Abstract
Low-phosphorus stress significantly impacts the development of maize kernels. In this study, the phosphor efficient maize genotype 082 and phosphor deficient maize genotype Ye107, were used to construct an F2:3 population. QTL mapping was then employed to determine the genetic basis of differences in the maize kernel traits of the two parents in a low-phosphorus environment. This analysis revealed several major QTL that control environmental impacts on kernel length, width, thickness, and weight. These QTL were detected in all three environments and were distributed on five genome segments of chromosomes 3, 5, 6, and 9, and some new kernel-trait QTL were also detected (eg: Qkwid6, Qkthi3, Qkwei9, and Qklen3-1). These environmentally insensitive QTL can be stably expressed in low phosphorus environments, indicating that they can lay a foundation for the breeding of high phosphorus utilization efficiency germplasm.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-023-01300-0.
Keywords: Maize, Low-phosphorus stress, Kernel traits, QTL mapping
Introduction
Maize (Zea mays L.) is an important ternary crop that is used for both human consumption and animal feed. Total global production of maize has increased dramatically over the last few decades and will need to at least double by 2050 in order to keep pace with population growth (Ci et al. 2011; Ray et al. 2013). The level of naturally occurring soil phosphorus is not sufficient to meet the needs of corn, thus forcing growers to apply chemical fertilizers and limiting maize productivity. In addition, the input of chemical phosphate fertilizer is expensive and results in significant pollution.
Yield is one of the most complex maize traits (Venkatesan 2005) and is composed of several factors, including both kernel and ear traits. Because of their significant impact on yield and quality, maize kernels have been studied extensively (Dai et al. 2021). The majority of kernel morphological traits are quantitative and result from multiple loci, which can be independently studied through analysis of quantitative trait loci (QTL) (Peleg et al. 2009). Through the examination of kernel length, kernel width, kernel thickness, and 200-kernel weight in a low-phosphorus environment, it is possible to better understand the underlying causes of differing maize yields. To explore the major QTL for kernel traits in low-phosphorus environments, we examined multiple underlying maize kernel traits, including kernel length, kernel width, kernel thickness, and 200-kernel weight. These traits were then mapped through QTL analysis to identify the underlying loci (Ribaut et al. 1997). Although a large number of QTL for maize yield components are listed on the MAIZEGDB and GRAMENE websites, few QTL related to kernel length and thickness have been reported (Peng et al. 2010). Analyzing the genetic basis of maize yield constituent factors and kernel-related traits in a low-phosphorus environment may be useful for improving maize phosphorus efficiency and yield to promote more sustainable and efficient agricultural development.
The rapid development of maize genomics technologies, including molecular markers (Li et al. 2003), has enabled the quantitative mapping of many agronomically important traits (Rex et al. 2008). For example, Zhang et al. (2008) and Wang et al. (2009) used bioinformatics to analyze yield-related QTL found across maize databases. With the help of the high-density maize genetic linkage map IBM Neighbors and molecular markers, these researchers integrated the QTL map of maize kernel-related traits. However, Peng et al. (2010) pointed out that due to the influence of factors such as population size and marker density, a large number of QTL studies cannot accurately identify the genetic basis of yield traits. Such approaches also have difficulty distinguishing single-locus from multigenic traits and have trouble separating traits with close linkage. Liu et al. (2014) studied F2:3 populations in five environments; they used composite interval mapping (CIM) for single-environment analysis and a mixed linear model-based CIM approach for joint analysis, identifying 55 and 28 kernel-related QTL, respectively. Nikolić et al. (2013) used CIM in Win QTL Cartographer v2.5 and identified five single-plant kernel QTL in an F3 population. Lan et al. (2018) measured maize kernel length, width, thickness, and weight in seven environments, and they used a fusion compound interval mapping method to locate QTL for yield-related traits, resulting in the detection of 52 QTL. Liu et al. (2020a, b) studied the genetic basis of maize kernel length, width, and thickness in seven environments; they detected 50 QTL controlling these traits, eight of which were detected in at least three environments. Bai (2014) phenotyped the kernel traits of maize over a two-year period and performed QTL analysis to identify associated loci. This led to the identification of 33 kernel-trait QTL, which were found on chromosomes 4–10. Qin et al. (2015) used the complete interval mapping method to identify 30 QTL for kernel length, kernel width, 100-kernel weight, and kernel length/width ratio under five environmental conditions. Mu (2018) used high-density genetic maps for QTL analysis of kernel length, kernel width, 100-kernel weight, and ear rows, locating QTL for each trait on the basis of multi-environment phenotype averages. There were 16 QTL loci located on chromosomes 2, 3, 4, 5, 8, 9, and 10, including two for kernel length, three for kernel width, four for 100-kernel weight, and seven for ear rows. Li (2010) detected seven QTL for 100-kernel weight in a low-phosphorus environment at two sites. Hou et al. (2009) studied changes in the quality traits of maize varieties screened based on 100-kernel weight under normal-phosphorus and low-phosphorus conditions and found that low-phosphorus stress had a greater impact on variation in phosphorus-sensitive kernel quality traits. Ren et al. (2015) detected 23 QTL that control maize yield traits under different phosphorus levels. This analysis showed that low-phosphorus stress mainly caused yield reduction by affecting 100-seed weight. Li et al. (2010) detected 33 QTL for maize kernel traits under low-phosphorus conditions. In the present work, kernel length, kernel width, kernel thickness, and 200-kernel weight were assessed under low-phosphorus conditions. A mapping population developed from two parents with different phosphorus use efficiencies was used to determine the genetic basis of changes in kernel traits under phosphorus stress, leading to the identification of multiple QTL. These findings have significant implications for marker-assisted breeding of maize with improved kernel traits and yield under low-phosphorus conditions.
Materials and methods
Plant material
An F2:3 population was constructed using the phosphorus-efficient genotype 082 and the phosphorus-inefficient genotype Ye107 (Chen et al. 2007; Qiu 2013). This population consisted of 180 F2:3 families, which were used for QTL mapping of kernel traits under low-phosphorus stress.
Field trials and trait measurements
The parents and F2:3 families were planted in Beibei (BB, 29.81°N, 106.36°E, 350 m altitude) and Hechuan (HC, 30.02°N, 106.15°E, 240 m altitude) in spring 2011 and in Beibei (E3) in spring 2012, respectively. In this study, a completely randomized block design was adopted, with 20 plants per plot and two replicates of each genotype, for a final planting density of 45,000 plants hm2.The mature maize plants were covered with bags for phenotypic analysis of the self-crossing plants.
