Toyoshima M, Matsuzaki C, Ueno M, Mizuno N, Nagatoshi Y, Imamura T, Miyago M, Tanaka K, Mise K, Tanaka T, Mizukoshi H, Mori M, Fujita Y. Draft genome sequence of an inbred line of Chenopodium quinoa an allotetraploid crop with great environmental adaptability and outstanding nutritional properties. DNA Res. 2016 doi: 10.1093/dnares/dsw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu S, Takano T. Two cysteine proteinase inhibitors from Arabidopsis thaliana, AtCYSa and AtCYSb, increasing the salt, drought, oxidation and cold tolerance. Plant Mol Biol. 2008;68(1–2):131–143. doi: 10.1007/s11103-008-9357-x. [DOI] [PubMed] [Google Scholar]
- Zou C, Chen A, Xiao L, Muller HM, Ache P, Haberer G, Zhang M, Jia W, Deng P, Huang R, Lang D, Li F, Zhan D, Wu X, Zhang H, Bohm J, Liu R, Shabala S, Hedrich R, Zhang H. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 2017;27(11):1327–1340. doi: 10.1038/cr.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.