The Beibei field block contained sandy loam soil with a pH of 6.6 and an organic matter content of 14.6 g kg−1. The total N, total P, and total K contents were 0.890 g kg−1, 0.761 g kg−1, and 14.2 g kg−1, respectively. The available N, available P, and available K contents were 8.9 mg kg−1, 3.3 mg kg−1, and 117 mg kg−1, respectively. The Hechuan field also contained sandy loam soil, with a pH of 7.3 and an organic matter content of 10.6 g kg−1. The total N, total P, and total K contents were 0.734 g kg−1, 0.987 g kg−1, and 14.1 g kg−1, respectively. The available N, available P, and available K contents were 5.1 mg kg−1, 3.5 mg kg−1, and 105 mg kg−1, respectively. According to the nutrient classification index of the second national soil survey, soils found in both the Beibei (BB) and Hechuan (HC) test sites were low in phosphorus. All fertilizers were applied at 120 kg hm−2, no phosphate fertilizer was applied, and other field management was consistent.
After maturity, 13 plants were collected from each plot, and the ears were dried before measurements were taken indoors. Kernel length (cm) (KLEN), kernel width (cm) (KWID), kernel thickness (cm) (KTHI), and 200-kernel weight (g) (KWEI) were measured. The phenotypic data for the four traits are all presented as the average value of 10 consecutive ears.
Statistical analysis of phenotypic data
IBM SPSS Statistics 26 software was used to analyze the significance of differences between parents and groups, the correlations between traits, and the normality of each trait. Generalized heritability was calculated as reported previously by Knapp et al. (1985):
where σ2G, σ2GE, and σ2E are estimates of genotype variance, interaction variance between genotype and environment, and experimental error variance, respectively, and n and r are the numbers of environments and replications, respectively.
Linkage map construction
In this study, the genomic DNA of F2:3 families and parents was extracted by CTAB method. The genetic map of F2 population was constructed by selecting 249 polymorphic SSR markers covering the whole maize genome from maize genome database. The JoinMap version 3.0 software was used to convert the recombination rate into graph distance units (cM).
SSR marker analysis
It includes PCR amplification, polyacrylamide gel electrophoresis, silver dyeing and other procedures. The steps are as follows: (1) PCR amplification: each amplification reaction (volume 10μL) consisted of DNA 1μL, primers (F + R) 1μL, Star Mix 5μL, Titrated water supplement 10μL. The amplification procedure was as follows: predenaturation 5 min at 94 °C,1 cycle; There were 35 cycles: denaturation for 40 s at 94 °C, annealing for 35 s at 58 °C (57, 58, 59,60), extension for 45 s at 72 °C; final extension at 72 °C for 10 min and store at 4 °C.
The gel used in this experiment was 10% non-denatured gel. The PCR amplified products were loaded into the wells of the gel, and 1 × TBE buffer was added into the electrophoresis tank for electrophoresis at 300 V and 100 mA.
Silver staining after electrophoresis was performed by removing the agarose gel from the glass plate and placing it in a fixing solution, and gently shaking it on a shaking bed for 12 min. Silver dyeing was performed by keeping the gel in 200 ml of 0.15% silver nitrate solution for 12 min. The gel was then washed with Na2S2O3 solution twice, 10 s each time. For development the gel was placed in the developer to develop color until the strip is clear. Finally, the film from the developer was placed on the light box, and the pictures were taken for preservation.
Amplification tape type statistics were recorded by reading the amplified fragments on the electrophoretic glue plate on the light box. At the same mobility position, the band type derived from parent 082 was denoted as "A", the band type derived from parent Ye107 was denoted as "B", the heterozygous band type was denoted as "H", and the missing band was denoted as "−". The case of ambiguous or not recorded band type was denoted as missing.
QTL mapping
QTL IciMapping software was used for QTL mapping. When performing QTL detection, a step size of 1 cM was selected, with the LOD threshold set manually to 2.5 and the significance level set to 0.05. According to the standards of Stuber et al. (1987) and Tuberosa et al. (1998), the mode of action of a gene can be classified as additive (A, 0 to 0.21), partially dominant (PD, 0.21 to 0.80), dominant (D, 0.81 to 1.20), or over dominant (OD, > 1.20). Taking qKLEN3-1 as an example of QTL nomenclature, KLEN represents kernel length (KWID, KTHI, and KWEI represent kernel width, kernel thickness, and 200-kernel weight respectively), the number 3 is the serial number of the chromosome, and -1 represents the number of the chromosome.
Results
Phenotypic analysis of traits related to parents and kernels
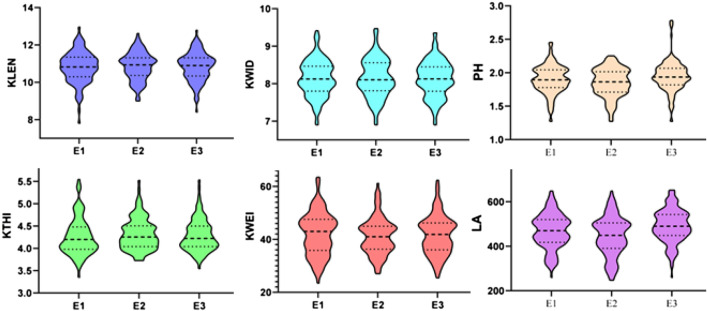
There were obvious differences in the phenotypes of kernel-related traits between the parents (Table 1). For example, the kernels of 082 were longer than those of Ye107, whereas the Ye107 kernels were wider, thicker, and had a higher 200-kernel weight. These differences in the parental lines were also seen in the F2:3 population (Table 2). For example, in the E1 environment, the average kernel length of the F2:3 family was 10.80 cm, and the range of variation was 5.17. The kernel-related traits of F2:3 families showed a continuous distribution in all three environments, with small skewness and kurtosis and phenotypic frequencies that were all normally distributed or approximately normal (Fig. 1). These characteristics imply that all traits surveyed were quantitative and could be studied further via QTL mapping. Kernel length, kernel width, kernel thickness, and 200-kernel weight all showed high heritability, with values of 0.89, 0.90, 0.81, and 0.87, respectively (Table 2). This implied that differences in the four kernel traits were controlled mainly by genetic factors rather than the environment.
Table 1.
Phenotypic expression of the parents
| Character | Parent | Mean | SDa | Max | Min |
|---|---|---|---|---|---|
| KLEN | 082 | 9.37 | 0.45 | 9.8 | 8.9 |
| Y107 | 7.93 | 0.29 | 8.1 | 7.6 | |
| KWID | 082 | 7.97 | 0.45 | 8.1 | 7.9 |
| Y107 | 8.70 | 0.29 | 9.1 | 8.3 | |
| KTHI | 082 | 5.87 | 0.35 | 6.2 | 5.5 |
| Y107 | 6.63 | 0.25 | 6.9 | 6.4 | |
| KWEI | 082 | 39.60 | 0.35 | 40.1 | 38.9 |
| Y107 | 50.07 | 0.25 | 51.1 | 49 |
aSD: standard deviation
Table 2.
Phenotypic expression and variance analysis of traits in the F2:3 population in three environments
| Traita | Environment | F2:3 families | Variance component | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | SDb | Skewness | Kurtosis | Gc | G × Ed | Ee | H2 | ||
| KLEN | E1 | 5.17 | 10.80 | 0.85 | − 0.46 | 1.02 | 2.210 | 0.010 | 0.019 | 0.89 |
| E2 | 3.62 | 10.86 | 0.74 | − 0.23 | − 0.28 | |||||
| E3 | 4.39 | 10.84 | 0.76 | − 0.32 | 0.34 | |||||
| KWID | E1 | 2.52 | 8.14 | 0.48 | 0.06 | 0.03 | 0.883 | 0.003 | 0.001 | 0.90 |
| E2 | 2.57 | 8.15 | 0.51 | 0.14 | − 0.11 | |||||
| E3 | 2.46 | 8.14 | 0.47 | − 0.04 | − 0.05 | |||||
| KTHI | E1 | 2.20 | 4.26 | 0.41 | 0.87 | 0.76 | 0.481 | 0.005 | 0.026 | 0.81 |
| E2 | 1.80 | 4.32 | 0.35 | 0.74 | 0.55 | |||||
| E3 | 1.99 | 4.29 | 0.36 | 0.84 | 0.91 | |||||
| KWEI | E1 | 40.09 | 42.15 | 8.12 | 0.11 | − 0.30 | 195.856 | 0.871 | 5.454 | 0.87 |
| E2 | 34.23 | 41.18 | 6.86 | 0.40 | 0.14 | |||||
| E3 | 36.93 | 41.63 | 7.30 | 0.27 | − 0.06 | |||||
aKLEN: 10-kernel length, KWID: 10-kernel width, KTHI: 10-kernel thickness, KWEI: 200-kernel weight, E1: Beibei 2011, E2: Hechuan 2011, E3: Beibei 2012
bSD: standard deviation
cG: genotype
dG × E: genotype × environment
eE: environment
Fig. 1.
Violin plots of phenotypic data for four kernel-related traits in the F2:3 populations in three low-phosphorus environments. (KLEN: 10-kernel length, KWID: 10-kernel width, KTHI: 10-kernel thickness, KWEI: 200-kernel weight, PH: plant height(m), LA: Leaf area; E1: Beibei, 2011; E2: Hechuan, 2011; E3: Beibei, 2012)
Correlation analysis of kernel-related traits
In all three environments, the kernel length, kernel width, and 200-kernel weight showed significant positive correlations, with kernel length and 200-kernel weight showing the highest correlation (0.39 to 0.53). Kernel length and kernel thickness, on the other hand, showed a significant negative correlation (P < 0.01). Kernel width and kernel thickness showed a significant positive correlation in E2 (P < 0.05), and there was also a slight positive correlation between kernel width and 200-kernel weight in E1 and E3 (0.48 to 0.54, P < 0.01). Kernel thickness and 200-kernel weight showed a significant positive correlation in E2 and E3 but only a weak positive correlation in E1 (Table 3).
Table 3.
Correlation analysis of KLEN, KWID, KTHI, and KWEI in the F2:3 population in three environments
| Environment | KLEN | KWID | KTHI | KWEI | |
|---|---|---|---|---|---|
| KLEN | E1 | 1 | |||
| E2 | 1 | ||||
| E3 | 1 | ||||
| KWID | E1 | 0.268** | 1 | ||
| E2 | 0.237* | 1 | |||
| E3 | 0.283** | 1 | |||
| KTHI | E1 | − 0.298** | 0.147 | 1 | |
| E2 | − 0.281** | 0.221* | 1 | ||
| E3 | − 0.284** | 0.120 | 1 | ||
| KWEI | E1 | 0.393** | 0.485** | 0.108 | 1 |
| E2 | 0.538** | 0.529** | 0.309** | 1 | |
| E3 | 0.490** | 0.540** | 0.228* | 1 |
KLEN: 10-kernel length, KWID: 10-kernel width, KTHI: 10-kernel thickness, KWEI: 200-kernel weight, E1: Beibei 2011, E2: Hechuan 2011, E3: Beibei 2012
*P < 0.05, **P < 0.01
Linkage map construction
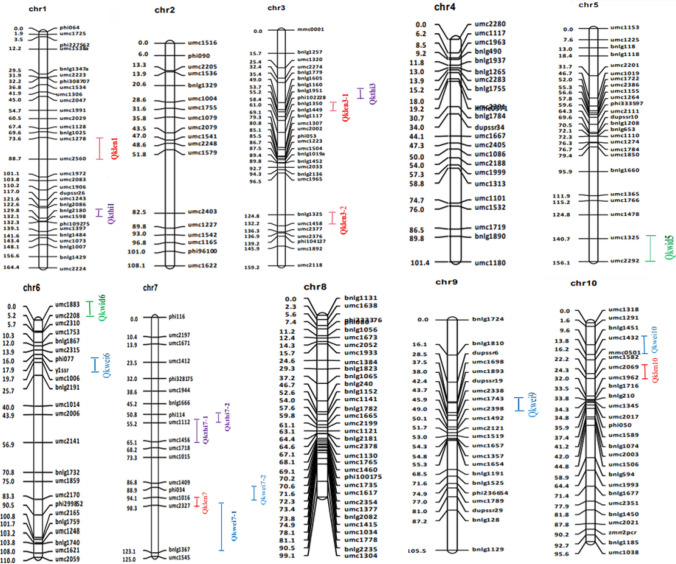
The genotypes of F2:3 populations were analyzed by 249 polymorphic SSR markers. Among them, parental 082 and Ye107 genes accounted for 45% and 55% of the population genetic composition, respectively, which was in line with the expected ratio of 1:1. The results indicated that the population was random and could be used to construct a genetic map. The total length of the F2:3 population map was 1224.521 cM, and the average distance between markers was 4.92 cM (Fig. 2).
Fig. 2.
Distribution of the QTL for all traits on the linkage map of the F2:3 population. The line segments indicate marker intervals of the QTL, and nodes of the line segments indicate positions of the flanking markers
QTL mapping of kernel-related traits in the F2:3 population
QTL analysis in all environments revealed a total of 27 QTL for the four kernel-related traits, which were distributed on chromosomes 1, 3, 5, 6, 7, 9, and 10. The LOD values of the QTL ranged from 2.5 to 5.3, explaining 8.11% to 18.02% of the phenotypic variation (Table 4).
Table 4.
Kernel trait QTLs identified in the F2:3 population by single-environment analysis
| Environment | Trait | QTL | Position | Flanking markers | LOD | PVEa (%) | Addb | Domc | Gene actiond |
|---|---|---|---|---|---|---|---|---|---|
| KLEN | |||||||||
| E1 | Qklen1 | 77 | umc1278-umc2560 | 2.64 | 9.26 | 0.387 | − 0.042 | A | |
| Qklen3-1 | 61 | bnlg1350-bnlg1449 | 3.65 | 11.56 | 0.417 | 0.227 | PD | ||
| Qklen3-2 | 132 | bnlg1325-umc1458 | 2.89 | 9.25 | 0.385 | 0.082 | PD | ||
| E2 | Qklen3-1 | 62 | bnlg1350-bnlg1449 | 2.90 | 11.36 | 0.371 | 0.120 | PD | |
| E3 | Qklen3-1 | 62 | bnlg1350-bnlg1449 | 3.26 | 11.53 | 0.393 | 0.101 | PD | |
| Qklen3-2 | 132 | bnlg1325-umc1458 | 2.75 | 9.38 | -0.345 | 0.021 | A | ||
| Qklen7 | 95 | umc1016-umc2327 | 2.53 | 8.30 | 0.289 | − 0.254 | D | ||
| Qklen10 | 32 | umc2069-umc1962 | 2.81 | 8.53 | 0.223 | 0.337 | OD | ||
| KWID | |||||||||
| E1 | Qkwid5 | 146 | umc1325-umc2292 | 2.73 | 11.27 | 0.227 | 0.143 | PD | |
| Qkwid6 | 1 | umc1883-umc2208 | 3.98 | 15.62 | − 0.239 | − 0.180 | PD | ||
| E2 | Qkwid5 | 143 | umc1325-umc2292 | 2.62 | 10.20 | 0.251 | 0.124 | PD | |
| Qkwid6 | 1 | umc1883-umc2208 | 3.58 | 14.25 | − 0.240 | − 0.189 | PD | ||
| E3 | Qkwid5 | 149 | umc1325-umc2292 | 2.83 | 11.96 | 0.219 | 0.159 | PD | |
| Qkwid6 | 0 | umc1883-umc2208 | 5.32 | 18.02 | − 0.254 | − 0.188 | PD | ||
| KTHI | |||||||||
| E1 | Qkthi3 | 56 | bnlg1951-phi102228 | 2.50 | 10.03 | − 0.193 | − 0.061 | PD | |
| E2 | Qkthi3 | 57 | bnlg1951-phi102228 | 3.43 | 13.16 | − 0.186 | − 0.069 | PD | |
| Qkthi7-1 | 59 | umc1112-umc1456 | 2.77 | 11.93 | − 0.152 | 0.123 | D | ||
| E3 | Qkthi1 | 130 | bnlg2180-umc1598 | 2.68 | 8.90 | − 0.105 | 0.157 | OD | |
| Qkthi3 | 57 | bnlg1951-phi102228 | 3.43 | 13.19 | − 0.189 | − 0.069 | PD | ||
| Qkthi7-2 | 51 | phi114-umc1112 | 2.88 | 9.59 | − 0.152 | − 0.103 | PD | ||
| KWEI | |||||||||
| E1 | Qkwei6 | 16 | phi077-y1ssr | 2.60 | 8.41 | − 2.893 | − 2.426 | D | |
| Qkwei9 | 46 | umc1743-umc2398 | 3.42 | 11.58 | − 4.084 | − 3.277 | PD | ||
| Qkwei10 | 16 | umc1432-mmc0501 | 2.85 | 9.54 | 0.747 | − 4.858 | OD | ||
| E2 | Qkwei7-1 | 107 | umc2327-bnlg1367 | 2.96 | 13.56 | 3.613 | − 1.633 | PD | |
| Qkwei9 | 46 | umc1743-umc2398 | 2.95 | 9.32 | − 3.354 | − 1.877 | PD | ||
| E3 | Qkwei7-2 | 94 | phi034-umc1016 | 2.61 | 8.11 | 3.227 | − 1.204 | PD | |
| Qkwei9 | 46 | umc1743-umc2398 | 3.18 | 10.16 | − 3.586 | − 2.450 | PD | ||
aPVE (%): percentage of phenotypic variance explained by the QTL
bAdd: additive effect of the QTL. Positive values indicate that the alleles responsible for increasing trait values were contributed by the 082 genotype, whereas negative values indicate that the alleles responsible for increasing trait values were contributed by Ye107 genotype
cDom: dominance effect of the QTL
dGene action: Gene mode of action
QTL mapping of kernel length
Eight QTL for kernel length were found on chromosomes 1, 3, 7, and 10. One of the QTL, Qklen3-1, was expressed in three environments and was located between markers bnlg1350 and bnlg1449 on chromosome 3. The LOD values of this QTL were 3.65, 2.90, and 3.26, and the contribution rates to the phenotype were 11.56%, 11.36%, and 11.53% in E1, E2, and E3, respectively. The modes of gene action were all partially dominant, and the synergistic genes all came from the female parent 082(Table 4).
QTL mapping of kernel width
Under the three environmental conditions, six kernel-width QTL were found on chromosomes 5 and 6. Qkwid5, located on chromosome 5, was delineated by markers umc1325 and umc2292. This QTL was expressed in all three environments with LOD values of 2.73, 2.62, 2.83 and contribution rates of 11.27%, 10.20%, and 11.96% in E1, E2, and E3, respectively (Table 4). The modes of gene action were all partially dominant, and the synergistic genes all came from the female parent 082. Qkwid6 was also detected in all three environments, and it was located between markers umc1883 and umc2208 on chromosome 6. Its LOD values were 3.98, 3.58, and 5.32, with phenotype contributions of 15.62%, 14.25%, and 18.02% in E1, E2, and E3, respectively (Table 4). The modes of gene action were all partially dominant, and the synergistic genes all came from the male parent Ye107.
QTL mapping of kernel thickness
Six kernel thickness QTL were detected on chromosomes 1, 3, and 7. Among them, Qkthi3 was expressed in all three environments; it was located between markers bnlg1951 and phi102228 on chromosome 3 and had LOD values of 2.50, 3.43, and 3.43 in E1, E2, and E3, respectively. The contribution rates to the phenotype were 10.03%, 13.16%, and 13.19% (Table 4). The modes of gene action were all partially dominant, and the synergistic genes all came from the male parent Ye107. Qkthi7-1 was located on chromosome 7 and was only detected in one environment, but its phenotypic contribution rate was 11.93%.
QTL mapping of 200-kernel weight
Under all environmental conditions, seven QTL for 200-kernel weight were found on chromosomes 6, 7, 9, and 10. Among them, Qkwei9 was located on chromosome 9 and was expressed in all three environments; it was located between umc1743 and umc2398, and its LOD values were 3.42, 2.95, and 3.18, with contribution rates of 11.58%, 9.32%, and 10.16% in E1, E2, and E3, respectively (Table 4). The modes of gene action were all partially dominant, and the synergistic genes all came from the male parent Ye107. Qkwei7-1, located on chromosome 7, also affected 200-kernel weight and had the highest phenotypic contribution rate (13.56%) among the detected QTL.
Joint analysis of QTL for kernel-related traits in the F2:3 population in all environments
A total of 63 QTL for the four traits were detected from all environments combined (Table 5). Some QTL had high reproducibility with different QTL detection methods. For example, Qkwid6, Qkthi3, and Qklen3-2 were detected in the same marker interval in 3, 3, and 2 single-environment analyses and in the joint analysis. Some QTL such as Qkwei7-1, which had a high contribution rate in the single-environment QTL analysis, were also detected in the same marker interval in the joint analysis. Qklen3-1, Qklen7, and Qkwei6 were detected in all three single-environment analyses, and JAQklen3-1, JAQklen7, and JAQkwei6 were detected in the joint analysis (bnlg1350, umc2327, phi077). Some QTL were also detected in the joint analysis but not in single-environment analyses. This may have been due to their small effect size, and such QTL may only be detected using larger populations.
Table 5.
Joint analysis of QTLs for kernel-related traits in the F2:3 populations in all environments
| Trait | Chra | QTL | Position | LeftMarker | RightMarker | LODb (A) | LODc (A by E) | PVEd | Adde |
|---|---|---|---|---|---|---|---|---|---|
| KLEN | 1 | JAQklen1-1 | 0 | phi064 | umc1725 | 2.528 | 0.008 | 1.27 | − 0.087 |
| 1 | JAQklen1-2 | 292 | umc1991 | umc2029 | 2.844 | 0.015 | 1.56 | 0.097 | |
| 1 | JAQklen1-3 | 418 | umc1128 | bnlg1025 | 3.052 | 0.006 | 1.55 | 0.097 | |
| 1 | JAQklen1-4 | 958 | umc2083 | umc1906 | 2.698 | 0.058 | 1.82 | 0.103 | |
| 1 | JAQklen1-5 | 2612 | bnlg1429 | umc2224 | 3.033 | 0.032 | 1.58 | 0.097 | |
| 2 | JAQklen2 | 340 | umc2248 | umc1579 | 3.147 | 0.021 | 1.64 | 0.098 | |
| 3 | JAQklen3-1 | 386 | phi102228 | bnlg1350 | 3.714 | 0.123 | 2.01 | 0.105 | |
| 3 | JAQklen3-3 | 535 | bnlg1117 | umc1307 | 2.556 | 0.267 | 1.55 | 0.088 | |
| 3 | JAQklen3-4 | 1134 | bnlg1019a | bnlg1452 | 7.576 | 0.278 | 5.16 | 0.180 | |
| 3 | JAQklen3-2 | 1548 | bnlg1325 | umc1458 | 4.290 | 0.520 | 2.18 | − 0.116 | |
| 3 | JAQklen3-2 | 1806 | umc1458 | umc2377 | 5.671 | 0.086 | 4.49 | − 0.166 | |
| 3 | JAQklen3-5 | 1944 | umc2377 | umc2376 | 5.571 | 0.255 | 4.24 | − 0.162 | |
| 5 | JAQklen5-1 | 39 | bnlg118 | bnlg1118 | 3.617 | 0.054 | 1.74 | 0.104 | |
| 5 | JAQklen5-2 | 824 | umc1274 | umc1784 | 5.117 | 0.114 | 2.48 | − 0.124 | |
| 6 | JAQklen6 | 0 | umc1883 | umc2208 | 2.829 | 0.053 | 1.37 | − 0.092 | |
| 7 | JAQklen7-1 | 403 | umc1718 | umc1015 | 3.023 | 0.010 | 1.48 | 0.096 | |
| 7 | JAQklen7 | 845 | umc2327 | bnlg1367 | 2.528 | 0.011 | 1.28 | 0.088 | |
| 9 | JAQklen9-1 | 111 | umc1698 | umc1893 | 4.542 | 0.269 | 3.32 | − 0.143 | |
| 9 | JAQklen9-2 | 206 | dupssr19 | umc2338 | 3.660 | 0.109 | 1.77 | − 0.105 | |
| 10 | JAQklen10-1 | 329 | phi050 | umc1589 | 7.670 | 0.200 | 3.85 | 0.154 | |
| KWID | 1 | JAQkwid1-1 | 61 | bnlg1347a | umc2223 | 2.499 | 0.013 | 2.16 | 0.071 |
| 1 | JAQkwid1-2 | 235 | umc2047 | umc1991 | 2.985 | 0.004 | 1.61 | 0.062 | |
| 1 | JAQkwid1-3 | 2612 | bnlg1429 | umc2224 | 2.742 | 0.031 | 1.59 | 0.061 | |
| 2 | JAQkwid2 | 911 | phi96100 | umc1622 | 3.557 | 0.120 | 2.07 | 0.068 | |
| 3 | JAQkwid3 | 1816 | umc2377 | umc2376 | 5.615 | 0.073 | 2.98 | − 0.084 | |
| 5 | JAQkwid5-1 | 584 | dupssr10 | bnlg1208 | 2.938 | 0.005 | 3.62 | − 0.093 | |
| 6 | JAQkwid6 | 0 | umc1883 | umc2208 | 4.794 | 0.006 | 2.54 | − 0.078 | |
| 6 | JAQkwid6-1 | 47 | bnlg1867 | umc2315 | 5.600 | 0.015 | 2.97 | − 0.084 | |
| 6 | JAQkwid6-2 | 101 | umc1006 | bnlg2191 | 4.230 | 0.011 | 2.29 | − 0.073 | |
| 6 | JAQkwid6-3 | 496 | umc1859 | umc2170 | 3.341 | 0.010 | 1.82 | 0.066 | |
| 6 | JAQkwid6-4 | 789 | umc2165 | bnlg1759 | 3.456 | 0.047 | 1.88 | 0.067 | |
| 8 | JAQkwid8-1 | 12 | phi233376 | phi080 | 4.623 | 0.010 | 2.70 | − 0.080 | |
| 8 | JAQkwid8-2 | 105 | umc1384 | bnlg1823 | 6.138 | 0.031 | 5.00 | − 0.109 | |
| 8 | JAQkwid8-3 | 1391 | bnlg2082 | umc1415 | 2.976 | 0.004 | 2.03 | 0.069 | |
| 9 | JAQkwid9 | 145 | umc1893 | dupssr19 | 3.147 | 0.009 | 3.33 | − 0.089 | |
| KTHI | 1 | JAQkthi1-1 | 7 | phi227562 | umc1538a | 2.685 | 0.032 | 1.54 | 0.046 |
| 1 | JAQkthi1-2 | 561 | umc1278 | umc2560 | 2.598 | 0.055 | 1.39 | 0.044 | |
| 3 | JAQkthi3-1 | 74 | umc2274 | bnlg1779 | 3.378 | 0.304 | 1.86 | − 0.051 | |
| 3 | JAQkthi3 | 287 | bnlg1951 | phi102228 | 5.114 | 0.113 | 4.22 | − 0.077 | |
| 3 | JAQkthi3-2 | 2092 | phi104127 | umc1892 | 2.913 | 0.019 | 1.54 | 0.047 | |
| 5 | JAQkthi5-1 | 38 | bnlg118 | bnlg1118 | 3.348 | 0.011 | 1.86 | − 0.051 | |
| 5 | JAQkthi5-2 | 281 | umc2386 | umc1155 | 2.971 | 0.161 | 1.75 | 0.047 | |
| 6 | JAQkthi6 | 254 | umc2006 | umc2141 | 3.586 | 0.033 | 2.86 | − 0.064 | |
| 7 | JAQkthi7 | 403 | umc1718 | umc1015 | 5.153 | 0.013 | 2.72 | − 0.062 | |
| 8 | JAQkthi8 | 160 | bnlg1065 | bnlg240 | 2.709 | 0.018 | 1.44 | − 0.045 | |
| 9 | JAQkthi9 | 380 | umc1492 | umc2121 | 3.040 | 0.027 | 2.45 | 0.058 | |
| 10 | JAQkthi10-1 | 120 | umc1962 | bnlg1716 | 5.230 | 0.056 | 2.76 | − 0.062 | |
| 10 | JAQkthi10-2 | 272 | umc2017 | phi050 | 3.047 | 0.060 | 2.20 | − 0.056 | |
| KWEI | 1 | JAQkwei6-1 | 750 | umc2560 | umc1972 | 3.944 | 0.045 | 2.04 | − 1.063 |
| 1 | JAQkwei6-2 | 1203 | dupssr26 | umc1243 | 4.257 | 0.266 | 2.20 | − 1.091 | |
| 1 | JAQkwei6-3 | 1859 | umc1397 | bnlg1484 | 2.737 | 0.219 | 1.47 | − 0.880 | |
| 3 | JAQkwei3 | 1816 | umc2377 | umc2376 | 4.988 | 0.037 | 2.51 | − 1.184 | |
| 5 | JAQkwei5-1 | 0 | umc1153 | umc1225 | 3.705 | 0.024 | 1.88 | 1.020 | |
| 5 | JAQkwei5-2 | 549 | dupssr10 | bnlg1208 | 2.833 | 0.008 | 2.56 | − 1.186 | |
| 5 | JAQkwei5-3 | 824 | umc1274 | umc1784 | 2.936 | 0.023 | 1.51 | − 0.916 | |
| 6 | JAQkwei6 | 52 | umc2315 | phi077 | 3.447 | 0.013 | 2.08 | − 1.073 | |
| 7 | JAQkwei7-1 | 957 | umc2327 | bnlg1367 | 2.569 | 0.083 | 2.16 | 1.092 | |
| 8 | JAQkwei8-1 | 115 | umc1384 | bnlg1823 | 2.550 | 0.007 | 1.69 | − 0.966 | |
| 8 | JAQkwei8-2 | 1394 | bnlg2082 | umc1415 | 2.654 | 0.027 | 1.42 | 0.882 | |
| 9 | JAQkwei9-1 | 159 | umc1893 | dupssr19 | 7.243 | 0.045 | 4.44 | − 1.549 | |
| 9 | JAQkwei9-2 | 678 | umc1654 | bnlg1191 | 3.653 | 0.118 | 3.14 | − 1.313 | |
| 10 | JAQkwei10-1 | 734 | umc2351 | bnlg1450 | 2.597 | 0.125 | 1.70 | − 0.946 | |
| 10 | JAQkwei10-2 | 987 | umc2021 | zmrr2pcr | 4.193 | 0.002 | 2.47 | − 1.167 |
aChr: chromosome
bLOD (A): LOD score for additive and dominant effects
cLOD (A by E): LOD score for additive and dominant by environment effects
dPVE (%): percentage of phenotypic variance explained by the QTL
eAdd: additive effect represents the average of the three additive effects of the QTL in each of the three environments
Discussion
Maize traits such as kernel length, kernel width, kernel thickness, and 200-kernel weight have high heritability and can easily be phenotyped, enabling examination of their underlying causes by QTL mapping (Yang et al. 2016; Liu et al. 2020a, b). However, in addition to QTL for kernel traits, we have previously detected QTL for phosphorus absorption capacity (PC), plant height (PH), plant shoot dry weight (PW), root dry weight (RW), leaf age (LA), table root dry weight (TW), and root length (RL) under phosphorus stress (Chen 2008). In this study, a maize F2:3 population of 180 families was constructed using the parents 082 and Ye107. In the single-environment analysis, target trait QTL with a contribution rate greater than 10% in at least one environment were considered to be primary QTL. If these QTL were detected in more than two environments, they were considered to be the main-effect QTL, regardless of environment. For example, Qklen3-1 was the main-effect QTL for kernel length, whereas Qkwid5 and Qkwid6 were the main-effect QTL for kernel width. The main-effect QTL for kernel thickness was Qkthi3, and the main-effect QTL for 200-kernel weight was Qkwei9. The detection of these QTL across all environments indicates that they were stably expressed in all low-phosphorus stress environments (Liu et al. 2020a, b). In this study, QTL for different traits were found in similar regions of the genome in the same environment, which probably reflect the phenomenon of " one cause and multiple effects " (Ma and Cao 2021).
In the three low-phosphorus stress environments, kernel width, kernel length, and 200-kernel weight were all relatively stable, and their heritability ranged from 87 to 90%. The heritability of kernel thickness was relatively low, suggesting that the environment had a greater influence on this trait. Similar results were obtained by Peng et al. (2010) and Qin et al. (2015). Correlation analysis revealed that kernel width and 200-kernel weight had the highest correlation, which was significantly higher than that between kernel width and kernel thickness, similar to the results of Wang et al. (2021).
In this study, one, two, one and one QTL of kernel length, kernel width, kernel thickness, and 200-kernel weight were detected in all environments, respectively. Qkwid5 (bin 5.0) on chromosome 5 and Qkwei9 (bin 9.03/9.04, Physical location: 117,367,859–117,454,573) on chromosome 9 were the main QTL controlling kernel width and 200-kernel weight, respectively. These results do not overlap the QTL intervals found by Yang et al. (2016), who detected QTL controlling the 10-kernel length width and 100-kernel weight on chromosomes 5 (bin 5.03) and 9 (bin 9.04, Physical location: 123,538,205–123,542,793). In addition, Lan et al. (2018) also found that the QTL controlling kernel width was located on chromosome 4 (bin 4.06) and chromosome 3 (bin 3.01/3.02). We also found that Qklen3-1(bin 3.06/3.08) and Qkthi3 (bin 3.06), the main effect QTL controlling kernel length and kernel thickness, were stably expressed in all three environments and were simultaneously localized to chromosome 3. These results were different locations from those of Zhou et al. (2020) and Raihan et al. (2016), which detected QTL controlling kernel length (bin 3.04) and kernel thickness (bin 3.05) on chromosome 3. Overall, the major QTL identified in this study were located at different locations on the same chromosome than those previously reported in low-phosphorus environments. Therefore, these novel QTL will help to understand the genetic composition of maize yield and lay the foundation for marker-assisted breeding of maize.
We performed QTL analysis in three low-phosphorus environments to improve the reliability and consistency of kernel-trait QTL detection. We found that main-effect QTL were detected repeatedly in all environments, whether analyzed single or jointly, while other QTL with unstable expression were not detected simultaneously. This is because the main QTL that can be detected in both single and joint environments have large contribution rate and high effect value. Some QTL that were not detected in the joint environment were micro-effect QTL, which were easily affected by environmental factors such as rain, temperature and topography, and could not be detected stably.
Conclusion
QTL were identified for all four maize kernel traits (length, width, thickness, and 200-kernel weight), and these QTL were stable across three different low-phosphorus conditions. A number of them coincided with previously reported QTL detected under different environmental stresses: Qklen3-1, which controls kernel length, Qkwid5 and Qkwid6, which control kernel width, Qkthi3, which controls kernel thickness, and Qkwei9, which controls 200-kernel weight. In addition, some new kernel-trait QTL were also detected. These results, in combination with previous work, provide guidance for the breeding of maize with desirable kernel traits that are stable across multiple different environments.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the Guizhou Provincial Science and Technology Plan Project (Qian Kehe Support [2022] key 026), Science and Technology Program of Guizhou Province (Guizhou Science and Technology Cooperation Foundation-ZK [2021] general 129) and the Scientific Research Fund for Introducing Talents of Guizhou University (Guizhou University Talent Basic Cooperation [2013] No. 27).
Abbreviations
- KLEN
10-Kernel length
- KWID
10-Kernel width
- KTHI
10-Kernel thickness
- KWEI
200-Kernel weight
- QTL
Quantitative trait loci
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bai GH (2014) Methods of kernel traits measurement and QTL mapping and analysis of association. Dissertation, Xinjiang Agricultural University. China
- Chen JY, Cai YL, Xu DL, Lu XG, Chen TQ. Maize genotype differences in phosphorus efficiency and relative biological characteristics and regression modeling analysis. Plant Nutr Fertil Sci. 2007;13(06):1068–1073. [Google Scholar]
- Chen JY (2008) QTL mapping of phosphorus efficiency and P-related traits in maize(Zea mays L.). Dissertation, Southwest University, China
- Ci XK, Li MS, Liang XL, Xie ZJ, Zhang DG, Li XH, Lu ZY, Ru GL, Bai L, Xie CX, Hao ZF, Zhang SH. Genetic contribution to advanced yield for maize hybrids released from 1970 to 2000 in China. Crop Sci. 2011;51(1):13–20. doi: 10.2135/cropsci2010.04.0207. [DOI] [Google Scholar]
- Dai DW, Ma ZY, Song RT. Maize kernel development. Mol Breeding. 2021;41:2. doi: 10.1007/S11032-020-01195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YN, Yang JC, Jiang HM, Zhang JF, Wu QY, Li J. Effect of low phosphorus press on grain p content and characters of different maize genotypes. J Nucl Agric Sci. 2009;23(02):327–333. [Google Scholar]
- Knapp EW, Fischer SF, Zinth W, Sander M, Kaiser W, Deisenhofer J, Michel H. Analysis of optical spectra from single crystals of Rhodopseudomonas viridis reaction centers. Proc Natl Acad Sci. 1985;82(24):8463–8467. doi: 10.1073/pnas.82.24.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan TR, He KH, Chang LG, Cui TT, Zhao ZX, Xue JQ, Liu JC. QTL mapping and genetic analysis for maize kernel size and weight in multi-environments. Euphytica. 2018;214(7):1–12. doi: 10.1007/s10681-018-2189-0. [DOI] [Google Scholar]
- Li Y, Wang TY, Shi YS, Song YC. Applications of genomics approaches in studies on maize germplasm. J Plant Genetic Resour. 2003;03:256–260. doi: 10.13430/j.cnki.jpgr.2003.03.016. [DOI] [Google Scholar]
- Li M, Guo XH, Zhang M, Wang XP, Zhang GD, Tian YC, Wang ZL. Mapping QTLs for grain yield and yield components under high and low phosphorus treatments in maize ( Zea mays L.) Plant Sci. 2010;178(5):454–462. doi: 10.1016/j.plantsci.2010.02.019. [DOI] [Google Scholar]
- Li M (2010) QTL Analysis for Grain Yield and its Components to Phosphorus Deficiency Tolerance in Maize. Dissertation, Shandong Agricultural University. China
- Liu Y, Wang LW, Sun CL, Zhang ZX, Zheng YL, Qiu FZ. Genetic analysis and major QTL detection for maize kernel size and weight in multi-environments. Theor Appl Genet. 2014;127(5):1019–1037. doi: 10.1007/s00122-014-2276-0. [DOI] [PubMed] [Google Scholar]
- Liu M, Tan XL, Yang Y, Liu P, Zhang XX, Zhang YC, Wang L, Hu Y, Ma LL, Li ZL, Zhang YL, Zou CY, Lin HJ, Gao SB, Lee M, Lübberstedt T, Pan GT, Shen YO. Analysis of the genetic architecture of maize kernel size traits by combined linkage and association mapping. Plant Biotechnol J. 2020;18(1):207–221. doi: 10.1111/pbi.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YH, Yi Q, Hou XB, Hu YF, Li YP, Yu GW, Liu HM, Zhang JJ, Huang YB. Identification of quantitative trait loci for kernel-related traits and the heterosis for these traits in maize (Zea mays L.) Mol Genet Genomics. 2020;295(1):121–133. doi: 10.1007/s00438-019-01608-1. [DOI] [PubMed] [Google Scholar]
- Ma J, Cao YY. Genetic dissection of grain yield of maize and yield-related traits through association mapping and genomic prediction. Front Plant Sci. 2021;12:690059–690059. doi: 10.3389/fpls.2021.690059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu ZS (2018) Genetic dissection of major QTL associated with kenel width and kernel weight in maize. Dissertation, Chinese Academy of Agricultural Sciences
- Nikolić A, Anđelković V, Dodig D, Mladenović-Drinić S, Kravić N, Ignjatović-Micić D. Identification of QTL-s for drought tolerance in maize, II: yield and yield components. Genetika. 2013;45(2):341–350. doi: 10.2298/GENSR1302341N. [DOI] [Google Scholar]
- Peleg Z, Fahima T, Krugman T, Abbo S, Yakir D, Korol AB, Saranga Y. Genomic dissection of drought resistance in durum wheat x wild emmer wheat recombinant inbreed line population. Plant Cell Environ. 2009;32(7):758–779. doi: 10.1111/j.1365-3040.2009.01956.x. [DOI] [PubMed] [Google Scholar]
- Peng B, Wang Y, Li YX, Liu C, Liu ZZ, Wang D, Tan WW, Zhang Y, Sun BC, Shi YS, Song YC, Wang TY, Li Y. QTL analysis for yield components and kernel-related traits in maize under different water regimes. Acta Agron Sin. 2010;36(11):1832–1842. doi: 10.3724/SP.J.1006.2010.01832. [DOI] [Google Scholar]
- Qin WW, Li YX, Li CH, Chen L, Wu X, Bai N, Shi YS, Song YC, Zhang DF, Wang TY, Li Y. QTL mapping for kernel related traits based on a high-density genetic map. Acta Agron Sin. 2015;41(10):1510–1518. doi: 10.3724/SP.J.1006.2015.01510. [DOI] [Google Scholar]
- Qiu HB (2013) Mapping of quantitative trait loci for acid phosphatase activity in maize. Dissertation Southwest University, China
- Raihan MS, Liu J, Huang J. Multi-environment QTL analysis of grain morphology traits and fine mapping of a kernel-width QTL in Zheng58 × SK maize population. Theor Appl Genet. 2016;129(8):1465–1477. doi: 10.1007/s00122-016-2717-z. [DOI] [PubMed] [Google Scholar]
- Ray DK, Mueller ND, West PC, Foley JA. Yield Trends are insufficient to double global crop production by 2050. PLoS ONE. 2013;8(6):e66428. doi: 10.1371/journal.pone.0066428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZY, Su SZ, Zhang SZ, Liu HL, Luo BW, Liu D, Wu L, Rong TZ, Gao SB. Characterization and QTL mapping of yield trait under two phosphorus regimes in maize. Acta Agric Boreali Sinica. 2015;30(01):9–14. [Google Scholar]
- Rex B. Molecular markers and selection for complex traits in plants: learning from the last 20 years. Crop Sci. 2008;48(5):1649–1664. doi: 10.2135/cropsci2008.03.0131. [DOI] [Google Scholar]
- Ribaut J-M, Jiang C, Gonzalez-de-Leon D, Edmeades GO, Hoisington DA. Identification of quantitative trait loci under drought conditions in tropical maize.2. Yield components and marker-assisted selection strategies. Theor Appl Genet. 1997;94(6–7):887–896. doi: 10.1007/s001220050492. [DOI] [PubMed] [Google Scholar]
- Stuber CW, Edwards M, Wendel J. Molecular marker-facilitated investigations of quantitative trait loci in maize. II. Factors influencing yield and its component traits. Crop Sci. 1987;27:639–648. doi: 10.2135/cropsci1987.0011183x002700040006x. [DOI] [Google Scholar]
- Tuberosa R, Sanguineti MC, Landi P, Salvi S, Casarini E, Conti S. RFLP mapping of quantitative trait loci controlling abscisic acid concentration in leaves of drought-stressed maize (Zea mays L) Theor Appl Genet. 1998;97(5–6):744–755. doi: 10.1007/s001220050951. [DOI] [Google Scholar]
- Venkatesan S. Control of seed size in plants. Proc Natl Acad Sci USA. 2005;102(50):17887–17888. doi: 10.1073/pnas.0509021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BT, Wu JY, Ding JQ, Xi ZY. Map integration of QTLs for grain yield and lts related traits in maize. Acta Agron Sin. 2009;35(10):1836–1843. doi: 10.3724/SP.J.1006.2009.01836. [DOI] [Google Scholar]
- Wang XT, Li BY, Yang Q, Dai ZJ, Hao JJ. QTLs Mapping for traits related with kernel in maize. J Henan Agric Sci. 2021;50(9):9–15. doi: 10.15933/j.cnki.1004-3268.2021.09.002. [DOI] [Google Scholar]
- Yang C, Zhang L, Jia AM, Rong TZ. Identification of QTL for maize grain yield and kernel-related traits. J Genet. 2016;95(2):239–247. doi: 10.1007/s12041-016-0628-z. [DOI] [PubMed] [Google Scholar]
- Zhang G, Zhu LY, Chen JT. Consensus map of the QTLs relevant to yield traits of maize and the development of functional candidate gene. Acta Agric Boreali Sinica. 2008;23(06):20–27. [Google Scholar]
- Zhou ZP, Li GL, Tan SY, Li DD, Thea MW, Wang XF, Chen SJ, Tobias W, Liu WX. A QTL atlas for grain yield and its component traits in maize ( Zea mays ) Plant Breed. 2020;139(3):562–574. doi: 10.1111/pbr.12809. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.